- 1College of Animal Science and Technology, Shandong Agricultural University, Tai'an, China
- 2Laboratory for Reproduction Health Institute of Biomedicine and Biotechnology Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen University Town, Shenzhen, China
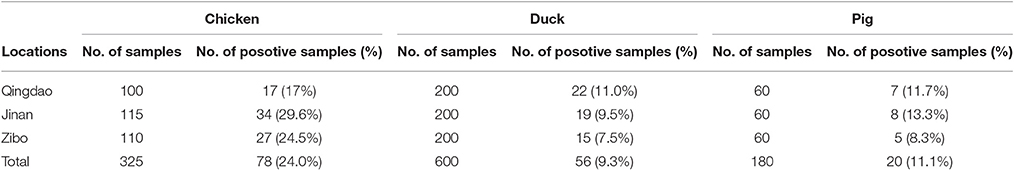
A total of 154 non-duplicate Salmonella isolates were recovered from 1,105 rectal swabs collected from three large-scale chicken farms (78/325, 24.0%), three large-scale duck farms (56/600, 9.3%) and three large-scale pig farms (20/180, 11.1%) between April and July 2016. Seven serotypes were identified among the 154 isolates, with the most common serotype in chickens and ducks being Salmonella enteritidis and in pigs Salmonella typhimurium. Antimicrobial susceptibility testing revealed that high antimicrobial resistance rates were observed for tetracycline (72.0%) and ampicillin (69.4%) in all sources. Class 1 integrons were detected in 16.9% (26/154) of these isolates and contained gene cassettes aadA2, aadA1, drfA1-aadA1, drfA12-aadA2, and drfA17-aadA5. Three β-lactamase genes were detected among the 154 isolates, and most of the isolates carried blaTEM−1(55/154), followed by blaPSE−1(14/154) and blaCTX−M−55 (11/154). Three plasmid-mediated quinolone resistance genes were detected among the 154 isolates, and most of the isolates carried qnrA (113/154), followed by qnrB (99/154) and qnrS (10/154). Fifty-four isolates carried floR among the 154 isolates. Multilocus sequence typing (MLST) analysis showed that nine sequence types (STs) were identified; ST11 was the most frequent genotype in chickens and ducks, and ST19 was identified in pigs. Our findings indicated that Salmonella was widespread, and the overuse of antibiotics in animals should be reduced considerably in developing countries.
Introduction
Salmonella is an important source of foodborne diseases that cause morbidity and mortality worldwide. Among 94 million cases of non-typhoid Salmonella infections, it was presumed that approximately 85% of the cases were induced by food origin Salmonella (Chiu et al., 2010). In China, Salmonella causes an estimated 22.2% of foodborne diseases (Wang et al., 2007). Many Salmonella serovars exist. More than 2,600 serovars are classified based on the reactivity of antisera to O and H antigens (Stevens et al., 2009), and the serovars from farms have a significant overlap with those causing illnesses in humans (Alcaine et al., 2006). Animals have been recognized as an important reservoir for Salmonella, and this pathogen can be transferred to humans via the food chain, posing a serious threat to human health (Vo et al., 2006).
The use of antimicrobials is important for the control and treatment of Salmonella. However, antimicrobial- and multidrug-resistant Salmonella strains have emerged, leading to treatment failure (Gong et al., 2013). The increasing prevalence of multidrug-resistance among Salmonella, not only against the front-line antimicrobials, chloramphenicol and trimethoprim/sulfamethoxazole but also against clinically important antimicrobial agents, such as β-lactams and fluoroquinolones, is also an emerging problem (Lunguya et al., 2013).
The spread of the antibiotic resistant potential in Salmonella is mainly attributed to integrons. Integrons are DNA elements, capable of capturing antimicrobial resistant genes and disseminating them using a mobile genetic element (MGE) such as a plasmid among bacteria. The class I integron is the most common integron type identified in multidrug-resistant (MDR) Salmonella and plays an important role in the dissemination of resistance genes among pathogens (Wright, 2010).
In developed countries, many surveys have been conducted at the molecular level to monitor the incidence of antibiotic-resistant Salmonella in animal farms (Melendez et al., 2010; Graciela et al., 2016). However, the extent of antibiotic-resistant Salmonella in many developing countries and the molecular mechanisms underlying this resistance remain unclear. Therefore, we selected large-scale animal farms as sample sites, collected swab samples, isolated Salmonella and characterized the molecular mechanisms of antimicrobial resistance.
Materials and Methods
Samples and Salmonella Isolation
From April to July 2016, rectal swabs were collected from healthy animals on farms in Qingdao, Jinan and Zibo regions in Shandong Province, China. All of the sampling sites were visited only once. In total, 1,105 samples were collected in a random manner from chickens (n = 325), ducks (n = 600), and pigs (n = 180). The samples were independently collected from individual animals, and the sample collection conformed to the cluster random sampling principle. Farms were chosen based on their scale with the following requirements: for chickens, the breeding stock was >150,000 heads; for ducks, the breeding stock was >100,000 heads, and for pigs, the breeding stock was >1,000 heads. The owners of each farm gave permission for rectal swab samples to be collected. The animals from which samples were extracted remained alive and did not undergo any surgery. Therefore, ethical approval was not required for the study because the sampling process did not harm the animals. All of the collected samples were transported in an ice box to our laboratory within 6 h for further bacteriological analysis.
