- College of Animal Science and Technology, Shandong Agricultural University, Tai’an, China
This study was designed to evaluate the protection mechanism of oral administration of Clostridium butyricum against Salmonella enteritidis (SE) colonization in broilers. In the current study, 180 one-day-old healthy Arbor Acres (AA) broilers were meanly grouped into three, with three replicates of 20 birds each. An negative control group was fed basal diet without SE challenge and a positive control (PC) group was fed the basal diet and challenged with SE [106 colony forming unit (CFU)/0.2 mL]. An experimental (EXP) group was fed the basal diet, orally administered with C. butyricum (106 CFU/mL) and challenged with SE (106 CFU/0.2 mL). The results showed that compared to the PC group, the SE loads in livers, spleens, and cecal contents of chickens in EXP group were significantly reduced (P < 0.05) except in spleens at the 2-day post-infection; the production of interferon-γ, interleukin (IL)-1β, IL-8, and tumor necrosis factor-α in the livers, spleens, and cecal tissues of chickens in EXP group were decreased to different extents. The results of quantitative real-time polymerase chain reaction further revealed that the inflammation of chickens in EXP group was alleviated by C. butyricum via down-regulating TLR4, MyD88, and NF-κB-dependent pathways. Collectively, these findings indicated that oral administration of C. butyricum could be a suitable alternative for preventing SE infection in broilers.
Introduction
Salmonella, as an important foodborne pathogen, can lead to serious infections in animals and humans worldwide (Mead et al., 1999; Scallan et al., 2011). Poultry have been recognized as an important reservoir for Salmonella (Chen and Jiang, 2014). Salmonella can cause high morbidity and mortality in poultry breeding industry, especially in young birds within 1 week age (Wigley et al., 2001; Vo et al., 2006). At the early stage of Salmonella infection, the production of cytokines, such as interferon (IFN)-γ, interleukin (IL)-1β, IL-8, and tumor necrosis factor (TNF)-α, is of utmost importance for controlling Salmonella growth and spread in the host body (Brown et al., 2006; Hu et al., 2015). In addition, Toll-like receptors (TLRs) can play a key role in the protection animals and humans against Salmonella infection, and they combat the pathogen through recognition of pathogen-associated molecular patterns (Akira and Takeda, 2004). TLR4, as one important member of the TLRs family, can recognize lipopolysaccharide (LPS) of Gram-negative bacteria and can activate nuclear factor-kappa B (NF-κB) through myeloid differentiation primary response protein 88 (MyD88), and therefore leading to cytokine secretion and inflammatory response (Kawai and Akira, 2007).
As for the combat against Salmonella infections, antimicrobials have been widely used in the clinical practice. However, the overuse and even abuse of antibiotics have contributed to the increasing and dissemination of drug-resistant Salmonella and have sparked a severe public health concern (Chiu et al., 2002; Tseng et al., 2014). Furthermore, antimicrobials can lead to the loss of commensal gastrointestinal microbiota and potentially to the overgrowth of pathogens (McDonald et al., 2016; Wischmeyer et al., 2016). Therefore, in the recent years, many researchers have been striving to find the substitutes of antimicrobials, and probiotics are being considered as one of the promising substitute for antimicrobials against Salmonella infections (Mathipa and Thantsha, 2017).
Probiotics have ability to provide protection effects for the host when administered in adequate amounts (Food and Agricultural Organization/World Health Organization [FAO/WHO], 2002). Numerous studies have showed that the use of probiotics is able to modulate mucosal immune functions, prevent bacterial translocation, and potentially suppress inflammatory cytokine production through modulating LPS-induced inflammation by binding to LPS or directly perturbing the MyD88 signaling pathway (Mainous et al., 1995; Kemgang et al., 2014).
Clostridium butyricum, a strictly anaerobic endospore-forming Gram-positive bacillus, could produce butyric acid. Compared to Lactobacillus and Bifidobacterium, C. butyricum is able to survive at lower pH and relatively higher bile concentrations (Okamoto et al., 2000; Zhang et al., 2016). Previous studies demonstrated that C. butyricum can inhibit pathogens propagation and spread in host body and therefore is considered as a potential substitute for antibiotics (Gao et al., 2012; Yang et al., 2012; Zhang et al., 2016). However, the protection mechanism of C. butyricum against Salmonella enteritidis (SE) colonization in broilers remains to be elucidated. This study was therefore conducted to better understand the protection mechanism by which C. butyricum protects chickens against SE infection.
Materials and Methods
Ethics Statement
All procedures were approved by the Animal Care and Use committee of Shandong Agricultural University (SDAUA-2016-016).
Bacterial Strains and Growth Conditions
Clostridium butyricum (AQQF01000149) was obtained as a gift from Dalian Sanyi Animal Medicine Company (China) and grown anaerobically at 37°C in liquid fermentation tank for 48 h. The concentration of C. butyricum was adjusted to 1 × 106 colony forming unit (CFU)/mL in sterile saline.
A virulent atrichia SE, a isolate from a diseased chicken, was obtained from the Avian Disease Centre of Shandong Agricultural University. While cultivating SE, single colony was picked from xylose lysine deoxycholate agar plate and transferred into a tube contained 5 mL tryptic soy broth and then incubated at 37°C for 12 h. The concentration of SE was adjusted to 1 × 106 CFU/mL in sterile saline.
