Neurofeedback for Tinnitus Treatment – Review and Current Concepts
- 1Neuroplasticity and Learning in the Healthy Aging Brain (HAB LAB), Department of Psychology, University of Zurich, Zurich, Switzerland
- 2University Research Priority Program ‘Dynamics of Healthy Aging’, University of Zurich, Zurich, Switzerland
- 3Department of Otorhinolaryngology, University Hospital of Zurich, Zurich, Switzerland
An effective treatment to completely alleviate chronic tinnitus symptoms has not yet been discovered. However, recent developments suggest that neurofeedback (NFB), a method already popular in the treatment of other psychological and neurological disorders, may provide a suitable alternative. NFB is a non-invasive method generally based on electrophysiological recordings and visualizing of certain aspects of brain activity as positive or negative feedback that enables patients to voluntarily control their brain activity and thus triggers them to unlearn typical neural activity patterns related to tinnitus. The purpose of this review is to summarize and discuss previous findings of neurofeedback treatment studies in the field of chronic tinnitus. In doing so, also an overview about the underlying theories of tinnitus emergence is presented and results of resting-state EEG and MEG studies summarized and critically discussed. To date, neurofeedback as well as electrophysiological tinnitus studies lack general guidelines that are crucial to produce more comparable and consistent results. Even though neurofeedback has already shown promising results for chronic tinnitus treatment, further research is needed in order to develop more sophisticated protocols that are able to tackle the individual needs of tinnitus patients more specifically.
Introduction
Subjective tinnitus has been described as the constant perception of an auditory sensation that does not correlate to any external acoustic stimulus (Stouffer and Tyler, 1990). It can be perceived as either pitch or noise-like sound and its perception may be unilateral, bilateral or spread out in the whole head (De Ridder et al., 2014b). In industrialized countries, roughly 10% of the population is affected by this stressful condition and many people suffer from sleeping or concentration problems, affected social interactions and psychological distress that can also lead to severe depression or anxiety impairments (Heller, 2003; Henry et al., 2005). The relatively large percentage of affected people, recently developed neuropsychological models, and the fact that, to date, no satisfactory potent treatment has been discovered may explain the increasing interest in tinnitus research. New findings on the pathophysiology of tinnitus have led to the development of several promising neuromodulatory techniques that have been shown to relieve symptoms of the chronic acoustic sensation and significantly increase quality of life for tinnitus sufferers (e.g., Eggermont and Roberts, 2004; Weisz et al., 2007a). One of them is neurofeedback, an already well-established form of neuropsychological treatment that recently enjoys great popularity due to its non-invasive nature, its long-lasting effects, its easy-handling and relatively low cost, as well as its rapid technological improvements. The purpose of this review is to summarize and discuss findings of neurofeedback studies for the treatment of chronic tinnitus. The focus is hereby laid on neurofeedback based on electrophysiological recordings with electroencephalography (EEG) or magnetoencephalography (MEG) but also a short summary of new innovative methods (e.g., real-time functional Magnetic Resonance Imaging, rt-fMRI) will be given. In a first step, an overview about popular models of tinnitus genesis will be provided, and studies investigating chronic tinnitus with EEG or MEG will be presented and critically discussed. Next, the development and history of neurofeedback will be briefly introduced and the different neurofeedback protocols used in tinnitus treatment summarized and evaluated. Finally, limitations of existing treatment studies will be discussed, and implications for future studies will be given.
Tinnitus Models and Electrophysiological Studies
Tinnitus was first assumed to be solely generated in the ear or by a dysfunction of the auditory nerve (Møller, 1984; Eggermont, 1990), but the focus of attention quickly shifted to the human brain after Jastreboff (1990) proposed what is nowadays known as the neurophysiological model of tinnitus. Even though some form of inner ear damage indeed seems to be a necessary prerequisite, Jastreboff (1990) suggested central processes in the auditory cortex, the limbic system, and prefrontal areas to be crucial for tinnitus genesis. Later models picked up this idea and tried to specify the neuroplastic alterations emerging after auditory deafferentation. In this context, an increase in central gain in subcortical structures of the auditory pathway (Noreña, 2011), reorganization of tonotopic maps in the primary auditory cortex (Mühlnickel et al., 1998), a thalamocortical dysrhythmia (Llinás et al., 1998, 1999, 2005; Weisz et al., 2007a) and changes in neural synchrony (Noreña and Eggermont, 2003; Seki and Eggermont, 2003; Eggermont and Roberts, 2004; Weisz et al., 2005), or a failing top-down noise-canceling mechanism (Rauschecker et al., 2010, 2015) have been discussed. Furthermore, global workspace models emphasize the importance of networks beyond the auditory system (De Ridder et al., 2014b), and frameworks of filling-in missing auditory information have been suggested in a Bayesian way (De Ridder et al., 2006, 2011, 2014a) or based on predictive coding (Sedley et al., 2016).
First Wave of Electrophysiological Studies
Apart from animal experiments, brain imaging and morphometry studies, the investigation of resting-state brain activity with electrophysiological methods, such as EEG or MEG, enjoys great popularity in tinnitus research (Adjamian, 2014). In order to pinpoint neural correlates of the ongoing tinnitus sensation, first studies compared spontaneous brain activity of tinnitus patients at rest with the one of healthy controls. In this context, most investigations focused on the analysis of neuronal oscillations separated into distinct frequency bands: delta (0.5–4 Hz), theta (4.5–8 Hz), alpha (8.5–12 Hz), beta (12.5–35 Hz), and gamma (35.5–80 Hz). Following this approach, early studies (Weisz et al., 2005, 2007b; Ashton et al., 2007; Kahlbrock and Weisz, 2008; Lorenz et al., 2009) found a relatively consistent pattern of enhanced activity in delta- and gamma frequencies, alongside with reduced amounts of alpha oscillations over temporal areas of tinnitus patients (for a review, see Schlee et al., 2008; Adjamian et al., 2009). These findings have been interpreted in the framework of the thalamocortical dysrhythmia model (TCD), originally proposed by Llinás et al.’s (1998, 1999, 2005) and later significantly refined by Weisz et al. (2007a) to the synchronization by loss of inhibition modulation (SLIM) model. Both models aim at sketching tinnitus genesis as the result of an imbalance between inhibition and excitation in thalamocortical circuits. Loss of sensory input (deafferentation) gives raise to low frequent self-oscillations of thalamic cells which activate the auditory cortex and can thus be measured as oscillations in a slow delta rhythm on the scalp. At the same time, input deprivation also leads to a downregulation of inhibitory mechanisms which is reflected in alpha desynchronization in the resting-state EEG or MEG. This decrease of inhibition is then proposed to lead to spontaneous synchronization of firing reflected in increasing activity in fast gamma oscillations. This pattern of increased resting-state delta and gamma and decreased alpha has thus been termed the neural signature of tinnitus, and gamma has been interpreted as the neuronal substrate of the sound percept itself.
Limitations of the Early Studies
One of the major flaws of these early studies, however, was that they did not consider that chronic tinnitus is a very heterogeneous phenomenon and can differ substantially between individuals. It has clearly been shown that the subjective experience of the chronic sound (intensity, pitch, location) as well as the related distress and comorbid symptoms vary considerably among sufferers (Landgrebe et al., 2010; Langguth et al., 2013; Weidt et al., 2016; van den Berge et al., 2017). In addition, the underlying neuroanatomical and neurophysiological alterations may be far from homogenous in the population of tinnitus patients. Instead of comparing tinnitus patients with healthy controls, more recent studies thus focused on differences within the tinnitus sample with the ultimate goal of identifying distinct subtypes of tinnitus and finding different forms of treatment for each of these subtypes.
Another issue that the earlier studies had to deal with is the fact that electrophysiological methods suffer from rather poor spatial resolution. In terms of neuroscience, the inverse problem describes the fact that signal as measured by electrodes or magnetometers on the scalp could be generated by infinite combinations of neuronal sources (Scherg and Berg, 1991). The described pattern of tinnitus-specific oscillations found in the earlier studies, even though measured over temporal areas, could therefore have been generated in (or significantly altered by) cell assemblies outside of the primary auditory cortex. Different source estimation algorithms have been developed in the recent past to solve this inverse problem as well as possible by applying different a priori assumptions. With these algorithms the source of a measured signal can be estimated and spatial resolution of resting-state EEG and MEG measurements significantly increased (Michel et al., 2004). Standardized Low Resolution Electromagnetic Tomography (sLORETA) (Pascual-Marqui, 2002) or beamformer algorithms (van Veen et al., 1997; Hillebrand et al., 2005; Grosse-Wentrup et al., 2009) are examples of fairly precise and therefore relatively popular source estimation techniques.
