- 1Division of Genetics and Molecular Biology, Faculty of Science, Institute of Biological Sciences, University of Malaya, Kuala Lumpur, Malaysia
- 2Novel Bacteria and Drug Discovery Research Group, School of Pharmacy, Monash University Malaysia, Bandar Sunway, Malaysia
- 3Center of Health Outcomes Research and Therapeutic Safety, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
Background
Vibrio parahaemolyticus is a Gram-negative bacterium that widely inhabits the marine and estuarine environments worldwide (Letchumanan et al., 2014). While the majority of strains isolated from environmental sources are innocuous members of marine microbiota, a small number of V. parahaemolyticus strains is capable of causing human illness and often associated with food borne gastroenteritis or diarrhea (Hazen et al., 2015; Raghunath, 2015). This organism has caused the highest number of seafood associated gastroenteritis cases in many countries including United States and Asian countries (Scallan et al., 2011; Newton et al., 2012).
In addition, there have been many reports of multidrug antibiotic resistance in V. parahaemolyticus worldwide (Odeyemi and Stratev, 2016). Our dependence on antibiotics to control this bacterial infections in humans, aquaculture, agriculture, veterinary medicine, and clinical setting has resulted in indiscriminate use which in turn led to the emergence of multidrug resistant strains in the biosphere (Letchumanan et al., 2015b, 2016a; Rao and Lalitha, 2015). Multidrug resistant V. parahaemolyticus strains have been isolated and detected from shrimp in Thailand (Yano et al., 2014), Malaysia (Al-Othrubi et al., 2011; Sani et al., 2013; Letchumanan et al., 2015a,c), and China (Peng et al., 2010; Xu et al., 2014). Resistance toward clinically used antibiotics will eventually hamper the treatment of bacterial infections in humans and potentially increase the fatality rate (Daniels et al., 2000). Therefore, monitoring Vibrio species in aquaculture surroundings is crucial for both human health and the aquaculture industry.
In order to gain better understanding of the multidrug resistance pattern, we studied the genome sequence of V. parahaemolyticus VP103 strain which was isolated from our previous study (Letchumanan et al., 2015a). V. parahaemolyticus VP103 strain was isolated from Penaeus indicus (Banana prawn) and originated from a fishery market in Malaysia. This strain exhibited multidrug resistance profiles toward 5/14 antibiotics tested. Based on the antibiotic susceptibility phenotype, the strain exhibited multiple-antibiotic resistance toward ampicillin, 3rd generation cephalosporins (cefotaxime and ceftazidime), and aminoglycosides (amikacin and kanamycin) (Letchumanan et al., 2015a).
This is a worrying situation as the antibiotic resistant profiles shown by V. parahaemolyticus VP103 include the recommended antimicrobial agents used in treatment of Vibrio spp. infections, including 3rd generation cephalosporin, fluoroquinolones, aminoglycosides, tetracycline, gentamicin, trimethoprim/sulfamethoxazole (Daniels and Shafaie, 2000; Shaw et al., 2014). Therefore, the whole genome sequence of V. parahaemolyticus VP103 was studied with respect to the multidrug resistance profiles to gain a better understanding of the antibiotic resistant patterns. The availability of this genome sequence of V. parahaemolyticus VP103 will aid as a basis for further in-depth analysis of the antibiotic resistance profile of V. parahaemolyticus.
Materials and Methods
Genome Sequencing and Assembly and Annotation
Genomic DNA of VP103 was extracted using Masterpure™ DNA purification kit (Epicenter, Illumina Inc, Madison, WI, USA) followed by RNase (Qiagen, USA) treatment (Ser et al., 2015; Letchumanan et al., 2016b). The DNA quality was quantified using NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA), and a Qubit version 2.0 fluorometer (Life Technologies, Carlsbad, CA, USA). Illumina sequencing library of genomic DNA was prepared using Nextera™ DNA Sample Preparation kit (Illumina, San Diego, CA, USA) and library quality was validated by a Bioanalyzer 2100 high sensitivity DNA kit (Agilent Technologies, Palo Alto, CA) prior to sequencing. The genome of VP103 strain was sequenced on MiSeq platform with MiSeq Reagent Kit 2 (2 × 250 bp Illumina Inc, San Diego, CA, USA). The trimmed sequences were de novo assembled with CLC Genomic Workbench version 5.1 (CLC Bio, Denmark).
