- 1Department of Physical Chemistry, Faculty of Pharmacy, Medical University of Gdansk, Gdansk, Poland
- 2Department of Inorganic Chemistry, Faculty of Pharmacy, Medical University of Gdansk, Gdansk, Poland
To get a better insight into the antimicrobial potency of short cationic lipopeptides, 35 new entities were synthesized using solid phase peptide strategy. All newly obtained lipopeptides were designed to be positively charged from +1 to +4. This was achieved by introducing basic amino acid - lysine - into the lipopeptide structure and had a hydrophobic fatty acid chain attached. Lipopeptides were subjected to microbiological tests using reference strains of Gram-negative bacteria: Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, Gram-positive bacteria: Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Enterococcus faecalis, and fungi: Candida albicans, Candida tropicalis, Aspergillus brasiliensis. The minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and minimal fungicidal concentration (MFC) were established for each strain. The toxicity toward human cells was determined by hemolysis tests via minimum hemolytic concentration (MHC) determination. The effect of the trifluoroacetic acid (TFA) counter ion on the antimicrobial activity of lipopeptides was also examined by its removing and performing the antimicrobial tests using counter ion-free compounds. The study shows that lipopeptides are more potent against Gram-positive than Gram-negative strains. It was revealed that positive charge equals at least +2 is a necessary condition to observe significant antimicrobial activity, but only when it is balanced with a proper length of hydrophobic fatty acid chain. The hemolytic activity of lipopeptides strongly depends on amino acid composition of the hydrophilic portion of the molecule as well as fatty acid chain length. Compounds endowed with a greater positive charge were more toxic to human erythrocytes. This should be considered during new lipopeptide molecules design. Our studies also revealed the TFA counter ion has no significant effect on the antimicrobial behavior of cationic lipopeptides.
Introduction
Most naturally occurring antimicrobial peptides are positively charged and their amino acid sequences form an amphipathic structure. These structural conditions seem to be mandatory for the occurrence of high levels of antimicrobial activity (Hancock and Diamond, 2000; Brown and Hancock, 2006; Hancock and Sahl, 2006). Therefore, antimicrobial peptide molecule design and the synthesis of positively charged antimicrobial peptide compounds are often achieved by introducing basic amino acids, e.g., lysine or arginine, into the sequence. In turn, the condition of amphipathicity is provided by introducing amino acids with hydrophobic side chains. It is also possible to create amphiphilic peptide-like molecule by replacing a few hydrophobic amino acid residues with one, also hydrophobic, fatty acid chain. (Makovitzki et al., 2006; Domalaon et al., 2016). A large group of potent antimicrobial lipopeptides have been generated this way. Some of them, e.g., surotomycin, dalbavancin and HB1345, are currently being developed by the pharmaceutical industry and are undergoing clinical trials (Greber and Dawgul, 2016). The emergence and spread of multidrug resistant (MDR) microorganisms causes that a development of a new antibiotics is nowadays one of the most important challenges of pharmaceutical chemistry (Ventola, 2015). Due to the issue of researching of an effective antibiotic therapies is urgent, we decided to synthesize a group of potentially antimicrobial cationic lipopeptides and study their antibacterial and antifungal potency. Our lipopeptides are divided into five groups differing in fatty acid chain length and cationic net charge. In the present study, we determined the antibacterial and antifungal potency of lipopeptides toward 11 reference strains of Gram-negatives: Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, Gram-positives: Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Enterococcus faecalis, and fungi: Candida albicans, Candida tropicalis, and Aspergillus brasiliensis. The minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and minimal fungicidal concentration (MFC) were established for each strain. The toxicity toward human cells was tested via minimum hemolytic concentration (MHC) determination. Solid phase peptide synthesis procedure, employed in this work, as well as chromatographic purification of lipopeptides requires use of trifluoroacetic acid. This means that cationic lipopeptides are obtained as trifluoroacetate salts. Due to the fact that trifluoroacetic ion is not a physiological and it is not present in the common microbial environment it may have an impact on microbial growth. We decided to study if there is a difference in antimicrobial activity of lipopeptides in the form of trifluoroacetic salts and lipopeptides in the form of bases. There is no other data published in this matter.
Materials and Methods
Chemicals
All chemicals and solvents for peptide synthesis were from Iris Biotech (Germany). The column for lipopeptide RP-HPLC purification (Nucleodur C8e/c, 100 Å, 5 μm, 250 × 10 mm) was from Macherey-Nagel (Germany). The analytical column for peptide characterization (Chromolith Performance RP-18e, 100 × 4.6 mm) was from Merck Millipore (Germany). The gradient grade acetonitrile was from Scharlau (Spain). Deionized water (18.2 M'Ω) was obtained using a Direct-Q 3 UV-R Ultrapure Water System, Merck-Millipore, (Germany). The ion exchange device for TFA removal was a VariPure IPE (Ion Pair Extraction), Agilent (USA).