The new focus on differences within the tinnitus population and the improvements in electrophysiological analysis methods have led to a veritable boom of resting-state tinnitus studies. Some investigations have confirmed the neuronal tinnitus code and auditory gamma as its major brain correlate by applying sLORETA (van der Loo et al., 2009; Moazami-Goudarzi et al., 2010; Vanneste et al., 2011a) or beamformer (Ortmann et al., 2011) source estimations to the measured signal, reporting correlations between tinnitus loudness and auditory gamma (van der Loo et al., 2009) or by performing intervention studies with acoustic coordinated reset (Tass et al., 2012; Adamchic et al., 2014a,b, 2017). Schlee et al. (2014), on the other hand, found decreased power (and variability) only for the lower (8–10 Hz) but not for the upper alpha band (10–12 Hz) and other studies failed completely to find the expected pattern in the auditory areas (Vanneste et al., 2011b, 2012; Song et al., 2013; Meyer et al., 2014; Zobay and Adjamian, 2015). Furthermore, two studies (Sedley et al., 2012; Sedley and Cunningham, 2013) discussed the possibility that auditory gamma oscillations could emerge as an attempt of the brain to suppress the tinnitus percept rather than causing it.
Tinnitus Network(s) and Areas Beyond the Auditory Cortex
In neuroscience, the gamma frequency range has also been debated as a binding medium connecting activity of various circuits to form a unified percept (Singer, 1993). Already Schlee et al. (2009) reported gamma-related abnormalities in a network with core regions in prefrontal, orbitofrontal, and parieto-occipital areas. Later the different parallel networks that may differentially contribute to the various tinnitus symptoms were described in more detail (De Ridder et al., 2011, 2014b; Vanneste and De Ridder, 2012). A tinnitus core network was proposed to generate the sound per se and code its intensity and location (holocranial, uni- or bilateral). Other networks were introduced as modulating the sound type (sine wave tone, hissing, ringing) as well as aversive states and feelings (e.g., distress or mood) of tinnitus (De Ridder et al., 2014b). An increased and persisting amount of gamma oscillations and coupling with slow-waves could thus suggest that activity of these widely-distributed brain networks is constantly bound together (synchronized), and a unified tinnitus percept is formed with its very own characteristics for each individual coded in the relevant sub-networks. In order to capture the tinnitus phenomenon in its entirety, areas outside of the central auditory regions therefore have to be considered. Furthermore, the specificity of the measured EEG-patterns has to be carefully validated as related disorders might produce similar findings (e.g., Joos et al., 2012; Meyer et al., 2017). These considerations are also relevant with regard to the development of neurofeedback protocols.
Apart from investigations comparing brain networks of tinnitus patients and healthy controls based on analyses with graph theory or machine learning algorithms (Mohan et al., 2016a,b, 2017a,b), a multitude of recent electrophysiological studies attempt to find specific correlates in neural networks for the different aspects of tinnitus (Adjamian, 2014; De Ridder et al., 2015; Eggermont, 2015; Elgoyhen et al., 2015). These studies mainly investigated tinnitus-related distress or loudness, but also covered tinnitus type, pitch, location/laterality, duration, age of onset, day-time awareness, or related problems such as hearing loss, hyperacusis, depression, or general quality of life (a detailed summary is provided in the Supplementary Materials). The most consistent findings are reported for tinnitus-related distress, which seems to be represented in a network ranging from structures of the limbic system (e.g., anterior cingulate cortex and amygdala) to prefrontal areas (e.g., dorsolateral prefrontal cortex), and also includes the insula. Altogether, however, the results of these studies are rather heterogeneous, and attempts of replication are scarce and partly fail to confirm previous findings (Pierzycki et al., 2015; Meyer et al., 2017). This can partially be explained by different EEG or MEG hardware used for resting-state recordings, different paradigms during the measurement [e.g., length of measurement, operationalization of tinnitus symptoms, or condition of resting-state (eyes open/closed) used for the analysis], different source estimation algorithms and data analysis procedures. To resolve this issue, scholars of the European research network TINNET1 are channeling their efforts to establishing general guidelines for (electrophysiological) tinnitus studies and collecting comparable data in a large database2. In order to tackle the problem of tinnitus heterogeneity, it is thus of utmost importance that future studies take these guidelines into consideration, report also null- or conflicting results and further also extend their focus to replicating previous findings.
Neurofeedback
Applying neurophysiological methods, neurofeedback is a non-invasive neuromodulation technique which records a subject’s neuronal activity, extracts relevant aspects of brain processes by means of real time signal processing and returns feedback to the subject as visual or auditory stimuli. The aim of neurofeedback is to change behavioral traits or medical conditions associated with altered neural activity as demonstrated for chronic tinnitus in the previous section. This is generally done by means of operant conditioning (i.e., rewarding of wanted, inhibiting of unwanted changes) whereby the subjects learn to voluntarily change their own brain activity in the desired direction.
A Brief History of Neurofeedback
In the early 1930’s and 1940’s, human studies already suggested the capability of the central nervous system to alter neural activity patterns by means of conditioning methods (Loomis et al., 1936; Jasper and Shagass, 1941). Later, Wyrwicka and Sterman (1968) were able to train cats to change their brain activity in a specific direction, and, shortly after that, the first study with human subjects in this context was published (Sterman and Friar, 1972). In the following years, neurofeedback was intensively tested and showed promising results mainly in treatment studies with epilepsy and attention deficit hyperactivity disorder (ADHD) (Lubar and Bahler, 1976; Lubar and Lubar, 1984). For ADHD, neurofeedback already found acceptance as alternative to established medication based treatment, due to its non-invasive character, the almost complete absence of any side-effects and high self-efficacy experienced by the subjects (Lubar et al., 1995; Lévesque et al., 2006; Arns et al., 2009; Gevensleben et al., 2009; Strehl et al., 2017). Apart from that, effectiveness and feasibility of neurofeedback are more and more investigated in the context of many other psychological disorders and neurological conditions ranging from the treatment of depression (Kelley et al., 2017), anxiety (Mennella et al., 2017), or autism (Datko et al., 2017) to stroke patients (Kober et al., 2017) and prevention of Alzheimer’s disease (Jiang et al., 2017). Today, quality control is an important aspect in the neurofeedback field. The Biofeedback Certification International Alliance (BCIA)3 certifies bio- and neurofeedback practitioners who meet certain requirements and the Association for Applied Psychophysiology and Biofeedback (AAPB)4 recently released the 3rd edition of Evidence-Based Practice in Biofeedback and Neurofeedback, a document that summarizes treatment efficacy for various disorders (Tan et al., 2016).
Common Neurofeedback Paradigms
Neurofeedback training of classical definitions of distinct frequency bands (i.e., delta, theta, alpha, beta, and gamma) are the most commonly used protocols in the current literature. The main field of frequency band neurofeedback is the treatment of ADHD, where often a combination of different frequencies is trained (Lofthouse et al., 2012). However, classic frequency band training has also been adapted for other disorders, most prominently anxiety or affective problems (Hammond, 2005). Importantly, neurofeedback training based on this paradigm ultimately depends on findings of fundamental research about disorder-specific neural alterations and can even be used to confirm or disprove these findings.
Sensorimotor rhythms (SMR) are defined as EEG oscillations in the lower beta range (12 – 20 Hz). They are generally measured over the sensorimotor cortex and proposed to originate from the ventrobasal nucleus in the thalamus (Howe and Sterman, 1972, 1973). Neurofeedback training based on SMR mainly found application in the treatment of epilepsy (Sterman and Egner, 2006) or ADHD (Monastra et al., 2002; Fuchs et al., 2003).
Slow cortical potentials (SCP’s) describe very slow oscillations in a range of 0.3–1.5 Hz. They describe slow, discrete, and continuous shifts (up to seconds) of the overall cortical distribution of electrical activity representing increased or decreased excitability of underlying neuronal structures. SCP’s are usually recorded with a single electrode in a central position (Cz) and are proposed to reflect cognitive or motor preparation (Hammond, 2011). Initially, SCP training was exclusively applied in trials with patients suffering from epilepsy (Rockstroh et al., 1993) but later also found application in the treatment of ADHD (Strehl et al., 2017).
Infra-low neurofeedback (ILN) relies on training of even slower brain oscillations, ranging from 0.001 to 1.5 Hz (Vanhatalo et al., 2004). Infra-low oscillations were shown to correlate with other frequency bands as well (Monastra et al., 2002). There is an overlap with SCP-based neurofeedback, which mainly differs in the recording of SCP’s with a single central electrode and thus a training of a more summarized potential over the whole head. Positive effects of ILN on different neurological conditions were reported in case reports (Legarda et al., 2011).
In z-score neurofeedback, the training protocol for an individual patient is based on previous recordings of EEG data and comparison to a healthy age-matched normative database (Thatcher, 2010). During the neurofeedback training, patients try to normalize their EEG patterns and minimize deviations from this control group. This NFB alternative is a rather data-driven technique, and some studies report successful treatment of various disorders (e.g., schizophrenia, addiction, ADHD, or personality, anxiety, and affective disorders) with z-score neurofeedback (Surmeli and Ertem, 2009; Surmeli et al., 2012; Simkin et al., 2014).
