- 1Toxoplasmosis Research Center, Mazandaran University of Medical Sciences, Sari, Iran
- 2Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran
- 3Department of Parasitology and Mycology, Sari Medical School, Mazandaran University of Medical Sciences, Sari, Iran
- 4Department of Pharmacology, School of Medicine, Iran University of Medical Sciences Tehran, Iran
- 5Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
The currently available anti-Toxoplasma agents have serious limitations. This systematic review was performed to evaluate drugs and new compounds used for the treatment of toxoplasmosis. Data was systematically collected from published papers on the efficacy of drugs/compounds used against Toxoplasma gondii (T. gondii) globally during 2006–2016. The searched databases were PubMed, Google Scholar, Science Direct, ISI Web of Science, EBSCO, and Scopus. One hundred and eighteen papers were eligible for inclusion in this systematic review, which were both in vitro and in vivo studies. Within this review, 80 clinically available drugs and a large number of new compounds with more than 39 mechanisms of action were evaluated. Interestingly, many of the drugs/compounds evaluated against T. gondii act on the apicoplast. Therefore, the apicoplast represents as a potential drug target for new chemotherapy. Based on the current findings, 49 drugs/compounds demonstrated in vitro half-maximal inhibitory concentration (IC50) values of below 1 μM, but most of them were not evaluated further for in vivo effectiveness. However, the derivatives of the ciprofloxacin, endochin-like quinolones and 1-[4-(4-nitrophenoxy) phenyl] propane-1-one (NPPP) were significantly active against T. gondii tachyzoites both in vitro and in vivo. Thus, these compounds are promising candidates for future studies. Also, compound 32 (T. gondii calcium-dependent protein kinase 1 inhibitor), endochin-like quinolones, miltefosine, rolipram abolish, and guanabenz can be repurposed into an effective anti-parasitic with a unique ability to reduce brain tissue cysts (88.7, 88, 78, 74, and 69%, respectively). Additionally, no promising drugs are available for congenital toxoplasmosis. In conclusion, as current chemotherapy against toxoplasmosis is still not satisfactory, development of well-tolerated and safe specific immunoprophylaxis in relaxing the need of dependence on chemotherapeutics is a highly valuable goal for global disease control. However, with the increasing number of high-risk individuals, and absence of a proper vaccine, continued efforts are necessary for the development of novel treatment options against T. gondii. Some of the novel compounds reviewed here may represent good starting points for the discovery of effective new drugs. In further, bioinformatic and in silico studies are needed in order to identify new potential toxoplasmicidal drugs.
Introduction
Toxoplasma gondii (T. gondii), an obligate intracellular, parasitic protozoan, is the etiologic agent of toxoplasmosis. About 30–50% of the world population is infected with the parasite, and it is the most prevalent infection among humans (Tenter et al., 2000; Flegr et al., 2014). Worldwide, over 1 billion people are estimated to be infected with T. gondii (Hoffmann et al., 2012). Its prevalence in some countries is high (e.g., Brazil, 77.5%; Sao Tome and Principe, 75.2%; Iran, 63.9%; Colombia, 63.5%; and Cuba, 61.8%) (Pappas et al., 2009). The annual incidence of congenital toxoplasmosis was estimated to be 190,100 cases globally (Torgerson and Mastroiacovo, 2013).
In the United States, the Centers for Disease Control and Prevention (CDC) reported that 22.5% of the population 12 years and older have been infected with Toxoplasma with 1.1 million new infections each year, making it the second most common cause of deaths due to foodborne diseases (an estimated 327 deaths) and the fourth leading cause of hospitalizations attributable to foodborne illness (an estimated 4428 hospitalizations). Also, an estimated 400–4000 infants are born with congenital toxoplasmosis in the United States each year (Jones et al., 2014).
T. gondii has three infectious stages of sporozoites (in oocysts), tachyzoites (rapidly multiplying form), and bradyzoites (tissue cyst form). Among them, tachyzoites are responsible for clinical manifestations and the acute phase of the disease. They are susceptible to the immune response of the host and to drug action. The resistant cyst form of the parasite is resistant to both the immune system and drugs (Hill and Dubey, 2002).
Acute toxoplasmosis in healthy individuals is usually subclinical and asymptomatic, but may lead to chronic infection. However, toxoplasmosis can lead to great morbidity and mortality rates in imunocompromised or congenitally infected individuals (Dubey and Jones, 2008; Ahmadpour et al., 2014). In AIDS patients, presence of the parasite causes necrotizing encephalitis and focal cerebral lesions in the central nervous system (CNS) from primary or recrudescent infection. In immunocompetent patients, latent toxoplasmosis occurs with the formation of cysts principally in the CNS (Martins-Duarte et al., 2006).
In the recent years, the development of well-tolerated and safe specific immunoprophylaxis against toxoplasmosis is a highly valuable goal for global disease control (Lim and Othman, 2014). Immunotherapeutics strategies for improving toxoplasmosis control could either be a vaccine which would induce strong protective immunity against toxoplasmosis, or passive immunization in cases of disease recrudescence. In the last few years, much progress has been made in vaccine research on DNA vaccination, protein vaccination, live attenuated vaccinations, and heterologous vaccination; while there were few candidates on passive immunization. New vaccine candidates have been tested, including in particular proteins from T. gondii ROP, MIC, and GRA organelles, multi-antigen vaccines, novel adjuvants but until now the researches could not access to a proper vaccine for prevention of toxoplasmosis in human (Zhang et al., 2013, 2015).
The recommended drugs for treatment or prophylaxis of toxoplasmosis are pyrimethamine and sulfadiazine. Unfortunately, these drugs have side effects such as neutropenia, severe drop of platelet count, thrombocytopenia, leucopenia, elevation in serum creatinine and serum liver enzymes, hematological abnormalities, and hypersensitivity reactions (Bosch-Driessen et al., 2002; Silveira et al., 2002; Schmidt et al., 2006). In addition, other drugs, such as azithromycin, clarithromycin, spiramycin, atovaquone, dapsone, and cotrimoxazole (trimethoprim-sulfamethoxazole), have been used for clinical toxoplasmosis. However, these drugs are poorly tolerated and have no effect on the bradyzoite form (Araujo and Remington, 1992; Petersen and Schmidt, 2003; Serranti et al., 2011).
In a clinical trial, 24% of sera positive women treated with spiramycin and pyrimethamine plus sulfadoxine combination delivered Toxoplasma infected infants in France (Bessières et al., 2009). Spiramycin monotherapy can be effective during the early stage of pregnancy to prevent prenatal transmission (Julliac et al., 2010). More than 50% of patients treated with spiramycin retained T. gondii DNA in blood and remained infected (Habib, 2008).
In recent years, studies have focused on finding safe drugs with novel mechanisms of action against T. gondii. Accordingly, there is an urgent need to evaluate new drugs based on novel and innovative therapeutic strategies against T. gondii that are both efficacious and nontoxic for humans (Rodriguez and Szajnman, 2012; Vanagas et al., 2012; Angel et al., 2013). Therefore, the goal of the present systematic review was to retrieve published studies related to in vitro and in vivo evaluation of drugs and compounds for the treatment of toxoplasmosis (2006–2016) in order to prepare comprehensive data for designing more accurate investigations in future.
Methodology
This review followed the preferred reporting items for systematic reviews (PRISMA) guidelines (Moher et al., 2009).
Literature Search, Study Selection, and Data Extraction
English databases, including PubMed, Science Direct, Scopus, Google Scholar, ISI Web of Science, and EBSCO, were systematically searched for papers on in vitro and in vivo evaluation of anti-Toxoplasma activities of drugs and compounds, published worldwide, from 2006 to 2016. The keywords included were: “Toxoplasmosis,” “T. gondii,” “Anti-Toxoplasma,” “Drug,” “Anticoccidial,” “Treatment,” “In vitro,” “In vivo,” and “Compound.”
Papers written in English were selected. Gray literature and abstracts of articles which were published in congresses were not explored. In addition, in order to avoid missing any articles, whole references of the papers were meticulously hand-searched. Among English articles found with the mentioned strategies, full text papers that used laboratory method both in vitro and in vivo were included.
Also, studies with at least one of the following criteria were excluded: (1) studies that were not relevant; (2) articles not available in English; (3) studies on treatments for ocular infection; (4) articles that were of review or descriptive study type; (5) articles which contained no eligible data; (6) case series reports; (7) the data were duplicated from other studies or we were unable to obtain them; (8) those that were on efficacy of anti-T. gondii medicines in humans; and (9) any drug with an IC50 value > 10 μM.
Data Collection
All the experimental studies that were carried out to evaluate the efficacy of either drugs or compounds against T. gondii both in vitro and in vivo were included, and replicates were excluded. The inclusion criteria for selection of in vitro studies were important information about medication used for the experiments, type of cells used for culture, identification of the Toxoplasma strain, laboratory methods used for assessing drug activities, and main results comprising of the 50% inhibitory concentration (IC50). We reported in vivo studies used animal models, Toxoplasma strain, route of infection, the treatment schedule (dosage, route of administration, duration of treatment), the criteria for assessing drug activity (mainly survival for acute toxoplasmosis, histology, and brain cyst burdens for chronic infection), and the main results.
Results
Analysis of the Included Literature
A total of 118 papers (83 studies in vitro, 59 in vivo, 27 both in vitro and in vivo) published from 2006 to 2016, were included in the systematic review. Figure 1 briefly shows the search process in this systematic review article.
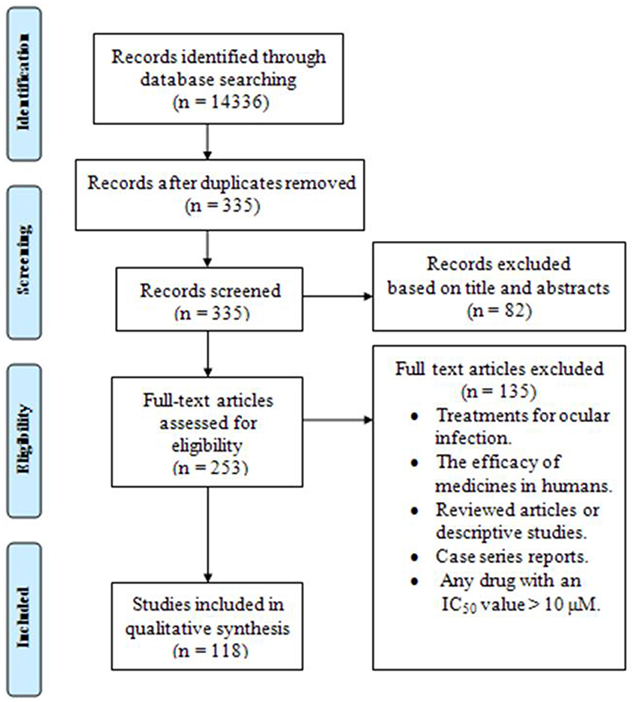
Figure 1. The PRISMA flow diagram of the search strategy, study selection, and data management procedure of in vitro and in vivo activities of anti-Toxoplasma drugs and compounds (2006–2016).