Peptide Synthesis and Purification
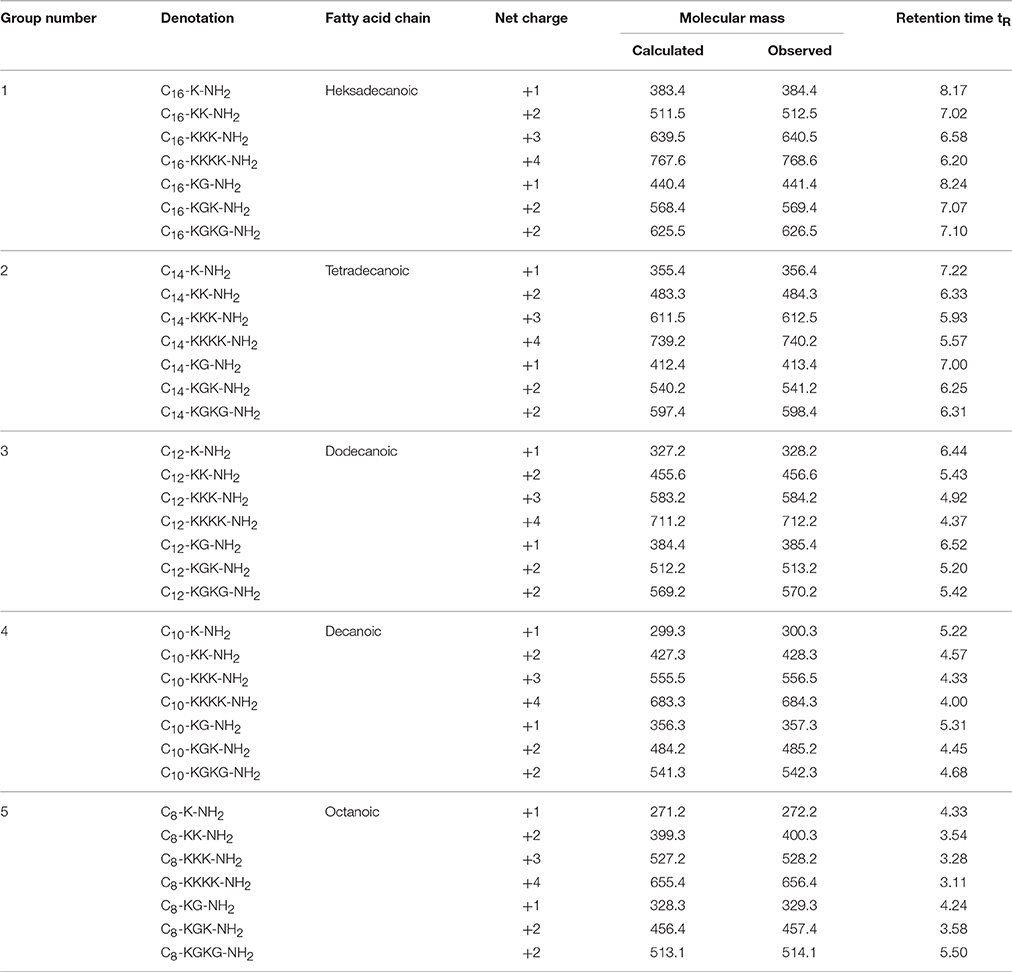
All lipopeptides were synthesized using the Fmoc/tBu procedure on Fmoc-Rink Amide AM resin (0.59 mmol/g) (Table 1). The Fmoc protecting group of amino acid was detached by 20% piperidine in DMF. Peptide bond formation was carried out with a 3-fold excess of Fmoc-protected amino acid dissolved in a DMF/DCM mixture (1:1 v/v) in the presence of 1% of Triton X-100 using DIC and HOBt as coupling reagents. Removal of the side chain protecting group and cleavage from the resin was achieved via 95% TFA with 2.5% of TIS and 2.5% of water for 1 h (Chan and White, 2000). The cleaved lipopeptides were precipitated and lyophilized. They were then purified by semi-preparative reverse-phase high performance liquid chromatography (RP-HPLC) with a Macharey Nagel C8 e/c column and eluted with a 20–60% linear gradient of phase B (phase A—0.1% TFA in water, phase B—0.1% TFA in ACN), at a flow rate of 3 mL/min, and analyzed at 214 nm.
Reversed-Phase Analysis of Lipopeptides
The lipopeptides were analyzed by RP-HPLC on a Chromolith Performance monolithic column with a linear gradient 2–98% phase B (where phase A was 0.1% TFA in water and phase B was 0.1% TFA in ACN), at a flow rate of 2 mL/min, and analyzed at 214 nm. Fractions with a purity greater than 95% were collected and freeze-dried on an Alpha 2–4 LD plus system (Christ, Germany). Lipopeptides were further characterized by matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF, Bruker, Germany).
Organisms and Antimicrobial Assay
The minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) were determined according to the procedure recommended by the Clinical Laboratory Standards Institute (CLSI). The following reference strains were tested: Gram-negative E. coli (ATCC 25922), K. pneumoniae (ATCC 700603), P. vulgaris (PCM 2668), P. aeruginosa (ATCC 9027), Gram-positive S. aureus (ATCC 25923), S. epidermidis (PCM 2118), B. subtilis (ATCC 6633), E. faecalis (ATCC 29212) and fungi C. albicans (ATCC 10231), C. tropicalis (PCM 2681), and A. brasiliensis (ATCC 16404). All the microorganisms were from the Polish Collection of Microorganisms (Wroclaw, Poland). MIC was determined using a microbroth dilution method with Mueller-Hinton (MH) broth for bacteria and Sabouraud Dextrose broth for fungi (Becton Dickinson, Le Pont de Claix, France). An initial inoculum of 105 cfu/mL was used for bacteria and 103 cfu/mL for fungi. Polypropylene 96-well plates (Kartell, Noviglio, Italy) were incubated for 18 h at 37°C for bacteria and for 48 h at 25°C for the tested fungi. MIC (μg/mL) was taken as the lowest concentration of the lipopeptide, at which the observable growth was inhibited. All the MIC wells which did not reveal turbidity were cultured on solid media: a Mueller-Hinton II Agar for bacteria and a Sabouraud 2% Glucose Agar for fungi. The lowest concentrations of lipopeptides that did not show any visible growth on the plates after 24 h of incubation at 37°C and 48 h of incubation at 25°C were recorded as MBCs and MFCs, respectively. The experiments were performed in triplicate on three different days.
Hemolysis
The hemolytic activity of the lipopeptides was determined by exposing human red blood cells (4% v/v) to tested compounds at graded concentrations. Red blood cells were obtained from a healthy donor. EDTA was used to prevent the blood clotting. The plasma was removed via centrifugation and the erythrocytes were washed three times with PBS. The red blood cells were suspended in PBS and incubated with different concentrations of lipopeptides at 37°C for 1 h and centrifuged for 5 min at 1000 × g. The supernatants were transferred to a sterile 96-well plate and hemoglobin release was measured with an Epoch microplate spectrophotometer (BioTek, USA) by recording the absorbance at 550 nm. A 0.1% Triton X-100 solution was used as the positive control (100% of hemolysis) and pure PBS as the negative (0% of hemolysis). Minimum hemolytic concentration (MHC) was taken as the lowest concentration which induced 10% of hemolysis.
