- 1Animal Microecology Institute, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China
- 2Chengdu Wildlife Institute, Chengdu Zoo, Chengdu, China
- 3Key Laboratory of Animal Disease and Human Health of Sichuan Province, Chengdu, China
In this work, we searched for an effective probiotic that can help control intestinal infection, particularly enterotoxigenic Escherichia coli K88 (ETEC) invasion, in giant panda (Ailuropoda melanoleuca). As a potential probiotic strain, Lactobacillus plantarum BSGP201683 (L. plantarum G83) was isolated from the feces of giant panda and proven beneficial in vitro. This study was aimed to evaluate the protective effect of L. plantarum G83 in mice challenged with ETEC. The mice were orally administered with 0.2 mL of PBS containing L. plantarum G83 at 0 colony-forming units (cfu) mL−1 (control; negative control, ETEC group), 5.0 × 108 cfu mL−1 (LDLP), 5.0 × 109 cfu mL−1 (MDLP), and 5.0 × 1010 cfu mL−1 (HDLP) for 14 consecutive days. At day 15, the mice (LDLP, MDLP, HDLP, and ETEC groups) were challenged with ETEC and assessed at 0, 24, and 144 h. Animal health status; chemical and biological intestinal barriers; and body weight were measured. Results showed that L. plantarum G83 supplementation protected the mouse gut mainly by attenuating inflammation and improving the gut microflora. Most indices significantly changed at 24 h after challenge compared to those at 0 and 144 h. All treatment groups showed inhibited plasma diamine oxidase activity and D-lactate concentration. Tight-junction protein expression was down-regulated, and interleukin (IL)-1β, IL-6, IL-8, TLR4, and MyD88 levels were up-regulated in the jejunum in the LDLP and MDLP groups. The number of the Enterobacteriaceae family and the heat-labile enterotoxin (LT) gene decreased (P < 0.05) in the colons in the LDLP and MDLP groups. All data indicated that L. plantarum G83 could attenuate acute intestinal inflammation caused by ETEC infection, and the low and intermediate doses were superior to the high dose. These findings suggested that L. plantarum G83 may serve as a protective probiotic for intestinal disease and merits further investigation.
Introduction
The giant panda is known as a rare, vulnerable species that is extremely popular worldwide. As a special herbivore, the giant panda has retained a typical carnivorous digestive system, which is easily afflicted by various intestinal diseases, especially ETEC infection which affects the intestinal barrier when the feed structure changes (Nataro and Kaper, 1998; Chen et al., 2015). Controlling this pathogen and its associated diarrhea heavily relies on antibiotics, of which their disadvantage could also threaten the animals. Probiotics are considered as one of the most effective alternatives to antibiotics because of their abilities to maintain or restore the normal microbiota, inhibit pathogen adhesion to intestinal walls, prevent inflammation, and protect intestinal barrier function (Geier et al., 2010; Archambaud et al., 2012; Yu et al., 2015).
Few reports are available on the role of probiotic strains in preventing ETEC invasion in giant panda. In a previous work, we isolated L. plantarum G83, a novel probiotic strain, from the feces of healthy captive giant panda. The microbe survived well at low pH, was tolerant to high bile-salt concentrations and resistant to antibiotics, and antagonized pathogenic bacteria in vitro (data not shown). For the next research step, the safety of this strain was assessed in vivo. Our laboratory also analyzed the intestinal microfloral structure of captive giant pandas at different age (Peng et al., 2016). The above-mentioned results served as the foundation for using the L. plantarum G83 as an antibiotic alternative and motivated the present study in mice. Similar to giant pandas, mice are omnivorous and easily infected by ETEC. Thus, mice are adopted as models for the study of the protective effects of L. plantarum G83 against ETEC invasion in giant panda.
Materials and Methods
Bacterial Strains and Preparation
L. plantarum G83 was grown in De Man, Rogosa, and Sharp broth at 37°C for 24 h. Meanwhile, the ETEC K88 strain (O8:H19:F4ac+, LT+, STa−, STb+) was obtained from the China Institute of Veterinary Drug Control (Beijing, China) and grown in Luria–Bertani broth containing 5% fetal bovine serum at 37°C for 12 h.
The L. plantarum G83 cells were harvested by centrifugation at 3,000 × g for 10 min at 4°C after growing in an anaerobic environment at 37°C for 24 h. The cell pellet was washed with sterile physiological saline twice. Finally, the cells were diluted to 5.0 × 108, 5.0 × 109, and 5.0 × 1010 colony-forming units (cfu) mL−1 in 200 μL of PBS for 14 consecutive days.
Meanwhile, the ETEC cells were harvested by centrifugation at 3,000 × g for 10 min at 4°C after being incubated at 37°C for 12 h with vigorous shaking. Next, the cell pellet was washed with sterile physiological saline twice. The cells were then diluted to 1 × 109 cfu mL−1 in PBS for the infectious challenge.
Animals and Experimental Design
Ninety male ICR mice (16 ± 2 g average weight) were purchased from Chengdu Dashuo Biological Institute (Chengdu, China) and fed with commercial chow. All mice were housed in a temperature- and humidity-controlled room with a 12 h light/dark cycle and allowed free access to food and water.
The animals were randomly divided into five groups of 18 mice each. Each group was given different treatments as follows: (1) oral administration of sterile PBS from day 1 to day 15 (CONT), (2) oral administration of sterile PBS from day 1 to day 14 followed by oral challenge with ETEC on day 15 (ETEC), (3) oral administration of low-dose L. plantarum G83 (5.0 × 108 cfu mL−1) from day 1 to day 14 followed by oral challenge with ETEC on day 15 (LDLP), (4) oral administration of intermediate-dose L. plantarum G83 (5.0 × 109 cfu mL−1) from day 1 to day 14 followed by oral challenge with ETEC on day 15 (MDLP), and (5) oral administration of high-dose L. plantarum G83 (5.0 × 1010 cfu mL−1) on day 1 to day 14 followed by oral challenge with ETEC on day 15 (HDLP). Simultaneously, individual body weights were recorded daily.
After blood sampling, the animals were killed by cervical dislocation. All animal experiments were performed in accordance with the guidelines for the care and use of laboratory animals approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (No. SYXKchuan 2014-187).
Blood Sample Analysis
At 0, 24, and 144 h after ETEC challenge, blood samples were collected from the orbital venous plexus. The leukocyte level and population distribution were detected through a PE-6800 VET Fully Auto-hematology Analyzer.
At 0, 24, and 144 h after ETEC challenge, the blood samples were centrifuged at 2,000 × g for 20 min at 4°C. The resultant sera were obtained and stored at −80°C. The levels of immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin G (IgG), D-lactate, and diamine oxidase (DAO) activity were then analyzed by using ELISA kits (Shanghai MLBIO Biotechnology Co. Ltd., China).
