- 1Departamento de Biotecnología de los Alimentos, Grupo de Biología de Sistemas en Levaduras de Interés Biotecnológico, Instituto de Agroquímica y Tecnología de los Alimentos (CSIC), Valencia, Spain
- 2Departament de Genètica, Universitat de València, Valencia, Spain
Saccharomyces cerevisiae is the most widespread microorganism responsible for wine alcoholic fermentation. Nevertheless, the wine industry is currently facing new challenges, some of them associate with climate change, which have a negative effect on ethanol content and wine quality. Numerous and varied strategies have been carried out to overcome these concerns. From a biotechnological point of view, the use of alternative non-Saccharomyces yeasts, yielding lower ethanol concentrations and sometimes giving rise to new and interesting aroma, is one of the trendiest approaches. However, S. cerevisiae usually outcompetes other Saccharomyces species due to its better adaptation to the fermentative environment. For this reason, we studied for the first time the use of a Saccharomyces kudriavzevii strain, CR85, for co-inoculations at increasing proportions and sequential inoculations, as well as the effect of aeration, to improve its fermentation performance in order to obtain wines with an ethanol yield reduction. An enhanced competitive performance of S. kudriavzevii CR85 was observed when it represented 90% of the cells present in the inoculum. Furthermore, airflow supply of 20 VVH to the fermentation synergistically improved CR85 endurance and, interestingly, a significant ethanol concentration reduction was achieved.
Introduction
Wine composition is the product of complex interactions among yeast and bacteria that take place in vineyards and wineries, although one yeast species, Saccharomyces cerevisiae, is generally the main microorganism responsible for winemaking process (Pretorius, 2000). Its vigorous fermentative capacity, even in the presence of oxygen (Crabtree effect), makes S. cerevisiae a very efficient ethanol producer, strategy that allows its imposition over the rest of the microbiota during fermentation due to the toxicity of this compound (Thomson et al., 2005; Piškur et al., 2006).
However, this high ethanol production capability may be disadvantageous taking into account the challenges currently faced by the wine industry. In the first place, global warming provokes a gap during grape ripening between phenolic maturity and sugar content. If grapes are harvested when the sugar content is appropriate but the phenolic maturity has not been reached, wines can show altered aroma, flavor, mouth feel, and astringency. On the contrary, if grapes are harvested when their phenolic maturity is the appropriate, their sugar contents are higher, giving rise to wines with increasing ethanol concentrations (Jones et al., 2005). This higher ethanol content is undesirable according to consumers' new demands, because affects flavor complexity sensing (Goldner et al., 2009), and its excessive consumption is harmful for health and road safety.
A variety of measures are taken at the different winemaking stages to overcome the problem of the higher ethanol levels in wines. These include new agronomical methods for grape cultivation (Intrigliolo and Castel, 2009), the use of mixed musts from grapes at different ripening stages (Kontoudakis et al., 2011), the use of engineered yeasts producing lower ethanol yields (Varela et al., 2012), or the partial dealcoholisation of wines by chemical or physical procedures (Gómez-Plaza et al., 1999; Pilipovik and Riverol, 2005; Diban et al., 2008; Hernández et al., 2010; Offeman et al., 2010; Belisario-Sánchez et al., 2012). However, some of these approaches have little impact on ethanol contents, negatively affect the quality of wine, are highly expensive industrial processes, or contravene the current regulations about the use of GMO.
In addition, a wide range of different biological strategies have been proposed to reduce alcohol contents in wines (Kutyna et al., 2010). The use of non-conventional yeast strains in winemaking stands out for its potential. Several non-Saccharomyces yeasts, usually in combination with S. cerevisiae, have been tested to reduce ethanol yields during wine fermentation (Comitini et al., 2011; Sadoudi et al., 2012; Contreras et al., 2014, 2015; Quirós et al., 2014; Ciani et al., 2016). Different strategies have been carried out to improve the fermentation performance of these non-Saccharomyces yeasts, such as, sequential inoculation or co-inoculation at increased proportions with S. cerevisiae, to provide new characteristics to the final wines (Andorrà et al., 2012; Gobbi et al., 2013; Izquierdo Cañas et al., 2014; Jolly et al., 2014; Loira et al., 2014; Canonico et al., 2016). Another approach to reduce alcohol content in wines is the supply of oxygen to the fermenters, under a controlled flowrate, to promote the respiratory consumption of sugars by these non-Saccharomyces yeasts (Gonzalez et al., 2013; Rodrigues et al., 2016). However, temperature under industrial winemaking conditions is generally close to 25°C, which does not allow for any of these alternative yeasts to survive the first hours of the process (Nissen and Arneborg, 2003; Torija, 2003; Pérez-Nevado et al., 2006; Williams et al., 2015).
Alternative Saccharomyces yeasts, such as, Saccharomyces kudriavzevii or S. uvarum, can help to solve some of the new challenges of the wine industry. These species exhibit physiological properties that are especially relevant during the winemaking process, such as, their good fermentative capabilities at low temperatures, resulting in wines with lower alcohol and higher glycerol amounts (Varela et al., 2016; Pérez-Torrado et al., 2017a). In the case of S. kudriavzevii, this species displays a different metabolic regulation concerning ethanol and glycerol syntheses (Arroyo-López et al., 2010; Oliveira et al., 2014; Pérez-Torrado et al., 2016). Moreover, it recently showed an ethanol reducing capability in mixed fermentation with S. cerevisiae at low temperatures (Alonso-del-Real et al., 2017). Again, temperature appears as the most important factor to determine the preponderance of S. cerevisiae during wine fermentation (Nissen and Arneborg, 2003; Torija, 2003; Pérez-Nevado et al., 2006; Arroyo-López et al., 2011; Salvado et al., 2011; Williams et al., 2015; Alonso-del-Real et al., 2017).