Experimental Design
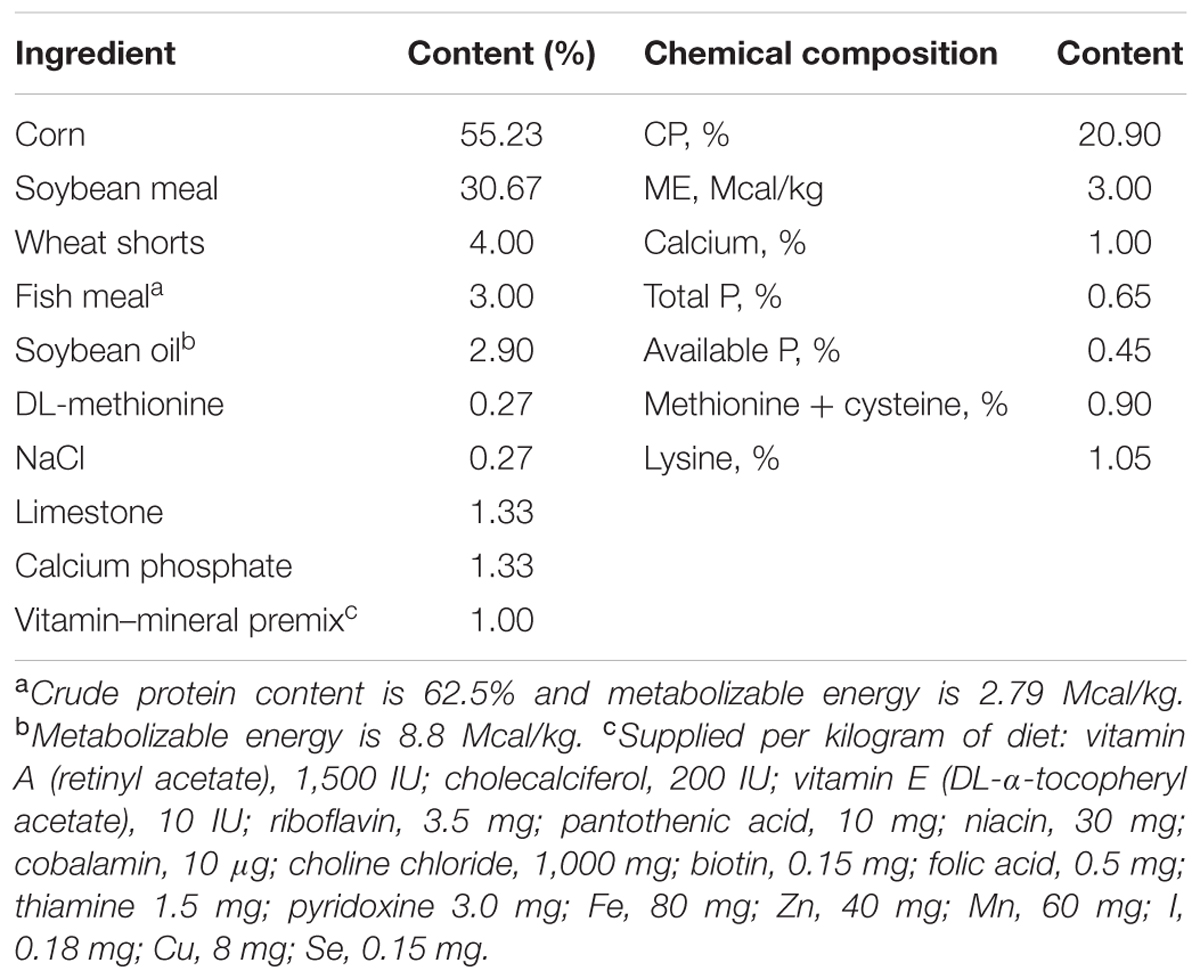
The experiment was performed in October, 2016. In total, 180 one-day-old healthy Arbor Acres (AA) chickens (negative for Salmonella) were bought from a hatchery in Xintai, China. Chickens were housed in metal cages and provided ad libitum with water and commercial starter diet in the animal room of Shandong Agricultural University. The temperature was maintained at 30°C at the first 3 days and gradually reduced to 28°C during the last days of the experiment. The nutrient levels of the basal diets met the nutritional requirement of the broilers (NRC, 1994) (Table 1), and the rearing duration lasted 2 weeks. The sanitation of raising environments were regularly cleaned for the health of chicken. Chickens were divided into three treatment groups in random manner: an negative control (NC) group, chickens were orally administrated 0.2 mL sterile saline per chick once every day through day 1 to day 7; a positive control (PC) group, chickens were challenged with 0.2 mL SE enrichment solution (106 CFU/0.2 mL) at the 8 day, and were given sterile saline (0.2 mL/chick) during day 1 to day 7; an experimental (EXP) group, chickens were given 0.2 mL C. butyricum enrichment solution (106 CFU/0.2 mL) once every day from 1 to 7 day, and at the 8 day, chickens were challenged with 0.2 mL SE enrichment solution (106 CFU/0.2 mL). For all groups, chickens were euthanized via cervical dislocation, and livers, spleens, as well as cecal tissues and contents were sampled at 2 and 6 days of post-infection. These samples were frozen at -80°C for further analyses.
SE Translocation
SE translocation to livers, spleens, and cecal contents of all SE-challenged groups was determined at 2 and 6 days of post-infection. Livers, spleens, and cecal contents were weighted, homogenized respectively and serially diluted 10-fold with sterile phosphate-buffered saline (1:10, w/v), and then screened on Brilliant Green Agar plates (Hopebio, Qingdao, China) to count the CFU of SE after incubation at 37°C for 24 h.
Quantitative Real-time Polymerase Chain Reaction
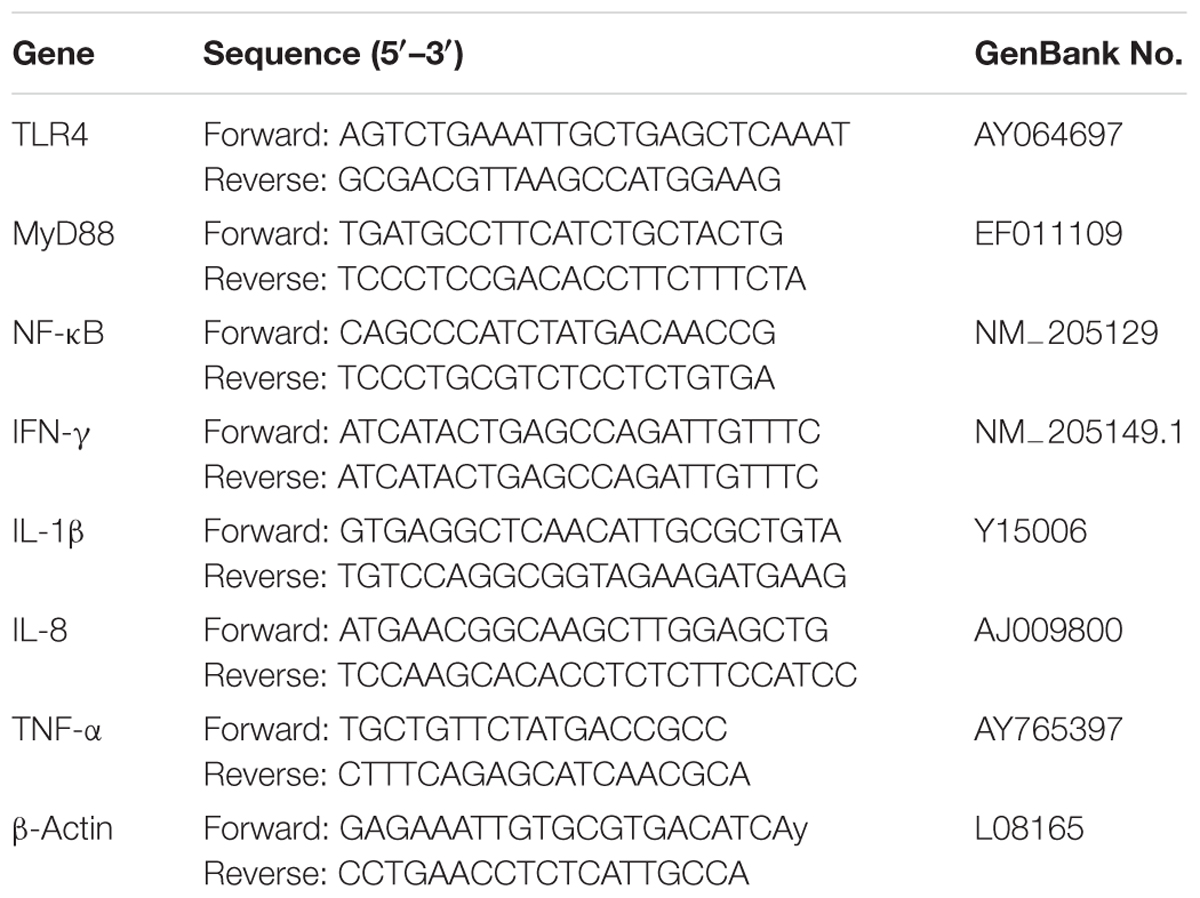
Quantitative real-time polymerase chain reaction (Q-PCR) was undertaken to relatively quantify the expression levels of cytokine genes including IFN-γ, IL-1β, IL-8, and TNF-α, and the gene expressions of the MyD88-dependent pathway of TLR4, MyD88, and NF-κB in the livers, spleens, and cecal tissues. At 2 and 6 days of post-infection, Trizol reagent (Invitrogen) was used to extract total RNA