- 1Key Laboratory of Animal Parasitology of Ministry of Agriculture, Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Shanghai, China
- 2Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou, China
Babesiosis caused by Babesia microti parasite is an emerging tick borne zoonotic disease that was confirmed recently in China. To understand the epidemiology characteristics of this emerging disease, infectivity of B. microti to domestic animals and ticks outside the genus Ixodes was evaluated in this study. Different domestic animals, chick, pig, goat, dog and the reference host rat were experimentally inoculated with B. microti-infected erythrocytes and the parasite infection was monitored daily by blood smear observation, real-time PCR detection, nested-PCR and special antibody responses during 55 days period. The results showed that rats infected with B. microti showed a typical sustained infection with strongly antibody responses; however, both goats and dogs infected with B. microti only showed transient antibody responses and the parasite was not found by blood smear observation or PCR; neither the parasite nor the special antibodies were detected in experimental chicks and pigs. On the other hand, the present study also experimentally investigated the infectivity of B. microti to Rhipicephalus haemaphysaloides, Haemaphysalis longicornis, and Hyalomma asiaticum three species of ticks outside the genus Ixodes and the transmission experiment of B. microti between H. longicornis ticks and mice. Results showed that B. microti can be detected in the nymph and adult of these species after molting from engorged tick fed on infected mice, but the parasite was not detected in larvae hatching from eggs of engorged female tick fed on the infected mice. Transmission of B. microti to mice by infected H. longicornis nymphs was confirmed. These results indicated that these domestic animals do not have reservoir competence for B. microti, however, three species of ticks out of the genus Ixodes, common in China were successfully infected by B. microti, with H. longicornis showing the potential of transmitting the parasite to the vertebrate host.
Introduction
Babesia microti are tick-borne protozoan parasites from the phylum Apicomplexa, which invade and replicate in RBCs of animals, including humans, causing zoonotic babesiosis (Gray et al., 2010). Thousands of cases of human babesiosis are reported in the world (Gray et al., 2010; Centers for Disease Control and Prevention [CDC], 2012; Fang et al., 2015), most caused by tick bites and also as a result of transferring infected blood; severe disease can result in susceptible patients, such as those who are splenectomized (Vannier and Krause, 2009). Most cases of babesiosis occur in the USA and parts of Europe, with fewer in Africa, Australia, Asia and other regions (Gray et al., 2010). In China, B. microti organisms have been found in local ticks (Sun et al., 2008), wild mice (Zhao et al., 2013) and human (Zhou X. et al., 2013) and was regarded as emerging public health threat (Fang et al., 2015; Vannier and Krause, 2015).
B. microti can infect a range of animal species, some of hosts were confirmed by experimental infection, and most of the others were deduced from the DNA detection in host samples or the blood feeding tick samples. Experimentally, some rodent species have shown susceptibility to infection with B. microti (Konopka and Sinski, 1996). Meanwhile intravenously inoculation B. microti showed the persistent infection with low parasitemia in rhesus macaques (van Duivenvoorde et al., 2010). In a recent study of reservoir competence in 14 wildlife host species (10 mammals and four birds), all species were regarded to be the reservoir hosts for B. microti by detecting blood feeding ticks on the hosts, which included rodents, raccoon and birds (Hersh et al., 2012). Infection with B. microti has also been observed in other wildlife mammal species, such as short tailed shrews (Blarina brevicauda) (Telford et al., 1990), eastern cottontail rabbits (Sylvilagus floridanus) (Spielman et al., 1981), and foxes (Birkenheuer et al., 2010). B. microti infection in birds has not been extensively studied; however, evidence of B. microti infection in birds had been discovered recently in Europe, which showed B. microti infection in engorged larval ticks that had been feeding on birds of several species (Franke et al., 2010a,b; Hildebrandt et al., 2010).
B. microti has long been known to infect both humans and rodents. Reservoir competence of wild animals for B. microti has been investigated extensively, however, the evidence whether B. microti can infect domestic animals such as pig, goat, dog and chick unclear yet. The Reservoir competence of domestic animals for B. microti will help advance understanding of the spread of human Babesiosis.
Tick species of the genus Ixodes are considered the primary vector of B. microti (Gray et al., 2010). Ixodes scapularis and I. ricinus are the main vectors of B. microti in the United States and Europe respectively (Oliveira and Kreier, 1979; Gray et al., 2002). In Asia, B. microti have been detected in questing tick I. ovatus and I. persulcatus (Sun et al., 2008; Zamoto-Niikura et al., 2012; Tuvshintulga et al., 2015), and a recent study confirmed I. persulcatus ticks as a vector for B. microti by experimental transmission (Zamoto-Niikura et al., 2016). In China, I. persulcatus is considered the primary vector of B. microti, which is distributed in the northeastern regions (Sun et al., 2008), however, up to date, B. microti infection case in China were all found in the south of China, where I. persulcatus ticks have not been found (Yao et al., 2012; Zhou X. et al., 2013). Therefore, competent vectors of B. microti in south of China are not yet well understood. Considering the dominant tick species in southern China, recently a preliminary experimental transmission of B. microti by Rhipicephalus haemaphysaloides ticks was carried out and found the transmission potential of this tick species (Li et al., 2016). B. microti has also been detected in ticks other than the species of genus Ixodes in the field (Wojcik-Fatla et al., 2012) and under experimental condition (Kusakisako et al., 2015). Therefore, more studies are needed on the transmission potentials of tick species from other genera.
