- Department of Plant Sciences, University of Oxford, Oxford, United Kingdom
Creating sustainable bioeconomies for the 21st century relies on optimizing the use of biological resources to improve agricultural productivity and create new products. Arbuscular mycorrhizae (phylum Glomeromycota) form symbiotic relationships with over 80% of vascular plants. In return for carbon, these fungi improve plant health and tolerance to environmental stress. This symbiosis is over 400 million years old and there are currently over 200 known arbuscular mycorrhizae, with dozens of new species described annually. Metagenomic sequencing of native soil communities, from species-rich meadows to mangroves, suggests biologically diverse habitats support a variety of mycorrhizal species with potential agricultural, medical, and biotechnological applications. This review looks at the effect of mycorrhizae on plant metabolism and how we can harness this symbiosis to improve crop health. I will first describe the mechanisms that underlie this symbiosis and what physiological, metabolic, and environmental factors trigger these plant-fungal relationships. These include mycorrhizal manipulation of host genetic expression, host mitochondrial and plastid proliferation, and increased production of terpenoids and jasmonic acid by the host plant. I will then discuss the effects of mycorrhizae on plant root and foliar secondary metabolism. I subsequently outline how mycorrhizae induce three key benefits in crops: defense against pathogen and herbivore attack, drought resistance, and heavy metal tolerance. I conclude with an overview of current efforts to harness mycorrhizal diversity to improve crop health through customized inoculum. I argue future research should embrace synthetic biology to create mycorrhizal chasses with improved symbiotic abilities and potentially novel functions to improve plant health. As the effects of climate change and anthropogenic disturbance increase, the global diversity of arbuscular mycorrhizal fungi should be monitored and protected to ensure this important agricultural and biotechnological resource for the future.
Introduction
Mutualistic symbiosis is the reciprocally beneficial relationship between two organisms and can have a profound effect on organism fitness, ecology, and evolution (Wade, 2007; Gilbert et al., 2015; Kiers and West, 2015). Symbioses abound across all forms of marine and terrestrial life, from whales (Cetacea) and barnacles (Coronula diadema) to fig wasps (Courtella wardi) and fig trees (Ficus carica) (Jousselin et al., 2003; Nogata and Matsumura, 2006). Some symbioses can also form among three partners, as seen in the exchange of nutrients among three-toed sloths (Bradypus spp.), pyralid moths (Cryptoses spp.), and algae (Trichophilus spp.) (Pauli et al., 2014). However, some of the most intriguing forms of symbioses happen in the microbial world (Keeling and Palmer, 2008). Bacteria and fungi are capable of invading host organisms, altering genetic and metabolic processes along the way (McFall-Ngai et al., 2013). To date, bacterial symbioses have received the most attention due to their complex relationships across many species across all trophic levels, from algae to plants and humans (McFall-Ngai and Ruby, 1991). Fungal symbioses have received less attention despite their key role in terrestrial nutrient exchange and recycling systems (Gadd, 2006; Emery et al., 2015). For example, some fungi (Neocallimastix spp.) colonize the guts of cows, helping them digest plant matter (Davies et al., 1993). Other species (ascomycete and basidiomycetes) form symbiotic relationships with cyanobacteria to form lichen, a composite organism (holobiont) that degrades organic and inorganic materials and serve as an important food-source for herbivores (Spribille et al., 2016).
Microbial symbioses have important ecological roles and can also be manipulated to increase the sustainability and resilience of global agricultural systems. To date, the agricultural use of naturally occurring symbioses has focused on promoting symbiosis between nitrogen-fixing bacteria (rhizobia) and legume plants to decrease inorganic nitrogen application on arable fields, which can lead to eutrophication and decline of soil microbial diversity over time (Matson et al., 1997). Arbuscular mycorrhizal fungi (AMF) form an equally important symbiosis with plants that is currently underexploited. This symbiosis is over 400 million years old and predates the symbiotic relationship between nitrogen-fixing bacteria and legumes. In the phylum Glomeromycota there are currently 200 known species from 10 families and many more are likely to be discovered in terrestrial landscapes rich in plant diversity and/or in extreme environments (Bever et al., 2001; Öpik et al., 2010; Ohsowski et al., 2012). In exchange for carbon, mycorrhizae provide plants with Phosphorus (P), minerals, and other nutrients to over 80% of vascular plants (Parniske, 2008). Major crops such as wheat and maize form symbioses with mycorrhizal fungi. The only crops that do not form this symbiosis are from the Brassicaceae and Papaveraceae families (Fester and Sawers, 2011). A growing body of greenhouse and field-scale experiments has shown the positive effect of mycorrhizal inoculation on crop productivity and resilience to environmental stress. In this review, I will provide an overview of our current knowledge of how mycorrhizal symbiosis effects plant metabolism and crop health and provide new insight into how synthetic biology could revolutionize how we harness this symbiosis in the future.
Mycorrhizal Infection and Host Metabolic Response
Arbuscular mycorrhizal symbiosis dramatically alters plant primary and secondary metabolism in affected roots (Figure 1). Upon infection, cells with arbuscules gradually emit enzymes that degrade or stop the suppression of plant cell wall materials (e.g., lignin) and suppress salicylic acid production (which decreases AMF symbiosis) (Smith and Gianinazzi-Pearson, 1988). A shared, permeable membrane is created between the arbuscule and host plant which allows for the exchange of nutrients. This membrane is comprised of three layers: the plant-derived periarbuscular membrane (PAM), the periarbuscular space (PAS) composed of plant and fungal-derived elements, and the fungal plasma membrane (Parniske, 2008). It contains enzymes capable of generating energy gradients for active, bi-directional transport of nutrients and compounds (Smith and Gianinazzi-Pearson, 1988). Infection causes specific physiological changes in host cells. The number of mitochondria increase threefold and migrate toward the arbuscule, the nucleus increases in size, and nuclear chromatin decondenses (allowing for increased transcriptional activity) (Gianinazzi-Pearson, 1996; Lohse et al., 2005). Plastids also increase in number and stromules become more abundant; they can move toward arbuscules, forming a net-like structure over the fungus (Buee et al., 2000; Lohse et al., 2005).
