- 1Institute of Fundamental Medicine and Biology, Kazan Federal University, Kazan, Russia
- 2Biofunctional Chemistry Laboratory, Alexander Butlerov Institute of Chemistry, Kazan Federal University, Kazan, Russia
- 3Center for Infectious Diseases and Infection Control, Jena University Hospital, Jena, Germany
- 4Biomedical Engineering Research Centre, Saint Petersburg Electrotechnical University, Saint Petersburg, Russia
The gram-positive opportunistic bacterium Staphylococcus aureus is one of the most common causatives of a variety of diseases including skin and skin structure infection or nosocomial catheter-associated infections. The biofilm formation that is an important virulence factor of this microorganism renders the antibiotic therapy ineffective, because biofilm-embedded bacteria exhibit strongly increased tolerance to antimicrobials. Here, we describe a novel 3-chloro-5(S)-[(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-4-[4-methylphenylsulfonyl]-2(5H)-furanone (F105), possessing a sulfonyl group and l-menthol moiety. Minimal inhibitory and bactericidal concentration values (MIC and MBC) of F105 were 10 and 40 mg/L, respectively, suggesting F105 biocidal properties. F105 exhibits pronounced activity against biofilm-embedded S. aureus and increases the efficacy of aminoglycosides (amikacin, gentamicin, and kanamycin) and benzalkonium chloride with fractional inhibitory concentration index values of 0.33–0.44 and 0.29, respectively, suggesting an alternative external treatment option, e.g., for wound infections. Moreover, low concentrations (0.5–1.3 mg/L) of F105 reduced the MICs of these antimicrobials twofold. By using confocal laser scanning microscopy and CFU counting, we show explicitly that F105 also restores the antimicrobial activity of gentamicin and ampicillin against S. aureus biofilms by several orders of magnitude. Biofilm structures were not destroyed but sterilized, with embedded cells being almost completely killed at twofold MBC. While F105 is quite toxic (CC50/MBC ratio 0.2), our data suggest that the F105 chemotype might be a promising starting point for the development of complex topical agents for combined anti-staphylococcal biofilm-therapies restoring the efficacy of some antibiotics against difficult to treat S. aureus biofilm.
Introduction
Formation of biofilms represents an important virulence factor of the Gram-positive opportunist Staphylococcus aureus (McCarthy et al., 2015; Naicker et al., 2016), one of the most common causatives of a variety of biofilm-associated diseases such as osteomyelitis, endocarditis, skin and skin structure infection (SSSI) as well as foreign body associated infections, commonly leading to the development of sepsis (Conlon, 2014). Therefore, infections caused by S. aureus are associated with increased morbidity and mortality (Bassetti et al., 2017).
Biofilms are complex three-dimensional microbial communities attached to a multitude of surfaces representing the preferred life-style of the bacteria in natural and artificial habitats (Donlan and Costerton, 2002; Bremer et al., 2011). In biofilms, bacterial cells are embedded in an extracellular matrix of organic polymers such as polysaccharides, peptides, and extracellular DNA that are synthesized and released by the microbes themselves (Lewis, 2001; Atshan et al., 2015; Fagerlund et al., 2016). The matrix drastically reduces the susceptibility to different outer stress factors (Donlan, 2002) indicated by up to 1000-fold higher tolerance to antimicrobials of the biofilm-embedded cells compared to their planktonic counterparts (Cosgrove et al., 2002; Sanchez-Vizuete et al., 2015). Treatment of chronic infections is often complicated due to the presence of bacterial biofilm on the surface of the wound that detain the healing process (Doll et al., 2016; Sharafutdinov et al., 2016; Roy et al., 2017). In addition, biofilms demonstrate increased robustness against the host immune system leading to recurrent and/or persistent chronic infections. In addition, the genotypic resistance of bacteria can increase the overall antimicrobial resistance of the biofilm-embedded bacteria providing the so called herd-protection for the susceptible co-occupants. The conjugative acquisition of methicillin resistance is also a strong limiting factor for the antimicrobial therapy efficacy of S. aureus caused infections. Infections by methicillin-resistant S. aureus strains (MRSA) have even poorer outcomes (Cosgrove and Fowler, 2008) requiring alternative therapeutic options.
In general, only very few antibiotics are capable of directly attacking bacterial biofilms. For example, rifampicin in combination with other antibiotics such as β-lactam is often used against persisting staphylococcal biofilms (Forrest and Tamura, 2010), because bacteria rapidly develop rifampicin resistance under treatment. Experimental data also suggest that ribosome-active antibiotics (such as linezolid or clindamycin) might be effective against staphylococcal biofilms because of their comparable suppressive effect on the expression of various virulence factors (Hodille et al., 2017). However, clinical evidence is still missing; thus, nowadays neither linezolid nor clindamycin are recommended for treatment of biofilm-associated staphylococcal infections.
Investigations on alternative treatment options against biofilm-associated infections is largely based upon the use of specialized agents (such as quaternary ammonium compounds, curcumin or chlorquinaldol) that in combinations with antibiotics provide high local drug concentrations avoiding systemic adverse effects (Kali et al., 2016; Percival et al., 2016; Bortolin et al., 2017). Among various compounds exhibiting antimicrobial and anti-biofilm activities, the derivates of 2(5H)-furanone have been intensively studied in last two decades. In the nature, furanones are known to exhibit many different functions, such as intra- and inter-species signaling and communication, attractant molecules and pheromones, antimicrobials, and anti-carcinogens (Fenske and Merzweiler, 1989; Donlan and Costerton, 2002; Bremer et al., 2011). Several studies have reported the ability of synthetic furanones to inhibit biofilm formation of various bacteria (Ren et al., 2001; Hentzer et al., 2002; Lonn-Stensrud et al., 2009; Fedorova et al., 2013). While many 2(5H)-furanone derivatives interfere with AI-II quorum-sensing systems of Gram-negative bacteria thereby blocking the biofilm growth (Ren et al., 2001), a number of furanones were shown to repress the biofilm formation by Bacillus subtilis and Staphylococci (Heck and Stuetz, 1988; Ren et al., 2004; Lonn-Stensrud et al., 2009; Kayumov et al., 2015a). In particular, Lonn-Stensrud et al. (2009) reported that (Z)-5-(bromomethylene)furan-2(5H)-one completely repressed the biofilm formation by S. epidermidis without any irritative or genotoxic effects. In contrast, brominated furanone increased the production of the extracellular matrix by S. aureus (Yujie et al., 2013) indicating no universality in the effects of these compounds on bacterial cells. Besides the biofilm repression effects, in several other studies furanone derivatives were reported to exhibit bactericidal activity against gram positive bacteria (Ren et al., 2004; Kitty et al., 2015).
It has been previously shown that the introduction of l-menthol moiety into carbamate derivatives significantly increases their anti-biofilm properties toward multidrug-resistant S. aureus (Rogers et al., 2010). Several other studies reported that sulfonyl-containing compounds efficiently repressed the growth and biofilm formation by Staphylococci (Meadows and Gervay-Hague, 2006; Kudryavtsev et al., 2009; Low et al., 2011). Therefore, we aimed to investigate the antimicrobial activity of a novel 2(5H)-furanone derivative (F105) possessing two pharmacophores, a sulfonyl group and an l-menthol moiety. We found synergy of F105 with aminoglycosides against planktonic S. aureus and demonstrated their attractive activity toward the biofilm-embedded bacteria.
Materials and Methods
Chemistry
3-Chloro-5(S)-[(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-4-[4-methylphenylsulfonyl]-2(5H)-furanone (F105) was synthesized in three steps. For the detailed description of compounds preparation and characterization, we refer to the Supplementary Data, available online. The stock solutions of F105 were prepared by diluting powders in pure DMSO (Sigma–Aldrich, Saint-Quentin Fallavier, France) at the concentration of 20 g/L. To solubilize the furanone at high concentrations in medium, pluoronic acid F-127 (Sigma–Aldrich) (10% stock solution in DMSO) was added to the final concentration of 0.1%. Working solutions were prepared in bacterial growth medium such that the final concentrations of DMSO 5% have been obtained, which was next verified to be non-toxic for the bacterial strains tested. All conventional antibiotics were purchased from Sigma.
Strains and Culture Conditions
A methicillin sensitive Staphylococcus aureus ATCC®29213 (MSSA) and a methicillin resistant S. aureus strain ATCC®43300 (MRSA) (both laboratory strains) as well as 10 clinical MRSA isolates provided by the Republican Clinical Hospital, Laboratory of Clinical Bacteriology in Kazan were used in this study (see Table 1). The bacterial strains were stored in 10% (V/V) glycerol stocks at -80°C and freshly streaked on blood agar plates (BD Diagnostics) following by their overnight growth at 35°C before use. Fresh colony material was used to adjust an optical density to 0.5 McFarland (equivalent to 108 cells/mL) in 0.9% NaCl solution that was used as a working suspension.
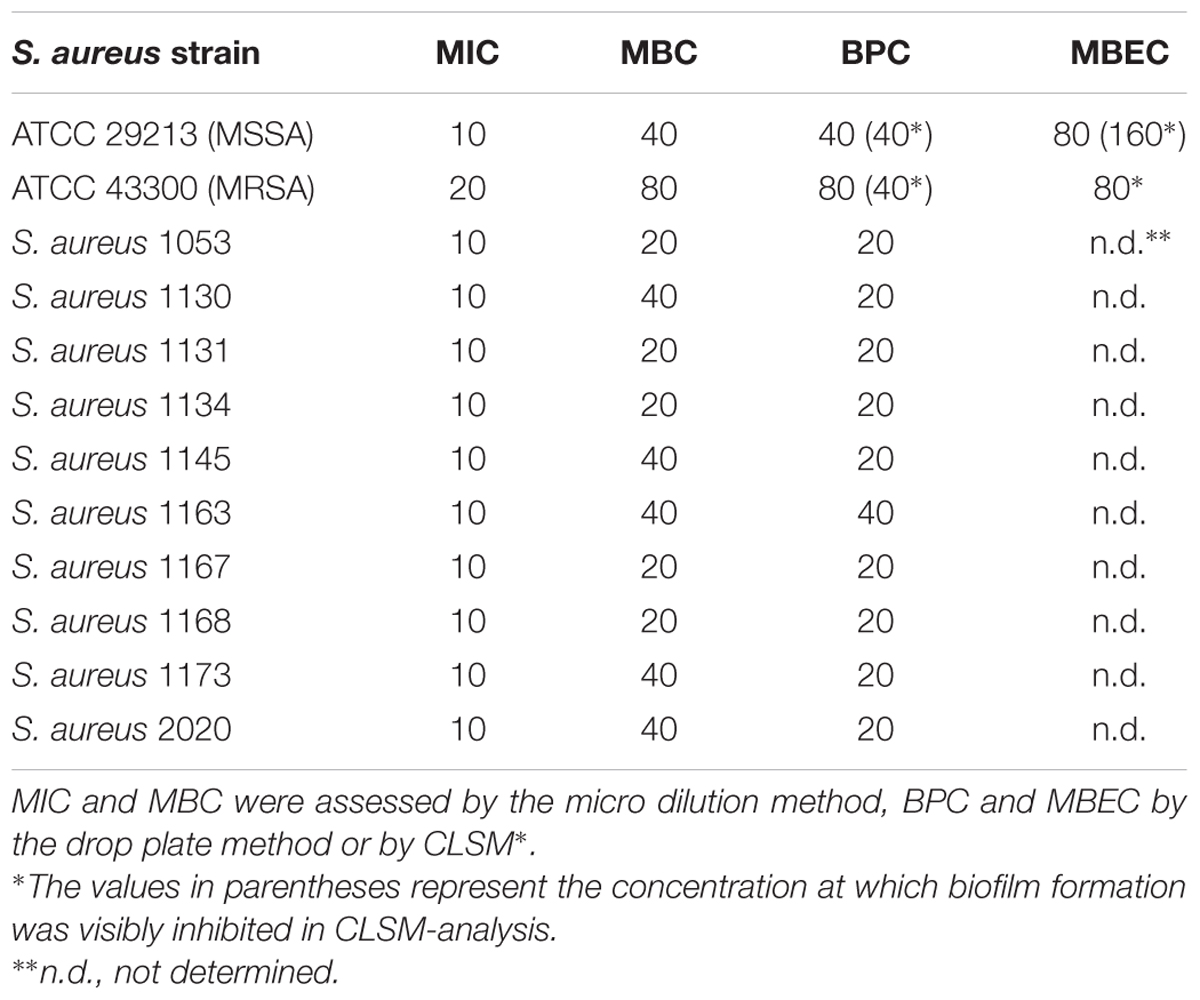
TABLE 1. The antimicrobial effects of F105 expressed as MIC, MBC, BPC and MBEC against S. aureus isolates in mg/L.
