Presence of Anticardiolipin Antibodies in Patients with Dementia: A Systematic Review and Meta-Analysis
- 1Human Genome Centre, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
- 2King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 3Enzymoics, Hebersham, NSW, Australia
- 4Novel Global Community Educational Foundation, Hebersham, NSW, Australia
- 5Division of Human Biology, School of Medicine, International Medical University, Bukit Jalil, Malaysia
- 6Department of Immunology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
Growing evidences are supporting towards the involvement of antiphospholipid antibodies [aPLs e.g., lupus anticoagulant (LA), anticardiolipin (aCL) and anti-β2-glycoprotein I (anti-β2-GPI) antibodies] in various neurological manifestations including migraine, epilepsy and dementia in the presence or absence of autoimmune diseases such as antiphospholipid syndrome or systemic lupus erythematosus. The aim of this systematic review and meta-analysis was to assess the presence of aPLs in dementia patients without a diagnosis of any autoimmune disease. Electronic databases (e.g., PubMed, Web of Science, Scopus, ScienceDirect and Google Scholar) were searched without any year or language restrictions and based on the inclusion criteria, nine prospective case-control studies assessing only aCL were included involving 372 dementia patients and 337 healthy controls. No studies were found to assess the presence of both LA or anti-β2-GPI. The study-specific odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using random-effects model. We observed the prevalence of aCL in dementia was higher (32.80%) than that of controls (9.50%) e.g., 3.45 times higher risk of presenting with dementia than the controls, and significant presence of aCL antibodies was detected in dementia patients compared to controls (OR: 4.94, 95% CI: 2.66 – 9.16, p < 0.00001; I2 = 32%, p = 0.16). Publication bias was not observed from Egger’s (p = 0.081) and Begg’s tests (p = 0.180). Based on the study quality assessment using modified Newcastle–Ottawa Scale for case-control studies, seven of nine studies were of high methodological quality scoring ≥ 7 (median value). In summary, aCL antibodies were significantly present in dementia patients suggesting that aCL antibodies are generated due to the autoimmune-derived effects of dementia or there might be a potential causative role of this autoantibody in dementia pathogenesis.
Introduction
Dementia is a clinical syndrome that encompasses a set of neurologic symptoms involving difficulties in memory, speaking, problem solving, and thinking abilities, leading to the impairments of personal and social life (Román, 2003; Burns and Iliffe, 2009). It is most common in elderly people where advanced age being the strongest risk factor. A prevalence of 7.1% among the aged population (>65 years old) has been reported (Prince et al., 2014), and the number of people with dementia worldwide is estimated at 47 million and is projected to increase over 131 million by 2050 (Prince et al., 2016). Worldwide, the total number of new cases of dementia each year amounts to approximately 7.7 million, indicating one new case every 4.1 s (Prince et al., 2015).
Among several types of dementia, Alzheimer’s disease (AD) and vascular dementia (VD) are most commonly observed (Dening and Babu Sandilyan, 2015; Robinson et al., 2015). AD accounts for 60% whereas VD accounts for almost 30% of the prevalence (Kalaria et al., 2008). In AD, neurodegeneration occurs due to abnormal extracellular deposition of insoluble plaques consisting of Aβ peptides and intraneuronal aggregates of twisted fibers consisting of tau proteins (Dening and Babu Sandilyan, 2015). VD occurs when blood circulation to the brain is compromised due to arterial disease resulting in reduced neuronal function and eventually neurons cell death (Dening and Babu Sandilyan, 2015). In AD patients, the synthesis of intra-blood-brain barrier (BBB) IgG was observed which indicates an involvement of immune-mediated mechanisms in the pathogenesis of AD (Blennow et al., 1990).
In previous years, researches have been conducted on autoimmune diseases including antiphospholipid syndrome (APS) which may have links with the risk of dementia development (Gomez-Puerta et al., 2005; Lin et al., 2016). A recent study conducted on 1.8 million hospital cases reported that patients with autoimmune disorders including APS and systemic lupus erythematosus (SLE) were 20% more likely to develop dementia (Wotton and Goldacre, 2017), suggesting an autoimmune-mediated pathogenesis of dementia. In APS, presence of antiphospholipid antibodies (aPLs) (autoantibodies which react against anionic phospholipids and proteins on plasma membranes) namely anticardiolipin (aCL) antibody, anti-β2-glycoprotein I (β2GPI) antibody and lupus anticoagulant (LA) are found persistently in high titers (Miyakis et al., 2006; Giannakopoulos and Krilis, 2013). Presence of aPLs in high titers was also observed in APS patients suffering from different neurologic disorders including dementia (Islam et al., 2016, 2017a). Dementia has been observed in up to 56% APS patients (Chapman et al., 2002; Gomez-Puerta et al., 2005), and a study on non-SLE patients with neurological symptoms showed that over 50% of the patients with high levels of aPLs developed dementia (Inzelberg et al., 1992). Furthermore, aPLs are associated with impaired cognitive function (Schmidt et al., 1995) and the frequency of cognitive dysfunction is high ranging between 19 and 40% in aPLs-positive asymptomatic patients (Jacobson et al., 1999; Kozora et al., 2013).
The pathogenesis of aPL-mediated dementia in APS is not entirely understood. Suggested mechanisms include aPLs-induced BBB disruption (Katzav et al., 2010), aPLs-related microvascular thrombosis (Asherson et al., 1987; Denburg et al., 1997), or a direct effect of aPLs on brain tissues (Appenzeller et al., 2012). Thrombotic events triggered by aPL might contribute to the multiple cerebral thrombotic symptoms and greater aggression to the brain (de Godoy et al., 2000). Besides thrombotic effects, inflammatory and immune effects may contribute to the development of cognitive dysfunction in the presence of aPLs (Katzav et al., 2011).
To date, the association of aPLs in patients with dementia remains inconclusive based on the primary studies conducted on small number of dementia subjects. Certain studies have shown that aCL was significantly (p < 0.05) present in dementia patients versus healthy controls, 27% vs. 0% (Juby and Davis, 1998) or 28% vs. 3% (Tan et al., 2001). However, other studies did not report such significant association of aCL positivity in dementia versus healthy subjects, 29% vs. 26.4% (de Godoy et al., 2012). Thus, a systematic review and meta-analysis on all the primary studies was conducted to bring together all evidences in this topic and synthesize a conclusive information about the presence of aPLs in dementia patients. In addition, subgroup analyses were performed to evaluate the presence of aCL in different types of dementia, distinct age ranges and patients in different geographical continents.
Materials and Methods
To conduct this meta-analysis, we followed the guidelines published by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group (Supplementary Table S1) (Stroup et al., 2000).
Study Selection Criteria
Studies were included if: (1) Study design was prospective case-control; (2) The aim of the study was to evaluate the existence of aPLs (LA, aCL, and anti-β2-GPI antibodies) in patients with dementia; (3) Dementia subjects were of any age, sex or race without any underlying autoimmune disorders such as APS or SLE.
Literature Search
A systematic literature search using ‘Advanced’ and ‘Expert’ search strategies of PubMed, Web of Science, Scopus, Science Direct, and Google Scholar databases was independently conducted by two researchers (MAI and FA), and the shortlisted studies were independently verified by KKW. There were no search year or language restrictions. Review articles, case reports, clinical trials, editorials, letters, and comments were excluded. Studies were also excluded if overlapping of identical study subjects was observed with other included studies from similar research group. To ensure that there were no potential papers overlooked, we examined the reference list of selected studies and reviewed publications that had cited the selected studies (via Google Scholar). The electronic search included both Medical Subject Heading (MeSH) in addition of appropriate keywords and combined with the Boolean operators (‘AND’ and ‘OR’). The following search terms were used: (antiphospholipid antibody OR antiphospholipid antibodies OR anticardiolipin antibody OR anticardiolipin antibodies OR lupus anticoagulant OR β2GPI OR β2-GPI OR β2glycoprotein OR β2-glycoprotein) AND (dementia OR Alzheimer OR Alzheimer’s). The final systematic search was conducted on 12th March 2017 (Supplementary Table S2).
Data Extraction, Management and Quality Assessment
Two researchers (MAI and FA) independently extracted the following data from each of the selected studies: first author and year (study ID), study design, country, number of dementia patients and controls (number of female patients and controls), types of dementia, mean age of dementia patients and controls, types and isotypes of tested aPLs, dementia diagnostic criteria, aPLs measurement techniques and cut-off values. To resolve any discrepancies such as unclear or missing data presentation, all authors took part in the discussion. If not resolved, we then contacted either the corresponding or the first author of the respective study for further clarifications. By using a modified version of the Newcastle–Ottawa Scale (NOS) (Islam et al., 2017b), quality of each of the selected studies was assessed; studies scoring above the median NOS value were considered as high quality (low risk of bias) and those scoring below the median value were considered as low quality (high risk of bias) (Wu et al., 2016).
Exploration of Heterogeneity and Publication Bias
Heterogeneity across studies was tested based on I2 statistics which indicates the percentage of variance attributable to study heterogeneity. Studies with I2< 40%, I2 = 40–75% or I2 > 75% was considered to have low, moderate or high heterogeneity (Harris et al., 2015). Three independent subgroup analyses were conducted as follows: (1) VD vs. dementia of the Alzheimer’s type (DAT); (2) Age ranged from 60 to 70 years vs. above 70 years old; (3) Subjects from Asia and Europe vs. North and South America. Additionally, L’Abbé plot was generated for the visual inspection of heterogeneity by using RStudio (version 1.0.136) software (metafor package, version 1.9-9) (Viechtbauer, 2010).
