- 1Department of Endodontology, Maurice and Gabriela Goldschleger School of Dental Medicine, Tel Aviv University, Tel Aviv, Israel
- 2Department of Molecular Genetics, Weizmann Institute of Science, Rehovot, Israel
Objectives: This work assesses different methods to interfere with Enterococcus faecalis biofilms formed on human dentin slabs.
Methods: First, methods are presented that select for small molecule inhibitors of biofilm targets using multi-well polystyrene biofilm plates. Next, we establish methodologies to study and interfere with biofilm formation on a medically relevant model, whereby biofilms are grown on human root dentin slabs.
Results: Non-conventional D-amino acid (D-Leucine) can efficiently disperse biofilms formed on dentin slabs without disturbing planktonic growth. Cation chelators interfere with biofilm formation on dentin slabs and polystyrene surfaces, and modestly impact planktonic growth. Strikingly, sodium hypochlorite, the treatment conventionally used to decontaminate infected root canal systems, was extremely toxic to planktonic bacteria, but did not eradicate biofilm cells. Instead, it induced a viable but non-culturable state in biofilm cells when grown on dentin slabs.
Conclusion: Sodium hypochlorite may contribute to bacterial persistence. A combination of the methods described here can greatly contribute to the development of biofilm inhibitors and therapies to treat Enterococcus faecalis infections formed in the root canal system.
Introduction
Bacterial biofilms are multicellular microbial communities that adhere to surfaces and interfaces (Kolter and Greenberg, 2006). The formation and maintenance of biofilms is dependent on the production of extracellular substances including proteins and exopolysaccharides that constitute the extracellular matrix. These extracellular matrices secure the bacteria together in a multicellular community (Parsek and Singh, 2003; Branda et al., 2005; Oppenheimer-Shaanan et al., 2013; Vlamakis et al., 2013). Biofilms offer the microbial communities shelter from environmental insults and assaults, attachment to a host and access to oxygen and nutrients (Costerton et al., 1987; Chen et al., 2012).
Microbial biofilms account for over 80% of microbial infections in the body (Costerton et al., 1999; Stewart, 2002; Mah et al., 2003; Fux et al., 2005; Oppenheimer-Shaanan et al., 2013), and are considered as a primary cause of apical periodontitis in teeth with infected root canal spaces (Haapasalo and Shen, 2012). Apical periodontitis is a relatively common dental pathology that involves an inflammatory reaction and destruction of tissues around the apex of a tooth-root. This is caused by microbial invasion and infection of the dental pulp, and biofilm colonization within the root canal system (Ricucci and Siqueira, 2010).
Gram-positive and facultative anaerobes are the most frequently isolated species within treated canals in teeth with persistent intra-radicular infections, with Enterococcus faecalis (E. faecalis) being the most prevalent (Zhang et al., 2015). In the root canal environment, E. faecalis bacterium plays an important role in bacterial biofilm formation, and therefore E. faecalis biofilms are considered to be an appropriate model for testing novel antimicrobial treatments (Meire et al., 2012; Du et al., 2013; Tay et al., 2015; Shlezinger et al., 2016).
One of the primary goals of root canal treatment is to eliminate bacteria from the root canal system in order to treat or prevent apical periodontitis (Kishen, 2012). However, bacteria within biofilm communities are 10-fold to 1000-fold more resistant to antimicrobial agents and antibiotics than planktonic (free-living) bacteria, and are also able to effectively evade the immune system (Costerton et al., 1999; Stewart, 2002; Mah et al., 2003; Fux et al., 2005; Oppenheimer-Shaanan et al., 2013; Tay et al., 2015). For these reasons bacterial biofilms pose a major obstacle to endodontic disinfection in root canal systems, and therefore methods to promote biofilm dispersal may ultimately improve the treatment outcome (Kishen, 2012; Meire et al., 2012; Du et al., 2013).
The key element in the elimination of intra-canal biofilms is the use of anti-microbial irrigating solutions during the root canal treatment. However, currently the most commonly used anti-microbial irrigation solution, sodium hypochlorite, has a limited ability to completely eliminate the biofilm from the root canal, sometimes leading to persistent infection. Thus, stressing the need to develop novel anti-microbial biofilm agents in order to achieve predictable, effective disinfection of the root canal system (Ricucci and Siqueira, 2010).
Small molecules that target the cell envelope were found to be efficient inhibitors for biofilms formed by Gram-positive bacteria, and to effectively disperse the biofilms. Recently, flavomycin, an antibiotic that inhibits transglycosylation directly by binding the transglycosylation domain of PBP enzymes (Dengler et al., 2011) was found to antagonize biofilm formation but not planktonic growth in the soil bacterium, Bacillus subtilis (Bucher et al., 2015). An additional group of cell-wall interfering agents that promote dispersal are non-canonical D-amino acids (Bucher et al., 2015, 2016). D-amino acids compete with D-Alanine for the fifth position in the B. subtilis pentapeptide, and interfere with transpeptidation (Lam et al., 2009; Cava et al., 2011; Lupoli et al., 2011) and transglycosylation (Lam et al., 2009). D-amino acids were found to inhibit and disperse biofilms without affecting planktonic growth in various model organisms (Kolodkin-Gal et al., 2010; Hochbaum et al., 2011; Yu et al., 2012; Sanchez et al., 2013; Li and Wang, 2014; Bucher et al., 2015, 2016; She et al., 2015), but to the best of our knowledge their efficacy on endodontic biofilms was never evaluated.
An additional method to induce dispersal of biofilms in various model organisms is the use of cation chelators. Iron is an essential component of many metabolically relevant proteins in living cells, and the maintenance of biofilms requires higher concentrations of iron than planktonic growth (Banin et al., 2005; Ramos et al., 2010; Kolodkin-Gal et al., 2013). The functional siderophore pyoverdin is required for biofilm maturation of P. aeruginosa, and its absence promotes disassembly (Banin et al., 2005). Moreover, lactoferrin, an innate immunity protein, was shown to disrupt P. aeruginosa biofilm formation by sequestering Fe(III) from siderophores (Singh et al., 2002), and chelation of cations by Ethylenediaminetetraacetic acid (EDTA) was demonstrated to disperse staphylococcal biofilms (Raad et al., 2003). Overall, chelation of cations in biofilm deformation could result in effective therapeutic strategies for eradication of medical biofilms. In root-canal treatment, EDTA is traditionally used as a chelating agent to remove calcium, demineralize and soften dentin, and to remove the “smear layer,” a surface film of debris containing dentin particles, remnants of vital or necrotic pulp tissue, and bacterial components, retained on the dentin and other surfaces after the root canal procedure (de Almeida et al., 2016).
