- 1Department of Biomedical and Molecular Sciences, Queen’s University, Kingston, ON, Canada
- 2Department of Molecular and Cellular Biology, University of Guelph, Guelph, ON, Canada
In Methanococcus maripaludis, the euryarchaeal archaellum regulator A (EarA) is required for the transcription of the fla operon, which is comprised of a series of genes which encode most of the proteins needed for the formation of the archaeal swimming organelle, the archaellum. In mutants deleted for earA (ΔearA), there is almost undetectable transcription of the fla operon, Fla proteins are not synthesized and the cells are non-archaellated. In this study, we have isolated a spontaneous mutant of a ΔearA mutant in which the restoration of the transcription and translation of the fla operon (using flaB2, the second gene of the operon, as a reporter), archaella formation and swarming motility were all restored even in the absence of EarA. Analysis of the DNA sequence from the fla promoter of this spontaneous mutant revealed a deletion of three adenines within a string of seven adenines in the transcription factor B recognition element (BRE). When the three adenine deletion in the BRE was regenerated in a stock culture of the ΔearA mutant, very similar phenotypes to that of the spontaneous mutant were observed. Deletion of the three adenines in the fla promoter BRE resulted in the mutant BRE having high sequence identity to BREs from promoters that have strong basal transcription level in Mc. maripaludis and Methanocaldococcus jannaschii. These data suggest that EarA may help recruit transcription factor B to a weak BRE in the fla promoter of wild-type cells but is not required for transcription from the fla promoter with a strong BRE, as in the three adenine deletion version in the spontaneous mutant.
Introduction
In the third domain of life, the Archaea, the transcription machinery is composed of a multi-subunit RNA polymerase that shares homology to the eukaryotic RNA polymerase II, as well as two general transcription factors: the TATA-box binding protein (TBP) and transcription factor B (TFB) (Bell and Jackson, 2001; Jun et al., 2011; Gehring et al., 2016). The corresponding DNA elements of a basal archaeal promoter includes a purine-rich transcription factor B recognition element (BRE), which is recognized by the TFB, immediately followed by a TATA box centered at a distance of 26/27 bp upstream of the transcription start site (TSS) (Soppa, 1999; Bartlett, 2005; Gehring et al., 2016). To initiate transcription, TBP first binds to TATA box. This is followed by the binding of TFB to the DNA-TBP complex by recognition of the BRE sequence (Bell et al., 1999) and, finally, the recruitment of RNA polymerase to initiate transcription (Bell and Jackson, 2001). Mutations in either the TATA box or BRE can decrease transcription levels by reducing recruitment of TBP and TFB (Bartlett, 2005).
Although Archaea use a eukaryote-like basal transcription machinery, the genome structure and its transcription regulation are more like that found in Bacteria. In Archaea, a cluster of genes is co-transcribed into a poly-cistronic mRNA under the control of a single promoter, which can be regulated by repressors and/or activators (Peeters et al., 2013). Transcriptional activators typically bind to sites located upstream of the BRE and help in the recruitment of TBP or TFB. In contrast, repressors can bind to either the promoter region where they interfere with TFB or TBP binding by steric hindrance, or downstream of the promoter, sometimes even after the TSS, to prevent RNA polymerase recruitment or transcription elongation (Bell, 2005; Peeters et al., 2013; Karr, 2014). Transcriptional activators are often associated with promoters that have TATA box or BRE sequences that deviate from consensus sequences (Ochs et al., 2012; Peeters et al., 2013). They are believed to help overcome poor binding of TBP or TFB to weak TATA and BRE sequences to activate transcription (Ouhammouch et al., 2003; Peng et al., 2009; Ochs et al., 2012).
The methanogen Methanococcus maripaludis is a member of the phylum Euryarchaeota and a model organism for studies in Archaea. Here, the fla operon, encoding the components of the archaeal swimming organelle, the archaellum (Jarrell and Albers, 2012; Albers and Jarrell, 2015), begins with flaB1-B3 encoding the three major structural proteins (archaellins), followed by the fla-associated genes flaC-J (Chaban et al., 2007). Transcription of the fla operon is controlled by the transcriptional activator EarA (Ding et al., 2016b). Deletion of earA results in almost undetectable transcription of the fla operon and a corresponding disappearance of FlaB2 protein and archaella production (Ding et al., 2016b). Immediately upstream of the BRE in the fla promoter, four 6 bp consensus sequences were identified as EarA binding sites. When all four EarA binding sites were eliminated in the genome of wild-type Mc. maripaludis, similar phenotypes were observed as in the ΔearA mutant (Ding et al., 2016b). Recently, we have shown that EarA homologs from selected archaellated methanogens could successfully complement the function of EarA in the Mc. maripaludis ΔearA mutant, indicating that the EarA regulatory model is likely widespread in the methanogen fla promoters (Ding et al., 2017).
In addition to the direct control of transcription of the fla operon by EarA, transcription of the fla operon was also found to be regulated under several growth conditions. Global transcriptome analysis of Mc. maripaludis showed that the transcription of the fla operon is up-regulated when H2 is limited and down-regulated under leucine starvation, for example (Hendrickson et al., 2008). In addition, we recently showed that transcription of the fla operon was severely impaired in cells grown at temperatures greater than 38°C (Ding et al., 2016a). The mechanism behind the regulation of the fla promoter under the above conditions, including any possible involvement of EarA or other putative transcriptional activators or repressors, is yet to be reported.
In this study, we isolated a spontaneous mutant of the ΔearA mutant in which transcription of the fla operon, production of archaellins and archaellation were all restored to near wild-type levels, despite the absence of EarA. Analysis of the fla promoter region of this mutant revealed a deletion of three adenines in the BRE. Recreation of the three adenine deletion in the original ΔearA mutant by molecular biology techniques resulted in very similar archaella-related phenotypes as observed in the spontaneous mutant. Examination of the fla promoter wild-type BRE and the three adenine deletion BRE revealed that the mutant BRE were highly similar to BRE sequences associated with promoters with strong basal transcription levels in both Mc. maripaludis and a related hyperthermophilic methanogen Methanocaldococcus jannaschii.
Materials and Methods
Strains and Culture Conditions
Methanococcus maripaludis Δhpt (Mm900) (Moore and Leigh, 2005), Mc. maripaludis ΔhptΔearA (ΔearA, Ding et al., 2016b) and mutant strains derived from them were routinely cultured in 120 mL sealed serum bottles containing 10 mL Balch medium III under a headspace of H2:CO2 (80:20) with shaking at 35°C (Balch et al., 1979). Escherichia coli TOP10 cells were cultured in Luria-Bertani (LB) broth or LB agar in the presence of 100 μg/mL ampicillin for plasmid selection at 37°C. Strains used in this study are listed in Table 1.
