Age-Related Difference in Functional Brain Connectivity of Mastication
- 1Department of Dentistry, School of Dentistry, National Yang-Ming University, Taipei, Taiwan
- 2School of Dentistry, Institute of Oral Biology, National Yang-Ming University, Taipei, Taiwan
- 3Division of Family Dentistry, Department of Stomatology, Taipei Veterans General Hospital, Taipei, Taiwan
- 4Division of Prosthodontics, Department of Stomatology, Taipei Veterans General Hospital, Taipei, Taiwan
- 5Division of Oral and Maxillofacial Surgery, Department of Stomatology, Taipei Veterans General Hospital, Taipei, Taiwan
The age-related decline in motor function is associated with changes in intrinsic brain signatures. Here, we investigated the functional connectivity (FC) associated with masticatory performance, a clinical index evaluating general masticatory function. Twenty-six older adults (OA) and 26 younger (YA) healthy adults were recruited and assessed using the masticatory performance index (MPI) and resting-state functional magnetic resonance imaging (rs-fMRI). We analyzed the rs-fMRI FC network related to mastication, which was constructed based on 12 bilateral mastication-related brain regions according to the literature. For the OA and the YA group, we identified the mastication-related hubs, i.e., the nodes for which the degree centrality (DC) was positively correlated with the MPI. For each pair of nodes, we identified the inter-nodal link for which the FC was positively correlated with the MPI. The network analysis revealed that, in the YA group, the FC between the sensorimotor cortex, the thalamus (THA) and the cerebellum was positively correlated with the MPI. Consistently, the cerebellum nodes were defined as the mastication-related hubs. In contrast, in the OA group, we found a sparser connection within the sensorimotor regions and cerebellum and a denser connection across distributed regions, including the FC between the superior parietal lobe (SPL), the anterior insula (aINS) and the dorsal anterior cingulate cortex (dACC). Compared to the YA group, the network of the OA group also comprised more mastication-related hubs, which were spatially distributed outside the sensorimotor regions, including the right SPL, the right aINS, and the bilateral dACC. In general, the findings supported the hypothesis that in OA, higher masticatory performance is associated with a widespread pattern of mastication-related hubs. Such a widespread engagement of multiple brain regions associated with the MPI may reflect an increased demand in sensorimotor integration, attentional control and monitoring for OA to maintain good mastication.
Introduction
The traditional view holds that the individual differences in masticatory ability are predominantly predisposed by oral conditions, such as the number of teeth lost, the amount of biting force and the saliva flow rate (Hatch et al., 2001; Ikebe et al., 2011). However, as a highly coordinated rhythmic and automatic movement, mastication is also regulated by the central nervous system (CNS; Avivi-Arber et al., 2011). Neuroimaging evidence has suggested that mastication is associated with the brain activity in the cortical sensory and motor regions, the subcortical regions, and the cerebellum (Onozuka et al., 2002; Quintero et al., 2013). Furthermore, in elderly healthy subjects, good masticatory ability, as indexed by higher masticatory performance, was associated with greater gray matter volume at the premotor cortex (PMC) and an increased resting-state functional connectivity (FC) with the cerebellum (Lin et al., 2015). These findings highlighted that the individual differences in masticatory ability are not only associated with peripheral oral conditions but also the intrinsic brain signatures related to motor control.
The age-related difference in the CNS mechanisms of mastication has remained unclear. Neuroimaging findings have suggested that aging is associated with different patterns of brain activation for motor control (Mattay et al., 2002; Onozuka et al., 2003; Heuninckx et al., 2005; Wu and Hallett, 2005). First, when performing a simple motor task (e.g., finger tapping), older adults (OA) showed widespread activation in a distributed network, including in the sensorimotor area, the premotor area, the rostral cingulate the prefrontal cortex, the subcortical regions and the cerebellum, compared to that in younger adults (YA; Mattay et al., 2002; Heuninckx et al., 2005; Wu and Hallett, 2005). Such a widespread activation may indicate an age-related compensation in motor control or enhanced cognitive demand during movement (Ward, 2006; Seidler et al., 2010). Second, in OA, central pattern generator (CPG)-maintained movements (e.g., chewing, swallowing and locomotion) are engaged with extended cortical activation, including the insula, the prefrontal cortex and the parietal cortex (Onozuka et al., 2003; Zwergal et al., 2012; Windel et al., 2015; Lotze et al., 2016). Finally, evidence from resting-state FC showed a pronounced age-related change in the sensorimotor network (Tomasi and Volkow, 2012; Chan et al., 2014; Song et al., 2014; Fujiyama et al., 2016). The findings suggested that as age increases, a complex interplay between motor, sensory and cognitive processing, via altered FC, may play a predominant role in mastication.
The current study aimed to qualitatively and quantitatively compare the pattern of FC networks associated with masticatory performance between older and younger healthy adults. We adopted a graph-based method to construct the mastication-related network, which was composed of 12 bilateral brain regions associated with mastication. We identified the mastication-related “hubs”, which showed a positive correlation between masticatory performance and the degree centrality (DC) of the network. We hypothesized that, in older people vs. younger people, a higher masticatory performance would be associated with a widespread pattern of mastication-related hubs.