The aim of this study was to evaluate the use of small molecules that were previously shown to inhibit and eradicate biofilms, for the elimination of E. faecalis biofilms grown on human dentin slabs, and compare their efficiency with sodium hypochlorite, a commonly used antimicrobial agent in root canal treatment.
Materials and Methods
Samples Preparation
Twenty freshly extracted single rooted fully developed intact human teeth were stored in 0.05% sodium hypochlorite solution. Informed consent was obtained from the extracted teeth donors.
This study was approved by the Tel Aviv university ethics committee.
The crowns of the selected teeth were removed in order to obtain multiple root specimens of 13 mm length, and the apical 3 mm of the root end was resected without a bevel using Zakaria high speed bur (Maillefer, Ballaigues, Switzerland). The root canal lumen was then enlarged to a minimum of 0.5 mm using low speed burs (Gates Glidden Drills, Dentsply Maillefer, Tulsa, OK, USA). The roots were embedded in self-cure acrylic repair material (UNIFAST Trad, GC America). To prepare the dentin slabs, the roots were cut perpendicular to the long axis of the root under water cooling with a diamond saw rotating at 500 rpm (Isomet, Buehler Ltd., Lake Bluff, IL, USA). Two dentin slabs of 1 mm thickness each were obtained from each root (Kuci et al., 2014), see Supplementary Figure S1. The specimens were then placed in small dishes and sterilized overnight using ethylene oxide gas (Brosco et al., 2010). For each of the indicated treatment at least nine independent dentin slabs were evaluated under the same conditions.
Strains and Media
All of the experiments were performed in a clinical isolate of Enterococcus faecalis 29212 (Minogue et al., 2014). To confirm reproducible results we evaluated biofilm formation of a single virulent strain on-top of artificial and biological surfaces.
The strains were routinely manipulated in LB broth (Difco), or in TSB broth (Difco), enriched with 0.5% glucose (Sigma) (Lopez and Kolter, 2010).
Biofilm Formation Assay
Cells were grown in LB from a single colony isolated over LB plates to a mid-logarithmic phase of growth (6 h at 37°C with shaking). To grow biofilms, 1.5 μl of starter culture was inoculated into the TSB glucose media in 96-well polystyrene plates and further incubated for 24 h. The growth media were either applied or not with one of the following substances: (i) A Sodium hypochlorite was added to the final concentration of 0.6% from a stock solution of sodium hypochlorite solution, reagent grade, 10–15% (sigma), (ii) D-Leucine (Sigma–Aldrich) was added to the final concentration of 2 mM from a stock solution of 76 mM D-Leucine in DDW, (iii) flavomycin (AK Scientific) was added to the final concentration of 2 μg/ml from a stock solution of 2 mg/ml (iv) EDTA (Sigma–Aldrich) was added to the final concentration of 0.5 mM from a stock solution of 50 mM (0.5 mM), and (v) 2-2′ bipyridyl (Sigma–Aldrich) was added to a final concentration of 10 μg/ml from a stock solution of 10 mg/ml in ethanol. In the case of the 96-well polystyrene plate, the crystal violet (Sigma–Aldrich) assay was performed as described by Friedman and Kolter (2004).
Growth Measurements
Cells were grown from a single colony isolated from LB plates to a stationary phase of growth (12 h at 37°C with shaking). The culture was then diluted 1:25 in 5 ml liquid TSB glucose medium (Thermo Scientific). Cells were grown with agitation at 37°C for 10 h in a growth chamber, and the optical density at 600 nm (OD600) was measured every 2 h. Cells were either grown in presence or absence of cell-wall inhibiting molecules, as indicated in the corresponding figure legend.
Determination of Cell Density and Live Cell Counts during Dentin Disk Colonization
Cells were grown in LB from a single colony isolated over LB plates to a mid-logarithmic phase of growth (6 h at 37°C with shaking). To grow biofilms, 1.5 μl of starter culture was inoculated into 6 ml TSB glucose media dispensed into a Petri dish containing the dentin disks. Cells were grown on-top of dentin disks as described above. Following 24 h of growth, the media was removed from the dentin disks, and the associated bacteria were incubated in different substances and treated further, as specified in the legends for each figure. Following incubation, the suspension solution was collected and further evaluated for the live cell counts as well as the biofilm fraction. The biofilm fraction was obtained by three washes of the dentin disks with phosphate buffer to the final volume of the suspension solution. To determine the number of live cells, cells were serially diluted in phosphate-buffered saline (PBS; Biological Industries, Israel), plated on LB plates, and colony forming units (CFU) were counted after incubation at 37°C overnight as done by us previously (Bucher et al., 2015).
Confocal Scanning Laser Microscopy and Live∖Dead Evaluation
To determine culture density and live cell counts of cells grown on the disks, cells were harvested from a dentin biofilm dispersal assay (described above): Cells were grown on-top of dentin disks as described above. Following 24 h of growth, the media was removed from the dentin disks, and the associated bacteria were incubated in different substances and treated further, as specified in the legends for each figure. Samples of biofilms grown for 24 h and treated as indicated were stained using LIVE/DEAD BacLight Bacterial Viability kit L-7012 for microscopy and quantitative assays (Molecular Probes, Eugene, OR, USA) containing separate vials of the two component dyes (SYTO 9 and propidium iodide in 1:1 mixture) in solution was used for staining of the biofilm following the manufacturer’s instructions. The excitation/emission maxima for these dyes is approximately 480–500 nm for the SYTO 9 stain and 490–635 nm for propidium iodide (Aziz et al., 2010). Fluorescence from the stained cell was viewed under a confocal laser scanning microscope (Leica TCS SP5, Leica Microsystems CMS GmbH Germany). Single channel and simultaneous dual-channel imaging was used to display green and red fluorescence (Zapata et al., 2008). Confocal laser scanning microscope images of the biofilms were acquired by the LAS AF software (version 2.6.0.7266; Leica Microsystems CMS GmbH) at a resolution of 512 × 512 pixels. The mounted specimens were observed using a X4 lens. Confocal LIVE/DEAD images were analyzed and quantitated using the above mentioned software (LAS AF; Zapata et al., 2008; Shen et al., 2010; Kuci et al., 2014). The specimens were coded for blind evaluation.
Statistical Methods
All studies were performed in duplicates or triplicates at least three separate and independent times. Data are expressed as average values ± standard deviations of the means. Parametric testing was performed after confirming that raw data were normally distributed. Data were analyzed by student’s t-test, used to determine if the set of treated versus the untreated control are different from each other (A paired t-test comparing two sets of measurements) differ and P values of less than 0.1 were considered significant.