Identification of a Spontaneous Mutant Strain (ΔearA-sp) Derived from ΔearA in Which the Expression of FlaB2 Was Restored
Immediately after its generation, the ΔearA mutant was streaked three times for purity, and one colony was grown overnight and frozen as the stock culture at -80°C. Western blot analysis confirmed the cessation of FlaB2 expression in the ΔearA strain at this stage (Ding et al., 2016a). The ΔearA strain was also maintained in the lab via weekly subculture in Balch medium III statically at 37°C. After 6 months of sub-culturing, western blotting experiments revealed that the expression of FlaB2 was restored. PCR experiments determined that this strain still had the deletion of earA, so the restoration of FlaB2 expression was not a result of strain contamination. The newly isolated strain was named as ΔearA-sp (sp for spontaneous).
Sequence Analysis of the fla Promoter Region in the ΔearA-sp Strain
The fla promoter region spanning from -348 bp upstream of the TSS of the fla promoter to 162 bp downstream of the TSS from the ΔearA-sp strain was PCR amplified using primer pair P1-For/P1-Rev (Table 2) and washed ΔearA-sp cells as template (Ding et al., 2016b). The sequence of the PCR products was aligned with the corresponding region of the Mc. maripaludis S2 genome (NCBI version CAF31274.1) using Clustal Omega to detect the presence of any mutation (Goujon et al., 2010; Sievers et al., 2011).
Construction of Plasmids Used for the Δ3A Mutant Strain Generation
A mutant strain harboring the same three adenine deletion in the fla promoter BRE region as found in the ΔearA-sp strain was generated in the ΔearA mutant that showed no production of FlaB2 by western blotting. Briefly, an ∼2 kb DNA fragment containing the fla promoter region missing the three adenines in the BRE was PCR amplified with primers P-fus-F and P-fus-R (Table 2) and washed ΔearA-sp strain cells as template. The PCR product was digested with BamHI and cloned into BamHI digested pCRPrtNeo (Moore and Leigh, 2005) to create plasmid pKJ1273. Sequencing of the insert in pKJ1273 confirmed the three adenine deletion in BRE and no other changes. To generate the Δ3A mutant strain, pKJ1273 was transformed into ΔearA using a PEG-based method (Tumbula et al., 1994). The transformation mixture was cultured overnight without selection and then sub-cultured in McCas medium containing 1 mg/ml of neomycin for selection of cells in which pKJ1273 was integrated into the genome. After two passages in medium with neomycin selection, cells were cultured in McCas medium without neomycin to allow a second recombination event that would excise the pCRPrtNeo vector backbone, and this culture was plated onto McCas agar with 250 μg/mL 8-azahypoxanthine to kill any cells in which the vector backbone had remained integrated. Single colonies were picked and cultured in Balch medium III for western blot analysis of FlaB2 expression. For colonies in which the FlaB2 expression was restored, PCR was conducted to amplify both the earA gene region and the fla promoter region using primers listed in Table 2 and washed cells as template. The size of the PCR amplicons of the earA gene region was analyzed by electrophoresis through 0.8% agarose gels to confirm the deletion in earA. The PCR products of the fla promoter region from seven colonies that produced FlaB2 and four colonies that did not produce FlaB2 were sequenced. One of the colonies that produced FlaB2 and contained the deletion of the targeted three adenines in the BRE region was restreaked for purity and designated as Δ3A.
Western Blot Analysis of FlaB2 Expression in Mc. maripaludis Strains
The presence of the archaellin FlaB2 in the wild-type and various mutant strains of Mc. maripaludis was analyzed by western blot with an anti-FlaB2 antibody as previously described (Chaban et al., 2007).
Quantitative RT-PCR (qRT-PCR) Analysis of the flaB2 Transcription Level in Mc. maripaludis Strains
Total RNA from an Mc. maripaludis overnight cell culture was extracted using a High Pure RNA Isolation Kit (Roche Life Science) following a modified Gram negative bacteria RNA extraction protocol with an additional DNase treatment using a TURBO DNA-free Kit (Ambion) at 37°C for 30 min. Ten nanograms of total RNA from each extraction was converted into cDNA using an iScriptTM cDNA Synthesis Kit (Bio-Rad) with random hexamer primers. To detect the transcript level of flaB2, gene specific primers were constructed to amplify flaB2 and the slp gene that encodes the S-layer protein (the latter was used as the reference) [Table 2, (Ding et al., 2016a)]. qRT-PCR experiments were performed as previously described (Ding et al., 2016a). Triplicates were included in each experiment, and three biological repeats were conducted.
Swarming Motility Analysis of Mc. maripaludis Strains on Semi-Solid Agar
Five microliters of overnight cell cultures of each Mc. maripaludis strain (OD600 normalized to 1.0) grown in Balch Medium III were stabbed into Balch Medium III plates containing 0.25% agar (w/v) (Ding et al., 2015). Plates were incubated anaerobically in a canister under an atmosphere of H2:CO2 (80:20) at 37°C for 4 days.
Electron Microscopy Analysis of Mc. maripaludis Strains
Cells grown overnight in Balch medium III were centrifuged and the pellets washed briefly with 2% NaCl (w/v), and resuspended in 2% NaCl. Cell resuspensions were loaded on 200-mesh carbon-coated copper grids. After adhesion to the grid for 1 min, cells were washed with 2% NaCl and then stained with 2% (w/v) phosphotungstic acid, pH 7.0. Samples were examined with a Philips CM-10 transmission electron microscope at 80 kV and images were taken with a SIS/Olympus Morada 11-megapixel charge-coupled device camera under standard operating conditions.
Results
Isolation and Identification of a Spontaneous Mutant of the ΔearA Strain in Which FlaB2 Expression Was Restored
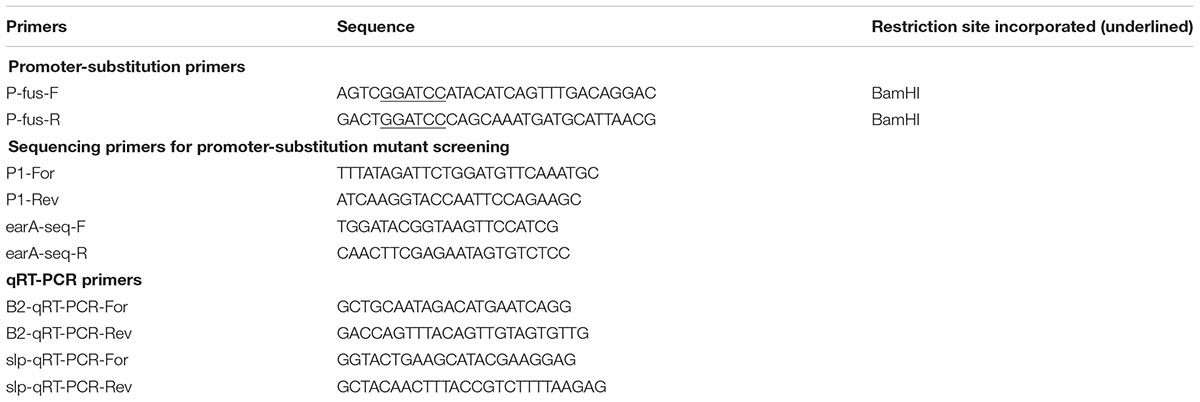
In Mc. maripaludis, the transcription of the fla operon is dependent on the transcription activator EarA (Ding et al., 2016b). In the absence of EarA, as in the ΔearA strain, the archaellin FlaB2 (encoded by the second gene in the fla operon) is not detected in western blots (Figure 1A) and cells are non-archaellated. However, continuous weekly transfer of the ΔearA strain for about 6 months resulted in the isolation of a mutant form of the ΔearA strain in which FlaB2 synthesis was restored (Figure 1B). This spontaneous mutant was designated as ΔearA-sp. The deletion of the earA gene in ΔearA-sp was still present, as confirmed by PCR analysis of this strain compared to the original ΔearA strain and Mm900 cells. As shown in Figure 1C, both ΔearA and the ΔearA-sp cells had the expected smaller amplicon size obtained in PCR using primers flanking the deletion area of earA compared with amplicons obtained using Mm900 or ΔflaB2 cells as template, ruling out the possibility that the restoration of FlaB2 in ΔearA-sp was due to contamination with the wild-type Mm900 strain or any other Mc. maripaludis strain with an intact earA.