Relative Quantitative Real-Time PCR
Total RNA was extracted from liquid-nitrogen-frozen jejuna using RNAiso plus (TaKaRa, Dalian, China) in accordance with the manufacturer's instructions. Meanwhile, RNA integrity and purity were assessed using 1% agarose gel electrophoresis and Nano Drop spectrophotometry (Nano Drop Technologies, Wilmington, DE, USA), respectively. Then, 1 μg of the total RNA was reverse transcribed using the PrimeScript® RT reagent Kit with gDNAEraser (TaKaRa, China) in compliance with the manufacturer's protocol. The generated cDNA was stored at −80°C until real-time PCR analysis.
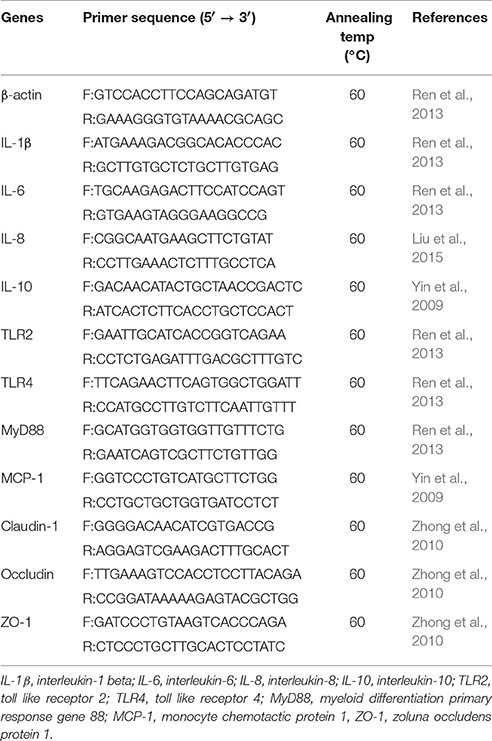
All the genes detected and specific primers used are listed in Table 1. Quantitative real-time PCR was performed using a CFX Connect™ Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA) with a SYBR Premix Ex Taq™ II PCR kit (TaKaRa, China). The PCR conditions were as follows: 95°C for 1 min, 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. Melting curve analyses were performed to monitor the purity of the PCR product. All reactions were run in triplicates. Relative gene expression levels were evaluated through the 2−ΔΔCt method, where ΔΔCt = (Ct, target  Ct, β−actin)treated group
Ct, β−actin)treated group  (Ct, target
(Ct, target  Ct, β−actin)control group.
Ct, β−actin)control group.
Real-Time PCR Quantification for Colon Microflora
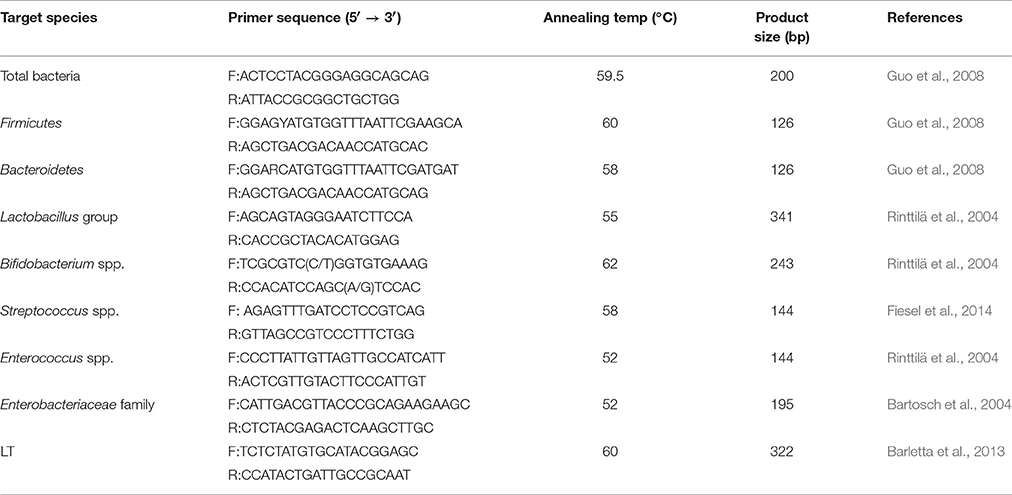
The sample of total DNA for colon microflora (n = 6, each group, randomly) was extracted by using the E.Z.N.A.® Stool DNA kit (Omega Biotechnology, USA). The DNA concentration was detected by a Nano Drop spectrophotometer (Nano Drop Technologies, Wilmington, DE, USA). The primers, annealing temperatures, and product sizes for the different bacterial and total bacterial quantifications were displayed in Table 2. The PCR conditions were as same as those stated under Relative Quantitative Real-time PCR but with different annealing temperatures. The same approach (Rinttilä et al., 2004) was also used to quantify the LT.
Statistical Analysis
All data were expressed as means and standard deviations. Statistical analysis was performed by one-way ANOVA in SPSS 19.0. Differences among treatments were compared using the Student-Newman-Keuls multiple comparison test. Statistical significance was set at P ≤ 0.05.
Results
Clinical Symptoms and Growth Performance
Before the ETEC challenge, no clinical sign was observed in the mice of any experimental group. After the ETEC challenge, only one mouse from the ETEC group presented with mild diarrhea, whereas most of the mice initially became dull and depressed and then lost weight. All groups showed no significant difference (P > 0.05) in body weight gain throughout the entire trial period (Figure 1). However, the mice given L. plantarum gained more body weight than those by others. After ETEC challenge, the ETEC, LDLP, MDLP, and HDLP groups presented similar weight changes (Figure 1).
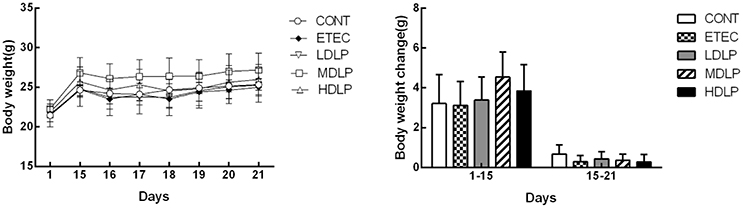
Figure 1. Dose effect of Lactobacillus plantarum on the body weight of mice during pre- and post-challenge periods (n = 6). CONT, control group, treatment with PBS at all period; ETEC, negative group, oral challenge with ETEC on day 15; LDLP, low dose (5.0 × 108 cfu mL−1) group; MDLP, intermediate dose (5.0 × 109 cfu mL−1) group; HDLP, high dose (5.0 × 1010 cfu mL−1) group; LDLP, MDLP, and HDLP groups were infected with ETEC at day 15. There was no significant (P > 0.05) in all groups.
Leukocyte Analysis
Figure 2 shows the changes in the number of leukocytes, lymphocytes, intermediate cells (including monocyte, eosinophils, and basophils), and granulocytes numbers in the five groups after ETEC challenge. At 24 h after ETEC challenge, the lymphocyte concentrations decreased (P < 0.05) and the granulocyte concentrations increased (P < 0.05) in the ETEC group and Lactobacillus-supplemented groups relative to those in the CONT group. No significant difference (P > 0.05) was noted among the ETEC group and the Lactobacillus-supplemented groups. At 0, 24, and 144 h after ETEC challenge, the levels of leukocyte, lymphocyte, intermediate cell, and granulocyte did not change significantly (P > 0.05) in all groups.
