- 1College of Plant Protection, Nanjing Agricultural University, Nanjing, China
- 2Key Laboratory of Monitoring and Management of Crop Diseases and Pest Insects, Ministry of Education, Nanjing, China
- 3Chongqing Key Laboratory of Economic Plant Biotechnology, College of Forestry & Life Science, Chongqing University of Arts and Sciences, Yongchuan, China
- 4Nord Reet UG, Greifswald, Germany
- 5Fachgebiet Phytomedizin, Institut für Agrar-und Gartenbauwissenschaften, Humboldt-Universität zu Berlin, Berlin, Germany
- 6College of Horticulture, Nanjing Agricultural University, Nanjing, China
Bacillus amyloliquefaciens FZB42 is a plant growth-promoting rhizobacterium that induces resistance to a broad spectrum of pathogens. This study analyzed the mechanism by which FZB42 restricts leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana. The oomycete foliar pathogen P. nicotianae is able to reopen stomata which had been closed by the plant innate immune response to initiate penetration and infection. Here, we showed that root colonization by B. amyloliquefaciens FZB42 restricted pathogen-mediated stomatal reopening in N. benthamiana. Abscisic acid (ABA) and salicylic acid (SA)-regulated pathways mediated FZB42-induced stomatal closure after pathogen infection. Moreover, the defense-related genes PR-1a, LOX, and ERF1, involved in the SA and jasmonic acid (JA)/ethylene (ET) signaling pathways, respectively, were overexpressed, and levels of the hormones SA, JA, and ET increased in the leaves of B. amyloliquefaciens FZB42-treated wild type plants. Disruption of one of these three pathways in N. benthamiana plants increased susceptibility to the pathogen. These suggest that SA- and JA/ET-dependent signaling pathways were important in plant defenses against the pathogen. Our data thus explain a biocontrol mechanism of soil rhizobacteria in a plant.
Introduction
Plants have evolved a variety of inducible defense mechanisms to protect themselves against pathogen attack. Well-studied, classic examples of induced resistance are: (1) the activation of systemic acquired resistance (SAR), triggered by infection with necrosis-inducing pathogens (Van Wees et al., 2008); and (2) rhizobacteria-induced systemic resistance (ISR), triggered by some non-pathogenic rhizobacteria (Van Loon et al., 1998; Hammerschmidt, 1999) such as plant growth-promoting rhizobacteria (PGPR) (Conrath et al., 2002).
The plant hormones jasmonic acid (JA), salicylic acid (SA), and ethylene (ET) are important signaling molecules in SAR and ISR (Glazebrook, 2001; Pieterse et al., 2014). SAR involves the SA-dependent signaling pathway. Initiation of SAR involves local and systemic increases in endogenously synthesized SA, which causes activation of the regulatory protein NPR1 and then NPR1-dependent expression of genes that encode pathogenesis-related (PR) proteins including PR-1a (Ward et al., 1991; Niu et al., 2011). Transgenic A. thaliana NahG plants expressing the bacterial nahG gene, which encodes the SA-degrading enzyme salicylate hydroxylase, are compromised in PR gene expression and SAR (Gaffney et al., 1993). In contrast, ISR requires the JA- and ET-pathways, and is associated with expression of defensin 1.2 (Pieterse et al., 1998; Van Oosten et al., 2008). However, dependence on both SA- and JA/ET-signaling pathways is also observed. For example, colonization of Arabidopsis roots by Trichoderma atroviride IMI 206040 trigger ISR by inducing the expression of SA and JA/ET pathways simultaneously to confer resistance against hemibiotrophic and necrotrophic phytopathogens (Salas-Marina et al., 2011).
Plant growth-promoting rhizobacteria-mediated ISR, induced by Bacillus spp. for example, has been demonstrated in many plant species including melon, bean, tomato, tobacco, and the model plant A. thaliana (Van Loon et al., 1998). García-Gutiérrez et al. (2013) demonstrated that B. subtilis UMAF6639 confers protection to melon plants against cucurbit powdery mildew by activation of JA- and SA-dependent defense responses. B. cereus AR156 has been shown to trigger ISR in A. thaliana by simultaneously activating the SA and JA/ET signaling pathways against Pseudomonas syringae pv. tomato DC3000 (Niu et al., 2011). In A. thaliana, volatile compounds acetoin and 2,3-butanediol produced by B. subtilis GB03 elicited ISR dependent on the ET signaling pathway (Ryu et al., 2004; Yi et al., 2016).
Entry of a pathogen into host tissue is a critical early step in infection. For foliar plant pathogens, natural surface openings, such as stomata, are important entry sites (Melotto et al., 2008). Melotto et al. (2006) and Kumar et al. (2012) have shown that the foliar bacterial pathogen P. syringae pv. tomato DC3000 can actively enter plant tissues through stomata, and that root colonization by the rhizobacterial species B. subtilis FB17 triggered the abscisic acid (ABA) and SA signaling pathways to restrict the stomatal-mediated pathogen entry of DC3000 in A. thaliana.
Phytophthora nicotianae, an oomycete, is a soil-borne, hemibiotrophic pathogen that infects 72 genera of (predominately solanaceous) plants (Hickman, 1958). In tobacco, it can cause black shank disease, symptoms of which include leaf wilting, root rot, stem blackening, and death (Scharte et al., 2005). Several studies have demonstrated that the hyphae of Phytophthora can enter the plant leaf via the stomata to initiate disease progression, and the fraction of open stomata strongly decreased after the early stage of P. nicotianae infection but reopened again at later stages of the infection that are associated with pathogen entry (Scharte et al., 2005; Hardham, 2007; Zhang et al., 2012).
Bacillus amyloliquefaciens FZB42 is a Gram-positive bacterium and a model for the study of plant-microbe interactions. This species is used commercially as a biofertilizer and a biocontrol agent (Chen et al., 2007). Treatment of plants with FZB42 enhances expression of defense genes involved in SA and ET pathways, and it reduces bottom rot caused by Rhizoctonia solani in lettuce (Chowdhury et al., 2015). The aims of the present work were: (1) to assess FZB42-induced protection against leaf disease in N. benthamiana caused by P. nicotianae; (2) to investigate FZB42-mediated stomatal closure and to explore the genetic mechanism; and (3) to identify the signaling pathways involved in plant defenses against P. nicotianae.