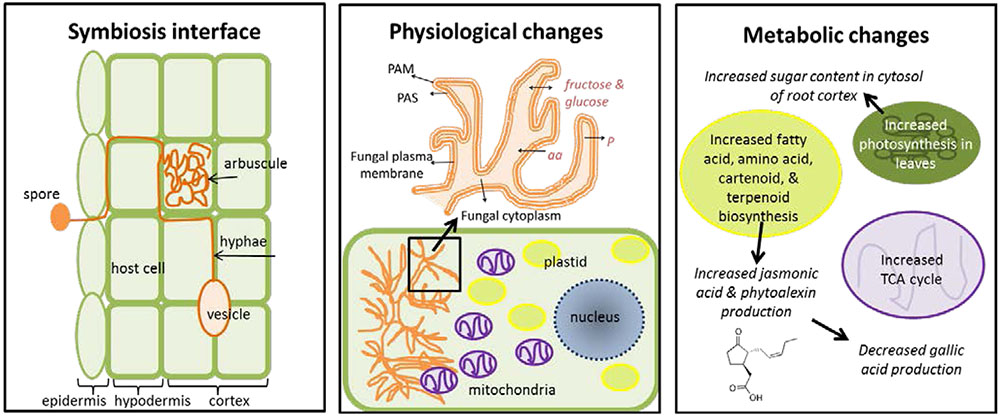
FIGURE 1. Overview of physiological and metabolic changes induced by mycorrhizal symbiosis with host plants. PAM, plant-derived periarbuscular membrane; PAS, the periarbuscular space; aa, amino acids; P, phosphorus.
These physiological changes trigger metabolic changes in root cortex cells. Increased numbers of mitochondria and plastids lead to increased energy production (from the TCA cycle) and production of plastid metabolites (fatty acids, amino acids, carotenoids, and terpenoids) respectively (Lohse et al., 2005; Jung et al., 2012). In the cytosol, sugar levels increase due to increased photosynthesis in the above-ground leaves, which favors high efflux rates between the arbuscule and host cell (Smith and Gianinazzi-Pearson, 1988; Berger et al., 2007; Gaude et al., 2015). Levels of jasmonic acid (derived from linoleic acid produced in the plastids) also increase and trigger the production of phytoalexins (defensive compounds). Most of these defensive compounds are nitrogen-rich alkaloids produced by plastids. As these metabolic changes occur, phosphorus is transferred from the mycorrhiza to the host cell in exchange for fatty acids, amino acids, and sugars (fructose and glucose) (Smith and Gianinazzi-Pearson, 1988). The production of anti-fungal compounds (e.g., gallic acid) by the host plant decreases (Gaude et al., 2015).
Foliar secondary metabolism also changes dramatically. Defensive compounds in foliar tissues increase. These compounds include rutin, p-hydroxybenzoic acid, antioxidants (flavonoids), and terpenoids (Copetta et al., 2006; Toussaint, 2007; Geneva et al., 2010; Zubek et al., 2015; Kapoor et al., 2016). Earlier studies suggested plant secondary metabolism increased due to increased access to nutrients (specifically, P) provided by the mycorrhizal-endosymbiont (Gupta et al., 2002; Smith et al., 2003). However, several recent experimental studies suggest that increased production of specific secondary compounds does not correlate with increased plant P content (Copetta et al., 2006; Toussaint, 2007). Instead, hormonal changes induced by AMF infection may trigger these metabolic changes. In addition, changes in root chemistry may also lead to the storage of these compounds in foliar tissues. For example, the increased production of glandular trichomes in Ocimum basilicum L. when inoculated by Gigaspora rosea as noted by Copetta et al. (2006) could be due to increased production of chloroplasts in infected roots. Potentially, sequestering additional volatiles in leaves protects the mycorrhizae living within the hosts’ roots from these bioactive compounds. Additionally, plants may circulate these compounds to where they are most needed.
Arbuscular mycorrhizal fungi symbiosis causes both global (species-independent) and local (species-specific) changes in plant metabolism. Global changes include increased production of amino acids (glutamic, aspartic, and asparagine acid); fatty acids (palmitic and oleic); secondary metabolites (phenyl alcohols, a linolenic acid, apocarotenoids, isoflavonoids); plant hormones (oxylipin, cytokinins, and jasmonic acid); activation of the oxylipin pathway; and increased sugar metabolism (Fernández et al., 2014; Gaude et al., 2015; Rivero et al., 2015). In contrast, levels of specific secondary compounds increase according to plant species identity. For example, Schweiger et al. (2014) found that inoculating Plantago lanceolata, P. major, Veronica chamaedrys, Medicago truncatula, and Poa annua with Rhizophagus irregularis caused 18–45% of each species core metabolomes and increased species-specific compounds (e.g., sorbitol in P. lanceolata). Different mycorrhizal fungi can also produce different metabolic effects. For example, repeated pot experiments have shown that Funneliformis mosseae causes more metabolic changes than R. irregularis (Rivero et al., 2015).
Impact on Crop Health
Arbuscular mycorrhizal fungi symbiosis can boost plant defenses against pathogens. Previous studies have reported mycorrhizal-induced protection against fungal (Alternaria, Fusarium, Phytophthora, Pythium, Rhizoctonia, and Verticillium), bacterial (Ralstonia solanacearum and Pseudomonas syringae), nematode (Pratylenchus and Meloidogyne), and insect (Otiorhynchus sulcatus) damage (Garcia-Garrido and Ocampo, 1989; de la Peña et al., 2006; Fritz et al., 2006; Pozo and Azcón-Aguilar, 2007; Jung et al., 2012). A recent meta-analysis by Veresoglou and Rillig (2012) suggest inoculation of crops with mycorrhiza reduces fungal infections by 30–42% and nematode infestations by 44–57%. This protection results from passive and active activation of plant secondary metabolism by AMF. Passively, AMF infection causes host plants to produce and store highly potent defensive compounds (alkaloids and terpenoids). These are stored in trichomes and vacuoles and can be released at will (Wink, 1993; Champagne and Boutry, 2016). More actively, external and internal fungal hyphae may sense pathogen effectors and other secondary compounds in the surrounding environment (soil and apoplast, respectively) and ‘warn’ host cells by producing lipo-chitooligosaccharides (LCOs) and short chito-oligosaccharides (Cos) (Kosuta et al., 2003; Maillet et al., 2011; Bonfante and Genre, 2015; Zipfel and Oldroyd, 2017). These messages may transmit through the host plant from cell to cell through the plasmodesmata.
