- 1Instituto Madrileño de Investigación y Desarrollo Rural, Agrario y Alimentario, Madrid, Spain
- 2Departament de Bioquímica i Biotecnologia, Facultat d’Enologia, Universitat Rovira i Virgili, Tarragona, Spain
There is an increasing trend toward understanding the impact of non-Saccharomyces yeasts on the winemaking process. Although Saccharomyces cerevisiae is the predominant species at the end of fermentation, it has been recognized that the presence of non-Saccharomyces species during alcoholic fermentation can produce an improvement in the quality and complexity of the final wines. A previous work was developed for selecting the best combinations between S. cerevisiae and five non-Saccharomyces (Torulaspora delbrueckii, Schizosaccharomyces pombe, Candida stellata, Metschnikowia pulcherrima, and Lachancea thermotolorans) native yeast strains from D.O. “Vinos de Madrid” at the laboratory scale. The best inoculation strategies between S. cerevisiae and non-Saccharomyces strains were chosen to analyze, by real-time quantitative PCR (qPCR) combined with the use of specific primers, the dynamics of inoculated populations throughout the fermentation process at the pilot scale using the Malvar white grape variety. The efficiency of the qPCR system was verified independently of the samples matrix, founding the inoculated yeast species throughout alcoholic fermentation. Finally, we can validate the positive effect of selected co-cultures in the Malvar wine quality, highlighting the sequential cultures of T. delbrueckii CLI 918/S. cerevisiae CLI 889 and C. stellata CLI 920/S. cerevisiae CLI 889 and, mixed and sequential cultures of L. thermotolerans 9-6C combined with S. cerevisiae CLI 889.
Introduction
Alcoholic fermentation is a complex ecological and biochemical process where a succession of yeasts of several genera and species are able to convert must sugars into ethanol and carbon dioxide, as well as into important secondary metabolites (Barata et al., 2012; Sun et al., 2014; Albergaria and Arneborg, 2016). Even though Saccharomyces species are present at a low frequency on the surface of healthy grapes, Saccharomyces cerevisiae is considered the primary microorganism in the fermentation process and it is widely used in oenology (Martini et al., 1996; Fleet, 2003). However, during the last decade, non-Saccharomyces yeasts species have been proposed for winemaking as they could contribute to the improvement of wine quality (Ciani et al., 2014; Wang et al., 2015; Masneuf-Pomarede et al., 2016; Puertas et al., 2016). Thus, a new trend has emerged in winemaking using starter cultures composed by non-Saccharomyces yeasts, together with S. cerevisiae or for sequential fermentation with S. cerevisiae.
Molecular methods are showing useful results for detection and faster identification of microorganisms throughout the wine elaboration process (Ivey and Phister, 2011). Classical microbiological methods involving isolation coupled with the enumeration of microbes by plating can lead to misinterpretation of the real number of microorganisms since these methods fail to detect viable but non-culturable (VBNC) organisms (Divol and Lonvaud-Funel, 2005; Quirós et al., 2009; Salma et al., 2013; Wang et al., 2016) and minor populations present are difficult to detect on plates (Cocolin et al., 2013; David et al., 2014). Instead, molecular techniques, generally named culture-independent methods, are used for the identification of microorganism directly in the system through the study of their DNA or RNA without the need for isolation and cultivation, reducing detection time (Andorrà et al., 2008). Real-time quantitative PCR (qPCR) has been widely used in wine for microorganism detection during wine elaboration (Rawsthorne and Phister, 2006; Andorrà et al., 2008, 2010; Tofalo et al., 2012; Wang et al., 2014), providing significant advantages as the low detection level, the speed by which assays are performed, and the ability to quantify yeasts present following alcoholic fermentation.
In a previous work of García et al. (2017), small-scale fermentations were elaborated to study the oenological characterization of five non-Saccharomyces native yeast species under several co-culture conditions in combination with selected strain of S. cerevisiae CLI 889 to improve the organoleptic properties of the regional Malvar wines. There, the best inoculation process was selected depending on the non-Saccharomyces strain inoculated. Preferred sequential inoculations were elaborated with S. cerevisiae CLI 889 in combination with Torulaspora delbrueckii CLI 918 that produced wines with a higher fruity and floral aroma and lower ethanol content; with Candida stellata CLI 920 that increased the aroma complexity and glycerol content; and, with Lachancea thermotolerans 9-6C, produced an increase in acidity and floral and ripe fruit aroma. In the case of Schizosaccharomyces pombe, sequential fermentation was selected according to its fruity aroma score obtained after tasting. However, mixed cultures of S. cerevisiae with Metschnikowia pulcherrima CLI 457 and L. thermotolerans 9-6C was chosen due to a lower volatile acidity observed in final wines. Moreover, an increase of glycerol and ripe fruit aroma in the case of M. pulcherrima was observed, and for L. thermotolerans mixed culture the freshness, citric aroma, and full body were the main aspects to verify at the pilot scale.
Regarding these results, the aim of this work is to study yeast population evolution using real-time PCR during pilot winemaking trials under the best inoculation strategies. Moreover, validation of their positive effect on wine fermentation and wine quality was observed in the previous laboratory scale study (García et al., 2017) using sensory analysis.
Materials and Methods
Yeast Strains
The non-Saccharomyces strains used in this study are T. delbrueckii CLI 918, S. pombe CLI 1079, C. stellata CLI 920, M. pulcherrima CLI 457 and L. thermotolerans 9-6C, and S. cerevisiae CLI 889 strain were previously isolated on the Madrid winegrowing region and selected and characterized in our laboratories based on some established and desirable oenological criteria (Arroyo, 2000; Cordero-Bueso et al., 2013, 2016).
Wine Fermentation and Sampling
The pilot winemaking (stainless steel tanks with 16 L of must) was performed at IMIDRA’s experimental cellar is located in the Madrid winegrowing region, Spain (40°31′ N, 3°17′ W and 610 m altitude). Grapes were collected from Malvar (Vitis vinifera cv.) white grape variety to elaborate the wines, which were obtained in accordance with the cellar standard practices for harvest. Musts were racked, homogenized, and dislodged statically at 4°C to clarify and be sulfited (50 ppm). Musts obtained from two different vineyards, Must I and Must II, showed 1095 and 1099 g L-1 of density, pH values were 3.05 and 3.15, titratable acidity (expressed as g L-1 of tartaric acid) was 5.7 and 4.8, and yeast assimilable nitrogen (YAN) values were 218 and 100 mgN L-1, respectively.
Triplicate fermentations were carried out in stainless steel tanks with 16 L of fresh Malvar must at a controlled temperature of 18°C without agitation and, the tanks were locked to maintain anaerobiosis throughout alcoholic fermentation (CO2 was released through a sterile Müller valve with 96% H2SO4). Tanks were inoculated with a pied de cuve until a concentration of 106 cells mL-1 of each yeast strain. These inocula were achieved by an overnight culture of the different yeast strains in sterile must of the same variety prepared away from the cellar. Preselected combinations between S. cerevisiae CLI 889 and the different non-Saccharomyces species were the best results in García et al. (2017). We named mixed fermentation when both strains are inoculated at the same time, and in sequential fermentation, the non-Saccharomyces culture was inoculated at first and the addition of S. cerevisiae takes place when the wine contains 5% alcohol (v/v). The trials tested in must I, were: sequential culture of T. delbrueckii CLI 918 and S. cerevisiae CLI 889 strains (s-Td/ScI); mixed culture of M. pulcherrima CLI 457 and S. cerevisiae CLI 889 strains (m-Mp/ScI); and pure culture of S. cerevisiae CLI 889 (p-ScI), culture considered as control. The combinations in Malvar must II were: sequential culture of S. pombe CLI 1079 and S. cerevisiae CLI 889 (s-Sp/ScII); sequential culture of C. stellata CLI 920 and S. cerevisiae CLI 889 (s-Cs/ScII); mixed culture of L. thermotolerans 9-6C and S. cerevisiae CLI 889 (m-Lt/ScII); sequential culture of L. thermotolerans 9-6C and S. cerevisiae CLI 889 (s-Lt/ScII); and pure culture of S. cerevisiae CLI 889 (p-ScII) as a control.
The fermentation process was monitored daily though density, °Baumé, and temperature measurements until constant density (lower than 1000 g L-1). Samples were taken for every tank during the vinification process. Samples (1 mL) for qPCR analyses were centrifuged and pellets were immediately cryo-preserved. For total yeast counts, samples were spread on yeast extract peptone dextrose (YPD) plates and on lysine agar medium [0.25% L-Lysine monohydrochloride (Sigma–Aldrich, St. Louis, MO, United States), 1.17% yeast carbon base (Difco, Detroit, MI, United States), and 2% agar, w/v], a selective medium for the differentiation of non-Saccharomyces yeast populations which does not support the growth of S. cerevisiae (Walters and Thiselton, 1953). One week after fermentation finished, the wines were bottled after racking and adding 50 ppm SO2.