Materials and Methods
Plants and Microorganisms
Nicotiana benthamiana seeds were surface sterilized for 5 min in 95% (v/v) ethanol, then for a further 5 min in 5% (w/v) NaClO. Seeds were washed thrice with sterile distilled water, then spread evenly on solid Murashige and Skoog medium (Murashige and Skoog, 1962) to germinate. Seedlings were transplanted to pots containing sterile vermiculite and grown for about 6 weeks in a greenhouse (light intensity 200 μE m-2 s-1, 50–60% relative humidity, 25°C) with a 16/8 h light/dark cycle. The bacterial strain B. amyloliquefaciens FZB42 was deposited as strain 10A6 in the culture collection of the Bacillus Genetic Stock Center (BGSC; The Ohio State University, Columbus, OH, United States). P. nicotianae was cultured at 24°C for 2 days on clarified V8-agar medium (von Broembsen and Deacon, 1996).
Phytophthora nicotianae Infection Assays
A hyphal plug of P. nicotianae (7 mm × 7 mm) was fixed on the surface of N. benthamiana leaves (Teng et al., 2014), and the samples were kept in the greenhouse conditions described above. Symptoms of disease were recorded after 48 h. The leaves were then placed in 100% ethanol. The rate of resistance was calculated after measurement of the diameter of P. nicotianae lesions: inhibition rate = [(diameter of control lesions-diameter of treatment lesions)/diameter of control lesions] × 100%.
Bacillus amyloliquefaciens FZB42 Root Inoculation
The rhizobacterial strain B. amyloliquefaciens FZB42 was maintained on Luria-Bertani medium plates. A single colony from a freshly streaked plate was used to grow overnight cultures that were adjusted to a final density of OD600 = 0.5 (106 colony forming units/mL). Root inoculation of FZB42 was performed by pipetting 5 ml of the bacterial suspension onto the roots of 6-week-old N. benthamiana plants. Controls were root-inoculated with sterile distilled water. To assess the effect of FZB42 and P. nicotianae on stomata, N. benthamiana plants were co-inoculated concurrently by FZB42 on the roots and P. nicotianae on the leaves.
Stomatal Aperture Measurements
Stomatal apertures in leaves of N. benthamiana plants inoculated with P. nicotianae, B. amyloliquefaciens FZB42, or co-inoculated with both were measured as described by Kumar et al. (2012). Images of stomatal apertures on epidermal strips were recorded (Olympus BX43 microscope [Tokyo, Japan] and cellSens Standard Software). At least 50 randomly selected stomatal apertures were measured in each treatment, and each assay was repeated three times.
Plant Hormone Content Determination
To determine ABA content (Fotopoulos et al., 2008), 1 g of freeze-dried, homogenized leaf tissue was extracted in 80% (v/v) methanol and stirred overnight at 4°C. After centrifuging twice at 4000 ×g for 20 min, the extracts were completely evaporated under vacuum and dissolved in water at pH 3.0. The solutions were partitioned with diethyl ether three times and then passed through anhydrous sodium sulfate. After evaporation of the apolar phase, the dry residue was dissolved in Tris-buffered saline (150 mM NaCl, 1 mM MgCl2, 50 mM Tris–HCl, pH 7.8), and the ABA was detected immunologically using a Phytodetek Kit (Agdia, Elkhart, IN, United States).
Free and SA-conjugated phytohormones were extracted from flash-frozen leaf tissue (1 g) and quantified according to Schuhegger et al. (2006). SA was detected using a Shimadzu RF 535 fluorescence detector at excitation and emission wavelengths of 305 and 407 nm, respectively.
For JA determination, leaves were flash-frozen in liquid nitrogen and tissue (1 g) was processed as described by Mueller and Brodschelm (1994). JA was quantified by gas chromatography-mass spectrometry (GC-MS; SSQ quadrupole instrument; Finnigan, United States) in negative ion chemical ionization mode with isobutane as the reactant gas. Dihydro JA-pentafluorobenzyl (PFB; m/z = 211) and [molecular anions-PFB]-ions of JA-PFB (m/z = 209) were monitored. JA levels were calculated from the GC peak areas of the selected ions.
The concentration of ET was determined from flash-frozen leaf tissue (1 g) according to the method of De Laat and Van Loon (1983).
RNA Isolation, RT-PCR, and qRT-PCR
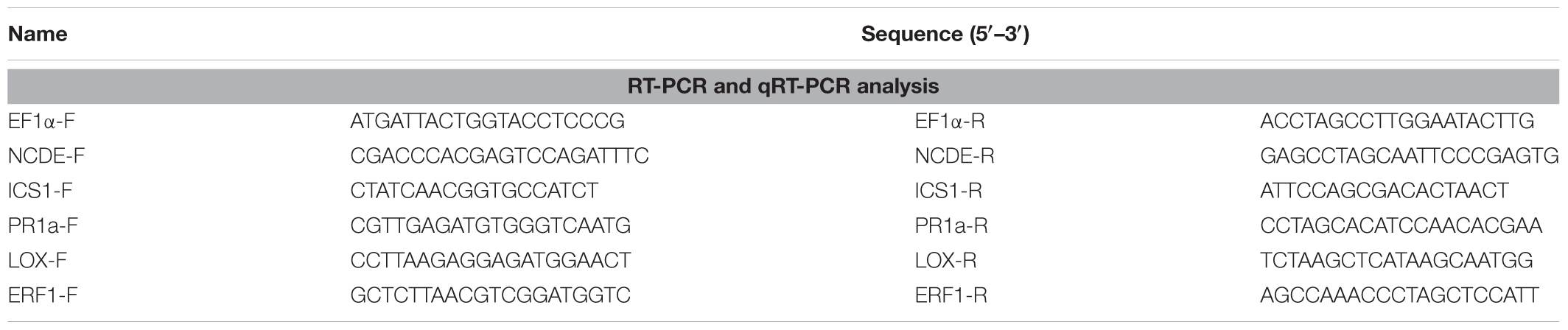
Total RNA was extracted from leaves of N. benthamiana according to the method of Wu et al. (2017) using a Plant RNA Kit (Omega Bio-Tek, United States). First-strand cDNA was synthesized using reverse transcriptase (TaKaRa Bio Inc., Dalian, China) and oligo(dT) primers. Reverse transcription PCR (RT-PCR) products were examined by agarose gel electrophoresis. Quantitative RT-PCR (qRT-PCR) was performed using SYBR Premix Ex Taq (TaKaRa) and an ABI 7500 Fast Real-Time PCR Detection System (Applied Biosystems, United States). PCR reactions were heated to 95°C for 3 min, followed by 30 cycles of 95°C for 30 s, 60°C for 30 s, and 60°C for 30 s. Gene expression in each sample was normalized to expression of N. benthamiana EF-1α, and relative expression levels calculated by the 2-ΔΔCT method (Livak and Schmittgen, 2001). Gene-specific PCR primers shown in Table 1 are same for RT-PCR and qRT-PCR analysis.