Removal of Trifluoroacetic Acid Counter Ion and FTIR Spectroscopy
Removal of trifluoroacetate counter ions was performed on VariPure IPE exchange columns. Before applying lipopeptides, ion exchange devices were conditioned as follows: 2 × 30 mL MeOH, 2 × 30 mL ACN, and 2 × 30 mL purified water. Previously lyophilized lipopeptides were dissolved in 10% acetonitrile and applied to ion exchange columns. Lipopeptides were eluted from the ion-exchange resin using a clean matrix. Thus obtained counter ion-free eluates were diluted with purified water and then subjected to a freeze-drying process. In order to confirm the absence of trifluoroacetate counter ions in the synthesized cationic lipopeptides, infrared spectroscopic studies were carried out. Infrared spectra were recorded on a Jasco FT/IR-410 spectrophotometer (Labor and Datentechnik GmbH, Germany) using the KBr tablet method. Tablets were prepared from 100 mg of potassium bromide (KBr) and 1.5 mg of the tested lipopeptide.
Results
Characterization of Bacteriostatic Properties
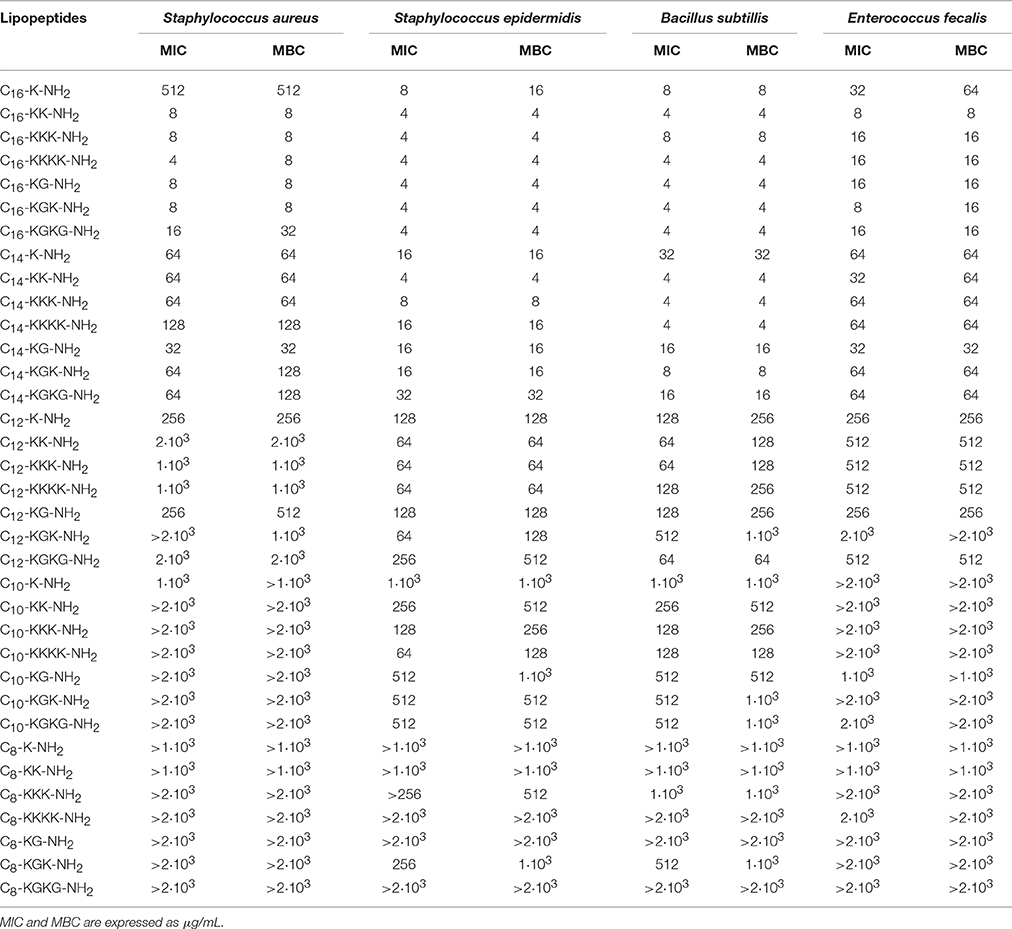
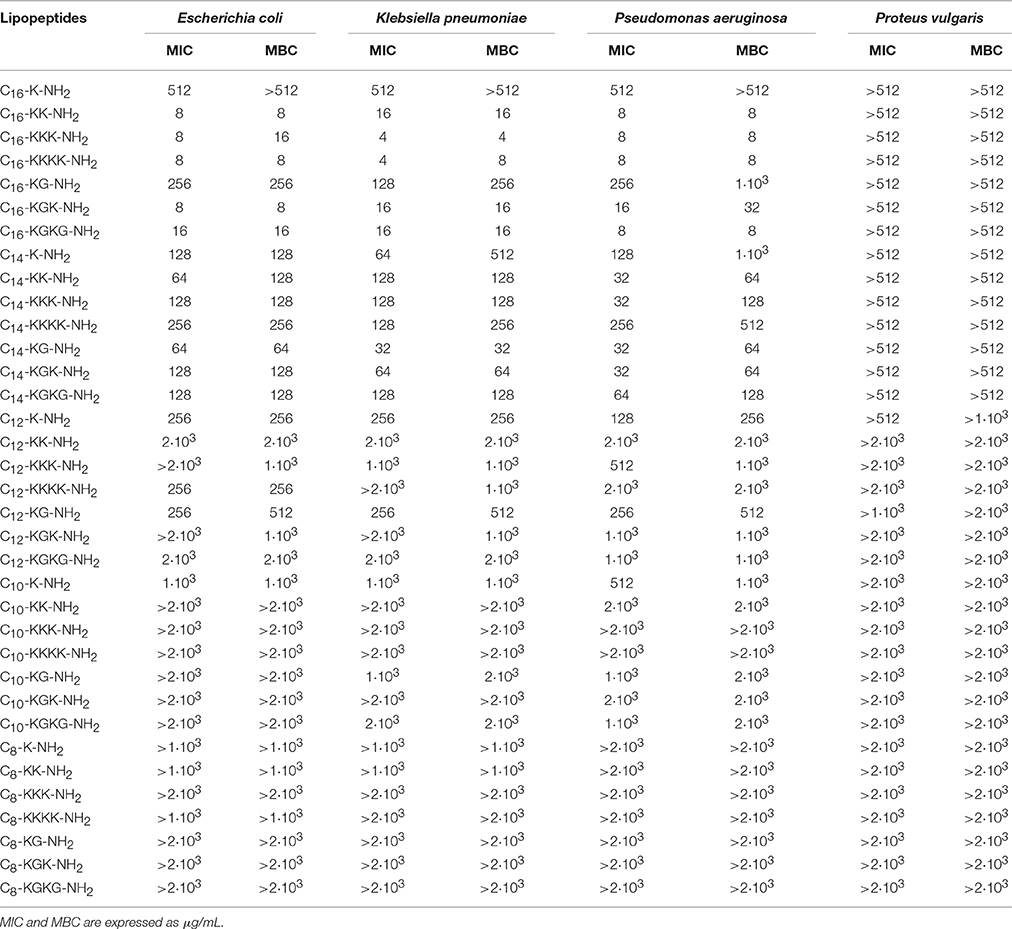
The antimicrobial tests indicated that lipopeptides containing hexadecanoic acid residue reveal high antibacterial activity toward Gram positives and Gram negatives; however, this activity is higher toward Gram positive bacteria (Tables 2, 3). The most potent bacteriostatic effect was observed with analogs containing more than one basic amino acid, that is C16-KK-NH2, C16-KKK-NH2, C16-KKKK-NH2, C16-KGK-NH2, and C16-KGKG-NH2. It was demonstrated that lipopeptides inhibit the growth of G+ and G− in the concentration range of 4–16 μg/mL. The exception is the P. vulgaris strain, which is resistant to the most active palmitate analogs and its MIC value reaches 512 μg/mL for all lipopeptide analogs. The lowest growth inhibition of G− was recorded for lipopeptides containing one basic amino acid: C16-K-NH2 and C16-KG-NH2. Results obtained for lipopeptides modified with tetradecanoic acid varied depending on the tested strain. The compounds exhibited relatively high antibacterial activity toward B. subtilis and S. epidermidis. The strongest bacteriostatic effect, from 4 to 8 μg/mL, was observed for the analogs C14-KK-NH2 and C14-KKK-NH2. Moderate bacteriostatic activity against the remaining Gram positive bacteria and Gram negatives was found (Tables 2, 3). However the activity toward gram-positive strains was higher in comparison to gram-negative strains. Lipopeptides modified with dodecanoic acid revealed rather poor bacteriostatic activity against tested G+ and G− strains (Tables 2, 3). Among all of the dodecanoic analogs, the highest growth inhibitory activity was perceived for lipopeptides containing one basic amino acid, i.e., C12-K-NH2 or C12-KG-NH2. MIC values determined for these compounds toward G+ and G− are in the range of 128–256 μg/mL, but for the strain P. vulgaris MIC was found at 512 μg/mL. Lipopeptides with two and three lysine residues (C12-KK-NH2, C12-KKK-NH2) are characterized by irregular activity. They are bacteriostatic to S. epidermidis and B. subtillis at a concentration of 64 μg/mL, but to S. aureus at a