Oligonucleotides
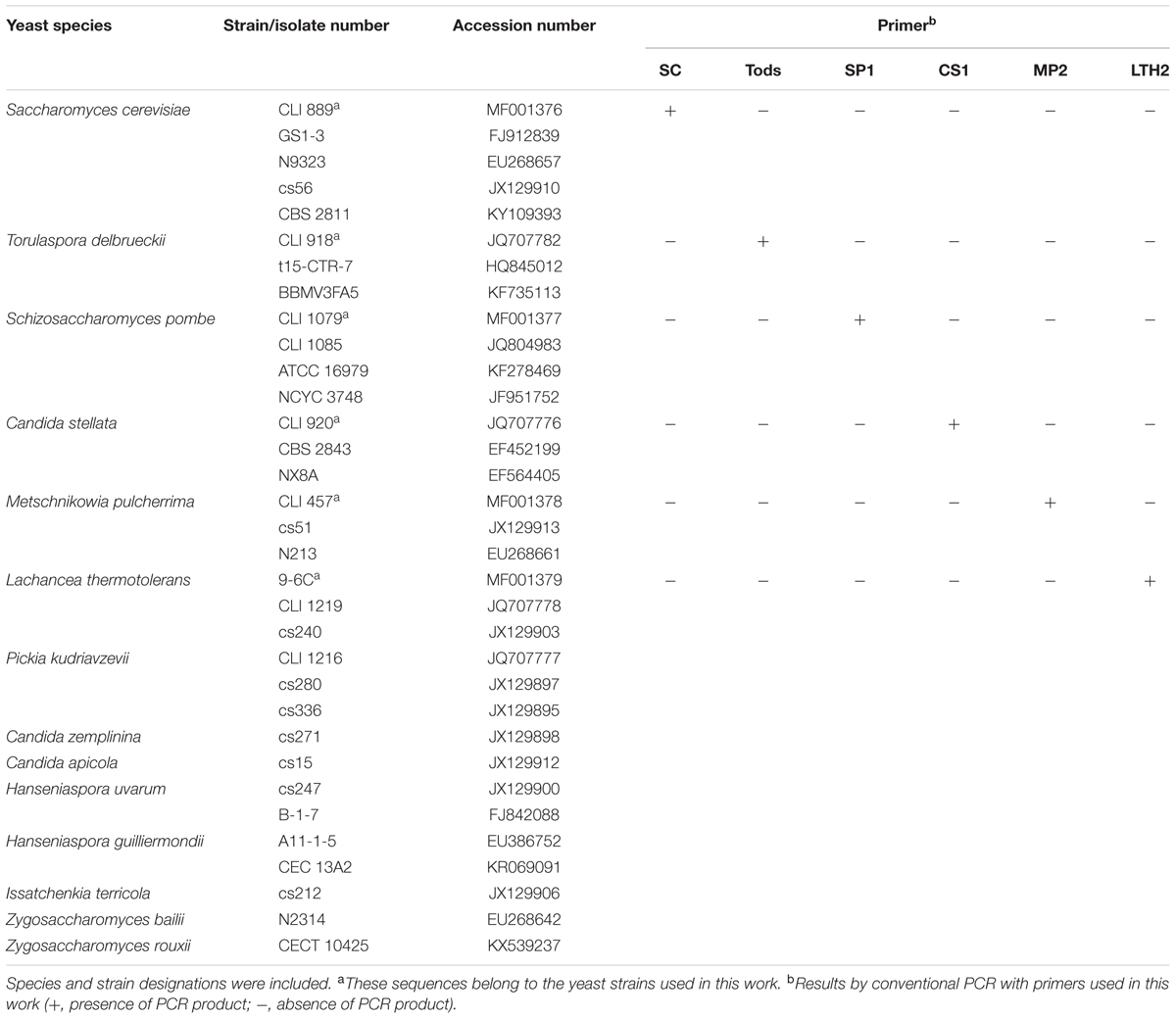
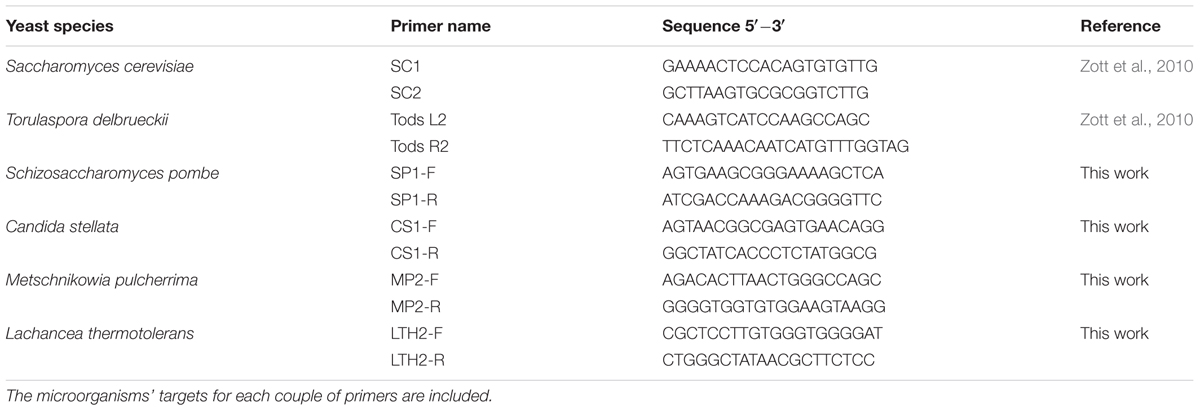
Specific-species primers were designed in this work from conserved sequences of the variable D1/D2 domains of the 26S rDNA gene. Generated sequences were aligned with sequences of strains of the same species (Table 1) available at the National Centre for Biotechnology Information (NCBI)1 using Clustal W multiple-sequence alignment (Thompson et al., 1994). The primer design was performed using the Primer3Plus program2. Furthermore, the properties of each primer were verified by NIST Primer Tools3. Primers used in this study (Table 2) were synthetized by TIB MOLBIOL (Berlin, Germany). Moreover, conventional and real-time PCR were carried out using a range of yeast species to verify the specificity of each primer set.
DNA Extraction and Real-Time PCR Assays
Yeast cell pellets were washed with sterile distilled water, and the pellets were resuspended in 700 μL of AP1 buffer (DNeasy Plant Mini Kit; QIAGEN, Valencia, CA, United States) and transferred in a 2-mL microcentrifuge tube containing 1 g of 0.5 mm-diameter glass beads. The tubes were shaken in a mixer mill (Retsch GmbH, Haan, Germany) for 3 min at the maximum rate and then centrifuged at 10,000 rpm for 1 min. Then, the supernatant was transferred to a sterile tube and purified using DNeasy Plant Mini Kit (QIAGEN) according to the manufacturer’s instructions.
qPCR was performed on an Applied Biosystems Prism 7500 sequence detection system (Applied Biosystems, Carlsbad, CA, United States). PCR amplification was conducted in optical-grade 96-well plates (Applied Biosystems) and each 25 μL reaction mixture containing 5 μL of DNA, 0.7 μM of each respective primer, and 12.5 μL of SYBR Green Master Mix (Roche Diagnostics GmbH, Mannheim, Germany). Each reaction was made in triplicate. The reaction conditions were an initial step at 95°C for 10 min and 40 cycles of 95°C for 15 s, 60°C for 1 min and 72°C for 30 s. The CT was determined automatically by the instrument. The coefficients of efficiency (E) were calculated using the formula E = (10-1/slope) – 1 (Higuchi et al., 1993).
Standard Curves
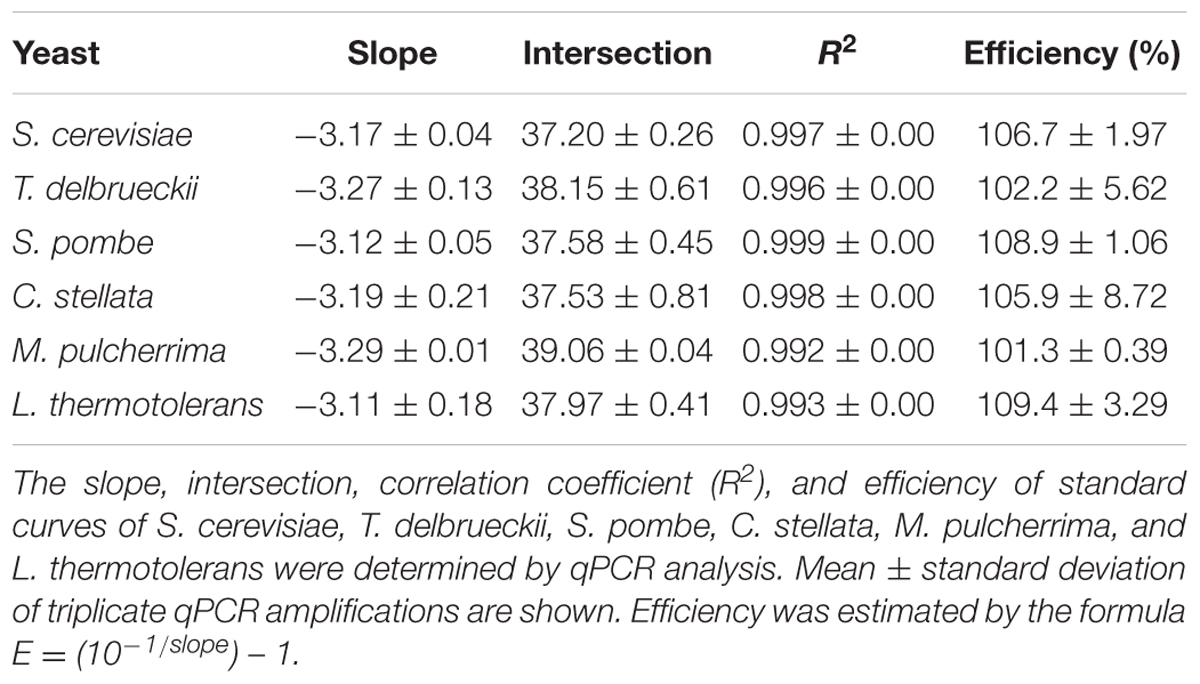
Standard curves for each yeast strain were created by plotting the cycle threshold (CT) values of the qPCR performed with dilution series of yeast cells (107 to 103 cells mL-1) against the log input cell mL-1 (ABI PRISM 7500 sequence detection system, Applied Biosystems). Standard curves were created for the six yeast strains used in this work.
Artificial Contamination of Wines
Commercial Tempranillo red wine and Malvar white wine, previously sterilized by filtration, and YPD liquid medium were artificially contaminated with T. delbrueckii CLI 918, at known concentrations (106 to 102 cells mL-1). After incubation of 24 h at 20°C, DNA was isolated as indicated before for qPCR analysis. Standard curves for quantification of samples and determination of amplification efficiency were constructed. These dilutions were also plated on YPD agar and incubated 1 week at 28°C to obtain the number of CFU per milliliter using an easySpiral® plater (Interscience, St. Nom, France).
Study of Saccharomyces cerevisiae at the Strain Level
Microsatellite multiplex PCR analysis was used to check the presence of S. cerevisiae CLI 889 in the different types of elaboration, using the highly polymorphic loci SC8132X, YOR267C, and SCPTSY7 (Vaudano and Garcia-Moruno, 2008). The analysis was performed according to Cordero-Bueso et al. (2011) and Tello et al. (2012).
Analytical Determination
Oenological parameters as alcohol degree, pH, volatile acidity, total acidity, reducing sugars, glycerol, malic acid, and lactic acid were measured by Fourier transform infrared spectroscopy in the laboratories of Liec Agroalimentaria S.L. (Manzanares, Spain). An accredited laboratory for physico-chemical analysis in wines to conform to UNE-EN ISO/IEC 17025:2005 rules. YAN was determined in must by the formol titration method (Gump et al., 2002).
Quantification of major volatile compounds was carried out in a GC Agilent 6850 with a FID detector equipped with a column DB-Wax (60 m × 0.32 mm × 0.5 μm film thickness) from J&W Scientific (Folsom, CA, United States). Analyses were done according to Gil et al. (2006) and Balboa-Lagunero et al. (2013).
Sensorial Analysis
The final wines were subjected to two sensory analyses, triangle tests (ISO 4120:2007) and descriptive analysis by a trained panel of seven skilled judges from the IMIDRA Institute. Using triangle tests, the judges determine if a sensory difference exists between the wines tested. Sensory descriptive analysis was based on the description of attributes of the wines though 15 aroma and taste descriptors, and the panelists were asked about their preferences. These attributes were estimated on basis a scale from 1 (low intensity) to 10 (high intensity) and total scores were obtained as the mean and standard deviation of seven evaluations (Arroyo et al., 2009; Balboa-Lagunero et al., 2013).
Statistical Analysis
Analysis of variance was carried out by an ANOVA Tukey test to determine significant differences (α = 0.05) between the samples with their respective fermentation control. PCA analysis was performed to identify the most influential oenological parameters and volatile compounds in the different types of cultures. The data were analyzed with SPSS Statistics 21.0 Software for Windows (SPSS, Inc., Chicago, IL, United States).
Results
Primer Design, Specificity and Sensitivity of qPCR
Primers proposed in this work were designed on the variable D1/D2 domains of 26S rDNA gene, amplifying products between 100 and 150 bp in length. Primers for the quantification of T. delbrueckii and S. cerevisiae strains were designed by Zott et al. (2010) from the region of internal transcribed spacers (ITSs) of the ribosomal DNA region. The other primers used were designed for this work according to those described in the material and methods sections. Sequences for all primers are listed on the Table 2.
Each pair of primers exhibited in silico specific homology to only species for which were designed. Additionally, conventional PCR was performed using purified DNA from the yeast species used in this study and different strains belonging to the yeasts species Candida vini, Wickerhamomyces anomalus, Zygosaccharomyces bailii, Meyerozyma guilliermondii, Pichia membranifaciens, Priceomyces carsonii, and Lachancea fermentati, the most usual species isolated during spontaneous fermentation of Malvar must in the experimental cellar of IMIDRA (Cordero-Bueso et al., 2013), which are also included in the IMIDRA Institute Collection. Amplifications were observed only for those species which the primers that were specifically developed (Table 1).
