- 1BioTechnology Institute, University of Minnesota – Twin Cities, Saint Paul, MN, USA
- 2Department of Microbiology, University of Minnesota – Twin Cities, Minneapolis, MN, USA
Reaching a depth of 713 m below the surface, the Soudan Underground Iron Mine (Soudan, MN, USA) transects a massive Archaean (2.7 Ga) banded iron formation, providing a remarkably accessible window into the terrestrial deep biosphere. Despite organic carbon limitation, metal-reducing microbial communities are present in potentially ancient anoxic brines continuously emanating from exploratory boreholes on Level 27. Using graphite electrodes deposited in situ as bait, we electrochemically enriched and isolated a novel halophilic iron-reducing Deltaproteobacterium, ‘Desulfuromonas soudanensis’ strain WTL, from an acetate-fed three-electrode bioreactor poised at +0.24 V (vs. standard hydrogen electrode). Cyclic voltammetry revealed that ‘D. soudanensis’ releases electrons at redox potentials approximately 100 mV more positive than the model freshwater surface isolate Geobacter sulfurreducens, suggesting that its extracellular respiration is tuned for higher potential electron acceptors. ‘D. soudanensis’ contains a 3,958,620-bp circular genome, assembled to completion using single-molecule real-time (SMRT) sequencing reads, which encodes a complete TCA cycle, 38 putative multiheme c-type cytochromes, one of which contains 69 heme-binding motifs, and a LuxI/LuxR quorum sensing cassette that produces an unidentified N-acyl homoserine lactone. Another cytochrome is predicted to lie within a putative prophage, suggesting that horizontal gene transfer plays a role in respiratory flexibility among metal reducers. Isolation of ‘D. soudanensis’ underscores the utility of electrode-based approaches for enriching rare metal reducers from a wide range of habitats.
Introduction
Microbial anaerobic respiration via metal reduction is a ubiquitous biogeochemical process in anoxic sediments and subsurface environments. Metal reducers are found in several Bacterial and Archaeal phyla, and are often recognizable by their genomic arsenal of redox proteins such as multiheme c-type cytochromes, that are implicated in transferring respiratory electrons across insulating biological membranes and cell walls to extracellular metal oxide particles. Many metal-reducing bacteria can also grow as biofilms using poised electrodes as terminal electron acceptors, a phenotype similar to the recently described phenomenon of syntrophic interspecies electron transfer (Rotaru et al., 2015). Because electrodes act as unlimited electron sinks, they are powerful tools for enrichment of microorganisms capable of respiring extracellular acceptors. Mixed communities and pure cultures reducing electrodes have been obtained from habitats as diverse as wastewater sludge (Fu et al., 2013), freshwater and marine sediments (Bond et al., 2002; Holmes et al., 2004b; Miceli et al., 2012), soda lakes (Zavarzina et al., 2006), and deep subsurface fluids (Greene et al., 2009). Electrodes have also been successfully used to enrich and isolate microorganisms capable of electrode oxidation (Rowe et al., 2014; Eddie et al., 2016). To our knowledge, in situ electrode-based approaches to obtain deep subsurface bacteria have not been demonstrated.
Along the southern edge of the Canadian shield, the Soudan Underground Mine in northern Minnesota’s Vermilion Range (Figure 1A) transects the Archaean Animikie ocean basin (Ojakangas et al., 2001), with the main mineshaft providing access to 2.7 Ga banded iron deposits. On its lowest level (Level 27; 713 m depth), exploratory boreholes allow access to calcium- and metal-rich deep subsurface brines (73–200 mS/cm) with low oxidation-reduction potentials, circumneutral pH levels, millimolar concentrations of ferrous iron, and dissolved organic carbon concentrations below detection (Edwards et al., 2006). Boreholes at this level represent access a deep subsurface environment free of recent surface water contamination and photosynthetic inputs, unperturbed by volcanic or tectonic activity. While metagenomic sequencing provides useful data regarding organism and well-characterized pathway abundance in such remote environments, genomic information alone cannot yet predict the phenotype or metabolic strategy of metal-reducing bacteria.
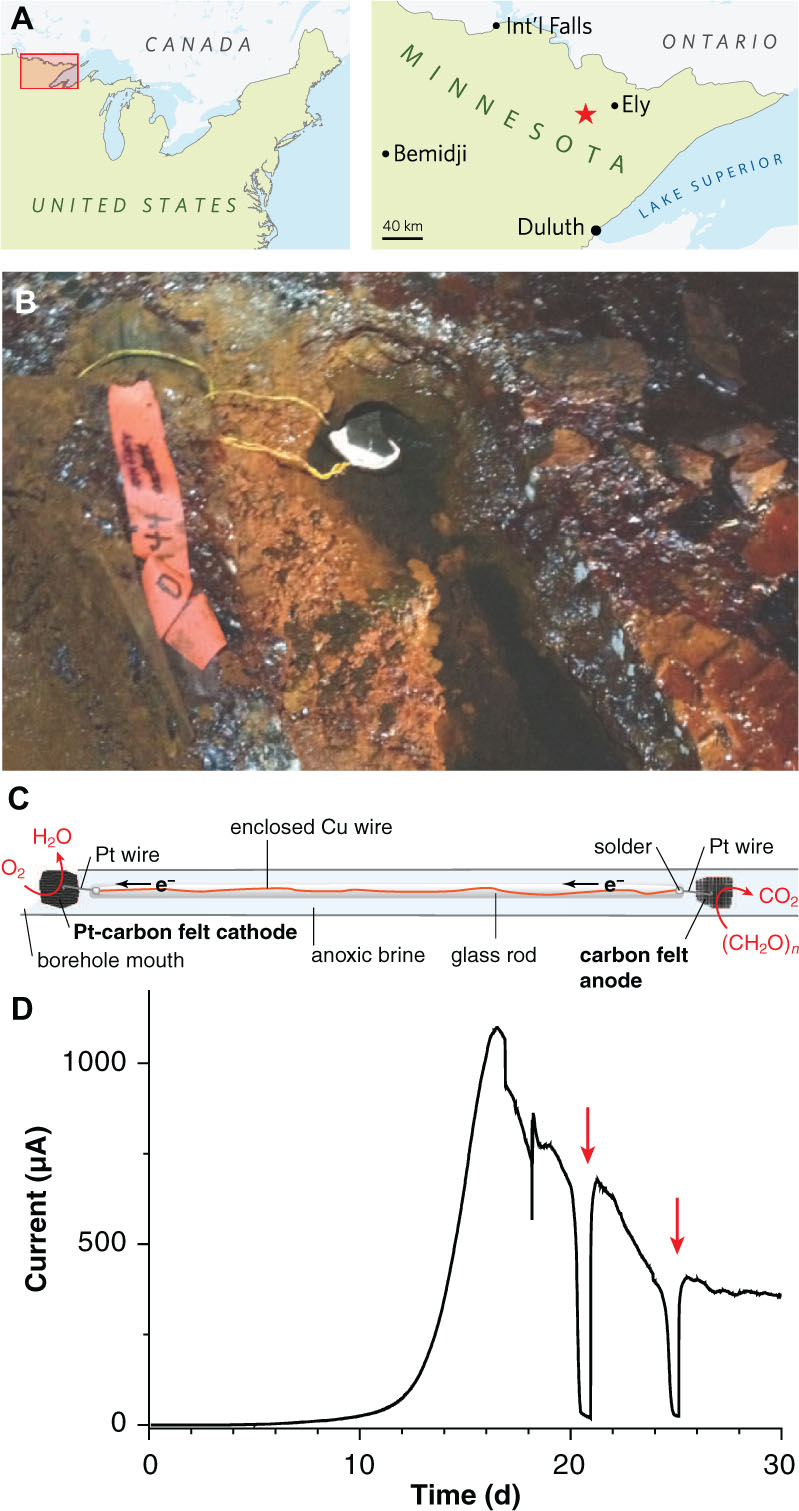
FIGURE 1. Electrodes for enriching novel metal reducers from the terrestrial deep subsurface. (A) Map showing the location of the Soudan Underground Iron Mine within Minnesota’s Vermilion Range, approximately 30 km WSW of the town of Ely. (B) Photograph of diamond drill hole (DDH) 944 located along the west tunnel of Level 27 (713 m depth below the surface). A white precipitate is visible coating the platinized carbon cathode. (C) Schematic diagram of the electrode apparatus used for in situ enrichment. (D) Chronoamperometry of a potentiostatically controlled laboratory bioreactor (12 cm2 total anode surface area) inoculated with the anode from the in situ enrichment. Red arrows indicate addition of acetate following substrate depletion.