concentration of 2 and 1 mg/mL, respectively. The compounds are practically inactive against strains G−. Lipopeptides modified with decanoic acid are characterized by either poor bacteriostatic activity against G+ and G− strains or a complete lack thereof (Tables 2, 3). Among these lipopeptides, some higher bacteriostatic effect against G+ was observed for analogs C10-KKK-NH2 and C10-KKKK-NH2. They are also moderately active toward S. epidermidis and B. subtillis, while S. aureus and E. fecalis strains revealed complete resistance to them. None of the obtained lipopeptides modified with decanoic acid inhibited the growth of tested Gram negatives. Only the C10-K-NH2 analog exhibited very poor activity toward the P. aeruginosa strain; MIC value was observed at 512 μg/mL. Lipopeptides with attached octanoic fatty acid are generally inactive against gram positives as well as gram negatives. Among the 7 synthesized compounds, bacteriostatic activity at the concentration of 256 μg/mL toward S. epidermidis was noticed for C8-KKK-NH2 and C8-KGK-NH2. Additionally, C8-KGK-NH2 was bacteriostatic to B. subtillis at the concentration of 512 μg/mL.
Characterization of Bactericidal Properties
The highest bactericidal activity was revealed for lipopeptides modified with hexadecanoic acid. C16-KK-NH2, C16-KKK-NH2, C16-KKKK-NH2, C16-KGK-NH2, and C16-KGKG-NH2 are bactericidal to G+ and G− in a concentration range similar to MIC, i.e., from 4 to 32 μg/mL. The bactericidal activity of the analogs containing one lysine residue (C16-K-NH2 or C16-KG-NH2) is distinctly lower. Although, S. epidermidis, B. subtillis and E. fecalis are already sensitive to these at a concentration of 4–64 μg/mL; unlike S. aureus, which is sensitive only at 512 μg/mL. For G− bacteria, much weaker bactericidal activity was observed. MBC values of compounds where the total net charge of the molecule is +1 reach 256–1·103 μg/mL. Selected myristates as well as palmitates exhibit good bactericidal activity. The obtained MBC values were usually as much as twice as high as the MICs. The strongest bactericidal effect was proven for analogs of C14-KK-NH2 and C14-KKK-NH2. The most sensitive strains are B. subtillis and S. epidermidis. MBC determined for these organisms is within the range of 4–8 μg/mL. MBC of the lipopeptides C12-K-NH2 and C12-KG-NH2 is only the same as MIC for S. epidermidis and E. fecalis. For strains of S. aureus and B. subtillis, MBC is twice as high as MIC. Lipopeptides containing two or three lysine residues (C12-KK-NH2, C12-KKK-NH2) are weakly bactericidal to all of the studied gram negatives. Most susceptible to C12-KKK-NH2 was P. aeruginosa; MBC was observed at 512 μg/mL. None of the decanoic analogs have any bactericidal activity against S. aureus and E. fecalis. Strains of S. epidermidis and B. subtillis are more sensitive, in particular to C10-KKK-NH2 and C10-KKKK-NH2, and MBC values determined for these compounds range 128–256 μg/mL. From MBC values it can be concluded that decanoic modified lipopeptides are not bactericidal toward the tested strains. The MBC test demonstrated that only C8-KKK-NH2 was bactericidal to S. epidermidis at the concentration of 512 μg/mL.