Determination of the Minimal Inhibitory (MIC) and the Minimal Bactericidal Concentrations (MBC) of F105
The MIC of furanone F105 was determined by the broth microdilution method in 96-well microtiter plates (Eppendorf) according to the EUCAST rules for antimicrobial susceptibility testing (Leclercq et al., 2013) with minor modifications to account for the decreased solubility of the compound. Briefly, the 108 cells/mL bacterial suspension was subsequently diluted 1:300 with Mueller-Hinton broth (MH) (Carl Roth GmbH, Germany), cation-adjusted with 20 mg/L Ca2+ and 10 mg/L Mg2+ and supplemented with various concentrations of F105 in microwell plates to obtain a 3 × 105 cells/mL suspension. To solubilize the furanone at high concentrations in medium, pluoronic acid F-127 (Sigma–Aldrich) (10% stock solution in DMSO) was added to the final concentration of 0.1%. The final concentration of DMSO was adjusted to 5% in all bacterial cultures. The concentrations of F105 ranged from 1.25 to 160 mg/L. Besides the usual double dilutions, additional concentrations were included in between. The cultures were incubated at 35°C for 24 h. The MIC was determined as the lowest concentration of furanone for which no visible bacterial growth could be observed after 24 h of incubation.
To determine the MBC, the CFU/mL were further evaluated in culture liquid from wells without visible growth. The F105 concentration reducing the number of viable cells by at least three orders of magnitude was considered as MBC according to the recommendation of the European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) (2000).
Synergy Testing by Checkerboard Assay
The checkerboard assay was performed similarly to the MIC testing in 96-well microtiter plates (Eppendorf). Each plate contained serial dilutions of F105 in 0.1% pluoronic acid F-127 and different antibiotics in a checkerboard fashion as described previously (Eliopoulos and Moellering, 1996). Briefly, the final concentrations of both compounds ranged from 1/16× to 4× MIC for F105 and from 1/256× to 4× MIC for the antibiotics. In total, 11 dilution steps of antibiotics and 7 dilution steps of F105 were analyzed. The microwell plates were incubated at 35°C for 24 h. Each test was performed in triplicate and included a growth control with neither antibiotic nor F105 addition. The fractional inhibitory concentration index (FICI) for each double combination was calculated as
The FICIs were counted from the concentrations in the first non-turbid well found in each row and column along the turbidity/non-turbidity interface and the lowest FICI value was used to characterize the synergy. For the FICI interpretation we refer to den Hollander et al. (1998) and Odds (2003): FICI < 0.5 corresponds to synergy, 0.5 < FICI < 4 corresponds to either additive effects or indifference, while FICI > 4 corresponds to antagonism.
Analysis of the Biofilm Prevention Concentration (BPC) of F105
To determine the BPC of F105, two methods have been applied, including the modified crystal-violet staining (Merritt et al., 2005) and the drop plate approach (Herigstad et al., 2001). Briefly, the bacterial culture was adjusted to 5 × 105 cells/mL in the MH broth and seeded into 24-well polystyrene culture plates (Eppendorf). F105 was added in serial dilutions to the final concentrations between 1.25 and 160 mg/L following by the cells growth under static conditions for 24 h at 35°C. For the crystal violet staining, the liquid culture was removed after 24 h of incubation and the plates were washed twice with PBS (pH 7.4) and dried. Then 1 ml of the 0.5% crystal violet solution (Sigma) in 96% ethanol was added per well, followed by 20 min incubation. Next the crystal violet solution was removed and the plate was washed 3 times with PBS. After 30 min air drying, 1 ml of 96% ethanol was added to resolubilize the bound crystal violet, and the absorbance was measured at 570 nm with the microplate reader Infinite 200 Pro (Tecan). Cell-free wells incubated with pure medium subjected to all staining manipulations were used as control.
Alternatively, the viability of cells was evaluated by the drop plate approach with minor modifications (Herigstad et al., 2001). Serial 10-fold dilutions from each well were prepared and 5 ml of suspension was dropped onto LB agar plates in five repeats. CFU/mL were counted and averaged from those drops containing 5–30 colonies. To evaluate the viability of biofilm-embedded cells, wells were washed several times with phosphate-buffered saline (PBS) to remove both non-adherent and detached cells. The washed biofilms were suspended in PBS by scratching the well bottoms with following treatment in a sonicator bath for 2 min at 20 kHz to favor the disintegration of bacterial clumps, and viable cells were counted by the drop plate method as described above.
Time-Kill Assay
Time-kill curves were obtained by growing MSSA in MH medium in the presence of F105 at concentrations of 0.5× MBC, 1× MBC, and 2× MBC. The starting bacterial suspension was adjusted to the 5 × 107 cells/mL concentration. Cells were then cultivated in 6-well microtiter plates without shaking at 35°C for 12 h and samples were taken every 4 h, serially diluted 1:10 and plated onto agar plates. The CFU/mL was counted after incubation at 35°C for at least 16 h.
Determination of Anti-biofilm Activity of F105 by CLSM
To additionally compare the BPC of MSSA and MRSA standard strains by a microscopic method, the biofilms were grown in the presence of F105 as descried above. To determine the minimal biofilm eradicating concentration (MBEC), the biofilms were grown without F105 under static conditions for 24 h at 35°C. Subsequently, the supernatants were carefully removed and the biofilms were treated with 500 μL of F105 solutions with various concentrations between 5 and 160 mg/L serially diluted in MH broth, and cultivation was continued for 24 h at 35°C. The effect of ampicillin, gentamicin, and benzalkonium chloride on the biofilm-embedded cells was analyzed similarly.
For both BPC and MBEC determination, the liquid cultures were carefully removed and the biofilms were washed once with 300 μl of 0.9% NaCl solution. The biofilms were stained using LIVE/DEAD BacLight Bacterial Viability Kit for microscopy (Life Technologies GmbH) according to manufacturer’s protocol. Stained biofilms were analyzed under vital conditions using an inverted confocal laser scanning microscope (CLSM) Carl Zeiss LSM 780 (Carl Zeiss AG) at green (522 nm) and red (635 nm) filters, respectively, using laser excitation at 490 nm as described previously (Klinger-Strobel et al., 2016; Baidamshina et al., 2017). An area of approximately 100 μm (X) × 100 μm (Y) was screened in 1 μm Z-intervals (Z-stack). The biofilm microscopy data were processed using ZEN 9.0 software (Carl Zeiss AG).
The BPC was considered as the furanone concentration at which the complete absence of the biofilm assessed with crystal violet staining was observed (Peeters et al., 2008). F105 concentrations reducing the viable cells by at least 3 orders of magnitude in the biofilm matrix were considered as MBEC.
Determination of F105 Cytotoxicity
Cytotoxicity of F105 was determined using the CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay (Promega) using MCF-7 cells. The cells were cultured in DMEM – Dulbecco’s Modified Eagle’s Medium (Sigma–Aldrich) supplemented with 10% FBS, 2 mM L-glutamine, 100 mg/L penicillin and 100 mg/L streptomycin. Cells were seeded in 96-well plates with the density of 3000 cells per well and left overnight to allow for the attachment. Cells were next cultured at 37°C and 5% CO2 in the presence of F105 at various concentrations from 1.25 to 160 mg/L. After 24 h of cultivation the cells were subjected to MTS-assay based on the cellular reduction of MTS (3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) by the mitochondrial dehydroxygenase using phenazinemethosulfate (PMS) as the electron coupling reagent. The MTS tetrazolium compound was bioreduced by viable cells into a colored formazan product, which was measured on Tecan Infinite 200Pro at 550 nm. The concentration required to inhibit cellular dehydrogenase activity by 50% (CC50 value) was calculated as recommended by the manufacturer.
The mutagenicity of F105 was evaluated in the Ames test with Salmonella typhimurium TA98, TA100 and TA102 strains as described in McCann and Ames (1976). The tested compound was considered to be mutagenic if the number of revertant colonies in the experiment was more than two times higher than that in the negative control (DMSO) and increased at higher F105 concentrations (OECD, 1997).
Data Analysis
All experiments were performed in biological triplicates with three repeats in each run. The data were analyzed and graphically visualized using GraphPad Prism version 6.00 for Windows (GraphPad Software, United States, www.graphpad.com). In each experiment, comparison against negative control has been performed using the non-parametric Kruskal–Wallis one-way analysis of variance test. Significant differences against respective controls were considered at p < 0.05 and are specified in the corresponding figure captions. Additionally, statistical significance of time-kill curves and dose-response curves have been assessed by linear regression analysis applied in log scales where appropriate. For the regression analysis data, shown confidence intervals also correspond to p < 0.05.