Isolation and identification of Salmonella were performed as described previously (Yan et al., 2010), with some modifications. Briefly, swabbing samples were placed into a sterile plastic bag containing 100 ml of buffered peptone water (BPW) and mixed vigorously for 3 min. The BPW mixture was then incubated for 24 h at 37°C for pre-enrichment. Approximately 1 ml of pre-enrichment cultures were incubated in 10 ml of selenite cysteine (SC) broth and 10 ml of rappaport-vassiliadis (RV) broth at 42°C for 24 h, respectively. After selective enrichment, a loop-full of SC and RV broth cultures were streaked onto xylose lysine tergitol 4 (XLT4) agar and incubated at 37°C overnight. A minimum of two presumptive Salmonella colonies was confirmed by PCR using a previously described method (Malorny et al., 2003).
Salmonella Serotyping
According to the manufacturer's instructions, the serogroup and serovars of Salmonella isolates were determined according to the Kauffmann-White scheme by slide agglutination with O and H antigens (Tianrun Bio-Pharmaceutical, Ningbo, China).
Antimicrobial Susceptibility Testing
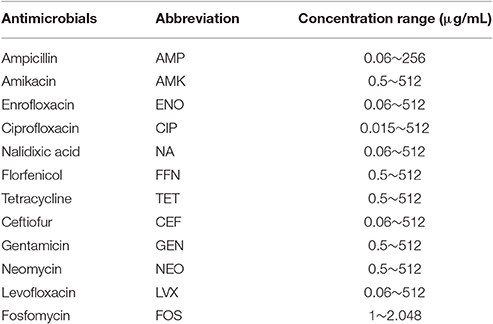
A minimal inhibition concentration (MIC) assay, as described by the Clinical and Laboratory Standard Institute (Clinical Laboratory Standards Institute, 2013), was used in this study to test the susceptibility of 12 commonly used antibiotics (Table 1), including ampicillin (AMP), amikacin (AMK), enrofloxacin (ENO), ciprofloxacin (CIP), nalidixic acid (NA), florfenicol (FFN), tetracycline (TET), ceftiofur (CEF), gentamicin (GEN), neomycin (NEO), levofloxacin (LVX), and fosfomycin (FOS). Escherichia coli (ATCC 25922) and Klebsiella pneumoniae (ATCC 700603) were used as the quality control strains in this study. Salmonella isolates resistant to more than three classes of antimicrobials were defined as MDR isolates.
Detection of Class I Integrons and Antimicrobial Resistance Genes
Bacterial DNA was extracted using a TIANamp Bacteria DNA Kit (Tiangen, Beijing, China) according to the manufacturer's instructions. Conserved primers were used for the detection and identification of class I integrons using previously described primers and procedures (Kerrnet et al., 2002). PCR screening for β-lactamase-encoding genes blaTEM, blaPSE−1, blaSHV, and blaCTX−M was performed as previously described (Li et al., 2013). Furthermore, PCR amplification was used to screen for plasmid-mediated quinolone resistance genes, qnrA, qnrB, qnrC, qnrD, and qnrS, which were the most frequently observed in China, using previously described primers (Ahmed et al., 2013). Finally, the florfenicol resistance gene, floR, was detected using previously described primers (Ahmed et al., 2013). The PCR products were purified and subsequently sequenced (Invitrogen, Beijing, China). The obtained DNA sequences were compared with those in GenBank using Basic Local Alignment Search Tool (BLAST).
MLST
Seven housekeeping genes (aroC, dnaN, hemD, hisD, purE, sucA, and thrA) were used to characterize Salmonella by MLST. MLST was performed as described online (http://mlst.warwick.ac.uk/mlst/dbs/Senterica/documents/primersEnterica_html). All polymerase chain reaction products were purified and sequenced (Invitrogen, Beijing, China), and the alleles and STs were assigned according to the MLST scheme at http://mlst.warwick.ac.uk/mlst/dbs/Senterica.
Data Analysis
The statistical package SPSS (version 15.0, SPSS, Chicago, IL, USA) was used to compare the prevalence and MDR resistance rate of Salmonella isolated from chickens, ducks and pigs, and a P-value less than 0.05 was considered significant.
Results
Prevalence and Serotypes of Salmonella
In this study, a total of 154 non-duplicate Salmonella isolates (154/1105, 13.9%) were recovered. From chickens, 78 Salmonella isolates were recovered (78/325, 24.0%) (Table 2), which was significantly higher than the Salmonella isolated from ducks and pigs (P < 0.05). Seventy-eight Salmonella isolates were divided into six serovars. The most common serovar was Salmonella enteritidis (69/78, 88.5%) (Table 3).