Characterization of Antifungal Properties
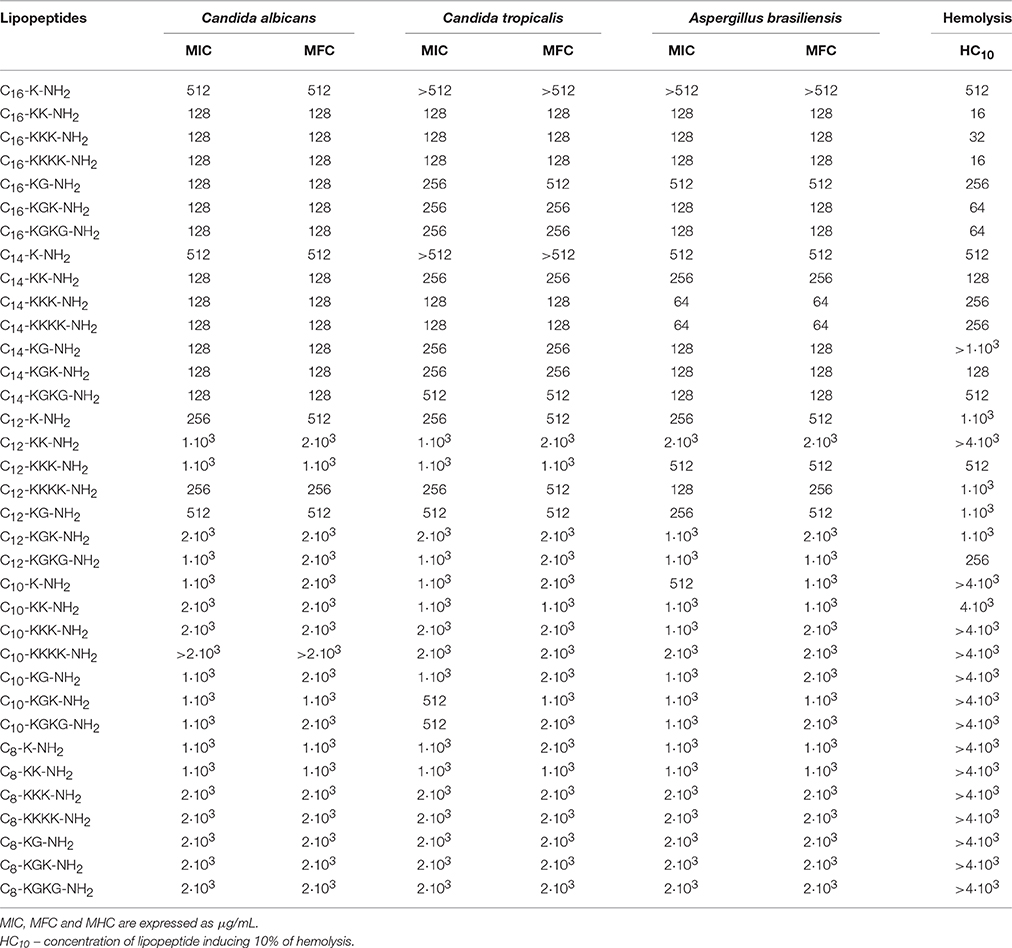
It was found that palmitic acid derived peptides are weakly active against Candida species and Aspergillus molds (Table 4). This was confirmed by MIC and MFC determination. Of the seven analogs, the best fungistatic properties were confirmed for C16-KK-NH2, C16-KKK-NH2, and C16-KKKK-NH2. Both the MIC and the MFC are 128 μg/mL. Analogs containing glycine in the sequence (i.e., C16-KG-NH2, C16-KGK-NH2, and C16-KGKG-NH2) show weaker activity against C. tropicalis and A. brasiliensis. The lowest antifungal activity among all hexadecanoic analogs was revealed for lipopeptides containing one lysine: C16-K-NH2. Lipopeptides modified with tetradecanoic acid revealed some fungistatic and fungicidal activity against Candida and Aspergillus species. The lowest MIC and MBC value, i.e., 64 μg/mL, toward A. brasiliensis was noted in two cases: C14-KKK-NH2 and C14-KKKK-NH2. These compounds inhibit the growth of C. albicans and C. tropicalis slightly less effectively, because they require a concentration twice as high—128 μg/mL (Table 4). It was found that, at the same concentrations, all the tetradecanoic lipopeptides are fungistatic and fungicidal to the tested strains of C. albicans, C. tropicalis and A. brasiliensis. It was observed that dodecanoic lipopeptides weakly inhibits the growth of yeasts of Candida and A. brasiliensis (Table 4). The most bacteriostatically efficient compound proved to be that containing four lysines—C12-KKKK-NH2; MIC of this compound is in the range 128–256 μg/mL. Nearly identical properties were demonstrated by C12-K-NH2. Other analogs did not show significant bacteriostatic activity toward the tested fungi species. The best MFC results were observed for C12-KKKK-NH2. Analogs C12-K-NH2 and C12-KG-NH2 presented the same fungicidal activity—512 μg/mL. Generally, decanoic lipopeptides revealed no fungistatic activity toward Candida species and A. brasiliensis (Table 4). Some very weak fungistatic effect at the concentration of 512 μg/mL was noticed for C10-KGK-NH2 and C10-KGKG-NH2 toward C. tropicalis and for C10-K-NH2 toward A. brasiliensis. All tested fungi were totally resistant to octanoic analogs, as can be seen from the high MIC and MFC values. Results of antimicrobial studies are presented in Tables 2–4.