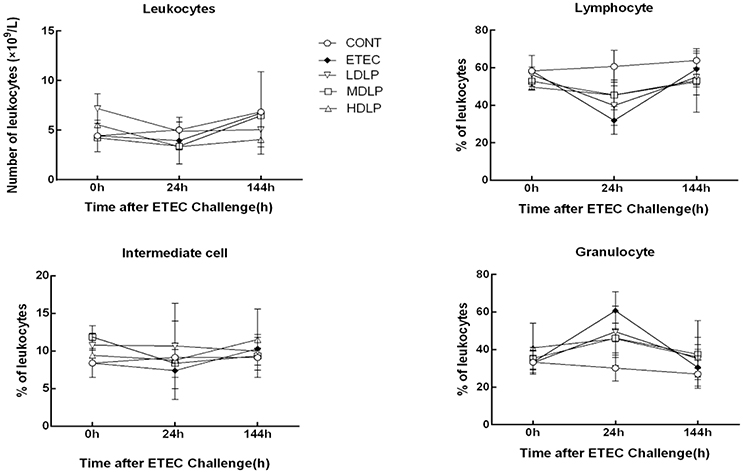
Figure 2. Dose effect of Lactobacillus plantarum on blood leukocyte count and population distribution of mice during pre- and post-challenge periods. CONT, control group, treatment with PBS at all period; ETEC, negative group, oral challenge with ETEC on day 15; LDLP, low dose (5.0 × 108 cfu mL−1) group; MDLP, intermediate dose (5.0 × 109 cfu mL−1) group; HDLP, high dose (5.0 × 1010 cfu mL−1) group; LDLP, MDLP, and HDLP groups were infected with ETEC at day 15. At 24 h after ETEC challenge, lymphocyte concentrations decreased (P < 0.05) and granulocyte concentrations increased (P < 0.05) in ETEC group and Lactobacillus-supplemented groups as compared to CONT group.
Serum DAO Activity and D-Lactate Concentration
The levels of DAO and D-lactate after ETEC challenge are shown in Figure 3. At 24 h after ETEC challenge, the DAO and D-lactate concentrations in the ETEC group were significantly higher (P < 0.05) than those in the CONT and MDLP groups but did not differ (P > 0.05) from those of the HDLP group. At 0 and 144 h after ETEC challenge, no difference (P > 0.05) in DAO and D-lactate concentrations was noted in all the groups.
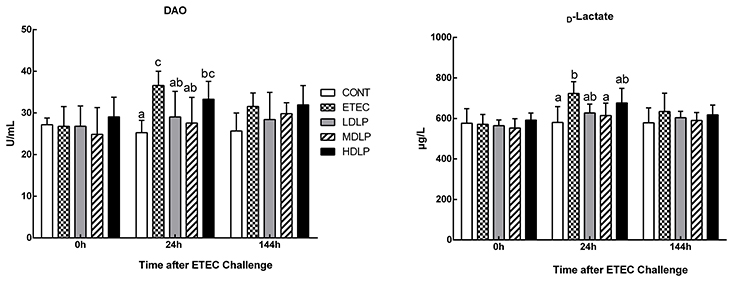
Figure 3. Dose effect of Lactobacillus plantarum on DAO activity and D-lactate concentration in serum of mice during pre- and post-challenge periods. CONT, control group, treatment with PBS at all period; ETEC, negative group, oral challenge with ETEC on day 15; LDLP, low dose (5.0 × 108 cfu mL−1) group; MDLP, intermediate dose (5.0 × 109 cfu mL−1) group; HDLP, high dose (5.0 × 1010 cfu mL−1) group; LDLP, MDLP, and HDLP groups were infected with ETEC at day 15. Bars with different letters are significantly different (P < 0.05). Bars share the same letters do not differ significantly (P > 0.05).
Effects of L. plantarum on the Serum Concentrations of IgG, IgA, and IgM
Before challenge, the serum IgA levels in the LDLP and MDLP groups significantly increased (P < 0.05) with respect to that in the CONT group. Meanwhile, the IgM concentrations in the MDLP and HDLP groups were higher (P < 0.05) than that in the CONT. However, only the IgG level in HDLP increased (P < 0.05) with respect to that in the CONT. At 24 h after ETEC challenge, the IgA and IgM contents in the LDLP, MDLP, and HDLP groups were higher (P < 0.05) than those in the CONT. At 144 h post-challenge, no significant difference in IgA, IgM, or IgG level (P > 0.05) was observed among the groups (Figure 4).
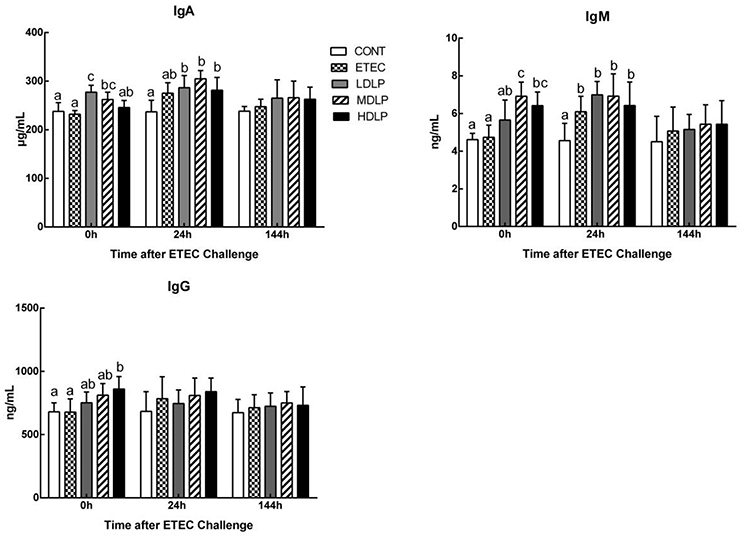
Figure 4. Dose effect of Lactobacillus plantarum on concentrations of IgA, IgM and IgG in serum of mice during pre- and post-challenge periods. CONT, control group, treatment with PBS at all period; ETEC, negative group, oral challenge with ETEC on day 15; LDLP, low dose (5.0 × 108 cfu mL−1) group; MDLP, intermediate dose (5.0 × 109 cfu mL−1) group; HDLP, high dose (5.0 × 1010 cfu mL−1) group; LDLP, MDLP, and HDLP groups were infected with ETEC at day 15. Bars with different letters are significantly different (P < 0.05). Bars share the same letters do not differ significantly (P > 0.05).
Effect of L. plantarum on the Expression of Tight Junction (TJ)-Associated Proteins in the Jejunum
Before challenge, the MDLP group exhibited an up-regulated expression (P < 0.05) of occludin and zonula occluden 1 (ZO-1), whereas the LDLP group showed only an increased expression (P < 0.05) of occludin, with respect to those in the CONT group. At 24 h after ETEC challenge, the expression of all the TJ-associated proteins was down-regulated, and the ETEC group achieved lower claudin-1 and occludin levels than those in the CONT group (P < 0.05). However, the difference among the CONT, LDLP, and MDLP groups was not significant (P > 0.05). At 144 h after challenge, the expression of claudin-1 and occludin in all groups was not significant (P > 0.05). The expression of ZO-1 in ETEC and HDLP groups was down-regulated (P < 0.05) compared to CONT group (Figure 5).