Materials and Methods
Protozoa, Ticks and Experimental Animals
The strain of B. microti was supplied by American Type Culture Collection (ATCC) under the number ATCCR PRA-99TM and was maintained in our laboratory by serial passage of parasitized erythrocytes in mice (Zhao et al., 2016). The colony of R. haemaphysaloides ticks was derived from water buffalo in Hubei Province in southern China (Zhou et al., 2006) and the colony of Hyalomma asiaticum ticks was derived from sheep in the Xinjiang Autonomous Region in northern China (Li et al., 2014). The colony of parthenogenetic Haemaphysalis longicornis ticks was derived from deer in the Shanghai Wildlife Park in China (Zhou J. et al., 2013). A single engorged female was used to establish each tick colony. Tick rearing was performed in the laboratory in a dark incubator at 25°C with 92% relative humidity and feeding on a New Zealand white rabbit. After three generations had been maintained under laboratory conditions, the colonies of ticks were established in Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Shanghai, China. According to general principles, sexual maturity animals were chosen for this study. Six-week-old KM mice (Shanghai Silaike Experimental Animal Co. Ltd.). Two-month-old SD rats (Shanghai Silaike Experimental Animal Co. Ltd.), Three-week-old Yellow hair chicks (Shanghai Songlian Experimental Animal Co. Ltd.), 2-month-old Changshang pigs (Shanghai Nongxi Pig Farm), 3-month-old Berger’s dogs (Shanghai Songlian Experimental Animal Co. Ltd.), and 1-month-old goats (Shanghai Songlian Experimental Animal Co. Ltd.) were used in this study. The experimental animals were treated following the approved guidelines from the Animal Care and Use Committee of the Shanghai Veterinary Research Institute (Approval number SHVRI-MO-0023), all surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Inoculation of B. microti-Infected Erythrocytes in Animals
Blood containing B. microti-infected erythrocytes were collected by cardiac puncture from mice infected with B. microti. After measuring the total erythrocyte count and infection rate, parasitized erythrocytes were adjusted to 1 × 108/mL by phosphate buffered saline (0.01M PBS, pH7.4). The B. microti-infected erythrocytes were then injected either intraperitoneally for rat or intravenously for chick, pig, dog, and goat with 1 × 107 parasitized erythrocytes, three individuals were infected for each kinds of domestic animals. Blood samples were then collected from the animals once every 5 days until 55 days after inoculation.
Microscopic Observation of B. microti in Blood Smears
Thin blood smears with Giemsa staining were used to examine Babesia parasites in infected red blood cells (RBCs) under a light microscope, a method that is currently used in most diagnostic laboratories (Mosqueda et al., 2012). The peripheral bloods from infected animals were taken, and then made thin blood smears with Giemsa staining for microscopic observation.
Detection of B. microti in Animal Blood by Quantitative PCR and Nested-PCR
DNA was extracted from 300-μl of EDTA preserved whole blood using the QIAamp DNA minikit blood and body fluids protocol (Qiagen) according to the manufacturer’s instructions and eluted in an equal volume of elution buffer. Extracted DNA (5 μl) was used as a template for quantitative PCR following the methodology (Rollend et al., 2013), Forward and reverse primers and probe sequences are: Bm18Sf-AACAGGCATTCGCCTTGAAT, Bm18Sr-CCAACT GCTCCTATTAACCATTACTCT, and Bm18Sp-6FAM-CTA CAGCATGGAATAATGA-MGBNFQ, respectively. The quantitative PCR was performed using ABI 7500 instruments. Nested-PCR was used to detect parasites in blood samples by a previously published protocol (Persing et al., 1992).
Detection of Antibody to B. microti in Host Animals after Infection
Whole blood samples were collected regularly from infected animals and the sera were collected and kept at -30°C. Enzyme-linked immunosorbent assay (ELISA) was carried out to detect the antibody responses against B. microti infection, which used the recombinant BmSA1 as the coated antigen (Luo et al., 2011). Briefly, 96-well microplates were coated with the antigen, glutathione S-transferase (GST)- BmSA1 and GST (negative control) at a concentration of 250 ng/well. Animal sera diluted at 1:100 with PBS containing 0.05% Tween 20 were used as the source of primary antibody and HRPO-conjugated anti-chicken IgG (ID, A30-107P) or anti-pig IgG (ID, A100-105P), or anti-goat IgG (ID, A50-100P), or anti-dog IgG (ID, A40-123P), or anti-rat IgG (ID, A110-105P) was used as the second antibody respectively (Bethyl Laboratories, United States). After addition of substrate [2,20-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid)], the optical density (OD) value was recorded at 415 nm by SpectraMax M5 (Molecular Devices, United States).
Blood Examination of the Infected Animals
EDTA-preserved blood was collected from infected animals as described above, blood examination was carried out using the Mindray BC-2800 Auto Hematology Analyzer (Mindray, China). Number of white blood cells (WBC), number of RBCs, hematocrit (HCT), and mean corpuscular volume (MCV) were checked.
Ticks Infection by Blood Feeding on Mice Infected with B. microti
Approximately 600 larvae were applied equally to feed on 3 mice with B. microti parasitemia of 20%. Each mouse was collared with a plastic cap with central drilling to prevent grooming and then ticks were allowed to feed to repletion on infected mice. Parts of engorged larvae ticks were pooled (10 larvae ticks) for B. microti qPCR detection at once; the other engorged larvae were allowed to molt in the dark at 25°C, 92% relative humidity for 2–4 weeks. Newly molted tick nymphs were collected (individual) and used for qPCR detection. In the same way, about 100 nymphs were applied equally to feed on 3 mice with B. microti parasitemia of 20%. Some of the engorged nymphs and newly molted adult ticks from the engorged nymphal were collected (individual) and used for B. microti qPCR detection respectively. Also, about 60 adult female ticks (add 60 adult male ticks for R. haemaphysaloides and H. asiaticum) were applied equally to feed on 10–30 mice with B. microti parasitemia of 20% depending on different species of ticks. Some of engorged female ticks (individual) and the pool (10 larvae ticks) of newly molted larval ticks from the single engorged female were collected and used for B. microti qPCR detection.