from livers, spleens, and cecal tissues according to the manufacturer’s instruction. Nano Drop 2000 spectrophotometer (Thermo Fisher Scientific, MA, United States) was used to determine the concentration and quality of total RNA. SuperScript III First Strand synthesis kit (Life Technologies, Carlsbad, CA, United States) was used to synthesize cDNA with 2 μg of total RNA. The cDNA was stored at -20°C. The Q-PCR was performed with SYBR Green master mix using 7500 Fast Real-Time PCR system (Applied Biosystems, Carlsbad, CA, United States). PCR conditions contained one cycle of 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s. Dissociation analysis of amplification products was performed at the end of each PCR to confirm the specificity of amplicon. The primers for real-time PCR are listed in Table 2. mRNA relative expression was calculated using the 2-ΔΔCt method.
Statistical Analysis
The one-way ANOVA and Student’s t-test of SPSS 15.0 (SPSS Inc., Chicago, IL, United States) were used to perform statistical analyses. The results were shown as mean ± standard deviations (SD). Differences were considered significant at P < 0.05.
Results
SE Translocation
The results of SE translocation showed that after 2 and 6 days post-infection, chickens in EXP group significantly reduced the viable count of SE compared to the PC group in the liver, spleen, and cecal content (P < 0.05), except in the spleen at 2 day post-infection (P > 0.05). In addition, SE was not detected in NC group (Table 3).
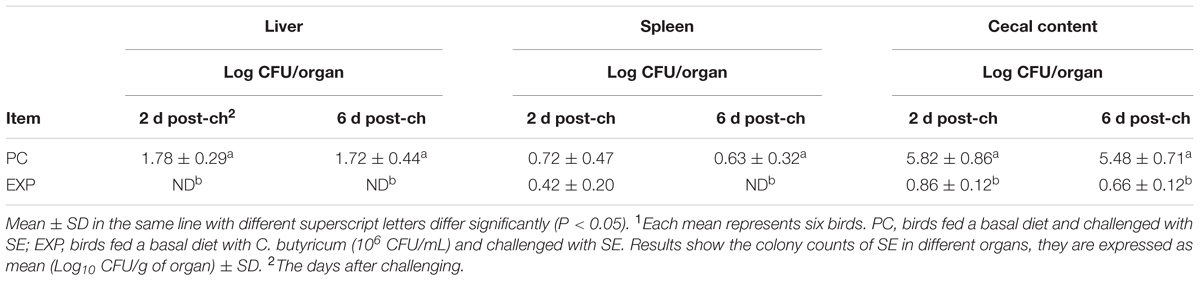
TABLE 3. Effect of C. butyricum on the reduction of SE counts in livers, spleens, and cecal contents of broilers1.
Gene Expression of Cytokines in the Liver
At 2-day post-infection, gene expression for pro-inflammatory cytokine TNF-α was significantly elevated in PC group compared to NC and EXP groups (P < 0.05), but no significant difference was found between NC and EXP groups (P > 0.05); with regard to IFN-γ, IL-1β, and IL-8 production, no significant difference was observed among EXP, PC, and NC groups (P > 0.05). At 6-day post-infection in the PC group, the gene expressions of IFN-γ, IL-1β, and TNF-α were elevated significantly (P < 0.05) compared to NC and EXP groups, but no difference was found between NC and EXP groups (P > 0.05); in terms of IL-8, no significant differences were found among EXP, NC, and PC groups (P > 0.05) (Table 4).
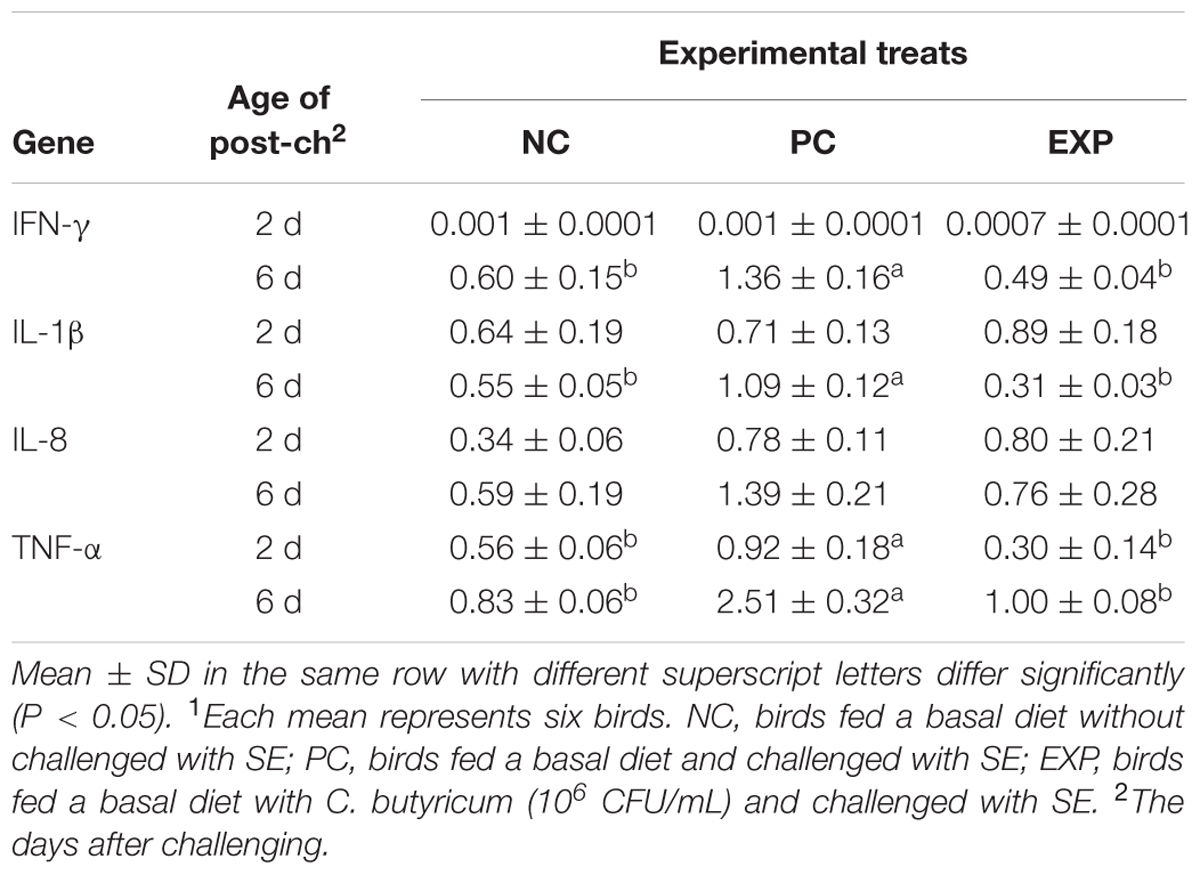
TABLE 4. Fold changes of cytokine gene expression in the livers of broilers after challenged with SE1.