Mechanisms of Action
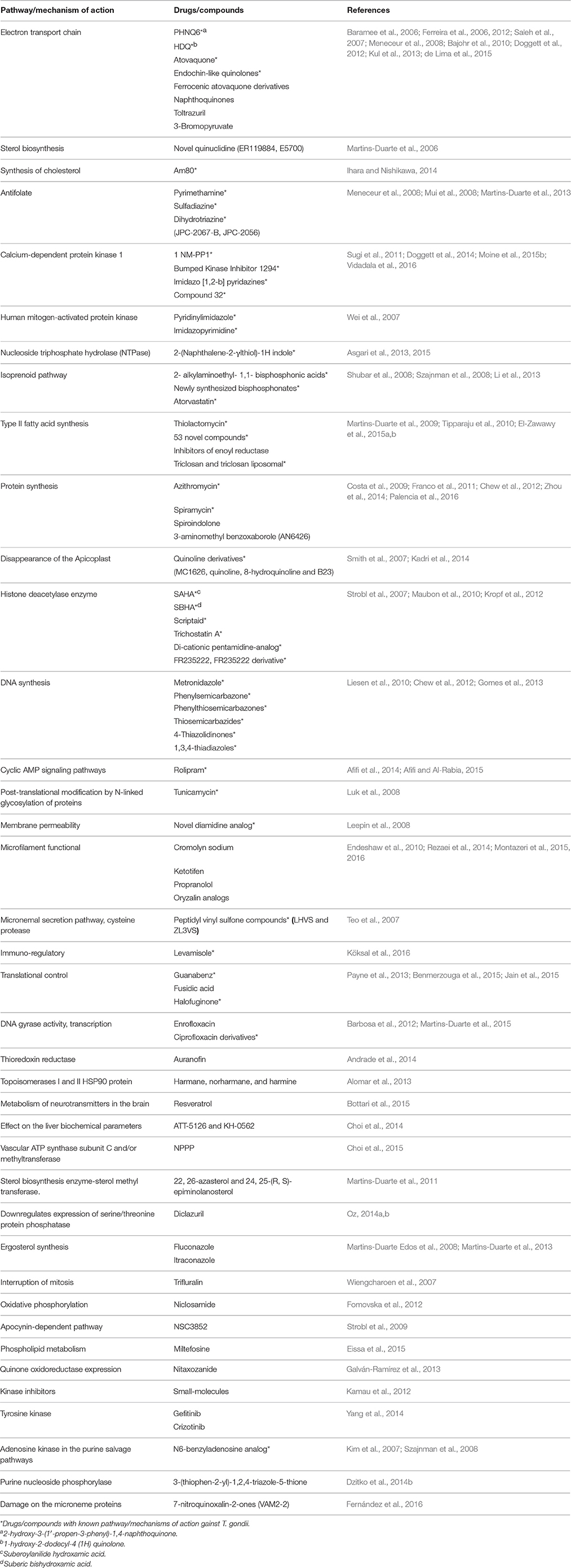
In the current systematic review, 80 clinically available drugs (Table 1) and several new compounds with more than 39 pathways/ mechanisms of action were evaluated against T. gondii in both in vitro and in vivo studies. Several target based drug screens were also identified against T. gondii include mitochondrial electron transport chain, calcium-dependent protein kinase 1, type II fatty acid synthesis, DNA synthesis, DNA replication, etc. (Table 2). Also, drugs/compounds with known mechanisms of action on life stages of T. gondii are shown in Figure 2. Our collective data indicated that many of the drugs/ compounds evaluated against T. gondii act on the apicoplast. Therefore, the apicoplast represents as a potential drug target for new chemotherapy.
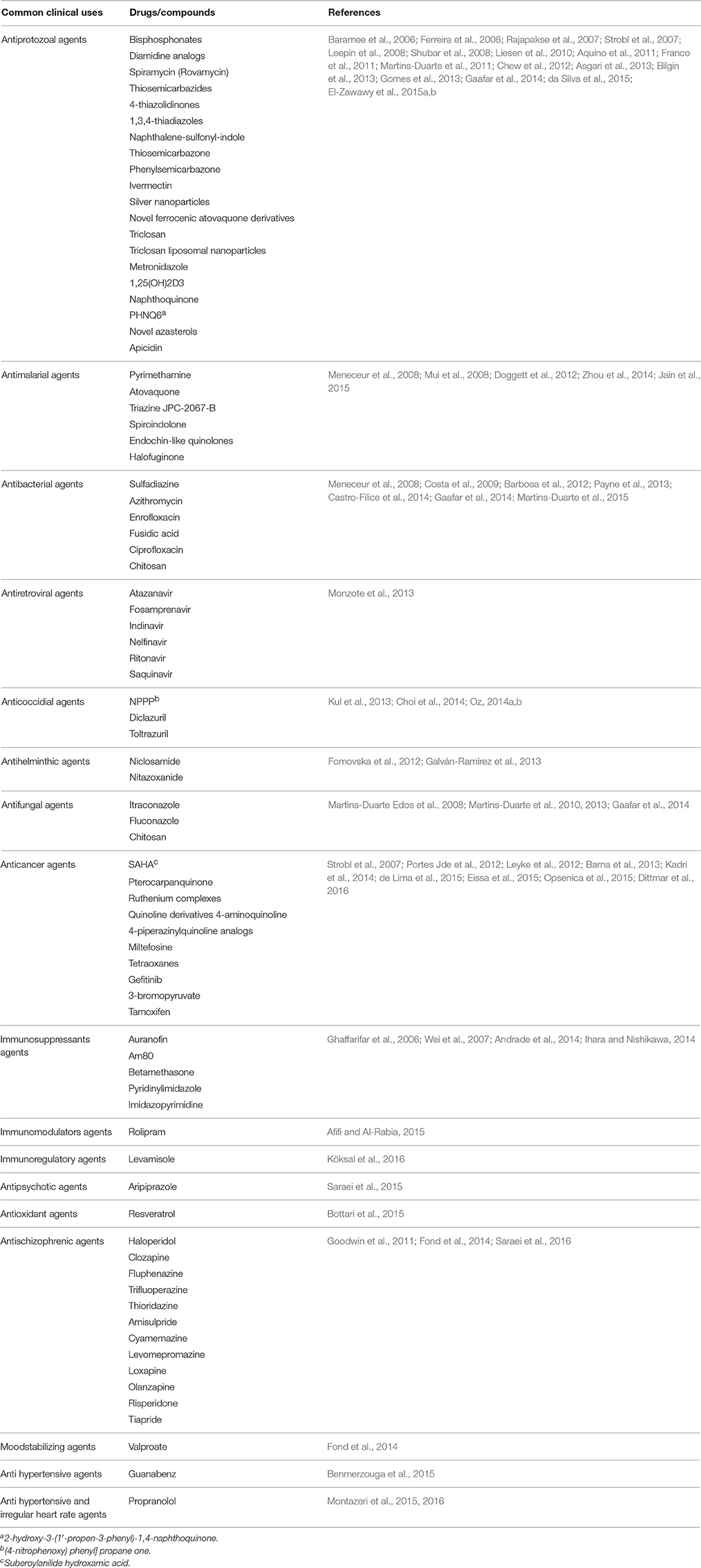
Table 1. Clinically available drugs/compounds evaluated against T. gondii in vitro and in vivo studies.
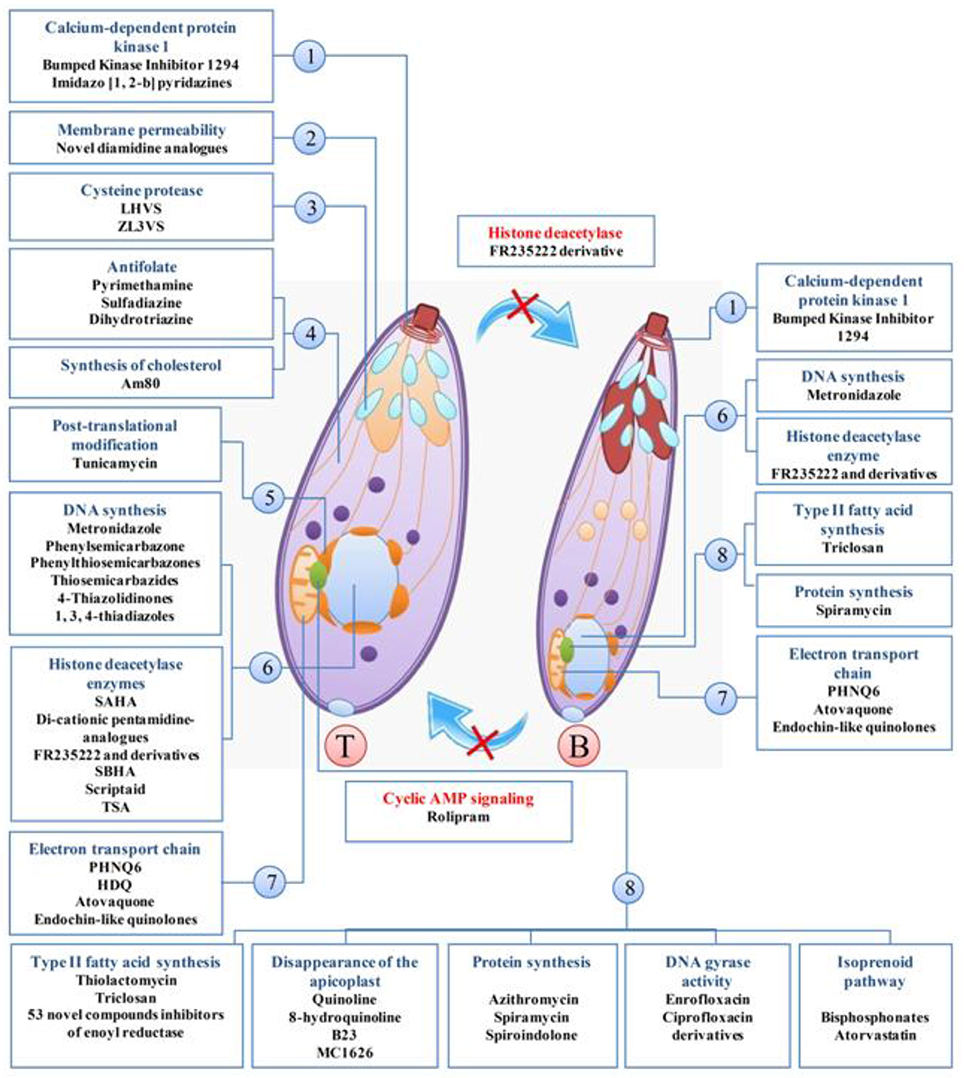
Figure 2. Drugs/compounds with known mechanisms of action on life stages of T. gondii, tachyzoites (T), and bradyzoites (B). 1, apical end; 2, Cell membrane; 3, microneme; 4, cytosol; 5, endoplasmic reticulum; 6, core; 7, mitochondria; 8, apicoplast.
The Investigated Strains
T. gondii has three main clonal lineages in population structure; type I (including a highly virulent RH strain), Type II (including ME49 and PRU, avirulent strains), and Type III (including avirulent strains like NED), which is correlated with virulence expression in mice (Howe and Sibley, 1995).