Genome Annotation
Gene prediction was carried out using Prodigal 2.6, while rRNA and tRNA were analyzed using RNAmmer and tRNAscan SE version 1.21 (Lowe and Eddy, 1997; Lagesen et al., 2007; Hyatt et al., 2010). Gene prediction and annotation were performed using Rapid Annotation Search Tool RAST, (Aziz et al., 2008). Antibiotic resistance genes were analyzed using antibiotic resistance genes-ANNOTation (ARG-ANNOT, Gupta et al., 2014).
Results
Genome Characteristics
The genome of V. parahaemolyticus VP103 consists of 4,988,425 bp with a mean genome coverage of 177.8-fold and with an average G + C content of 53.37% (Table 1). A total of 4820 genes was predicted of which 4648 genes were identified as protein coding genes. There are 91 RNA genes consisting of 10 rRNAs and 81 tRNAs. This genome sequence data of VP103 strain sequenced under this study has been deposited in DDBJ/EMBL/GenBank under Accession No. LBDB00000000. The version described in this paper is the first version, LBDB01000000. The genome sequences data are available in FASTA, annotated GenBank flat file, graphical, and ASN.1 formats.
Virulence and Antimicrobial Resistance Genes
The analysis obtained from RAST server revealed 573 subsystems (Figure 1). The annotated genome has 97 genes responsible for resistance to antibiotic and toxic compounds including 19 genes for multidrug resistance efflux pumps, 4 genes for Beta-lactamase, 4 genes for multiple antibiotic resistance MAR locus, and 2 genes for aminoglycosides adenylyltransferase. The hemolysin gene was present in V. parahaemolyticus VP103 strain genome. The genome analysis on ARG-ANNOT noted the presences of β-lactam resistant gene, bla gene within the genome at 99% similarities when compared to other V. parahaemolyticus strains. The phenotypic resistance shown by V. parahaemolyticus VP103 toward ampicillin, cefotaxime, and ceftazidime is closely related to the gene coding Beta-lactamase in the genome. The gene coding aminoglycosides adenylyltransferase of V. parahaemolyticus VP103 confers resistance phenotypic observed toward amikacin and kanamycin.
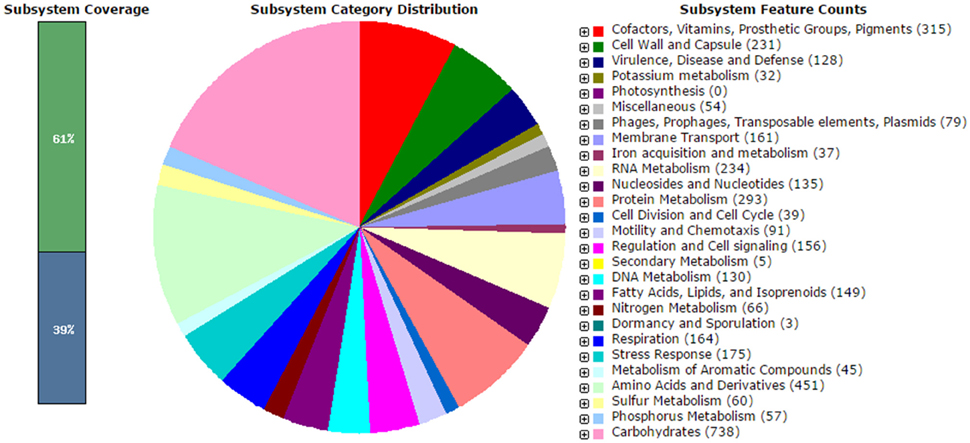
Figure 1. Subsystem category distribution of Vibrio parahaemolyticus VP103 (based on RAST annotation server).
Multidrug resistance profile seen in the phenotype and genes of V. parahaemolyticus VP103 genome illustrates how extensive antibiotics have been used in the aquaculture. Although antibiotics namely oxytetracycline, tetracycline, quinolone, sulphonamides, and trimethoprim are allowed in the Asian aquaculture industry (Rico et al., 2012; Yano et al., 2014), the extensive use of these antimicrobials has led to emergence of multidrug resistant strains in the environment. As the efficiency of clinical antibiotics has declined, the extensive use of antibiotics in the aquaculture and humans are in distress conditions due to spread of multidrug resistant strains (Letchumanan et al., 2015b). This situation is a definite cause of concern and warrants more stringent surveillance in the use of antibiotics. In summary, the whole genome sequence of V. parahaemolyticus VP103 will be useful in future studies to determine antimicrobial resistance and virulence attributes as well as mechanisms that enhance its environmental or host fitness.