Statistical Analysis
At least five replicates were performed for each experiment. Data were evaluated using one-way analysis of variance and Fisher’s least significant difference tests in SPSS software v. 16.0 (Chicago, IL, United States).
Results
B. amyloliquefaciens FZB42 Treatment Induced Resistance to P. nicotianae Infection in N. benthamiana Plants
Bacillus amyloliquefaciens FZB42 was tested for its capacity to trigger resistance to the oomycete pathogen P. nicotianae. N. benthamiana roots were inoculated with a suspension of B. amyloliquefaciens FZB42 cells. Leaves were inoculated with P. nicotianae. Disease symptoms were assessed 48 h after P. nicotianae infection by comparing the sizes of the lesions. Plants inoculated with P. nicotianae showed typical symptoms of Phytophthora infection; the leaves were water-soaked at 48 h post-inoculation. However, inoculation of roots with B. amyloliquefaciens FZB42 resulted in a significant reduction (P < 0.01, n ≥ 5) in the lesion size compared with control plants treated with P. nicotianae alone; the inhibition rate of FZB42 in controlling the leaf disease caused by P. nicotianae was 60.09% (Figure 1), which suggests that pretreatment with FZB42 can provide enhanced resistance to P. nicotianae.
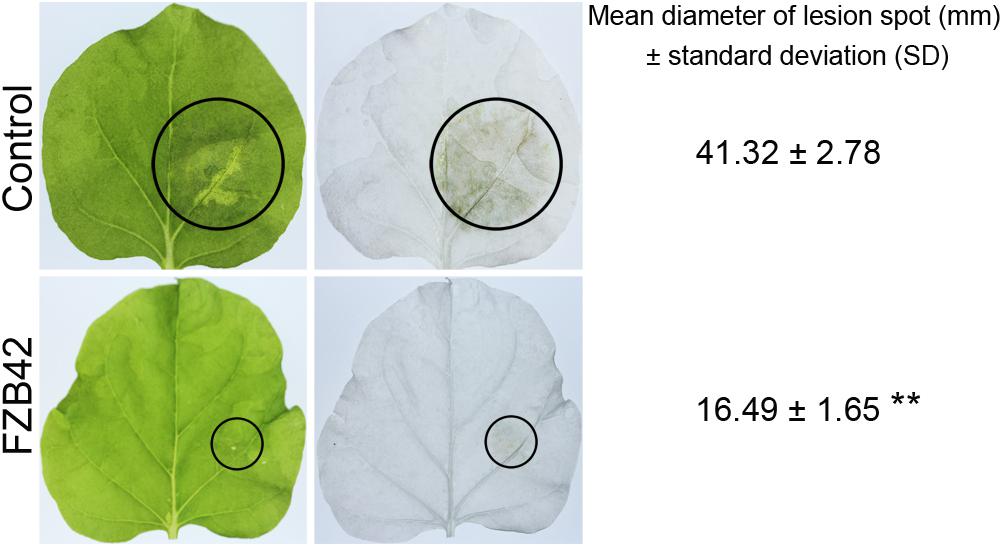
FIGURE 1. Root-associated Bacillus amyloliquefaciens FZB42 induces resistance against the foliar pathogen Phytophthora nicotianae in Nicotiana benthamiana. Photographs of the lesions were taken 48 h post-inoculation, and the lesion diameters (black circles) were measured. Both the original and ethanol-bleached images are shown. ∗∗Highly significant difference compared with the control (P < 0.01, n ≥ 5).
FZB42 Treatment Caused Stomatal Closure in N. benthamiana
Previous studies have shown that root-associated B. subtilis restricts the stomate-mediated entry of the foliar pathogen P. syringae pv. tomato DC3000 in A. thaliana (Kumar et al., 2012). To investigate whether stomatal closure contributes to FZB42-mediated defense, roots of N. benthamiana were inoculated with FZB42 and stomatal apertures were subsequently measured by microscopic evaluation of freshly prepared epidermal peels. We observed that root inoculation with B. amyloliquefaciens FZB42 resulted in a decrease in the mean size of the stomatal aperture 3 and 9 h post-inoculation, while stomata in the control leaves remained open over the entire investigation period (Figures 2A,B). P. nicotianae triggered stomatal closure 3 h after inoculation, and that the stomata reopened by 9 h post-inoculation (Figures 2A,B). Because both FZB42 and P. nicotianae can influence the stomata, stomatal apertures were evaluated after co-inoculating N. benthamiana plants with FZB42 on the roots and P. nicotianae on the leaves. At 3 h post-inoculation, FZB42 and P. nicotianae had no significant difference on reducing the stomatal apertures compared with either FZB42 or P. nicotianae inoculation alone. Surprisingly, at 9 h post co-inoculation, B. amyloliquefaciens FZB42 prevented the reopening of stomata by disrupting the effect of P. nicotianae infection (Figures 2A,B).