However, none of the techniques used to favor the growth of non-Saccharomyces yeasts, such as, co-inoculation, sequential inoculation, or microoxigenation, have been applied to S. kudriavzevii species to favor their presence during wine fermentation. In this work, we first analyzed the presence of S. kudriavzevii during co-fermentation with a S. cerevisiae wine strain under different aeration conditions to select the most suitable one. Next, we studied the effect of S. kudriavzevii enrichment in the inoculum with and without external oxygen supply, and finally the effect of sequential inoculation of the strains.
Materials and Methods
Yeast and Growth Media
The commercial S. cerevisiae strain T73 (Lalvin T73 from Lallemand Monteral, Canada), was used as a conventional wine strain. S. kudriavzevii CR85, a natural isolate from oak tree bark in Agudo, Ciudad Real province, Spain, was selected as the non-conventional, quality enhancer candidate yeast according to its physiological properties. In a recent study, CR85 was shown to be the S. kudriavzevii strain with better fermentation kinetics, despite the high genomic homogeneity among that species (Peris et al., 2016).
Synthetic must (SM, Rossignol et al., 2003) was used in microvinification experiments, with 100 g/L glucose and 100 g/L fructose. YPD medium (2% glucose, 2% peptone, 1% yeast extract) was used for overnight growth of precultures.
Synthetic Must Fermentations
First, in order to determine the best aeration condition, fermentations of 200 mL SM were carried out by a S. cerevisiae and S. kudriavzevii co-inoculum (ratio 1:1) at four different aeration conditions throughout the process: 1 VVH, 5 VVH, 10 VVH, and 20 VVH taking in account the previous data from non-conventional yeasts (Morales et al., 2015). Secondly, different ratios S. cerevisiae/S. kudriavzevii (1:1, 3:7, and 1:9) were used in further 200 mL SM fermentations, both in anaerobiosis and with an air flow rate of 20 VVH during the first 48 h. Also, a condition in which S. cerevisiae was inoculated after 24 h in a proportion of 1% with respect to S. kudriavzevii was also considered. Single cultures of S. cerevisiae and S. kudriavzevii were taken as control for fermentation. In addition, a bottle containing distilled water and another one with water and 5% (v/v) ethanol were set as control for evaporation and ethanol loss due to aeration.
Aeration system is composed of a compressed air generator, 3.1 mm internal diameter silicon tubes, 0.2 μm pore-size filters, a flow meter and a set of flow regulators (one for each bottle) as depicted in Supplementary Figure 1. All the experiments were conducted in triplicate at 25°C with gentle shaking (100 rpm) and an initial inoculation with an OD600 of 0.2. The fermentation process was monitored through weight loss. Yeast cells were collected at different moments during fermentation and kept at −20°C to determine the proportion of both yeast species by QPCR, according to Alonso-del-Real et al. (2017). Supernatants of the samples were also stored at −20°C for the analysis of wine composition by HPLC.
HPLC Analysis and Data Treatment
Sugars (glucose and fructose), glycerol, ethanol, and acetic acid from the fermentation at different time point samples were determined by HPLC (Thermo Fisher Scientific, Waltham, MA, USA) using a refraction index detector and a HyperREZTM XP Carbohydrate H + 8 μm column (Thermo Fisher Scientific) equipped with a HyperREZTM XP Carbohydrate Guard (Thermo Fisher Scientific). Samples were 3-fold diluted, filtered through a 0.22-μm nylon filter (Symta, Madrid, Spain) and injected in duplicate. The analysis conditions were: eluent, 1.5 mM of H2SO4; 0.6 ml min-1 flux and a 50°C oven temperature.
Water and ethanol losses were considered as lineal with respect to time. Deviation factors were dimensioned in bottles with 5% (w/v) ethanol in 400 mL water, and bottles with 400 mL of water, all them with air supply (20 VVH). Water mass loss followed a lineal equation (R2 = 0.99569):
where y refers to weight loss due to H2O evaporation in bottles with only water and t refers to time.
where y refers to weight loss due to H2O and ethanol evaporation in bottles with 5% (w/v) ethanol and t refers to time. HPLC measures of the last were taken at different time points. We observed that ethanol loss followed a lineal function, and that a subtraction of the equation for ethanol bottle minus the one for water bottle, very precisely predicted HPLC results. The calculation was done following Equations (3–5):
where F1 is factor 1 for ethanol correction (% h−1), a1 is the slope of Equation (1), a2 is the slope of Equation (2), and 20 is the value for the total mass of ethanol weighted for 400 mL of solution.
where F2 is factor 2 for ethanol correction (%), t is the time corresponding to an assessed value and EHPLC is the HPLC measure for ethanol concentration.
where EC is corrected ethanol concentration (%).
The rest of compounds in our system were assumed as nonvolatile, however, their concentration values were considered as affected by water and ethanol volume losses. To calculate this concentration factor, the density of must was considered to be equal to the density of water. HPLC values for glucose, fructose, glycerol and acetic acid were corrected using the following equation:
where CC is the corrected concentration for the compound.
Fermentations were tested for the significant differences among them with an ANOVA using the one-way ANOVA module of the Statistica 7.0 software. The concentrations of glucose, fructose, glycerol, ethanol, and acetic acid obtained by HPLC were introduced as the dependent variables. Means were grouped using the Tukey HSD test (α = 0.05).