Gene Expression of Cytokines in the Spleen
At 2-day post-infection, gene expression for IFN-γ was significantly increased in PC group compared to NC and EXP groups (P < 0.05), but no significant difference was found between NC and EXP groups (P > 0.05); in addition, no significant differences were observed in the IL-1β and TNF-α productions among EXP, PC, and NC groups (P > 0.05); the expression of IL-8 was significantly mounted in the PC group compared to EXP group (P < 0.05), but no significant difference was observed between EXP and NC groups (P > 0.05), and the same change was found between PC and NC groups (P > 0.05). At 6-day post-infection, gene expressions of IFN-γ, IL-1β, and IL-8 were elevated significantly in the PC group compared to NC and EXP groups (P < 0.05), but no significant difference was found between NC and EXP groups (P > 0.05); additionally, no significant difference in the TNF-α production was found among EXP, NC, and PC groups (P > 0.05) (Table 5).
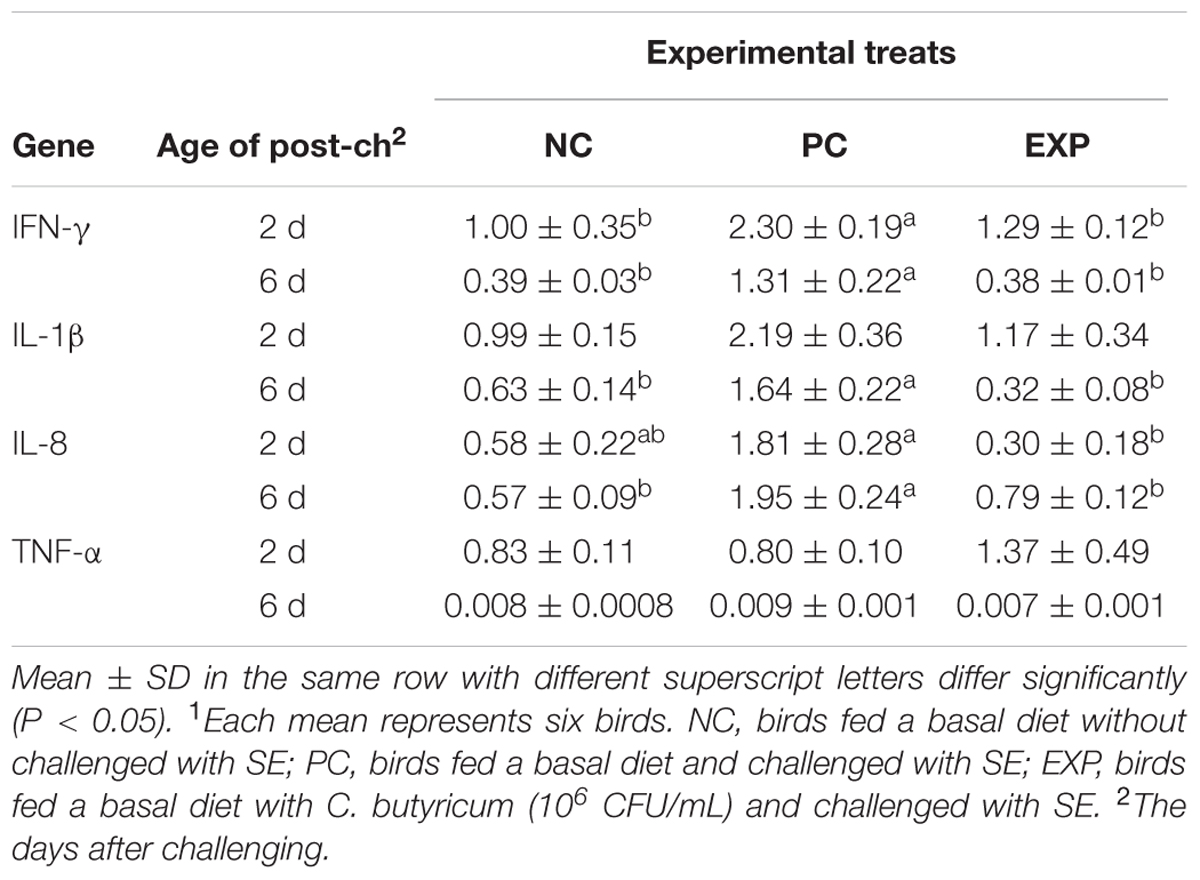
TABLE 5. Fold changes of cytokine gene expression in the spleens of broilers after challenged with SE1.
Gene Expression of Cytokines in the Cecal Tissues
At 2-day post-infection, gene expression for IL-1β was significantly elevated in PC group compared to NC and EXP groups (P < 0.05), but no significant difference was found between NC and EXP groups (P > 0.05); with regard to IFN-γ, IL-8, and TNF-α production, no significant difference was observed among EXP, PC, and NC groups (P > 0.05). At 6-day post-infection, gene expressions of IFN-γ, IL-1β, and IL-8 were elevated significantly in PC group compared to NC and EXP groups (P < 0.05), but no significant difference was found between NC and EXP groups (P > 0.05); of note, no significant difference in the TNF-α was found among EXP, NC, and PC groups (P > 0.05) (Table 6).
