Variants in SNCA Gene Are Associated with Parkinson’s Disease Risk and Cognitive Symptoms in a Brazilian Sample
- 1Memory Studies Laboratory, Physiology Department, Universidade Federal do Rio Grande do Norte, Natal, Brazil
- 2Molecular Biology and Gene Expression Laboratory, Universidade Federal de Alagoas, Arapiraca, Brazil
- 3Medicine Department, Universidade Federal do Rio Grande do Norte, Natal, Brazil
- 4Bioscience Department, Universidade Federal de Sergipe, Itabaiana, Brazil
- 5Department of Cell Biology, Embryology and Genetics, Universidade Federal de Santa Catarina, Florianópolis, Brazil
- 6Biosciences Department, Universidade Federal de São Paulo, Santos, Brazil
- 7Faculty of Medicine, Universidade Federal de Alagoas, Maceió, Brazil
- 8Behavioral Neuroscience Laboratory, Pharmacology Department, Universidade Federal de São Paulo, São Paulo, Brazil
Genetic susceptibility contributes to the etiology of sporadic Parkinson’s Disease (PD) and worldwide studies have found positive associations of polymorphisms in the alpha-synuclein gene (SNCA) with the risk for PD. However, little is known about the influence of variants of SNCA in individual traits or phenotypical aspects of PD. Further, there is a lack of studies with Latin-American samples. We evaluated the association between SNCA single nucleotide polymorphisms (single nucleotide polymorphisms, SNPs – rs2583988, rs356219, rs2736990, and rs11931074) and PD risk in a Brazilians sample. In addition, we investigated their potential interactions with environmental factors and specific clinical outcomes (motor and cognitive impairments, depression, and anxiety). A total of 105 PD patients and 101 controls participated in the study. Single locus analysis showed that the risk allele of all SNPs were more frequent in PD patients (p < 0.05), and the associations of SNPs rs2583988, rs356219, and rs2736990 with increased PD risk were confirmed. Further, the G-rs356219 and C-rs2736990 alleles were associated with early onset PD. T-rs2583988, G-rs356219 and C-2736990 alleles were significantly more frequent in PD patients with cognitive impairments than controls in this condition. In addition, in a logistic regression model, we found an association of cognitive impairment with PD, and the practice of cognitive activity and smoking habits had a protective effect. This study shows for the first time an association of SNCA polymorphism and PD in a South-American sample. In addition, we found an interaction between SNP rs356219 and a specific clinical outcome, i.e., the increased risk for cognitive impairment in PD patients.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease. This condition mainly affects motor function, but also causes non-motor symptoms (Fahn, 2003; Wirdefeldt et al., 2011; Pihlstrøm et al., 2013). Among other neurological sings, the cardinal features of this disorder are bradykinesia, rigidity and resting tremor. In parallel, impairment of executive functions and the presence of apathy, anxiety, and depression are the main neuropsychiatric manifestations in PD patients (Rodriguez-Oroz et al., 2009). The onset of PD is usually after 50 years old, and a sharp increase of the incidence is seen after the age of 60 (1% of the population; Lau and Breteler, 2006). Although PD’s etiology remains unclear, the interaction between genetic and environmental substrates has been associated with the development of the disease (Lau and Breteler, 2006; Wirdefeldt et al., 2011). Among those environmental factors, several studies pointed the inverse correlation between cigarette smoking and PD risk (Allam et al., 2004; Li et al., 2015). On the other hand, history of professional pesticide exposure, rural living or well water drinking were reported to increase PD risk (Semchuk et al., 1991; Firestone et al., 2005). In addition, physical activity (Paillard et al., 2015; Shih et al., 2016), cognitive reserve (Hindle et al., 2014, 2015) and caffeine intake (Costa et al., 2010) are suggested as protective factors, but with insufficiently consistent results.
Genome-wide association studies (GWAS) have identified single nucleotide polymorphisms (SNP) in many candidate genes that contribute to PD susceptibility such as microtubule-associated protein tau (MAPT), leucine-rich repeat kinase (LRRK2) and alpha-synuclein (SNCA) (Mata et al., 2011; Satake et al., 2009; Simón-Sánchez et al., 2009; Sharma et al., 2012). The relevance of SNCA variations for PD risk is already well established through linkage and GWAS studies. Moreover, certain polymorphisms of SNCA are among the major risk factors for sporadic PD (Simón-Sánchez et al., 2009) and have been correlated with increased plasmatic levels of alpha-synuclein (Mata et al., 2010).
The presynaptic protein alpha-synuclein is the major component of the Lewy body, which is the pathological hallmark of PD (Spillantini et al., 1997, 1998; Trojanowski and Lee, 1998; Xu et al., 2015). The physiological function of alpha-synuclein implicates molecular mechanisms of dopaminergic neurotransmission such as regulation of oxidative stress, maintenance of synaptic function and neuronal trafficking (Schapira, 2007; Bendor et al., 2013; Eisbach and Outeiro, 2013). The overexpression of alpha-synuclein reduces tyrosine hydroxylase activity and dopamine release (Perez et al., 2002; Ozansoy and Basak, 2012), disrupts microtubule-dependent trafficking (Lee et al., 2006), increases oxidative by complex I mitochondrial dysfunction (Mullin and Schapira, 2013; Wu-Chou et al., 2013) and impairs neurotransmitter storage which leads to cytoplasmic accumulation (Lotharius and Brundin, 2002). Mutant protein can result in increased dopamine intracytoplasmic concentration, which contributes to raise the sensitivity to dopamine toxicity by reactive oxygen species generation (Tabrizi et al., 2000).
Case-control studies in different populations have also found associations between several SNCA polymorphisms and increased risk of PD. For example, the dinucleotide repeat REP1 located in SNCA promoter (SNCA-Rep1) and the 3′ untranslated region (UTR) variants have been broadly investigated (Pals et al., 2004; Hu et al., 2010; Ritz et al., 2012). Variations in these regions may confer susceptibility to PD by altering transcription factor binding sites (Chiba-Falek and Nussbaum, 2001; Chiba-Falek et al., 2003) and generating or destroying microRNA target sites, which in turn modifies gene expression (Wang et al., 2008; Sotiriou et al., 2009; Mccarthy et al., 2011). Several investigations had focused on the association between SNCA SNPs and PD in ethnic groups, most of them performed in Caucasian and Asian populations. Hence, the results may be applicable only for these groups (Han et al., 2015). To our knowledge, no studies have investigated associations of SNCA polymorphisms in South American populations.
Currently, there is much interest in the search for clinical predictors of motor and non-motor symptoms in PD. Notwithstanding, most of the gene association studies are limited to genetic risk factor data. Importantly, the consequences of genetic variability on clinicalphenotypes, as well the interaction between genetic and environmental substrates, are poorly elucidated. Few studies pointed weak or absence of associations with clinical outcomes such as motor impairment (Ritz et al., 2012; Markopoulou et al., 2014), anxiety, or depression (Verbaan et al., 2008; Guo et al., 2014; Chen W. et al., 2015; Cheng et al., 2016), sleep, and autonomic disorders (Verbaan et al., 2008; Chen W. et al., 2015), and cognitive impairments (Verbaan et al., 2008; Guo et al., 2014; Chen W. et al., 2015; Chen Y.P. et al., 2015; Cheng et al., 2016; Wang et al., 2016). Thus, there is little information concerning motor and non-motor symptoms assessment, lifestyle and environmental expositions. Gene-environmental studies investigate whether environmental factors such as smoking habits and coffee consumption could modify genetic associations with PD (Gao et al., 2012; Miyake et al., 2012; Ritz et al., 2012; Trotta et al., 2012). However, studies that investigate possible SNCA polymorphisms associations with specific clinical aspects of PD are inconclusive. Therefore, the elucidation of the genetic contribution to clinical phenotypes remains a challenge. In this respect, the description of genetic predictors and their relationship with other etiological factors and clinical outcomes is determinant to improve the knowledge of pathophysiological pathways and help to target the best therapeutic program.
In the present study, we investigated possible interactions between polymorphisms in the SNCA gene and PD in a Brazilian sample, and examined potential associations between these polymorphisms and environmental factors and specific clinical outcomes.
Subjects and Methods
Patients and Controls
The unrelated sample consisted of 105 PD patients and 101 control subjects recruited from Onofre Lopes University Hospital, in Rio Grande do Norte (Northeastern – Brazil) from June, 2013 to November, 2014. PD was diagnosed by a neurologist according to UK Parkinson’s Disease Society Brain Bank Clinical Criteria (Hughes, 2004). Recruitment of control subjects was conducted in the same hospital at other departments than the neurology department. Controls were subjects from the general population without neurological disease and family history of PD. The groups were matched by age and sex. This study was approved by the ethical committee of Onofre Lopes University Hospital (protocol number 04261012.5.1001.5292). All the patients and controls were requested to sign the written informed consent.
Clinical Assessment
Case and control subjects filled out a set of eight questionnaires. Information of demographic variables and medical history, such as sex, age, education level, age at PD onset and disease duration were obtained in the baseline interview. Family history was considered positive until second-degree relatives. A structured questionnaire of environmental factors delivered information about risk (pesticide exposition, living in rural areas and well-water consumption) and protective (smoking habits, coffee intake, physical exercises and cognitive activities) factors. History of smoking was defined on basis of self-report as never vs. ever having smoked at least once a day for at least one year (Miyake et al., 2012). Coffee consumption was assessed on basis of self-report and consumption was defined as more than two coffee cups per day (Trotta et al., 2012). Similarly, self-reported physical exercises or cognitive actives (i.e., reading, crossword puzzles, card games, chess, and others) were considered when carried out at least once a week.
The evaluation of different clinical aspects comprised the application of the following inventories: (1) PD motor symptoms were assessed by Unified Parkinson’s Disease Rating Scale (UPDRS I, II, and III) (Fahn and Elton, 1987), Hoehn & Yahr scale (HY) (Hoehn and Yahr, 1967) and Schwab and England Activities Daily Living Scale (SE); (2) emotional status was assessed by Beck Depression Inventory (BDI) (Beck et al., 1988b) with a cut-off score of 10 to detect depression (Tröster et al., 1995) and Beck Anxiety Inventory (BAI) (Beck et al., 1988a) with a cut-off score of 10 to detect anxiety (Julian, 2011). The severities of depression and anxiety were determined, according to the following scores, respectively: moderate (BDI: 19–29 points; BAI: 20–30 points) and severe (BDI: higher than 29 points; BAI: higher than 30 points); (3) Mini Mental State Examination (Folstein et al., 1975) and Frontal Assessment Battery (FAB) (Beato et al., 2007) assessed cognitive functions. Cognitive impairment was defined by the application of the cut- off MMSE scores taking the educational level into consideration: 20 for illiterate, 25 for lower education (1 to 4 years), 27 for middle education (5 to 8 years), and 28 for high education (greater than 8 years) (Brucki et al., 2003). All clinical evaluations were conducted during the “on” state of levodopa treatment.