To determine the standard curves qPCR, YPD cultures of each strain containing 107 cells mL-1 were serially diluted 10-fold until 103 cells mL-1 and DNA were extracted from 1 ml of each dilution. The DNA was then amplified by qPCR and standard curves were constructed. The slope, intersection, correlation coefficient (R2), and efficiency of the standard curves obtained are shown in Table 3. The assays were linear over five orders of magnitude and, the detection limit for all yeast species was 103 cells mL-1.
Quantification in Artificially Contaminated Wines
To study the influence of the wine matrix on the efficiency of the real-time PCR system, standard curves using artificial contaminated wines with T. delbrueckii CLI 918 strain were obtained from white (Malvar) and red (Tempranillo) wines, and YPD (control) cultures (Figure 1). T. delbrueckii CLI 918 strain was used to study this influence. Detection limits for all curves were 102 cells mL-1 being linear over five orders of magnitude. The correlation coefficients, slopes, and efficiencies of the amplification of standard curves are shown in Figure 1. It could be possible to observe that the efficiency of qPCR in red wine is lower than white wine and YPD medium, however the differences observed were not statistically significant (p < 0.05). This type of analysis was also done for other yeast species used in this study (data not shown) and the results agreed with the T. delbrueckii trial.
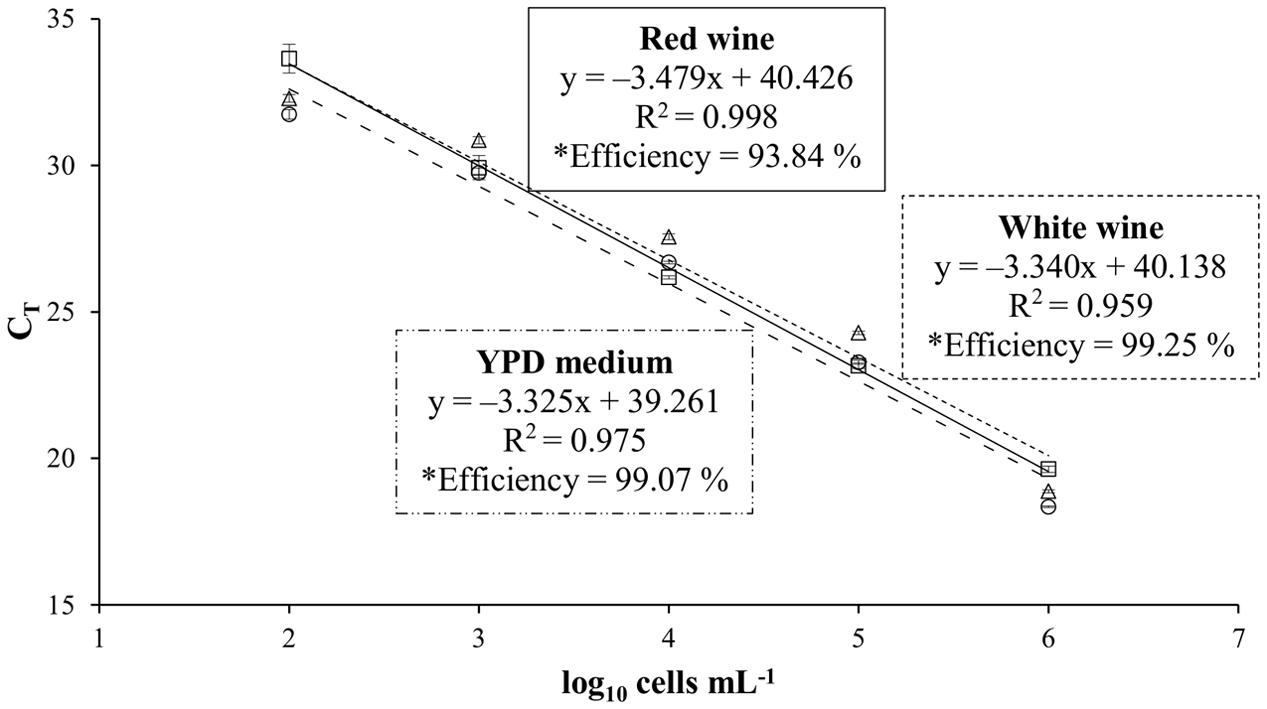
FIGURE 1. Standard curves obtained for Torulaspora delbrueckii CLI 918 from YPD culture (○, –..–), white wine (∆, - - - -), and red wine (□, —). CT values of standard curves from YPD medium, white wine and red wine are the averages of three individual repetitions. ∗Efficiency was estimated by the formula E = (10-1/slope) – 1.
Yeast Inoculated Population Analysis by qPCR during Alcoholic Fermentation
qPCR analysis was used to analyze the dynamics of five non-Saccharomyces yeasts inoculated, revealing that they were present throughout the alcoholic fermentation. A culture-dependent technique on YPD plates were used to follow the evolution of total cultivable yeasts (Figure 2).
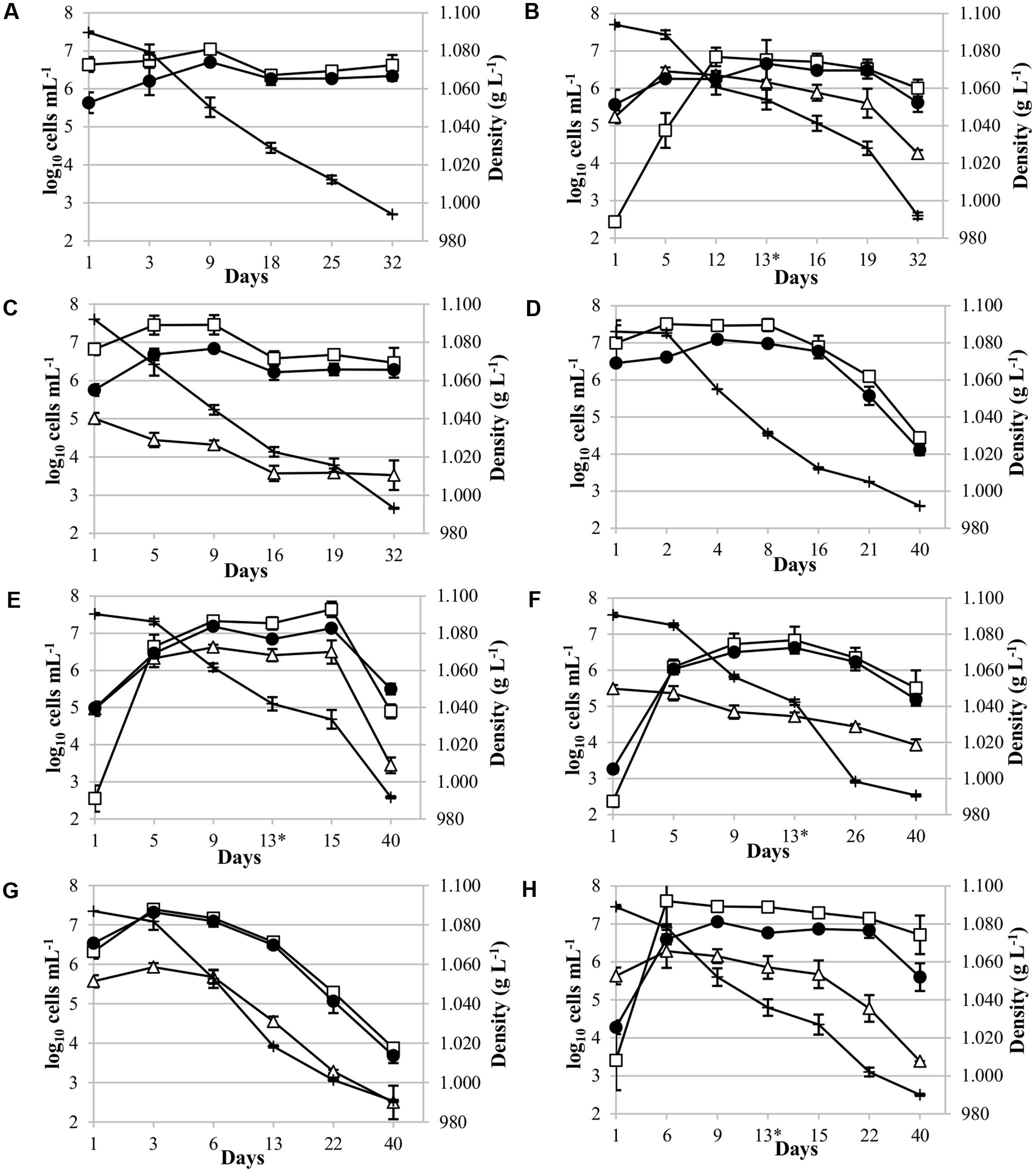
FIGURE 2. Yeast population dynamics during Malvar fermentation. Results, expressed as log10 cells mL-1, were obtained using YPD culture media (●) and qPCR analysis [(□) for Saccharomyces cerevisiae; (∆) for non-Saccharomyces strains] and, density values (+) expressed in g L-1. Species population analyzed: (A) Pure culture of S. cerevisiae CLI 889, control p-ScI; (B) sequential culture of T. delbrueckii CLI 918 and S. cerevisiae CLI 889 (s-Td/ScI); (C) mixed culture of Metschnikowia pulcherrima CLI 457 and S. cerevisiae CLI 889 (m-Mp/ScI); (D) pure culture of S. cerevisiae, control p-ScII; (E) sequential culture of Schizosaccharomyces pombe CLI 1079 and S. cerevisiae CLI 889 (s-Sp/ScII); (F) sequential culture of Candida stellata CLI 920 and S. cerevisiae CLI 889 (s-Cs/ScII); (G) mixed culture of Lachancea thermotolerans 9-6C and S. cerevisiae CLI 889 (m-Lt/ScII); and (H) sequential culture of L. thermotolerans 9-6C and S. cerevisiae CLI 889 (s-Lt/ScII). Asterisk in graphics indicates the day of inoculation of S. cerevisiae strain in sequential cultures.
Pure cultures of S. cerevisiae CLI 889 (p-ScI and p-ScII) used as controls in the fermentations with must I and must II presented different population dynamics. The control p-ScI slowly started to ferment, achieving the highest population at day 9, its fermentation finished with a population of 2.5 × 106 cells mL-1 after 32 days (Figure 2A). Instead, p-ScII culture reached the greatest population on the second day of fermentation, finishing with 2.7 × 104 cells mL-1 after 40 days (Figure 2D). The amount of sugar daily transformed in these pure cultures when 50% of the sugar content had been consumed (V50) was higher in p-ScII (V50: 16.23) than p-ScI (V50: 13.30); finally, the p-ScI culture ended the fermentation with 9.86 g L-1 of reducing sugars and 13.5% (v/v) of ethanol, while p-ScII was able to consume the sugars present in the grape must and finished with 13.0% (v/v) of ethanol (Supplementary Table S1).