Materials and Methods
Study Group
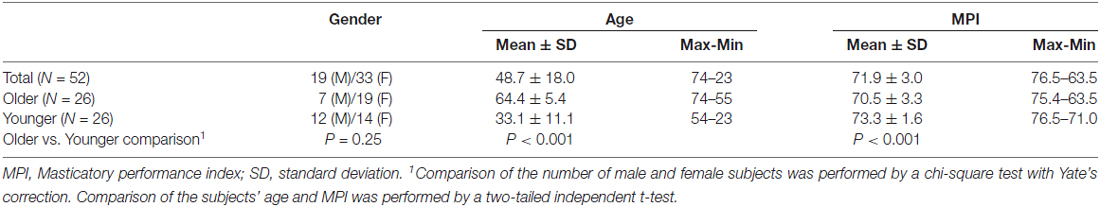
Fifty-two healthy adults (33 female/19 male) aged 48.7 ± 18.0 (mean ± standard deviation) years old (y/o) were recruited. To investigate the age-related effect on masticatory performance and the related brain signatures, we median-split the participants into a subgroup of OA (>54.5 y/o, n = 26) and a subgroup of YA (<54.5 y/o, n = 26). Among the 52 participants, 21 were investigated in our previous study on the brain signatures associated with masticatory performance (Lin et al., 2015). The following exclusion criteria were applied: (1) having a history of major physical or psychiatric disorders, including epilepsy, major depression, schizophrenia or neurovascular diseases; (2) having a history of brain injury or receiving brain surgery; and (3) being unable to undergo MRI due to physical or psychological contraindications. The participants were recruited via advertisements in local community centers and the Taipei Veterans General Hospital. The study was approved by the Institutional Review Board of the National Yang Ming University and Taipei Veterans General Hospital, Taiwan. Written informed consent was provided by all participants before the experiment was initiated.
Clinical and Neuroimaging Assessment
We adopted the colorimetric method based on color-changeable chewing gum (Hayakawa et al., 1998) for assessing individual masticatory function. After the MRI scan, each participant was asked to chew a piece of color-changeable chewing gum (Lotte Co. Ltd., Tokyo, Japan), which is designed for assessing masticatory performance (Hama et al., 2014), for 3 min. The chewed gum was flattened and scanned as a digitalized image (Schimmel et al., 2007), and the masticatory performance index (MPI) was quantified as the color change (ΔE) in the L*a*b* chromatic system (Hama et al., 2014) of the image.
Acquisition and Preprocessing of Imaging Data
Resting-state functional MRI (rs-fMRI) was performed at the 3T MRI Laboratory of National Yang-Ming University using a 3 Tesla Siemens MRI scanner (Siemens Magnetom Tim Trio, Erlangen, Germany). The rs-fMRI images were acquired using a gradient echo EPI (Echo Planar Imaging) T2* weighted sequence ([TR] = 2000 ms, [TE] = 20 ms, matrix size = 64 × 64 × 40, voxel size = 3.4 × 3.4 × 3.4 mm3 and 183 volumes in total). During scanning, the participants were instructed to be relaxed but remain awake, keeping their eyes open and fixing on a cross symbol on the screen. Pre-processing of the rs-fMRI data was performed using the Data Processing Assistant for Resting-State fMRI (Chao-Gan and Yu-Feng, 2010) and the Resting-State fMRI Data Analysis Toolkit (Song et al., 2011), according to the pipeline from a previous study (Lin et al., 2014). (1) The first three scans were discarded due to magnetic saturation effects. (2) The imaging data were corrected for slice-timing order, realigned for head movement, and normalized to the Montreal Neurological Institute (MNI) template. (3) The time series was detrended and bandpass filtered (0.01–0.08 Hz) to extract the low-frequency oscillating components contributing to intrinsic FC. (4) Spurious or non-specific effects were removed by a regression model, with the following covariates: (a) the six movement parameters obtained from the image realignment procedure; (b) the mean signal within the lateral ventricles; and (c) the mean signal within the deep white matter. We did not perform a regression for the whole-brain global signal due to the debate on its effect (Murphy et al., 2009). The processed imaging data were subsequently used for the network analysis.
Analysis of the Effect of Head Motion
Because head motion can significantly influence the results of the network analysis (Van Dijk et al., 2012), we compared the amount of head motion between the OA and the YA groups during the rs-fMRI scan. The motion parameters were extracted from the image realignment of the rs-fMRI data. For each participant, a head motion index was quantified as the mean displacement (i.e., the translation/rotation in the ith time point relative to the (i − 1)th time point), based on the method reported in a previous study (Liu et al., 2008). The head motion index was calculated for both translational motion and rotational motion. Between-group differences in the translational motion and rotational motion were examined using a two-tailed two-sample t test. We find a significant between-group (OA vs. YA) difference in the translation motion (OA > YA, two-tailed two-sample t test, p = 0.017) but no rotational motion (two-tailed two-sample t test, p = 0.38) during all scans. All participants showed a maximal displacement of <1.5 mm in translation and <1.5° in rotation.
Graph-Based Network Analysis
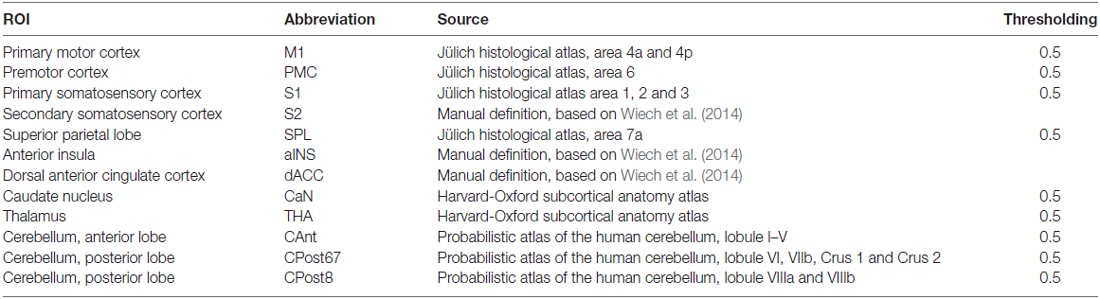
(a) Definition of the nodes: we assigned the nodes as the 12 brain ROIs related to motor control or mastication, based on a review of the literature (Figure 1 and Table 1). In addition to the primary motor cortex (M1), we included the secondary motor cortex, i.e., the PMC (including the supplementary motor area) and the dorsal anterior cingulate cortex (dACC, being part of the cingulate motor area; Paus, 2001). We included the subcortical regions, i.e., the thalamus (THA) and the caudate nucleus (CaN), due to their activation related to mastication in OA (Onozuka et al., 2003). We also included brain regions associated with sensory and cognitive processing, including the superior parietal lobe (SPL), the anterior insula (aINS) and the primary and secondary somatosensory cortices (S1/S2), due to their roles in sensorimotor integration and attention control in OA (Ward, 2006; Seidler et al., 2010). Finally, we included three sub-regions of the cerebellum: the anterior lobe (CAnt), lobule VI/VII of the posterior lobe (CPost67) and lobule VIII of the posterior lobe (CPost8), due to their connectivity with the cortical regions. The anterior and posterior sub-divisions contribute to sensorimotor and cognitive-affective processing, respectively (Stoodley and Schmahmann, 2010; Stoodley et al., 2012).