DNA Extraction and Genotyping
Genomic DNA was extracted from EDTA-containing peripheral blood samples (commercial kit FlexiGene® DNA kit, Qiagen, Germany). Genotyping of SNPs rs2583988 (C > T), rs356219 (A > G), rs2736990 (T > C), and rs11931074 (G > T) in SNCA gene was performed using real-time TaqMan® polymerase chain reaction assay according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, United States). Four samples were lost due to poor quality of DNA. 104 PD patients and 98 controls were successfully genotyped.
Statistical Analysis
Non-parametric data were presented as median [minimum value; maximum value]. Inventories scores were compared between groups with Mann-Whitney and Kruskal-Wallis given the nonparametric nature of the data. X2 statistics (Fisher exact test) was used to compare categorical data, calculation of frequency significance and odds ratio (OR). Parametric data were presented as mean and standard deviation. T-student independent test was used to assess differences in mean age at interview between PD and control groups. Calculations of Hardy–Weinberg equilibrium, linkage disequilibrium (LD), estimation of haplotypes and haplotype frequency were performed by the software Snpstat1. Crude odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate risk size for the heterozygotes and homozygotes for the risk alleles by binary logistic regression analysis, adjusted for age and gender. The statistical power calculation was performed using G ∗ Power statistical program. Statistical significance was defined when p-values < 0.05.
Results
Study Population
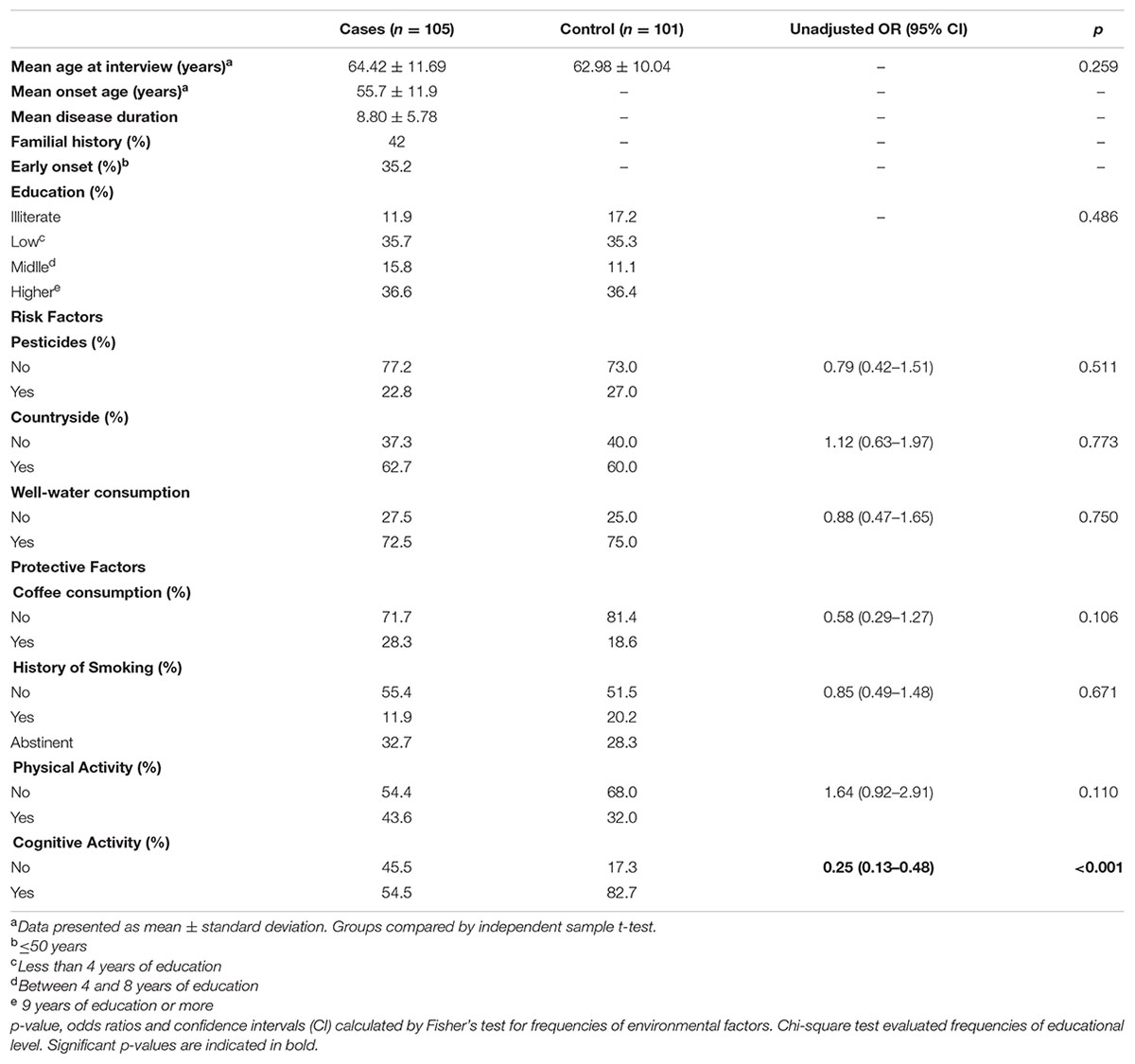
Two hundred and six subjects participated in the study. In PD group (n = 105, 73 men and 32 women) the mean age was 64.42 years (range: 37–89 years) and in control group (n = 101, 68 men and 33 women) it was 62.98 years (range: 38–88 years), without significant difference (U = 4632.00, p = 0.260) (Table 1). The majority of PD and control subjects were married, living in urban area and had basic education (35.7% of cases and 35.3% of controls had less than four years of education). There were no differences in percent of subjects in each level of education between groups (p = 0.486). Most of PD cases (81.4%) were retired while 49% of controls subjects had a work activity.
PD Group’s Profile
The mean age of PD onset was 55.7 ± 11.9 years (range 30–87), with 37 patients (35.2%) classified as early onset (≤50 years). The mean of disease duration was 8.80 ± 5.78 years (range 1–32) and of treatment duration was 5.6 ± 4.5 years (range 0–21). Seventy patients (69.3%) describe their first symptom as involuntary tremors. A positive family history was reported by 45 patients (42.9%). Those cases were categorized as “familial”, the remaining subjects as “sporadic” (Table 1).
Frequencies of exposition to environmental factors are shown in Table 1. We found a similar frequency of exposure to risk factors (pesticide contact, living in countryside and well-water consumption) and protective factors (coffee consumption, smoking habits, and physical activities) between groups, without significant associations with PD risk. Although most of the subjects reported living in countryside (62.7% in cases and 60% in controls), there were few reports of pesticide use in agriculture (22.8% in cases and 27% in controls). There was a low frequency of reported coffee consumption (28.3% in cases and 18.6% in controls) and current smokers (11.9% in cases and 20.2% in controls) in our sample. We found a higher frequency of practice of cognitive activities in the control group, and this factor showed a strong protective effect against PD (OR = 0.25; 95% CI = 0.13–0.48).
Clinical Assessment
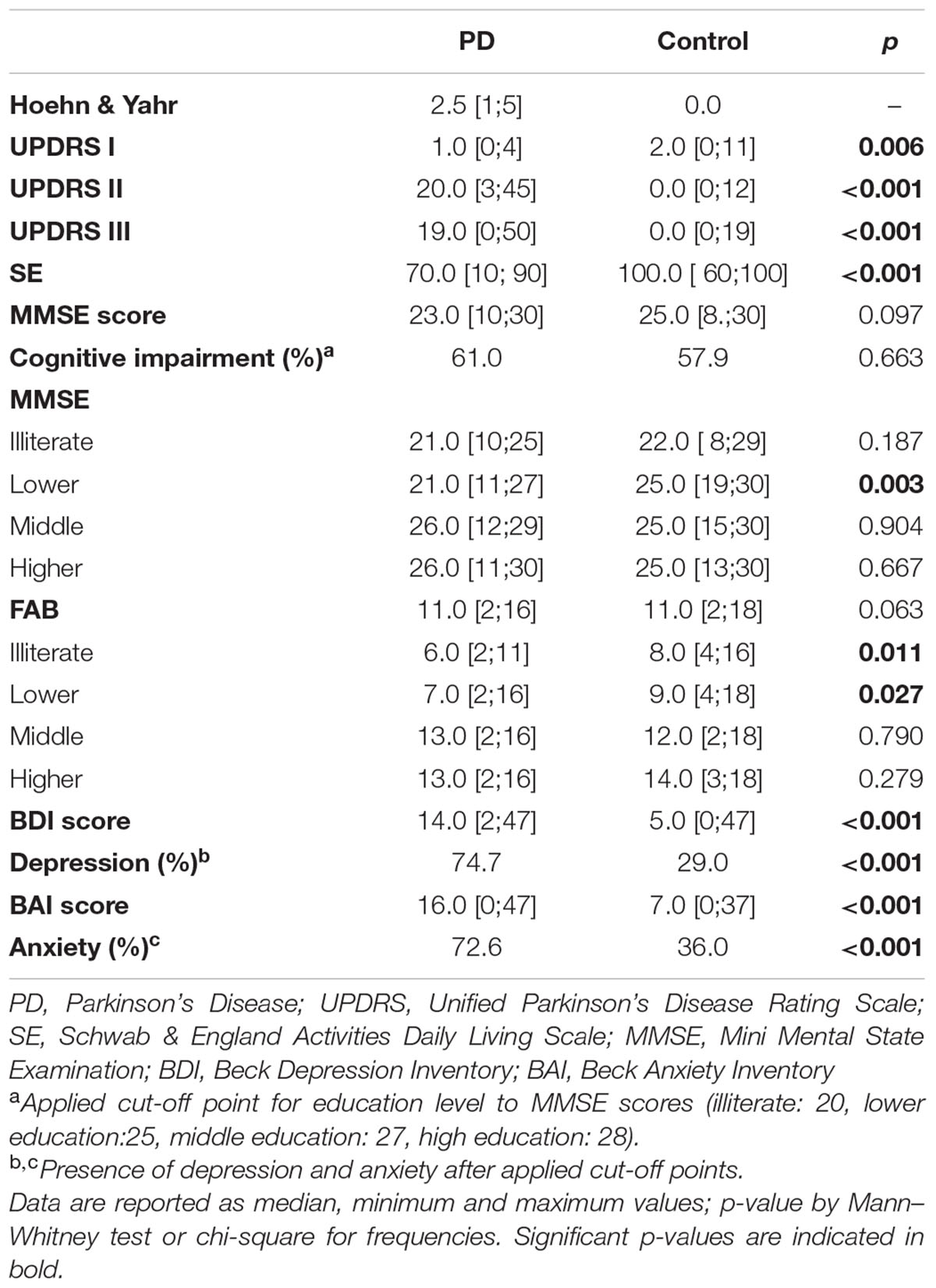
Performances of PD and control group in the clinical assessment are described in Table 2. Median of disease progression measured by Hoehn & Yahr scale was 2.5 [1–5], and 53% of the patients were in stages III to V. Assessment of motor activity measured by UDPRS III indicated a significant motor impairment in PD group [19.0 [0–50] versus 0.0 [0–19]; U = 181.0, p < 0.001]. PD patients also showed greater difficulty to perform daily living activities (20.0 [3–45 versus 0.0 [0–12]) when evaluated by UDPRS II (U = 82.5, p < 0.001). In addition, patients presented a reduction of functional independence (70.0 [10–90] versus 100.0 [60–100]; U = 296.0, p < 0001).