Regarding to mixed cultures (Figure 2C for M. pulcherrima and 2G for L. thermotolerans), on Figure 2C it could be possible to observe a small increase of S. cerevisiae population until day 9, after that a decrease and a maintenance in its population were observed. In contrast, M. pulcherrima population decreased from the beginning of the fermentation, finishing with three orders of magnitude lower than its control (p-ScI) at the end of fermentation after 32 days of vinification. The density values decreased to day 16, when the slow decrease of density coincided in time with the population stabilization of M. pulcherrima and S. cerevisiae. In the case of L. thermotolerans mixed fermentation (Figure 2G), there was an increase of this yeast population at the beginning, and after 6 days, a decrease was observed. In the whole fermentation process, the S. cerevisiae population was higher than L. thermotolerans population. The growth profile of S. cerevisiae in this mixed culture (Figure 2G) shows a high similarity with its control p-ScII (Figure 2D). Both cases on mixed fermentations, the fermentation takes the same time to reduce the density than the controls, and the residual sugars in final wines were also similar to their respective controls.
For sequential cultures, S. cerevisiae CLI 889 strain was inoculated at day 13 (represented by the asterisk in the graphics). It is worth noting that the native S. cerevisiae population increased between four and five orders of magnitude during the beginning of sequential fermentations, however, an improvement of the fermentation rate has been observed after S. cerevisiae inoculation (Figures 2B,E,F,H). After microsatellites multiplex PCR analysis to check the presence of S. cerevisiae CLI 889 strain from its day of inoculation (day 13) over another S. cerevisiae presented in the cellar environment, we found that the microsatellite pattern of the strain inoculated was exhibited by all the isolates analyzed. In Figure 2B it is possible to observe that the highest concentration of T. delbrueckii CLI 918 was achieved after 5 days, remaining at this level during the alcoholic fermentation, and finishing with the greatest final concentration in comparison with the other non-Saccharomyces tested in the sequential cultures. Although this fermentation takes the same length that its control, they need 32 days to reduce the density to lower than 1000 g L-1, the amount of residual sugars is different, showing lower concentrations for the sequential inoculation than its control (Supplementary Table S1). In the S. pombe/S. cerevisiae sequential culture (Figure 2E), an increment of S. cerevisiae population after S. cerevisiae CLI 889 inoculation can be observed. The S. pombe CLI 1079 population is maintained high during the fermentation even after S. cerevisiae is added. At the end of vinification, this non-Saccharomyces strain finished with approximately one order of magnitude less than S. cerevisiae population. C. stellata CLI 920 which seemed to be less competitive in this type of inoculation, presented a number of cells two orders of magnitude lower than S. cerevisiae from the day 9 (Figure 2F). This strain in sequential fermentation (Figure 2F) presented its higher counts after the first 24 h, then started to decrease until the end of fermentation (day 40). In this case, however, the inoculation of the S. cerevisiae strain produces an improvement of the fermentation rate, showing on Figure 2F a high reduction on the density, but the amount of S. cerevisiae was not changed. L. thermotolerans in sequential culture (Figure 2H) remained at high and relatively stable cell levels until day 15 when its population decreased more quickly, ending with three orders of magnitude less than S. cerevisiae at the end of fermentation, probably due to S. cerevisiae CLI 889 inoculation at day 13, which also produced a decrease of density.
Analytical Determination of Wines
The main oenological parameters analyzed are listed in Supplementary Table S1, which shows that sequential fermentations produced wines so different to their control. Most of the cases the differences involve three or more parameters, while on mixed fermentations the differences with respect to the controls are reduced to a few parameters. Although the differences observed in the ethanol produced among the different fermentations exhibited a significant difference, these differences are lower than 0.5% (v/v), having no consideration for establish differences due to this parameter. However, the differences with respect to the control can be observed using other parameters, such as glycerol or malic acid. Volatile compounds analyzed (Supplementary Table S2) do not show significant differences on single compounds, but they have been observed when clusters of compounds have been conducted.
To confirm the differences among pure cultures of S. cerevisiae (p-ScI and p-ScII, considered as controls) and co-culture-fermented wines, a principal component analysis (PCA) was elaborated (Figure 3) from all data obtained from the analysis of oenological parameters and volatile compounds (Supplementary Tables S1, S2). The first two principal components, PC1 and PC2 accounted for 72.23% of total variance (Figure 3). PC2, which is mostly formed by volatile compounds (the impact of each parameter on the component is indicated in brackets) as ethyl isovalerate (0.971), ethyl-3-hydroxybutyrate (0.949), 1-butanol (0.914), isoamyl acetate (0.750), ethyl butyrate (0.614), and ethyl hexanoate (0.606), allowed us to differentiate the different types of culture with non-Saccharomyces species in combination with the S. cerevisiae strain, while the main parameters for PC1 were hexanoic acid (0.989), octanoic acid (0.982), 1-hexanol (0.979), isovaleric acid (0.965), diacetyle (0.961), isoamyl alcohol (0.929), β-phenylethyl alcohol (0.927), isobutanol (0.901), and pH (0.876), differentiating the cultures elaborated with Malvar must I and must II. This PCA confirmed the evidence given by the analytical assays, making it possible to confirm a higher similarity between mixed cultures and their respective controls in contrast with the greater differences found in sequential cultures (Figure 3).
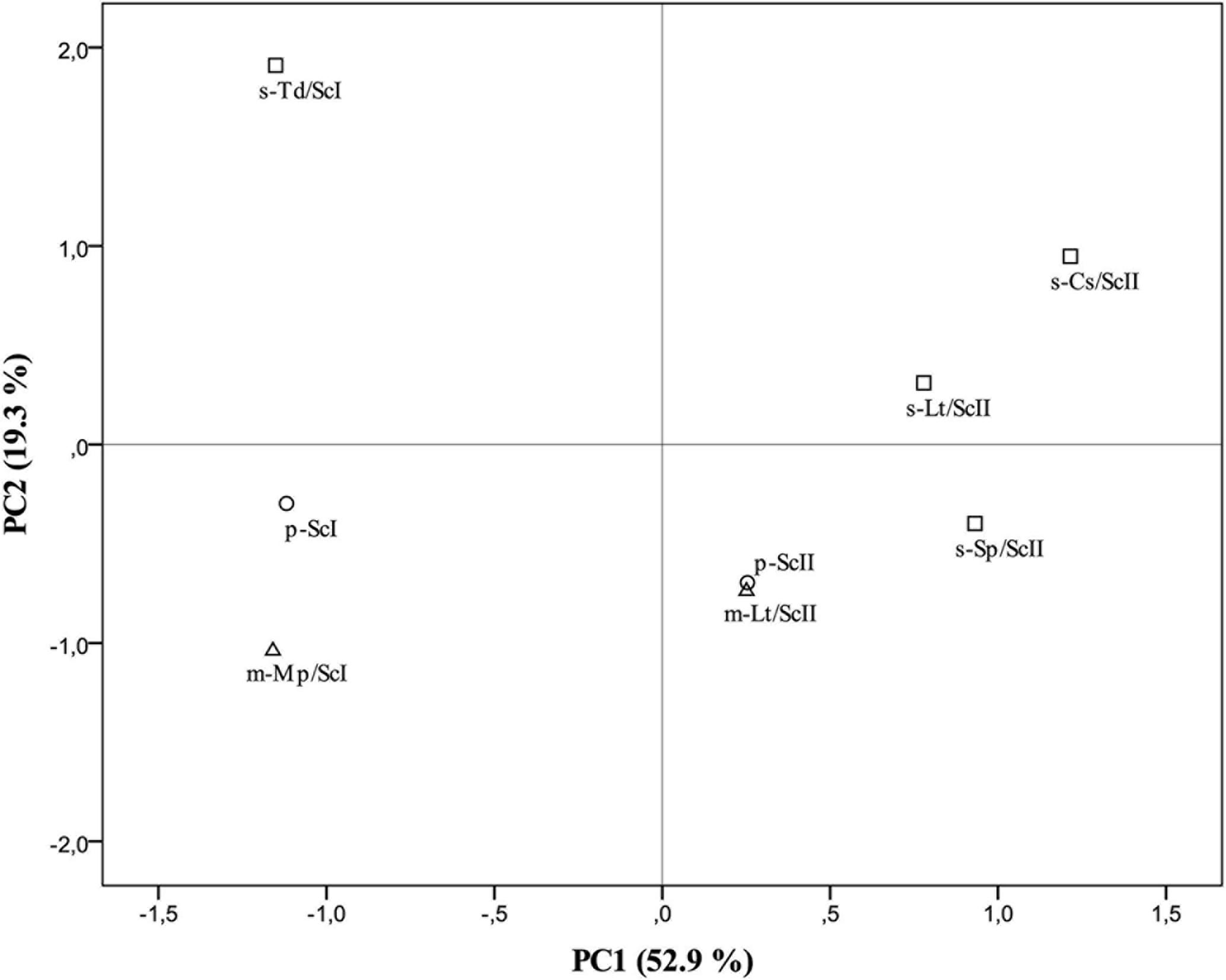
FIGURE 3. Results of the principal component analysis (PCA) performed on the oenological parameters and volatile compound data. Mean of triplicates wine samples derived from pure cultures of S. cerevisiae (p-ScI and p-ScII), sequential culture of T. delbrueckii and S. cerevisiae (s-Td/ScI), mixed culture of M. pulcherrima and S. cerevisiae (m-Mp/ScI), sequential culture of S. pombe and S. cerevisiae (s-Sp/ScII), sequential culture of C. stellata and S. cerevisiae (s-Cs/ScII), mixed (m-Lt/ScII) and sequential (s-Lt/ScII) cultures of L. thermotolerans and S. cerevisiae in the plane formed by the two first principal components. Pure cultures represented by circles (○), mixed cultures by triangles (∆), and sequential cultures by squares (□).
Sensory Profile of the Produced Wines
Wines elaborated were tested by skilled judges from the IMIDRA Institute as the sensorial panel. For fermentations conducted with must I, all panelists were able to distinguish sequential culture of T. delbrueckii from the control with a 0.1% significance level by triangle tests. In the case of mixed culture of M. pulcherrima/S. cerevisiae, tasters differentiated this type of inoculation with respect to the control with a 5% significance level (data not shown). Most panelists considered the sequential culture of T. delbrueckii as the best one wine due to its higher aroma intensity, overall quality, and its fruity and floral aroma; also, they denoted its bitter taste (Figure 4A). The mixed culture of M. pulcherrima/S. cerevisiae was described by tasters for its acid and alcoholic character (Figure 4A), but also residual sugars in this fermentation (Supplementary Table S1) were detected. The aroma was described by tasters as ripe fruit and banana but, in general, this wine was described as not intense and its lower concentration of volatile compounds compared to the rest of wines can also be seen (Supplementary Table S2).
