The Role of Insulin-Like Growth Factor 1 in the Progression of Age-Related Hearing Loss
- 1“Alberto Sols” Biomedical Research Institute CSIC-UAM, Madrid, Spain
- 2Centro de Investigación Biomédica en Red de Enfermedades Raras, Instituto de Salud Carlos III, Madrid, Spain
- 3Hospital La Paz Institute for Health Research (IdiPAZ), Madrid, Spain
- 4Otorhinolaryngology Department, Hospital La Paz, Madrid, Spain
Aging is associated with impairment of sensorial functions and with the onset of neurodegenerative diseases. As pari passu circulating insulin-like growth factor 1 (IGF-1) bioavailability progressively decreases, we see a direct correlation with sensory impairment and cognitive performance in older humans. Age-related sensory loss is typically caused by the irreversible death of highly differentiated neurons and sensory receptor cells. Among sensory deficits, age-related hearing loss (ARHL), also named presbycusis, affects one third of the population over 65 years of age and is a major factor in the progression of cognitive problems in the elderly. The genetic and molecular bases of ARHL are largely unknown and only a few genes related to susceptibility to oxidative stress, excitotoxicity, and cell death have been identified. IGF-1 is known to be a neuroprotective agent that maintains cellular metabolism, activates growth, proliferation and differentiation, and limits cell death. Inborn IGF-1 deficiency leads to profound sensorineural hearing loss both in humans and mice. IGF-1 haploinsufficiency has also been shown to correlate with ARHL. There is not much information available on the effect of IGF-1 deficiency on other human sensory systems, but experimental models show a long-term impact on the retina. A secondary action of IGF-1 is the control of oxidative stress and inflammation, thus helping to resolve damage situations, acute or made chronic by aging. Here we will review the primary actions of IGF-1 in the auditory system and the underlying molecular mechanisms.
IGF System, Upstream Regulation, and Downstream IGF-1 Signaling
The mammalian IGF system is comprised of insulin-like growth factors (IGF), receptors and binding proteins (IGFBP). IGFs and insulin are small polypeptides produced as pre-pro-peptides that can bind the insulin (IR) and IGF-1 (IGF1R) tyrosine kinase receptors. IGFs also bind the cation-independent mannose-6-phosphatase IGF-2 receptor (IGF2R; Foulstone et al., 2005). The biological actions of IGFs are primarily mediated by binding to the IGF1R, a heterotetramer with extracellular IGF binding domains and intracellular tyrosine kinase domains. The carboxy-terminal domain has docking sites for intracellular substrates (IRS1-4/SHC; Laviola et al., 2007) that, in turn, bind and activate a network of intracellular signaling molecules. Factor-receptor interactions are modulated by binding proteins (IGFBPs) and associated proteases. In plasma, IGFBPs carry IGFs and these regulate their half-life, distribution and biological actions. IGFBPs control the bioavailability of IGF-1 to its receptors by competing with receptors for free factors (Firth and Baxter, 2002).
The growth hormone (GH) is a peptide hormone secreted by somatotroph cells of the pituitary gland. GH stimulates the growth of all body tissues, thus its level rises progressively during childhood and peaks at puberty. IGF-1 is secreted by the liver as a result of stimulation by GH, which binds to its receptor, GHR, inducing homodimerization and initiating signal transduction through receptor-associated Janus kinase (JAK) 2 (Herrington et al., 2000). Activation of signal transducers and transcription activators of STAT5b family members is critical for regulating liver IGF-1 gene expression (Davey et al., 2001), as well as for the transcriptional regulation of other IGF system genes, including those coding for IGFBP3 and IGFALS. ALS is a secreted hepatic protein that can be found freely circulating or forming a ternary complex with IGF-1 and IGFBP-3, thus prolonging the half-life of IGF-1 and decreasing its availability to tissues (Boisclair et al., 2001).
At the cell surface, IGF-1 binds to high affinity IGF1R, inducing autophosphorylation and allowing docking of the receptor substrates IRS-1 to IRS-4, Grb2-associated binder 1 (Gab-1) and the Src homology 2 domain containing protein (SHC). Activated docking proteins subsequently recruit cytoplasmic components of downstream signaling pathways, including the MAPK (RAF-MEK-ERK1/2 and p38) and the PI3K-AKT pathways, and transduce the IGF signaling (Siddle, 2011). Depending on the cellular context, IGF-1 regulates different processes. For example, IGF-1 modulates gene expression in chondrocytes (Yang et al., 2017), protein synthesis in osteoblasts (Guo et al., 2017), cell cycle in enterocytes (Van Landeghem et al., 2015), metabolism in adipocytes (Chang et al., 2016), survival in cochlear hair cells (Yamahara et al., 2017) and autophagy in otic neural precusors (Aburto et al., 2012b). IGF1R can also translocate to the nucleus, activate transcription and regulate gene expression (Sehat et al., 2010).
Analysis of downstream signaling in the deaf Igf1−/− mouse has further contributed toward understanding its cochlear actions and demonstrated that an IGF-1 deficit is associated with activation of the p38 MAPK pathway (related to the cellular response to stress). RAF-ERK1/2 and AKT activity (cell proliferation and survival) are also impaired (Sanchez-Calderon et al., 2010) but the overall autophagic flux is unaffected (de Iriarte Rodríguez et al., 2015b; Magariños et al., 2017). Further analysis of IGF-1 signaling has been carried out by studying null mice for downstream targets such as IRS1 (Tang et al., 2017), IRS2, PTP1B (Murillo-Cuesta et al., 2012), GRF1 (Fernández-Medarde et al., 2009), and CRAF (De Iriarte Rodriguez et al., 2015a). Cochlear comparative transcriptomics have unveiled the role of FoxM1 and FoxP3 forkhead box transcription factors, and myocyte enhancer factor-2 (MEF-2) in inner ear development as well as IGF-1 cochlear actions (Sanchez-Calderon et al., 2010). The impact of IGF-1 deficiency depends on the tissue and organs studied. In the Igf1−/− mouse bone, cell survival and AKT signaling are gravely affected, but RAF kinase-mediated proliferation is not (Rodríguez-de La Rosa et al., 2014). In the mouse retina, however, IGF-1 deficit causes impairment of the autophagic flux, leading to increased inflammation, apoptosis and age-associated blindness (Rodriguez-de La Rosa et al., 2012; Arroba et al., 2016). Finally, analysis of the Igf1−/− vestibule has confirmed the role of p38 and added new players such as p53 and microRNAs (Rodríguez-de la Rosa et al., 2015).
In summary, IGF-1 has a role in brain development and maturation (Dyer et al., 2016). Later in life, bioactive IGF-1 circulating levels are reduced, a trend that has been associated with human frailty and cognitive decline (Vestergaard et al., 2014). IGF-1 deficiency leads to increased inflammation and to the failure of intracellular cell renewal mechanisms. This is critical in the inner ear because, as discussed below, none of the main cell types essential for hearing regenerate (Mittal et al., 2017).
Inner Ear Development, Adult Anatomy, and Age-Associated Degeneration
The inner ear develops from the otic ectodermal placode that invaginates to form the otic vesicle. This transitory embryonic structure contains the information required to generate most cell types of the adult inner ear, including the sensory cells and the auditory-vestibular neurons (Kelly and Chen, 2009; Magariños et al., 2012, 2014; Burns et al., 2015). Development of the inner ear is tightly regulated by intrinsic and extrinsic factors (Sanchez-Calderon et al., 2007; Gálvez et al., 2017). Among these factors, IGF-1 promotes proliferation and survival of otic progenitor cells, supports neurogenesis and facilitates late differentiation in species from fish to humans (Ayaso et al., 2002; Schlueter et al., 2007; Zou et al., 2009; Aburto et al., 2012a; Varela-Nieto et al., 2013; Tafra et al., 2014). IGF-1 plays a key role in brain development and maintenance of stem cells (Nieto-Estévez et al., 2016).