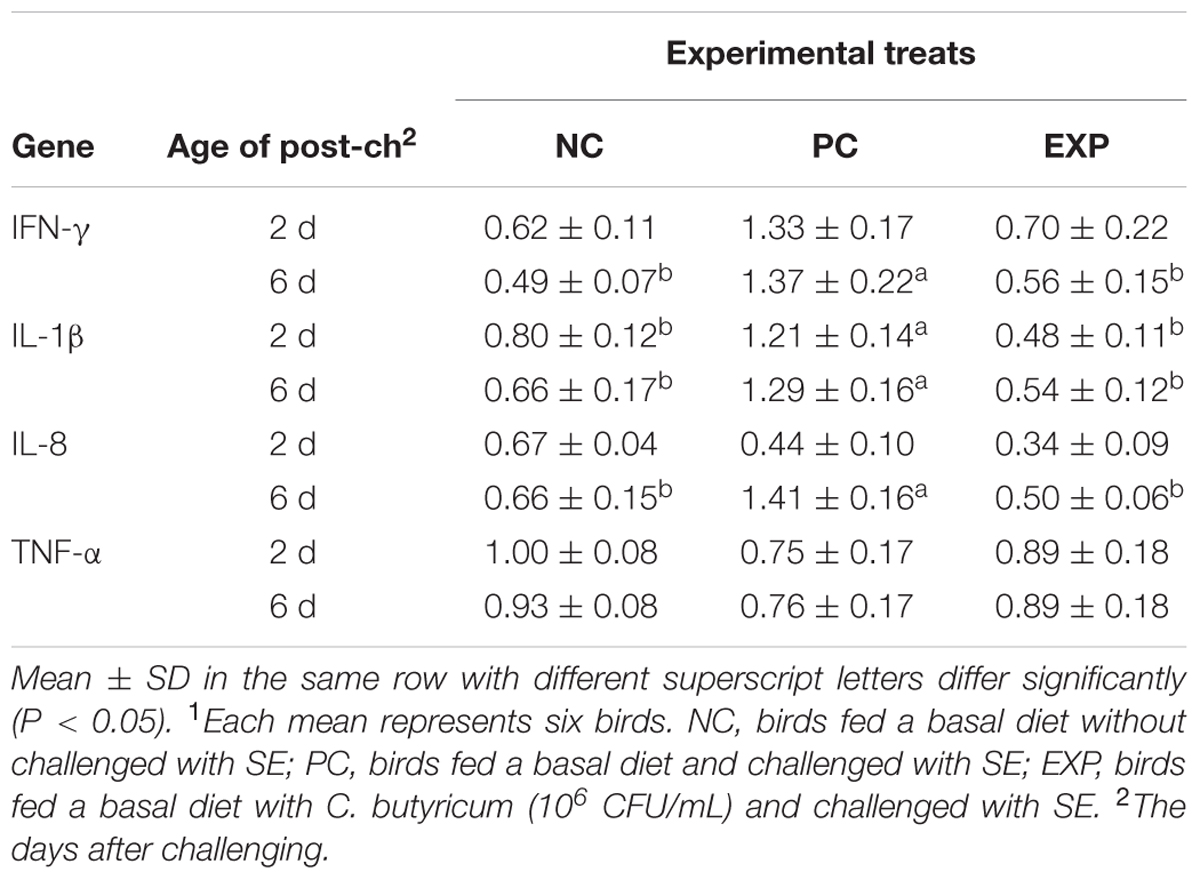
TABLE 6. Fold changes of cytokine gene expression in the cecal tissues of broilers after challenged with SE1.
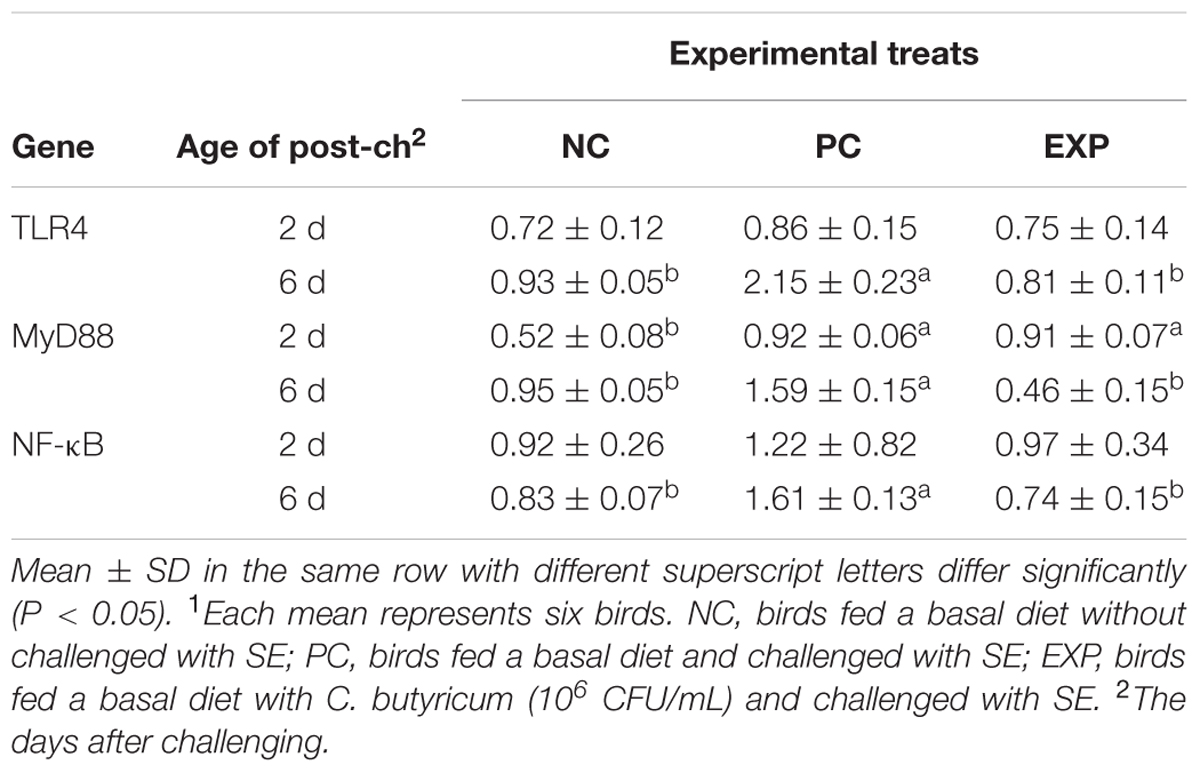
Expression of Genes of the MyD88-Dependent Pathway in Liver, Spleen, and Cecal Tissues
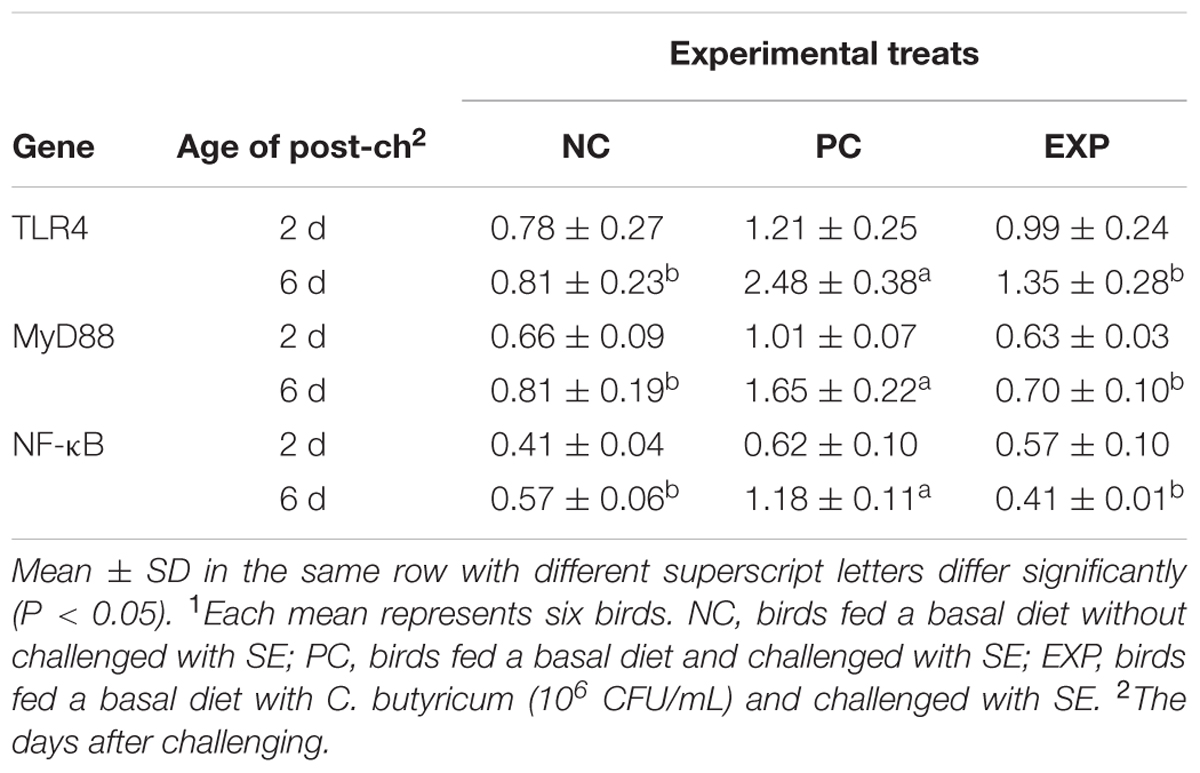
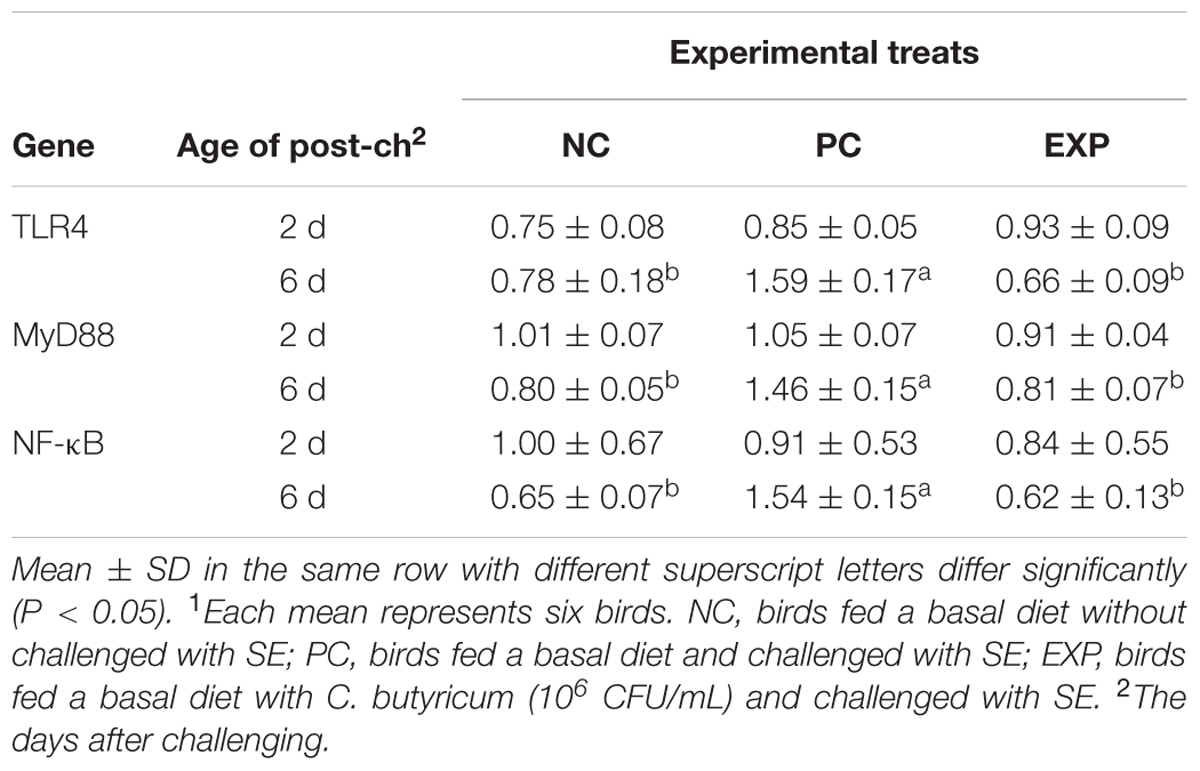
At 2-day post-infection, no significant difference was observed between EXP and PC groups with regard to the production of TLR4, MyD88, and NF-κB in liver, spleen, and cecal tissues (P > 0.05). However, at 6-day post-infection, gene expressions for TLR4, MyD88, and NF-κB in liver, spleen, and cecal tissues were elevated significantly (P < 0.05) in the PC group compared to NC and EXP groups (P < 0.05), but no significant differences were found between EXP and NC groups (P > 0.05) (Tables 7–9).
