- 1Virology Laboratory, Basil Hetzel Institute for Translational Medicine, Discipline of Surgery, University of Adelaide, Adelaide, SA, Australia
- 2Molecular Mucosal Vaccine Immunology Group, The John Curtin School of Medical Research, The Australian National University, Canberra, ACT, Australia
Human immunodeficiency virus (HIV)-1 has infected >75 million individuals globally, and, according to the UN, is responsible for ~2.1 million new infections and 1.1 million deaths each year. Currently, there are ~37 million individuals with HIV infection and the epidemic has already resulted in 35 million deaths. Despite the advances of anti-retroviral therapy (ART), a cost-effective vaccine remains the best long-term solution to end the HIV-1 epidemic especially given that the vast majority of infected individuals live in poor socio-economic regions of the world such as Sub-Saharan Africa which limits their accessibility to ART. The modest efficacy of the RV144 Thai trial provides hope that a vaccine for HIV-1 is possible, but as markers for sterilizing immunity are unknown, the design of an effective vaccine is empirical, although broadly cross-reactive neutralizing antibodies (bNAb) that can neutralize various quasispecies of HIV-1 are considered crucial. Since HIV-1 transmission often occurs at the genito-rectal mucosa and is cell-associated, there is a need to develop vaccines that can elicit CD8+ T cell immunity with the capacity to kill virus infected cells at the genito-rectal mucosa and the gut. Here we discuss the recent progress made in developing T cell-mediated vaccines for HIV-1 and emphasize the need to elicit mucosal tissue-resident memory CD8+ T (CD8+ Trm) cells. CD8+ Trm cells will likely form a robust front-line defense against HIV-1 and eliminate transmitter/founder virus-infected cells which are responsible for propagating HIV-1 infections following transmission in vast majority of cases.
Introduction
Since the discovery of human immunodeficiency virus (HIV)-1 as the causative agent of the acquired immunodeficiency syndrome (AIDS), significant progress has been made to thwart the worldwide spread of the virus despite many setbacks. The “Berlin Patient” highlighted that a cure for HIV/AIDS is possible through bone marrow transplantation (Hütter et al., 2009). However, the excessive cost of this procedure and the lack of compatible donors severely limit the application of this cure strategy. The use of anti-retroviral therapy (ART) remains the most feasible medical intervention to suppress the virus in patients. Furthermore, early treatment of infected individuals with ART can prevent HIV-1 transmission to uninfected individuals (Cohen et al., 2011) while the daily use of anti-retroviral drugs Truvada (i.e., for pre-exposure prophylaxis) by individuals can successfully prevent acquisition of HIV-1 (Molina et al., 2015). Unfortunately, it is estimated that more than half of the HIV-infected individuals are undiagnosed and 3/5 people infected with this virus do not have access to ART. This hinders the ability of ART to control the epidemic and given that ART does not eliminate latent reservoirs of HIV-1, strict and life-long adherence of ART is required for the therapy to be effective in patients.
A vaccine is essential to complement the efforts of ART to bring an end to HIV/AIDS especially in HIV endemic regions (e.g., Sub-Saharan Africa) manifested by the inability of patients to access ART adequately due to their poor socio-economic status. The main limitations to the development of an effective HIV-1 vaccine include the ability of the virus to mutate rapidly to facilitate immune escape and its ability to infect, deplete and establish viral reservoirs in CD4+ T cells. Despite these limitations, vaccines that elicit (i) broadly neutralizing antibodies (bNAb), (ii) non-neutralizing antibodies that direct virus-infected cells for destruction, or (iii) CD8+ T cell immunity have emerged as attractive targets for the future of HIV-1 vaccine development. In this brief review, we will discuss the avenues and challenges for developing an effective T cell-mediated vaccine for HIV-1 with a particular focus on CD8+ T cell immunity.
Why Do We Need A Vaccine Which Will Elicit CD8+ T Cell Immunity?
There are several reasons which support the development of a vaccine to elicit CD8+ T cell immunity against HIV-1.
1. Elite controllers of HIV-1 and simian immunodeficiency virus (SIV) provide valuable clues for vaccine development because these individuals are able to suppress the virus to undetectable levels for prolonged periods in the absence of ART. Although enhanced activity of anti-viral CD8+ T cells is not always predictive of elite control of infection (Blankson, 2010), numerous studies in humans and macaques suggest that elite control strongly correlates with- or is dependent on- anti-viral CD8+ T cell responses that target conserved regions of SIV and HIV-1 proteins (Pontesilli et al., 1998; Saez-Cirion et al., 2007; Wijesundara et al., 2011; Chowdhury et al., 2015).
2. Anti-viral CD8+ T cells can directly kill virus-infected cells and can establish residency at sites where HIV-1 will likely be encountered during transmission (see below). Given that most HIV-1 infections are initiated by a single variant (i.e., transmitted/founder virus) (Zhu et al., 1993; Keele et al., 2008), the presence of HIV-specific CD8+ T cells at transmission sites will likely be crucial for reducing the viral set point and thereby reducing the reservoir size.