The adult inner ear comprises the cochlea and the vestibular organ, which are responsible for hearing and equilibrium, respectively. The organ of Corti in the cochlea contains highly specialized hair cells (inner -IHC- and outer -OHC-), which transform mechanical sounds into electrochemical signals that are conveyed to the brain by the VII vestibulocochlear cranial nerve (Stephenson, 2012). Sound induces the movement of hair cell stereocilia, causing the opening of ion channels, an influx of K+ ions and depolarization. Depolarization of IHC results in glutamate release and synapse with 10–30 afferent auditory bipolar neurons. Meanwhile, OHCs amplify the incoming sound stimulation and enhance frequency selectivity of the cochlear response (Fettiplace and Kim, 2014; Reichenbach and Hudspeth, 2014). The organ of Corti is connected to the brain by two types of neurons in the spiral ganglion (SGN; Coate and Kelley, 2013). Type I and type II neurons innervate IHC and OHC respectively. Of these two kinds of neurons, type I is the most abundant (95%). The SGN axons form the cochlear branch of the vestibulocochlear nerve and connect the peripheral spiral ganglia with the cochlear nuclei at the brainstem. Sound information progresses in a complex multisynaptic, parallel, and ascendant pathway from the cochlea through the brainstem nuclei to the auditory cortex, preserving the tonotopic organization (Tsukano et al., 2017).
Expression levels of IGF-1 and its high affinity receptor, IGF1R, are elevated during late cochlear development, decline significantly after birth, but baseline expression is maintained throughout the organism's life (Murillo-Cuesta et al., 2011; Okano et al., 2011). In the mouse cochlea, IGF-1 is clearly detected in spiral ganglion neurons and stria vascularis, and its expression is modulated with aging (Riva et al., 2007). IGF binding proteins are also expressed in the developing ear and throughout life (Okano and Kelley, 2013). Finally, IGF system elements are expressed in the vestibular system over a similar time course (Degerman et al., 2013; Rodríguez-de la Rosa et al., 2015).
During aging, peripheral and central auditory structures degenerate, leading to ARHL (Fetoni et al., 2011). The primary pathological alterations observed in mouse models include progressive degeneration and loss of HC and SGN (Bao and Ohlemiller, 2010; Bowl and Dawson, 2015), as well as changes in the central auditory pathway. Typically, presbycusis debuts with loss of OHC, mainly in basal cochlear regions (high frequencies), and extends toward the apex and IHC (Ohlemiller and Gagnon, 2004). OHC defects result in a moderate increase of the hearing threshold, whereas defects in IHC or the auditory neurons can lead to profound deafness (Ouda et al., 2015). Swelling of the afferent nerve terminals and a decrease in the density of their associated ribbon synapses causes a synaptopathy that is sometimes the primary defect (Wan and Corfas, 2015).
The stria vascularis is a three-cell layer structure within the cochlea that maintains the K+ concentration and the endocochlear potential (Magariños et al., 2012). During aging, the stria vascularis shows disorganization and atrophy, with loss of marginal cells and progressive merging of strial capillaries (Ohlemiller, 2009). In addition, thinning, degeneration of fibrocytes and loss of capillaries are observed in the spiral ligament (Hequembourg and Liberman, 2001). In human cohorts, decreased age-related-IGF-1 bioavailability correlates with progression of hearing impairment (Lassale et al., 2017), as will be discussed in depth below. Studies carried out with Igf1−/− and Igf1−/+ mice confirmed this trend and showed acceleration in the damage of the neural structures and stria vascularis (Riquelme et al., 2010).
The molecular mechanisms underlying ARHL that have been described include redox imbalance, accumulation of mitochondrial DNA damage, and excitotoxicity, leading to apoptotic and necrotic cell death (Menardo et al., 2012; Wong and Ryan, 2015). Interestingly, experimental models indicate that anti-oxidant therapy and control of micronutrients could prevent or ameliorate ARHL (Fetoni et al., 2009; Guastini et al., 2011; Ding et al., 2016). These mechanisms are similar to those involved in drug- and noise-induced hearing loss (Frisina et al., 2016; Kalinec et al., 2017).
The central auditory system shows alterations that could be secondary to the diminished input from the damaged periphery. These include modifications in the expression of calcium binding proteins (parvalbumin, calbindin and calretinin, and glutamate-decarboxylase), atrophy of the gray and white matter and changes in the content of some metabolites (Ouda et al., 2015). However, a minimal age-related decline in the total number of neurons in central auditory structures has been reported. IGF-1 expression is modulated by cochlear damage in the auditory central pathway (Alvarado et al., 2007) and its deficit also impacts neurotransmission in the cochlear nucleus (Fuentes-Santamaria et al., 2013, 2016).
To summarize, IGF system elements are expressed in the inner ear and central auditory pathway, and their expression is regulated by damage and age in different species. The importance of this system in hearing loss has been further proven by the study of human mutations and human cohorts as described below.
Association of the IGF System with Human Genetic Deafness and Age-Related Hearing Loss
ARHL is a multifactorial process that results in cochlear damage over the span of a life. Noise, ototoxic agents, trauma, vascular insults, metabolic changes, hormones, diet, immune system, and genetic predisposition are all contributing factors (Gates and Mills, 2005; Fetoni et al., 2011). Given the increase in the average age of the population as well as in noxious environmental agents, the impact of ARHL is continuously growing.
The relationship between nutrition and ARHL is an emerging interdisciplinary field and recent evidence points to vitamin imbalances and high fat diets as risk factors (Partearroyo et al., 2017). In addition, the genetic study of ARHL is an expanding field that has rendered several gene candidates thus far (Salminen and Kaarniranta, 2010; Op De Beeck et al., 2011; Fransen et al., 2015; Koffler et al., 2015).