Publication bias was visually assessed by using funnel plots. Moreover, Egger’s regression test (Egger et al., 1997) and Begg’s test (Begg and Mazumdar, 1994) was conducted to further assess publication bias with random-effects model. Publication bias was considered significant if p < 0.05. Funnel plot was illustrated with the metafor package, version 1.9-9 (Viechtbauer, 2010).
Statistical Analyses of Meta-Analysis
Random-effects model was used to conduct this meta-analysis. Odds ratio (OR) was used to evaluate the comorbid association of the presence of aPLs in dementia patients compared to controls where p < 0.05 was considered significant. RevMan (Cochrane Collaboration, software version 5.3.5) (The Cochrane Collaboration, 2014) was used to generate the forest plot.
Results
Study Selection
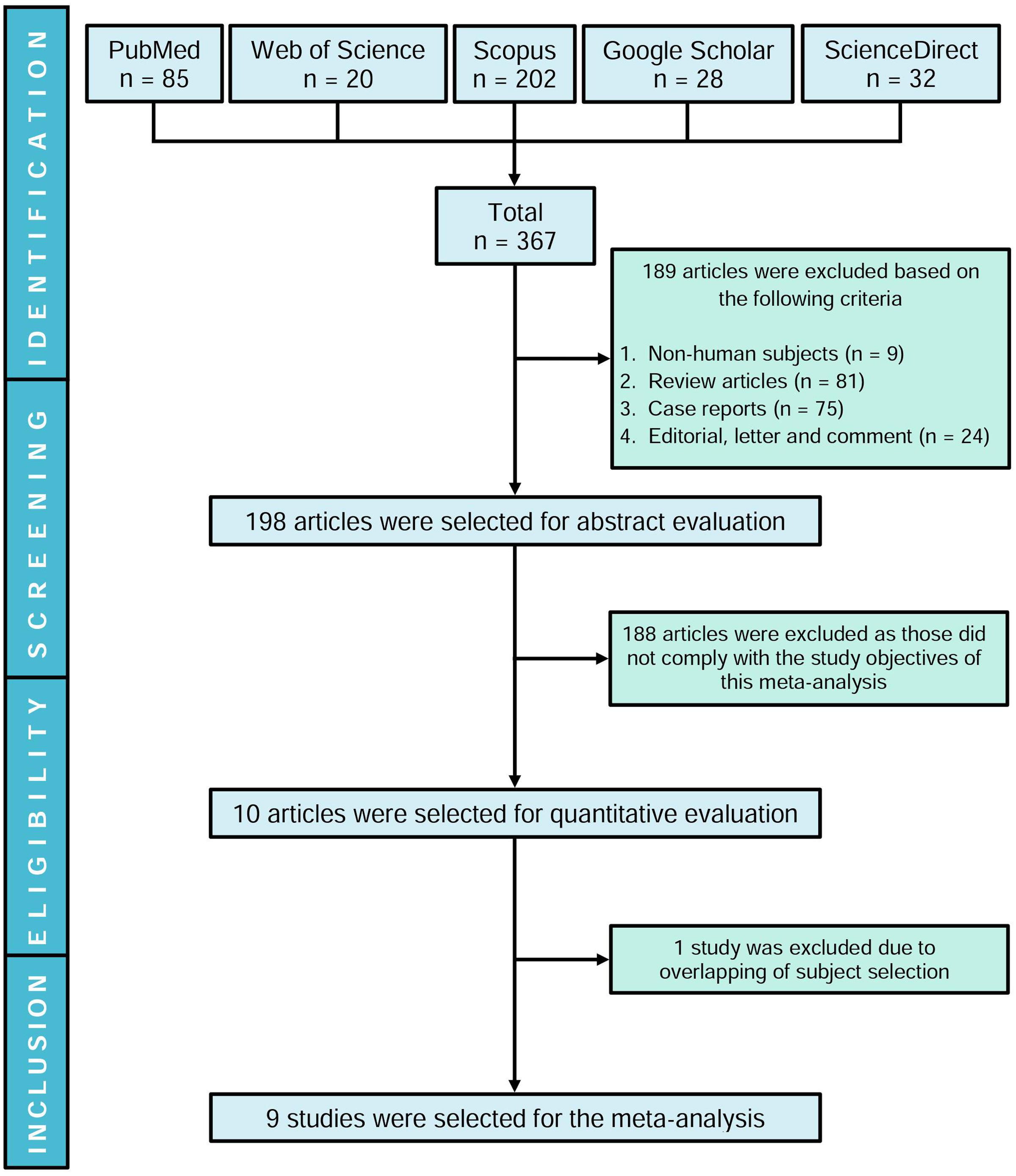
Our initial search yielded 367 articles where 189 studies were excluded and the remaining 198 articles were evaluated based on title and abstract. Ten studies were shortlisted following the inclusion criteria, and one article (Juby et al., 1995) was excluded due to overlapping of identical study subjects with another eligible study (Juby and Davis, 1998). Therefore, nine studies were included in this meta-analysis (Figure 1). Although initially the aim was to evaluate the presence of aPLs in dementia patients, based on our search strategies and systematic review, we could not find studies evaluating LA or anti-β2GPI except for aCL.
Study Characteristics and Quality Assessment
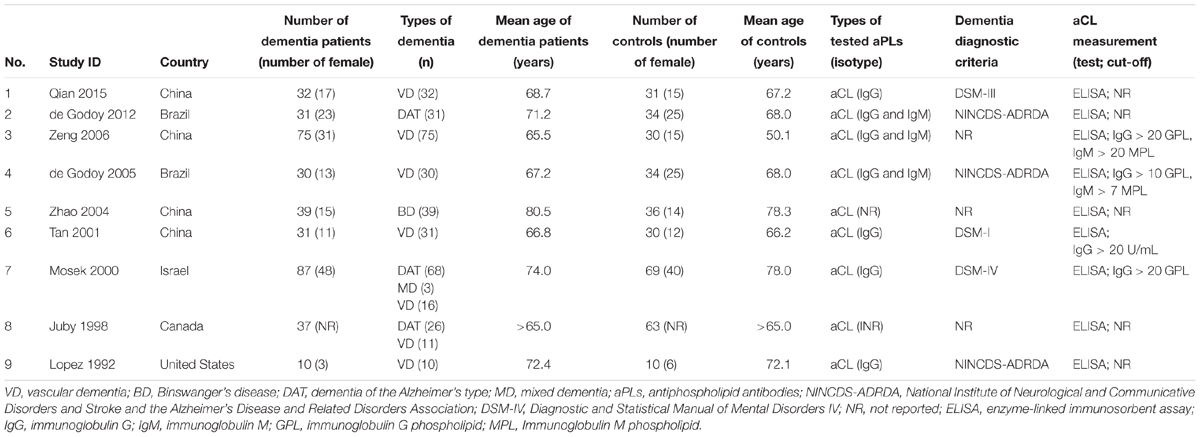
Among the included studies, four were from China (Tan et al., 2001; Zhao and Tan, 2004; Zeng et al., 2006; Qian et al., 2015), two were from Brazil (de Godoy et al., 2005, 2012), and the remaining three studies were from Israel (Mosek et al., 2000), Canada (Juby and Davis, 1998), and United States (Lopez et al., 1992). Across the nine studies, there were 709 subjects (dementia patients: n = 372; controls: n = 337) in total. All of the studies were designed as case-control and evaluated the presence of aCL as the only aPLs in dementia patients compared to controls. In particular, six of the studies were on VD (Lopez et al., 1992; Tan et al., 2001; Zhao and Tan, 2004; de Godoy et al., 2005; Zeng et al., 2006; Qian et al., 2015) and two on DAT (Juby and Davis, 1998; de Godoy et al., 2012) and one study with both VD and DAT patients (Mosek et al., 2000). The age range of the dementia patients and controls was 65–80.5 and 50.1–78.3 years, respectively. Table 1 summarizes the major characteristics of the included studies.
Quality assessment of the included studies by using NOS for case-control studies is shown in Table 2. The median score of NOS was 7. Among the nine studies, seven studies were of high quality (low risk of bias) scoring ≥ 7 (Lopez et al., 1992; Juby and Davis, 1998; Mosek et al., 2000; Tan et al., 2001; de Godoy et al., 2005, 2012; Qian et al., 2015) and two studies were of low quality (high risk of bias) scoring < 7 (Zhao and Tan, 2004; Zeng et al., 2006).
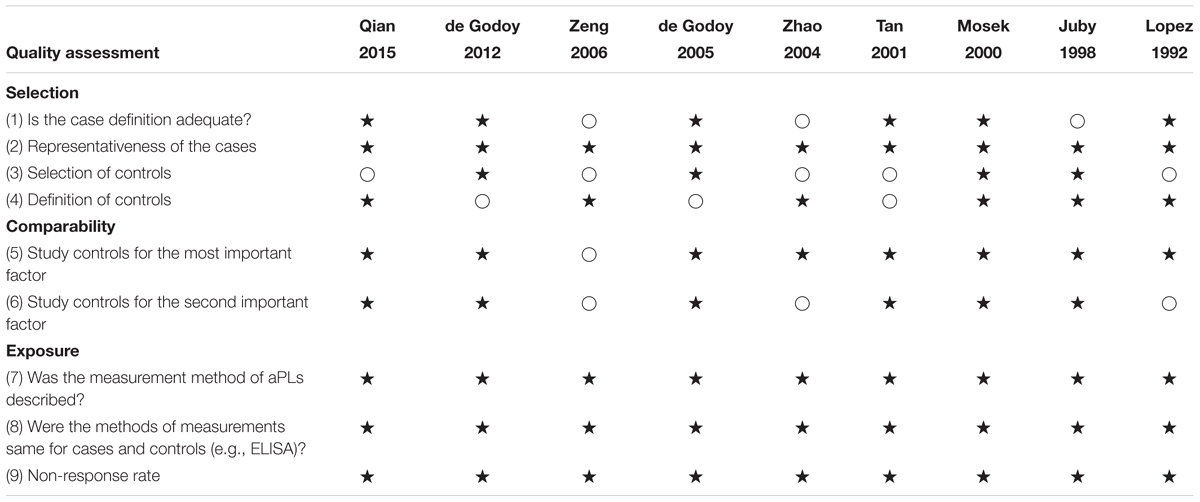
TABLE 2. Risk of bias assessment of the included studies according to the modified Newcastle-Ottawa Scale (NOS).