3. Passive transfer studies and vector-based immunoprophylaxis studies suggest that bNAb will be very effective in protection against HIV-1 (Balazs et al., 2011; Scheid et al., 2016; Caskey et al., 2017). Non-neutralizing antibodies and bNAb with the capacity to direct killing of cell-associated forms of HIV-1 have also been identified (Haynes et al., 2012; Malbec et al., 2013; Bruel et al., 2016). Some recent progress has also been made to develop a vaccine which will elicit bNAb (Pauthner et al., 2017). However, a significant conceptual breakthrough is still needed to develop a vaccination strategy to elicit high titres of potent bNAb and non-neutralizing antibodies especially at HIV-1 transmission sites. Furthermore, to be effective in preventing cell-to-cell transmission by eliminating cell-associated forms of the virus at transmission sites, antibody-based vaccine strategies will probably require sufficient numbers of innate effector cells (e.g., natural killer cells) to be present at transmission sites prior to a transmission event.
4. Therapeutic vaccines against HIV-1 and SIV have failed to demonstrate efficacy. However, a recent study demonstrated that a vaccination regimen which elicited broad CD8+ T cell immunity to SIV Gag, Pol, and envelope (Env) proteins protected SIV-infected macaques undergoing treatment interruption (Borducchi et al., 2016). Following on from this landmark study, a “kick and kill” approach (Shan et al., 2012) was exploited to increase the therapeutic efficacy of a recombinant modified vaccinia virus Ankara (MVA) vaccine in the ongoing BCN02-Romi (NCT02616874) proof of concept study. The findings presented by Beatriz Mothe at the 2017 Conference on Retroviruses and Opportunistic Infections in Seattle suggest that CD8+ T cell responses targeting conserved regions of HIV-1 were involved in protection.
The Importance of Mucosal Immunity for HIV-1 Vaccine Efficacy
HIV-1 is first encountered at the mucosa (genito-rectal tract), and the gastro-intestinal tract is the major site of HIV replication and CD4+ T cell depletion (Veazey and Lackner, 1998). It is well-established that a vaccine delivered mucosally can induce long-lasting immune responses at these mucosal sites, as systemic vaccination rarely induces optimal sustained mucosal immunity (Belyakov and Ahlers, 2009; Fouda et al., 2013; Sun et al., 2013). Mucosa-associated lymphoid tissues are linked to the “common mucosal immune system,” whereby antigenic stimulation at one site results in both local and distant mucosal immunity. According to the site of mucosal vaccination (i.e., oral, nasal, rectal, or vaginal), the immunity generated at different sites can be vastly different, mainly due to the unique antigen presentation mechanisms (which is dependent on the type of antigen presenting cells present at different antigen-sampling sites) and the homing markers they acquire at the local mucosa (Trivedi and Ranasinghe, 2015; Trivedi et al., 2015). For example, a vaccine delivered intranasally will induce mucosal immunity at the lung, genito-rectal tract and also the gastro-intestinal tract, whereas rectal delivery will only induce immunity in the rectum and the some instances in the intestine (Ranasinghe et al., 2011; De Rose et al., 2014; Tomusange et al., 2016a). However, different adjuvants and cytokines have the capacity to promote T cell migration to the mucosa by modulating the initial antigen presenting cell environment at the vaccination site (Xi et al., 2012; Trivedi et al., 2014). This may explain how some viral vectors (i.e., pox viral vector-based vaccines) delivered systemically can induce mucosal immunity (Stevceva et al., 2002; Marlin et al., 2017).
Despite that HIV-1 is transmitted mainly through mucosal surfaces, no mucosal vaccine has yet entered efficacy trials in humans, although a few phase I trials have been conducted (Leroux-Roels et al., 2013; Nyombayire et al., 2017). Studies in non-human primate models have demonstrated that mucosal CD8+ T cell responses can better control a mucosal viral challenge while systemic CD8+ T cell immunity is not fully effective against mucosal infection (Kent et al., 2005; Belyakov et al., 2006; Ferre et al., 2009). Several studies in animal models from our laboratories suggest that mucosal vaccination strategies can enhance the quality, viz. avidity and polyfunctionality, of HIV-specific CD8+ T cells and protection against surrogate HIV-1 challenge models (Ranasinghe et al., 2007, 2013, 2014). High quality CD8+ T cells can recognize and respond to low densities of peptide-major histocompatibility class I complexes, thereby eliminating infected cells at very early stages of infection (Alexander-Miller et al., 1996; Wijesundara and Ranasinghe, 2012). Several studies in humans suggest that high quality CD8+ T cell responses correlate with elite control of HIV-1 infection (Betts et al., 2006; Almeida et al., 2007; Berger et al., 2011). Consequently mucosal vaccination strategies are desirable to achieve protective outcomes against HIV-1 infections especially given that mucosal immunisations can elicit high quality mucosal CD8+ T cell responses. It is also likely that a mucosal vaccination regimen could be exploited to elicit tissue resident memory CD8+ T cells in the genito-rectal mucosa, which will likely form a front-line defense against HIV-1 as discussed below.
Implications for Eliciting Tissue Resident Memory CD8+ T Cells
The optimum protective outcomes following vaccination are achieved if immunity is established at sites where pathogens are initially encountered or migrate to in order to establish persistence or cause disease. Several recent discoveries in mice suggest that the presence of CD8+ tissue resident memory T (Trm) cells significantly enhance or are obligatory for protection against infections resulting from influenza virus, herpes simplex virus (HSV), vaccinia virus (VV), and Plasmodium berghei exposure (Fernandez-Ruiz et al., 2016; Mueller and Mackay, 2016). CD8+ Trm cells reside in tissues for the lifespan of the individual and the expression of CD69, CD103 (Mueller and Mackay, 2016), and/or high expression of CD11a (McNamara et al., 2017) are unique biomarkers that can be used to identify these cells in tissues of mice.