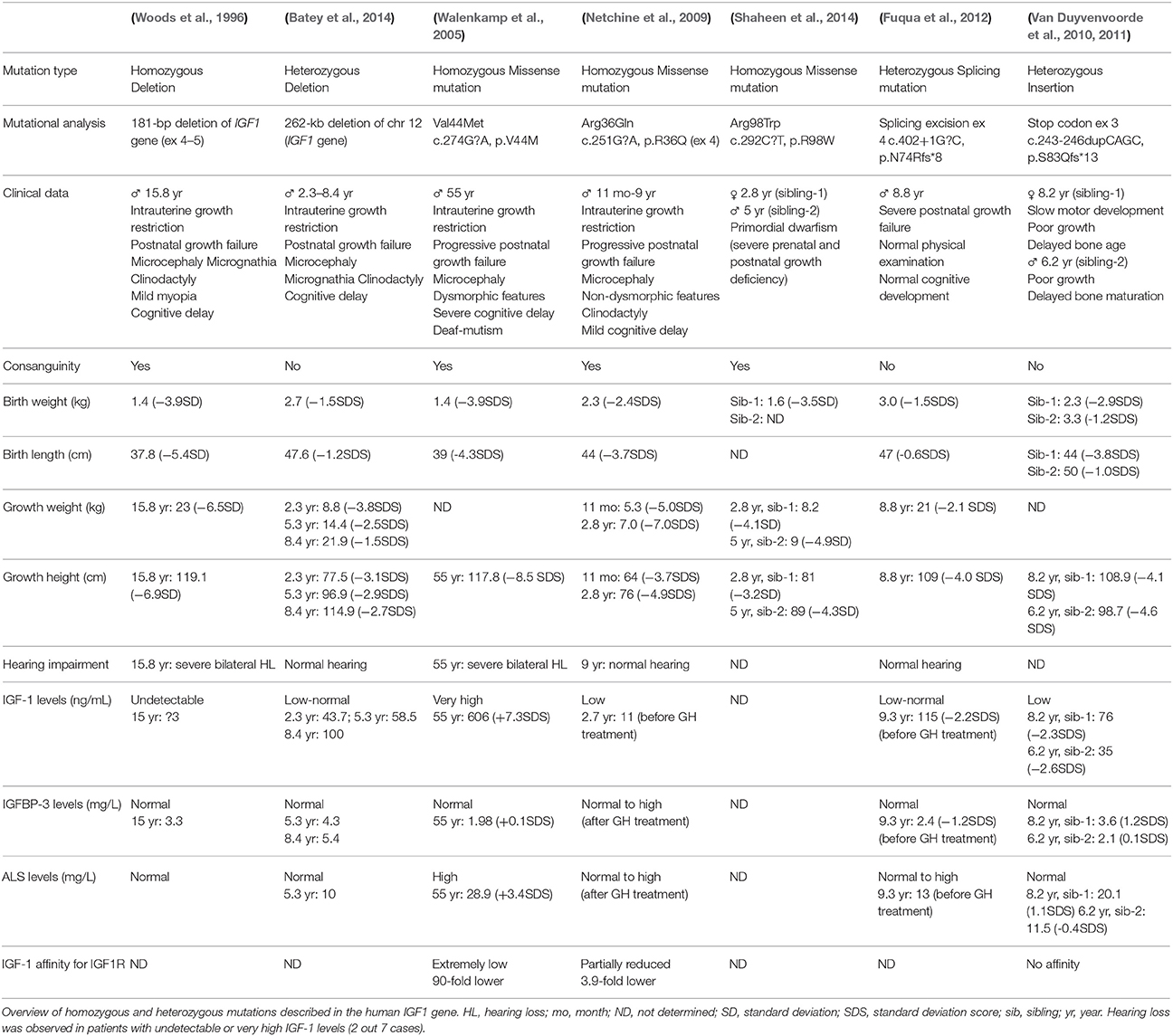
Mutations in human genes coding for IGF-1, IGF1R, and other members of the GH/IGF-1 system cause rare diseases (Tables 1, 2), which normally have an early onset. Patients show growth retardation and frequent microcephaly. Interestingly, only when IGF-1 actions are totally impaired do the affected patients show syndromic hearing loss (Table 1). Indeed, of the 7 mutations described in young humans, hearing phenotype of 2 patients have not been reported (Van Duyvenvoorde et al., 2010, 2011; Shaheen et al., 2014) and 3 show low to normal IGF-1 levels, which show reduced affinity for IGF1R binding in the case tested, and normal hearing (Netchine et al., 2009; Fuqua et al., 2012; Batey et al., 2014). No data have been published on the evolution of hearing loss associated with aging that these patients present. Finally, 2 patients showed severe hearing loss which was associated in one case with total absence of circulating IGF-1 (Woods et al., 1996) and in the other with an extremely low binding affinity (Walenkamp et al., 2005). Accordingly, haploinsufficient IGF-1 availability causes growth retardation and has been associated with short adult stature and hearing loss in other genetic syndromes such as Noonan's or Turner' syndromes (Barrenäs et al., 2000, 2005b; Welch and Dawes, 2007; El Bouchikhi et al., 2016).
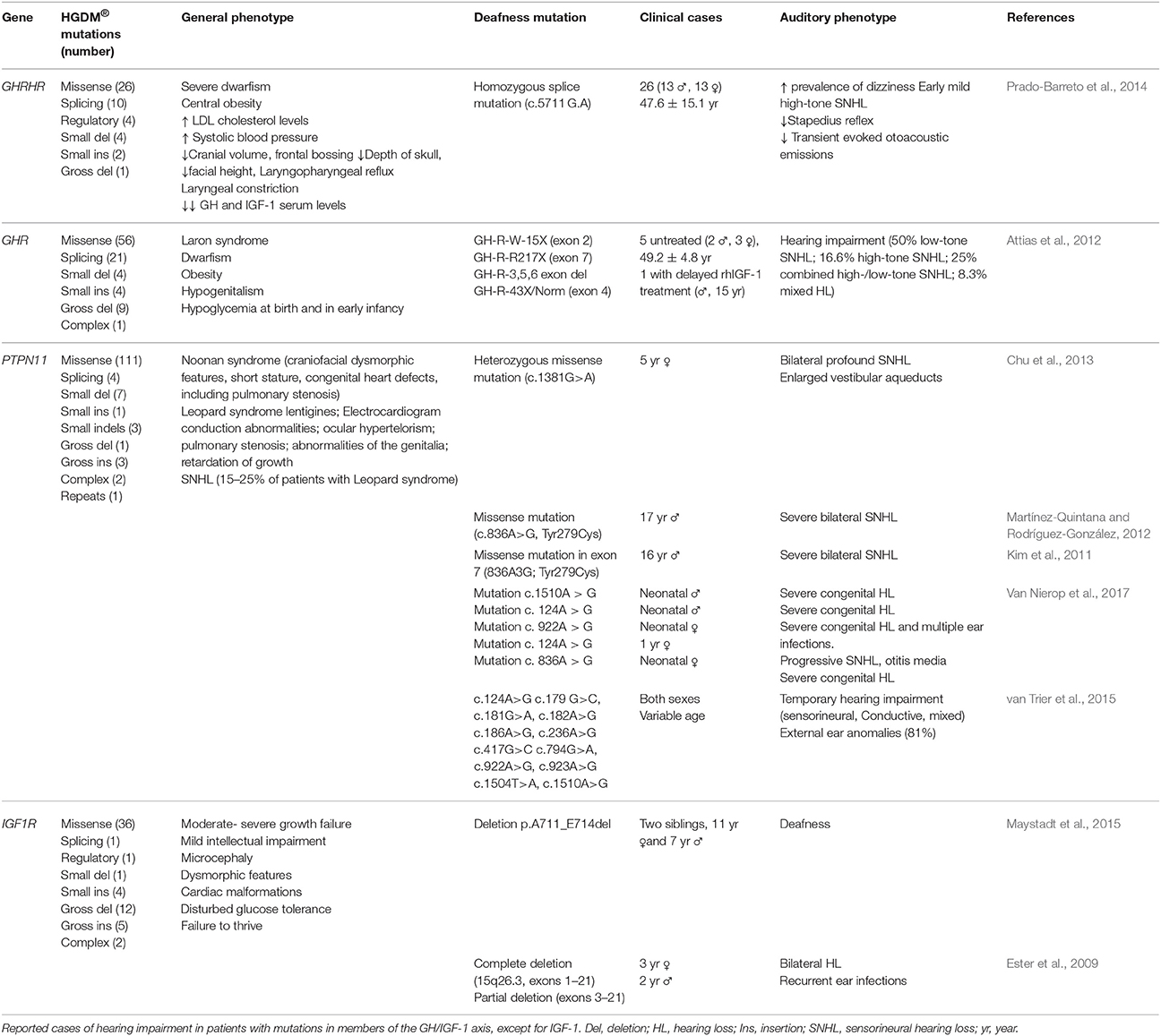
Human mutations in the gene coding for the high affinity receptor IGF1R are even less frequent and normally found in heterozygosis (Table 2; Abuzzahab et al., 2003; Ester et al., 2009; Klammt et al., 2011; Fang et al., 2012; Gannagé-Yared et al., 2013; Prontera et al., 2015). Reduced bioactive IGF-1 levels caused by mutations in related genes, such as those coding for GH and the GHR, are also associated with hearing loss (Table 1; Giordano et al., 2015; Muus et al., 2017). In general, the authors have not done a thorough study of sensory functions, including hearing. However, it is worth noting that Laron's syndrome patients have been studied in depth and show early onset of ARHL (Attias et al., 2012). Laron's syndrome is an autosomal recessive human disorder characterized by mutations in the GHR that cause insensitivity to GH stimuli and, in turn, extremely low IGF-1 synthesis in the liver. Patients have a short stature, among other characteristics, and normal hearing at young ages but develop early onset ARHL, which could be prevented by IGF-1 treatment. Still, much work is needed to fully understand the relationship between genes associated with short stature (Baron et al., 2015; Wit et al., 2016) and hearing loss.