Results
Synthesis of Furanone F105
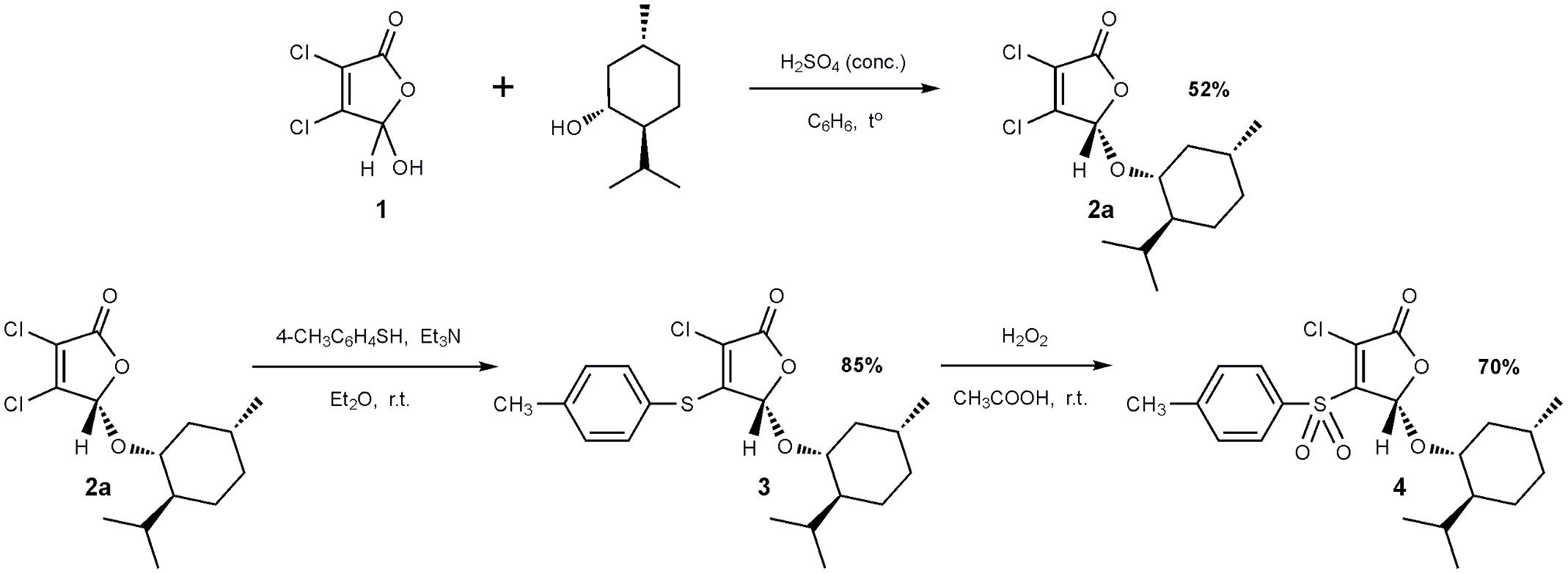
The 2(5H)-furanone derivative F105 (4) was synthesized in three steps from commercially available mucochloric acid 1 (see Figure 1). In the first stage, a mixture of diastereomers 2a + 2b was obtained in the reaction of mucochloric acid 1 with l-menthol in the presence of catalytic amounts of concentrated sulfuric acid as described previously (Fenske and Merzweiler, 1989). The pure stereoisomer 2a with S-configuration of the carbon atom C5 of the γ-lactone ring was isolated in 52% yield after two recrystallizations from hexane (Figure 1).
Next a thiolation reaction of the isolated stereoisomer 2a under basic catalysis was carried out. It is well-known that in the presence of triethylamine reactions of 5-alkoxy-2(5H)-furanones with thiols proceed with the regioselective substitution of the chlorine atom in the fourth position of the lactone ring (Kurbangalieva et al., 2007; Latypova et al., 2014). The reaction was performed in diethyl ether at room temperature with the equimolar ratio of furanone 2a, p-thiocresol and triethylamine resulting in the novel optically pure thioether 3 with 85% yield. Thioether 3 was further subjected to oxidation to the corresponding sulfone 4 using a recently developed selective method (Latypova et al., 2014). The compound 3 was treated with 10-fold excess of 33% hydrogen peroxide in acetic acid at room temperature and the novel optically pure sulfonyl derivative of 2(5H)-furanone 4 (studied compound F105, Figure 1) was isolated in the form of colorless crystals in 70% yield. The structure of compounds 2–4 was characterized in detail by IR and NMR spectroscopy (Supplementary Figures S1–S5).
Antimicrobial Activity of F105 on Planktonic S. aureus
The antimicrobial properties of F105 were determined on both methycillin-susceptible (MSSA) and -resistant (MRSA) S. aureus strains ATCC29213 and ATCC43300, respectively. The minimal inhibitory concentration (MIC) of F105 for MSSA was found to be 10 mg/L (25 μM), and 20 mg/L (50 μM) for MRSA (see Table 1). The minimal bactericidal concentration (MBC) value of F105 was found to be 40 mg/L in MSSA and 80 mg/L in MRSA. The time-kill curves revealed that all cells of MSSA exposed to F105 at concentration of 2× MBC were killed within 8 h of treatment (Figure 2A). Alternatively, 1× MBC of F105 led to the reduction in the number of viable cells by three orders of magnitude within 12 h. The dose-response curves (Figure 2B) confirm the concentration-dependence of F105 effect on cells viability explicitly suggesting that F105 exhibits biocidal activity. Interestingly, our earlier results indicate that the analogs of F105 lacking either sulfonyl group (compound 3 on the Figure 1) or l-menthol moiety (F70, 3-chloro-5-hydroxy-4-[(4-methylphenylsulfonyl)]-2(5H)-furanone) exhibited no activity against S. aureus (Latypova et al., 2014; Kayumov et al., 2015a) (data not shown) suggesting the requirement of both functional groups for bactericidal activity, while in other works the presence of either sulfonyl group or l-menthol moiety increased antibacterial effects (Meadows and Gervay-Hague, 2006; Kudryavtsev et al., 2009; Rogers et al., 2010; Low et al., 2011).
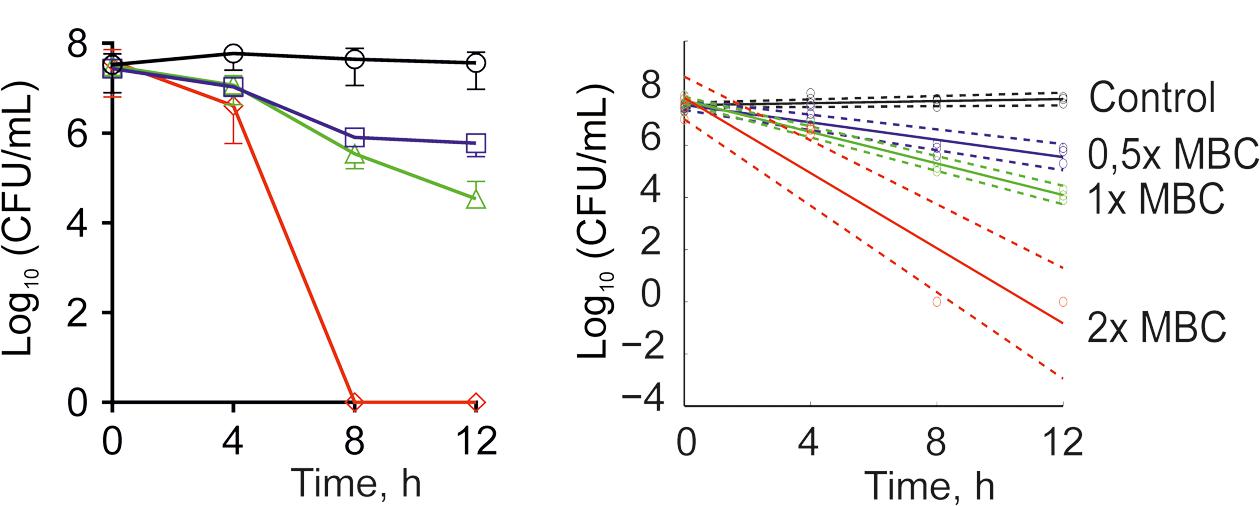
FIGURE 2. Time-kill curves of F105 against MSSA cells. The numbers of CFU/mL were calculated by using drop plate approach (A) and respective dose-response curves were plotted providing residual CFU/mL as a function of exposition time (B). F105 is presented in different concentrations: untreated cells (black); 0.5× MBC (20 mg/L, blue); 1× MBC (40 mg/L, green); 2× MBC (80 mg/L, red). Full lines denote regression lines, while dashed lines denote corresponding 95% confidence intervals.
We additionally assessed both MIC and MBC in 10 clinical isolates exhibiting a MRSA phenotype. Both values ranged within the MIC and MBC found in the MSSA laboratory standard strain with all MICs at 10 mg/L, and MBC at 20 mg/L (n = 5) or 40 mg/L (n = 5) (Table 1).
Synergistic Effects of F105 with Other Antimicrobials on S. aureus Planktonic Cells
The synergism of F105 in combination with various antibiotics on the bacterial growth was analyzed by the checkerboard assay (Table 2 and Supplementary Table S1). Thereby synergism of F105 was observed when combined with aminoglycosides. Particularly, the FICI values for F105 were determined to be 0.33 ± 0.04 in combination with amikacin, 0.33 ± 0.16 with gentamicin and 0.44 ± 0.17 with kanamycin. Besides aminoglycosides, strong synergy has been observed also for benzalkonium chloride with FICI of 0.29 ± 0.09. Indifferent effects, but with relatively low FICI (between 0.51 and 0.75) indicating enhancement of antimicrobial activity were observed for F105 in combination with daptomycin, rifampicin, ciprofloxacin, tetracycline, and linezolid; whereas the only additive effects were observed for combinations of F105 with erythromycin and vancomycin. For deeper analysis of F105 synergy with antimicrobials, the combined MICs of the antibiotic/F105-mixtures were plotted (as isoboles) and the effective concentrations of F105 (EC50) leading to the twofold reduction of antibiotic’s MIC were calculated (Table 2 and Supplementary Figure S6). The EC50 values of F105 in combinations with amikacin, gentamycin, and kanamycin were determined to be 0.7, 0.7, and 1.3 mg/L, respectively. Only 0.5 mg/L of F105 was required to reduce the MIC of benzalkonium chloride twofold. For other studied antibiotics EC50 values of F105 were in the range of 4.3 and 9.5 mg/L.