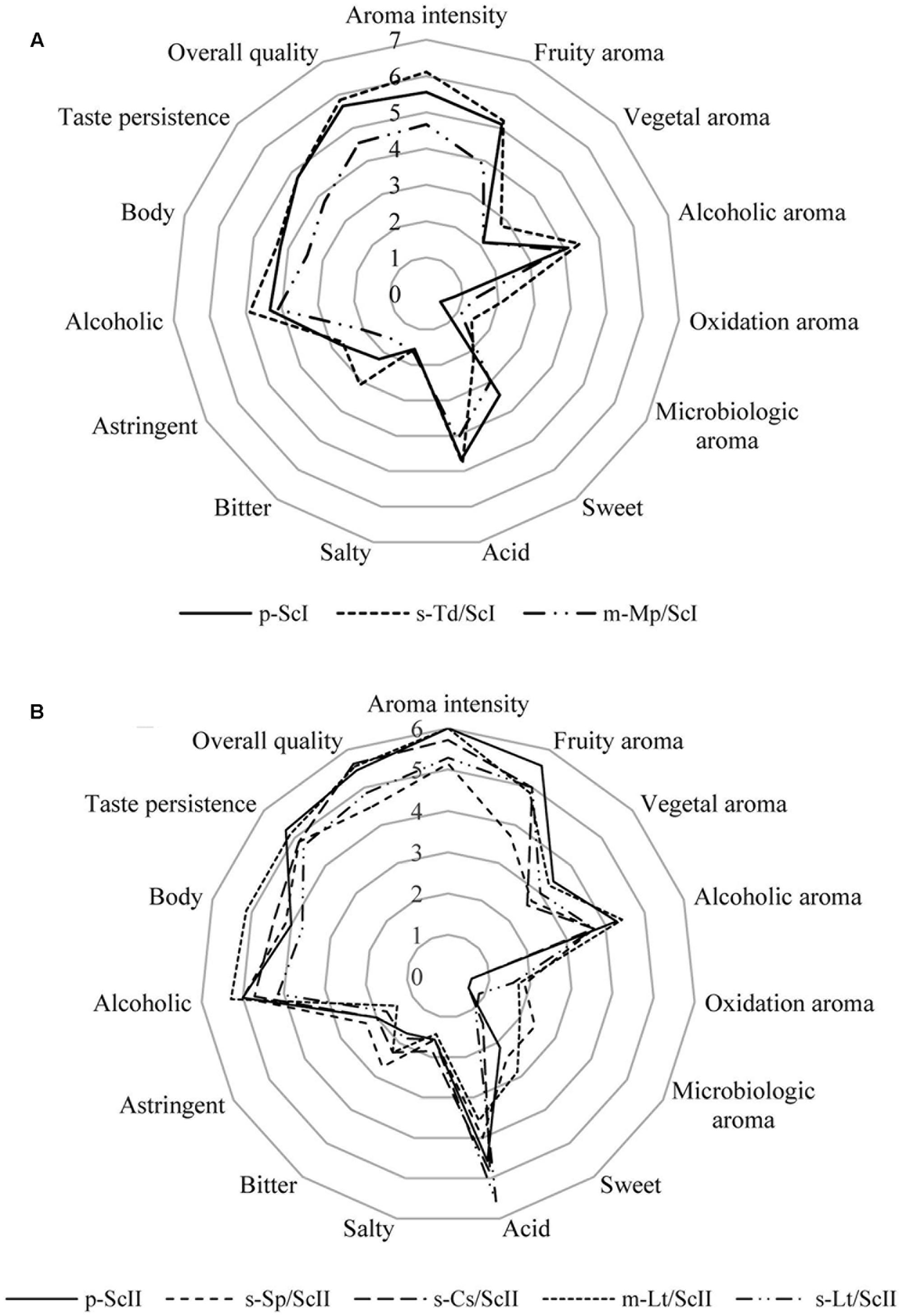
FIGURE 4. Cobweb diagrams of mean sensory scores of wines made with Saccharomyces and non-Saccharomyces combinations. (A) Cobweb graph of wines: p-ScI, s-Td/ScI, and m-Mp/ScI; and (B) cobweb graph of wines: p-ScII, s-Sp/ScII, s-Cs/ScII, m-Lt/ScII, and s-Lt/ScII. Abbreviations related with the type of culture employed and the yeast strains are explained in Figure 2.
Furthermore, on fermentations with must II, tasters were able to differentiate the sequential culture of S. pombe/S. cerevisiae (s-Sp/ScII), the sequential culture of C. stellata/S. cerevisiae (s-Cs/ScII), and the mixed culture of L. thermotolerans/S. cerevisiae (s-Lt/ScII) from the control with a 5% significance level by triangle tests; and, the sequential culture of L. thermotolerans/S. cerevisiae was differentiated with a 1% significance level through the same tests (data not shown). However, there was no clear preference on sensorial analysis; three of the seven panelists preferred the sequential culture of C. stellata/S. cerevisiae, and two of them chose the mixed culture of L. thermotolerans/S. cerevisiae, while the other two preferred the sequential culture of L. thermotolerans by descriptive analysis.
Sequential culture of C. stellata was described by tasters as a wine with a pleasant fruity (green apple, grapefruit) and floral aroma; it was denoted as fresh and full-bodied on the palate (Figure 4B).
Lachancea thermotolerans in sequential and mixed cultures were well-accepted by tasters (Figure 4B). The mixed culture was noted for an intense flavor, balanced acidity, and alcohol with slight sweetness and full body. Its aroma was described as lemon, apple, and nut notes and high aroma intensity. Instead, the sequential culture of L. thermotolerans presented the highest acidity of all wines (Figure 4B) due to its higher lactic acid content (Supplementary Table S1). Tasters highlighted its fruity (ripe fruit) and floral aroma and freshness on the palate.
Finally, tasters noted that sequential culture of S. pombe/S. cerevisiae did not improve the organoleptic characteristics to Malvar wines (Figure 4B). This wine was described as acid and bitter, low aromatic intensity with citric notes probably due to ethyl octanoate, and ethyl hexanoate volatile compounds (Supplementary Table S2). Additionally, microbiological aroma was detected by tasters in this culture.
Discussion
In this study, we quantified the evolution of inoculated non-Saccharomyces and Saccharomyces populations during alcoholic fermentation in different combinations between strains of different species in a natural must of a white grape Malvar variety. A rapid culture-independent qPCR method for detection and enumeration of different yeasts was applied in Malvar wine fermentations. Four pairs of primers were designed in this work into the variable D1/D2 domains of the 26S ribosomal DNA gene to the strains S. pombe CLI 1079, C. stellata CLI 920, M. pulcherrima CLI 457, and L. thermotolerans 9-6C; this region has previously been used to develop qPCR methods for several yeasts (Andorrà et al., 2010; Albertin et al., 2014). Two other pair of primers were designed by Zott et al. (2010) to the ITS region of rDNA, and this region is widely used in yeast species identification due to the high degree of interspecies sequence variations (Esteve-Zarzoso et al., 1999; Schoch et al., 2012). These qPCR species-specific primers showed an excellent specificity with all wine yeasts tested and did not amplify other representative wine species. Moreover, standard curves elaborated with the different yeast strains presented high efficiencies, and good detection limits, we enumerated the concentration of 103 cells mL-1, and the trials were linear over five orders of magnitude.
The T. delbrueckii CLI 918 strain has been utilized to study the matrix influence in the efficiency of qPCR system. Our results were able to show that the matrix of red wine influences on the PCR amplification or on the DNA extraction and purification, due presumably to its much higher proportion of polyphenols. It is known that wine is a complex matrix that presents various PCR inhibitors (Zoecklein et al., 1999; Phister and Mills, 2003), such as major compounds as polyphenols, tannins, and polysaccharides. The efficiency obtained on qPCR analysis from red wine is lower than from white wine and YPD medium, although these values are similar in all cases and without statistical significance. Some authors have reported problems of amplification with DNA isolated directly from wine (Phister and Mills, 2003; Martorell et al., 2005). The assay performed here helped to check that the wine matrix did not significantly influence in the efficiency of the qPCR analysis. According to our results the construction of standard curves in different matrices do not substantially modify the results, and any matrix can be used to quantify the yeast populations from wine fermentation.
It had long been considered that the non-Saccharomyces yeasts are present at the beginning of alcoholic fermentation, being replaced by S. cerevisiae which has a high capacity to take over the process. In this work, the dynamics of five non-Saccharomyces yeasts in co-inoculation with S. cerevisiae have been analyzed, revealing that these non-Saccharomyces species were present throughout the fermentation process. If they are present during fermentation we expected contribution to the chemical and sensory attributes of the final wines. However, even though these five non-Saccharomyces strains were present during fermentation, S. cerevisiae was the most abundant yeast under any of the co-cultures tested at the end of the fermentations. Different mechanisms have been described to explain the dominance of S. cerevisiae over other competitors during wine fermentation, i.e., cell-to-cell contact (Nissen et al., 2003); competition for nutrients (Taillandier et al., 2014; Kemsawasd et al., 2015b; Lleixà et al., 2016); secretion of toxic compounds (Pérez-Nevado et al., 2006; Branco et al., 2015; Ramírez et al., 2015; Wang et al., 2016), or changes in the medium (Goddard, 2008; Salvadó et al., 2011). These effects caused by S. cerevisiae metabolite production and changes in the medium could provide an explanation for the decrease of M. pulcherrima and C. stellata and the increase and persistence of T. delbrueckii, S. pombe, and L. thermotolerans belong to the fermentation, due to their higher fermentative power (García et al., 2017) in relation to the amount of alcohol produced by the yeast species (Lopes et al., 2006) and, therefore, related to their alcohol tolerance (Ciani et al., 2016). In the case of L. thermotolerans, the enhancement of total acidity produced by this species can also influence in the growth of S. cerevisiae and other yeast species. However, the sensibility to these toxic compounds has been described as species- and strain-specific (Wang et al., 2016).
The multi-starter fermentations, combining both non-Saccharomyces yeasts and S. cerevisiae species able to complete the fermentation, are being studied in depth. All these yeast interaction studies have been increased to explain yeast–yeast interactions and their underlying mechanisms in the increasing use of controlled mixed cultures (Ciani et al., 2010; Ciani and Comitini, 2015). These studies have also been driven by the presence of viable and non-culturable microorganisms in wine samples (Millet and Lonvaud-Funel, 2000; Divol and Lonvaud-Funel, 2005), and may have a false idea about the number of non-Saccharomyces species on microbiological methods based on plating (Serpaggi et al., 2012; Wang et al., 2016). In this study, the counts obtained by qPCR were contrasted with plating in YPD non-selective medium and LYS medium (data not shown), a selective medium for non-Saccharomyces yeasts. Generally, the yeast populations observed in LYS agar were higher than those obtained by qPCR. This greater growth on LYS medium, could be explained by the growth of other non-Saccharomyces yeasts present in the non-sterile Malvar must. This fact is in agreement with the results obtained by Phister and Mills (2003) in a Dekkera bruxellensis study.
Differences on the evolution of Saccharomyces and non-Saccharomyces yeasts have been observed depending on the type of inoculation. In the mixed culture of M. pulcherrima/S. cerevisiae, the M. pulcherrima CLI 457 population started to decrease at 24 h in contrast with the increase of S. cerevisiae counts studied by qPCR. The antagonist effect of M. pulcherrima on several yeasts, including S. cerevisiae, which leads to delays in the fermentation, has been studied (Nguyen and Panon, 1998; Türkel and Ener, 2009). This phenomenon was due to a killer effect linked to pulcherrimin pigment produced by M. pulcherrima strains, Türkel and Ener (2009) found three strains of M. pulcherrima (UMY12, UMY14, and UMY15) that produce the same amount of the pigment pulcherrimin, but their antimicrobial activities showed important variations. Different distinct biotypes within the M. pulcherrima species with respect the pulcherrimin production were identified by Pallmann et al. (2001). However, it has recently been described a difficulty in classifying Metschnikowia fructicola species since this species is not distinguishable from Metschnikowia andauensis and other species of the M. pulcherrima clade because of a possible heterogeneity of rRNA repeats (Cordero-Bueso et al., 2017). For this reason, we keep the original designation for this yeast strain, keeping the same yeast species name described on the published document by Arroyo et al. (2010). However, the variable D1/D2 domain of this strain was sequenced by Macrogen to be identified with 99% of sequence identity as M. pulcherrima and its sequence included in GenBank Database (accession number MF001378). Our results showed that the mixed culture of M. pulcherrima/S. cerevisiae finished with a high level of reducing sugars, in the same way that happened in co-cultures with these strains in laboratory scale fermentations (García et al., 2017), so it could be possible that the M. pulcherrima CLI 457 strain had a negative effect on the fermentative capacity of the S. cerevisiae CLI 889 strain. Instead, sequential fermentation with T. delbrueckii and S. cerevisiae finished with sugar values lower than 4 g L-1 as at laboratory level (García et al., 2017). Therefore, the fermentative capacity of T. delbrueckii in the first days seems to influence in the low sugar content of final wines, independently of the scale of fermentations, which is in agreement with results obtained by Puertas et al. (2016).
