Mild Cognitive Impairment Is Not “Mild” at All in Altered Activation of Episodic Memory Brain Networks: Evidence from ALE Meta-Analysis
- 1Center on Aging Psychology, Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China
- 2Laboratory for Functional Connectome and Development, Key Laboratory of Behavioral Science, Institute of Psychology, Chinese Academy of Sciences, Beijing, China
- 3University of Chinese Academy of Sciences, Beijing, China
The present study conducted a quantitative meta-analysis aiming at assessing consensus across the functional neuroimaging studies of episodic memory in individuals with amnestic mild cognitive impairment (aMCI) and elucidating consistent activation patterns. An activation likelihood estimation (ALE) was conducted on the functional neuroimaging studies of episodic encoding and retrieval in aMCI individuals published up to March 31, 2015. Analyses covered 24 studies, which yielded 770 distinct foci. Compared to healthy controls, aMCI individuals showed statistically significant consistent activation differences in a widespread episodic memory network, not only in the bilateral medial temporal lobe and prefrontal cortex, but also in the angular gyrus, precunes, posterior cingulate cortex, and even certain more basic structures. The present ALE meta-analysis revealed that the abnormal patterns of widespread episodic memory network indicated that individuals with aMCI may not be completely “mild” in nature.
Introduction
Mild cognitive impairment (MCI) is a state where individuals display certain form of cognitive dysfunction, but still maintain the intact ability to perform basic daily activities. MCI is generally considered as a transitional stage between normal aging and clinical dementia (Petersen, 2004). A meta-analysis reported that the annual conversion rate from MCI to dementia is approximately 5–10% (Mitchell and Shiri Feshki, 2009), which is obviously higher than the incidence rates from normal elderly to dementia (1–2% per year) (Petersen, 2004). According to Petersen (2004), the MCI individuals with memory impairment are described as amnestic MCI (aMCI) and without memory impairment as non-amnestic MCI (naMCI). Furthermore, if memory is the only impaired domain, the aMCI individuals are then classified into the aMCI-single domain; if other domains besides memory—such as language, attention/executive function, or visuospatial skills, etc.—are impaired as well, such aMCI individuals are classified into the aMCI-multiple domain. Approximately 80% of individuals with aMCI progress to Alzheimer's disease (AD) which is the most common form of dementia after a clinical follow-up of 6 years (Petersen, 2004). Thus, aMCI individuals have been receiving increasing attention.
Episodic memory is one of the earliest cognitive functions which are impaired in both early AD (Petersen et al., 1999; Perri et al., 2007) and aMCI (Bäckman et al., 2005). Impairment of episodic memory may precede dementia by as many as 10 years during when a diagnosis of aMCI may be applicable (Dannhauser et al., 2008). Typically, episodic memory is measured by tests that require knowledge of a prior episode, such as free recall, cued recall, or recognition tests (Yonelinas, 2001). Therefore, episodic memory impairment may arise from a deficiency in encoding information and/or retrieving previously stored information. And both encoding and retrieval success are associated with activation in the medial temporal lobe (MTL), prefrontal cortex (PFC), and parietal regions (Diana et al., 2007; Spaniol et al., 2009).
Task-related neuroimaging studies with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have been increasingly conducted to examine episodic memory function in individuals with aMCI, because the future diagnostic system and successful treatment require standardized imaging inclusion criteria and isolating imaging markers which can predict the disease. Unfortunately, it is difficult to achieve consensus in activation patterns within episodic memory studies, although existing findings indicate that individuals with aMCI showed comparable fMRI test-retest reproducibility to those of healthy controls (Clément and Belleville, 2009). For instance, some studies have observed increased activation in the MTL during episodic memory encoding among aMCI individuals relative to normal controls (Dickerson et al., 2004, 2005; Kircher et al., 2007). Other studies, however, found decreased activation in the MTL during similar encoding tasks (Mandzia et al., 2009; Hanseeuw et al., 2011). Regarding the pattern of activity in neocortical areas, there is an absence of consensus in the literature of individuals with aMCI. In some studies, MTL dysfunction went with concomitant increases in PFC activity, parietal or other sites in both encoding (Heun et al., 2007; Clément and Belleville, 2012) and retrieval (Jin et al., 2012). This leads to the possibility that such changes may represent compensatory increases as a result of the MTL dysfunction. However, such findings are not universal. In other studies, individuals with aMCI showed less encoding activation in certain regions of frontal and parietal lobes (Johnson et al., 2006; Machulda et al., 2009 for retrieval). These aforementioned inconsistencies may reflect differences in participant samples, task paradigms, and methodologies across studies, which resulted in inconsistent conclusions regarding consistent brain activation patterns.
The activation likelihood estimation (ALE) is a quantitative meta-analytic procedure that has been frequently used to examine the stereotactic brain coordinates most consistently active across studies (Schwindt and Black, 2009; Browndyke et al., 2013). With an ALE analysis (Browndyke et al., 2013) evaluated inconsistent results in the episodic memory encoding literature assessing individuals with MCI and AD. However, their meta-analysis covered very few studies of individuals with MCI (8 studies as of December 31, 2009). The recently burgeoning literature assessing neural correlates of episodic memory among aMCI individuals has created an interest within this area. Additionally, it is worth noting that despite the large number of neuroimaging studies concerning episodic memory retrieval in aMCI, to our knowledge, there is no meta-analyses addressing consensus activation patterns within this participant group. Accordingly, by using a quantitative ALE meta-analysis, the goal of the present study was to establish consistently robust patterns during both memory encoding and retrieval across several studies assessing episodic memory in MCI individuals. Considering the difference of dysfunction between individuals with aMCI and naMCI, and the seldom studies of individuals with naMCI, only studies with aMCI participants are contained in the present Meta-analysis, including both single domain and multiple domain aMCI.
Methods
Literature Collection and Criteria
An initially broad and thorough literature search was implemented on PubMed, Web of Knowledge, and EBSCO (PsyINFO, PsycARTICLES, PsycCRITIQUES, PsycEXTRA, and PsycTESTS) searchesto collect functional imaging studies assessing individuals with aMCI using the following key words: (functional Magnetic Resonance Imaging OR fMRI OR positron emission tomography OR PET) AND (mild cognitive impairment OR MCI) AND (memory OR recognition OR recall). These searches were confined to articles published between January 1, 1990 (which was early enough for searching the studies on MCI) and March 31, 2015, which yielded 2059 unique research or review articles.
Within these studies, only those that met the following criteria were examined and taken into consideration: (1) performing the diagnosis of aMCI according to Petersen et al. (2001); Petersen (2004); or Winblad et al. (2004) was; (2) reporting PET or fMRI results of episodic encoding and/or retrieval paradigms compared to baseline task(s); (3) describing results of independent groups (MCIs and matched controls) or between-group comparisons based on a whole-brain analysis; and (4) using standard stereotactic coordinates to list peaks of significant activation (Talairach and Tournoux, 1988) or Montreal Neurologic Institute (MNI) space.
According to the criteria mentioned above, all articles were reviewed by two independent raters (Pengyun Wang and Lijuan Huo). After applying the first two inclusion criteria, 2018 unrelated articles were excluded and 41 related articles were left. These articles were then subject to further consideration on the basis of inclusion Criteria three and four. Thirteen studies did not meet the criteria three, for their results based on a priori cortical ROIs, and either did not conduct whole-brain voxel-wise analyses, simply reporting differences in activation only in certain specific areas (Dickerson et al., 2004, 2005; Johnson et al., 2006, 2008; Sandstrom et al., 2006; Mevel et al., 2007; Xu et al., 2007; Chao et al., 2009; Yassa et al., 2010; Miettinen et al., 2011; Putcha et al., 2011; Trivedi et al., 2011; Bakker et al., 2015). Such an analysis may partially emphasize some regions and ignore others. Two studies were ruled out, because their stereotactic results were not reported (Dhanjal et al., 2013; Dhanjal and Wise, 2014). Another two studies were excluded because they did not reveal any differences in activation between individual with aMCI and normal controls (Parra et al., 2013; Nicholas et al., 2014). As emphasized above, only individuals with aMCI (including both aMCI-single and aMCI-multiple domain) were included in the current meta analysis. In the study of Machulda et al. (2009), for example, participants of naMCI were excluded, but the ones of aMCI were included.
A final set of 24 studies (publication dates range from 2006 to 2013) was included in the current analysis, yielding 770 distinct foci for the ALE meta-analysis (Table 1).