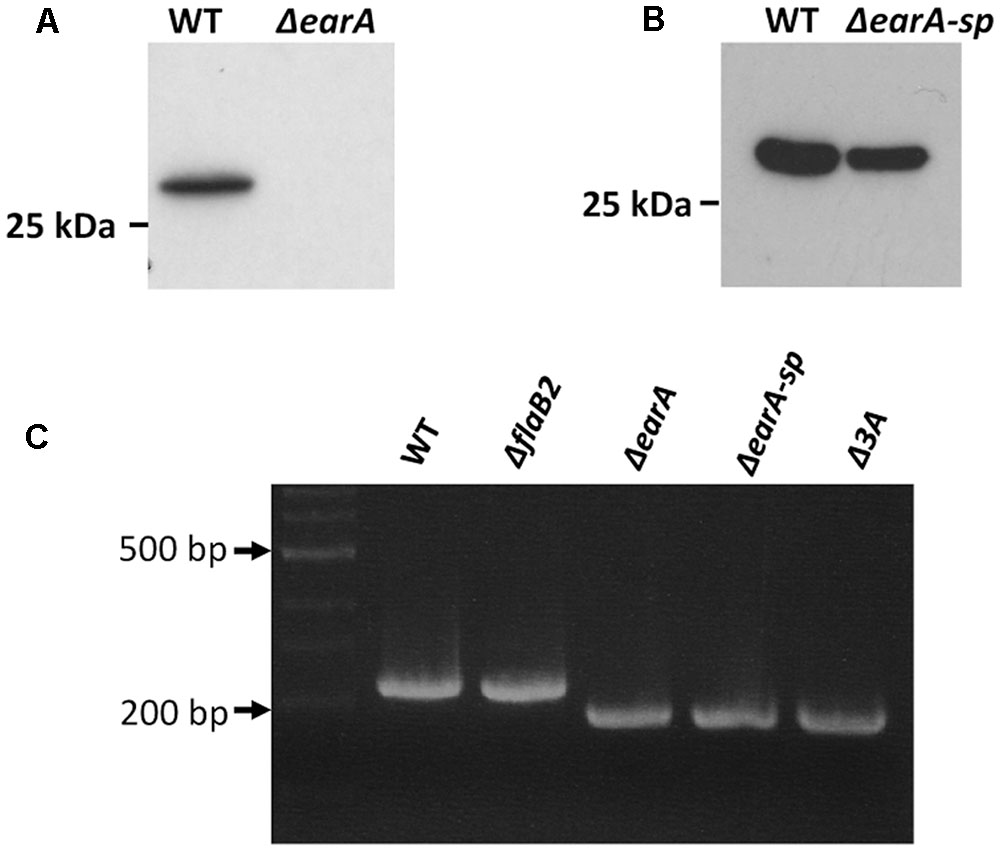
FIGURE 1. Western blot analysis of wild-type cells, the ΔearA strain and a spontaneous mutant of the ΔearA strain, ΔearA-sp, in which the expression of FlaB2 was restored. (A) Wild-type cells (Mm900) produce FlaB2 readily detected in western blots using FlaB2-specific antibodies. In the ΔearA strain, where the gene encoding the transcriptional activator EarA required for the transcription of the fla operon has been deleted, no FlaB2 was detected. (B) In the spontaneous mutant ΔearA-sp, the expression of FlaB2 was restored. (C) Confirmation of the deletion of earA in ΔearA-sp and Δ3A mutants. PCR products obtained using ΔearA, ΔearA-sp and Δ3A mutant cells as templates with primers amplifying the flanking area of the earA gene were smaller than those obtained using wild-type and ΔflaB2 cells as template with the same primer pair, confirming that earA was deleted in the ΔearA-sp and Δ3A mutants.
As an initial step in an effort to determine how these cells had regained the ability to transcribe the fla operon genes without EarA, we amplified and sequenced a ∼500 bp region encompassing the fla promoter from ΔearA-sp [from -348 nt to +162 nt with respect to the TSS; (Ding et al., 2016b)]. Analysis of the sequencing data showed that the four EarA binding sites (Ding et al., 2016b) upstream of the fla promoter remained intact, as did the TATA box, but in a stretch of seven adenines in the BRE found immediately upstream of the TATA box, three out of the seven adenines were missing in the ΔearA-sp strain (Figure 2). No other changes were found in the sequence of the PCR product amplified from the fla promoter region in the ΔearA-sp strain.
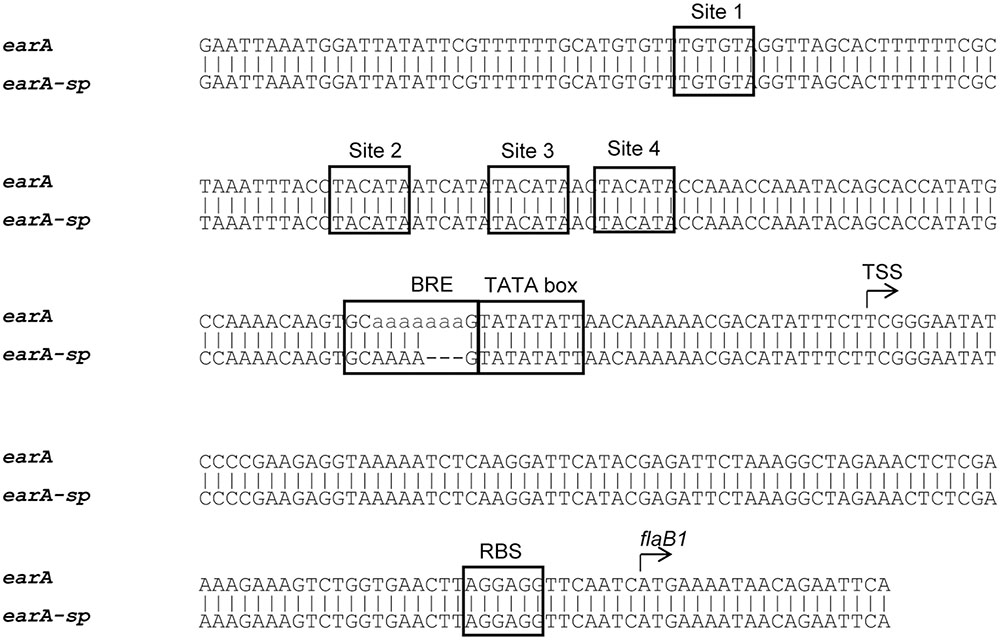
FIGURE 2. DNA sequence alignment of the fla promoter region from the ΔearA and ΔearA-sp strains showed a three adenine deletion in the BRE region. The four EarA binding sites (sites 1–4), BRE, TATA box, and ribosome binding site (RBS) are boxed in the figure. The transcription start site (TSS) and the start codon of the first gene in the fla operon, flaB1, are also indicated.