Results
Determining the Air Flow Conditions Favoring S. kudriavzevii Presence in Mixed Fermentations with S. cerevisiae
A controlled aeration system feeding a set of fermentations co-inoculated with S. cerevisiae and S. kudriavzevii in a ratio 1:1 with 4 different air flow rates: 1, 5, 10, and 20 VVH was installed. Figure 1 shows a clear disadvantage of S. kudriavzevii even in the presence of an external oxygen input. However air flow rate seems to have an influence on the time that S. kudriavzevii can remain in the culture in substantial proportions, and thus, can have a more relevant role during fermentation. The percentage of S. kudriavzevii was higher than 30% during the first 48 h in fermentations performed with air flows of 10 and 20 VVH. However, after 48 h of fermentation a faster decline of the S. kudriavzevii population is observed, which suggests that aeration only favors S. kudriavzevii growth at the beginning of the fermentations.
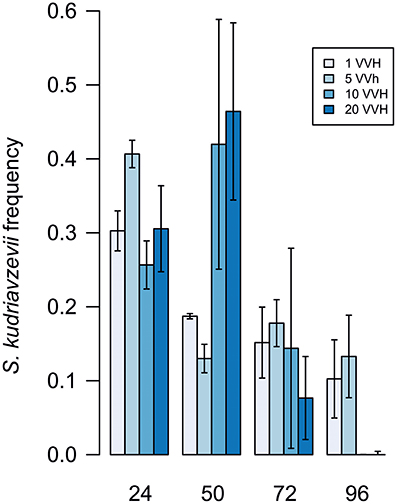
Figure 1. S. kudriavzevii frequency under different aeration conditions. Values are mean of three replicates. Error lines represent standard deviations.
Assaying Different S. cerevisiae/S. kudriavzevii Inoculation Proportions in Fermentations with and without Air Supply
According to these previous data, aeration was applied only for short periods (48 h) for subsequent fermentations because longer aeration time does not favor growth of S. kudriavzevii, and also could increase the final acetic acid concentrations in wines, due to respiration (Salmon, 2006). To test whether a higher inoculation from the beginning of the fermentation, in combination with aeration, could improve S. kudriavzevii's competitive performance, starters composed by S. cerevisiae/S. kudriavzevii proportions of 1:3 and 1:9 in were inoculated into fermentations supplied with an air flow rate of 20 VVH during the first 48 h. Fermentations in the same conditions without aeration were also included to analyze the effect of the yeast species proportions alone.
There were significant differences between aerated and non-aerated fermentations. First, there is a considerable reduction of the fermentation time at which all sugars were totally consumed. Whereas unaerated fermentations took 10 days to finish, aerated fermentations took only 7 days. Second, a clear effect on the maximum cell density was observed, thus, single cultures of S. cerevisiae and S. kudriavzevii with air supply reached OD600 values around 25, however OD600 values for single cultures without aeration were around 20 and 15, respectively.
Regarding yeast proportion changes during fermentations, the initial inoculum proportion of 1:3 shows a slight increase of the frequency of S. kudriavzevii at the final fermentation stage due to limited air supply (Figures 2A,B). However, this inoculation ratio does not provide, with respect to the 1:1 proportion a clear competition advantage for S. kudriavzevii. However, when the inoculation proportion was 1:9 and without aeration (Figure 2C), S. kudriavzevii is able to remain at frequencies higher than 40% for 4 days, although at the end, is outcompeted by S. cerevisiae. Strikingly, the addition of the oxygen supply to inoculation proportions of 1:9 seems to provide a favorable environment for S. kudriavzevii imposition (Figure 2D).
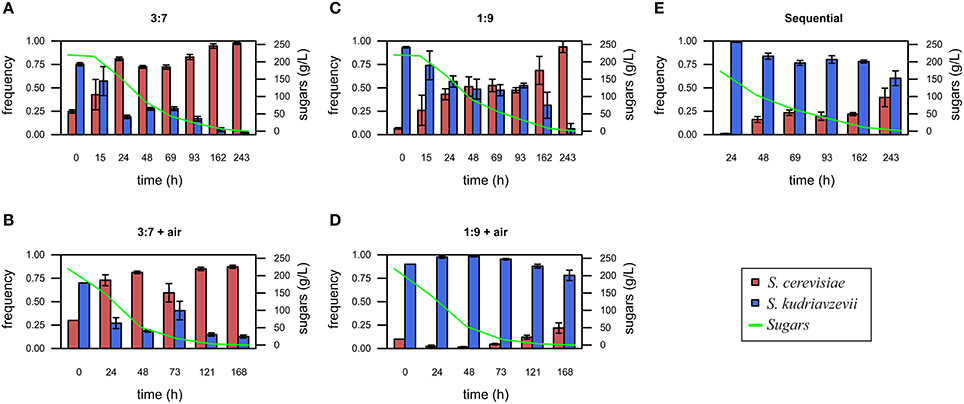
Figure 2. Saccharomyces cerevisiae and S. kudriavzevii frequency during fermentation under different conditions: inoculum proportion 3:7 without air (A), inoculum proportion 3:7 with aeration during the first 48 h (B), inoculum proportion 1:9 without air (C), inoculum proportion 1:9 with aeration during the first 48 h (D), and sequential inoculation (E). Values are mean for 3 replicates. Error bars represent standard deviations. The sum of glucose and fructose concentrations in the must at every time point was also shown.