Some authors have reported the competition mechanisms between L. thermotolerans and S. cerevisiae in mixed culture. Hansen et al. (2001) found that oxygen increases the competitiveness between L. thermotolerans CBS 2803 and S. cerevisiae Saint Georges S101 strains in mixed culture. In the same way, Nissen et al. (2004) concluded that S. cerevisiae Saint Georges S101 is able to grow and ferment more efficiently under oxygen-limited conditions present during wine fermentation in comparison with L. thermotolerans CBS 2803 and T. delbrueckii CBS 3085. Although other previous studies (Nissen and Arneborg, 2003; Nissen et al., 2003) showed that the death of L. thermotolerans in mixed culture with S. cerevisiae was induced by a cell-to-cell contact mediated mechanism with the same strains used by Nissen et al. (2004). Finally, Kemsawasd et al. (2015a) concluded that cell-to-cell contact and antimicrobial peptides play a combined role in the death of L. thermotolerans CBS 2803 in mixed fermentation with S. cerevisiae Saint Georges S101 strain. Our strain of L. thermotolerans in mixed culture showed a loss of viability most pronounced, although both populations decreased during fermentation process from day 3.
In sequential cultures, the S. cerevisiae population found in Malvar wine in the first 24 h of fermentation were low, between 102 and 103 cells mL-1. It can be seen that native Saccharomyces yeasts of the cellar environmental started to grow on the following days, but when S. cerevisiae CLI 889 was inoculated (day 13), this strain causes a progressive fall in the density until the end of sequential fermentations. It is well-known that S. cerevisiae yeasts are very competitive and normally dominates the fermentation due to its fast growth, efficient glucose competition, good ability to produce ethanol, and a higher tolerance to environmental stresses (Piškur et al., 2006). In this study, the growth of S. cerevisiae CLI 889 after its inoculation may have been affected by environmental factors, such as a low controlled temperature (18°C) during the fermentation process, a different availability of nutrients in the musts, and a wine elaboration without the addition of nutrients. After microsatellites multiplex analysis, the presence of the inoculated S. cerevisiae CLI 889 strain at the end of fermentation together with other S. cerevisiae strains could be confirmed; although in sequential culture, S. cerevisiae CLI 889 was found in lower percentage than in mixed cultures at the end of fermentation.
Nutrient content of the musts can modulate the yeast populations, the time of fermentation and secondary metabolites produced during alcoholic fermentation (Beltran et al., 2005; Andorrà et al., 2012; Kemsawasd et al., 2015b). In grape must, nitrogen is considered the main limiting nutrient for optimized growth and good fermentation performance (Bisson, 1999). We could observe when Malvar must II was used in the elaboration of wines, the fermentation length was increased in the cultures (40 days) compared to the elaborations with must I that finished in 32 days; the higher YAN content of must I (218 mgN L-1) than must II (100 mgN L-1) could have influence in the fermentation rate in agreement with other studies (Bely et al., 1990; Monteiro and Bisson, 1992; Beltran et al., 2005). Medina et al. (2012) noticed a negative effect of non-Saccharomyces yeasts on nutrient availability for S. cerevisiae reducing its ability to grow, especially when it was sequentially inoculated. In the tested sequential fermentations, it could be possible that the YAN consumption by non-Saccharomyces would explain the slow growth of S. cerevisiae CLI 889, although S. cerevisiae population was eventually greater at final of fermentation in all cases, since it is well-known that S. cerevisiae strains show a favorable adaptation to the nitrogen-limited wine fermentation environment (Marsit et al., 2015). Additionally, a higher alcohols production (isobutanol, isoamyl alcohol, metionol, and β-phenylethyl alcohol) has been noted in fermentations elaborated with Malvar must II. This is related to the nitrogen concentration, the less nitrogen there is available in the fermentation medium, the more higher alcohols are produced (Beltran et al., 2005; Andorrà et al., 2012). The higher alcohols, along with glycerol, are the end-products of reductive pathway alternatives to the ethanol products. However, we did not detect in all co-cultures a significant decrease in the ethanol content with regard to their controls. Other volatile compounds as acetates, ethyl esters, and 1-propanol have also presented positive correlation with the level of nitrogen in the fermentation process (Rapp and Versini, 1995), this correlation can be observed for most of these compounds when the wines were elaborated with must I.
In terms of glycerol content, we can confirm the use of the tested non-Saccharomyces strains provides an enhancement of glycerol both at laboratory scale and at the pilot scale with the exception of L. thermotolerans 9-6C that did not produce high concentrations with respect to its controls at both scales. It is well-known that several non-Saccharomyces yeasts can considerably increase the glycerol concentrations in wine (Soden et al., 2000; Cominiti et al., 2011; Englezos et al., 2015; Benito et al., 2016b). Glycerol is one of the major compounds produced during wine fermentation, and it is important in yeast metabolism for regulating the redox potential in the cell (Prior et al., 2000). This compound contributes to mouth-feel, sweetness, and complexity in wines (Ciani and Maccarelli, 1998), but its production is usually linked to increased acetic acid production (Prior et al., 2000). In our results, the volatile acidity values measured as grams per liter of acetic acid, were kept low, especially at the pilot scale, with a particular decline in volatile acidity produced by T. delbrueckii CLI 918 in sequential culture.
In respect of the oenological parameters studied, the behavior of the yeast strains and the wine styles were similar regardless of the scale of fermentation tested. However, due to the type of vinification being different, some parameters changed at the pilot scale. Most of the wines can be considered as dry since their sugar content was less than 4 g L-1 at final of fermentation (Belitz and Grosch, 1999), with the exception of pure culture of S. cerevisiae p-ScI and mixed culture of M. pulcherrima/S. cerevisiae (m-Mp/ScI) (Supplementary Table S1). Generally, volatile acidity values are lower for all co-cultures in this work.
Sequential culture of T. delbrueckii/S. cerevisiae (s-Td/ScI), in comparison with its control (p-ScI), was distinguished for a significant decrease in volatile acidity (0.34 g L-1) and an increase of glycerol content (Supplementary Table S1). In relation with aromatic compounds, sequential culture of T. delbrueckii presented higher concentration of β-phenylethyl alcohol, and esters, such as ethyl butyrate, ethyl isovalerate, isoamyl acetate, ethyl hexanoate, and 2-phenylethyl acetate (Supplementary Table S2) associated with the fruity and floral character of this wine.
In relation with cultures elaborated with L. thermotolerans 9-6C and S. cerevisiae CLI 889, the effect of L. thermotolerans on oenological and sensorial properties of wines (increase of lactic acid, glycerol, and β-phenylethyl alcohol) depends on the way of inoculation with S. cerevisiae (Kapsopoulou et al., 2007; Gobbi et al., 2013). We observed a higher lactic acid and β-phenylethyl alcohol content in sequential culture due to L. thermotolerans 9-6C growth before S. cerevisiae CLI 889 inoculation. L. thermotolerans seems to be dominant over S. cerevisiae due to the significant enhancement in total acidity and, consequently, a decrease of pH. In contrast, this behavior appears to be softened in mixed culture. This result contrasts with other studies (Gobbi et al., 2013; Benito et al., 2016a) that also observed this pattern of competitiveness in the different inoculation strategies with L. thermotolerans and S. cerevisiae.
Our results showed that C. stellata CLI 920, along with L. thermotolerans 9-6C, are strains that produce lactic acid and, therefore, they increase the total acidity, both at the laboratory scale using sterile Malvar must and at the pilot scale. This production could be related with the higher concentration of ethyl lactate observed in both sequential inoculations since this ester is produced by esterification from acid lactic and ethanol (Inaba et al., 2009; Delgado et al., 2010). Higher concentrations of ethyl lactate after the use of co-cultures with L. thermotolerans and S. cerevisiae have been documented by other authors (Cominiti et al., 2011; Gobbi et al., 2013; Benito et al., 2015, 2016a).
In relation to the S. pombe strain, we tested in this work the S. pombe CLI 1079 yeast strain instead of the CLI 1085 strain used at laboratory scale due to the low growth capacity of this latest strain, that did impossible a successful pied de cuve at the pilot scale. The S. pombe CLI 1079 in sequential culture were able to finish the fermentation with residual sugars less than 4 g L-1; this strain presented a low consumption of the malic acid at the pilot scale, ending the fermentation with 1.00 g L-1 of malic acid, a value slightly lower than its control (p-ScII). Additionally, glycerol content was higher than the control. This culture presented an elevated concentration of β-phenylethyl alcohol and the highest values of alcohols. Volatile compounds associated with cheese and butter aromas were higher in sequential culture of S. pombe than the control p-ScII.
Conclusion
We can confirm that the inoculation strategies conducted at the laboratory scale produce a notable improvement in the quality of regional Malvar wines at the pilot scale also. Tasters were able to distinguish the different elaborations with respect the controls and most appreciated wines by tasting panel were those elaborated in sequential cultures with T. delbrueckii CLI 918/S. cerevisiae CLI 889 and C. stellata CLI 920/S. cerevisiae CLI 889 and, mixed and sequential cultures with L. thermotolerans 9-6C in combination with the S. cerevisiae CLI 889 strain. Sequential cultures have produced more different wines with respect to the controls, providing organoleptic properties associated with the non-Saccharomyces strains, but more studies need to be carried out varying the moment of inoculation of S. cerevisiae strain in these cultures to prevent native S. cerevisiae growth on musts, and the reduction of the fermentation time. This work provides the basis for the implementation of new biotechnological strategies for improving Malvar wine quality and it can be tested in commercial wineries.
Author Contributions
MG, TA, and BE-Z designed the experiments, analyzed the results, discussion of the results and wrote the manuscript. MG, JC, and JMC performed experiments and analyzed results.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the project RM2010-00009-C03-01 funded by INIA (Instituto Nacional de Investigación Agraria y Alimentaria). MG thanks the IMIDRA for his grant.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.02520/full#supplementary-material
Footnotes
- ^ http://www.ncbi.nlm.nih.gov
- ^ http://www.primer3plus.com
- ^ https://www-s.nist.gov/dnaAnalysis/primerToolsPage.do
References
Albergaria, H., and Arneborg, N. (2016). Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 100, 2035–2046. doi: 10.1007/s00253-015-7255-0
Albertin, W., Miot-Sertier, C., Bely, M., Marullo, P., Coulon, J., Moine, V., et al. (2014). Oenological prefermentation practices strongly impact yeast population dynamics and alcoholic fermentation kinetics in Chardonnay grape must. Int. J. Food Microbiol. 178, 87–97. doi: 10.1016/j.ijfoodmicro.2014.03.009
Andorrà, I., Berradre, M., Mas, A., Esteve-Zarzoso, B., and Guillamón, J. M. (2012). Effect of mixed culture fermentations on yeast populations and aroma profile. LWT Food Sci. Technol. 49, 8–13. doi: 10.1016/j.lwt.2012.04.008
Andorrà, I., Esteve-Zarzoso, B., Guillamón, J. M., and Mas, A. (2010). Determination of viable wine yeast using DNA binding dyes and quantitative PCR. Int. J. Food Microbiol. 144, 257–262. doi: 10.1016/j.ijfoodmicro.2010.10.003
Andorrà, I., Landi, S., Mas, A., Guillamón, J. M., and Esteve-Zarzoso, B. (2008). Effect of oenological practices on microbial populations using culture-independent techniques. Food Microbiol. 25, 849–856. doi: 10.1016/j.fm.2008.05.005
Arroyo, T. (2000). Estudio de la Influencia de Diferentes Tratamientos Enológicos en la Evolución de la Microbiota y en la Calidad de Los Vinos Elaborados con la Variedad “Airén”, en la D.O. “Vinos de Madrid”. Master’s thesis, University of Alcalá, Alcalá de Henares.