Total score of MMSE (PD: 23[10-30] versus Control: 25.0 [8–30]) and FAB PD: 11.0 [2–16] versus control: 11.0 [2–18]) (Table 2), as well as the presence of cognitive impairment (61% in PD and 57.9% in control group) did not differ between groups [χ2(1) = 0.243; p = 0.663]. However, we observed a significant lower score of MMSE in PD group after the stratification of data by educational levels, which indicates a larger cognitive impairment in the subgroup of PD patients with lower education compared to controls with the same level of education (21.0 [11–27] versus 25.0 [19–30]) (U = 336.50, p = 0.003). Similar effect of education level was found after the stratification of FAB scores. Regarding emotional evaluation, PD group had significantly higher scores in BDI (11.0 [2–47] versus 5.0 [4–74]) and BAI (16.0 [0–47] versus 7.0 [0–37]) than the control group. Furthermore, levels of depression and anxiety were significantly more severe in the PD group (BDI: 24.2% moderate and 10.5% severe; BAI: 26.3% moderate and 9.5% severe, respectively), which resulted in a higher frequency of depression [74.7% versus 2%; χ2(1) = 40.971, p < 0.001] and anxiety symptoms [72.6% versus 36.0%; χ2(1) = 26.305, p < 0.001] in the PD group.
SNP Assessment
Deviation from Hardy-Weinberg equilibrium was not observed for any of the SNPs. 104 participants in PD group and 98 in control group were genotyped for all SNPs. Linkage disequilibrium (LD) structure of SNCA indicates a significant pairwise value of LD between rs356219 and rs2736990 (r2 = 0.888, D′ = 0.988, p < 0.001). Allele and genotype distributions of SNPs in patients and controls are summarized in Table 3.
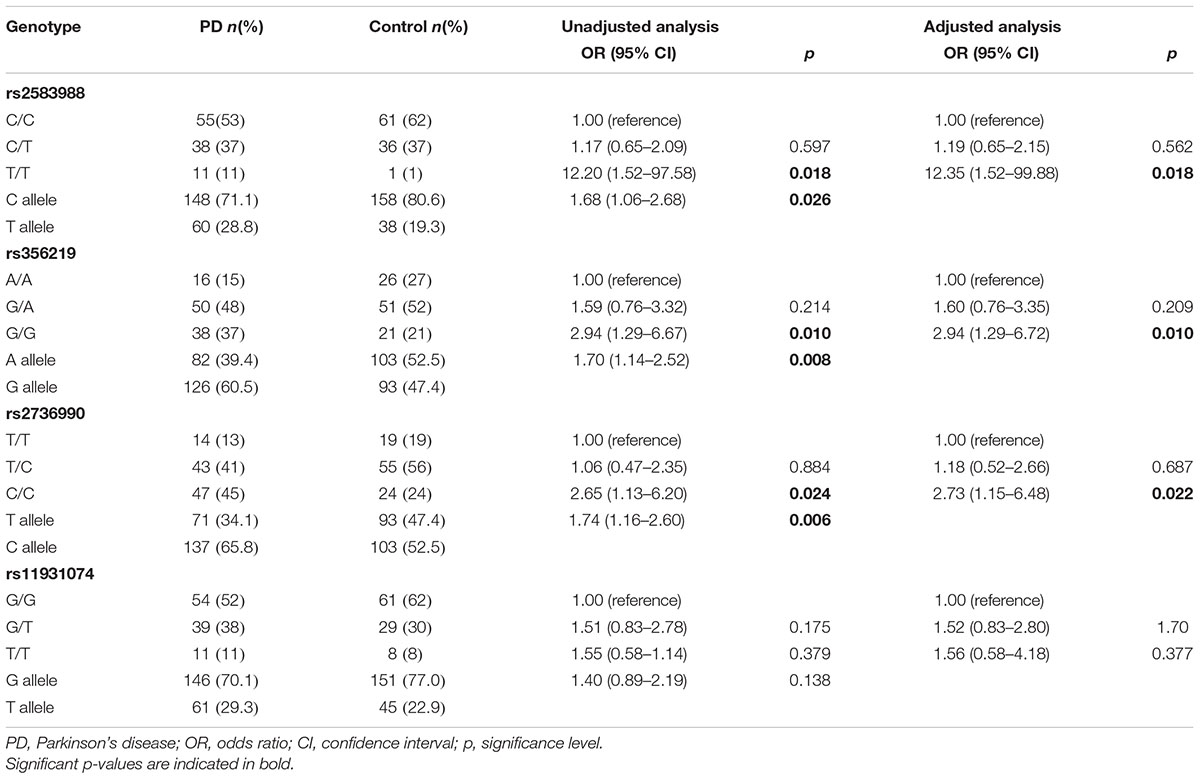
TABLE 3. Genotypic and allelic frequencies of single nucleotide polymorphisms (SNPs) in alpha-synuclein gene (SNCA) gene in PD cases (n = 104) and controls (n = 98).
Single locus analysis of SNPs rs2583988, rs356219, rs2736990, and rs11931074 showed that the risk alleles of each SNP, as well as homozygotes for these alleles, were more frequent in PD patients compared to controls. Logistic regression analysis confirmed a significant association between risk genotypes and PD for rs2583988 (OR = 12.20, 95%IC: 1.52–97.58, p = 0.018), rs356219 (OR = 2.94, 95%IC: 1.29–6.67, p = 0.010), rs2736990 (OR = 2.65, 95%IC: 1.16–2.60, p = 0.024), that remained significant after correction for the covariates age and sex. There was no significant difference in genotype distribution for rs11931074 between patients and controls (OR = 1.55, 95% IC: 0.58–1.14, p = 0.379). Statistical power calculation applied to the sample size used in this research indicate a detection of a gene-disease association for SNPs rs2583988, rs356219, rs2736990 with values of OR higher than 2.60, with an accuracy between 68 and 85% under the recessive model.
Age at disease onset was not different between the three genotypes for the SNPs evaluated (data not shown). However, frequency analyses of risk allele in patients with early disease onset (EOPD) indicated a significant higher frequency of G-rs356219 and C-rs2736990 in patients when compared to controls (p = 0.040; p = 0.028, respectively), with OR indicating an increased risk of 1.82 (95%IC: 1.05–3.14) and 1.88 (95%IC: 1.07–3.29), respectively (Table 4).
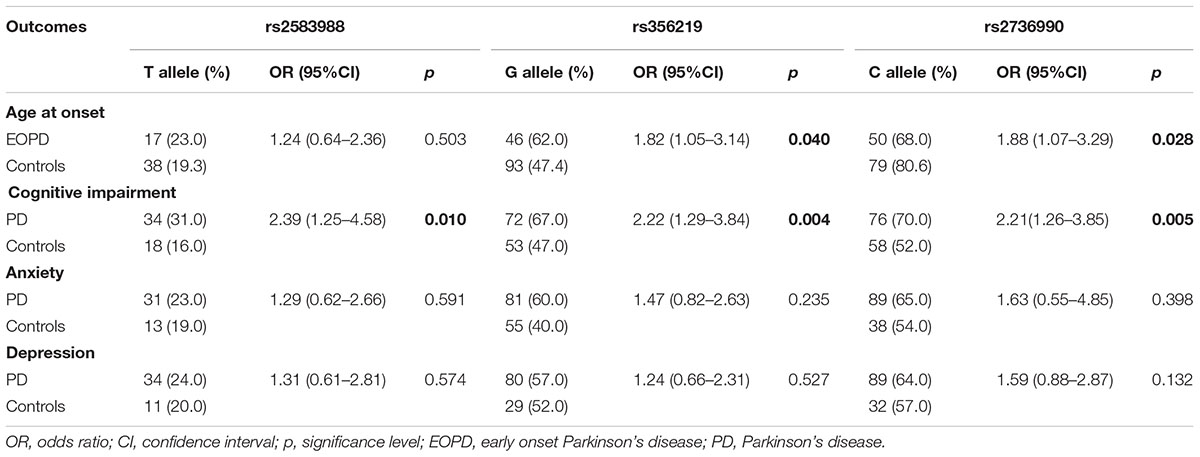
TABLE 4. Comparisons of risk allele frequencies between case and control with non-motor clinical outcomes.
The frequencies of risk alleles in cases and control groups considering only the subjects that presented cognitive impairment, anxiety and depression by clinical assessment were described in Table 4. There were no differences in the frequency of risks alleles between groups considering presence of depression and anxiety. However, the risk alleles T-rs2583988, G-rs356219, and C-2736990 had higher frequencies in patients (31, 67, and 70%, respectively) than controls (16, 47, and 52%) with cognitive impairments (ORs = 2.21 to 2.39). When patients were stratified by genotypes, Kruskal-Wallis test detected no differences in the scores of disease progression (HY stage), daily living activities (UPDRS-II score) or motor assessment (UPDRS-III score) between genotypes in each SNP and assessment (data not shown).
Analyses restricted to patients were performed to investigate whether risk genotypes influenced clinical outcomes (cognitive impairments, anxiety and depression). Table 5 shows the significant and marginal associations with each SNP in SNCA by binary logistic regression. There was no significant association with motor impairment. Both rs356219 heterozygotes (OR = 4.74, 95% CI: 1.27–17.75, p < 0.05) and homozygotes (OR = 5.74, 95% CI: 1.42-23.21, p < 0.05) had significantly increased risk for cognitive impairment, while the CT-rs2736990 (OR = 3.87, 95% IC: 0.97–15.36, p = 0.054) and CC-rs2736990 (OR = 3.84, 95% IC: 0.96–15.30, p = 0.056) genotypes presented a trend toward to increased risk of the same outcome. No association of rs2583988 genotypes were found for this outcome. Conversely, TT-rs2583988 genotype significantly reduced the risk of depression (OR = 0.21, 95% CI: 0.04–0.97, p = 0.046) and had a marginal significance for reduced risk of anxiety (OR = 0.24, 95% CI: 0.05–1.07, p = 0.061). There was no significant association for rs2583988 heterozygote genotype and anxiety or depression. The SNPs rs356219 and rs2736990 had no association with these outcomes.