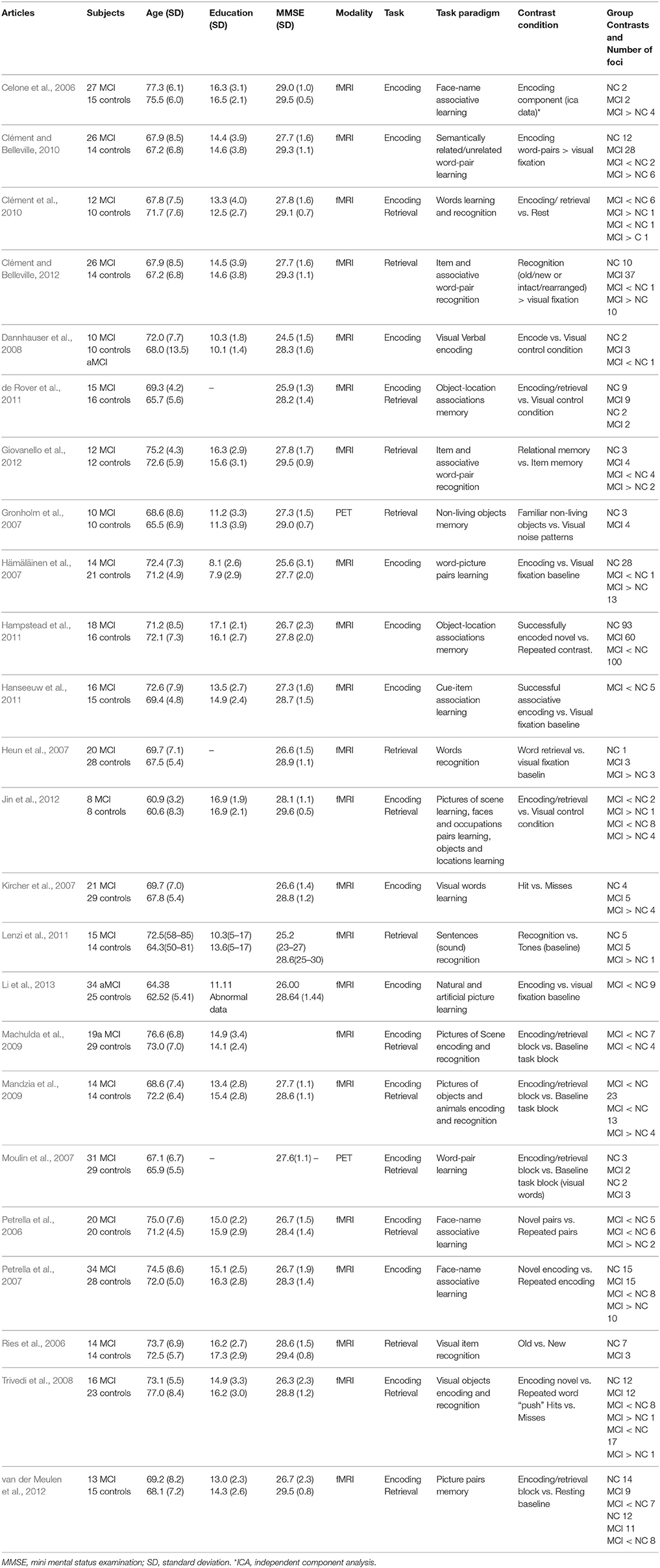
Table 1. Characteristics of studies included in the meta-analysis of fMRI studies of mild cognitive impairment.
ALE Analysis
The software GingerALE 2.3 (Turkeltaub et al., 2002; Eickhoff et al., 2009) was used to conduct these ALE meta-analyses. To allow for direct comparisons of spatial brain coordinates across studies, relevant foci in the included studies were converted from the Talairach and Tournoux (1988) atlas into MNI space, using the Lancaster transform (Lancaster et al., 2007) implemented in the GingerALE software (www.brainmap.org/ale/). The activation foci were then modeled as the center of a 10-mm3 full width-at-half-maximum Gaussian sphere. The ALE statistical test represents the probability that a voxel contains at least one of the activation foci. The GingerALE software compares the resultant ALE maps to the averaged map from 5000 permutations of an identical number of foci placed randomly throughout the brain, controlling the false discovery rate alpha cut-off of 0.05 during the multiple comparisons (Laird et al., 2005). A cluster threshold with a minimum volume of 100 mm3 was applied. ALE analysis clusters were required to have contributing spatial coordinates from a minimum of two independent studies shown in Table 1. The results of these ALE analyses were viewed using the MRIcroN (http://www.nitrc.org/projects/mricron/). The template was “Colin27_T1_seg_MNI” (http://www.brainmap.org/ale/).
Eight separate ALE analyses were performed in two ways. First, analyses were run for individuals with aMCI and healthy controls separately, by computing the activated foci during encoding and retrieval separately. Then, the ALE analyzed the attenuated (healthy controls > individuals with aMCI) and hyperactivated (individuals with aMCI > healthy controls) brain foci during encoding and retrieval separately.
Results
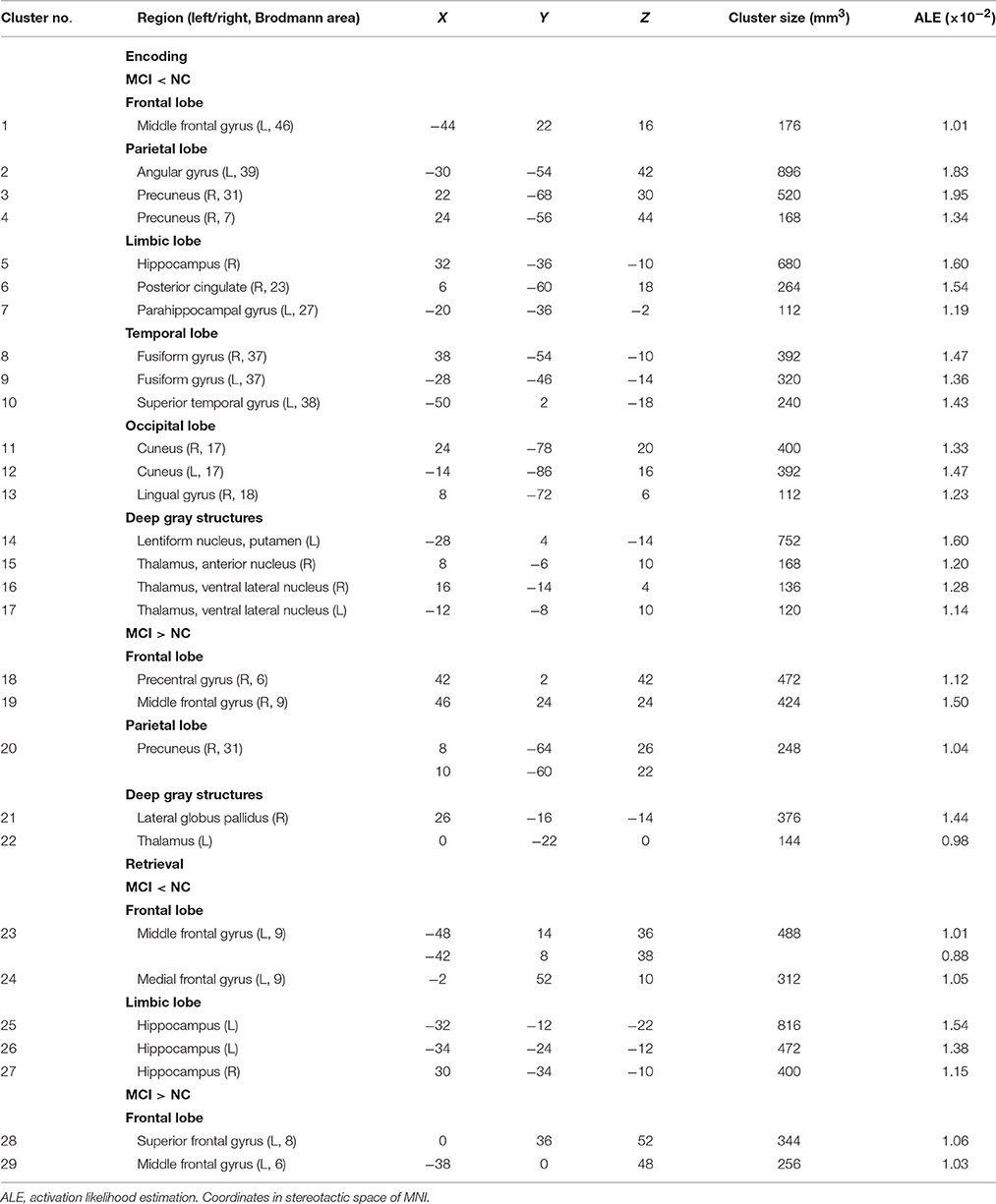
Peak MNI coordinates, Brodmann areas (BA), and cluster sizes of significant ALE regions are summarized in Table 2 (group difference between aMCI individuals and healthy controls during encoding and retrieval respectively) and Supplementary Table 1 (within group activation of individuals with aMCI and healthy controls during encoding and retrieval respectively). The ALE values showed in these two tables are the maximum activation likelihood estimates for individual statistically significant clusters. Thresholded ALE spatial maps for regions of general difference between aMCI individuals and healthy controls were presented in Figure 1 for encoding and Figure 2 for retrieval.
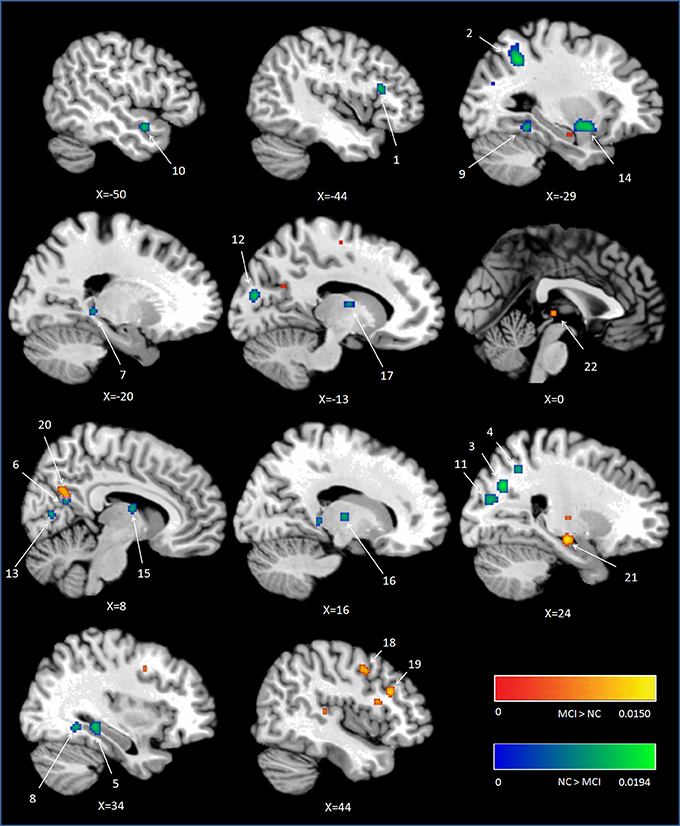
Figure 1. Cluster results of ALE comparison analysis between individuals with aMCI and healthy controls across encoding studies. MCI, mild cognitive impairment; NC, normal control.