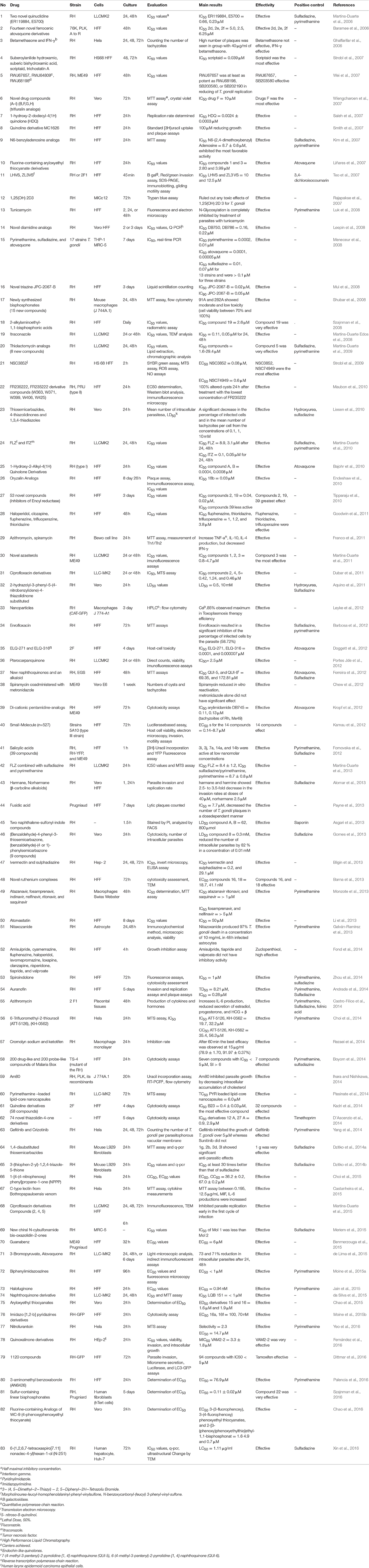
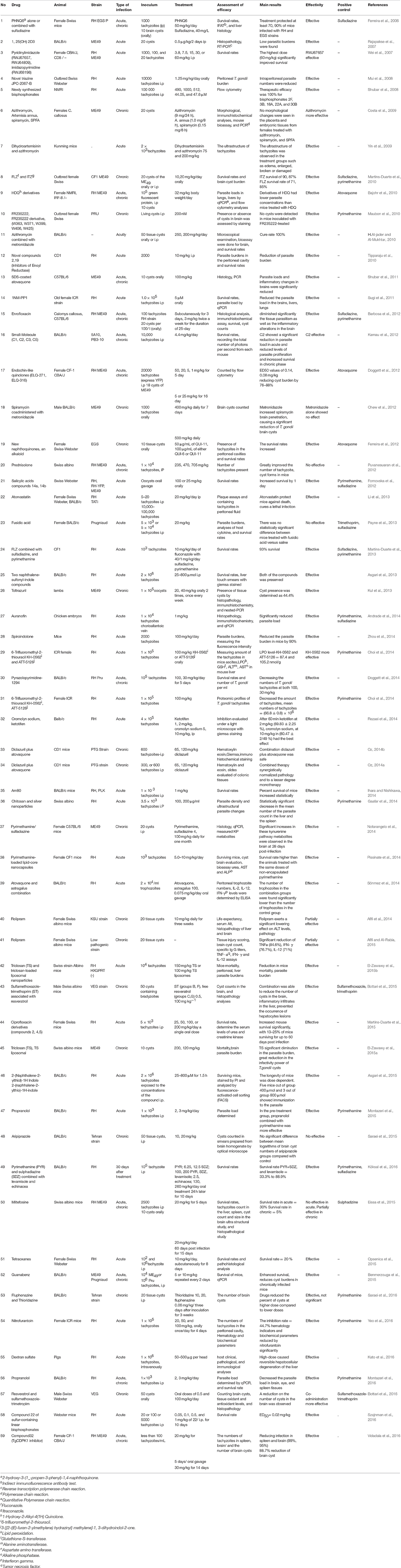
In vitro and in vivo screening methods were used of type I T. gondii (mostly RH strain; 76 studies in vitro, and 36 in vivo). Because type I RH strain is highly virulent in mice, causing 100% mortality, but types II and III are relatively less virulent. Although in some studies, ME49 (7 studies in vitro, and 17 in vivo), Prugniaud, EGS, and VEG strains were used, which showed that the outcome of infections depends on the challenge dose and on the genotype of the host (Szabo and Finney, 2016). Details about the investigated strains in vitro and in vivo are shown in Tables 3, 4, respectively.
Cell Culture
The cell cultures used in in vitro studies were mostly human foreskin fibroblast (HFF; 39 studies), LLCMK2 (12 studies), Vero (11 studies), Hela (6 studies), mouse macrophage cell line (J774A.1) (5 studies), and MRC-5 (2 studies; Table 3).
Laboratory Animals
T. gondii can infect most warm-blooded animals, and is studied in different animal models depending on the nature of the investigation (Szabo and Finney, 2016). The animal model used in studies was mostly mice (16 studies BALB/c and 19 studies Swiss-Webster). In murine models of acute toxoplasmosis, some medicines were protective even when administered at low dosages. But some drugs despite of their excellent in vitro activity were poorly protective in murine models with acute toxoplasmosis (Payne et al., 2013).
Diagnostic Tests and Evaluation Methods
The present review outlines the results of in vitro screening methods including morphological assay, incorporation of [3H] uracil assay, plaque assays, enzyme-linked immunosorbent assay (ELISA), colorimetric micro titer assay (b-galactosidase assay), flow cytometric quantification assay, and cell viability assay. Numerous versions of fluorescent proteins have been expressed in T. gondii (Kim et al., 2001). The reporter genes used in vitro and in vivo studies were the green fluorescent protein (GFP) and yellow fluorescent protein (YFP). Parasites expressing fluorescent proteins can also be analyzed and sorted by flow cytometry. This technology used for drugs screening in 10 studies.
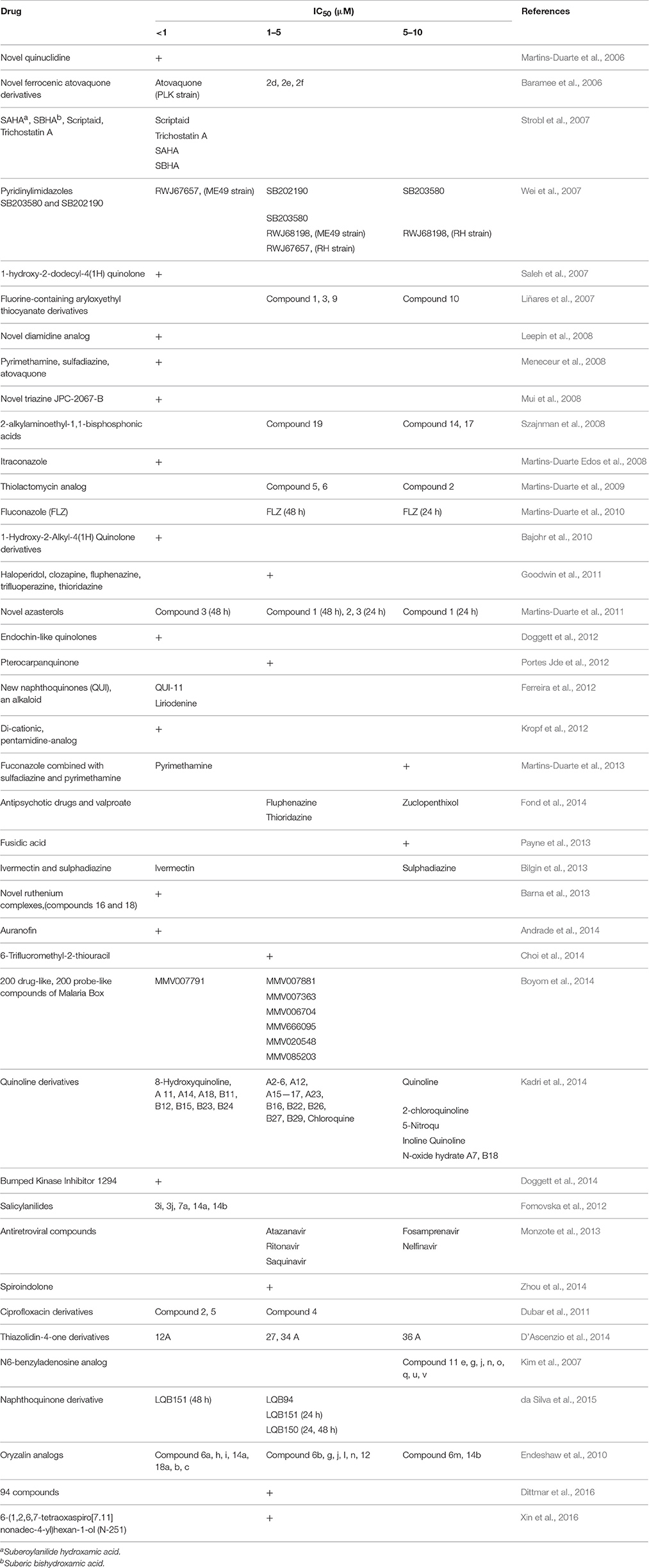
Details about the diagnostic methods and drug dosage under in vivo conditions are shown in Table 4. Also, a comprehensive list of drugs/compounds evaluated against T. gondii with regard to IC50 is illustrated in Table 5.
Discussion
The aim of this systematic review was to investigate the in vitro and in vivo effects of anti-Toxoplasma drugs and synthetic compounds, from 2006 to 2016. The current anti-T. gondii chemotherapy is deficient; as it is not well-tolerated by immunocompromised patients and cannot completely eradicate tissue cysts produced by the parasite (Rodriguez and Szajnman, 2012). Therefore, developing new, safe, effective, and well-tolerated drugs with novel mechanisms of action could be a global priority (Lai et al., 2012). An ideal drug for prophylaxis and/or treatment of toxoplasmosis would show effective penetration and concentration in the placenta, transplacental passage, parasiticidal properties vs. the different parasitic stages, penetration into cysts, and distribution in the main sites. No available drug fulfills these criteria (Derouin et al., 2000; Montoya and Liesenfeld, 2004).
Thus, the findings of the present systematic review article encourage and support more accurate investigations for future to select new anti-Toxoplasma drugs and strategies in designing new targets with specific activity against the parasite.
Activities of Anti-Toxoplasma Clinically Available Drugs
With growing parasite resistance to therapeutic drugs and in the absence of a vaccine, to increase the effectiveness of drugs, various changes have been made in construction of the clinically available medicines. Thus, the activity of new formulations of clinically available drugs against T. gondii should be evaluated to find alternative treatments for toxoplasmosis (da Cunha et al., 2010).
Interestingly, encapsulation of pyrimethamine improved the efficacy and tolerability of this drug against acute toxoplasmosis in mice and can be considered as an alternative for reducing the dose and side effects of pyrimethamine (Pissinate et al., 2014). Recently, researchers reported that computational analysis of biochemical differences between human and T. gondii dihydrofolate reductase enabled the design of inhibitors with both improved potency and selectivity against T. gondii (Welsch et al., 2016). El-Zawawy et al. reported that incorporating triclosan into in the lipid bilayer of liposomes allowed its use in lower doses, which in turn, reduced its biochemical adverse effects (El-Zawawy et al., 2015b). In another study, sodium dodecyl sulfate (SDS)-coated atovaquone nanosuspensions (ANSs) considerably increased the therapeutic efficacy against experimentally reactivated and acquired toxoplasmosis by improving passage of gastrointestinal and blood-brain barriers. Accordingly, coating of ANSs with SDS may improve the treatment of toxoplasmic encephalitis and other cerebral diseases (Shubar et al., 2011).
Also, various studies showed that a number of drugs were investigated for the mechanisms of action summarized in Table 2 and Figure 2. One study discussing the metabolic differences between the host and the parasite noted that dihydrofolate reductase, isoprenoid pathway, and T. gondii histone deacetylase are promising molecular targets (Rodriguez and Szajnman, 2012).
Novel triazine JPC-2067-B (4, 6-diamino-1, 2-dihydro-2, 2-dimethyl-1-(3′(2-chloro-, 4-trifluoromethoxyphenoxy)propyloxy)-1, 3, 5-triazine), the anti-folate medicines, is highly effective against T. gondii with an IC50 of 0.02 μM, which is more efficacious than pyrimethamine and has in vitro cidal activity. Additionally, pro-drug JPC-2056 (1-(3′-(2-chloro-4-trifluoromethoxyphenyloxy) propyl oxy)-5-isopropylbiguanide) is effective in vivo when administered orally (Mui et al., 2008). Moreover, histone deacetylase is potentially a very important drug target in T. gondii, since scriptaid and trichostatin A had the highest effect against T. gondii tachyzoite proliferation with the IC50 of 0.039 and 0.041 μM, respectively (Strobl et al., 2007). For promising anti- T. gondii drugs/compounds, assessment of their ability to control parasite growth is a key step in drug development (McFarland et al., 2016).
A large number of research papers suggested that the apicoplast represents a potential drug target for new chemotherapy, as it is essential to the parasite and it is absent in host cells. Functions of the apicoplast include fatty acid synthesis, protein synthesis, DNA replication, electron transport, and heme biosynthesis (Yung and Lang-Unnasch, 2004). Some of the drugs evaluated against T. gondii are shown to act in the apicoplast such as thiolactomycin, triclosan (TS), azithromycin, fusidic acid, ciprofloxacin, and quinoline derivatives (Costa et al., 2009; Martins-Duarte et al., 2009, 2015; Payne et al., 2013; Kadri et al., 2014; El-Zawawy et al., 2015b).