Functional magnetic resonance imaging (fMRI) was introduced to the field of neurofeedback to obtain a better spatial resolution. Real-time acquisition of blood oxygenation level dependent (BOLD) signals demonstrates increased neural activity according to higher oxygen supply to active neurons (Ogawa et al., 1990). Although newer to the field, a large quantity of clinical treatment studies already focused on the use of real-time fMRI neurofeedback (Sulzer et al., 2013). The higher spatial resolution of fMRI neurofeedback, however, does not come without limitations. Increased blood oxygenation can be measured only after a delay of several seconds and is an indirect correlate of underlying neuronal processes. Compared to electrophysiological methods, the temporal resolution of fMRI is thus rather poor, and fast fluctuations cannot be captured accordingly and used for the feedback. Additionally, it is questionable if an MRI-scanner is a favorable setting to perform neurofeedback because of the limited space and the loud constant background noise. For tinnitus patients, this is a huge drawback, in particular in those individuals suffering from additional hyperacusis.
To address the poor spatial resolution of single- or multi-electrode EEG and MEG recordings, neurofeedback techniques have also been combined with source estimation algorithms. Congedo et al. (2004) introduced the first tomographic neurofeedback protocol based on the inverse solution technique LORETA (Pascual-Marqui et al., 1994). This approach has subsequently been intensely tested mainly in the context of ADHD treatment (Cannon et al., 2006, 2007, 2009, 2014; Koberda et al., 2012, 2013) and has recently been further refined (Congedo, 2006; Pllana and Bauer, 2011; Kopřivová et al., 2013; Bauer and Pllana, 2014; White et al., 2014).
Neurofeedback and Tinnitus: Existing Studies
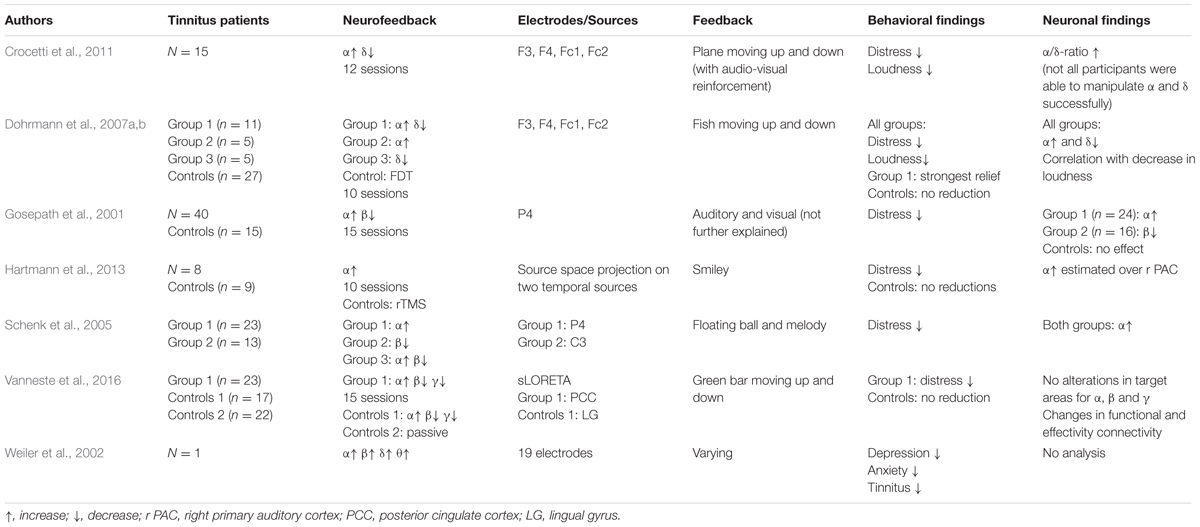
Presently, only a handful of studies investigated the efficacy of neurofeedback in the treatment of chronic tinnitus according to standard searching tools such as PubMed5. An overview is provided in Table 1.
In the first study in this context published by Gosepath et al. (2001), 40 patients suffering from chronic tinnitus and 15 control subjects underwent neurofeedback training. The training protocol included alpha training (8–13 Hz) alongside with a reduction of beta oscillations (14–30 Hz). While one group of patients (n = 24) was able to only increase their alpha activity, the effects of the other group (n = 16) were limited to the decrease of beta oscillations. All patients, however, reported to be less disturbed by their tinnitus after the training, indicated by significant decrement in scores of the tinnitus questionnaire (TQ) (Goebel and Hiller, 1994). Control subjects underwent identical trainings but without real-time feedback and did thus not show any changes in alpha or beta activity. Schenk et al. (2005) aimed at replicating the findings from Gosepath et al. (2001) with the aforementioned protocol. Before assigning them to different study groups, participants underwent baseline EEG-recordings at rest and during a stress test. Participants (n = 40) were assigned to three different groups according to their results. Twenty-three subjects showing decreased alpha activity under stress were allocated to a first group and set to train alpha activity (8–13 Hz) in the subsequent neurofeedback training. The second group consisted of 13 patients with increased beta activity in the stress condition and their treatment protocol thus aimed at the decreasing of beta oscillations (14–30 Hz). Four patients could not be assigned to either of the aforementioned groups according to their spontaneous brain activity and hence were allocated in a third group that had to increase alpha and decrease beta activity simultaneously. Subjects of the first group were able to increase their alpha activity, whereas subjects of the second group failed to significantly decrease their amount of beta oscillations. Surprisingly, also subjects of the second group showed increases in alpha activity even though it was not intended with the feedback. Reduced subjective tinnitus distress in terms of a reduction of TQ scores was reported for both groups. The third group was excluded from data analysis due to its small size.
A third rather explorative study shall briefly be mentioned. In a case report, Weiler et al. (2002) used z-score neurofeedback for one patient with bilateral tinnitus. The feedback protocol was based on EEG recordings prior to the training where decreased delta, theta, alpha and beta activities compared to 20 control subjects had been observed. The results indicated a normalization of depressive and anxiety symptoms and the patient reported that tinnitus was only occasionally present. However, no comparisons of pre–post changes in EEG patterns have been drawn in this study.
Even though these three first attempts to treat tinnitus with neurofeedback seemed to be promising, they should not be over-interpreted. First, the training-protocols were chosen rather arbitrarily and not based on previous findings of tinnitus-specific neural abnormalities. Moreover, the fact that patients of all groups reported significant improvements in tinnitus-related distress, regardless of their actual alterations of neural activity, speaks in favor of unspecific effects of the neurofeedback training. Especially the unintended increase of alpha activity in the second group of the study by Schenk et al. (2005) suggests that a general relaxation effect might have had a bigger impact than the actual neurofeedback protocol. In general, these first three studies rather aimed at helping their patients relax and reduce their general level of stress, and it is thus not surprising that reduced distress was reported after the training. However, since knowledge about the origins of tinnitus was still rare at this time, these studies can clearly be seen as pioneering works in the treatment of tinnitus with neurofeedback.
The TCD-model by Llinás et al. (1999, 2005) and the proposition of the neural signature of tinnitus (Weisz et al., 2007a) gave rise to new and potentially more appropriate neurofeedback protocols. Dohrmann et al. (2007a,b) developed their neurofeedback protocols by reference to these findings and aimed at an increasing of alpha and a decreasing of delta activity. Twenty-one patients suffering from chronic tinnitus were included into their study and further assigned to three different treatment groups (see Table 1). For the neurofeedback application 4 fronto-central electrodes (F3, F4, Fc1, and Fc2) were chosen because the recorded signal is most likely generated in the auditory cortex according to the authors. For a forth group of tinnitus patients (n = 27) frequency discrimination training (FDT) was applied aiming at a change of hearing-loss induced cortical map reorganization. Data analysis showed a significantly increased ratio between alpha and delta activity for the three neurofeedback groups suggesting an increase of alpha alongside with a decrease of delta over temporal auditory regions. These alterations were also correlated with a significant decline of tinnitus loudness for tinnitus patients. Subjects who were able to modify both bands simultaneously in the desired way showed the strongest relief from tinnitus compared to other groups (i.e., subgroups of patients with only alpha-, only delta-, or no change). Furthermore, the training generally resulted in a reduction of tinnitus related distress that was still notable even 6 months after the termination of the training. No statistically meaningful effects regarding tinnitus loudness or distress were found in the FDT group. In order to replicate these findings, Crocetti et al. (2011) conducted a study with 15 normal hearing tinnitus patients and tried to train them in decreasing delta and increasing alpha frequency bands. Even though no significant differences between pre- and post-training EEG patterns have been found, the results suggested an obvious trend toward an increasing alpha/delta ratio. In addition, scores evaluated with the Tinnitus Handicap Questionnaire (THI) (Newman et al., 1996) indicated significant improvements, which were maintained after the end of the training period.
All in all, these two studies suggested the protocol of upregulating alpha and downregulating delta to be a highly promising approach in tinnitus treatment. However, the surface-based nature of the neurofeedback application by simply using four electrodes on the scalp could not ensure that the brain activity used for the feedback indeed originated in the auditory areas. To address this problem, Hartmann et al. (2013) used a 32-channel EEG system and projected the recorded activity on the surface to eight regional dipole-sources, of which two were situated in the temporal cortex. Eight subjects of this investigation received neurofeedback treatment to train an increase of alpha power and nine subjects were treated with repetitive transcranial magnetic stimulation (rTMS). With the completion of the training, only patients of the neurofeedback group showed improved tinnitus distress scores. In comparison to the control group with rTMS treatment, they achieved significantly ameliorated scores in the TQ. Additionally, a comparison of MEG resting-state activity before and after treatment combined with spatial filtering based on a LCMV beamformer algorithm (van Veen et al., 1997) revealed a significant increase of alpha activity over the right primary auditory cortex. According to Hartmann et al. (2013) this proves that alpha activity can be systematically altered in the primary auditory cortex which helps restore the disturbed excitatory–inhibitory balance of tinnitus patients.