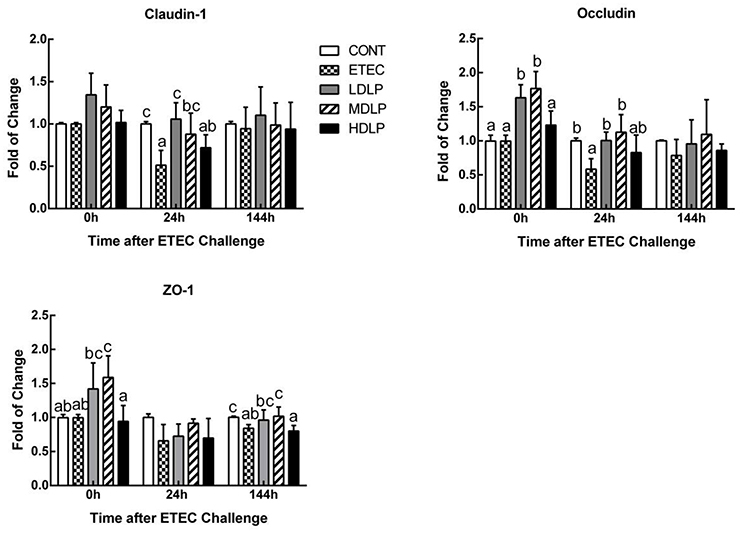
Figure 5. Dose effect of Lactobacillus plantarum on tight junction proteins mRNA expression in jejunum of mice during pre- and post-challenge periods. CONT, control group, treatment with PBS at all period; ETEC, negative group, oral challenge with ETEC on day 15; LDLP, low dose (5.0 × 108 cfu mL−1) group; MDLP, intermediate dose (5.0 × 109 cfu mL−1) group; HDLP, high dose (5.0 × 1010 cfu mL−1) group; LDLP, MDLP, and HDLP groups were infected with ETEC at day 15. Bars with different letters are significantly different (P < 0.05). Bars share the same letters do not differ significantly (P > 0.05).
Effect of L. plantarum on the Expression of Cytokines and Toll-Like Receptors (TLRs) in the Jejunum
Interleukin (IL)-1β and IL-8 levels in the HDLP group obviously rose (P < 0.05) relative to those of the CONT group at 0 h after ETEC challenge. At 24 h after ETEC challenge, the IL-1β, IL-8, IL-6, TLR4, and MyD88 levels in the ETEC group were significantly higher (P < 0.05) than those of the CONT, LDLP, and MDLP groups. Meanwhile, the IL-8 contents did not significantly change (P > 0.05) between the ETEC and HDLP groups. At 144 h, the IL-1β expression in the ETEC group was higher (P < 0.05) than those of the other groups (Figure 6).
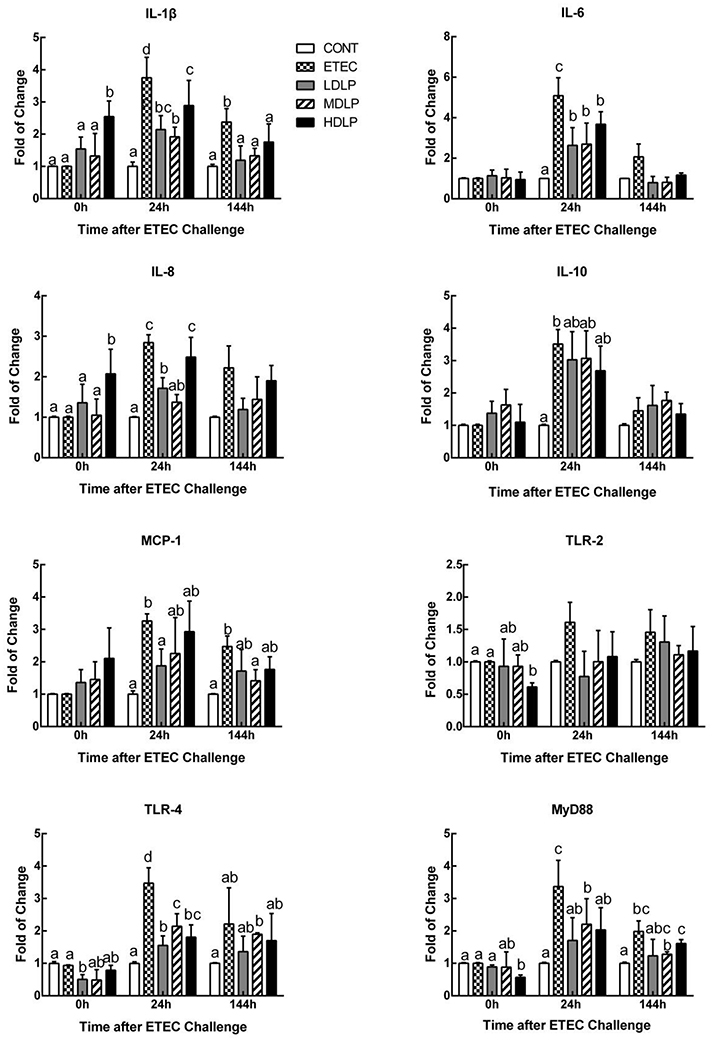
Figure 6. Dose effect of Lactobacillus plantarum on cytokines and Toll-like receptor mRNA expression in jejunum of mice during pre- and post-challenge periods. CONT, control group, treatment with PBS at all period; ETEC, negative group, oral challenge with ETEC on day 15; LDLP, low dose (5.0 × 108 cfu mL−1) group; MDLP, intermediate dose (5.0 × 109 cfu mL−1) group; HDLP, high dose (5.0 × 1010 cfu mL−1) group; LDLP, MDLP, and HDLP groups were infected with ETEC at day 15. Bars with different letters are significantly different (P < 0.05). Bars share the same letters do not differ significantly (P > 0.05).
Microbial Analysis in the Colon
At 0 h, the Lactobacillus concentration of the HDLP group and the Bifidobacterium spp. content of the MDLP group was significantly higher (P < 0.05) compared to the CONT group. Meanwhile, the Bacteroidetes level in the LDLP, MDLP, and HDLP group and the Enterobacteriaceae level in the MDLP decreased (P < 0.05). At 24 h, the Enterobacteriaceae concentration in the ETEC group grew (P < 0.05) relative to those of the other groups. At 144 h, all bacterial concentrations apart from Bacteroidetes and Lactobacillus did not considerably vary among the groups (Figure 7).