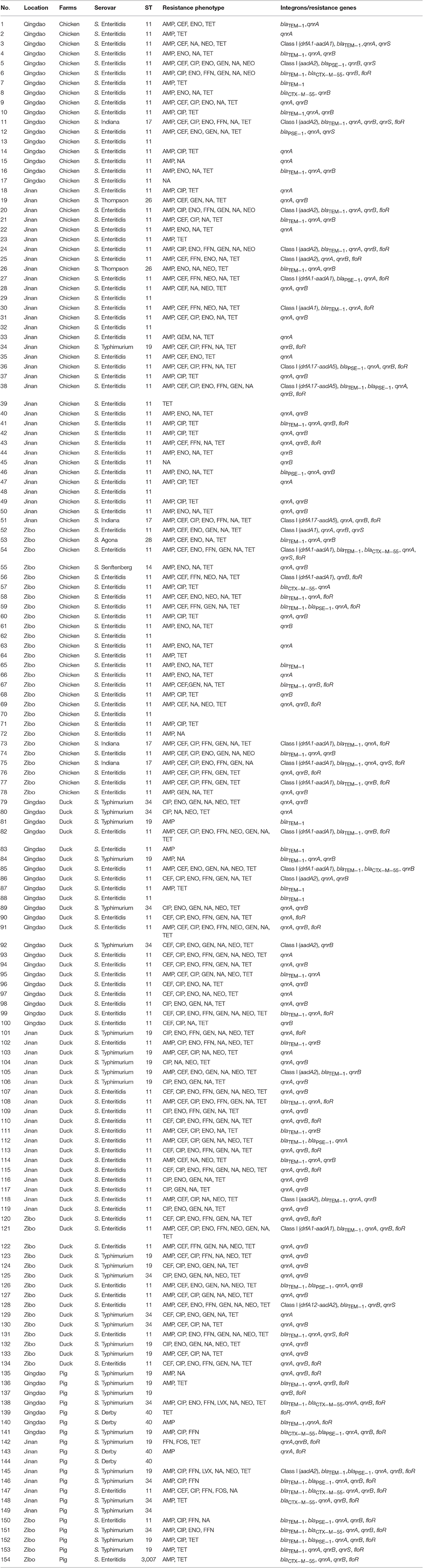
Table 3. Resistance phenotype, ST, incidence of class I integron, and resistance gens in Salmonella isolated from animals in farms.
From ducks, 56 Salmonella isolates were recovered (56/600, 9.3%) (Table 2), and they were divided into two serovars. The most common serovar was Salmonella enteritidis (38/56, 67.9%) (Table 3).
From pigs, 20 Salmonella isolates were recovered (20/180, 11.1%) (Table 2), and they were divided into three serovars. The most common serovar was Salmonella typhimurium (13/20, 65.0%) (Table 3).
Antimicrobial Susceptibility Testing
Among 78 isolates from chickens, they were susceptible to amikacin, levofloxacin and fosfomycin. Most isolates were resistant to ampicillin (69/78, 88.5%) and tetracycline (61/78, 78.2%). In addition, 63 isolates (63/78, 80.8%) exhibited MDR (Table 3).
Among 56 isolates from ducks, they were susceptible to amikacin, levofloxacin and fosfomycin. Most isolates were resistant to tetracycline (52/56, 92.9%) and ciprofloxacin (45/56, 80.4%). In addition, 50 isolates (50/56, 89.3%) exhibited MDR (Table 3), which was significantly higher than the Salmonella isolated from chickens and pigs (P < 0.05).
Among 20 isolates from pigs, they were susceptible to amikacin and levofloxacin. Most isolates were resistant to ampicillin (15/20, 75.0%) and tetracycline (9/20, 45.0%). In addition, 9 isolates (9/20, 45.0%) exhibited MDR (Table 3).
Characteristics of Class I Integrons and Antimicrobial Resistance Genes
Among the 78 isolates recovered from chickens, 17 isolates (17/78, 21.8%) contained four groups of resistance gene cassettes, consisting of drfA1-aadA1 (1.7 kb, n = 7), aadA2 (1.2 kb, n = 5), drfA17-aadA5 (2 kb, n = 3), and aadA1 (1.2 kb, n = 2). Three β-lactamase genes were detected among the isolates, and blaTEM−1 (n = 25) was the most commonly isolated β-lactamase gene, followed by blaPSE−1 (n = 7) and blaCTX−M−55 (n = 4). Three plasmid-mediated quinolone resistance genes were detected among the isolates. qnrA (n = 53) was the most commonly isolated plasmid-mediated quinolone resistance gene, followed by qnrB (n = 44) and qnrS (n = 7). In addition, 23 isolates carried floR (Table 3).