Finally, two recently published neurofeedback studies shall be mentioned. Milner et al. (2015) used SCP neurofeedback training in a case report and could show decreased tinnitus pitch and loudness as well as a reduction of delta and theta frequencies over left hemispheric fronto-temporal and temporo-occipital electrodes which they interpret as a normalization of tinnitus-specific activity. Vanneste et al. (2016) applied neurofeedback combined with sLORETA source estimation to a group of 58 tinnitus patients. A first group (n = 23) of this study received alpha-up training, and beta- and gamma-down training whereby the feedback was limited on the activity that was estimated to originate over the posterior cingulate cortex (PCC). A second group of 17 tinnitus patients received the same training but for activity over the lingual gyrus and a third group (n = 18) did not receive any treatment at all. Decreased tinnitus distress was only found for the PCC-group but no significant changes in any frequency bands were found in the trained areas. However, decreased cross-frequency coupling (i.e., alpha to beta and alpha to gamma power nesting) in the PCC and changes in functional and effective connectivity between PCC and different areas of the distress network suggest a specific effect of this training.
Finally, even though this review mainly focuses on neurofeedback based on electrophysiological recordings, it shall be noted that also real-time fMRI protocols are currently being developed and tested for tinnitus treatment with promising results (Haller et al., 2010, 2013; Emmert et al., 2017). In their investigations, the auditory cortex of tinnitus patients is first precisely localized thanks to the good spatial resolution of fMRI, and, subsequently, neurofeedback training aiming at reducing auditory BOLD activity provided. Even though this protocol leads to the intended neuronal alterations, no significant effects on tinnitus symptoms have been reported (Emmert et al., 2017).
Limitations of Neurofeedback Training Studies
Currently, the AAPB rates the efficacy of chronic tinnitus treatment with neurofeedback as possibly efficacious (level 2) (Tan et al., 2016). Although various neurofeedback training protocols showed promising results in treatment of several neurological disorders, there still remain limitations and open issues which need to be addressed. In particular, EEG- and MEG-based neurofeedback studies are often criticized about the low spatial resolution of electrophysiological recordings. Despite more refined source estimation algorithms, an uncertainty about the precision of the estimation remains, which is especially important when changes in frequency bands are considered as primary outcome measures. Studies that are able to verify specific effects in the brain areas of interest are still scarce and successful improvements of certain symptoms are thus often criticized to be the mere result of unspecific placebo effects (Thibault et al., 2016, 2017). Expectations of researcher and participant, the treatment condition in general (e.g., taking time off from a busy work schedule) and interactions with the practitioner (such as, the simple meeting with a clinician) can contribute greatly to the improvement of psychological symptoms. This problem is especially predominant in the context of chronic tinnitus therapy where most participants turn to neurofeedback hopefully after repeatedly being told by their doctors that nothing can be done to treat tinnitus and having undergone a wide variety of (sometimes rather questionable) treatments on their own.
One way to resolve this issue is to improve study designs and conduct double-blind trials with control groups using a form of sham neurofeedback. In this context, Thibault et al. (2016) suggest the use of prerecorded feedback of other participants, feedback of another disease-unrelated brain area, or inverse feedback protocols that reward unwanted and inhibit wanted changes of brain activity. The use of sham-control is, however, difficult to establish in clinical neurofeedback trials because of several reasons. First, participation in neurofeedback treatment studies requires considerable investments in time and energy on the part of participants as they generally have to attend multiple training sessions over the course of several weeks. Furthermore, in sham-controlled clinical studies, participants always enter a trial with some form of expectation and hope to be part of the treatment group. Absent success after the first training sessions may lead to a misleading belief that they instead have been assigned to the control group which negatively affects their motivation and further success in the training process (Strehl et al., 2017). These drawbacks of placebo-controlled trials have to be considered and alleviated with appropriate designs, such as a cross-over approach where one group of participants receives sham training first while the other starts with verum treatment. In a second step the protocols are swapped so that both groups undergo sham- as well as verum-neurofeedback. In this context several authors point to the importance of a systematic investigation of non-specific factors in neurofeedback studies (Friedrich et al., 2014; Sitaram et al., 2017; Thibault et al., 2017). Appropriate knowledge about the factors favoring and the ones hindering success in neurofeedback treatment can indeed lead to a better understanding of the actual mode of action of neurofeedback as well as help improve the treatment setting in order to optimize therapy outcomes for patients.
A major flaw of previous neurofeedback studies is that most of them settle for reporting positive effects of their trained protocol. It is known, however, that there is a wide variability among the efficacy of neurofeedback treatment for different subjects. While some are able to successfully self-regulate their neural activity in the desired way and show improvements of corresponding symptoms (responders), others fail to do so (non-responders) (Friedrich et al., 2014). This issue was described as neurofeedback inefficacy by Alkoby et al. (2017) who provide a thorough review about this currently existing topic. In their publication, they chose 20 papers published after 2010 at random and found that only two of them reported the actual number of responders and non-responders in their studies. This, of course, hampers a proper evaluation of the feasibility of a given neurofeedback protocol for the treatment of a certain disorder. For one thing, positive effects of the training might be concealed or confounded by the negative results of non-responders in the clinical trial. Furthermore, information provided about responder and non-responder groups helps define and analyze factors for success or failure of the protocol. That is, by means of a thorough investigation of the attributes of responders and non-responders, predictors for (un-) successful neurofeedback can be identified, which can be used to improve training protocols for future patients.
Another issue in this context is the high heterogeneity among outcome measures and definitions to appropriately measure success or failure used in previous neurofeedback studies. On the one hand, it can be useful to use a wide variety of outcome measures in a clinical study in order to account for changes which might not be anticipated in the first place. For instance, it can be important to measure the general level of stress of tinnitus patients as the positive effects of neurofeedback could also be explained by a decrease of the general stress condition of the patient. However, guidelines need to be established which suggest the use of certain questionnaires or tests for a given field of interest to which scholars can relate when planning an investigation [substantial work in the tinnitus field is currently being done by Hall et al. (2016) in this context]. This will limit the amount of different outcome measures in clinical trials, promote the use of well-established and validated questionnaires, and foster direct comparability between findings of different investigations. Additionally, guidelines in the context of neurofeedback treatment need to answer the question as to what can be regarded as successful or unsuccessful training and how to distinguish responders from non-responders. Is it already sufficient that a given symptom simply changes over the course of a training in a positive way or does it have to improve by a certain amount (e.g., an increase by certain points in a questionnaire score)? What, on the other hand, needs to happen to and in between brain circuits? How and how much does neural activity have to be altered by the neurofeedback treatment so that an individual can be labeled as a responder? Even though some publications already tried to postulate criteria or guidelines (Gruzelier, 2014; Rogala et al., 2016; Enriquez-Geppert et al., 2017), many open issues remain in this regard.
Conclusion
In this review, we summarized and discussed the current state of electrophysiological brain research in the field of chronic tinnitus as well as recent advances of neurofeedback treatment. Up to date, only a handful of studies exist that investigated feasibility of neurofeedback protocols for chronic tinnitus patients. While the first studies in this context rather focused on creating a general state of relaxation for the subject, later trials considered tinnitus-specific alterations in brain activity based on comparisons of EEG or MEG resting-state recordings between tinnitus patients and healthy controls. The main region of interest in these studies was the auditory cortex, and fairly good results have been achieved following this approach. With the newer developments in tinnitus research and the numerous investigations dealing with differences within the tinnitus population, which take into account the substantial amount of heterogeneity amongst tinnitus sufferers, also other potential tinnitus-related brain areas can be targeted in future neurofeedback studies. A good example in this regard is the recent publication by Vanneste et al. (2016) where the posterior cingulate cortex as part of the tinnitus distress network has been targeted. Furthermore, this investigation is the only neurofeedback study in the context of chronic tinnitus treatment to date that included a control group with training of a tinnitus-irrelevant brain area in its design.
To sum up, even though often criticized in the recent past, results of current studies suggest that neurofeedback seems to be a promising method for efficient tinnitus treatment and may enjoy great popularity in the future. The ultimate goal may be to develop different neurofeedback alternatives for a given subgroup of tinnitus sufferers or even establish neurofeedback on an individualized basis for each patient. In this context, multi-location and multi-frequency neurofeedback protocols with adequate source estimation algorithms, which are able to train multiple brain networks in power and maybe even connectivity changes simultaneously, can be seen as the gold standard for future neurofeedback protocols. At the moment, however, there still exist several challenges that need to be overcome. A general issue are technological aspects of electrophysiological measurements (e.g., the limited spatial precision of resting-state EEG recordings) and neurofeedback applications (e.g., the implementation of connectivity-based neurofeedback protocols) that need to be improved. Regarding the treatment of chronic tinnitus in particular, results of existing fundamental studies are still too heterogeneous in order to suffice for the development of more sophisticated neurofeedback protocols. One possibility to resolve this latter issue is by means of the establishment of general guidelines about adequate symptom assessment, measurement paradigms, and analysis methods. In this way, more coherent and comparable results should be published in order to lead to a better understanding of tinnitus heterogeneity and its underlying alterations in brain networks that could be tackled by future neurofeedback protocols. Additionally, this urgent need for guidelines has been shown to be an open issue in the field of clinical neurofeedback research in general. Clarity is needed about how to separate responders from non-responders, and which outcome domains and measurements are best suited to do so. Furthermore, also non-specific effects of the training have to be taken into account and systematic investigations about the most (or least) favorable neurofeedback settings and treatment conditions are needed.