As part of a larger effort to understand the diversity of metal-reducing bacteria, and obtain isolates capable of bioprocessing in high-ionic strength reactors, here we describe isolation and initial phenotypic characterization of a new subsurface isolate ‘Desulfuromonas soudanensis’ WTL, enriched by in situ electrodes placed directly in a Soudan Mine borehole. Measurements of Fe(III) reduction and electron transfer rates indicate that this isolate is adapted for coupling oxidation of organic matter to reduction of higher-potential electron acceptors than surface isolates. Its complete circular chromosome is limited in cytochrome abundance compared to most described metal-reducing Deltaproteobacteria from freshwater surface sites or aquifers, and at least one of these cytochromes is embedded within a putative prophage, suggesting that virus-mediated horizontal gene transfer contributes to respiratory flexibility in the environment. The ‘D. soudanensis’ genome encodes LuxI/LuxR quorum-sensing circuitry, and medium from ‘D. soudanensis’ cultures activates an N-acyl-L-homoserine lactone (AHL)-sensing indicator strain (Zhu et al., 2003). These findings underscore the effectiveness of microbial electrochemistry as an alternative to classical enrichment approaches to obtain rare organisms from the deep terrestrial subsurface for reference genome sequencing and physiological characterization.
Materials and Methods
Media and Culture Conditions
Soudan Mine medium (SM-1X) used for enrichment and isolation contained the following (per liter of deionized water): CaCl2 ⋅ 2H2O, 22.1 g; MgCl2 ⋅ 6H2O, 15.3 g; NaCl, 15.8 g; MgSO4 ⋅ 7H2O, 0.01 g; NH4Cl, 1.0 g; KH2PO4, 0.05 g; sodium acetate, 1.64 g; NaHCO3, 1.8 g; non-chelated trace minerals (Marsili et al., 2008), 10 ml; Wolfe’s vitamins, 10 ml. SM-0.5X medium, used for characterization and routine growth of the pure culture, contained half the concentration of chloride salts. When fumarate was the electron acceptor, fumaric acid was added to 40 mM final concentration and titrated to pH 6.0–6.1 with 50% (w/v) NaOH before adding other ingredients. When SM-0.5X medium was used for electrode-based growth of pure cultures, it contained 50 mM additional NaCl in lieu of fumarate. Fe(III)-reducing cultures contained either ferric citrate (55 mM), poorly crystalline iron(III) oxide (∼100 mM; Levar et al., 2014), or schwertmannite (∼20 mM). Schwertmannite was prepared by addition of 5.2 ml 30% H2O2 to 1 L of 10 g/l FeSO4 ⋅ 7H2O and stirred overnight, and was collected by centrifugation followed by 3 rinses with deionized water. In all cases, Soudan Mine medium was prepared containing all ingredients except chloride salts and bicarbonate, brought to 0.7 times the final volume (i.e., 700 ml for 1 L), adjusted to pH 6.8 before adding NaHCO3, bubbled with oxygen-free 80:20 N2:CO2 and autoclaved in butyl rubber-stoppered tubes or serum bottles. A separate salt solution containing 73.5 g CaCl2 ⋅ 2H2O, 50.8 g MgCl2 ⋅ 6H2O, and 52.6 g NaCl per liter was bubbled with Ar and autoclaved separately. Upon cooling, this salt solution was aseptically and anaerobically added to basal SM medium to achieve the desired final volume.
In Situ Electrode Enrichment
A ∼40-cm length of Cu wire was threaded into glass tubing, and soldered to Pt wire near the end of the hollow tube. The glass was fused shut at either end, leaving a short length of Pt wire exposed so that electrodes could be attached to each end and so that the whole device could be autoclaved and incubated in the mine without any copper wire exposure (Figure 1C). One Pt lead was connected to a ∼25-cm2 piece of graphite felt as the anode; the other was attached to an equal area of platinized carbon cloth (BASF Fuel Cell Co., Somerset, NJ, USA) as the cathode. To remove impurities, electrodes were soaked sequentially in 70% EtOH, 1 N HCl, and 1 N NaOH, with diH2O rinses in between prior to autoclaving. The sterile rod containing the anode was slid gently down diamond drill hole (DDH) 944, located along the West Drift of Level 27 of the Soudan Underground Mine (Soudan, MN, USA; 47°49′24″ N, 92°14′14″ W) and held in place such that the cathode was exposed to the oxic opening of the borehole. After 2 months of incubation in situ, the anode was retrieved and small sections used to inoculate laboratory electrode enrichments as described below. Samples of DDH 944 borehole water were also brought directly to the laboratory and used to inoculate reactors containing poised electrodes. However, out of eight attempts (two separate sampling events), no laboratory reactors produced current above background levels after up to 60 days of incubation.
Electrochemical Methods
Anode sections from in situ mine enrichments were transferred to 100-ml glass cylindrical electrochemical cells (BASi, West Lafayette, IN, USA) containing SM-1X electrode medium and four separate planar graphite electrodes (12 cm2 total surface area), a Pt counter electrode, and an Ag/AgCl reference electrode as previously described (Marsili et al., 2008). Electrodes were poised at +0.24 V vs. standard hydrogen electrode (SHE) with a potentiostat (Bio-Logic, Knoxville, TN, USA) and bioreactors were incubated in a 20°C circulating water bath in the dark under constant magnetic stirring and flushing with humidified 80:20 N2:CO2 scrubbed free of O2. Cyclic voltammograms (CVs) were collected at 1 mV/s scan rate with the second of two scans reported. CVs were compared to those of G. sulfurreducens (Chan et al., 2015) grown at 30°C as previously described (Marsili et al., 2008).
Isolation and Cultivation
Enrichments were serially diluted in SM-1X medium containing 20 mM acetate and 100 mM Fe(III)-oxide, and the highest dilutions yielding Fe(II)-production at 24°C in the dark under an 80:20 N2:CO2 atmosphere were diluted further in 40 mM fumarate-containing medium. High dilutions (10-6) were streaked for isolation with fumarate as the electron acceptor in an anaerobic glovebag (Coy Laboratory Products, Grass Lake, MI, USA) on vitamin-free SM-0.5X medium solidified with 0.9% (w/v) Bacto agar (Difco) amended with 0.5 mM cysteine, and incubated under a 75:20:5 N2:CO2:H2 atmosphere in an anaerobic jar (Almore International, Beaverton, OR, USA) containing a basket of Pt catalyst recharge pellets (Microbiology International, Frederick, MD, USA). After 3 weeks of incubation at 24°C, isolated pink colonies were picked into 0.5 ml SM-0.5X medium containing 0.5 mM cysteine and subsequently transferred into 10-ml culture tubes. Possible contamination was assessed by fluorescence microscopy of DAPI-stained cells and by plating aerobically on either unbuffered SM agar or LB agar and anaerobically (75:20:5 N2:CO2:H2) on LB agar buffered with 1.8 g/l NaHCO3. SM-0.5X medium with 20 mM acetate and 40 mM fumarate was used for routine cultivation at 24°C. Growth with fumarate was determined by increase in OD600 and Fe(III) reduction was measured by the FerroZine assay. Axenic cultures were stored at -80°C in 10% (v/v) dimethyl sulfoxide, and a clonal population of revived ‘D. soudanensis’ cells used to generate the genomic sequence was deposited in the German Collection of Microorganisms and Cell Cultures (DSMZ) as strain 101009. Because this enrichment survived unplanned oxygen exposures and temperature changes due to power outages, the isolate was dubbed strain WTL for “will to live.”
Electron Donor Utilization
‘D. soudanensis’ cells grown with fumarate were inoculated (1:2 inoculum, as cells reached an OD600 of ∼0.25) into magnetically stirred conical bioreactors containing a single graphite electrode poised at +0.24 V at 24°C as described previously (Marsili et al., 2008). To remove residual acetate, the potentiostat was paused and spent growth medium replaced twice with donor-free medium under a constant stream of 80:20 N2:CO2. Electrodes were then re-poised at +0.24 V for at least 30 min to obtain a steady background current in the absence of donors. The following electron donors were tested individually to 5 mM final concentration unless noted: lactate, ethanol, methanol, formate, glycerol, pyruvate, citrate, succinate, propionate, butyrate, glucose (2 mM), and benzoate (0.25 mM). If a substrate yielded an increase above background current, after 2 h medium was replaced twice again before testing another substrate; otherwise, up to three substrates were added cumulatively before 5 mM acetate was added to re-establish current production.
Genome Sequencing, Assembly, and Annotation
Thirty micrograms high MW genomic DNA (gDNA) was pooled from duplicate 10-ml stationary phase fumarate-grown ‘D. soudanensis’ cultures using a DNeasy Blood & Tissue kit (Qiagen). gDNA was quantified with Qubit (Life Technologies) and prepared for PacBio sequencing using standard 10-kb insert protocols. SMRTbellTM templates were size-selected at a 7-kbp cutoff using Blue Pippin electrophoresis (Sage Science). Long sequencing reads were collected from a total of 8 SMRT cells (P4-C2 chemistry, 120-min movies) on a PacBio RS II instrument (Mayo Clinic, Rochester, MN, USA) yielding 1.7 Gbp of raw data (mean read length 4,663 bp; N50 6,613 bp). Assembly was performed with HGAP v.3 (Chin et al., 2013) in SMRT Analysis v. 2.2 with a 10-kb minimum subread length to provide ∼100× coverage. Unambiguous circularity of the resulting ∼4-Mbp contig was confirmed via dot plot (Gepard v. 1.30) and self-complementary ends were manually trimmed. A crude initial annotation was generated with Prokka v. 1.10 (Seemann, 2014) to locate a single copy of dnaA upstream of several DnaA boxes predicted with Ori-Finder (Gao and Zhang, 2008). The manually reoriented contig was then polished using the entire PacBio dataset (∼427 × coverage) to QV > 50 before a final polishing step with 100 × coverage of 250-bp paired-end Illumina reads using breseq v. 0.26 (Deatherage and Barrick, 2014) followed by Pilon v. 1.10 (Walker et al., 2014). The final 3,958,620-bp assembly was uploaded for automated annotation via the IMG/ER pipeline1. Putative multiheme c-type cytochromes (≥3 Cxx(x)CH motifs) were identified with a Python script2, and Demerec gene abbreviations were assigned based on bi-directional best hits between ‘D. soudanensis’ and Geobacter sulfurreducens PCA (GenBank accession no. NC_002939.5) as identified by GET_HOMOLOGUES (2015-05-29 release; Contreras-Moreira and Vinuesa, 2013) at 30% identity and 90% query coverage. The manually curated IMG annotation was then submitted to GenBank (accession numbers provided below) to ensure consistency of genetic features across public databases.