Transmission of B. microti to Mice by Infected H. longicornis Nymphs
Ten KM mice were infested with 60 nymphs each, which were previously infected with B. microti as larvae. Approximately 50 μl tail bloods of mice were collected for parasite detection every 3 days starting from day 7 to day 30 after tick infestation. When no B. microti infection was detected after blood samples were microscopically examined, each mouse was sacrificed, bled and a fresh mice was inoculated with the blood. The monitoring process was conducted again as mentioned above for another 30 days until positive mice were found.
For each blood sample, a thin blood smear was stained with Giemsa-stain in order to examine parasites by microscope. Forty-five microliters of blood were used for DNA extraction, and qPCR was performed to detect B. microti infection. Blood collection was stopped when the mice were proved to be infected upon microscopic examination.
Data Analysis
Differences in the positive rate of ticks were tested by Chi-square, which was performed using SPSS 20.0 software. A probability P-value < 0.05 was considered statistically significant.
Results
Observation of Parasitemia by Blood Smears
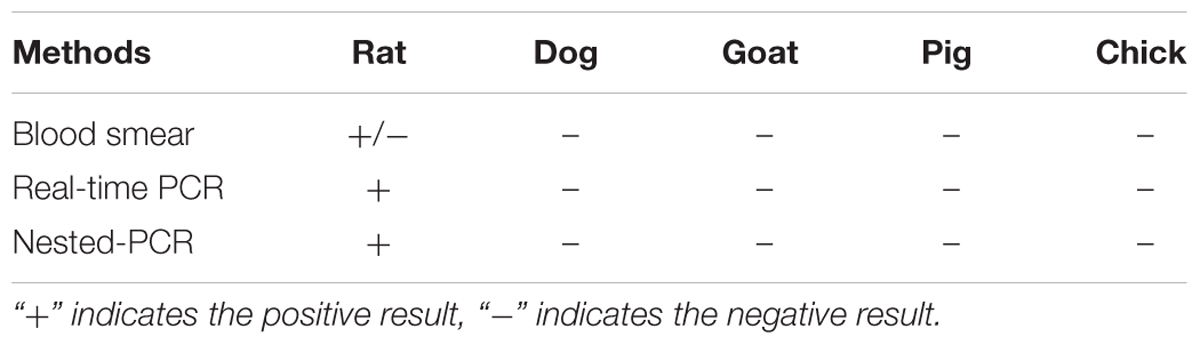
Following infection with B. microti, parasitemia increased rapidly in rats, parasites were observed in RBCs 1 day after infection, with parasitemia peaking at 29.1% at day 6, then declining rapidly to <1% at day 11 post-infection (Supplementary Figure S1). Although it was difficult to detect parasites in RBCs of infected rats after day 11 post-infection, a few parasites could still be observed over the following few days. By contrast, no parasites were observed in the RBCs of dogs, goat, pigs and chicks at any time in the experiment periods (data not shown).
Detection of the Parasite DNA by Quantitative PCR and Nested-PCR
As shown in Table 1, the parasite DNA could not be detected by quantitative PCR and nested-PCR in the blood samples of all host animals from 5 to 55 days after infection except the rat. The copy numbers of target gene of B. microti were never less than 103 at any point during the experiment in the rat in real-time PCR (Supplementary Figure S2). However, B. microti did not to be detected in the blood samples of dogs, goats, pigs and chicks after 5 days following initial infection by both qPCR and nested-PCR (data not shown).
Antibody Detection of Host Animals after Infections
As shown in Figure 1, compared with rat serum before the infection, the specific antibodies against B. microti were detectable in rats as early as 10 days post-infection and a significant antibodies levels were found on 20 days post-infection, peaking at 30 days post-infection (OD > 0.8) and then kept high levels until the end of experiments. In dogs and goats, unexpectedly, antibody response was quick. Significant antibody levels were found on 10 days post-infection, peaking at 15 days post-infection and then antibody responses decreased quickly and disappeared on 20 and 30 days post-infection for goats and dogs respectively. By contrast, all serum samples from the experimental pigs and chicks showed no any specific antibody responses against B. microti.