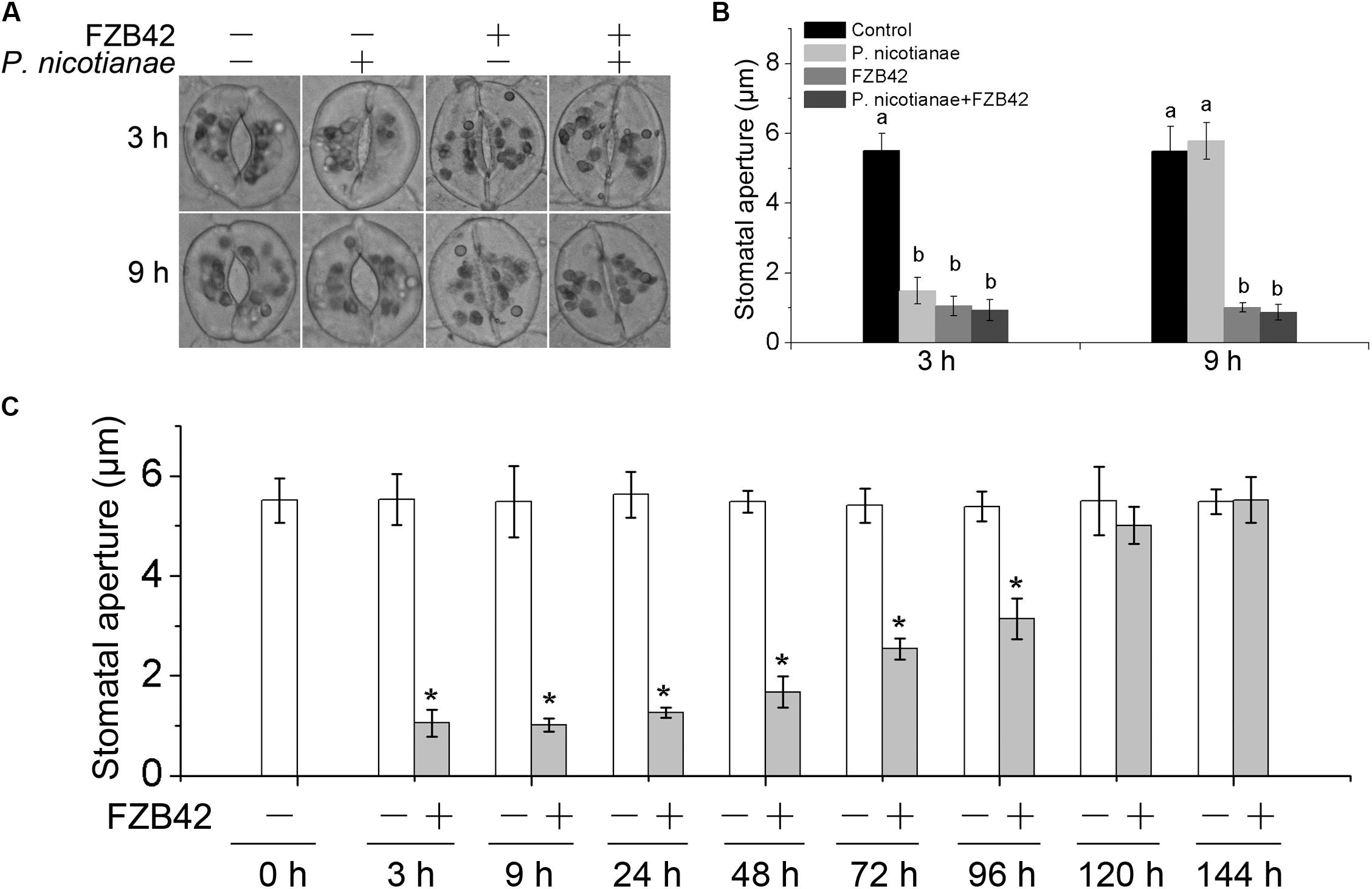
FIGURE 2. Root inoculation with B. amyloliquefaciens FZB42 causes stomatal closure in N. benthamiana. (A) Micrographs of stomata in leaves of N. benthamiana inoculated singly with P. nicotianae, B. amyloliquefaciens FZB42, or both (co-inoculated). (B) Stomatal aperture sizes in leaves of N. benthamiana inoculated with P. nicotianae, FZB42, or co-inoculated, at 3 and 9 h post-inoculation. Different letters indicate that the means are statistically significantly different at P < 0.05. (C) Stomatal aperture sizes in N. benthamiana after FZB42 root inoculation measured over a time period of 144 h. Data represent means ± standard deviation (SD) in B,C. ∗Significant difference compared with the control (P < 0.05; n = 50).
Since FZB42 treatment can specifically keep stomata closed, we root-inoculated N. benthamiana plants with FZB42 and examined the sizes of stomatal apertures over a 144-h time course. Interestingly, we found that closure of stomata induced by inoculation of roots with B. amyloliquefaciens FZB42 was only a transient response by the plant. The stomatal aperture sizes decreased to ∼1 μm by 3 h after inoculation with strain FZB42, but then there was a gradual increase in the stomatal aperture sizes starting around 24 h (Figure 2C). At 120 h post-inoculation, the sizes of the stomatal apertures were similar to those observed in the control. These data indicated that FZB42-mediated stomatal closure in N. benthamiana was transient.
ABA and SA Are Required for FZB42-Mediated Stomatal Closure
Abscisic acid plays a major role in closure of stomata in response to water stress and pathogen challenge (Acharya and Assmann, 2009; Sawinski et al., 2013; Su et al., 2017). Therefore, to investigate the involvement of ABA in B. amyloliquefaciens FZB42-mediated closure of stomata, we measured the stomatal aperture sizes in N. benthamiana abi1 plants that have a dominant negative mutation in a phosphatase, which impairs ABA transduction during stomatal regulation (Leyman et al., 2000). In the abi1 mutants, FZB42-mediated closure of stomata was disrupted 3, 9, and 24 h after the addition of B. amyloliquefaciens (Figure 3A); thus, ABA is required for FZB42-induced stomatal closure. Simultaneously, we measured the mRNA levels of the ABA biosynthetic gene nced1, which encodes a 9-cis-epoxycarotenoid dioxygenase that catalyzes the key step in ABA biosynthesis (Tan et al., 1997), and the ABA contents in the leaves of wild type plants at 3, 9, and 24 h after root inoculation with FZB42. There was a significant increase in ABA content (Figure 3B), and an increase in transcription of the nced1 gene (Supplementary Figure S1) compared to non-inoculated control. Our data also showed a marked reduction in ABA content in N. benthamiana infected by P. nicotianae, while the ABA content remained high during co-inoculation with P. nicotianae and FZB42 (Figure 3B).