Drought tolerance also increases under inoculation with AMF. This may be due to increased production and accumulation of the sugar trehalose in affected plant cells. Trehalose forms a gel-like substance that attaches to cellular compartments and stabilizes lipid bilayers (Müller et al., 1995; Richards et al., 2002; Lunn et al., 2014). During desiccation organelles remain intact and can spring back to life under favorable environmental conditions (Wingler, 2002). For example, Adams et al. (1990) showed that Selaginella lepidophylla’s ability to withstand long-term desiccation was due to high levels of trehalose which formed 12.5% of plant body mass. As many high plants do not produce trehalose, this sugar may potentially be supplied by fungal endosymbionts. Trehalose has been detected in the roots of trees and vascular plants inoculated with ectomycorrhizal and arbuscular mycorrhiza (Müller et al., 1995). Increased trehalose production alters plant carbohydrate metabolism by decreasing sugar and starch levels (Wagner et al., 1986). Providing this benefit to host plants thus comes at a cost to mycorrhizae and emphasizes the mutualism of this symbiosis.
Arbuscular mycorrhizal fungi can also increase tolerance to heavy metals in crops. The reasons for this are still unclear. Potentially, the chitin and melanin found in the cell walls of AMF hyphae may bind metals in the surrounding soil and/or in the host plant (Morley and Gadd, 1995; Ruscitti et al., 2011; Eisenman and Casadevall, 2012). Melanin in particular is well-known for its ability to protect fungi from a variety of harsh environmental conditions, including nuclear radiation (Zhdanova et al., 2000). Non-mycorrhizal fungi (primarily Aspergillus, Phanerochaete chrys, and Trichoderma) have been shown to absorb and incorporate into their cell walls up to 90% of metal ions from soil contaminated with cadmium (Mohsenzadeh and Shahrokhi, 2014), silver (Aksu, 2001), uranium (Wang and Zhou, 2005), and lead (Jianlong et al., 2001). Fungi also use chelating proteins (e.g., phytochelatins and metallothioneins) and metabolites (e.g., oxalate) to deactivate the toxicity of metals (Tomsett, 1993; Sayer and Gadd, 1997). This absorption often triggers changes in fungal metabolism and can cause fungi to change color (e.g., orange and black) as new compounds are produced (Baldrian, 2003). In ectomycorrhiza, Blaudez et al. (2000) have shown that metal ions are deposited throughout the cell wall (50%), cytoplasm (30%), and vacuole (20%). This highlights that multiple mechanisms may be used by mycorrhiza to immobilize metal toxins. The hyphae of ectomycorrhizae exposed to heavy metals also seem to proliferate (Darlington and Rauser, 1988) suggesting they may confer some fitness advantage to fungi. Mycorrhizae may also alter host metabolism to respond to metal toxicity. Shabani et al. (2016) have shown that inoculation of Festuca arundinacea with Funneliformis mosseae increased the transcription of host metallothioneins and ABC transporters (which aid in the excretion of toxins) in nickel-contaminated soil.
Inoculum, Synthetic Biology and the Development of Fungal Chasses
Over the past 20 years most attempts to harness AMF diversity to improve crop health have focused on increasing fungal diversity by encouraging plant species diversity (in native habitats like grasslands). In addition, a number of initiatives have sought to create AMF inoculum optimized to increase plant growth. This inoculum often comprises of soil taken from (presumably, fungal-rich) habitats and transferred to arable fields. Although the species-composition of native mycorrhizal communities may vary globally depending on local vegetation, elevation, climate, and soil chemistry, most AMF have low host-specificity (Lee et al., 2013; Veresoglou and Rillig, 2014; Peay et al., 2016). Since AMF readily form symbiosis with multiple plant species, mycorrhizae isolated from one location have the potentially to successfully colonize plants at other sites. Stahl et al.’s (1988) research established that native strains of AMF from non-disturbed sagebrush grasslands increased the biomass and tissue phosphorus content of vegetation planted on reclaimed coal pits in Wyoming. Native soil inocula rich in mycorrhizae have increased the productivity and vegetation cover of American prairies (Richter and Stutz, 2002), Belgian species-rich grasslands (Torrez et al., 2016), and land reclaimed from Mercury mining in California (Emam, 2016). Some mycorrhizae have more beneficial traits than others and in the future inoculum containing these species could be developed. For example, three AMF with hyphae 10x longer than usual mycorrhiza are Acaulospora laevis (10.55 cm), Glomus calospora (12.3 cm), and Glomus tenue (14.2 cm) (Smith and Gianinazzi-Pearson, 1988). Longer hyphae could increase fungal phosphorus uptake and transfer to plant hosts, increasing the production of beneficial phytoalexins and plant growth. In addition, metagenomic research on the microbial communities of biodiverse and/or extreme environments suggests there are many other AMF species which could be exploited further. For example, three new species of AMF (Diversispora omaniana, Septoglomus nakheelum, and Rhizophagus arabicus spp. nov.) were identified in 2014 from environmental samples in the Arabian desert and could be propagated as inoculum for crops grown in arid regions in other parts of the world (Symanczik et al., 2014).
Synthetic biology could draw upon arbuscular mycorrhizal diversity to increase the effect of AMF on plant health (Figure 2). Synthetic biology can increase the expression of native host genes by altering transcription rates or by inserting new genes from foreign organisms (Khalil and Collins, 2010). In addition, specific traits could be selected for and expressed in modified fungal chasses. To date, synthetic biology initiatives have focused on using filamentous fungi to produce high value compounds (anti-tumor and antibiotics mainly) (Mattern et al., 2015; Xiao and Zhong, 2016). Currently, the biosynthetic pathways of 197 compounds linked to 779 nucleotide records from 174 fugal species are known and can be accessed via a public database1 (Li et al., 2016). The majority of these genes (98%) come from the Ascomycota family and there is a strong bias toward Aspergillus.