In T. gondii, FAS-II enzymes are present in the apicoplast and are essential for its survival. The key enzyme in this process is the ENR enzyme, which is not found in mammals (Surolia and Surolia, 2001). This enzyme catalyzes the last reductive step of the type II FAS pathway. The TS, which inhibits type II FAS, significantly reduced mice mortality, parasite burden, as well as viability and infectivity of tachyzoites and cysts harvested from infected treated mice and their brains. Accordingly, TS is proved as an effective, promising, and safe preventive drug against acute and chronic murine toxoplasmosis. Liposomal formulation of TS enhanced its efficacy and allowed its use at a lower dose (Surolia and Surolia, 2001; El-Zawawy et al., 2015a,b). Among apicoplast pathways, DNA replication is an important potential chemotherapeutic target. Fluoroquinolones are the known DNA replication inhibitors that target prokaryotic type II topoisomerases (Collin et al., 2011). In two studies, researchers showed that derivatives of the antibiotic ciprofloxacin, a fluoroquinolone, are active against T. gondii tachyzoites both in vitro and in vivo (Neville et al., 2015). While all mice treated with ciprofloxacin died by day 10 post-infection, some mice treated with ciprofloxacin derivatives remained alive for at least 60 days, suggesting that ciprofloxacin derivatives cured T. gondii infection in treated mice (Dubar et al., 2011; Martins-Duarte et al., 2015).
Anti-Toxoplasma Activities of New Synthetic Compounds
There are numerous reports on efficacy of many new synthetic compounds with a focus on identifying drug candidates with innovative and acceptable profiles against T. gondii. The anti-coccidial effect of 1-[4-(4-nitrophenoxy) phenyl] propane-1-one (NPPP), a synthetic compound, was studied both in vitro and in vivo. Treatment with NPPP showed anti-Toxoplasma activity in vitro with a lower EC50 value than pyrimethamine. In ICR mice infected with T. gondii, oral administration of NPPP for 4 days showed statistically significant anti-Toxoplasma activity with lower number of tachyzoites than those of the negative control (Choi et al., 2015).
In a study by Kadri et al. anti-Toxoplasma properties of 58 newly synthesized quinoline compounds were evaluated. A significant improvement in anti-Toxoplasma effect among quinoline derivatives was detected in B11, B12, B23, and B24. Among these compounds, B23 was the most effective compound with the IC50 value of < 1 μM, displaying its anti-Toxoplasma effects and ability to cause the disappearance of the apicoplast (40–45% of the parasites lost their apicoplasts; Kadri et al., 2014).
In a study by Boyom et al. the strategy adopted was to repurpose the open access Malaria Box to identify chemical series active against T. gondii. The results showed that the most interesting compound was MMV007791, a piperazine acetamide, which has an IC50 of 0.19 μM. This compound is novel for its anti-Toxoplasma activity, and of course, further studies on the rates and mechanisms of compound action will elucidate these considerations (Boyom et al., 2014).
Tetraoxanes, anti-cancer molecules, were tested in vivo against T. gondii. Subcutaneous, administration of a 10 mg/kg/day dose of derivative 21, for 8 days allowed the survival of 20% of infected mice, demonstrating the high potential of tetraoxanes for the treatment of T. gondii (Opsenica et al., 2015).
In another study by Moine et al. researchers evaluated in vitro anti-T. gondii activity of 51 compounds with a biphenylimidazoazine scaffold. Eight of these compounds displayed highly potent activity against T. gondii growth in vitro, with 50% effective concentration (EC50) below 1 mM, without demonstrating cytotoxic effects on human fibroblastic cell at equivalent concentrations. However, these compounds have to be evaluated in animal models so as to confirm their in vivo activity (Moine et al., 2015a).
Several pathways were characterized and shown to differ significantly from those of the mammalian host cells, thus, revealing an attractive area for therapeutic intervention. 1-Hydroxy-2-Alkyl-4 (1H) quinolone derivatives inhibit the fourth step of the essential de novo synthesis of pyrimidine, which uses ubiquinol reduction as an electron sink for dihydroorotate oxidation (Saleh et al., 2007). Also, newly synthesized bisphosphonates interfere with the mevanolate pathway, which leads to the synthesis of sterols and polyisoprenoid compounds that are important for parasite survival (Shubar et al., 2008).
Interestingly, Kamau et al. identified novel kinases that are integral to essential pathways, elucidating their mechanism of action and ultimately, identifying new drug targets (Kamau et al., 2012). In that study, 527 compounds were evaluated in vitro; also, they assessed the impact of the inhibitory compounds C1, C2, C3, and C5 in mouse models of toxoplasmosis. C2 was found quite effective in decreasing the parasite burden and increasing mice survival. These results should be considered with caution, since there are a number of factors are at play in whether a compound will be in vivo effective, such as solubility in vivo, access to different tissues, and host metabolic processes (Kamau et al., 2012). In a recent study, Dittmar et al. screened a collection of 1,120 compounds, 94 of which were blocked parasite replications with IC50 of <5 μM. These data suggest that tamoxifen restricts Toxoplasma growth by inducing xenophagy or autophagic destruction of this parasite (Dittmar et al., 2016). According to a new study, in silico screening is useful, particularly in the identification of molecular targets in the laboratory. Fernandez et al. synthesized VAM2 compounds (7-nitroquinoxalin-2-ones), based on the design obtained from an in silico prediction with the software TOMOCOMD-CARDD. From the group of VAM2 compounds, Fernandez et al. chose VAM2-2 with an IC50 of 3.3 μM against T. gondii. However, more studies are required to evaluate its effect on the cysts formed by of the parasite and in animal models of toxoplasmosis (Fernández et al., 2016).
Activity of Drugs, Compounds, and Combined Therapy against Cysts
An ideal drug against toxoplasmosis should not only be effective against the proliferative stage of the parasite but also exert dual activity against the tissue cyst stage and penetration into cysts (Benmerzouga et al., 2015). Currently, there is no approved therapy that eliminates the tissue cysts responsible for chronic infection (Innes, 2010). Derouin reported that among the drugs commonly used in humans, only atovaquone and azithromycin were found effective after long-term incubation. Besides, arpinocid-N-oxyde, an anticoccidial for veterinary use, was efficient at a high dosage (Derouin, 2005).
Recently, investigators have focused on guanabenz for in vivo studies, as guanabenz inhibitor of eIF2a dephosphorylation, is already an food and drug administration (FDA) approved drug and has excellent solubility with good penetration into the CNS. The results of that study show that guanabenz (5 mg/kg/day) not only protects mice against acute toxoplasmosis, but also reduces 69% of the number of brain cysts in chronically infected animals. This finding suggested that guanabenz can be repurposed into an effective antiparasitic with a unique ability to diminish tissue cysts in the brain (Benmerzouga et al., 2015).
Another study showed that miltefosine had no efficacy in controlling acute toxoplasmosis after 5 days of treatment; however, a 15-day treatment against the established chronic stage led to a 78% reduction of cysts in the brain. Additionally, the remaining cysts were noticeably smaller upon microscopic examination, suggesting that the drug effectively penetrates the blood-brain barrier, and that extension of treatment time may produce greater effects (Eissa et al., 2015).
In another study by Maubon et al. FR235222 and its derivatives were identified as new lead compounds for use against acute and chronic toxoplasmosis both in vitro and in vivo. In vivo experiments indicated that FR235222, as a histone deacetylase inhibitor, is able to access the bradyzoites within the cyst. The ability of FR235222 to permeate the membrane wall is a major advantage for crossing the blood-brain barrier and CNS tissue, where Toxoplasma cysts are located. This opens a promising way to develop drugs that are selective against Toxoplasma and those that have sterilizing activity, especially in patients with cysts, who are at risk for reactivating acute toxoplasmosis (patients with HIV infection, hematological malignancies, or transplantation). Still, effectiveness of FR235222 against chronically infected mice remains to be directly demonstrated in vivo (Maubon et al., 2010).
In a new study Vidadala et al. identified compounds 32 (T. gondii calcium-dependent protein kinase 1 inhibitor) a promising lead for the development of a new antitoxoplasmosis therapy. Compounds 32 is CNS-penetrant and highly effective in acute and latent mouse models of T. gondii infection, significantly reducing brain cysts by 88.7% (Vidadala et al., 2016).
Many studies reported anti- Toxoplasma effects of different drugs combination with novel compounds. The compound 2-hydroxy-3-(1′-propen-3-phenyl)-1, 4-naphthoquinone (PHNQ6), (50 mg/kg/day) combined with sulfadiazine showed reduction or elimination of brain cysts in vivo (Ferreira et al., 2006). In another study that coadministered spiramycin and metronidazole, spiramycin, did not reach effective concentrations in the brain due to the presence of the efflux transporters multidrug-resistant protein 2, and P-glycoprotein. Metronidazole increased brain penetration of spiramycin, causing a significant reduction of T. gondii brain cysts, with potential clinical translatability for chronic toxoplasmosis treatment. According to the reports, combination therapy leads to faster recovery, less relapse, lower doses of drugs, and fewer side effects of the disease. Furthermore, such combinations are highly promising to develop a drug that is able to eliminate the cyst stage of the parasite, and thus, efficiently impairs relapse of the disease (Chew et al., 2012; Martins-Duarte et al., 2013).
Activity of Drugs against Congenital Toxoplasmosis
In pregnant women, current toxoplasmosis treatment is based on the administration of spiramycin or a drug combination such as sulphadiazine-pyrimethamine-folinic acid (SPFA) in cases of confirmed fetal infection. However, these drugs are not well-tolerated and present many adverse effects due to their toxic effects to the host (Degerli et al., 2003).
Degerli et al. evaluated the effectiveness of azithromycin, artemisia annua infusion, spiramycin, and SPFA in Calomys callosus, such as model of congenital toxoplasmosis. The results demonstrated that the treatment of pregnant C. callosus with azithromycin was effective for inhibiting the vertical transmission of T. gondii ME49 strain, suggesting that it may be an alternative drug of choice for the treatment of congenital infection, since it is able to inhibit fetal infection and offers new perspectives for the treatment of congenital toxoplasmosis. Azithromycin is one of the new generation macrolides with numerous advantages. Mechanism of action of azithromycin is based on the inhibition of protein synthesis in both T. gondii tachyzoite and bradyzoite stages (Degerli et al., 2003), but it may present limited effectiveness against T. gondii, requiring high drug concentrations (Costa et al., 2009). In another study, Oz et al. reported that combined atovaquone and diclazuril therapy is a novel synergistic prophylactic and therapeutic approach to fetal maternal toxoplasmosis (Oz, 2014a). Atovaquone, an inhibitor of mitochondrial electron-transport processes, is an FDA-approved toxoplasmosis therapy but not for use in congenital toxoplasmosis treatment (Oz, 2014a). Another compound, diclazuril, and its related benzeneacetonitriles have long been used in the treatment and prevention of poultry and livestock coccidiosis. In addition, it is known to be a safe compound at therapeutic dose levels (Assis et al., 2010).
Adverse Effects of Drugs
However, anti-Toxoplasma effects of drugs/compounds were reported in many trials, but prednisolone increased the number of tachyzoites and bradyzoites in immunosuppressed infected mice (Puvanesuaran et al., 2012). In addition, betamethasone can escalate the invasion of tachyzoites, in cell culture. It could be suggested that patients under prolonged use of betamethasone and prednisolone should be protected against T. gondii infection. Also, if individuals receiving betamethasone are infected with T. gondii, interferon-gamma may be used to reduce the invasion of tachyzoites (Ghaffarifar et al., 2006).
Conclusions
As current chemotherapy against toxoplasmosis is still not satisfactory, the development of well-tolerated and safe specific immunoprophylaxis in relaxing the need of dependence on chemotherapeutics is a highly valuable goal for global disease control. Immunotherapeutics strategies for improving toxoplasmosis control could either be a vaccine which would induce strong protective immunity against toxoplasmosis, or passive immunization in cases of disease recrudescence. However, with the increasing number of high-risk individuals, such as immunocompromised patients, and absence of a proper vaccine, continued efforts are necessary for the development of novel treatment options against T. gondii. Some of the novel compounds reviewed here may represent good starting points for the discovery of effective new drugs. In further bioinformatic and in silico studies are needed in order to identify new potential toxoplasmicidal drugs.
Author Contributions
AD and MS conceived the idea for this review. MM and SS searched the databases for potentially eligible articles based on their titles and abstracts. AD and MM participated in the study design and wrote the manuscript. SM and EA critically reviewed the manuscript. All authors read and approved the final manuscript for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We also would like to thank of financial support by Vice Chancellors for Research of Mazandaran University of Medical Sciences, Sari, Iran (Grant number: 2085).
References
Al-jader, Z. H., and Al-Mukhtar, M. A. (2010). Synergistic activity of azithromycin combined with metronidazole against toxoplasmosis in experimental mice. Irq. J. Pharm. 10, 1–4.