Phylogenomic Classification and Genomic Analyses
FASTA nucleotide sequences for all publicly available Desulfuromonas, Desulfuromusa, Geoalkalibacter, Geobacter, Geopsychrobacter, and Pelobacter genomes (26 total) were downloaded from NCBI or IMG/ER and analyzed with PhyloSift v. 1.0.1 (Darling et al., 2014). A concatenated alignment of 40 conserved single-copy marker genes was used to generate an unrooted maximum likelihood tree in FigTree v. 1.4.0 with Desulfovibrio vulgaris Hildenborough as the outgroup.
Community Analysis
Community gDNA harvested from unenriched Soudan brine (DDH 944), and a scraped section of electrode-enriched biofilms was isolated using a PowerWater Kit (Mo Bio, Carlsbad, CA, USA). 16S rRNA genes were PCR amplified with phased V3–V4 primers as described previously (Bartram et al., 2011) and sequenced with 150-bp paired-end Illumina reads on a HiSeq 2000 (>22 M reads/sample). Raw reads were quality trimmed, filtered, merged, aligned, clustered at 97% identity, and taxonomically assigned using mothur (Schloss et al., 2009) against the SILVA release 115 reference database. In addition, merged reads were mapped to the full-length 16S rRNA gene of ‘D. soudanensis’ using bowtie2 v. 2.2.6 and mapped read counts were extracted from the resulting SAM files.
Nucleotide Accession Numbers
Nucleotide sequence and annotation are available from GenBank (accession no. CP010802) and IMG/ER (GOLD analysis project Ga0069009). Raw reads and base modification data have been uploaded to the NCBI Sequence Read Archive under BioProject PRJNA272946.
Results
Electrodes Enrich Rare Taxa from Subsurface Brine
To enrich potential metal reducers within Soudan Mine boreholes, sterile carbon cloth anodes were placed in the anoxic zone and connected to platinized cathodes in the oxic zone ∼40 cm above, near the mouth of the borehole (Figures 1B,C). After 2 months of in situ incubation, 1 cm2 anode subsamples were transferred under anaerobic conditions to laboratory reactors containing acetate and poised graphite electrodes (+0.24 V, 20°C). Within 16 days, an exponential increase in anodic current, doubling every ∼1 day was observed, reaching a maximum of 92 μA/cm2 (Figure 1D). Acetate addition after starvation periods immediately rescued current production (Figure 1D, red arrows), and transfer of scraped biofilm material from this initial enrichment to new reactors resulted in a similar growth pattern.
Amplicons from bacterial V3–V4 16S rRNA regions were generated to compare DNA recovered from the original borehole fluids with electrode-enriched communities. While Deltaproteobacteria sequences were among the least abundant lineages (<0.2%) in native borehole (DDH 944) brine, these sequences increased ∼100-fold during laboratory electrode enrichments (20–22% relative abundance). In contrast, heterotrophic Marinobacter linages were equally abundant in both native and enriched samples (Bonis and Gralnick, 2015). With successive transfers, a Desulfuromonas sequence present at < 0.02% relative abundance in the original anoxic fluid samples grew to comprise nearly 100% of the electrode Deltaproteobacteria. Other lineages linked to metal reduction, such as Acidobacteria (Mehta-Kolte and Bond, 2012) and the Peptococcaceae family of Firmicutes (Wrighton et al., 2011) were also present in brines, but they were not enriched. Inoculating borehole water collected on two separate sampling events directly into laboratory reactors did not produce any current-producing enrichments (two separate field sampling events, n = 8 reactors).
Pure Cultures of ‘D. soudanensis’ Grow on Electrodes and Reduce Fe(III)
An isolate was obtained from electrode enrichments by first diluting in Fe(III)-oxide medium, then plating using fumarate as the electron acceptor, and was named ‘Desulfuromonas soudanensis’ strain WTL, though molecular and phenotypic characterizations (described below) are not sufficient for formal recognition of a species designation. This strain demonstrated 96% full-length 16S rRNA gene sequence identity to Desulfuromonas sp. WB3 (enriched from a subsurface aquifer using As(V) as the electron acceptor; Osborne et al., 2015), D. carbonis (enriched from a coal bed gas well using Fe(III); An and Picardal, 2015), and D. michiganensis (enriched from river sediment using tetrachloroethene; Sung et al., 2003) (GenBank accession nos. KM452745.1, KJ776405.2, and NR_114607.1, respectively). Reads mapping with 100% identity to the 16S rRNA gene of the isolated ‘D. soudanensis’ were present in the original DDH 944 brine DNA samples, albeit at extremely low relative abundance. While original enrichments were conducted at 20°C and at a salinity reflecting borehole conditions (∼70 mS/cm conductivity), ‘D. soudanensis’ grew more rapidly at 24°C and at 50% lower salt concentrations. These more favorable conditions were used for all subsequent characterization.
The maximum doubling time of ‘D. soudanensis’ pure cultures growing on poised electrodes (+0.24 V vs. SHE) was 13.2 ± 0.05 h (n = 5; Figure 2A). Based on current densities (58 ± 18 μA/cm2; n = 6), and attached protein levels (52 ± 11 μg/cm2; n = 5), the specific respiration rate of ‘D. soudanensis’ was calculated to be 1.03 μA/μg protein (±0.06; n = 5). This current:protein ratio, which was significantly lower than the 2–4 μA/μg ratios reported for G. sulfurreducens at various growth stages, agreed with the relatively slow doubling time of ‘D. soudanensis’. In addition, even with excess electron donor, biofilms reached a plateau at protein levels that suggested an inability to form multilayer biofilms on the electrode surface (10–20 μm thick biofilms typically correspond to attached protein of ∼500 μg/cm2; Marsili et al., 2008).
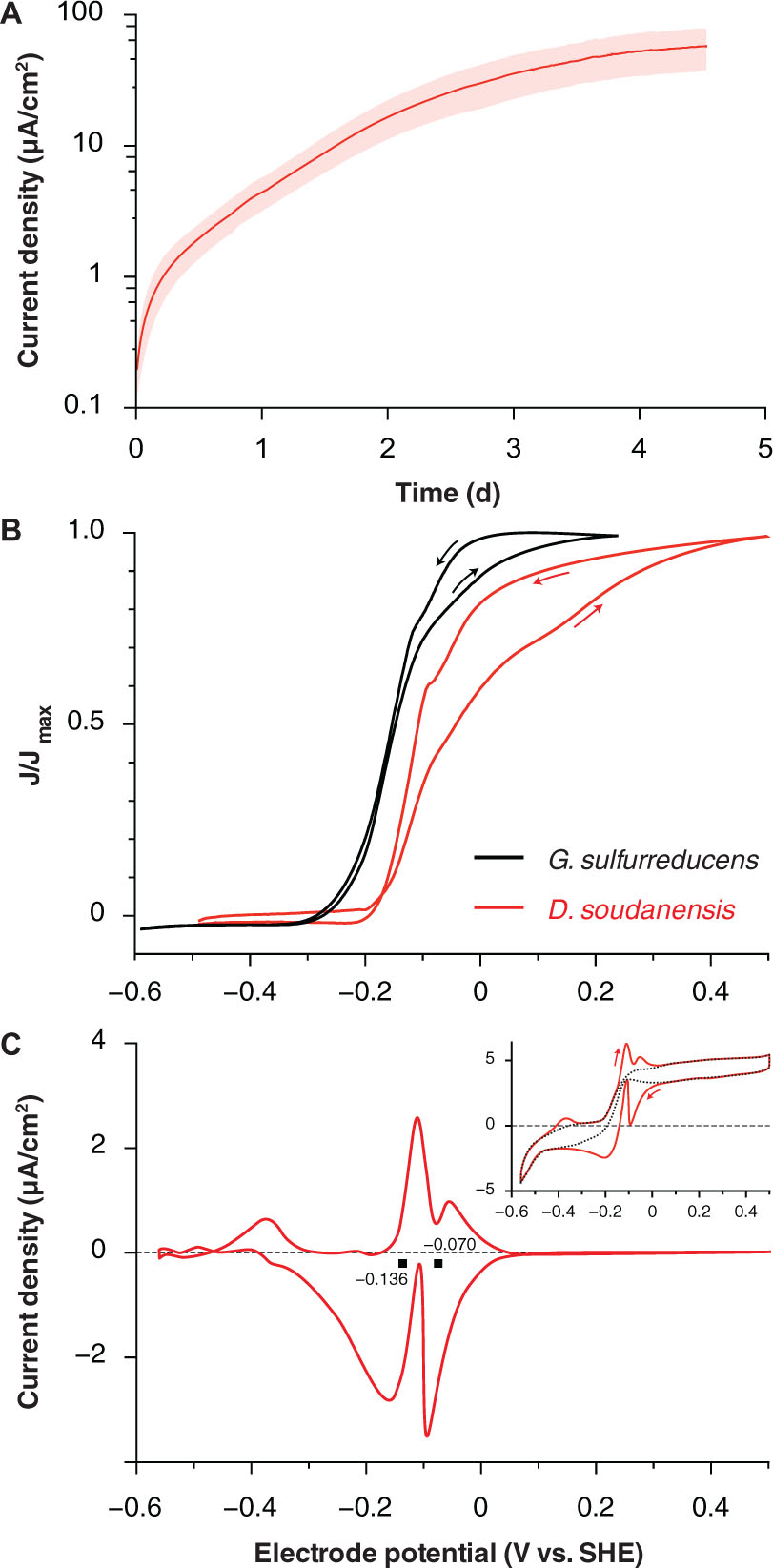
FIGURE 2. Electrochemical characterization of pure cultures of ‘Desulfuromonas soudanensis’ WTL. (A) Chronoamperometry of potentiostatically controlled acetate-fed 10-ml bioreactors (+0.24 V vs. SHE) containing a single 3 cm2 graphite electrode. All electrode experiments were inoculated 1:2 with late log phase cultures pre-grown under electron acceptor limitation. The red trace and pink area show the mean and standard deviation, respectively, of six independent biological replicates. Estimates of doubling time were calculated against the linear portion of the curve from 0.7 to 2 d. (B) Comparison of low scan rate (1 mV/s) cyclic voltammograms of Geobacter sulfurreducens (Chan et al., 2015; black trace) and ‘D. soudanensis’ WTL (red trace). Current density on the y-axis is normalized against its maximum value for either organism. (C) Baseline subtracted single-turnover cyclic voltammogram of an established ‘D. soudanensis’ biofilm starved of acetate. Black squares denote the midpoint potentials of the two redox processes observed, and the inset shows the raw data (red trace) and polynomial function (black trace) used for baseline subtraction.
