- 1Department of Ecology and Evolutionary Biology, Tulane University, New Orleans, LA, United States
- 2Department of Biological Sciences, University of Pittsburgh, Pittsburgh, PA, United States
Defense against pathogens is one of many benefits that bacteria provide to animal hosts. A clearer understanding of how changes in the environment affect the interactions between animals and their microbial benefactors is needed in order to predict the impact and dynamics of emerging animal diseases. Due to its dramatic effects on the physiology of animals and their pathogens, temperature may be a key variable modulating the level of protection that beneficial bacteria provide to their animal hosts. Here we investigate how temperature and the makeup of the skin microbial community affect the susceptibility of amphibian hosts to infection by Batrachochytrium dendrobatidis (Bd), one of two fungal pathogens known to cause the disease chytridiomycosis. To do this, we manipulated the skin bacterial communities of susceptible hosts, northern cricket frogs (Acris crepitans), prior to exposing these animals to Bd under two different ecologically relevant temperatures. Our manipulations included one treatment where antibiotics were used to reduce the skin bacterial community, one where the bacterial community was augmented with the antifungal bacterium, Stenotrophomonas maltophilia, and one in which the frog’s skin bacterial community was left intact. We predicted that frogs with reduced skin bacterial communities would be more susceptible (i.e., less resistant to and/or tolerant of Bd infection), and frogs with skin bacterial communities augmented with the known antifungal bacterium would be less susceptible to Bd infection and chytridiomycosis. However, we also predicted that this interaction would be temperature dependent. We found a strong effect of temperature but not of skin microbial treatment on the probability and intensity of infection in Bd-exposed frogs. Whether temperature affected survival; however, it differed among our skin microbial treatment groups, with animals having more S. maltophilia on their skin surviving longer at 14 but not at 26°C. Our results suggest that temperature was the predominant factor influencing Bd’s ability to colonize the host (i.e., resistance) but that the composition of the cutaneous bacterial community was important in modulating the host’s ability to survive (i.e., tolerate) a heavy Bd infection.
Introduction
That there exist mutualistic and even symbiotic relationships between animals and microbes has long been understood, yet it is only recently that we have come to appreciate how common and influential these relationships can be for ecological processes that play out across taxa and environments (reviewed in McFall-Ngai et al., 2013). While effects of temperature on microbial growth and community structure in soil and other environmental samples have been well-documented (e.g., Jansson and Tas, 2014; Piquet et al., 2016), how temperature variation affects animal–microbe interactions is less well-understood. Empirical data exist for a few hosts and their bacterial symbionts (e.g., sponges: Webster et al., 2008; Fan et al., 2013; sea anemone: Fraune et al., 2016), yet the range of taxa and types of interactions under which this relationship with temperature has been explored remains small (Carey and Duddleston, 2014). Studies that broaden this range of hosts and ecological contexts are needed to clarify how temperature affects animal interactions with microbes, and how these effects may impact wildlife responses to environmental stressors such as climate change and habitat modification.
Defense against infection is one important service that microbes can provide for their animal hosts (McFall-Ngai et al., 2013; Clavel et al., 2017) and changes in temperature can affect the potential of microbes to cause disease or help their hosts resist infections (Daskin and Alford, 2012). For example, elevated ocean temperatures increase the expression of virulence genes in the bacterial pathogen Vibrio shiloi, which induces bleaching in the coral Oculina patagonica (Rosenberg and Ben-Halm, 2002). On the flip side, the ability of ascidians to defend themselves against pathogens is likely impacted by effects of temperature on their community of symbiotic microbes (Tianero et al., 2015); bacterial symbionts provide these animals with their diverse repertoire of defensive secondary metabolites, some of which have antimicrobial and antiviral properties (Paul et al., 1990). Temperature may be a key player in determining the health benefits that symbiotic microbes bestow upon their animal hosts, especially for ectotherms (Kohl and Yahn, 2016; Ferguson, 2017), though clear empirical examples appear to be limited to handful of invertebrates (Tianero et al., 2015; Ferguson, 2017). Amphibians are another taxon useful for investigating the effects of temperature on microbial symbioses, as several aspects of the amphibian immune system are known to function in a temperature-dependent manner (Maniero and Carey, 1997; Rollins-Smith and Woodhams, 2012) and the cutaneous bacteria of amphibians are known to be important to their defense against other skin microbes, including pathogenic fungi in the genus Batrachochytrium (Harris et al., 2009a).
Chytridiomycosis, the disease caused by the chytrid fungi Batrachochytrium dendrobatidis (Bd) and Batrachochytrium salamandrivorans, has been implicated in global amphibian declines (Berger et al., 1998; Lips et al., 2006; Rachowicz et al., 2006; Martel et al., 2013). Because Batrachochytrium salamandrivorans has only recently been described (Martel et al., 2013), less is known about its potential consequences for host populations (but see Martel et al., 2014). Bd, however, is known to disrupt electrolyte transport across frog skin, which can cause cardiac arrest, the mechanism of mortality (Voyles et al., 2009). Not all amphibians are equally at risk of infection. Hosts found in consistently cool, wet habitats in both temperate and tropical regions appear particularly vulnerable to Bd-related declines (Berger et al., 2016). Bd infection dynamics have also been correlated with climate and seasonality (Berger et al., 2004; Woodhams and Alford, 2005; Bishop et al., 2009; Rohr and Raffel, 2010), with infections often peaking in early spring (Kriger and Hero, 2007; Longcore et al., 2007; Rothermel et al., 2008). Variation in susceptibility to chytridiomycosis also exists within and among host species (Tobler and Schmidt, 2010; Martel et al., 2014), with some species requiring a higher pathogen load in order to become sick than others (Berger et al., 2004). This could be caused by differences among strains of Bd, as some are more pathogenic than others (Retallick and Miera, 2007; Farrer et al., 2011). Differences in susceptibility among hosts (Woodhams et al., 2007a) and populations (Savage and Zamudio, 2011) could also reflect intrinsic or temperature-driven differences in host immunity (reviewed in Rowley and Alford, 2010; Rollins-Smith and Woodhams, 2012).
Amphibian hosts have several potential lines of defense against Batrachochytrium pathogens. Antimicrobial peptides (AMPs), produced in the granular glands of the skin of some amphibians and secreted in mucus, have been shown to inhibit the growth of Bd in vitro (Rollins-Smith et al., 2006; Ramsey et al., 2010). Antibody and lymphocyte production is also stimulated by Bd exposure in some host species, suggesting the potential for an acquired immune response to this pathogen (Ramsey et al., 2010; McMahon et al., 2014). Variation in body temperature among individuals has also been correlated with the probability of Bd infection (Richards-Zawacki, 2010; Rowley and Alford, 2013; Roznik et al., 2015). Amphibians may also elevate their body temperature above normal by selecting warmer microhabitats, thereby inducing a behavioral fever (Sherman and Stephens, 1998; Woodhams et al., 2003; Sherman, 2008; Richards-Zawacki, 2010). This elevated temperature presumably enhances the immune response (Maniero and Carey, 1997; Rollins-Smith and Woodhams, 2012) though the effectiveness of such a fever in combatting Bd infection has not been demonstrated empirically.
Another component of amphibian defense against pathogens is the skin microbiome. The mucus on frog skin is home to a rich community of bacteria (McKenzie et al., 2012; Kueneman et al., 2013). While there is also variation within frog species (Lauer et al., 2008), cutaneous bacterial communities have been found to differ more among amphibian species than among individuals and/or environments (McKenzie et al., 2012; Becker et al., 2014). This suggests that innate differences in skin bacterial communities among species could contribute to differences in susceptibility to chytridiomycosis (McKenzie et al., 2012). Some members of amphibian skin bacterial communities are known to produce substances with antifungal capabilities. This was first demonstrated in two North American salamander species by Harris et al. (2006), who isolated skin bacteria and challenged Bd to grow in the presence of those isolates in vitro. Since then, a large and growing number of bacteria found on amphibian skin have been shown to inhibit the growth of Bd (Woodhams et al., 2015). It is believed to be metabolites produced by these bacteria that inhibit Bd growth and confer resistance and/or tolerance to infection in amphibian hosts (Brucker et al., 2008a,b; Harris et al., 2009a; Becker et al., 2015). Support for this idea comes from in vitro studies where the metabolites produced by a variety of bacteria isolated from frog mucus inhibit the growth of Bd (Daskin et al., 2014; Woodhams et al., 2015).
Laboratory exposure studies provide further evidence that bacteria found on frog skin can contribute to variation in susceptibility to chytridiomycosis (Harris et al., 2009b; Woodhams et al., 2014). For example, adding Janthinobacterium lividum, a bacterium that produces the Bd-inhibitory metabolite violacein, to the skin of R. muscosa decreased the risk of mortality after Bd exposure (Harris et al., 2009a). Correlations between skin bacterial communities and susceptibility to chytridiomycosis have been documented in wild populations as well (Woodhams et al., 2007b). Given this, efforts are underway to develop probiotics that could be applied to frogs to protect them from Bd in the wild (Bletz et al., 2013). Probiotic approaches may prove to be the most plausible solution for in situ conservation of species that are threatened with extinction due to Bd, but their effectiveness may be dependent upon environmental conditions, including temperature (Woodhams et al., 2014).
Temperature is known to affect the growth (Gaddad and Rodgi, 1987; Pietikainen et al., 2005) and antifungal metabolite production (Noaman et al., 2004; Ripa et al., 2009; Kariluoto et al., 2010) of bacteria. There is also some evidence that the bacteria found on frog skin produce anti-Bd metabolites better at some temperatures than others. Daskin et al. (2014) found that the cell free supernatants from frog skin bacteria cultured at cooler temperatures were less effective at inhibiting the growth of Bd in vitro. Robak (2016) also found that antifungal metabolites produced by frog skin bacteria were more effective at inhibiting Bd growth at higher temperatures, but in this study, the temperature at which the metabolites were produced was less important than the temperature at which the growth challenge assay was performed. If this temperature dependence of anti-Bd activity is a general phenomenon, it could contribute to the observed correlations between frog body temperatures and Bd infection (Richards-Zawacki, 2010; Rowley and Alford, 2013; Roznik et al., 2015) and between climatic variation and chytridiomycosis (Berger et al., 2004; Woodhams and Alford, 2005; Rohr and Raffel, 2010). It also suggests that development of a successful probiotic treatment for Bd infected animals will require information on how the chosen bacteria would function in natural environments, where conditions such as temperature vary in space and time. In the Robak (2016) study, one bacterium of interest, Stenotrophomonas maltophilia, was found to produce metabolites that inhibit the growth of Bd in vitro at 14, 20, and 26°C, although the extent of inhibition was greatest at 20°C. While its products effectively inhibit Bd growth across a range of ecologically relevant temperatures in culture, it is not known whether (1) S. maltophilia presence on the skin would protect frogs from Bd infection and/or chytridiomycosis, or whether (2) the relationship between temperature and protection on hosts would mirror what was seen for Bd growth in vitro.