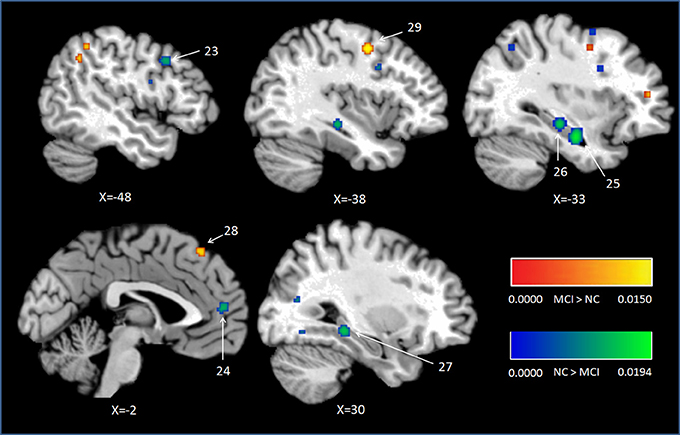
Figure 2. Cluster results of ALE comparison analysis between individuals with aMCI and controls across retrieval studies. MCI, mild cognitive impairment; NC, normal control.
Encoding
NC
Eleven of the twenty-four fMRI episodic memory studies reported activated foci in healthy controls alone. The current analysis included a total of 194 foci and 216 healthy controls. During encoding, NC demonstrated elevated activation likelihood in a range of prefrontal, parietal, limbic, and some other cortical sites. Within the frontal lobe, elevated values were showed in the bilateral DL-PFC (L, BA9; R, BA46), regions of the dorsal lateral surface in the right precentral gyrus (BA6), left medial frontal gyrus (BA6), and left superior frontal gyrus (BA6). Increased activation likelihood in the limbic lobe was observed in the bilateral parahippocampal gyrus (R, L, BA27 and L, BA36). The peaks of these clusters were located in the bilateral entorhinal cortex and the left perirhinal cortex. Increased activation likelihood in the parietal lobe was seen in the bilateral precuneus (BA7 and BA19). Additional areas of high likelihood were found in the left fusiform gyrus (BA37), right medial globus pallidus, left sub-lobar thalamus, left thalamus, left insular cortex (BA13), left amygdala, right lingual gyrus (BA18), right cuneus (BA17), right middle occipital gyrus (BA19), and two clusters in bilateral cerebellum. See Supplementary Table 1.
MCI
Eleven of the twenty-four fMRI episodic memory studies reported activated foci in aMCI individuals alone. The analysis included a total of 177 foci and 225 individuals with aMCI. During encoding, individuals with aMCI showed large areas of prefrontal activation, with multiple clusters in the right DL-PFC (BA9), regions of the dorsal lateral surface in bilateral precentral gyrus (BA6), and left medial frontal gyrus (BA6). Increased activation likelihood in the limbic lobe was observed in the bilateral entorhinal (BA27, 28) and perirhinal cortex (BA36), while no hippocampal peaks were seen. Parietal involvement was limited to the area of the right superior parietal lobule (BA7) and bilateral precuneus (BA7). Additional peaks were found bilaterally in the left sub-lobar thalamus pulvinar, superior temporal gyrus (BA22, 38), left fusiform gyrus (BA37), bilateral lingual gyrus (BA18), bilateral middle occipital gyrus (L, BA18; R, BA19), right cuneus (BA17), and cerebellum. See Supplementary Table 1.
MCI < Controls
Fourteen studies with 184 foci provided information about attenuated brain activation of individuals with aMCI when performing the episodic encoding processing relative to healthy controls. The ALE analysis indicated that individuals with aMCI showed consistently lower activation likelihood in a range of sites in frontal, parietal and limbic lobe, including the left DLPFC (Cluster No. 1 in Tabel 2), left angular gyrus (BA39), right precuneus (BA31/7), right hippocampus, right posterior cingulate (BA23), and left parahippocampal gyrus (BA27). Three temporal peaks referred to bilateral fusiform gyrus (BA37) and left superior temporal gyrus (BA38). Other peaks were observed in the bilateral cuneus (BA17), lingual gyrus (BA18) and a few deep gray structures in the left lentiform nucleus and bilateral thalamus. See Table 2 and Figure 1.
MCI > Controls
Eight studies reported 40 brain foci with higher activation in aMCI individuals in the current ALE analysis. The results indicated that individuals with aMCI also demonstrated greater activation likelihood compared to healthy controls in several regions. Two prefrontal involved the right DLPFC (BA9) and a region of the dorsal surface in the right precentral gyrus (BA6). Other peaks were observed in the right precuneus (BA31), the right lateral globus pallidus, and left thalamus. See Table 2 and Figure 1.
Retrieval
NC
Nine fMRI episodic retrieval studies reported activated foci in healthy controls alone. The analysis contained a total of 45 foci and 152 healthy controls. At retrieval, increased activation likelihood in NC were seen in the medial part of right superior frontal gyrus (BA6), left precuneus (BA7), and left middle temporal gyrus (BA39). Additional peaks were found in the right cuneus (BA18), right extra-nuclear (BA7), and part of left cerebellum. See Supplementary Table 1.
MCI
Nine studies reported activated foci in individuals with aMCI alone. The ALE analysis included a total of 72 foci and 156 individuals with aMCI. At retrieval, increased activation likelihood in individuals with aMCI were oberserved in the medial part of right superior frontal gyrus (BA6) and bilateral precuneus (BA7). Additional peaks were found in the right lingual gyrus (BA18), right claustrum, and part of right cerebellum. See Supplementary Table 1.
MCI < Controls
Nine studies with 63 foci provided information aboutattenuated brain activation of individuals with aMCI when performing the episodic retrieval processing compared to healthy controls. The ALE analysis indicated that individuals with aMCI demonstrated lower activation likelihood relative to controls within left areas of the DLPFC (Cluster No. 23 in Table 2), mPFC (Cluster No. 24 in Table 2), and the bilateral hippocampus. See Table 2 and Figure 2.
MCI > Controls
Nine studies reported 28 brain foci with higher activation in individuals with aMCI in the current ALE analysis. Compared to healthy controls, aMCI individuals demonstrated greater activation likelihood only in two prefrontal areas, the left middle frontal gyrus (BA6) and left superior frontal gyrus (BA8). See Table 2 and Figure 2.
Discussion
For aMCI and NC respectively, the results of the present study demonstrated that both of the two groups showed elevated activation likelihood during encoding, involving DLPFC, dorsal frontal cortex, precuneus, parahippocampal gyrus, fusiform gyrus, lingual gyrus, cuneus and certain more basic structures. During retrieval, the consistent activations in both aMCI and NC were observed in the dorsal frontal cortex, the precuneus, the cuneus and some regions in sub-lobar and cerebellum.
What was mainly concerned in the present meta-analysis, however, was the group differences between aMCI individuals and the healthy controls. Compared to healthy controls, individuals with aMCI showed statistically significant consistent activation differences in a widespread episodic memory network, not only in the bilateral medial temporal lobe and prefrontal cortex, but also in the angular gyrus, precunes, posterior cingulate cortex, and even some more basic structures such as the thalamus, fusiform gyrus, and cuneus.
MTL Structures
The results on episodic encoding in the present meta-analysis were inconsistent with the previous founding by Browndyke et al. (2013). Specifically, in the present study, individuals with aMCI showed reliably lower activation likelihood in the right hippocampus and left parahippocampal gyrus compared to normal controls. These regions belong to MTL, which is crucial to episodic encoding. In line with the findings of meta-analysis in AD patients (Schwindt and Black, 2009), the result of present study indicated that the lower activation in MTL structures during encoding processing led to memory impairment. In contrast, Browndyke et al. (2013) found that aMCI individuals showed higher activation likelihood within a region near the right perirhinal cortex (BA35) during memory encoding. They proposed that this may reflect an increase or overreliance on familiarity-based processing during episodic encoding in MCI, not necessarily being able to benefit successful memory retrieval. The present meta-analysis differed from that of Browndyke et al. (2013) mainly in the literature included in the ALE analysis. In respect of the MTL region, comparing with Browndyke et al.'s research, the present study included more studies which reported lower activation foci during encoding. Specifically, in the present study, five studies (Celone et al., 2006; Hämäläinen et al., 2007; Kircher et al., 2007; Trivedi et al., 2008; Clément and Belleville, 2010) reported seven elevated activation foci in individuals with aMCI in the MTL structure during encoding. Browndyke et al. (2013), however, included four of them except one (Clément and Belleville, 2010). With respect to the lower activation foci in aMCI in the MTL structure, six studies (Trivedi et al., 2008; Mandzia et al., 2009; Hampstead et al., 2011; Hanseeuw et al., 2011; Jin et al., 2012; van der Meulen et al., 2012) were analyzed in the present study providing nine foci. Browndyke et al. (2013), however, included only three studies (Johnson et al., 2006, 2008; Trivedi et al., 2008) with five foci. The first two were excluded in the present study because of their ROI analysis method. Moreover, these two studies did not lead to any difference in the MTL region between the present study and the meta-analysis by Browndyke et al. (2013), because neither of them found any areas where individuals with MCI had elevated activation during encoding. In short, far more lower activation foci, which were found in the recently years, were contained in the present meta-analysis. Therefore, the different results may be due to the insufficient number of studies examined in the previous meta-analysis (Browndyke et al., 2013). The results of the present study are relatively more reliable.