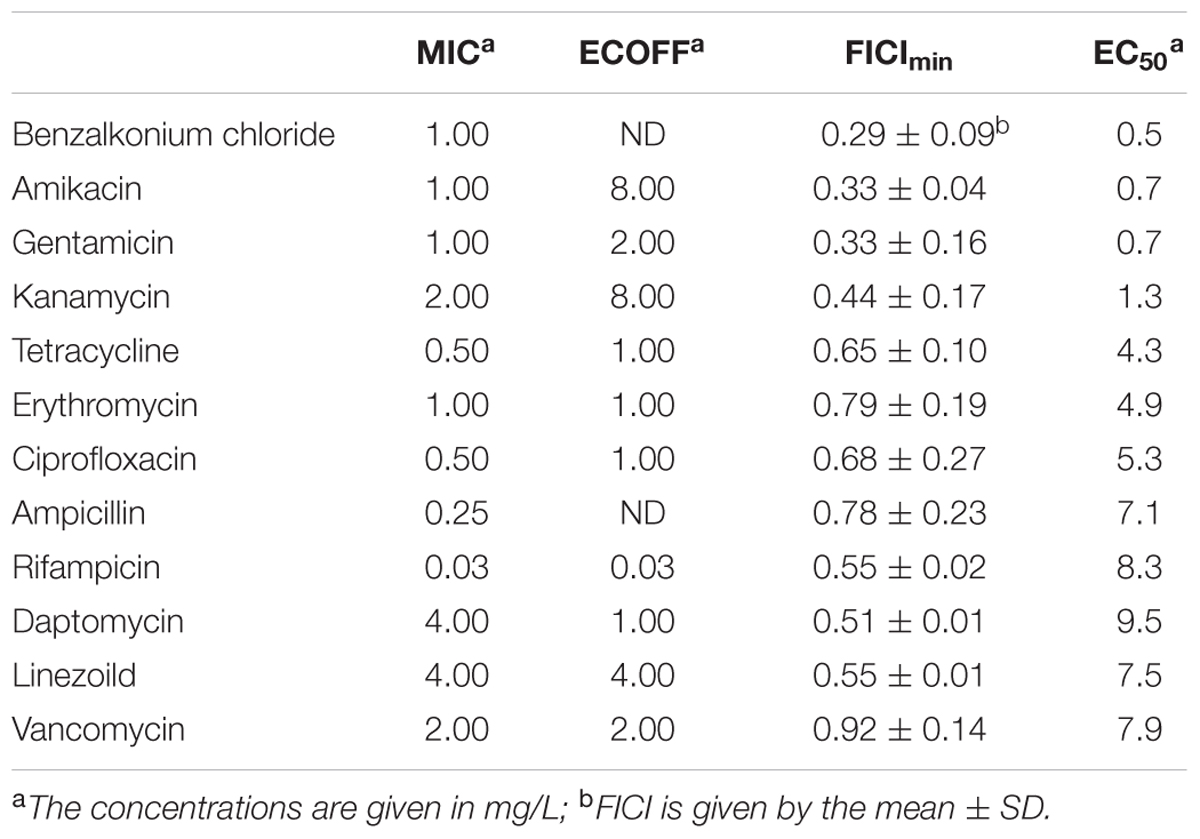
TABLE 2. MIC and ECOFF values of various antibiotics against MSSA, FICI values of those antibiotics combined with F105 and EC50 of F105 reducing twice the MIC of the appropriate antibiotic.
Anti-biofilm Activity of F105
Since the anti-biofilm activity of furanones was reported for some compounds (Ren et al., 2001; Hentzer et al., 2002; Lonn-Stensrud et al., 2009; Fedorova et al., 2013), the biofilm preventing concentration (BPC) of F105 for the MSSA laboratory standard strain was assessed first by two different methods. The crystal violet staining method revealed that F105 completely inhibited the biofilm formation of the MSSA at the concentration of 20 mg/L (Figure 3A); whereas, applying direct counting of viable cell in the biofilm the BPC was found to be 40 mg/L (Figure 3B and Table 1). Since the amount of viable planktonic cells (swimming cells) also decreased by three orders of magnitude at 20 mg/L of F105, we suggested that the biofilm suppression was rather the consequence of cell growth repression. The CLSM analysis confirmed the BPC of F105 of 40 mg/L for MSSA (Figure 4), thus we estimated that these direct methods are more adequate compared to the indirect crystal violet staining. The determined BPC of MRSA was 80 mg/L according to the CFU/ml counting and 40 mg/L according to the CLSM (Table 1 and Figure 4). The BPC was measured by the drop plate method in the clinical MRSA isolates resulting in 20 mg/L in nine and 40 mg/L in one isolate (Table 1).
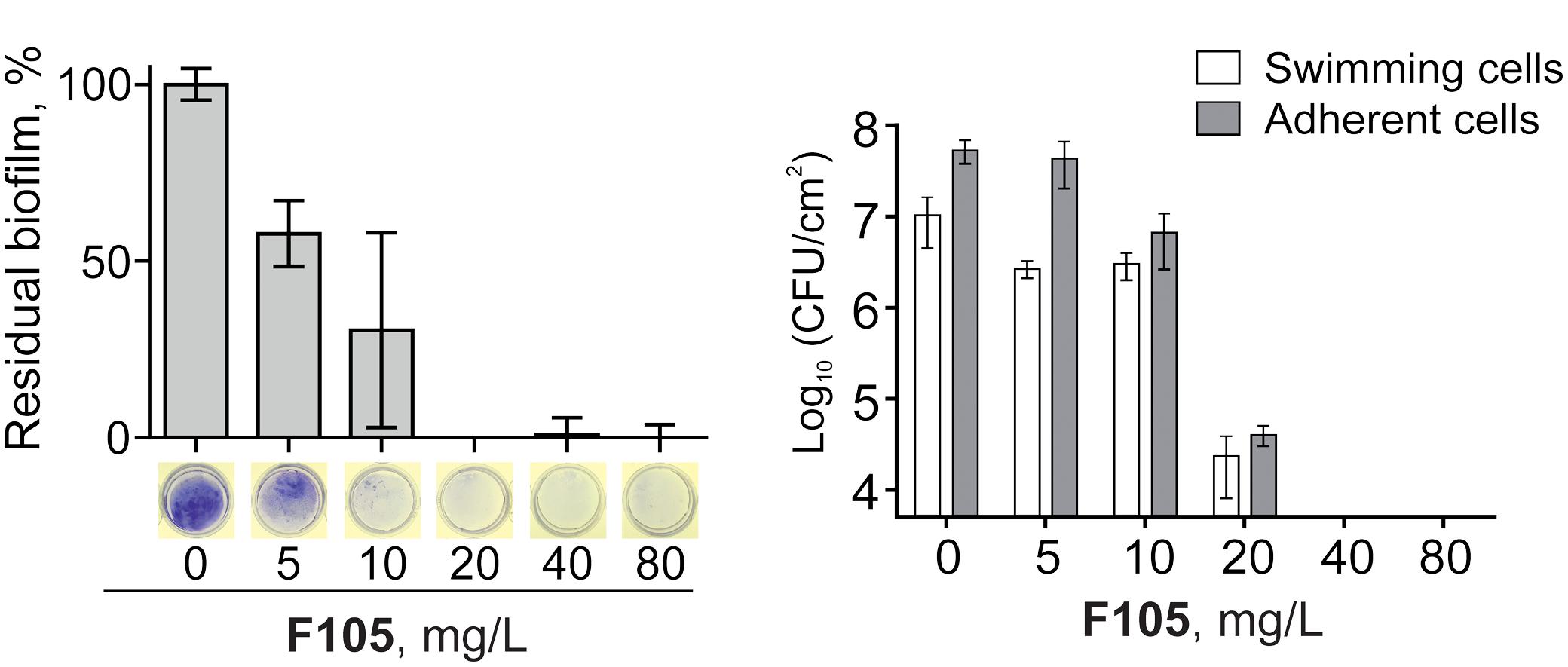
FIGURE 3. Effect of F105 on the biofilm formation by MSSA. Cells were incubated for 24 h in the presence of different concentrations of F105, the biofilms were quantified after crystal-violet staining (A) and CFU counting (B); thereby the crystal-violet method correlates with the biomass and CFU/mL with viable cells.
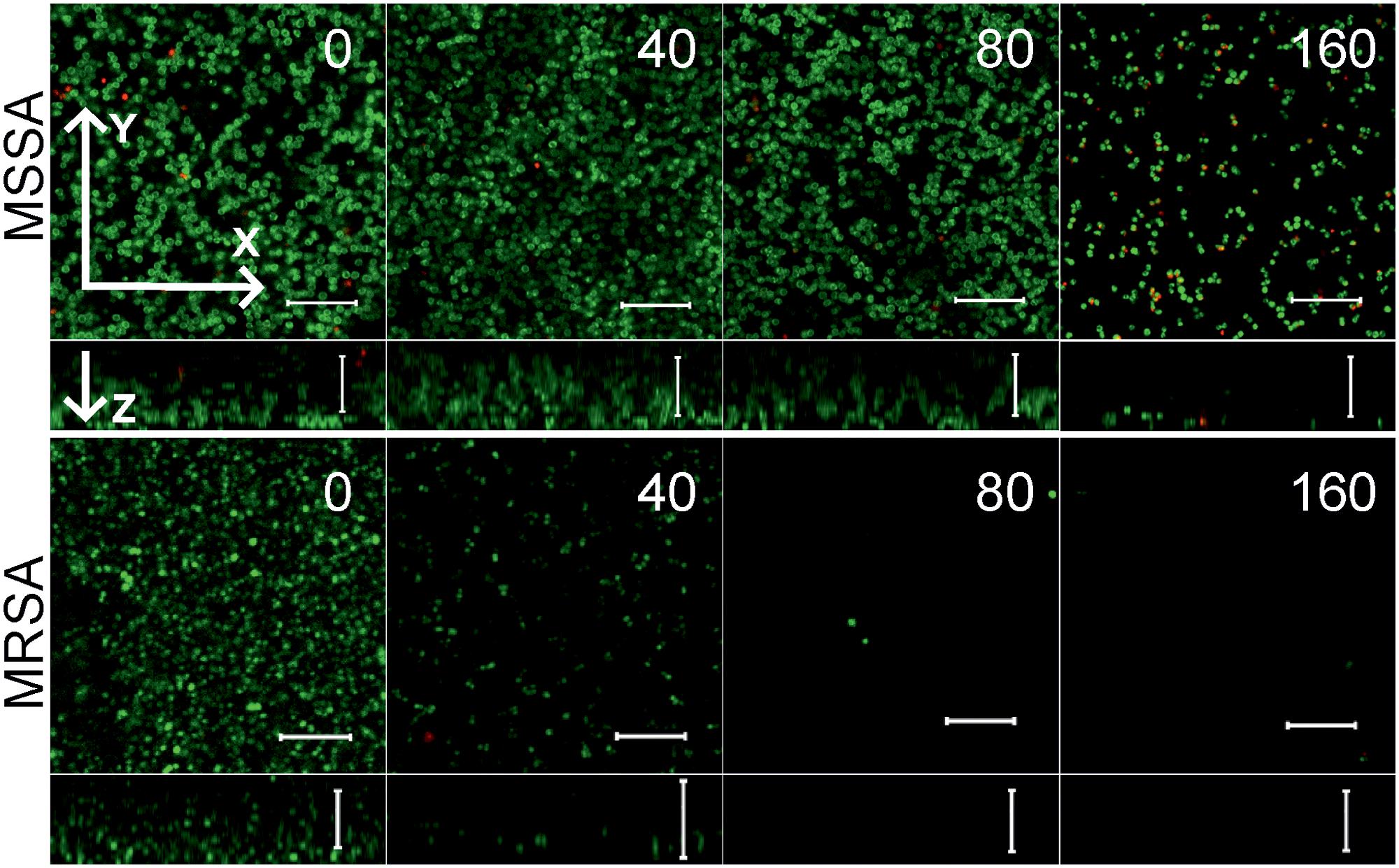
FIGURE 4. The biofilm-preventing activity (BPC) of F105 against MSSA (upper) and MRSA (lower) analyzed by CLSM. F105 was added prior the inoculation with following cell grow for 24 h. The images show a plan view on a basal biofilm layer (indicated by X and Y axis) and a cross section thought the biofilm (Z axis). The scale bars indicate 10 μm.