Afifi, M. A., and Al-Rabia, M. W. (2015). The immunomodulatory effects of rolipram abolish drug-resistant latent phase of Toxoplasma gondii infection in a murine model. J. Microsc. Ultrastruct. 3, 86–91. doi: 10.1016/j.jmau.2014.12.001
Afifi, M. A., Jiman-Fatani, A. A., Al-Rabia, M. W., and Al-Hussainy, N. H. (2014). Application of a phosphodiesterase-4 (PDE4) inhibitor to abort chronic toxoplasmosis and to mitigate consequential pathological changes. J. Microsc. Ultrastruct. 2, 94–99. doi: 10.1016/j.jmau.2014.05.002
Ahmadpour, E., Daryani, A., Sharif, M., Sarvi, S., Aarabi, M., Mizani, A., et al. (2014). Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta-analysis. J. Infect. Dev. Ctries. 8, 1503–1510. doi: 10.3855/jidc.4796
Alomar, M. L., Rasse-Suriani, F. A., Ganuza, A., Cóceres, V. M., Cabrerizo, F. M., and Angel, S. O. (2013). In vitro evaluation of β-carboline alkaloids as potential anti-Toxoplasma agents. BMC Res. Notes 6:193. doi: 10.1186/1756-0500-6-193
Andrade, R. M., Chaparro, J. D., Capparelli, E., and Reed, S. L. (2014). Auranofin is highly efficacious against Toxoplasma gondii in vitro and in an in vivo experimental model of acute toxoplasmosis. PLoS Negl. Trop. Dis. 8:e2973. doi: 10.1371/journal.pntd.0002973
Angel, S. O., Matrajt, M., and Echeverria, P. C. (2013). A review of recent patents on the protozoan parasite HSP90 as a drug target. Recent Pat. Biotechnol. 7, 2–8. doi: 10.2174/1872208311307010002
Aquino, T. M., Nascimento, A. A., Spacov, I. C., Carvalho, C. S., Lima, V. T., Alves, A. Q., et al. (2011). Synthesis, anti-Toxoplasma gondii and antimicrobial activities of 2-hydrazolyl-3-phenyl-5-(4-nitrobenzylidene)-4-thiazolidinone substituted derivatives. Lat. Am. J. Pharm. 30, 1567–1573. doi: 10.1016/j.bmc.2007.09.025
Araujo, F. G., and Remington, J. S. (1992). Recent advances in the search for new drugs for treatment of toxoplasmosis. Int. J. Antimicrob. Agents 1, 153–164. doi: 10.1016/0924-8579(92)90002-9
Asgari, Q., Keshavarz, H., Rezaeian, M., Motazedian, M. H., Shojaee, S., Mohebali, M., et al. (2013). Direct effect of two naphthalene-sulfonyl-indole compounds on Toxoplasma gondii tachyzoite. J. Parasitol. Res. 2013:716976. doi: 10.1155/2013/716976
Asgari, Q., Keshavarz, H., Rezaeian, M., Sadeghpour, H., Miri, R., and Motazedian, M. H. (2015). Anti-toxoplasma activity of 2-(naphthalene-2-γlthiol)-1H indole. Iran. J. Parasitol. 10, 171–180. Available online at: http://ijpa.tums.ac.ir
Assis, R. C., Luns, F. D., Beletti, M. E., Assis, R. L., Nasser, N. M., Faria, E. S., et al. (2010). Histomorphometry and macroscopic intestinal lesions in broilers infected with Eimeriaacervulina. Vet. Parasitol. 168, 185–189. doi: 10.1016/j.vetpar.2009.11.017
Bajohr, L. L., Ma, L., Platte, C., Liesenfeld, O., Tietze, L. F., Groß, U., et al. (2010). In vitro and in vivo activities of 1-hydroxy-2-alkyl-4 (1H) quinolone derivatives against Toxoplasma gondii. Antimicrob. Agents Chemother. 54, 517–521. doi: 10.1128/AAC.01001-09
Baramee, A., Coppin, A., Mortuaire, M., Pelinski, L., Tomavo, S., and Brocard, J. (2006). Synthesis and in vitro activities of ferrocenic aminohydroxynaphthoquinones against Toxoplasma gondii and Plasmodium falciparum. Bioorg. Med. Chem. 14, 1294–1302. doi: 10.1016/j.bmc.2005.09.054
Barbosa, B. F., Gomes, A. O., Ferro, E. A., Napolitano, D. R., Mineo, J. R., and Silva, N. M. (2012). Enrofloxacin is able to control Toxoplasma gondii infection in both in vitro and in vivo experimental models. Vet. Parasitol. 187, 44–52. doi: 10.1016/j.vetpar.2011.12.039
Barna, F., Debache, K., Vock, C. A., Küster, T., and Hemphill, A. (2013). In vitro effects of novel ruthenium complexes in Neospora caninum and Toxoplasma gondii tachyzoites. Antimicrob. Agents Chemother. 57, 5747–5754. doi: 10.1128/AAC.02446-12
Benmerzouga, I., Checkley, L. A., Ferdig, M. T., Arrizabalaga, G., Wek, R. C., and Sullivan, W. J. (2015). Guanabenz repurposed as an antiparasitic with activity against acute and latent toxoplasmosis. Antimicrob. Agents Chemother. 59, 6939–6945. doi: 10.1128/AAC.01683-15
Bessières, M., Berrebi, A., Cassaing, S., Fillaux, J., Cambus, J., Berry, A., et al. (2009). Diagnosis of congenital toxoplasmosis: prenatal and neonatal evaluation of methods used in Toulouse University Hospital and incidence of congenital toxoplasmosis. Mem. Inst. Oswaldo Cruz 104, 389–392. doi: 10.1590/S0074-02762009000200038
Bilgin, M., Yıldırım, T., and Hökelek, M. (2013). In vitro effects of ivermectin and sulphadiazine on Toxoplasma gondii. Balkan Med. J. 30, 19. doi: 10.5152/balkanmedj.2012.098
Bosch-Driessen, L. H., Verbraak, F. D., Suttorp-Schulten, M. S., van Ruyven, R. L., Klok, A. M., Hoyng, C. B., et al. (2002). A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am. J. Ophthalmol. 134, 34–40. doi: 10.1016/S0002-9394(02)01537-4
Bottari, N. B., Baldissera, M. D., Tonin, A. A., Rech, V. C., Alves, C. B., D'Avila, F., et al. (2016). Synergistic effects of resveratrol (free and inclusion complex) and sulfamethoxazole-trimetropim treatment on pathology, oxidant/antioxidant status and behavior of mice infected with Toxoplasma gondii. Microb. Pathog. 95, 166–174. doi: 10.1016/j.micpath.2016.04.002
Bottari, N. B., Baldissera, M. D., Tonin, A. A., Rech, V. C., Nishihira, V. S., Thomé, G. R., et al. (2015). Sulfamethoxazole-trimethoprim associated with resveratrol for the treatment of toxoplasmosis in mice: influence on the activity of enzymes involved in brain neurotransmission. Microb. Pathog. 79, 17–23. doi: 10.1016/j.micpath.2015.01.001
Boyom, F. F., Fokou, P. V., Tchokouaha, L. R., Spangenberg, T., Mfopa, A. N., Kouipou, R. M., et al. (2014). Repurposing the open access malaria box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob. Agents Chemother. 58, 5848–5854. doi: 10.1128/AAC.02541-14
Castanheira, L., Naves de Souza, D. L., Silva, R. J., Barbosa, B., Mineo, J. R., Tudini, K. A., et al. (2015). Insights into anti-parasitism induced by a C-type lectin from Bothrops pauloensis venom on Toxoplasma gondii. Int. J. Biol. Macromol. 74, 568–574. doi: 10.1016/j.ijbiomac.2014.11.035
Castro-Filice, L. S., Barbosa, B. F., Angeloni, M. B., Silva, N. M., Gomes, A. O., Alves, C. M., et al. (2014). Azithromycin is able to control Toxoplasma gondii infection in human villous explants. J. Transl. Med. 12:132. doi: 10.1186/1479-5876-12-132
Chao, M. N., Li, C., Storey, M., Falcone, B. N., Szajnman, S. H., Bonesi, S. M., et al. (2016). Activity of fluorine-containing analogues of WC−9 and structurally related analogues against two intracellular parasites: Trypanosoma cruzi and Toxoplasma gondii. ChemMedChem 11, 2690–2702. doi: 10.1002/cmdc.201600505
Chao, M. N., Matiuzzi, C. E., Storey, M., Li, C., Szajnman, S. H., Docampo, R., et al. (2015). Aryloxyethyl thiocyanates are potent growth inhibitors of Trypanosoma cruzi and Toxoplasma gondii. ChemMedChem 10, 1094–1108. doi: 10.1002/cmdc.201500100
Chew, W. K., Segarra, I., Ambu, S., and Mak, J. W. (2012). Significant reduction of brain cysts caused by Toxoplasma gondii after treatment with spiramycin coadministered with metronidazole in a mouse model of chronic toxoplasmosis. Antimicrob. Agents Chemother. 56, 1762–1768. doi: 10.1128/AAC.05183-11
Choi, H. J., Lee, J. H., Yeo, S. J., Kaewintajuk, K., Yi, K. Y., Kim, S., et al. (2015). Evaluation of anti-coccidial effects of 1-[4-(4-nitrophenoxy) phenyl] propane-1-one and identification of its potential target proteins in Toxoplasma gondii. Arch. Pharm. Res. 38, 752–760. doi: 10.1007/s12272-014-0400-y
Choi, H. J., Yu, S. T., Lee, K. I., Choi, J. K., Chang, B. Y., Kim, S. Y., et al. (2014). 6-trifluoromethyl-2-thiouracil possesses anti-Toxoplasma gondii effect in vitro and in vivo with low hepatotoxicity. Exp. Parasitol. 143, 24–29. doi: 10.1016/j.exppara.2014.05.002
Collin, F., Karkare, S., and Maxwell, A. (2011). Exploiting bacterial DNA gyrase as a drug target: current state and perspectives. Appl. Microbiol. Biotechnol. 92, 479–497. doi: 10.1007/s00253-011-3557-z
Costa, I. N., Angeloni, M. B., Santana, L. A., Barbosa, B. F., Silva, M. C., Rodrigues, A. A., et al. (2009). Azithromycin inhibits vertical transmission of Toxoplasma gondii in Calomys callosus (Rodentia: Cricetidae). Placenta 30, 884–890. doi: 10.1016/j.placenta.2009.08.002
da Cunha, E. F., Ramalho, T. C., Mancini, D. T., Fonseca, E., and Oliveira, A. A. (2010). New approaches to the development of anti-protozoan drug candidates: a review of patents. J. Braz. Chem. Soc. 21, 1787–1806. doi: 10.1590/S0103-50532010001000002
da Silva, L. L. R., de Araujo Portes, J., de Araújo, M. H., Silva, J. L., Rennó, M. N., Netto, C. D., et al. (2015). Further evidence that naphthoquinone inhibits Toxoplasma gondii growth in vitro. Parasitol. Int. 64, 622–631. doi: 10.1016/j.parint.2015.08.010
D'Ascenzio, M., Bizzarri, B., De Monte, C., Carradori, S., Bolasco, A., Secci, D., et al. (2014). Design, synthesis and biological characterization of thiazolidin-4-one derivatives as promising inhibitors of Toxoplasma gondii. Eur. J. Med. Chem. 86, 17–30. doi: 10.1016/j.ejmech.2014.08.046
de Lima, L. P., Seabra, S. H., Carneiro, H., and Barbosa, H. S. (2015). Effect of 3-bromopyruvate and atovaquone on infection during in vitro interaction of Toxoplasma gondii and LLC-MK2 cells. Antimicrob. Agents Chemother. 59, 5239–5249. doi: 10.1128/AAC.00337-15
Degerli, K., Kilimcioglu, A. A., Kurt, Ö., Tamay, A. T., and Özbilgin, A. (2003). Efficacy of azithromycin in a murine toxoplasmosis model, employing a Toxoplasma gondii strain from Turkey. Acta Trop. 88, 45–50. doi: 10.1016/S0001-706X(03)00194-3
Derouin, F. (2005). Systematic Search and Analysis of Preclinical Published Data on In vitro and In vivo Activities of Antitoxoplasma Drugs (Panel 2: Treatment Issues). Bordeaux: The Eurotoxo Group.