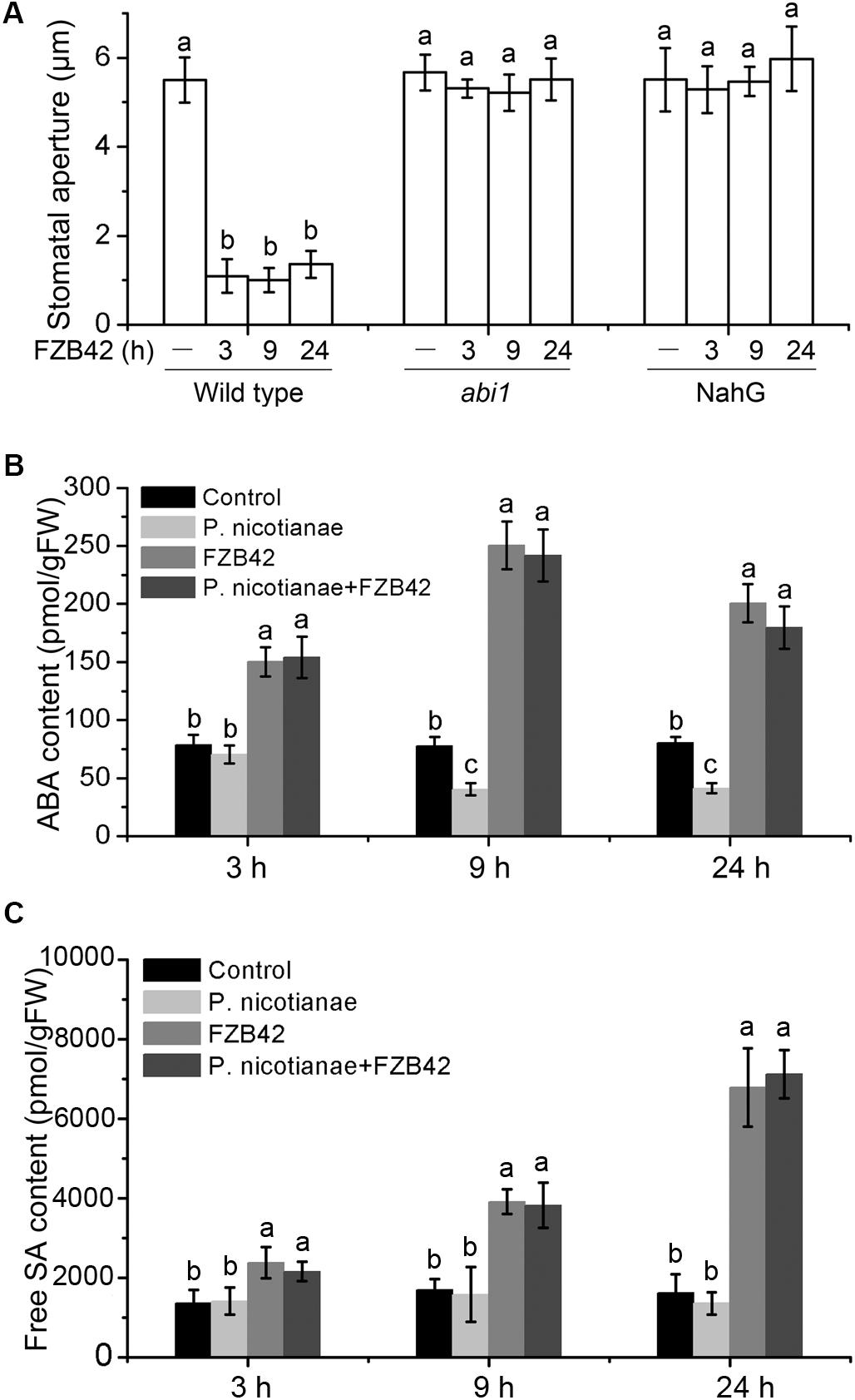
FIGURE 3. B. amyloliquefaciens FZB42 mediates the closure of stomata through abscisic acid (ABA) and salicylic acid (SA) pathways. (A) Stomatal aperture sizes in wild-type N. benthamiana, and transgenic abi1 and NahG plants after inoculation of roots with B. amyloliquefaciens FZB42. (B) Total ABA content in N. benthamiana leaves inoculated with P. nicotianae, FZB42, or co-inoculated, 3, 9, and 24 h post-inoculation. (C) Content of free SA in N. benthamiana leaves after inoculation with P. nicotianae, FZB42, or both, 3, 9, and 24 h post-inoculation. Data are means ± SD. Different letters indicate significant differences (P < 0.05; n ≥ 5). FW, fresh weight.
Salicylic acid signaling acts upstream of ABA signaling in B. subtilis-triggered stomatal closure in A. thaliana (Zeng and He, 2010; Kumar et al., 2012). Therefore, we determined if SA is also involved in FZB42-mediated closure of stomata in N. benthamiana. To achieve this, after root inoculation with FZB42 we measured the stomatal apertures in transgenic plants expressing the bacterial nahG gene; these plants do not accumulate SA. NahG plants showed disrupted FZB42-mediated stomatal closure 3, 9, and 24 h after root inoculation (Figure 3A). We also measured the levels of free and conjugated SA in wild type leaves, and the transcriptional levels of the ICS1 gene (which is involved in biosynthesis of SA) (Zhu et al., 2014). The SA content was significantly elevated (Figure 3C and Supplementary Figure S2), and ICS1 expression was upregulated after FZB42 treatment compared to control or P. nicotianae treatment (Supplementary Figure S3). Collectively, these findings indicate that SA and ABA are both involved in FZB42-mediated stomatal closure in response to P. nicotianae.
The SA-and JA/ET-Dependent Signaling Pathways Are Important in Plant Defenses Against P. nicotianae
To investigate the potential signal transduction pathways involved in FZB42-mediated resistance, we analyzed the expression of the SA-responsive gene PR-1a, the JA synthesis-related gene LOX, and the ET-responsive gene ERF1 in the leaves of wild type N. benthamiana plants in response to FZB42 treatment alone, to P. nicotianae inoculation alone, and to FZB42 treatment combined with P. nicotianae inoculation. At 24 h following treatment with FZB42, expression of the PR-1a, LOX and ERF1 genes was evident in wild type plants (Figure 4A). We observed similar gene expression patterns in N. benthamiana plants treated with B. amyloliquefaciens FZB42 then challenged with P. nicotianae. In plants inoculated with P. nicotianae alone, expression of the three marker genes was either very low or not detectable (Figure 4A). Results from transgenic NahG, JA signaling-related gene COI1-silenced and ET signaling-related gene EIN2-silenced N. benthamiana plants (Shibata et al., 2010) also have stated this (Figure 4A and Supplementary Figure S4). qRT-RCR analysis also showed that transcription of the PR-1a, LOX, and ERF1 genes was significantly upregulated after treatment with FZB42 and P. nicotianae (Supplementary Figure S5). Meanwhile, after treatment with B. amyloliquefaciens FZB42, the free and conjugated SA contents were elevated between four- and sixfold (Figure 3C and Supplementary Figure S2), and the JA and ET contents increased five and three-fold, respectively (Figure 4B). On the other hand, reduced resistance of transgenic NahG, COI1- and EIN2-silenced plants was scored by visible development of disease symptoms and inhibition rate (Supplementary Table S1). These data show that SA-and JA/ET- dependent signaling pathways were important in plant defenses against P. nicotianae.