Among the 56 isolates recovered from ducks, eight isolates (8/56, 14.3%) contained three groups of resistance gene cassettes, consisting of aadA2 (1.2 kb, n = 4), drfA1-aadA1 (1.7 kb, n = 3), and drfA12-aadA2 (2 kb, n = 1). Three β-lactamase genes were detected among the isolates. blaTEM−1 was the most commonly isolated β-lactamase gene (n = 20), followed by blaPSE−1(n = 2) and blaCTX−M−55 (n = 1). Three plasmid-mediated quinolone resistance genes were detected among the isolates. qnrA was the most commonly isolated plasmid-mediated quinolone resistance gene (n = 44), followed by qnrB (n = 40) and qnrS (n = 2). In addition, 13 isolates carried floR (Table 3).
Among the 20 isolates recovered from pigs, one isolate (1/20, 5.0%) contained one group of a resistance gene cassette, consisting of aadA2 (1.2 kb, n = 1). Three β-lactamase genes were detected among the isolates. blaTEM−1 was the most commonly isolated β-lactamase gene (n = 10), followed by blaCTX−M−55 (n = 6) and blaPSE−1 (n = 5). Three plasmid-mediated quinolone resistance genes were detected among the isolates. qnrA was the most commonly isolated plasmid-mediated quinolone resistance gene (n = 16), followed by qnrB (n = 15) and qnrS (n = 1). In addition, 18 isolates carried floR (Table 3).
MLST
A total of nine STs among the 154 isolates were found. ST11 was the most common ST in both chickens and ducks, and it was represented by 69 and 38 Salmonella isolates, respectively. ST19 was the most common ST in pigs, and it was represented by eight Salmonella isolates (Table 3). The STs in this study were correlated with specific serovars, such as ST11 with Salmonella enteritidis, ST19 and ST34 with Salmonella typhimurium, and ST40 with Salmonella derby.
Discussion
In this study, Salmonella spp. were recovered from chickens, ducks and pigs in Qingdao, Jinan and Zibo regions. For the chickens, the prevalence (24.0%) was significantly higher than that reported in Shanghai, China (4.5%) (Liu et al., 2010) but was lower than that reported from chicken farms in Egypt (41.0%) (Hanem et al., 2017). The prevalence (9.3%) in ducks was similar to that obtained from duck farms in Sichuan province (12.0%) (Li et al., 2013) but was lower than those reported in Penang, Malaysia (39.0%) (Adzitey et al., 2012), and in South Korea (65.2%) (Cha et al., 2013). For pigs, the occurrence ratio (11.1%) was similar to those reported in previous studies of Salmonella spp. in food products of animal origin in China (Jiang et al., 2006; Li et al., 2013) but was higher than that reported from conventional farms (3.5%) in Korea (Migma et al., 2015). Data on the prevalence of Salmonella in different studies were difficult to compare based on differences in regions, collection seasons, sample types, isolation methodologies, culture methods, culture media, and environmental conditions.
For serotyping, a total of seven serovars were found among the 154 isolates, including six from chickens, two from ducks, and three from pigs. The most common serotype in chickens and ducks was Salmonella enteritidis. This result was consistent with those from Shanxi province (Yang et al., 2010), but it was different from other reports that the dominant serotype in chicken farms was Salmonella Colindale in Chad (Tabo et al., 2013). The most common serotype in duck farms was Salmonella typhimurium (Martelli et al., 2016). The dominant serotype in pigs was Salmonella typhimurium, which was the most common serovar isolated from humans and it can lead to severe human and animal diseases (Deng et al., 2012), but it was different from other studies, where the dominant serotype in pig farms was Salmonella IIIb in Henan province (Kuang et al., 2015), and Salmonella derby in England and Wales (Miller et al., 2011). The difference in dominant serotype among animals may be due to differences in the pathogenicity of two serovars, geographical regions and diversities (Volf et al., 2010; European Centre for Disease Prevention Control, 2013).
Antimicrobial resistance in Salmonella is a threat to human public health. As shown in Table 3, the high rates of antimicrobial resistance were against tetracycline (72.0%) and ampicillin (69.4%) in all sources, which was similar to reports of Salmonella isolates from Africa, in which chickens exhibited resistance to tetracycline (93.0%) and ampicillin (47.0%) (Zishiri et al., 2016). These high resistance rates are due to its wide use in animal feed and were consistent with other reports (Piras et al., 2011; Shao, 2011; Bai et al., 2015). In addition, resistance to ciprofloxacin in 35.9% of chickens, 80.4% of ducks, and 30.0% of pigs deserves our attention because resistance to this antimicrobial agent may lead to the delay or failure of fluoroquinolone therapies (Van et al., 2007). In this study, all of the isolates were susceptible to amikacin, which may be because this antimicrobial is not used for therapeutic purposes in veterinary medicine or as a growth promoter in conventional animal fattening, and the result was consistent with other reports (Eva et al., 2015). In this study, MDR Salmonella isolates were frequently observed among chickens, ducks and pigs. In addition, MDR Salmonella is serotype-dependent (Clemente et al., 2014): the data provided evidence that Salmonella indiana, Salmonella typhimurium and Salmonella enteritidis were strongly associated with MDR phenotypes. Of particular concern, MDR strains could transfer to humans via animal or animal-derived products and pose a great risk to public health (Rosangela et al., 2016).