Sequential inoculation is one of the most common strategies proposed for the preservation of non-dominant microorganisms during food fermentations (Gobbi et al., 2013; Contreras et al., 2014; Loira et al., 2014). In the present study, this strategy was also applied by inoculating a set of bottles only with S. kudriavzevii at the beginning, and adding S. cerevisiae after 24 h in a proportion of 1%. In this case, S. cerevisiae was able to increase its frequency to 40% at the end of the fermentations (Figure 2E).
As a summary of these results, the use of aeration has a slight impact on the relative competitive fitness of S. kudriavzevii when inoculated at equal proportions with S. cerevisiae. However, highly biased proportions of S. kudriavzevii, as well as sequential inoculations, can extend the presence of this less competitive species of interest to promote its impact in the fermentation process. Nevertheless, the combination of aeration and biased inoculation synergistically improves S. kudriavzevii presence during fermentation.
Effect of the Different Inoculation-Aeration Strategies on the Final Fermentation Product
To determine if these strategies really improve wine fermentations, the final wine composition was evaluated by HPLC analysis. First, it is important to remark that in all assayed conditions fermentations were finished with the consumption of all sugars present in the original must, except for fermentations performed only with single cultures of S. kudriavzevii (Table 1), and under aeration, fructose was totally consumed.
Glycerol concentrations were clearly higher in all conditions in which S. kudriavzevii is present, compared to fermentations performed only with the reference S. cerevisiae wine strain, except for the 1:1 proportion with aeration. This glycerol production increase was especially relevant in fermentations with sequential inoculation (Table 1).
Ethanol reduction was accomplished in fermentations with microaeration (up to 1.9% v/v less) and with sequential inoculation (Table 1). However, the ethanol reduction achieved by increasing respiration rate had the counterpart of an acetic acid content increase between 0.5 and 0.7 g/L in bottles under limited aeration, which was not observed in non-aerated fermentations.
Discussion
In the last century, alcohol abuse became considered as one of the most important health problems in the world, and promoted new behavioral strategies against alcohol consumption. In addition, because of global warming, in wine-growing regions with a Mediterranean climate there is excessive ripening of the grape, which produces musts with a higher concentration of sugars (Jones et al., 2005), and hence, higher alcohol yields, implying a higher tax burden, which makes wines less competitive, and a rejection by the consumer for health reasons, road safety, etc.
Therefore, wine industry must respond to these challenges posed both by new consumer demands and by changes in the composition and properties of the grape must due to climate change. These demands have a significant impact on the quality and acceptance of the final wines and require improvements in the enological practices, among which the development of new yeast starters exhibiting lower ethanol yields during wine fermentation is of chief importance.
Different approaches in the use of yeast starters have been proposed to reduce alcohol contents in wines (Schmidtke et al., 2012; Varela et al., 2015). They include controlled aeration, starter strain proportion adjustment, or inoculation of dominant yeast species after a non-Saccharomyces yeast of interest (Comitini et al., 2011; Sadoudi et al., 2012; Contreras et al., 2014, 2015; Quirós et al., 2014; Ciani et al., 2016). In the present study, we adapted these strategies to foster a Saccharomyces non-cerevisiae strain (S. kudriavzevii CR85) presence in synthetic must fermentation. This yeast had been proved to foster decreased ethanol content, and also to increase fermentation kinetics and glycerol concentration in a 1:1 inoculum proportion with S. cerevisiae under low temperatures conditions. In contrast, this effect was not found under regular red winemaking temperatures (Alonso-del-Real et al., 2017), probably due to some of the already proposed competition mechanisms, such as, antimicrobial GAPDH-derived peptides produced by S. cerevisiae (Branco et al., 2016), lower sulfite tolerance and efflux capacity (Pérez-Torrado et al., 2017b), or early nutrient depletion by S. cerevisiae (Fleet, 2003). However, the results reported in the present work show that S. kudriavzevii presence during an important period of the fermentation was achieved at regular industrial temperatures.
Although S. kudriavzevii and S. cerevisiae show long-term Crabtree effect, the carbon flux ratio between respiration and fermentation under aerobic conditions seem to be slightly higher in S. kudriavzevii CR85 compared to S. cerevisiae T73 (our unpublished data). Thus, an external oxygen supply to a fermentation co-inoculated with these two yeast species may benefit S. kudriavzevii growth. Nevertheless, high oxygen levels can deteriorate important compounds of must, originating undesired metabolites correlated to respiration such as acetic acid (Salmon, 2006). Therefore, a fine tuning of the amount of oxygen introduced into the system seems to be critical for the final wine quality. A wide range of airflow rates, from 2.4 to 60 VVH have been used at laboratory scale (Vilanova et al., 2007; Shekhawat et al., 2016). Nevertheless, an air flow rate of 20 VVH has been showed to be on the top limit for acetic acid production when applied to S. cerevisiae microvinification (Morales et al., 2015), therefore the screening for the most suitable condition was performed always below this value.
S. kudriavzevii performance under air supply conditions was observed to improve its competitive fitness against S. cerevisiae (Arroyo-López et al., 2011; Alonso-del-Real et al., 2017). Our results suggest, though, that despite maintaining an air supply during the whole fermentation, after 48 h, S. kudriavzevii was outcompeted by S. cerevisiae. This, together with the fact that an aerobic environment produces a higher acetic acid accumulation up to 70%, led us to reduce aeration just for the first 48 h of fermentation for the successive experiments. Nevertheless, it is noteworthy that, as observed by Moruno et al. (1993) and later confirmed by Beltrán et al. (2008), synthetic and natural musts have different impact on the final product composition, acetic acid levels are much higher for synthetic must, as can also be observed for our aerated conditions. Thus, due to laboratory experimental conditions, acetic acid values obtained in the present work are high even for non-aerated synthetic must fermentations performed with the S. cerevisiae wine strain, compared to natural must fermentation under industrial conditions (0.35 g/L). Therefore, acetic acid levels produced during fermentations with air supply could still be under the limits of regulation (~1 g/L) and consumers' acceptance when tested at industrial scale.