(b) Definition of the edges: we defined the edges of the FC network as the inter-nodal strength of the association of the time series, according to the following procedure. (1) We extracted the time series from all voxels within each node and calculated the mean regional time series. (2) For each pair of nodes, we calculated the Pearson correlation coefficient (r) between their regional mean time series. (3) The r value was converted to a z score using Fisher’s r-to-z transformation to improve the normality, followed by transformation to its absolute value. The absolute value of the z score was indexed as the weight of the network edge. Based on this procedure, an undirected and weighted network composed of 24 nodes and 276 edges was obtained for each participant.
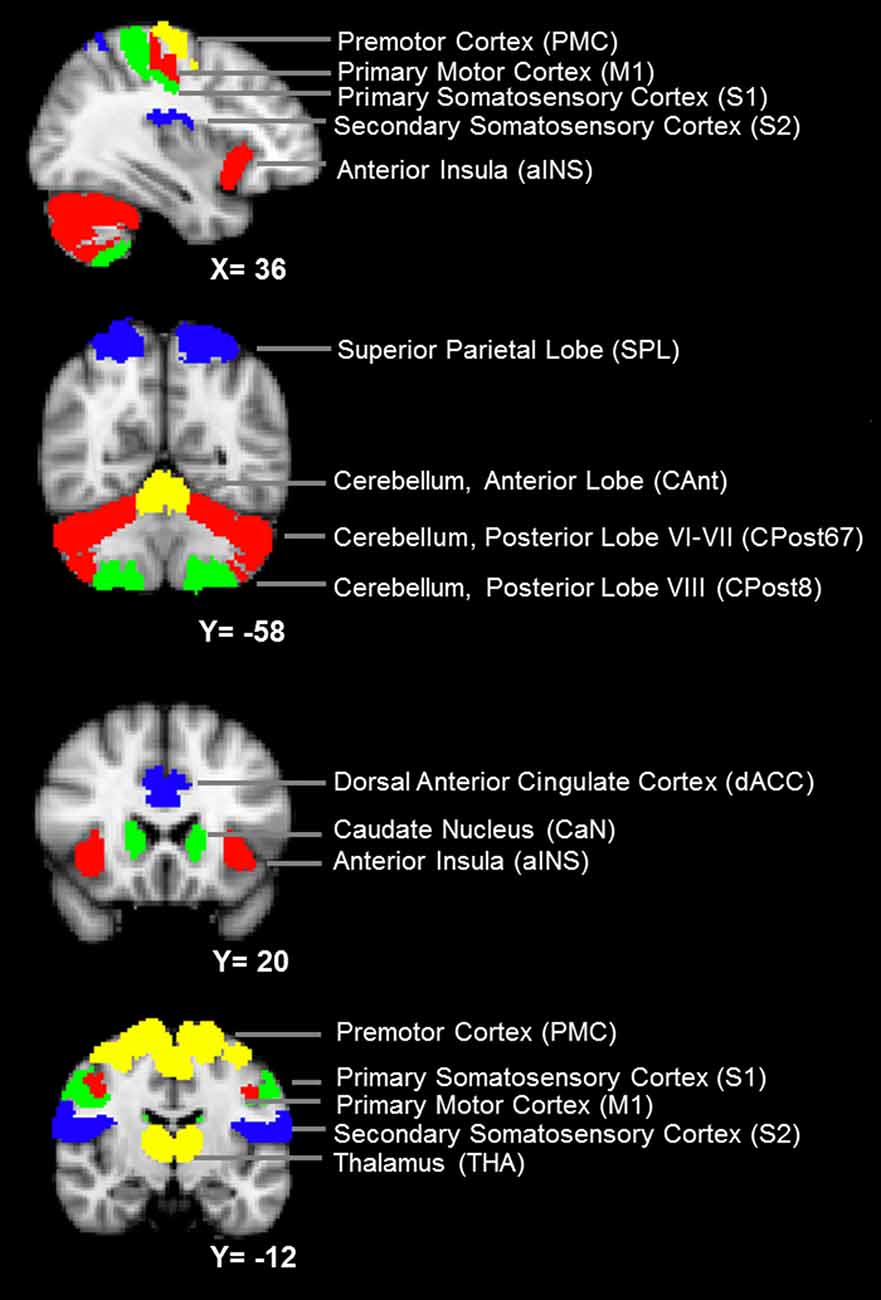
Figure 1. The nodes of the mastication-related network. See Table 1 for a detailed definition of the regions of interest (ROIs) of the nodes.
Definition of the ROIs
The ROI of a node was designed according to anatomical probabilistic atlases or manually defined based on an independent group of subjects (Table 1 and Figure 1). M1, PMC, S1, SPL were defined according to the Jülich Histological Atlas (Eickhoff et al., 2007). CaN and THA were defined according to the Harvard-Oxford Subcortical Structural Atlas (Desikan et al., 2006). The cerebellum lobes (CAnt, CPost67 and CPost8) were defined according to the Probabilistic Cerebellar Atlas (Diedrichsen et al., 2009). These nine ROIs were designed, using FSLView1, by thresholding the probabilistic map with a group threshold of 0.5 (Table 1). S2, aINS and dACC were defined manually, according to the methods published in a previous study (Wiech et al., 2014; Table 1). The bilateral ROIs of a node were independently defined, and, therefore, 24 nodes were included in the network.