In this study, we examined the effect of temperature on the ability of skin microbes to protect an amphibian host against Bd infection and chytridiomycosis. To do this, we manipulated the skin bacterial communities of northern cricket frogs (Acris crepitans), a species known to be susceptible to chytridiomycosis (Zippel and Tabaka, 2008; Sonn et al., 2017) and either exposed them to Bd or sham-exposed them and housed half of each group at 14°C and the other half at 26°C. These temperatures were chosen as they are within the range of body temperatures that this host experiences during times of the year when they are infected with Bd in the wild (Sonn, 2016) and because susceptibility to chytridiomycosis in this host has been shown to differ between these two temperatures (Sonn et al., 2017). Within each temperature and exposure group (Bd vs. sham), frog skin bacterial communities were either (1) maintained intact, (2) reduced with antibiotics, or (3) augmented by inoculation with S. maltophilia (family Xanthomonadaceae, order Xanthomonadales). This Gram-negative bacterium found on frog skin, as well as in water, soil, and plant samples from a wide variety of environments and geographic regions (Denton and Kerr, 1998), has been demonstrated to inhibit Bd growth in vitro (Robak, 2016). We predicted that Bd-exposed frogs at the lower temperature would be more susceptible to chytridiomycosis, which we defined as having a greater Bd load (Voyles et al., 2009), decreased survival (Voyles et al., 2009), a higher prevalence of Bd infection (Vredenburg et al., 2010), or a lower body condition (Retallick and Miera, 2007; Murphy et al., 2011). We also predicted that frogs with their bacterial communities reduced would be more susceptible, and frogs with S. maltophilia added would be less susceptible to chytridiomycosis than frogs with intact skin microbial communities (Harris et al., 2009a), but that the effect of S. maltophilia on Bd susceptibility would be temperature dependent.
Materials and Methods
Animal Husbandry
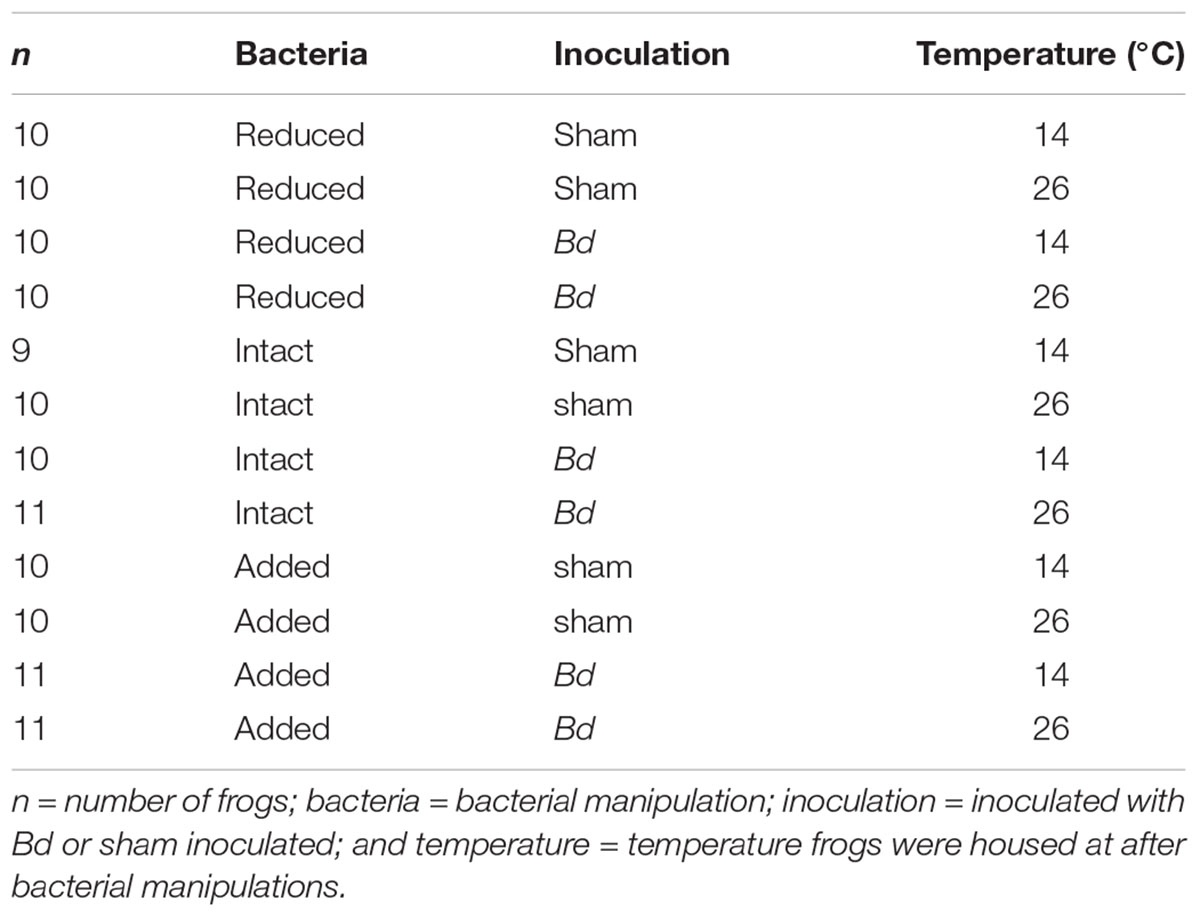
In February 2016, we collected 122 A. crepitans frogs from Tulane University’s F. Edward Hebert Riverside Research Center near Belle Chasse, LA, United States (WGS84: 29.8852489, -89.9694904) and placed them individually into cylindrical plastic enclosures (15 cm tall, 11 cm diameter with ventilated lids) containing a 2.5 cm depth of filtered tap water. While the previous infection history of these individual frogs was not known, Bd had been detected, sometimes at greater than 50% prevalence, in this population (Brannelly et al., unpublished data). To clear any potential Bd infections, we heat-treated the animals by holding them at 30°C in an environmental chamber (Conviron, Adaptis; 12 h light/dark cycle) for 10 days (Chatfield and Richards-Zawacki, 2011). Given the high prevalence of Bd in this population and our observation that individuals frequently gain and lose infections in the wild (Brannelly et al., unpublished data), we assumed that most or all of these animals were likely exposed to Bd prior to this study, and if any immunoprotective effects of prior-exposure existed, they did not preclude animals from becoming re-infected. However, our study design did not permit us to control for immunoprotective effects of prior Bd-exposure explicitly. After heat treatment, we tested the frogs for Bd following the swabbing and quantitative polymerase chain reaction (qPCR) assay protocols described below. After heat-treatment and during bacterial manipulations, we housed the frogs at 20°C. To get to this temperature, we lowered the temperature gradually over a period of 28 h. We assigned animals haphazardly to temperature, bacterial manipulation, and exposure groups, with each combination of temperature, bacterial manipulation, and exposure containing either 9, 10, or 11 animals. After bacterial manipulation, frogs were housed at either 14 or 26°C and were either inoculated with Bd or sham inoculated (Table 1). We fed the frogs ad libitum on 2 week-old crickets and provided them with a clean enclosure and fresh water every 7 days. We cleaned the enclosures with a 10% bleach solution and allowed them to dry completely before reuse. We wore a clean pair of nitrile gloves when handling each frog. We carried out this study in accordance with the recommendations of Tulane University’s Institutional Animal Care and Use Committee (IACUC, Protocol No. 0391R2).
Animal Monitoring
We monitored frogs daily for the following clinical signs of chytridiomycosis: lethargy, inappetence, loss of righting reflex, excessive skin sloughing, abnormal posture, and cutaneous erythema (Berger et al., 2005). To test for Bd infection and the presence of S. maltophilia on frog skin, we rinsed the frogs in filtered tap water and then swabbed the skin by rubbing a rayon tipped swab (MWE 113, Medical Wire and Equipment, Co., United Kingdom) five times over the dorsum, venter, each side of the body, and the bottom of each foot. This was done once each week starting on day 6. Snout-vent length (SVL), measured to the nearest 0.1 mm with a dial calipers, and mass, measured with a scale to the nearest 0.01 g, were recorded weekly, starting on day -1, the day prior to the first round of Bd inoculations (Table 2). We used residual mass as our index of body condition and we calculated this using the line of best fit from a linear regression between SVL and body mass for all frogs on day -1 (Supplementary Figure S1). The predicted mass of each frog, based on this pre-experiment regression was then subtracted from the actual mass each week to get a residual value, which reflects body condition relative to the mean for a frog of that size prior to Bd exposure (Jakob et al., 2011). Frogs were euthanized by bath in tricaine methane sulfonate (MS-222, pH 7) at the conclusion of the experiment.
Skin Microbe Quantification
We extracted genomic DNA from skin swabs using the Qiagen DNeasy Blood and Tissue kit, following the protocol for animal tissues with two modifications: (1) we incubated swabs for just 1 h, vortexing and spinning them in a centrifuge after 30 and 60 min of incubation; (2) we eluted samples twice with 100 μL of elution buffer instead of once with 200 μL. We then used a qPCR assay, performed on an Applied Biosystems 7500 system, to quantify the amount of Bd [in plasmid equivalents (PEs)]. We followed the protocol of Boyle et al. (2004) with the following modifications: (1) 0.7 μL of bovine serum albumin (Applied Biosystems) was added to each well prior to amplification (Garland et al., 2010) and (2) a sevenfold dilution series of Bd plasmid standards (Pisces Molecular, Boulder, CO, United States) was included in each run. For S. maltophilia, we quantified colony forming units (cfus) per swab using the same qPCR reaction cycling conditions and reagent concentrations as for Bd, but with primers and probes from Rios-Licea et al. (2010). For S. maltophilia, we generated a sevenfold dilution series of cfu standards by making serial dilutions of DNA extracted from a sample containing 5 × 106 cfus of S. maltophilia. We ran qPCRs on all swab samples in singlicate and considered animals positive for Bd if the qPCR result indicated that one or more copies of Bd DNA (i.e., ≥1 PE) were present in the reaction. Animals were considered to have become infected if they tested positive for Bd on one or more weekly swab samples. We converted Bd and S. maltophilia loads per 5 μL reaction volume to whole swab loads and then log-transformed these values prior to statistical analysis.