Discussion
In the present study, compared with the PC group, the levels of SE recovered from liver, spleen, and cecal contents were reduced in 1-day-old chickens fed C. butyricum for seven consecutive days, which was in agreement with previous reports (Berndt et al., 2007; Tanedjeu et al., 2016). However, the results were different from another study which indicated that the Salmonella burden in cecal contents was not affected by probiotic treatments while Salmonella infections in liver and spleen were reduced (Yang et al., 2014). The differences may be associated with the types of probiotics used, breed and age of chickens, as well as rearing environments.
IFN-γ is a Th1 cytokine that stimulates macrophages to secret oxidants with antimicrobial activities and is produced by natural killer cells and T-lymphocytes (Alam et al., 2002). In this study, C. butyricum significantly decreased SE-induced IFN-γ expression level, which was similar to the report that pretreatment of 1-day-old chickens with probiotics could significantly reduce IFN-γ expression level in Salmonella infection period (Chen et al., 2012).
IL-1β is a major mediator of inflammation in birds and mammals, primarily produced by monocytes, tissue macrophages, and enterocytes (Bar-Shira and Friedman, 2006). In this study, C. butyricum significantly decreased SE-induced IL-1β expression level, which was consistent with a previous report which showed that treating Salmonella-infected chicks with Lactobacillus strains could significantly down-modulate the expression level of IL-1β (Chen et al., 2012).
IL-8, as an important member of the chemokines, has chemotactic activity and shares similar structure to cytokines (Baggiolini et al., 1997). The results in this study showed that C. butyricum could significantly reduce mRNA level of IL-8, which was also observed in a previous report (Yi et al., 2016).
LPS-induced TNF-α factor, as one kind of vital indicator for evaluating inflammatory response in chickens, can produce the inflammatory response in chickens when infected with pathogens (Feng et al., 2016). In the present study, C. butyricum significantly decreased SE-induced the mRNA level of TNF-α in the liver, which was consistent with the report that Lactobacillus rhamnosus may decrease Escherichia coli-induced TNF-α expression level (Liu et al., 2016).
TLR4 plays an essential role in the innate immune response and hence is likely to be involved in young chickens at risk of Salmonella infection (Li et al., 2010). In the study, C. butyricum suppressed inflammation by down-regulating TLR4, MyD88, and NF-κB-dependent pathways in chickens with SE infection on day 6 post-infection, which is consistent with the report that indicated that probiotics can decrease pro-inflammatory cytokine levels by inhibiting the expression of TLR4, MyD88, and NF-κB-dependent pathways in LPS-induced macrophages and in mice (Song et al., 2015; Yi et al., 2015).
Although there was a limitation in this study (the 2-week rearing period of SE infection experiment was relatively short), these findings indicated that C. butyricum can decrease SE infection by down-regulating cytokine gene expression, and can inhibit inflammation by down-regulating TLR4, MyD88, and NF-κB-dependent pathways. Collectively, C. butyricum could be a potential probiotics against SE infection in broiler chickens.
Author Contributions
SS, HL, and XZ designed the work. XZ, JY, and LW raised animals. XZ and JY collected samples. XZ analyzed and interpreted data. XZ drafted the article. SS and HL critically reviewed the article.
Funding
This work was supported by the National key R&D project (2016YFD0501608 and 2016 YFD0500510); Taishan Scholar Program (201511023); Funds of Shandong “Double Tops” program.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Akira, S., and Takeda, K. (2004). Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511. doi: 10.1038/nri1391
Alam, M. S., Akaike, T., Okamoto, S., Kubota, T., Yoshitake, J., Sawa, T., et al. (2002). Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect. Immun. 70, 3130–3142. doi: 10.1128/IAI.70.6.3130-3142.2002
Baggiolini, M., Dewald, B., and Moser, B. (1997). Human chemokines: an update. Annu. Rev. Immunol. 15, 675–705. doi: 10.1146/annurev.immunol.15.1.675
Bar-Shira, E., and Friedman, A. (2006). Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev. Comp. Immunol. 30, 930–941. doi: 10.1016/j.dci.2005.12.002
Berndt, A., Wilhelm, A., Jugert, C., Pieper, J., Sachse, K., and Methner, U. (2007). Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 75, 5993–6007. doi: 10.1128/IAI.00695-07
Brown, S. P., Cornell, S. J., Sheppard, M., Grant, A. J., Maskell, D. J., and Mastroeni, P. (2006). Intracellular demography and the dynamics of Salmonella enterica infections. PLoS Biol. 4:e349. doi: 10.1371/journal.pbio.0040349
Chen, C. Y., Tsen, H. Y., Lin, C. L., Yu, B., and Chen, C. S. (2012). Oral administration of a combination of select lactic acid bacteria strains to reduce the Salmonella invasion and inflammation of broiler chicks. Poult. Sci. 91, 2139–2147. doi: 10.3382/ps.2012-02237
Chen, Z., and Jiang, X. (2014). Microbiological safety of chicken litter or chicken litter-based organic fertilizers: a review. Agriculture 4, 1–29. doi: 10.3390/agriculture4010001
Chiu, C. H., Wu, T. L., Su, L. H., Chu, C., Chia, J. H., Kuo, A. J., et al. (2002). The emergence in Taiwan of fluoroquinolone resistance in Salmonella enterica serotype Choleraesuis. N. Engl. J. Med. 346, 413–419. doi: 10.1056/NEJMoa012261
Feng, J. C., Wang, L. H., Zhou, L. X., Yang, X., and Zhao, X. (2016). Using in vitro immunomodulatory properties of Lactic Acid Bacteria for selection of Probiotics against Salmonella infection in broiler chicks. PLoS ONE 11:e0147630. doi: 10.1371/journal.pone.0147630
Food and Agricultural Organization/World Health Organization [FAO/WHO] (2002). Report of a Joint FAO/WHO Expert Consultation on Guidelines for the Evaluation of Probiotics in Food. London, ON: FAO/WHO of the United Nations.