Results
Evaluation of Biofilm Inhibitors on Planktonic Growth and Biofilm Formation on Polystyrene Surfaces
Systematic evaluation of small molecule biofilm inhibitors was performed on planktonic growth (Figure 1) and biofilm formation, according to the Microtiter Dish Biofilm Formation Assay (Friedman and Kolter, 2004). Three categories of biofilm inhibitors were tested: (i) Small molecules that target the cell envelope: Flavomycin, an antibiotic that inhibits transglycosylation directly by binding the transglycosylation domain of PBP enzymes (Dengler et al., 2011); and D-Leucine, a non-canonical D-amino acid, which competes with D-Alanine for the fifth position in the B. subtilis pentapeptide (Bucher et al., 2015), and interferes with transpeptidation (Lam et al., 2009; Cava et al., 2011; Lupoli et al., 2011) and transglycosylation (Lam et al., 2009). (ii) Cation chelators: EDTA, a chelating agent that sequesters a variety of polyvalent cations such as calcium; and 2,2′-bipyridyl, an organic bidentate chelating ligand, forming complexes with many transition metals, with a strong affinity to iron. (iii) Sodium hypochlorite (bleach) – commonly used concentrations between 0.5 and 6% for irrigation in root canal treatments (Bystrom and Sundqvist, 1985; Gomes et al., 2001; Haapasalo et al., 2014).
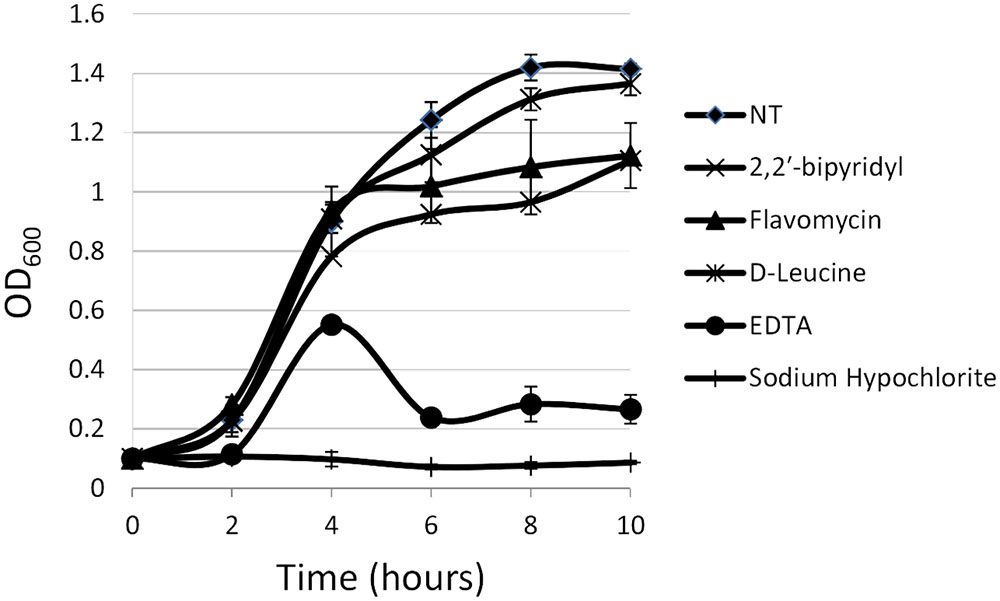
FIGURE 1. Analyzing the effects of small molecules on planktonic growth of Enterococcus faecalis. Growth of strain 29212 was assessed at 37°C with shaking in liquid TSB-glucose medium (untreated) or applied with the following: Sodium hypochlorite (0.6%), D-Leucine (2 mM), flavomycin (2 μg/ml), EDTA (0.5 mM), and 2-2′ bipyridyl (10 μg/ml). Growth was monitored by measuring OD600. Results are averages of three independent experiment performed in duplicates and their standard deviations.
For planktonic growth, sodium hypochlorite turned out to be most toxic, eliminating E. faecalis growth altogether (Figure 1), EDTA, flavomycin and 2-2′ bipyridyl inhibited (reversibly) bacterial growth at indicated concentrations, and D-Leucine at concentrations of up to 2 mg/ml had little or no effect on planktonic growth.
In contrast, the results from the assay on biofilm formation in polystyrene wells were as following: the mildly toxic 2-2′ bipyridyl and flavomycin had a modest inhibitory effect on biofilm formation, while EDTA (mildly toxic), D-Leucine (non-toxic), and sodium hypochlorite (highly toxic) inhibited biofilm formation comparably and significantly. These results indicate that inhibition of biofilm formation may not be directly correlated to inhibition of planktonic growth.
Evaluation of Biofilm Inhibitors Enterococcus faecalis Biofilms Formed on a Root-Dentin Model
In order to establish a more ecological dentin model, root dentin slabs of 1 mm thickness each were cut as described in the section “Materials and Methods” (Supplementary Figure S1). The sterile dentin disk was then inoculated with E. faecalis and further incubated in biofilm media. As shown, E. faecalis cells formed a thick biofilm on the dentin slab within 24 h. Once a biofilm was established, we used the several biofilm inhibitors that proved to effectively inhibit biofilm formation on-top of polystyrene plates (Figure 2) and evaluated their effect on dispersing root-dentin associated biofilms. For this purpose, we first scored the remaining biofilm using the Live/Dead BacLight Viability Kit (Figure 3). The outcome of the application of biofilm inhibitors to an established dentin-associated biofilm differed dramatically between treatments. Surprisingly, the sodium hypochlorite treatment, found to be most toxic to planktonic growth, had little effect on removal of the biofilm biomass, and only modestly impacted the overall viability of the biofilm cells. The concentration of flavomycin which halted planktonic growth and biofilm formation (Figures 1 and 2) had no impact on established biofilms (Supplementary Figure S2), and chelation of cations by EDTA had little or no effect on the overall biofilm’s biomass. However, EDTA treatment significantly increased the proportion of dead cells (Figures 3 and 4). Specific chelation of iron by 2-2′ bipyridyl greatly increased cell death within dentin-associated biofilms (Figures 3 and 4). The most efficient treatment was D-Leucine, as it significantly dispersed the biofilm’s biomass (Figure 3) and modestly increased the fraction of dead cells compared with the control. Importantly, the D-Leucine treatment was found to be effective in sub-toxic concentration compatible with endodontic therapy.