To further analyze, whether F105 not only inhibits the biofilm formation but also exhibits activity against already established biofilms, 24 h-old S. aureus biofilms of the MSSA and MRSA laboratory standard strains were treated with different concentrations of F105. The CLSM analysis of the MBEC indicated that the treatment with F105 did not lead to any visibly remarkable decrease of the biofilm thickness (Figure 5), while the ratio of dead/viable cell increased significantly in the concentration dependent manner. Biofilm-embedded cells of MRSA were nearly completely killed at 80 mg/L of F105, whereas MSSA cells were killed at 160 mg/L. However, the CFU/mL of the planktonic cells and the adherent cells of MSSA biofilm cultures revealed a reduction in the number of viable cells by three orders of magnitude at 80 mg/L of F105 for both attached and detached cells (Figure 6) indicating that CLSM results vary depending on the screened section and the CFU/mL values are more reliable.
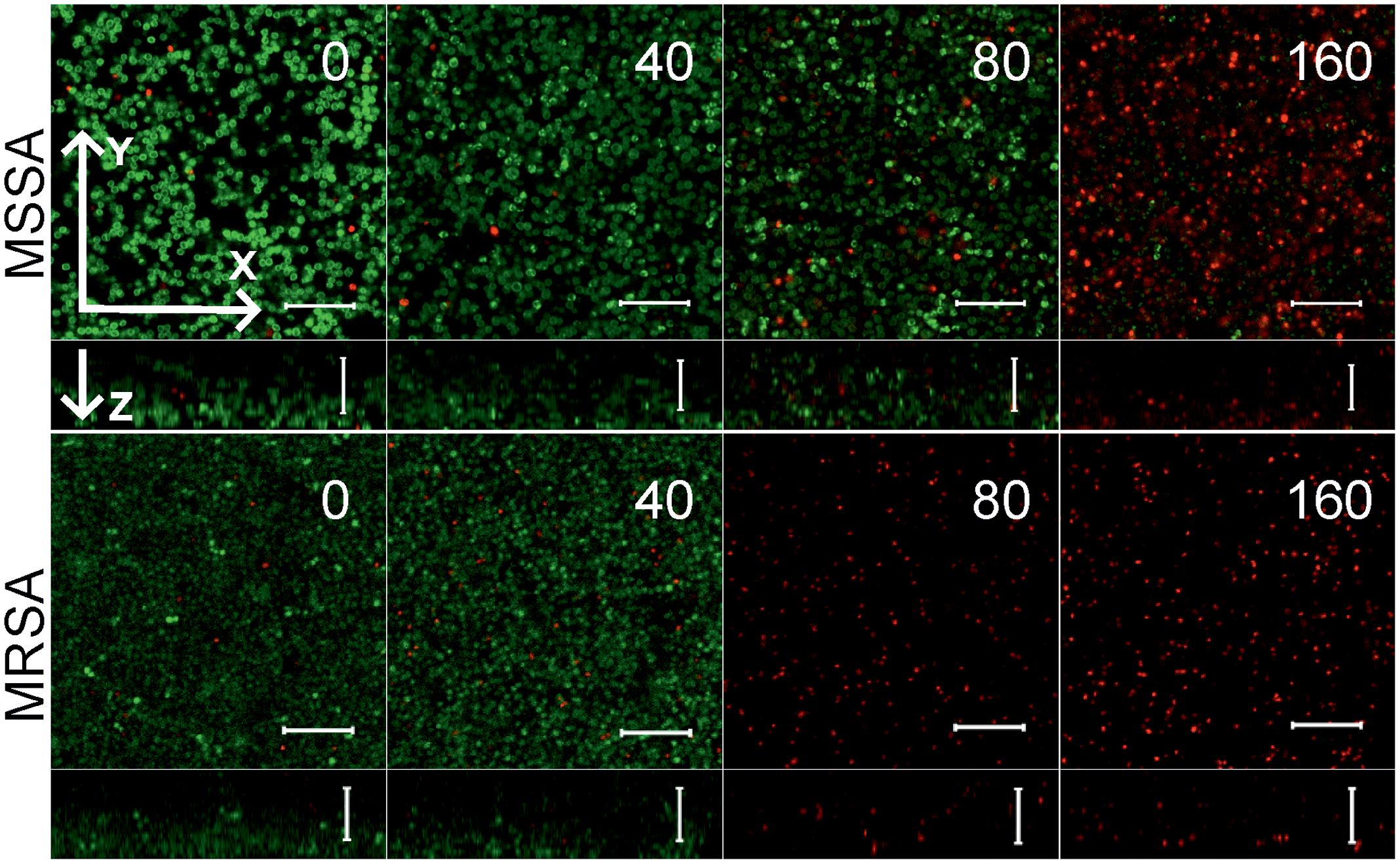
FIGURE 5. The antimicrobial activity of F105 against biofilm-embedded MSSA (upper) and MRSA (lower) analyzed by CLSM. F105 was added to established 24 h old biofilms with following 24 h cultivation. The scale bars indicate 10 μm.
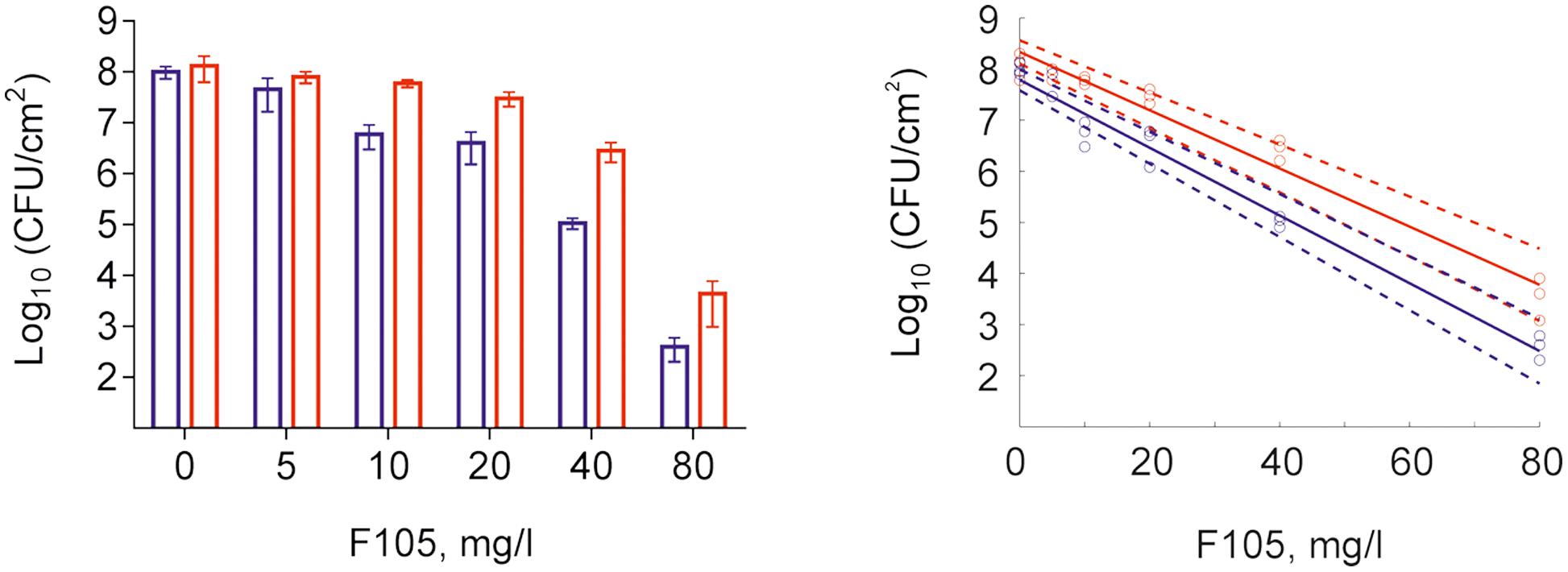
FIGURE 6. Effect of F105 on MSSA cells viability in culture liquid (blue) and within the 24 h old biofilm layer (red) (A) and corresponding dose-response curves (B) estimated by linear regression in the logarithmic scale (full lines) with 95% confidence intervals for the regression coefficients (dashed lines) of swimming (planctonic and detached) and adherent cells. F105 was added to 24 h old biofilms with additional 24 h cultivation. Significant differences with control could be observed at concentrations from 10 mg/L and above for swimming cells as well as from 20 mg/L and above for adherent cells by Kruskal–Wallis test at p < 0.05.
To compare the biofilm activity of F105 against other antimicrobials, similar experiments were performed with ampicillin, gentamicin, and benzalkonium chloride (see Supplementary Table S2 for MBC values). A fourfold MBC of benzalkonium chloride was required to reduce the number of viable biofilm-embedded cells by three orders of magnitude, while even 16-fold MBC of gentamicin or ampicillin led to only 10-fold decrease of viable cells in the biofilm (Figure 7). In marked contrast, twofold MBC of F105 reduced the viable cells of the MSSA biofilm by more than four orders of magnitude (Figure 7 and Supplementary Figure S7).
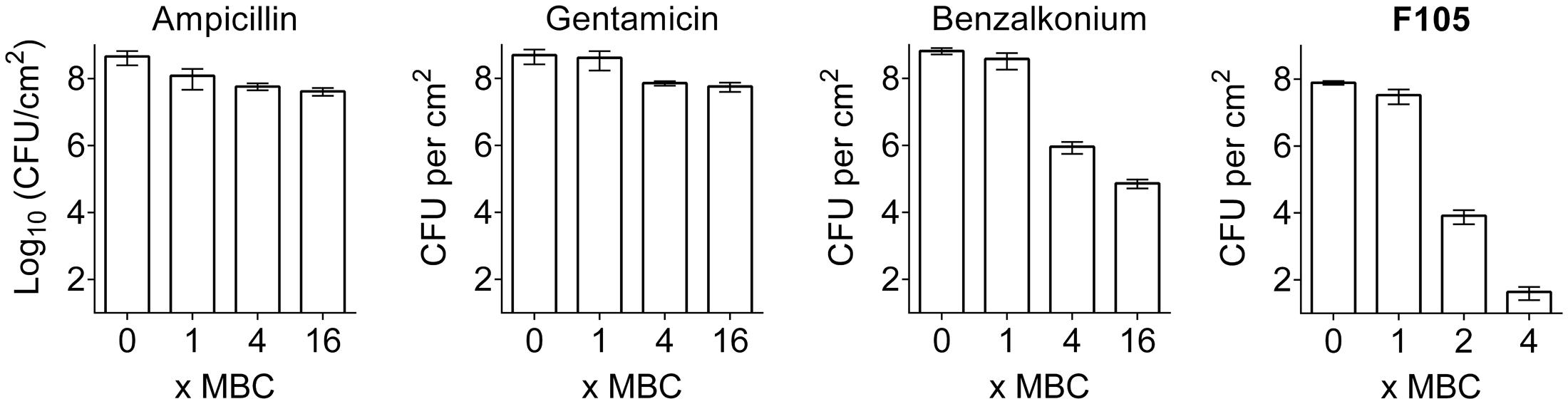
FIGURE 7. The comparison of antimicrobial activity of F105 and various antibiotics against biofilm embedded MSSA cells. Antimicrobials were added in wells with established 24 h old biofilms and incubation was continued for 24 h at 35°C. The concentrations are given in relative units as X-fold MBC, which were as follows: 8 mg/L for ampicillin, 4 mg/L for gentamycin, 2 mg/L for benzalkonium, and 40 mg/L for F105.