Bacterial Manipulations
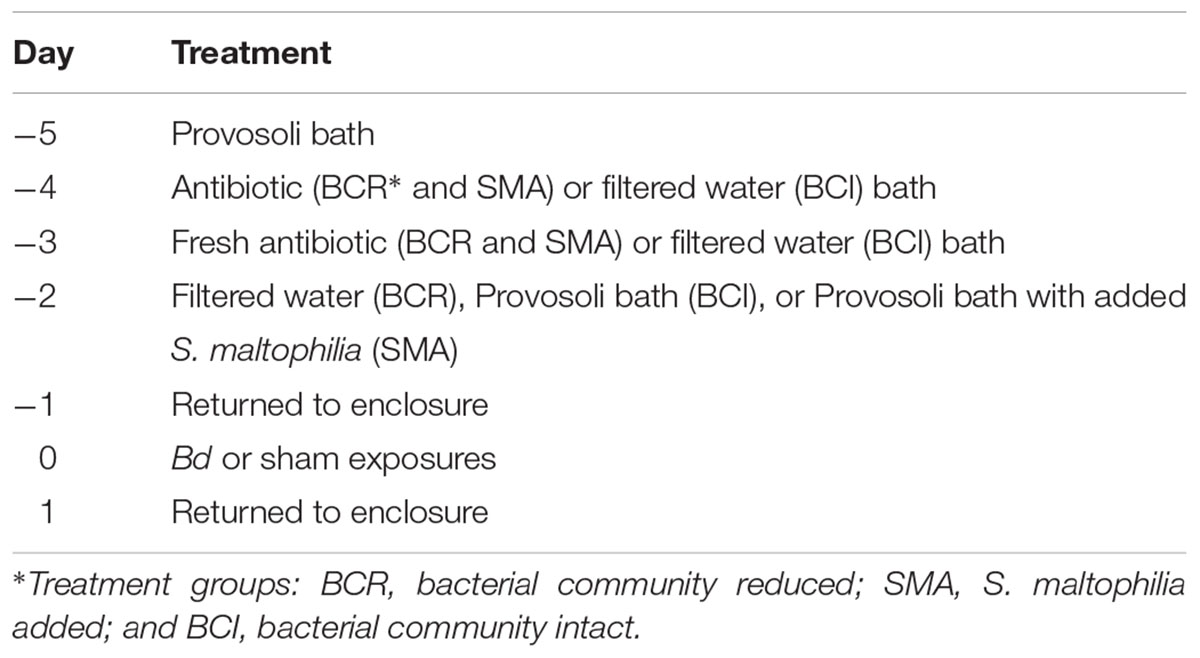
Our skin bacterial community manipulations took place over 4 days and ended on the day prior to the first Bd (or sham) exposure (see Table 2). On day -5, we placed frogs in a bath of 30 mL modified Provosoli medium (Wyngaard and Chinnappa, 1982) for 24 h to collect native cutaneous bacteria. On day -4, we removed the frogs from their Provosoli baths and placed these baths in a refrigerator. Animals in the “reduced” bacterial community and “S. maltophilia added” treatments were moved directly into a second bath, this time containing 30 mL of an antibiotic cocktail that targets both Gram-negative and Gram-positive bacteria (24 mg/L cephalexin, 14.5 mg/L sulfamethazine, 2.9 mg/L trimethoprim, 100 mg/L streptomycin, and 105 I.U./L penicillin) for 24 h (following Holden et al., 2015). Frogs in the “intact” bacterial community treatment were instead moved into a bath containing 30 mL of filtered tap water for 24 h. On day -3, we placed the frogs into either a fresh antibiotic cocktail bath with the same composition as the first (“reduced” and “S. maltophilia added” treatments) or into a filtered water bath (“intact” treatment) for 24 h. On day -2, we rinsed all frogs with filtered tap water and frogs in the “reduced” treatment group were placed in a bath containing 30 mL of filtered water for 24 h. Frogs in the “intact” treatment were placed back in their Provosoli baths (from day -4). Frogs in the “S. maltophilia added” treatment were placed back in their Provosoli baths also, but only after adding 2 × 108 S. maltophilia to the bath. We left the frogs in these baths for 24 h. On day -1, we rinsed all frogs with filtered tap water and placed them in clean enclosures.
Bd Exposure
To prepare inoculum for Bd exposures, we grew Bd (JEL412 isolated from a Sachatamia ilex frog in Panama in 2005 and provided by Dr. Joyce Longcore) on 1% tryptone agar plates for 7 days, at which point we harvested zoospores by flooding plates with 5 mL of deionized water. We then exposed frogs individually by placing them in a bath of 2 × 106 zoospores suspended in 30 mL filtered tap water for 12 h. We carried out sham exposures in the same way except that 1% tryptone plates without Bd were flooded. We exposed frogs in this way weekly throughout the experiment, starting on day 0.
Data Analysis
We used generalized linear mixed models (GLMMs) to test for significant effects of bacterial manipulations, temperature, and an interaction between temperature and bacterial manipulation on the log-transformed Bd and S. maltophilia loads on Bd-exposed frogs. Our model contained fixed effects of temperature, bacterial community manipulation (“reduced,” “intact,” or “S. maltophilia added”), and the interaction between those variables with day as a repeated measure. For these and all following GLMMs, we used a first-order autoregressive covariance type for repeated effects and residuals, assumed a non-normal distribution, and used a Satterthwaite approximation for degrees of freedom. A second GLMM with these same fixed effect plus exposure group (Bd- vs. sham-exposed) allowed us to test for significant main and interactive effects of temperature, bacterial manipulation, and Bd exposure on body condition. We used a third GLMM to test whether temperature, bacterial treatment, or their interaction affected the probability that Bd-exposed frogs became infected (yes/no). This model used a binary logistic distribution and the events/trials syntax, with infection as the event and day as the trial. To test for significant differences in survival among treatments (bacterial manipulations, temperatures, and exposure groups), we used a Cox regression with log-transformed Bd load included as a covariate. For significant effects, we used a Kaplan–Meier survival analysis to compute Tarone–Ware pairwise comparisons among groups.
As an alternative analytical approach, since S. maltophilia loads did not always differ among our bacterial community manipulation groups (see section “Results”), we repeated the analyses for Bd load, body condition, probability of infection (yes/no), and survival described above, but this time replacing the fixed effect of bacterial community manipulation (“reduced,” “intact,” or “S. maltophilia added”) with the covariate log-transformed S. maltophilia load (as determined by qPCR). These models included only Bd-exposed animals. When we had significant interaction effects with temperature in our survival analysis, we used separate Cox regressions for each experimental temperature (with Bonferroni-corrected p-values) for post hoc comparisons since Kaplan–Meier survival analyses cannot handle continuous factors like S. maltophilia load. All analyses were performed in IBM SPSS Statistics (v 23).
Results
Stenotrophomonas maltophilia was detected on all frogs from all three bacterial manipulation groups and both temperatures throughout the experiment, with the exception of two frogs at 26°C that each tested negative in one weekly sample: one frog from the “S. maltophilia added” group tested negative on day 41 and one from the “intact” bacteria group tested negative on day 55 (Supplementary Figures S2A,B). The amount of S. maltophilia on the skin differed among temperature (GLMM: F1,239 = 30.608, P < 0.001) and bacterial manipulation (GLMM: F2,239 = 5.538, P = 0.004) groups, but the interaction between temperature and bacterial manipulation was not significant (GLMM: F2,239 = 1.618, P = 0.200). Frogs at 14°C had greater S. maltophilia loads than frogs at 26°C and frogs in the “intact” bacterial treatment had lower S. maltophilia loads than frogs in the “reduced” and “S. maltophilia added” groups (Tukey LSD: t239 ≥ 2.960, P ≤ 0.003, Supplementary Figure S3). There was no significant difference in S. maltophilia load between frogs in the “reduced” and “S. maltophilia added” treatments (Tukey LSD: t239 = 0.047, P = 0.962), suggesting that our cocktail of antibiotics did not reduce S. maltophilia abundance on frog skin.
All animals in Bd-exposed treatment groups had a positive qPCR result for Bd on at least one week of the experiment (Supplementary Figures S2C,D) and were therefore considered to have become infected. However, at 26°C, our Bd-exposed frogs tended to test positive for Bd only once or twice during the initial weeks of the experiment and then clear their infections. None of the sham-exposed animals ever tested positive for Bd. Bd-exposed frogs at 14°C had a greater probability of testing positive for Bd on any given swab than did Bd-exposed frogs at 26°C (GLMM: F1,106 = 90.731, P < 0.001; Figure 1A). There was no significant effect of bacterial manipulation (GLMM: F2,106 = 0.097, P = 0.908) and no significant interaction between temperature and bacterial manipulation (GLMM: F2,106 = 0.151, P = 0.860) on the weekly probability of Bd infection. When S. maltophilia load replaced bacterial manipulation in our model, the result was similar: neither S. maltophilia load (GLMM: F1,184 = 1.174, P = 0.280) nor the interaction between S. maltophilia load and temperature (GLMM: F1,193 = 1.528, P = 0.218) was significant predictors of Bd infection in weekly swab samples.