Author Contributions
Each author has provided substantial contributions to warrant authorship. Contributions are as follows: DG and CT equally contributed to the conception, draft and revision of the paper and are sharing first-authorship. MM, PN, and TK contributed to conception, critically revising and final approval of the manuscript.
Funding
The authors disclose the following financial support for research, authorship, and/or publication of this article: ‘Velux Stiftung’, ‘Zürcher Stiftung für das Hören (ZSFH)’, ‘Fonds zur Förderung des akademischen Nachwuchses (FAN) des Zürcher Universitätsvereins (ZUNIV)’, University Research Priority Program ‘Dynamics of Healthy Aging’ of the University of Zurich.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with the authors MM and TK.
Acknowledgments
The authors are further indebted to the TINNET - COST Action BM1306 ‘Better Understanding the Heterogeneity of Tinnitus to Improve and Develop New Treatments’ for providing a network, which allows exchange of knowledge among tinnitus researchers in Europe. During the work on his dissertation, DG was a pre-doctoral fellow of LIFE (International Max Planck Research School on the Life Course; participating institutions: MPI for Human Development, Humboldt-Universität zu Berlin, Freie Universität Berlin, University of Michigan, University of Virginia, University of Zurich).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2017.00386/full#supplementary-material
Footnotes
- ^ http://tinnet.tinnitusresearch.net/
- ^ https://www.tinnitus-database.de/
- ^ http://www.bcia.org
- ^ https://www.aapb.org
- ^ https://www.ncbi.nlm.nih.gov/pubmed/
References
Adamchic, I., Langguth, B., Hauptmann, C., and Tass, P. A. (2014a). Abnormal cross-frequency coupling in the tinnitus network. Front. Neurosci. 8:284. doi: 10.3389/fnins.2014.00284
Adamchic, I., Toth, T., Hauptmann, C., and Tass, P. A. (2014b). Reversing pathologically increased EEG power by acoustic coordinated reset neuromodulation. Hum. Brain Mapp. 35, 2099–2118. doi: 10.1002/hbm.22314
Adamchic, I., Toth, T., Hauptmann, C., Walger, M., Langguth, B., Klingmann, I., et al. (2017). Acute effects and after-effects of acoustic coordinated reset neuromodulation in patients with chronic subjective tinnitus. Neuroimage Clin. 15, 541–558. doi: 10.1016/j.nicl.2017.05.017
Adjamian, P. (2014). The application of electro- and magneto-encephalography in tinnitus research - methods and interpretations. Front. Neurol. 5:228. doi: 10.3389/fneur.2014.00228
Adjamian, P., Sereda, M., and Hall, D. A. (2009). The mechanisms of tinnitus. Perspectives from human functional neuroimaging. Hear. Res. 253, 15–31. doi: 10.1016/j.heares.2009.04.001
Alkoby, O., Abu-Rmileh, A., Shriki, O., and Todder, D. (2017). Can we predict who will respond to neurofeedback? A review of the inefficacy problem and existing predictors for successful EEG neurofeedback learning. Neuroscience doi: 10.1016/j.neuroscience.2016.12.050 [Epub ahead of print].
Arns, M., De Ridder, S., Strehl, U., Breteler, M., and Coenen, A. (2009). Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin. EEG Neurosci. 40, 180–189. doi: 10.1177/155005940904000311
Ashton, H., Reid, K., Marsh, R., Johnson, I., Alter, K., and Griffiths, T. D. (2007). High frequency localised “hot spots” in temporal lobes of patients with intractable tinnitus: a quantitative electroencephalographic (QEEG) study. Neurosci. Lett. 426, 23–28. doi: 10.1016/j.neulet.2007.08.034
Bauer, H., and Pllana, A. (2014). EEG-based local brain activity feedback training - tomographic neurofeedback. Front. Hum. Neurosci. 8:1005. doi: 10.3389/fnhum.2014.01005
Cannon, R. L., Baldwin, D. R., Diloreto, D. J., Phillips, S. T., Shaw, T. L., and Levy, J. J. (2014). LORETA neurofeedback in the precuneus: operant conditioning in basic mechanisms of self-regulation. Clin EEG Neurosci. 45, 238–248. doi: 10.1177/1550059413512796
Cannon, R. L., Congedo, M., Lubar, J. F., and Hutchens, T. (2009). Differentiating a network of executive attention: loreta neurofeedback in anterior cingulate and dorsolateral prefrontal cortices. Int. J. Neurosci. 119, 404–441. doi: 10.1080/00207450802480325
Cannon, R. L., Lubar, J. F., Congedo, M., Thornton, K., Towler, K., and Hutchens, T. (2007). The effects of neurofeedback training in the cognitive division of the anterior cingulate gyrus. Int. J. Neurosci. 117, 337–357. doi: 10.1080/00207450500514003
Cannon, R. L., Lubar, J. F., Gerke, A., Thornton, K., Hutchens, T., and McCammon, V. (2006). EEG spectral-power and coherence: LORETA neurofeedback training in the anterior cingulate gyrus. J. Neurother. 10, 5–31. doi: 10.1300/J184v10n01_02
Congedo, M. (2006). Subspace projection filters for real-time brain electromagnetic imaging. IEEE Trans. Biomed. Eng. 53, 1624–1634. doi: 10.1109/TBME.2006.878055
Congedo, M., Lubar, J. F., and Joffe, D. (2004). Low-resolution electromagnetic tomography neurofeedback. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 387–397. doi: 10.1109/TNSRE.2004.840492
Crocetti, A., Forti, S., and Del Bo, L. (2011). Neurofeedback for subjective tinnitus patients. Auris Nasus Larynx 38, 735–738. doi: 10.1016/j.anl.2011.02.003
Datko, M., Pineda, J. A., and Muller, R.-A. (2017). Positive effects of neurofeedback on autism symptoms correlate with brain activation during imitation and observation. Eur. J. Neurosci. doi: 10.1111/ejn.13551 [Epub ahead of print].
De Ridder, D., Elgoyhen, A. B., Romo, R., and Langguth, B. (2011). Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc. Natl. Acad. Sci. U.S.A. 108, 8075–8080. doi: 10.1073/pnas.1018466108
De Ridder, D., Fransen, H., Francois, O., Sunaert, S., Kovacs, S., and van de Heyning, P. (2006). Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Otolaryngol. 126, 50–53. doi: 10.1080/03655230600895580
De Ridder, D., Vanneste, S., and Freeman, W. (2014a). The Bayesian brain: phantom percepts resolve sensory uncertainty. Neurosci. Biobehav. Rev. 44, 4–15. doi: 10.1016/j.neubiorev.2012.04.001
De Ridder, D., Vanneste, S., Weisz, N., Londero, A., Schlee, W., Elgoyhen, A. B., et al. (2014b). An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci. Biobehav. Rev. 44, 16–32. doi: 10.1016/j.neubiorev.2013.03.021
De Ridder, D., Vanneste, S., Langguth, B., and Llinás, R. R. (2015). Thalamocortical dysrhythmia: a theoretical update in tinnitus. Front. Neurol. 6:124. doi: 10.3389/fneur.2015.00124
Dohrmann, K., Elbert, T., Schlee, W., and Weisz, N. (2007a). Tuning the tinnitus percept by modification of synchronous brain activity. Restor. Neurol. Neurosci. 25, 371–378.