Cyclic voltammetry of ‘D. soudanensis’ biofilms revealed a ∼100-mV more positive shift in the potential triggering current flow from bacteria, as well as the midpoint, and saturation potentials of electron flux compared to G. sulfurreducens (Figure 2B). Single-turnover cyclic voltammetry resolved two major redox processes centered at -0.136 and -0.070 V vs. SHE (Figure 2C), similar to other Geobacter biofilms where reversible oxidation/reduction midpoint potentials are slightly more negative than catalytic turnover voltammetry (Marsili et al., 2010; Strycharz-Glaven and Tender, 2012).
Electrochemical data showing a shift in the catalytic wave toward more positive redox potential suggested that ‘D. soudanensis’ may be adapted to harvest energy by coupling oxidation of organic electron donors to reduction of extracellular acceptors with higher redox potentials than those typically encountered by other bacteria previously characterized on electrodes. In experiments with insoluble Fe(III)-oxides, Fe(II) accumulation increased exponentially with a doubling time of 12.7 ± 1.1 h (n = 6) when freshly prepared schwertmannite, an acceptor with a predicted midpoint potential near +0.1 V SHE, was provided (Figure 3A; Thamdrup, 2000). Poorly crystalline Fe(III) oxide, predicted to have a redox potential near or below 0 V, supported a slower Fe(II) accumulation rate (15.7 ± 1.0 h, n = 3) (Figure 3B). With soluble acceptors, ‘D. soudanensis’ showed poor Fe(III) reduction in ferric citrate medium, and growth slowed after accumulation of >3 mM Fe(II) (Figure 3C). With fumarate as the electron acceptor, cells grew exponentially, demonstrating a maximum doubling time of 13.3 ± 0.6 h (n = 3) when acetate was the electron donor, and fumarate fermentation was observed in donor-free controls (Figure 3D).
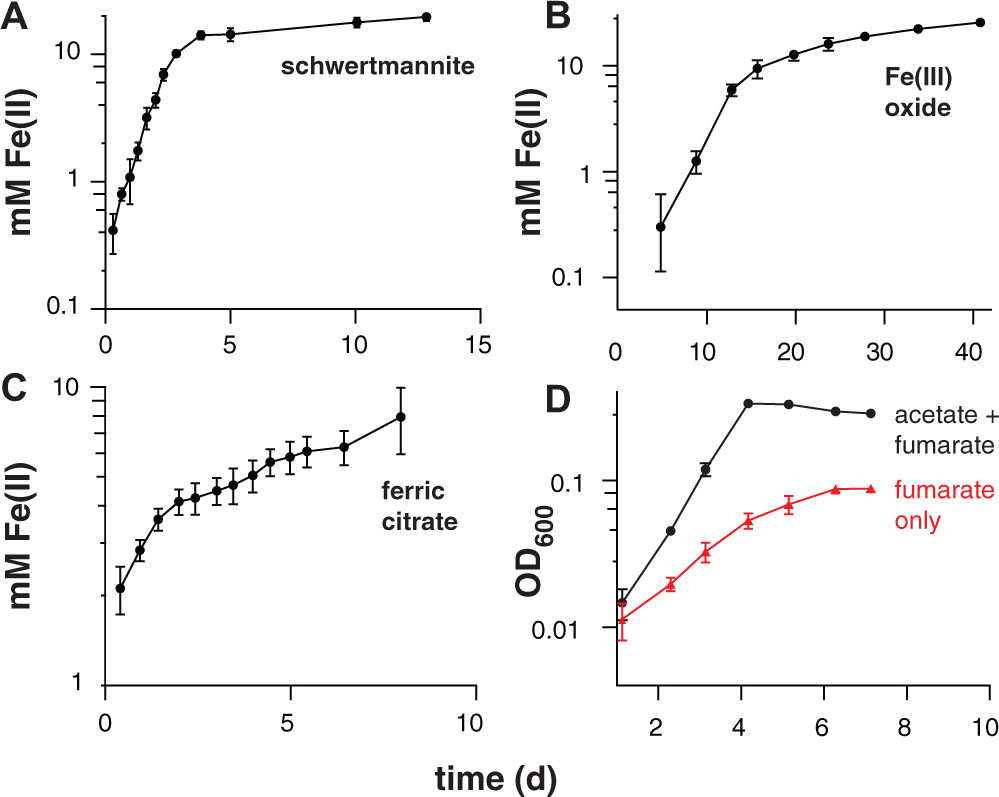
FIGURE 3. Iron(III) reduction and growth of pure cultures of ‘Desulfuromonas soudanensis’ WTL on various electron acceptors with 20 mM acetate as the electron donor. All plots are representative of three independent biological replicates. Accumulation of ferrous iron over time in incubations with either ∼20 mM schwertmannite (A), ∼100 mM iron(III) oxide (B), or 55 mM ferric citrate (C). In all cases Fe(III) reduction slowed once Fe(II) concentrations reached 10 mM regardless of available Fe(III). (D) Growth comparison of ‘D. soudanensis’ cultures grown either with acetate plus 40 mM fumarate (black trace) or fumarate only (red trace).
Genomic Features
‘D. soudanensis’ contains a single 3,958,620-bp circular chromosome (Figure 4A) with similar G+C content (61.19%) to other Desulfuromonas spp., and represents the first complete Desulfuromonas genome. The genome encodes 3,419 protein-coding genes, 46 pseudogenes, 55 tRNAs, and 4 RNA polymerase sigma factors, and includes an exact tandem duplication of the 16S-5S-23S rRNA operon. Genes for assimilatory sulfate reduction, respiratory/dissimilatory nitrate reduction to ammonia, and N2 fixation are present, and ‘D. soudanensis’ appears prototrophic for all amino acids, vitamins, and cofactors. Central metabolism in ‘D. soudanensis’ includes a non-oxidative pentose phosphate pathway and Embden–Meyerhof–Parnas glycolysis/gluconeogenesis, linked by at least two putative pyruvate:ferredoxin oxidoreductases and one pyruvate dehydrogenase to a complete TCA cycle that includes the eukaryotic-like citrate synthase characteristic of other Geobacter and Desulfuromonas strains (Bond et al., 2005).