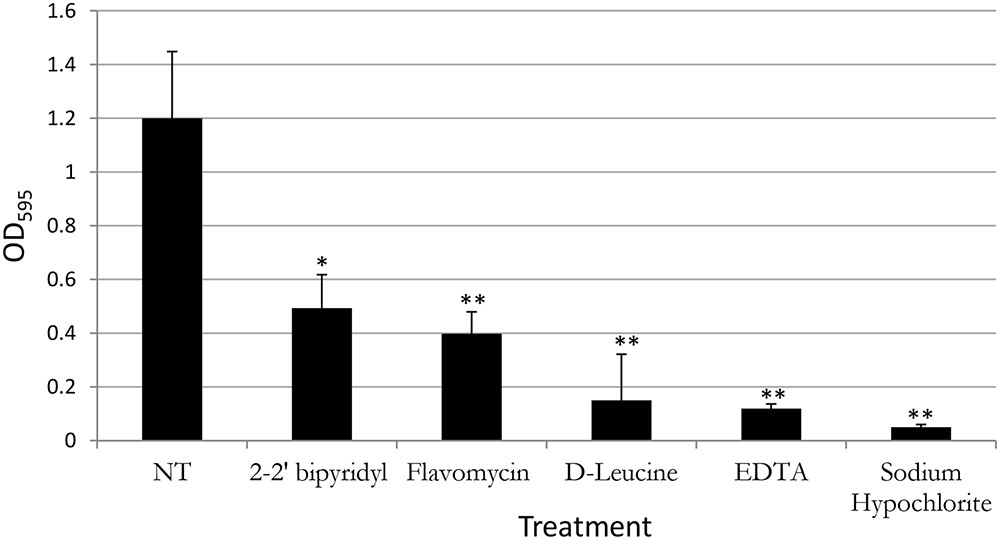
FIGURE 2. Analyzing the effects of small molecules on biofilm formation of E. faecalis in a microplate model. Single colony of Strain 29212 was grown at 37°C with shaking in liquid TSB-glucose medium to a mid-logarithmic stage. Cells were diluted 1:100 into a fresh medium (untreated) or applied with the following small molecules: Sodium hypochlorite (0.6%), D-Leucine (2 mM), flavomycin (2 μg/ml), EDTA (0.5 mM), and 2-2′ bipyridyl (10 μg/ml). Cultures were split into a 96-well polystyrene plate, 100 μL in each well, and further incubated at 37°C for 24 h. Biofilm formation was assessed by crystal violet staining as described in “Material and Methods.” Results are averages of three independent experiments performed with five repeats. P value was calculated using a student’s t-test. ∗P < 0.1, ∗∗P < 0.05, compared with the untreated control.
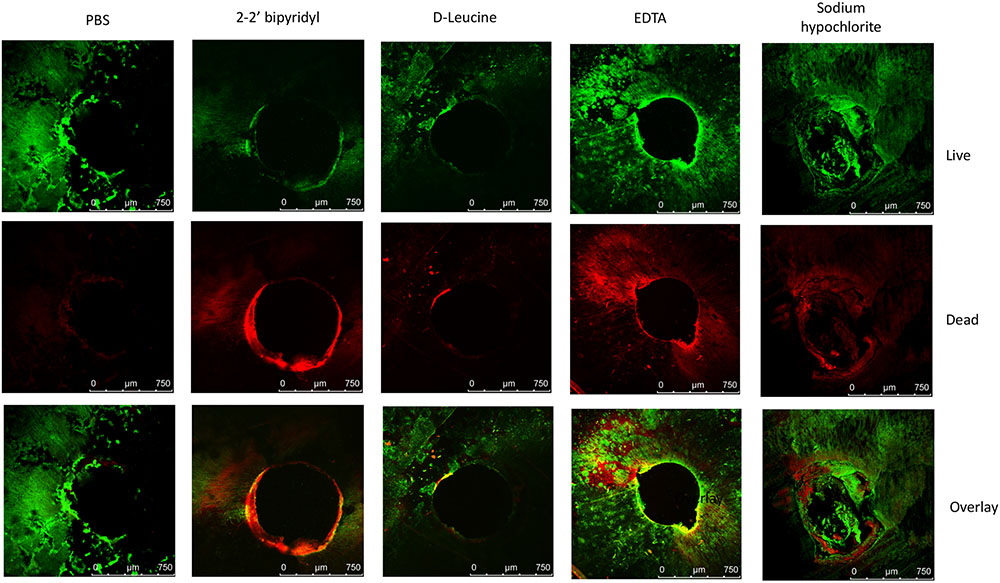
FIGURE 3. Analyzing the effects of small molecules on pre-established biofilms of E. faecalis on human dentin disks. Single colony of Strain 29212 was grown at 37°C with shaking in liquid TSB-glucose medium to a mid-logarithmic stage. Cells were diluted 1:100 into fresh medium in polystyrene plates, containing a fixed dentin disk. Following 24 h of incubation cells were applied with the following solutions, phosphate-buffered saline (PBS), or a PBS solution applied with the following substances: Sodium hypochlorite (0.6%), D-Leucine (2 mM), flavomycin (2 μg/ml), EDTA (0.5 mM), and 2-2′ bipyridyl (10 μg/ml) for 2 h. Cells were washed, stained with BacLight Bacterial Viability kit and imaged as described in the section “Materials and Methods.” ∗P < 0.1, ∗∗P < 0.05, compared with the PBS treatment.