Despite the acetic acid increase, ethanol reduction is notable for the aerated fermentations, in concordance with previous studies (Morales et al., 2015; Shekhawat et al., 2016), and similar to ethanol reductions obtained in other works in which similar co-inoculation strategies with non-Saccharomyces yeasts have been followed (Contreras et al., 2015; Ciani et al., 2016; Englezos et al., 2016). However, this is the first study in which S. kudriavzevii was used to reduce ethanol yields, which, together with a recent study on the sequential inoculation of S. uvarum and S. cerevisiae (Varela et al., 2016), opens new approaches to the use of other Saccharomyces species. These species, in addition to their ethanol metabolic characteristics, also provide richer aroma profiles to wine (Stribny et al., 2015).
The analysis of the non-aerated fermentations also showed a slight ethanol yield reductions clearly correlated with the S. kudriavzevii proportions during the fermentation process under the different assayed conditions. Moreover, there also is a clear direct correlation between S. kudriavzevii proportions and glycerol production, another desirable enological characteristic of importance for wine quality because it contributes to wine body and astringency masking (Jolly et al., 2014). Glycerol and ethanol metabolism has been proven to differ in S. kudriavzevii with respect to S. cerevisiae (Arroyo-López et al., 2010; Pérez-Torrado et al., 2016). In fact, cryotolerant Saccharomyces species, such as, S. kudriavzevii and S. uvarum, have been proven to produce wines and ciders with higher glycerol contents than S. cerevisiae (Bertolini et al., 1996; Masneuf-Pomarède et al., 2010; Peris et al., 2016; González Flores et al., 2017), so their use could be of great interest for wine industry.
Among the strategies followed to favor S. kudriavzevii growth against S. cerevisiae, the co-inoculation with a proportion of S. cerevisiae lower than 10% and the sequential inoculation showed the more promising results. Air supply showed a synergistic effect in proportion S. cerevisiae/S. kudriavzevii 1:9, whereas it did not have a significant impact on the rest of the assayed inoculum proportions. These results agree with the fact that S. cerevisiae is better adapted to anaerobic conditions such as, wine fermentation, and air supply produces an imbalance in this environment, which promotes S. kudriavzevii survival. According to our results, it also seems feasible that a certain threshold in S. cerevisiae cell density is necessary to trigger S. kudriavzevii lack of viability. This also agrees with the previous observations indicating that the viability of a competitor strain is affected by its interaction with S. cerevisiae due to cell-to-cell contacts (Nissen et al., 2003; Arneborg et al., 2005; Branco et al., 2016; Pérez-Torrado et al., 2017b), or by microenvironment modifications produced by S. cerevisiae (Goddard, 2008). A rise in temperature due to the higher fermentative rate of S. cerevisiae (Goddard, 2008) can affect S. kudriavzevii viability (Arroyo-López et al., 2011).
In summary, the most promising results were obtained from the combination of different strategies for promoting S. kudriavzevii prevalence during wine fermentation, such as, co-inoculation with a low proportion of S. cerevisiae (<10%) or sequential inoculation together with limited aeration, resulting in an ethanol yield reduction as well as a higher glycerol production. Aeration requires costly additional technology, but it is already implemented in the wine industry (Vivas and Glories, 1996; Vidal and Aagaard, 2008) to improve wine quality by accelerating the transformations of phenols reducing the astringency.
Finally, these results have to be confirmed in real grape must to evaluate not only the effect of aeration on yeast physiology but also a potential effect on sensory profile. In addition, lower aeration rates can also be tested at industrial scale, particularly for S. cerevisiae/S. kudriavzevii proportions lower than 1:9. In addition a deeper understanding of the interactions among Saccharomyces yeasts, are also needed in order to finely tune the optimal use of these tools to reduce ethanol contents in wine.