Construction of a Δ3A Mutant in Which the Three Adenine Deletion in the BRE Was Recreated
It is possible that mutations other than the three adenine deletion in the fla promoter region could have occurred elsewhere in the genome of ΔearA-sp that were solely, or partially, responsible for the restoration of FlaB2 production. To explore if the three adenine deletion detected in the fla promoter region in the ΔearA-sp strain alone would result in the restoration of expression of FlaB2 in the absence of EarA, a mutant which carried the same three adenine deletion mutation in the fla promoter region as that in ΔearA-sp, was generated from the original stock ΔearA strain that did not synthesize FlaB2. Since the size difference in the fla promoter region of ΔearA and the generated three adenine mutant would be only three nucleotides, we did not try to screen mutants by PCR analysis. Instead, we used western blotting to screen for FlaB2 production, since if the deletion of the three adenines was responsible for restoration of transcription of the fla operon, transformants bearing this deletion would be readily identified from transformants that had retained the wild-type seven adenine sequence in the BRE region. Western blotting of a random number of transformant colonies appearing on 8-azahypoxanthine plates identified both ones that did and did not synthesize detectable amounts of FlaB2. The sequence of the fla promoter of four colonies where FlaB2 production was detected and seven colonies in which FlaB2 production was not detected were determined. In each of the colonies in which no FlaB2 was detected by western blotting, a wild-type fla promoter sequence, i.e., with seven consecutive adenines in the BRE, was found. In each of the four colonies that were found to produce FlaB2, the fla promoter was identical to the wild-type sequence except for the three adenine deletion in the BRE (data not shown). One of the transformant colonies that produced FlaB2 and had the three adenine deletion in the BRE was designated Δ3A and studied further. As shown in Figure 3A, FlaB2 production in the ΔearA-sp strain was near wild-type levels. In contrast, in the Δ3A cells, the expression level of FlaB2 was lower than that from the ΔearA-sp strain. PCR analysis of the Δ3A cells confirmed that these cells still possessed the deletion in earA (Figure 1C).
FIGURE 3. The translation and transcription of flaB2 was restored in the ΔearA-sp and Δ3A mutants. (A) Western blot analysis showed that FlaB2 was expressed in both ΔearA-sp and Δ3A mutants, as well as in the wild-type cells but not in the ΔearA mutant or in a mutant deleted for flaB2. (B) The transcription of flaB2 was restored in ΔearA-sp and Δ3A strains, as detected by qRT-PCR experiments. While transcripts for flaB2 were barely detectable in the ΔearA mutant, flaB2 transcription in the ΔearA-sp and Δ3A strains exceeded that of wild-type cells. Error bar shows standard derivation from nine data sets from three biological repeats, each of which were performed with triplicates.
Transcription of flaB2 in the ΔearA-sp and Δ3A Strains Was Restored
Restoration of FlaB2 synthesis in the ΔearA-sp and Δ3A strains as demonstrated by the western blot results indicated that transcription of flaB2 was occurring in both mutant strains. A direct measure of the transcript level of flaB2 in these two mutants as well as control strains was obtained in qRT-PCR experiments (Figure 3B). As expected, flaB2 transcripts were not detected in the ΔflaB2 strain and were barely detectable in the ΔearA strain. In contrast, the transcription level of flaB2 was increased over 4-fold and 2.5-fold in the ΔearA-sp and the Δ3A strains, respectively, compared to that detected in wild-type cells. The relatively higher transcription level of flaB2 in the ΔearA-sp cells compared to the Δ3A cells was consistent with production of FlaB2 in the two strains detected in the western blot. However, the production of FlaB2 in the Δ3A cells was lower than in wild-type cells even though flaB2 transcription was higher.
ΔearA-sp and Δ3A Strains Were Archaellated
qRT-PCR and western blot analyses demonstrated that transcription and translation of flaB2 had been restored in the ΔearA-sp and Δ3A strains. To determine if the transcription and translation of the entire fla operon was restored in the two mutant stains resulting in assembly of archaella, cells were examined by electron microscopy. As shown in Figure 4, archaella were observed on the cell surface of both ΔearA-sp and Δ3A cells, as well as the wild-type cells, but not on ΔflaB2 or ΔearA cells.
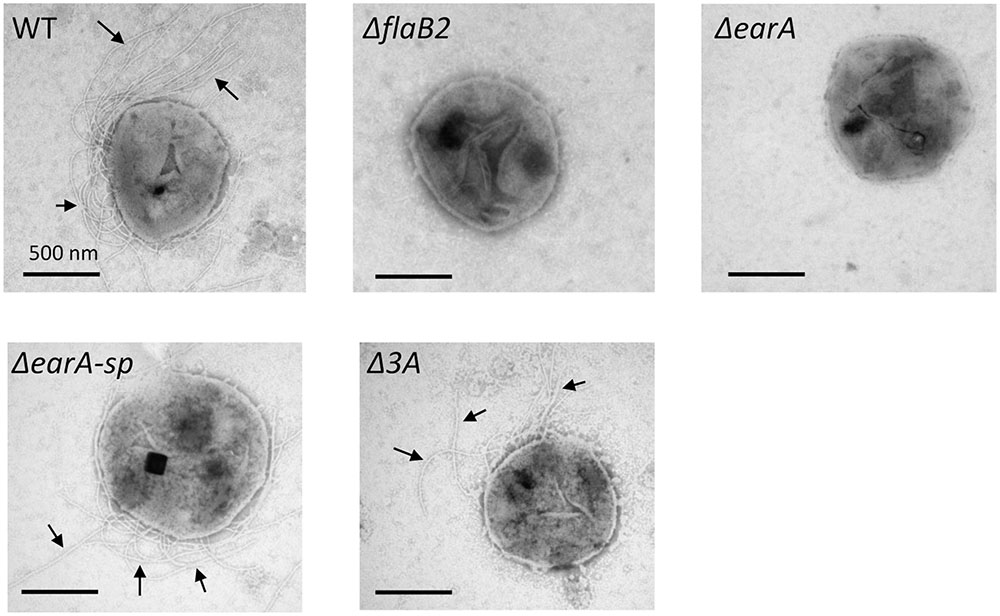
FIGURE 4. Electron micrographs illustrating archaella on the surface of ΔearA-sp and Δ3A mutants, as well as wild-type cells. As expected, the ΔflaB2 and ΔearA mutants were non-archaellated. Bars equal 500 nm.