Finally, epidemiologic studies of aging cohorts have shown a relationship between bioactive IGF-1 and hearing loss (Lassale et al., 2017). Furthermore, there is increasing evidence linking these two factors with cognitive decline and onset of neurodegenerative diseases (Dik et al., 2003; Watanabe et al., 2005; Fortunato et al., 2016; Lassale et al., 2017; Wrigley et al., 2017), dementia (Peracino, 2014; Su et al., 2017), depression (Van Varsseveld et al., 2015; Kim et al., 2017), short stature (Barrenäs et al., 2005a; Crowe et al., 2011), cardiovascular pathologies (Burgers et al., 2011; Tan et al., 2017), and aging (Bainbridge and Wallhagen, 2014; Vestergaard et al., 2014).
These data lead us to pose the following questions: (i) which cochlear processes are IGF-1-dependent and dramatically impaired during aging? And (ii) is there a potential for prevention and repair interventions based on IGF-1 targets? Insight into the first question has been obtained from the study of the Igf1−/− mouse that shows profound syndromic bilateral sensorineural hearing loss (Cediel et al., 2006). The main cellular alterations reported were delayed postnatal development, a significant decrease in the number and size of auditory neurons, aberrant innervation patterns, increased neural apoptosis, deficits in myelination and increased efficacy of glutamatergic synapses (Camarero et al., 2001, 2002; Sanchez-Calderon et al., 2007; Fuentes-Santamaria et al., 2016). Igf1−/− mice develop further cellular degeneration with aging. As bioactive IGF-1 levels decrease, the heterozygous mice also show an accelerated hearing loss secondary to degeneration of the SGN (Riquelme et al., 2010). Our findings support the idea that IGF-1 levels may hold predictive value for the stratification of ARHL and, secondary to hearing loss, for cognitive decline. Regarding the aforementioned mechanisms, IGF-1 is a neurotrophic factor with anti-inflammatory actions. It is anti-apoptotic and favors cell renewal.
In this context, IGF-1 emerges as a potential protector of the inner ear. Studies in animal models have shown that local application of recombinant human IGF-1 (rhIGF-I) protects the cochlea from functional and histologic losses induced by aminoglycoside ototoxicity and noise exposure (Iwai et al., 2006; Yamahara et al., 2015). IGF-1 rescued hair cells from apoptosis by downregulating pro-apoptotic gene expression and regulating glucose transporters (Yamahara et al., 2015). Similarly, IGF-1 and substance P protect vestibular hair cells against neomycin ototoxicity (Yoshida et al., 2015). Recombinant IGF-1 therapy has been approved to increase linear growth, and therefore height, in humans, spurring an increasing interest in the potential use of IGF-1 for the treatment of hearing loss (Yamahara et al., 2015). IGF-1 potential has been studied in patients with sudden sensorineural hearing loss who were resistant to treatment with systemic glucocorticoids (Nakagawa et al., 2012, 2014). A recent study with 120 patients concluded that treatment with topical IGF-1 therapy had significant effects on hearing recovery depending on their age (< 60 years) and early initiation of salvage treatment (Nakagawa et al., 2016). Prolonged activation of IGF1R by treatment with IGF-1 or analogs might have undesired secondary effects, thus their potential use to delay aging maybe limited. However available data highlight the interest of exploring IGF-1 downstream targets as drug candidates for ARHL.
Author Contributions
LR-dlR, LL, MC, SM-C, IV-N: drafted the work and wrote the manuscript; LR-dlR, SM-C, and IV-N: revised the manuscript. All authors approved the manuscript in its final form.
Funding
This work was supported by the European Commission FP7-PEOPLE-2013-IAPP TARGEAR, Spanish MINECO/European Social Funds (FEDER) SAF2014-53979-R and CIBERER-ISCiii MODCELANI_CRISPR ER16P5AC761, and FENOCRISPR ER17P5AC7612 to IV-N. LR-dlR and SM-C are supported by FEDER/CIBERER contracts.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We warmly thank our present and former colleagues in the Neurobiology of Hearing group for sharing information and for useful discussions.
References
Aburto, M. R., Magariños, M., Leon, Y., Varela-Nieto, I., and Sanchez-Calderon, H. (2012a). AKT signaling mediates IGF-I survival actions on otic neural progenitors. PLoS ONE 7:e30790. doi: 10.1371/journal.pone.0030790
Aburto, M. R., Sánchez-Calderón, H., Hurlé, J. M., Varela-Nieto, I., and Magariños, M. (2012b). Early otic development depends on autophagy for apoptotic cell clearance and neural differentiation. Cell Death Dis. 3:e394. doi: 10.1038/cddis.2012.132
Abuzzahab, M. J., Schneider, A., Goddard, A., Grigorescu, F., Lautier, C., Keller, E., et al. (2003). IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N. Engl. J. Med. 349, 2211–2222. doi: 10.1056/NEJMoa010107
Alvarado, J. C., Fuentes-Santamaria, V., Franklin, S. R., Brunso-Bechtold, J. K., and Henkel, C. K. (2007). Synaptophysin and insulin-like growth factor-1 immunostaining in the central nucleus of the inferior colliculus in adult ferrets following unilateral cochlear removal: a densitometric analysis. Synapse 61, 288–302. doi: 10.1002/syn.20373
Arroba, A. I., Rodríguez-De La Rosa, L., Murillo-Cuesta, S., Vaquero-Villanueva, L., Hurlé, J. M., Varela-Nieto, I., et al. (2016). Autophagy resolves early retinal inflammation in Igf1-deficient mice. Dis. Model. Mech. 9, 965–974. doi: 10.1242/dmm.026344
Attias, J., Zarchi, O., Nageris, B. I., and Laron, Z. (2012). Cochlear hearing loss in patients with Laron syndrome. Eur. Arch. Otorhinolaryngol. 269, 461–466. doi: 10.1007/s00405-011-1668-x
Ayaso, E., Nolan, C. M., and Byrnes, L. (2002). Zebrafish insulin-like growth factor-I receptor: molecular cloning and developmental expression. Mol. Cell. Endocrinol. 191, 137–148. doi: 10.1016/S0303-7207(02)00083-7
Bainbridge, K. E., and Wallhagen, M. I. (2014). Hearing loss in an aging American population: extent, impact, and management. Annu. Rev. Pub. Heal. 35, 139–152. doi: 10.1146/annurev-publhealth-032013-182510
Bao, J., and Ohlemiller, K. K. (2010). Age-related loss of spiral ganglion neurons. Hear. Res. 264, 93–97. doi: 10.1016/j.heares.2009.10.009
Baron, J., Sävendahl, L., De Luca, F., Dauber, A., Phillip, M., Wit, J. M., et al. (2015). Short and tall stature: a new paradigm emerges. Nat. Rev. Endocrinol. 11, 735–746. doi: 10.1038/nrendo.2015.165
Barrenäs, M., Landin-Wilhelmsen, K., and Hanson, C. (2000). Ear and hearing in relation to genotype and growth in Turner syndrome. Hear. Res. 144, 21–28. doi: 10.1016/S0378-5955(00)00040-X
Barrenäs, M. L., Bratthall, A., and Dahlgren, J. (2005a). The association between short stature and sensorineural hearing loss. Hear. Res. 205, 123–130. doi: 10.1016/j.heares.2005.03.019
Barrenäs, M. L., Jonsson, B., Tuvemo, T., Hellstrom, P. A., and Lundgren, M. (2005b). High risk of sensorineural hearing loss in men born small for gestational age with and without obesity or height catch-up growth: a prospective longitudinal register study on birth size in 245,000 Swedish conscripts. J. Clin. Endocrinol. Metab. 90, 4452–4456. doi: 10.1210/jc.2005-0385
Batey, L., Moon, J. E., Yu, Y., Wu, B., Hirschhorn, J. N., Shen, Y., et al. (2014). A novel deletion of IGF1 in a patient with idiopathic short stature provides insight Into IGF1 haploinsufficiency. J. Clin. Endocrinol. Metab. 99, E153–E159. doi: 10.1210/jc.2013-3106
Boisclair, Y. R., Rhoads, R. P., Ueki, I., Wang, J., and Ooi, G. T. (2001). The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J. Endocrinol. 170, 63–70. doi: 10.1677/joe.0.1700063
Bowl, M. R., and Dawson, S. J. (2015). The mouse as a model for age-related hearing loss - a mini-review. Gerontology 61, 149–157. doi: 10.1159/000368399
Burgers, A. M., Biermasz, N. R., Schoones, J. W., Pereira, A. M., Renehan, A. G., Zwahlen, M., et al. (2011). Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J. Clin. Endocrinol. Metab. 96, 2912–2920. doi: 10.1210/jc.2011-1377
Burns, J. C., Kelly, M. C., Hoa, M., Morell, R. J., and Kelley, M. W. (2015). Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat. Commun. 6:8557. doi: 10.1038/ncomms9557
Camarero, G., Avendano, C., Fernandez-Moreno, C., Villar, A., Contreras, J., de Pablo, F., et al. (2001). Delayed inner ear maturation and neuronal loss in postnatal Igf-1-deficient mice. J. Neurosci. 21, 7630–7641.