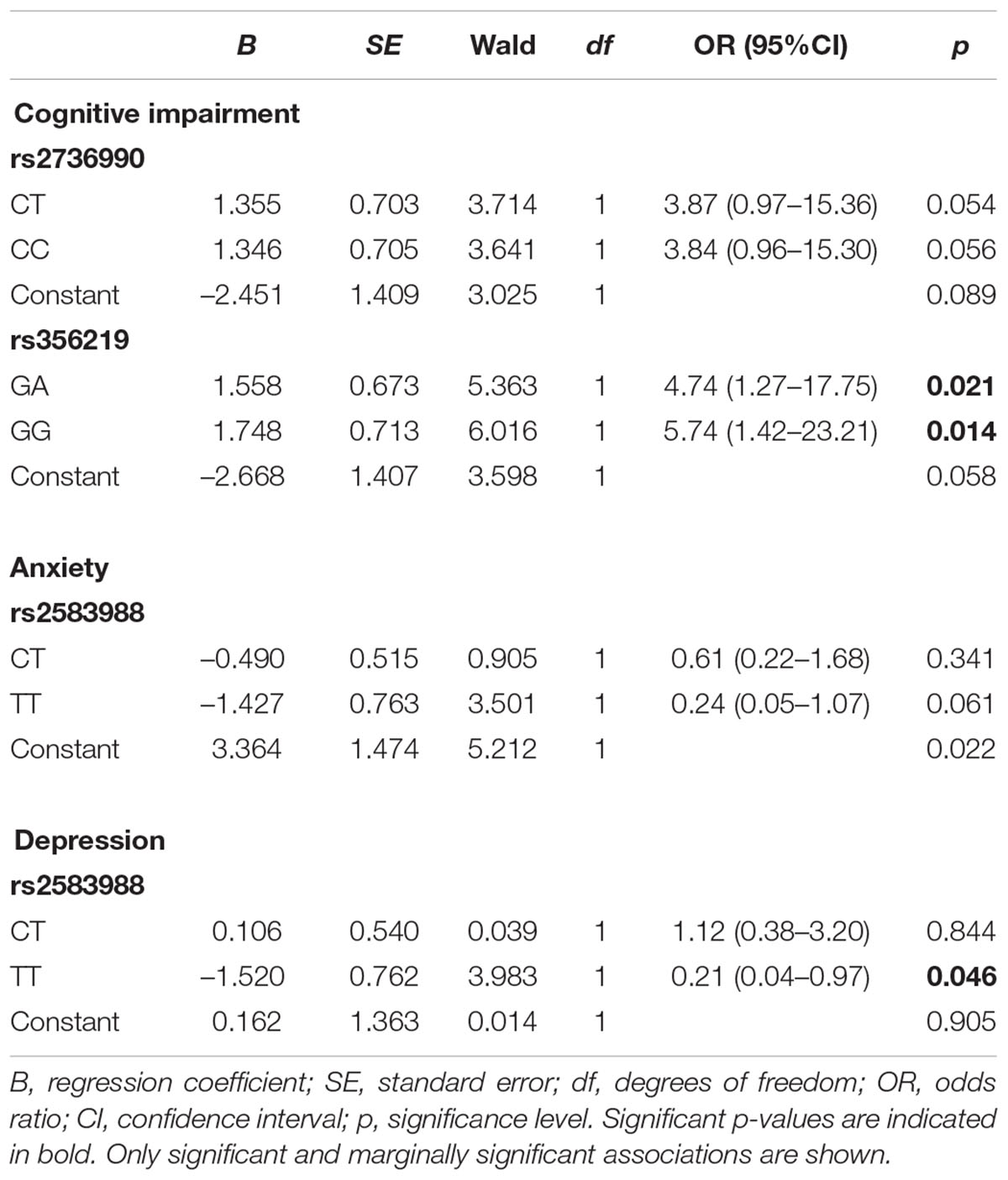
TABLE 5. Binary logistic regression for risk genotypes predicting cognitive, anxiety, and depression in PD patients.
The best logistic regression models (with case or control as dependent variable) performed to investigate genotypes, clinical aspects and environmental factors in prediction of disease are represented in Table 6. TT-rs2583988 (OR = 16.25, 95%IC: 1.72–152.97, p = 0.015) and CC-rs2736990 (OR = 2.37, 95%IC: 1.10–5.03, p = 0.026) risk genotypes were associated with increased PD risk. There was no significant improvement in the fit of the model by addition of rs356219 and rs11931074. Higher scores in BDI (OR = 1.16, 95%IC: 1.10–1.22, p < 0.001) and presence of cognitive impairment (OR 2.27, 95%IC: 1.03–4.98, p = 0.040) were directly associated with increased risk of disease. Conversely, practice of cognitive activity (OR = 0.37, 95%IC: 0.16–0.84, p = 0.019) and smoking habits (OR = 0.45, 95%IC: 0.21–0.97, p = 0.042) had a protective effect against PD.
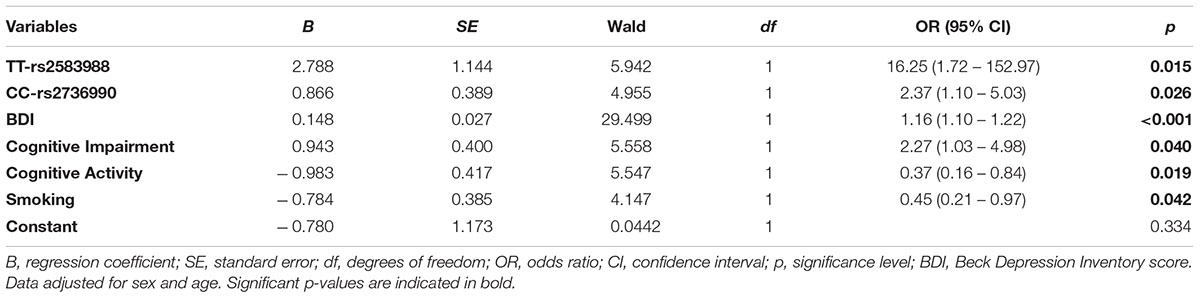
TABLE 6. Binary logistic regression model for SNPs genotypes, clinical variables, and environmental factors predicting PD.
Logistic regression analysis revealed an association with the number of risk alleles and the presence of disease (OR = 1.27, 95% CI = 1.06–1.53, p = 0.009), indicating a cumulative effect. Finally, haplotype analysis result in two blocks with significant association with PD. Table 7 shows the estimated frequency of each haplotype for cases and controls when all four markers are considered. Estimated odds ratios and associated 95% CIs indicate that haplotype T-rs2583988 + G-rs356219 + C-rs2736990 + T-rs11931074 had a greater risk for PD (OR = 2.51, 95%IC: 1.37–4.58, p = 0.003) than CGCT haplotype (OR = 1.85, 95%IC: 1.12–3.05, p = 0.017).
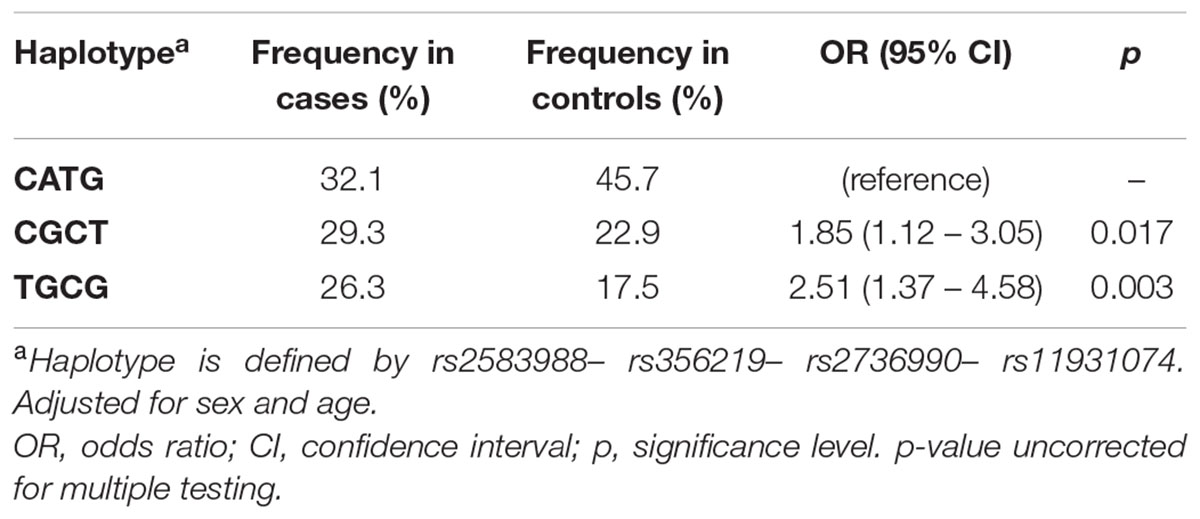
TABLE 7. Estimated haplotype frequencies for SNPs rs2583988, rs356219, rs2736990, and rs11931074 in SNCA gene.
Discussion
The present study investigated the association between four variants of the SNCA gene and clinical outcomes in Parkinson’s disease patients compared to controls in a Brazilian sample. The risk alleles and the risk genotypes were significantly more frequent in cases than in controls and the PD risk associations for SNPs rs2583988, rs356219, and rs2736990 were confirmed for the homozygote genotype. Furthermore, rs356219 and rs2736990 demonstrated a similar frequency in cases, corroborating the positive correlation for the pairs rs356219 and rs2736990. Carriers of risk allele T-rs2583988, G-rs356219, and C-rs2736990 were significantly more frequent in cases than in controls with cognitive impairments. Regression analysis suggested associations between risk alleles and clinical outcomes and the contribution of environmental factors for PD risk.
Parkinson’s disease is a neurodegenerative, chronic, and progressive disease characterized by the presence of motor (tremor, bradykinesia, rigidity, and postural instability) and non-motor (mood disturbs, cognitive alterations, sleep, and autonomic dysfunctions) symptoms (Lauterbach, 2004; Wirdefeldt et al., 2011). Studies show higher prevalence of cognitive deficits and depression in PD (Reijnders et al., 2009; Kehagia et al., 2010) and there is a clinical association between the two kinds of symptoms (Chagas et al., 2014). Our PD sample presented moderate motor impairment, higher prevalence of anxiety and depression symptoms and a similar frequency of cognitive impairment compared to control group. Cognitive impairment is a usual finding in elderly population (Ward et al., 2012) which can explain the higher frequency of this outcome in controls, and also highlight a difficulty in identifying typical cognitive alterations of PD. Furthermore, we observed an effect of educational levels on our results. The patients with lower education presented a larger degree of cognitive impairment than controls with this same educational level. In a longitudinal study, Hindle et al. (2015) demonstrated that PD patients with a higher cognitive reserve had a better performance in cognitive tests. In addition, educational experiences are essential to attenuate age-related cognitive decline, and a major protective factor in dementia (Stern, 2009). Similarly, the practice of cognitive activities was associated with decreased risk for PD in our regression model.