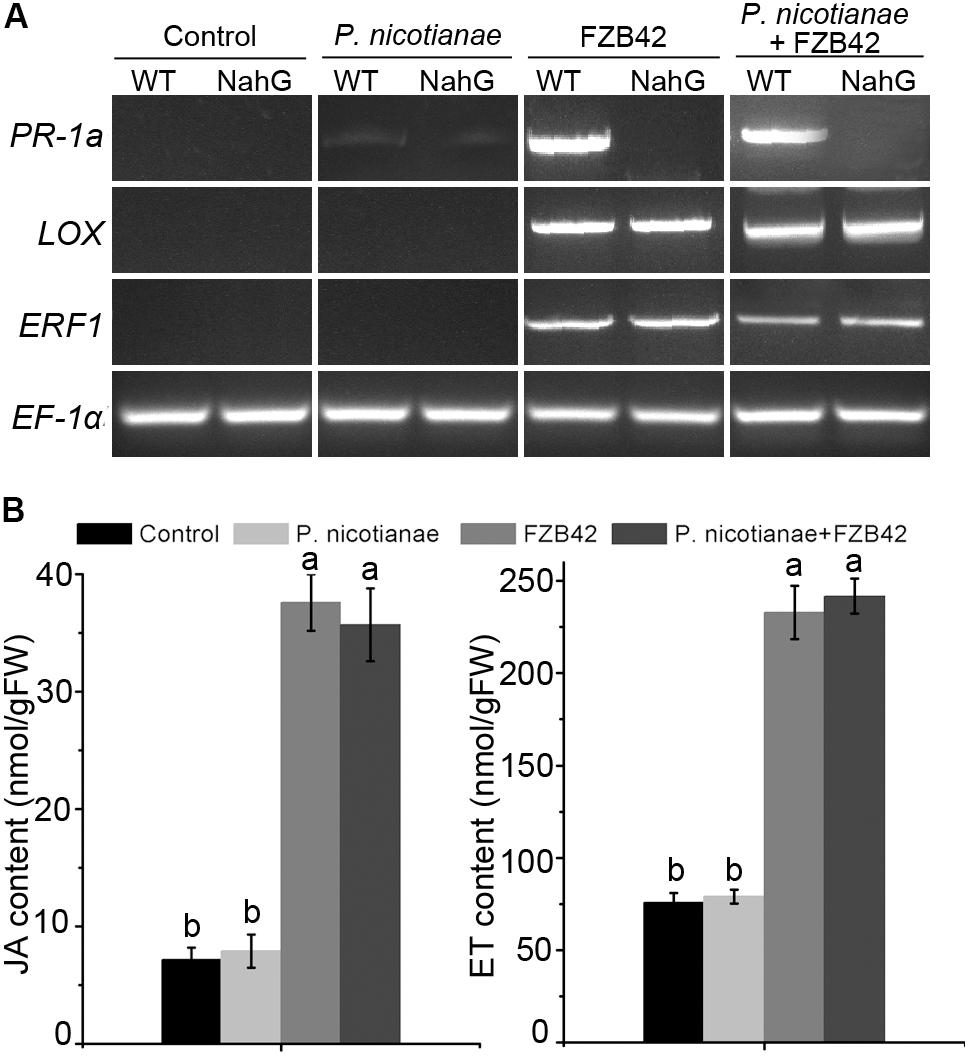
FIGURE 4. The SA-and jasmonic acid (JA)/ethylene (ET)-dependent signaling pathways are important in plant defenses against P. nicotianae in N. benthamiana. (A) Expression of the PR-1a, LOX, and ERF1 genes in N. benthamiana wild-type and NahG plants after inoculation with P. nicotianae, FZB42, or co-inoculation with both. (B) Levels of JA and ET in N. benthamiana leaves after inoculation of roots with B. amyloliquefaciens FZB42. Different letters indicate significant differences at P < 0.05. FW, fresh weight.
Discussion
A product based on B. amyloliquefaciens FZB42 is commercially available (Chen et al., 2007), and although it is used widely as a biocontrol agent, the complicated mechanisms underlying its actions remain to be elucidated. In this study, we characterized the biocontrol mechanism by investigating the FZB42-mediated resistance to P. nicotianae in N. benthamiana at the phenotypic, cellular, and molecular levels.
Applying FZB42 to the roots of N. benthamiana plants reduced the severity of the disease caused by P. nicotianae and inhibited proliferation of the pathogen in the leaves, even though B. amyloliquefaciens FZB42 only colonizes the roots. Chowdhury et al. (2015) showed that FZB42 treatment can enhance the defense response in lettuce against the fungal pathogen R. solani.
Stomata sense plant pathogens and close in their presence (Melotto et al., 2006). P. nicotianae infects leaves via stomatal entry, and the reopening of stomata is associated with an increased pathogen concentration in infected plants (Scharte et al., 2005; Zhang et al., 2012). Here, root colonization by B. amyloliquefaciens FZB42 restricted pathogen-mediated stomatal reopening in N. benthamiana. Meanwhile, FZB42-mediated stomatal closure was transient.
Abscisic acid and SA play a critical role in closure of stomata (Acharya and Assmann, 2009). Our findings also suggest that both ABA and SA are required for the FZB42-mediated closure of stomata. Similarly, Kumar et al. (2012) showed that in A. thaliana, root-inoculation of B. subtilis FB17 invokes ABA and SA signaling pathways that close light-adapted stomata. Previous studies have demonstrated that ABA/SA-stimulated reactive oxygen species (ROS) production mediated by NADPH oxidases and a peroxidase-catalyzed reaction, respectively, may lead to elevation of cytosolic Ca2+, thereby inducing stomatal closure (Pei et al., 2000; Khokon et al., 2011). Here, we showed using the specific fluorescent dye dihydrorhodamine 123 that a ROS burst was observed in N. benthamiana after inoculation of roots with FZB42 (Supplementary Figure S6).
As well as stomatal defense, hormonal signaling pathways were also important in plant defenses against the pathogen. Expression of the marker genes PR-1a, LOX, and ERF1 involved in the SA, JA/ET signaling pathways was up-regulated in N. benthamiana wild-type plants. The levels of the plant hormones SA, JA, and ET also increased. Simultaneously, transgenic NahG, COI1- and EIN2-silenced plants showed a significant reduction in resistance against P. nicotianae. Hence, our data demonstrated that SA and JA/ET signaling pathways play a crucial role in plant defenses against P. nicotianae, which agree with results of Niu et al. (2011) and Chowdhury et al. (2015).
Conclusion
Stomatal closure and SA, JA/ET signaling pathways are essential for the rhizobacterial species B. amyloliquefaciens FZB42 to protect the plant from infection by the foliar pathogen P. nicotianae (Figure 5). These results provide a deeper understanding of the efficiency of biocontrol agents that affect the entry of a pathogen into its host, although future research has to confirm whether Bacillus has similar effect on necrotrophic pathogens.