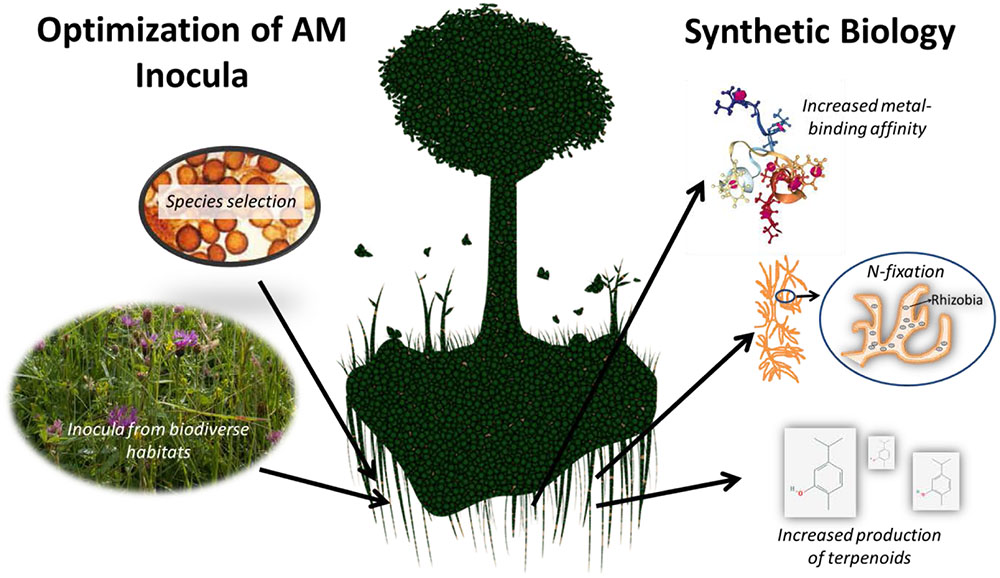
FIGURE 2. Mycorrhizal symbiosis can be harnessed for agriculture by optimizing soil inocula and through synthetic biology. Image credits (left to right): spores of Glomus spp. (IVAM); tree and root image (modified) (CC0 Public Domain); metallothionein from sea urchin (Strongylocentrotus purpuratus) (PDB ID 1QJK; Riek et al., 1999); carvacrol (PubChem). All other images belong to the author.
Currently, there is little to no research on the use of mycorrhizae in synthetic biology, either to improve crop health or to perform advanced biological functions (e.g., mycoremediation). To initiate this process R. irregularis (formerly Glomus intraradices) could serve as an initial chassis because it is the only mycorrhizae with a fully sequenced genome (Franz Lang and Hijri, 2009; Tisserant et al., 2013). This extensive knowledge of this mycorrhizal host genome could overcome the challenges bioengineering fungi face including (1) the location of biosynthetic gene clusters (BGCs) on multiple loci and (2) the control of BGCs by shared cis-regulatory elements (van der Lee and Medema, 2016). Promoters and other regulators of gene expression (e.g., transcription factors) currently developed for use in Aspergillus niger and Penicillium chrysogenum could be trialed out in these mycorrhizae (Polli et al., 2016; Wanka et al., 2016). Potential genetic targets in R. irregularis for manipulation are listed in Table 1.
Potential applications of synthetically modified mycorrhizae include increased phosphorus uptake, increased production of economically valuable terpenoids (e.g., antibiotic monoterpenes such as carvacrol) in host plants, and potentially even nitrogen-fixation. The latter could be achieved by modifying AMF metabolism or by engineering N-fixing bacteria to engage in symbiosis with specific AMF strains (Manchanda and Garg, 2007). Burkholderia spp. engage with Gigaspora and Scutellospora with as many as 250,000 bacteria per spore (Bianciotto et al., 2000; Artursson et al., 2006), indicating that mycorrhiza have a natural capacity to engage in symbiosis with bacteria. In addition, introducing metallothioneins from other fungi, bacteria, or even higher eukaryotes such as sea urchins into mycorrhiza could improve their ability to protect host plants from metal-contaminated soils. As mycorrhizae do not sexually reproduce, there is little chance these genetic changes would enter into native mycorrhizal gene pools (Pawlowska, 2005). These steps could usher in a revolution in the use of mycorrhiza in synthetic biology.
Conclusion
Arbuscular mycorrhizal symbioses with plants hold immense promise for the development of more sustainable agricultural systems (Gosling et al., 2006; Garg and Chandel, 2010). Fungi are already extensively used in biotechnology to produce antibiotics, anti-cancer drugs, pigments, bioethanol, and biomaterials (Bennett, 1998; Adrio and Demain, 2003; Cragg et al., 2015; Haneef et al., 2017). To date, arbuscular mycorrhizae have received less attention, despite their dramatic effect on plant metabolism and host resilience to environmental stresses. This fusion of plant and fungal endophyte increases the production of specific plant secondary metabolism products (e.g., fatty acids and terpenoids) and redirects the products of plant primary metabolism (e.g., fructose and glucose) to the fungal partner. This symbiosis also appears to increase the tolerance of crops to pathogen, water, and heavy metal stresses through a variety of mechanisms. Advances in metagenomic sequencing will allow us to promote native AMF diversity while boosting crop fitness (Lumini et al., 2011). The tools and techniques provided by synthetic biology may also lead to new innovations in how these symbioses function and the benefits provided to host plants. Future research should focus on identifying key mycorrhizal genes that affect plant growth and begin experimenting with genetic modification of potential chasses AMF, specifically R. irregularis. Rising climate change and anthropogenic disturbance of native ecosystems may harm the diversity and functioning of AMF across the world (Fitter et al., 2000; Antoninka et al., 2009; Classen et al., 2015; French et al., 2017). Conservation efforts must now extend below the soil if we are to ensure the preservation of this resource for the future (Bodelier, 2011).