Derouin, F., Jacqz-Aigrain, E., Thulliez, P., Couvreur, J., and Leport, C. (2000). Cotrimoxazole for prenatal treatment of congenital toxoplasmosis? Parasitol. Today 16, 254–256. doi: 10.1016/S0169-4758(00)01667-7
Dittmar, A. J., Drozda, A. A., and Blader, I. J. (2016). Drug repurposing screening identifies novel compounds that effectively inhibit Toxoplasma gondii growth. mSphere 1, e00042-15. doi: 10.1128/mSphere.00042-15
Doggett, J. S., Nilsen, A., Forquer, I., Wegmann, K. W., Jones-Brando, L., Yolken, R. H., et al. (2012). Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc. Natl. Acad. Sci. U.S.A. 109, 15936–15941. doi: 10.1073/pnas.1208069109
Doggett, J. S., Ojo, K. K., Fan, E., Maly, D. J., and Van Voorhis, W. C. (2014). Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob. Agents Chemother. 58, 3547–3549. doi: 10.1128/AAC.01823-13
Dubar, F., Wintjens, R., Martins-Duarte, É. S., Vommaro, R. C., de Souza, W., Dive, D., et al. (2011). Ester prodrugs of ciprofloxacin as DNA-gyrase inhibitors: synthesis, antiparasitic evaluation and docking studies. Medchemcomm 2, 430–435. doi: 10.1039/c1md00022e
Dubey, J. P., and Jones, J. L. (2008). Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 38, 1257–1278. doi: 10.1016/j.ijpara.2008.03.007
Dzitko, K., Paneth, A., Plech, T., Pawełczyk, J., Stączek, P., Stefañska, J., et al. (2014a). 1, 4-disubstituted thiosemicarbazide derivatives are potent inhibitors of Toxoplasma gondii proliferation. Molecules 19, 9926–9943. doi: 10.3390/molecules19079926
Dzitko, K., Paneth, A., Plech, T., Pawełczyk, J., Wêgliñska, L., and Paneth, P. (2014b). Triazole-based compound as a candidate to develop novel medicines to treat toxoplasmosis. Antimicrob. Agents Chemother. 58, 7583–7585. doi: 10.1128/AAC.03832-14
Eissa, M. M., Barakat, A. M., Amer, E. I., and Younis, L. K. (2015). Could miltefosine be used as a therapy for toxoplasmosis? Exp. Parasitol. 157, 12–22. doi: 10.1016/j.exppara.2015.06.005
El-Zawawy, L. A., El-Said, D., Mossallam, S. F., Ramadan, H. S., and Younis, S. S. (2015a). Preventive prospective of triclosan and triclosan-liposomal nanoparticles against experimental infection with a cystogenic ME49 strain of Toxoplasma gondii. Acta Trop. 141, 103–111. doi: 10.1016/j.actatropica.2014.09.020
El-Zawawy, L. A., El-Said, D., Mossallam, S. F., Ramadan, H. S., and Younis, S. S. (2015b). Triclosan and triclosan-loaded liposomal nanoparticles in the treatment of acute experimental toxoplasmosis. Exp. Parasitol. 149, 54–64. doi: 10.1016/j.exppara.2014.12.007
Endeshaw, M. M., Li, C., de Leon, J., Yao, N., Latibeaudiere, K., Premalatha, K., et al. (2010). Synthesis and evaluation of oryzalin analogs against Toxoplasma gondii. Bioorg. Med. Chem. Lett. 20, 5179–5183. doi: 10.1016/j.bmcl.2010.07.003
Ferreira, R. A., de Oliveira, A. B., Gualberto, S. A., Miguel Del Corral, J. M., Fujiwara, R. T., Gazzinelli Guimarães, P. H., et al. (2012). New naphthoquinones and an alkaloid with in vitro activity against Toxoplasma gondii RH and EGS strains. Exp. Parasitol. 132, 450–457. doi: 10.1016/j.exppara.2012.09.003
Ferreira, R. A., Oliveira, A. B., Ribeiro, M. F., Tafuri, W. L., and Vitor, R. W. (2006). Toxoplasma gondii: in vitro and in vivo activities of the hydroxynaphthoquinone 2-hydroxy-3-(1′-propen-3-phenyl)-1, 4-naphthoquinone alone or combined with sulfadiazine. Exp. Parasitol. 113, 125–129. doi: 10.1016/j.exppara.2005.12.006
Flegr, J., Prandota, J., Sovièková, M., and Israili, Z. H. (2014). Toxoplasmosis–a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS ONE 9:e90203. doi: 10.1371/journal.pone.0090203
Fomovska, A., Wood, R. D., Mui, E., Dubey, J. P., Ferreira, L. R., Hickman, M. R., et al. (2012). Salicylanilide inhibitors of Toxoplasma gondii. J. Med. Chem. 55, 8375–8391. doi: 10.1021/jm3007596
Fond, G., MacGregor, A., Tamouza, R., Hamdani, N., Meary, A., Leboyer, M., et al. (2014). Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur. Arch. Psychiatry Clin. Neurosci. 264, 179–183. doi: 10.1007/s00406-013-0413-4
Franco, P. S., Gomes, A. O., Barbosa, B. F., Angeloni, M. B., Silva, N. M., Teixeira-Carvalho, A., et al. (2011). Azithromycin and spiramycin induce anti-inflammatory response in human trophoblastic (BeWo) cells infected by Toxoplasma gondii but are able to control infection. Placenta 32, 838–844. doi: 10.1016/j.placenta.2011.08.012
Gaafar, M. R., Mady, R. F., Diab, R. G., and Shalaby, T. I. (2014). Chitosan and silver nanoparticles: promising anti-toxoplasma agents. Exp. Parasitol. 143, 30–38. doi: 10.1016/j.exppara.2014.05.005
Galván-Ramírez Mde, L., Jiménez, J. M. D., Pérez, L. R. R., Troyo-Sanroman, R., Ramírez-Herrera, M., and García-Iglesias, T. (2013). Effect of nitaxozanide and pyrimethamine on astrocytes infected by Toxoplasma gondii in vitro. Arch. Med. Res. 44, 415–421. doi: 10.1016/j.arcmed.2013.07.002
Ghaffarifar, F., Asl, A. D., Sharifi, Z., Ghasemi, S., Solhjoo, K., and Mohammadi, S. R. (2006). The effect of betamethasone and IFN-γ on replication of Toxoplasma gondii (RH Strain) and nitric oxide production in HeLa cell culture. Iran. J. Allergy Asthma Immunol. 5, 75–78.
Gomes, M. A. G., Carvalho, L. P., Rocha, B. S., Oliveira, R. R., de Melo, E. J., and Maria, E. J. (2013). Evaluating anti-Toxoplasma gondii activity of new serie of phenylsemicarbazone and phenylthiosemicarbazones in vitro. Med. Chem. Res. 22, 3574–3580. doi: 10.1007/s00044-012-0347-9
Goodwin, D. G., Strobl, J. S., and Lindsay, D. S. (2011). Evaluation of five antischizophrenic agents against Toxoplasma gondii in human cell cultures. J. Parasitol. 97, 148–151. doi: 10.1645/G.E.2536.1
Habib, F. (2008). Post-treatment assessment of acute Toxoplasma infection during pregnancy. J. Obstet. Gynaecol. (Lahore). 28, 593–595. doi: 10.1080/01443610802344332
Hill, D., and Dubey, J. (2002). Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 8, 634–640. doi: 10.1046/j.1469-0691.2002.00485.x
Hoffmann, S., Batz, M. B., and Morris, J. G. Jr. (2012). Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J. Food Protect. 75, 1292–1302. doi: 10.4315/0362-028X.JFP-11-417
Howe, D. K., and Sibley, L. D. (1995). Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172, 1561–1566. doi: 10.1093/infdis/172.6.1561
Ihara, F., and Nishikawa, Y. (2014). Synthetic retinoid Am80 controls growth of intracellular Toxoplasma by inhibiting acquisition and synthesis of cholesterol in macrophages. J. Protozool. Res. 24, 1–10.
Innes, E. A. (2010). Vaccination against Toxoplasma gondii: an increasing priority for collaborative research? Expert Rev. Vaccines 9, 1117–1119. doi: 10.1586/erv.10.113
Jain, V., Yogavel, M., Oshima, Y., Kikuchi, H., Touquet, B., Hakimi, M. A., et al. (2015). Structure of prolyl-tRNA synthetase-halofuginone complex provides basis for development of drugs against malaria and toxoplasmosis. Structure 23, 819–829. doi: 10.1016/j.str.2015.02.011
Jones, J. L., Parise, M. E., and Fiore, A. E. (2014). Neglected parasitic infections in the United States: toxoplasmosis. Am. J. Trop. Med. Hyg. 90, 794–799. doi: 10.4269/ajtmh.13-0722
Julliac, B., Théophile, H., Begorre, M., Richez, B., and Haramburu, F. (2010). Side effects of spiramycin masquerading as local anesthetic toxicity during labor epidural analgesia. Int. J. Obstet. Anesth. 19, 331–332. doi: 10.1016/j.ijoa.2010.03.002
Kadri, D., Crater, A. K., Lee, H., Solomon, V. R., and Ananvoranich, S. (2014). The potential of quinoline derivatives for the treatment of Toxoplasma gondii infection. Exp. Parasitol. 145, 135–144. doi: 10.1016/j.exppara.2014.08.008
Kamau, E. T., Srinivasan, A. R., Brown, M. J., Fair, M. G., Caraher, E. J., and Boyle, J. P. (2012). A focused small-molecule screen identifies 14 compounds with distinct effects on Toxoplasma gondii. Antimicrob. Agents Chemother. 56, 5581–5590. doi: 10.1128/AAC.00868-12
Kato, K., Murata, Y., Horiuchi, N., Inomata, A., Terkawi, M. A., Ishiwa, A., et al. (2016). Dextran sulfate inhibits acute Toxoplama gondii infection in pigs. Parasit. Vectors 9, 134. doi: 10.1186/s13071-016-1421-9
Kim, K., Eaton, M. S., Schubert, W., Wu, S., and Tang, J. (2001). Optimized expression of green fluorescent protein in Toxoplasma gondii using thermostable green fluorescent protein mutants. Mol. Biochem. Parasitol. 113, 309–313. doi: 10.1016/S0166-6851(01)00212-2
Kim, Y. A., Sharon, A., Chu, C. K., Rais, R. H., Al Safarjalani, O. N., Naguib, F. N., et al. (2007). Synthesis, biological evaluation and molecular modeling studies of N 6-benzyladenosine analogues as potential anti-toxoplasma agents. Biochem. Pharmacol. 73, 1558–1572. doi: 10.1016/j.bcp.2007.01.026
Köksal, Z. Ş., Yanik, K., Bilgin, K., Yılmaz, E. M., and Hokelek, M. (2016). In vivo efficacy of drugs acting on Toxoplasma gondii combined with immunomodulators. 69, 113–117. Jpn. J. Infect. Dis. doi: 10.7883/yoken.JJID.2015.023
Kropf, C., Debache, K., Rampa, C., Barna, F., Schorer, M., Stephens, C. E., et al. (2012). The adaptive potential of a survival artist: characterization of the in vitro interactions of Toxoplasma gondii tachyzoites with di-cationic compounds in human fibroblast cell cultures. Parasitology 139, 208–220. doi: 10.1017/S0031182011001776
Kul, O., Yildiz, K., Ocal, N., Freyre, A., Deniz, A., Karahan, S., et al. (2013). In-vivo efficacy of toltrazuril on experimentally induced Toxoplasma gondii tissue cysts in lambs: a novel strategy for prevention of human exposure to meat-borne toxoplasmosis. Res. Vet. Sci. 94, 269–276. doi: 10.1016/j.rvsc.2012.08.001