ΔearA-sp and Δ3A Strains Had Swarming Motility
To further determine if the archaella observed on ΔearA-sp and Δ3A cells were functional, swarming motility assays were performed. Overnight cultures of ΔearA-sp, Δ3A, as well as Mm900, ΔflaB2, and ΔearA strains were inoculated onto semi-solid Balch medium III agar. After incubation at 37°C for 4 days Mm900, ΔearA-sp and Δ3A cells were clearly motile although the motility of the Δ3A cells was less than the other two strains (Figure 5). The non-archaellated strains, ΔflaB2 and ΔearA, remained at the inoculation spot, as expected. The swarming data are consistent with data from western blot, qRT-PCR, and EM analyses.
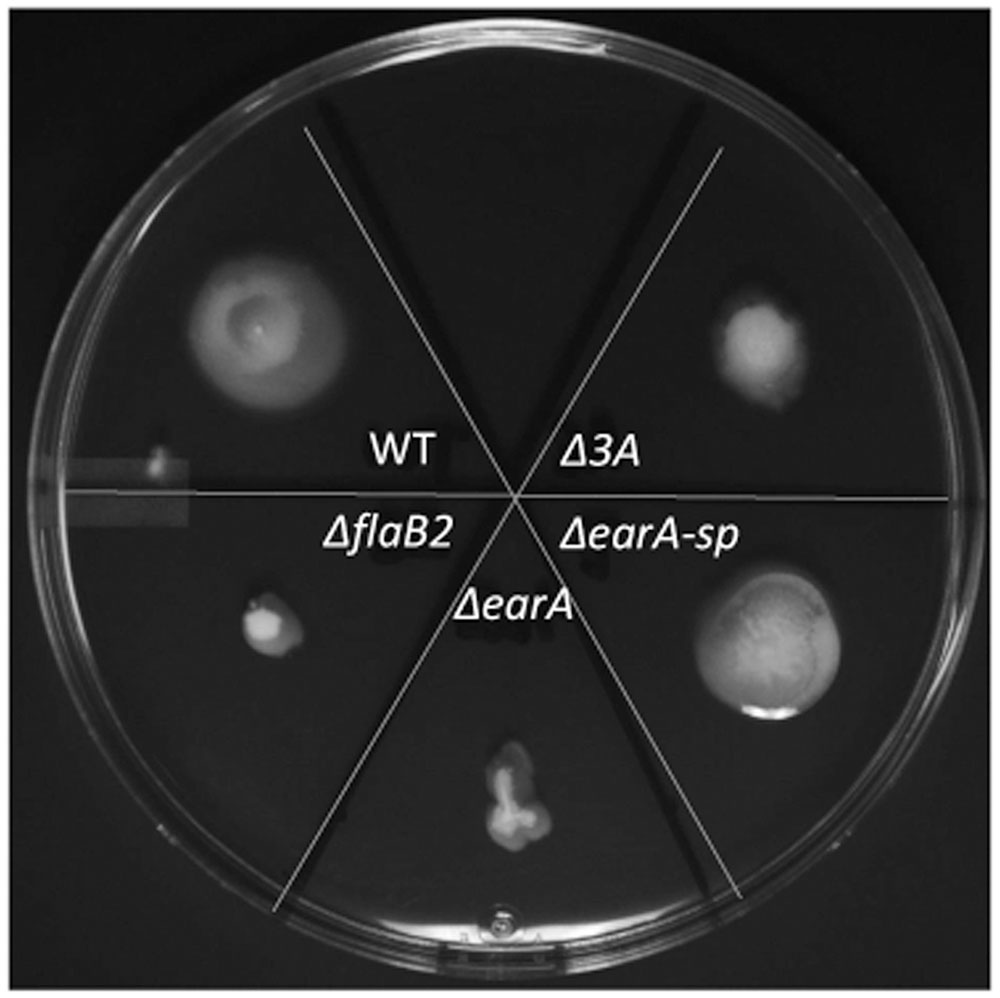
FIGURE 5. Swarming assay demonstrating the motility of the ΔearA-sp and Δ3A mutants. Overnight cell cultures were normalized with respect to their OD600 and the same amount of cells were inoculated onto Balch medium III plates containing 0.25% agar and incubated for 4 days at 37°C.
Discussion
Previous studies have shown that the euryarchaeal archaellum regulator EarA was critical for transcription of the fla operon in Mc. maripaludis via its binding to at least one of four consensus sequences located immediately upstream of the BRE and TATA box of the fla promoter. In a ΔearA mutant, transcription of the fla operon is barely detectable and cells are non-archaellated (Ding et al., 2016b). In this study, we have isolated a spontaneous mutant of a ΔearA strain in which the transcription of the fla operon and archaellation were restored. Analysis of the DNA sequence of the fla promoter region in this mutant, designated ΔearA-sp, revealed a deletion of three adenines in the BRE region. Recreation of the three adenine deletion in the stock strain of the ΔearA mutant also led to restoration of fla operon transcription and archaellation, indicating that this small deletion in the BRE overcame the requirement for EarA for activation of transcription of the fla operon. However, the expression of FlaB2 detected by western blotting was lower in the recreated strain than in the spontaneous mutant ΔearA-sp, suggesting that the three adenine deletion may not be the sole change in the ΔearA-sp strain affecting transcription of the fla operon. However, it seems clear from our studies on the directed mutant Δ3A strain, that the deletion of three adenines in the BRE of the fla operon promoter is sufficient on its own to result in all the phenotypes related to archaellation observed in the spontaneous mutant.
Since there is virtually no transcription detected from the native fla promoter if earA is deleted, it suggests that the fla promoter is intrinsically very weak or inactive. Two key elements that determine promoter strength in Archaea are the sequences of the TATA box and BRE (Bartlett, 2005). The TATA box is the site of binding of the TATA-binding protein TBP while the BRE sequence is the site of binding for TFB (Peeters et al., 2013). While relatively few transcriptional activators have been studied in Archaea, the mechanism of activation in these limited studies has been shown to involve recruitment of TBP or TFB to the TATA box or BRE (Karr, 2014). Consensus TATA box sequences vary for different subgroups of Archaea and mutations in the TATA box can reduce transcription efficiency (Soppa, 1999; Bartlett, 2005; van de Werken et al., 2006). For protein promoters in Mcc. jannaschii, the TATA box was determined to be TWTATATA (where W = A or T) (Zhang et al., 2009), very similar to the TTTATATA proposed previously for the promoters of stable RNA genes in Methanococcus vannielii (Thomm and Wich, 1988) and featuring the methanogen characteristic of strict alterations of T and A in contrast to TATA boxes in other major archaeal groups (Soppa, 1999). One of the best-studied archaeal transcriptional activators, Ptr2 of Mcc. jannaschii, binds to multiple sequences upstream of BRE in the rubredoxin 2 gene and has its stimulatory effect due to direct recruitment of TBP (Ouhammouch et al., 2003, 2005). Adding binding sites for Ptr2 upstream of heterologous promoters with sub-optimal TATA box sequences resulted in significant transcriptional activation (Ouhammouch et al., 2005). Analysis of the TATA box of the fla operon in Mc. maripaludis revealed a strong identity to the consensus sequence, including the alternating T and A stretch TATATAT, suggesting binding of TBP should not be impaired.