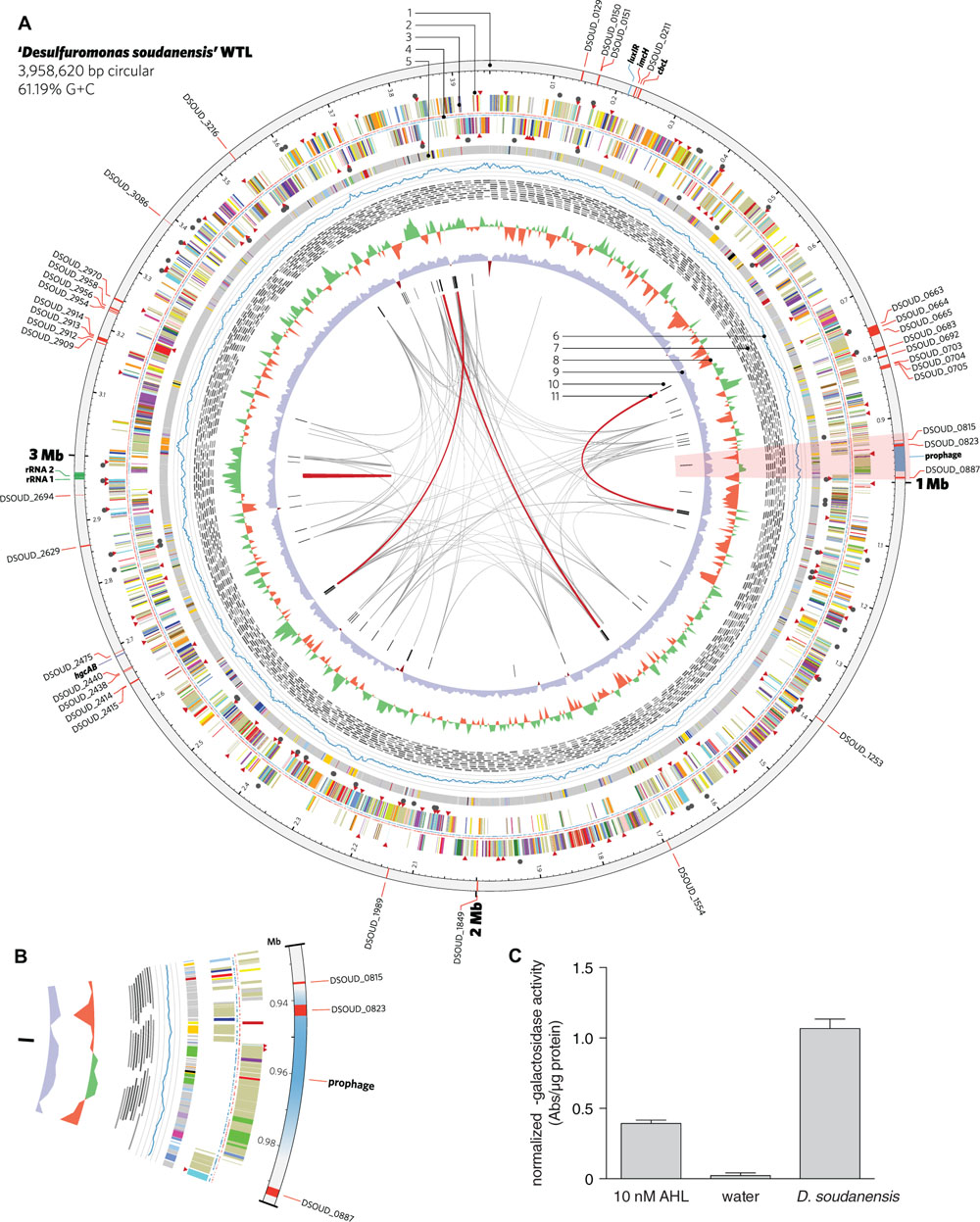
FIGURE 4. Features of the complete genome of ‘Desulfuromonas soudanensis’ WTL. (A) Circular representation of the genome generated in Circos v. 0.64 (Krzywinski et al., 2009). Rings are numbered moving from the outermost inward as follows: 1, location and locus tags of multiheme c-type cytochromes (red), rRNA operon duplication (green), and other features (blue); 2, putative sensor histidine kinases (red triangles) and response regulators (black circles); 3, protein coding sequences colored by COG category; 4, locations of methylated DNA bases identified by PacBio sequencing on the plus (red) and minus (blue) strands; 5, regions of putative horizontal gene transfer as predicted in IMG/ER, where gray is closest BLAST homology to other Deltaproteobacteria; 6, mapped read coverage (range 200–700x); 7, mapping positions of the longest reads in the PacBio dataset; 8, G+C skew in a 5-kbp windows; 9, G+C content (blue >50%; maroon <50%); 10, putative viral protein coding genes; 11, links showing repetitive sequence at 95% identity (gray, > 500 bp; red, >2 kbp). (B) Zoomed region of the area highlighted in pink in (A) showing the location of a putative prophage integrated into the ‘D. soudanensis’ genome. (C) Concentrated ethyl acetate extract of ‘D. soudanensis’ supernatant triggers LacZ activity in an acyl-L-homoserine lactone indicator Agrobacterium tumefaciens strain (Zhu et al., 2003). LacZ activity in this strain is compared to a negative control (water) and to a positive control containing 10 nM authentic N-3-oxohexanoyl-L-homoserine lactone. Specific activity = ΔA420/μg protein.
The ‘D. soudanensis’ genome is dense with chemosensory and regulatory features, including 68 two-component histidine kinases, 97 transcriptional response regulators, 15 methyl-accepting chemotaxis proteins, 27 putative diguanylate cyclases and 16 predicted GEMM cis-regulatory cyclic di-GMP (or cyclic AMP-GMP) riboswitches. Genes linked to growth in a metal-rich environment were also prevalent, such as an hgcAB gene cluster encoding mercury methylation (DSOUD_2468-2469; Parks et al., 2013), a Czc cobalt-zinc-cadmium exporter (DSOUD_2899-2901), CopA-family copper resistance system (DSOUD_1515–1516), and a cluster containing a glutaredoxin-dependent arsenate reductase, arsenite transporter, and an ArsR-family repressor (DSOUD_3330–3333).
A region encoding a LuxI-like acyl homoserine lactone synthase and a LuxR family transcriptional regulator is present in the vicinity of the encoded ImcH and CbcL homologs (Figure 4A), suggesting possible quorum-sensing abilities in this organism. Because production of AHL has never been described for any metal-reducing Desulfuromonas or Geobacter isolate, ‘D. soudanensis’ was cultivated to ∼0.5 OD600, cell-free supernatants were extracted with ethyl acetate, and dried extracts were resuspended in water. Extracts prepared from ‘D. soudanensis’ cultures produced positive results for AHL-like compounds, using an indicator Agrobacterium strain (Zhu et al., 2003). Protein-normalized levels of beta-galactosidase activity resulting from addition of the ‘D. soudanensis’ AHL were similar to positive control containing 10 nM N-3-oxohexanoyl-L-homoserine lactone, while an AHL-free control showed no activity (Figure 4C).
‘D. soudanensis’ Carries a Reduced Repertoire of Multiheme Cytochromes
‘D. soudanensis’ possesses 38 putative multiheme c-type cytochromes (three or more Cxx(x)CH heme-binding motifs), a value about half of what is typically seen in Geobacter strains. Two of these proteins are homologs to inner membrane cytochromes ImcH and CbcL of G. sulfurreducens (DSOUD_0207 and DSOUD_0214; 39% and 71% BLAST identity, respectively), which have been implicated in transfer of electrons out of the quinone pool and into the periplasm (Levar et al., 2014; Zacharoff et al., 2016). Two inner membrane cytochromes were components of a putative NrfH/NrfA nitrite-to-ammonia respiration pathway. While most Geobacter species contain three to five triheme PpcA-like periplasmic cytochromes, only one (DSOUD_3086) was present in ‘D. soudanensis’. Only two outer membrane ‘conduit’ clusters consisting of multiheme cytochromes, lipoprotein cytochromes, and putative β-barrel proteins were identified (DSOUD_0702-0705, DSOUD_2909-2915), comprising seven cytochromes. At least 4 cytochromes were predicted to have extracellular localization, and one of these (DSOUD_0664) contains 69 heme-binding motifs. One-third of the putative multiheme cytochromes identified in ‘D. soudanensis’ had no obvious homologs in available databases, even when a recently obtained genome sequence was included (‘Ca. D. biiwaabikowi’; NCBI BioProject accession PRJNA316855).
Bioinformatic predictions using VirSorter (Roux et al., 2015) and phiSpy (Akhter et al., 2012) identified a region of the ‘D. soudanensis’ genome bearing viral signatures such as a phage-like repressor and integrases, along with sheath, tail, and baseplate proteins. Within this predicted prophage genome, a large number of predicted horizontally transferred genes were present, and these were predicted to encode a triheme cytochrome, an 11-heme predicted lipoprotein cytochrome, and an MtrC family decaheme cytochrome (Figure 4B).
Electrodes Confirm Genomic Predictions for Substrate Utilization
When biofilms were starved and washed free of exogenous acetate, the real-time response to addition of various electron donors could be used as an indicator of the ability of ‘D. soudanensis’ to metabolize that compound. Current increased immediately upon additions of lactate, ethanol, or pyruvate (Figure 5), consistent with genomic predictions for lactate dehydrogenase (DSOUD_0819), alcohol dehydrogenase (DSOUD_1067 and DSOUD_1075), and multiple mechanisms feeding pyruvate into the TCA cycle. H2 injected into the reactor phase also caused an increase in current (data not shown), in agreement with multiple [Ni-Fe] uptake hydrogenases encoded on the genome. In contrast, no electrochemical response was observed for methanol, glycerol, or glucose (Figure 5), agreeing with the lack of genes for activation, catabolism, and/or membrane transport of these substrates. Other substrates, including formate, citrate, succinate, propionate, butyrate, and benzoate were tested but failed to elicit a respiratory response within 30 min.