Arroyo, T., Cordero, G., Serrano, A., and Valero, E. (2010). “β-Glucosidase production by non-Saccharomyces yeasts isolated from vineyard,” in Proceedings of the 12th Weurman Symposium Expression of Multidisciplinary Flavour Science, eds I. Blank, M. Wüst, and C. Yeretzian (Winterthur: ZHAW), 359–362.
Arroyo, T., Lozano, J., Cabellos, J. M., Gil-Diaz, M., Santos, J. P., and Horrillo, C. (2009). Evaluation of wine aromatic compounds by a sensory human panel and an electronic nose. J. Agric. Food Chem. 57, 11543–11549. doi: 10.1021/jf902109y
Balboa-Lagunero, T., Arroyo, T., Cabellos, J. M., and Aznar, M. (2013). Yeast selection as a tool for reducing key oxidation notes in organic wines. Food Res. Int. 53, 252–259. doi: 10.1016/j.foodres.2013.04.006
Barata, A., Malfeito-Ferreira, M., and Loureiro, V. (2012). The microbial ecology of wine grape berries. Int. J. Food Microbiol. 153, 243–259. doi: 10.1016/j.ijfoodmicro.2011.11.025
Belitz, H. D., and Grosch, W. (1999). “Carbohydrates,” in Food Chemistry, eds H.-D. Belitz, W. Grosch, and P. Schieberle, (Berlin: Springer), 248–339. doi: 10.1007/978-3-662-07281-3
Beltran, G., Esteve-Zarzoso, B., Rozès, N., Mas, A., and Guillamón, J. M. (2005). Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 53, 996–1002. doi: 10.1021/jf0487001
Bely, M., Sablayrolles, J. M., and Barre, P. (1990). Description of alcoholic fermentation kinetics: its variability and significance. Am. J. Enol. Vitic. 41, 319–324.
Benito,Á., Calderón, F., Palomero, F., and Benito, S. (2016a). Quality and composition of Airén wines fermented by sequential inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 54, 135–144. doi: 10.17113/ftb.54.02.16.4220
Benito,Á., Jeffares, D., Palomero, F., Calderón, F., Bai, F. Y., Bähler, J., et al. (2016b). Selected Schizosaccharomyces pombe strains have characteristics that are beneficial for winemaking. PLOS ONE 11:e0151102. doi: 10.1371/journal.pone.0151102
Benito, S., Hofmann, T., Laier, M., Lochbühler, B., Schüttler, A., Ebert, K., et al. (2015). Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 241, 707–717. doi: 10.1007/s00217-015-2497-8
Branco, P., Viana, T., Albergaria, H., and Arneborg, N. (2015). Antimicrobial peptides (AMPs) produced by Saccharomyces cerevisiae induce alterations in the intracellular pH, membrane permeability and culturability of Hanseniaspora guilliermondii cells. Int. J. Food Microbiol. 205, 112–118. doi: 10.1016/j.ijfoodmicro.2015.04.015
Ciani, M., Canonico, L., Oro, L., Comitini, F., and Vita, S. (2014). Sequential fermentation using non-Saccharomyces yeasts for the reduction of alcohol content in wine. BIOWeb Conf. 3, 2014–2016. doi: 10.1051/bioconf/20140302015
Ciani, M., and Comitini, F. (2015). Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 1, 1–6. doi: 10.1016/j.cofs.2014.07.001
Ciani, M., Comitini, F., Mannazzu, I., and Domizio, P. (2010). Controlled mixed culture fermentation: a new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 10, 123–133. doi: 10.1111/j.1567-1364.2009.00579.x
Ciani, M., and Maccarelli, F. (1998). Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 14, 199–203. doi: 10.1023/A:1008825928354
Ciani, M., Morales, P., Comitini, F., Tronchoni, J., Canonico, L., Curiel, J. A., et al. (2016). Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 7:642. doi: 10.3389/fmicb.2016.00642
Cocolin, L., Alessandria, V., Dolci, P., Gorra, R., and Rantsiou, K. (2013). Culture independent methods to assess the diversity and dynamics of microbiota during food fermentation. Int. J. Food Microbiol. 167, 29–43. doi: 10.1016/j.ijfoodmicro.2013.05.008
Cominiti, F., Gobbi, M., Domizio, P., Romani, C., Lencioni, L., Mannazzu, I., et al. (2011). Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 28, 873–882. doi: 10.1016/j.fm.2010.12.001
Cordero-Bueso, G., Arroyo, T., Serrano, A., Tello, J., Aporta, I., Vélez, M. D., et al. (2011). Influence of the farming system and vine variety on yeast communities associated with grape berries. Int. J. Food Microbiol. 145, 132–139. doi: 10.1016/j.ijfoodmicro.2010.11.040
Cordero-Bueso, G., Esteve-Zarzoso, B., Cabellos, J. M., Gil-Díaz, M., and Arroyo, T. (2013). Biotechnological potential of non-Saccharomyces yeasts isolated during spontaneous fermentations of Malvar (Vitis vinifera cv. L.). Eur. Food Res. Technol. 236, 193–207. doi: 10.1007/s00217-012-1874-9
Cordero-Bueso, G., Esteve-Zarzoso, B., Gil-Díaz, M., García, M., Cabellos, J., and Arroyo, T. (2016). Improvement of Malvar wine quality by use of locally-selected Saccharomyces cerevisiae strains. Fermentation 2:7. doi: 10.3390/fermentation2010007
Cordero-Bueso, G., Mangieri, N., Maghradze, D., Foschino, R., Valdetara, F., Cantoral, J. M., et al. (2017). Wild grape-associated yeasts as promising biocontrol agents against Vitis vinifera fungal pathogens. Front. Microbiol. 8:2025. doi: 10.3389/fmicb.2017.02025
David, V., Terrat, S., Herzine, K., Claisse, O., Rousseaux, S., Tourdot-Maréchal, R., et al. (2014). High-throughput sequencing of amplicons for monitoring yeast biodiversity in must and during alcoholic fermentation. J. Ind. Microbiol. Biotechnol. 41, 811–821. doi: 10.1007/s10295-014-1427-2
Delgado, P., Sanz, M. T., Beltrán, S., and Núñez, L. A. (2010). Ethyl lactate production via esterification of lactic acid with ethanol combined with pervaporation. Chem. Eng. J. 165, 693–700. doi: 10.1016/j.cej.2010.10.009
Divol, B., and Lonvaud-Funel, A. (2005). Evidence for viable but nonculturable yeasts in botrytis-affected wine. J. Appl. Microbiol. 99, 85–93. doi: 10.1111/j.1365-2672.2005.02578.x
Englezos, V., Rantsiou, K., Torchio, F., Rolle, L., Gerbi, V., and Cocolin, L. (2015). Exploitation of the non-Saccharomyces yeast Starmerella bacillaris (synonym Candida zemplinina) in wine fermentation: physiological and molecular characterizations. Int. J. Food Microbiol. 199, 33–40. doi: 10.1016/j.ijfoodmicro.2015.01.009
Esteve-Zarzoso, B., Belloch, C., Uruburu, F., and Querol, A. (1999). Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 49, 329–337. doi: 10.1099/00207713-49-1-329
Fleet, G. H. (2003). Yeast interactions and wine flavour. Int. J. Food Microbiol. 86, 11–22. doi: 10.1016/S0168-1605(03)00245-9
García, M., Arroyo, T., Crespo, J., Cabellos, J. M., and Esteve-Zarzoso, B. (2017). Use of native non-Saccharomyces strain: a new strategy in D.O. “Vinos de Madrid” (Spain) wines elaboration. Eur. J. Food Sci. Technol. 5, 1–31.
Gil, M., Cabellos, J. M., Arroyo, T., and Prodanov, M. (2006). Characterization of the volatile fraction of young wines from the Denomination of Origin “Vinos de Madrid” (Spain). Anal. Chim. Acta 563, 145–153. doi: 10.1016/j.aca.2005.11.060
Gobbi, M., Comitini, F., Domizio, P., Romani, C., Lencioni, L., Mannazzu, I., et al. (2013). Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: a strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 33, 271–281. doi: 10.1016/j.fm.2012.10.004
Goddard, M. R. (2008). Quantifying the complexities of Saccharomyces cerevisiae’s ecosystem engineering via fermentation. Ecology 89, 2077–2082. doi: 10.2307/41739278
Gump, B. H., Zoecklein, B. W., Fugelsang, K. C., and Whiton, R. S. (2002). Comparison of analytical methods for prediction of prefermentation nutritional status of grape juice. Am. J. Enol. Vitic. 53, 325–329.
Hansen, E. H., Nissen, P., Sommer, P., Nielsen, J. C., and Arneborg, N. (2001). The effect of oxygen on the survival of non-Saccharomyces yeasts during mixed culture fermentations of grape juice with Saccharomyces cerevisiae. J. Appl. Microbiol. 91, 541–547. doi: 10.1046/j.1365-2672.2001.01426.x
Higuchi, R., Fockler, C., Dollinger, G., and Watson, R. (1993). Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology 11, 1026–1030. doi: 10.1038/nbt0993-1026
Inaba, C., Maekawa, K., Morisaka, H., Kuroda, K., and Ueda, M. (2009). Efficient synthesis of enantiomeric ethyl lactate by Candida antarctica lipase B (CALB)-displaying yeasts. Appl. Microbiol. Biotechnol. 83, 859–864. doi: 10.1007/s00253-009-1931-x
Ivey, M. L., and Phister, T. G. (2011). Detection and identification of microorganisms in wine: a review of molecular techniques. J. Ind. Microbiol. Biotechnol. 38, 1619–1634. doi: 10.1007/s10295-011-1020-x
Kapsopoulou, K., Mourtzini, A., Anthoulas, M., and Nerantzis, E. (2007). Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 23, 735–739. doi: 10.1007/s11274-006-9283-5
Kemsawasd, V., Branco, P., Almeida, M. G., Caldeira, J., Albergaria, H., and Arneborg, N. (2015a). Cell-to-cell contact and antimicrobial peptides play a combined role in the death of Lachanchea thermotolerans during mixed-culture alcoholic fermentation with Saccharomyces cerevisiae. FEMS Microbiol. Lett. 362:fnv103. doi: 10.1093/femsle/fnv103
Kemsawasd, V., Viana, T., Ardö, Y., and Arneborg, N. (2015b). Influence of nitrogen sources on growth and fermentation performance of different wine yeast species during alcoholic fermentation. Appl. Microbiol. Biotechnol. 99, 10191–10207. doi: 10.1007/s00253-015-6835-3
Lleixà, J., Manzano, M., Mas, A., Portillo, M., and del, C. (2016). Saccharomyces and non-Saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front. Microbiol. 7:1959. doi: 10.3389/fmicb.2016.01959
Lopes, C. A., Rodríguez, M. E., Querol, A., Bramardi, S., and Caballero, A. C. (2006). Relationship between molecular and enological features of Patagonian wine yeasts: relevance in selection protocols. World J. Microbiol. Biotechnol. 22, 827–833. doi: 10.1007/s11274-005-9110-4
Marsit, S., Mena, A., Bigey, F., Sauvage, F. X., Couloux, A., Guy, J., et al. (2015). Evolutionary advantage conferred by an eukaryote-to-eukaryote gene transfer event in wine yeasts. Mol. Biol. Evol. 32, 1695–1707. doi: 10.1093/molbev/msv057
Martini, A., Ciani, M., and Scorzetti, G. (1996). Direct enumeration and isolation of wine yeasts from grape surfaces. Am. J. Enol. Vitic. 47, 435–440.