Consistent deficits in activation within MTL structures were observed among individuals with aMCI during retrieval. The peaks of the clusters were located in bilateral hippocampus as showed in Figure 2, the clusters with lower activation likelihood involved large regions in bilateral hippocampus of aMCI individuals. It is well established that hippocampus play a critical role in episodic memory retrieval (Diana et al., 2007; Spaniol et al., 2009). The dysfunction of hippocampus in individuals with aMCI impaired the access of information which has been stored previously.
In short, areas in MTL structures showed lower activation among individuals with aMCI compared to healthy controls during both encoding and retrieval processing. It is reported that the earliest brain changes in MCI, as measured by volume loss, occur in the hippocampus and entorhinal cortex of the MTL (Masdeu et al., 2005). Considering the crucial function of MTL in episodic memory (Eichenbaum et al., 2007), these findings suggest that volumetric and functional reductions in MTL have a significant impact on the episodic encoding and retrieval impairments observed in MCI.
Frontal Regions
The results of current meta-analysis indicated that individuals with aMCI showed different patterns in encoding and retrieval phases in frontal regions. In encoding phase, healthy controls demonstrated greater activation likelihood in the left DLPFC (BA46) relative to individuals with aMCI. In the meanwhile, individuals with aMCI showed elevated likelihood in the right dorsal frontal cortex (BA 6) and the right DLPFC (BA 9). In retrieval phase, lower activation likelihood in the left DLPFC and mPFC, but greater likelihood in the left dorsal frontal cortex (BA 6 and 8) were found in individuals with aMCI compared to controls.
In the present ALE analysis, the greater activity of right DLPFC during encoding in the aMCI individuals is possible to be a compensation for their lower activity of left DLPFC. Converging evidence indicates that the DLPFC specifically contributes to successful memory formation through its role in building relation among items (Dolan and Fletcher, 1997; Murray and Ranganath, 2007; Blumenfeld et al., 2011). In the present meta-analysis, the two foci contributing to the cluster of left DLPFC (lower likelihood in individuals with aMCI) during encoding were reported by two papers (Petrella et al., 2006; Dannhauser et al., 2008), while the contributors to the cluster in right DLPFC (greater likelihood in individuals with aMCI) by another study (Clément and Belleville, 2010). In the last study, the participants with aMCI were divided into two groups of different levels of cognitive impairment (aMCI higher-cognition and aMCI lower-cognition) according to a split-median of their scores on the Mattis Dementia Rating Scale (MDRS), which is an abbreviated neuropsychological scale that covers a wide range of cognitive functions. The aMCI higher-cognition participants showed more activity in these two areas in right DLPFC relative to healthy controls. As argued by the authors, the aMCI higher-cognition participants achieved comparable performances of healthy controls. This suggested that their additional right DLPFC activations reflected compensatory mechanisms. In contrast, the aMCI lower-cognition participants in this study exhibited lower activity in right DLPFC, and they did not exhibited additional activations in right or left DLPFC when comparing to the controls. In short, the result of the present ALE analysis argues that the greater activity of right DLPFC in the aMCI individuals is possible to be a compensation for their lower activity of left DLPFC and the MTL region during encoding episodic information, especially for the aMCI with higher-cognition. This explanation is in line with the degeneration models, which propose that there is a trade-off between the accumulation of lesions and the ability for the neural system to exhibit compensation (Friston and Price, 2003; Cabeza and Dennis, 2012). Furthermore, this change of activity showed in the dominant (left) DLPFC and compensatory (right) DLPFC was also in agreement with the phenomenon of increased bilaterality in frontal areas, which is clearly established in normal aging [see review of Craik and Rose, 2012 and the ‘scaffolding theory of aging and cognition’ (STAC) Park and Reuter-Lorenz, 2009].
The role of DLPFC has also been demonstrated to provide top–down input to the medial temporal lobe in support of retrieval (Tomita et al., 1999), such as monitoring the outcome of retrieval attempts (Fletcher et al., 1998; Fletcher and Henson, 2001). Recently some research suggested that intentional retrieval was associated with increased activation in DLPFC (Kompus et al., 2011). Moreover, attempts to stop memory retrieval are also associated with greater activation of lateral prefrontal cortex than attempts to retrieve memories (for review, see Anderson and Huddleston, 2012). A DLPFC-cingulate-parietal-hippocampal network has been demonstrated to exhibit strongly correlated activity during retrieval suppression. Individuals who were able to suppress memory retrieval exhibited tighter coupling between the key nodes in this network than individuals who were not (Paz-Alonso et al., 2013). In the present study, the lower activation likelihood in left DLPFC during memory retrieval indicated the deficit of aMCI individuals in top–down memory retrieval control which is related to their insufficient performance of episodic memory.
However, we also observed additional activations both during encoding and retrieval in the dorsal frontal cortex that was usually not reported as being involved in verbal episodic tasks. Those regions are localized in premotor cortex and supplementary motor cortex regions [i.e., the right precentral gyrus (BA6), superior frontal gyrus (BA8) and middle frontal gyrus (L, 6)]. These new activations may represent the recruitment of additional compensatory networks for the disrupted function in DLPFC. The additional activations in the superior frontal gyrus (BA8) are more possible to agree with this hypothesis. It has been found that insufficient engagement of the superior frontal gyrus (BA8) may allow goal-irrelevant information access to working memory and to be encoded into long-term memory (Minamoto et al., 2012). Considering the dysfunctional inhibition of distracting information in AD (Baddeley et al., 2001; Amieva et al., 2004), individuals with aMCI may need more effort to regulate this form of attentional control. Alternatively, as suggested by Jin et al., the elevated activation in precentral gyrus and superior motor area in individuals with aMCI may be caused by the active control state which is not memory relevant (Jin et al., 2012). However, considering the memory task procedures used in the included studies of current meta-analysis, participants performed almost the equal active control effort in both memory processing and baseline task. Therefore, the differences between active control efforts had been counteracted by the baseline contrasting. The compensatory hypothesis may explain results of the present meta-analysis more reasonably.
Individuals with aMCI also showed lower activation likelihood in left mPFC during retrieval, which is a key region in memory network, especially in memory retrieval and consolidation (Preston and Eichenbaum, 2013). Complementary studies support the idea that the mPFC acquires representations of behavioral contexts to control memory retrieval. The interactions between the mPFC and hippocampus may support the ability to create contextual representations, and use these representations to retrieve the memories that conform to a given context (for review, see Preston and Eichenbaum, 2013). The deactivation of mPFC in aMCI individuals can be one of the most critical reasons which lead to their retrieval deficits.
In short, the current study exhibited aMCI's deficits in the left DLPFC during both encoding and retrieval, revealing greater activation in the right DLPFC and right dorsal frontal cortex during encoding, and the left dorsal frontal cortex during retrieval. These findings are different from the pattern found in AD patients (Schwindt and Black, 2009), which presented greater activity in the DL-PFC and VL-PFC but less activity in the anterior PFC regions and dorsal frontal cortex during both encoding and retrieval. Given that the increased frontal cortex activity during episodic memory is considered as compensation for the MTL dysfunction (Grady et al., 2005), these results suggest that individuals with aMCI are quite likely touse a distinctive compensatory network in frontal cortex during episodic memory relative to AD patients.
Parietal Region
As part of the medial posterior parietal cortex, the precuneus, especially the dorsal subregions of precuneus, has been acknowledged playing a central role in a wide spectrum of highly integrated tasks, including visuo-spatial imagery, episodic memory retrieval (Lundstrom et al., 2003, 2005; Dörfel et al., 2009) and self-processing operations (see Cavanna and Trimble, 2006; Cavanna, 2007 for review). Although the contribution of precuneus to successful encoding has received relatively little attention, it was still found that precuneus is involved in allocentric encoding of spatial locations (Frings et al., 2006). Furthermore, the ventral subregion of precuneus showed greater activity during resting as compared to responding to an external task (Fransson and Marrelec, 2008), and is wildly accepted as part of the default mode network (Zhang and Li, 2012). Some studies argued that the ventral subregion of precuneus (next to the posterior cingulate cortex) deactivated during successful encoding processes (Daselaar et al., 2004; Vannini et al., 2011). A recent study found that this deactivation reversed to higher activation in preclinical stage of AD compared to controls when performing a visual encoding memory task. In addition, there was a tendency negative correlation between the activations of this region and the task performance (Rami et al., 2012). In the present study, lower activations in the dorsal precuneus (region 3 and 4 in Figure 1) were observed but elevated activations in the ventral precuneus (region 20 in Figure 1) in aMCI individuals compared to controls during encoding. According to the findings of previous studies mentioned above, this aberrant activation pattern can be detrimental to individuals with aMCI and be responsible for their episodic memory dysfunction.