Assessment of aCL Presence by Meta-Analysis
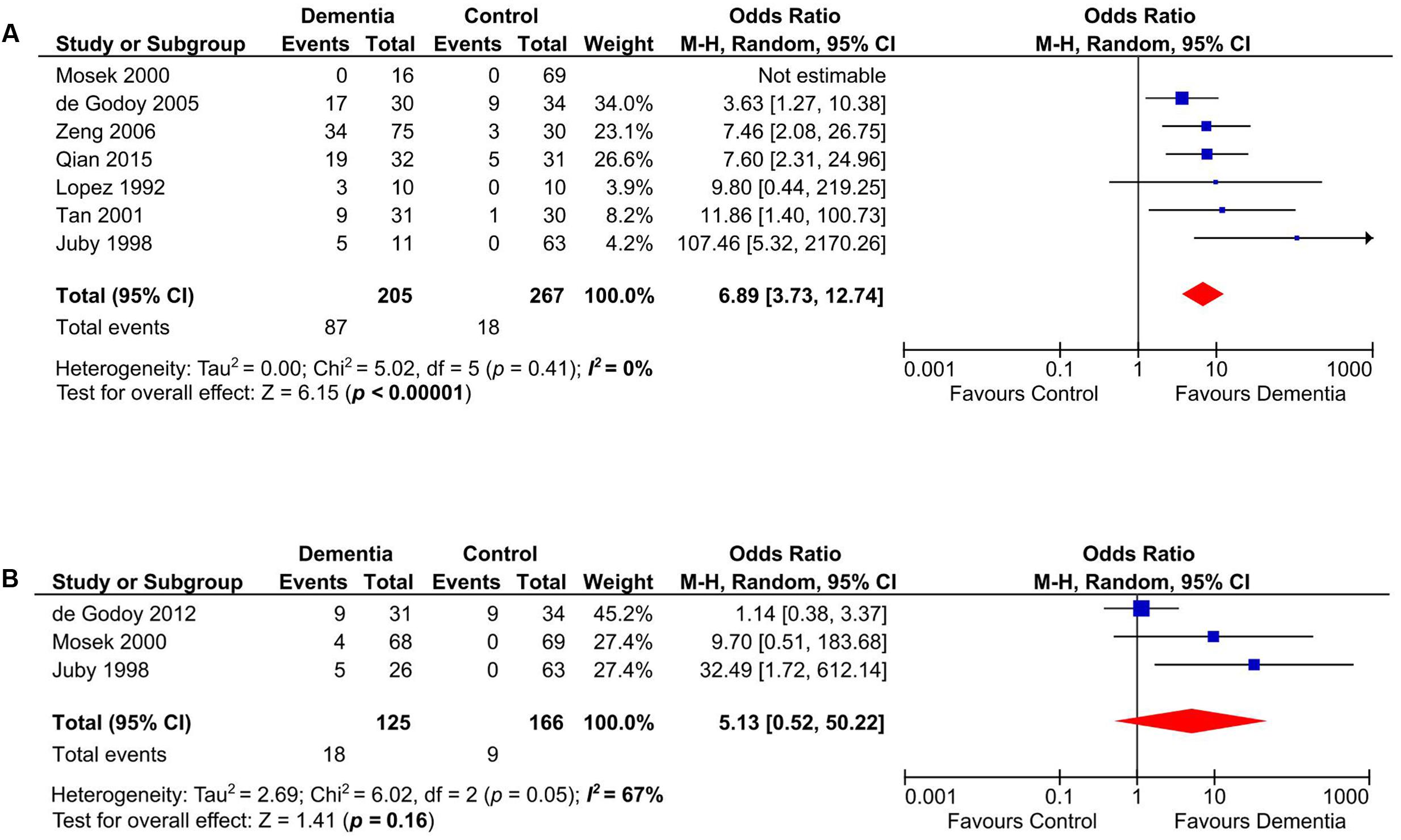
Presence of aCL in dementia patients was highly significant compared to controls (OR: 4.94, 95% CI: 2.66–9.16, p < 0.00001; I2 = 32%, p = 0.16) (Figure 2). aCL was present in 32.80% of dementia patients and 9.50% in controls corresponding to 3.45 times higher probability to present with dementia than controls.
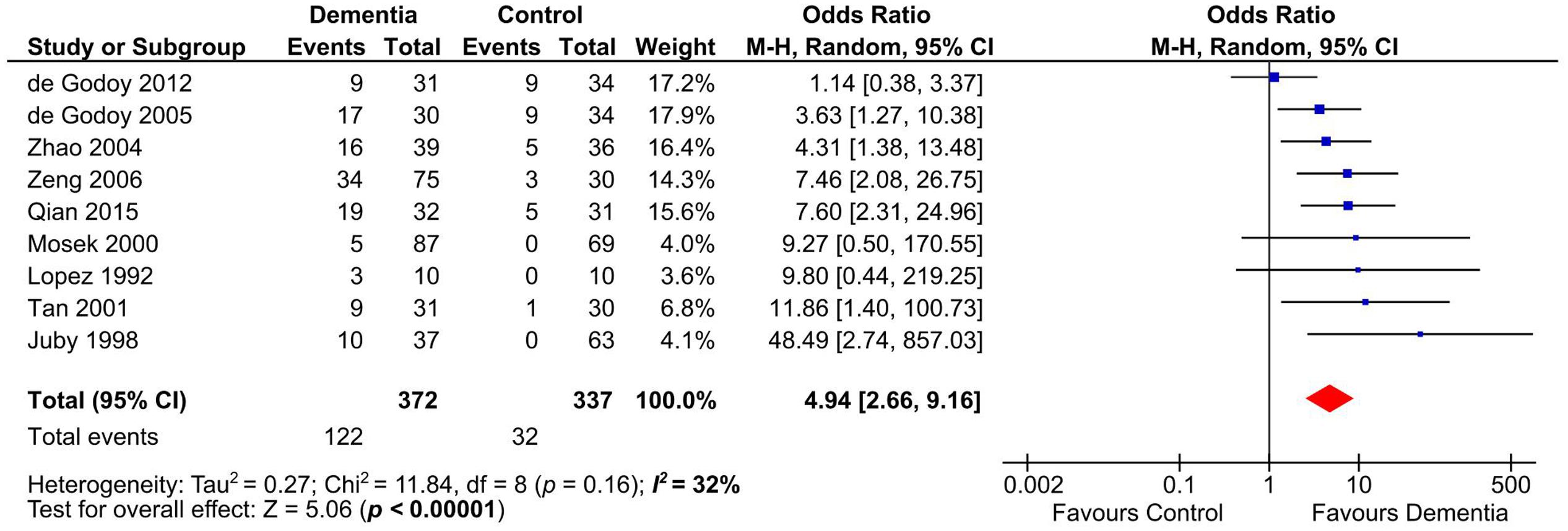
FIGURE 2. Forest plot representing the presence of anticardiolipin (aCL) antibodies in dementia patients compared to controls.
Subgroup Analyses on VD and DAT
Our meta-analysis on studies that evaluated the presence of aCL in patients with VD (n = 472) (Lopez et al., 1992; Juby and Davis, 1998; Mosek et al., 2000; Tan et al., 2001; de Godoy et al., 2005; Zeng et al., 2006; Qian et al., 2015) indicated that aCL was significantly present in VD patients as compared to healthy controls (OR: 6.89, 95% CI: 3.73–12.74, p < 0.00001; I2 = 0%, p = 0.41) (Figure 3A). For studies that measured aCL antibodies in DAT patients (n = 291) (Juby and Davis, 1998; Mosek et al., 2000; de Godoy et al., 2012), aCL was not significantly associated with DAT (OR: 5.13, 95% CI: 0.52–50.22, p = 0.16; I2 = 67%, p = 0.05) (Figure 3B).
Subgroup Analyses on Different Age Ranges
Anticardiolipin was significantly present in dementia subjects of age groups between 60 and 70 years old (n = 293) (Tan et al., 2001; de Godoy et al., 2005; Zeng et al., 2006; Qian et al., 2015) (OR: 5.99, 95% CI: 3.16–11.35, p < 0.00001; I2 = 0%, p = 0.67) (Figure 4A) and those more than 70 years old (n = 316) (Lopez et al., 1992; Mosek et al., 2000; Zhao and Tan, 2004; de Godoy et al., 2012) (OR: 2.92, 95% CI: 1.06–8.06, p = 0.04; I2 = 33%, p = 0.21) (Figure 4B).