The vast majority of HIV-1 transmissions occur through sexual intercourse and heterosexual intercourse accounts for an estimated 70–80% of global HIV-1 transmissions. Thus, the appearance of HIV-specific CD8+ Trm cells in the rectal or cervicovaginal mucosa will likely contribute to protection during initial exposure to HIV-1 resulting from sexual transmission (Figure 1). The first study to describe the use of a vaccination regimen to elicit vaginal CD8+ Trm cells used a “prime and pull” strategy against HSV-2 (Shin and Iwasaki, 2012). The strategy involved priming the CD8+ T cells with a replication defective HSV-2 subcutaneously and then “pulling” the primed cells into the vaginal mucosa following intravaginal (i.vag) topical application of the CXCL9 and CXCL10. A few studies since have used i.vag delivery of human papillomavirus pseudovirions (HPV PsV) to induce the formation of cervicovaginal CD8+ Trm cells which correlated with protection against intravaginal challenge with VV and HSV (Çuburu et al., 2012, 2015). The cervicovaginal CD8+ Trm cells that were established following i.vag immunisations with HPV PsV express CD44, CD69, CXCR3, CD49a (α1 integrin), and CD103 (Çuburu et al., 2015).
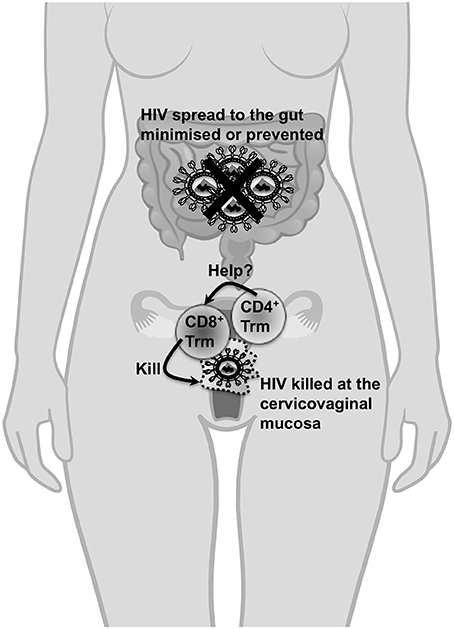
Figure 1. The importance of cervicovaginal Trm cells in protection against HIV-1. Cervicovaginal Trm cells will migrate through the cervicovaginal tissues for the lifespan of an individual and do not need to be recruited to the cervix or the vagina unlike other memory T cell subsets (which could take days to be recruited to these sites following primary exposure) during HIV-1 exposure. Thus, the presence of CD8+ Trm cells in cervicovaginal tissues is crucial to kill transmitted cell-associated forms of HIV and nascent infected cells resulting from cell-free virus during transmission. Most importantly, the transmitter/founder virus which initiates the HIV infection will likely be most vulnerable for destruction when CD8+ Trm cells are present at the cervicovaginal mucosa during primary exposure. Consequently, the likelihood of the virus spreading systemically, especially to the gut where a vast majority of CD4+ T cells are depleted during acute infection, is minimized or prevented. Furthermore, the presence of these Trm cells in this instance is also expected to significantly reduce the viral set point to an extent that it is unlikely for the infected individual to transmit the virus. It is unclear whether CD4+ Trm cells form in the cervicovaginal mucosa, whether they can act as targets for HIV infection or whether they will provide essential help to CD8+ Trm cells. The protective role of CD8+ Trm cells will likely apply to scenarios where HIV-1 is exposed in the rectum although there are no studies to demonstrate how these cells can be elicited in this site.
These studies provide promise that vaccines can be exploited to elicit CD8+ Trm cells in the genito-rectal mucosa for protection against HIV-1 but several challenges remain in order to achieve this goal. Ideally, an HIV-1 vaccine will not require i.vag delivery as studies in macaques have highlighted that it will be difficult for vaccine vectors to penetrate the epithelium barrier of the vagina (Xu et al., 2013). Even in mice, i.vag delivery of HPV PsV required the use of progestins (Depo-Provera) to reduce the thickness of the vaginal epithelium and ensure PsV infection of cervicovaginal keratinocytes. Although a few recent studies suggest that expression of CD69 and CD103 could be used to characterize CD8+ Trm cells in the vagina of humans (Moylan et al., 2016; Posavad et al., 2017), the markers that uniquely represent these cells in the genito-rectal mucosa in macaques and humans are poorly characterized or unclear.
CD4+ T cell help allows dendritic cells to effectively prime effector CD8+ T cell responses and facilitate entry of effector CD8+ T cells into the female reproductive tract and ensure long-term survival of memory CD8+ T cells (Laidlaw et al., 2016). It is likely that a vaccine will need to elicit CD4+ T cell helper responses for efficient induction and survival of CD8+ Trm cells in cervicovaginal tissues. However, CD4+ T cells are major targets of HIV-1 infection and provision of cytokines such as interleukin-15 and type I interferons can be used to overcome the requirement of CD4+ T cell help for priming and survival of CD8+ T cells (Laidlaw et al., 2016). The presence/contribution of vaginal CD4+ Trm cells in protection against- or pathogenesis of- HIV infection is not well-understood (Figure 1). A better understanding of the requirement of CD4+ T helper cell responses for eliciting long-term CD8+ Trm cell responses in the reproductive tract and the rectum is necessary for rational HIV-1 vaccine design.