Camarero, G., Villar, M. A., Contreras, J., Fernández-Moreno, C., Pichel, J. G., Avendaño, C., et al. (2002). Cochlear abnormalities in insulin-like growth factor-1 mouse mutants. Hear. Res. 170, 2–11. doi: 10.1016/S0378-5955(02)00447-1
Cediel, R., Riquelme, R., Contreras, J., Díaz, A., and Varela-Nieto, I. (2006). Sensorineural hearing loss in insulin-like growth factor I-null mice: a new model of human deafness. Eur. J. Neurosci. 23, 587–590. doi: 10.1111/j.1460-9568.2005.04584.x
Chang, H. R., Kim, H. J., Xu, X., and Ferrante, A. W. Jr. (2016). Macrophage and adipocyte IGF1 maintain adipose tissue homeostasis during metabolic stresses. Obesity 24, 172–183. doi: 10.1002/oby.21354
Chu, H. S., Chung, H. S., Ko, M. H., Kim, H. J., Ki, C. S., Chung, W. H., et al. (2013). Syndromic hearing loss in association with PTPN11-related disorder: the experience of cochlear implantation in a child with LEOPARD syndrome. Clin. Exp. Otorhinolaryngol. 6, 99–102. doi: 10.3342/ceo.2013.6.2.99
Coate, T. M., and Kelley, M. W. (2013). Making connections in the inner ear: recent insights into the development of spiral ganglion neurons and their connectivity with sensory hair cells. Semin. Cell Dev. Biol. 24, 460–469. doi: 10.1016/j.semcdb.2013.04.003
Crowe, F. L., Key, T. J., Allen, N. E., Appleby, P. N., Overvad, K., Grønbæk, H., et al. (2011). A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1-2 and-3 in the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann. Hum. Biol. 38, 194–202. doi: 10.3109/03014460.2010.507221
Davey, H. W., Xie, T., Mclachlan, M. J., Wilkins, R. J., Waxman, D. J., and Grattan, D. R. (2001). STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology 142, 3836–3841. doi: 10.1210/endo.142.9.8400
Degerman, E., Rauch, U., Lindberg, S., Caye-Thomasen, P., Hultgårdh, A., and Magnusson, M. (2013). Expression of insulin signalling components in the sensory epithelium of the human saccule. Cell Tissue. Res. 352, 469–478. doi: 10.1007/s00441-013-1614-x
de Iriarte Rodríguez, R., Magariños, M., Pfeiffer, V., Rapp, U. R., and Varela-Nieto, I. (2015a). C-Raf deficiency leads to hearing loss and increased noise susceptibility. Cell. Mol. Life Sci. 72, 3983–3998. doi: 10.1007/s00018-015-1919-x
de Iriarte Rodríguez, R., Pulido, S., Rodríguez-de la Rosa, L., Magariños, M., and Varela-Nieto, I. (2015b). Age-regulated function of autophagy in the mouse inner ear. Hear. Res. 330, 39–50. doi: 10.1016/j.heares.2015.07.020.
Dik, M. G., Pluijm, S. M., Jonker, C., Deeg, D. J., Lomecky, M. Z., and Lips, P. (2003). Insulin-like growth factor I (IGF-I) and cognitive decline in older persons. Neurobiol. Aging 24, 573–581. doi: 10.1016/S0197-4580(02)00136-7
Ding, D., Jiang, H., Chen, G. D., Longo-Guess, C., Muthaiah, V. P., Tian, C., et al. (2016). N-acetyl-cysteine prevents age-related hearing loss and the progressive loss of inner hair cells in gamma-glutamyl transferase 1 deficient mice. Aging 8, 730–750. doi: 10.18632/aging.100927
Dyer, A. H., Vahdatpour, C., Sanfeliu, A., and Tropea, D. (2016). The role of insulin-like growth factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 325, 89–99. doi: 10.1016/j.neuroscience.2016.03.056
El Bouchikhi, I., Belhassan, K., Moufid, F. Z., Iraqui Houssaini, M., Bouguenouch, L., Samri, I., et al. (2016). Noonan syndrome-causing genes: molecular update and an assessment of the mutation rate. Int. J. Pediatr. Adol. Med. 3, 133–142. doi: 10.1016/j.ijpam.2016.06.003
Ester, W. A., van Duyvenvoorde, H. A., de Wit, C. C., Broekman, A. J., Ruivenkamp, C. A., Govaerts, L. C., et al. (2009). Two short children born small for gestational age with insulin-like growth factor 1 receptor haploinsufficiency illustrate the heterogeneity of its phenotype. J. Clin. Endocrinol. Metab. 94, 4717–4727. doi: 10.1210/jc.2008-1502
Fang, P., Cho, Y. H., Derr, M. A., Rosenfeld, R. G., Hwa, V., and Cowell, C. T. (2012). Severe short stature caused by novel compound heterozygous mutations of the insulin-like growth factor 1 receptor (IGF1R). J. Clin. Endocrinol. Metab. 97, E243–E247. doi: 10.1210/jc.2011-2142
Fernández-Medarde, A., Barhoum, R., Riquelme, R., Porteros, A., Núñez, A., de Luis, A., et al. (2009). RasGRF1 disruption causes retinal photoreception defects and associated transcriptomic alterations. J. Neurochem. 110, 641–652. doi: 10.1111/j.1471-4159.2009.06162.x
Fetoni, A. R., Piacentini, R., Fiorita, A., Paludetti, G., and Troiani, D. (2009). Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL). Brain Res. 1257, 108–116. doi: 10.1016/j.brainres.2008.12.027
Fetoni, A. R., Picciotti, P. M., Paludetti, G., and Troiani, D. (2011). Pathogenesis of presbycusis in animal models: a review. Exp. Gerontol. 46, 413–425. doi: 10.1016/j.exger.2010.12.003
Fettiplace, R., and Kim, K. X. (2014). The physiology of mechanoelectrical transduction channels in hearing. Physiol. Rev. 94, 951–986. doi: 10.1152/physrev.00038.2013
Firth, S. M., and Baxter, R. C. (2002). Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 23, 824–854. doi: 10.1210/er.2001-0033
Fortunato, S., Forli, F., Guglielmi, V., De Corso, E., Paludetti, G., Berrettini, S., et al. (2016). A review of new insights on the association between hearing loss and cognitive decline in ageing. Acta Otorhinolaryngol. Ital. 36, 155–166. doi: 10.14639/0392-100X-993
Foulstone, E., Prince, S., Zaccheo, O., Burns, J. L., Harper, J., Jacobs, C., et al. (2005). Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J. Pathol. 205, 145–153. doi: 10.1002/path.1712
Fransen, E., Bonneux, S., Corneveaux, J. J., Schrauwen, I., Di Berardino, F., White, C. H., et al. (2015). Genome-wide association analysis demonstrates the highly polygenic character of age-related hearing impairment. Eur. J. Hum. Genet. 23, 110–115. doi: 10.1038/ejhg.2014.56