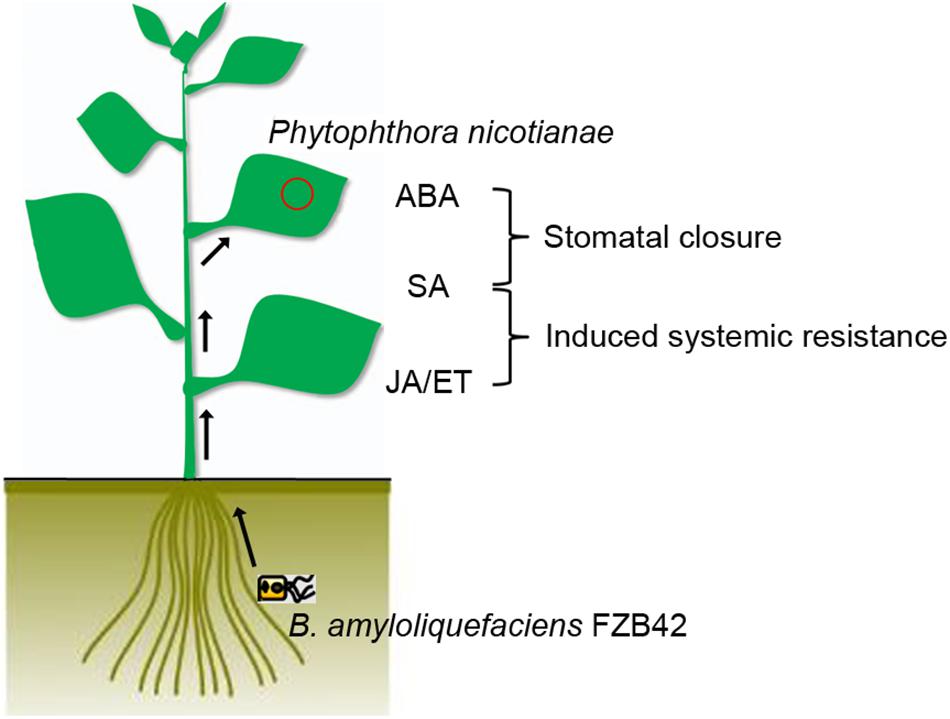
FIGURE 5. Model of the signal transduction cascade for stomatal defense and hormonal-regulated pathways after FZB42 root inoculation in N. benthamiana. After root inoculation with B. amyloliquefaciens FZB42, FZB42 mediates guard cell closure through ABA and SA. Meanwhile, concurrent expression of a large set of the SA- and JA/ET-responsive genes in the leaves was activated, suggesting the SA-, JA-, and ET-dependent signaling pathways were also important in plant defenses against P. nicotianae.
Author Contributions
LW, HW, ZW, and XG conceived and designed the experiments. LW and ZH performed of the most experiments. XL, LM, and QG performed the quantitative real time-PCR. RB supplied B. amyloliquefaciens strains. LW and JL analyzed data and wrote the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China [grant number 2017YFD0200400], the National Natural Science Foundation of China [grant number 31701833], the Natural Science Foundation of Jiangsu Province [grant number BK20170712] and the China Postdoctoral Science Foundation [grant number 2016M601834].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00847/full#supplementary-material
References
Acharya, B. R., and Assmann, S. M. (2009). Hormone interactions in stomatal function. Plant Mol. Biol. 69, 451–462. doi: 10.1007/s11103-008-9427-0
Chen, X. H., Koumoutsi, A., Scholz, R., Eisenreich, A., Schneider, K., Heinemeyer, I., et al. (2007). Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 25, 1007–1014. doi: 10.1038/nbt1325
Chowdhury, S. P., Uhl, J., Grosch, R., Alquéres, S., Pittroff, S., Dietel, K., et al. (2015). Cyclic lipopeptides of Bacillus amyloliquefaciens subsp. plantarum colonizing the lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Mol. Plant Microbe Interact. 28, 984–995. doi: 10.1094/MPMI-03-15-0066-R
Conrath, U., Pieterse, C. M., and Mauch-Mani, B. (2002). Priming in plant–pathogen interactions. Trends Plant Sci. 7, 210–216. doi: 10.1016/S1360-1385(02)02244-6
De Laat, A. M. M., and Van Loon, L. C. (1983). The relationship between stimulated ethylene production and symptom expression in virus-infected tobacco leaves. Physiol. Plant Pathol. 22, 261–273. doi: 10.1016/S0048-4059(83)81014-5
Fotopoulos, V., De Tullio, M. C., Barnes, J., and Kanellis, A. K. (2008). Altered stomatal dynamics in ascorbate oxidase over-expressing tobacco plants suggest a role for dehydroascorbate signalling. J. Exp. Bot. 59, 729–737. doi: 10.1093/jxb/erm359
Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., et al. (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754–754. doi: 10.1126/science.261.5122.754
García-Gutiérrez, L., Zeriouh, H., Romero, D., Cubero, J., Vicente, A., and Pérez-García, A. (2013). The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate- and salicylic acid- dependent defence responses. Microb. Biotechnol. 6, 264–274. doi: 10.1111/1751-7915.12028
Glazebrook, J. (2001). Genes controlling expression of defense responses in Arabidopsis-2001 status. Curr. Opin. Plant Biol. 4, 301–308. doi: 10.1016/S1369-5266(00)00177-1
Hammerschmidt, R. (1999). Induced disease resistance: how do induced plants stop pathogens? Physiol. Mol. Plant Pathol. 55, 77–84. doi: 10.1006/pmpp.1999.0215
Hardham, A. R. (2007). Cell biology of plant–oomycete interactions. Cell. Microbiol. 9, 31–39. doi: 10.1111/j.1462-5822.2006.00833.x
Khokon, A. R., Okuma, E., Hossain, M. A., Munemasa, S., Uraji, M., Nakamura, Y., et al. (2011). Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 34, 434–443. doi: 10.1111/j.1365-3040.2010.02253.x
Kumar, A. S., Lakshmanan, V., Caplan, J. L., Powell, D., Czymmek, K. J., Levia, D. F., et al. (2012). Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata. Plant J. 72, 694–706. doi: 10.1111/j.1365-313X.2012.05116.x
Leyman, B., Geelen, D., and Blatt, M. R. (2000). Localization and control of expression of Nt-Syr1, a tobacco snare protein. Plant J. 24, 369–382. doi: 10.1046/j.1365-313x.2000.00886.x
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTCt method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Melotto, M., Underwood, W., and He, S. Y. (2008). Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 46, 101–122. doi: 10.1146/annurev.phyto.121107.104959
Melotto, M., Underwood, W., Koczan, J., Nomura, K., and He, S. Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980. doi: 10.1016/j.cell.2006.06.054
Mueller, M. J., and Brodschelm, W. (1994). Quantification of jasmonic acid by capillary gas chromatography-negative chemical ionization-mass spectrometry. Anal. Biochem. 218, 425–435. doi: 10.1006/abio.1994.1202
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Niu, D. D., Liu, H. X., Jiang, C. H., Wang, Y. P., Wang, Q. Y., Jin, H. L., et al. (2011). The plant growth–promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Mol. Plant Microbe Interact. 24, 533–542. doi: 10.1094/MPMI-09-10-0213
Pei, Z. M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. doi: 10.1038/35021067
Pieterse, C. M., Van Wees, S. C., Van Pelt, J. A., Knoester, M., Laan, R., Gerrits, H., et al. (1998). A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10, 1571–1580. doi: 10.1105/tpc.10.9.1571