The 6–7 nucleotide long BRE sequences are the major site of binding for TFB, with positions -3 and -6 of BRE (relative to the TATA box) showing the strongest specificity determinants (Qureshi and Jackson, 1998; Littlefield et al., 1999). There are no BRE consensus sequences reported for halophiles and methanogens (van de Werken et al., 2006). However, in Mcc. jannaschii, a hyperthermophilic relative of Mc. maripaludis, two studies have identified promoter sequences on a whole genome basis (Li et al., 2008; Zhang et al., 2009). The first study used the binding of TBP and TFB in EMSA studies to identify promoters (Li et al., 2008). These studies had a strong bias for strong promoters, especially for promoters of tRNA genes with only small percentage of promoters for protein genes being retrieved. These studies led to the identification of an extended BRE element sequence of 9–10 nucleotides (MRCCGAAAAG where M = A, C and R = A, G). The second study focused on identification of promoters for protein-encoding genes (Zhang et al., 2009). It was found for Mcc. jannaschii protein gene promoters that there was a greater variability in the BRE than in the TATA box (Zhang et al., 2009). The identified promoters for protein-encoding genes were shown to bind the general transcription factors less tightly than tRNA gene promoters. Notably, base frequencies at several BRE positions considered important for TFB binding were significantly different from the in vitro selected promoters (mostly for tRNA genes) in the earlier study. Examination of the BRE sequences in both protein-encoding genes and tRNA genes revealed that most had internal stretches of 3–5 adenines, far less than the seven adenines in the wild-type fla operon promoter. Interestingly, the fla operon BRE element has a G at position -1, the most commonly found base at that position in the strong tRNA gene BRE (Figure 6A), while in protein-encoding genes the most common base at -1 is C (Zhang et al., 2009).

FIGURE 6. Promoter sequence analysis of the wild-type and mutated fla promoters and other archaeal promoters. (A) Promoter sequences of fla promoter (fla), mutated fla promoter with the three adenine deletion in the BRE (Sp/3A), glnA promoter (glnA), and nifH promoter (nifH) from Methanococcus maripaludis, as well as the conserved promoter sequence from the tRNAlys gene of Methanocaldococcus jannaschii (Mja). (B) BRE/TATA box sequences of the fla operon promoters of selected Methanococcales.
The wild-type version of the BRE of the fla operon promoter, with its stretch of seven adenines, does not show strong sequence identity to what may be considered strong BRE sequences as reported for Mcc. jannaschii. On the other hand, it is apparent that the three adenine deletion version found in the ΔearA-sp mutant much more closely aligns with BRE sequences found in strong tRNA gene promoters of Mcc. jannaschii (Figure 6A). In addition, the mutated fla promoter in the ΔearA-sp strain shares high sequence identity with two studied promoter sequences in Mc. maripaludis, namely the nitrogen-regulated glnA and nifH promoters (Figure 6A) (Cohen-Kupiec et al., 1997, 1999). Both glnA and nifH promoters are regulated via the repressor NrpR, which binds to the nif operators located downstream of the TATA boxes just after the TSS in the two promoters leading to repression of transcription under ammonia growth conditions (Cohen-Kupiec et al., 1997, 1999; Lie et al., 2005). Both nifH and glnA expression is very low when cells are grown on ammonia and NrpR binds but high expression is observed under conditions of diazotrophic growth where NrpR does not bind or in a strain where nrpR has been deleted (Cohen-Kupiec et al., 1997, 1999; Lie and Leigh, 2003; Lie et al., 2005). This indicated that the basal transcription level of the two promoters was strong, suggesting that TFB and TBP in Mc. maripaludis could recognize BRE and the TATA box of these two promoters and initiate transcription (Cohen-Kupiec et al., 1997, 1999). The high sequence identity of the three adenine deletion BRE of the ΔearA-sp strain with that of the glnA and nifH promoters, as well as the BRE of the highly expressed tRNA genes of Mcc. jannaschii likely explains why the pre-initiation complex could be formed with the mutated fla promoter without the aid of EarA. The qRT-PCR results (Figure 3B) suggest that the wild-type fla operon promoter even with EarA is not as strong as the three adenine deletion version in the absence of EarA.
Studies in several archaea have indicated that promoters containing non-conserved BRE sequences can be weak or even inactive (Peng et al., 2009, 2011; Marschaus and Pfeifer, 2012; Ochs et al., 2012). Replacement of the BRE of inducible promoters with a BRE from strong promoters, for example, can greatly increase the transcription from the resulting chimeric promoter. In Sulfolobus solfataricus, transcription from the arabinose promoter is induced in the presence of arabinose, via an unidentified factor that binds to a consensus ara-box sequence located immediately upstream of the BRE and TATA box (Lubelska et al., 2006; Peng et al., 2011). When the BRE from the arabinose promoter was replaced with the strong BRE from the Sulfolobus shibatae viral (SSV) T6 promoter (Qureshi and Jackson, 1998), the resulting chimeric promoter was now constitutive and not regulated by the ara-box element (Peng et al., 2009). The apparent mechanism of transcription activation of the ara-box binding factor is thought to be by recruitment of TFB to a weak BRE (Peng et al., 2009, 2011). In Pyrococcus furiosus, transcription from the pf1089 promoter is activated by PF1088 (Transcription factor B recruitment factor 1, TFB-RF1). This activation is dependent on the weak BRE of the pf1089 promoter and is not observed if the pf1089 promoter BRE is replaced with the BRE from the strong gdh promoter (Spitalny and Thomm, 2003; Ochs et al., 2012). Electrophoretic mobility shift assays further revealed that the transcription activation of the wild-type pf1089 promoter was by the recruitment of TFB via TFB-RF1, thereby overcoming the weak BRE.
We have recently shown that EarA homologs are commonly found in the Euryarchaota and that EarA proteins from numerous methanogens can rescue the defects in archaellation in a Mc. maripaludis ΔearA strain (Ding et al., 2017). As shown in Figure 6B, examination of the fla promoter regions in selected archaellated Methanococcales containing an earA homolog revealed BRE sequences identical, or very similar, to that in Mc. maripaludis, i.e., with a string of seven adenines as in Methanococcus voltae, Methanococcus vannielii, and Methanothermococcus thermolithotrophicus or seven adenines in a stretch of eight nucleotides in the BRE of the fla promoter of Mcc. jannaschii. It would appear that in all these cases, the fla promoter requires the presence of EarA proteins to overcome weak BRE sequences, presumably to aid in the recruitment of TFB, as found for transcriptional activators TFB-RF1 and the ara-box binding factor.
