- 1Health Science Research Centre, Department of Life Sciences, Whitelands College, University of Roehampton, London, United Kingdom
- 2Department of the Science of Agriculture, Food and Environment, University of Foggia, Foggia, Italy
Recently, an innovative gluten detoxification method called Gluten FriendlyTM (GF) has been developed. It induces structural modifications, which abolish the antigenic capacity of gluten and reduce the in vitro immunogenicity of the most common epitopes involved in celiac disease, without compromising the nutritional and technological properties. This study investigated the in vitro effects of GF bread (GFB) on the fecal microbiota from healthy and celiac individuals by a three-stage continuous fermentative system, which simulates the colon (vessel 1, proximal colon; vessel 2, transverse colon; and vessel 3, distal colon), as well as on the production of short chain fatty acids (SCFA, acetate, propionate, butyrate). The system was fed with GFB and the changes in microbiota through fluorescence in situ hybridization and in SCFA content were assessed. GFB exerted beneficial modulations such as bifidogenic effects in each compartment of the model both with healthy- and celiac-derived samples, as well as growth in Clostridium clusters XIVa+b in celiac-derived samples. Furthermore, increased levels of acetic acid were found in vessel 1 inoculated with the fecal microbiota of healthy individuals, as well as acetic and propionic in vessel 1 and 2 with celiac-derived samples. In addition, the use of multivariate approaches showed that the supplementation of GFB could result in a different modulation of the fecal microbiota and SCFA, as a function of initial equilibrium.
Introduction
The composition and the metabolism of human microbiota play crucial roles in human health. Microbial colonization of the gastrointestinal tract varies widely, with the large intestine having not only the highest density of microbes in terms of bacterial cells per gram but also the most metabolically active organ (Turnbaugh et al., 2006). Genetics, mode of birth, infant feeding patterns, antibiotic usage, sanitary living conditions, and long-term dietary habits contribute to shaping the composition of the gut microbiome. Diet clearly has been considered as a major driver for changes in the compositional and functional relationship between microbiota and the host (Muegge et al., 2011). The fermentative but also (anaerobic) respiratory bacterial metabolism of dietary components produces an extraordinary chemical diversity in the large intestine with protective (e.g., short chain fatty acids [SCFAs]) or detrimental (e.g., hydrogen sulfite, phenol, p-cresol, or bile acids) effects on the disease development (Flint, 2012). There have been numerous attempts to identify a “core” microbiota, usually defined as bacterial taxa that are shared between 95% of individuals tested. Identification of a core microbiome is important for defining a “normal” healthy state from which major variations may indicate a dysbiotic system that can result from or contribute to disease development.
Recent reports have shown how the intestinal microbiota in celiac patients might be altered and influenced underlying mucosal immune response. Celiac disease (CD) is a chronic immune-mediated enteropathy triggered by the ingestion of gluten, the water-insoluble protein fraction in wheat, rye, and barley, in patients who are HLA-DQ2 or HLA-DQ8 positive. Approximately 30% of the general population carry the HLA-DQ2/8 CD susceptibility genes; however, only 2–5% of these individuals will go on to develop CD, suggesting that additional environmental factors contribute to disease development. Generally, gluten-free diet alleviates many of the symptoms, but somewhat surprisingly, it does not completely restore healthy microbiota profiles (Wacklin et al., 2014).
Recently, a new and innovative gluten detoxification method has been developed (PCT/IB2013/000797) (Lamacchia et al., 2013, 2015a). It is usually referred to as Gluten FriendlyTM (GF) and lies in the application of microwave energy for few seconds to hydrated wheat kernels. This technology induces structural modifications to endosperm components which abolish the antigenic capacity of gluten and dramatically reduce in vitro the immunogenicity of the most common epitopes involved in CD (Lamacchia et al., 2015b, 2016), without compromising both the nutritional and technological properties necessary to process flour into bread, pasta, and other baked goods. Recently, Landriscina et al. (2017) confirmed the effect of this new technology on the immunogenic epitopes. They combined microscopy with immunodetection with specific antibodies for gliadins, γ-gliadins, LMW subunits, and antigenic epitopes to gain a better understanding of the technology at a molecular level. Cross-reactivity toward the antibodies recognizing almost the entire range of gluten proteins as well as the antigenic epitopes through the sequences QQSF, QQSY, PEQPFPQGC, and QQPFP was significantly reduced.
This study aimed to investigate the impact of the administration of GF bread (GFB) on the fecal microbiota of healthy and celiac volunteers using a three-stage continuous culture colonic model system. This is a useful tool to monitor the ecology and metabolic activities of the microbiota in the proximal, transverse, and distal colon, in relation to different environmental conditions, dietary intervention, and the administration of drugs and antimicrobials. The influence of GFB on the fecal microflora and SCFA production [acetate, propionate (PRO), butyrate] was determined.
Materials and Methods
Substrate
Grain of treated wheat (according to the patented method PCT n. PCT/IB2013/000797) was milled commercially and kindly supplied by Casillo Group S.p.A1. The treatment was performed as follows: 100 g of cleaned wheat grains was dampened until reaching 15–18% humidity, which was measured by a halogen thermal balance (Mettler Toledo, HB43-S, Switzerland), and subjected to rapid heating via microwaves (Delonghi, Italy; approximately 1 min between 1000 and 750 watts), followed by slow evaporation of the water. The rapid heating and subsequent slow evaporation of the water was repeated until reaching a final temperature of 80–90°C, which was measured by a thermal camera (Fluke, i20 Model, Italy), and a moisture degree of 13–13.5% in the wheat grains.
After microwave treatment, the wheat kernels were cooled and dried at room temperature (24°C) for 12–24 h and then ground using an automatic laboratory mill MCKA (Bühler AG, Uzwil, Switzerland; diameter of grid 118–180 μm) (Bevilacqua et al., 2016).
Gluten Friendly bread was prepared according to the bread-making process (100 g wheat flour, 66 mL water, 1.33 g yeast, 1 g salt) to the Chorleywood Bread Process (Bevilacqua et al., 2016).
Simulated In Vitro Human Digestion
Prior to being added to the in vitro colonic system, GFB was digested in vitro, under appropriate conditions according to the procedures described by Maccaferri et al. (2012) to mimic mouth, stomach, and intestine’s condition.
Fecal Samples Collection and Inocula Preparation
Fecal samples were obtained from two healthy and two celiac donors (male and female aged 30–50 years old) who were free of any metabolic and gastrointestinal diseases, were not taking probiotic or prebiotic supplements, and had not taken antibiotics 6 months before fecal sample donation. All donors have provided written informed consent and filled in a standard questionnaire to collect information regarding health status, drug use, clinical anamnesis, and lifestyle factors. The University of Reading Research Ethics Committee (UREC 15/20) approved this study in accordance with the Declaration of Helsinki. Sample size was in accordance with previous studies (Bevilacqua et al., 2016). Fecal samples were placed in an anaerobic jar (AnaeroJarTM 2.5 L, Oxoid Ltd.) including a Gas-Generating Kit (AnaeroGenTM, Oxoid Ltd.) in order to reproduce anaerobic conditions inside the chamber. An aliquot of 20 g of samples was diluted in 100 mL anaerobic PBS (0.1 mol/L phosphate buffer solution, pH 7.4, w/w) and homogenized (Stomacher 400; Seward, West Sussex, UK) for 2 min at 240 paddle beats per minute. Samples were added to anaerobic fermenters within 15 min of voiding.