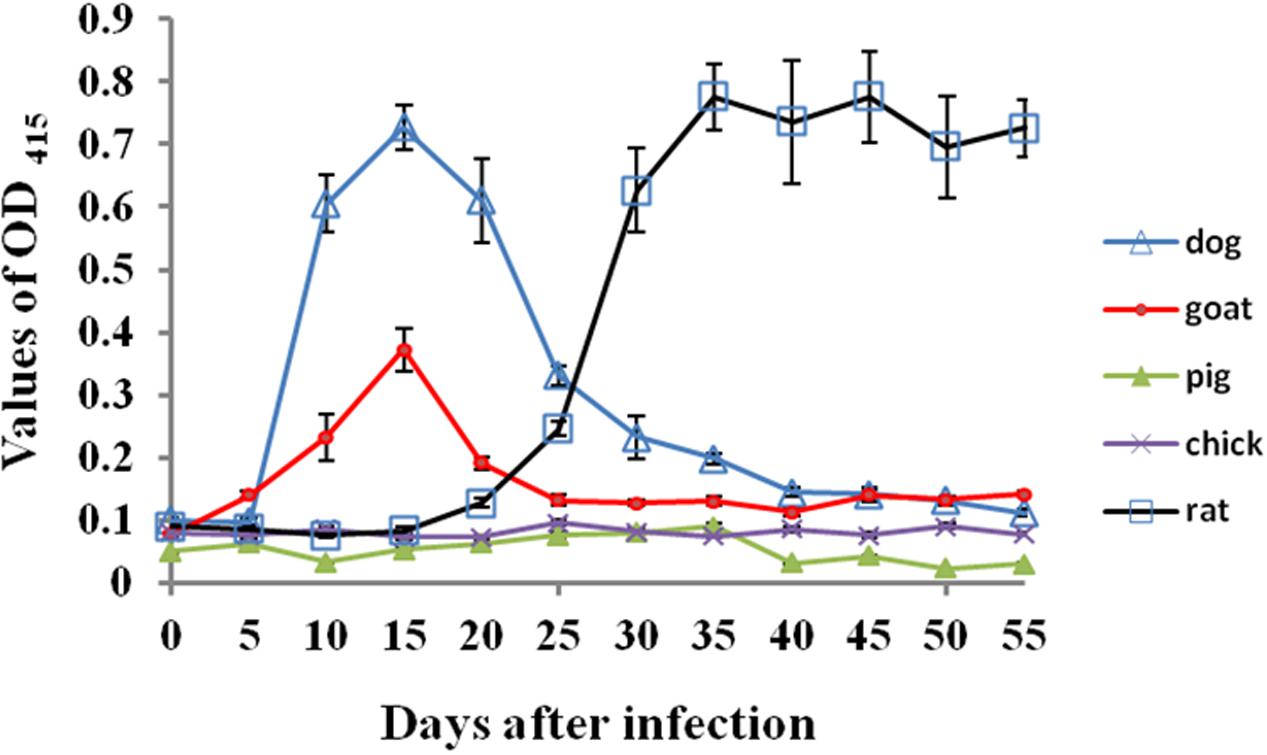
FIGURE 1. The specific antibody responses against B. microti in different host animals post-infection. The error bars represent three biological replicates.
Blood Examination of the Infected Animals
As shown in Figure 2, during early infection in rats, the values of RBC and HCT were below the value of the normal range at day 5 post-infection. The numbers of RBCs of infected rats increased at 10 days post-infection and returned to the normal range at 15 days; the values of HCT returned to the normal range at 10 days post-infection. The numbers of leukocytes increased rapidly at day 5 post-infection, peaking at day 10 post-infection which was eight times the maximum normal value (20.9 × 109 /L), returning to within the normal range at day 15 post-infection. The mean value of MCV of infected rats was also higher than the normal range at day 10 post-infection and returned normal at day 15 post-infection. By contrast, no significant changes from normal values were recorded in the levels of RBC, HCT, WBC and MCV in experimental infected dogs, goats, pigs and chicks (Supplementary Figures S3–S6).
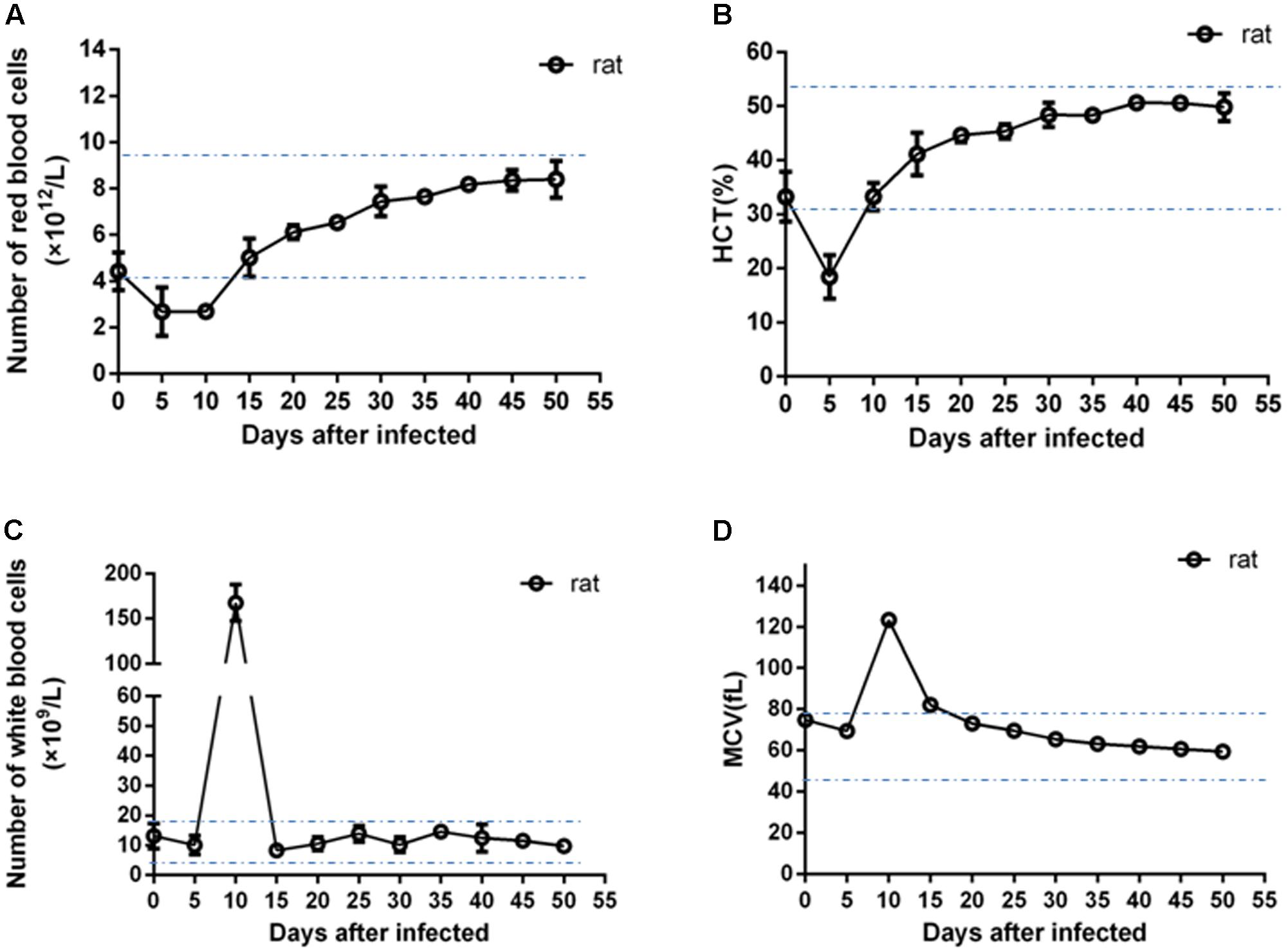
FIGURE 2. Blood examination of the infected rats. The error bars represent three biological replicates. The dotted lines showed the minimum and maximum values of normal ranges. (A) Values of number of red blood cells; (B) values of hematocrit (HCT); (C) values of number of white blood cells; (D) values of mean corpuscular volume (MCV).