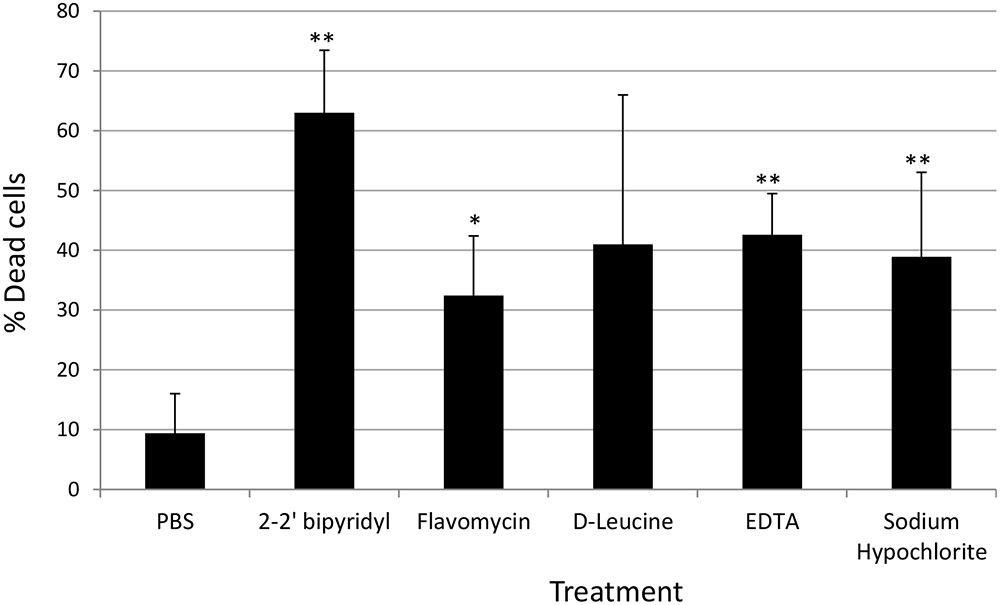
FIGURE 4. Analyzing the effects of small molecules on the viability of E. faecalis biofilm cells grown on human dentin disks. Single colony of Strain 29212 was grown at 37°C with shaking in liquid TSB-glucose medium to a mid-logarithmic stage. Cells were diluted 1:100 into a fresh medium in polystyrene plates, containing a fixed dentin disk. Following 24 h of incubation cells were applied with the following solutions, PBS, or a PBS solution applied with the following substances: Sodium hypochlorite (0.6%), D-Leucine (2 mM), flavomycin (2 μg/ml), EDTA (0.5 mM), and 2-2′ bipyridyl (10 μg/ml) for 2 h. Cells were washed and imaged as described in the section “Materials and Methods.” The number of cells stained in PI (Dead), and the number of cells stained with fluorescein was calculated as described in the section “Materials and Methods.” Results are averages of two independent experiments performed with at least three repeats. P value was calculated using a student’s t-test. ∗P < 0.1, ∗∗P < 0.05, compared with the PBS treatment.
To further evaluate the viability of the E. faecalis following different treatments, we assessed the replicative cell counts from the treated biofilms and the growth media. Strikingly, though the conservation of the biofilm biomass following sodium hypochlorite treatment was evident between different experiments (Figure 3), very few culturable cells could be eluted from the dentin disks and the inoculation media (Figure 5). In contrast, replicative cell counts from other treatments correlated better with the confocal examination. This result could be an indication that the sodium hypochlorite treatment is promoting a viable but not culturable state (VBNC) in root-associated biofilms.
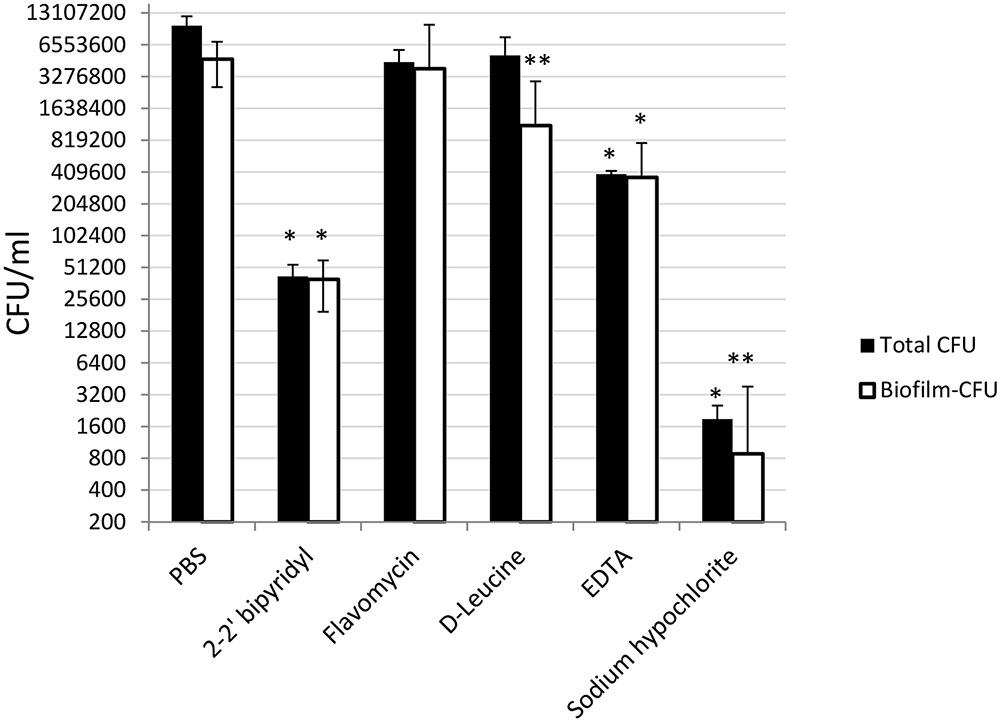
FIGURE 5. Analyzing the effects of small molecules on the cultivability of E. faecalis biofilm cells grown on human dentin disks. Single colony of Strain 29212 was grown at 37°C with shaking in liquid TSB-glucose medium to a mid-logarithmic stage. Cells were diluted 1:100 into a fresh medium in polystyrene plates, containing a fixed dentin disk. Following 24 h of incubation, cells were applied with the following solutions, PBS, or a PBS solution applied with the following substances: Sodium hypochlorite (0.6%), D-Leucine (2 mM), flavomycin (2 μg/ml), EDTA (0.5 mM), and 2-2′ bipyridyl (10 μg/ml) for 2 h. Cells were then obtained by rigorous pipetting and cultures as described in the section “Materials and Methods.” Results are averages of two independent experiments performed with at-least four repeats. ∗P < 0.1, ∗∗P < 0.05, compared with the PBS treatment.
Discussion
Enterococcus faecalis is a commensal Gram-positive microorganism residing within the gastrointestinal tract. Nonetheless, it can cause life-threatening infections such as endocarditis, bacteremia, urinary tract infection, and meningitis (Khalifa et al., 2015), and is especially problematic in hospitals where antibiotic resistance is developed (Deshpande et al., 2007). In addition, E. faecalis is frequently recovered from persistent infections associated with root canal treatment failures (Zapata et al., 2008), and can result in chronic or acute inflammation and destruction of the tissues surrounding the tip of the tooth-root with subsequent development of abscesses. Despite meticulous mechanical and chemical preparation during root canal treatment, infection may persist (Zhang et al., 2015), in most of the treated and filled root canals, and in some cases may lead to treatment failure and further complications (Molander et al., 1998). To date, the available therapeutic tools to efficiently and predictably eradicate intra-canal E. faecalis biofilm infection are limited (Paganelli et al., 2012). Biofilms may pose a severe health threat, since at this phase bacteria become inaccessible to antibacterial agents and the body’s immune system (Bryers, 2008; Wang et al., 2012). The penetration failure may be associated with various factors, including the extracellular matrix encapsulating the biofilm cells, and multidrug resistance development of bacteria within the biofilm (Bryers, 2008).