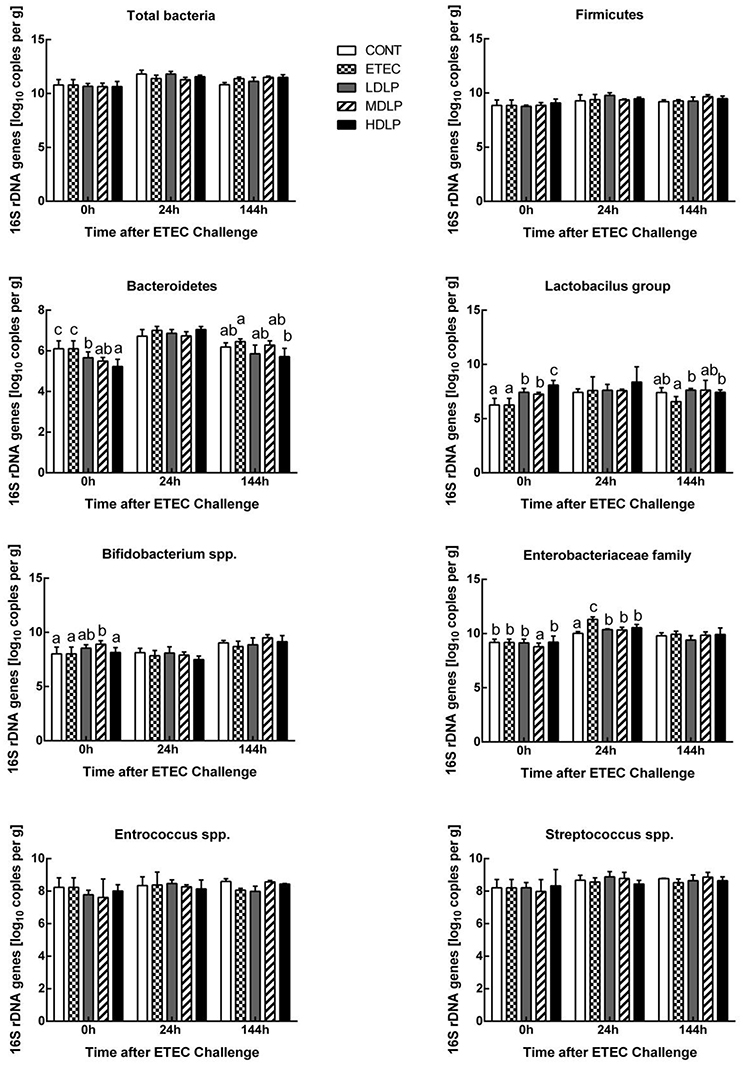
Figure 7. Dose effect of Lactobacillus plantarum on composition of the mice colonic microbiota during pre- and post-challenge periods. CONT, control group, treatment with PBS at all period; ETEC, negative group, oral challenge with ETEC on day 15; LDLP, low dose (5.0 × 108 cfu mL−1) group; MDLP, intermediate dose (5.0 × 109 cfu mL−1) group; HDLP, high dose (5.0 × 1010 cfu mL−1) group; LDLP, MDLP, and HDLP groups were infected with ETEC at day 15. Bars with different letters are significantly different (P < 0.05). Bars share the same letters do not differ significantly (P > 0.05).
Effect of L. plantarum on the LT Gene Levels in the Colon
The LT levels in the colon of the ETEC group were significantly higher (P < 0.05) than those of the other groups. Moreover, LT was not detected at 0 and 144 h after challenge (Figure 8).
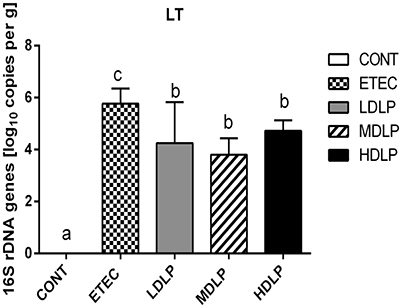
Figure 8. Amount of LT gene in colon of mice at 24 h after ETEC challenge. CONT, control group, treatment with PBS at all period; ETEC, negative group, oral challenge with ETEC on day 15; LDLP, low dose (5.0 × 108 cfu mL−1) group; MDLP, intermediate dose (5.0 × 109 cfu mL−1) group; HDLP, high dose (5.0 × 1010 cfu mL−1) group; LDLP, MDLP, and HDLP groups were infected with ETEC at day 15. Bars with different letters are significantly different (P < 0.05). Bars share the same letters do not differ significantly (P > 0.05).
Discussion
Severe gut disease can damage host health. However, in giant panda, this kind of disease must be prevented and treated. The relationship between probiotics and improved gut health has received considerable scientific interest for more than a century. To protect animal guts, scientists have shown that probiotics, such as Lactobacillus, Bifidobacterium, and Bacillus, prevent gut bacterial disease (Barba-Vidal et al., 2017; Nishida et al., 2017; Wu et al., 2017). In the present study, L. plantarum G83 was isolated from the feces of healthy giant panda and confirmed beneficial in vitro. This current work aimed to evaluate the protective effect of L. plantarum G83 in mice challenged with ETEC. To accomplish this objective, we assessed mice for their weights, as well as chemical and biological intestinal barriers.
In the present study, the weights and gain ratios in the ETEC group were certainly lower than those of the other groups. However, the growth performance did not significantly change in the mice supplemented with L. plantarum G83 either before or after ETEC infection. This result was similar to those of previous works (Nguyen et al., 2007; Wang et al., 2009, 2016) but can otherwise be explained by the shortage of weight gain evaluation period for weight gain. After ETEC infection, the mice only showed weight loss and slow growth rather than the clear clinical signs of diarrhea and death. Such presentation may be due to the differences in sensitivity of the model animal and in virulence of the strain (Duchet-Suchaux et al., 1990; Porter et al., 2011). These results revealed that the strain did not obviously affect the gain weight within a short period.
Maintaining the intestinal epithelial barrier integrity is important for the defense against pathogen invasion and inflammation (Fasano and Shea-Donohue, 2005). In this regard, plasma DAO and D-lactate have been proposed as circulating markers for the extent of damage and repair of the intestinal mucosa (Wijtten et al., 2011; Zhao et al., 2014). These markers can reflect the intestinal permeability and barrier function in peripheral blood (Ewaschuk et al., 2005). Furthermore, TJs, such as claudin-1, occludin and ZO-1, have been proven to seal the lateral intercellular space and achieve an intact layer of epithelial cells that guard against pathogens (Groschwitz and Hogan, 2009; Suzuki, 2013). In the present study, the intermediate- and low-dose groups notably inhibited the increase in plasma DAO and D-lactate levels after ETEC infection. ETEC invasion has been reported to increase the release of DAO and D-lactate in the plasma and damage the intestinal epithelial cell membrane (Roselli et al., 2007; Yang et al., 2014; Xun et al., 2015). Hence, our results indicated that the strain exerts a protective effect on the host during ETEC invasion. Different doses of L. plantarum G83 prevented ETEC-induced membrane damage by inhibiting the delocalization of ZO-1 and consequently raising occludin and claudin-1 amounts. This effect is consistent with that observed by Roselli et al. (2007), who found higher occludin and ZO-1 mRNA expression in piglets fed with Lactobacillus sobrius than in those not fed with the strain. In the former piglets, ETEC-induced membrane damage was prevented by inhibiting the rearrangement of F-actin and dephosphorylation of occludin. As Mennigen and Bruewer (2009) reported, there is a close link between probiotics and regulation of intestinal permeability and barrier function. The above-mentioned findings collectively indicated that L. plantarum G83 exerts a protective effect against ETEC-induced membrane damage.