Frisina, R. D., Ding, B., Zhu, X., and Walton, J. P. (2016). Age-related hearing loss: prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons. Aging 8, 2081–2099. doi: 10.18632/aging.101045
Fuentes-Santamaria, V., Alvarado, J. C., Gabaldon-Ull, M. C., and Manuel Juiz, J. (2013). Upregulation of insulin-like growth factor and interleukin 1β occurs in neurons but not in glial cells in the cochlear nucleus following cochlear ablation. J. Comp. Neurol. 521, 3478–3499. doi: 10.1002/cne.23362
Fuentes-Santamaria, V., Alvarado, J. C., Rodriguez-De La Rosa, L., Murillo-Cuesta, S., Contreras, J., Juiz, J. M., et al. (2016). IGF-1 deficiency causes atrophic changes associated with upregulation of VGluT1 and downregulation of MEF2 transcription factors in the mouse cochlear nuclei. Brain Struct. Funct. 221, 709–734. doi: 10.1007/s00429-014-0934-2
Fuqua, J. S., Derr, M., Rosenfeld, R. G., and Hwa, V. (2012). Identification of a novel heterozygous IGF1 splicing mutation in a large kindred with familial short stature. Horm. Res. Paediatr. 78, 59–66. doi: 10.1159/000337249
Gálvez, H., Abelló, G., and Giraldez, F. (2017). Signaling and transcription factors during inner ear development: the generation of hair cells and otic neurons. Front. Cell Dev. Biol. 5:21. doi: 10.3389/fcell.2017.00021
Gannagé-Yared, M. H., Klammt, J., Chouery, E., Corbani, S., Mégarbané, H., Abou Ghoch, J., et al. (2013). Homozygous mutation of the IGF1 receptor gene in a patient with severe pre- and postnatal growth failure and congenital malformations. Eur. J. Endocrinol. 168, K1–K7. doi: 10.1530/EJE-12-0701
Gates, G. A., and Mills, J. H. (2005). Presbycusis. Lancet 366, 1111–1120. doi: 10.1016/S0140-6736(05)67423-5
Giordano, M., Gertosio, C., Pagani, S., Meazza, C., Fusco, I., Bozzola, E., et al. (2015). A 5.8 Mb interstitial deletion on chromosome Xq21.1 in a boy with intellectual disability, cleft palate, hearing impairment and combined growth hormone deficiency. BMC Med. Genet. 16:74. doi: 10.1186/s12881-015-0220-z
Guastini, L., Mora, R., Dellepiane, M., Santomauro, V., Giorgio, M., and Salami, A. (2011). Water-soluble coenzyme Q10 formulation in presbycusis: long-term effects. Acta Otolaryngol. 131, 512–517. doi: 10.3109/00016489.2010.539261
Guo, Y., Tang, C. Y., Man, X. F., Tang, H. N., Tang, J., Zhou, C. L., et al. (2017). Insulin-like growth factor-1 promotes osteogenic differentiation and collagen I alpha 2 synthesis via induction of mRNA-binding protein LARP6 expression. Dev. Growth Differ. 59, 94–103. doi: 10.1111/dgd.12342
Hequembourg, S., and Liberman, M. C. (2001). Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J. Assoc. Res. Otolaryngol. 2, 118–129. doi: 10.1007/s101620010075
Herrington, J., Smit, L. S., Schwartz, J., and Carter-Su, C. (2000). The role of STAT proteins in growth hormone signaling. Oncogene 19, 2585–2597. doi: 10.1038/sj.onc.1203526
Iwai, K., Nakagawa, T., Endo, T., Matsuoka, Y., Kita, T., Kim, T. S., et al. (2006). Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope 116, 529–533. doi: 10.1097/01.mlg.0000200791.77819.eb
Kalinec, G. M., Lomberk, G., Urrutia, R. A., and Kalinec, F. (2017). Resolution of cochlear inflammation: novel target for preventing or ameliorating drug-, noise- and age-related hearing loss. Front. Cell. Neurosci. 11:192. doi: 10.3389/fncel.2017.00192
Kelly, M. C., and Chen, P. (2009). Development of form and function in the mammalian cochlea. Curr. Opin. Neurobiol. 19, 395–401. doi: 10.1016/j.conb.2009.07.010
Kim, J., Kim, M. R., Kim, H. J., Lee, K. A., and Lee, M. G. (2011). LEOPARD syndrome with PTPN11 gene mutation showing six cardinal symptoms of LEOPARD. Ann. Dermatol. 23, 232–235. doi: 10.5021/ad.2011.23.2.232
Kim, S. Y., Kim, H. J., Park, E. K., Joe, J., Sim, S., and Choi, H. G. (2017). Severe hearing impairment and risk of depression: a national cohort study. PLoS ONE 12:e0179973. doi: 10.1371/journal.pone.0179973
Klammt, J., Kiess, W., and Pfäffle, R. (2011). IGF1R mutations as cause of SGA. Best Pract. Res. Clin. Endocrinol. Metab. 25, 191–206. doi: 10.1016/j.beem.2010.09.012
Koffler, T., Ushakov, K., and Avraham, K. B. (2015). Genetics of hearing loss: syndromic. Otolaryngol. Clin. North Am. 48, 1041–1061. doi: 10.1016/j.otc.2015.07.007
Lassale, C., Batty, G. D., Steptoe, A., and Zaninotto, P. (2017). Insulin-like Growth Factor 1 in relation to future hearing impairment: findings from the English Longitudinal Study of Ageing. Sci. Rep. 7, 4212. doi: 10.1038/s41598-017-04526-7
Laviola, L., Natalicchio, A., and Giorgino, F. (2007). The IGF-I signaling pathway. Curr. Pharm. Des. 13, 663–669. doi: 10.2174/138161207780249146
Magariños, M., Contreras, J., Aburto, M. R., and Varela-Nieto, I. (2012). Early development of the vertebrate inner ear. Anat. Rec. 295, 1775–1790. doi: 10.1002/ar.22575
Magariños, M., Contreras, J., and Varela-Nieto, I. (2014). “Early development of the vertebrate inner ear,” in Development of Auditory and Vestibular Systems, eds. R. Romand and I. Varela-Nieto (Kidlington: Academic Press), 1–30.
Magariños, M., Pulido, S., Aburto, M. R., de Iriarte Rodríguez, R., and Varela-Nieto, I. (2017). Autophagy in the vertebrate inner ear. Front. Cell Dev. Biol. 5:56. doi: 10.3389/fcell.2017.00056
Martínez-Quintana, E., and Rodríguez-González, F. (2012). LEOPARD Syndrome Caused by Tyr279Cys Mutation in the PTPN11 Gene. Mol. Syndromol. 2, 251–253. doi: 10.1159/000335995
Maystadt, I., Andrew, S.F., De Schepper, J., Wauters, N., Benoît, V., Joset, P., et al. (2015). “Novel Homozygous IGF1 Receptor (IGF1R) mutation, p.A711_E714del, in two siblings with severe growth failure, congenital malformations, deafness and insulin insensitivity,” in Endocrine Society's 97th Annual Meeting and Expo (San Diego).