Environmental factors have been related with PD in epidemiologic researches (Lau and Breteler, 2006; Wirdefeldt et al., 2011). Toxins as MPTP, herbicide paraquat and pesticide rotenone are selective complex I inhibitor and induce neuronal degeneration demonstrated in vivo (Betarbet et al., 2000; Lima et al., 2012) and in vitro (Chun et al., 2001; Uversky et al., 2002; Giordano et al., 2012) studies. Studies on the association between environmental toxins and the risk for PD present inconsistent results when rural living, pesticide use and well-water consumption are assessed (Allam et al., 2005; Firestone et al., 2005). In contrast, habits as cigarette smoking (Allam et al., 2004; Li et al., 2015) and caffeine intake (Costa et al., 2010) have been linked to protective effects, even though the mechanisms underlying these protective effects remain to be clarified. In our study, the frequencies of exposure to environmental factors were similar in cases and controls, which prevented significant associations with the disease. However, cognitive activities as reading, playing cards, board games and crossword puzzles were more frequent in controls, which suggests a protective role. To our knowledge, history of cognitive stimulation through leisure cognitive activities as a protective factor to PD has not been previously described in the literature. In this respect, the association of cognitive activities and a possible decreased risk of PD described in the present study is a new finding. However, this conclusion is limited because our protocol did not allow a precise description of type, frequency, intensity, and other aspects of the self-reported cognitive activities. It is worth mention that a recent experimental study showed that environmental stimulation facilitated motor recovery and prevented cognitive impairment in a mice model of PD (Campêlo et al., 2017). Thus, although not conclusive, the present finding encourages investigations regarding the protective role of cognitive activities in PD, possibly by prospective clinical studies.
Despite the consistent importance of environmental factors in PD etiology, most genetic association studies have not incorporated gene-environmental interactions in the researches. The interaction of smoking habits and coffee consumption and SNCA variations have been investigated, but had provided inconsistent results (De Palma et al., 1998; Gao et al., 2012; Miyake et al., 2012; Ritz et al., 2012; Trotta et al., 2012). SNPs rs2583988 and rs356219 had no association with coffee drinking and cigarette smoking when investigated in an Italian sample (Trotta et al., 2012). Similarly, the SNPs rs2736990 and rs11931074 did not demonstrate significant results in a North American study (Gao et al., 2012). However, a study in a Japanese sample reported addictive interactions between SNP rs356219 and smoking for increased risk of PD in subjects with GG- rs356219 genotype who had never smoked (Miyake et al., 2012). One of the biological mechanisms proposed to explain the protective effect of smoking is that nicotine inhibits alpha-synuclein fibrillation and stabilizes soluble oligomeric forms (Hong et al., 2009). In our sample, the logistic regression model including cognitive activities and smoking habits within TT-rs2583988 and CC-rs2736990 genotypes revealed a protective effect for PD. Taking together, these results suggest that nicotine might neutralize the detrimental effect of the risk-associated genotypes of rs2583988 and rs27366990, and a higher cognitive stimulation might be protective against PD in individuals with the risk genotypes.
Genetic data supports the role of alpha-synuclein in the pathogenic process of PD. Duplications and triplications of SNCA and a higher production of alpha-synuclein correlate with disease severity (Chartier-Harlin et al., 2004; Ibáñez et al., 2004). However, these mutations of SNCA are rare and the role of common variants are investigated as modifiers of PD susceptibility. Case-control studies have linked SNCA to sporadic PD susceptibility using SNPs analysis. Two major linkage disequilibrium blocks in SNCA gene had been proposed (Mueller et al., 2005; Myhre et al., 2008): a 5′ block that extends to promoter-enhancer region to exon 4 and a 3′ block that comprises intron, 3′ untranslated region, and the 3′ end region of the gene. Associations between SNPs rs2583988 in 5′ region (Pals et al., 2004; Winkler et al., 2007; Heckman et al., 2012; Trotta et al., 2012), rs2736990 in intron 4 (Mata et al., 2011; Heckman et al., 2012; Miyake et al., 2012; Alieva et al., 2013; Guo et al., 2014; Davila-Ortiz de Montellano et al., 2016), rs356219 (Lazzarini et al., 1994; Mata et al., 2010, 2011; Botta-Orfila et al., 2011; Wider et al., 2011; Trotta et al., 2012; Brockmann et al., 2013; Emelyanov et al., 2013), and rs11931074 (Gao et al., 2012; Wu-Chou et al., 2013) in 3′ end were demonstrated by recent studies. A meta-analysis confirmed the risk association of rs2583988, rs356219, and rs11931074 variants and PD susceptibility, performed in dominant and recessive genetic models (Han et al., 2015).
Of notice, the majority of these studies were performed in Caucasian and Asian populations. Data from Latin American populations are quite recent (Davila-Ortiz de Montellano et al., 2016; García et al., 2016), and show associations for rs3857059, rs356220, rs356203, rs7684318, and rs2736990 variants and PD in Mexican samples. Further, no prior study investigated these associations in Brazilians, or even in a South American population. Our findings were in agreement with the literature, indicating a higher PD risk for homozygote genotypes in our sample for all SNPs studied, except for rs11931074 that had no significant association with the disease. The Brazilian population is one of the most heterogeneous populations in the world and such ethnical heterogeneity creates a particular background. Variations in our findings in comparison to other studies can be explained by differences in the genetic backgrounds (Dahodwala et al., 2009). Although this feature can be a limitation due to the lack of a straight biological category, several countries in Latin America and worldwide have a multiethnic profile, which reinforces the need of genetic association studies in heterogeneous populations.
Genetic polymorphisms may contribute to specific disease characteristics and play an important role in phenotypic diversity of PD. Age at onset of PD is a predictor of progression and mortality (Wirdefeldt et al., 2011) and has been associated with multiple SNPs in several studies (Rajput et al., 2009; Yu et al., 2010; Botta-Orfila et al., 2012; Brockmann et al., 2013; Pan et al., 2013; Cardo et al., 2014; Huang et al., 2015). We found a significantly higher frequency of the G-rs356219 and C-rs2736990 risk alleles in patients with earlier onset compared to controls. Similarly, case-control studies in Germanic (Brockmann et al., 2013) and Chinese (Pan et al., 2012) samples showed that G-rs356219 and C-rs2736990 alleles significantly contributed to earlier age onset. In contrast, a lack of association was observed in Italians (Trotta et al., 2012) and North Americans (Mata et al., 2011; Heckman et al., 2012), which suggest the participation of other modifying factors in age onset across different populations. Therefore, the contributions of these polymorphisms to age onset remain unclear. We speculate that increased expression of alpha-synuclein protein may result in an early manifestation of PD symptoms.
Studies have investigated the functional effects of the different SNCA SNPs on gene expression in brain tissues and protein levels in blood samples, but the results were inconsistent (Fuchs et al., 2008; Linnertz et al., 2009; Mccarthy et al., 2011; Alieva et al., 2013; Cardo et al., 2014). For example, a study with a transgenic mouse model demonstrated that REP1 variants in 5′ region affect the regulation of transcriptional activity (Cronin et al., 2009). Human studies demonstrated reduction of SNCA-mRNA levels in brain tissues and protein levels in blood in the absence of REP1 risk allele (Fuchs et al., 2008; Linnertz et al., 2009). Despite some evidence of correlations between rs2583988 and REP1 variants (Pals et al., 2004; Winkler et al., 2007; Myhre et al., 2008), no significant associations of rs2583988 were found for assessment of blood protein levels (Fuchs et al., 2008) or SNCA-mRNA (Fuchs et al., 2008; Linnertz et al., 2009; Alieva et al., 2013).
For the intronic SNP rs2736990, an investigation of gene expression revealed a trend toward lower levels of SNCA-mRNA in blood samples of PD patients (Alieva et al., 2013), but in healthy subjects the T allele was correlated with higher levels of the isoform SNCA112-mRNA in frontal cortex tissue samples.
SNPs rs356219 and rs11931074 are part of 3′ block and variants in this region may affect post-transcriptional regulation factors, such as biding sites of mRNA, and impair RNA processing or stability and gene or protein expression (Linnertz et al., 2009; Venda et al., 2010; Elbaz et al., 2011; Mccarthy et al., 2011). For rs356219, the heterozygote genotype was correlated with higher levels of mRNA in substantia nigra of PD cases (Fuchs et al., 2008). Further, G allele carriers presented higher levels of the SNCA112-mRNA isoform in frontal cortex (Mccarthy et al., 2011). However, a divergent result was found by Linnertz et al. (2009) that showed a correlation between higher levels of SNCA-mRNA and the protective allele A.
As mentioned, literature supports that polymorphisms in the SNCA gene increase genetic susceptibility to sporadic PD and suggests an increased expression of alpha-synuclein. Despite disease severity (rapid cognitive decline, severe non-motor symptoms, more widespread neurodegeneration and faster disease progression) is related to increased alpha-synuclein expression in familial PD (Venda et al., 2010), there is a lack of evidence regarding the SNCA variants’ influence on specific clinical outcomes. Previous studies that assessed associations between polymorphisms and motor impairment and disease progression (Ritz et al., 2012; Markopoulou et al., 2014; Cheng et al., 2016; Davis et al., 2016; Wang et al., 2016), cognitive functions (Goris et al., 2007; Guo et al., 2014; Markopoulou et al., 2014; Chen W. et al., 2015; Wang et al., 2016) and presence or absence of anxiety and depression (Guo et al., 2014; Chen W. et al., 2015; Cheng et al., 2016; Dan et al., 2016) present poorly consistent results.
In our study, no associations were observed regarding the distribution of risk allele and presence of depression or anxiety. Nevertheless, here we indicate that the TT- rs2583988 genotype has a protective effect against these outcomes in patients. In contrast, a study with a Chinese sample found a decreased risk of depression in carriers of REP1 risk homozygote genotype and a correlation with UPDRS part II score, motor fluctuation and female sex in prediction of PD depression (Dan et al., 2016).
Concerning cognitive aspects, T-rs2583988, C-rs2736990, and G-rs356219 risk alleles were more frequent in cases than controls with cognitive impairment, indicating a greater risk of this outcome in PD. Furthermore, we found an association of cognitive impairment with risk of PD in the logistic regression model. An unexpected found for REP1 genotypes was described in a longitudinal North American study: PD cases with higher REP1 scores were associated with better motor function and reduced risk of cognitive impairments (Markopoulou et al., 2014). These data highlight a possible dual effect or time-dependent role for SNCA variants.