The appearance of the ΔearA-sp mutant was surprising to us. The isolation of the original ΔearA mutant arose after it was discovered that, after repeated transfers in the lab, mutants carrying deletions of various fla or agl genes required for assembly of archaella stopped transcription of the fla operon and the fla operon reporter protein FlaB2 could not be detected in western blots (Vandyke et al., 2009; Ding et al., 2016b). It was determined that in at least some of these mutants, the reason for the cessation of fla operon transcription was a reading-frame shift mutation in earA. We reasoned that in these strains that carried mutations in fla or agl genes necessary for archaella assembly, it was an advantage to no longer synthesize several proteins, some of which, like archaellins, were required in large amounts when they could not be assembled in archaella. This led to a selective advantage for those cells that had stopped transcription of the fla operon, as in the earA mutants. Thus, it is not obvious to us why a mutation in the ΔearA that would restore transcription of the fla operon would arise and outgrow the original ΔearA mutant. The answer may lie in the presence of an additional mutation(s) in the ΔearA-sp strain that could be revealed by comparison of the complete sequence of the ΔearA and ΔearA-sp strains.
In this study, a spontaneous mutant with restored FlaB2 expression was isolated from a ΔearA mutant, indicating that in the spontaneous mutant the need for the transcriptional activator EarA for the transcription of the fla promoter was bypassed. Analysis of the DNA sequence in the fla promoter region from the spontaneous mutant revealed a three adenine deletion in the BRE region in the fla promoter. Sequence alignment showed that the mutated BRE in the fla promoter shares high similarity with BREs from strong promoters in methanogens, indicating that with this mutated BRE, the transcription initiation of the fla promoter could be conducted with components of the basal pre-initiation complex. We believe this is the first report of spontaneous mutation in the promoter region that overcomes the need for a transcriptional activator and it emphasizes the key role played by the BRE in promoter strength in Archaea.
Author Contributions
Conceived and designed experiments: YD, AB, CK, and KJ. Performed the experiments: YD, AB, CK, and KJ. Analyzed the data: YD, AB, CK, and KJ. Wrote the paper: YD, AB, CK, and KJ.
Funding
This research was funded by Discovery Grants from The Natural Sciences and Engineering Research Council of Canada (NSERC) to KJ and CK.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Albers, S. V., and Jarrell, K. F. (2015). Archaellum moves Archaea with distinction. Microbe 10, 283–288. doi: 10.1128/microbe.10.283.1
Balch, W. E., Fox, G. E., Magrum, L. J., Woese, C. R., and Wolfe, R. S. (1979). Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43, 260–296.
Bartlett, M. S. (2005). Determinants of transcription initiation by archaeal RNA polymerase. Curr. Opin. Microbiol. 8, 677–684. doi: 10.1016/j.mib.2005.10.016
Bell, S. D. (2005). Archaeal transcriptional regulation–variation on a bacterial theme? Trends Microbiol. 13, 262–265.
Bell, S. D., and Jackson, S. P. (2001). Mechanism and regulation of transcription in archaea. Curr. Opin. Microbiol. 4, 208–213. doi: 10.1016/S1369-5274(00)00190-9
Bell, S. D., Kosa, P. L., Sigler, P. B., and Jackson, S. P. (1999). Orientation of the transcription preinitiation complex in archaea. Proc. Natl. Acad. Sci. U.S.A. 96, 13662–13667. doi: 10.1073/pnas.96.24.13662
Chaban, B., Ng, S. Y., Kanbe, M., Saltzman, I., Nimmo, G., Aizawa, S. I., et al. (2007). Systematic deletion analyses of the fla genes in the flagella operon identify several genes essential for proper assembly and function of flagella in the archaeon, Methanococcus maripaludis. Mol. Microbiol. 66, 596–609. doi: 10.1111/j.1365-2958.2007.05913.x
Cohen-Kupiec, R., Blank, C., and Leigh, J. A. (1997). Transcriptional regulation in Archaea: in vivo demonstration of a repressor binding site in a methanogen. Proc. Natl. Acad. Sci. U.S.A. 94, 1316–1320. doi: 10.1073/pnas.94.4.1316
Cohen-Kupiec, R., Marx, C. J., and Leigh, J. A. (1999). Function and regulation of glnA the methanogenic archaeon Methanococcus maripaludis. J. Bacteriol. 181, 256–261.
Ding, Y., Berezuk, A., Khursigara, C. M., and Jarrell, K. F. (2017). Phylogenetic distribution of the euryarchaeal archaellum regulator EarA and complementation of a Methanococcus maripaludis ΔearA mutant with heterologous earA homologues. Microbiology doi: 10.1099/mic.0.000464 [Epub ahead of print].