Dohrmann, K., Weisz, N., Schlee, W., Hartmann, T., and Elbert, T. (2007b). Neurofeedback for treating tinnitus. Prog. Brain Res. 166, 473–485. doi: 10.1016/S0079-6123(07)66046-4
Eggermont, J. J. (1990). On the pathophysiology of tinnitus; a review and a peripheral model. Hear. Res. 48, 111–123. doi: 10.1016/0378-5955(90)90202-Z
Eggermont, J. J. (2015). The auditory cortex and tinnitus – a review of animal and human studies. Eur. J. Neurosci. 41, 665–676. doi: 10.1111/ejn.12759
Eggermont, J. J., and Roberts, L. E. (2004). The neuroscience of tinnitus. Trends Neurosci. 27, 676–682. doi: 10.1016/j.tins.2004.08.010
Elgoyhen, A. B., Langguth, B., De Ridder, D., and Vanneste, S. (2015). Tinnitus: perspectives from human neuroimaging. Nat. Rev. Neurosci. 16, 632–642. doi: 10.1038/nrn4003
Emmert, K., Kopel, R., Koush, Y., Maire, R., Senn, P., van de Ville, D., et al. (2017). Continuous vs. intermittent neurofeedback to regulate auditory cortex activity of tinnitus patients using real-time fMRI - A pilot study. Neuroimage. Clin. 14, 97–104. doi: 10.1016/j.nicl.2016.12.023
Enriquez-Geppert, S., Huster, R. J., and Herrmann, C. S. (2017). EEG-Neurofeedback as a tool to modulate cognition and behavior: a review tutorial. Front. Hum. Neurosci. 11:51. doi: 10.3389/fnhum.2017.00051
Friedrich, E. V. C., Wood, G., Scherer, R., and Neuper, C. (2014). Mind over brain, brain over mind: cognitive causes and consequences of controlling brain activity. Front. Hum. Neurosci. 8:348. doi: 10.3389/fnhum.2014.00348
Fuchs, T., Birbaumer, N., Lutzenberger, W., Gruzelier, J. H., and Kaiser, J. (2003). Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: a comparison with methylphenidate. Appl. Psychophysiol. Biofeedback 28, 1–12. doi: 10.1023/A:1022353731579
Gevensleben, H., Holl, B., Albrecht, B., Vogel, C., Schlamp, D., Kratz, O., et al. (2009). Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J. Child Psychol. Psychiatry 50, 780–789. doi: 10.1111/j.1469-7610.2008.02033.x
Goebel, G., and Hiller, W. (1994). Tinnitus-Fragebogen (TF). Standardinstrument zur Graduierung des Tinnitusschweregrades. Ergebnisse einer Multicenterstudie mit dem Tinnitus-Fragebogen (TF) [The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire]. HNO 42, 166–172. doi: 10.1007/s00106-013-2798-9
Gosepath, K., Nafe, B., Ziegler, E., and Mann, W. J. (2001). Neurofeedback in der Therapie des Tinnitus [Neurofeedback in therapy of tinnitus]. HNO 49, 29–35. doi: 10.1007/s001060050704
Grosse-Wentrup, M., Liefhold, C., Gramann, K., and Buss, M. (2009). Beamforming in noninvasive brain-computer interfaces. IEEE Trans. Biomed. Eng. 56, 1209–1219. doi: 10.1109/TBME.2008.2009768
Gruzelier, J. H. (2014). EEG-neurofeedback for optimising performance. III. A review of methodological and theoretical considerations. Neurosci. Biobehav. Rev. 44, 159–182. doi: 10.1016/j.neubiorev.2014.03.015
Hall, D. A., Haider, H., Szczepek, A. J., Lau, P., Rabau, S., Jones-Diette, J., et al. (2016). Systematic review of outcome domains and instruments used in clinical trials of tinnitus treatments in adults. Trials 17, 270. doi: 10.1186/s13063-016-1399-9
Haller, S., Birbaumer, N., and Veit, R. (2010). Real-time fMRI feedback training may improve chronic tinnitus. Eur. Radiol. 20, 696–703. doi: 10.1007/s00330-009-1595-z
Haller, S., Kopel, R., Jhooti, P., Haas, T., Scharnowski, F., Lovblad, K.-O., et al. (2013). Dynamic reconfiguration of human brain functional networks through neurofeedback. Neuroimage 81, 243–252. doi: 10.1016/j.neuroimage.2013.05.019
Hammond, D. C. (2005). Neurofeedback with anxiety and affective disorders. Child Adolesc. Psychiatr. Clin. N. Am. 14, 105. doi: 10.1016/j.chc.2004.07.008
Hammond, D. C. (2011). What is Neurofeedback. An Update. J. Neurother. 15, 305–336. doi: 10.1080/10874208.2011.623090
Hartmann, T., Lorenz, I., Müller, N., Langguth, B., and Weisz, N. (2013). The effects of neurofeedback on oscillatory processes related to tinnitus. Brain Topogr. 27, 149–157. doi: 10.1007/s10548-013-0295-9
Heller, A. J. (2003). Classification and epidemiology of tinnitus. Otolaryngol. Clin. N. Am. 36, 239–248. doi: 10.1016/S0030-6665(02)00160-3
Henry, J. A., Dennis, K. C., and Schechter, M. A. (2005). General review of tinnitus: prevalence, mechanisms, effects, and management. J. Speech Lang. Hear. Res. 48, 1204–1235. doi: 10.1044/1092-4388(2005/084)
Hillebrand, A., Singh, K. D., Holliday, I. E., Furlong, P. L., and Barnes, G. R. (2005). A new approach to neuroimaging with magnetoencephalography. Hum. Brain Mapp. 25, 199–211. doi: 10.1002/hbm.20102
Howe, R. C., and Sterman, M. B. (1972). Cortical-subcortical EEG correlates of suppressed motor behavior during sleep and waking in the cat. Electroencephalogr. Clin. Neurophysiol. 32, 681–695. doi: 10.1016/0013-4694(72)90104-6
Howe, R. C., and Sterman, M. B. (1973). Somatosensory system evoked potentials during waking behavior and sleep in the cat. Electroencephalogr. Clin. Neurophysiol. 34, 605–618. doi: 10.1016/0013-4694(73)90006-0
Jasper, H., and Shagass, C. (1941). Conditioning of the occipital alpha rhythm in man. J. Exp. Psychol. 28, 373–388. doi: 10.1037/h0056139
Jastreboff, P. J. (1990). Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci. Res. 8, 221–254. doi: 10.1016/0168-0102(90)90031-9
Jiang, Y., Abiri, R., and Zhao, X. (2017). Tuning up the old brain with new tricks: attention training via neurofeedback. Front. Aging Neurosci. 9:52. doi: 10.3389/fnagi.2017.00052
Joos, K., Vanneste, S., and De Ridder, D. (2012). Disentangling depression and distress networks in the tinnitus brain. PLOS ONE 7:e40544. doi: 10.1371/journal.pone.0040544.g001
Kahlbrock, N., and Weisz, N. (2008). Transient reduction of tinnitus intensity is marked by concomitant reductions of delta band power. BMC Biol. 6:4. doi: 10.1186/1741-7007-6-4
Kelley, N. J., Hortensius, R., Schutter, D. J. L. G., and Harmon-Jones, E. (2017). The relationship of approach/avoidance motivation and asymmetric frontal cortical activity. A review of studies manipulating frontal asymmetry. Int. J. Psychophysiol. 119, 19–30. doi: 10.1016/j.ijpsycho.2017.03.001
Kober, S. E., Schweiger, D., Reichert, J. L., Neuper, C., and Wood, G. (2017). Upper Alpha based neurofeedback training in chronic stroke: brain plasticity processes and cognitive effects. Appl. Psychophysiol. Biofeedback 42, 69–83. doi: 10.1007/s10484-017-9353-5
Koberda, J. L., Koberda, P., Bienkiewicz, A. A., Moses, A., and Koberda, L. (2013). Pain management using 19-Electrode Z-Score LORETA neurofeedback. J. Neurother. 17, 179–190. doi: 10.1080/10874208.2013.813204
Koberda, J. L., Moses, A., Koberda, L., and Koberda, P. (2012). Cognitive enhancement using 19-Electrode Z-Score neurofeedback. J. Neurother. 16, 224–230. doi: 10.1080/10874208.2012.705769
Kopřivová, J., Congedo, M., Razska, M., Praško, J., Brunovský, M., and Horáček, J. (2013). Prediction of treatment response and the effect of independent component neurofeedback in obsessive-compulsive disorder: a randomized, sham-controlled, and double-blind study. Neuropsychobiology 67, 210–223. doi: 10.1159/000347087
Landgrebe, M., Zeman, F., Koller, M., Eberl, Y., Mohr, M., Reiter, J., et al. (2010). The Tinnitus Research Initiative (TRI) database: a new approach for delineation of tinnitus subtypes and generation of predictors for treatment outcome. BMC Med. Inform. Decis. Mak. 10:42. doi: 10.1186/1472-6947-10-42
Langguth, B., Kreuzer, P. M., Kleinjung, T., and De Ridder, D. (2013). Tinnitus. Causes and clinical management. Lancet Neurol. 12, 920–930. doi: 10.1016/S1474-4422(13)70160-1
Legarda, S. B., McMahon, D., Othmer, S., and Othmer, S. (2011). Clinical neurofeedback. Case studies, proposed mechanism, and implications for pediatric neurology practice. J. Child Neurol. 26, 1045–1051. doi: 10.1177/0883073811405052
Lévesque, J., Beauregard, M., and Mensour, B. (2006). Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Neurosci. Lett. 394, 216–221. doi: 10.1016/j.neulet.2005.10.100