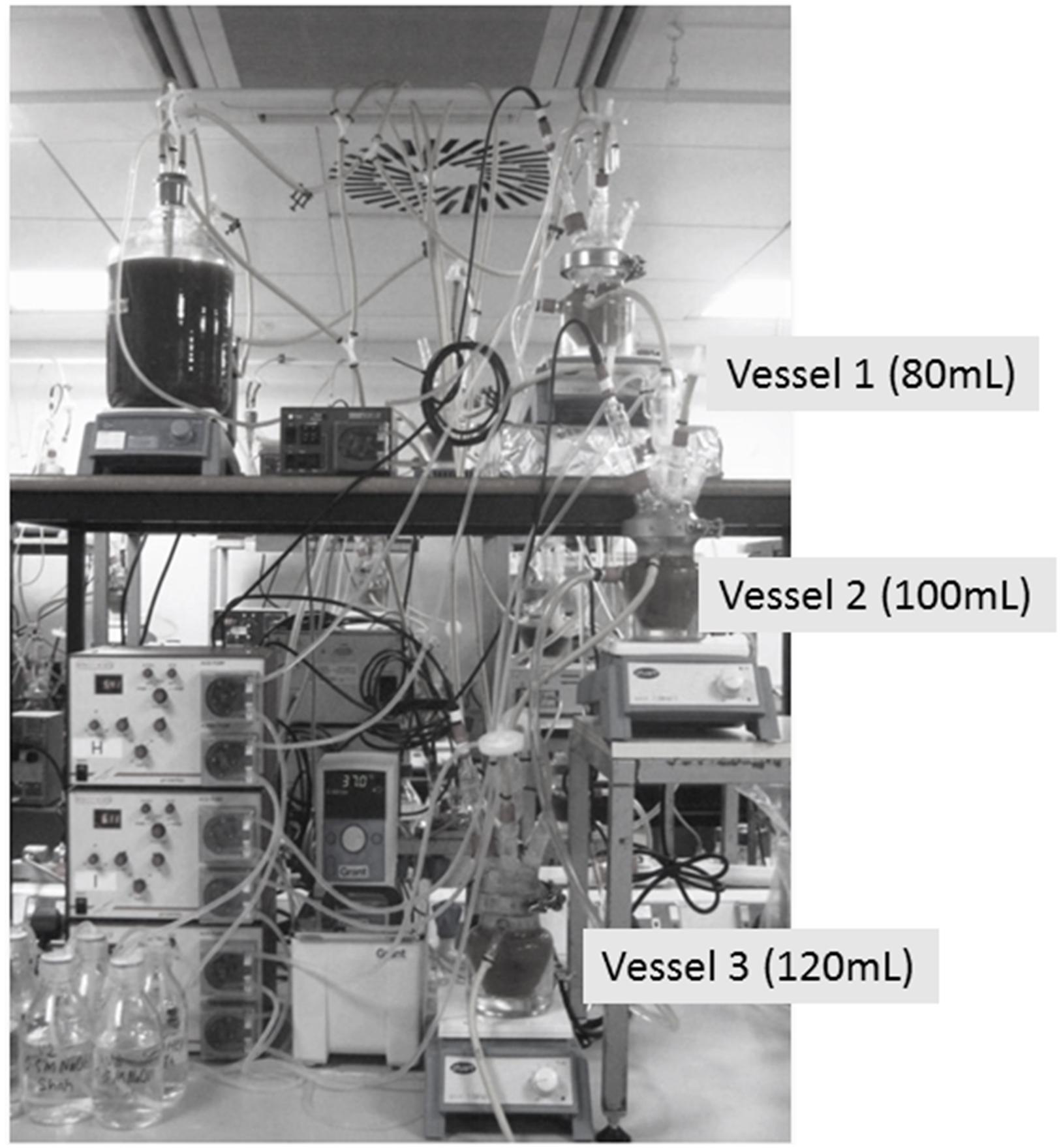
Three Stage-Continuous Culture Gut Model System
Physicochemical conditions in the colon were replicated in a three-stage continuous system, comprised of a cascade of three glass fermenters of increasing working volume connected in series. A small-scale version of the validated system by Macfarlane et al. (1998) has been used in this study, as suggested by Grimaldi et al. (2017). The gut model is often used to resemble the complexity and diversity of the intestinal microbiota and simulates the proximal [vessel 1 (V1), 80 mL, pH 5.5], transverse [vessel 2 (V2), 100 mL, pH 6.2], and distal colon [vessel 3 (V3), 120 mL, pH 6.8] (Figure 1); the vessels were filled with fecal homogenate from healthy and celiac volunteers (20%) and a complex colonic growth medium (80%). The growth medium contained the following ingredients: starch, 5 g/L; mucin, 4 g/L; casein, 3 g/L; peptone water, 5 g/L; tryptone water, 5 g/L; bile salts, 0.4 g/L; yeast exact, 4.5 g/L; FeSO4, 0.005 g/L; NaCl, 4.5 g/L; KCl, 4.5 g/L; KH2PO4, 0.5 g/L; MgSO4 × 7H2O, 1.25 g/L; CaCl2 × 6H2O, 0.15 g/L; NaHCO3, 1.5 g/L; Tween 80, 1 mL; hemin, 0.05 g/L; and cysteine HCl, 0.8 g/L. Following inoculation, the colonic model was run as a batch culture for 24 h to stabilize the bacterial populations prior to the initiation of medium flow. After 24 h (T0), the medium flow was initiated and the system was ran for eight full volume turnovers to allow for steady state to be achieved (SS1) assessed through stabilization of the SCFA profiles (±5%); the duration of each turnover was 48 h, thus this first step was done for 384 h. The flow rate of the system was 6.25 mL/h. The system was run for further eight volume turnovers upon which steady-state 2 (SS2) was achieved; daily 3.75 mL of digested GFB was added. Each steady state was confirmed by stabilization of SCFAs profiles over 3 consecutive days. SS2 was achieved after 384 h; the whole experiments took place for 792 h. Each vessel was magnetically stirred and continually sparged with oxygen free nitrogen gas. Temperature (37°C) was maintained by a water-cooling system and culture pH was controlled automatically through the addition of 1 N NaOH or HCl. Aliquots of 4.5 mL were removed at SS1 and SS2 from each vessel (V1, V2, and V3).