Regarding motor aspects, a cohort study with American patients (Ritz et al., 2012) demonstrated an increased risk of faster decline of motor function in carriers of the REP1 263bp promoter variant and G-rs356165 allele. In our transversal study, we did not found correlations between genotypes and severity of motor symptoms in the patients.
Our study had some limitations. Environmental data were self-reported, which could provide a misclassification. Further, the MMSE used to assess cognitive impairment may not be the limited number of patients, the power analysis indicated a moderate statistical power. Irrespective, it would be interesting to expand the sample to reinforce the results. Indeed, the few conflicting results may be explained by the small sample size and differences in genetic background among different ethnic populations, as mentioned.
Conclusion
This study confirms the association between PD and SNCA SNPs and haplotypes in a Brazilian population. Further, the data provide evidence that the SNCA variants are associated with increased risk of cognitive impairment in PD patients. Therefore, our results encourage the investigation of associations between genetic variants in SNCA and specific clinical outcomes. Phenotypic studies and functional assays, with a large sample and different ethnicities should be performed to confirm and specify the nature of the associations.
Ethics Statement
This study was carried out in accordance with the recommendations of Comissão Nacional de Ética em Pesquisa (CONEP), Brazil, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the ethical committee of Onofre Lopes University Hospital (protocol number 04261012.5.1001.5292).
Author Contributions
CC and RS designed the research and wrote the paper. CC, FC, LO, AS-N, and PT collected the data. CC, DdS, and TdA conducted molecular assays. CC. analyzed the data. JS, GI, AR, and CdO contributed with theoretical support and data analysis.
Funding
The research was supported by fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant # 402054/2010-5), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Apoio à Pesquisa do Estado do Rio Grande do Norte (FAPERN/PRONEX) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grant #2015/12308-5).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
The authors would like to thank Ywilliane Meurer for helpful suggestions and Valter Andrade Neto for technical support.
Footnotes
References
Alieva, A. K., Shadrina, M. I., Filatova, E. V., Ustinova, V. V., Fedotova, E. Y., Karabanov, A., et al. (2013). Polymorphisms in the SNCA gene: association with the risk of development of the sporadic form of Parkinson’s disease and the level of SNCA gene expression in peripheral blood of patients from Russia. Neurosci. Med. 4, 208–214. doi: 10.4236/nm.2013.44032
Allam, M., Del Castillo, A., and Navajas, R. (2005). Parkinson’s disease risk factors: genetic, environmental, or both? Neurol. Res. 27, 206–208. doi: 10.1179/016164105x22057
Allam, M. F., Campbell, M. J., Hofman, A., Del Castillo, A. S., and Fernandez-Crehuet Navajas, R. (2004). Smoking and Parkinson’s disease: systematic review of prospective studies. Mov. Disord. 19, 614–621. doi: 10.1002/mds.20029
Beato, R., Nitrini, R., Formigoni, A., and Caramelli, P. (2007). Brazilian version of the Frontal Assessment Battery (FAB): preliminary data on administration to healthy elderly. Dement. Neuropsychol. 1, 59–65.
Beck, A. T., Epstein, N., Brown, G., and Steer, R. A. (1988a). An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 56, 893–897. doi: 10.1037/0022-006X.56.6.893
Beck, A. T., Steer, R. A., and Garbin, M. G. (1988b). Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 8, 77–100. doi: 10.1016/0272-7358(88)90050-5
Bendor, J., Logan, T., and Edwards, R. H. (2013). The function of α-synuclein. Neuron 79, 1044–1066. doi: 10.1016/j.neuron.2013.09.004
Betarbet, R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V., and Greenamyre, J. T. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 3, 1301–1306. doi: 10.1038/81834
Botta-Orfila, T., Ezquerra, M., Pastor, P., Fernández-Santiago, R., Pont-Sunyer, C., Compta, Y., et al. (2012). Age at onset in LRRK2-associated PD is modified by SNCA variants. J. Mol. Neurosci. 48, 245–247. doi: 10.1007/s12031-012-9820-7
Botta-Orfila, T., Ezquerra, M., Ríos, J., Fernández-Santiago, R., Cervantes, S., Samaranch, L., et al. (2011). Lack of interaction of SNCA and MAPT genotypes in Parkinson’s disease. Eur. J. Neurol. 18:e32. doi: 10.1111/j.1468-1331.2010.03245.x
Brockmann, K., Schulte, C., Hauser, A., Lichtner, P., Huber, H., Maetzler, W., et al. (2013). SNCA: major genetic modifier of age at onset of Parkinson’s disease. Mov. Disord. 28, 1217–1221. doi: 10.1002/mds.25469
Brucki, S. M. D., Nitrin, R., Caramelli, P., Bertolucci, P. H. F., and Okamoto, I. H. (2003). Sugestões para o uso do mini-exame do estado mental no Brasil. Arq. Neuropsiquiatr. 61, 777–781. doi: 10.1590/S0004-282X2003000500014
Campêlo, C. L. C., Santos, J. R., Silva, A. F., Dierschnabel, A. L., Pontes, A., Cavalcante, J. S., et al. (2017). Exposure to an enriched environment facilitates motor recovery and prevents short-term memory impairment and reduction of striatal BDNF in a progressive pharmacological model of parkinsonism in mice. Behav. Brain Res. 328, 138–148. doi: 10.1016/j.bbr.2017.04.028
Cardo, L. F., Coto, E., Mena, L., Ribacoba, R., Mata, I. F., Menéndez, M., et al. (2014). Alpha-synuclein transcript isoforms in three different brain regions from Parkinson’s disease and healthy subjects in relation to the SNCA rs356165/rs11931074 polymorphisms. Neurosci. Lett. 562, 45–49. doi: 10.1016/j.neulet.2014.01.009
Chagas, M. H. N., Moriyama, T. S., Felício, A. C., Sosa, A. L., Bressan, R. A., and Ferri, C. P. (2014). Depression increases in patients with Parkinson’ s disease according to the increasing severity of the cognitive impairment. Arq. Neuropsiquiatr. 72, 426–429. doi: 10.1590/0004-282X20140049
Chartier-Harlin, M. C., Kachergus, J., Roumier, C., Mouroux, V., and Douay, X. (2004). Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364, 1167–1169. doi: 10.1016/S0140-6736(04)17103-1
Chen, W., Kang, W. Y., Chen, S., Wang, Y., Xiao, Q., Wang, G., et al. (2015). Hyposmia correlates with SNCA variant and non-motor symptoms in Chinese patients with Parkinson’s disease. Park. Relat. Disord. 21, 610–614. doi: 10.1016/j.parkreldis.2015.03.021
Chen, Y. P., Wei, Q. Q., Ou, R. W., Cao, B., Chen, X. P., Zhao, B., et al. (2015). Genetic variants of SNCA are associated with susceptibility to Parkinson’s disease but not amyotrophic lateral sclerosis or multiple system atrophy in a Chinese population. PLoS ONE 10:e0133776. doi: 10.1371/journal.pone.0133776
Cheng, L., Wang, L., Li, N. N., Yu, W. J., Sun, X. Y., Li, J. Y., et al. (2016). SNCA rs356182 variant increases risk of sporadic Parkinson’s disease in ethnic Chinese. J. Neurol. Sci. 368, 231–234. doi: 10.1016/j.jns.2016.07.032
Chiba-Falek, O., and Nussbaum, R. L. (2001). Effect of allelic variation at the NACP-Rep1 repeat upstream of the α-synuclein gene (SNCA) on transcription in a cell culture luciferase reporter system. Hum. Mol. Genet. 10, 3101–3109. doi: 10.1093/hmg/10.26.3101
Chiba-Falek, O., Touchman, J. W., and Nussbaum, R. L. (2003). Functional analysis of intra-allelic variation at NACP-Rep1 in the alpha-synuclein gene. Hum. Genet. 113, 426–431. doi: 10.1007/s00439-003-1002-9
Chun, H. S., Gibson, G. E., DeGiorgio, L. A., Zhang, H., Kidd, V. J., and Son, J. H. (2001). Dopaminergic cell death induced by MPP+, oxidant and specific neurotoxicants shares the common molecular mechanism. J. Neurochem. 76, 1010–1021. doi: 10.1046/j.1471-4159.2001.00096.x
Costa, J., Lunet, N., Santos, C., Santos, J., and Vaz-Carneiro, A. (2010). Caffeine exposure and the risk of Parkinson’s disease: a systematic review and meta-analysis of observational studies. J. Alzheimers Dis. 20(Suppl. 1), S221–S238. doi: 10.3233/JAD-2010-091525
Cronin, K. D., Ge, D., Manninger, P., Linnertz, C., Rossoshek, A., Orrison, B. M., et al. (2009). Expansion of the Parkinson disease-associated SNCA-Rep1 allele upregulates human α-synuclein in transgenic mouse brain. Hum. Mol. Genet. 18, 3274–3285. doi: 10.1093/hmg/ddp265
Dahodwala, N., Siderowf, A., Xie, M., Noll, E., Stern, M., and Mandell, D. (2009). Racial differences in the diagnosis of Parkinson’s disease. Mov. Disord. 24, 1200–1205. doi: 10.1002/mds.22557
Dan, X., Wang, C., Zhang, J., Gu, Z., Zhou, Y., Ma, J., et al. (2016). Association between common genetic risk variants and depression in Parkinson’s disease: A dPD study in Chinese. Parkinsonism Relat. Disord. 33, 122–126. doi: 10.1016/j.parkreldis.2016.09.029
Davila-Ortiz de Montellano, D. J., Rodriguez-Violante, M., Fresan, A., Monroy-Jaramillo, N., and Yescas-Gomez, P. (2016). Frequency of single nucleotide polymorphisms and alpha-synuclein haplotypes associated with sporadic Parkinson’s disease in the Mexican population. Rev. Neurol. 63, 345–350.