Quantifying Network Degree Centrality
Our main hypothesis focused on the age-related difference in the mastication-related network. We calculated the graph index DC to quantify the importance of a node in the network. DC has been widely used to examine the local centrality of a node (Zuo et al., 2012). A higher DC value indicates that the node is highly connected and is a “hub” in the network. DC was calculated for each node by summing up the edge weights between this node and all of the other nodes.
Statistical Analyses
(a) We identified the mastication-related hub, i.e., the node for which the DC was significantly positively correlated with the MPI. Partial correlation analyses were performed to investigate the correlation between the MPI and DC for each node, controlling for age and gender, across all participants in each age subgroup.
(b) For each node of the mastication-related network, we screened for the other nodes (n = 23) for which the FC with the target node was significantly correlated with the MPI, controlling for age and gender. Due to the exploratory nature of the analysis, we did not adjust the alpha value for multiple testing.
Results
Demographic and Clinical Profiles of the Study Group
The demographic and clinical data are shown in Table 2. The mean MPI in the OA group was significantly lower than that in the YA group (OA: 70.5 ± 3.3, YA: 73.3 ± 1.6, two-tailed two-sample t-test p < 0.001). There was no significant gender difference in the number of subjects (χ2(1) = 1.33, two-tailed p = 0.25). Across all participants (n = 52), age was significantly negatively correlated with the MPI (Pearson’s correlation coefficient r = −0.54, two-tailed p < 0.001). Specifically, within the OA group (n = 26), age was significantly negatively correlated with the MPI (r = −0.53, two-tailed p = 0.006), consistent with previous findings (Ikebe et al., 2011; Figure 2). In contrast, within the YA group, the correlation between age and the MPI was not statistically significant (Figure 2). The findings revealed that masticatory performance did not decline gradually as age increased. It showed a pattern of late-life decrease, i.e., starting to decline significantly during older age but not during younger age.
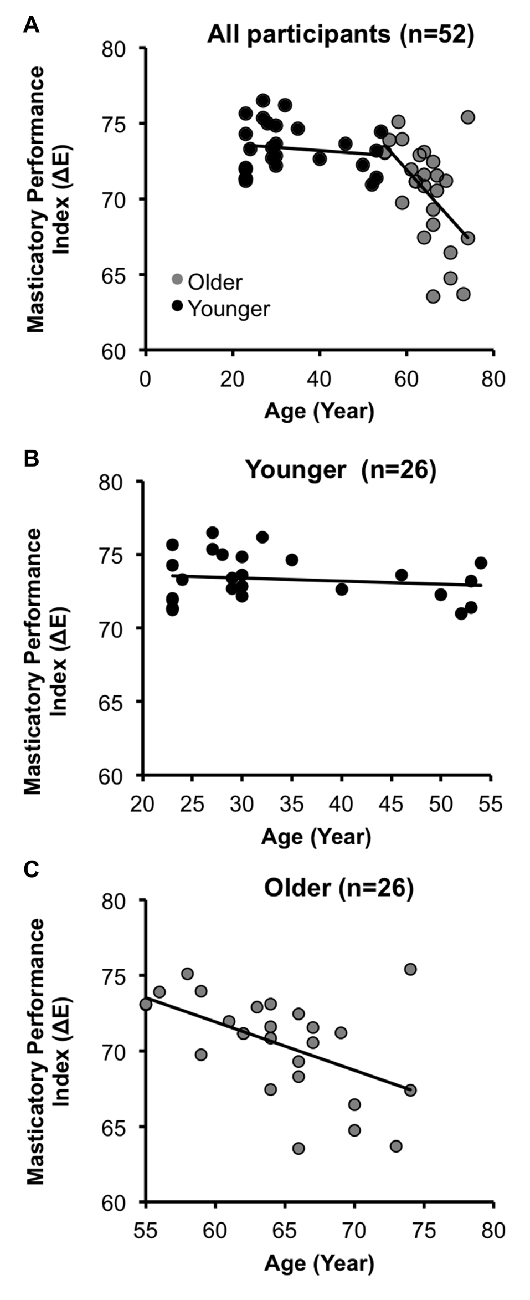
Figure 2. Age-related decline in masticatory performance. (A) Across all participants (N = 52), the masticatory performance index (MPI) decreased with increasing age. (B) In the younger adult group (YA), the correlation between the MPI and age was insignificant. (C) In the older adult group (OA), the MPI was significantly negatively correlated with age.
Identification of the Mastication-Related Hub
In the YA subgroup, we found the following mastication-related hubs, i.e., the nodes that showed a positive correlation between DC and the MPI: the left CAnt (r = 0.42, p = 0.032) and right CPost8 (r = 0.42, p = 0.034). In the OA subgroup, we found the following mastication-related hubs: the left PMC (r = 0.39, p = 0.048), left S2 (r = 0.41, p = 0.037), right S2 (r = 0.45, p = 0.021), right SPL (r = 0.48, p = 0.14), right aINS (r = 0.43, p = 0.027), left dACC (r = 0.45, p = 0.021) and right dACC (r = 0.42, p = 0.034).
Network Analysis
In the YA subgroup, we found a dense connection between the sensorimotor nodes (M1, PMC, S1 and S2), and a dense connection between the subcortical and cerebellum nodes. Notably, the cerebellum nodes showed a dense connection with the cortical sensorimotor nodes, including FC between the bilateral M1 and the right CPost8 and FC between the bilateral S1 and the left CAnt (Figure 3). In contrast, in the OA subgroup, the sensorimotor nodes were less connected (except for the bilateral S2). The cerebellum nodes also showed less connection. A dense connection was found between the sensorimotor nodes and the other cortical nodes, including the right SPL, the bilateral aINS and the dACC (Figure 3).