Pieterse, C. M., Zamioudis, C., Berendsen, R. L., Weller, D. M., Van Wees, S. C., and Bakker, P. A. (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375. doi: 10.1146/annurev-phyto-082712-102340
Ryu, C. M., Farag, M. A., Hu, C. H., Reddy, M. S., Kloepper, J. W., and Paré, P. W. (2004). Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134, 1017–1026. doi: 10.1104/pp.103.026583
Salas-Marina, M. A., Silva-Flores, M. A., Uresti-Rivera, E. E., Castro-Longoria, E., Herrera-Estrella, A., and Casas-Flores, S. (2011). Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 131, 15–26. doi: 10.1007/s10658-011-9782-6
Sawinski, K., Mersmann, S., Robatzek, S., and Böhmer, M. (2013). Guarding the green: pathways to stomatal immunity. Mol. Plant Microbe Interact. 26, 626–632. doi: 10.1094/MPMI-12-12-0288-CR
Scharte, J., Schön, H., and Weis, E. (2005). Photosynthesis and carbohydrate metabolism in tobacco leaves during an incompatible interaction with Phytophthora nicotianae. Plant Cell Environ. 28, 1421–1435. doi: 10.1111/j.1365-3040.2005.01380.x
Schuhegger, R., Ihring, A., Gantner, S., Bahnweg, G., Knappe, C., Vogg, G., et al. (2006). Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 29, 909–918. doi: 10.1111/j.1365-3040.2005.01471.x
Shibata, Y., Kawakita, K., and Takemoto, D. (2010). Age-related resistance of Nicotiana benthamiana against hemibiotrophic pathogen Phytophthora infestans requires both ethylene-and salicylic acid-mediated signaling pathways. Mol. Plant Microbe Interact. 23, 1130–1142. doi: 10.1094/MPMI-23-9-1130
Su, J., Zhang, M., Zhang, L., Sun, T., Liu, Y., Lukowitz, W., et al. (2017). Regulation of stomatal immunity by interdependent functions of a pathogen-responsive MPK3/MPK6 cascade and abscisic Acid. Plant Cell 29, 526–542. doi: 10.1105/tpc.16.00577
Tan, B. C., Schwartz, S. H., Zeevaart, J. A., and McCarty, D. R. (1997). Genetic control of abscisic acid biosynthesis in maize. Proc. Natl. Acad. Sci. U.S.A. 94, 12235–12240. doi: 10.1073/pnas.94.22.12235
Teng, W., Zhang, H., Wang, W., Li, D., Wang, M., Liu, J., et al. (2014). ALY proteins participate in multifaceted Nep1Mo-triggered responses in Nicotiana benthamiana and Arabidopsis thaliana. J. Exp. Bot. 65, 2483–2494. doi: 10.1093/jxb/eru136
Van Loon, L. C., Bakker, P., and Pieterse, C. M. (1998). Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36, 453–483. doi: 10.1146/annurev.phyto.36.1.453
Van Oosten, V. R., Bodenhausen, N., Reymond, P., Van Pelt, J. A., Van Loon, L. C., Dicke, M., et al. (2008). Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis. Mol. Plant Microbe Interact. 21, 919–930. doi: 10.1094/MPMI-21-7-0919
Van Wees, S. C., Van der Ent, S., and Pieterse, C. M. (2008). Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 11, 443–448. doi: 10.1016/j.pbi.2008.05.005
von Broembsen, S. L., and Deacon, J. W. (1996). Effects of calcium on germination and further zoospore release from zoospore cysts of Phytophthora parasitica. Mycol. Res. 100, 1498–1504. doi: 10.1016/S0953-7562(96)80085-2
Ward, E. R., Uknes, S. J., Williams, S. C., Dincher, S. S., Wiederhold, D. L., Alexander, D. C., et al. (1991). Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 3, 1085–1094. doi: 10.1105/tpc.3.10.1085
Wu, L., Wu, H., Chen, L., Zhang, H., Gao, X. (2017). Induction of systemic disease resistance in Nicotiana benthamiana by the cyclodipeptides cyclo (L-Pro-L-Pro) and cyclo (D-Pro-D-Pro). Mol. Plant Pathol. 18, 67–74. doi: 10.1111/mpp.12381
Yi, H. S., Ahn, Y. R., Song, G. C., Ghim, S. Y., Lee, S., Lee, G., et al. (2016). Impact of a bacterial volatile 2, 3-butanediol on Bacillus subtilis rhizosphere robustness. Front. Microbiol. 28:993. doi: 10.3389/fmicb.2016.00993
Zeng, W., and He, S. Y. (2010). A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 153, 1188–1198. doi: 10.1104/pp.110.157016
Zhang, H. J., Li, D. Q., Wang, M. F., Liu, J. W., Teng, W. J., Cheng, B. P., et al. (2012). The Nicotiana benthamiana mitogen-activated protein kinase cascade and WRKY transcription factor participate in Nep1Mo-triggered plant responses. Mol. Plant Microbe Interact. 25, 1639–1653. doi: 10.1094/MPMI-11-11-0293
Keywords: Bacillus amyloliquefaciens FZB42, stomata, ABA, ISR, Nicotiana benthamiana
Citation: Wu L, Huang Z, Li X, Ma L, Gu Q, Wu H, Liu J, Borriss R, Wu Z and Gao X (2018) Stomatal Closure and SA-, JA/ET-Signaling Pathways Are Essential for Bacillus amyloliquefaciens FZB42 to Restrict Leaf Disease Caused by Phytophthora nicotianae in Nicotiana benthamiana. Front. Microbiol. 9:847. doi: 10.3389/fmicb.2018.00847
Received: 05 June 2017; Accepted: 12 April 2018;
Published: 27 April 2018.
Edited by:
Brigitte Mauch-Mani, University of Neuchâtel, SwitzerlandReviewed by:
Pierre Pétriacq, Université de Bordeaux, FranceYoun-Sig Kwak, Gyeongsang National University, South Korea
Copyright © 2018 Wu, Huang, Li, Ma, Gu, Wu, Liu, Borriss, Wu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Wu, wzh@njau.edu.cn Xuewen Gao, gaoxw@njau.edu.cn
 Liming Wu
Liming Wu Ziyang Huang1
Ziyang Huang1 Qin Gu
Qin Gu Huijun Wu
Huijun Wu Jia Liu
Jia Liu Rainer Borriss
Rainer Borriss Xuewen Gao
Xuewen Gao