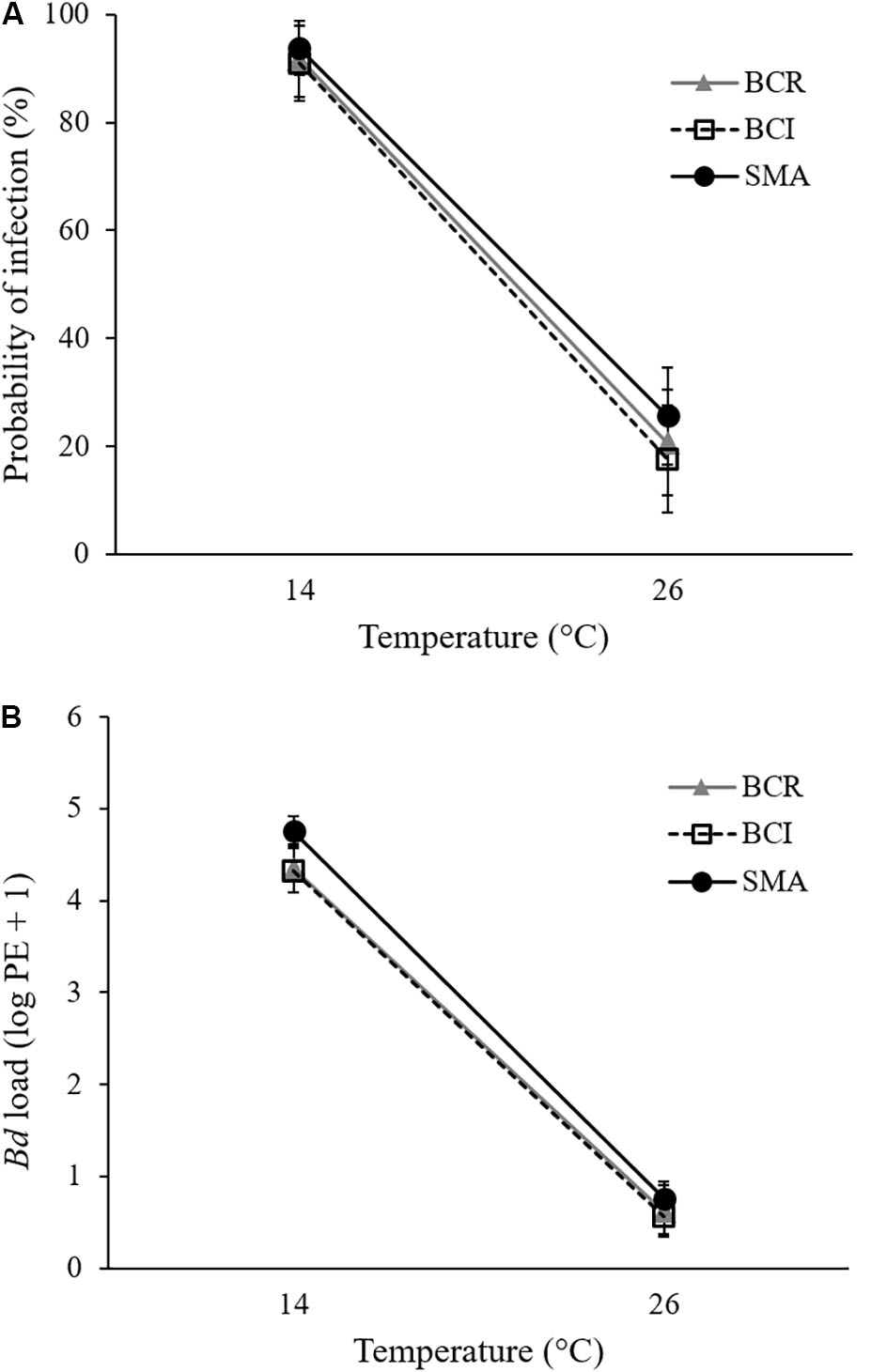
FIGURE 1. Mean (± SE) of (A) probability of infection and (B) Bd load, measured as log10 of plasmid equivalents (PEs) for Bd-exposed frogs at two treatment temperatures and three bacterial manipulations (BCR, bacterial community reduced; BCI, bacterial community intact; and SMA, S. maltophilia added) over the 9 weeks of the experiment.
For pathogen load, we found a significant effect of temperature (GLMM: F1,175 = 446.463, P < 0.001) but not of bacterial manipulation (GLMM: F2,190 = 1.521, P = 0.221) and the interaction between temperature and bacterial manipulation was not significant (GLMM: F2,190 = 0.254, P = 0.776). Bd-exposed animals at 14°C had greater Bd loads than those at 26°C (Figure 1B and Supplementary Figures S2C,D). When S. maltophilia load replaced bacterial manipulation in our model, the effect of temperature remained significant (GLMM: F1,197 = 4.659, P = 0.032) but the main effect of S. maltophilia load (GLMM: F1,188 = 4.559, P = 0.034) and the interaction between S. maltophilia load and temperature (GLMM: F1,186 = 6.924, P = 0.009) were also significant. Post hoc linear regressions (Figure 2) showed that the relationship between S. maltophilia load and Bd load on swabs was positive at 14°C (R2 = 0.171, B = 0.572, F1,99 = 20.466, P < 0.001) but non-significant at 26°C (R2 = 0.003, F1,100 = 0.350, P = 0.555).
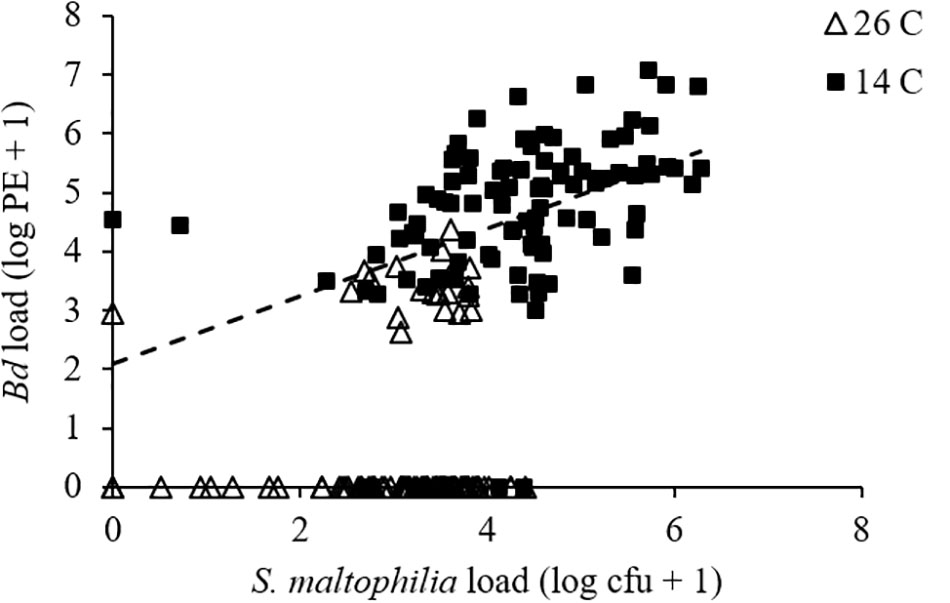
FIGURE 2. Relationship between Bd load, measured as log10 of PEs and S. maltophilia load, measured as log10 of colony forming units (cfus) for Bd-exposed frogs. Line is the least-squares line of best fit (y = 0.5722x + 2.0977; R2 = 0.171) for frogs at 14°C. The relationship was non-significant at 26°C.
Neither temperature (GLMM: F1,672 = 2.827, P = 0.093), bacterial manipulation (GLMM: F1,672 = 0.212, P = 0.809), nor exposure group (Bd vs. sham, GLMM: F1,672 = 0.083, P = 0.773) had a significant effect on body condition and there were no significant two- or three-way interactions between temperature, bacterial manipulation, and exposure group (GLMM: F2,672 ≤ 2.483, P ≥ 0.084). Results were similar when S. maltophilia load replaced bacterial manipulation in our model (GLMM: all F1,201 ≤ 1.702, all P ≥ 0.194). Body condition did change over the course of the experiment, though (GLMM, day: F9,672 = 1.950, P = 0.043; Supplementary Figure S4), with frogs increasing in body condition during the initial 3 weeks of the experiment (Tukey HSD: all pairwise t672 ≤ 1.834, P ≥ 0.05 except for day -1 vs. days 6, 13, and 20, which had t672 ≥ 2.464, P ≤ 0.014).
Clinical signs of chytridiomycosis and mortality were observed in Bd-exposed animals beginning 8 days after the initial Bd exposure. By the end of the experiment, 62 days after the first exposure, only five Bd-exposed frogs survived. Four of these were from 26°C treatments (one from each of the “intact” and “reduced” bacterial treatments and two from the “S. maltophilia added” treatment). The only surviving frog at 14°C was from the “reduced” bacterial community treatment (Supplementary Figures S2E,F). Mortality was low in sham-infected frogs (≤2 deaths per group), no clinical signs of chytridiomycosis were observed, and there were no significant differences in survival with respect to temperature or bacterial treatment (Tarone–Ware: X2 ≤ 1.108, P ≥ 0.293).
Our final Cox regression model (overall model: χ2 = 143.135, P < 0.001) included the effect of exposure group (Wald1 = 71.712, P < 0.001), with Bd-exposed frogs having lower survival than sham-exposed animals, and the interaction between bacterial manipulation and temperature (Wald2 = 7.453, P = 0.024) but not the main effects of temperature (Wald1 = 0.122, P = 0.727) or bacterial manipulation (Wald2 = 3.393, P = 0.183) as significant predictors of survival. Considering only the Bd-exposed animals, Bd load was not a significant predictor of survival (Wald1 = 0.153, P = 0.695). Frogs in the “S. maltophilia added” treatment survived longer than frogs with either “intact” or “reduced” bacterial communities at 26°C (Tarone–Ware: χ2 ≥ 5.123, P ≤ 0.023). At 14°C, Bd-exposed frogs in the “S. maltophilia added” treatment survived longer than those in the “intact” bacterial community treatment (Tarone–Ware: χ2 = 11.392, P ≤ 0.001), but not significantly longer than frogs in the “reduced” bacteria treatment (Tarone–Ware: χ2 = 0.017, P ≤ 0.896; Figure 3). When S. maltophilia load replaced bacterial manipulation in our Cox regression model, only the interaction between S. maltophilia load and temperature was significant (Wald1 = 5.852, P = 0.016). Separate Cox regressions for animals at 14 versus 26°C showed that odds of mortality in Bd-exposed frogs decreased with increasing S. maltophilia load at 14°C (B = -0.547, Wald1 = 5.731, corrected P = 0.034) but at 26°C, S. maltophilia load was not a significant predictor of mortality (Wald1 = 2.128, corrected P = 0.290).