Martorell, P., Querol, A., and Ferna, M. T. (2005). Rapid identification and enumeration of Saccharomyces cerevisiae cells in wine by real-time PCR. Appl. Environ. Microbiol. 71, 6823–6830. doi: 10.1128/AEM.71.11.6823
Masneuf-Pomarede, I., Bely, M., Marullo, P., and Albertin, W. (2016). The genetics of non-conventional wine yeasts: current knowledge and future challenges. Front. Microbiol. 6:1563. doi: 10.3389/fmicb.2015.01563
Medina, K., Boido, E., Dellacassa, E., and Carrau, F. (2012). Growth of non-Saccharomyces yeasts affects nutrient availability for Saccharomyces cerevisiae during wine fermentation. Int. J. Food Microbiol. 157, 245–250. doi: 10.1016/j.ijfoodmicro.2012.05.012
Millet, V., and Lonvaud-Funel, A. (2000). The viable but non-culturable state of wine micro-organisms during storage. Lett. Appl. Microbiol. 30, 136–141. doi: 10.1046/j.1472-765x.2000.00684.x
Monteiro, F. F., and Bisson, L. F. (1992). Nitrogen supplementation of grape juice. I. Effect on amino acid utilization during fermentation. Am. J. Enol. Vitic. 41, 1–10.
Nguyen, H. V., and Panon, G. (1998). The yeast Metschnikowia pulcherrima has an inhibitory effect against various yeast species. Sci. Aliments 18, 515–526. doi: 10.1016/j.fm.2014.11.013
Nissen, P., and Arneborg, N. (2003). Characterization of early deaths of non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Arch. Microbiol. 180, 257–263. doi: 10.1007/s00203-003-0585-9
Nissen, P., Neilsen, D., and Arneborg, N. (2004). The relative glucose uptake abilities of non-Saccharomyces yeasts play a role in their coexistence with Saccharomyces cerevisiae in mixed cultures. Appl. Microbiol. Biotechnol. 64, 543–550. doi: 10.1007/s00253-003-1487-0
Nissen, P., Nielsen, D., and Arneborg, N. (2003). Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell-cell contact-mediated mechanism. Yeast 20, 331–341. doi: 10.1002/yea.965
Pallmann, C. L., Brown, J. A., Olineka, T. L., Cocolin, L., Mills, D. A., and Bisson, L. F. (2001). Use of WL medium to profile native flora fermentations. Am. J. Enol. Vitic. 52, 198–203.
Pérez-Nevado, F., Albergaria, H., Hogg, T., and Girio, F. (2006). Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 108, 336–345. doi: 10.1016/j.ijfoodmicro.2005.12.012
Phister, T. G., and Mills, D. A. (2003). Real-time PCR assay for detection and enumeration of Dekkera bruxellensis in wine. Appl. Environ. Microbiol. 69, 7430–7434. doi: 10.1128/AEM.69.12.7430
Piškur, J., Rozpedowska, E., Polakova, S., Merico, A., and Compagno, C. (2006). How did Saccharomyces evolve to become a good brewer? Trends Genet. 22, 183–186. doi: 10.1016/j.tig.2006.02.002
Prior, B. A., Toh, T. H., Jolly, N., Baccari, C., and Mortimer, R. K. (2000). Impact of yeast breeding for elevated glycerol production on fermentative activity and metabolite formation in Chardonnay wine. S. Afr. J. Enol. Vitic. 21, 92–99.
Puertas, B., Jiménez, M. J., Cantos-Villar, E., Cantoral, J. M., and Rodriguez, M. E. (2016). Use of Torulaspora delbrueckii and Saccharomyces cerevisiae in semi-industrial sequential inoculation to improve quality of Palomino and Chardonnay wines in warm climates. J. Appl. Microbiol. 122, 733–746. doi: 10.1111/jam.13375
Quirós, C., Herrero, M., García, L. A., and Díaz, M. (2009). Quantitative approach to determining the contribution of viable-but-nonculturable subpopulations to malolactic fermentation processes. Appl. Environ. Microbiol. 75, 2977–2981. doi: 10.1128/AEM.01707-08
Ramírez, M., Velázquez, R., Maqueda, M., López-Piñeiro, A., and Ribas, J. C. (2015). A new wine Torulaspora delbrueckii killer strain with broad antifungal activity and its toxin-encoding double-stranded RNA virus. Front. Microbiol. 6:983. doi: 10.3389/fmicb.2015.00983
Rapp, A., and Versini, G. (1995). Influence of nitrogen compounds in grapes on aroma compounds of wines. Dev. Food Sci. 37, 1659–1694. doi: 10.1016/S0167-4501(06)80257-8
Rawsthorne, H., and Phister, T. G. (2006). A real-time PCR assay for the enumeration and detection of Zygosaccharomyces bailii from wine and fruit juices. Int. J. Food Microbiol. 112, 1–7. doi: 10.1016/j.ijfoodmicro.2006.05.003
Salma, M., Rousseaux, S., Sequeira-Le Grand, A., Divol, B., and Alexandre, H. (2013). Characterization of the viable but nonculturable (VBNC) state in Saccharomyces cerevisiae. PLOS ONE 8:e77600. doi: 10.1371/journal.pone.0077600
Salvadó, Z., Arroyo-López, F. N., Barrio, E., Querol, A., and Guillamón, J. M. (2011). Quantifying the individual effects of ethanol and temperature on the fitness advantage of Saccharomyces cerevisiae. Food Microbiol. 28, 1155–1161. doi: 10.1016/j.fm.2011.03.008
Schoch, C. L., Seifert, K. A., Huhndorf, S., Robert, V., Spouge, J. L., Levesque, C. A., et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. U.S.A. 109, 6241–6246. doi: 10.1073/pnas.1117018109
Serpaggi, V., Remize, F., Recorbet, G., Gaudot-Dumas, E., Sequeira-Le Grand, A., and Alexandre, H. (2012). Characterization of the “viable but nonculturable” (VBNC) state in the wine spoilage yeast Brettanomyces. Food Microbiol. 30, 438–447. doi: 10.1016/j.fm.2011.12.020
Soden, A., Francis, I. L., Oakey, H., and Henschke, P. A. (2000). Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust. J. Grape Wine Res. 6, 21–30. doi: 10.1111/j.1755-0238.2000.tb00158.x
Sun, S. Y., Gong, H. S., Jiang, X. M., and Zhao, Y. P. (2014). Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae on alcoholic fermentation behavior and wine aroma of cherry wines. Food Microbiol. 44, 15–23. doi: 10.1016/j.fm.2014.05.007
Taillandier, P., Lai, Q. P., Julien-Ortiz, A., and Brandam, C. (2014). Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in wine fermentation: influence of inoculation and nitrogen content. World J. Microbiol. Biotechnol. 30, 1959–1967. doi: 10.1007/s11274-014-1618-z
Tello, J., Cordero-Bueso, G., Aporta, I., Cabellos, J. M., and Arroyo, T. (2012). Genetic diversity in commercial wineries: effects of the farming system and vinification management on wine yeasts. J. Appl. Microbiol. 112, 302–315. doi: 10.1111/j.1365-2672.2011.05202.x
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Tofalo, R., Schirone, M., Corsetti, A., and Suzzi, G. (2012). Detection of Brettanomyces spp. in red wines using real-time PCR. J. Food Sci. 77, 545–549. doi: 10.1111/j.1750-3841.2012.02871.x
Türkel, S., and Ener, B. (2009). Isolation and characterization of new Metschnikowia pulcherrima strains as producers of the antimicrobial pigment pulcherrimin. Z. Naturforsch. C 64, 405–410. doi: 10.1515/znc-2009-5-618
Vaudano, E., and Garcia-Moruno, E. (2008). Discrimination of Saccharomyces cerevisiae wine strains using microsatellite multiplex PCR and band pattern analysis. Food Microbiol. 25, 56–64. doi: 10.1016/j.fm.2007.08.001
Walters, L. S., and Thiselton, M. R. (1953). Utilization of lysine by yeasts. J. Inst. Brew. 59, 401–404. doi: 10.1002/j.2050-0416.1953.tb02736.x
Wang, C., Esteve-Zarzoso, B., and Mas, A. (2014). Monitoring of Saccharomyces cerevisiae, Hanseniaspora uvarum, and Starmerella bacillaris (synonym Candida zemplinina) populations during alcoholic fermentation by fluorescence in situ hybridization. Int. J. Food Microbiol. 191, 1–9. doi: 10.1016/j.ijfoodmicro.2014.08.014
Wang, C., Mas, A., and Esteve-Zarzoso, B. (2015). Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 206, 67–74. doi: 10.1016/j.ijfoodmicro.2015.04.022
Wang, C., Mas, A., and Esteve-Zarzoso, B. (2016). The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 7:502. doi: 10.3389/fmicb.2016.00502
Zoecklein, B., Fugelsang, K. C., Gump, B., and Nury, F. S. (1999). Wine Analysis and Production. New York, NY: Aspen Publishers. doi: 10.1007/978-1-4757-6967-8
Keywords: qPCR, native yeast, non-Saccharomyces, Saccharomyces cerevisiae, multi-starter fermentation, Malvar wine, sensorial analysis
Citation: García M, Esteve-Zarzoso B, Crespo J, Cabellos JM and Arroyo T (2017) Yeast Monitoring of Wine Mixed or Sequential Fermentations Made by Native Strains from D.O. “Vinos de Madrid” Using Real-Time Quantitative PCR. Front. Microbiol. 8:2520. doi: 10.3389/fmicb.2017.02520
Received: 31 July 2017; Accepted: 04 December 2017;
Published: 20 December 2017.
Edited by:
Pedro Miguel Izquierdo Cañas, Instituto de la Vid y el Vino de Castilla-La Mancha, SpainReviewed by:
Silvana Vero, University of the Republic, UruguayEsther Rodríguez, University of Cádiz, Spain
Copyright © 2017 García, Esteve-Zarzoso, Crespo, Cabellos and Arroyo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Braulio Esteve-Zarzoso, braulio.esteve@urv.cat Teresa Arroyo, teresa.arroyo@madrid.org
 Margarita García
Margarita García Braulio Esteve-Zarzoso
Braulio Esteve-Zarzoso Julia Crespo
Julia Crespo Juan M. Cabellos1
Juan M. Cabellos1 Teresa Arroyo
Teresa Arroyo