Lai, B. S., Witola, W. H., El Bissati, K., Zhou, Y., Mui, E., Fomovska, A., et al. (2012). Molecular target validation, antimicrobial delivery, and potential treatment of Toxoplasma gondii infections. Proc. Natl. Acad. Sci. U.S.A. 109, 14182–14187. doi: 10.1073/pnas.1208775109
Leepin, A., Stüdli, A., Brun, R., Stephens, C. E., Boykin, D. W., and Hemphill, A. (2008). Host cells participate in the in vitro effects of novel diamidine analogues against tachyzoites of the intracellular apicomplexan parasites Neospora caninum and Toxoplasma gondii. Antimicrob. Agents Chemother. 52, 1999–2008. doi: 10.1128/AAC.01236-07
Leyke, S., Köhler-Sokolowska, W., Paulke, B. R., and Presber, W. (2012). Effects of nanoparticles in cells infected by Toxoplasma gondii. e-Polymers 12, 647–663. doi: 10.1515/epoly.2012.12.1.647
Li, Z. H., Ramakrishnan, S., Striepen, B., and Moreno, S. N. (2013). Toxoplasma gondii relies on both host and parasite isoprenoids and can be rendered sensitive to atorvastatin. PLoS Pathog. 9:e1003665. doi: 10.1371/journal.ppat.1003665
Liñares, G. G., Gismondi, S., Codesido, N. O., Moreno, S. N., Docampo, R., and Rodriguez, J. B. (2007). Fluorine-containing aryloxyethyl thiocyanate derivatives are potent inhibitors of Trypanosoma cruzi and Toxoplasma gondii proliferation. Bioorg. Med. Chem. Lett. 17, 5068–5071. doi: 10.1016/j.bmcl.2007.07.012
Liesen, A. P., de Aquino, T. M., Carvalho, C. S., Lima, V. T., de Araújo, J. M., de Lima, J. G., et al. (2010). Synthesis and evaluation of anti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1, 3, 4-thiadiazoles. Eur. J. Med. Chem. 45, 3685–3691. doi: 10.1016/j.ejmech.2010.05.017
Lim, S. S., and Othman, R. Y. (2014). Recent advances in Toxoplasma gondii immunotherapeutics. Korean J. Parasitol. 52, 581–593. doi: 10.3347/kjp.2014.52.6.581
Luk, F. C., Johnson, T. M., and Beckers, C. J. (2008). N-linked glycosylation of proteins in the protozoan parasite Toxoplasma gondii. Mol. Biochem. Parasitol. 157, 169–178. doi: 10.1016/j.molbiopara.2007.10.012
Martins-Duarte Edos, S., De Souza, W., and Vommaro, R. C. (2008). Itraconazole affects Toxoplasma gondii endodyogeny. FEMS Microbiol. Lett. 282, 290–298. doi: 10.1111/j.1574-6968.2008.01130.x
Martins-Duarte, É. S., de Souza, W., and Vommaro, R. C. (2013). Toxoplasma gondii: the effect of fluconazole combined with sulfadiazine and pyrimethamine against acute toxoplasmosis in murine model. Exp. Parasitol. 133, 294–299. doi: 10.1016/j.exppara.2012.12.011
Martins-Duarte, E. S., Dubar, F., Lawton, P., da Silva, C. F., Soeiro Mde, N., de Souza, W., et al. (2015). Ciprofloxacin derivatives affect parasite cell division and increase the survival of mice infected with Toxoplasma gondii. PLoS ONE 10:e0125705. doi: 10.1371/journal.pone.0125705
Martins-Duarte, E. S., Jones, S. M., Gilbert, I. H., Atella, G. C., de Souza, W., and Vommaro, R. C. (2009). Thiolactomycin analogues as potential anti-Toxoplasma gondii agents. Parasitol. Int. 58, 411–415. doi: 10.1016/j.parint.2009.08.004
Martins-Duarte, É. S., Lemgruber, L., de Souza, W., and Vommaro, R. C. (2010). Toxoplasma gondii: fluconazole and itraconazole activity against toxoplasmosis in a murine model. Exp. Parasitol. 124, 466–469. doi: 10.1016/j.exppara.2009.12.011
Martins-Duarte, É. S., Lemgruber, L., Lorente, S. O., Gros, L., Magaraci, F., Gilbert, I. H., et al. (2011). Evaluation of three novel azasterols against Toxoplasma gondii. Vet. Parasitol. 177, 157–161. doi: 10.1016/j.vetpar.2010.11.034
Martins-Duarte, É. S., Urbina, J. A., de Souza, W., and Vommaro, R. C. (2006). Antiproliferative activities of two novel quinuclidine inhibitors against Toxoplasma gondii tachyzoites in vitro. J. Antimicrob. Chemother. 58, 59–65. doi: 10.1093/jac/dkl180
Maubon, D., Bougdour, A., Wong, Y. S., Brenier-Pinchart, M. P., Curt, A., Hakimi, M. A., et al. (2010). Activity of the histone deacetylase inhibitor FR235222 on Toxoplasma gondii: inhibition of stage conversion of the parasite cyst form and study of new derivative compounds. Antimicrob. Agents Chemother. 54, 4843–4850. doi: 10.1128/AAC.00462-10
McFarland, M. M., Zach, S. J., Wang, X., Potluri, L.-P., Neville, A. J., Vennerstrom, J. L., et al. (2016). Review of experimental compounds demonstrating anti-toxoplasma activity. Antimicrob. Agents Chemother. 60, 7017–7034. doi: 10.1128/AAC.01176-16
Meneceur, P., Bouldouyre, M. A., Aubert, D., Villena, I., Menotti, J., Sauvage, V., et al. (2008). In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob. Agents Chemother. 52, 1269–1277. doi: 10.1128/AAC.01203-07
Meriem, B., Hajira, B., Radia, B., Malika, B., and Med Reda, D. (2015). In vitro and in vivo potential effect of the N-acylsulfonamide Bis-oxazolidi-2-ones on Toxoplasma gondii. Biomed. Biol. Eng. 2. Available online at: scholar.waset.org/1999.16/24904
Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G., and The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. doi: 10.1136/bmj.b2535
Moine, E., Denevault-Sabourin, C., Debierre-Grockiego, F., Silpa, L., Gorgette, O., Barale, J. C., et al. (2015a). A small-molecule cell-based screen led to the identification of biphenylimidazoazines with highly potent and broad-spectrum anti-apicomplexan activity. Eur. J. Med. Chem. 89, 386–400. doi: 10.1016/j.ejmech.2014.10.057
Moine, E., Dimier-Poisson, I., Enguehard-Gueiffier, C., Logé, C., Pénichon, M., Moiré, N., et al. (2015b). Development of new highly potent imidazo [1, 2-b] pyridazines targeting Toxoplasma gondii calcium-dependent protein kinase 1. Eur. J. Med. Chem. 105, 80–105. doi: 10.1016/j.ejmech.2015.10.004
Montazeri, M., Daryani, A., Ebrahimzadeh, M., Ahmadpour, E., Sharif, M., and Sarvi, S. (2015). Effect of propranolol alone and in combination with pyrimethamine on acute murine toxoplasmosis. Jundishapur J. Microbiol. 8:e22572. doi: 10.5812/jjm.22572
Montazeri, M., Ebrahimzadeh, M. A., Ahmadpour, E., Sharif, M., Sarvi, S., and Daryani, A. (2016). Evaluation of propranolol effect on experimental acute and chronic toxoplasmosis using quantitative PCR. Antimicrob. Agents Chemother. 60, 7128–7133. doi: 10.1128/AAC.01323-16
Montoya, J. G., and Liesenfeld, O. (2004). Toxoplasmosis. Lancet 363, 1965–1976. doi: 10.1016/S0140-6736(04)16412-X
Monzote, L., Rodríguez, M., Alfonso, Y., and Cox, R. (2013). Antiretroviral activity of protease inhibitors against Toxoplasma gondii. Rev. Inst. Med. Trop. S. Paulo 55, 65–67. doi: 10.1590/S0036-46652013000100012
Mui, E. J., Schiehser, G. A., Milhous, W. K., Hsu, H., Roberts, C. W., Kirisits, M., et al. (2008). Novel triazine JPC-2067-B inhibits Toxoplasma gondii in vitro and in vivo. PLoS Negl. Trop. Dis. 2:e190. doi: 10.1371/journal.pntd.0000190
Neville, A. J., Zach, S. J., Wang, X., Larson, J. J., Judge, A. K., Davis, L. A., et al. (2015). Clinically available medicines demonstrating anti-toxoplasma activity. Antimicrob. Agents Chemother. 59, 7161–7169. doi: 10.1128/AAC.02009-15
Notarangelo, F. M., Wilson, E. H., Horning, K., Thomas, M., Harris, T., Fang, Q., et al. (2014). Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: implications for schizophrenia. Schizophr. Res. 152, 261–267. doi: 10.1016/j.schres.2013.11.011
Opsenica, D., Radivojević, J., Matić, I., Štajner, T., KneŽević-Ušaj, S., Djurković-Djaković, O., et al. (2015). Tetraoxanes as inhibitors of Apicomplexan parasites Plasmodium falciparum and Toxoplasma gondii and anti-cancer molecules. J. Serb. Chem. Soc. 80, 1339–1359. doi: 10.2298/JSC150430063O
Oz, H. S. (2014a). Novel synergistic protective efficacy of atovaquone and diclazuril on fetal-maternal toxoplasmosis. Int. J. Clin. Med. 5, 921. doi: 10.4236/ijcm.2014.515124
Oz, H. S. (2014b). Toxoplasmosis complications and novel therapeutic synergism combination of diclazuril plus atovaquone. Front. Microbiol. 5:484. doi: 10.3389/fmicb.2014.00484
Palencia, A., Liu, R. J., Lukarska, M., Gut, J., Bougdour, A., Touquet, B., et al. (2016). Cryptosporidium and Toxoplasma parasites are inhibited by a benzoxaborole targeting leucyl-tRNA synthetase. Antimicrob. Agents Chemother. 60, 5817–5827. doi: 10.1128/AAC.00873-16
Pappas, G., Roussos, N., and Falagas, M. E. (2009). Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 39, 1385–1394. doi: 10.1016/j.ijpara.2009.04.003
Payne, A. J., Neal, L. M., and Knoll, L. J. (2013). Fusidic acid is an effective treatment against Toxoplasma gondii and Listeria monocytogenes in vitro, but not in mice. Parasitol. Res. 112, 3859–3863. doi: 10.1007/s00436-013-3574-1
Petersen, E., and Schmidt, D. R. (2003). Sulfadiazine and pyrimethamine in the postnatal treatment of congenital toxoplasmosis: what are the options? Exp. Rev. Anti-Infect. Ther. 1, 175–182. doi: 10.1586/14787210.1.1.175
Pissinate, K., dos Santos Martins-Duarte, É., Schaffazick, S. R., de Oliveira, C. P., Vommaro, R. C., Guterres, S. S., et al. (2014). Pyrimethamine-loaded lipid-core nanocapsules to improve drug efficacy for the treatment of toxoplasmosis. Parasitol. Res. 113, 555–564. doi: 10.1007/s00436-013-3715-6
Portes Jde, A., Netto, C. D., da Silva, A. J. M., Costa, P. R. R., DaMatta, R. A., dos Santos, T. A. T., et al. (2012). A new type of pterocarpanquinone that affects Toxoplasma gondii tachyzoites in vitro. Vet. Parasitol. 186, 261–269. doi: 10.1016/j.vetpar.2011.11.008
Puvanesuaran, V. R., Nowroji, K., Sreenivasan, S., Noordin, R., and Balakrishnan, V. (2012). Use of prednisolone to aid propagation of Toxoplasma gondii in mice. Eur. Rev. Med. Pharmacol. Sci. 16, 1028–1032.