Influence of the Presence of TFA on the Antimicrobial Activity
The most promising compounds, i.e., lipopeptides containing palmitic and myristic fatty acid residues, were tested after removal of counter ions to determine whether this influences their antimicrobial activity. We noticed some differences in the activity of compounds toward various strains. The determined antimicrobial activity was usually either the same for compounds with and without TFA or 2-fold higher/ lower, depending on the compounds and strain tested. For S. aureus, palmitic analogs without TFA (with the exception of C16-K-NH2) were twice as active as compounds containing TFA in both MIC and MBC assays. MICs obtained for C14-KK-NH2 and C14-KKK-NH2 were also twice as low as those obtained for lipopeptides after TFA removal. For the compounds C14-KGK-NH2 and C14-KGKG-NH2 the same difference was observed, but for the MBC values. Similar results were obtained with the E. faecalis strain. For almost all palmitic analogs without counter ions, the bacteriostatic and bactericidal concentrations were twice as low as peptides containing TFA. For myristic analogs, we did not observe any discrepancies in the activity of compounds both before and after removal of the counter ion. Bactericidal activity of the majority of palmitic analogs without TFA against E. coli was twice as high as peptides with a counter ion. MIC values were different for palmitic analogs containing glycine and more than one lysine residues. Bacteriostatic activity was also higher for myrisitic analogs: C14-K-NH2, C14-KK-NH2, and C14-KKK-NH2. For K. pneumoniae, differences between the activity of the compounds after counter ion removal were noticed only for a few compounds. Obtained MBCs were lower for lipopeptides without TFA, i.e., C16-K-NH2, C16-KKKK-NH2, and C14-KKKK-NH2, while for compounds C16-KGK-NH2, C16-KGKG-NH2, and C14-KG-NH2 we observed a decrease in antibacterial activity after removal of TFA. A decrease in the activity of compounds without counter ions was observed for the majority of the tested myristic analogs in MIC and MBC tests with C. albicans.
Hemolysis
Lytic activity against human erythrocytes was measured to assess the toxicity of lipopeptides to eukaryotic cells. Lipopeptides containing a hexadecanoic chain displayed strong hemolysis. The strongest toxicity was demonstrated by palmitic derivatives containing more than one lysine residue: C16-KK-NH2, C16-KKKNH2, and C16-KKKK-NH2 (MHC 16–32 μg/mL). Compounds with the same cationic charge additionally containing a glycine residue showed slightly reduced lytic activity (MHC 64 μg/mL). Among the group of lipopeptides with a tetradecanoic acid, two analogs—C14-KK-NH2 and C14-KGK-NH2—were established as moderately hemolytic. These lead to lysis of red blood cells at the concentration of 128 μg/mL. Compounds containing 3 or 4 basic amino acid destroy erythrocytes at a higher concentration, i.e., only at 256 μg/mL. The rest of the synthesized lipopeptides are very weakly hemolytic or display no hemolysis at all within the range of the tested concentrations. The study shows that the hemolytic activity of lipopeptides increases with the length of the attached hydrophobic chain. Among the synthesized lipopeptides, the greatest hemolytic activity was observed for hexadecanoic modified lipopeptides. For those lipopeptides comprising a fragment of octanoic acid, hemolysis was not observed, even at a concentration of 4·103 μg/mL. Furthermore, within the group of lipopeptides with a hexadecanoic fatty acid a strong dependence of the hemolytic activity on amino acid composition of the hydrophilic portion of the molecule was observed. Compounds endowed with a greater positive charge were more toxic to human erythrocytes.
TFA Removal and Antibacterial Activity of Lipopeptides without a Counter Ion
Since the most significant antimicrobial activities were observed for lipopeptides containing hexadecanoic and tetradecanoic hydrocarbon chains, only those two groups of compounds were subjected to TFA counter ion removal to test the influence of TFA presence on the antimicrobial activity of lipopeptides themselves. Obtained FTIR spectra confirmed the absence of TFA counter ions, thus proving that the process of removing the counter ions via a VariPure IPE ion-exchange column ran properly. Figure 1 presents IR spectra of lipopeptides before (green line) and after (blue line) TFA removal. There are visible bands derived from the stretching vibration of CF2 and CF3 groups within the range of 1350–1120 cm−1 (green line). However, there is no such band in the sample subjected to removal of the counter ion.
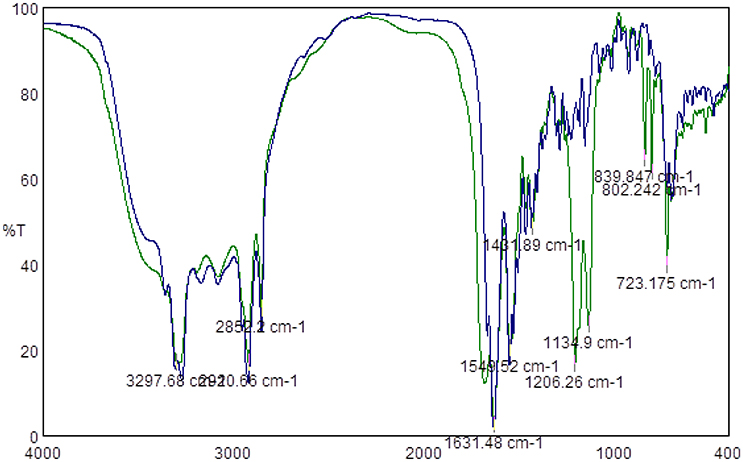
Figure 1. Example of FTIR spectra of lipopeptide C16-KK-NH2 before TFA counter ion removal (Green Line) and after TFA counter ion removal (blue line).