Menardo, J., Tang, Y., Ladrech, S., Lenoir, M., Casas, F., Michel, C., et al. (2012). Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid. Redox Signal. 16, 263–274. doi: 10.1089/ars.2011.4037
Mittal, R., Nguyen, D., Patel, A. P., Debs, L. H., Mittal, J., Yan, D., et al. (2017). Recent advancements in the regeneration of auditory hair cells and hearing restoration. Front. Mol. Neurosci. 10:236. doi: 10.3389/fnmol.2017.00236
Murillo-Cuesta, S., Camarero, G., González-Rodríguez, A., Rodríguez de La Rosa, L., Burks, D. J., Avendano, C., et al. (2012). Insulin receptor substrate 2 (IRS2)-deficient mice show sensorineural hearing loss that is delayed by concomitant protein tyrosine phosphatase 1B (PTP1B) loss of function. Mol. Med. 18, 260–269. doi: 10.2119/molmed.2011.00328
Murillo-Cuesta, S., Rodriguez-De La Rosa, L., Cediel, R., Lassaletta, L., and Varela-Nieto, I. (2011). The role of insulin-like growth factor-I in the physiopathology of hearing. Front. Mol. Neurosci. 4:11. doi: 10.3389/fnmol.2011.00011
Muus, J. S., Weir, F. W., Kreicher, K. L., Bowlby, D. A., Discolo, C. M., and Meyer, T. A. (2017). Hearing loss in children with growth hormone deficiency. Int. J. Pediatr. Otorhinolaryngol. 100, 107–113. doi: 10.1016/j.ijporl.2017.06.037
Nakagawa, T., Kumakawa, K., Usami, S., Hato, N., Tabuchi, K., Takahashi, M., et al. (2014). A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med. 12:219. doi: 10.1186/s12916-014-0219-x
Nakagawa, T., Ogino-Nishimura, E., Hiraumi, H., Sakamoto, T., Yamamoto, N., and Ito, J. (2012). Audiometric outcomes of topical IGF1 treatment for sudden deafness refractory to systemic steroids. Otol. Neurotol. 33, 941–946. doi: 10.1097/MAO.0b013e31825f251a
Nakagawa, T., Yamamoto, M., Kumakawa, K., Usami, S., Hato, N., Tabuchi, K., et al. (2016). Prognostic impact of salvage treatment on hearing recovery in patients with sudden sensorineural hearing loss refractory to systemic corticosteroids: a retrospective observational study. Auris Nasus Larynx 43, 489–494. doi: 10.1016/j.anl.2015.12.004
Netchine, I., Azzi, S., Houang, M., Seurin, D., Perin, L., Ricort, J. M., et al. (2009). Partial primary deficiency of insulin-like growth factor (IGF)-I activity associated with IGF1 mutation demonstrates its critical role in growth and brain development. J. Clin. Endocrinol. Metab. 94, 3913–3921. doi: 10.1210/jc.2009-0452
Nieto-Estévez, V., Oueslati-Morales, C. O., Li, L., Pickel, J., Morales, A. V., and Vicario-Abejón, C. (2016). Brain insulin-like growth factor-i directs the transition from stem cells to mature neurons during postnatal/adult hippocampal neurogenesis. Stem Cells 34, 2194–2209. doi: 10.1002/stem.2397
Ohlemiller, K. K. (2009). Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 1277, 70–83. doi: 10.1016/j.brainres.2009.02.079
Ohlemiller, K. K., and Gagnon, P. M. (2004). Cellular correlates of progressive hearing loss in 129S6/SvEv mice. J. Comp. Neurol. 469, 377–390. doi: 10.1002/cne.11011
Okano, T., and Kelley, M. W. (2013). Expression of insulin-like growth factor binding proteins during mouse cochlear development. Dev. Dyn. 242, 1210–1221. doi: 10.1002/dvdy.24005
Okano, T., Xuan, S., and Kelley, M. W. (2011). Insulin-like growth factor signaling regulates the timing of sensory cell differentiation in the mouse cochlea. J. Neurosci. 31, 18104–18118. doi: 10.1523/JNEUROSCI.3619-11.2011
Op De Beeck, K., Schacht, J., and Van Camp, G. (2011). Apoptosis in acquired and genetic hearing impairment: the programmed death of the hair cell. Hear. Res. 281, 18–27. doi: 10.1016/j.heares.2011.07.002
Ouda, L., Profant, O., and Syka, J. (2015). Age-related changes in the central auditory system. Cell Tissue Res. 361, 337–358. doi: 10.1007/s00441-014-2107-2
Partearroyo, T., Vallecillo, N., Pajares, M. A., Varela-Moreiras, G., and Varela-Nieto, I. (2017). Cochlear homocysteine metabolism at the crossroad of nutrition and sensorineural hearing loss. Front. Mol. Neurosci. 10:107. doi: 10.3389/fnmol.2017.00107
Peracino, A. (2014). Hearing loss and dementia in the aging population. Audiol. Neurootol. 19(Suppl. 1), 6–9. doi: 10.1159/000371595
Prado-Barreto, V. M., Salvatori, R., Santos Júnior, R. C., Brandão-Martins, M. B., Correa, E. A., Garcez, F. B., et al. (2014). Hearing status in adult individuals with lifetime, untreated isolated growth hormone deficiency. Otolaryngol. Head Neck Surg. 150, 464–471. doi: 10.1177/0194599813517987
Prontera, P., Micale, L., Verrotti, A., Napolioni, V., Stangoni, G., and Merla, G. (2015). A new homozygous iGF1R variant defines a clinically recognizable incomplete dominant form of SHORT syndrome. Hum. Mutat. 36, 1043–1047. doi: 10.1002/humu.22853
Reichenbach, T., and Hudspeth, A. J. (2014). The physics of hearing: fluid mechanics and the active process of the inner ear. Rep. Prog. Phys. 77:076601. doi: 10.1088/0034-4885/77/7/076601
Riquelme, R., Cediel, R., Contreras, J., la Rosa Lourdes, R. D., Murillo-Cuesta, S., Hernandez-Sanchez, C., et al. (2010). A comparative study of age-related hearing loss in wild type and insulin-like growth factor I deficient mice. Front. Neuroanat. 4:27. doi: 10.3389/fnana.2010.00027
Riva, C., Donadieu, E., Magnan, J., and Lavieille, J. P. (2007). Age-related hearing loss in CD/1 mice is associated to ROS formation and HIF target proteins up-regulation in the cochlea. Exp. Gerontol. 42, 327–336. doi: 10.1016/j.exger.2006.10.014
Rodriguez-de La Rosa, L., Fernandez-Sanchez, L., Germain, F., Murillo-Cuesta, S., Varela-Nieto, I., de la Villa, P., et al. (2012). Age-related functional and structural retinal modifications in the Igf1-/- null mouse. Neurobiol. Dis. 46, 476–485. doi: 10.1016/j.nbd.2012.02.013
Rodríguez-de La Rosa, L., López-Herradón, A., Portal-Núñez, S., Murillo-Cuesta, S., Lozano, D., Cediel, R., et al. (2014). Treatment with N- and C-terminal peptides of parathyroid hormone-related protein partly compensate the skeletal abnormalities in IGF-I deficient mice. PLoS ONE9:e87536. doi: 10.1371/journal.pone.0087536
Rodríguez-de la Rosa, L., Sánchez-Calderón, H., Contreras, J., Murillo-Cuesta, S., Falagan, S., Avendaño, C., et al. (2015). Comparative gene expression study of the vestibular organ of the Igf1 deficient mouse using whole-transcript arrays. Hear. Res. 330, 62–77. doi: 10.1016/j.heares.2015.08.016
Salminen, A., and Kaarniranta, K. (2010). Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NF-kappaB signaling. Cell. Signal. 22, 573–577. doi: 10.1016/j.cellsig.2009.10.006
Sanchez-Calderon, H., Milo, M., Leon, Y., and Varela-Nieto, I. (2007). A network of growth and transcription factors controls neuronal differentation and survival in the developing ear. Int. J. Dev. Biol. 51, 557–570. doi: 10.1387/ijdb.072373hs
Sanchez-Calderon, H., Rodriguez-De la Rosa, L., Milo, M., Pichel, J. G., Holley, M., and Varela-Nieto, I. (2010). RNA microarray analysis in prenatal mouse cochlea reveals novel IGF-I target genes: implication of MEF2 and FOXM1 transcription factors. PLoS ONE 5:e8699. doi: 10.1371/journal.pone.0008699