During infection, innate or acquired immunity is activated. Immunocytes then play a key role in the innate and adaptive immune responses. Immunocyte number and variation reflect the immunity or infection status (Demissie et al., 2013; Hunt et al., 2015). Furthermore, the immunocyte induction of cytokine and antibody production participates in immune regulation and the inflammatory response (Baehner, 1975; Cassatella, 1999). One signaling pathway that promotes the expression of pro-inflammatory genes is the TLR- and MyD88-dependent pathways (Zughaier et al., 2005). In the current study, the L. plantarum G83 strain did not significantly alter the leukocyte amount and variation in the mouse peripheral blood among the groups at different time points. Besides the granulocyte, the amount of leukocytes, lymphocytes, and intermediate cells decreased at 24 h after ETEC inoculation. This effect may have been caused by the high expression of IL-8 and MCP-1, which take part in the immunocytes migration to the site of infection in the intestine. Such results were similar to those of previous reports (Zhu et al., 2014). Lactobacillus supplementation enhanced serum IgG, IgM, and IgA levels and indicated that probiotics could stimulate systemic or mucosal antibody response (Frece et al., 2006; Mizumachi et al., 2009). In the present study, the L. plantarum G83 obviously increased the IgA, IgM, and IgG levels before or after ETEC challenge. IgA has an important role in the protection of mucosal surfaces against pathogens (Galdeano and Perdigon, 2004). The secretory IgA of intestinal mucosa will be detected in next study. Low and intermediate doses of the strain also decreased the mRNA levels of IL-1β, −6, and −8 in the jejunum, probably by modifying the immunocyte profile. Early studies confirmed that lactobacilli lower serum IL-6 concentrations after acute ETEC challenge in piglets (Zhang et al., 2010). The aforementioned observations suggested that inflammation was ameliorated in the LDLP and MDLP groups and were consistent with those of previous studies where probiotics exerted dose-dependent effects (Mileti et al., 2009). Lee et al. (2012) found that high-dose L. plantarum CJLP243 (108–1010 cfu kg−1) most substantially improved the growth and health performance of weaning pigs, especially those with bacterium-induced acute bowel inflammation. A recent trial of piglets challenged with F4+ ETEC demonstrated that administering LGG (1012 cfu day−1) failed to prevent F4+ ETEC infection. This failure was probably due to the disruption of microbial and inflammatory responses by excessive probiotic levels (Li et al., 2012). Same probiotic in different doses have different modulation effect on T-cell immune response and mucosal IgA response (Wen et al., 2012, 2015). Thus, only intermediate- and low-dose L. plantarum G83 positively affects acute inflammatory bowel disease.
Besides promoting nutritional digestion, intestinal microflora is also involved in host immunity and pathology. Bacterial infections or enteritis, antibiotic treatment, and immunosuppression may alter the population, quantity, or habitat of gut microflora and may lead to the excessive growth of opportunistic pathogens as well as microbial dysbiosis (Bouhnik et al., 2004; Taur and Pamer, 2013). Numerous studies showed that probiotics can regulate and maintain the balance of intestinal flora by enhancing indigenous bacterial colonization and/or competitive exclusion to battle pathogens (Gareau et al., 2010). The present results revealed that administering L. plantarum G83 not only increased the abundance of Lactobacillus and Bifidobacterium, but also decreased the number of Bacteroidetes and Enterobacteriaceae in the colon. Our results were also similar to those of the reports where LGG and Lactobacillus Bar13 greatly reduced the colonization of pathogenic E. coli and Salmonella (Collado et al., 2007; Candela et al., 2008). Probiotics secrete some bacteriocin that can kill or inhibit pathogens (Nakamura et al., 2013; Pandey et al., 2013). Furthermore, Lactobacillus and Bifidobacterium can also create a biomembrane on intestinal mucosa to serve as a barrier against colonizing pathogen and improve gut immunity (Collado et al., 2008; Jones and Versalovic, 2009; Chen et al., 2014). Probiotics can also compete with harmful bacteria for nutrition (Elli et al., 2000; Hooper, 2009). Overall, our results demonstrated that L. plantarum G83 is beneficial to host health.
Conclusion
The probiotic L. plantarum G83 had a positive effect against pathogens mainly by chemical barrier and biological barrier, not only increasing the abundance of Lactobacillus and Bifidobacterium, but modulating immune response. In addition, different dose of L. plantarum G83 had various effects in modulating immune response. The results of this research may suggest that there is an opportunity to develop a new probiotic product for use in giant panda. And more research is needed for fully guarantee the safety and efficacy of this strain.
Author Contributions
Designed the experiments: QL, XN, and ZP. Conceived and supervised the study: XN, QW, ZP, and LN. Performed the experiments: QL, YZ, and HS. Data analyzed by: QL, ZP, KP, and BJ. Wrote the manuscript: QL, ZP, and DZ. Proofread the manuscript: DZ and HW. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The present study was supported by National Natural Science Foundation of China (No. 31672318) and the Funded Project of Chengdu Giant Panda Breeding Research Foundation (No. CPF2014-15 and CPF2015-06).
References
Archambaud, C., Nahori, M. A., Soubigou, G., Bécavin, C., Laval, L., Lechat, P., et al. (2012). Impact of lactobacilli on orally acquired listeriosis. Proc. Natl. Acad. Sci. U.S.A. 109, 16684–16689. doi: 10.1073/pnas.1212809109
Baehner, R. L. (1975). Microbe ingestion and killing by neutrophils: normal mechanisms and abnormalities. Clin. Haematol. 4, 609–633.
Barba-Vidal, E., Castillejos, L., López-Colom, P., Rivero Urgell, M., Moreno Mu-oz, J. A., and Martín-Orúe, S. M. (2017). Evaluation of the probiotic strain Bifidobacterium longum subsp. Infantis CECT 7210 capacities to improve health status and fight digestive pathogens in a piglet model. Front. Microbiol. 8:533. doi: 10.3389/fmicb.2017.00533
Barletta, F., Ochoa, T. J., and Cleary, T. G. (2013). Multiplex Real-Time PCR (MRT-PCR) for Diarrheagenic. Methods Mol. Biol. 943, 307–314. doi: 10.1007/978-1-60327-353-4_21
Bartosch, S., Fite, A., Macfarlane, G. T., and McMurdo, M. E. (2004). Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 70, 3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004
Bouhnik, Y., Raskine, L., Simoneau, G., Vicaut, E., Neut, C., Flourié, B., et al. (2004). The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am. J. Clin. Nutr. 80:1658.