In this study, our results related to the incidence of class I integrons (26/154, 16.9%) were similar to the report in Sichuan (Li et al., 2013) but were higher than those reported in the USA, as class I integrons were identified in only 2.8% of the Salmonella isolates from bulk milk and milk filters (Van et al., 2013). In the present study, the incidence of class I integrons was significantly higher in Salmonella from chickens (21.8%) than Salmonella from pigs (5.0%). In addition, in this study, the Salmonella isolates carrying class I integrons included Salmonella enteritidis, typhimurium and indiana.
Production of β-lactamases is considered to be the main mechanism of resistance in Gram-negative bacteria to overcome penicillin-derived antibiotics, and the blaTEM and blaCTX−M ESBLs can hydrolyse third and fourth generation cephalosporins. In this study, a total of three β-lactamase genes were detected among the Salmonella isolates recovered from chickens, ducks and pigs: blaTEM−1, blaPSE−1, and blaCTX−M−55. Most of the isolates carried blaTEM−1, which was similar to the report in South Africa that blaTEM−1 was the most commonly identified β-lactamase gene in Salmonella isolates from food-producing animals (Igbinosa, 2015). In addition, in this study, most isolates carried blaTEM−1 and blaCTX−M−55, which confer resistance to ampicillin.
Quinolones are the first choice for the treatment of invasive and systemic salmonellosis that occurs in humans and animals (Dimitrov et al., 2007). A total of three quinolone resistance genes were detected among the Salmonella isolates recovered from chickens, ducks and pigs: qnrA, qnrB and qnrS. qnrA was the most commonly isolated plasmid-mediated quinolone resistance gene consistent with a report in Henan, where qnrA, qnrB and qnrS were identified in Salmonella strains isolated from retail food with an incidence of 46.6, 12.7, and 19.5%, respectively (Yang et al., 2013). It is well known that qnr genes confer only low-level resistance to fluoroquinolones, and accumulation of quinolone resistance-determining region (QRDR) mutations is necessary for S. enterica to be resistant to fluoroquinolone, especially ciprofloxacin (Eaves et al., 2004). In this study, most Salmonella isolates containing a plasmid-mediated quinolone resistance gene were resistant to ciprofloxacin, nalidixic acid and gentamicin.
Florfenicol, a new chemosynthesis broad spectrum antibiotic of chloramphenicol analogs, is a fluorinated derivative of thiamphenicol. It is not approved for human use. In this study, floR was identified in 35.1% of Salmonella strains isolated from chickens, ducks and pigs, which was significantly higher than that reported in Egypt (1.0%) (Ahmed and Shimamoto, 2012). In addition, floR was identified in 90.0% of Salmonella strains isolated from pigs in this study. In this study, most Salmonella isolates containing the floR gene were resistant to florfenicol.
MLST results reveal that a total of nine STs were identified in this study. ST11 was the most frequent genotype that was recovered in chickens and ducks, and ST19 was the most frequent genotype that was recovered in pigs. ST11 belongs to Salmonella enteritidis, and ST19 belongs to Salmonella typhimurium; they all have continually been reported to cause human salmonellosis in recent years (Cai et al., 2016; Kang et al., 2017). In addition, our results revealed that the MLST patterns were generally associated with serotypes and provided a reliable prediction of the Salmonella serovars, which was consistent with previous research (Achtman et al., 2012).
Conclusion
The prevalence of Salmonella was higher in the animal farms. Moreover, many serovars reported in humans and MDR Salmonella were recovered in this study. The high rates of MDR Salmonella, class I integrons and antibiotic resistance gene positive isolates detected suggest that measures must be taken to facilitate the reasonable use of antimicrobials in animal husbandry. Therefore, continuous surveillance of Salmonella and associated antimicrobial resistance in Salmonella of animals is essential to detect emerging Salmonella serovars and associated resistance genes.
Author Contributions
SS, XZ, contributed to the conception of the study; WC, XZ; contributed significantly to analysis and manuscript preparation; XZ, performed the data analyses and wrote the manuscript; XZ, JY, BZ: helped perform the analysis with constructive discussions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National key R&D project (2016YFD0501608) and (2016 YFD0500510). Taishan Scholars Program (201511023); Funds of Shandong “Double Tops” program.