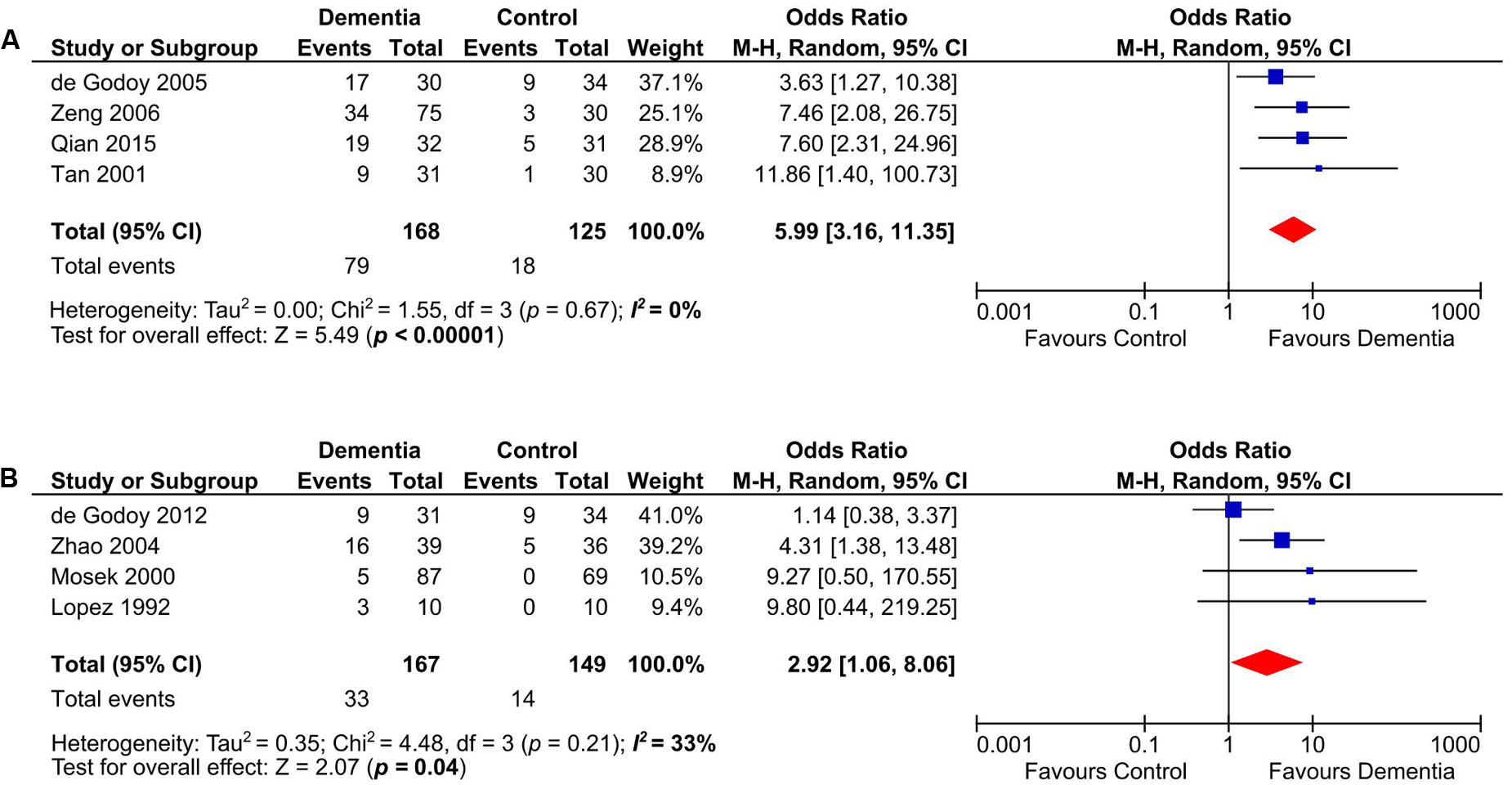
FIGURE 4. Subgroup analysis of aCL in demetia patients with age range of 60–70 years (A) and above 70 years old (B).
Subgroup Analyses on Patients in Different Continents
Anticardiolipin antibody was significantly present in Asian and European dementia patients (n = 460) (Mosek et al., 2000; Tan et al., 2001; Zhao and Tan, 2004; Zeng et al., 2006; Qian et al., 2015) (OR: 6.64, 95% CI: 3.50–12.62, p < 0.00001; I2 = 0%, p = 0.91) (Figure 5A), as well as in North and South American dementia subjects (n = 249) (Lopez et al., 1992; Juby and Davis, 1998; de Godoy et al., 2005, 2012) (OR: 4.06, 95% CI: 1.04–15.84, p = 0.04; I2 = 62%, p = 0.05) (Figure 5B).
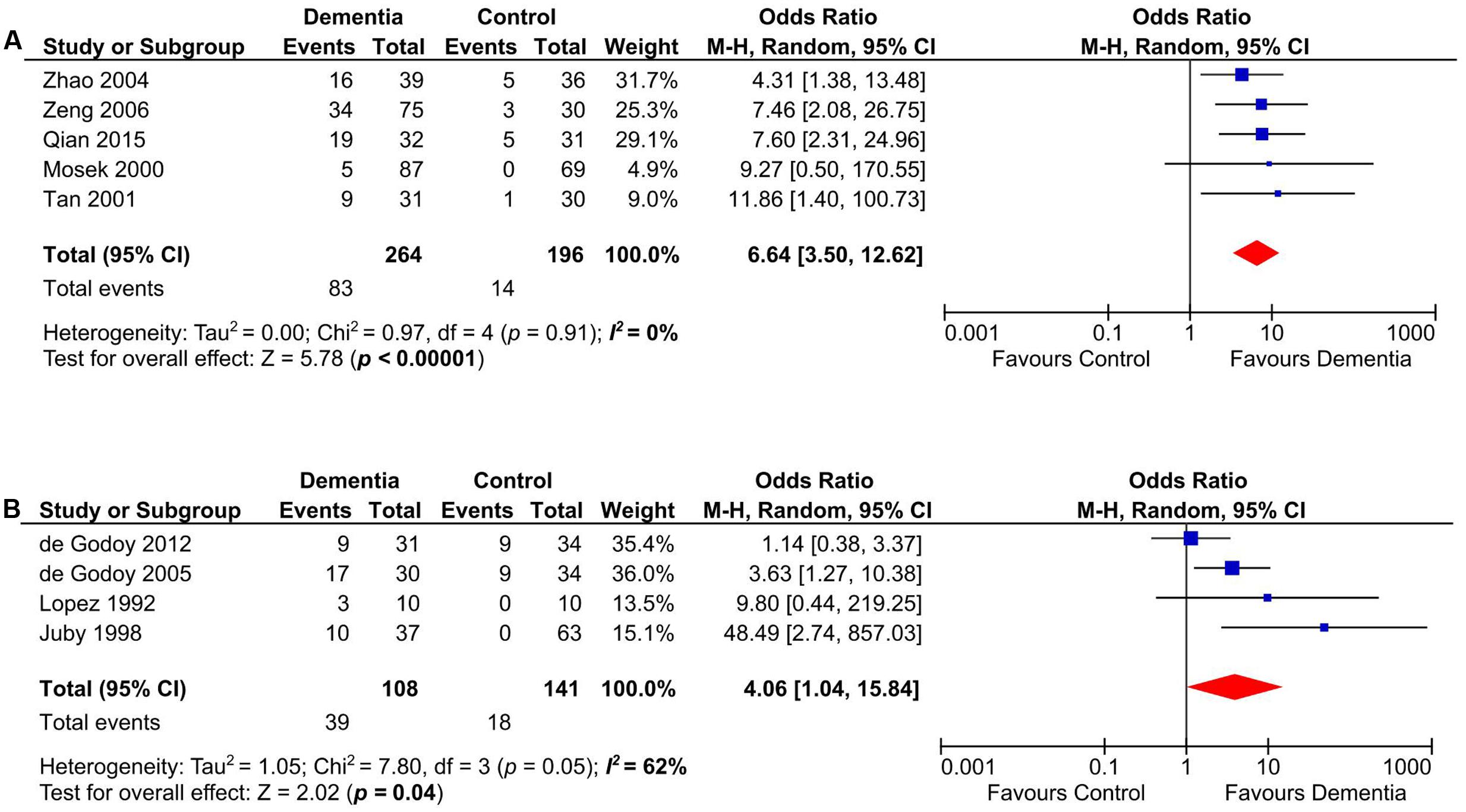
FIGURE 5. Subgroup analysis of aCL in Asian and European (A), North and South American (B) dementia populations.