Vectors and Immunogens: Lessons from Humans and Macaques
The failures of the only phase IIb/phase III trials (STEP, HVTN 503, and HVTN 505) to test the efficacy of a vaccine designed to elicit CD8+ T cells (Buchbinder et al., 2008; Hammer et al., 2013) have highlighted that the choice of vectors and immunogens are important determinants of vaccine efficacy in humans. In summary, the outcomes of these trials suggest that irrespective of the circumcision status of vaccine recipients and the presence of pre-existing immunity to Adenovirus serotype 5 (Ad5), the Ad5 vector used for vaccination is ineffective in humans. The exact reasons for the lack of efficacy in these trials is not known. However, eminent researchers have predicted that this could be due to the presence of Ad5-specific CD4+ T cells at mucosal sites (Barouch, 2010) and immunodominant CD8+ T cell responses directed to the highly variable Env protein (McMichael and Koff, 2014).
After the failures of the Ad5 vaccine, cytomegalovirus (CMV), Adenovirus serotype 26 (Ad26), Adenovirus serotype 35 (Ad35), and MVA vectors have emerged as extremely promising candidates for eliciting protective T cell responses against HIV-1. Vaccination of macaques with rhesus CMV encoding SIV Gag, Pol, Env, Rev, Nef, and Tat proteins provided superior and long-term control of SIVmac251 infection in 5/12 intra-rectally challenged animals (Hansen et al., 2011). Protection in this study was associated with robust effector memory CD8+ T cell responses and CD8+ T cell immunity targeting a broad-range of non-classical Major Histocompatibility Class-II restricted Gag epitopes (Hansen et al., 2011, 2013). Thus, although CMV may not represent an ideal vector with respect to safety of use, the strategy suggests that protection against persistent infection is possible, provided the CD8+ T cell responses are sufficiently robust and broad.
An alternative strategy that has gained considerable momentum since its development is the use of 2-valent mosaic antigens for vaccination (Barouch et al., 2010). The 2-valent mosaic antigens encompass native HIV Gag, Pol, and Env sequences that are most conserved amongst the isolates of clades B and C, and include as many CD4+ and CD8+ T cell epitopes as possible. Vaccines encoding mosaic antigens are capable of increasing the breadth of T cell responses to HIV Gag from clades A, B, and C whilst not compromising humoral immunity to Env from these clades (Barouch et al., 2010). Furthermore, vaccination of uninfected macaques with Ad26, Ad35, and MVA mosaic vaccines can elicit broad T cell responses and protective polyfunctional antibody responses especially when a liposome-based Env protein boost was used prior to SIV challenge (Barouch et al., 2010, 2013, 2015). Recently, a landmark therapeutic vaccination study exploited the Ad26 and MVA mosaic vaccines in a regimen involving a toll like receptor 7 agonist to increase the breadth of T cell responses to SIV Gag, Pol, and Env (Borducchi et al., 2016). In this study, the increased breadth of SIV-specific T cell immunity rather than humoral immunity correlated with partial protection and the regimen also allowed 3/9 vaccinated macaques to completely control SIVmac251 infection (Borducchi et al., 2016).
There may well also be a place for DNA vaccines especially given their recent success in the clinic (Trimble et al., 2015). We have recently shown that a novel cytolytic DNA vaccine which encodes a mutant form of perforin in addition to the immunogen is more immunogenic than a canonical DNA vaccine (Gargett et al., 2014). We have also shown that a DNA vaccine encoding Tat generated anti-Tat neutralizing antibodies (NAb) that inhibited the function of Tat in an in vitro transactivation assay (Tomusange et al., 2016b). As recent clinical trials reported that a clade B Tat vaccine induced cross-clade anti-Tat NAb in patients treated with ART, increased CD4+ T cell counts and reduced the viral load (Ensoli et al., 2016; Loret et al., 2016), some consideration should be given to combining a Gag/Pol/Env T cell vaccine with a vaccine designed to elicit anti-Tat NAb. Overall, the studies discussed above suggest that a vaccine vector designed to elicit protective T cell immunity against HIV-1 will need to encode as many conserved T cell immunogens of the virus as possible.
Future Directions
Although there are no correlates of protection from HIV-1, the induction of bNAb is considered to be the most desirable outcome for a HIV-1 vaccine, but a vaccine able to elicit such antibodies has not yet been developed for humans. However, many successful live attenuated virus vaccines (e.g., measles and yellow fever virus) that elicit protective NAb also elicit T cell immunity (Querec et al., 2006). HIV-1 employs multiple strategies to evade NAb (Schiffner et al., 2013) and cell to cell transmission is effective (Smith and Derdeyn, 2017). This suggests that an effective HIV-1 NAb vaccine regimen should also elicit CD8+ killer T cells, most likely against a broad range of highly conserved viral antigens as discussed previously. Thus, it is expected that a vaccine which will elicit heterotypic immunity, viz. bNAb and CD8+ T cell immunity, targeting conserved viral epitopes will be highly protective, but there are significant challenges to achieve this goal (Table 1). Perhaps the most urgent need with respect to a T cell-mediated vaccine is to develop a vaccine with the ability to not only induce systemic immune responses but also induce killer CD8+ T cell immunity at genito-rectal mucosa and the gut. In this regard, a vaccine with the capacity to elicit CD8+ Trm cells in the genito-rectal mucosa and the gut represents a plausible and a rational path for HIV-1 vaccine design in the future.