Author Contributions
KF conceived and wrote the manuscript.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Adams, R. P., Kendall, E., and Kartha, K. K. (1990). Comparison of free sugars in growing and desiccated plants of Selaginella lepidophylla. Biochem. Syst. Ecol. 18, 107–110. doi: 10.1016/0305-1978(90)90044-G
Adrio, J. L., and Demain, A. L. (2003). Fungal biotechnology. Int. Microbiol. Off. J. Span. Soc. Microbiol. 6, 191–199. doi: 10.1007/s10123-003-0133-0
Aksu, Z. (2001). Equilibrium and kinetic modelling of cadmium(II) biosorption by C. vulgaris in a batch system: effect of temperature. Sep. Purif. Technol. 21, 285–294. doi: 10.1016/S1383-5866(00)00212-4
Antoninka, A., Wolf, J. E., Bowker, M., Classen, A. T., and Johnson, N. C. (2009). Linking above- and belowground responses to global change at community and ecosystem scales. Glob. Change Biol. 15, 914–929. doi: 10.1111/j.1365-2486.2008.01760.x
Artursson, V., Finlay, R. D., and Jansson, J. K. (2006). Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ. Microbiol. 8, 1–10. doi: 10.1111/j.1462-2920.2005.00942.x
Baldrian, P. (2003). Interactions of heavy metals with white-rot fungi. Enzyme Microb. Technol. 32, 78–91. doi: 10.1016/S0141-0229(02)00245-4
Bennett, J. W. (1998). Mycotechnology: the role of fungi in biotechnology1. J. Biotechnol. 66, 101–107. doi: 10.1016/S0168-1656(98)00133-3
Berger, S., Sinha, A. K., and Roitsch, T. (2007). Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J. Exp. Bot. 58, 4019–4026. doi: 10.1093/jxb/erm298
Bever, J. D., Schultz, P. A., Pringle, A., and Morton, J. B. (2001). Arbuscular mycorrhizal fungi: more diverse than meets the eye, and the ecological tale of why the high diversity of ecologically distinct species of arbuscular mycorrhizal fungi within a single community has broad implications for plant ecology. BioScience 51, 923–931. doi: 10.1641/0006-3568(2001)051[0923:AMFMDT]2.0.CO;2
Bianciotto, V., Lumini, E., Lanfranco, L., Minerdi, D., Bonfante, P., and Perotto, S. (2000). Detection and identification of bacterial endosymbionts in arbuscular mycorrhizal fungi belonging to the family Gigasporaceae. Appl. Environ. Microbiol. 66, 4503–4509. doi: 10.1128/AEM.66.10.4503-4509.2000
Blaudez, D., Botton, B., and Chalot, M. (2000). Cadmium uptake and subcellular compartmentation in the ectomycorrhizal fungus Paxillus involutus. Microbiology 146, 1109–1117. doi: 10.1099/00221287-146-5-1109
Bodelier, P. L. E. (2011). Toward understanding, managing, and protecting microbial ecosystems. Front. Microbiol. 2:80. doi: 10.3389/fmicb.2011.00080
Bonfante, P., and Genre, A. (2015). Arbuscular mycorrhizal dialogues: do you speak ‘plantish’ or ‘fungish’? Trends Plant Sci. 20, 150–154. doi: 10.1016/j.tplants.2014.12.002
Buee, M., Rossignol, M., Jauneau, A., Ranjeva, R., and Bécard, G. (2000). The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol. Plant Microbe Interact. MPMI 13, 693–698. doi: 10.1094/MPMI.2000.13.6.693
Champagne, A., and Boutry, M. (2016). Proteomics of terpenoid biosynthesis and secretion in trichomes of higher plant species. Biochim. Biophys. Acta BBA 1864, 1039–1049. doi: 10.1016/j.bbapap.2016.02.010
Classen, A. T., Sundqvist, M. K., Henning, J. A., Newman, G. S., Moore, J. A. M., Cregger, M. A., et al. (2015). Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: what lies ahead? Ecosphere 6, 1–21. doi: 10.1890/ES15-00217.1
Copetta, A., Lingua, G., and Berta, G. (2006). Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 16, 485–494. doi: 10.1007/s00572-006-0065-6
Cragg, S. M., Beckham, G. T., Bruce, N. C., Bugg, T. D., Distel, D. L., Dupree, P., et al. (2015). Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 29, 108–119. doi: 10.1016/j.cbpa.2015.10.018
Darlington, A. B., and Rauser, W. E. (1988). Cadmium alters the growth of the ectomycorrhizal fungus Paxillus involutus: a new growth model accounts for changes in branching. Can. J. Bot. 66, 225–229. doi: 10.1139/b88-038
Davies, D., Theodorou, M., Lawrence, M., and Trinci, A. (1993). Distribution of anaerobic fungi in the digestive-tract of cattle and their survival in feces. J. Gen. Microbiol. 139, 1395–1400. doi: 10.1099/00221287-139-6-1395
de la Peña, E., Echeverría, S. R., van der Putten, W. H., Freitas, H., and Moens, M. (2006). Mechanism of control of root-feeding nematodes by mycorrhizal fungi in the dune grass Ammophila arenaria. New Phytol. 169, 829–840. doi: 10.1111/j.1469-8137.2005.01602.x
Eisenman, H. C., and Casadevall, A. (2012). Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 93, 931–940. doi: 10.1007/s00253-011-3777-2
Emam, T. (2016). Local soil, but not commercial AMF inoculum, increases native and non-native grass growth at a mine restoration site. Restor. Ecol. 24, 35–44. doi: 10.1111/rec.12287
Emery, S. M., Bell-Dereske, L., and Rudgers, J. A. (2015). Fungal symbiosis and precipitation alter traits and dune building by the ecosystem engineer, Ammophila breviligulata. Ecology 96, 927–935. doi: 10.1890/14-1121.1
Fernández, I., Merlos, M., López-Ráez, J. A., Martínez-Medina, A., Ferrol, N., Azcón, C., et al. (2014). Defense related phytohormones regulation in arbuscular mycorrhizal symbioses depends on the partner genotypes. J. Chem. Ecol. 40, 791–803. doi: 10.1007/s10886-014-0473-6
Fester, T., and Sawers, R. (2011). Progress and challenges in agricultural applications of arbuscular mycorrhizal fungi. Crit. Rev. Plant Sci. 30, 459–470. doi: 10.1080/07352689.2011.605741
Fitter, A. H., Heinemeyer, A., and Staddon, P. L. (2000). The impact of elevated CO2 and global climate change on arbuscular mycorrhizas: a mycocentric approach. New Phytol. 147, 179–187. doi: 10.1046/j.1469-8137.2000.00680.x
Franz Lang, B., and Hijri, M. (2009). The complete Glomus intraradices mitochondrial genome sequence – a milestone in mycorrhizal research. New Phytol. 183, 3–6. doi: 10.1111/j.1469-8137.2009.02885.x
French, K. E., Tkacz, A., and Turnbull, L. A. (2017). Conversion of grassland to arable decreases microbial diversity and alters community composition. Appl. Soil Ecol. 110, 43–52. doi: 10.1016/j.apsoil.2016.10.015
Fritz, M., Jakobsen, I., Lyngkjaer, M. F., Thordal-Christensen, H., and Pons-Kühnemann, J. (2006). Arbuscular mycorrhiza reduces susceptibility of tomato to Alternaria solani. Mycorrhiza 16, 413–419. doi: 10.1007/s00572-006-0051-z
Gadd, G. M. (2006). Fungi in Biogeochemical Cycles, British Mycological Society Symposium Series; 24. Cambridge: Cambridge University Press.