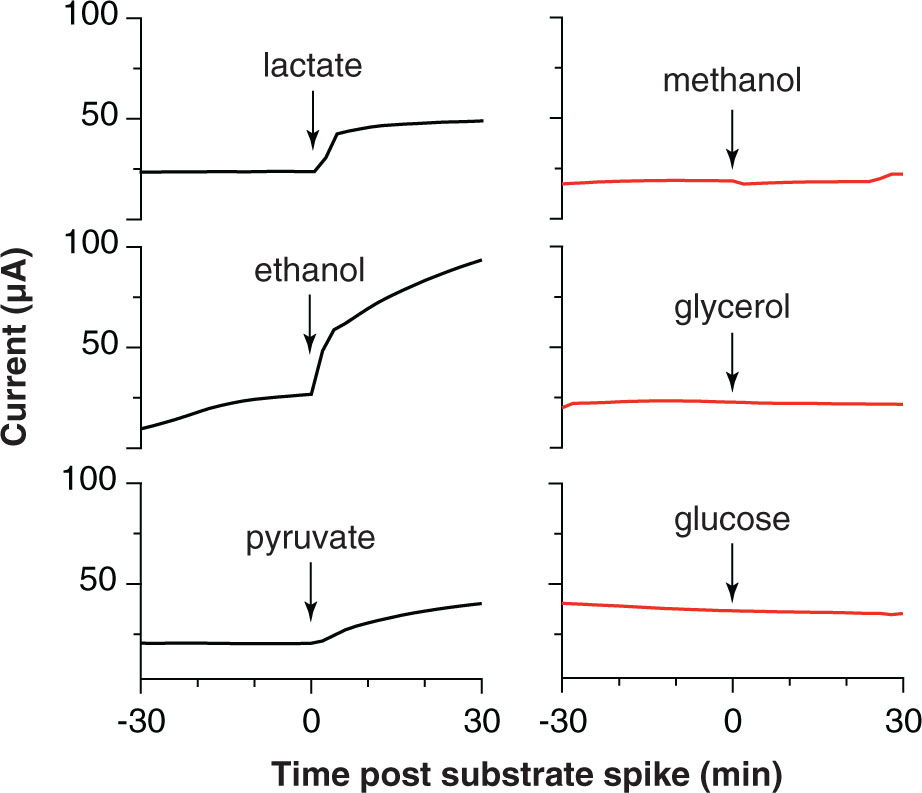
FIGURE 5. Respiratory responses of starved, pre-established ‘Desulfuromonas soudanensis’ WTL biofilms to addition of various electron donors. All compounds were added to a final concentration of 5 mM except glucose (2 mM) into electrode bioreactors that were constantly poised at +0.24 V vs. SHE.
Discussion
By first enriching bacteria using anodes placed directly in anoxic Soudan Iron Mine borehole brine fluids, this study was able to isolate ‘D. soudanensis’ WTL, a new metal- and electrode-respiring bacterium. In contrast, parallel attempts to directly inoculate laboratory reactors with borehole fluid samples consistently failed to obtain positive enrichments. As DNA surveys estimate ‘D. soudanensis’ is present at less than 0.02% of organisms in borehole fluids, it is likely that samples from this ∼103 cell/ml environment contain, at best, a few ‘D. soudanensis’ cells, even if they are concentrated via filtration. The rarity of this organism underscores the value of a selective bottleneck such as that created by an electrode, which provides a consistent electron sink to support multiple doublings and increase cell numbers in situ.
Electrodes also enable real-time measurements of respiration rates in response to environmental or electrochemical perturbations, and this allowed screening a panel of utilizable electron donors (Figure 5) as well as quickly surveying redox potentials supporting extracellular respiration. The higher redox potential preferred by ‘D. soudanensis’ (Figure 2B) is unique from characterized Geobacter strains, and provides evidence that the electron acceptor supporting growth of this organism in its natural environment is different from what supports better-characterized freshwater isolates. ‘D. soudanensis’ does have genes for inner membrane cytochromes (ImcH and CbcL) implicated in setting the redox potential preferences of G. sulfurreducens, raising the question of whether these cytochromes have altered redox potential windows, or if additional adaptations drive this preference (Levar et al., 2014; Zacharoff et al., 2016). Based on the recent observation that growth of thick multilayer biofilms on electrodes is correlated with the ability to form syntrophic associations with other bacteria (such as methanogens; Rotaru et al., 2015), the thin biofilms of ‘D. soudanensis’ suggest a more isolated, or non-syntrophic lifestyle.
Long read DNA sequencing has enabled cost-effective reconstruction of complete, reference-quality microbial genomes (Koren et al., 2013; Koren and Phillippy, 2015), even in this case where ‘D. soudanensis’ possessed a tandem 16S-5S-23S rRNA operon duplication, as well as repetitive transposases, integrases, and recombinases (Figure 4A, internal links). The ‘D. soudanensis’ genome represents the first complete genome from the Desulfuromonas genus, despite the type strain, D. acetoxidans DSM 684, having been first reported 40 years ago (Pfennig and Biebl, 1976). 278 proteins encoded in the ‘D. soudanensis’ genome have no obvious homologs (>30% threshold) in other metal-reducing Deltaproteobacteria, and among the 127 of these proteins with predicted functional annotation, a LuxI family N-acyl homoserine lactone synthase bearing only 24% BLAST identity to a gene in G. uraniireducens was identified. As ‘D. soudanensis’ produces AHL-like compounds in vivo (Figure 4C), this finding suggests a previously undocumented role for quorum sensing in the broader context of Deltaproteobacteria, even in organic carbon-limited subsurface environments, where small aggregates of ‘D. soudanensis’ may need to respond to changes in flow rates or environmental conditions (Connell et al., 2010).
Though ‘D. soudanensis’ only encodes roughly half as many c-type cytochromes as freshwater Geobacter spp., 11 of its 38 cytochromes are still unique among known Deltaproteobacteria. Poor conservation of outer surface cytochromes was recognized in early comparisons of Geobacter genomes (Butler et al., 2010) and remains a pattern among metal-reducing bacteria in general. A new possibility suggested by the ‘D. soudanensis’ genome is that phage-mediated horizontal gene transfer contributes to cytochrome diversity. The closest homolog to the 11-heme cytochrome predicted to lie within a prophage (Figure 4B) is another cytochrome (33% amino acid identity) from Geopsychrobacter electrodophilus, an organism isolated from a marine sediment fuel cell in Jersey City, NJ, USA (Holmes et al., 2004b). When we analyzed all available metal-reducing Deltaproteobacterial genomes for viral signatures, Gps. electrodophilus was the only other genome that contained a cytochrome located within a putative prophage. Remarkably, both the cytochrome and prophage are different than those of ‘D. soudanensis’. Taken together, these findings suggest that viral transfer may accelerate exchange of cytochromes between metal-reducing bacteria. However, until phage particles can be recovered from these organisms or their environment, this remains a speculation.
The taxonomy of metal-reducing Deltaproteobacteria is still being resolved, particularly among isolates recovered from saline environments. While freshwater isolates are consistently named as Geobacter spp., marine isolates are often given genus designations that reflect isolation or enrichment conditions. Holmes et al. (2004a) proposed the family Geobacteraceae to include Geobacter, Desulfuromonas, Desulfuromusa, Pelobacter, and Malonomonas. Kuever et al. (2005; corrig. 2006) described two family names with standing in Bergey’s Manual, originally placing Geobacter, Geoalkalibacter, Geothermobacter, and Geopsychrobacter in the Geobacteraceae family, and assigning all other genera to the Desulfuromonadaceae family, based on D. acetoxidans as the type strain (Pfennig and Biebl, 1976). Phylogenomic analysis supports an early evolutionary divergence between freshwater and marine strains (Figure 6), with 26 sequenced genomes separated into two distinct clades. Notably, this separation also tracks with per-genome cytochrome abundance, where freshwater strains contain much higher multiheme cytochrome content. One hypothesis for lower cytochrome abundance is that it reflects a reduced diversity of extracellular acceptors experienced by Desulfuromonas-like strains in saline or deep habitats, due to increased sulfide levels or lower overall iron availability.