Author Contributions
DW conceived the initial draft of the manuscript. CR, BG, and EG revised many parts of the manuscript, and contributed to finalize the manuscript.
Funding
The following grants have supported the work conducted in our laboratories and cited in the manuscript: From the National Health and Medical Research Council (NHMRC): grants APP1026293 (EG), APP525431 (CR), APP543139 (EG), and APP543143 (EG). From the Australian Centre for HIV and Hepatitis Virology Research, CR received an EOI grant.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Hospital Research Foundation supports an early career fellowship for DW. The image of the human torso with the reproductive tract and the intestine in Figure 1 was purchased from Schutterstock and drawn by artists Skalapendra and Marochkina Anastasiia.
References
Alexander-Miller, M. A., Leggatt, G. R., and Berzofsky, J. A. (1996). Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. U.S.A. 93, 4102–4107.
Almeida, J. R., Price, D. A., Papagno, L., Arkoub, Z. A., Sauce, D., Bornstein, E., et al. (2007). Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204, 2473–2485. doi: 10.1084/jem.20070784
Balazs, A. B., Chen, J., Hong, C. M., Rao, D. S., Yang, L., and Baltimore, D. (2011). Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481, 81–84. doi: 10.1038/nature10660
Barouch, D. H. (2010). Novel adenovirus vector-based vaccines for HIV-1. Curr. Opin. HIV AIDS 5, 386–390. doi: 10.1097/COH.0b013e32833cfe4c
Barouch, D. H., Alter, G., Broge, T., Linde, C., Ackerman, M. E., Brown, E. P., et al. (2015). Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349, 320–324. doi: 10.1126/science.aab3886
Barouch, D. H., O'Brien, K. L., Simmons, N. L., King, S. L., Abbink, P., Maxfield, L. F., et al. (2010). Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat. Med. 16, 319–323. doi: 10.1038/nm.2089
Barouch, D. H., Stephenson, K. E., Borducchi, E. N., Smith, K., Stanley, K., McNally, A. G., et al. (2013). Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155, 531–539. doi: 10.1016/j.cell.2013.09.061
Belyakov, I. M., and Ahlers, J. D. (2009). What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J. Immunol. 183, 6883–6892. doi: 10.4049/jimmunol.0901466
Belyakov, I. M., Kuznetsov, V. A., Kelsall, B., Klinman, D., Moniuszko, M., Lemon, M., et al. (2006). Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood 107, 3258–3264. doi: 10.1182/blood-2005-11-4374
Berger, C. T., Frahm, N., Price, D. A., Mothe, B., Ghebremichael, M., Hartman, K. L., et al. (2011). High-functional-avidity cytotoxic T lymphocyte responses to HLA-B-restricted Gag-derived epitopes associated with relative HIV control. J. Virol. 85, 9334–9345. doi: 10.1128/JVI.00460-11
Betts, M. R., Nason, M. C., West, S. M., De Rosa, S. C., Migueles, S. A., Abraham, J., et al. (2006). HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107, 4781–4789. doi: 10.1182/blood-2005-12-4818
Blankson, J. N. (2010). Effector mechanisms in HIV-1 infected elite controllers: highly active immune responses? Antiviral Res. 85, 295–302. doi: 10.1016/j.antiviral.2009.08.007
Borducchi, E. N., Cabral, C., Stephenson, K. E., Liu, J., Abbink, P., Ng'ang'a, D., et al. (2016). Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540, 284–287. doi: 10.1038/nature20583
Bruel, T., Guivel-Benhassine, F., Amraoui, S., Malbec, M., Richard, L., Bourdic, K., et al. (2016). Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat. Commun. 7:10844. doi: 10.1038/ncomms10844
Buchbinder, S. P., Mehrotra, D. V., Duerr, A., Fitzgerald, D. W., Mogg, R., Li, D., et al. (2008). Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372, 1881–1893. doi: 10.1016/S0140-6736(08)61591-3
Caskey, M., Schoofs, T., Gruell, H., Settler, A., Karagounis, T., Kreider, E. F., et al. (2017). Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat. Med. 23, 185–191. doi: 10.1038/nm.4268
Chowdhury, A., Hayes, T. L., Bosinger, S. E., Lawson, B. O., Vanderford, T., Schmitz, J. E., et al. (2015). Differential impact of in vivo CD8+ T lymphocyte depletion in controller versus progressor simian immunodeficiency virus-infected macaques. J. Virol. 89, 8677–8686. doi: 10.1128/JVI.00869-15
Cohen, M. S., Chen, Y. Q., McCauley, M., Gamble, T., Hosseinipour, M. C., Kumarasamy, N., et al. (2011). Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 365, 493–505. doi: 10.1056/NEJMoa1105243
Çuburu, N., Graham, B. S., Buck, C. B., Kines, R. C., Pang, Y. Y., Day, P. M., et al. (2012). Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J. Clin. Invest. 122, 4606–4620. doi: 10.1172/JCI63287
Çuburu, N., Wang, K., Goodman, K. N., Pang, Y. Y., Thompson, C. D., Lowy, D. R., et al. (2015). Topical herpes simplex virus 2 (HSV-2) vaccination with human papillomavirus vectors expressing gB/gD ectodomains induces genital-tissue-resident memory CD8+ T cells and reduces genital disease and viral shedding after HSV-2 challenge. J. Virol. 89, 83–96. doi: 10.1128/JVI.02380-14
De Rose, R., Kent, S. J., and Ranasinghe, C. (2014). “Prime-boost vaccination: Impact on the HIV-1 vaccine field,” in Novel Approaches and Strategies for Biologics, Vaccines and Cancer Therapies, eds M. Singh and M. Salnikova (Cambridge, MA: Elsevier, Inc), 289–313.