Gao, Q., Qi, L., Wu, T., and Wang, J. (2012). Ability of Clostridium butyricum to inhibit Escherichia coli-induced apoptosis in chicken embryo intestinal cells. Vet. Microbiol. 160, 395–402. doi: 10.1016/j.vetmic.2012.06.009
Hu, J. L., Yu, H., Raveendra, R. K., Shayan, S., Steve, W. C., Xie, M. Y., et al. (2015). Modulation of cytokine gene expression by selected Lactobacillus isolates in the ileum, caecal tonsils and spleen of Salmonella-challenged broilers. Avian Pathol. 44, 463–469. doi: 10.1080/03079457.2015.1086725
Kawai, T., and Akira, S. (2007). TLR signaling. Semin. Immunol. 19, 24–32. doi: 10.1016/j.smim.2006.12.004
Kemgang, S. T., Kapila, S., Shanmugam, V. P., and Kapila, R. (2014). Cross-talk between probiotic lactobacilli and host immune system. J. Appl. Microbiol. 117, 303–319. doi: 10.1111/jam.12521
Li, P., Xia, P. G., Wen, J., Zheng, M. Q., Chen, J. L., Zhao, J. P., et al. (2010). Up-regulation of the MyD88-dependent pathway of TLR signaling in spleen and caecum of young chickens infected with Salmonella serovar Pullorum. Vet. Microbiol. 143, 346–351. doi: 10.1016/j.vetmic.2009.12.008
Liu, M. C., Wu, Q., Wang, M. L., Fu, Y. H., and Wang, J. F. (2016). Lactobacillus rhamnosus GR-1 limits Escherichia coli-Induced inflammatory responses via attenuating MyD88-Dependent and MyD88-Independent pathway activation in bovine endometrial epithelial cells. Inflammation 39, 1483–1494. doi: 10.1007/s10753-016-0382-7
Mainous, M. R., Ertel, W., Chaudry, I. H., and Deitch, E. A. (1995). The gut: a cytokine-generating organ in systemic inflammation? Shock 4, 193–199. doi: 10.1097/00024382-199509000-00007
Mathipa, M. G., and Thantsha, M. S. (2017). Probiotic engineering: towards development of robust probiotic strains with enhanced functional properties and for targeted control of enteric pathogens. Gut Pathog. 9, 28. doi: 10.1186/s13099-017-0178-9
McDonald, D., Ackermann, G., Khailova, L., Baird, C., Heyland, D., Kozar, R., et al. (2016). Extreme dysbiosis of the microbiome in critical illness. mSphere 1:e199–e216. doi: 10.1128/mSphere.00199-16
Mead, P. S., Slutsker, L., Dietz, V., McCaig, L. F., Bresee, J. S., Shapiro, C., et al. (1999). Food-related illness and death in the United States. Emerg. Infect. Dis. 5, 607–625. doi: 10.3201/eid0505.990502
Okamoto, T., Sasaki, M., Tsujikawa, T., Fujiyama, Y., Bamba, T., and Kusunoki, M. (2000). Preventive efficacy of butyrate enemas and oral administration of Clostridium butyricum M588 in dextran sodium sulfate-induced colitis in rats. J. Gastroenterol. 35, 341–346. doi: 10.1007/s005350050358
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 17, 7–15. doi: 10.3201/eid1701.P11101
Song, D., Zong, X., Zhang, H., Wang, T., Yi, H., Luan, C., et al. (2015). Antimicrobial peptide Cathelicidin-BF prevents intestinal barrier dysfunction in a mouse model of endotoxemia. Int. Immunopharmacol. 25, 141–147. doi: 10.1016/j.intimp.2015.01.017
Tanedjeu, S. K., Suman, K., Venkatesa, P. S., Srinu, R., and Rajeev, K. (2016). Fermented milk with probiotic Lactobacillus rhamnosus S1K3 (MTCC5957) protects mice from Salmonella by enhancing immune and nonimmune protection mechanisms at intestinal mucosal level. J. Nutr. Biochem. 30, 62–73. doi: 10.1016/j.jnutbio.2015.11.018
Tseng, C. S., Yen, Y. C., Chang, C. C., and Hsu, Y. M. (2014). Polymorphism of gene cassette promoter variants of class 1 integron harbored in S. Choleraesuis and Typhimurium isolated from Taiwan. Biomedicine 4, 1–6. doi: 10.7603/s40681-014-0020-3
Vo, A. T., Van, D. E., Fluit, A. C., Heck, M. E., Verbruggen, A., Maas, H. M., et al. (2006). Distribution of Salmonella enterica serovars from humans, livestock and meat in Vietnam and the dominance of Salmonella Typhimurium phage type 90. Vet. Microbiol. 113, 153–158. doi: 10.1016/j.vetmic.2005.10.034
Wigley, P., Berchieri, J. R., Page, A. K. L., Smith, A. L., and Barrow, P. A. (2001). Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infect. Immun. 69, 7873–7879. doi: 10.1128/IAI.69.12.7873-7879.2001
Wischmeyer, P. E., McDonald, D., and Knight, R. (2016). Role of the microbiome, probiotics, and ‘dysbiosis therapy’ in critical illness. Curr. Opin. Crit. Care 22, 347–353. doi: 10.1097/MCC.0000000000000321
Yang, C. M., Cao, G. T., Ferket, P. R., Liu, T. T., Zhou, L., Zhang, L., et al. (2012). Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 91, 2121–2129. doi: 10.3382/ps.2011-02131
Yang, X. J., Brisbin, J., Yu, H., Wang, Q., Yin, F. G., Zhang, Y. G., et al. (2014). Selected lactic acid-producing bacterial isolates with the capacity to reduce Salmonella translocation and virulence gene expression in chickens. PLoS ONE 9:e93022. doi: 10.1371/journal.pone.0093022
Yi, H., Zhang, L., Gan, Z., Xiong, H., Yu, C., Du, H., et al. (2016). High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 6, 25679. doi: 10.1038/srep25679
Yi, H. B., Hu, W. Y., Chen, S., Lu, Z. Q., and Wang, Y. Z. (2015). Cathelicidin-BF suppresses intestinal in?ammation by inhibiting the nuclear factor-kappa B signaling pathway and enhancing the phagocytosis of immune cells via STAT-1 in weanling piglets. Int. Immunopharmacol. 28, 61–69. doi: 10.1016/j.intimp.2015.05.034
Zhang, L., Zhang, L. L., Zhan, X. A., Zeng, X. F., Zhou, L., Cao, G. T., et al. (2016). Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 7, 107–115. doi: 10.1186/s40104-016-0061-4
Keywords: AA broilers, oral administration, S. enteritidis, C. butyricum, Q-PCR
Citation: Zhao X, Yang J, Wang L, Lin H and Sun S (2017) Protection Mechanism of Clostridium butyricum against Salmonella Enteritidis Infection in Broilers. Front. Microbiol. 8:1523. doi: 10.3389/fmicb.2017.01523
Received: 12 April 2017; Accepted: 28 July 2017;
Published: 09 August 2017.
Edited by:
Rebeca Martín, INRA Centre Jouy-en-Josas, FranceReviewed by:
Zhao Chen, Clemson University, United StatesKiiyukia Matthews Ciira, Mount Kenya University, Kenya
Alessandra De Cesare, Università di Bologna, Italy
Copyright © 2017 Zhao, Yang, Wang, Lin and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhong Sun, jqybfkyjs@163.com Hai Lin, hailin@sdau.edu.cn
 Xiaonan Zhao
Xiaonan Zhao Jie Yang
Jie Yang Shuhong Sun
Shuhong Sun