Garcia-Garrido, J. M., and Ocampo, J. A. (1989). Effect of VA mycorrhizal infection of tomato on damage caused by Pseudomonas syringae. Soil Biol. Biochem. 21, 165–167. doi: 10.1016/0038-0717(89)90027-8
Garg, N., and Chandel, S. (2010). Arbuscular mycorrhizal networks: process and functions. A review. Agron. Sustain. Dev. 30, 581–599. doi: 10.1051/agro/2009054
Gaude, N., Bortfeld, S., Erban, A., Kopka, J., and Krajinski, F. (2015). Symbiosis dependent accumulation of primary metabolites in arbuscule-containing cells. BMC Plant Biol. 15:234. doi: 10.1186/s12870-015-0601-7
Geneva, M. P., Stancheva, I. V., Boychinova, M. M., Mincheva, N. H., and Yonova, P. A. (2010). Effects of foliar fertilization and arbuscular mycorrhizal colonization on Salvia officinalis L. growth, antioxidant capacity, and essential oil composition. J. Sci. Food Agric. 90, 696–702. doi: 10.1002/jsfa.3871
Gianinazzi-Pearson, V. (1996). Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. Plant Cell 8, 1871–1883. doi: 10.1105/tpc.8.10.1871
Gilbert, S. F., Bosch, T. C. G., and Ledón-Rettig, C. (2015). Eco-Evo-Devo: developmental symbiosis and developmental plasticity as evolutionary agents. Nat. Rev. Genet. 16, 611–622. doi: 10.1038/nrg3982
Gosling, P., Hodge, A., Goodlass, G., and Bending, G. D. (2006). Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 113, 17–35. doi: 10.1016/j.agee.2005.09.009
Gupta, M. L., Prasad, A., Ram, M., and Kumar, S. (2002). Effect of the vesicular–arbuscular mycorrhizal (VAM) fungus Glomus fasciculatum on the essential oil yield related characters and nutrient acquisition in the crops of different cultivars of menthol mint (Mentha arvensis) under field conditions. Bioresour. Technol. 81, 77–79. doi: 10.1016/S0960-8524(01)00109-2
Haneef, M., Ceseracciu, L., Canale, C., Bayer, I. S., Heredia-Guerrero, J. A., and Athanassiou, A. (2017). Advanced materials from fungal mycelium: fabrication and tuning of physical properties. Sci. Rep. 7:41292. doi: 10.1038/srep41292
Jianlong, W., Xinmin, Z., Decai, D., and Ding, Z. (2001). Bioadsorption of lead(II) from aqueous solution by fungal biomass of Aspergillus niger. J. Biotechnol. 87, 273–277. doi: 10.1016/S0168-1656(00)00379-5
Jousselin, E., Rasplus, J.-Y., Kjellberg, F., and May, G. (2003). Convergence and coevolution in a mutualism: evidence from a molecular phylogeny of ficus. Evolution 57, 1255–1269. doi: 10.1554/02-445
Jung, S. C., Martinez-Medina, A., Lopez-Raez, J. A., and Pozo, M. J. (2012). Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 38, 651–664. doi: 10.1007/s10886-012-0134-6
Kapoor, R., Anand, G., Gupta, P., and Mandal, S. (2016). Insight into the mechanisms of enhanced production of valuable terpenoids by arbuscular mycorrhiza. Phytochem. Rev. 1–16. doi: 10.1007/s11101-016-9486-9
Keeling, P. J., and Palmer, J. D. (2008). Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 9, 605–618. doi: 10.1038/nrg2386
Khalil, A. S., and Collins, J. J. (2010). Synthetic biology: applications come of age. Nat. Rev. Genet. 11, 367–379. doi: 10.1038/nrg2775
Kiers, E. T., and West, S. A. (2015). Evolving new organisms via symbiosis. Science 348, 392–394. doi: 10.1126/science.aaa9605
Kosuta, S., Chabaud, M., Lougnon, G., Gough, C., Dénarié, J., Barker, D. G., et al. (2003). A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiol. 131, 952–962. doi: 10.1104/pp.011882
Lee, E.-H., Eo, J.-K., Ka, K.-H., and Eom, A.-H. (2013). Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology 41, 121–125. doi: 10.5941/MYCO.2013.41.3.121
Li, Y. F., Tsai, K. J., Harvey, C. J., Li, J. J., Ary, B. E., Berlew, E. E., et al. (2016). Comprehensive curation and analysis of fungal biosynthetic gene clusters of published natural products. Fungal Genet. Biol. 89, 18–28. doi: 10.1016/j.fgb.2016.01.012
Lohse, S., Schliemann, W., Ammer, C., Kopka, J., Strack, D., and Fester, T. (2005). Organization and metabolism of plastids and mitochondria in arbuscular mycorrhizal roots of Medicago truncatula. Plant Physiol. 139, 329–340. doi: 10.1104/pp.105.061457
Lumini, E., Bianciotto, V., Orgiazzi, A., Borriello, R., and Bonfante, P. (2011). “Metagenomics applied to arbuscular mycorrhizal fungal communities,” in Metagenomics Applied to Arbuscular Mycorrhizal Fungal Communities., Metagenomics: Current Innovations and Future Trends, ed. M. Diana (Norwich: Caister Academic Press), 159–176.