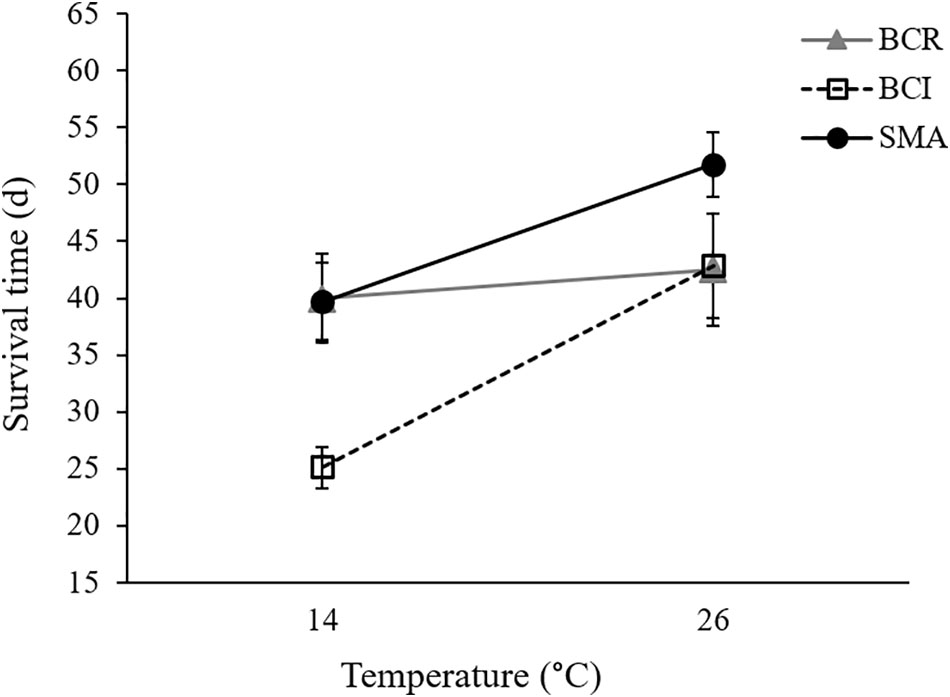
FIGURE 3. Mean (± SE) survival time for Bd-exposed frogs in three bacterial manipulation groups (BCR, bacterial community reduced; BCI, bacterial community intact; and SMA, S. maltophilia added) at 14 and 26°C.
Discussion
We examined temperature’s effect on the ability of skin microbes to protect a susceptible frog species against Bd infection and chytridiomycosis. Given that amphibian immune function is reduced at low temperatures (Rollins-Smith and Woodhams, 2012), and that Bd infections and chytridiomycosis are more prevalent in animals with cooler body temperatures (Rowley and Alford, 2013) and in cool climates (Berger et al., 2004; Woodhams and Alford, 2005; Rohr and Raffel, 2010), we predicted that temperature would affect the susceptibility of A. crepitans to Bd infection and chytridiomycosis in this experiment. Specifically, we predicted that, as in a previous study (Sonn et al., 2017), Bd-exposed frogs housed under colder conditions would have higher pathogen loads, a greater likelihood of becoming infected, lower body condition, and/or lower survival. We also predicted that frogs treated with antibiotics prior to Bd exposure would be more susceptible, and frogs with the anti-Bd bacterium S. maltophilia added to their skin would be less susceptible to infection and/or disease than animals with an un-manipulated bacterial community. And finally, we predicted that the effects of S. maltophilia on Bd susceptibility would be temperature dependent. Some, but not all, of these predictions were upheld.
Temperature had an effect on the susceptibility of Bd-exposed frogs, with animals housed at 14°C having a greater likelihood of infection (Figure 1A) and also having greater pathogen loads (Figure 1B and Supplementary Figures S2C,D) than frogs housed at 26°C. This is consistent with idea that amphibian immune responses are often improved at higher temperatures (Maniero and Carey, 1997; Rollins-Smith and Woodhams, 2012). It is also consistent with the observations that in nature, (1) individual frogs with higher body temperatures are less likely to be infected with Bd (Richards-Zawacki, 2010; Rowley and Alford, 2013) and (2) disease variables and risk of Bd-related declines often reach a peak in cool seasons and climates (Berger et al., 2004; Woodhams and Alford, 2005; Kriger and Hero, 2006; Longcore et al., 2007; Rohr and Raffel, 2010). This result is also consistent with a recent laboratory exposure study where the susceptibility of A. crepitans to chytridiomycosis was found to be inversely related to temperature (Sonn et al., 2017). In contrast to that study, however, the present study showed no significant main effect of temperature on the body condition or survival of Bd-exposed animals. There were, however, significant interactions between temperature and bacterial manipulation and between temperature and S. maltophilia load for survival, suggesting that the effect of temperature on the survival of Bd-exposed frogs depended on their cutaneous bacterial community.
While we predicted we would see significant main effects of bacterial manipulation on several indices of susceptibility to Bd infection and chytridiomycosis, the only variable we measured that appears to have been affected by our bacterial manipulations was survival, which differed in a temperature-dependent manner across our bacterial manipulation groups. On average, Bd-exposed frogs in the “reduced” bacterial treatment survived equally well at 14 and 26°C whereas frogs with “intact” or “S. maltophilia added” bacterial communities survived longer at the higher temperature (Figure 3). Frogs in the “S. maltophilia added” treatment at 26°C had the greatest mean survival time of all Bd-exposed groups.
Stenotrophomonas maltophilia has been found not only on frog skin, but also in water, soil, and plant samples from a wide variety of environments and geographic regions (Denton and Kerr, 1998). It is known to inhibit the growth of a broad range of plant (e.g., Elad et al., 1994; Kobayashi et al., 1995; Berg et al., 1996), and even some human (e.g., Candida spp., Aspergillus fumigatus; Kerr, 1996) fungal pathogens. Stenotrophomonas species have been isolated from amphibian skin on several continents (North America: Woodhams et al., 2007b; South America: Flechas et al., 2012; Australia: Woodhams et al., 2015) and S. maltophilia (Flechas et al., 2012; Robak, 2016) and some of its congeners (Woodhams et al., 2015) have been shown to inhibit Bd growth in vitro. We chose S. maltophilia for use in this study because our previous work suggested that S. maltophilia inhibits Bd growth in vitro across a range of temperatures (Robak, 2016). However, despite having higher average S. maltophilia loads on their skin during this experiment, we did not see a lower probability of infection or lower pathogen load on Bd-exposed animals in our “S. maltophilia added” treatment, compared with animals in our “intact” bacterial treatment. Importantly though, we did see greater survival in the “S. maltophilia added” treatment, compared to our “intact” treatment animals at both 14 and 26°C. This suggests that augmentation of the cutaneous S. maltophilia population may yield benefits for Bd-exposed frogs across a range of temperatures.
We attempted to reduce the number and diversity of bacteria present on the skins of frogs in our “reduced” bacterial treatment via bath in a cocktail of antibiotics. However, our qPCR assays suggest that animals in our “reduced” treatments maintained as much S. maltophilia on their skins as animals in our “S. maltophilia added” treatments. It is possible that our antibiotic baths failed to reduce the bacterial communities on the frogs that received them. However, S. maltophilia is known to be naturally resistant to many broad-spectrum antibiotics (Denton and Kerr, 1998), so the large populations of this bacterial species on the skins of frogs in our “reduced” treatments could also be explained by the growth of S. maltophilia (and potentially other antibiotic-resistant bacteria) after the removal of their more antibiotic-susceptible competitors. The lower concentrations of S. maltophilia maintained by frogs with putatively more diverse skin communities in our “intact” treatments are consistent with this explanation. If S. maltophilia does indeed augment the host’s ability to tolerate a heavy Bd infection, this could explain why frogs in our “reduced” treatment, which maintained high S. maltophilia loads on their skin, especially at 14°C, survived as long as frogs in our “S. maltophilia added” treatment at that temperature. However, in that case, it is not clear why our “reduced” treatment frogs did not receive the same survival benefit as our “S. maltophilia added” frogs at 26°C.
At 14°C, but not at 26°C, frogs with abundant S. maltophilia on their skin survived repeated exposures to Bd longer than frogs with “intact” skin bacterial communities where S. maltophilia was present, but less abundant. Interestingly, though S. maltophilia (Denton and Kerr, 1998) and other bacteria isolated from A. crepitans grow faster in vitro at 26 than at 14°C (Robak, 2016), frogs in all three of our bacterial treatment groups maintained more S. maltophilia on their skin at 14 than at 26°C (Supplementary Figure S3). It is not clear what caused this difference between in vitro and in vivo growth of S. maltophilia or whether the pattern holds for other members of the microbial community on amphibian skin. Interestingly, the temperature optimal for Bd growth on A. crepitans (Sonn et al., 2017) and other amphibian hosts (Cohen et al., 2017) also commonly differs from that of growth in culture.
While augmentation with a known anti-Bd bacterium was associated with longer survival in Bd-exposed animals at both 14 and 26°C, our results also support our prediction of temperature-dependent effects of beneficial skin microbes. For example, at 14°C, there was a positive relationship between S. maltophilia load and the odds of survival in Bd-exposed frogs. At this temperature, frogs in the “S. maltophilia added” and “reduced” bacterial community treatments, which had greater S. maltophilia loads (Supplementary Figure S3), survived Bd infections longer than did animals in the “intact” bacterial community treatment (Figure 3 and Supplementary Figure S2E). We observed this difference in survival, despite the fact that Bd loads were similarly high among animals in all three bacterial treatment groups, suggesting that at 14°C, the load of S. maltophilia on the skin affected the animals’ ability to survive with (i.e., tolerate) a heavy Bd infection. In contrast, at 26°C, the relationship between S. maltophilia load and survival was not significant.
The mechanism by which tolerance of Bd infection is modulated by S. maltophilia remains unclear. This bacterium’s inhibition of phytopathogenic fungal growth has been linked to its production the antifungal secondary metabolites pyrrolnitrin (Kerr, 1996) and maltophilin (Jakobi et al., 1996). Interestingly, S. maltophilia also exhibits chitinolytic activity (Kobayashi et al., 1995). Chitin is an important part of cell wall structure stability for Bd and other chytrid fungi, and drugs that interfere with chitin synthesis have been shown to inhibit Bd growth in vitro (Holden et al., 2014). It seems likely that this chytinolytic activity plays a role in the effect that heavy loads of S. maltophilia on the skin had on the survival of our Bd-exposed hosts. At 14°C, S. maltophilia load was positively associated with survival in our Bd-exposed frogs. However, at 26°C, we saw no significant relationship between S. maltophilia load and survival. This could be because the S. maltophilia loads on our 26°C frogs never reached the levels that they did on animals at 14°C. Perhaps some threshold load of S. maltophilia is needed before the benefits of this microbe can be seen and the higher temperature prevented frogs in our 26°C treatments from reaching this threshold? It could also be that heavy Bd infections facilitate frogs sustaining large populations of S. maltophilia on the skin, as we found a positive relationship between Bd load and S. maltophilia load at 14°C (Figure 2). In this case, the lack of an effect of S. maltophilia on the survival of Bd-exposed animals at 26°C could be explained by the lower average Bd loads these animals experienced (Figure 1B).