Cytotoxicity and Mutagenicity
No mutagenicity of F105 was detected in the Ames test using Salmonella typhimurium TA98, TA100, and TA102 strains at the furanone concentrations up to 80 mg/L without (Supplementary Table S3) and with (Supplementary Table S4) metabolic activation. The cytotoxicity test provided with CC50 values of 40.3 mg/L for MCF-7 cells (a human breast adenocarcinoma cell line) but only 8.1 mg/L for skin fibroblast suggesting that the current structure of F105 might be too toxic for direct use for systemic therapeutic in humans and requires further modification of the structure in order to reduce its toxicity and improve its solubility.
Discussion
The biofilm formation by the methicillin-resistant S. aureus cells on wounds and surfaces contacting with different tissues makes bacteria inaccessible to both antimicrobials and the immune system of the host. The discovery of the natural furanones derivatives exhibiting biofilm suppression activity (Ren et al., 2001, 2004) gave the rise to investigations of these compounds as anti-biofilm agents (Brackman and Coenye, 2015). While many 2(5H)-furanone derivatives interfere with AI-II quorum-sensing systems of Gram-negative bacteria blocking the biofilm formation (Ren et al., 2001; Hentzer et al., 2003), a number of furanones were shown to be active against gram-positive S. epidermidis and B. subtilis (Heck and Stuetz, 1988; Ren et al., 2004; Lonn-Stensrud et al., 2009; Kayumov et al., 2015a; Trizna et al., 2015).
Here, we have shown that 3-chloro-5(S)-[(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-4-[4-methylphenylsulfonyl]-2(5H)-furanone (F105), carrying sulfonyl and l-menthol moieties, exhibits antibacterial activity against both planktonic and biofilm-embedded S. aureus. There were some reasons, why we have chosen these modifications to improve the antimicrobial activity of this furanone derivative. In particular, menthol has been shown previously to exhibit biofilm inhibiting and biofilm eliminating effects (Kifer et al., 2016) and it is one of the most effective terpenes used to enhance the dermal penetration of pharmaceuticals (Kamatou et al., 2013). Moreover, introduction of the l-menthol moiety into carbamate derivatives significantly increases their anti-biofilm properties toward MRSA (Rogers et al., 2010). It could be also shown that l-menthol increased CH2 stretching frequencies on either side of lipid transition and lowed the Tm by ∼2–8°C suggesting that l-menthol disrupts the interlamellar hydrogen-bonding network at the polar head group region and increases the hydration levels of the model lipid system, most probably by forming new aqueous channels (Narishetty and Panchagnula, 2005). Therefore, it could be speculated that l-menthol moiety acts similarly in the biofilm and facilitates the diffusion of F105 into the biofilm matrix and probably through the cell membrane. Several researchers reported that sulfonyl-containing compounds effectively repressed the growth of Staphylococci (Meadows and Gervay-Hague, 2006; Kudryavtsev et al., 2009; Low et al., 2011) suggesting antibacterial activity of the sulfonyl moiety (Mohan et al., 2014; Thirukovela et al., 2017). In contrast, the analogs of F105 lacking either sulfonyl group (compound 3 on the Figure 1) or l-menthol moiety (3-chloro-5-hydroxy-4-[(4-methylphenylsulfonyl)]-2(5H)-furanone) exhibited no activity against S. aureus (Latypova et al., 2014; Kayumov et al., 2015a) suggesting the requirement of both functional groups for bactericidal activity of F105.
The MIC was twofold higher (20 mg/L) in the MRSA compared to the MSSA. This difference between the MSSA and MRSA laboratory strains might be attributed to the alternation of the peptidoglycan composition due to the methicillin resistance as described previously (de Jonge and Tomasz, 1993), which might hamper efficient cell penetration, while we did not investigate this hypothesis explicitly. The reduced susceptibility might be also related to the SCCmec cassette that harbor, besides the methicillin-resistance determinants (mecA allels), up to more than 50 open reading frames. While some of these ORFs are known to be responsible for various resistances (e.g., the cadDX or arsRBC and arsDARBC operons for cadmium and arsenate resistance) (Li et al., 2011) the other are of unknown functions and might also influence the susceptibility to F105. However, this has not been analyzed in this work and is currently only a hypothetical assumption. The analysis of clinical MRSA isolates also indicates that there might be in general variability in the MIC between the strains, because 9/10 clinical MRSA isolates showed an MIC of F105 of 10 mg/L. Thus we assume that 10–20 mg/L F105 (corresponding to 25–50 μM) might be the effective MIC-range in many staphylococci. Comparing F105 to other furanone derivatives, which MICs in different S. aureus strains were reported to range between 4.65 mg/L (15 μM) (Kuehl et al., 2009) and 16 mg/L (107 μM) (Lattmann et al., 2005), the antimicrobial activity of F105 is moderate. However, further experiments in a broad range of clinical isolates will be needed to determine the MIC distributions of various furanones in the staphylococcal population to draw conclusion of the ecological cut-off (ECOFF), which separates the wild-type population from resistant isolate.
Comparing to other furanone derivatives and antimicrobials, the effect of F105 in established biofilms was remarkably strong and exceeded the activity of conventional antibiotics by several orders of magnitude. This could be attributed to the assumption that, leaving the biofilm structure almost unchanged, F105 easily penetrates the extracellular biofilm matrix and kills the matrix-embedded cells, in marked contrast to other antibiotics like gentamicin, ampicillin, chloramphenicol, or vancomycin (Parra-Ruiz et al., 2010; Singh et al., 2010; Kayumov et al., 2015b; Trizna et al., 2016). There are only few antibiotics with specific mechanisms of action, which show some activity in S. aureus biofilms, e.g., rifampin and to a lesser extent daptomycin or fluoroquinolones (Garcia et al., 2013; Siala et al., 2014; Stein et al., 2016). Rifampin inhibits RNA translation, fluoroquinolones inhibit DNA transcription and amplification, while daptomycin perforates the bacterial cell membrane. The mechanism of F105 action remains so far elusive. Our preliminary screening of the potential molecular targets of the F105 by LC–MS mass spectrometry analysis indicated that the intracellular levels of many proteins in S. aureus either decreased or increased when growing at 0.5x MIC of F105. Most of those proteins are enzymes involved in different cellular metabolic processes (Supplementary Tables S5, S6), while it remains currently unclear, whether these processes are directly (by interaction with specific regulators) or indirectly (e.g., in course of general stress response) impacted by F105. These data provide no evidence that F105 targets quorum sensing-depending processes and thus it remains rather unlikely that the observed intra-biofilm killing is conferred by quorum sensing inhibition alone. We also cannot explain the opposite biofilm-killing efficacy of F015 on both MRSA and MSSA laboratory strains comparing to the MIC and MBC. We can only hypothesize that this effect might also originate from intra-species variability and differentially expressed F105 targets.
No mutagenicity of F105 was detected in the Ames test using Salmonella typhimurium TA98, TA100 and TA102 strains at the furanone concentrations up to 80 mg/L without (Supplementary Table S3) and with (Supplementary Table S4) metabolic activation. The cytotoxicity test provided with CC50 values of 40.3 mg/L for MCF-7 cells (a human breast adenocarcinoma cell line) but only 8.1 mg/L for the skin fibroblast suggesting that the current structure of F105 might be too toxic for direct use in systemic therapeutics in humans and requires further modification of the structure in order to lower its toxicity and improve its solubility. The nearest derivative of F105 carrying arylsulfanyl substituent instead of arylsulfonyl group (compound 3 from the scheme 1) or lacking the l-menthol moiety (sulfone F70; Kayumov et al., 2015a) demonstrated higher CC50 values (32 and 45 mg/L, respectively), but exhibited no antibacterial activity even at concentrations up to 256 mg/L. These data suggest that the cytotoxicity of F105 presumably originates from the chlorinated 2(5H)-furanone fragment (Ren et al., 2004; Kitty et al., 2015) rather than from either sulfonyl or l-menthol moieties which are responsible for antibacterial effects of the studied compound.
Since the number of patients with skin wounds with MRSA infections and MRSA-related hospitalizations and deaths are continually increasing (Kalita et al., 2015), the synergy of F105 with aminoglycosides and benzalkonium chloride makes it an attractive starting point for the development of therapeutic strategies for the skin wounds treatment. Furthermore, some biocides with relatively high cytotoxicity are widely used in practice as disinfectants or local antiseptics, with miramistin or benzalkonium chloride, two biocides belonging to quaternary ammonium salts being a prominent example. Both are cytotoxic in direct contact with typical human cells, but the compounds are known as effective antiseptics for the local treatment of infected wounds with low side effects (Bernstein, 2000; Fromm-Dornieden et al., 2015). In particular, the CC50 value of benzalkonium chloride for the normal human fibroblasts was reported to be 6.7 mg/L, with CC50/MBC ratio of 0.05 (Damour et al., 1992). For F105 the CC50/MBC ratio was found to be 0.2, suggesting its higher or comparable therapeutic index in comparison with benzalkonium chloride, which is widely used as a biocide for outer treatment (Akimitsu et al., 1999; Bernstein, 2000). Taking in account that already 0.5–0.7 mg/L of F105 (∼15-fold less than CC50) decreases the MICs of aminoglycosides and benzalkonium chloride twofold, and the ability of F105 to target the biofilm-embedded Staphylococci, its chemotype looks as attractive tool for combination with antimicrobials to reduce their therapeutic concentrations, as well as to decrease their side effects and to enhance the efficacy of treatment of both planktonic and biofilm-embedded bacteria.
Author Contributions
IS, ET, DB, RS, LL, and ER performed the experiments. AKa, AKu, MK-S, RF, and OM conducted the experiments. IS, MP, MB, AKa, AKu, and OM analyzed the results. IS, AKa, AKu, and OM prepared figures and graphs and wrote the manuscript. AKa, AKu, MB, OM, and MP revised the manuscript. All the authors read and approved the final version of the manuscript.