The angular gyrus has been reported consistent activations in a variety of tasks (see Seghier, 2013 for review), particularly during successful episodic memory retrieval (e.g., Vilberg and Rugg, 2008; Spaniol et al., 2009). As reviewed by Rugg and Vilberg (2013), evidence from resting state connectivity and DTI tractography (Uddin et al., 2010; Sestieri et al., 2011), especially the findings that the performance of recollection-based recognition, is associated with enhanced connectivity between the angular gyrus and hippocampus (McCormick et al., 2010), indicating that the angular gyrus may play an important role in the memory network despite many different theories (e.g., bottom-up attentional re-orienting, episodic buffer, episodic convergence zone). In the present study, however, individuals with aMCI demonstrated lower activation likelihood in this region not during retrieval but the encoding stage. As a part of the default mood network, some studies reported that the deactivation of angular gyrus during encoding is beneficial for the memory performance (Daselaar et al., 2009; Uncapher and Wagner, 2009). Nevertheless, there are also opposite findings suggesting that the left angular gyrus activity is greater during successful vs. unsuccessful episodic encoding (Maillet and Rajah, 2014). Elman and colleagues demonstrated the dynamic changes in angular gyrus during encoding. The angular gyrus activity decreased when the stimulus initially presented and increased during an elaborative representational encoding process (Elman et al., 2013). The lower activation likelihood of left angular gyrus during episodic encoding in aMCI individual in the present study indicated the deficit of this representational process which results in their memory impairment. Alternatively, this lower activation likelihood during encoding may be a compensative inhibition because of its role of default mood network. Due to the ALE analysis technique, the present study cannot fully prove which explanaiton is more reasonable. From the tasks perspective (intentional encoding) used in the studies which provided the foci (i.e., Machulda et al., 2009; Hampstead et al., 2011), the first hypothesis is more plausible.
Other Regions
Relative to healthy controls, individual with aMCI demonstrated lower activation likelihood in the anterior portion of left superior temporal gyrus (BA 38) during encoding. The lateral temporal lobes are not the key structures for episodic encoding and retrieval processes, but these regions are reported to be important for semantic knowledge representation. Particularly, the portions in anterior temporal lobes (BA 38) have been suggested as “hubs” which converge the distributed attributes to a common set of semantic representations, regardless of the task (see Patterson et al., 2007 for a review). Recent studies showed the interaction between the episodic memory and semantic memory network during lexicalization (similar with a lexical episodic memory task) with the superior temporal gyrus involved in the novel words memory (Takashima et al., 2014). For AD patients, the superior temporal gyrus is among one of the first areas affected by the disease (Ding et al., 2009), and it had been found that the activity in this portion during memory encoding predicted better performance on measures of cognitive status across AD patients (Diamond et al., 2007). Thus, the deactivation of superior temporal gyrus in aMCI individuals in the present study may reflect impairment of semantic knowledge processing during episodic encoding. This is in line with studies demonstrating that this region is related to the semantic deficit in MCI participants (Vandenbulcke et al., 2007; Clark et al., 2014).
In encoding conditions, portions of the posterior cingulate cortex (PCC) showed less likelihood of activity among aMCI individuals than controls in the present study. As a part of the memory retrieval network, the PCC involves in elaborative retrieval and evaluation of self-referential information (Shannon and Buckner, 2004; Wheeler and Buckner, 2004; Rugg and Vilberg, 2013). Several studies have observed the dysfunctional lower activity in PCC in MCI during episodic retrieval (Johnson et al., 2006; Ries et al., 2006); the deficit of PCC during encoding in MCI, however, was rarely reported. An ALE meta-analysis reported that the PCC was significantly less activated during encoding in early AD patients than controls (Schwindt and Black, 2009). As a transitional stage, individuals with MCI have volumetric and metabolic decline in PCC (Nestor et al., 2003), and the level of metabolism and regional blood flow in PCC were able to predict the conversion to AD (Chételat et al., 2003). Therefore, the less activation likelihood among aMCI individuals in PCC during encoding may reflect the dysfunction in the representational encoding process and result in their following elaborative retrieval.
Individuals with aMCI also showed lower activation likelihood in the left fusiform gyrus, bilateial cuneus, left putamen, and right thalamus, as well as elevated activation likelihood in the right lateral globus pallidus and a portion of left thalamus during encoding. These results were similar as the situation in AD patients (Schwindt and Black, 2009). These regions are usually not reported as key nodes involving in either episodic encoding or retrieval mode. However, a successful episodic encoding is subserved by more widespread cortical regions, not only the key notes (MTL, PFC, areas of posterior parietal), but also the more fundamental structures such as the thalamus, fusiform gyrus, and cuneus (Patterson et al., 2007; Akanuma et al., 2009). These differences may prove more basic task-specific processing or the pathological dysfunction observed in individuals with aMCI.
A Widespread Episodic Memory Network Impairment
As reviewed by Shimamura (2014), PFC as an executive-control system, selects and updates information of sensory, conceptual, and emotional features that constitute an episodic memory. Then, the MTL binds the features as an encapsulated memory to make each item of episodic memory information distinct or separable from others. For retrieval, neuroimaging studies have shown a general network, including the MTL structure, retrosplenial/posterior cingulate, ventral posterior parietal cortex (vPPC), and mPFC (Rugg and Vilberg, 2013). Retrieval typically starts within PFC which facilitates the search through memory and activates pertinent event features. The MTL functions by activating event features through relational bindings (Shimamura, 2014). Because of their connections with the hippocampus and parahippocampal crotex, the PCC and mPFC may play a role in the processing of contextual information (Kveraga et al., 2011; Aggleton, 2012). The ventral posterior parietal cortex centered on the angular gyrus is also a part of the retrieval network due to its interconnection with the MTL and posterior cingulate cortex (Uddin et al., 2010; Sestieri et al., 2011), although its exact role has not been confirmed (see review Rugg and Vilberg, 2013; Shimamura, 2014).
The present study indicated a broad damaged network of episodic memory in aMCI individuals, which involves all the core structures in encoding, retrieval, and some more basic brain structures. Although the additional activations both during encoding and retrieval in the dorsal frontal cortex could refer to a form of compensation mechanism, this is still not a normal situation in contrast to healthy older adults. The original hypothesis which attributed the memory impairment in aMCI specifically to degeneration of the MTL structure (Petersen et al., 2001) is now viewed as incomplete: functional brain imaging revealed hypometabolism not only in the bilateral MTL, but also in the PFC, angular gyrus, precunes, PCC, and several more basic structures such as the thalamus, fusiform gyrus, and cuneus, which apparently constitute a complicated network that is crucial for the formation and representation of new memories.
In addition, as indicated by several studies, the connectivity between the key nodes of the mnemonic network (such as PFC, MTL, posterior parietal cortex) is very important to memory process (Ranganath et al., 2005). This connectivity even plays a significant role in compensating for reduced regional activity during successful memory processing in aging. For instance, Oh and Jagust (2013) reported that cognitively normal older adults without β-amyloid deposition (a prominent feature of Alzheimer's disease associated with neural alterations and episodic memory decline) showed a reduced regional brain activation with increased task-related connectivity (compared with young adults) between parahippocampal gyrus and prefrontal cortex, and the degree of connectivity was related to memory performance. However, cognitively normal older adults with β-amyloid deposition showed no such increased task-related network connectivity. Due to the limitations of the ALE technique, the present study is unfortunately only able to describe the differences of brain activation between aMCI individuals and healthy controls. Recent studies have proved that the functional connectivity within this mnemonic network is declined in individuals with aMCI during resting state, which is associated with their memory impairment (Li et al., 2013; Dunn et al., 2014). The future research probably requires the investigation of the functional connectivity characters within this mnemonic network in individuals with aMCI during episodic encoding and retrieval processing.
Limitations
Firstly, due to the specificity of our research objective and technique, we were forced to ignore a number of factors that varied across the included papers. The limitations of the present meta-analysis were largely related to the variety of task paradigms related to episodic memory across the included studies (as shown in Table 1), such as the stimuli, baseline contrast, paradigm design (i.e., block vs. event-related), or statistical method (i.e., univariate vs. multivariate). As mentioned in the similar ALE meta-analysis (Schwindt and Black, 2009; Browndyke et al., 2013), we were unable to control the potential confound of effort and task difficulty between groups across studies due to the technique.
Secondly, participant characteristics such as age, gender, and disease severity were other uncontrollable factors. Especially the subtypes and severity of the cognitive impairment in aMCI individuals across the papers were important issues and could have a significant impact on the pattern of activation. Although, only the studies with aMCI participants were included in the present study, it was hardly impossible to control the single and multiple dysfunctions or the severity of impairment in some cognitive functions. The heterogeneous nature of the individuals with aMCI leads us to be cautious with our interpretations, for the activation in various brain regions may be a time of dynamic change between increases and decreases with the cognitive impairment progress in individuals with aMCI (Celone et al., 2006; Clément and Belleville, 2010). As reviewed by Gainotti et al. (2014), the spread of the neurofibrillary tangles from the subcortical noradrenergic structures to the perirhinal/entorhinal cortices and to the hippocampus may be the substrate of the sequence of semantic and episodic memory disorders spanning from the early subclinical to the aMCI stage, and other cognitive defects and AD become apparent when it spreads to the neocortical associative areas. Unfortunately, due to the limited literatures, it was impossible to divide the aMCI individuals into several subtypes according to the severity of their cognitive impairment.
Thirdly, a meta-analysis study revealed that individuals with MCI affected structurally in the (trans-) entorhinal and hippocampal regions (Schroeter et al., 2009), however, majority of the studies included in the presents analysis did not account for brain atrophy in interpreting activation differences, except several such as (Lenzi et al., 2011).
Conclusion
Despite a number of challenges inherent in functional imaging of the individuals with aMCI, the present ALE meta-analysis encouragingly reveals that certain findings are consistent across the episodic memory literature and laboratories. Individuals with aMCI definitively demonstrated an abnormal pattern in a widespread episodic memory network, not only in the bilateral MTL, but also in the PFC, angular gyrus, precunes, PCC, and even some more basic structures such as the thalamus, fusiform gyrus, and cuneus. The results of current ALE meta-analysis further support that the abnormal condition in the functional brain network of aMCI individuals may not be “mild” at all, but even more severe in nature.