References
Achtman, M., Wain, J., Weill, F. X., Nair, S., Zhou, Z., Sangal, V., et al. (2012). Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog. 8:e1002776. doi: 10.1371/journal.ppat.1002776
Adzitey, F., Rusul, R., and Huda, N. (2012). Prevalence and antibiotic resistance of Salmonella serovars in ducks, duck rearing and processing environments in Penang, Malaysia. Food Res. Int. 45, 947–952. doi: 10.1016/j.foodres.2011.02.051
Ahmed, A. M., and Shimamoto, T. (2012). Genetic analysis of multiple antimicrobial resistance in Salmonella isolated from diseased broilers in Egypt. Microbiol. Immunol. 56, 254–261. doi: 10.1111/j.1348-0421.2012.00429.x
Ahmed, A. M., Shimamoto, T., and Shimamoto, T. (2013). Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int. J. Med. Microbiol. 303, 475–483. doi: 10.1016/j.ijmm.2013.06.009
Alcaine, S. D., Soyer, Y., Warnick, L. D., Su, W. L., Sukhnanand, S., Richards, J., et al. (2006). Multilocus sequence typing supports the hypothesis that cow- and human-associated Salmonella isolates represent distinct and overlapping populations. Appl. Environ. Microb. 72, 7575–7585. doi: 10.1128/AEM.01174-06
Bai, L., Lan, R. T., Zhang, X. L., Cui, S. H., Xu, J., Guo, Y. C., et al. (2015). Prevalence of Salmonella isolates from chicken and pig slaughter houses and emergence of ciprofloxacin and cefotaxime co-resistant, S.enterica Serovar Indiana in Henan, China. PLoS ONE 10:e0144532. doi: 10.1371/journal.pone.0144532
Cai, Y. Q., Tao, J., Jiao, Y., Fei, X., Zhou, L., Wang, Y., et al. (2016). Phenotypic characteristics and genotypic correlation between Salmonella isolates from a slaughterhouse and retail markets in Yangzhou, China. Int. J. Food Microb. 222, 56–64. doi: 10.1016/j.ijfoodmicro.2016.01.020
Cha, S. Y., Kang, M., Yoon, R. H., Park, C. K., Moon, O. K., and Jang, H. K. (2013). Prevalence and antimicrobial susceptibility of Salmonella isolates in Pekin ducks from South Korea. Comp. Immunol. Microbiol. Infect. Dis. 36, 473–479. doi: 10.1016/j.cimid.2013.03.004
Chiu, L. H., Chiu, C. H., Horn, Y. M., Chiou, C. S., Lee, C. Y., Yeh, C. M., et al. (2010). Characterization of 13 multi-drug resistant Salmonella serovars from different broiler chickens associated with those of human isolates. BMC Microb. 10:86. doi: 10.1186/1471-2180-10-86
Clemente, L., Correia, I., Themudo, P., Neto, I., Canica, M., and Bernardo, F. (2014). Antimicrobial susceptibility of Salmonella enterica isolates from healthy breeder and broiler flocks in Portugal. Vet. J. 200, 276–281. doi: 10.1016/j.tvjl.2014.02.007
Clinical and Laboratory Standards Institute. (2013). Performance Standards for Antimicrobial Susceptibility Testing: Twentieth-third Informational Supplement M100-S23. Wayne, PA: CLSI.
Deng, X., Ran, L., Wu, S., Ke, B., He, D., Yang, X., et al. (2012). Laboratory-based surveillance of non-typhoidal Salmonella infections in Guangdong Province, China. Foodborne Pathog. Dis. 9, 305–312. doi: 10.1089/fpd.2011.1008
Dimitrov, T., Udo, E. E., Albaksami, O., Kilani, A. A., and Shehab, E. M. R. (2007). Ciprofloxacin treatment failure in a case of typhoid fever caused by Salmonella enterica serotype Paratyphi A with reduced susceptibility to ciprofloxacin. J. Med. Microbiol. 56, 277–279. doi: 10.1099/jmm.0.46773-0
Eaves, D. J., Randall, L., Gray, D. T., Buckley, A., Woodward, M. J., White, A. P., et al. (2004). Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob. Agents Chemother. 48, 4012–4015. doi: 10.1128/AAC.48.10.4012-4015.2004
European Centre for Disease Prevention and Control. (2013). Annual Epidemiological Report Reporting on 2011 Surveillance Data and 2012 Epidemic Intelligence Data. 103–108.
Eva, D., Ana, J. B., Jose, A. A., Maria, A. F., and Isabel, E. (2015). Pravalence and antimicrobial resistance of Listeria monocytogenes and Salmonella strains isolated in ready-to-eat foods in Eastern Spain. Food Control 47, 120–125. doi: 10.1016/j.foodcont.2014.06.043
Gong, J., Xu, M., Zhu, C., Miao, J., Liu, X., Xu, B., et al. (2013). Antimicrobial resistance, presence of integrons and biofilm formation of Salmonella pullorum isolates from eastern China (1962-2010). Avian Pathol. 42, 290–294. doi: 10.1080/03079457.2013.788129
Graciela, V. L., Geovana, B. M., Marisa, C., and Stefan, S. (2016). Antimicrobial resistance and class1 integron-associated gene cassettes in Salmonella enterica Serovar typhimurium isolated from pigs at slaughter and abattoir enviroment. Vet. Microbiol. 194, 84–92. doi: 10.1016/j.vetmic.2016.04.020
Hanem, E. S., Amin, T., Abd, E. E., Moshira, E. A., Fares, E. K., Trudi, G., et al. (2017). Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 9:8. doi: 10.1186/s13099-017-0157-1
Igbinosa, I. H. (2015). Prevalence and detection of antibiptic-resistant determinant in Salmonella isolated from food-producing animals. Trop. Anim. Health Prod. 47, 37–43. doi: 10.1007/s11250-014-0680-8
Jiang, Z. L., Lv, S. L., Tang, Z. Z., Li, H., Wang, H., and Chen, G. (2006). Surveillance of Salmonella in food in Guangxi during 2002~2005. Pract. Prevent. Med. 64, 23–26.