Discussion
The antimicrobial activity of synthetic cationic lipopeptides is widely studied and many structures have already been described. For example, ultrashort lipopeptides very similar to ours, composed of lysine, glycine, alanine, leucine and isoleucine acylated with dodecanoic, tetradecanoic, and hexadecanoic acids were synthesized. For each of the ultrashort lipopeptides, one amino acid of D-configuration was introduced. The best MIC values were obtained for the compound C16-KGG-[D]-K-NH2. This was found to be active toward G− and G+ tested strains (Makovitzki et al., 2006). We obtained similar results for palmitic derivatives. The compounds exhibited very potent antibacterial activity, but unfortunately they turned out to be also the most toxic to human erythrocytes. Due to their hemolytic activity only topical application can be considered. However, in some previous studies the compound C16-KK-NH2 exhibited toxicity toward human keratinocytes HaCaT exposed to its microbiologically active concentrations (Barańska-Rybak et al., 2013). Therefore, further consideration of this compound should be as a topical agent applied on uninjured skin, e.g., eradication of bacterial colonization, or as a preservative for dermatological drugs or cosmetics. For abovementioned ultrashort lipopeptides, similarly to our results, strong hemolytic activity, at the level of 8% at 20 μM, was observed. It seems that these lipopeptides do not discriminate effectively between bacterial and eukaryotic cells. Some better results have been obtained for myristic residues. We observed some discrimination between various microbial species, which would suggest some additional mechanism of antibacterial action. However, the toxicity is still at a relatively high level and to improve the selectivity of the lipopeptide antibiotic structure modifications are needed. A possible solution is also the use of a combination of active lipopeptides with conventional antibiotics, which would probably allow the use of lower concentrations of both substances (Barchiesi et al., 2007; Cirioni et al., 2007; Simonetti et al., 2009). According to the literature, the anti-infective activity of C16-KK-NH2 has been studied utilizing the rat model, where the compound demonstrated effectiveness against induced staphylococcal infections, at the same time without causing harmful side effects in the test animals. Kamysz et al. studied the activity of C16-KK-NH2 and C16-KK-OH against gram-positive cocci resistant to methicillin and vancomycin. The tested compounds showed high antimicrobial activity against these strains and a slightly synergistic effect in combination with amoxicillin, imipenem and vancomycin (Kamysz et al., 2007). The antibacterial activity of selected lipopeptides was also determined against clinical isolates of S. aureus. The tested compounds, i.e., C16-KK-NH2, C16-KKK-NH2, C16-KKKK-NH2, C16-KG-NH2 and C16-KGK-NH2, showed high antistaphylococcal activity against all tested strains, including those resistant to chloramphenicol, erythromycin and penicillin. G. Sarig et al. received a lipopeptide composed of three residues of lysine and two hydrocarbon chains (C12-KK-C12-K-NH2), wherein one of the hydrophobic chains separated the amino acid sequence into two parts. For this compound, a very high antimicrobial activity against S. aureus strains was found (2.5–5 μg/mL) and a weaker one against E. coli (≥ 40 μg/mL) (Sarig et al., 2010).
There is not much available data in the literature regarding the impact of counter ions on the biological activity of antimicrobial peptides obtained via chemical synthesis. However, Gaussier et. al. revealed that replacement of trifluoroacetic acid with HCl counter ions did not significantly affect the antimicrobial activity of pediocin PA-1 (Gaussier et al., 2002). In our study, the removal of TFA either caused no change in activity or the results differed by one well (Table 5). In the majority of cases, the obtained MICs and MBCs were lower for lipopeptides without counter ions. This might be a result of the different molecular weights of the two peptide forms, rather than being a direct influence on antimicrobial action. However, in a few cases peptides containing TFA were slightly more active toward K. pneumoniae and C. albicans, which would suggest some positive influence on antimicrobial activity. However, further study with the use of other counter ions and more microbial species is needed to clarify this issue.
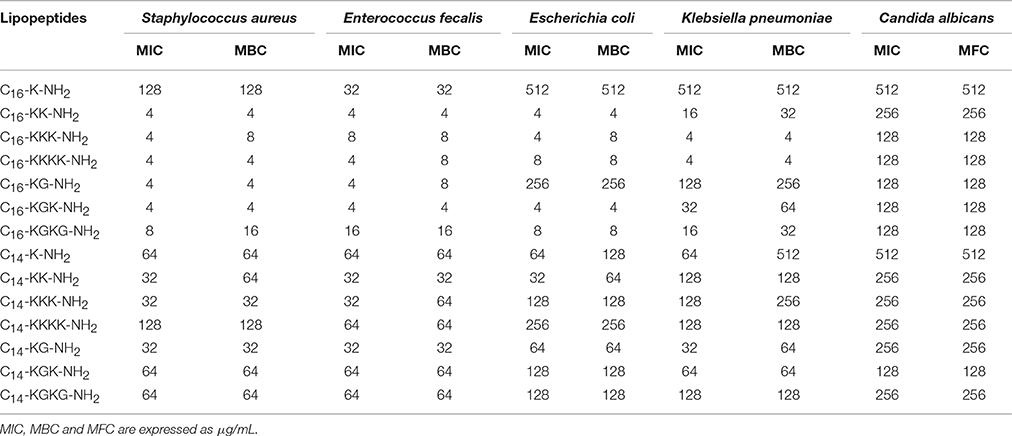
Table 5. Antimicrobial activity of lipopeptides without TFA counter ion, expressed as minimum inhibitory concentration and minimum bactericidal/fungicidal concentration.
Conclusions
We have demonstrated that the antimicrobial activity of short cationic lipopeptides is strongly dependent on the net charge of the lipopeptide molecule. A higher antibacterial activity is displayed by lipopeptides with a net charge from +2 to +4 and a hexadecanoic fatty acid chain attached. This clearly suggests that a antimicrobial peptide molecule needs proper balance between a hydrophilic and hydrophobic entity to effectively interact with a bacterial membrane. Our studies also clarify that the presence of a TFA counter ion has no significant impact on the antimicrobial activity of the tested lipopeptides.