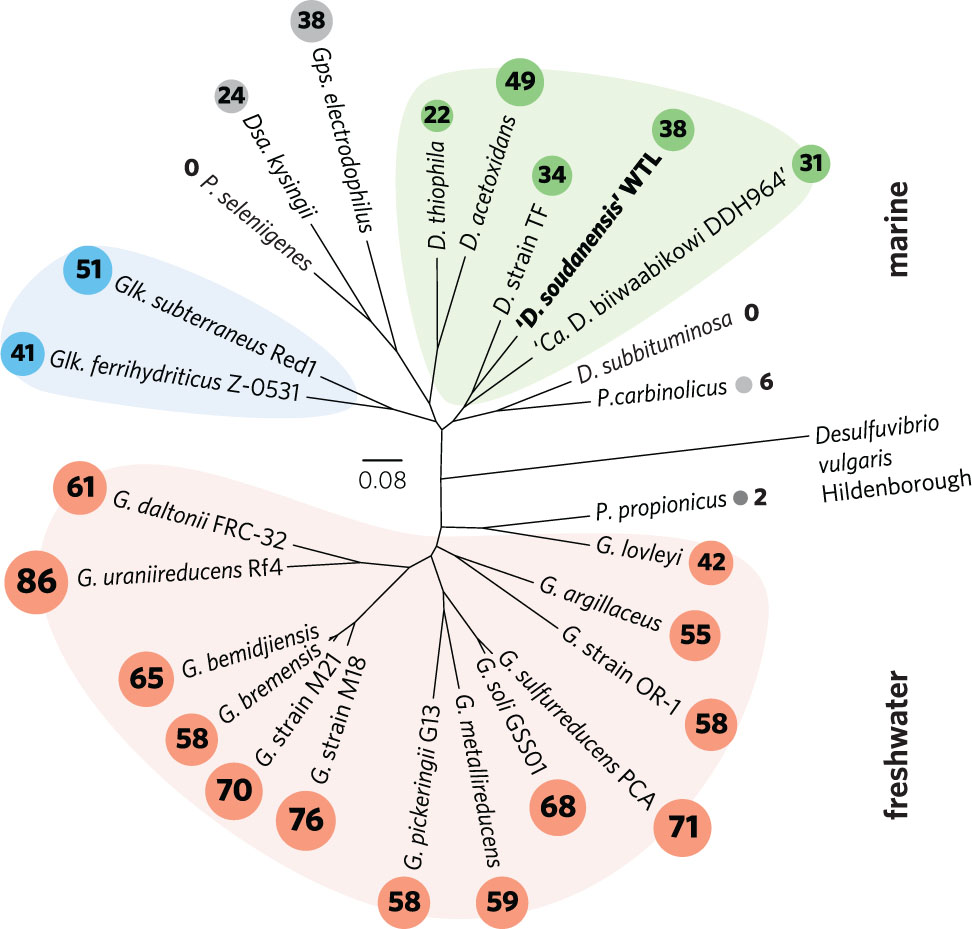
FIGURE 6. Outgroup-rooted phylogenetic tree of Geobacteraceae and Desulfuromonadaceae family members with sequenced genomes. The tree was constructed from an alignment of a concatenated set of 40 conserved single-copy marker genes in PhyloSift. Colored backgrounds are shown to group coherent genera: pink – Geobacter, blue – Geoalkalibacter, and green – Desulfuromonas. ‘D. soudanensis’ WTL is shown in bold. Multiheme c-type cytochrome counts are shown in proportionally sized circles. Genus abbreviations: D, Desulfuromonas; Dsa, Desulfuromusa; G, Geobacter; Glk, Geoalkalibacter; Gps, Geopsychrobacter; P, Pelobacter.
While this initial phenotypic characterization is not sufficient to establish a species designation with official standing, genomic analyses allow us to place ‘D. soudanensis’ WTL in phylogenetic context. Based on whole-genome phylogeny, ‘D. soudanensis’ falls into a separate clade with Desulfuromonas sp. TF (isolated using an electrode as the electron acceptor) and ‘Ca. Desulfuromonas biiwaabikowi DDH964’ (a genome recovered from another Soudan electrode enrichment, NCBI BioProject Accession PRJNA316855). This clade contains bacteria isolated from saline and subsurface sites, but is phylogenetically distinct from the two known halophilic Geoalkalibacter spp. (isolated from an oil well and soda lake) (Zavarzina et al., 2006; Greene et al., 2009). The fact that each new metal-reducing isolate within this class shares a similar core anaerobic physiology, yet contains a highly variable collection of redox and sensory proteins, suggests that niches defined by salinity, pH, and mineralogy are driving divergence within the Geobacter-Desulfuromonas clusters. Continued efforts to recover isolates and genomes from a wide diversity of habitats, using surfaces such as electrodes to target specific abilities, will aid in understanding the extent and origin of this diversity.
Author Contributions
ZS designed the study and performed in situ enrichments. CC performed AHL extractions and activity assays. JG and DB supervised research activities. JB performed isolation, genome sequencing, and electrochemical experiments, and wrote the manuscript. All authors reviewed the manuscript.
Funding
This work was supported by the Minnesota Environment and Natural Resources Trust Fund grant 089-E2. CC was supported by grant N000141210308 from the Office of Naval Research.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Minnesota Department of Natural Resources and Jim Essig, Soudan Mine Underground State Park Manager, for providing logistical support during sampling trips. We thank Vuong Nguyen for performing the AHL LacZ activity assays. We also thank Karl Oles (Mayo Clinic Bioinformatics Core) for performing PacBio sequencing, and the Minnesota Supercomputing Institute for providing high-performance computing resources to support PacBio genome assembly and bioinformatics analyses.
Footnotes
References
Akhter, S., Aziz, R. K., and Edwards, R. A. (2012). PhiSpy: a novel algorithm for finding prophages in bacterial genomes that combines similarity- and composition-based strategies. Nucleic Acids Res. 40:e126. doi: 10.1093/nar/gks406
An, T. T., and Picardal, F. W. (2015). Desulfuromonas carbonis sp. nov., an Fe(III)-, S0- and Mn(IV)-reducing bacterium isolated from an active coalbed methane gas well. Int. J. Syst. Evol. Microbiol. 65, 1686–1693. doi: 10.1099/ijs.0.000159
Bartram, A. K., Lynch, M. D. J., Stearns, J. C., Moreno-Hagelsieb, G., and Neufeld, J. D. (2011). Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end Illumina reads. Appl. Environ. Microbiol. 77, 3846–3852. doi: 10.1128/AEM.02772-10
Bond, D. R., Holmes, D. E., Tender, L. M., and Lovley, D. R. (2002). Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295, 483–485. doi: 10.1126/science.1066771
Bond, D. R., Mester, T., Nesbø, C. L., Izquierdo-Lopez, A. V., Collart, F. L., and Lovley, D. R. (2005). Characterization of citrate synthase from Geobacter sulfurreducens and evidence for a family of citrate synthases similar to those of eukaryotes throughout the Geobacteraceae. Appl. Environ. Microbiol. 71, 3858–3865. doi: 10.1128/AEM.71.7.3858-3865.2005
Bonis, B. M., and Gralnick, J. A. (2015). Marinobacter subterrani, a genetically tractable neutrophilic Fe(II)-oxidizing strain isolated from the Soudan Iron Mine. Front. Microbiol. 6:719. doi: 10.3389/fmicb.2015.00719
Butler, J. E., Young, N. D., and Lovley, D. R. (2010). Evolution of electron transfer out of the cell: comparative genomics of six Geobacter genomes. BMC Genomics 11:40. doi: 10.1186/1471-2164-11-40
Chan, C. H., Levar, C. E., Zacharoff, L., Badalamenti, J. P., and Bond, D. R. (2015). Scarless genome editing and stable inducible expression vectors for Geobacter sulfurreducens. Appl. Environ. Microbiol. 81, 7178–7186. doi: 10.1128/AEM.01967-15
Chin, C.-S., Alexander, D. H., Marks, P., Klammer, A. A., Drake, J., Heiner, C., et al. (2013). Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 10, 563–569. doi: 10.1038/nmeth.2474
Connell, J. L., Wessel, A. K., Parsek, M. R., Ellington, A. D., Whiteley, M., and Shear, J. B. (2010). Probing prokaryotic social behaviors with bacterial “lobster traps.” mBio 1:e00202-10. doi: 10.1128/mBio.00202-10
Contreras-Moreira, B., and Vinuesa, P. (2013). GET_HOMOLOGUES, a versatile software package for scalable and robust microbial pangenome analysis. Appl. Environ. Microbiol. 79, 7696–7701. doi: 10.1128/AEM.02411-13
Darling, A. E., Jospin, G., Lowe, E., Matsen, F. A., Bik, H. M., and Eisen, J. A. (2014). PhyloSift: phylogenetic analysis of genomes and metagenomes. PeerJ 2:e243. doi: 10.7717/peerj.243
Deatherage, D. E., and Barrick, J. E. (2014). Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 1151, 165–188. doi: 10.1007/978-1-4939-0554-6_12
Eddie, B. J., Wang, Z., Malanoski, A. P., Hall, R. J., Oh, S. D., Heiner, C., et al. (2016). Description of ‘Candidatus Tenderia electrophaga,’ an uncultivated electroautotroph from a biocathode enrichment. Int. J. Syst. Evol. Microbiol. doi: 10.1099/ijsem.0.001006 [Epub ahead of print].