Rajapakse, R., Uring-Lambert, B., Andarawewa, K. L., Rajapakse, R. P., Abou-Bacar, A., Marcellin, L., et al. (2007). 1, 25 (OH) 2D3 inhibits in vitro and in vivo intracellular growth of apicomplexan parasite Toxoplasma gondii. J. Steroid Biochem. Mol. Biol. 103, 811–814. doi: 10.1016/j.jsbmb.2006.12.058
Rezaei, F., Ebrahimzadeh, M. A., Daryani, A., Sharif, M., Ahmadpour, E., and Sarvi, S. (2014). The inhibitory effect of cromolyn sodium and ketotifen on Toxoplasma gondii entrance into host cells in vitro and in vivo. J. Parasit. Dis. 40, 1001–1005. doi: 10.1007/s12639-014-0623-3
Rivera Fernández, N., Castelán, M. M., Pozos, S. G., Flores, C. J. R., González, R. M., de León, C. T. G., et al. (2016). A new type of quinoxalinone derivatives affects viability, invasion, and intracellular growth of Toxoplasma gondii tachyzoites in vitro. Parasitol. Res. 115, 2081–2096. doi: 10.1007/s00436-016-4953-1
Rodriguez, J. B., and Szajnman, S. H. (2012). New antibacterials for the treatment of toxoplasmosis; a patent review. Expert Opin. Ther. Pat. 22, 311–333. doi: 10.1517/13543776.2012.668886
Saleh, A., Friesen, J., Baumeister, S., Gross, U., and Bohne, W. (2007). Growth inhibition of Toxoplasma gondii and Plasmodium falciparum by nanomolar concentrations of 1-hydroxy-2-dodecyl-4 (1H) quinolone, a high-affinity inhibitor of alternative (type II) NADH dehydrogenases. Antimicrob. Agents Chemother. 51, 1217–1222. doi: 10.1128/AAC.00895-06
Saraei, M., Ghaderi, Y., Mosavi, T., Shahnazi, M., Nassiri-Asl, M., and Jahanihashemi, H. (2016). The effect of fluphenazine and thioridazine on Toxoplasma gondii in vivo. Iran. J. Parasitol. 11, 226–231. Available online at: http://tums.ac.ir
Saraei, M., Samadzadeh, N., Khoeini, J., Shahnazi, M., Nassiri-Asl, M., and Jahanihashemi, H. (2015). In vivo anti-Toxoplasma activity of aripiprazole. Iran. J. Basic Med. Sci. 18, 938. doi: 10.22038/ijbms.2015.5219
Schmidt, D. R., Hogh, B., Andersen, O., Hansen, S. H., Dalhoff, K., and Petersen, E. (2006). Treatment of infants with congenital toxoplasmosis: tolerability and plasma concentrations of sulfadiazine and pyrimethamine. Eur. J. Pediatr. 165, 19–25. doi: 10.1007/s00431-005-1665-4
Serranti, D., Buonsenso, D., and Valentini, P. (2011). Congenital toxoplasmosis treatment. Eur. Rev. Med. Pharmacol. Sci. 15, 193–198.
Shubar, H. M., Lachenmaier, S., Heimesaat, M. M., Lohman, U., Mauludin, R., Mueller, R. H., et al. (2011). SDS-coated atovaquone nanosuspensions show improved therapeutic efficacy against experimental acquired and reactivated toxoplasmosis by improving passage of gastrointestinal and blood–brain barriers. J. Drug Target. 19, 114–124. doi: 10.3109/10611861003733995
Shubar, H. M., Mayer, J. P., Hopfenmüller, W., and Liesenfeld, O. (2008). A new combined flow-cytometry-based assay reveals excellent activity against Toxoplasma gondii and low toxicity of new bisphosphonates in vitro and in vivo. J. Antimicrob. Chemother. 61, 1110–1119. doi: 10.1093/jac/dkn047
Silveira, C., Belfort, R. Jr., Muccioli, C., Holland, G. N., Victora, C. G., Horta, B. L., et al. (2002). The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am. J. Ophthalmol. 134, 41–46. doi: 10.1016/S0002-9394(02)01527-1
Smith, A. T., Livingston, M. R., Mai, A., Filetici, P., Queener, S. F., and Sullivan, W. J. (2007). Quinoline derivative MC1626, a putative GCN5 histone acetyltransferase (HAT) inhibitor, exhibits HAT-independent activity against Toxoplasma gondii. Antimicrob. Agents Chemother. 51, 1109–1111. doi: 10.1128/AAC.01256-06
Sönmez, N., Büyükbaba Boral, O., Kaşali, K., and Tekeli, F. (2014). [Effects of atovaquone and astragalus combination on the treatment and IL-2, IL-12, IFN-γ levels on mouse models of acute toxoplasmosis]. Mikrobiyol. Bul. 48, 639–651. doi: 10.5578/mb.8025
Strobl, J. S., Cassell, M., Mitchell, S. M., Reilly, C. M., and Lindsay, D. S. (2007). Scriptaid and suberoylanilide hydroxamic acid are histone deacetylase inhibitors with potent anti-Toxoplasma gondii activity in vitro. J. Parasitol. 93, 694–700. doi: 10.1645/GE-1043R.1
Strobl, J. S., Seibert, C. W., Li, Y., Nagarkatti, R., Mitchell, S. M., Rosypal, A. C., et al. (2009). Inhibition of Toxoplasma gondii and Plasmodium falciparum infections in vitro by NSC3852, a redox active antiproliferative and tumor cell differentiation agent. J. Parasitol. 95, 215–223. doi: 10.1645/GE-1608.1
Sugi, T., Kato, K., Kobayashi, K., Kurokawa, H., Takemae, H., Gong, H., et al. (2011). 1NM-PP1 treatment of mice infected with Toxoplasma gondii. J. Vet. Med. Sci. 73, 1377–1379. doi: 10.1292/jvms.11-0085
Surolia, N., and Surolia, A. (2001). Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 7, 167–173. doi: 10.1038/84612
Szabo, E. K., and Finney, C. A. (2016). Toxoplasma gondii: one organism, multiple models. Trends Parasitol. doi: 10.1016/j.pt.2016.11.007. [Epub ahead of print].
Szajnman, S. H., Galaka, T., Li, Z.-H., Li, C., Howell, N. M., Chao, M. N., et al. (2016). 1. In vitro and in vivo activity of sulfur-containing linear bisphosphonates against apicomplexan parasites. Antimicrob. Agents Chemother. doi: 10.1128/AAC.01590-16. [Epub ahead of print].
Szajnman, S. H., García Liñares, G. E., Li, Z.-H., Jiang, C., Galizzi, M., Bontempi, E. J., et al. (2008). Synthesis and biological evaluation of 2-alkylaminoethyl-1, 1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii targeting farnesyl diphosphate synthase. Bioorg. Med. Chem. 16, 3283–3290. doi: 10.1016/j.bmc.2007.12.010
Tenter, A. M., Heckeroth, A. R., and Weiss, L. M. (2000). Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30, 1217–1258. doi: 10.1016/S0020-7519(00)00124-7
Teo, C. F., Zhou, X. W., Bogyo, M., and Carruthers, V. B. (2007). Cysteine protease inhibitors block Toxoplasma gondii microneme secretion and cell invasion. Antimicrob. Agents Chemother. 51, 679–688. doi: 10.1128/AAC.01059-06
Tipparaju, S. K., Muench, S. P., Mui, E. J., Ruzheinikov, S. N., Lu, J. Z., Hutson, S. L., et al. (2010). Identification and development of novel inhibitors of Toxoplasma gondii enoyl reductase. J. Med. Chem. 53, 6287–6300. doi: 10.1021/jm9017724
Torgerson, P. R., and Mastroiacovo, P. (2013). The global burden of congenital toxoplasmosis: a systematic review. Bull. World Health Organ. 91, 501–508. doi: 10.2471/BLT.12.111732
Vanagas, L., Jeffers, V., Bogado, S. S., Dalmasso, M. C., Sullivan, W. J. Jr., and Angel, S. O. (2012). Toxoplasma histone acetylation remodelers as novel drug targets. Expert Rev. Anti Infect. Ther. 10, 1189–1201. doi: 10.1586/eri.12.100
Vidadala, R. S. R., Rivas, K. L., Ojo, K. K., Hulverson, M. A., Zambriski, J. A., Bruzual, I., et al. (2016). Development of an orally available and Central Nervous System (CNS) penetrant Toxoplasma gondii calcium-dependent protein Kinase 1 (Tg CDPK1) inhibitor with minimal human Ether-a-go-go-Related Gene (hERG) activity for the treatment of toxoplasmosis. J. Med. Chem. 59, 6531–6546. doi: 10.1021/acs.jmedchem.6b00760
Wei, S., Daniel, B. J., Brumlik, M. J., Burow, M. E., Zou, W., Khan, I. A., et al. (2007). Drugs designed to inhibit human p38 mitogen-activated protein kinase activation treat Toxoplasma gondii and Encephalitozoon cuniculi infection. Antimicrob. Agents Chemother. 51, 4324–4328. doi: 10.1128/AAC.00680-07
Welsch, M. E., Zhou, J., Gao, Y., Yan, Y., Porter, G., Agnihotri, G., et al. (2016). Discovery of potent and selective leads against Toxoplasma gondii dihydrofolate reductase via structure-based design. ACS Med. Chem. Lett. 7, 1124–1129. doi: 10.1021/acsmedchemlett.6b00328
Wiengcharoen, J., O., Hanley, R., Armstrong, T., Best, W., Sukthana, Y., and Thompson, R. A. (2007). Novel drug compounds against Neospora caninum and Toxoplasma gondii in vitro. Southeast Asian J. Trop. Med. Public Health 38, 15.
Xin, C. F., Kim, H. S., Sato, A., Lee, H. J., Lee, Y. W., Pyo, K. H., et al. (2016). In vitro inhibition of Toxoplasma gondii by the anti-malarial candidate, 6-(1, 2, 6, 7-tetraoxaspiro [7.11] nonadec-4-yl) hexan-1-ol. Parasitol. Int. 65, 494–499. doi: 10.1016/j.parint.2016.06.013
Yang, Z., Ahn, H. J., and Nam, H. W. (2014). Gefitinib inhibits the growth of Toxoplasma gondii in HeLa cells. Korean J. Parasitol. 52:439. doi: 10.3347/kjp.2014.52.4.439
Yeo, S. J., Jin, C., Kim, S., and Park, H. (2016). In vitro and in vivo effects of nitrofurantoin on experimental toxoplasmosis. Korean J. Parasitol. 54:155. doi: 10.3347/kjp.2016.54.2.155
Yin, W. D., Gao, Q. C., Liu, X. D, and Tang, H. W. (2009). [In vivo effect of dihydroartemisinin and azithromycin on the ultrastructure of Toxoplasma gondii tachyzoites]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 27, 4325–4327.
Yung, S. C., and Lang-Unnasch, N. (2004). “Targeting the Toxoplasma gondii apicoplast for chemotherapy,” in Opportunistic Infections: Toxoplasma, Sarcocystis, and Microsporidia, eds D. S. Lindsay and L. M. Weiss (Boston, MA: Springer), 39–49.
Zhang, N. Z., Chen, J., Wang, M., Petersen, E., and Zhu, X. Q. (2013). Vaccines against Toxoplasma gondii: new developments and perspectives. Expert Rev. Vaccines 12, 1287–1299. doi: 10.1586/14760584.2013.844652
Zhang, N. Z., Wang, M., Xu, Y., Petersen, E., and Zhu, X. Q. (2015). Recent advances in developing vaccines against Toxoplasma gondii: an update. Expert Rev. Vaccines 14, 1609–1621. doi: 10.1586/14760584.2015.1098539
Keywords: Toxoplasma gondii, toxoplasmosis, drugs, compounds, in vitro, in vivo
Citation: Montazeri M, Sharif M, Sarvi S, Mehrzadi S, Ahmadpour E and Daryani A (2017) A Systematic Review of In vitro and In vivo Activities of Anti-Toxoplasma Drugs and Compounds (2006–2016). Front. Microbiol. 8:25. doi: 10.3389/fmicb.2017.00025
Received: 01 September 2016; Accepted: 05 January 2017;
Published: 20 January 2017.
Edited by:
Octavio Luiz Franco, Universidade Católica de Brasília, BrazilReviewed by:
Osmar Nascimento Silva, Universidade Católica Dom Bosco, BrazilNasib Singh, Eternal University, India
Copyright © 2017 Montazeri, Sharif, Sarvi, Mehrzadi, Ahmadpour and Daryani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Daryani, daryanii@yahoo.com
 Mahbobeh Montazeri
Mahbobeh Montazeri Mehdi Sharif1,3
Mehdi Sharif1,3 Shahabeddin Sarvi
Shahabeddin Sarvi Saeed Mehrzadi
Saeed Mehrzadi Ehsan Ahmadpour
Ehsan Ahmadpour Ahmad Daryani
Ahmad Daryani