Heterogeneity and Publication Bias
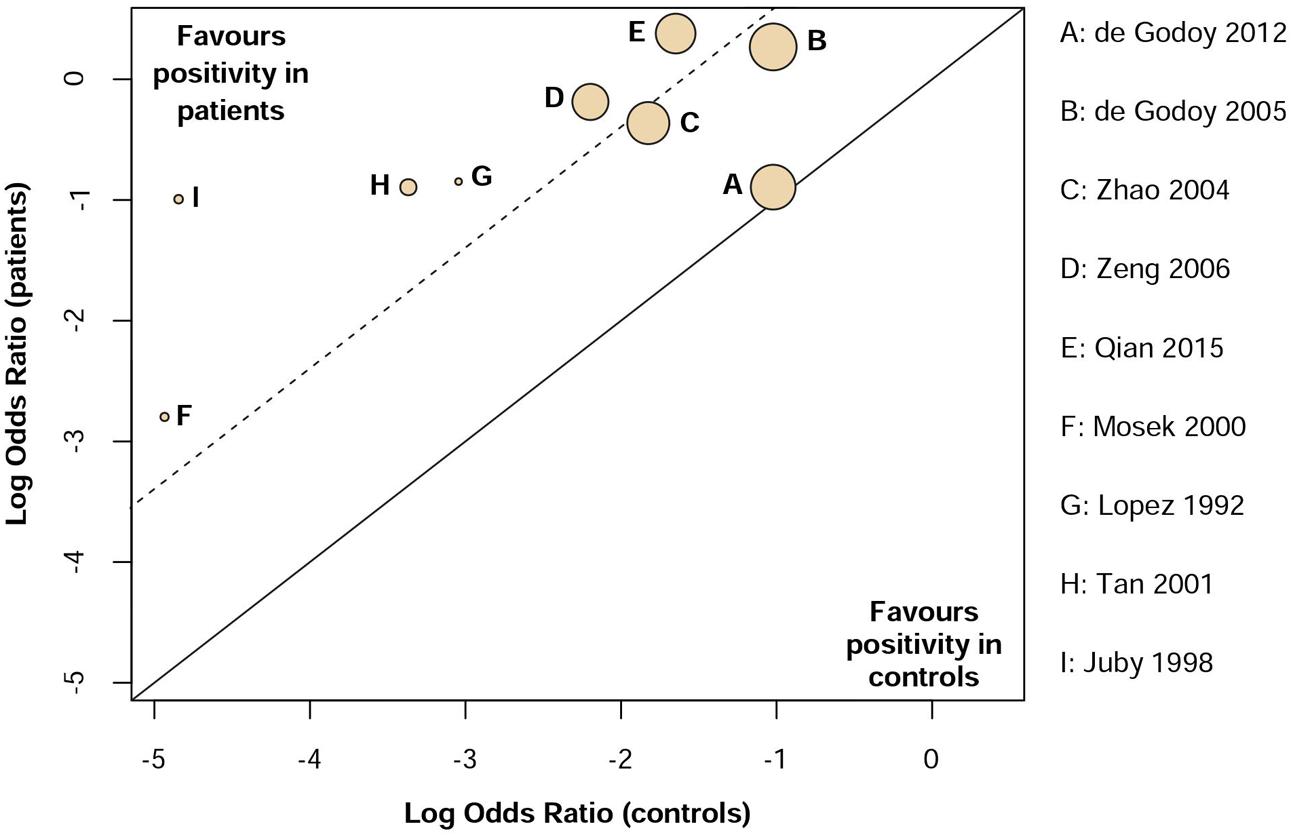
Low heterogeneity was observed (I2 = 32%) in assessing aCL in dementia patients compared to controls. Additionally, visual inspection of L’Abbé plot (Figure 6) demonstrated no substantial heterogeneity.
Visual assessment of funnel plot (Figure 7) showed that the studies were distributed asymmetrically, suggesting the presence of some publication bias. Begg’s test was not significant (p = 0.180), however, there was a trend toward significance for Egger’s regression (p = 0.081).
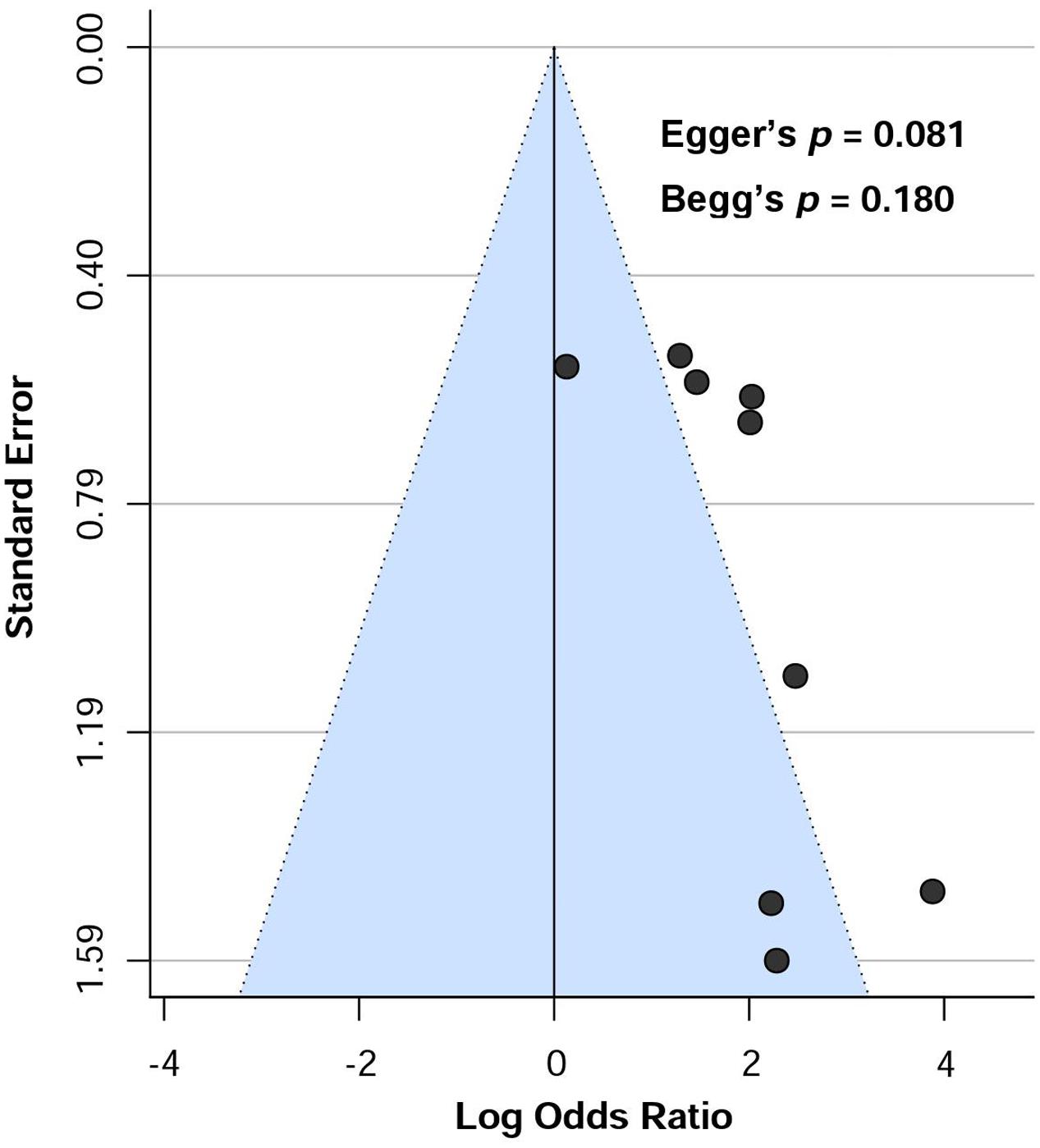
FIGURE 7. Funnel plot showing the risk of publication bias assessing the presence of aCL antibodies in dementia patients.
Discussion
In this study, based on the meta-analysis of nine shortlisted studies (372 dementia patients and 337 healthy controls), we validated the fact that aCL antibodies were significantly present in dementia patients as compared to healthy subjects, thus resolving previous conflicting reports on their associations. Our observation based on the meta-analysis on case-control studies was also supported by some cohort studies demonstrating the association of aCL positivity with cognitive decline and impaired motor function as follows: (1) Jacobson et al. (1999) reported that the frequency of impaired neuropsychologic performance was significantly higher among young individuals with aPLs (n = 27) as compared with controls (p < 0.01). (2) Primary data of a longitudinal study demonstrated that aCL was positive up to 19% of subjects with impaired cognitive and motor function (Arvanitakis et al., 2012); (3) Another cohort study on normal population (n = 1895) without neurological disease including dementia indicated that aCL-positive subjects performed worst on the Mini Mental State Examination (MMSE) cognitive scale, suggesting an aCL-mediated mechanism in cognitive decline (Homayoon et al., 2014).
In addition, our subgroup analyses showed that aCL was significantly present in patients with VD but not DAT. aCL has been observed to be associated with stroke development (Levine et al., 1987; Muir et al., 1994; Janardhan et al., 2004). It has been hypothesized that aCL-induced thrombotic events may contribute to multiple cerebral thrombotic symptoms and exert greater aggression to the brain (de Godoy et al., 2000). aCL antibodies may exert VD via vascular events similar with that seen in aCL-associated strokes (Tan et al., 2001).