Ensoli, B., Nchabeleng, M., Ensoli, F., Tripiciano, A., Bellino, S., Picconi, O., et al. (2016). HIV-Tat immunization induces cross-clade neutralizing antibodies and CD4(+) T cell increases in antiretroviral-treated South African volunteers: a randomized phase II clinical trial. Retrovirology 13:34. doi: 10.1186/s12977-016-0261-1
Fernandez-Ruiz, D., Ng, W. Y., Holz, L. E., Ma, J. Z., Zaid, A., Wong, Y. C., et al. (2016). Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity 45, 889–902. doi: 10.1016/j.immuni.2016.08.011
Ferre, A. L., Hunt, P. W., Critchfield, J. W., Young, D. H., Morris, M. M., Garcia, J. C., et al. (2009). Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 113, 3978–3989. doi: 10.1182/blood-2008-10-182709
Fouda, G. G., Amos, J. D., Wilks, A. B., Pollara, J., Ray, C. A., Chand, A., et al. (2013). Mucosal immunization of lactating female rhesus monkeys with a transmitted/founder HIV-1 envelope induces strong Env-specific IgA antibody responses in breast milk. J. Virol. 87, 6986–6999. doi: 10.1128/JVI.00528-13
Gargett, T., Grubor-Bauk, B., Garrod, T. J., Yu, W., Miller, D., Major, L., et al. (2014). Induction of antigen-positive cell death by the expression of perforin, but not DTa, from a DNA vaccine enhances the immune response. Immunol. Cell Biol. 92, 359–367. doi: 10.1038/icb.2013.93
Hammer, S. M., Sobieszczyk, M. E., Janes, H., Karuna, S. T., Mulligan, M. J., Grove, D., et al. (2013). Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 369, 2083–2092. doi: 10.1056/NEJMoa1310566
Hansen, S. G., Ford, J. C., Lewis, M. S., Ventura, A. B., Hughes, C. M., Coyne-Johnson, L., et al. (2011). Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473, 523–527. doi: 10.1038/nature10003
Hansen, S. G., Sacha, J. B., Hughes, C. M., Ford, J. C., Burwitz, B. J., Scholz, I., et al. (2013). Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340:1237874. doi: 10.1126/science.1237874
Haynes, B. F., Gilbert, P. B., McElrath, M. J., Zolla-Pazner, S., Tomaras, G. D., Alam, S. M., et al. (2012). Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366, 1275–1286. doi: 10.1056/NEJMoa1113425
Hütter, G., Nowak, D., Mossner, M., Ganepola, S., Müssig, A., Allers, K., et al. (2009). Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698. doi: 10.1056/NEJMoa0802905
Keele, B. F., Giorgi, E. E., Salazar-Gonzalez, J. F., Decker, J. M., Pham, K. T., Salazar, M. G., et al. (2008). Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U.S.A. 105, 7552–7557. doi: 10.1073/pnas.0802203105
Kent, S. J., Dale, C. J., Ranasinghe, C., Stratov, I., De Rose, R., Chea, S., et al. (2005). Mucosally-administered human-simian immunodeficiency virus DNA and fowlpoxvirus-based recombinant vaccines reduce acute phase viral replication in macaques following vaginal challenge with CCR5-tropic SHIVSF162P3. Vaccine 23, 5009–5021. doi: 10.1016/j.vaccine.2005.05.032
Laidlaw, B. J., Craft, J. E., and Kaech, S. M. (2016). The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat. Rev. Immunol. 16, 102–111. doi: 10.1038/nri.2015.10
Leroux-Roels, G., Maes, C., Clement, F., van Engelenburg, F., van den Dobbelsteen, M., Adler, M., et al. (2013). Randomized phase I: safety, immunogenicity and mucosal antiviral activity in young healthy women vaccinated with HIV-1 Gp41 P1 peptide on virosomes. PLoS ONE 8:e55438. doi: 10.1371/journal.pone.0055438
Loret, E. P., Darque, A., Jouve, E., Loret, E. A., Nicolino-Brunet, C., Morange, S., et al. (2016). Intradermal injection of a Tat Oyi-based therapeutic HIV vaccine reduces of 1.5 log copies/mL the HIV RNA rebound median and no HIV DNA rebound following cART interruption in a phase I/II randomized controlled clinical trial. Retrovirology 13:21. doi: 10.1186/s12977-016-0251-3
Malbec, M., Porrot, F., Rua, R., Horwitz, J., Klein, F., Halper-Stromberg, A., et al. (2013). Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J. Exp. Med. 210, 2813–2821. doi: 10.1084/jem.20131244
Marlin, R., Nugeyre, M. T., Tchitchek, N., Parenti, M., Hocini, H., Benjelloun, F., et al. (2017). Modified vaccinia virus ankara vector induces specific cellular and humoral responses in the female reproductive tract, the main HIV portal of entry. J. Immunol. 199, 1923–1932. doi: 10.4049/jimmunol.1700320