Davis, A. A., Andruska, K. M., Benitez, B. A., Racette, B. A., Perlmutter, J. S., and Cruchaga, C. (2016). Variants in GBA, SNCA, and MAPT influence Parkinson disease risk, age at onset, and progression. Neurobiol. Aging 37, 209.e1–209.e7. doi: 10.1016/j.neurobiolaging.2015.09.014
De Palma, G., Mozzoni, P., Mutti, A., Calzetti, S., and Negrotti, A. (1998). Case-control study of interactions between genetic and environmental factors in Parkinson’s disease. Lancet 352, 19–26. doi: 10.1016/S0140-6736(05)61332-3
Eisbach, S. E., and Outeiro, T. F. (2013). Alpha-Synuclein and intracellular trafficking: impact on the spreading of Parkinson’s disease pathology. J. Mol. Med. 91, 693–703. doi: 10.1007/s00109-013-1038-9
Elbaz, A., Ross, O. A., Ioannidis, J. P. A., Soto-Ortolaza, A. I., Moisan, F., Aasly, J., et al. (2011). Independent and joint effects of the MAPT and SNCA genes in Parkinson disease. Ann. Neurol. 69, 778–792. doi: 10.1002/ana.22321
Emelyanov, A., Andoskin, P., Yakimovskii, A., Usenko, T., Nuzhnyi, E., Nikolaev, M., et al. (2013). SNCA, LRRK2, MAPT polymorphisms and Parkinson’s disease in Russia. Parkinsonism Relat. Disord. 19, 1064–1065. doi: 10.1016/j.parkreldis.2013.06.003
Fahn, S. (2003). Description of Parkinson’s disease as a clinical syndrome. Ann. N. Y. Acad. Sci. 991, 1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x
Fahn, S., and Elton, R. (1987). “Members of the UPDRS development committee. the unified Parkinson’s disease rating scale,” in Recent Developments in Parkinson’s Disease, eds S. Fahn, C. D. Marsden, D. B. Calne, and M. Goldstein (Florham Park, NJ: Mcmellam Health Care Information), 153–163.
Firestone, J. A., Smith-Weller, T., Franklin, G., Swanson, P., Longstrech, W. T., and Checkoway, H. (2005). Pesticides and risk of Parkinson disease. Arch. Gerontol. Geriatr. 62, 91–95. doi: 10.1001/archneur.62.1.91
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Fuchs, J., Tichopad, A., Golub, Y., Munz, M., Schweitzer, K. J., Wolf, B., et al. (2008). Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB J. 22, 1327–1334. doi: 10.1096/fj.07-9348com
Gao, J., Nalls, M. A., Shi, M., Joubert, B. R., Hernandez, D. G., Huang, X., et al. (2012). An exploratory analysis on gene-environment interactions for Parkinson disease. Neurobiol. Aging 33, 2528.e1–2528.e6. doi: 10.1016/j.neurobiolaging.2012.06.007
García, S., Chavira-Hernández, G., Gallegos-Arreola, M. P., Dávila-Maldonado, L., García Martínez, F., Montes Almanza, L. A., et al. (2016). The rs3857059 variant of the SNCA gene is associated with Parkinson’s disease in Mexican Mestizos. Arq. Neuropsiquiatr. 74, 445–449. doi: 10.1590/0004-282X20160061
Giordano, S., Lee, J., Darley-Usmar, V. M., and Zhang, J. (2012). Distinct effects of rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on cellular bioenergetics and cell death. PLoS ONE 7:e44610. doi: 10.1371/journal.pone.0044610
Goris, A., Williams-Gray, C. H., Clark, G. R., Foltynie, T., Lewis, S. J. G., Brown, J., et al. (2007). Tau and α-synuclein in susceptibility to, and dementia in, Parkinson’s disease. Ann. Neurol. 62, 145–153. doi: 10.1002/ana.21192
Guo, X. Y., Chen, Y. P., Song, W., Zhao, B., Cao, B., Wei, Q. Q., et al. (2014). SNCA variants rs2736990 and rs356220 as risk factors for Parkinson’s disease but not for amyotrophic lateral sclerosis and multiple system atrophy in a Chinese population. Neurobiol. Aging 35, 2882.e1–2882.e6. doi: 10.1016/j.neurobiolaging.2014.07.014
Han, W., Liu, Y., Mi, Y., Zhao, J., Liu, D., et al. (2015). Alpha-synuclein (SNCA) polymorphisms and susceptibility to Parkinson’s disease: a meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 168, 123–134. doi: 10.1002/ajmg.b.32288
Heckman, M. G., Soto-Ortolaza, A. I., Diehl, N. N., Carrasquillo, M. M., Uitti, R. J., Wszolek, Z. K., et al. (2012). Evaluation of the role of SNCA variants in survival without neurological disease. PLoS ONE 7:e42877. doi: 10.1371/journal.pone.0042877
Hindle, J. V., Hurt, C. S., Burn, D. J., Brown, R. G., Samuel, M., Wilson, K. C., et al. (2015). The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson’s disease - a longitudinal cohort study. Int. J. Geriatr. Psychiatry 31, 13–23. doi: 10.1002/gps.4284
Hindle, J. V., Martyr, A., and Clare, L. (2014). Cognitive reserve in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat. Disord. 20, 1–7. doi: 10.1016/j.parkreldis.2013.08.010
Hoehn, M., and Yahr, M. (1967). Parkinsonism: onset, progression, and mortality. Neurology 17, 427–442. doi: 10.1212/WNL.17.5.427
Hong, D.-P., Fink, A. L., and Uversky, V. N. (2009). Smoking and Parkinson’s disease: Does nicotine affect alpha-synuclein fibrillation? Biochim. Biophys. Acta 1794, 282–290. doi: 10.1016/j.bbapap.2008.09.026
Hu, F.-Y., Hu, W.-B., Liu, L., Yu, L.-H., Xi, J., He, X.-H., et al. (2010). Lack of replication of a previously reported association between polymorphism in the 3’UTR of the alpha-synuclein gene and Parkinson’s disease in Chinese subjects. Neurosci. Lett. 479, 31–33. doi: 10.1016/j.neulet.2010.05.022
Huang, Y., Wang, G., Rowe, D., Wang, Y., Kwok, J. B. J., Xiao, Q., et al. (2015). SNCA gene, but not MAPT, influences onset age of Parkinson’s disease in Chinese and Australians. Biomed. Res. Int. 2015:135674. doi: 10.1155/2015/135674
Hughes, R. N. (2004). The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 28, 497–505. doi: 10.1016/j.neubiorev.2004.06.006
Ibáñez, P., Bonnet, A., Débarges, B., Lohmann, E., Tison, F., Pollak, P., et al. (2004). Causal relation between α-synuclein gene duplication and familial Parkinson’s disease. Lancet 364, 1169–1171. doi: 10.1016/S0140-6736(04)17104-3
Julian, L. J. (2011). Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 63, S467–S472. doi: 10.1002/acr.20561
Kehagia, A., Barker, R. A., and Robbins, T. W. (2010). Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 9, 1200–1213. doi: 10.1016/S1474-4422(10)70212-X
Lau, L. M. L., and Breteler, M. M. B. (2006). Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535. doi: 10.1016/S1474-4422(06)70471-9
Lauterbach, E. C. (2004). The neuropsychiatry of Parkinson’s disease and related disorders. Psichatr. Clin. North Am. 27, 801–825. doi: 10.1016/j.psc.2004.07.001
Lazzarini, A., Myers, R., Zimmerman, T. J., Mark, M., Golbe, L., Sage, J., et al. (1994). A clinical genetic study of Parkinson’s disease: evidence for dominant transmission. Neurology 44, 499–506. doi: 10.1212/WNL.44.3_Part_1.499
Lee, H.-J., Khoshaghideh, F., Lee, S., and Lee, S.-J. (2006). Impairment of microtubule-dependent trafficking by overexpression of alpha-synuclein. Eur. J. Neurosci. 24, 3153–3162. doi: 10.1111/j.1460-9568.2006.05210.x
Li, X., Li, W., Liu, G., Shen, X., and Tang, Y. (2015). Association between cigarette smoking and Parkinson’s disease: a meta-analysis. Arch. Gerontol. Geriatr. 61, 510–516. doi: 10.1016/j.archger.2015.08.004
Lima, M. M. S., Andersen, M. L., Reksidler, A. B., Ferraz, A. C., Vital, M. A. B. F., and Tufik, S. (2012). Paradoxical sleep deprivation modulates tyrosine hydroxylase expression in the nigrostriatal pathway and attenuates motor deficits induced by dopaminergic depletion. CNS Neurol. Disord. Drug Targets 11, 359–368. doi: 10.2174/187152712800792839
Linnertz, C., Saucier, L., Ge, D., Cronin, K. D., Burke, J. R., Jeffrey, N., et al. (2009). Genetic regulation of α-Synuclein mRNA expression in various human brain tissues. PLoS ONE 4:e7480. doi: 10.1371/journal.pone.0007480
Lotharius, J., and Brundin, P. (2002). Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum. Mol. Genet. 11, 2395–2407. doi: 10.1093/hmg/11.20.2395
Markopoulou, K., Biernacka, J. M., Armasu, S. M., Anderson, K. J., Ahlskog, J. E., Chase, B. A., et al. (2014). Does α-synuclein have a dual and opposing effect in preclinical vs. clinical Parkinson’s disease? Parkinsonism Relat. Disord. 20, 584–589. doi: 10.1016/j.parkreldis.2014.02.021
Mata, I., Shi, M., Agarwal, P., Chung, K., Edwards, K., Factor, S., et al. (2010). SNCA variant associated with Parkinson disease and plasma α-Synuclein level. Arch. Neurol. 67, 1350–1356. doi: 10.1001/archneurol.2010.279
Mata, I., Yearout, D., Alvarez, V., Coto, E., de Mena, L., Ribacoba, R., et al. (2011). Replication of MAPT and SNCA, but not PARK16-18, as susceptibility genes for Parkinson’s disease. Mov. Disord. 26, 819–823. doi: 10.1002/mds.23642
Mccarthy, J. J., Linnertz, C., Saucier, L., Burke, J. R., Hulette, C. M., Welsh-Bohmer, K. A., et al. (2011). The effect of SNCA 3’ region on the levels of SNCA-112 splicing variant. Neurogenetics 12, 59–64. doi: 10.1007/s10048-010-0263-4.The
Miyake, Y., Tanaka, K., Fukushima, W., Kiyohara, C., Sasaki, S., Tsuboi, Y., et al. (2012). SNCA polymorphisms, smoking, and sporadic Parkinson’s disease in Japanese. Parkinsonism Relat. Disord. 18, 557–561. doi: 10.1016/j.parkreldis.2012.02.016
Mueller, J. C., Fuchs, J., Hofer, A., Zimprich, A., Lichtner, P., Illig, T., et al. (2005). Multiple regions of α-synuclein are associated with Parkinson’s disease. Ann. Neurol. 57, 535–541. doi: 10.1002/ana.20438
Mullin, S., and Schapira, A. (2013). α-synuclein and mitochondrial dysfunction in Parkinson’s disease. Mol. Neurobiol. 47, 587–597. doi: 10.1007/s12035-013-8394-x
Myhre, R., Toft, M., Kachergus, J., Hulihan, M. M., Aasly, J. O., Klungland, H., et al. (2008). Multiple alpha-synuclein gene polymorphisms are associated with Parkinson’s disease in a Norwegian population. Acta Neurol. Scand. 118, 320–327. doi: 10.1111/j.1600-0404.2008.01019.x
Ozansoy, M., and Basak, A. N. (2012). The central theme of Parkinson’s disease: α-synuclein. Mol. Neurobiol. 47, 460–465. doi: 10.1007/s12035-012-8369-3
Paillard, T., Rolland, Y., and de Souto Barreto, P. (2015). Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: a narrative review. J. Clin. Neurol. 11, 212–219. doi: 10.3988/jcn.2015.11.3.212
Pals, P., Lincoln, S., Manning, J., Heckman, M., Skipper, L., Hulihan, M., et al. (2004). Alpha-synuclein promoter confers susceptibility to Parkinson’s disease. Ann. Neurol. 56, 591–595. doi: 10.1002/ana.20268
Pan, F., Ding, H., Dong, H., Ye, M., Liu, W., Cui, G., et al. (2013). Association of polymorphism in rs2736990 of the alpha-synuclein gene with Parkinson’s disease in a Chinese population. Neurol. India 61, 360–364. doi: 10.4103/0028-3886.117595
Pan, F., Dong, H., Ding, H., Ye, M., Liu, W., Wu, Y., et al. (2012). SNP rs356219 of the α-synuclein (SNCA) gene is associated with Parkinson’s disease in a Chinese Han population. Parkinsonism Relat. Disord. 18, 632–634. doi: 10.1016/j.parkreldis.2012.01.025
Perez, R. G., Waymire, J. C., Lin, E., Liu, J. J., Guo, F., and Zigmond, M. J. (2002). A role for alpha-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 22, 3090–3099.