Kang, M. S., Oh, Y. J., Kwon, Y. K., Lee, D. Y., Jeong, O. M., Choi, B. K., et al. (2017). Public health significance of major genotypes of Salmonella enterica serovar Enteritidis present in both human and chicken isolates in Korea. Res. Vet. Sci. 112, 125–131. doi: 10.1016/j.rvsc.2017.02.010
Kerrnet, M. B., Klemmensen, T., Frimodt-Moller, N., and Espersen, F. (2002). Susceptibility of danish Escherichia coli strains isolated from urinary tract infections and bacteraemia, and distribution of sul genes conferring sulphonamide resistance. J. Antimicrob. Chemother. 50, 513–516. doi: 10.1093/jac/dkf164
Kuang, X. H., Hao, H. H., Dai, M. H., Wang, Y. L., Ahmad, I., Liu, Z. L., et al. (2015). Serotypes and antimicrobial susceptibility of Salmonella spp. isolated from farm animals in China. Front. Microbiol. 6:602. doi: 10.3389/fmicb.2015.00602
Li, R. C., Lai, J., Wang, Y., Liu, S. L., Li, Y., Liu, K. Y., et al. (2013). Prevalence and characterization of Salmonella species isolated from pigs, ducks and chickens in Sichuan Province, China. Int. J. Food Microbiol. 163, 14–18. doi: 10.1016/j.ijfoodmicro.2013.01.020
Liu, W. B., Chen, J., Huang, Y. Y., Liu, B., and Shi, X. M. (2010). Serotype, genotype, and antimicrobial susceptibility profiles of Salmonella from Chicken Farms in Shanghai. J. Food Protect. 73, 562–567. doi: 10.4315/0362-028X-73.3.562
Lunguya, O., Lejon, V., Phoba, M. F., Bertrand, S., Vanhoof, R., Glupczynski, Y., et al. (2013). Antimicrobial resistance in invasive nontyphoid Salmonella from the Democratic Republic of the Congo: emergence of decreased fluoroquinolone susceptibility and extended-spectrum beta lactamases. PLoS Negl. Trop. Dis. 7:e2103. doi: 10.1371/journal.pntd.0002103
Malorny, B., Hoorfar, J., Hugas, M., Heuvelink, A., Fach, P., Ellerbroek, L., et al. (2003). Interlaboratory diagnostic accuracy of a Salmonella specific PCR-based method. Int. J. Food Microbiol. 89, 241–249. doi: 10.1016/S0168-1605(03)00154-5
Martelli, F., Birch, C., and Davies, R. H. (2016). Observations on the distribution and control of Salmonella in commercial duck hatcheries in the UK. Avian Pathol. 45, 261–266. doi: 10.1080/03079457.2016.1146820
Melendez, S. N., Hanning, I., Han, J., Nayak, R., Clement, A. R., Wooming, A., et al. (2010). Salmonella enterica isolates from pasture-raised poultry exhibit antimicrobial resistance and class I integrons. J. Appl. Microbiol. 109, 1957–1966. doi: 10.1111/j.1365-2672.2010.04825.x
Migma, D. T., Mamata, G., Hyang, M. N., Dong, C. M., Kima, S. R., Jang, G. C., et al. (2015). Prevalence and characterization of Salmonella in pigs from conventional and organic farms and first report of S. serovar 1,4,[5],12:i:- from Korea. Vet. Microbiol. 178, 119–124. doi: 10.1016/j.vetmic.2015.05.005
Miller, A. J., Twomey, D. F., Davies, R. H., Teale, C. J., Williamson, S. M., Reichel, R., et al. (2011). Salmonella serovars and antimicrobial resistance patterns on a sample of high seroprevalence pig farms in England and Wales. Zoonoses Public Health. 56, 137–144. doi: 10.1111/j.1863-2378.2011.01402.x
Piras, F., Brown, D. J., Meloni, D., Mureddu, A., and Mazzette, R. (2011). Investigation of Salmonella enterica in Sardinian slaughter pigs: prevalence, serotype and genotype characterization. Int. J. Food Microbiol. 151, 201–209. doi: 10.1016/j.ijfoodmicro.2011.08.025
Rosangela, E. Z., Camila, L., Ana, P. P., Mallu, J. S., Ricardo, A. P. S., Cibeli, V., et al. (2016). Multidrug resistance and ESBL-producing Salmonella spp. Isolated from broiler processing plants. Braz. J. Microbiol. 47, 191–195. doi: 10.1016/j.bjm.2015.11.021
Shao, K. (2011). Study on Types and Active Surveillance of Foodborne Salmonella spp. Master's thesis, Shandong Province.