Although dementia has been observed to comorbid with aPLs, the causative role of aPLs in dementia is still inconclusive. The BBB is an interface comprising of endothelial cells, astrocyte end-feet and pericytes, separating the brain involving neurons, blood vessels and glial cells from the circulatory system (Ballabh et al., 2004; Capani et al., 2016). BBB protects the central nervous system (CNS) by blocking the entry of harmful substances while allowing the transport of essential molecules (Lee et al., 2016). Interestingly, aCL antibodies have shown a direct binding affinity towards astrocytes in an in vivo study (Sun et al., 1992). aCL could inhibit the proliferation of astrocytes that ultimately distort the structure and function of BBB in addition of eliciting thrombus formation in the brain’s blood vessels (Yu et al., 1991; Sun et al., 1992). Direct binding of aPLs to brain tissues (Kent et al., 1997; Caronti et al., 1998a,b; Kent et al., 2000) after BBB disruption might be a potential pathogenic mechanism of dementia development.
aPLs mainly react with phospholipids, phospholipid-protein complexes, and phospholipid-binding proteins (Fischer et al., 2007; Misasi et al., 2015). Brain tissues comprising of gray and white-matter contain high proportion of phospholipids (especially on brain cell membranes) such as phosphatidylcholine, phosphatidylserine, phosphatidylinositol, and sphingomyelin (Hamberger and Svennerholm, 1971; Kwee and Nakada, 1988; Calderon et al., 1995) which may become the target of aCL antibodies. In an in vivo study, aCL antibodies were found to bind with only brain tissues when compared with liver tissues (Sun et al., 1992). In addition, through a damaged BBB, aCL antibodies were also found to diffuse from blood circulation to CNS as aCL antibodies were simultaneously found in the cerebrospinal fluids of different neurologic disorders such as multiple sclerosis, neurosyphilis and Guillain–Barré syndrome (Harris et al., 1985). Thus, there might exist a selective mechanism of aCL antibody binding with phospholipids of brain tissues, contributing to dementia pathogenesis.
Past studies have shown that aPL-positive subjects for more than 20 years had higher risk of developing dementia (Mosek et al., 2000; Chapman et al., 2002). In transgenic animal model of AD, prolonged exposure of aPLs in the brain was found to generate AD-like pathology including accumulation of amyloid peptides, formation of mature amyloid plaque as well as development of behavioral and cognitive changes (Katzav et al., 2011). The researchers proposed that BBB break down might occur via inflammation, coagulation and direct antibody binding such as aPLs (Katzav et al., 2011).
In patients with VD, cognitive impairments occur due to cerebrovascular disease and ischemic or hemorrhagic brain injury (Iemolo et al., 2009). Increasing evidences of BBB dysfunction have been observed in stroke patients and cerebrovascular incidents are believed to play significant roles in VD development (Ueno et al., 2015; van de Haar et al., 2015). On the other hand, altered BBB was also observed in VD without brain infarcts, suggesting infarction-beyond pathogenesis of VD via BBB disruption (Wallin et al., 1990). Cerebrovascular events have been observed in patients exhibiting aCL antibodies and thought to contribute in the pathogenesis via triggering thrombotic events (Kushner, 1990; de Godoy et al., 2000; Brey et al., 2002). Chapman et al. (1999) reported that IgG aCL antibodies could disrupt neuronal function via permeabilization and depolarization of brain synaptoneurosomes by direct action on nerve terminals. Therefore, subsequent chronic permeabilization could lead to irreversible damage and neuronal loss which might explain our findings of significant presence of aCL in patients with dementia. Therefore, in terms of VD, aCL antibodies might have indirect pathogenic contributions via either developing cerebrovascular events or direct immune-mediated mechanisms.
Blood-brain barrier dysfunction was reported in a group of demented patients (AD = 56; VD = 29) with white-matter changes without evidences of stroke (Wallin et al., 2000). This study concludes that BBB dysfunction might be linked with vasculature and tissue damage. Interestingly, a significant association was observed (p < 0.05) between the presence of aCL antibodies and cerebral damage in white-matter of neuropsychiatric SLE patients (Steens et al., 2006). Another study reported that reduced white-matter volume was associated with the presence of aPLs in SLE patients with cognitive impairment (Appenzeller et al., 2007). Therefore, besides BBB, white-matter region could be a potential target of aCL antibodies in the pathogenesis of dementia.
In both AD and VD, oxidative stress is an established phenomenon to be involved in the pathogenesis (Cervellati et al., 2014; Luca et al., 2015; Alam et al., 2016; Islam et al., 2017c). In an experimental mouse model, aCL antibodies were significantly associated with decreased paraoxonase activity and reduced nitric oxide levels (Alves et al., 2005), which suggests the involvement of aCL in inducing oxidative stress.
Several limitations should be noted in this meta-analysis. Firstly, the number of included studies (n = 9) in the meta-analysis was relatively low. However, aCL was significantly associated with dementia patients regardless of age ranges (60–70 vs. above 70 years old) nor patients from different geographical continents (Asian and European vs. North and South Americans), suggesting the reproducibility of aCL-dementia association across different age groups and nationalities. Secondly, the cut-off values of aCL antibody positivity were different from one study to another. Thirdly, the diagnostic criteria followed to confirm dementia varied across the studies. Finally, although neither Begg’s nor Egger’s tests showed significant publication bias, visual inspection of the funnel plot demonstrated asymmetrical distribution of included studies showing a trend towards publication bias. This discrepancy was possibly due assessment of heterogeneity using lower number of studies (<10).
Although a few case-reports and cohort studies reported the presence (Inzelberg et al., 1992; Kurita et al., 1994; Van Horn et al., 1996; Ciubotaru et al., 2002) or absence (Friedman, 2011; De Maeseneire et al., 2014) of anti-β2-GPI and LA in dementia patients, to the best of our knowledge, no case-control studies assessing anti-β2-GPI and LA in dementia patients have been conducted. In addition, the role of aCL antibodies in the pathogenic mechanisms of dementia remains unclear, and further research is required to establish the potential involvement of aCL antibodies in the pathogenesis of dementia.
Conclusion
Anticardiolipin antibodies were significantly present in dementia patients compared to healthy controls, underscoring the potential to screen aCL-positive subjects for early symptoms of neurological impairment and dementia, as well as suggesting the important role of aCL antibodies in the pathogenesis of dementia.
Author Contributions
MAI and KKW conceived and designed the study. MAI and FA searched the databases and KKW participated in the study selection process. MAI, FA and KKW analyzed and interpreted the data. MAI, FA and KKW drafted the manuscript. THS, SHG and MAK critically edited, reviewed and approved the final version of the submitted manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the Universiti Sains Malaysia (USM) Vice-Chancellor Award (2015/2016) and the USM Global Fellowship (2014/2015) awarded to MAI and FA, respectively, to pursue their Ph.D. degrees. Research by KKW was supported by Research University (RU) grant (1001/PPSP/813054). For the English translation of the Chinese papers, we would like to thank Ms. Zichen Zhang and Mr. Md. Ahsanul Kabir Khan. Additionally, we are thankful to Frontiers for approving the publication fee waiver request.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fnagi.2017.00250/full#supplementary-material
References
Alam, F., Islam, M. A., Sasongko, T. H., and Gan, S. H. (2016). Type 2 diabetes mellitus and Alzheimer’s disease: bridging the pathophysiology and management. Curr. Pharm. Des. 22, 4430–4442. doi: 10.2174/1381612822666160527160236
Alves, J. D., Mason, L., Ames, P., Chen, P., Rauch, J., Levine, J., et al. (2005). Antiphospholipid antibodies are associated with enhanced oxidative stress, decreased plasma nitric oxide and paraoxonase activity in an experimental mouse model. Rheumatology 44, 1238–1244. doi: 10.1093/rheumatology/keh722
Appenzeller, S., Bonilha, L., Rio, P. A., Li, L. M., Costallat, L. T. L., and Cendes, F. (2007). Longitudinal analysis of gray and white matter loss in patients with systemic lupus erythematosus. Neuroimage 34, 694–701. doi: 10.1016/j.neuroimage.2006.09.029
Appenzeller, S., Lapa, A. T., De Carvalho, J. F., Peres, F. A., and Shoenfeld, Y. (2012). Cognitive dysfunction and antiphospholipid antibodies. Curr. Rheumatol. Rep. 14, 95–98. doi: 10.1007/s11926-011-0224-4
Arvanitakis, Z., Brey, R. L., Rand, J. H., Schneider, J. A., Leurgans, S. E., Yu, L., et al. (2012). Antiphospholipid antibodies, brain infarcts, and cognitive and motor decline in aging (ABICMA): design of a community-based, longitudinal, clinical-pathological study. Neuroepidemiology 40, 73–84. doi: 10.1159/000342761
Asherson, R., Mercey, D., Phillips, G., Sheehan, N., Gharavi, A., Harris, E., et al. (1987). Recurrent stroke and multi-infarct dementia in systemic lupus erythematosus: association with antiphospholipid antibodies. Ann. Rheum. Dis. 46, 605–611. doi: 10.1136/ard.46.8.605
Ballabh, P., Braun, A., and Nedergaard, M. (2004). The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1–13. doi: 10.1016/j.nbd.2003.12.016
Begg, C. B., and Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. doi: 10.2307/2533446
Blennow, K., Wallin, A., Davidsson, P., Fredman, P., Gottfries, C.-G., and Svennerholm, L. (1990). Intra-blood-brain-barrier synthesis of immunoglobulins in patients with dementia of the Alzheimer type. Alzheimer Dis. Assoc. Disord. 4, 79–86. doi: 10.1097/00002093-199040200-00002
Brey, R. L., Stallworth, C. L., Mcglasson, D. L., Wozniak, M. A., Wityk, R. J., Stern, B. J., et al. (2002). Antiphospholipid antibodies and stroke in young women. Stroke 33, 2396–2401. doi: 10.1161/01.STR.0000031927.25510.D1
Calderon, R., Attema, B., and Devries, G. (1995). Lipid composition of neuronal cell bodies and neurites from cultured dorsal root ganglia. J. Neurochem. 64, 424–429. doi: 10.1046/j.1471-4159.1995.64010424.x
Capani, F., Quarracino, C., Caccuri, R., and Sica, R. E. (2016). Astrocytes as the main players in primary degenerative disorders of the human central nervous system. Front. Aging Neurosci. 8:45. doi: 10.3389/fnagi.2016.00045
Caronti, B., Calderaro, C., Alessandri, C., Conti, F., Tinghino, R., Pini, C., et al. (1998a). Serum anti-β2-glycoprotein I antibodies from patients with antiphospholipid antibody syndrome bind central nervous system cells. J. Autoimmun. 11, 425–429. doi: 10.1006/jaut.1998.0214
Caronti, B., Pittoni, V., Palladini, G., and Valesini, G. (1998b). Anti-β2-glycoprotein I antibodies bind to central nervous system. J. Neurol. Sci. 156, 211–219. doi: 10.1016/S0022-510X(98)00027-6
Cervellati, C., Romani, A., Seripa, D., Cremonini, E., Bosi, C., Magon, S., et al. (2014). Oxidative balance, homocysteine, and uric acid levels in older patients with Late Onset Alzheimer’s Disease or Vascular Dementia. J. Neurol. Sci. 337, 156–161. doi: 10.1016/j.jns.2013.11.041
Chapman, J., Abu-Katash, M., Inzelberg, R., Yust, I., Neufeld, M. Y., Vardinon, N., et al. (2002). Prevalence and clinical features of dementia associated with the antiphospholipid syndrome and circulating anticoagulants. J. Neurol. Sci. 203, 81–84. doi: 10.1016/S0022-510X(02)00271-X
Chapman, J., Cohen-Armon, M., Shoenfeld, Y., and Korczyn, A. (1999). Antiphospholipid antibodies permeabilize and depolarize brain synaptoneurosomes. Lupus 8, 127–133. doi: 10.1191/096120399678847524
Ciubotaru, C. R., Esfahani, F., Benedict, R. H., Wild, L. M., and Baer, A. N. (2002). Chorea and rapidly progressive subcortical dementia in antiphospholipid syndrome. J. Clin. Rheumatol. 8, 332–339. doi: 10.1097/00124743-200212000-00010
de Godoy, J. M. P., De Godoy, M. R. P., Cipulo, J. P., and Tognola, V. A. (2005). Vascular dementia and anticardiolipin antibodies. Med. Sci. Monit. 11, 430–433.