Author Contributions
JA, GC, EB, and AQ conceived and designed the experiments. JA, AC, and GC performed the experiments. JA, EB, and AQ analyzed the data and wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
JA was supported by a FPI grant from the Ministerio de Economía y Competitividad, Spain (ref. BES-2013-066434) and GC was supported by National Council for Scientific and Technological Development (CNPq), Brazil. This work was funded by grants AGL2015-67504-C3-1-R and AGL2015-67504-C3-3-R from the Spanish Government and FEDER to AQ and EB, respectively, and by a PROMETEO grant (ref. PROMETEOII/2014/042) from Generalitat Valenciana, Spain to AQ.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.02087/full#supplementary-material
References
Alonso-del-Real, J., Lairón-Peris, M., Barrio, E., and Querol, A. (2017). Effect of temperature on the prevalence of Saccharomyces non cerevisiae species against a S. cerevisiae wine strain in wine fermentation: competition, physiological fitness, and influence in final wine composition. Front. Microbiol. 8:150. doi: 10.3389/fmicb.2017.00150
Andorrà, I., Berradre, M., Mas, A., Esteve-Zarzoso, B., and Guillamón, J. M. (2012). Effect of mixed culture fermentations on yeast populations and aroma profile. LWT Food Sci. Technol. 49, 8–13. doi: 10.1016/j.lwt.2012.04.008
Arneborg, N., Siegumfeldt, H., Andersen, G. H., Nissen, P., Daria, V. R., Rodrigo, P. J., et al. (2005). Interactive optical trapping shows that confinement is a determinant of growth in a mixed yeast culture. FEMS Microbiol. Lett. 245, 155–159. doi: 10.1016/j.femsle.2005.03.008
Arroyo-López, F. N., Pérez-Torrado, R., Querol, A., and Barrio, E. (2010). Modulation of the glycerol and ethanol syntheses in the yeast Saccharomyces kudriavzevii differs from that exhibited by Saccharomyces cerevisiae and their hybrid. Food Microbiol. 27, 628–637. doi: 10.1016/j.fm.2010.02.001
Arroyo-López, F. N., Pérez-Través, L., Querol, A., and Barrio, E. (2011). Exclusion of Saccharomyces kudriavzevii from a wine model system mediated by Saccharomyces cerevisiae. Yeast 28, 423–435. doi: 10.1002/yea.1848
Belisario-Sánchez, Y. Y., Taboada-Rodríguez, A., Marín-Iniesta, F., Iguaz-Gainza, A., and López-Gómez, A. (2012). Aroma recovery in wine dealcoholization by SCC distillation. FEMS Microbiol. Lett. 5, 2529–2539. doi: 10.1007/s11947-011-0574-y
Beltrán, G., Novo, M., Guillamón, J. M., Mas, A., and Rozès, N. (2008). Effect of fermentation temperature and culture media on the yeast lipid composition and wine volatile compounds. Int. J. Food Microbiol. 121, 169–177. doi: 10.1016/j.ijfoodmicro.2007.11.030
Bertolini, L., Zambonelli, C., Giudici, P., and Castellari, L. (1996). Higher alcohol production by cryotolerant Saccharomyces strains. Am. J. Enol. Vitic. 47, 343–345.
Branco, P., Francisco, D., Monteiro, M., Almeida, M. G., Caldeira, J., Arneborg, N., et al. (2016). Antimicrobial properties and death-inducing mechanisms of saccharomycin, a biocide secreted by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 101, 159–171. doi: 10.1007/s00253-016-7755-6
Canonico, L., Agarbati, A., Comitini, F., and Ciani, M. (2016). Torulaspora delbrueckii in the brewing process: a new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 56, 45–51. doi: 10.1016/j.fm.2015.12.005
Ciani, M., Morales, P., Comitini, F., Tronchoni, J., Canonico, L., Curiel, J. A., et al. (2016). Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 7:642. doi: 10.3389/fmicb.2016.00642
Comitini, F., Gobbi, M., Domizio, P., Romani, C., Lencioni, L., Mannazzu, I., et al. (2011). Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 28, 873–882. doi: 10.1016/j.fm.2010.12.001
Contreras, A., Hidalgo, C., Henschke, P. A., Chambers, P. J., Curtin, C., and Varela, C. (2014). Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 80, 1670–1678. doi: 10.1128/AEM.03780-13
Contreras, A., Hidalgo, C., Schmidt, S., Henschke, P. A., Curtin, C., and Varela, C. (2015). The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 205, 7–15. doi: 10.1016/j.ijfoodmicro.2015.03.027
Diban, N., Athes, V., Bes, M., and Souchon, I. (2008). Ethanol and aroma compounds transfer study for partial dealcoholization of wine using membrane contactor. J. Memb. Sci. 311, 136–146. doi: 10.1016/j.memsci.2007.12.004
Englezos, V., Torchio, F., Cravero, F., Marengo, F., Giacosa, S., Gerbi, V., et al. (2016). Aroma profile and composition of Barbera wines obtained by mixed fermentations of Starmerella bacillaris (synonym Candida zemplinina) and Saccharomyces cerevisiae. LWT Food Sci. Technol. 73, 567–575. doi: 10.1016/j.lwt.2016.06.063
Fleet, G. H. (2003). Yeast interactions and wine flavour. Int. J. Food Microbiol. 86, 11–22. doi: 10.1016/S0168-1605(03)00245-9
Gobbi, M., Comitini, F., Domizio, P., Romani, C., Lencioni, L., Mannazzu, I., et al. (2013). Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: a strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 33, 271–281. doi: 10.1016/j.fm.2012.10.004