Llinás, R. R., Ribary, U., Contreras, D., and Pedroarena, C. (1998). The neuronal basis for consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1841–1849. doi: 10.1098/rstb.1998.0336
Llinás, R. R., Ribary, U., Jeanmonod, D., Kronberg, E., and Mitra, P. P. (1999). Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. U.S.A. 96, 15222–15227. doi: 10.1073/pnas.96.26.15222
Llinás, R. R., Urbano, F. J., Leznik, E., Ramírez, R. R., and van Marle, H. J. F. (2005). Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 28, 325–333. doi: 10.1016/j.tins.2005.04.006
Lofthouse, N., Arnold, L. E., Hersch, S., Hurt, E., and DeBeus, R. (2012). A review of neurofeedback treatment for pediatric ADHD. J. Atten. Disord. 16, 351–372. doi: 10.1177/1087054711427530
Loomis, A. L., Harvey, E. N., and Hobart, G. (1936). Electrical potentials of the human brain. J. Exp. Psychol. 19, 249–279. doi: 10.1037/h0062089
Lorenz, I., Müller, N., Schlee, W., Hartmann, T., and Weisz, N. (2009). Loss of alpha power is related to increased gamma synchronization—A marker of reduced inhibition in tinnitus? Neurosci. Lett. 453, 225–228. doi: 10.1016/j.neulet.2009.02.028
Lubar, J. F., and Bahler, W. W. (1976). Behavioral management of epileptic seizures following EEG biofeedback training of the sensorimotor rhythm. Biofeedback Self Regul. 1, 77–104. doi: 10.1007/BF00998692
Lubar, J. F., Swartwood, M. O., Swartwood, J. N., and O’Donnell, P. H. (1995). Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self Regul. 20, 83–99. doi: 10.1007/BF01712768
Lubar, J. O., and Lubar, J. F. (1984). Electroencephalographic biofeedback of SMR and beta for treatment of attention deficit disorders in a clinical setting. Biofeedback Self Regul. 9, 1–23. doi: 10.1007/BF00998842
Mennella, R., Patron, E., and Palomba, D. (2017). Frontal alpha asymmetry neurofeedback for the reduction of negative affect and anxiety. Behav. Res. Ther. 92, 32–40. doi: 10.1016/j.brat.2017.02.002
Meyer, M., Luethi, M. S., Neff, P., Langer, N., and Büchi, S. (2014). Disentangling tinnitus distress and tinnitus presence by means of EEG power analysis. Neural Plast. 2014, 1–13. doi: 10.1155/2014/468546
Meyer, M., Neff, P., Grest, A., Hemsley, C., Weidt, S., and Kleinjung, T. (2017). EEG oscillatory power dissociates between distress- and depression-related psychopathology in subjective tinnitus. Brain Res. 1663, 194–204. doi: 10.1016/j.brainres.2017.03.007
Michel, C. M., Murray, M. M., Lantz, G., Gonzalez, S., Spinelli, L., and Grave de Peralta, R. (2004). EEG source imaging. Clin. Neurophysiol. 115, 2195–2222. doi: 10.1016/j.clinph.2004.06.001
Milner, R., Lewandowska, M., Ganc, M., Cieśla, K., Niedziałek, I., and Skarżyñski, H. (2015). Slow cortical potential neurofeedback in chronic tinnitus therapy: a case report. Appl. Psychophysiol. Biofeedback 41, 225–249. doi: 10.1007/s10484-015-9318-5
Moazami-Goudarzi, M., Michels, L., Weisz, N., and Jeanmonod, D. (2010). Temporo-insular enhancement of EEG low and high frequencies in patients with chronic tinnitus. BMC Neurosci. 11:40. doi: 10.1186/1471-2202-11-40
Mohan, A., De Ridder, D., and Vanneste, S. (2016a). Emerging hubs in phantom perception connectomics. Neuroimage Clin. 11, 181–194. doi: 10.1016/j.nicl.2016.01.022
Mohan, A., De Ridder, D., and Vanneste, S. (2016b). Graph theoretical analysis of brain connectivity in phantom sound perception. Sci. Rep. 6:19683. doi: 10.1038/srep19683
Mohan, A., De Ridder, D., and Vanneste, S. (2017a). Robustness and dynamicity of functional networks in phantom sound. Neuroimage 146, 171–187. doi: 10.1016/j.neuroimage.2016.04.033
Mohan, A., Moreno, N., Song, J.-J., De Ridder, D., and Vanneste, S. (2017b). Evidence for behaviorally segregated, spatiotemporally overlapping subnetworks in phantom sound perception. Brain Connect. 7, 197–210. doi: 10.1089/brain.2016.0459
Monastra, V. J., Monastra, D. M., and George, S. (2002). The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of attention-deficit/hyperactivity disorder. Appl. Psychophysiol. Biofeedback 27, 231–249. doi: 10.1023/A:1021018700609
Mühlnickel, W., Elbert, T., Taub, E., and Flor, H. (1998). Reorganization of auditory cortex in tinnitus. Proc. Natl. Acad. Sci. U.S.A. 95, 10340–10343. doi: 10.1073/pnas.95.17.10340
Newman, C. W., Jacobson, G. P., and Spitzer, J. B. (1996). Development of the tinnitus handicap inventory. Arch. Otolaryngol. Head Neck Surg. 122, 143–148. doi: 10.1001/archotol.1996.01890140029007
Noreña, A. J. (2011). An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci. Biobehav. Rev. 35, 1089–1109. doi: 10.1016/j.neubiorev.2010.11.003
Noreña, A. J., and Eggermont, J. J. (2003). Changes in spontaneous neural activity immediately after an acoustic trauma. Implications for neural correlates of tinnitus. Hear. Res. 183, 137–153. doi: 10.1016/S0378-5955(03)00225-9
Ogawa, S., Lee, T. M., Kay, A. R., and Tank, D. W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. U.S.A. 87, 9868–9872. doi: 10.1073/pnas.87.24.9868
Ortmann, M., Müller, N., Schlee, W., and Weisz, N. (2011). Rapid increases of gamma power in the auditory cortex following noise trauma in humans. Eur. J. Neurosci. 33, 568–575. doi: 10.1111/j.1460-9568.2010.07542.x
Pascual-Marqui, R. D. (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 24, 5–12.
Pascual-Marqui, R. D., Michel, C. M., and Lehmann, D. (1994). Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 18, 49–65. doi: 10.1016/0167-8760(84)90014-X
Pierzycki, R. H., McNamara, A. J., Hoare, D. J., and Hall, D. A. (2015). Whole scalp resting state EEG of oscillatory brain activity shows no parametric relationship with psychoacoustic and psychosocial assessment of tinnitus: a repeated measures study. Hear. Res. 331, 101–108. doi: 10.1016/j.heares.2015.11.003
Pllana, A., and Bauer, H. (2011). BEM-based SMS-LORETA – an advanced method to localize multiple simultaneously active sources in the cerebral cortex. arXiv:1106.2679
Rauschecker, J. P., Leaver, A. M., and Mühlau, M. (2010). Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66, 819–826. doi: 10.1016/j.neuron.2010.04.032
Rauschecker, J. P., May, E. S., Maudoux, A., and Ploner, M. (2015). Frontostriatal gating of tinnitus and chronic pain. Trends Cogn. Sci. 19, 567–578. doi: 10.1016/j.tics.2015.08.002
Rockstroh, B., Elbert, T., Birbaumer, N., Wolf, P., Düchting-Röth, A., Reker, M., et al. (1993). Cortical self-regulation in patients with epilepsies. Epilepsy Res. 14, 63–72. doi: 10.1016/0920-1211(93)90075-I
Rogala, J., Jurewicz, K., Paluch, K., Kublik, E., Cetnarski, R., and Wrobel, A. (2016). The Do’s and Don’ts of neurofeedback training: a review of the controlled studies using healthy adults. Front. Hum. Neurosci. 10:301. doi: 10.3389/fnhum.2016.00301
Schenk, S., Lamm, K., Gündel, H., and Ladwig, K.-H. (2005). Neurofeedbackgestütztes EEG-alpha- und EEG-beta-training. Wirksamkeit in der Therapie des chronisch-dekompensierten Tinnitus [Neurofeedback-based EEG alpha and EEG beta training. Effectiveness in patients with chronically decompensated tinnitus]. HNO 53, 29–37. doi: 10.1007/s00106-004-1066-4
Scherg, M., and Berg, P. (1991). Use of prior knowledge in brain electromagnetic source analysis. Brain Topogr. 4, 143–150. doi: 10.1007/BF01132771
Schlee, W., Dohrmann, K., Hartmann, T., Lorenz, I., Müller, N., Elbert, T., et al. (2008). Assessment and modification of the tinnitus-related cortical network. Semin. Hear. 29, 270–287. doi: 10.1055/s-0028-1082033
Schlee, W., Müller, N., Hartmann, T., Keil, J., Lorenz, I., and Weisz, N. (2009). Mapping cortical hubs in tinnitus. BMC Biol. 7:80. doi: 10.1186/1741-7007-7-80
Schlee, W., Schecklmann, M., Lehner, A., Kreuzer, P. M., Vielsmeier, V., Poeppl, T. B., et al. (2014). Reduced variability of auditory alpha activity in chronic tinnitus. Neural Plast. 2014:436146. doi: 10.1155/2014/436146
Sedley, W., and Cunningham, M. O. (2013). Do cortical gamma oscillations promote or suppress perception? An under-asked question with an over-assumed answer. Front. Hum. Neurosci. 7:595. doi: 10.3389/fnhum.2013.00595
Sedley, W., Friston, K. J., Gander, P. E., Kumar, S., and Griffiths, T. D. (2016). An integrative tinnitus model based on sensory precision. Trends Neurosci. 39, 799–812. doi: 10.1016/j.tins.2016.10.004