In this study several biofilm inhibitors and dispersing agents were evaluated for their ability to combat E. faecalis infection on dentin slabs mimicking E. faecalis root canal infections. Surprisingly, sodium hypochlorite, the commonly used anti-bacterial irrigation solution for treatment of root canal infections failed to reduce the biofilm biomass on dentin disks (Figure 3), though it was most efficient in reducing the replicative properties of the biofilm’s cells (Figure 5).
In addition, our results may imply the induction of a VBNC state in enterococcus biofilms treated with sodium hypochlorite. The entry of bacteria into a state described as VBNC has been reported repeatedly for a large number of bacterial species, and among them several Gram-positive bacteria, including bacteria that reside in the oral cavity (Hiyari and Bennett, 2011; E et al., 2015). A bacterium in the VBNC state has been defined as a cell which can be demonstrated to be metabolically active, while being incapable of undergoing the sustained cellular division required for growth in or on a medium normally supporting growth of that cell (Koch, 1997). Importantly, the presence of E. faecalis on dentin slabs following a treatment with sodium hypochlorite may explain the resistance of E. faecalis biofilms to the currently used treatment protocols, and involvement in treatment failure with persistent infections following root canal treatments (Zhang et al., 2015).
In contrast, the anti-biofilm treatment D-Leucine efficiently dispersed dentin-associated biofilms with little effect on the viability of the biofilm cells. The biocompatibility of D-amino acids is especially promising as they were non-cytotoxic to human osteoblasts at concentrations less than 50 mmol/L, 25 times more than the required concentration for biofilm inhibition (Harmata et al., 2015) and were non-toxic when orally administrated (Tsume et al., 2014). Inducing dispersal by a sub-toxic concentrations of an anti-biofilm agent is of high interest (Kolodkin-Gal et al., 2010; Romero and Kolter, 2011; Bucher et al., 2015, 2016), as it is expected to reduce the selective pressure for the success of resistant mutants. Indeed toxic concentrations of D-amino acids were shown to select for mutants that carry various alleles of resistance (Leiman et al., 2015).
The iron chelator 2-2′ bipyridyl efficiently induced cell death within dentin-associated biofilms, but did not change the overall dentin-associated biomass. 2-2′ bipyridyl can inhibit Fe2+ containing enzymes at 10-8 M. However, in the concentrations used in our study it is a widely used ligand (Kaes et al., 2000), and may be appropriate for endocarditis treatment. Thus, our overall result highlights the need and the potential for combination therapies in root canal biofilm infections.
Author Contributions
IK-G, ER, and IT designed experiments. NS, SE, and IK-G performed the experiments. NS, SE, and IK-G contributed materials and reagents. IK-G, SE, ER, and IT wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer TMBR and handling Editor declared their shared affiliation and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgment
This research was supported by the ISF I-CORE grant 152/1, Mr. and Mrs. Dan Kane, Ms. Lois Rosen, by the Larson Charitable Foundation, by Ruth and Herman Albert Scholars Program for New Scientists, by the Ilse Katz Institute for Materials Sciences and Magnetic Resonance Research grant, by the Ministry of Health grant 712376 for alternative research methods, and by the France-Israel Cooperation – Maimonide-Israel Research Program grant 3-13021, and by the Israeli Science Foundation (No. 119/16). IK-G is a recipient of the Rowland and Sylvia Career Development Chair.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.02055/full#supplementary-material
References
Aziz, A., Parmar, D., Mcnaughton, A., and Tompkins, G. R. (2010). Bacterial viability determination in a dentinal tubule infection model by confocal laser scanning microscopy. Methods Mol. Biol. 666, 141–150. doi: 10.1007/978-1-60761-820-1_10
Banin, E., Vasil, M. L., and Greenberg, E. P. (2005). Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. U.S.A. 102, 11076–11081. doi: 10.1073/pnas.0504266102
Branda, S. S., Vik, S., Friedman, L., and Kolter, R. (2005). Biofilms: the matrix revisited. Trends Microbiol. 13, 20–26. doi: 10.1016/j.tim.2004.11.006
Brosco, V. H., Bernardineli, N., Torres, S. A., Consolaro, A., Bramante, C. M., De Moraes, I. G., et al. (2010). Bacterial leakage in obturated root canals-part 2: a comparative histologic and microbiologic analyses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109, 788–794. doi: 10.1016/j.tripleo.2009.11.036
Bucher, T., Kartvelishvily, E., and Kolodkin-Gal, I. (2016). Methodologies for studying B. subtilis biofilms as a model for characterizing small molecule biofilm inhibitors. J. Vis. Exp. doi: 10.3791/54612
Bucher, T., Oppenheimer-Shaanan, Y., Savidor, A., Bloom-Ackermann, Z., and Kolodkin-Gal, I. (2015). Disturbance of the bacterial cell wall specifically interferes with biofilm formation. Environ. Microbiol. Rep. 7, 990–1004. doi: 10.1111/1758-2229.12346
Bystrom, A., and Sundqvist, G. (1985). The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int. Endod. J. 18, 35–40. doi: 10.1111/j.1365-2591.1985.tb00416.x
Cava, F., De Pedro, M. A., Lam, H., Davis, B. M., and Waldor, M. K. (2011). Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids. EMBO J. 30, 3442–3453. doi: 10.1038/emboj.2011.246
Chen, Y., Cao, S., Chai, Y., Clardy, J., Kolter, R., Guo, J. H., et al. (2012). A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol. Microbiol. 85, 418–430. doi: 10.1111/j.1365-2958.2012.08109.x