Candela, M., Perna, F., Carnevali, P., Vitali, B., Ciati, R., Gionchetti, P., et al. (2008). Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 125, 286–292. doi: 10.1016/j.ijfoodmicro.2008.04.012
Cassatella, M. A. (1999). Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73, 369–509. doi: 10.1016/S.0065-2776(08)60791-9
Chen, X. I., Yin, M., Wang, X., Pu, Z., Ma, Y., and Chen, Z. (2015). Isolation and preliminary identification of intestinal pathogens of captive giant panda (In Chinese). J. Mianyang Normal Univ. (陈希文, 尹苗, 王雄清, 蒲中慧, 马缨, and 陈紫娟 (2015). 圈养大熊猫肠道致病菌的分离与初步鉴定.绵阳师范学 院学报, 1–7). doi: 10.3969/j.issn.1672-612x.2015.02.001
Chen, X. Y., Woodward, A., Zijlstra, R. T., and Gänzle, M. G. (2014). Exopolysaccharides synthesized by Lactobacillus reuteri protect against enterotoxigenic Escherichia coli in piglets. Appl. Environ. Microbiol. 80, 5752. doi: 10.1128/AEM.01782-14
Collado, M. C., Grześkowiak, Ł., and Salminen, S. (2007). Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr. Microbiol. 55, 260–265. doi: 10.1007/s00284-007-0144-8
Collado, M. C., Isolauri, E., and Salminen, S. (2008). Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol. Lett. 285, 58–64. doi: 10.1111/j.1574-6968.2008.01211.x
Demissie, D. E., Kaplan, S. L., Romero, J. R., Leake, J. A., Barson, W. J., Halasa, N. B., et al. (2013). Altered neutrophil counts at diagnosis of invasive meningococcal infection in children. Pediatr. Infect. Dis. J. 32, 1070–1072. doi: 10.1097/INF.0b013e31829e31f1
Duchet-Suchaux, M., Le Maitre, C., and Bertin, A. (1990). Differences in susceptibility of inbred and outbred infant mice to enterotoxigenic Escherichia coli of bovine, porcine and human origin. J. Med. Microbiol. 31, 185–190. doi: 10.1099/00222615-31-3-185
Elli, M., Zink, R., Rytz, A., Reniero, R., and Morelli, L. (2000). Iron requirement of Lactobacillus spp. in completely chemically defined growth media. J. Appl. Microbiol. 88, 695–703. doi: 10.1046/j.1365-2672.2000.01013.x
Ewaschuk, J. B., Naylor, J. M., and Zello, G. A. (2005). D-lactate in human and ruminant metabolism. J. Nutr. 135, 1619–1625.
Fasano, A., and Shea-Donohue, T. (2005). Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2, 416–422. doi: 10.1038/ncpgasthep0259
Fiesel, A., Gessner, D. K., Most, E., and Eder, K. (2014). Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 10:196. doi: 10.1186/s12917-014-0196-5
Frece, J., Kos, B., Beganović, J., Vuković, S., and Šušković, J. (2006). In vivo testing of functional properties of three selected probiotic strains. World J. Microbiol. Biotechnol. 21, 1401–1408. doi: 10.1007/s11274-005-5741-8
Galdeano, C., and Perdigon, G. (2004). Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 97:673. doi: 10.1111/j.1365-2672.2004.02353.x
Gareau, M. G., Sherman, P. M., and Walker, W. A. (2010). Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 7, 503–514. doi: 10.1038/nrgastro.2010.117
Geier, M. S., Mikkelsen, L. L., Torok, V. A., Allison, G. E., Olnood, C. G., Boulianne, M., et al. (2010). Comparison of alternatives to in-feed antimicrobials for the prevention of clinical necrotic enteritis. J. Appl. Microbiol. 109, 1329–1338. doi: 10.1111/j.1365-2672.2010.04758.x
Groschwitz, K. R., and Hogan, S. P. (2009). Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 124, 21–22. doi: 10.1016/j.jaci.2009.05.038
Guo, X., Xia, X., Tang, R., Zhou, J., Zhao, H., and Wang, K. (2008). Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 47, 367–373. doi: 10.1111/j.1472-765X.2008.02408.x
Hooper, L. V. (2009). Do symbiotic bacteria subvert host immunity? Nat. Rev. Microbiol. 7, 367–374. doi: 10.1038/nrmicro2114
Hunt, L., Gupta-Wright, A., Simms, V., Tamba, F., Knott, V., Tamba, K., et al. (2015). Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect. Dis. 15, 1292–1299. doi: 10.1016/S1473-3099(15)00144-9
Jones, S. E., and Versalovic, J. (2009). Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 9:935. doi: 10.1186/1471-2180-9-35
Lee, J. S., Awji, E. G., Lee, S. J., Tassew, D. D., Park, Y. B., Park, K. S., et al. (2012). Effect of Lactobacillus plantarum CJLP243 on the growth performance and cytokine response of weaning pigs challenged with enterotoxigenic Escherichia coli. J. Anim. Sci. 90, 3709–3717. doi: 10.2527/jas.2011-4434
Li, X. Q., Zhu, Y-H., Zhang, H-F., Yue, Y., Cai, Z-X., Lu, Q-P., et al. (2012). Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: intestinal microbiota and immune imbalances. PLoS ONE 7:e40666. doi: 10.1371/journal.pone.0040666
Liu, H., French, B. A., Nelson, T. J., Li, J., Tillman, B., and French, S. W. (2015). IL-8 signaling is up regulated in alcoholic hepatitis and DDC fed mice with Mallory denk bodies (MDBs) present. Exp. Mol. Pathol. 99, 320–325. doi: 10.1016/j.yexmp.2015.08.002
Mennigen, R., and Bruewer, M. (2009). Effect of probiotics on intestinal barrier function. Ann. N. Y. Acad. Sci. 1165, 183–189. doi: 10.1111/j.1749-6632.2009.04059.x
Mileti, E., Matteoli, G., Iliev, I. D., and Rescigno, M. (2009). Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS ONE 4:e7056. doi: 10.1371/journal.pone.0007056
Mizumachi, K., Aoki, R., Ohmori, H., Saeki, M., and Kawashima, T. (2009). Effect of fermented liquid diet prepared with Lactobacillus plantarum LQ80 on the immune response in weaning pigs. Animal 3, 670–676. doi: 10.1017/S1751731109003978
Nakamura, K., Arakawa, K., Kawai, Y., Yasuta, N., Chujo, T., Watanabe, M., et al. (2013). Food preservative potential of gassericin A-containing concentrate prepared from cheese whey culture supernatant of Lactobacillus gasseri LA39. Anim. Sci. J. 84, 144–149. doi: 10.1111/j.1740-0929.2012.01048.x
Nataro, J. P., and Kaper, J. B. (1998). Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11, 142.