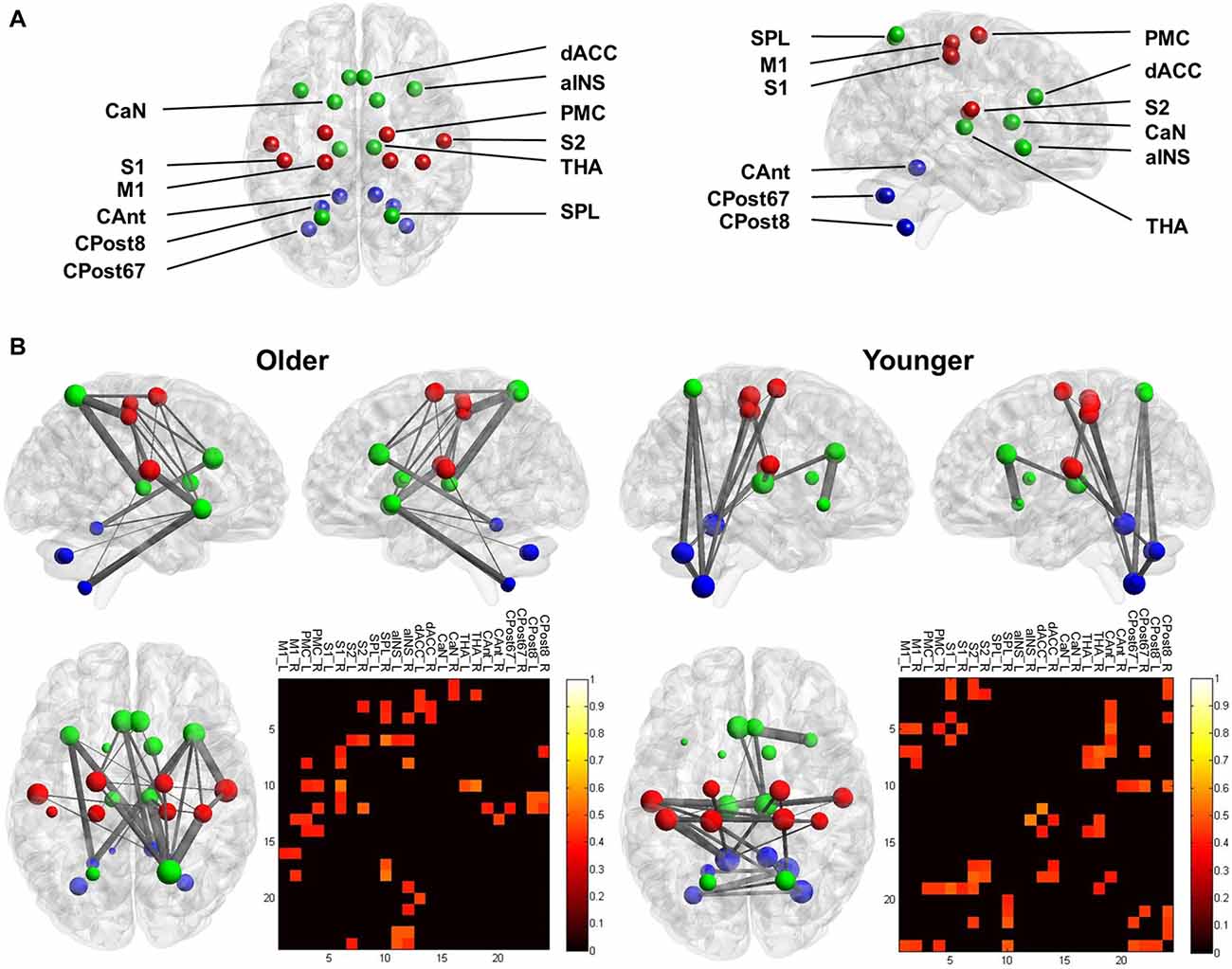
Figure 3. Functional connectivity (FC) of the mastication-related network. (A) The nodes that comprised the network. For visualization, the sensorimotor nodes (S1, PMC, S1 and S2) are colored red. The cerebellum nodes (CAnt, CPost67 and CPost8) are colored blue. All other cortical and subcortical nodes are colored green. See Table 1 for the abbreviation of each node. (B) The mastication-related FC network. The line between each node represents the FC that is statistically significant. Line thickness denotes the strength of association between the FC and the MPI. The pattern of the FC is displayed from the sagittal view (upper left and right) and the dorsal view (lower left) for both the younger and older groups. The lower right panel displays the matrix of FC between all pairs of nodes. The color denotes the strength of association between the FC and the MPI, quantified by Pearson’s correlation coefficient. Only the links with a significant correlation (p < 0.05) are displayed.
Discussion
Previous studies have identified brain activation of the cerebellum and the motor cortex when participants were chewing (Onozuka et al., 2002; Quintero et al., 2013). In the current study, we further investigated the brain signatures associated with masticatory performance, a widely used index for evaluating the masticatory function of elderly people. In the YA group, the network analysis revealed that FC between the sensorimotor cortex, the THA and the cerebellum was positively correlated with the MPI (Figure 3). The findings suggested that younger people may automatically chew food without additional effort for monitoring or regulating their movement. Consistently, we found that the cerebellum, a key component for automatic control of movement (Ramnani, 2014), was identified as mastication-related hubs. In contrast, in the OA group, the network analysis revealed a sparser connection within the sensorimotor regions and cerebellum and a denser connection across distributed regions (Figure 3). Compared to the YA group, the OA group also comprised more mastication-related hubs, which were spatially distributed outside of the sensorimotor regions, including the right SPL, the right aINS, the left dACC and the right dACC. In general, the findings confirmed our main hypothesis that in OA, higher masticatory performance is associated with a more widespread pattern of mastication-related hubs compared to that observed in YA.