Short Chain Fatty Acids (SCFAs) Analysis by HPLC
The production of acetic, propionic, and butyric acids in the fermentations was determined by HPLC (Merck, NJ, United States) equipped with RI detection. The column used was an ion-exclusion REZEX-ROA organic acid column (Phenomenex Inc., United Kingdom) and temperature maintained at 84°C. Sulfuric acid in HPLC-grade H2O (0.0025 mmol/L) was used as the eluent, and the flow rate was maintained at 0.5 mL/min. Aliquots of 1 mL collected from each vessel in microcentrifuge tubes were centrifuged at 1136 × g for 10 min to remove all particulate matter. The supernatants were then filtered using 0.22 μm low protein binding Durapore polyvinylidene fluoride (PVDF) membranes (Millex; EMD Millipore, Billerica, MA, United States). Twenty microliters of each sample was injected with a run of 45 min. Peaks were integrated using the Atlas Lab Managing Software (Thermo Lab Systems, Mainz, Germany). Quantification of the samples was obtained through calibration curves of acetic, propionic, and butyric acids in concentrations ranging between 12.5 and 100 mM.
In Vitro Enumeration of Bacterial Populations by Fluorescence In Situ Hybridization (FISH) Analysis
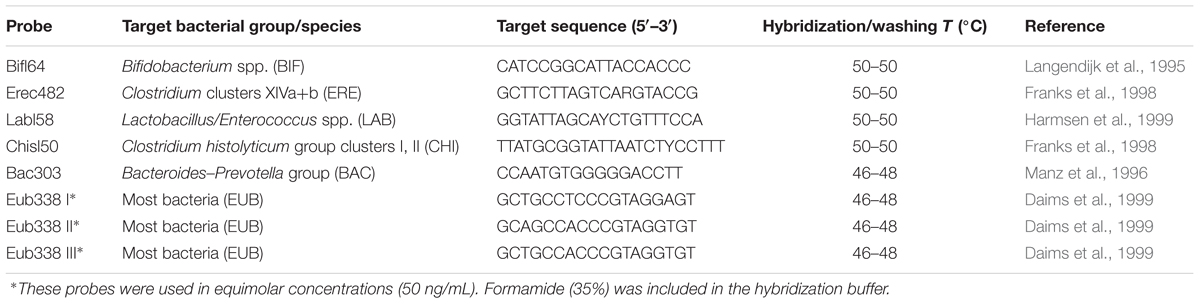
Fluorescence in situ hybridization analysis was performed as described by Costabile et al. (2014). Briefly, aliquots (375 μL) of gut model samples were fixed in three volumes of ice-cold 4% (w/v) paraformaldehyde for 4 h at 4°C. They were then centrifuged at 13,000 × g for 5 min and washed twice in 1 mL of sterile PBS. The cells were again pelleted by centrifugation and re-suspended in 150 μL of sterile PBS, to which 150 μL of ethanol was added. Samples were then vortexed and stored at -20°C until used in hybridizations. For the hybridizations, all probes were commercially synthesized and 5′-labeled with the fluorescent dye (Sigma-Aldrich, St. Louis, MO, United States). The probes used in this study are detailed in Table 1.
Statistical Analysis
The analyses were performed on two independent batches (two healthy donors and two celiac subjects); for each batch, the experiments were done in triplicate. Bacterial counts and SCFAs were statistically analyzed using paired t-tests to assess the effect of the same treatment at different time points. Significant differences were defined at p < 0.05. All analyses were performed using GraphPad Prism 6.0.
Furthermore, FISH and SCFAs data were standardized and reported as increase/decrease of SS2 (steady state at the end of the assay) relative to SS1 (steady state before the addition of GFB); each donor was treated as a separate “sample.”
First, a principal component analysis was run by using all SCFA and microbial groups as leading variables. The analysis was done following the traditional approach of PCA to qualitatively study the effect of the supplementation of GFB on each donor in each vessel.
Thereafter, the PCA was modified and run through the approach of the Multivariate Statistical Process Control/NIPALS algorithm (non-linear iterative partial least square); the number of interactions was set to 50. For this second analysis, the leading variables were either standardized SCFA or standardized microbial counts. The main outputs of this second approach are the standardized plots, showing the cases (i.e., each donor per each vessel), the line of the leading variables (the microbial groups for the first analysis and the SCFAs for the second analysis) and for each variable the minimum and maximum values. The benefit of this approach is that it allows the reader to point out both a qualitatively and a quantitatively grouping of cases. PCA and modified PCA were done through the software Statistica for Windows, ver. 12.0 (Statsoft, Tulsa, OK, United States).
Results
Bacterial Enumeration and SCFA
Changes in the bacterial composition are in Figure 2; these results focused on the global effect of GFB, without considering a possible variability linked to donors. A significant increase in numbers of bacteria within the Bifidobacterium genus was found; fecal microbiota of celiac donors showed a significant increase in this population from 8.42 to 8.90 log CFU/mL (p < 0.05) and from 8.60 to 9.20 log CFU/mL (p < 0.05) in V2 and V3, respectively, transverse and distal colon. In the fecal samples of healthy volunteers, there was a significant increase in numbers of bifidobacteria (BIF) in V3, from 7.90 to 8.40 log CFU/mL (p < 0.05). Significant increases were also found in numbers of bacteria in all vessels for celiac volunteers in Clostridium cluster population, from 8.85 to 9.50 (V1), from 9.1 to 9.60 (V2), and from 9.00 to 9.50 log CFU/mL (V3) (p < 0.05) (ERE).