Author Contributions
KG: Conception and design of work, performing the experiments, collecting the literature, scientific description of the comments, corresponding author; MD: Co-operation in performing the experiments, collecting the literature, co-operation in the manuscript preparation; WK and WS: Co-operation in the manuscript preparation. All authors critically revised the manuscript and approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by The National Science Centre, Poland; under grant 2012/05/B/ST5/00281 and by the Ministry of Science and Higher Education of the Republic of Poland, from the quality—promoting subsidy under the Leading National Research Centre (KNOW) programme for the years 2012–2017.
Abbreviations
AMP, antimicrobial peptide; Boc, tert-butoxycarbonyl; CFU, colony forming units; CLSI, Clinical and Laboratory Standards Institute; DCM, dichloromethane; DIC, diisopropylcarbodiimide; DMF, N,N-dimethylformamide; Fmoc, 9-fluorenylmethoxycarbonyl, G−, Gram-negative bacteria; G+, Gram positive bacteria; HOBt, 1-hydroxybenzotriazole; MBC, minimum bactericidal concentration; MFC, minimum fungicidal concentration; MIC, minimum inhibitory concentration; TFA, trifluoroacetic acid; TIS, triisopropylosilane.
References
Barańska-Rybak, W., Pikula, M., Dawgul, M., Kamysz, W., Trzonkowski, P., and Roszkiewicz, J. (2013). Safety profile of antimicrobial peptides: camel, citropin, protegrin, temporin a and lipopeptide on HaCaT keratinocytes. Acta Pol. Pharm. 70, 795–801.
Barchiesi, F., Giacometti, A., Cirioni, O., Arzeni, D., Silvestri, C., Kamysz, W., et al. (2007). In vitro activity of the synthetic lipopeptide PAL-Lys-Lys-NH2 alone and in combination with antifungal agents against clinical isolates of Cryptococcus neoformans. Peptides 28, 1509–1513. doi: 10.1016/j.peptides.2007.07.010
Brown, K. L., and Hancock, R. E. W. (2006). Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 18, 24–30. doi: 10.1016/j.coi.2005.11.004
Chan, W. C., and White, P. D. (2000). Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford: Oxford University Press.
Cirioni, O., Giacometti, A., Ghiselli, R., Kamysz, W., Silvestri, C., Orlando, F., et al. (2007). The lipopeptides Pal–Lys–Lys–NH2 and Pal–Lys–Lys soaking alone and in combination with intraperitoneal vancomycin prevent vascular graft biofilm in a subcutaneous rat pouch model of staphylococcal infection. Peptides 28, 1299–1303. doi: 10.1016/j.peptides.2007.03.017
Domalaon, R., Findlay, B., Ogunsina, M., Arthur, G., and Schweizer, F. (2016). Ultrashort cationic lipopeptides and lipopeptoids: evaluation and mechanistic insights against epithelial cancer cells. Peptides 84, 58–67. doi: 10.1016/j.peptides.2016.07.007
Gaussier, H., Morency, H., Lavoie, M. C., and Subirade, M. (2002). Replacement of trifluoroacetic acid with HCl in the hydrophobic purification steps of pediocin PA-1: a structural effect. Appl. Environ. Microbiol. 68, 4803–4808. doi: 10.1128/AEM.68.10.4803-4808.2002
Greber, K. E., and Dawgul, M. (2016). Antimicrobial peptides under clinical trials. Curr. Top. Med. Chem. 17, 620–628. doi: 10.2174/1568026616666160713143331
Hancock, R. E. W., and Diamond, G. (2000). The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8, 402–410. doi: 10.1016/S0966-842X(00)01823-0
Hancock, R. E. W., and Sahl, H. G. (2006). Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24, 1551–1557. doi: 10.1038/nbt1267
Kamysz, W., Silvestri, C., Cirioni, O., Giacometti, A., Licci, A., Della Vittoria, A., et al. (2007). In vitro activities of the lipopeptides palmitoyl (Pal)-Lys-Lys-NH2 and Pal-Lys-Lys alone and in combination with antimicrobial agents against multiresistant gram-positive cocci. Antimicrob. Agents Chemother. 51, 354–358. doi: 10.1128/AAC.00344-06
Makovitzki, A., Avrahami, D., and Shai, Y. (2006). Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. U.S.A. 103, 15997–16002. doi: 10.1073/pnas.0606129103
Sarig, H., Livne, L., Held-Kuznetsov, V., Zaknoon, F., Ivankin, A., Gidalevitz, D., et al. (2010). A miniature mimic of host defense peptides with systemic antibacterial efficacy. FASEB J. 24, 1904–1913. doi: 10.1096/fj.09-149427
Simonetti, O., Arzeni, D., Ganzetti, G., Silvestri, C., Cirioni, O., Gabrielli, E., et al. (2009). In vitro activity of the lipopeptide derivative (Pal-Lys-Lys-NH), alone and in combination with antifungal agents, against clinical isolates of dermatophytes. Br. J. Dermatol. 161, 249–252. doi: 10.1111/j.1365-2133.2009.09166.x
Keywords: lipopeptides, antimicrobial activity, MIC, MBC, TFA counter ion, fatty acid chain length
Citation: Greber KE, Dawgul M, Kamysz W and Sawicki W (2017) Cationic Net Charge and Counter Ion Type as Antimicrobial Activity Determinant Factors of Short Lipopeptides. Front. Microbiol. 8:123. doi: 10.3389/fmicb.2017.00123
Received: 05 December 2016; Accepted: 18 January 2017;
Published: 01 February 2017.
Edited by:
Jack Wong, Chinese University of Hong Kong, Hong KongReviewed by:
Osmar Nascimento Silva, Universidade Católica Dom Bosco, BrazilMichael Kogut, USDA Agricultural Research Service, USA
Copyright © 2017 Greber, Dawgul, Kamysz and Sawicki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katarzyna E. Greber, greber@gumed.edu.pl
 Katarzyna E. Greber
Katarzyna E. Greber Malgorzata Dawgul2
Malgorzata Dawgul2