Funding
This work was supported by the Russian Science Foundation, grant no. 15-14-00046 (AKa), by the Federal Ministry of Education and Research (BMBF, Germany), grant nos. 01KI1501 and 13GW0096D (MWP, OM); by the German Research Foundation, grant no. PL320/3-1 (MWP, MK-S), by the Ministry of Education and Science of Russia, assignment no. 2.5475.2017/6.7 (MB) and by the German Academic Exchange Service (DAAD), personal reference no. 91531398 (IS). This work was partially funded by the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities, assignment no. 6.7743.2017/6.7 (RF). This study was partially performed in the framework of the Russian Government Program of Competitive Development of Kazan Federal University.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
We thank Prof. Dr. Albert Rizvanov for providing the MCF7 and human fibroblasts 464 cell lines.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.02246/full#supplementary-material
References
Akimitsu, N., Hamamoto, H., Inoue, R., Shoji, M., Akamine, A., Takemori, K., et al. (1999). Increase in resistance of methicillin-resistant Staphylococcus aureus to beta-lactams caused by mutations conferring resistance to benzalkonium chloride, a disinfectant widely used in hospitals. Antimicrob. Agents Chemother. 43, 3042–3043.
Atshan, S. S., Shamsudin, M. N., Sekawi, Z., Lung, L. T. T., Barantalab, F., Liew, Y. K., et al. (2015). Comparative proteomic analysis of extracellular proteins expressed by various clonal types of Staphylococcus aureus and during planktonic growth and biofilm development. Front. Microbiol. 6:524. doi: 10.3389/fmicb.2015.00524
Baidamshina, D. R., Trizna, E. Y., Holyavka, M. G., Bogachev, M. I., Artyukhov, V. G., Akhatova, F. S., et al. (2017). Targeting microbial biofilms using Ficin, a nonspecific plant protease. Sci. Rep. 7:46068. doi: 10.1038/srep46068
Bassetti, M., Carnelutti, A., and Righi, E. (2017). The role of methicillin-resistant Staphylococcus aureus in skin and soft tissue infections. Curr. Opin. Infect. Dis. 30, 150–157. doi: 10.1097/qco.0000000000000353
Bernstein, I. L. (2000). Is the use of benzalkonium chloride as a preservative for nasal formulations a safety concern? A cautionary note based on compromised mucociliary transport. J. Allergy Clin. Immunol. 105, 39–44. doi: 10.1016/s0091-6749(00)90175-1
Bortolin, M., Bidossi, A., De Vecchi, E., Avveniente, M., and Drago, L. (2017). In vitro antimicrobial activity of chlorquinaldol against microorganisms responsible for skin and soft tissue infections: comparative evaluation with gentamicin and fusidic acid. Front. Microbiol. 8:10. doi: 10.3389/fmicb.2017.01039
Brackman, G., and Coenye, T. (2015). Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 21, 5–11. doi: 10.2174/1381612820666140905114627
Bremer, F., Grade, S., Kohorst, P., and Stiesch, M. (2011). In vivo biofilm formation on different dental ceramics. Quintessence Int. 42, 565–574.
Conlon, B. P. (2014). Staphylococcus aureus chronic and relapsing infections: evidence of a role for persister cells an investigation of persister cells, their formation and their role in S. aureus disease. Bioessays 36, 991–996. doi: 10.1002/bies.201400080
Cosgrove, S. E., and Fowler, V. G. Jr. (2008). Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46, S386–S393. doi: 10.1086/533595
Cosgrove, S. E., Kaye, K. S., Eliopoulous, G. M., and Carmeli, Y. (2002). Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162, 185–190. doi: 10.1001/archinte.162.2.185
Damour, O., Hua, S. Z., Lasne, F., Villain, M., Rousselle, P., and Collombel, C. (1992). Cytotoxicity evaluation of antibiotics on cultured human fibroblasts ans kreatinocytes. Burns 18, 479–485. doi: 10.1016/0305-4179(92)90180-3
de Jonge, B. L., and Tomasz, A. (1993). Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus aureus grown in the presence of methicillin: functional role for penicillin-binding protein 2A in cell wall synthesis. Antimicrob. Agents Chemother. 37, 342–346. doi: 10.1128/AAC.37.2.342
den Hollander, J. G., Mouton, J. W., and Verbrugh, H. A. (1998). Use of pharmacodynamic parameters to predict efficacy of combination therapy by using fractional inhibitory concentration kinetics. Antimicrob. Agents Chemother. 42, 744–748.
Doll, K., Jongsthaphongpun, K. L., Stumpp, N. S., Winkel, A., and Stiesch, M. (2016). Quantifying implant-associated biofilms: comparison of microscopic, microbiologic and biochemical methods. J. Microbiol. Methods 130, 61–68. doi: 10.1016/j.mimet.2016.07.016
Donlan, R. M. (2002). Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8, 881–890. doi: 10.3201/eid0809.020063
Donlan, R. M., and Costerton, J. W. (2002). Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167–193. doi: 10.1128/cmr.15.2.167-193.2002
Eliopoulos, G., and Moellering, R. C. Jr. (1996). “Antimicrobial combinations,” in Antibiotics in Laboratory Medicine, 4th Edn, ed. V. Lorian (Baltimore, MD: The Williams & Wilkins Co), 330–396.
European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). (2000). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 6, 503–508. doi: 10.1046/j.1469-0691.2000.00149.x
Fagerlund, A., Langsrud, S., Heir, E., Mikkelsen, M. I., and Moretro, T. (2016). Biofilm matrix composition affects the susceptibility of food associated staphylococci to cleaning and disinfection agents. Front. Microbiol. 7:856. doi: 10.3389/fmich.2010.00856
Fedorova, K. P., Scharafutdinov, I. S., Turbina, E. Y., Bogachev, M. I., Ilinskaja, O. N., and Kayumov, A. R. (2013). C-terminus of transcription factor TnrA from Bacillus subtilis controls DNA-binding domain activity but is not required for dimerization. Mol. Biol. 47, 293–298. doi: 10.1134/S0026893313020052
Fenske, D., and Merzweiler, K. (1989). Synthesis of a new chiral phosphine ligand. J. Chem. Sci. 44, 879–883.
Forrest, G. N., and Tamura, K. (2010). Rifampin combination therapy for nonmycobacterial infections. Clin. Microbiol. Rev. 23, 14–34. doi: 10.1128/cmr.00034-09
Fromm-Dornieden, C., Rembe, J. D., Schafer, N., Bohm, J., and Stuermer, E. K. (2015). Cetylpyridinium chloride and miramistin as antiseptic substances in chronic wound management - prospects and limitations. J. Med. Microbiol. 64, 407–414. doi: 10.1099/jmm.0.000034
Garcia, L. G., Lemaire, S., Kahl, B. C., Becker, K., Proctor, R. A., Denis, O., et al. (2013). Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 68, 1455–1464. doi: 10.1093/jac/dkt072
Heck, R., and Stuetz, A. (1988). Antimycotic-6-phenyl-2-hexen-4-ynamines. U.S. Patent No. EP 0254677 A1.
Hentzer, M., Riedel, K., Rasmussen, T. B., Heydorn, A., Andersen, J. B., Parsek, M. R., et al. (2002). Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148, 87–102. doi: 10.1099/00221287-148-1-87
Hentzer, M., Wu, H., Andersen, J. B., Riedel, K., Rasmussen, T. B., Bagge, N., et al. (2003). Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22, 3803–3815. doi: 10.1093/emboj/cdg366
Herigstad, B., Hamilton, M., and Heersink, J. (2001). How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 44, 121–129. doi: 10.1016/s0167-7012(00)00241-4
Hodille, E., Rose, W., Diep, B. A., Goutelle, S., Lina, G., and Dumitrescu, O. (2017). The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin. Microbiol. Rev. 30, 887–917. doi: 10.1128/CMR.00120-16
Kali, A., Bhuvaneshwar, D., Charles, P. M., and Seetha, K. S. (2016). Antibacterial synergy of curcumin with antibiotics against biofilm producing clinical bacterial isolates. J. Basic Clin. Pharm. 7, 93–96. doi: 10.4103/0976-0105.183265
Kalita, S., Devi, B., Kandimalla, R., Sharma, K. K., Sharma, A., Kalita, K., et al. (2015). Chloramphenicol encapsulated in poly-epsilon-caprolactone-pluronic composite: nanoparticles for treatment of MRSA-infected burn wounds. Int. J. Nanomed. 10, 2971–2984. doi: 10.2147/ijn.s75023
Kamatou, G. P., Vermaak, I., Viljoen, A. M., and Lawrence, B. M. (2013). Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry 96, 15–25. doi: 10.1016/j.phytochem.2013.08.005
Kayumov, A. R., Khakimullina, E. N., Sharafutdinov, I. S., Trizna, E. Y., Latypova, L. Z., Hoang Thi, L., et al. (2015a). Inhibition of biofilm formation in Bacillus subtilis by new halogenated furanones. J. Antibiotics 68, 297–301. doi: 10.1038/ja.2014.143
Kayumov, A. R., Nureeva, A. A., Trizna, E. Y., Gazizova, G. R., Bogachev, M. I., Shtyrlin, N. V., et al. (2015b). New derivatives of pyridoxine exhibit high antibacterial activity against biofilm-embedded staphylococcus cells. Biomed Res. Int. 2015:890968. doi: 10.1155/2015/890968
Kifer, D., Muzinic, V., and Klaric, M. S. (2016). Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 69, 689–696. doi: 10.1038/ja.2016.10
Kitty, H., Samuel, K., Daniel, C., Renxun, C., Mark, W., and Naresh, K. (2015). “Development of fimbrolides, halogenated furanones and their derivatives as antimicrobial agents,” in Antibacterial Surfaces, ed. R. C. Elena Ivanova (Cham: Springer International Publishing), 149–170.