Author Contributions
PW coded, analyzed and interpreted data, drafted the manuscript. JL conceived the idea, designed the study, and participated in writing up and revising the manuscript. LH, HL, and RL assisted coding and data analysis. All authors reviewed the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31271108, 30911120494, 31070916, 31671157, and 31400895); the National Science and Technology Pillar Program of China (2009BAI77B03); the Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX2-EW-J-8), CAS/SAFEA International Partnership Program for Creative Research Team (Y2CX131003), the Institute of psychology, Chinese Academy of Sciences (111000C038, Y3CX151005), the Pioneer Initiative of the Chinese Academy of Sciences, Feature Institutes Program, TSS-2015-06, and CAS Key Laboratory of Mental Health, Institute of Psychology (KLMH2014ZK02, KLMH2014ZG10).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fnagi.2016.00260/full#supplementary-material
References
Aggleton, J. P. (2012). Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci. Biobehav. Rev. 36, 1579–1596. doi: 10.1016/j.neubiorev.2011.09.005
Akanuma, N., Reed, L. J., Marsden, P. K., Jarosz, J., Adachi, N., Hallett, W. A., et al. (2009). Hemisphere-specific episodic memory networks in the human brain: a correlation study between intracarotid amobarbital test and [18f] fdg-pet. J. Cogn. Neurosci. 21, 605–622. doi: 10.1162/jocn.2009.21035
Amieva, H. L. N., Phillips, L. H., Della Sala, S., and Henry, J. D. (2004). Inhibitory functioning in Alzheimer's disease. Brain 127, 949–964. doi: 10.1093/brain/awh045
Anderson, M. C., and Huddleston, E. (2012). “Towards a cognitive and neurobiological model of motivated forgetting,” in True and False Recovered Memories, ed R. Belli (New York, NY: Springer Press), 53–120.
Bäckman, L., Jones, S., Berger, A.-K., Laukka, E. J., and Small, B. J. (2005). Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology 19, 520. doi: 10.1037/0894-4105.19.4.520
Baddeley, A. D., Baddeley, H. A., Bucks, R. S., and Wilcock, G. K. (2001). Attentional control in Alzheimer's disease. Brain 124, 1492–1508. doi: 10.1093/brain/124.8.1492
Bakker, A., Albert, M. S., Krauss, G., Speck, C. L., and Gallagher, M. (2015). Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 7, 688–698. doi: 10.1016/j.nicl.2015.02.009
Blumenfeld, R. S., Parks, C. M., Yonelinas, A. P., and Ranganath, C. (2011). Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. J. Cogn. Neurosci. 23, 257–265. doi: 10.1162/jocn.2010.21459
Browndyke, J. N., Giovanello, K., Petrella, J., Hayden, K., Chiba-Falek, O., Tucker, K. A., et al. (2013). Phenotypic regional functional imaging patterns during memory encoding in mild cognitive impairment and Alzheimer's disease. Alzheimers. Dement. 9, 284–294. doi: 10.1016/j.jalz.2011.12.006
Cabeza, R., and Dennis, N. A. (2012). “Frontal lobes and aging: deterioration and compensation,” in Principles Frontal Lobe Function, eds D. Stuss and R. Knight (New York, NY: Oxford University Press), 628–652.
Cavanna, A. E. (2007). The precuneus and consciousness. CNS Spectr. 12, 545–552. doi: 10.1017/S1092852900021295
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583. doi: 10.1093/brain/awl004
Celone, K. A., Calhoun, V. D., Dickerson, B. C., Atri, A., Chua, E. F., Miller, S. L., et al. (2006). Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J. Neurosci. 26, 10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006
Chao, L. L., Pa, J., Duarte, A., Schuff, N., Weiner, M. W., Kramer, J. H., et al. (2009). Patterns of cerebral hypoperfusion in amnestic and dysexecutive MCI. Alzheimer Dis. Assoc. Disord. 23, 245–252. doi: 10.1097/WAD.0b013e318199ff46
Chételat, G., Desgranges, B., de la Sayette, V., Viader, F., Eustache, F., and Baron, J.-C. (2003). Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology 60, 1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6
Clark, D. G., Wadley, V. G., Kapur, P., Deramus, T. P., Singletary, B., Nicholas, A. P., et al. (2014). Lexical factors and cerebral regions influencing verbal fluency performance in MCI. Neuropsychologia 54, 98–111. doi: 10.1016/j.neuropsychologia.2013.12.010
Clément, F., and Belleville, S. (2009). Test-retest reliability of fMRI verbal episodic memory paradigms in healthy older adults and in persons with mild cognitive impairment. Hum. Brain Mapp. 30, 4033–4047. doi: 10.1002/hbm.20827
Clément, F., and Belleville, S. (2010). Compensation and disease severity on the memory-related activations in mild cognitive impairment. Biol. Psychiatry 68, 894–902. doi: 10.1016/j.biopsych.2010.02.004
Clément, F., and Belleville, S. (2012). Effect of disease severity on neural compensation of item and associative recognition in mild cognitive impairment. J. Alzheimers. Dis. 29, 109–123. doi: 10.3233/JAD-2012-110426
Clément, F., Belleville, S., and Mellah, S. (2010). Functional neuroanatomy of the encoding and retrieval processes of verbal episodic memory in MCI. Cortex 46, 1005–1015. doi: 10.1016/j.cortex.2009.07.003
Craik, F. I., and Rose, N. S. (2012). Memory encoding and aging: a neurocognitive perspective. Neurosci. Biobehav. Rev. 36, 1729–1739. doi: 10.1016/j.neubiorev.2011.11.007
Dannhauser, T. M., Shergill, S. S., Stevens, T., Lee, L., Seal, M., Walker, R. W., et al. (2008). An fMRI study of verbal episodic memory encoding in amnestic mild cognitive impairment. Cortex 44, 869–880. doi: 10.1016/j.cortex.2007.04.005
Daselaar, S. M., Prince, S. E., and Cabeza, R. (2004). When less means more: deactivations during encoding that predict subsequent memory. Neuroimage 23, 921–927. doi: 10.1016/j.neuroimage.2004.07.031
Daselaar, S. M., Prince, S. E., Dennis, N. A., Hayes, S. M., Kim, H., and Cabeza, R. (2009). Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front. Hum. Neurosci. 3:13. doi: 10.3389/neuro.09.013.2009
de Rover, M., Pironti, V. A., McCabe, J. A., Acosta-Cabronero, J., Arana, F. S., Morein-Zamir, S., et al. (2011). Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia 49, 2060–2070. doi: 10.1016/j.neuropsychologia.2011.03.037
Dhanjal, N. S., Warren, J. E., Patel, M. C., and Wise, R. J. S. (2013). Auditory cortical function during verbal episodic memory encoding in Alzheimer's disease. Ann. Neurol. 73, 294–302. doi: 10.1002/ana.23789
Dhanjal, N. S., and Wise, R. J. S. (2014). Frontoparietal cognitive control of verbal memory recall in Alzheimer's disease. Ann. Neurol. 76, 241–251. doi: 10.1002/ana.24199
Diamond, E. L., Miller, S., Dickerson, B. C., Atri, A., DePeau, K., Fenstermacher, E., et al. (2007). Relationship of fMRI activation to clinical trial memory measures in Alzheimer disease. Neurology 69, 1331–1341. doi: 10.1212/01.wnl.0000277292.37292.69
Diana, R. A., Yonelinas, A. P., and Ranganath, C. (2007). Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 11, 379–386. doi: 10.1016/j.tics.2007.08.001
Dickerson, B. C., Salat, D. H., Bates, J. F., Atiya, M., Killiany, R. J., Greve, D. N., et al. (2004). Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 56, 27–35. doi: 10.1002/ana.20163
Dickerson, B. C., Salat, D. H., Greve, D. N., Chua, E. F., Rand-Giovannetti, E., Rentz, D. M., et al. (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. doi: 10.1212/01.wnl.0000171450.97464.49
Ding, S. L., Van Hoesen, G. W., Cassell, M. D., and Poremba, A. (2009). Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J. Comp. Neurol. 514, 595–623. doi: 10.1002/cne.22053
Dolan, R. J., and Fletcher, P. C. (1997). Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature 388, 582–585. doi: 10.1038/41561
Dörfel, D., Werner, A., Schaefer, M., von Kummer, R., and Karl, A. (2009). Distinct brain networks in recognition memory share a defined region in the precuneus. Eur. J. Neurosci. 30, 1947–1959. doi: 10.1111/j.1460-9568.2009.06973.x
Dunn, C. J., Duffy, S. L., Hickie, I. B., Lagopoulos, J., Lewis, S. J., Naismith, S. L., et al. (2014). Deficits in episodic memory retrieval reveal impaired default mode network connectivity in amnestic mild cognitive impairment. Neuroimage Clin. 4, 473–480. doi: 10.1016/j.nicl.2014.02.010
Eichenbaum, H., Yonelinas, A. R., and Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123. doi: 10.1146/annurev.neuro.30.051606.094328
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., and Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926. doi: 10.1002/hbm.20718
Elman, J. A., Rosner, Z. A., Cohn-Sheehy, B. I., Cerreta, A. G., and Shimamura, A. P. (2013). Dynamic changes in parietal activation during encoding: implications for human learning and memory. Neuroimage 82, 44–52. doi: 10.1016/j.neuroimage.2013.05.113
Fletcher, P. C., and Henson, R. N. A. (2001). Frontal lobes and human memory. Brain 124, 849–881. doi: 10.1093/brain/124.5.849
Fletcher, P. C., Shallice, T., Frith, C. D., Frackowiak, R. S., and Dolan, R. J. (1998). The functional roles of prefrontal cortex in episodic memory. II. Retrieval. Brain 121, 1249–1256.