All but one of the Bd-exposed frogs in our 14°C treatments became heavily infected with Bd during our experiment and most died after exhibiting clinical signs of chytridiomycosis. Manipulation of the bacterial community appears to have affected the survival time of heavily infected frogs (i.e., tolerance) but not the likelihood of infection (i.e., resistance). However, the infections we observed in Bd-exposed frogs at 26°C were generally light and often transient. In many cases, death at this temperature was preceded by one or more weekly skin swabs that tested negative for the presence of Bd. It seems unlikely that these animals at 26°C were dying of chytridiomycosis, though they exhibited similar clinical signs to animals that died with high Bd loads in the 14°C treatments. However, survival of the Bd-exposed frogs was significantly lower than sham-exposed animals at both temperatures, suggesting that mortality was due to Bd exposure and not another pathogen or husbandry-related cause.
While it is not uncommon in Bd exposure studies for hosts to remain uninfected or clear Bd infections (e.g., Ramsey et al., 2010; Brannelly et al., 2012), we are not aware of other published studies where high mortality was seen in animals with transient and generally low-intensity Bd infections. We can think of two plausible explanations for the mortality experienced by our Bd-exposed animals at 26°C, both of which are related to our having exposed these animals repeatedly to high concentrations of this pathogen.
First, resisting infection can be costly (Dallas et al., 2016), especially if it involves activation of the immune system and/or stress response. Given that amphibian immune function is often temperature dependent (Rollins-Smith and Woodhams, 2012), the cost of resisting infection may depend upon temperature as well. Though this topic remains understudied, evidence for a cost of resisting Bd infection exists for newts (Cheatsazan et al., 2013) and tadpoles (Gabor et al., 2017). If such a cost exists for A. crepitans, it could explain the mortality we saw in Bd-exposed frogs at 26°C, though in that case, it is perhaps surprising that we did not see a decline in body condition in these animals. On the contrary, both Bd- and sham-exposed frogs at 26°C gained body condition over the course of the study and at no point in the experiment, there was a difference in body condition between these two exposure groups (Tukey HSD: t672 ≤ 1.895, P ≥ 0.058).
Second, Bd is known to produce and release toxic factors that cause pathology and mortality in crayfish, even in the absence of infection (McMahon et al., 2013). Bd is also known to produce a toxic factor or factors that inhibit immune responses to Bd in vitro (Fites et al., 2014) and possibly also in vivo (Ellison et al., 2014). While it is not clear whether the pathology and mortality in crayfish and immune inhibition in amphibians are generated by the same or different toxic factors, it seems likely that this fungus, like many others (Bondy and Pestka, 2000), produces toxins capable of affecting the fitness of amphibian hosts, perhaps even in the absence of an active infection in the skin (e.g., in tadpoles: Blaustein et al., 2005). While we cannot definitively attribute the mortality seen in our 26°C treatment to a toxin, if Bd does produce a substance capable of causing mortality in amphibians, our methods may have been more likely to produce this effect than the methods of other similar studies. We exposed frogs weekly by bath to small volumes of water containing millions of zoospores whereas other studies have tended to use fewer exposures and lower concentrations of Bd (e.g., reviewed in Kilpatrick et al., 2009). Not much is known about the frequency of exposure or the concentration of Bd in natural environments, so it is unclear whether our results would be expected to hold in the wild. However, a mark-recapture study of Louisiana A. crepitans suggests that repeated exposure and cycles of clearance and re-infection are common (Brannelly et al., unpublished data). We suggest that the potential for mortality due to toxin exposure rather than skin infection in amphibians exposed to Bd deserves further study.
Our results demonstrate that both temperature and the makeup of the skin bacterial community can impact the susceptibility of amphibian hosts to chytridiomycosis. Temperature’s main effects were on the likelihood (i.e., resistance) and magnitude of infection whereas the skin microbial community affected the host’s ability to survive a heavy infection (i.e., tolerance). Frogs at 14°C survived longer, despite large Bd burdens, when they harbored large populations of the antifungal bacterium S. maltophilia on their skin. Survival of frogs with S. maltophilia-enhanced skin communities was also longer at 26°C, though at this temperature, survival was not correlated with S. maltophilia load and exposure to, rather than infection with, Bd seems to have been the main cause of mortality. Whether this sort of interaction between temperature and the protection that bacteria provide against animal pathogens is common remains to be seen. Given its importance to the physiology of all three players, we predict that temperature may have especially strong impacts on the interactions of ectotherm hosts and their bacterial communities with fungal pathogens (Fisher et al., 2012; Carey and Duddleston, 2014; Daskin et al., 2014).
Author Contributions
MR and CR-Z designed the study. MR conducted the lab work and wrote the first draft of the manuscript. CR-Z revised it for publication. Both authors analyzed and interpreted the data and approved the manuscript’s content.
Funding
This work was funded by grants from the National Science Foundation (Award No. 1649443) and Louisiana Board of Regents [Award No. LEQSF (2011-14)-RD-A-26] to CR-Z.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Michael Blum, David Heins, and Louise Rollins-Smith for feedback on earlier drafts. Permission to collect A. crepitans was provided by the Louisiana Department of Wildlife and Fisheries.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00410/full#supplementary-material
References
Becker, M. H., Richards-Zawacki, C. L., Gratwicke, B., and Belden, L. K. (2014). The effect of captivity on the cutaneous bacterial community of the critically endangered Panamanian golden frog (Atelopus zeteki). Biol. Conserv. 176, 199–206. doi: 10.1016/j.biocon.2014.05.029
Becker, M. H., Walke, J. B., Cikanek, S., Savage, A. E., Mattheus, N., Santiago, C. N., et al. (2015). Composition of symbiotic bacteria predicts survival in Panamanian golden frogs infected with a lethal fungus. Proc. R. Soc. Lond. B Biol. Sci. 282:20142881. doi: 10.1098/rspb.2014.2881
Berg, G., Marten, P., and Balling, G. (1996). Stenotrophomonas maltophilia in the rhizosphere of oil-seed rape – occurrence, characterization, and interaction with phytopathogenic fungi. Microbiol. Res. 151, 19–27. doi: 10.1016/S0944-5013(96)80051-6
Berger, L., Marantelli, G., Skerratt, L. F., and Speare, R. (2005). Virulence of the amphibian chytrid fungus Batrachochytrium dendrobatidis varies with the strain. Dis. Aquat. Organ. 68, 47–50. doi: 10.3354/dao068047
Berger, L., Roberts, A. A., Voyles, J., Longcore, J. E., Murray, K. A., and Skerratt, L. F. (2016). History and recent progress on chytridiomycosis in amphibians. Fungal Ecol. 19, 89–99. doi: 10.1016/j.funeco.2015.09.007
Berger, L., Speare, R., Daszak, P., Green, D. E., Cunningham, A. A., Goggin, C. L., et al. (1998). Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. U.S.A. 95, 9031–9036. doi: 10.1073/pnas.95.15.9031
Berger, L., Speare, R., Hines, H. B., Marantelli, G., Hyatt, A. D., McDonald, K. R., et al. (2004). Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust. Vet. J. 82, 434–439. doi: 10.1111/j.1751-0813.2004.tb11137.x
Bishop, P. J., Speare, R., Poulter, R., Butler, M., Speare, B. J., Hyatt, A., et al. (2009). Elimination of the amphibian chytrid fungus Batrachochytrium dendrobatidis by Archey’s frog Leiopelma archeyi. Dis. Aquat. Organ. 84, 9–15. doi: 10.3354/dao02028
Blaustein, A., Romansic, J., Scheessele, E., Han, B., Pessier, A., and Longcore, J. (2005). Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batrachochytrium dendrobatidis. Conserv. Biol. 19, 1460–1468. doi: 10.1111/j.1523-1739.2005.00195.x
Bletz, M. C., Loudon, A. H., Becker, M. H., Bell, S. C., Woodhams, D. C., Minbiole, K. P. C., et al. (2013). Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecol. Lett. 16, 807–820. doi: 10.1111/ele.12099
Bondy, G. S., and Pestka, J. J. (2000). Immunomodulation by fungal toxins. J. Toxicol. Environ. Health B Crit. Rev. 3, 109–143. doi: 10.1080/109374000281113
Boyle, D. G., Boyle, D. B., Olsen, V., Morgan, J. A. T., and Hyatt, A. D. (2004). Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Organ. 60, 141–148. doi: 10.3354/dao060141
Brannelly, L. A., Chatfield, M. W. H., and Richards-Zawacki, C. L. (2012). Field and laboratory studies of the susceptibility of the green treefrog (Hyla cinerea) to Batrachochytrium dendrobatidis infection. PLoS One 7:e38473. doi: 10.1371/journal.pone.0038473
Brucker, R. M., Baylor, C. M., Walters, R. L., Lauer, A., Harris, R. N., and Minbiole, K. P. C. (2008a). The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J. Chem. Ecol. 34, 39–43.