Ticks Infection by Blood Feeding on Mice Infected with B. microti
Tick larvae were fed on the mice infected with B. microti for 3–5 days, 600–650 larvae of H. longicornis, R. haemaphysaloides and H. asiaticum were engorged and collected respectively. As shown in Table 2, engorged larvae pools of three species of ticks were all 100% positive (20/20) by PCR, which indicated that the larvae ticks had ingested B. microti successfully. However, positive rates of molted ticks (nymphs) were 93.3, 86.7, and 83.3% for H. longicornis, R. haemaphysaloides and H. asiaticum respectively, which showed no significant difference between different species of ticks.
About 150 engorged nymphs were collected from infected mice after feeding of H. longicornis, R. haemaphysaloides, and H. asiaticum. All 20 of the engorged nymphs tested by PCR were positive, however, positive rates of molted ticks (adult) were 70, 55, and 50% for H. longicornis, R. haemaphysaloides, and H. asiaticum respectively (Table 3), which showed significant difference between H. longicornis and the other two species of ticks. This result may suggest the different ability for transmission in this developmental stage.
About 50 engorged females of each tick species were collected from infected mice after feeding. All 20 of the engorged female ticks tested by PCR were positive, but no positive larvae tick pools from single female ticks were found in all tested samples in H. longicornis, R. haemaphysaloides, and H. asiaticum (Table 4).
Transmission of B. microti to Mice by Infected H. longicornis Nymphs
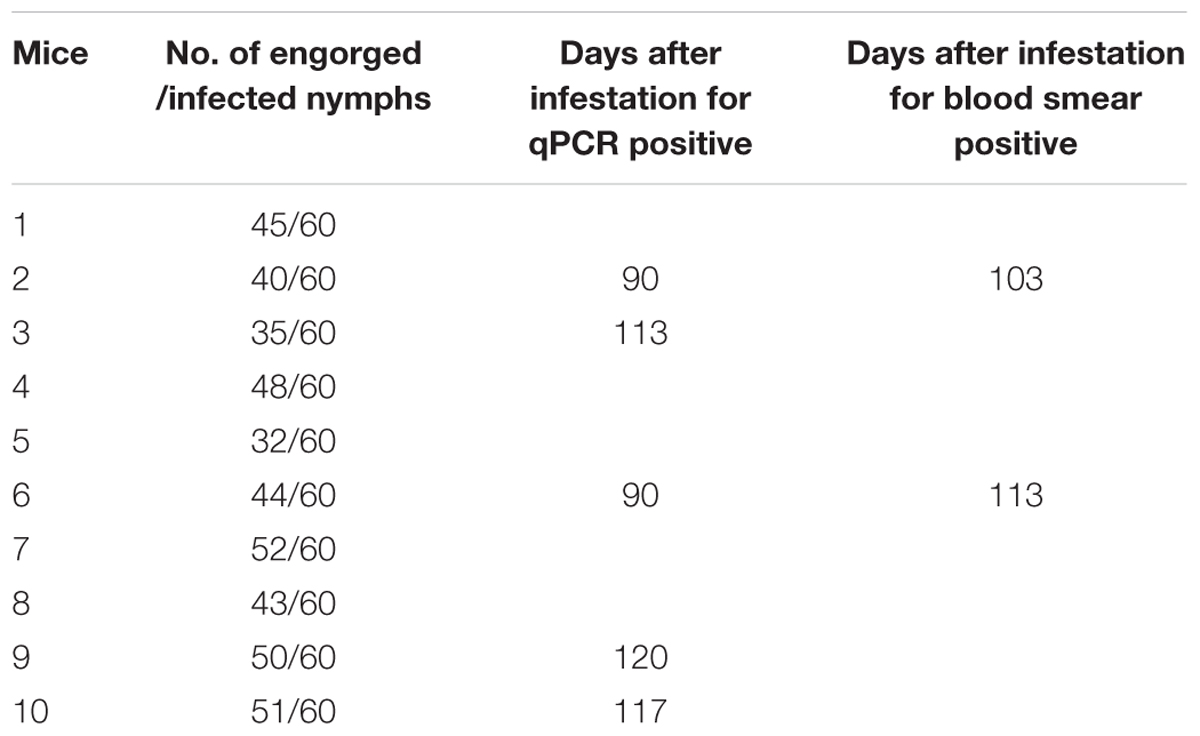
As shown in Table 5, most of infected nymphs had successfully engorged on mice between 3 and 5 days. However, B. microti was not detected in mice in the first 30 days after tick infestation and the second 30 days after collected blood was blindly passed by inoculation. Parasites were detected by qPCR in 2 out of 10 mice in the third 30 days after blood passage. The parasite was not found in the blood smears of mice until the fourth 30 days after blind passage of blood (Supplementary Figure S7). It took about 4 month to firstly detect B. microti infection after tick infestation test.