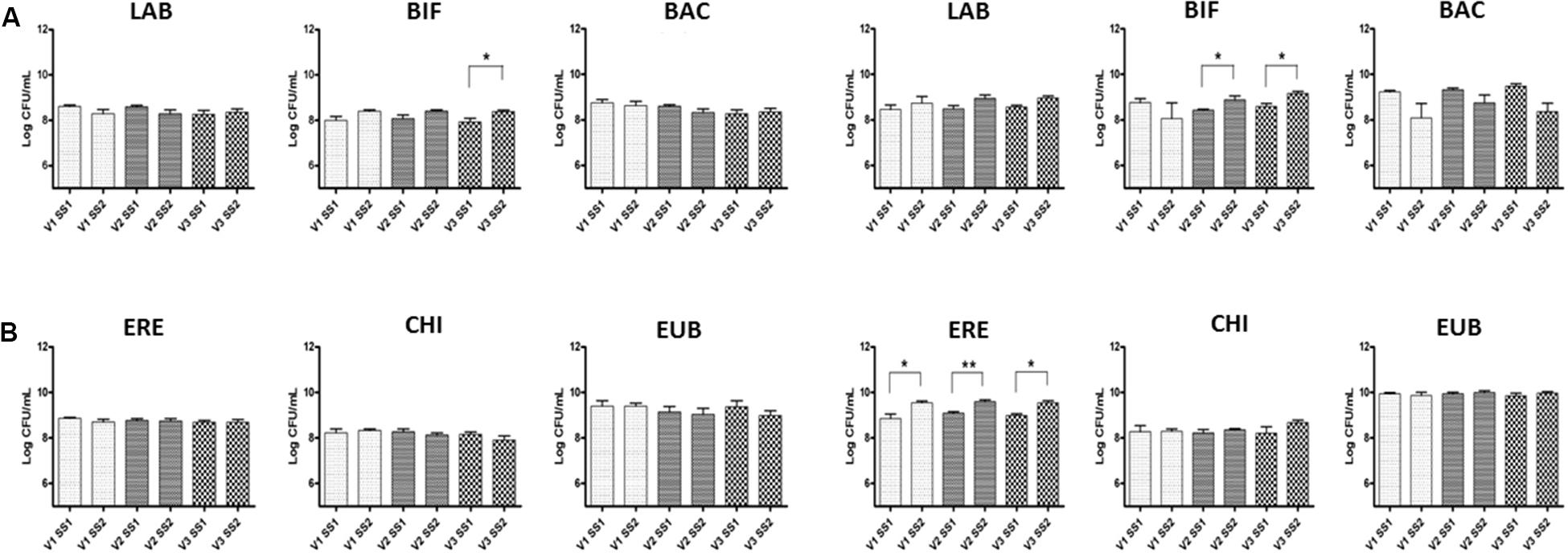
FIGURE 2. Bacterial groups in the culture broth recovered from the three different vessels (V1, V2, and V3) of the colonic model system before (SS1) and after (SS2) GFB supplementation. Inoculation with fecal slurries from healthy (A) and celiac donors (B). Results are reported as means of the data of two models ± SEM (n = 2). SS1, steady state 1; SS2, steady state 2; GFB, Gluten Friendly bread; LAB, Lactobacillus–Enterococcus (Lab158); BIF, bifidobacteria (Bif164); BAC, Bacteroides–Prevotella (Bac303); ERE, Clostridium clusters XIVa+b (Erec482); CHI, Clostridium group clusters I, II (Chis 150); and EUB, bacteria (Eub 338 I, 338 II, 338 III).
The results of SCFAs are in Figure 3. The fermentation of GFB by the fecal microbiota of healthy donors showed significant differences of acetic acid in all vessels, from 28.80 to 22.10 (V1) (p < 0.01), from 44.40 to 56.94 (V2) (p < 0.01), and from 46.00 to 76.50 mM (V3) (p < 0.001), respectively; butyrate also increased. Regarding the fecal microbiota of celiac volunteers, significant increases of propionic production were found in V1 and V2, from 45.10 to 69.20 (p < 0.01) and from 50.80 to 70.20 mM (p < 0.05), respectively; also, acetic acid showed a significant increase in V1, from 41.20 to 89.00 mM (p < 0.01).
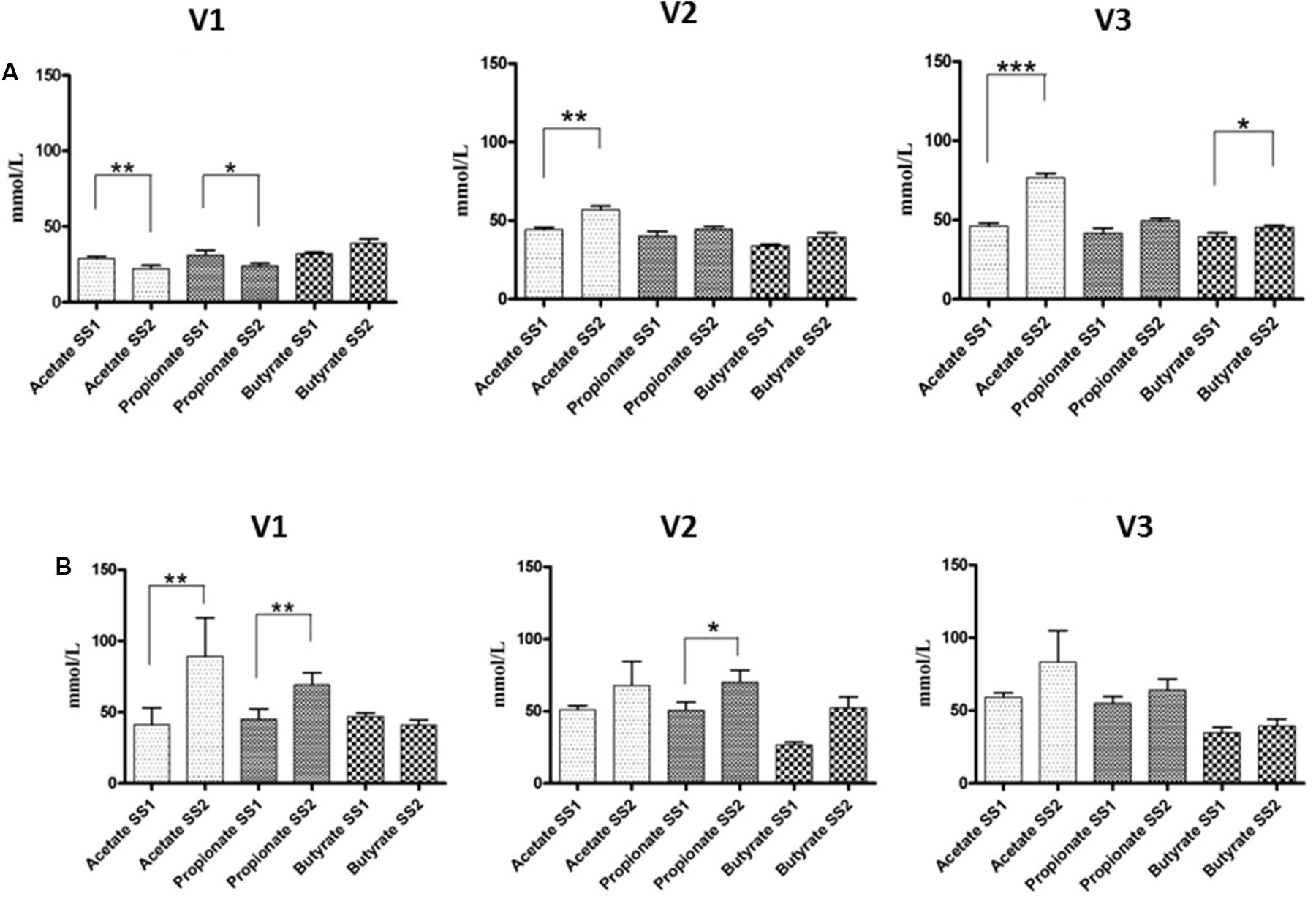
FIGURE 3. SCFA concentrations in the culture broths recovered from the three different vessels (V1, V2, and V3) of the colonic model system before (SS1) and after (SS2) GFB supplementation. Inoculation with fecal slurries from healthy (A) and celiac donors (B). Results are reported as means of the data of two models ± SEM (n = 2). SS1, steady state 1; SS2, steady state 2; GFB, Gluten Friendly bread.