Brucker, R. M., Harris, R. N., Schwantes, C. R., Gallaher, T. N., Flaherty, D. C., Lam, B. A., et al. (2008b). Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J. Chem. Ecol. 34, 1422–1429. doi: 10.1007/s10886-008-9555-7
Carey, H. V., and Duddleston, K. N. (2014). Animal-microbial symbioses in changing environments. J. Therm. Ecol. 44, 78–84. doi: 10.1016/j.jtherbio.2014.02.015
Chatfield, M. W. H., and Richards-Zawacki, C. L. (2011). Elevated temperature as a treatment for Batrachochytrium dendrobatidis infection in captive frogs. Dis. Aquat. Organ. 94, 235–238. doi: 10.3354/dao02337
Cheatsazan, H., de Almedia, A. P., Russell, A. F., and Bonneaud, C. (2013). Experimental evidence for a cost of resistance to the fungal pathogen Batrachochytrium dendrobatidis for the palmate newt, Lissotriton helveticus. BMC Ecol. 13:27. doi: 10.1186/1472-6785-13-27
Clavel, T., Gomes-Neto, J. C., Lagkouvardos, I., and Ramer-Tait, A. E. (2017). Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomes. Immunol. Rev. 279, 8–22. doi: 10.1111/imr.12578
Cohen, J. M., Venesky, M. D., Sauer, E. L., Civitello, D. J., McMahon, T. A., Roznik, E. A., et al. (2017). The thermal mismatch hypothesis explains host susceptibility to an emerging infectious disease. Ecol. Lett. 20, 184–193. doi: 10.1111/ele.12720
Dallas, T., Holtackers, M., and Drake, J. M. (2016). Costs of resistance and infection by a generalist pathogen. Ecol. Evol. 6, 1737–1744. doi: 10.1002/ece3.1889
Daskin, J. H., and Alford, R. A. (2012). Context-dependent symbioses and their potential roles in wildlife diseases. Proc. R. Soc. Lond. B Biol. Sci. 279, 1457–1465. doi: 10.1098/rspb.2011.2276
Daskin, J. H., Bell, S. C., Schwarzkopf, L., and Alford, R. A. (2014). Cool temperatures reduce antifungal activity of symbiotic bacteria of threatened amphibians—implications for disease management and patterns of decline. PLoS One 9:e100378. doi: 10.1371/journal.pone.0100378
Denton, M., and Kerr, K. G. (1998). Microbial and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 11, 57–80.
Elad, Y., Koelh, J., and Fokkema, N. J. (1994). Control of infection and sporulation of Botrytis cinerea on bean and tomato by saprophytic bacteria and fungi. Eur. J. Plant Pathol. 100, 315–336. doi: 10.1007/BF01876443
Ellison, A. R., Savage, A. E., DiRenzo, G. V., Langhammer, P., Lips, K. R., and Zamudio, K. R. (2014). Fighting a losing battle: vigorous immune response countered by pathogen suppression of host defenses in the chytridiomycosis-susceptible frog Atelopus zeteki. G3 4, 1275–1289. doi: 10.1534/g3.114.010744
Fan, L., Liu, M., Simister, R., Webster, N. S., and Thomas, T. (2013). Marine microbial symbiosis heats up: the phylogenetic and functional response of a sponge holobiont to thermal stress. ISME J. 7, 991–1002. doi: 10.1038/ismej.2012.165
Farrer, R. A., Weinert, L. A., Bielby, J., Garner, T. W., Balloux, F., Clare, F., et al. (2011). Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl. Acad. Sci. U.S.A. 108, 18732–18736. doi: 10.1073/pnas.1111915108
Ferguson, L. V. (2017). Thermal Biology of Insect Immunity and Host-microbe Interactions. Doctoral dissertation, University of Western Ontario, London, ON.
Fisher, M. C., Hank, D. A., Briggs, C. J., Brownstein, J. S., Madoff, L. C., McCraw, S. L., et al. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. doi: 10.1038/nature10947
Fites, J. S., Reinert, L. K., Chappell, T. M., and Rollins-Smith, L. A. (2014). Inhibition of local immune responses by the frog-killing fungus Batrachochytrium dendrobatidis. Infect. Immun. 82, 4698–4706. doi: 10.1128/IAI.02231-14
Flechas, S. V., Sarmiento, C., Cardenas, M. E., Medina, E. M., Restrepo, S., and Amezquita, A. (2012). Surviving chytridiomycosis: differential anti-Batrachochytrium dendrobatidis activity in bacterial isolates from three lowland species of Atelopus. PLoS One 7:e44832. doi: 10.1371/journal.pone.0044832
Fraune, S., Foret, S., and Reitzel, A. M. (2016). Using Nematostella vectensis to study the interactions between genome, epigenome, and bacteria in a changing environment. Front. Mar. Sci. 3:148. doi: 10.3389/fmars.2016.00148
Gabor, C., Forsburg, Z., Voros, J., Serrano-Laguna, C., and Bosch, J. (2017). Differences in chytridiomycosis infection costs between two amphibian species from Central Europe. Amphib. Reptilia 38, 250–256. doi: 10.1163/15685381-00003099
Gaddad, S. M., and Rodgi, S. S. (1987). The effect of temperature on the growth and biochemical activities of Escherichia coli in sewage. Environ. Pollut. 43, 313–321. doi: 10.1016/0269-7491(87)90184-9
Garland, S., Baker, A., Phillott, A. D., and Skerratt, L. F. (2010). BSA reduces inhibition in a TaqMan assay for the detection of Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 92, 113–116. doi: 10.3354/dao02053
Harris, R. N., Brucker, R. M., Walke, J. B., Becker, M. H., Schwantes, C. R., Flaherty, D. C., et al. (2009a). Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 3, 818–824. doi: 10.1038/ismej.2009.27
Harris, R. N., James, T. Y., Lauer, A., Simon, M. A., and Patel, A. (2006). Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth 3, 53–56. doi: 10.1007/s10393-005-0009-1
Harris, R. N., Lauer, A., Simon, M. A., Banning, J. L., and Alford, R. A. (2009b). Addition of antifungal skin bacteria to salamanders ameliorates the effects of chytridiomycosis. Dis. Aquat. Organ. 83, 11–16. doi: 10.3354/dao02004
Holden, W. M., Fites, J. S., Reinert, L. K., and Rollins-Smith, L. A. (2014). Nikkomycin Z is an effective inhibitor of the chytrid fungus linked to global amphibian declines. Fungal Biol. 118, 48–60. doi: 10.1016/j.funbio.2013.11.001
Holden, W. M., Hanlon, S. M., Woodhams, D. C., Chappell, T. M., Wells, H. L., Glisson, S. M., et al. (2015). Skin bacteria provide early protection for newly metamorphosed southern leopard frogs (Rana sphenocephala) against the frog-killing fungus, Batrachochytrium dendrobatidis. Biol. Conserv. 187, 91–102. doi: 10.1016/j.biocon.2015.04.007
Jakob, E. M., Marrshall, S. D., and Uetz, G. W. (2011). Estimating fitness: a comparison of body condition indices. Oikos 77, 61–67. doi: 10.2307/3545585
Jakobi, M., Windelmann, G., Kaiser, D., Kempter, C., Jung, G., Berg, G., et al. (1996). Maltophilin: a new antifungal compound produced by Stenotrophomonas maltophilia R3089. J. Antibiot. 49, 1101–1104. doi: 10.7164/antibiotics.49.1101
Jansson, J. K., and Tas, N. (2014). The microbial ecology of permafrost. Nat. Rev. Microbiol. 12, 414–425. doi: 10.1038/nrmicro3262
Kariluoto, S., Edelmann, M., Herranen, M., Lampi, A., Shmelev, A., Salovaara, H., et al. (2010). Production of folate by bacteria isolated from oat bran. Int. J. Food Microbiol. 143, 41–47. doi: 10.1016/j.ijfoodmicro.2010.07.026
Kerr, J. R. (1996). Inhibition of growth of fungi pathogenic to man by Stenotrophomonas maltophilia. J. Med. Microbiol. 45, 380–382. doi: 10.1099/00222615-45-5-380
Kilpatrick, A. M., Briggs, C. J., and Daszak, P. (2009). The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol. Evol. 25, 109–118. doi: 10.1016/j.tree.2009.07.011
Kobayashi, D. Y., Guglielmoni, M., and Clark, B. B. (1995). Isolation of the chitinolytic bacteria Xanthomonas maltophilia and Serratia marcescens as biological control agents for summer patch disease of turf grass. Soil Biol. Biochem. 27, 1479–1487. doi: 10.1016/0038-0717(95)00062-J
Kohl, K. D., and Yahn, J. (2016). Effects of environmental temperature on the gut microbial communities of tadpoles. Environ. Microbiol. 18, 1561–1565. doi: 10.1111/1462-2920.13255
Kriger, K. M., and Hero, J. M. (2006). Survivorship in wild frogs infected with chytridiomycosis. EcoHealth 3, 171–177. doi: 10.1007/s10393-006-0027-7
Kriger, K. M., and Hero, J. M. (2007). Large-scale seasonal variation in the prevalence and severity of chytridiomycosis. J. Zool. 271, 352–359.