Fransson, P., and Marrelec, G. (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage 42, 1178–1184. doi: 10.1016/j.neuroimage.2008.05.059
Frings, L., Wagner, K., Quiske, A., Schwarzwald, R., Spreer, J., Halsband, U., et al. (2006). Precuneus is involved in allocentric spatial location encoding and recognition. Exp. Brain Res. 173, 661–672. doi: 10.1007/s00221-006-0408-8
Friston, K. J., and Price, C. J. (2003). Degeneracy and redundancy in cognitive anatomy. Trends Cogn. Sci. 7, 151–152. doi: 10.1016/S1364-6613(03)00054-8
Gainotti, G., Quaranta, D., Vita, M. G., and Marra, C. (2014). Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer's disease. J. Alzheimer's Dis. 38, 481–495. doi: 10.3233/JAD-130881
Giovanello, K. S., De Brigard, F., Hennessey Ford, J., Kaufer, D. I., Burke, J. R., Browndyke, J. N., et al. (2012). Event-related functional magnetic resonance imaging changes during relational retrieval in normal aging and amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 18, 886–897. doi: 10.1017/s1355617712000689
Grady, C. L., McIntosh, A. R., and Craik, F. I. (2005). Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia 43, 1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016
Gronholm, P., Rinne, J. O., Vorobyev, V. A., and Laine, M. (2007). Neural correlates of naming newly learned objects in MCI. Neuropsychologia 45, 2355–2368. doi: 10.1016/j.neuropsychologia.2007.02.003
Hämäläinen, A., Pihlajamäki, M., Tanila, H., Hänninen, T., Niskanen, E., Tervo, S., et al. (2007). Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol. Aging 28, 1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008
Hampstead, B. M., Stringer, A. Y., Stilla, R. F., Amaraneni, A., and Sathian, K. (2011). Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia 49, 2349–2361. doi: 10.1016/j.neuropsychologia.2011.04.008
Hanseeuw, B., Dricot, L., Kavec, M., Grandin, C., Seron, X., and Ivanoiu, A. (2011). Associative encoding deficits in amnestic mild cognitive impairment: a volumetric and functional MRI study. Neuroimage 56, 1743–1748. doi: 10.1016/j.neuroimage.2011.03.034
Heun, R., Freymann, K., Erb, M., Leube, D. T., Jessen, F., Kircher, T. T., et al. (2007). Mild cognitive impairment (MCI) and actual retrieval performance affect cerebral activation in the elderly. Neurobiol. Aging 28, 404–413. doi: 10.1016/j.neurobiolaging.2006.01.012
Jin, M., Pelak, V. S., Curran, T., Nandy, R. R., and Cordes, D. (2012). A preliminary study of functional abnormalities in aMCI subjects during different episodic memory tasks. Magn. Reson. Imaging 30, 459–470. doi: 10.1016/j.mri.2011.12.014
Johnson, S. C., Schmitz, T. W., Asthana, S., Gluck, M. A., and Myers, C. (2008). Associative learning over trials activates the hippocampus in healthy elderly but not mild cognitive impairment. Aging Neuropsychol. Cogn. 15, 129–145. doi: 10.1080/13825580601139444
Johnson, S. C., Schmitz, T. W., Moritz, C. H., Meyerand, M. E., Rowley, H. A., Alexander, A. L., et al. (2006). Activation of brain regions vulnerable to Alzheimer's disease: the effect of mild cognitive impairment. Neurobiol. Aging 27, 1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017
Kircher, T. T., Weis, S., Freymann, K., Erb, M., Jessen, F., Grodd, W., et al. (2007). Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J. Neurol. Neurosurg. Psychiatry 78, 812–818. doi: 10.1136/jnnp.2006.104877
Kompus, K., Eichele, T., Hugdahl, K., and Nyberg, L. (2011). Multimodal imaging of incidental retrieval: the low route to memory. J. Cogn. Neurosci. 23, 947–960. doi: 10.1162/jocn.2010.21494
Kveraga, K., Ghuman, A. S., Kassam, K. S., Aminoff, E. A., Hämäläinen, M. S., Chaumon, M., and Bar, M. (2011). Early onset of neural synchronization in the contextual associations network. Proc. Natl. Acad. Sci. U.S.A. 108, 3389–3394. doi: 10.1073/pnas.1013760108
Laird, A. R., Fox, P. M., Price, C. J., Glahn, D. C., Uecker, A. M., Lancaster, J. L., et al. (2005). ALE meta? analysis: controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 25, 155–164. doi: 10.1002/hbm.20136
Lancaster, J. L., Tordesillas Gutiérrez, D., Martinez, M., Salinas, F., Evans, A., Zilles, K., et al. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 28, 1194–1205. doi: 10.1002/hbm.20345
Lenzi, D., Serra, L., Perri, R., Pantano, P., Lenzi, G. L., Paulesu, E., et al. (2011). Single domain amnestic MCI: a multiple cognitive domains fMRI investigation. Neurobiol. Aging 32, 1542–1557. doi: 10.1016/j.neurobiolaging.2009.09.006
Li, R., Yu, J., Zhang, S., Bao, F., Wang, P., Huang, X., et al. (2013). Bayesian network analysis reveals alterations to default mode network connectivity in individuals at risk for Alzheimer's disease. PLoS ONE 8:e82104. doi: 10.1371/journal.pone.0082104
Lundstrom, B. N., Ingvar, M., and Petersson, K. M. (2005). The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 27, 824–834. doi: 10.1016/j.neuroimage.2005.05.008
Lundstrom, B. N., Petersson, K. M., Andersson, J., Johansson, M., Fransson, P., and Ingvar, M. (2003). Isolating the retrieval of imagined pictures during episodic memory: activation of the left precuneus and left prefrontal cortex. Neuroimage 20, 1934–1943. doi: 10.1016/j.neuroimage.2003.07.017
Machulda, M. M., Senjem, M. L., Weigand, S. D., Smith, G. E., Ivnik, R. J., Boeve, B. F., et al. (2009). Functional magnetic resonance imaging changes in amnestic and nonamnestic mild cognitive impairment during encoding and recognition tasks. J. Int. Neuropsychol. Soc. 15, 372–382. doi: 10.1017/S1355617709090523
Maillet, D., and Rajah, M. N. (2014). Dissociable roles of default-mode regions during episodic encoding. Neuroimage 89, 244–255. doi: 10.1016/j.neuroimage.2013.11.050
Mandzia, J. L., McAndrews, M. P., Grady, C. L., Graham, S. J., and Black, S. E. (2009). Neural correlates of incidental memory in mild cognitive impairment: an fMRI study. Neurobiol. Aging 30, 717–730. doi: 10.1016/j.neurobiolaging.2007.08.024
Masdeu, J. C., Zubieta, J. L., and Arbizu, J. (2005). Neuroimaging as a marker of the onset and progression of Alzheimer's disease. J. Neurol. Sci. 236, 55–64. doi: 10.1016/j.jns.2005.05.001
McCormick, C., Moscovitch, M., Protzner, A. B., Huber, C. G., and McAndrews, M. P. (2010). Hippocampal-neocortical networks differ during encoding and retrieval of relational memory: Functional and effective connectivity analyses. Neuropsychologia 48, 3272–3281. doi: 10.1016/j.neuropsychologia.2010.07.010
Mevel, K., Desgranges, B., Baron, J. C., Landeau, B., De la Sayette, V., Viader, F., et al. (2007). Detecting hippocampal hypometabolism in Mild Cognitive Impairment using automatic voxel-based approaches. Neuroimage 37, 18–25. doi: 10.1016/j.neuroimage.2007.04.048
Miettinen, P. S., Pihlajäm ki, M., Jauhiainen, A. M., Niskanen, E., Hänninen, T., Vanninen, R., and Soininen, H. (2011). Structure and function of medial temporal and posteromedial cortices in early Alzheimer's disease. Eur. J. Neurosci. 34, 320–330. doi: 10.1111/j.1460-9568.2011.07745.x
Minamoto, T., Osaka, M., Engle, R. W., and Osaka, N. (2012). Incidental encoding of goal irrelevant information is associated with insufficient engagement of the dorsal frontal cortex and the inferior parietal cortex. Brain Res. 1429, 82–97. doi: 10.1016/j.brainres.2011.10.034
Mitchell, A. J., and Shiri Feshki, M. (2009). Rate of progression of mild cognitive impairment to dementia-meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 119, 252–265. doi: 10.1111/j.1600-0447.2008.01326.x
Moulin, C. J. A., Laine, M., Rinne, J. O., Kaasinen, V., Sipilä, H., Hiltunen, J., et al. (2007). Brain function during multi-trial learning in mild cognitive impairment: a PET activation study. Brain Res. 1136, 132–141. doi: 10.1016/j.brainres.2006.12.021
Murray, L. J., and Ranganath, C. (2007). The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J. Neurosci. 27, 5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007
Nestor, P. J., Fryer, T. D., Ikeda, M., and Hodges, J. R. (2003). Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer's disease). Eur. J. Neurosci. 18, 2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x
Nicholas, C. R., Okonkwo, O. C., Bendlin, B. B., Oh, J. M., Asthana, S., Rowley, H. A., et al. (2014). Posteromedial hyperactivation during episodic recognition among people with memory decline: findings from the WRAP study. Brain Imaging Behav. 9, 690–702. doi: 10.1007/s11682-014-9322-z
Oh, H., and Jagust, W. J. (2013). Frontotemporal network connectivity during memory encoding is increased with aging and disrupted by beta-amyloid. J. Neurosci. 33, 18425–18437. doi: 10.1523/JNEUROSCI.2775-13.2013
Park, D. C., and Reuter-Lorenz, P. (2009). The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 60:173. doi: 10.1146/annurev.psych.59.103006.093656
Parra, M. A., Pattan, V., Wong, D., Beaglehole, A., Lonie, J., Wan, H. I., et al. (2013). Medial temporal lobe function during emotional memory in early Alzheimer's disease, mild cognitive impairment and healthy ageing: an fMRI study. BMC Psychiatry 13:76. doi: 10.1186/1471-244X-13-76