Multivariate Approach
The last approach was a focus on the multivariate differences induced by the supplementation of GFB. The results were reported as increase/decrease in FISH, and SCFAs (difference SS2-SS1). Figure 4 shows the Principal Component Analysis on FISH and SCFA variations; all acids and microbial counts were used as leading variables. A difference from the previous approach (Figures 2, 3) was the use of each donor as a separate sample to highlight the differences due to the different fecal microbiota (effect of “donor”). Figure 4A shows the projection of the leading variables. Bifidobacteria (BIF) and Bacteroides/Prevotella (BAC) were mostly related to the factor 2 (the correlation coefficients were 0.918 and 0.874, respectively), whereas Lactobacillus/Enterococcus (LAB), Clostridium (EREC), and propionate (PRO) were related to the factor 1 (correlation coefficients ranging from 0.804 to 0.934). This first PCA suggests an effect of GFB on the whole ecosystem (fecal microbiota and produced acids); however, this effect could be also affected by the initial situation of the system, in this analysis expressed as “the donor.” GFB exerted a strong effect on the fecal microbiota of the celiac donor 2, as suggested by the shift from c2–v1 (acids and fecal microbiota of the celiac donor 2 in the first vessel) to c2–v2 and c2–v3. The increase of BIF played a major role for this increase. On the other hand, the same effect was not found in the vessels inoculated with the fecal microbiota of the celiac donor 1. A modification of the system was also found in the vessels inoculated with the fecal microbiota of healthy donor 1, as a slight shift toward right was found (from v1 to v2–v3).
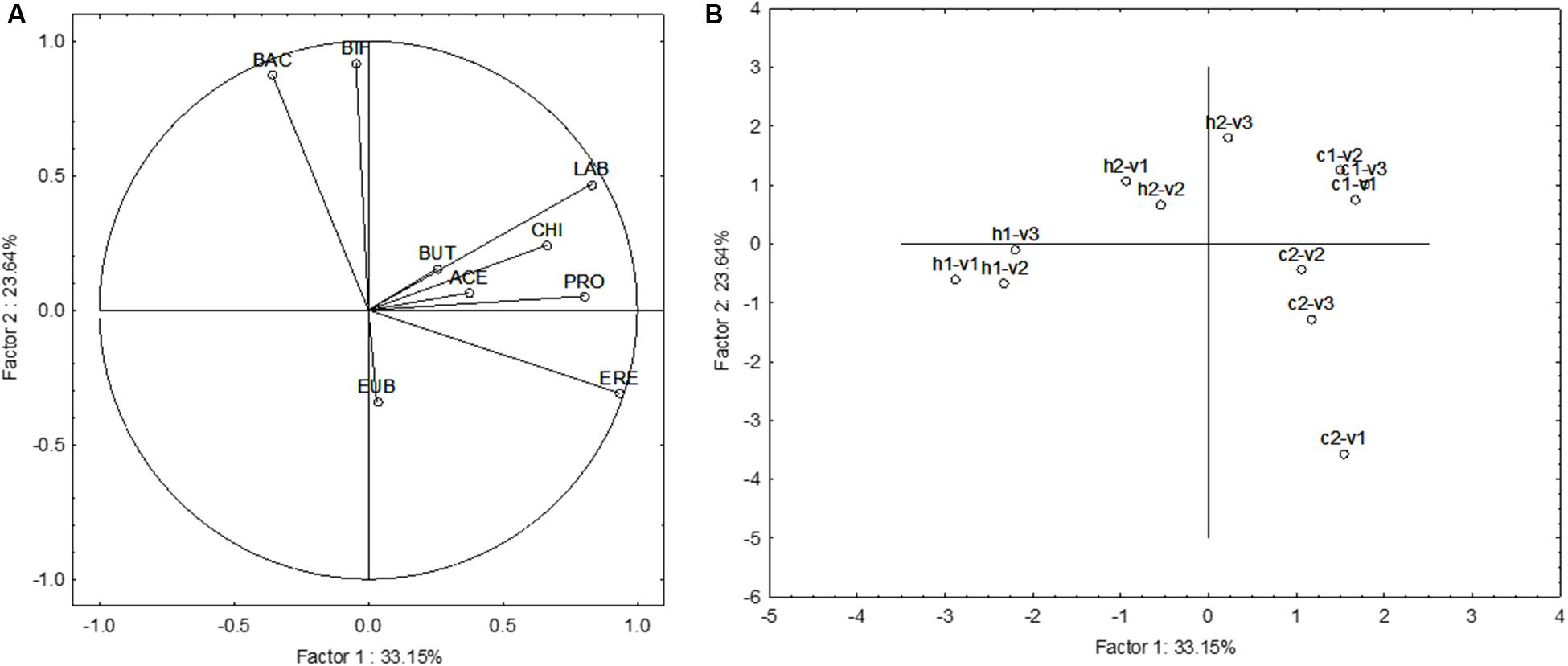
FIGURE 4. Principal Component Analysis on the increase of FISH counts and SCFA after the supplementation of GFB; the gut models were inoculated with the fecal slurries from healthy (letter H) and celiac donors (letter C). V1, V2, and V3, vessels of the colonic model and 1 and 2, donors 1 or donors 2; LAB, Lactobacillus–Enterococcus (Lab158); BIF, bifidobacteria (Bif164); BAC, Bacteroides–Prevotella (Bac303); ERE, Clostridium clusters XIVa+b (Erec482); CHI, Clostridium group clusters I, II (Chis 150); EUB, bacteria (Eub 338 I, 338 II, 338 III); BUT, butyrate; ACE, acetate; and PRO, propionate. (A) The projection of the variables, whereas (B) reports case projection.
This first approach suggests that GFB could induce a shift and a modification of the ecosystem; however, due to the high number of the leading variables (nine), the accounted variability explained by the statistic is quite low (ca. 57%). Therefore, a second approach was used by using either the data of FISH and on SCFA; in addition, PCA was modified with NIPALS algorithm to achieve quantitative data for each vessel and see what is the effect of the supplementation on the evolution from V1 to V2 and V3 and simulate what could happen from a region to another of the colon.
Figure 5A shows the results for the FISH. In the fecal microbiota of the healthy donor 1, the effect of the supplementation could be seen in the vessel 1, without differences from the vessels 2 and 3. On the other hand, the fecal microbiota of the healthy donor 2 experienced some differences, as from V1 to V2–V3 there was an effect on BIF, with a slight increase (ca. 0.4 log CFU/mL).