Discussion
As the infection reference for B. microti inoculation, the rats showed a typical infection course throughout the experimental periods similar to mice or hamster infection (Konopka and Sinski, 1996; Ike et al., 2005). B. microti can persist in rats over long time and with high antibody response levels. Moreover, during early stages of B. microti infection, the numbers of RBC and HCT of infected rats were significantly lower than the normal range, and the number of MCV was higher than the normal range, which suggests that rats would display symptoms of anemia. In addition, a significant finding was that the number of WBC increased to several times the normal value in rats at 10 days post-infection.
However, this study showed that the goat, pig, dog, and chick were not infected with B. microti by systematic surveys on parasitological observation, DNA detection, and blood examination for physiology indexes. Although goats and dogs infected with B. microti showed transient antibody responses, the parasite was not found by blood smear observation or PCR; this transient antibody response might be caused by the dog and goat immune system against the inoculated parasites, but not in the chick and pig.
The intravenously inoculation is the common method for B. microti experimental infection (van Duivenvoorde et al., 2010), in this study, considering the convenience and reliability for rats, B. microti-infected erythrocytes were injected intraperitoneally. This inoculation route difference between rat and other domestic animals is not relative with successful infection.
Some reports have suggested that B. microti-like parasites exhibit infectivity in dogs (Zahler et al., 2000; Camacho et al., 2001), however, the evidence whether B. microti can infect dogs or not was not clear yet. In the present experiment, we examined the inoculation of mouse-derived B. microti in dogs. A transient antibody response was found in the early time after parasites inoculation. However, the following evidences (parasitological observation, DNA detection, blood examination for physiology indexes) strongly suggested that dogs were not infected with this parasite in present study.
Evidence of B. microti infection in wild birds had recently been discovered in engorged larval ticks (or newly molted nymphs) that had been feeding on birds of several species (Franke et al., 2010b; Hildebrandt et al., 2010; Capligina et al., 2014). Unexpectedly, chicks as domestic birds were not proved to be infected in these studies. The B. microti DNA was only detected in 5 days post inoculation samples, while possible inoculation remnant was not found in any other time point in whole experimental course. Meantime, there were no significant antibody responses against B. microti infection, both microscopic observation and blood examination showed negative infection in chicks.
Ticks genera other than the genus Ixodes, such as Rhipicephalus genus, Haemaphysalis genus are very common and widespread in southern China. Dozens of human infections with B. microti have been reported recently, especially in southern China (Vannier and Krause, 2015). The vectors of this parasite in these areas are not well understood, and the transmission capability of ticks outside the genus Ixodes for B. microti need to be elucidated. In this study, the three species of tick from three genera were tested and all showed the transmission potential competence. Our results confirmed recent report on the potential vector of R. haemaphysaloides (Li et al., 2016). Moreover, according to positive rate of molted ticks (nymphal and adult), H. longicornis tick is more potentially competent for B. microti transmission, which is confirmed by the transmission tests of B. microti to mice by infected H. longicornis nymphs. This result showed some different from the report on vector potential of H. longicornis ticks for B. microti (Kusakisako et al., 2015), which indicate that B. microti can infect to this kind of ticks, but the transmission was not confirmed. The unsuccessful transmission experiment might be involved in many factors of study; one of the key factors may come from the short period (0–40 days) to detect the parasite. In this study, B. microti positive detection was occurred firstly on the 90th days after tick infestation by qPCR and the 103th days after tick infestation by blood smear examination. Because blood collection was stopped when the mice were proved to be infected upon microscopic examination, more positive infection might be found later than 120 days after infection. To our knowledge, this is the first experiment report to show vector competence of the H. longicornis tick for B. microti transmission.
Conclusion
We explored the infectivity of B. microti parasites in domestic animals and ticks other than the genus Ixodes, and found that dogs, goat, pigs and chicks were not the reservoir hosts, however, ticks investigated in this study R. haemaphysaloides,H. longicornis and H. asiaticum all showed potential competence for B. microti transmission.
Author Contributions
JZ directed the project, participated in the design of the study, and helped write manuscript. JW and JC performed the laboratory tests, analyzed the data, and wrote the manuscript. YZ participated in the animal experiments. HG and HZ participated in data interpretation.
Funding
Project support was provided by the “National Key Basic Research Program (973 program) of China” (Grant No. 2015CB150300).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
We thank Dr. Ibrahim Hassan for English correction of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.01915/full#supplementary-material
FIGURE S1 | Parasitemia in the infected rats. The error bars represent three biological replicates.
FIGURE S2 | DNA detection in the infected rats. The error bars represent three biological replicates.
FIGURE S3 | Blood examination of the infected chicken. The error bars represent three biological replicates.
FIGURE S4 | Blood examination of the infected pig. The error bars represent three biological replicates.
FIGURE S5 | Blood examination of the infected dog. The error bars represent three biological replicates.
FIGURE S6 | Blood examination of the infected goat. The error bars represent three biological replicates.
FIGURE S7 | The parasites in the blood smear of mouse experimentally transmitted by H. longicornis nymphs. The arrows indicated the parasites in RBC of mouse.