Goddard, M. R. (2008). Quantifying the complexities of Saccharomyces cerevisiae's ecosystem engineering via fermentation. Ecology 89, 2077–2082. doi: 10.1890/07-2060.1
Goldner, M. C., Zamora, M. C., Lira, P. D. L., Gianninoto, H., and Bandoni, A. (2009). Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J. Sens. Stud. 24, 243–257. doi: 10.1111/j.1745-459X.2009.00208.x
Gómez-Plaza, E., López-Nicolás, J. M., López-Roca, J. M., and Martinez-Cutillas, A. (1999). Dealcoholization of wine. Behaviour of the aroma components during the process. LWT Food Sci. Technol. 32, 384–386. doi: 10.1006/fstl.1999.0565
González Flores, M., Rodríguez, M. E., Oteiza, J. M., Barbagelata, R. J., and Lopes, C. A. (2017). Physiological characterization of Saccharomyces uvarum and Saccharomyces eubayanus from Patagonia and their potential for cidermaking. Int. J. Food Microbiol. 249, 9–17. doi: 10.1016/j.ijfoodmicro.2017.02.018
Gonzalez, R., Quirós, M., and Morales, P. (2013). Yeast respiration of sugars by non-Saccharomyces yeast species: a promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci. Technol. 29, 55–61. doi: 10.1016/j.tifs.2012.06.015
Hernández, E., Raventós, M., Auleda, J. M., and Ibarz, A. (2010). Freeze concentration of must in a pilot plant falling film cryoconcentrator. Innov. Food Sci. Emerg. Technol. 11, 130–136. doi: 10.1016/j.ifset.2009.08.014
Intrigliolo, D. S., and Castel, J. R. (2009). Response of Vitis vinifera cv. “Tempranillo” to partial rootzone drying in the field: water relations, growth, yield and fruit and wine quality. Agric. Water Manage. 96, 282–292. doi: 10.1016/j.agwat.2008.08.001
Izquierdo Cañas, P. M., García-Romero, E., Heras Manso, J. M., and Fernández-González, M. (2014). Influence of sequential inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae in the quality of red wines. Eur. Food Res. Technol. 239, 279–286. doi: 10.1007/s00217-014-2220-1
Jolly, N. P., Varela, C., and Pretorius, I. S. (2014). Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 14, 215–237. doi: 10.1111/1567-1364.12111
Jones, G. V., White, M. A., Cooper, O. R., and Storchmann, K. (2005). Climate change and global wine quality. Clim. Change 73, 319–343. doi: 10.1007/s10584-005-4704-2
Kontoudakis, N., Esteruelas, M., Fort, F., Canals, J. M., and Zamora, F. (2011). Use of unripe grapes harvested during cluster thinning as a method for reducing alcohol content and pH of wine. Aust. J. Grape Wine Res. 17, 230–238. doi: 10.1111/j.1755-0238.2011.00142.x
Kutyna, D. R., Varela, C., Henschke, P. A., Chambers, P. J., and Stanley, G. A. (2010). Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci. Technol. 21, 293–302. doi: 10.1016/j.tifs.2010.03.004
Loira, I., Vejarano, R., Bañuelos, M. A., Morata, A., Tesfaye, W., Uthurry, C., et al. (2014). Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT Food Sci. Technol. 59, 915–922. doi: 10.1016/j.lwt.2014.06.019
Masneuf-Pomarède, I., Bely, M., Marullo, P., Lonvaud-Funel, A., and Dubourdieu, D. (2010). Reassessment of phenotypic traits for Saccharomyces bayanus var. uvarum wine yeast strains. Int. J. Food Microbiol. 139, 79–86. doi: 10.1016/j.ijfoodmicro.2010.01.038
Morales, P., Rojas, V., Quirós, M., and Gonzalez, R. (2015). The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 99, 3993–4003. doi: 10.1007/s00253-014-6321-3
Moruno, E. G., Delfini, C., Pessione, E., and Giunta, C. (1993). Factors affecting acetic acid production by yeasts in strongly clarified grape musts. Microbios 74, 249–256.
Nissen, P., and Arneborg, N. (2003). Characterization of early deaths of non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Arch. Microbiol. 180, 257–263. doi: 10.1007/s00203-003-0585-9
Nissen, P., Nielsen, D., and Arneborg, N. (2003). Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell-cell contact-mediated mechanism. Yeast 20, 331–341. doi: 10.1002/yea.965
Offeman, R. D., Franqui-Espiet, D., Cline, J. L., Robertson, G. H., and Orts, W. J. (2010). Extraction of ethanol with higher carboxylic acid solvents and their toxicity to yeast. Sep. Purif. Technol. 72, 180–185. doi: 10.1016/j.seppur.2010.02.004
Oliveira, B. M., Barrio, E., Querol, A., and Pérez-Torrado, R. (2014). Enhanced enzymatic activity of glycerol-3-phosphate dehydrogenase from the cryophilic Saccharomyces kudriavzevii. PLoS ONE 9:e87290. doi: 10.1371/journal.pone.0087290
Pérez-Nevado, F., Albergaria, H., Hogg, T., and Gírio, F. (2006). Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 108, 336–345. doi: 10.1016/j.ijfoodmicro.2005.12.012
Pérez-Torrado, R., Barrio, E., and Querol, A. (2017a). Alternative yeasts for winemaking: Saccharomyces non- cerevisiae and its hybrids. Crit. Rev. Food Sci. Nutr. doi: 10.1080/10408398.2017.1285751. [Epub ahead of print].