Author Contributions
The experiments, data analysis and manuscript writing were performed by VL and HS, while KC, BG, and LL provided vital guidance and technical support. LL founded the research project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by PVC Award Grant (Project No. PVC-ECR-2016), External Industry Grant (Biotek Abadi Vote No. GBA-808813), MOSTI eScience funds (Project No. 06-02-10-SF0300) awarded to LL, and a University of Malaya for High Impact Research Grant (UM-MOHE HIR Nature Microbiome Grant No. H-50001-A000027 and No. A000001-50001) and PPP Grant (PG090-2015B) awarded to KC.
References
Al-Othrubi, S. M., Alfizah, H., Son, R., Humin, N., and Rahaman, J. (2011). Rapid detection and E-test antimicrobial susceptibility testing of Vibrio parahaemolyticus isolated from seafood and environmental sources in Malaysia. Saudi Med. J. 32, 400–406.
Aziz, R. K., Bartels, D., Best, A. A., DeJongh, M., Disz, T., Edwards, R. A., et al. (2008). The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75
Daniels, N. A., MacKinnon, L., Bishop, R., Altekruse, S., Ray, B., Hammond, R. M., et al. (2000). Vibrio parahaemolyticus infections in the United States, 1973–1998. J. Infect. Dis. 181, 1661–1666. doi: 10.1086/315459
Daniels, N. A Shafaie (2000). A review of pathogenic Vibrio infections for clinicians. J. Infect. Medic. 17, 665–685.
Gupta, S. K., Padmanabhan, B. R., Diene, S. M., Rojas, R. L., Kemf, M., Landraud, L., et al. (2014). ARG-ANNOT, a new bioinformatics tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 58, 212–220. doi: 10.1128/AAC.01310-13
Hazen, T. H., Lafon, P. C., Garrett, N. M., Lowe, T. M., Silberger, D. J., Rowe, L. A., et al. (2015). Insights into the environmental reservoir of pathogenic Vibrio parahaemolyticus using comparative genomics. Front. Microbiol. 6:204. doi: 10.3389/fmicb.2015.00204
Hyatt, D., Chen, G. L., Locascio, P. F., Land, M. L., Larimer, F. W., and Hauser, L. J. (2010). Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119
Lagesen, K., Hallin, P., Rodland, E. A., Staerfeldt, H. H., Rognes, T., and Ussery, D. W. (2007). RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35, 3100–3108. doi: 10.1093/nar/gkm160
Letchumanan, V., Chan, K.-G., and Lee, L.-H. (2014). Vibrio parahaemolyticus: a review on the pathogenesis, prevalence and advance molecular identification techniques. Front. Microbiol. 5:705. doi: 10.3389/fmicb.2014.00705
Letchumanan, V., Chan, K.-G., and Lee, L.-H. (2015b). An insight of traditional plasmid curing in Vibrio species. Front. Microbiol. 6:735. doi: 10.3389/fmicb.2015.00735
Letchumanan, V., Chan, K.-G., Pusparajah, P., Saokaew, S., Duangjai, A., Goh, B.-H., et al. (2016a). Insights into bacteriophage application in controlling Vibrio species. Front. Microbiol. 7:1114. doi: 10.3389/fmicb.2016.01114
Letchumanan, V., Ser, H.-L., Tan, W.-S., Ab Mutalib, N.-S., Goh, B.-H., Chan, K.-G., et al. (2016b). Genome sequence of Vibrio parahaemolyticus VP152 strain isolated from Penaeus indicus in Malaysia. Front. Microbiol. 7:1410. doi: 10.3389/fmicb.2016.01410
Letchumanan, V., Pusparajah, P., Loh, T. H. T., Yin, W.-F., Lee, L.-H., and Chan, K.-G. (2015c). Occurrence and antibiotic resistance of Vibrio parahaemolyticus from shellfish in Selangor, Malaysia. Front. Microbiol. 6:1417. doi: 10.3389/fmicb.2015.01417
Letchumanan, V., Yin, W.-F., Lee, L.-H., and Chan, K.-G. (2015a). Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 6:33. doi: 10.3389/fmicb.2015.00033
Lowe, T. M., and Eddy, S. R. (1997). tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 955–964. doi: 10.1093/nar/25.5.0955
Newton, A., Kendall, M., Vugia, D. J., Henao, O. L., and Mahon, B. E. (2012). Increasing rates of vibriosis in the United States, 1996-2010: review of surveillance data from 2 systems. Clin. Infect. Dis. 54, 391–395. doi: 10.1093/cid/cis243
Odeyemi, O. A., and Stratev, D. (2016). Occurrence of antimicrobial resistant or pathogenic Vibrio parahaemolyticus in seafood. A mini review. Rev. Med. Vet. 167, 93–98.