References
Birkenheuer, A. J., Horney, B., Bailey, M., Scott, M., Sherbert, B., Catto, V., et al. (2010). Babesia microti-like infections are prevalent in North American foxes. Vet. Parasitol. 172, 179–182. doi: 10.1016/j.vetpar.2010.05.020
Camacho, A. T., Pallas, E., Gestal, J. J., Guitián, F. J., Olmeda, A. S., Goethert, H. K., et al. (2001). Infection of dogs in north-west Spain with a Babesia microti-like agent. Vet. Rec. 149, 552–555. doi: 10.1136/vr.149.18.552
Capligina, V., Salmane, I., Keiss, O., Vilks, K., Japina, K., Baumanis, V., et al. (2014). Prevalence of tick-borne pathogens in ticks collected from migratory birds in Latvia. Ticks Tick Borne Dis. 5, 75–81. doi: 10.1016/j.ttbdis.2013.08.007
Centers for Disease Control and Prevention [CDC] (2012). Babesiosis surveillance - 18 States, 2011. MMWR Morb. Mortal. Wkly. Rep. 6, 505–509.
Fang, L. Q., Liu, K., Li, X. L., Liang, S., Yang, Y., Yao, H. W., et al. (2015). Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect. Dis. 15, 1467–1479. doi: 10.1016/S1473-3099(15)00177-2
Franke, J., Fritzsch, J., Tomaso, H., Straube, E., Dorn, W., and Hildebrandt, A. (2010a). Coexistence of pathogens in host-seeking and feeding ticks within a single natural habitat in central Germany. Appl. Environ. Microbiol. 76, 6829–6836. doi: 10.1128/AEM.01630-10
Franke, J., Meier, F., Moldenhauer, A., Straube, E., Dorn, W., and Hildebrandt, A. (2010b). Established and emerging pathogens in Ixodes ricinus ticks collected from birds on a conservation island in the Baltic Sea. Med. Vet. Entomol. 24, 425–432. doi: 10.1111/j.1365-2915.2010.00905.x
Gray, J., von Stedingk, L. V., Gurtelschmid, M., and Granstrom, M. (2002). Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J. Clin. Microbiol. 40, 1259–1263. doi: 10.1128/JCM.40.4.1259-1263.2002
Gray, J., Zintl, A., Hildebrandt, A., Hunfeld, K. P., and Weiss, L. (2010). Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 1, 3–10. doi: 10.1016/j.ttbdis.2009.11.003
Hersh, M. H., Tibbetts, M., Strauss, M., Ostfeld, R. S., and Keesing, F. (2012). Reservoir competence of wildlife host species for Babesia microti. Emerg. Infect. Dis. 18, 1951–1957. doi: 10.3201/eid1812.111392
Hildebrandt, A., Franke, J., Meier, F., Sachse, S., Dorn, W., and Straube, E. (2010). The potential role of migratory birds in transmission cycles of Babesia spp., Anaplasma phagocytophilum, and Rickettsia spp. Ticks Tick Borne Dis. 1, 105–107. doi: 10.1016/j.ttbdis.2009.12.003
Ike, K., Murakami, T., Komatsu, T., Uchida, Y., and Imai, S. (2005). Susceptibility of Chinese hamsters (Cricetulus griseus) to the infection of Babesia microti. J. Vet. Med. Sci. 67, 333–336. doi: 10.1292/jvms.67.333
Konopka, E., and Sinski, E. (1996). Experimental infection of mice with Babesia microti: characterization of parasitemia. Wiad. Parazytol. 42, 395–406.
Kusakisako, K., Maeda, H., Galay, R. L., Matsuo, T., Tsujio, M., Umemiya-Shirafuji, R., et al. (2015). The vector potential of Haemaphysalis longicornis ticks for Babesia microti parasites under experimental condition. J. Protozool. Res. 25, 8–17.
Li, C., Cao, J., Zhou, Y., Zhang, H., Gong, H., and Zhou, J. (2014). The midgut bacterial flora of laboratory-reared hard ticks, Haemaphysalis longicornis, Hyalomma asiaticum, and Rhipicephalus haemaphysaloides. J. Integr. Agric. 13, 1766–1771. doi: 10.1016/S2095-3119(13)60517-1
Li, L. H., Zhu, D., Zhang, C. C., Zhang, Y., and Zhou, X. N. (2016). Experimental transmission of Babesia microti by Rhipicephalus haemaphysaloides. Parasit. Vectors 9, 231. doi: 10.1186/s13071-016-1517-2
Luo, Y., Jia, H., Terkawi, M. A., Goo, Y. K., Kawano, S., Ooka, H., et al. (2011). Identification and characterization of a novel secreted antigen 1 of Babesia microti and evaluation of its potential use in enzyme-linked immunosorbent assay and immunochromatographic test. Parasitol. Int. 60, 119–125. doi: 10.1016/j.parint.2010.11.001
Mosqueda, J., Olvera-Ramirez, A., Aguilar-Tipacamu, G., and Canto, G. J. (2012). Current advances in detection and treatment of babesiosis. Curr. Med. Chem. 19, 1504–1518. doi: 10.2174/092986712799828355
Oliveira, M. R., and Kreier, J. P. (1979). Transmission of Babesia microti using various species of ticks as vectors. J. Parasitol. 65, 816–817. doi: 10.2307/3280370
Persing, D. H., Mathiesen, D., Marshall, W. F., Telford, S. R., Spielman, A., Thomford, J. W., et al. (1992). Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 30, 2097–2103.