It has been widely accepted that motor function declines with aging (Ward, 2006). As shown in our behavioral findings, the decreased masticatory performance was associated with the increasing age, especially during late life (Figure 2). Cumulating evidence has suggested that the CNS plays a key role in the age-related decline of motor control. When performing a simple motor task (e.g., finger tapping) or rhythmic movement (e.g., chewing), older people show a more widespread engagement of multiple brain regions compared to that in younger people. In addition to the activation of M1, older people show more co-activation in cortical and subcortical regions (Mattay et al., 2002; Onozuka et al., 2003; Heuninckx et al., 2005; Wu and Hallett, 2005; Zwergal et al., 2012; Windel et al., 2015). For example, when performing a simple flexion-extension movement, older subjects showed an increased activity in the premotor area, the rostral cingulate and the prefrontal cortex, suggesting an increased cognitive monitoring of motor performance (Heuninckx et al., 2005). When performing paced finger movements, older subjects showed a greater extent of activation in the sensorimotor cortex, the premotor area (including the supplementary motor area), and the cerebellum (Mattay et al., 2002; Wu and Hallett, 2005). Importantly, research on human chewing, a movement maintained by CPG, also revealed an age-related difference in the activation pattern. Older subjects showed increased activity in the prefrontal area and decreased activity in the somatosensory cortex and the cerebellum (Onozuka et al., 2003). All of the findings suggest that in OA, motor control is associated with a widespread engagement of multiple brain regions.
According to the compensation theory, OA increasingly rely on cognitive processes for motor control (Seidler et al., 2010). The extended engagement of multiple brain regions may reflect the increased demand of multi-sensory integration, attentional control, or enhanced cognitive efforts for movement in OA (Ward, 2006; Seidler et al., 2010). Mastication is automatically maintained and rhythmically executed by the CPG of the brainstem (Avivi-Arber et al., 2011). Mastication can be automatically achieved without one’s overt attention. However, it also requires feedback and monitoring from the sensorimotor cortex. Our findings suggested that in OA, the FC between the sensorimotor cortex and other brain regions, rather than the FC within the sensorimotor cortex, shows a greater contribution to mastication. The extended engagement of the brain may indicate the distinct experience of chewing in older people. During mastication, OA need to monitor their oral status continually, perhaps due to poorer oral conditions (e.g., having fewer teeth to chew well). Consistently, we found a positive correlation between the MPI and bilateral PMC-right SPL connectivity, right PMC-aINS connectivity right S1-bilateral aINS connectivity and right S1-SPL connectivity (Figure 3). The aINS, as part of the salience network of resting-state FC, plays a predominant role in awareness of bodily perception (Menon and Uddin, 2010). The SPL plays a key role in maintaining an internal representation of the body’s state and contributes to sensorimotor integration (Wolpert et al., 1998). The finding may indicate that the OA need to overtly monitor the progress of mastication and intraoral perception (e.g., the size of food particles). This feedback information would be essential to maintain mastication.
Our findings echoed the recent neuroimaging evidence that revealed that the functional brain signature reflects an age-related difference. For example, increased age is associated with an increased long-range FC density (Tomasi and Volkow, 2012) and network efficiency (Song et al., 2014) in the sensorimotor network. The sensorimotor network also showed a pronounced decrease in segregation and increase in between-network connection (Chan et al., 2014). The age-related changes suggest that in OA, the ability to execute a motor task relies on the function of brain regions outside of the sensorimotor network, such as the SPL, the aINS and subcortical regions. The engagement of these regions may reflect increased feedback or “compensation” to facilitate the motor task (Ward, 2006; Seidler et al., 2010). One potential explanation of the age-related FC changes is brain plasticity, i.e., “an intrinsic property of the nervous system retained throughout a lifespan” (Pascual-Leone et al., 2005) that can be molded by long-term experience, such as motor exercise and sensory experience. Accumulating evidence has shown that changes in the FC network reflect changes in motor or sensory experience (Kelly and Castellanos, 2014). For example, changes in the connectivity of the SPL and the PMC were found in subjects who received long-term training in a coordination task, which demands integration of perceptual, motor and cognitive abilities (Demirakca et al., 2016). Compared to training of motor tasks, mastication is a more ordinary and common exercise for our daily life, and, therefore, alterations in masticatory function may reshape the related brain signatures.
We considered the following limitations crucial for interpreting the current findings. First, our conclusion is limited to the representativeness of the study group, which included all physically healthy OA with generally good oral health. We purposely excluded OA with motor or cognitive deficits, which may confound the results of the masticatory performance assessment or neuroimaging. The trade-off is that our conclusion may not reflect the broader spectrum of clinical cases in which people with extremely poor masticatory functions are commonly seen. Second, we did not make a direct and quantitative comparison of the brain connectivity between the YA and the OA groups. Instead, all of the investigation on FC was performed within the subgroups. We avoided a direct between-age subgroup comparison for two reasons: (a) there exists a significant age-related difference in brain anatomy (Good et al., 2001); and (b) our findings showed a significant difference in head translational motion between the age subgroups. These factors may confound the results of a direct comparison in FC between the age subgroups. Third, in the experimental design, we did not aim to clarify the causal link between the pattern of brain connectivity and masticatory performance. The positive correlation between DC and the MPI may indicate that individuals with such a brain organization can better adopt themselves to chew well. Conversely, it may indicate that the individuals who tried to chew well would gain more experience in chewing, resulting in re-organization of brain connectivity (i.e., an effect of brain plasticity). To clarify the causal link would require a further longitudinal investigation. Finally, though we focused on the cortical, subcortical and cerebellar changes related to mastication, it should not be ignored that mastication is predominantly maintained by the CPG of the brainstem. We did not directly assess the activity of the CPG due to the difficulty in precisely recording the signal from the brainstem (Brooks et al., 2013). Therefore, our results are limited to the pattern of cortical, subcortical and cerebellar signatures related to mastication.