Lunn, J. E., Delorge, I., Figueroa, C. M., Van Dijck, P., and Stitt, M. (2014). Trehalose metabolism in plants. Plant J. 79, 544–567. doi: 10.1111/tpj.12509
Maillet, F., Poinsot, V., André, O., Puech-Pagès, V., Haouy, A., Gueunier, M., et al. (2011). Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 469, 58–63. doi: 10.1038/nature09622
Manchanda, G., and Garg, N. (2007). Endomycorrhizal and rhizobial symbiosis: how much do they share? J. Plant Interact. 2, 79–88. doi: 10.1080/17429140701558000
Matson, P. A., Parton, W. J., Power, A. G., and Swift, M. J. (1997). Agricultural intensification and ecosystem properties. Science 277, 504–509. doi: 10.1126/science.277.5325.504
Mattern, D. J., Valiante, V., Unkles, S. E., and Brakhage, A. A. (2015). Synthetic biology of fungal natural products. Front. Microbiol. 6:775. doi: 10.3389/fmicb.2015.00775
McFall-Ngai, M., Hadfield, M. G., Bosch, T. C. G., Carey, H. V., Domazet-Lošo, T., Douglas, A. E., et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. U.S.A. 110, 3229–3236. doi: 10.1073/pnas.1218525110
McFall-Ngai, M. J., and Ruby, E. G. (1991). Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science 254, 1491–1494. doi: 10.1126/science.1962208
Mohsenzadeh, F., and Shahrokhi, F. (2014). Biological removing of Cadmium from contaminated media by fungal biomass of Trichoderma species. J. Environ. Health Sci. Eng. 12:102. doi: 10.1186/2052-336X-12-102
Morley, G. F., and Gadd, G. M. (1995). Sorption of toxic metals by fungi and clay minerals. Mycol. Res. 99, 1429–1438. doi: 10.1016/S0953-7562(09)80789-2
Müller, J., Boller, T., and Wiemken, A. (1995). Trehalose and trehalase in plants: recent developments. Plant Sci. 112, 1–9. doi: 10.1016/0168-9452(95)04218-J
Nogata, Y., and Matsumura, K. (2006). Larval development and settlement of a whale barnacle. Biol. Lett. 2, 92–93. doi: 10.1098/rsbl.2005.0409
Ohsowski, B. M., Klironomos, J. N., Dunfield, K. E., and Hart, M. M. (2012). The potential of soil amendments for restoring severely disturbed grasslands. Appl. Soil Ecol. 60, 77–83. doi: 10.1016/j.apsoil.2012.02.006
Öpik, M., Vanatoa, A., Vanatoa, E., Moora, M., Davison, J., Kalwij, J. M., et al. (2010). The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 188, 223–241. doi: 10.1111/j.1469-8137.2010.03334.x
Parniske, M. (2008). Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 6, 763–775. doi: 10.1038/nrmicro1987
Pauli, J. N., Mendoza, J. E., Steffan, S. A., Carey, C. C., Weimer, P. J., and Peery, M. Z. (2014). A syndrome of mutualism reinforces the lifestyle of a sloth. Proc. R. Soc. B 281, 20133006. doi: 10.1098/rspb.2013.3006
Pawlowska, T. E. (2005). Genetic processes in arbuscular mycorrhizal fungi. FEMS Microbiol. Lett. 251, 185–192. doi: 10.1016/j.femsle.2005.08.007
Peay, K. G., Kennedy, P. G., and Talbot, J. M. (2016). Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 14, 434–447. doi: 10.1038/nrmicro.2016.59
Polli, F., Meijrink, B., Bovenberg, R. A. L., and Driessen, A. J. M. (2016). New promoters for strain engineering of Penicillium chrysogenum. Fungal Genet. Biol. 89, 62–71. doi: 10.1016/j.fgb.2015.12.003
Pozo, M. J., and Azcón-Aguilar, C. (2007). Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 10, 393–398. doi: 10.1016/j.pbi.2007.05.004
Richards, A. B., Krakowka, S., Dexter, L. B., Schmid, H., Wolterbeek, A. P. M., Waalkens-Berendsen, D. H., et al. (2002). Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 40, 871–898. doi: 10.1016/S0278-6915(02)00011-X
Richter, B. S., and Stutz, J. C. (2002). Mycorrhizal inoculation of big sacaton: implications for grassland restoration of abandoned agricultural fields. Restor. Ecol. 10, 607–616. doi: 10.1046/j.1526-100X.2002.01041.x
Riek, R., Prêcheur, B., Wang, Y., Mackay, E. A., Wider, G., Güntert, P., et al. (1999). NMR structure of the sea urchin (Strongylocentrotus purpuratus) metallothionein MTA. J. Mol. Biol. 291, 417–428. doi: 10.1006/jmbi.1999.2967
Rivero, J., Gamir, J., Aroca, R., Pozo, M. J., and Flors, V. (2015). Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 6:598. doi: 10.3389/fmicb.2015.00598
Ruscitti, M., Arango, M., Ronco, M., and Beltrano, J. (2011). Inoculation with mycorrhizal fungi modifies proline metabolism and increases chromium tolerance in pepper plants (Capsicum annuum L.). Braz. J. Plant Physiol. 23, 15–25. doi: 10.1590/S1677-04202011000100004
Sayer, J. A., and Gadd, G. M. (1997). Solubilization and transformation of insoluble inorganic metal compounds to insoluble metal oxalates by Aspergillus niger. Mycol. Res. 101, 653–661. doi: 10.1017/S0953756296003140
Schweiger, R., Baier, M. C., Persicke, M., and Müller, C. (2014). High specificity in plant leaf metabolic responses to arbuscular mycorrhiza. Nat. Commun. 5:3886. doi: 10.1038/ncomms4886