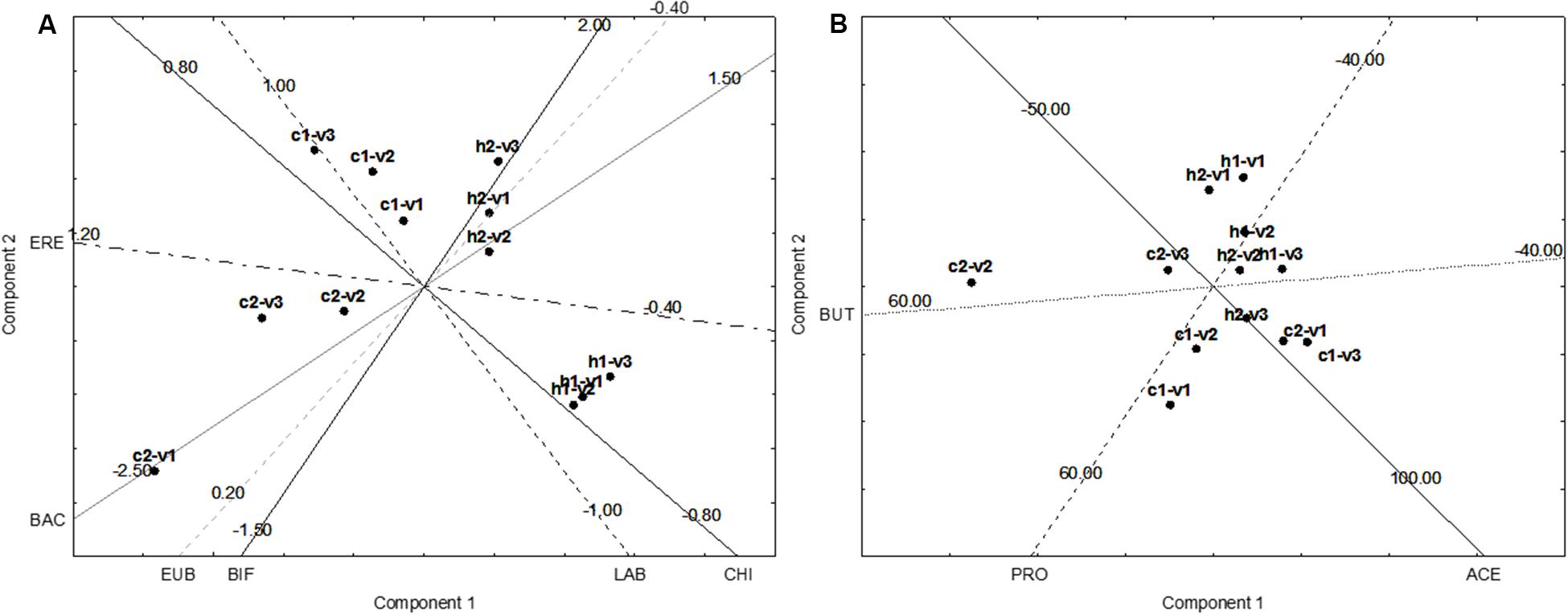
FIGURE 5. Principal Component Analysis on the increase of FISH counts and SCFA after the supplementation of GFB; the gut models were inoculated with the fecal slurries from healthy (letter H) and celiac donors (letter C). V1, V2, and V3, vessels of the colonic model; 1 and 2, donors 1 or donors 2. The numbers on the projection of the leading variables represent the quantitative data (minimum, central point, or maximum). (A) Effect on FISH counts. LAB, Lactobacillus–Enterococcus (Lab158); BIF, bifidobacteria (Bif164); BAC, Bacteroides–Prevotella (Bac303); ERE, Clostridium clusters XIVa+b (Erec482); CHI, Clostridium group clusters I, II (Chis 150); and EUB, bacteria (Eub 338 I, 338 II, 338 III). (B) Effect on SCFA. BUT, butyrate; ACE, acetate; PRO, propionate. (A) The projection of the variables, whereas (B) reports case projection.
The most important differences were found for the fecal microbiota of the celiac donors: in the donor 2, there was a shift from vessel 1 to vessels 2 and 3, probably due to BIF. In the vessel 1, there was a decrease of BIF (ca. 1 log CFU/mL), while in the vessels 2 and 3 BIF were restored with a mean decrease of 0.0–0.2 log CFU/mL.
The effect on the fecal microbiota of the celiac donor 1 was different. A slight effect on BIF was found in the vessel 1 and maintained in the vessels 2 and 3 (0.5–0.8 log CFU/mL), but a shift was found due to the different effect on Lactobacillus/Enterococcus and Clostridium cluster XIVa+b, as in the vessel 1 the increase was not significant (0.2 log CFU/mL), but in the vessel 3 the mean increase was 0.6–0.8 log CFU/mL. The difference in lactobacilli population was not significant when the mean between the two donors was done; on the other hand, a preliminary t-test revealed that on the donor 1 it was significant (p < 0.01).
Figure 5B shows the NIPALS approach for the SCFA; the main difference was in c2–v2 (vessel 2 inoculated with the fecal microbiota of the celiac donor 2). In this vessel, there was a strong increase of butyrate (ca. 45–50 mmol/L).
Discussion
The human colon contains a wide range of bacterial communities, distributed in hundreds of distinct species, and the balance among them plays an important role in health and disease (Holzapfel et al., 1998; Rigottier-Gois et al., 2003). Although a consensus for what constitutes a core gut microbiome has been elusive, one report suggested that an international cohort of 39 individuals could be assigned to one of three distinct clusters or “enterotypes” based on metagenomic sequences. Arumugam et al. (2011) have found that each cluster was dominated by a particular bacterial genus (Bacteroides, Prevotella, and Ruminococcus) with positive or negative associations with a number of other genera in the community. Many authors have found differences in the composition of the intestinal microbiota between CD patients and healthy volunteers (Collado et al., 2007; Sanz et al., 2007; Di Cagno et al., 2009; De Palma et al., 2010; Nistal et al., 2012; Sellitto et al., 2012). In fact, CD patients frequently present altered intestinal bacterial groups such as Bifidobacterium, Lactobacillus, Bacteroides, Prevotella, Staphylococcus, and Escherichia (Collado et al., 2007; Sanz et al., 2007; De Palma et al., 2010; Iebba et al., 2011; Sellitto et al., 2012). Mainly, a reduction in Bifidobacterium population diversity in CD patients was also found (Sanz et al., 2007; Collado et al., 2008), along with an increase of Bacteroides and Clostridium leptum groups (Collado et al., 2008).
Gluten exerts negative effects in terms of immunological response in CD; moreover, there are some evidences on possible changes induced on the colon microbiota. Bevilacqua et al. (2016) used the batch cultures approach to study the effect of the supplementation of either control or GFB on the microbiota of healthy and celiac volunteers. They found that the addition of control bread to the batches containing the microbiota of celiac volunteers determined a strong and significant decrease of BIF. On the other hand, the supplementation of GFB induced a partial restoration of BIF and lactobacilli, with a change of the whole system (microbiota+SCFA). These results were found within 48 h; thus, in the present study stool samples were collected from celiac and healthy volunteers, in order to better understand the differences in microbiota composition and metabolic activity induced by GFB after a prolonged supplementation and in a system able to simulate the whole colon.