Costerton, J. W., Cheng, K. J., Geesey, G. G., Ladd, T. I., Nickel, J. C., Dasgupta, M., et al. (1987). Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41, 435–464. doi: 10.1146/annurev.mi.41.100187.002251
Costerton, J. W., Stewart, P. S., and Greenberg, E. P. (1999). Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. doi: 10.1126/science.284.5418.1318
de Almeida, J., Hoogenkamp, M., Felippe, W. T., Crielaard, W., and Van Der Waal, S. V. (2016). Effectiveness of EDTA and modified salt solution to detach and kill cells from Enterococcus faecalis biofilm. J. Endod. 42, 320–323. doi: 10.1016/j.joen.2015.11.017
Dengler, V., Meier, P. S., Heusser, R., Berger-Bachi, B., and Mccallum, N. (2011). Induction kinetics of the Staphylococcus aureus cell wall stress stimulon in response to different cell wall active antibiotics. BMC Microbiol. 11:16. doi: 10.1186/1471-2180-11-16
Deshpande, L. M., Fritsche, T. R., Moet, G. J., Biedenbach, D. J., and Jones, R. N. (2007). Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 58, 163–170. doi: 10.1016/j.diagmicrobio.2006.12.022
Du, T., Shi, Q., Shen, Y., Cao, Y., Ma, J., Lu, X., et al. (2013). Effect of modified nonequilibrium plasma with chlorhexidine digluconate against endodontic biofilms in vitro. J. Endod. 39, 1438–1443. doi: 10.1016/j.joen.2013.06.027
E, J., Jiang, Y. T., Yan, P. F., and Liang, J. P. (2015). Biological changes of Enterococcus faecalis in the viable but nonculturable state. Genet. Mol. Res. 14, 14790–14801. doi: 10.4238/2015.November.18.44
Friedman, L., and Kolter, R. (2004). Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51, 675–690. doi: 10.1046/j.1365-2958.2003.03877.x
Fux, C. A., Costerton, J. W., Stewart, P. S., and Stoodley, P. (2005). Survival strategies of infectious biofilms. Trends Microbiol. 13, 34–40. doi: 10.1016/j.tim.2004.11.010
Gomes, B. P. F. A., Ferraz, C. C. R., Vianna, M. E., Berber, V. B., Teixeira, F. B., and Souza, F. J. (2001). In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int. Endod. J. 34, 424–428. doi: 10.1046/j.1365-2591.2001.00410.x
Haapasalo, M., Shen, Y., Wang, Z., and Gao, Y. (2014). Irrigation in endodontics. Br. Dent. J. 216, 299–303. doi: 10.1038/sj.bdj.2014.204
Haapasalo, M., and Shen, Y. A. (2012). Current therapeutic options for endodontic biofilms. Endod. Topics 22, 79–98. doi: 10.1111/j.1601-1546.2012.00281.x
Harmata, A. J., Ma, Y., Sanchez, C. J., Zienkiewicz, K. J., Elefteriou, F., Wenke, J. C., et al. (2015). D-amino acid inhibits biofilm but not new bone formation in an ovine model. Clin. Orthop. Relat. Res. 473, 3951–3961. doi: 10.1007/s11999-015-4465-9
Hiyari, S., and Bennett, K. M. (2011). Dental diagnostics: molecular analysis of oral biofilms. J. Dent. Hyg. 85, 256–263.
Hochbaum, A. I., Kolodkin-Gal, I., Foulston, L., Kolter, R., Aizenberg, J., and Losick, R. (2011). Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J. Bacteriol. 193, 5616–5622. doi: 10.1128/JB.05534-11
Kaes, C., Katz, A., and Hosseini, M. W. (2000). Bipyridine: the most widely used ligand. A review of molecules comprising at least two 2,2′-bipyridine units. Chem. Rev. 100, 3553–3590.
Khalifa, L., Brosh, Y., Gelman, D., Coppenhagen-Glazer, S., Beyth, S., Poradosu-Cohen, R., et al. (2015). Targeting Enterococcus faecalis biofilms with phage therapy. Appl. Environ. Microbiol. 81, 2696–2705. doi: 10.1128/AEM.00096-15
Kishen, A. (2012). Advanced therapeutic options for endodontic biofilms. Endod. Topics 22, 99–123. doi: 10.1111/j.1601-1546.2012.00284.x
Koch, A. L. (1997). Microbial physiology and ecology of slow growth. Microbiol. Mol. Biol. Rev. 61, 305–318.
Kolodkin-Gal, I., Elsholz, A. K., Muth, C., Girguis, P. R., Kolter, R., and Losick, R. (2013). Respiration control of multicellularity in Bacillus subtilis by a complex of the cytochrome chain with a membrane-embedded histidine kinase. Genes Dev. 27, 887–899. doi: 10.1101/gad.215244.113
Kolodkin-Gal, I., Romero, D., Cao, S., Clardy, J., Kolter, R., and Losick, R. (2010). D-amino acids trigger biofilm disassembly. Science 328, 627–629. doi: 10.1126/science.1188628
Kolter, R., and Greenberg, E. P. (2006). Microbial sciences: the superficial life of microbes. Nature 441, 300–302. doi: 10.1038/441300a
Kuci, A., Alacam, T., Yavas, O., Ergul-Ulger, Z., and Kayaoglu, G. (2014). Sealer penetration into dentinal tubules in the presence or absence of smear layer: a confocal laser scanning microscopic study. J. Endod. 40, 1627–1631. doi: 10.1016/j.joen.2014.03.019
Lam, H., Oh, D. C., Cava, F., Takacs, C. N., Clardy, J., De Pedro, M. A., et al. (2009). D-amino acids govern stationary phase cell wall remodeling in bacteria. Science 325, 1552–1555. doi: 10.1126/science.1178123
Leiman, S. A., Richardson, C., Foulston, L., Elsholz, A. K., First, E. A., and Losick, R. (2015). Identification and characterization of mutations conferring resistance to D-amino acids in Bacillus subtilis. J. Bacteriol. 197, 1632–1639. doi: 10.1128/JB.00009-15
Li, J., and Wang, N. (2014). Foliar application of biofilm formation-inhibiting compounds enhances control of citrus canker caused by Xanthomonas citri subsp. citri. Phytopathology 104, 134–142. doi: 10.1094/PHYTO-04-13-0100-R
Lopez, D., and Kolter, R. (2010). Functional microdomains in bacterial membranes. Genes Dev. 24, 1893–1902. doi: 10.1101/gad.1945010
Lupoli, T. J., Tsukamoto, H., Doud, E. H., Wang, T. S., Walker, S., and Kahne, D. (2011). Transpeptidase-mediated incorporation of D-amino acids into bacterial peptidoglycan. J. Am. Chem. Soc. 133, 10748–10751. doi: 10.1021/ja2040656
Mah, T. F., Pitts, B., Pellock, B., Walker, G. C., Stewart, P. S., and O’toole, G. A. (2003). A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426, 306–310. doi: 10.1038/nature02122