Patterson, K., Nestor, P. J., and Rogers, T. T. (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 8, 976–987. doi: 10.1038/nrn2277
Paz-Alonso, P. M., Bunge, S. A., Anderson, M. C., and Ghetti, S. (2013). Strength of Coupling within a mnemonic control network differentiates those who can and cannot suppress memory retrieval. J. Neurosci. 33, 5017–5026. doi: 10.1523/JNEUROSCI.3459-12.2013
Perri, R., Serra, L., Carlesimo, G. A., and Caltagirone, C. (2007). Amnestic mild cognitive impairment: difference of memory profile in subjects who converted or did not convert to Alzheimer's disease. Neuropsychology 21, 549–558. doi: 10.1037/0894-4105.21.5.549
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194. doi: 10.1111/j.1365-2796.2004.01388.x
Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., et al. (2001). Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992. doi: 10.1001/archneur.58.12.1985
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., and Kokmen, E. (1999). Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308. doi: 10.1001/archneur.56.3.303
Petrella, J. R., Krishnan, S., Slavin, M. J., Tran, T. T., Murty, L., and Doraiswamy, P. M. (2006). Mild cognitive impairment: evaluation with 4-T functional MR imaging. Radiology 240, 177–186. doi: 10.1148/radiol.2401050739
Preston, A. R., and Eichenbaum, H. (2013). Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 23, R764–R773. doi: 10.1016/j.cub.2013.05.041
Petrella, J. R., Wang, L., Krishnan, S., Slavin, M. J., Prince, S. E., Tran, T. T., et al. (2007). Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology 245, 224–235. doi: 10.1148/radiol.2451061847
Putcha, D., Brickhouse, M., O'Keefe, K., Sullivan, C., Rentz, D., Marshall, G., et al. (2011). Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults. J. Neurosci. 31, 17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011
Rami, L., Sala-Llonch, R., Solé Padullés, C., Fortea, J., Olives, J., Llad, A., et al. (2012). Distinct functional activity of the precuneus and posterior cingulate cortex during encoding in the preclinical stage of Alzheimer's disease. J. Alzheimer's Dis. 31, 517–526. doi: 10.3233/JAD-2012-120223
Ranganath, C., Heller, A., Cohen, M. X., Brozinsky, C. J., and Rissman, J. (2005). Functional connectivity with the hippocampus during successful memory formation. Hippocampus 15, 997–1005. doi: 10.1002/hipo.20141
Ries, M. L., Schmitz, T. W., Kawahara, T. N., Torgerson, B. M., Trivedi, M. A., and Johnson, S. C. (2006). Task-dependent posterior cingulate activation in mild cognitive impairment. Neuroimage 29, 485–492. doi: 10.1016/j.neuroimage.2005.07.030
Rugg, M. D., and Vilberg, K. L. (2013). Brain networks underlying episodic memory retrieval. Curr. Opin. Neurobiol. 23, 255–260. doi: 10.1016/j.conb.2012.11.005
Sandstrom, C. K., Krishnan, S., Slavin, M. J., Tran, T. T., Doraiswamy, P. M., and Petrella, J. R. (2006). Hippocampal atrophy confounds template-based functional MR imaging measures of hippocampal activation in patients with mild cognitive impairment. AJNR Am. J. Neuroradiol. 27, 1622–1627.
Schroeter, M. L., Stein, T., Maslowski, N., and Neumann, J. (2009). Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage 47, 1196–1206. doi: 10.1016/j.neuroimage.2009.05.037
Schwindt, G. C., and Black, S. E. (2009). Functional imaging studies of episodic memory in Alzheimer's disease: a quantitative meta-analysis. Neuroimage 45, 181–190. doi: 10.1016/j.neuroimage.2008.11.024
Seghier, M. L. (2013). The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19, 43–61. doi: 10.1177/1073858412440596
Sestieri, C., Corbetta, M., Romani, G. L., and Shulman, G. L. (2011). Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J. Neurosci. 31, 4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011
Shannon, B. J., and Buckner, R. L. (2004). Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J. Neurosci. 24, 10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004
Shimamura, A. P. (2014). Remembering the past neural substrates underlying episodic encoding and retrieval. Curr. Dir. Psychol. Sci. 23, 257–263. doi: 10.1177/0963721414536181
Spaniol, J., Davidson, P. S., Kim, A. S., Han, H., Moscovitch, M., and Grady, C. L. (2009). Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 47, 1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028
Takashima, A., Bakker, I., van Hell, J. G., Janzen, G., and McQueen, J. M. (2014). Richness of information about novel words influences how episodic and semantic memory networks interact during lexicalization. Neuroimage 84, 265–278. doi: 10.1016/j.neuroimage.2013.08.023
Talairach, J., and Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging. Stuttgart: Theime Press.
Tomita, H., Ohbayashi, M., Nakahara, K., Hasegawa, I., and Miyashita, Y. (1999). Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature 401, 699–703. doi: 10.1038/44372
Trivedi, M. A., Murphy, C. M., Goetz, C., Shah, R. C., Gabrieli, J. D., Whitfield-Gabrieli, S., et al. (2008). fMRI activation changes during successful episodic memory encoding and recognition in amnestic mild cognitive impairment relative to cognitively healthy older adults. Dement. Geriatr. Cogn. Disord. 26, 123–137. doi: 10.1159/000148190
Trivedi, M. A., Stoub, T. R., Murphy, C. M., George, S., deToledo-Morrell, L., Shah, R. C., et al. (2011). Entorhinal cortex volume is associated with episodic memory related brain activation in normal aging and amnesic mild cognitive impairment. Brain Imaging Behav. 5, 126–136. doi: 10.1007/s11682-011-9117-4
Turkeltaub, P. E., Eden, G. F., Jones, K. M., and Zeffiro, T. A. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16, 765–780. doi: 10.1006/nimg.2002.1131
Uddin, L. Q., Supekar, K., Amin, H., Rykhlevskaia, E., Nguyen, D. A., Greicius, M. D., et al. (2010). Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb. Cortex 20, 2636–2646. doi: 10.1093/cercor/bhq011
Uncapher M. R. Wagner A. D. (2009). Posterior Parietal Cortex and Episodic Encoding: Insights from fMRI Subsequent Memory Effects and Dual-attention Theory [Online]. Available online at: http://www.sciencedirect.com/science/article/pii/S1074742708002049 (Accessed 2, 91).
Vandenbulcke, M., Peeters, R., Dupont, P., Van Hecke, P., and Vandenberghe, R. (2007). Word reading and posterior temporal dysfunction in amnestic mild cognitive impairment. Cereb. Cortex 17, 542–551. doi: 10.1093/cercor/bhj179
van der Meulen, M., Lederrey, C., Rieger, S. W., van Assche, M., Schwartz, S., Vuilleumier, P., et al. (2012). Associative and semantic memory deficits in amnestic mild cognitive impairment as revealed by functional magnetic resonance imaging. Cogn. Behav. Neurol. 25, 195–215. doi: 10.1097/WNN.0b013e31827de67f
Vannini, P., O'Brien, J., O'Keefe, K., Pihlajamäki, M., Laviolette, P., and Sperling, R. A. (2011). What goes down must come up: role of the posteromedial cortices in encoding and retrieval. Cereb. Cortex 21, 22–34. doi: 10.1093/cercor/bhq051
Vilberg, K. L., and Rugg, M. D. (2008). Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia 46, 1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004
Wheeler, M. E., and Buckner, R. L. (2004). Functional-anatomic correlates of remembering and knowing. Neuroimage 21, 1337–1349. doi: 10.1016/j.neuroimage.2003.11.001
Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., et al. (2004). Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 256, 240–246. doi: 10.1111/j.1365-2796.2004.01380.x
Xu, G., Antuono, P. G., Jones, J., Xu, Y., Wu, G., Ward, D., et al. (2007). Perfusion fMRI detects deficits in regional CBF during memory-encoding tasks in MCI subjects. Neurology 69, 1650–1656. doi: 10.1212/01.wnl.0000296941.06685.22
Yassa, M. A., Stark, S. M., Bakker, A., Albert, M. S., Gallagher, M., and Stark, C. E. (2010). High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage 51, 1242–1252. doi: 10.1016/j.neuroimage.2010.03.040
Yonelinas, A. P. (2001). Components of episodic memory: the contribution of recollection and familiarity. Philos. Trans. R. Soc. B Biol. Sci. 356, 1363–1374. doi: 10.1098/rstb.2001.0939
Keywords: mild cognitive impairment, episodic memory, encoding, retrieval, activation likelihood estimation
Citation: Wang P, Li J, Li H-J, Huo L and Li R (2016) Mild Cognitive Impairment Is Not “Mild” at All in Altered Activation of Episodic Memory Brain Networks: Evidence from ALE Meta-Analysis. Front. Aging Neurosci. 8:260. doi: 10.3389/fnagi.2016.00260
Received: 06 March 2016; Accepted: 19 October 2016;
Published: 07 November 2016.
Edited by:
Rodrigo Orlando Kuljiš, University of Miami School of Medicine, USAReviewed by:
Guido Gainotti, Policlinico Gemelli, ItalyRamesh Kandimalla, Texas Tech University, USA
Copyright © 2016 Wang, Li, Li, Huo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Li, lijuan@psych.ac.cn
 Pengyun Wang
Pengyun Wang Juan Li
Juan Li Hui-Jie Li
Hui-Jie Li Lijuan Huo
Lijuan Huo Rui Li
Rui Li