Klinger-Strobel, M., Suesse, H., Fischer, D., Pletz, M. W., and Makarewicz, O. (2016). A novel computerized cell count algorithm for biofilm analysis. PLOS ONE 11:e0154937. doi: 10.1371/journal.pone.0154937
Kudryavtsev, K. V., Bentley, M. L., and McCafferty, D. G. (2009). Probing of the cis-5-phenyl proline scaffold as a platform for the synthesis of mechanism-based inhibitors of the Staphylococcus aureus sortase SrtA isoform. Bioorg. Med. Chem. 17, 2886–2893. doi: 10.1016/j.bmc.2009.02.008
Kuehl, R., Al-Bataineh, S., Gordon, O., Luginbuehl, R., Otto, M., Textor, M., et al. (2009). Furanone at subinhibitory concentrations enhances staphylococcal biofilm formation by luxS repression. Antimicrob. Agents Chemother. 53, 4159–4166. doi: 10.1128/aac.01704-08
Kurbangalieva, A. R., Devyatova, N. F., Bogdanov, A. V., Berdnikov, E. A., Mannafov, T. G., Krivolapov, D. B., et al. (2007). Synthesis of novel arylthio derivatives of mucochloric acid. Phosphorus Sulfur Silicon Relat. Elem. 182, 607–630. doi: 10.1080/10426500601015989
Lattmann, E., Dunn, S., Niamsanit, S., and Sattayasai, N. (2005). Synthesis and antibacterial activities of 5-hydroxy-4-amino-2(5H)-furanones. Bioorg. Med. Chem. Lett. 15, 919–921. doi: 10.1016/j.bmcl.2004.12.051
Latypova, L. Z., Saigitbatalova, E. S., Chulakova, D. R., Lodochnikova, O. A., Kurbangalieva, A. R., Berdnikov, E. A., et al. (2014). Sulfides, sulfones, and sulfoxides of the furan-2(5H)-one series. synthesis and structure. Russ. J. Organ. Chem. 50, 521–534. doi: 10.1134/s1070428014040149
Leclercq, R., Canton, R., Brown, D. F., Giske, C. G., Heisig, P., MacGowan, A. P., et al. (2013). EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect 19, 141–160. doi: 10.1111/j.1469-0691.2011.03703.x
Lewis, K. (2001). Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45, 999–1007. doi: 10.1128/AAC.45.4.999-1007.2001
Li, S. S., Skov, R. L., Han, X., Larsen, A. R., Larsen, J., Sorum, M., et al. (2011). Novel types of staphylococcal cassette chromosome mec elements identified in clonal complex 398 methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 55, 3046–3050. doi: 10.1128/aac.01475-10
Lonn-Stensrud, J., Landin, M. A., Benneche, T., Petersen, F. C., and Scheie, A. A. (2009). Furanones, potential agents for preventing Staphylococcus epidermidis biofilm infections? J. Antimicrob. Chemother. 63, 309–316. doi: 10.1093/jac/dkn501
Low, E., Kim, B., Francavilla, C., Shiau, T. P., Turtle, E. D., O’Mahony, D. J. R., et al. (2011). Structure stability/activity relationships of sulfone stabilized N,N-dichloroamines. Bioorg. Med. Chem. Lett. 21, 3682–3685. doi: 10.1016/j.bmcl.2011.04.084
McCann, J., and Ames, B. N. (1976). A simple method for detecting environmental carcinogens as mutagens. Ann. N. Y. Acad. Sci. 271, 5–13. doi: 10.1111/j.1749-6632.1976.tb23086.x
McCarthy, H., Rudkin, J. K., Black, N. S., Gallagher, L., O’Neill, E., and O’Gara, J. P. (2015). Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 5:1. doi: 10.3389/fcimb.2015.00001
Meadows, D. C., and Gervay-Hague, J. (2006). Vinyl sulfones: synthetic preparations and medicinal chemistry applications. Med. Res. Rev. 26, 793–814. doi: 10.1002/med.20074
Merritt, J. H., Kadouri, D. E., and O’Toole, G. A. (2005). Growing and analyzing static biofilms. Curr. Protoc. Microbiol. Chapter 1, Unit 1B. 1. doi: 10.1002/9780471729259.mc01b01s00
Mohan, N. R., Sreenivasa, S., Manojkumar, K. E., Rao, T. M. C., Thippeswamy, B. S., and Suchetan, P. A. (2014). Synthesis, antibacterial, anthelmintic and anti-inflammatory studies of novel methylpyrimidine sulfonyl piperazine derivatives. J. Braz. Chem. Soc. 25, 1012–1020. doi: 10.5935/0103-5053.20140073
Naicker, P. R., Karayem, K., Hoek, K. G. P., Harvey, J., and Wasserman, E. (2016). Biofilm formation in invasive Staphylococcus aureus isolates is associated with the clonal lineage. Microb. Pathog. 90, 41–49. doi: 10.1016/j.micpath.2015.10.023
Narishetty, S. T., and Panchagnula, R. (2005). Effect of L-menthol and 1,8-cineole on phase behavior and molecular organization of SC lipids and skin permeation of zidovudine. J. Control. Release 102, 59–70. doi: 10.1016/j.jconrel.2004.09.016
Odds, F. C. (2003). Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. doi: 10.1093/jac/dkg301
Parra-Ruiz, J., Vidaillac, C., Rose, W. E., and Rybak, M. J. (2010). Activities of high-dose daptomycin, vancomycin, and moxifloxacin alone or in combination with clarithromycin or rifampin in a novel in vitro model of Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 54, 4329–4334. doi: 10.1128/aac.00455-10
Peeters, E., Nelis, H. J., and Coenye, T. (2008). Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 72, 157–165. doi: 10.1016/j.mimet.2007.11.010
Percival, S. L., Finnegan, S., Donelli, G., Vuotto, C., Rimmer, S., and Lipsky, B. A. (2016). Antiseptics for treating infected wounds: efficacy on biofilms and effect of pH. Crit. Rev. Microbiol. 42, 293–309. doi: 10.3109/1040841x.2014.940495
Ren, D. C., Bedzyk, L. A., Setlow, P., England, D. F., Kjelleberg, S., Thomas, S. M., et al. (2004). Differential gene expression to investigate the effect of (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone on Bacillus subtilis. Appl. Environ. Microbiol. 70, 4941–4949. doi: 10.1128/aem.70.8.4941-4949.2004
Ren, D. C., Sims, J. J., and Wood, T. K. (2001). Inhibition of biofilm formation and swarming of Escherichia coli by (5Z)-4-bromo-5(bromomethylene)-3-butyl-2(5H)-furanone. Environ. Microbiol. 3, 731–736. doi: 10.1046/j.1462-2920.2001.00249.x.
Rogers, S. A., Whitehead, D. C., Mullikin, T., and Melander, C. (2010). Synthesis and bacterial biofilm inhibition studies of ethyl N-(2-phenethyl) carbamate derivatives. Organ. Biomol. Chem. 8, 3857–3859. doi: 10.1039/c0ob00063a
Roy, R., Tiwari, M., Donelli, G., and Tiwari, V. (2017). Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence doi: 10.1080/21505594.2017.1313372 [Epub ahead of print].
Sanchez-Vizuete, P., Orgaz, B., Aymerich, S., Le Coq, D., and Briandet, R. (2015). Pathogens protection against the action of disinfectants in multispecies biofilms. Front. Microbiol. 6:705. doi: 10.3389/fmicb.2015.00705
Sharafutdinov, I., Shigapova, Z., Baltin, M., Akhmetov, N., Bogachev, M., and Kayumov, A. (2016). HtrA protease from Bacillus subtilis suppresses the bacterial fouling of the rat skin injuries. Bionanoscience 6, 564–567. doi: 10.1007/s12668-016-0281-2
Siala, W., Mingeot-Leclercq, M. P., Tulkens, P. M., Hallin, M., Denis, O., and Van Bambeke, F. (2014). Comparison of the antibiotic activities of daptomycin, vancomycin, and the investigational fluoroquinolone delafloxacin against biofilms from Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 58, 6385–6397. doi: 10.1128/AAC.03482-14
Singh, R., Ray, P., Das, A., and Sharma, M. (2010). Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 65, 1955–1958. doi: 10.1093/jac/dkq257
Stein, C., Makarewicz, O., Forstner, C., Weis, S., Hagel, S., Loffler, B., et al. (2016). Should daptomycin-rifampin combinations for MSSA/MRSA isolates be avoided because of antagonism? Infection 44, 499–504. doi: 10.1007/s15010-016-0874-2
Thirukovela, N. S., Kankala, S., Kankala, R. K., Paidakula, S., Gangula, M. R., Vasam, C. S., et al. (2017). Regioselective synthesis of some new 1,4-disubstituted sulfonyl-1,2,3-triazoles and their antibacterial activity studies. Med. Chem. Res. 26, 2190–2195. doi: 10.1007/s00044-017-1926-6
Trizna, E., Latypova, L., Kurbangalieva, A., Bogachev, M., Kayumov, A., et al. (2016). 2(5H)-Furanone derivatives as inhibitors of staphylococcal biofilms. BioNanoScience 6, 423–426. doi: 10.1007/s12668-016-0258-1
Trizna, E. Y., Khakimullina, E. N., Latypova, L. Z., Kurbangalieva, A. R., Sharafutdinov, I. S., Evtyugin, V. G., et al. (2015). Thio derivatives of 2(5H)-furanone as inhibitors against Bacillus subtilis biofilms. Acta Naturae 7, 102–107
Keywords: 2(5H)-furanones, sulfones, Staphylococcus aureus, MRSA, biofilm, antimicrobials synergism
Citation: Sharafutdinov IS, Trizna EY, Baidamshina DR, Ryzhikova MN, Sibgatullina RR, Khabibrakhmanova AM, Latypova LZ, Kurbangalieva AR, Rozhina EV, Klinger-Strobel M, Fakhrullin RF, Pletz MW, Bogachev MI, Kayumov AR and Makarewicz O (2017) Antimicrobial Effects of Sulfonyl Derivative of 2(5H)-Furanone against Planktonic and Biofilm Associated Methicillin-Resistant and -Susceptible Staphylococcus aureus. Front. Microbiol. 8:2246. doi: 10.3389/fmicb.2017.02246
Received: 12 July 2017; Accepted: 31 October 2017;
Published: 20 November 2017.
Edited by:
Sara María Soto, ISGlobal, SpainReviewed by:
Giovanna Batoni, University of Pisa, ItalyDipankar Ghosh, Jawaharlal Nehru University, India
Copyright © 2017 Sharafutdinov, Trizna, Baidamshina, Ryzhikova, Sibgatullina, Khabibrakhmanova, Latypova, Kurbangalieva, Rozhina, Klinger-Strobel, Fakhrullin, Pletz, Bogachev, Kayumov and Makarewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Airat R. Kayumov, kairatr@yandex.ru Oliwia Makarewicz, oliwia.makarewicz@med.uni-jena.de
†These authors have contributed equally to this work as senior authors.
 Irshad S. Sharafutdinov
Irshad S. Sharafutdinov Elena Y. Trizna
Elena Y. Trizna Diana R. Baidamshina
Diana R. Baidamshina Maria N. Ryzhikova
Maria N. Ryzhikova Regina R. Sibgatullina
Regina R. Sibgatullina Alsu M. Khabibrakhmanova
Alsu M. Khabibrakhmanova Liliya Z. Latypova
Liliya Z. Latypova Almira R. Kurbangalieva
Almira R. Kurbangalieva Elvira V. Rozhina
Elvira V. Rozhina Mareike Klinger-Strobel3
Mareike Klinger-Strobel3 Rawil F. Fakhrullin
Rawil F. Fakhrullin Mathias W. Pletz
Mathias W. Pletz Mikhail I. Bogachev
Mikhail I. Bogachev Airat R. Kayumov
Airat R. Kayumov Oliwia Makarewicz
Oliwia Makarewicz