Sedley, W., Teki, S., Kumar, S., Barnes, G. R., Bamiou, D.-E., and Griffiths, T. D. (2012). Single-subject oscillatory gamma responses in tinnitus. Brain 135, 3089–3100. doi: 10.1093/brain/aws220
Seki, S., and Eggermont, J. J. (2003). Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear. Res. 180, 28–38. doi: 10.1016/S0378-5955(03)00074-1
Simkin, D. R., Thatcher, R. W., and Lubar, J. F. (2014). Quantitative EEG and neurofeedback in children and adolescents: anxiety disorders, depressive disorders, comorbid addiction and attention-deficit/hyperactivity disorder, and brain injury. Child Adolesc. Psychiatr. Clin. N. Am. 23, 427–464. doi: 10.1016/j.chc.2014.03.001
Singer, W. (1993). Synchronization of cortical activity and its putative role in information processing and learning. Annu. Rev. Physiol. 55, 349–374. doi: 10.1146/annurev.physiol.55.1.349
Sitaram, R., Ros, T., Stoeckel, L., Haller, S., Scharnowski, F., Lewis-Peacock, J., et al. (2017). Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 18, 86–100. doi: 10.1038/nrn.2016.164
Song, J.-J., Punte, A. K., De Ridder, D., Vanneste, S., and van de Heyning, P. (2013). Neural substrates predicting improvement of tinnitus after cochlear implantation in patients with single-sided deafness. Hear. Res. 299, 1–9. doi: 10.1016/j.heares.2013.02.001
Sterman, M. B., and Egner, T. (2006). Foundation and practice of neurofeedback for the treatment of epilepsy. Appl. Psychophysiol. Biofeedback 31, 21–35. doi: 10.1007/s10484-006-9002-x
Sterman, M. B., and Friar, L. (1972). Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr. Clin. Neurophysiol. 33, 89–95. doi: 10.1016/0013-4694(72)90028-4
Stouffer, J. L., and Tyler, R. S. (1990). Characterization of tinnitus by tinnitus patients. J. Speech Hear. Disord. 55, 439–453. doi: 10.1044/jshd.5503.439
Strehl, U., Aggensteiner, P., Wachtlin, D., Brandeis, D., Albrecht, B., Arana, M., et al. (2017). Neurofeedback of slow cortical potentials in children with attention-deficit/hyperactivity disorder: a multicenter randomized trial controlling for unspecific effects. Front. Hum. Neurosci. 11:135. doi: 10.3389/fnhum.2017.00135
Sulzer, J., Haller, S., Scharnowski, F., Weiskopf, N., Birbaumer, N., Blefari, M., et al. (2013). Real-time fMRI neurofeedback: progress and challenges. Neuroimage 76, 386–399. doi: 10.1016/j.neuroimage.2013.03.033
Surmeli, T., and Ertem, A. (2009). QEEG guided neurofeedback therapy in personality disorders: 13 case studies. Clin. EEG Neurosci. 40, 5–10. doi: 10.1177/155005940904000107
Surmeli, T., Ertem, A., Eralp, E., and Kos, I. H. (2012). Schizophrenia and the efficacy of qEEG-guided neurofeedback treatment: a clinical case series. Clin. EEG Neurosci. 43, 133–144. doi: 10.1177/1550059411429531
Tan, G., Shaffer, F., Lyle, R., and Teo, I. (2016). Evidence-Based Practice in Biofeedback and Neurofeedback, 3rd Edn. Wheat Ridge, CO: AAPB.
Tass, P. A., Adamchic, I., Freund, H.-J., von Stackelber, T., and Hauptmann, C. (2012). Counteracting tinnitus by acoustic coordinated reset neuromodulation. Restor. Neurol. Neurosci. 30, 137–159. doi: 10.3233/RNN-2012-110218
Thatcher, R. W. (2010). Neuropsychiatry and quantitative electroencephalography (qEEG) in the 21st Century. Neuropsychiatry 1, 495–514. doi: 10.2217/npy.11.45
Thibault, R. T., Lifshitz, M., and Raz, A. (2016). The self-regulating brain and neurofeedback. Experimental science and clinical promise. Cortex 74, 247–261. doi: 10.1016/j.cortex.2015.10.024
Thibault, R. T., Lifshitz, M., and Raz, A. (2017). Neurofeedback or neuroplacebo? Brain 140, 862–864. doi: 10.1093/brain/awx033
van den Berge, M. J. C., Free, R. H., Arnold, R., De Kleine, E., Hofman, R., van Dijk, J., et al. (2017). Cluster analysis to identify possible subgroups in tinnitus patients. Front. Neurol. 8:115. doi: 10.3389/fneur.2017.00115
van der Loo, E., Gais, S., Congedo, M., Vanneste, S., Plazier, M., Menovsky, T., et al. (2009). Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLOS ONE 4:e7396. doi: 10.1371/journal.pone.0007396
van Veen, B. D., van Drongelen, W., Yuchtman, M., and Suzuki, A. (1997). Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 44, 867–880. doi: 10.1109/10.623056
Vanhatalo, S., Palva, J. M., Holmes, M. D., Miller, J. W., Voipio, J., and Kaila, K. (2004). Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc. Natl. Acad. Sci. U.S.A. 101, 5053–5057. doi: 10.1073/pnas.0305375101
Vanneste, S., and De Ridder, D. (2012). The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front. Syst. Neurosci. 6:31. doi: 10.3389/fnsys.2012.00031
Vanneste, S., Joos, K., and De Ridder, D. (2012). Prefrontal cortex based sex differences in tinnitus perception. Same tinnitus intensity, same tinnitus distress, different mood. PLOS ONE 7:e31182. doi: 10.1371/journal.pone.0031182
Vanneste, S., Joos, K., Ost, J., and De Ridder, D. (2016). Influencing connectivity and cross-frequency coupling by real-time source localized neurofeedback of the posterior cingulate cortex reduces tinnitus related distress. Neurobiol. Stress (in press). doi: 10.1016/j.ynstr.2016.11.003
Vanneste, S., van de Heyning, P., and de Ridder, D. (2011a). Contralateral parahippocampal gamma-band activity determines noise-like tinnitus laterality: a region of interest analysis. J. Neurosci. 199, 481–490. doi: 10.1016/j.neuroscience.2011.07.067
Vanneste, S., van de Heyning, P., and De Ridder, D. (2011b). The neural network of phantom sound changes over time. A comparison between recent-onset and chronic tinnitus patients. Eur. J. Neurosci. 34, 718–731. doi: 10.1111/j.1460-9568.2011.07793.x
Weidt, S., Delsignore, A., Meyer, M., Rufer, M., Peter, N., Drabe, N., et al. (2016). Which tinnitus-related characteristics affect current health-related quality of life and depression? A cross-sectional cohort study. Psychiatry Res. 237, 114–121. doi: 10.1016/j.psychres.2016.01.065
Weiler, E. W., Brill, K., Tachiki, K. H., and Schneider, D. (2002). Neurofeedback and quantitative electroencephalography. Int. Tinnitus J. 8, 87–93.
Weisz, N., Dohrmann, K., and Elbert, T. (2007a). The relevance of spontaneous activity for the coding of the tinnitus sensation. Prog. Brain Res. 166, 61–70. doi: 10.1016/S0079-6123(07)66006-3
Weisz, N., Müller, S., Schlee, W., Dohrmann, K., Hartmann, T., and Elbert, T. (2007b). The neural code of auditory phantom perception. J. Neurosci. 27, 1479–1484. doi: 10.1523/JNEUROSCI.3711-06.2007
Weisz, N., Moratti, S., Meinzer, M., Dohrmann, K., and Elbert, T. (2005). Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLOS Med. 2:e153. doi: 10.1371/journal.pmed.0020153
White, D. J., Congedo, M., and Ciorciari, J. (2014). Source-based neurofeedback methods using EEG recordings: training altered brain activity in a functional brain source derived from blind source separation. Front. Behav. Neurosci. 8:373. doi: 10.3389/fnbeh.2014.00373
Wyrwicka, W., and Sterman, M. B. (1968). Instrumental conditioning of sensorimotor cortex EEG spindles in the waking cat. Physiol. Behav. 3, 703–707. doi: 10.1016/0031-9384(68)90139-X
Keywords: tinnitus, phantom perception, EEG, plasticity, heterogeneity, neurofeedback, frequency bands, alpha band
Citation: Güntensperger D, Thüring C, Meyer M, Neff P and Kleinjung T (2017) Neurofeedback for Tinnitus Treatment – Review and Current Concepts. Front. Aging Neurosci. 9:386. doi: 10.3389/fnagi.2017.00386
Received: 31 July 2017; Accepted: 09 November 2017;
Published: 01 December 2017.
Edited by:
Berthold Langguth, University of Regensburg, GermanyReviewed by:
Daniel Llano, University of Illinois at Urbana–Champaign, United StatesAndrea Crocetti, Independent Consultant, Milan, Italy
Copyright © 2017 Güntensperger, Thüring, Meyer, Neff and Kleinjung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tobias Kleinjung, tobias.kleinjung@usz.ch
 Dominik Güntensperger
Dominik Güntensperger Christian Thüring
Christian Thüring Martin Meyer
Martin Meyer Patrick Neff
Patrick Neff Tobias Kleinjung
Tobias Kleinjung