de Godoy, J. M. P., De Godoy, M. R. P., Godoy, M. F., Tognola, W. A., and Fernandes, M. R. (2000). Anticorpo anticardiolipina: fator de risco para demência vascular? HB Cient. 7, 105–107.
de Godoy, M. R. P., Tognola, W. A., and Cipullo, J. P. (2012). Alzheimer’s disease and anticardiolipin antibodies. J. Phlebol. Lymphol. 5, 19–21.
De Maeseneire, C., Duray, M., Rutgers, M., and Gille, M. (2014). Neurological presentations of the antiphospholipid syndrome: three illustrative cases. Acta Neurol. Belg. 114, 117–123. doi: 10.1007/s13760-013-0275-6
Denburg, S. D., Carbotte, R. M., Ginsberg, J. S., and Denburg, J. A. (1997). The relationship of antiphospholipid antibodies to cognitive function in patients with systemic lupus erythematosus. J. Int. Neuropsychol. Soc. 3, 377–386.
Dening, T., and Babu Sandilyan, M. (2015). Dementia: definitions and types. Nurs. Stand. 29, 37–42. doi: 10.7748/ns.29.37.37.e9405
Egger, M., Smith, G. D., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Fischer, M. J., Rauch, J., and Levine, J. S. (2007). The antiphospholipid syndrome. Semin. Nephrol. 27, 35–46. doi: 10.1016/j.semnephrol.2006.09.006
Friedman, J. H. (2011). Dementia, ataxia and parkinsonism associated with the antiphospholipid syndrome. Parkinsonism Relat. Disord. 17, 215–216. doi: 10.1155/2013/316495
Giannakopoulos, B., and Krilis, S. A. (2013). The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 368, 1033–1044. doi: 10.1056/NEJMra1112830
Gomez-Puerta, J., Cervera, R., Calvo, L., Gómez-Ansón, B., Espinosa, G., Claver, G., et al. (2005). Dementia associated with the antiphospholipid syndrome: clinical and radiological characteristics of 30 patients. Rheumatology 44, 95–99. doi: 10.1093/rheumatology/keh408
Hamberger, A., and Svennerholm, L. (1971). Composition of gangliosides and phospholipids of neuronal and glial cell enriched fractions. J. Neurochem. 18, 1821–1829. doi: 10.1111/j.1471-4159.1971.tb09587.x
Harris, D. M., Rantalainen, T., Muthalib, M., Johnson, L., and Teo, W.-P. (2015). Exergaming as a viable therapeutic tool to improve static and dynamic balance among older adults and people with idiopathic Parkinson’s disease: a systematic review and meta-analysis. Front. Aging Neurosci. 7:167. doi: 10.3389/fnagi.2015.00167
Harris, E., Gharavi, A., Mackworth-Young, C., Patel, B., Derue, G., and Hughes, G. (1985). Lupoid sclerosis: a possible pathogenetic role for antiphospholipid antibodies. Ann. Rheum. Dis. 44, 281–283. doi: 10.1136/ard.44.4.281
Homayoon, N., Schwingenschuh, P., Hofer, E., Katschnig-Winter, P., and Schmidt, R. (2014). Anticardiolipin antibodies are associated with cognitive dysfunction in stroke-free individuals. Eur. J. Neurol. 21, 427–432. doi: 10.1111/ene.12316
Iemolo, F., Duro, G., Rizzo, C., Castiglia, L., Hachinski, V., and Caruso, C. (2009). Pathophysiology of vascular dementia. Immun. Ageing 6:13. doi: 10.1186/1742-4933-6-13
Inzelberg, R., Bornstein, N., Reider, I., and Korczyn, A. (1992). The lupus anticoagulant and dementia in non-SLE patients. Dement. Geriatr. Cogn. Disord. 3, 140–145. doi: 10.1159/000107009
Islam, M. A., Alam, F., Gan, S. H., Cavestro, C., and Wong, K. K. (2017a). Coexistence of antiphospholipid antibodies and cephalalgia. Cephalalgia 1–13. doi: 10.1177/0333102417694881
Islam, M. A., Alam, F., and Wong, K. K. (2017b). Comorbid association of antiphospholipid antibodies and migraine: a systematic review and meta-analysis. Autoimmun. Rev. 16, 512–522. doi: 10.1016/j.autrev.2017.03.005
Islam, M. A., Khandker, S. S., Alam, F., Khalil, M. I., Kamal, M. A., and Gan, S. H. (2017c). Alzheimer’s disease and natural products: future regimens emerging from nature. Curr. Top. Med. Chem. 17, 1408–1428. doi: 10.2174/1568026617666170103163054
Islam, M. A., Alam, F., Kamal, M. A., Wong, K. K., Sasongko, T. H., and Gan, S. H. (2016). ‘Non-criteria’ neurologic manifestations of antiphospholipid syndrome: a hidden kingdom to be discovered. CNS Neurol. Disord. Drug Targets 15, 1253–1265. doi: 10.2174/1871527315666160920122750
Jacobson, M. W., Rapport, L. J., Keenan, P. A., Coleman, R. D., and Tietjen, G. E. (1999). Neuropsychological deficits associated with antiphospholipid antibodies. J. Clin. Exp. Neuropsychol. 21, 251–264. doi: 10.1076/jcen.21.2.251.931
Janardhan, V., Wolf, P. A., Kase, C. S., Massaro, J. M., D’agostino, R. B., Franzblau, C., et al. (2004). Anticardiolipin antibodies and risk of ischemic stroke and transient ischemic attack. Stroke 35, 736–741. doi: 10.1161/01.STR.0000117575.48205.2D
Juby, A., Davis, P., Genge, T., and Mcelhaney, J. (1995). Anticardiolipin antibodies in two elderly subpopulations. Lupus 4, 482–485. doi: 10.1177/096120339500400611
Juby, A. G., and Davis, P. (1998). Prevalence and disease associations of certain autoantibodies in elderly patients. Clin. Invest. Med. 21, 4–11.
Kalaria, R. N., Maestre, G. E., Arizaga, R., Friedland, R. P., Galasko, D., Hall, K., et al. (2008). Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 7, 812–826. doi: 10.1016/S1474-4422(08)70169-8
Katzav, A., Faust-Socher, A., Kvapil, F., Michaelson, D. M., Blank, M., Pick, C. G., et al. (2011). Antiphospholipid syndrome induction exacerbates a transgenic Alzheimer disease model on a female background. Neurobiol. Aging 32, 272–279. doi: 10.1016/j.neurobiolaging.2009.02.007
Katzav, A., Shoenfeld, Y., and Chapman, J. (2010). The pathogenesis of neural injury in animal models of the antiphospholipid syndrome. Clin. Rev. Allergy Immunol. 38, 196–200. doi: 10.1007/s12016-009-8154-x
Kent, M., Alvarez, F., Vogt, E., Fyffe, R., Ng, A., and Rote, N. (1997). Monoclonal antiphosphatidylserine antibodies react directly with feline and murine central nervous system. J. Rheumatol. 24, 1725–1733.
Kent, M. N., Alvarez, F. J., Ng, A.-K., and Rote, N. S. (2000). Ultrastructural localization of monoclonal antiphospholipid antibody binding to rat brain. Exp. Neurol. 163, 173–179. doi: 10.1006/exnr.2000.7358
Kozora, E., Erkan, D., Zhang, L., Zimmerman, R., Ramon, G., Ulug, A., et al. (2013). Cognitive dysfunction in antiphospholipid antibody (aPL)-negative systemic lupus erythematosus (SLE) versus aPL-positive non-SLE patients. Clin. Exp. Rheumatol. 32, 34–40.