Pihlstrøm, L., Axelsson, G., Anne, K., Dizdar, N., Fardell, C., Forsgren, L., et al. (2013). Supportive evidence for 11 loci from genome-wide association studies in Parkinson’s disease. Neurobiol. Aging 34, 1708.e7–1708.e13. doi: 10.1016/j.neurobiolaging.2012.10.019
Rajput, A., VIlariño-Güell, C., Rajput, M. L., Ross, O. A., Soto-Ortolaza, A. I., Lincoln, S. J., et al. (2009). Alpha-synuclein polymorphisms are associated with Parkinson’s disease in a Saskatchewan population. Mov. Disord. 24, 2411–2414. doi: 10.1002/mds.22768
Reijnders, J., Ehrt, U., Lousberg, R., Aarsland, D., and Leentjens, A. (2009). The association between motor subtypes and psychopathology in Parkinson’s disease. Parkinsonism Relat. Disord. 15, 379–382. doi: 10.1016/j.parkreldis.2008.09.003
Ritz, B., Rhodes, S. L., Bordelon, Y., and Bronstein, J. (2012). α-Synuclein genetic variants predict faster motor symptom progression in idiopathic Parkinson disease. PLoS ONE 7:e36199. doi: 10.1371/journal.pone.0036199
Rodriguez-Oroz, M. C., Jahanshahi, M., Krack, P., Litvan, I., Macias, R., Bezard, E., et al. (2009). Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 8, 1128–1139. doi: 10.1016/S1474-4422(09)70293-5
Satake, W., Nakabayashi, Y., Mizuta, I., Hirota, Y., Ito, C., Kubo, M., et al. (2009). Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 41, 1303–1307. doi: 10.1038/ng.485
Schapira, A. H. V. (2007). Mitochondrial dysfunction in Parkinson’s disease. Cell Death Differ. 14, 1261–1266. doi: 10.1038/sj.cdd.4402160
Semchuk, K. M., Love, E. J., and Lee, R. G. (1991). Parkinson’s disease and exposure to rural environmental factors: a population based case-control study. Can. J. Neurol. Sci. 18, 279–286. doi: 10.1017/S0317167100031826
Sharma, M., Ioannidis, J. P. A., Aasly, J. O., Annesi, G., Brice, A., Van Broeckhoven, C., et al. (2012). Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology 79, 659–667. doi: 10.1212/WNL.0b013e318264e353
Shih, I., Liew, Z., Krause, N., and Ritz, B. (2016). Lifetime occupational and leisure time physical activity and risk of Parkinson’s disease. Parkinsonism Relat. Disord. 28, 112–117. doi: 10.1016/j.parkreldis.2016.05.007
Simón-Sánchez, J., Schulte, C., Bras, J. M., Sharma, M., Gibbs, J. R., Berg, D., et al. (2009). Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 41, 1308–1312. doi: 10.1038/ng.487
Sotiriou, S., Gibney, G., Baxevanis, A. D., and Nussbaum, R. L. (2009). A single nucleotide polymorphism in the 3′UTR of the SNCA gene encoding alpha-synuclein is a new potential susceptibility locus for Parkinson disease. Neurosci. Lett. 461, 196–201. doi: 10.1016/j.neulet.2009.06.034
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M., and Goedert, M. (1998). α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473. doi: 10.1073/pnas.95.11.6469
Spillantini, M. G., Schmidt, M. L., Lee, V. M.-Y., Trojanowski, J. Q., and Goedert, M. R. J. (1997). α-synuclein in Lewy bodies. Nature 388, 839–840. doi: 10.1038/42166
Stern, Y. (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004
Tabrizi, S. J., Orth, M., Wilkinson, J. M., Taanman, J. W., Warner, T. T., Cooper, J. M., et al. (2000). Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum. Mol. Genet. 9, 2683–2689. doi: 10.1093/hmg/9.18.2683
Trojanowski, J. Q., and Lee, V. M.-Y. (1998). Aggregation of neurofilament and α-synuclein proteins in Lewy bodies: implications for the pathogenesis of Parkinson disease and Lewy body dementia. Arch. Neurol. 55, 151–152. doi: 10.1001/archneur.55.2.151
Tröster, A. I., Stalp, L. D., Paolo, A. M., Fields, J. A., and Koller, W. C. (1995). Neuropsychological impairment in Parkinson’s disease with and without depression. Arch. Neurol. 52, 1164–1169. doi: 10.1001/archneur.1995.00540360042014
Trotta, L., Guella, I., Soldà, G., Sironi, F., Tesei, S., Canesi, M., et al. (2012). SNCA and MAPT genes: independent and joint effects in Parkinson disease in the Italian population. Parkinsonism Relat. Disord. 135, 257–262. doi: 10.1016/j.parkreldis.2011.10.014
Uversky, V. N., Li, J., Bower, K., and Fink, A. L. (2002). Synergistic effects of pesticides and metals on the fibrillation of alpha-synuclein: implications for Parkinson’s disease. Neurotoxicology 23, 527–536. doi: 10.1016/S0161-813X(02)00067-0
Venda, L. L., Cragg, S. J., Buchman, V. L., and Wade-Martins, R. (2010). α-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 33, 559–568. doi: 10.1016/j.tins.2010.09.004
Verbaan, D., Boesveldt, S., Van Rooden, S. M., Visser, M., Marinus, J., MacEdo, M. G., et al. (2008). Is olfactory impairment in Parkinson disease related to phenotypic or genotypic characteristics? Neurology 71, 1877–1882. doi: 10.1212/01.wnl.0000336651.48596.c7
Wang, G., Huang, Y., Chen, W., Chen, S., Wang, Y., Qin, X., et al. (2016). Variants in the SNCA gene associate with motor progression while variants in the MAPT gene associate with the severity of Parkinson’s disease. Parkinsonism Relat. Disord. 24, 89–94. doi: 10.1016/j.parkreldis.2015.12.018
Wang, G., Van Der Walt, J. M., Mayhew, G., Li, Y., Zu, S., Scott, W. K., et al. (2008). Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am. J. Hum. Gentetics 82, 283–289. doi: 10.1016/j.ajhg.2007.09.021
Ward, A., Arrighi, H. M., Michels, S., and Cedarbaum, J. M. (2012). Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 8, 14–21. doi: 10.1016/j.jalz.2011.01.002
Wider, C., Vilariño-Güell, C., Heckman, M. G., Jasinska-Myga, B., Ortolaza-Soto, A. I., Diehl, N. N., et al. (2011). SNCA, MAPT, and GSK3B in Parkinson disease: a gene-gene interaction study. Eur. J. Neurol. 18, 876–881. doi: 10.1111/j.1468-1331.2010.03297.x
Winkler, S., Hagenah, J., Lincoln, S., Heckman, M., Hugarvoll, K., Lohmann-Hedrich, K., et al. (2007). Alpha-Synuclein and Parkinson disease susceptibility. Neurology 69, 1745–1750. doi: 10.1212/01.wnl.0000275524.15125.f4
Wirdefeldt, K., Adami, H.-O., Cole, P., Trichopoulos, D., and Mandel, J. (2011). Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur. J. Epidemiol. 26(Suppl. 1), S1–S58. doi: 10.1007/s10654-011-9581-6
Wu-Chou, Y.-H., Chen, Y.-T., Yeh, T.-H., Chang, H.-C., Weng, Y.-H., Lai, S.-C., et al. (2013). Genetic variants of SNCA and LRRK2 genes are associated with sporadic PD susceptibility: a replication study in a Taiwanese cohort. Parkinsonism Relat. Disord. 19, 251–255. doi: 10.1016/j.parkreldis.2012.10.019
Xu, W., Tan, L., and Yu, J. (2015). The link between the SNCA gene and parkinsonism. Neurobiol. Aging 36, 1505–1518. doi: 10.1016/j.neurobiolaging.2014.10.042
Keywords: Parkinson’s disease, alpha-synuclein, SNCA gene, polymorphism, cognitive impairment, clinical assessment, Brazil
Citation: Campêlo CLC, Cagni FC, de Siqueira Figueredo D, Oliveira LG Jr., Silva-Neto AB, Macêdo PT, Santos JR, Izídio GS, Ribeiro AM, de Andrade TG, de Oliveira Godeiro C Jr. and Silva RH (2017) Variants in SNCA Gene Are Associated with Parkinson’s Disease Risk and Cognitive Symptoms in a Brazilian Sample. Front. Aging Neurosci. 9:198. doi: 10.3389/fnagi.2017.00198
Received: 22 November 2016; Accepted: 02 June 2017;
Published: 20 June 2017.
Edited by:
Lucas Sedeño, Institute of Cognitive Neurology (INECO), ArgentinaReviewed by:
Arianna Bellucci, University of Brescia, ItalyYamile Bocanegra, University of Antioquia, Colombia
Copyright © 2017 Campêlo, Cagni, de Siqueira Figueredo, Oliveira, Silva-Neto, Macêdo, Santos, Izídio, Ribeiro, de Andrade, de Oliveira Godeiro and Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Regina H. Silva, reginahsilva@gmail.com
 Clarissa L.C Campêlo
Clarissa L.C Campêlo Fernanda C. Cagni1
Fernanda C. Cagni1  José R. Santos
José R. Santos Geison S. Izídio
Geison S. Izídio Alessandra M. Ribeiro
Alessandra M. Ribeiro Regina H. Silva
Regina H. Silva