Kueneman, J. G., Parfrey, L. W., Woodhams, D. C., Archer, H. M., Knight, R., and McKenzie, V. J. (2013). The amphibian skin-associated microbiome across species, space and life history stages. Mol. Ecol. 23, 1238–1250. doi: 10.1111/mec.12510
Lauer, A., Simon, M. A., Banning, J. L., Lam, B. A., and Harris, R. N. (2008). Diversity of cutaneous bacteria with antifungal activity isolated from female four-toed salamanders. ISME J. 2, 145–157. doi: 10.1038/ismej.2007.110
Lips, K. R., Brem, F., Brenes, R., Reeve, J. D., Alford, R. A., Voyles, J., et al. (2006). Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl. Acad. Sci. U.S.A. 103, 3165–3170. doi: 10.1073/pnas.0506889103
Longcore, J. R., Longcore, J. E., Pessier, A. P., and Halteman, W. A. (2007). Chytridiomycosis widespread in anurans of northeastern United States. J. Wildl. Manage. 71, 435–444. doi: 10.2193/2006-345
Maniero, G. D., and Carey, C. (1997). Changes in selected aspects of immune function in the leopard frog, Rana pipiens, associated with exposure to cold. J. Comp. Physiol. B 167, 256–263. doi: 10.1007/s003600050072
Martel, A., Blooi, M., Adriaensen, C., Van Rooij, P., Beukema, W., Fisher, M. C., et al. (2014). Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346, 630–631. doi: 10.1126/science.1258268
Martel, A., Spitzen-van der Slujis, A., Blooi, M., Bert, W., Ducatell, R., Fisher, M. C., et al. (2013). Batrachochytrium salamandrivorans sp nov causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. U.S.A. 110, 15325–15329. doi: 10.1073/pnas.1307356110
McFall-Ngai, M., Hadfield, M. G., Bosch, T. C. G., Carey, H. V., Comazet-Loso, T., Douglas, A. E., et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. U.S.A. 110, 3229–3236. doi: 10.1073/pnas.1218525110
McKenzie, V. J., Bowers, R. M., Fierer, N., Knight, R., and Lauber, C. L. (2012). Co-habiting amphibian species harbor unique skin bacterial communities in wild populations. ISME J. 6, 588–596. doi: 10.1038/ismej.2011.129
McMahon, T. A., Brannelly, L. A., Chatfield, M. W. H., Johnson, P. T., Joseph, M. B., and McKenzie, V. J. (2013). Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in crayfish in the absence of infection. Proc. Natl. Acad. Sci. U.S.A. 110, 210–215. doi: 10.1073/pnas.1200592110
McMahon, T. A., Sears, B. F., Venesky, M. D., Bessler, S. M., Brown, J. M., Deutsch, K., et al. (2014). Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511, 224–227. doi: 10.1038/nature13491
Murphy, P. J., St.-Hilaire, S., and Corn, P. S. (2011). Temperature, hydric environment, and prior pathogen exposure alter the experimental severity of chytridiomycosis in boreal toads. Dis. Aquat. Organ. 95, 31–42. doi: 10.3354/dao02336
Noaman, N. H., Fattah, A., Khaleafa, M., and Zaky, S. H. (2004). Factors affecting antimicrobial activity of Synechococcus leopoliensis. Microbiol. Res. 159, 395–402. doi: 10.1016/j.micres.2004.09.001
Paul, V. J., Lindquist, N., and Fenical, W. (1990). Chemical defenses of the tropical ascidian Atapozoa sp. and its nudibranch predators Nembrotha spp. Mar. Ecol. Prog. Ser. 59, 109–118. doi: 10.3354/meps059109
Pietikainen, J., Pettersson, M., and Baath, E. (2005). Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol. Ecol. 52, 49–58. doi: 10.1016/j.femsec.2004.10.002
Piquet, A. M.-T., Maat, D. S., Confurius-Guns, V., Sintes, E., Herndl, J., van de Poll, W. H., et al. (2016). Springtime dynamics, productivity and activity of prokaryotes in two Arctic fjords. Polar Biol. 39, 1749–1763. doi: 10.1007/s00300-015-1866-x
Rachowicz, L. J., Knapp, R. A., Morgan, J. A. T., Stice, M. J., Vredenburg, V. T., Parker, J. M., et al. (2006). Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology 87, 1671–1683. doi: 10.1890/0012-9658(2006)87[1671:EIDAAP]2.0.CO;2
Ramsey, J. P., Reinert, L. K., Harper, L. K., Woodhams, D. C., and Rollins-Smith, L. A. (2010). Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun. 78, 3981–3992. doi: 10.1128/IAI.00402-10
Retallick, R. W. R., and Miera, V. (2007). Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Dis. Aquat. Organ. 75, 201–207. doi: 10.3354/dao075201
Richards-Zawacki, C. L. (2010). Thermoregulatory behavior affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc. R. Soc. Lond. B Biol. Sci. 277, 519–528. doi: 10.1098/rspb.2009.1656
Rios-Licea, M. M., Bosques, F. J., Arroliga, A. C., Galindo-Galindo, J. O., and Garza-Gonzalez, E. (2010). Quadruplex real-time quantitative PCR assay for the detection of pathogen related to late-onset ventilator-associated pneumonia. A preliminary report. J. Microbiol. Methods 81, 232–234. doi: 10.1016/j.mimet.2010.03.018
Ripa, F. A., Nikkon, F., Zaman, S., and Khondkar, P. (2009). Optimal conditions for antimicrobial metabolites production from a new Streptomyces sp. RUPA-08PR isolated from Bangladeshi soil. Mycobiology 37, 211–214. doi: 10.4489/MYCO.2009.37.3.211
Robak, M. J. (2016). Effects of Temperature on Amphibian Resistance and Susceptibility to Chytridiomycosis. Doctoral dissertation, Tulane University, New Orleans, LA.
Rohr, J. R., and Raffel, T. R. (2010). Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc. Natl. Acad. Sci. U.S.A. 107, 8269–8274. doi: 10.1073/pnas.0912883107
Rollins-Smith, L. A., and Woodhams, D. C. (2012). “Amphibian immunity: staying in tune with the environment,” in Ecoimmunology, eds G. E. Demas and R. J. Nelson (Oxford: Oxford University Press), 92–143.
Rollins-Smith, L. A., Woodhams, D. C., Reinert, L. K., Vredenburg, V. T., Briggs, C. J., Nielsen, P. F., et al. (2006). Antimicrobial peptide defense of the mountain yellow-legged frog (Rana muscosa). Dev. Comp. Immunol. 30, 831–842. doi: 10.1016/j.dci.2005.10.005
Rosenberg, E., and Ben-Halm, Y. (2002). Microbial diseases of corals and global warming. Environ. Microbiol. 6, 318–326. doi: 10.1046/j.1462-2920.2002.00302.x
Rothermel, B. B., Walls, S. C., Mitchell, J. C., Dodd, C. K., Irwin, L. K., Green, D. E., et al. (2008). Widespread occurrence of the amphibian chytrid fungus Batrachochytrium dendrobatidis in the southeastern USA. Dis. Aquat. Organ. 82, 3–18. doi: 10.3354/dao01974
Rowley, J. J. L., and Alford, R. A. (2010). “Factors affecting interspecific variation in susceptibility to disease in amphibians,” in Amphibian Biology. Amphibian Decline: Diseases, Parasites, Maladies and Pollution, Vol. 8, eds H. Heatwole and J. W. Wilkinson (Baulkham Hills, NSW: Surrey Beatty and Sons), 3053–3066.
Rowley, J. J. L., and Alford, R. A. (2013). Hot bodies protect amphibians against chytrid infection in nature. Sci. Rep. 3:1515. doi: 10.1038/srep01515
Roznik, E. A., Sapsford, S. J., Pike, D. A., Schwarzkopf, L., and Alford, R. A. (2015). Natural disturbance reduces disease risk in endangered rainforest frog populations. Sci. Rep. 5:13472. doi: 10.1038/srep13472
Savage, A. E., and Zamudio, K. R. (2011). MHC genotypes associate with resistance to a frog-killing fungus. Proc. Natl. Acad. Sci. U.S.A. 108, 16705–16710. doi: 10.1073/pnas.1106893108
Sherman, E. (2008). Thermal biology of newts (Notophthalmus viridescens) chronically infected with a naturally occurring pathogen. J. Therm. Biol. 33, 27–31. doi: 10.1016/j.jtherbio.2007.09.005
Sherman, E., and Stephens, A. (1998). Fever and metabolic rate in the toad Bufo marinus. J. Therm. Biol. 23, 49–52. doi: 10.1016/S0306-4565(97)00045-4
Sonn, J. S. (2016). The Influence of Temperature on Chytridiomycosis in Northern Cricket Frogs (Acris crepitans). Doctoral dissertation, Tulane University, New Orleans, LA.
Sonn, J. S., Berman, S., and Richards-Zawacki, C. L. (2017). The influence of temperature on chytridiomycosis in vivo. EcoHealth 14, 762–770. doi: 10.1007/s10393-017-1269-2
Tianero, M. D. B., Kwan, J. C., Wyche, T. P., Presson, A. P., Koch, M., Barrows, L. R., et al. (2015). Species specificity of symbiosis and secondary metabolism in ascidians. ISME J. 9, 615–628. doi: 10.1038/ismej.2014.152
Tobler, U., and Schmidt, B. R. (2010). Within- and among-population variation in chytridiomycosis-induced mortality in the toad Alytes obstetricians. PLoS One 5:e10927. doi: 10.1371/journal.pone.0010927
Voyles, J., Young, S., Berger, L., Campbell, C., Voyles, W. F., Dinudom, A., et al. (2009). Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326, 582–585. doi: 10.1126/science.1176765
Vredenburg, V. T., Knapp, R. A., Tunstall, T. S., and Briggs, C. J. (2010). Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl. Acad. Sci. U.S.A. 107, 9688–9694. doi: 10.1073/pnas.0914111107
Webster, N. S., Cobb, R. E., and Negri, A. P. (2008). Temperature thresholds for bacterial symbiosis with a sponge. ISME J. 2, 830–842. doi: 10.1038/ismej.2008.42
Woodhams, D. C., and Alford, R. A. (2005). Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conserv. Biol. 19, 1449–1459. doi: 10.1111/j.1523-1739.2005.004403.x
Woodhams, D. C., Alford, R. A., Antwis, R. E., Archer, H., Becker, M. H., Belden, L. K., et al. (2015). Antifungal isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens. Ecology 96:595. doi: 10.1890/14-1837.1
Woodhams, D. C., Alford, R. A., and Marantelli, G. (2003). Emerging disease of amphibians cured by elevated body temperature. Dis. Aquat. Organ. 55, 65–67. doi: 10.3354/dao055065
Woodhams, D. C., Ardipradja, K., Alford, R. A., Marantelli, G., Reinert, L. K., and Rollins-Smith, L. A. (2007a). Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim. Conserv. 10, 409–417. doi: 10.1111/j.1469-1795.2007.00130.x
Woodhams, D. C., Brandt, H., Baumgartner, S., Kielgast, J., Kupfer, E., Tobler, U., et al. (2014). Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS One 9:e96375. doi: 10.1371/journal.pone.0096375
Woodhams, D. C., Vredenburg, V. T., Simon, M., Billheimer, D., Shakhtour, B., Shyr, Y., et al. (2007b). Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biol. Conserv. 138, 390–398. doi: 10.1016/j.biocon.2007.05.004
Wyngaard, G. A., and Chinnappa, C. C. (1982). “General biology and cytology of Cyclopoids,” in Developmental Biology of Freshwater Invertebrates, eds F. W. Harrison and R. R. Crowden (New York, NY: Liss), 485–533.
Keywords: Acris crepitans, amphibian chytridiomycosis, antifungal, bioaugmentation, host–pathogen interactions, skin microbes, Stenotrophomonas maltophilia
Citation: Robak MJ and Richards-Zawacki CL (2018) Temperature-Dependent Effects of Cutaneous Bacteria on a Frog’s Tolerance of Fungal Infection. Front. Microbiol. 9:410. doi: 10.3389/fmicb.2018.00410
Received: 09 November 2017; Accepted: 21 February 2018;
Published: 07 March 2018.
Edited by:
Reid Harris, James Madison University, United StatesReviewed by:
Ross Andrew Alford, James Cook University, AustraliaAlessandro Catenazzi, Southern Illinois University Carbondale, United States
Copyright © 2018 Robak and Richards-Zawacki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Corinne L. Richards-Zawacki, cori.zawacki@pitt.edu
 Matthew J. Robak
Matthew J. Robak Corinne L. Richards-Zawacki
Corinne L. Richards-Zawacki