Meire, M. A., Coenye, T., Nelis, H. J., and De Moor, R. J. (2012). Evaluation of Nd:YAG and Er:YAG irradiation, antibacterial photodynamic therapy and sodium hypochlorite treatment on Enterococcus faecalis biofilms. Int. Endod. J. 45, 482–491. doi: 10.1111/j.1365-2591.2011.02000.x
Minogue, T. D., Daligault, H. E., Davenport, K. W., Broomall, S. M., Bruce, D. C., Chain, P. S., et al. (2014). Complete genome assembly of Enterococcus faecalis 29212, a laboratory reference strain. Genome Announc. 2, e968–e914. doi: 10.1128/genomeA.00968-14
Molander, A., Reit, C., Dahlen, G., and Kvist, T. (1998). Microbiological status of root-filled teeth with apical periodontitis. Int. Endod. J. 31, 1–7. doi: 10.1046/j.1365-2591.1998.t01-1-00111.x
Oppenheimer-Shaanan, Y., Steinberg, N., and Kolodkin-Gal, I. (2013). Small molecules are natural triggers for the disassembly of biofilms. Trends Microbiol. 21, 594–601. doi: 10.1016/j.tim.2013.08.005
Paganelli, F. L., Willems, R. J., and Leavis, H. L. (2012). Optimizing future treatment of enterococcal infections: attacking the biofilm? Trends Microbiol. 20, 40–49. doi: 10.1016/j.tim.2011.11.001
Parsek, M. R., and Singh, P. K. (2003). Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57, 677–701. doi: 10.1146/annurev.micro.57.030502.090720
Raad, I., Chatzinikolaou, I., Chaiban, G., Hanna, H., Hachem, R., Dvorak, T., et al. (2003). In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob. Agents Chemother. 47, 3580–3585. doi: 10.1128/AAC.47.11.3580-3585.2003
Ramos, I., Dietrich, L. E., Price-Whelan, A., and Newman, D. K. (2010). Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res. Microbiol. 161, 187–191. doi: 10.1016/j.resmic.2010.01.003
Ricucci, D., and Siqueira, J. F. Jr. (2010). Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod 36, 1277–1288. doi: 10.1016/j.joen.2010.04.007
Romero, D., and Kolter, R. (2011). Will biofilm disassembly agents make it to market? Trends Microbiol. 19, 304–306. doi: 10.1016/j.tim.2011.03.003
Sanchez, C. J. Jr., Prieto, E. M., Krueger, C. A., Zienkiewicz, K. J., Romano, D. R., Ward, C. L., et al. (2013). Effects of local delivery of d-amino acids from biofilm-dispersive scaffolds on infection in contaminated rat segmental defects. Biomaterials 34, 7533–7543. doi: 10.1016/j.biomaterials.2013.06.026
She, P., Chen, L., Liu, H., Zou, Y., Luo, Z., Koronfel, A., et al. (2015). The effects of d-Tyrosine combined with amikacin on the biofilms of Pseudomonas aeruginosa. Microb. Pathog. 86, 38–44. doi: 10.1016/j.micpath.2015.07.009
Shen, Y., Stojicic, S., and Haapasalo, M. (2010). Bacterial viability in starved and revitalized biofilms: comparison of viability staining and direct culture. J. Endod. 36, 1820–1823. doi: 10.1016/j.joen.2010.08.029
Shlezinger, M., Houri-Haddad, Y., Coppenhagen-Glazer, S., Resch, G., Que, Y. A., Beyth, S., et al. (2016). Phage therapy: a new horizon in the antibacterial treatment of oral pathogens. Curr. Top. Med. Chem. doi: 10.2174/1568026616666160930145649 [Epub ahead of print].
Singh, P. K., Parsek, M. R., Greenberg, E. P., and Welsh, M. J. (2002). A component of innate immunity prevents bacterial biofilm development. Nature 417, 552–555. doi: 10.1038/417552a
Stewart, P. S. (2002). Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292, 107–113. doi: 10.1078/1438-4221-00196
Tay, C. X., Quah, S. Y., Lui, J. N., Yu, V. S., and Tan, K. S. (2015). Matrix metalloproteinase inhibitor as an antimicrobial agent to eradicate Enterococcus faecalis biofilm. J. Endod. 41, 858–863. doi: 10.1016/j.joen.2015.01.032
Tsume, Y., Incecayir, T., Song, X., Hilfinger, J. M., and Amidon, G. L. (2014). The development of orally administrable gemcitabine prodrugs with D-enantiomer amino acids: enhanced membrane permeability and enzymatic stability. Eur. J. Pharm. Biopharm. 86, 514–523. doi: 10.1016/j.ejpb.2013.12.009
Vlamakis, H., Chai, Y., Beauregard, P., Losick, R., and Kolter, R. (2013). Sticking together: building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 11, 157–168. doi: 10.1038/nrmicro2960
Wang, Q. Q., Zhang, C. F., Chu, C. H., and Zhu, X. F. (2012). Prevalence of Enterococcus faecalis in saliva and filled root canals of teeth associated with apical periodontitis. Int. J. Oral Sci. 4, 19–23. doi: 10.1038/ijos.2012.17
Yu, C., Wu, J. J., Contreras, A. E., and Li, Q. L. (2012). Control of nanofiltration membrane biofouling by Pseudomonas aeruginosa using D-tyrosine. J. Membr. Sci. 423, 487–494. doi: 10.1016/j.memsci.2012.08.051
Zapata, R. O., Bramante, C. M., De Moraes, I. G., Bernardineli, N., Gasparoto, T. H., Graeff, M. S., et al. (2008). Confocal laser scanning microscopy is appropriate to detect viability of Enterococcus faecalis in infected dentin. J. Endod. 34, 1198–1201. doi: 10.1016/j.joen.2008.07.001
Keywords: biofilms, dentin, viable but non-culturable (VBNC) state, D-amino acids, root canal therapy
Citation: Rosen E, Tsesis I, Elbahary S, Storzi N and Kolodkin-Gal I (2016) Eradication of Enterococcus faecalis Biofilms on Human Dentin. Front. Microbiol. 7:2055. doi: 10.3389/fmicb.2016.02055
Received: 16 September 2016; Accepted: 07 December 2016;
Published: 26 December 2016.
Edited by:
Octavio Luiz Franco, Universidade Católica de Brasília, BrazilReviewed by:
Suzana Meira Ribeiro, Universidade Católica Dom Bosco, BrazilTaia Maria Berto Rezende, Universidade Católica de Brasília, Brazil
Copyright © 2016 Rosen, Tsesis, Elbahary, Storzi and Kolodkin-Gal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eyal Rosen, dr.eyalr@post.tau.ac.il Ilana Kolodkin-Gal, ilana.kolodkin-gal@weizmann.ac.il
†These authors have contributed equally to this work.
 Eyal Rosen
Eyal Rosen Igor Tsesis1†
Igor Tsesis1† Shlomo Elbahary
Shlomo Elbahary Ilana Kolodkin-Gal
Ilana Kolodkin-Gal