Clinically, declined masticatory performance is related to worsening peripheral conditions, such as a decreased number of teeth, decreased saliva flow rate, or the reduction in biting force or masticatory muscle volume (Hatch et al., 2001; Ikebe et al., 2011). Our findings highlighted that, in addition to these peripheral factors, individual differences in the brain signatures may contribute to the age-related decline in masticatory performance. Decreased abilities in sensorimotor integration, attentional control or attentional monitoring, which are commonly age-related (Hedden and Gabrieli, 2004), may also contribute to the decline in the masticatory performance in OA. The findings suggest that, in addition to maintaining good oral health or installing a suitable dental prosthesis, training and exercise to increase oral motor and sensory functions play an important role in mastication. As age increases, a widespread engagement of cortical, subcortical and cerebellar regions may reflect an increased demand in sensorimotor integration, attentional control and monitoring for OA to chew.
Author Contributions
CL initiated, conceived, performed the study, analyzed data and drafted the manuscript. CW, SW, DC and WL conceived the study and performed the study. H-HL performed the study. All authors have participated in discussion and writing of the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewers and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
The study was funded by the Ministry of Science and Technology of Taiwan (103-2314-B-010-025-MY3). This work was supported in part by the 3T MRI Core Facility in National Yang-Ming University.
Footnotes
References
Avivi-Arber, L., Martin, R., Lee, J. C., and Sessle, B. J. (2011). Face sensorimotor cortex and its neuroplasticity related to orofacial sensorimotor functions. Arch. Oral Biol. 56, 1440–1465. doi: 10.1016/j.archoralbio.2011.04.005
Brooks, J. C., Faull, O. K., Pattinson, K. T., and Jenkinson, M. (2013). Physiological noise in brainstem fMRI. Front. Hum. Neurosci. 7:623. doi: 10.3389/fnhum.2013.00623
Chan, M. Y., Park, D. C., Savalia, N. K., Petersen, S. E., and Wig, G. S. (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proc. Natl. Acad. Sci. U S A 111, E4997–E5006. doi: 10.1073/pnas.1415122111
Chao-Gan, Y., and Yu-Feng, Z. (2010). DPARSF: A MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13. doi: 10.3389/fnsys.2010.00013
Demirakca, T., Cardinale, V., Dehn, S., Ruf, M., and Ende, G. (2016). The exercising brain: changes in functional connectivity induced by an integrated multimodal cognitive and whole-body coordination training. Neural Plast. 2016:8240894. doi: 10.1155/2016/8240894
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Diedrichsen, J., Balsters, J. H., Flavell, J., Cussans, E., and Ramnani, N. (2009). A probabilistic MR atlas of the human cerebellum. Neuroimage 46, 39–46. doi: 10.1016/j.neuroimage.2009.01.045
Eickhoff, S. B., Paus, T., Caspers, S., Grosbras, M. H., Evans, A. C., Zilles, K., et al. (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36, 511–521. doi: 10.1016/j.neuroimage.2007.03.060
Fujiyama, H., Van Soom, J., Rens, G., Gooijers, J., Leunissen, I., Levin, O., et al. (2016). Age-related changes in frontal network structural and functional connectivity in relation to bimanual movement control. J. Neurosci. 36, 1808–1822. doi: 10.1523/JNEUROSCI.3355-15.2016
Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N., Friston, K. J., and Frackowiak, R. S. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14, 21–36. doi: 10.1006/nimg.2001.0786
Hama, Y., Kanazawa, M., Minakuchi, S., Uchida, T., and Sasaki, Y. (2014). Reliability and validity of a quantitative color scale to evaluate masticatory performance using color-changeable chewing gum. J. Med. Dent. Sci. 61, 1–6.
Hatch, J. P., Shinkai, R. S., Sakai, S., Rugh, J. D., and Paunovich, E. D. (2001). Determinants of masticatory performance in dentate adults. Arch. Oral Biol. 46, 641–648. doi: 10.1016/s0003-9969(01)00023-1
Hayakawa, I., Watanabe, I., Hirano, S., Nagao, M., and Seki, T. (1998). A simple method for evaluating masticatory performance using a color-changeable chewing gum. Int. J. Prosthodont. 11, 173–176.
Hedden, T., and Gabrieli, J. D. (2004). Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 5, 87–96. doi: 10.1038/nrn1323
Heuninckx, S., Wenderoth, N., Debaere, F., Peeters, R., and Swinnen, S. P. (2005). Neural basis of aging: the penetration of cognition into action control. J. Neurosci. 25, 6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005
Ikebe, K., Matsuda, K., Kagawa, R., Enoki, K., Yoshida, M., Maeda, Y., et al. (2011). Association of masticatory performance with age, gender, number of teeth, occlusal force and salivary flow in Japanese older adults: is ageing a risk factor for masticatory dysfunction? Arch. Oral Biol. 56, 991–996. doi: 10.1016/j.archoralbio.2011.03.019
Kelly, C., and Castellanos, F. X. (2014). Strengthening connections: functional connectivity and brain plasticity. Neuropsychol. Rev. 24, 63–76. doi: 10.1007/s11065-014-9252-y
Lin, C.-S., Ku, H.-L., Chao, H.-T., Tu, P.-C., Li, C.-T., Cheng, C.-M., et al. (2014). Neural network of body representation differs between transsexuals and cissexuals. PLoS One 9:e85914. doi: 10.1371/journal.pone.0085914
Lin, C. S., Wu, S. Y., Wu, C. Y., and Ko, H. W. (2015). Gray matter volume and resting-state functional connectivity of the motor cortex-cerebellum network reflect the individual variation in masticatory performance in healthy elderly people. Front. Aging Neurosci. 7:247. doi: 10.3389/fnagi.2015.00247
Liu, Y., Liang, M., Zhou, Y., He, Y., Hao, Y., Song, M., et al. (2008). Disrupted small-world networks in schizophrenia. Brain 131, 945–961. doi: 10.1093/brain/awn018
Lotze, M., Domin, M., and Kordass, B. (2016). Symmetry of fMRI activation in the primary sensorimotor cortex during unilateral chewing. Clin. Oral Investig. 1–7. doi: 10.1007/s00784-016-1858-4 [Epub ahead of print].