McMichael, A. J., and Koff, W. C. (2014). Vaccines that stimulate T cell immunity to HIV-1: the next step. Nat. Immunol. 15, 319–322. doi: 10.1038/ni.2844
McNamara, H. A., Cai, Y., Wagle, M. V., Sontani, Y., Roots, C. M., Miosge, L. A., et al. (2017). Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci. Immunol. 2:eaaj1996. doi: 10.1126/sciimmunol.aaj1996
Molina, J. M., Capitant, C., Spire, B., Pialoux, G., Cotte, L., Charreau, I., et al. (2015). On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N. Engl. J. Med. 373, 2237–2246. doi: 10.1056/NEJMoa1506273
Moylan, D. C., Goepfert, P. A., Kempf, M. C., Saag, M. S., Richter, H. E., Mestecky, J., et al. (2016). Diminished CD103 (alphaEbeta7) expression on resident T cells from the female genital tract of HIV-positive women. Pathog. Immun. 1, 371–387. doi: 10.20411/pai.v1i2.166
Mueller, S. N., and Mackay, L. K. (2016). Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89. doi: 10.1038/nri.2015.3
Nyombayire, J., Anzala, O., Gazzard, B., Karita, E., Bergin, P., Hayes, P., et al. (2017). First-in-human evaluation of the safety and immunogenicity of an intranasally administered replication-competent sendai virus-vectored HIV Type 1 gag vaccine: induction of potent T-cell or antibody responses in prime-boost regimens. J. Infect. Dis. 215, 95–104. doi: 10.1093/infdis/jiw500
Pauthner, M., Havenar-Daughton, C., Sok, D., Nkolola, J. P., Bastidas, R., Boopathy, A. V., et al. (2017). Elicitation of robust tier 2 Neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46, 1073–1088.e6. doi: 10.1016/j.immuni.2017.05.007
Pontesilli, O., Klein, M. R., Kerkhof-Garde, S. R., Pakker, N. G., de Wolf, F., Schuitemaker, H., et al. (1998). Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J. Infect. Dis. 178, 1008–1018.
Posavad, C. M., Zhao, L., Dong, L., Jin, L., Stevens, C. E., Magaret, A. S., et al. (2017). Enrichment of herpes simplex virus type 2 (HSV-2) reactive mucosal T cells in the human female genital tract. Mucosal Immunol. 10, 1259–1269. doi: 10.1038/mi.2016.118
Querec, T., Bennouna, S., Alkan, S., Laouar, Y., Gorden, K., Flavell, R., et al. (2006). Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 203, 413–424. doi: 10.1084/jem.20051720
Ranasinghe, C., Eyers, F., Stambas, J., Boyle, D. B., Ramshaw, I. A., and Ramsay, A. J. (2011). A comparative analysis of HIV-specific mucosal/systemic T cell immunity and avidity following rDNA/rFPV and poxvirus-poxvirus prime boost immunisations. Vaccine 29, 3008–3020. doi: 10.1016/j.vaccine.2011.01.106
Ranasinghe, C., Trivedi, S., Stambas, J., and Jackson, R. J. (2013). Unique IL-13Ralpha2-based HIV-1 vaccine strategy to enhance mucosal immunity, CD8(+) T-cell avidity and protective immunity. Mucosal Immunol. 6, 1068–1080. doi: 10.1038/mi.2013.1
Ranasinghe, C., Trivedi, S., Wijesundara, D. K., and Jackson, R. J. (2014). IL-4 and IL-13 receptors: roles in immunity and powerful vaccine adjuvants. Cytokine Growth Factor Rev. 25, 437–442. doi: 10.1016/j.cytogfr.2014.07.010
Ranasinghe, C., Turner, S. J., McArthur, C., Sutherland, D. B., Kim, J. H., Doherty, P. C., et al. (2007). Mucosal HIV-1 pox virus prime-boost immunization induces high-avidity CD8+ T cells with regime-dependent cytokine/granzyme B profiles. J. Immunol. 178, 2370–2379. doi: 10.4049/jimmunol.178.4.2370
Saez-Cirion, A., Lacabaratz, C., Lambotte, O., Versmisse, P., Urrutia, A., Boufassa, F., et al. (2007). HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U.S.A. 104, 6776–6781. doi: 10.1073/pnas.0611244104
Scheid, J. F., Horwitz, J. A., Bar-On, Y., Kreider, E. F., Lu, C. L., Lorenzi, J. C., et al. (2016). HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535, 556–560. doi: 10.1038/nature18929
Schiffner, T., Sattentau, Q. J., and Dorrell, L. (2013). Development of prophylactic vaccines against HIV-1. Retrovirology 10:72. doi: 10.1186/1742-4690-10-72
Shan, L., Deng, K., Shroff, N. S., Durand, C. M., Rabi, S. A., Yang, H. C., et al. (2012). Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36, 491–501. doi: 10.1016/j.immuni.2012.01.014
Shin, H., and Iwasaki, A. (2012). A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467. doi: 10.1038/nature11522