Peng, F. M., Jiang, D. Y., Ruan, H. H., Liu, H. Q., and Zhou, L. P. (2010). Pathogenic investigation on a food poisoning induced by Vibrio parahaemolyticus. Prev. Med. Trib. 16, 746–747.
Raghunath, P. (2015). Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus. Front. Microbiol. 5:805. doi: 10.3389/fmicb.2014.00805
Rao, B. M., and Lalitha, K. V. (2015). Bacteriophage for aquaculture: are they beneficial or inimical. Aquaculture 437, 146–154. doi: 10.1016/j.aquaculture.2014.11.039
Rico, A., Satapornvanit, K., Haque, M. M., Min, J., Nguyen, P. T., Telfer, T., et al. (2012). Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: a critical review. Rev. Aqua. 4, 75–93. doi: 10.1111/j.1753-5131.2012.01062.x
Sani, N. A., Ariyawansa, S., Babji, A. S., and Hashim, J. K. (2013). The risk assessment of Vibrio parahaemolyticus in cooked black tiger shrimps (Penaeus monodon) in Malaysia. Food Cont. 31, 546–552. doi: 10.1016/j.foodcont.2012.10.018
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., and Roy, S. L. (2011). Foodborne illness acquired in the United States e major pathogens. Emerg. Infect. Dis. 17, 7–15. doi: 10.3201/eid1701.P11101
Ser, H. L., Tan, W. S., Cheng, H. J., Yin, W. F., Chan, K. G., and Lee, L. H. (2015). Draft genome of amylolytic actinobacterium, Sinomonas humi MUSC 117T isolated from intertidal soil. Mar. Gen. 24, 209–210. doi: 10.1016/j.margen.2015.05.012
Shaw, K. S Rosenberg Goldstein, R, E., He, X., Jacobs, J. M., Crump, B. C., et al. (2014). Antimicrobial susceptibility of Vibrio vulnificus and Vibrio parahaemolyticus recovered from recreational and commercial areas of Cheaspeake Bay and Maryland coastal bay. PLoS ONE 9, 1–11. doi: 10.1371/journal.pone.0089616
Xu, X., Wu, Q., Zhang, J., Cheng, J., Zhang, S., and Wu, K. (2014). Prevalence, pathogenicity, and serotypes of Vibrio parahaemolyticus in shrimp from Chinese retail markets. Food Cont. 46, 81–85. doi: 10.1016/j.foodcont.2014.04.042
Keywords: Vibrio parahaemolyticus, seafood, genome, Penaeus indicus, antibiotic resistance
Citation: Letchumanan V, Ser H-L, Chan K-G, Goh B-H and Lee L-H (2016) Genome Sequence of Vibrio parahaemolyticus VP103 Strain Isolated from Shrimp in Malaysia. Front. Microbiol. 7:1496. doi: 10.3389/fmicb.2016.01496
Received: 29 June 2016; Accepted: 07 September 2016;
Published: 21 September 2016.
Edited by:
Dongsheng Zhou, Beijing Institute of Microbiology and Epidemiology, ChinaReviewed by:
Adrian Canizalez-Roman, Autonomous University of Sinaloa, MexicoPendru Raghunath, Texila American University, Guyana
Copyright © 2016 Letchumanan, Ser, Chan, Goh and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bey-Hing Goh, goh.bey.hing@monash.edu
Learn-Han Lee, lee.learn.han@monash.edu
 Vengadesh Letchumanan
Vengadesh Letchumanan Hooi-Leng Ser
Hooi-Leng Ser Kok-Gan Chan
Kok-Gan Chan Bey-Hing Goh
Bey-Hing Goh Learn-Han Lee
Learn-Han Lee