Mattay, V. S., Fera, F., Tessitore, A., Hariri, A. R., Das, S., Callicott, J. H., et al. (2002). Neurophysiological correlates of age-related changes in human motor function. Neurology 58, 630–635. doi: 10.1212/wnl.58.4.630
Menon, V., and Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. doi: 10.1007/s00429-010-0262-0
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B., and Bandettini, P. A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905. doi: 10.1016/j.neuroimage.2008.09.036
Onozuka, M., Fujita, M., Watanabe, K., Hirano, Y., Niwa, M., Nishiyama, K., et al. (2002). Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J. Dent. Res. 81, 743–746. doi: 10.1177/154405910208101104
Onozuka, M., Fujita, M., Watanabe, K., Hirano, Y., Niwa, M., Nishiyama, K., et al. (2003). Age-related changes in brain regional activity during chewing: a functional magnetic resonance imaging study. J. Dent. Res. 82, 657–660. doi: 10.1177/154405910308200817
Pascual-Leone, A., Amedi, A., Fregni, F., and Merabet, L. B. (2005). The plastic human brain cortex. Annu. Rev. Neurosci. 28, 377–401. doi: 10.1146/annurev.neuro.27.070203.144216
Paus, T. (2001). Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2, 417–424. doi: 10.1038/35077500
Quintero, A., Ichesco, E., Myers, C., Schutt, R., and Gerstner, G. E. (2013). Brain activity and human unilateral chewing: an fMRI study. J. Dent. Res. 92, 136–142. doi: 10.1177/0022034512466265
Ramnani, N. (2014). Automatic and controlled processing in the corticocerebellar system. Prog. Brain Res. 210, 255–285. doi: 10.1016/b978-0-444-63356-9.00010-8
Schimmel, M., Christou, P., Herrmann, F., and Müller, F. (2007). A two-colour chewing gum test for masticatory efficiency: development of different assessment methods. J. Oral Rehabil. 34, 671–678. doi: 10.1111/j.1365-2842.2007.01773.x
Seidler, R. D., Bernard, J. A., Burutolu, T. B., Fling, B. W., Gordon, M. T., Gwin, J. T., et al. (2010). Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 34, 721–733. doi: 10.1016/j.neubiorev.2009.10.005
Song, J., Birn, R. M., Boly, M., Meier, T. B., Nair, V. A., Meyerand, M. E., et al. (2014). Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect. 4, 662–676. doi: 10.1089/brain.2014.0286
Song, X.-W., Dong, Z.-Y., Long, X.-Y., Li, S.-F., Zuo, X.-N., Zhu, C.-Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6:e25031. doi: 10.1371/journal.pone.0025031
Stoodley, C. J., and Schmahmann, J. D. (2010). Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46, 831–844. doi: 10.1016/j.cortex.2009.11.008
Stoodley, C. J., Valera, E. M., and Schmahmann, J. D. (2012). Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59, 1560–1570. doi: 10.1016/j.neuroimage.2011.08.065
Tomasi, D., and Volkow, N. D. (2012). Aging and functional brain networks. Mol. Psychiatry 17, 549–558. doi: 10.1038/mp.2011.81
Van Dijk, K. R., Sabuncu, M. R., and Buckner, R. L. (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438. doi: 10.1016/j.neuroimage.2011.07.044
Ward, N. S. (2006). Compensatory mechanisms in the aging motor system. Ageing Res. Rev. 5, 239–254. doi: 10.1016/j.arr.2006.04.003
Wiech, K., Jbabdi, S., Lin, C. S., Andersson, J., and Tracey, I. (2014). Differential structural and resting state connectivity between insular subdivisions and other pain-related brain regions. Pain 155, 2047–2055. doi: 10.1016/j.pain.2014.07.009
Windel, A. S., Mihai, P. G., and Lotze, M. (2015). Neural representation of swallowing is retained with age. A functional neuroimaging study validated by classical and Bayesian inference. Behav. Brain Res. 286, 308–317. doi: 10.1016/j.bbr.2015.03.009
Wolpert, D. M., Goodbody, S. J., and Husain, M. (1998). Maintaining internal representations: the role of the human superior parietal lobe. Nat. Neurosci. 1, 529–533. doi: 10.1038/2245
Wu, T., and Hallett, M. (2005). The influence of normal human ageing on automatic movements. J. Physiol. 562, 605–615. doi: 10.1113/jphysiol.2004.076042
Zuo, X. N., Ehmke, R., Mennes, M., Imperati, D., Castellanos, F. X., Sporns, O., et al. (2012). Network centrality in the human functional connectome. Cereb. Cortex 22, 1862–1875. doi: 10.1093/cercor/bhr269
Keywords: aging, magnetic resonance imaging, mastication, functional connectivity, graph theory
Citation: Lin C, Wu C, Wu S, Lin H-H, Cheng D and Lo W (2017) Age-Related Difference in Functional Brain Connectivity of Mastication. Front. Aging Neurosci. 9:82. doi: 10.3389/fnagi.2017.00082
Received: 01 January 2017; Accepted: 16 March 2017;
Published: 03 April 2017.
Edited by:
P. Hemachandra Reddy, Texas Tech University Health Sciences Center, USAReviewed by:
Sabarish Ramachandran, Texas Tech University Health Sciences Center, USAYang-soo Yoon, Texas Tech University Health Sciences Center, USA
Copyright © 2017 Lin, Wu, Wu, Lin, Cheng and Lo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chia-shu Lin, winzlin@ym.edu.tw
 Chia-shu Lin
Chia-shu Lin Ching-yi Wu
Ching-yi Wu Shih-yun Wu1,3
Shih-yun Wu1,3  Dong-hui Cheng
Dong-hui Cheng