Shabani, L., Sabzalian, M. R., and Pour, S. M. (2016). Arbuscular mycorrhiza affects nickel translocation and expression of ABC transporter and metallothionein genes in Festuca arundinacea. Mycorrhiza 26, 67–76. doi: 10.1007/s00572-015-0647-2
Smith, S., and Gianinazzi-Pearson, V. (1988). Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 221–244. doi: 10.1146/annurev.pp.39.060188.001253
Smith, S. E., Smith, F. A., and Jakobsen, I. (2003). Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 133, 16–20. doi: 10.1104/pp.103.024380
Spribille, T., Tuovinen, V., Resl, P., Vanderpool, D., Wolinski, H., Aime, M. C., et al. (2016). Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 353, 488–492. doi: 10.1126/science.aaf8287
Stahl, P. D., Williams, S. E., and Christensen, M. (1988). Efficacy of native vesicular-arbuscular mycorrhizal fungi after severe soil disturbance. New Phytol. 110, 347–354. doi: 10.1111/j.1469-8137.1988.tb00271.x
Symanczik, S., Błaszkowski, J., Chwat, G., Boller, T., Wiemken, A., and Al-Yahya’ei, M. N. (2014). Three new species of arbuscular mycorrhizal fungi discovered at one location in a desert of Oman: Diversispora omaniana, Septoglomus nakheelum and Rhizophagus arabicus. Mycologia 106, 243–259. doi: 10.3852/106.2.243
Tisserant, E., Malbreil, M., Kuo, A., Kohler, A., Symeonidi, A., Balestrini, R., et al. (2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. U.S.A. 110, 20117–20122. doi: 10.1073/pnas.1313452110
Tomsett, B. A. (1993). “Genetic and molecular biology of metal tolerance in fungi,” in Stress Tolerance of Fungi, ed. D. H. Jennings (New York: Marcel Dekker), 69–95.
Torrez, V., Ceulemans, T., Mergeay, J., de Meester, L., and Honnay, O. (2016). Effects of adding an arbuscular mycorrhizal fungi inoculum and of distance to donor sites on plant species recolonization following topsoil removal. Appl. Veg. Sci. 19, 7–19. doi: 10.1111/avsc.12193
Toussaint, J.-P. (2007). Investigating physiological changes in the aerial parts of AM plants: what do we know and where should we be heading? Mycorrhiza 17, 349–353. doi: 10.1007/s00572-007-0133-6
van der Lee, T. A. J., and Medema, M. H. (2016). Computational strategies for genome-based natural product discovery and engineering in fungi. Fungal Genet. Biol. 89, 29–36. doi: 10.1016/j.fgb.2016.01.006
Veresoglou, S. D., and Rillig, M. C. (2012). Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol. Lett. 8, 214–217. doi: 10.1098/rsbl.2011.0874
Veresoglou, S. D., and Rillig, M. C. (2014). Do closely related plants host similar arbuscular mycorrhizal fungal communities? A meta-analysis. Plant Soil 377, 395–406. doi: 10.1007/s11104-013-2008-2
Wade, M. J. (2007). The co-evolutionary genetics of ecological communities. Nat. Rev. Genet. 8, 185–195. doi: 10.1038/nrg2031
Wagner, W., Wiemken, A., and Matile, P. (1986). Regulation of fructan metabolism in leaves of barley (Hordeum vulgare L. cv Gerbel). Plant Physiol. 81, 444–447. doi: 10.1104/pp.81.2.444
Wang, M., and Zhou, Q. (2005). Single and joint toxicity of chlorimuron-ethyl, cadmium, and copper acting on wheat Triticum aestivum. Ecotoxicol. Environ. Saf. 60, 169–175. doi: 10.1016/j.ecoenv.2003.12.012
Wanka, F., Cairns, T., Boecker, S., Berens, C., Happel, A., Zheng, X., et al. (2016). Tet-on, or Tet-off, that is the question: advanced conditional gene expression in Aspergillus. Fungal Genet. Biol. 89, 72–83. doi: 10.1016/j.fgb.2015.11.003
Wingler, A. (2002). The function of trehalose biosynthesis in plants. Phytochemistry 60, 437–440. doi: 10.1016/S0031-9422(02)00137-1
Xiao, H., and Zhong, J.-J. (2016). Production of useful terpenoids by higher-fungus cell factory and synthetic biology approaches. Trends Biotechnol. 34, 242–255. doi: 10.1016/j.tibtech.2015.12.007
Zhdanova, N. N., Zakharchenko, V. A., Vember, V. V., and Nakonechnaya, L. T. (2000). Fungi from Chernobyl: mycobiota of the inner regions of the containment structures of the damaged nuclear reactor. Mycol. Res. 104, 1421–1426. doi: 10.1017/S0953756200002756
Zipfel, C., and Oldroyd, G. E. D. (2017). Plant signalling in symbiosis and immunity. Nature 543, 328–336. doi: 10.1038/nature22009
Keywords: fungal diversity, endosymbiosis, agriculture, synthetic biology, microbial-plant communication, bioprotectants
Citation: French KE (2017) Engineering Mycorrhizal Symbioses to Alter Plant Metabolism and Improve Crop Health. Front. Microbiol. 8:1403. doi: 10.3389/fmicb.2017.01403
Received: 06 May 2017; Accepted: 11 July 2017;
Published: 21 July 2017.
Edited by:
Magdalena Frac, Institute of Agrophysics (PAN), PolandReviewed by:
Bharath Prithiviraj, Brooklyn College and CUNY Advanced Science Research Center, United StatesFrédérique Reverchon, Instituto de Ecología A.C., Mexico
Copyright © 2017 French. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine E. French, katherine.french@plants.ox.ac.uk; katherine.french@wadham.ac.uk
 Katherine E. French
Katherine E. French