Ding, Y., Lau, Z., Logan, S. M., Kelly, J. F., Berezuk, A., Khursigara, C. M., et al. (2016a). Effects of growth conditions on archaellation and N-glycosylation in Methanococcus maripaludis. Microbiology 162, 339–350. doi: 10.1099/mic.0.000221
Ding, Y., Nash, J., Berezuk, A., Khursigara, C. M., Langelaan, D. N., Smith, S. P., et al. (2016b). Identification of the first transcriptional activator of an archaellum operon in a euryarchaeon. Mol. Microbiol. 102, 54–70. doi: 10.1111/mmi.13444
Ding, Y., Uchida, K., Aizawa, S. I., Murphy, K., Berezuk, A., Khursigara, C. M., et al. (2015). Effects of N-glycosylation site removal in archaellins on the assembly and function of archaella in Methanococcus maripaludis. PLoS ONE 10:e0116402. doi: 10.1371/journal.pone.0116402
Gehring, A. M., Walker, J. E., and Santangelo, T. J. (2016). Transcription regulation in Archaea. J. Bacteriol. 198, 1906–1917. doi: 10.1128/JB.00255-16
Goujon, M., McWilliam, H., Li, W., Valentin, F., Squizzato, S., Paern, J., et al. (2010). A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 38, W695–W699. doi: 10.1093/nar/gkq313
Hendrickson, E. L., Liu, Y., Rosas-Sandoval, G., Porat, I., Soll, D., Whitman, W. B., et al. (2008). Global responses of Methanococcus maripaludis to specific nutrient limitations and growth rate. J. Bacteriol. 190, 2198–2205. doi: 10.1128/JB.01805-07
Jarrell, K. F., and Albers, S. V. (2012). The archaellum: an old motility structure with a new name. Trends Microbiol. 20, 307–312. doi: 10.1016/j.tim.2012.04.007
Jun, S. H., Reichlen, M. J., Tajiri, M., and Murakami, K. S. (2011). Archaeal RNA polymerase and transcription regulation. Crit. Rev. Biochem. Mol. Biol. 46, 27–40. doi: 10.3109/10409238.2010.538662
Karr, E. A. (2014). Transcription regulation in the third domain. Adv. Appl. Microbiol. 89, 101–133. doi: 10.1016/B978-0-12-800259-9.00003-2
Li, E., Reich, C. I., and Olsen, G. J. (2008). A whole-genome approach to identifying protein binding sites: promoters in Methanocaldococcus (Methanococcus) jannaschii. Nucleic Acids Res. 36, 6948–6958. doi: 10.1093/nar/gkm499
Lie, T. J., and Leigh, J. A. (2003). A novel repressor of nif and glnA expression in the methanogenic archaeon Methanococcus maripaludis. Mol. Microbiol. 47, 235–246. doi: 10.1046/j.1365-2958.2003.03293.x
Lie, T. J., Wood, G. E., and Leigh, J. A. (2005). Regulation of nif expression in Methanococcus maripaludis: roles of the euryarchaeal repressor NrpR, 2-oxoglutarate, and two operators. J. Biol. Chem. 280, 5236–5241. doi: 10.1074/jbc.M411778200
Littlefield, O., Korkhin, Y., and Sigler, P. B. (1999). The structural basis for the oriented assembly of a TBP/TFB/promoter complex. Proc. Natl. Acad. Sci. U.S.A. 96, 13668–13673. doi: 10.1073/pnas.96.24.13668
Lubelska, J. M., Jonuscheit, M., Schleper, C., Albers, S. V., and Driessen, A. J. (2006). Regulation of expression of the arabinose and glucose transporter genes in the thermophilic archaeon Sulfolobus solfataricus. Extremophiles 10, 383–391. doi: 10.1007/s00792-006-0510-7
Marschaus, L., and Pfeifer, F. (2012). A dual promoter region with overlapping activator sequences drives the expression of gas vesicle protein genes in haloarchaea. Microbiology 158, 2815–2825. doi: 10.1099/mic.0.060178-0
Moore, B. C., and Leigh, J. A. (2005). Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J. Bacteriol. 187, 972–979. doi: 10.1128/JB.187.3.972-979.2005
Ochs, S. M., Thumann, S., Richau, R., Weirauch, M. T., Lowe, T. M., Thomm, M., et al. (2012). Activation of archaeal transcription mediated by recruitment of transcription factor B. J. Biol. Chem. 287, 18863–18871. doi: 10.1074/jbc.M112.365742
Ouhammouch, M., Dewhurst, R. E., Hausner, W., Thomm, M., and Geiduschek, E. P. (2003). Activation of archaeal transcription by recruitment of the TATA-binding protein. Proc. Natl. Acad. Sci. U.S.A. 100, 5097–5102. doi: 10.1073/pnas.0837150100
Ouhammouch, M., Langham, G. E., Hausner, W., Simpson, A. J., El-Sayed, N. M., and Geiduschek, E. P. (2005). Promoter architecture and response to a positive regulator of archaeal transcription. Mol. Microbiol. 56, 625–637. doi: 10.1111/j.1365-2958.2005.04563.x
Peeters, E., Peixeiro, N., and Sezonov, G. (2013). Cis-regulatory logic in archaeal transcription. Biochem. Soc. Trans. 41, 326–331. doi: 10.1042/BST20120312
Peng, N., Ao, X., Liang, Y. X., and She, Q. (2011). Archaeal promoter architecture and mechanism of gene activation. Biochem. Soc. Trans. 39, 99–103. doi: 10.1042/BST0390099
Peng, N., Xia, Q., Chen, Z., Liang, Y. X., and She, Q. (2009). An upstream activation element exerting differential transcriptional activation on an archaeal promoter. Mol. Microbiol. 74, 928–939. doi: 10.1111/j.1365-2958.2009.06908.x
Qureshi, S. A., and Jackson, S. P. (1998). Sequence-specific DNA binding by the S. shibatae TFIIB homolog, TFB, and its effect on promoter strength. Mol. Cell 1, 389–400. doi: 10.1016/S1097-2765(00)80039-8
Sievers, F., Wilm, A., Dineen, D., Gibson, T. J., Karplus, K., Li, W., et al. (2011). Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539. doi: 10.1038/msb.2011.75
Soppa, J. (1999). Normalized nucleotide frequencies allow the definition of archaeal promoter elements for different archaeal groups and reveal base-specific TFB contacts upstream of the TATA box. Mol. Microbiol. 31, 1589–1592. doi: 10.1046/j.1365-2958.1999.01274.x
Spitalny, P., and Thomm, M. (2003). Analysis of the open region and of DNA-protein contacts of archaeal RNA polymerase transcription complexes during transition from initiation to elongation. J. Biol. Chem. 278, 30497–30505. doi: 10.1074/jbc.M303633200
Thomm, M., and Wich, G. (1988). An archaebacterial promoter element for stable RNA genes with homology to the TATA box of higher eukaryotes. Nucleic Acids Res. 16, 151–163. doi: 10.1093/nar/16.1.151
Tumbula, D. L., Makula, R. A., and Whitman, W. B. (1994). Transformation of Methanococcus maripaludis and identification of a PstI-like restriction system. FEMS Microbiol. Lett. 121, 309–314. doi: 10.1111/j.1574-6968.1994.tb07118.x
van de Werken, H. J., Verhees, C. H., Akerboom, J., de Vos, W. M., and van der Oost, J. (2006). Identification of a glycolytic regulon in the archaea Pyrococcus and Thermococcus. FEMS Microbiol. Lett. 260, 69–76. doi: 10.1111/j.1574-6968.2006.00292.x
Vandyke, D. J., Wu, J., Logan, S. M., Kelly, J. F., Mizuno, S., Aizawa, S. I., et al. (2009). Identification of genes involved in the assembly and attachment of a novel flagellin N-linked tetrasaccharide important for motility in the archaeon Methanococcus maripaludis. Mol. Microbiol. 72, 633–644. doi: 10.1111/j.1365-2958.2009.06671.x
Keywords: BRE deletion, archaellum, EarA, promoter, fla operon, archaea
Citation: Ding Y, Berezuk A, Khursigara CM and Jarrell KF (2017) Bypassing the Need for the Transcriptional Activator EarA through a Spontaneous Deletion in the BRE Portion of the fla Operon Promoter in Methanococcus maripaludis. Front. Microbiol. 8:1329. doi: 10.3389/fmicb.2017.01329
Received: 19 April 2017; Accepted: 30 June 2017;
Published: 17 July 2017.
Edited by:
Julie Anne Maupin-Furlow, University of Florida, United StatesReviewed by:
Hua Xiang, Institute of Microbiology (CAS), ChinaEveline Peeters, Vrije Universiteit Brussel, Belgium
Copyright © 2017 Ding, Berezuk, Khursigara and Jarrell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ken F. Jarrell, jarrellk@queensu.ca
 Yan Ding
Yan Ding Alison Berezuk
Alison Berezuk Cezar M. Khursigara
Cezar M. Khursigara Ken F. Jarrell
Ken F. Jarrell