Significant increases in bifidobacterial numbers were observed in response to GFB in the fecal microbiota of healthy and celiac donors. BIF are recognized as one of the most important bacterial groups associated with human health, providing beneficial effects in the large intestine (Gibson and Wang, 1994; Russel et al., 2011); the positive effect on bifidobacterial, as well as the slight effect on the lactic acid bacteria in the fecal microbiota of the celiac donors 1, confirmed previous results (Bevilacqua et al., 2016).
The positive effect of GFB on some bacteria could be attributed to the changes in the secondary and the tertiary structures of the proteins, as suggested by SDS page, the increase in cysteine amounts, and protein aggregation at SEM (Lamacchia et al., 2016; Landriscina et al., 2017). These changes could be responsible of the reduction of the cross-reactivity toward some antibodies (Landriscina et al., 2017), as well as on a different exposition and arrangement of charges, as postulated previously (Bevilacqua et al., 2016). This different arrangement of charges could be the primary factor leading to a positive effect on some Gram-positive bacteria, like BIF.
The GFB fermentation also increased Clostridium cluster XIVa. This cluster contains saccharolytic species which can produce large concentrations of beneficial SCFAs from sugars, such as butyrate. Clostridial clusters IV and XIVa have gained a lot of attention during the last years because of their contribution to gut homeostasis, by preserving gut barrier functions and exerting immunomodulatory and anti-inflammatory properties (Velasquez-Manoff, 2015). In addition, some species of the cluster, like Faecalibacterium prausnitzii, could produce anti-inflammatory peptides (Quévrain et al., 2016).
The SCFAs produced by gut microbiota in the colon have an important role. Butyric acid is often associated as an energy source for the epithelial cells and acetic acid plays an important role in controlling inflammation and controlling pathogen invasion (Russel et al., 2013). In addition, other effects attributed to butyrate are the improvement of the gut barrier function by stimulation of the formation of mucin, antimicrobial peptides, and tight-junction proteins, the interaction with the immune system and the reduction of the oxidative stress in the colon (Rivière et al., 2016). Therefore, the increase of butyrate found in the vessel 2 inoculated with the microbiota of a celiac donor could be of interest and deserves further efforts and experiments.
Moreover, the increase of acetic and propionic acids is interesting as they also act as anti-inflammatory compounds, could play a role in the stimulation of the immune system, and decrease the pH of the colon (indirect antimicrobial effect) (Rivière et al., 2016).
The multivariate approaches and the NIPALS algorithm also suggest that the effect of GFB could also rely on the initial equilibrium of the fecal microbiota and the positive effect could result in a different modulation of the microorganisms and SCFA; in the present study, in the fecal microbiota of a celiac donor a pronounced effect on BIF was found, while the fecal microbiota of the other celiac donor experienced a significant effect on Lactobacillus/Enterococcus.
In conclusion, this in vitro work provides encouraging findings supporting the utilization of GF products with a positive effect on the fecal microbiota and SCFAs from celiac donors. Generally, GFB induced increases in bifidobacterial counts and a positive modulation of SCFAs, with an increase of butyrate, acetate, and propionate. In addition, the use of multivariate approaches showed that the supplementation of GFB could result in a different modulation of the fecal microbiota and SCFA, as a function of initial equilibrium of the microbiota.
The experiments were done on fecal slurries from two healthy and two celiac donors, thus the results are as a kind of preliminary evidence and should be confirmed and validated on a larger number of samples and by an in vivo human intervention study. However, the most important achievement of this paper is a new horizon to be explored and studied: the possibility of the modulation of colon microbiota by GFB.
Author Contributions
AB, MC, MS, CL, and AC conceived this study; AC and CL provided valuable input for this study’s design and data analyses; TB-M, IG-S, and LL performed the fermentation analyses; MC, LP, and MS helped in the analysis; AB performed the statistical analyses; TB-M, AB, and AC wrote the paper; and all authors edited and approved the final manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 732640.
Disclaimer
This publication reflects only the author’s view and the Agency is notresponsible for any use that may be made of the information it contains.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LV and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Footnotes
References
Arumugam, M., Raes, J., Pelletier, E., Le Paslier, D., Yamada, T., Mende, D. R., et al. (2011). Enterotypes of the human gut microbiome. Nature 473, 174–180. doi: 10.1038/nature09944
Bevilacqua, A., Costabile, A., Bergillos-Meca, T., Gonzalez, I., Landriscina, L., Ciuffreda, E., et al. (2016). Impact of Gluten-friendlyTM bread on the metabolism and function of in vitro gut microbiota in healthy human and coeliac subjects. PLOS ONE 11:e0162770. doi: 10.1371/journal.pone.0162770
Collado, M. C., Calabuig, M., and Sanz, Y. (2007). Differences between the faecal microbiota of coeliac infants and healthy controls. Curr. Issues Intest. Microbiol. 8, 9–14.
Collado, M. C., Donat, E., Ribes-Koninckx, C., Calabuig, M., and Sanz, Y. (2008). Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 8:232. doi: 10.1186/1471-2180-8-232
Costabile, A., Santarelli, S., Claus, S. P., Sanderson, J., Hudspith, B. N., Brostoff, J., et al. (2014). Effect of breadmaking process on in vitro gut microbiota parameters in irritable bowel syndrome. PLOS ONE 9:e111225. doi: 10.1371/journal.pone.0111225
Daims, H., Brühl, A., Amann, R., Schleifer, K. H., and Wagner, M. (1999). The domain specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22, 434–444. doi: 10.1016/S0723-2020(99)80053-8
De Palma, G., Cinova, J., Stepankova, R., Tuckova, L., and Sanz, Y. (2010). Pivotal advance: bifidobacteria and gram-negative bacteria differentially influence immune responses in the proinflammatory milieu of celiac disease. J. Leukoc. Biol. 87, 765–778. doi: 10.1189/jlb.0709471
Di Cagno, R., Rizzello, C. G., Gagliardi, F., Ricciuti, P., Ndagijimana, M., Francavilla, R., et al. (2009). Different faecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl. Environ. Microbiol. 75, 3963–3971. doi: 10.1128/AEM.02793-08
Franks, A. H., Harmsen, H. J. M., Raangs, G. C., Jansen, G. J., Schut, F., and Welling, G. W. (1998). Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64, 3336–3345.
Flint, H. J. (2012). The impact of nutrition on the human microbiome. Nutr. Rev. 70(Suppl 1), S10–S13. doi: 10.1111/j.1753-4887.2012.00499.x
Gibson, G. R., and Wang, X. (1994). Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 77, 412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x
Grimaldi, R., Cela, D., Swann, J. R., Vulevic, J., Gibson, G. R., Tzortzis, G., et al. (2017). In vitro fermentation of β-GOS: impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol. Ecol. 93:fiw233. doi: 10.1093/femsec/fiw233
Harmsen, H. J. M., Elfferich, P., Schut, F., and Welling, G. W. (1999). A 16S rRNA targeted probe for detection of lactobacilli and enterococci in faecal samples by fluorescent in situ hybridization. Microb. Ecol. Health Dis. 9, 3–12.