Stevens, M. P., Humphrey, T. J., and Maskell, D. J. (2009). Molecular insights into farm animal and zoonotic Salmonella infections. Philos. Trans. R. Soc. London. 364, 2709–2723. doi: 10.1098/rstb.2009.0094
Tabo, D. A., Diguimbaye, C. D., Granier, S. A., Moury, F. Q., Brisabois, A., Elgroud, R., et al. (2013). Prevalence and antimicrobial resistance of non-typhoidal Salmonella serotypes isolated from laying hens and broiler chicken farms in N'Djamena, Chad. Vet. Microbiol. 166, 293–298. doi: 10.1016/j.vetmic.2013.05.010
Van, K. J. S., Sonnier, J., Zhao, S., and Karns, J. S. (2013). Antimicrobial resistance of Salmonella enterica isolates from bulk tank milk and milk filters in the United States. J. Food Prot. 76, 18–25. doi: 10.4315/0362-028X.JFP-12-263
Van, T. T. H., Moutafis, G., Istivan, T., Tran, L. T., and Coloe, P. J. (2007). Detection of Salmonella spp. in retail raw food samples from Vietnam and characterization of their antibiotic resistance. Appl. Environ. Microb. 73, 6885–6890. doi: 10.1128/AEM.00972-07
Vo, A. T., Van, D. E., Fluit, A. C., Heck, M. E., Verbruggen, A., Maas, H. M., et al. (2006). Distribution of Salmonella enterica serovars from humans, livestock and meat in Vietnam and the dominance of Salmonella typhimurium phage type 90. Vet. Microbiol. 113, 153–158. doi: 10.1016/j.vetmic.2005.10.034
Volf, J., Havlickova, H., Hradecka, H., Ondrackova, P., Matiasovic, J., Faldyna, M., et al. (2010). Epidemiology and interaction of Salmonella enterica serovar Derby, Infantis and Typhimurium with porcine alveolar macrophages. Vet. Microbiol. 146, 105–110. doi: 10.1016/j.vetmic.2010.04.031
Wang, S. J., Duan, H. L., Zhang, W., and Li, J. (2007). Analysis of bacterial foodborne disease outbreaks in China between 1994 and 2005. FEMS Immunol. Med. Microbiol. 51, 8–13. doi: 10.1111/j.1574-695X.2007.00305.x
Wright, G. D. (2010). Antibiotic resistance in the environment: a link to the clinic? Curr. Opin. Microbiol. 13, 589–594. doi: 10.1016/j.mib.2010.08.005
Yan, H., Li, L., Alam, M. J., Shinoda, S., Miyoshi, S., and Shi, L. (2010). Prevalence and antimicrobial resistance of Salmonella in retail foods in northern China. Int. J. Food Microbiol. 143, 230–234. doi: 10.1016/j.ijfoodmicro.2010.07.034
Yang, B., Qiao, L., Zhang, X., Cui, Y., Xia, X., Cui, S., et al. (2013). Serotyping, antimicrobial susceptibility, pulse field gel electrophoresis analysis of Salmonella isolates from retail foods in Henan Province, China. Food Control 32, 228–235. doi: 10.1016/j.foodcont.2012.11.022
Yang, B., Qu, D., Zhang, X., Shen, J., Cui, S., Shi, Y., et al. (2010). Prevalence and characterization of Salmonella serovars in retail meats of marketplace in Shaanxi, China. Int. J. Food Microbiol. 141, 63–72. doi: 10.1016/j.ijfoodmicro.2010.04.015
Keywords: Salmonella, antimicrobial susceptibility, class 1 integron, antimicrobial resistance gene, MLST
Citation: Zhao X, Yang J, Zhang B, Sun S and Chang W (2017) Characterization of Integrons and Resistance Genes in Salmonella Isolates from Farm Animals in Shandong Province, China. Front. Microbiol. 8:1300. doi: 10.3389/fmicb.2017.01300
Received: 31 March 2017; Accepted: 27 June 2017;
Published: 12 July 2017.
Edited by:
Axel Cloeckaert, Institut National de la Recherche Agronomique (INRA), FranceReviewed by:
Sebastian Guenther, Freie Universität Berlin, GermanySéamus Fanning, University College Dublin, Ireland
Copyright © 2017 Zhao, Yang, Zhang, Sun and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhong Sun, jqybfkyjs@163.com
Weishan Chang, changweishan@yeah.net
 Xiaonan Zhao1
Xiaonan Zhao1 Shuhong Sun
Shuhong Sun