Pérez-Torrado, R., Oliveira, B. M., Zemancikova, J., Sychrová, H., and Querol, A. (2016). Alternative glycerol balance strategies among Saccharomyces species in response to winemaking stress. Front. Microbiol. 7:435. doi: 10.3389/fmicb.2016.00435
Pérez-Torrado, R., Rantsiou, K., Perrone, B., Navarro-Tapia, E., Querol, A., and Cocolin, L. (2017b). Ecological interactions among Saccharomyces cerevisiae strains: insight into the dominance phenomenon. Sci. Rep. 7:43603. doi: 10.1038/srep43603
Peris, D., Pérez-Través, L., Belloch, C., and Querol, A. (2016). Enological characterization of Spanish Saccharomyces kudriavzevii strains, one of the closest relatives to parental strains of winemaking and brewing Saccharomyces cerevisiae x S. kudriavzevii hybrids. Food Microbiol. 53, 31–40. doi: 10.1016/j.fm.2015.07.010
Pilipovik, M. V., and Riverol, C. (2005). Assessing dealcoholization systems based on reverse osmosis. J. Food Eng. 69, 437–441. doi: 10.1016/j.jfoodeng.2004.08.035
Piškur, J., Rozpedowska, E., Polakova, S., Merico, A., and Compagno, C. (2006). How did Saccharomyces evolve to become a good brewer? Trends Genet. 22, 183–186. doi: 10.1016/j.tig.2006.02.002
Pretorius, I. S. (2000). Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16, 675–729. doi: 10.1002/1097-0061(20000615)16:8<675::AID-YEA585>3.0.CO;2-B
Quirós, M., Rojas, V., Gonzalez, R., and Morales, P. (2014). Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 181, 85–91. doi: 10.1016/j.ijfoodmicro.2014.04.024
Rodrigues, A. J., Raimbourg, T., Gonzalez, R., and Morales, P. (2016). Environmental factors influencing the efficacy of different yeast strains for alcohol level reduction in wine by respiration. LWT Food Sci. Technol. 65, 1038–1043. doi: 10.1016/j.lwt.2015.09.046
Rossignol, T., Dulau, L., Julien, A., and Blondin, B. (2003). Genome-wide monitoring of wine yeast gene expression during alcoholic fermentation. Yeast 20, 1369–1385. doi: 10.1002/yea.1046
Sadoudi, M., Tourdot-Maréchal, R., Rousseaux, S., Steyer, D., Gallardo-Chacón, J. J., Ballester, J., et al. (2012). Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 32, 243–253. doi: 10.1016/j.fm.2012.06.006
Salmon, J.-M. (2006). Interactions between yeast, oxygen and polyphenols during alcoholic fermentations: practical implications. LWT Food Sci. Technol. 39, 959–965. doi: 10.1016/j.lwt.2005.11.005
Salvado, Z., Arroyo-Lopez, F. N., Barrio, E., Querol, A., and Guillamon, J. M. (2011). Quantifying the individual effects of ethanol and temperature on the fitness advantage of Saccharomyces cerevisiae. Food Microbiol. 28, 1155–1161. doi: 10.1016/j.fm.2011.03.008
Schmidtke, L. M., Blackman, J. W., and Agboola, S. O. (2012). Production technologies for reduced alcoholic wines. J. Food Sci. 77, 25–41. doi: 10.1111/j.1750-3841.2011.02448.x
Shekhawat, K., Bauer, F. F., and Setati, M. E. (2016). Impact of oxygenation on the performance of three non-Saccharomyces yeasts in co-fermentation with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 101, 2479–2491. doi: 10.1007/s00253-016-8001-y
Stribny, J., Gamero, A., Pérez-Torrado, R., and Querol, A. (2015). Saccharomyces kudriavzevii and Saccharomyces uvarum differ from Saccharomyces cerevisiae during the production of aroma-active higher alcohols and acetate esters using their amino acidic precursors. Int. J. Food Microbiol. 205, 41–46. doi: 10.1016/j.ijfoodmicro.2015.04.003
Thomson, J. M., Gaucher, E. A., Burgan, M. F., De Kee, D. W., Li, T., Aris, J. P., et al. (2005). Resurrecting ancestral alcohol dehydrogenases from yeast. Nat. Genet. 37, 630–635. doi: 10.1038/ng1553
Torija, M. (2003). Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 80, 47–53. doi: 10.1016/S0168-1605(02)00144-7
Varela, C., Dry, P. R., Kutyna, D. R., Francis, I. L., Henschke, P. A., Curtin, C. D., et al. (2015). Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine Res. 21, 670–679. doi: 10.1111/ajgw.12187
Varela, C., Kutyna, D. R., Solomon, M. R., Black, C. A., Borneman, A., Henschke, P. A., et al. (2012). Evaluation of gene modification strategies for the development of low-alcohol-wine Yeasts. Appl. Environ. Microbiol. 78, 6068–6077. doi: 10.1128/AEM.01279-12
Varela, C., Sengler, F., Solomon, M., and Curtin, C. (2016). Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 209, 57–64. doi: 10.1016/j.foodchem.2016.04.024
Vidal, S., and Aagaard, O. (2008). Oxygen management during vinifi cation and storage of Shiraz wine. Aust. New Zeal. Wine Ind. J. 23, 56–63.
Vilanova, M., Cortés, S., Santiago, J. L., Martínez, C., and Fernández, E. (2007). Aromatic compounds in wines produced during fermentation: effect of three red cultivars. Int. J. Food Prop. 10, 867–875. doi: 10.1080/10942910601161615
Vivas, N., and Glories, Y. (1996). Role of oak wood ellagitannins in the oxidation process of red wines during aging. Am. J. Enol. Vitic. 47, 103–107.
Keywords: Saccharomyces yeast, wine fermentation, ethanol reduction, fermentation oxygenation, starter cultures
Citation: Alonso-del-Real J, Contreras-Ruiz A, Castiglioni GL, Barrio E and Querol A (2017) The Use of Mixed Populations of Saccharomyces cerevisiae and S. kudriavzevii to Reduce Ethanol Content in Wine: Limited Aeration, Inoculum Proportions, and Sequential Inoculation. Front. Microbiol. 8:2087. doi: 10.3389/fmicb.2017.02087
Received: 08 September 2017; Accepted: 11 October 2017;
Published: 25 October 2017.
Edited by:
Aline Lonvaud, Université de Bordeaux, FranceReviewed by:
Cristian A. Varela, Australian Wine Research Institute, AustraliaGiuseppe Spano, University of Foggia, Italy
Copyright © 2017 Alonso-del-Real, Contreras-Ruiz, Castiglioni, Barrio and Querol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amparo Querol, aquerol@iata.csic.es
†Present Address: Gabriel L. Castiglioni, Department of Food Engineering, School of Agronomy, Federal University of Goiás, Goiania, Brazil
 Javier Alonso-del-Real
Javier Alonso-del-Real Alba Contreras-Ruiz1,2
Alba Contreras-Ruiz1,2 Eladio Barrio
Eladio Barrio Amparo Querol
Amparo Querol