Nguyen, T. D., Kang, J. H., and Lee, M. S. (2007). Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 113, 358–361. doi: 10.1016/j.ijfoodmicro.2006.08.015
Nishida, S., Ishii, M., Nishiyama, Y., Abe, S., Ono, Y., and Sekimizu, K. (2017). Lactobacillus paraplantarum 11-1 isolated from rice bran pickles activated innate immunity and improved survival in a silkworm bacterial infection model. Front. Microbiol. 8:436. doi: 10.3389/fmicb.2017.00436
Pandey, N., Malik, R. K., Kaushik, J. K., and Singroha, G. (2013). Gassericin A: a circular bacteriocin produced by Lactic acid bacteria Lactobacillus gasseri. World J. Microbiol. Biotechnol. 29, 1977–1987. doi: 10.1007/s11274-013-1368-3
Peng, Z., Zeng, D., Wang, Q., Niu, L., Ni, X., Zou, F., et al. (2016). Decreased microbial diversity and Lactobacillus group in the intestine of geriatric giant pandas (Ailuropoda melanoleuca). World J. Microbiol. Biotechnol. 32, 1–10. doi: 10.1007/s11274-016-2034-3
Porter, C. K., Riddle, M. S., Tribble, D. R., Louis Bougeois, A., McKenzie, R., Isidean, S. D., et al. (2011). A systematic review of experimental infections with enterotoxigenic Escherichia coli (ETEC). Vaccine 29, 5869–5885. doi: 10.1016/j.vaccine.2011.05.021
Ren, W., Liu, S., Chen, S., Zhang, F., Li, N., Yin, J., et al. (2013). Dietary L-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids 45, 947–955. doi: 10.1007/s00726-013-1551-8
Rinttilä, T., Kassinen, A., Malinen, E., Krogius, L., and Palva, A. (2004). Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 97, 1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x
Roselli, M., Finamore, A., Britti, M. S., Konstantinov, S. R., Smidt, H., de Vos, W. M., et al. (2007). The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J. Nutr. 137, 2709–2716.
Suzuki, T. (2013). Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 70, 631–659. doi: 10.1007/s00018-012-1070-x
Taur, Y., and Pamer, E. G. (2013). The intestinal microbiota and susceptibility to infection in immunocompromised patients. Curr. Opin. Infect. Dis. 26, 332–337. doi: 10.1097/QCO.0b013e3283630dd3
Wang, K., Qi, Y., Yi, S., Pei, Z., Pan, N., and Hu, G. (2016). Mouse duodenum as a model of inflammation induced by enterotoxigenic Escherichia coli K88. J. Veter. Res. 60, 19–23. doi: 10.1515/jvetres-2016-0004
Wang, Y., Xu, N., Xi, A., Ahmed, Z., Zhang, B., and Bai, X. (2009). Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 84, 341–347. doi: 10.1007/s00253-009-2012-x
Wen, K., Li, G., Bui, T., Liu, F., Li, Y., Kocher, J., et al. (2012). High dose and low dose Lactobacilli acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine 30, 1198–1207. doi: 10.1016/j.vaccine.2011.11.107
Wen, K., Liu, F., Li, G., Bai, M., Kocher, J., Yang, X., et al. (2015). Lactobacillus rhamnosus GG dosage affects the adjuvanticity and protection against rotavirus diarrhea in gnotobiotic pigs. J. Pediatr. Gastroenterol. Nut. 60:834. doi: 10.1097/MPG.0000000000000694
Wijtten, P. J., van der Meulen, J., and Verstegen, M. W. (2011). Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 105, 967–981. doi: 10.1017/S0007114510005660
Wu, Y., Wang, Y., Zhou, H., Wang, B., Sun, Q., Fu, A., et al. (2017). ProbioticBacillus amyloliquefaciensSC06 induces autophagy to protect against pathogens in macrophages. Front. Microbiol. 8:469. doi: 10.3389/fmicb.2017.00469
Xun, W., Shi, L., Zhou, H., Hou, G., Cao, T., and Zhao, C. (2015). Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int. Immunopharmacol. 27, 46–52. doi: 10.1016/j.intimp.2015.04.038
Yang, K. M., Jiang, Z. Y., Zheng, C. T., Wang, L., and Yang, X. F. (2014). Effect of on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic K88. J. Anim. Sci. 92, 1496–14503. doi: 10.2527/jas.2013-6619
Yin, M., Zhang, L., Sun, X. M., Mao, L. F., and Pan, J. (2009). Lack of apoE causes alteration of cytokines expression in young mice liver. Mol. Biol. Rep. 37, 2049–2054. doi: 10.1007/s11033-009-9660-x
Yu, Q., Yuan, L., Deng, J., and Yang, Q. (2015). Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front. Cell. Infect. Microbiol. 5:26. doi: 10.3389/fcimb.2015.00026
Zhang, Z., Xu, Y. Q., Liu, H. Y., Lai, T., Ma, J. L., Wang, J. F., et al. (2010). Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: effects on diarrhoea incidence, faecal microflora and immune responses. Vet. Microbiol. 141, 142–148. doi: 10.1016/j.vetmic.2009.09.003
Zhao, L., Luo, L., Jia, W., Xiao, J., Huang, G., Tian, G., et al. (2014). Serum diamine oxidase as a hemorrhagic shock biomarker in a rabbit model. PLoS ONE 9:e102285. doi: 10.1371/journal.pone.0102285
Zhong, W., Zhao, Y., McClain, C. J., Kang, Y. J., and Zhou, Z. (2010). Inactivation of hepatocyte nuclear factor-4α mediates alcohol-induced downregulation of intestinal tight junction proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 299, 1251–1254. doi: 10.1152/ajpgi.00515.2009
Zhu, Y. H., Li, X. Q., Zhang, W., Zhou, D., Liu, H. Y., Wang, J. F., et al. (2014). Dose-dependent effects of Lactobacillus rhamnosus on serum interleukin-17 production and intestinal T-cell responses in pigs challenged with Escherichia coli. Appl. Environ. Microbiol. 80, 1787–1798. doi: 10.1128/AEM.03668-13
Keywords: Lactobacillus plantarum BSGP201683, enterotoxigenic Escherichia coli K88, immune response, intestinal barrier, gut microflora
Citation: Liu Q, Ni X, Wang Q, Peng Z, Niu L, Wang H, Zhou Y, Sun H, Pan K, Jing B and Zeng D (2017) Lactobacillus plantarum BSGP201683 Isolated from Giant Panda Feces Attenuated Inflammation and Improved Gut Microflora in Mice Challenged with Enterotoxigenic Escherichia coli. Front. Microbiol. 8:1885. doi: 10.3389/fmicb.2017.01885
Received: 01 July 2017; Accepted: 14 September 2017;
Published: 26 September 2017.
Edited by:
Kuldeep Dhama, Indian Veterinary Research Institute (IVRI), IndiaReviewed by:
Muhammad Zubair Shabbir, University of Veterinary and Animal Sciences, PakistanLijuan Yuan, Virginia Tech, United States
Konstantinos Papadimitriou, Agricultural University of Athens, Greece
Copyright © 2017 Liu, Ni, Wang, Peng, Niu, Wang, Zhou, Sun, Pan, Jing and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Zeng, zend@sicau.edu.cn
†Joint first authors.
 Qian Liu
Qian Liu Xueqin Ni1†
Xueqin Ni1† Dong Zeng
Dong Zeng