Rollend, L., Bent, S. J., Krause, P. J., Usmani-Brown, S., Steeves, T. K., States, S. L., et al. (2013). Quantitative PCR for detection of Babesia microti in Ixodes scapularis ticks and in human blood. Vector Borne Zoonotic Dis. 13, 784–790. doi: 10.1089/vbz.2011.0935
Spielman, A., Etkind, P., Piesman, J., Ruebush, T. K., Juranek, D. D., and Jacobs, M. S. (1981). Reservoir hosts of human babesiosis on Nantucket Island. Am. J. Trop. Med. Hyg. 30, 560–565. doi: 10.4269/ajtmh.1981.30.560
Sun, Y., Liu, G., Yang, L., Xu, R., and Cao, W. (2008). Babesia microti-like rodent parasites isolated from Ixodes persulcatus (Acari: Ixodidae) in Heilongjiang Province, China. Vet. Parasitol. 156, 333–339. doi: 10.1016/j.vetpar.2008.05.026
Telford, S. R., Mather, T. N., Adler, G. H., and Spielman, A. (1990). Short-tailed shrews as reservoirs of the agents of Lyme disease and human babesiosis. J. Parasitol. 76, 681–683. doi: 10.2307/3282982
Tuvshintulga, B., Sivakumar, T., Battsetseg, B., Narantsatsaral, S. O., Enkhtaivan, B., Battur, B., et al. (2015). The PCR detection and phylogenetic characterization of Babesia microti in questing ticks in Mongolia. Parasitol. Int. 64, 527–532. doi: 10.1016/j.parint.2015.07.007
van Duivenvoorde, L. M., Voorberg-van der Wel, A., van der Werff, N. M., Braskamp, G., Remarque, E. J., Kondova, I., et al. (2010). Suppression of Plasmodium cynomolgi in rhesus macaques by coinfection with Babesia microti. Infect. Immun. 78, 1032–1039. doi: 10.1128/IAI.00921-09
Vannier, E., and Krause, P. J. (2009). Update on babesiosis. Interdiscip. Perspect. Infect. Dis. 2009:984568. doi: 10.1155/2009/984568
Vannier, E., and Krause, P. J. (2015). Babesiosis in China, an emerging threat. Lancet Infect. Dis. 15, 137–139. doi: 10.1016/S1473-3099(14)71062-X
Wojcik-Fatla, A., Bartosik, K., Buczek, A., and Dutkiewicz, J. (2012). Babesia microti in adult Dermacentor reticulatus ticks from eastern Poland. Vector Borne Zoonotic Dis. 12, 841–843. doi: 10.1089/vbz.2011.0904
Yao, L. N., Ruan, W., Zeng, C. Y., Li, Z. H., Zhang, X., Lei, Y. L., et al. (2012). Pathogen identification and clinical diagnosis for one case infected with Babesia [in Chinese]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 30, 118–121.
Zahler, M., Rinder, H., Schein, E., and Gothe, R. (2000). Detection of a new pathogenic Babesia microti-like species in dogs. Vet. Parasitol. 89, 241–248. doi: 10.1016/S0304-4017(00)00202-8
Zamoto-Niikura, A., Morikawa, S., Hanaki, K. I., Holman, P. J., and Ishihara, C. (2016). Ixodes persulcatus ticks as a vector for Babesia microti U.S. lineage in Japan. Appl. Environ. Microbiol. 82, 6624–6632. doi: 10.1128/AEM.02373-16
Zamoto-Niikura, A., Tsuji, M., Qiang, W., Nakao, M., Hirata, H., and Ishihara, C. (2012). Detection of two zoonotic Babesia microti lineages, the Hobetsu and U.S. lineages, in two sympatric tick species, Ixodes ovatus and Ixodes persulcatus, respectively, in Japan. Appl. Environ. Microbiol. 78, 3424–3430. doi: 10.1128/AEM.00142-12
Zhao, S., Gong, H., Zhou, Y., Zhang, H., Cao, J., and Zhou, J. (2016). Identification of a thioredoxin reductase from Babesia microti during mammalian infection. Parasitol. Res. 115, 3219–3227. doi: 10.1007/s00436-016-5084-4
Zhao, X. G., Li, H., Sun, Y., Zhang, Y. Y., Jiang, J. F., Liu, W., et al. (2013). Dual infection with Anaplasma phagocytophilum and Babesia microti in a Rattus norvegicus, China. Ticks Tick Borne Dis. 4, 399–402. doi: 10.1016/j.ttbdis.2013.04.002
Zhou, J., Gong, H., Zhou, Y., Xuan, X., and Fujisaki, K. (2006). Identification of a glycine-rich protein from the tick Rhipicephalus haemaphysaloides and evaluation of its vaccine potential against tick feeding. Parasitol. Res. 100, 77–84. doi: 10.1007/s00436-006-0243-7
Zhou, J., Zhou, Y., Cao, J., Zhang, H., and Yu, Y. (2013). Distinctive microRNAs profiles in the salivary glands of Haemaphysalis longicornis related to tick blood-feeding. Exp. Appl. Acarol. 59, 339–349. doi: 10.1007/s10493-012-9604-3
Keywords: Babesia microti, experimental infection, domestic animals, tick, Haemaphysalis longicornis
Citation: Wu J, Cao J, Zhou Y, Zhang H, Gong H and Zhou J (2017) Evaluation on Infectivity of Babesia microti to Domestic Animals and Ticks Outside the Ixodes Genus. Front. Microbiol. 8:1915. doi: 10.3389/fmicb.2017.01915
Received: 06 May 2017; Accepted: 20 September 2017;
Published: 06 October 2017.
Edited by:
Bang Shen, Huazhong Agricultural University, ChinaReviewed by:
Longxian Zhang, Henan Agricultural University, ChinaTatsunori Masatani, Kagoshima University, Japan
Junya Yamagishi, Hokkaido University, Japan
Min Liao, Zhejiang University, China
Copyright © 2017 Wu, Cao, Zhou, Zhang, Gong and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlin Zhou, jinlinzhou@shvri.ac.cn
†These authors have contributed equally to this work.
 Jiajun Wu
Jiajun Wu Jie Cao1†
Jie Cao1† Houshuang Zhang
Houshuang Zhang Haiyan Gong
Haiyan Gong Jinlin Zhou
Jinlin Zhou