Holzapfel, W. H., Haberer, P., Snel, J., Schillinger, U., and Huis in’t Veld, J. H. (1998). Overview of gut flora and probiotics. Int. J. Food Microbiol. 41, 85–101. doi: 10.1016/S0168-1605(98)00044-0
Iebba, V., Aloi, M., Civitelli, F., and Cucchiara, S. (2011). Gut microbiota and pediatric disease. Dig. Dis. 29, 531–539. doi: 10.1159/000332969
Lamacchia, C., Di Luccia, A., and Gianfrani, C. (2013). Method for the detoxification of gluten proteins from grains of cereals. Patent No WO 2014053891 A1.
Lamacchia, C., Di Luccia, A., and Gianfrani, C. (2015a). “Method for the detoxification of gluten proteins from grains of cereals,” in Proceedings of the BioVariaCongress, Europe’s Top Technologies, Munchen. doi: 10.4172/2157-7110.S1.017
Lamacchia, C., Di Luccia, A., and Gianfrani, C. (2015b). Metodo per la detossificazione delle proteine del glutine dalle granaglie dei cereali. Italian Patent No 0001414717.
Lamacchia, C., Landriscina, L., and D’Agnello, P. (2016). Changes in wheat kernels proteins induced by microwave treatment. Food Chem. 197, 634–640. doi: 10.1016/j.foodchem.2015.11.016
Landriscina, L., D’Agnello, P., Bevilacqua, A., Corbo, M. R., Sinigaglia, M., and Lamacchia, C. (2017). Impact of gluten-friendlyTM technology on wheat kernel endosperm and gluten protein structure in seeds by light and electron microscopy. Food Chem. 221, 1258–1268. doi: 10.1016/j.foodchem.2016.11.031
Langendijk, P. S., Schut, F., Jansen, G. J., Raangs, G. C., Kamphuis, G. R., Wilkinson, M. H., et al. (1995). Quantitative fluorescent in situ hybridisation of Bifidobacterium spp. with genus specific 16S rRNA targeted probes and its application in faecal samples. Appl. Environ. Microbiol. 61, 3069–3075.
Maccaferri, S., Klinder, A., Cacciatore, S., Chitarrari, R., Honda, H., Luchinat, C., et al. (2012). In vitro fermentation of potential prebiotic flours from natural sources: impact on the human colonic microbiota and metabolome. Mol. Nutr. Food Res. 56, 1342–1352. doi: 10.1002/mnfr.201200046
Macfarlane, G. T., Macfarlane, S., and Gibson, G. R. (1998). Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb. Ecol. 35, 180–187. doi: 10.1007/s002489900072
Manz, W., Amann, R., Ludwig, W., Vancanneyt, M., and Schleifer, K. H. (1996). Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology 142, 1097–1106. doi: 10.1099/13500872-142-5-1097
Muegge, B. D., Kuczynski, J., Knights, D., Clemente, J. C., González, A., Fontana, L., et al. (2011). Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332, 970–974. doi: 10.1126/science.1198719
Nistal, E., Caminero, A., Herràn, A. R., Arias, L., Vivas, S., de Morales, J. M., et al. (2012). Differences of small intestinal bacteria populations in adults and children with/without celiac disease: effect of age, gluten diet, and disease. Inflamm. Bowel Dis. 18, 649–656. doi: 10.1002/ibd.21830
Quévrain, E., Maubert, M. A., Michin, C., Chain, F., Marquant, R., Tailhades, J., et al. (2016). Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commercial bacterium deficient in Crohn’s disease. Gut 65, 415–425. doi: 10.1136/gutjnl-2014-307649
Rigottier-Gois, L., Rochet, V., Garrec, N., Suau, A., and Doré, J. (2003). Enumeration of Bacteroides species in human faeces by fluorescent in situ hybridisation combined with flow cytometry using 16S rRNA probes. Syst. Appl. Microbiol. 26, 110–118. doi: 10.1078/072320203322337399
Rivière, A., Selak, M., Lantin, D., Leroy, F., and De Vuyst, L. (2016). Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 7:979. doi: 10.3389/fmicb.2016.00979
Russel, W. R., Hoyles, L., Flint, H. J., and Dumas, M. (2013). Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 16, 246–254. doi: 10.1016/j.mib.2013.07.002
Russel, D. A., Rossa, R. P., Fitzgerald, G. F., and Stantona, C. (2011). Metabolic activities and propionic potential of bifidobacteria. Int. J. Food Microbiol. 149, 88–105. doi: 10.1016/j.ijfoodmicro.2011.06.003
Sanz, Y., Sánchez, E., Marzotto, M., Calabuig, M., Torriani, S., and Dellaglio, F. (2007). Differences in faecal bacterial communities in coeliac and healthy children as detected by PCR and denaturing gradient gel electrophoresis. FEMS Immunol. Med. Microbiol. 51, 562–568. doi: 10.1111/j.1574-695X.2007.00337.x
Sellitto, M., Bai, G., Serena, G., Fricke, W. F., Sturgeon, C., Gajer, P., et al. (2012). Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLOS ONE 7:e33387. doi: 10.1371/journal.pone.0033387
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., and Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. doi: 10.1038/nature05414
Velasquez-Manoff, M. (2015). Gut microbiome: the peacekeepers. Nature 518, S3–S11. doi: 10.1038/518S3a
Keywords: Gluten Friendly bread, fecal microbiota, celiac, healthy, gut model
Citation: Costabile A, Bergillos-Meca T, Landriscina L, Bevilacqua A, Gonzalez-Salvador I, Corbo MR, Petruzzi L, Sinigaglia M and Lamacchia C (2017) An In Vitro Fermentation Study on the Effects of Gluten FriendlyTM Bread on Microbiota and Short Chain Fatty Acids of Fecal Samples from Healthy and Celiac Subjects. Front. Microbiol. 8:1722. doi: 10.3389/fmicb.2017.01722
Received: 04 April 2017; Accepted: 24 August 2017;
Published: 07 September 2017.
Edited by:
Rosalba Lanciotti, Università di Bologna, ItalyCopyright © 2017 Costabile, Bergillos-Meca, Landriscina, Bevilacqua, Gonzalez-Salvador, Corbo, Petruzzi, Sinigaglia and Lamacchia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmela Lamacchia, carmela.lamacchia@unifg.it
 Adele Costabile
Adele Costabile Triana Bergillos-Meca1
Triana Bergillos-Meca1 Loretta Landriscina
Loretta Landriscina Antonio Bevilacqua
Antonio Bevilacqua Maria R. Corbo
Maria R. Corbo Leonardo Petruzzi
Leonardo Petruzzi