Kurita, A., Hasunuma, T., Mochio, S., Shimada, T., Isogai, Y., and Kurahashi, T. (1994). A young case with multi-infarct dementia associated with lupus anticoagulant. Intern. Med. 33, 373–375. doi: 10.2169/internalmedicine.33.373
Kushner, M. J. (1990). Prospective study of anticardiolipin antibodies in stroke. Stroke 21, 295–298. doi: 10.1161/01.STR.21.2.295
Kwee, I. L., and Nakada, T. (1988). Phospholipid profile of the human brain: 31P NMR spectroscopic study. Magn. Reson. Med. 6, 296–299. doi: 10.1002/mrm.1910060307
Lee, C.-Y., Dallérac, G., Ezan, P., Anderova, M., and Rouach, N. (2016). Glucose tightly controls morphological and functional properties of astrocytes. Front. Aging Neurosci. 8:82. doi: 10.3389/fnagi.2016.00082
Levine, S. R., Kim, S., Deegan, M. J., and Welch, K. (1987). Ischemic stroke associated with anticardiolipin antibodies. Stroke 18, 1101–1106. doi: 10.1161/01.STR.18.6.1101
Lin, Y. R., Chou, L. C., Chen, H. C., Liou, T. H., Huang, S. W., and Lin, H. W. (2016). Increased risk of dementia in patients with systemic lupus erythematosus: a nationwide population-based cohort study. Arthritis Care Res. 68, 1774–1779. doi: 10.1002/acr.22914
Lopez, O. L., Rabin, B. S., Huff, F. J., Rezek, D., and Reinmuth, O. M. (1992). Serum autoantibodies in patients with Alzheimer’s disease and vascular dementia and in nondemented control subjects. Stroke 23, 1078–1083. doi: 10.1161/01.STR.23.8.1078
Luca, M., Luca, A., and Calandra, C. (2015). The role of oxidative damage in the pathogenesis and progression of Alzheimer’s disease and vascular dementia. Oxid. Med. Cell. Longev. 2015:504678. doi: 10.1155/2015/504678
Misasi, R., Capozzi, A., Longo, A., Recalchi, S., Lococo, E., Alessandri, C., et al. (2015). “New” antigenic targets and methodological approaches for refining laboratory diagnosis of antiphospholipid syndrome. J. Immunol. Res. 2015:858542. doi: 10.1155/2015/858542
Miyakis, S., Lockshin, M. D., Atsumi, T., Branch, D. W., Brey, R. L., Cervera, R., et al. (2006). International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 4, 295–306. doi: 10.1111/j.1538-7836.2006.01753.x
Mosek, A., Yust, I., Treves, T., Vardinon, N., Korczyn, A., and Chapman, J. (2000). Dementia and antiphospholipid antibodies. Dement. Geriatr. Cogn. Disord. 11, 36–38. doi: 10.1159/000017211
Muir, K. W., Squire, I. B., Lees, K., and Alwan, W. (1994). Anticardiolipin antibodies in an unselected stroke population. Lancet 344, 452–456. doi: 10.1016/S0140-6736(94)91775-2
Prince, M., Comas-Herrera, A., Knapp, M., Guerchet, M., and Karagiannidou, M. (2016). World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia - Coverage, Quality and Costs now and in the Future. London: Alzheimer’s Disease International.
Prince, M., Knapp, M., Guerchet, M., Mccrone, P., Prina, M., Comas-Herrera, A., et al. (2014). Dementia UK: Update. London: Alzheimer’s Society.
Prince, M., Wimo, A., Guerchet, M., Ali, G., Wu, Y., and Prina, M. (2015). World Alzheimer’s Report 2015: The Global Impact of Dementia. Alzheimer’s Disease International - An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer’s Disease International.
Qian, W., Yuzhen, X., Chunhua, Q., Ailing, Y., and Yunlin, L. (2015). Investigation of relations between anti-cardiolipin antibodies and intima membrane thickness, carotid artery stenosis in patients with vascular dementia. Chin. J. Brain Dis. Rehabil. 5, 80–83.
The Cochrane Collaboration (2014). Review Manager (Revman) [Computer Program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre.
Robinson, L., Tang, E., and Taylor, J.-P. (2015). Dementia: timely diagnosis and early intervention. BMJ 350:h3029. doi: 10.1136/bmj.h3029
Román, G. C. (2003). Vascular dementia: distinguishing characteristics, treatment, and prevention. J. Am. Geriatr. Soc. 51, 296–304. doi: 10.1046/j.1532-5415.5155.x
Schmidt, R., Auer-Grumbach, P., Fazekas, F., Offenbacher, H., and Kapeller, P. (1995). Anticardiolipin antibodies in normal subjects. Stroke 26, 749–754. doi: 10.1161/01.STR.26.5.749
Steens, S. C., Bosma, G. P., Steup-Beekman, G. M., Le Cessie, S., Huizinga, T. W., and Van Buchem, M. A. (2006). Association between microscopic brain damage as indicated by magnetization transfer imaging and anticardiolipin antibodies in neuropsychiatric lupus. Arthritis Res. Ther. 8, R38. doi: 10.1186/ar1892
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283, 2008–2012. doi: 10.1001/jama.283.15.2008
Sun, K., Liu, W., Tsai, C.-Y., Liao, T.-S., Lin, W., and Yu, C. (1992). Inhibition of astrocyte proliferation and binding to brain tissue of anticardiolipin antibodies purified from lupus serum. Ann. Rheum. Dis. 51, 707–712. doi: 10.1136/ard.51.6.707
Tan, L., Su, J., and Liu, Z. (2001). Study on the correlation between antiphospholipid antibodies and vascular dementia. Shandong Med. J. 41, 3–5.
Ueno, M., Chiba, Y., Matsumoto, K., Murakami, R., Fujihara, R., Kawauchi, M., et al. (2015). Blood-brain barrier damage in vascular dementia. Neuropathology 36, 115–124. doi: 10.1111/neup.12262
van de Haar, H. J., Burgmans, S., Hofman, P. A., Verhey, F. R., Jansen, J. F., and Backes, W. H. (2015). Blood–brain barrier impairment in dementia: current and future in vivo assessments. Neurosci. Biobehav. Rev. 49, 71–81. doi: 10.1016/j.neubiorev.2014.11.022
Van Horn, G., Arnett, F. C., and Dimachkie, M. M. (1996). Reversible dementia and chorea in a young woman with the lupus anticoagulant. Neurology 46, 1599–1603. doi: 10.1212/WNL.46.6.1599
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. doi: 10.18637/jss.v036.i03
Wallin, A., Blennow, K., Fredman, P., Gottfries, C., Karlsson, I., and Svenner-Holm, L. (1990). Blood brain barrier function in vascular dementia. Acta Neurol. Scand. 81, 318–322. doi: 10.1111/j.1600-0404.1990.tb01562.x
Wallin, A., Sjögren, M., Edman,Å., Blennow, K., and Regland, B. (2000). Symptoms, vascular risk factors and blood-brain barrier function in relation to CT white-matter changes in dementia. Eur. Neurol. 44, 229–235. doi: 10.1159/000008242
Wotton, C. J., and Goldacre, M. J. (2017). Associations between specific autoimmune diseases and subsequent dementia: retrospective record-linkage cohort study, UK. J. Epidemiol. Community Health doi: 10.1136/jech-2016-207809 [Epub ahead of print].
Wu, G. C., Liu, H. R., Leng, R. X., Li, X. P., Li, X. M., Pan, H. F., et al. (2016). Subclinical atherosclerosis in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun. Rev. 15, 22–37. doi: 10.1016/j.autrev.2015.10.002
Yu, C.-L., Sun, K.-H., Tsai, C.-Y., and Wang, S.-R. (1991). Inhibitory effects of anticardiolipin antibodies on lymphocyte proliferation and neutrophil phagocytosis. Ann. Rheum. Dis. 50, 903–908. doi: 10.1136/ard.50.12.903
Zeng, A.-Y., Feng, J.-J., and Lan, W. (2006). Studies on correlation of anticardiolipin antibodies and multi-infarct dementia. Acta Med. Sin. 19, 635–636.
Keywords: dementia, Alzheimer’s disease, antiphospholipid antibodies, anticardiolipin antibodies, systematic review, meta-analysis
Citation: Islam MA, Alam F, Kamal MA, Gan SH, Sasongko TH and Wong KK (2017) Presence of Anticardiolipin Antibodies in Patients with Dementia: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 9:250. doi: 10.3389/fnagi.2017.00250
Received: 01 April 2017; Accepted: 14 July 2017;
Published: 02 August 2017.
Edited by:
Catarina Oliveira, University of Coimbra, PortugalReviewed by:
Wei Wang, Stowers Institute for Medical Research, United StatesVassiliki Nikoletopoulou, Institute of Molecular Biology and Biotechnology, Greece
Copyright © 2017 Islam, Alam, Kamal, Gan, Sasongko and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md. Asiful Islam, ayoncx70@yahoo.com Kah Keng Wong, kahkeng@usm.my
 Md. Asiful Islam
Md. Asiful Islam Fahmida Alam
Fahmida Alam Mohammad Amjad Kamal
Mohammad Amjad Kamal Siew Hua Gan
Siew Hua Gan Teguh Haryo Sasongko
Teguh Haryo Sasongko Kah Keng Wong
Kah Keng Wong