Edwards, R. A., Rodriguez-Brito, B., Wegley, L., Haynes, M., Breitbart, M., Peterson, D. M., et al. (2006). Using pyrosequencing to shed light on deep mine microbial ecology. BMC Genomics 7:57. doi: 10.1186/1471-2164-7-57
Fu, Q., Kobayashi, H., Kawaguchi, H., Wakayama, T., Maeda, H., and Sato, K. (2013). A thermophilic Gram-negative nitrate-reducing bacterium, Calditerrivibrio nitroreducens, exhibiting electricity generation capability. Environ. Sci. Technol. 47, 12583–12590. doi: 10.1021/es402749f
Gao, F., and Zhang, C.-T. (2008). Ori-Finder: a web-based system for finding oriC s in unannotated bacterial genomes. BMC Bioinformatics 9:79. doi: 10.1186/1471-2105-9-79
Greene, A. C., Patel, B. K. C., and Yacob, S. (2009). Geoalkalibacter subterraneus sp. nov., an anaerobic Fe(III)- and Mn(IV)-reducing bacterium from a petroleum reservoir, and emended descriptions of the family Desulfuromonadaceae and the genus Geoalkalibacter. Int. J. Syst. Evol. Microbiol. 59, 781–785. doi: 10.1099/ijs.0.001537-0
Holmes, D. E., Nevin, K., and Lovley, D. (2004a). Comparison of 16S rRNA, nifD, recA, gyrB, rpoB and fusA genes within the family Geobacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 54, 1591–1599. doi: 10.1099/ijs.0.02958-0
Holmes, D. E., Nicoll, J. S., Bond, D. R., and Lovley, D. R. (2004b). Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell. Appl Environ. Microbiol. 70, 6023–6030. doi: 10.1128/AEM.70.10.6023-6030.2004
Koren, S., Harhay, G. P., Smith, T. P., Bono, J. L., Harhay, D. M., Mcvey, D. S., et al. (2013). Reducing assembly complexity of microbial genomes with single-molecule sequencing. Genome Biol. 14:R101. doi: 10.1186/gb-2013-14-9-r101
Koren, S., and Phillippy, A. M. (2015). One chromosome, one contig: complete microbial genomes from long-read sequencing and assembly. Curr. Opin. Microbiol. 23, 110–120. doi: 10.1016/j.mib.2014.11.014
Krzywinski, M., Schein, J., Birol, I., Connors, J., Gascoyne, R., Horsman, D., et al. (2009). Circos: an information aesthetic for comparative genomics. Genome Res. 19, 1639–1645. doi: 10.1101/gr.092759.109
Kuever, J., Rainey, F. A., and Widdel, F. (2005). “Family I. Desulfuromonaceae fam. nov. (Desulfuromonadaceae corrig. Kuever et al. 2006),” in Bergey’s Manual of Systematic Bacteriology, 2nd Edn, eds D. J. Brenner, N. R. Krieg, J. T. Staley, and G. M. Garrity (New York, NY: Springer).
Levar, C. E., Chan, C. H., Mehta-Kolte, M. G., and Bond, D. R. (2014). An inner membrane cytochrome required only for reduction of high redox potential extracellular electron acceptors. mBio 5:e02034-14. doi: 10.1128/mBio.02034-14
Marsili, E., Rollefson, J. B., Baron, D. B., Hozalski, R. M., and Bond, D. R. (2008). Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Appl. Environ. Microbiol. 74, 7329–7337. doi: 10.1128/AEM.00177-08
Marsili, E., Sun, J., and Bond, D. R. (2010). Voltammetry and growth physiology of Geobacter sulfurreducens biofilms as a function of growth stage and imposed electrode potential. Electroanal 22, 865–874. doi: 10.1002/elan.200800007
Mehta-Kolte, M. G., and Bond, D. R. (2012). Geothrix fermentans secretes two different redox-active compounds to utilize electron acceptors across a wide range of redox potentials. Appl. Environ. Microbiol. 78, 6987–6995. doi: 10.1128/AEM.01460-12
Miceli, J. F., Parameswaran, P., Kang, D.-W., Krajmalnik-Brown, R., and Torres, C. I. (2012). Enrichment and analysis of anode-respiring bacteria from diverse anaerobic inocula. Environ. Sci. Technol. 46, 10349–10355. doi: 10.1021/es301902h
Ojakangas, R. W., Morey, G. B., and Southwick, D. L. (2001). Paleoproterozoic basin development and sedimentation in the Lake Superior region, North America. Sediment. Geol. 14, 319–341. doi: 10.1016/S0037-0738(01)00081-1
Osborne, T. H., McArthur, J. M., Sikdar, P. K., and Santini, J. M. (2015). Isolation of an arsenate-respiring bacterium from a redox front in an arsenic-polluted aquifer in West Bengal, Bengal Basin. Environ. Sci. Technol. 49, 4193–4199. doi: 10.1021/es504707x
Parks, J. M., Johs, A., Podar, M., Bridou, R., Hurt, R. A., Smith, S. D., et al. (2013). The genetic basis for bacterial mercury methylation. Science 339, 1332–1335. doi: 10.1126/science.1230667
Pfennig, N., and Biebl, H. (1976). Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch. Microbiol. 110, 3–12. doi: 10.1007/BF00416962
Rotaru, A.-E., Woodard, T. L., Nevin, K. P., and Lovley, D. R. (2015). Link between capacity for current production and syntrophic growth in Geobacter species. Front. Microbiol. 6:744. doi: 10.3389/fmicb.2015.00744
Roux, S., Enault, F., Hurwitz, B. L., and Sullivan, M. B. (2015). VirSorter: mining viral signal from microbial genomic data. PeerJ 3:e985. doi: 10.7717/peerj.985
Rowe, A. R., Chellamuthu, P., Lam, B., Okamoto, A., and Nealson, K. H. (2014). Marine sediments microbes capable of electrode oxidation as a surrogate for lithotrophic insoluble substrate metabolism. Front. Microbiol. 5:784. doi: 10.3389/fmicb.2014.00784
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Seemann, T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. doi: 10.1093/bioinformatics/btu153
Strycharz-Glaven, S. M., and Tender, L. M. (2012). Study of the mechanism of catalytic activity of G. sulfurreducens biofilm anodes during biofilm growth. ChemSusChem 5, 1106–1118. doi: 10.1002/cssc.201100737
Sung, Y., Ritalahti, K. M., Sanford, R. A., Urbance, J. W., Flynn, S. J., Tiedje, J. M., et al. (2003). Characterization of two tetrachloroethene-reducing, acetate-oxidizing anaerobic bacteria and their description as Desulfuromonas michiganensis sp. nov. Appl. Environ. Microbiol. 69, 2964–2974. doi: 10.1128/AEM.69.5.2964-2974.2003
Thamdrup, B. (2000). “Bacterial manganese and iron reduction in aquatic sediments,” in Advances in Microbial Ecology, ed. B. Schink (New York, NY: Springer), 41–84.
Walker, B. J., Abeel, T., Shea, T., Priest, M., Abouelliel, A., Sakthikumar, S., et al. (2014). Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 9:e112963. doi: 10.1371/journal.pone.0112963
Wrighton, K. C., Thrash, J. C., Melnyk, R. A., Bigi, J. P., Byrne-Bailey, K. G., Remis, J. P., et al. (2011). Evidence for direct electron transfer by a Gram-positive bacterium isolated from a microbial fuel cell. Appl. Environ. Microbiol. 77, 7633–7639. doi: 10.1128/AEM.05365-11
Zacharoff, L., Chan, C. H., and Bond, D. R. (2016). Reduction of low potential electron acceptors requires the CbcL inner membrane cytochrome of Geobacter sulfurreducens. Bioelectrochemistry 107, 7–13. doi: 10.1016/j.bioelechem.2015.08.003
Zavarzina, D. G., Kolganova, T. V., Boulygina, E. S., Kostrikina, N. A., Tourova, T. P., and Zavarzin, G. A. (2006). Geoalkalibacter ferrihydriticus gen. nov. sp. nov., the first alkaliphilic representative of the family Geobacteraceae, isolated from a soda lake. Mikrobiologiia 75, 673–682. doi: 10.1134/S0026261706060099
Zhu, J., Chai, Y., Zhong, Z., Li, S., and Winans, S. C. (2003). Agrobacterium bioassay strain for ultrasensitive detection of N-acylhomoserine lactone-type quorum-sensing molecules: detection of autoinducers in Mesorhizobium huakuii. Appl. Environ. Microbiol. 69, 6949–6953. doi: 10.1128/AEM.69.11.6949-6953.2003
Keywords: metal reduction, electrode, subsurface, Desulfuromonas, PacBio, complete genome
Citation: Badalamenti JP, Summers ZM, Chan CH, Gralnick JA and Bond DR (2016) Isolation and Genomic Characterization of ‘Desulfuromonas soudanensis WTL’, a Metal- and Electrode-Respiring Bacterium from Anoxic Deep Subsurface Brine. Front. Microbiol. 7:913. doi: 10.3389/fmicb.2016.00913
Received: 01 April 2016; Accepted: 27 May 2016;
Published: 21 June 2016.
Edited by:
Kelly Wrighton, The Ohio State University, USAReviewed by:
Matthew Schrenk, Michigan State University, USAElizaveta Bonch-Osmolovskyaya, Winogradsky Institute of Microbiology, Russian Academy of Sciences, Russia
Copyright © 2016 Badalamenti, Summers, Chan, Gralnick and Bond. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel R. Bond, dbond@umn.edu
†Present address: Zarath M. Summers, ExxonMobil Research and Engineering Company, Annandale, NJ, USA
 Jonathan P. Badalamenti
Jonathan P. Badalamenti Zarath M. Summers
Zarath M. Summers Chi Ho Chan
Chi Ho Chan Jeffrey A. Gralnick
Jeffrey A. Gralnick Daniel R. Bond
Daniel R. Bond