Smith, S. A., and Derdeyn, C. A. (2017). New connections: cell-to-cell HIV-1 transmission, resistance to broadly neutralizing antibodies, and an envelope sorting motif. J. Virol. 91:e00149-17. doi: 10.1128/JVI.00149-17
Stevceva, L., Alvarez, X., Lackner, A. A., Tryniszewska, E., Kelsall, B., Nacsa, J., et al. (2002). Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8(+) T-cell responses in mucosal tissues of macaques. J. Virol. 76, 11659–11676. doi: 10.1128/JVI.76.22.11659-11676.2002
Sun, C., Chen, Z., Tang, X., Zhang, Y., Feng, L., Du, Y., et al. (2013). Mucosal priming with a replicating-vaccinia virus-based vaccine elicits protective immunity to simian immunodeficiency virus challenge in rhesus monkeys. J. Virol. 87, 5669–5677. doi: 10.1128/JVI.03247-12
Tomusange, K., Wijesundara, D., Gummow, J., Garrod, T., Li, Y., Gray, L., et al. (2016b). A HIV-Tat/C4-binding protein chimera encoded by a DNA vaccine is highly immunogenic and contains acute EcoHIV infection in mice. Sci. Rep. 6:29131. doi: 10.1038/srep29131
Tomusange, K., Wijesundara, D., Gummow, J., Wesselingh, S., Suhrbier, A., Gowans, E. J., et al. (2016a). Mucosal vaccination with a live recombinant rhinovirus followed by intradermal DNA administration elicits potent and protective HIV-specific immune responses. Sci. Rep. 6:36658. doi: 10.1038/srep36658
Trimble, C. L., Morrow, M. P., Kraynyak, K. A., Shen, X., Dallas, M., Yan, J., et al. (2015). Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 386, 2078–2088. doi: 10.1016/S0140-6736(15)00239-1
Trivedi, S., and Ranasinghe, C. (2015). The influence of immunization route, tissue microenvironment, and cytokine cell milieu on HIV-specific CD8+ T cells measured using fluidigm dynamic arrays. PLoS ONE 10:e0126487. doi: 10.1371/journal.pone.0126487
Trivedi, S., Jackson, R. J., and Ranasinghe, C. (2014). Different HIV pox viral vector-based vaccines and adjuvants can induce unique antigen presenting cells that modulate CD8 T cell avidity. Virology 468–470, 479–489. doi: 10.1016/j.virol.2014.09.004
Trivedi, S., Neeman, T., Jackson, R. J., Ranasinghe, R., Jack, C., and Ranasinghe, C. (2015). Identification of biomarkers to measure HIV-specific mucosal and systemic CD8(+) T-cell immunity using single cell Fluidigm 48.48 Dynamic arrays. Vaccine 33, 7315–7327. doi: 10.1016/j.vaccine.2015.10.085
Veazey, R. S., and Lackner, A. A. (1998). The gastrointestinal tract and the pathogenesis of AIDS. AIDS 12(Suppl. A), S35–S42.
Wijesundara, D. K., and Ranasinghe, C. (2012). “Prime boost regimens for enhancing immunity: magnitude, quality of mucosal and systemic gene vaccines,”in Gene Vaccines, eds J. Thalmer, R. Weiss, and S. Scheiblhofer (New York, NY: Landes Bioscience/Springer Science+Business Media), 183–204.
Wijesundara, D. K., Jackson, R. J., Ramshaw, I. A., and Ranasinghe, C. (2011). Human immunodeficiency virus-1 vaccine design: where do we go now? Immunol. Cell Biol. 89, 367–374. doi: 10.1038/icb.2010.118
Xi, Y., Day, S. L., Jackson, R. J., and Ranasinghe, C. (2012). Role of novel type I interferon epsilon in viral infection and mucosal immunity. Mucosal Immunol. 5, 610–622. doi: 10.1038/mi.2012.35
Xu, H., Wang, X., and Veazey, R. S. (2013). Mucosal immunology of HIV infection. Immunol. Rev. 254, 10–33. doi: 10.1111/imr.12072
Keywords: human immunodeficiency virus, HIV-1, HIV vaccine, mucosal immunity, T cell, tissue resident memory, CD8+ T cell, vagina
Citation: Wijesundara DK, Ranasinghe C, Grubor-Bauk B and Gowans EJ (2017) Emerging Targets for Developing T Cell-Mediated Vaccines for Human Immunodeficiency Virus (HIV)-1. Front. Microbiol. 8:2091. doi: 10.3389/fmicb.2017.02091
Received: 30 August 2017; Accepted: 11 October 2017;
Published: 25 October 2017.
Edited by:
Michael W. Cho, Iowa State University, United StatesReviewed by:
Kazutaka Terahara, National Institute of Infectious Diseases (NIID), JapanMasaaki Miyazawa, Kindai University, Japan
Copyright © 2017 Wijesundara, Ranasinghe, Grubor-Bauk and Gowans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danushka K. Wijesundara, danushka.wijesundara@adelaide.edu.au
 Danushka K. Wijesundara
Danushka K. Wijesundara Charani Ranasinghe
Charani Ranasinghe Branka Grubor-Bauk
Branka Grubor-Bauk Eric J. Gowans
Eric J. Gowans