Schlueter, P. J., Peng, G., Westerfield, M., and Duan, C. (2007). Insulin-like growth factor signaling regulates zebrafish embryonic growth and development by promoting cell survival and cell cycle progression. Cell Death Differ. 14, 1095–1105. doi: 10.1038/sj.cdd.4402109
Sehat, B., Tofigh, A., Lin, Y., Trocmé, E., Liljedahl, U., Lagergren, J., et al. (2010). SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Sci. Signal. 3:ra10. doi: 10.1126/scisignal.2000628
Shaheen, R., Faqeih, E., Ansari, S., Abdel-Salam, G., Al-Hassnan, Z. N., Al-Shidi, T., et al. (2014). Genomic analysis of primordial dwarfism reveals novel disease genes. Genome Res. 24, 291–299. doi: 10.1101/gr.160572.113
Siddle, K. (2011). Signalling by insulin and IGF receptors: supporting acts and new players. J. Mol. Endocrinol. 47, R1–R10. doi: 10.1530/JME-11-0022
Stephenson, L. (2012). Structure and innervation of the cochlea and organ of corti. J. Vis. Commun. Med. 35, 159. doi: 10.3109/08039488.2012.747176
Su, P., Hsu, C. C., Lin, H. C., Huang, W. S., Yang, T. L., Hsu, W. T., et al. (2017). Age-related hearing loss and dementia: a 10-year national population-based study. Eur. Arch. Otorhinolaryngol. 274, 2327–2334. doi: 10.1007/s00405-017-4471-5
Tafra, R., Brakus, S. M., Vukojevic, K., Kablar, B., Colovic, Z., and Saraga-Babic, M. (2014). Interplay of proliferation and proapoptotic and antiapoptotic factors is revealed in the early human inner ear development. Otol. Neurotol. 35, 695–703. doi: 10.1097/MAO.0000000000000210
Tan, H. E., Lan, N. S. R., Knuiman, M. W., Divitini, M. L., Swanepoel, D. W., Hunter, M., et al. (2017). Associations between cardiovascular disease and its risk factors with hearing loss-A cross-sectional analysis. Clin. Otolaryngol. doi: 10.1111/coa.12936
Tang, C. Y., Man, X. F., Guo, Y., Tang, H. N., Tang, J., Zhou, C. L., et al. (2017). IRS-2 partially compensates for the insulin signal defects in IRS-1-/- mice mediated by miR-33. Mol. Cells 40, 123–132. doi: 10.14348/molcells.2017.2228
Tsukano, H., Horie, M., Ohga, S., Takahashi, K., Kubota, Y., Hishida, R., et al. (2017). Reconsidering tonotopic maps in the auditory cortex and lemniscal auditory thalamus in mice. Front. Neural Circuits 11:14. doi: 10.3389/fncir.2017.00014
Van Duyvenvoorde, H. A., Van Doorn, J., Koenig, J., Gauguin, L., Oostdijk, W., Wade, J. D., et al. (2011). The severe short stature in two siblings with a heterozygous IGF1 mutation is not caused by a dominant negative effect of the putative truncated protein. Growth Horm. IGF Res. 21, 44–50. doi: 10.1016/j.ghir.2010.12.004
van Duyvenvoorde, H. A., van Setten, P. A., Walenkamp, M. J., van Doorn, J., Koenig, J., Gauguin, L., et al. (2010). Short stature associated with a novel heterozygous mutation in the insulin-like growth factor 1 gene. J. Clin. Endocrinol. Metab. 95, E363–E367. doi: 10.1210/jc.2010-0511
Van Landeghem, L., Santoro, M. A., Mah, A. T., Krebs, A. E., Dehmer, J. J., Mcnaughton, K. K., et al. (2015). IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J. 29, 2828–2842. doi: 10.1096/fj.14-264010
Van Nierop, J. W. I., Van Trier, D. C., Van Der Burgt, I., Draaisma, J. M. T., Mylanus, E. A. M., Snik, A. F., et al. (2017). Cochlear implantation and clinical features in patients with Noonan syndrome and Noonan syndrome with multiple lentigines caused by a mutation in PTPN11. Int. J. Pediatr. Otorhinolaryngol. 97, 228–234. doi: 10.1016/j.ijporl.2017.04.024
van Trier, D. C., van Nierop, J., Draaisma, J. M., van Der Burgt, I., Kunst, H., Croonen, E. A., et al. (2015). External ear anomalies and hearing impairment in Noonan Syndrome. Int. J. Pediatr. Otorhinolaryngol. 79, 874–878. doi: 10.1016/j.ijporl.2015.03.021
van Varsseveld, N. C., van Bunderen, C. C., Sohl, E., Comijs, H. C., Penninx, B. W., Lips, P., et al. (2015). Serum insulin-like growth factor 1 and late-life depression: a population-based study. Psychoneuroendocrinology 54, 31–40. doi: 10.1016/j.psyneuen.2015.01.014
Varela-Nieto, I., Murillo-Cuesta, S., Rodríguez-de la Rosa, L., Lassatetta, L., and Contreras, J. (2013). IGF-I deficiency and hearing loss: molecular clues and clinical implications. Pediatr. Endocrinol. Rev. 10, 460–472.
Vestergaard, P. F., Hansen, M., Frystyk, J., Espelund, U., Christiansen, J. S., Jørgensen, J. O., et al. (2014). Serum levels of bioactive IGF1 and physiological markers of ageing in healthy adults. Eur. J. Endocrinol. 170, 229–236. doi: 10.1530/EJE-13-0661
Walenkamp, M. J., Karperien, M., Pereira, A. M., Hilhorst-Hofstee, Y., van Doorn, J., Chen, J. W., et al. (2005). Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J. Clin. Endocrinol. Metab. 90, 2855–2864. doi: 10.1210/jc.2004-1254
Wan, G., and Corfas, G. (2015). No longer falling on deaf ears: mechanisms of degeneration and regeneration of cochlear ribbon synapses. Hear. Res. 329, 1–10. doi: 10.1016/j.heares.2015.04.008
Watanabe, T., Miyazaki, A., Katagiri, T., Yamamoto, H., Idei, T., and Iguchi, T. (2005). Relationship between serum insulin-like growth factor-1 levels and Alzheimer's disease and vascular dementia. J. Am. Geriatr. Soc. 53, 1748–1753. doi: 10.1111/j.1532-5415.2005.53524.x
Welch, D., and Dawes, P. J. (2007). Childhood hearing is associated with growth rates in infancy and adolescence. Pediatr. Res. 62, 495–498. doi: 10.1203/PDR.0b013e3181425869
Wit, J. M., Oostdijk, W., Losekoot, M., van Duyvenvoorde, H. A., Ruivenkamp, C. A., and Kant, S. G. (2016). Mechanisms in endocrinology: novel genetic causes of short stature. Eur. J. Endocrinol. 174, R145–R173. doi: 10.1530/EJE-15-0937
Wong, A. C., and Ryan, A. F. (2015). Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci. 7:58. doi: 10.3389/fnagi.2015.00058
Woods, K. A., Camacho-Hübner, C., Savage, M. O., and Clark, A. J. (1996). Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N. Engl. J. Med. 335, 1363–1367. doi: 10.1056/NEJM199610313351805
Wrigley, S., Arafa, D., and Tropea, D. (2017). Insulin-like growth factor 1: at the crossroads of brain development and aging. Front. Cell. Neurosci. 11:14. doi: 10.3389/fncel.2017.00014
Yamahara, K., Nakagawa, T., Ito, J., Kinoshita, K., Omori, K., and Yamamoto, N. (2017). Netrin 1 mediates protective effects exerted by insulin-like growth factor 1 on cochlear hair cells. Neuropharmacology 119, 26–39. doi: 10.1016/j.neuropharm.2017.03.032
Yamahara, K., Yamamoto, N., Nakagawa, T., and Ito, J. (2015). Insulin-like growth factor 1: a novel treatment for the protection or regeneration of cochlear hair cells. Hear. Res. 330, 2–9. doi: 10.1016/j.heares.2015.04.009
Yang, Z. Q., Zhang, H. L., Duan, C. C., Geng, S., Wang, K., Yu, H. F., et al. (2017). IGF1 regulates RUNX1 expression via IRS1/2: implications for antler chondrocyte differentiation. Cell Cycle 16, 522–532. doi: 10.1080/15384101.2016.1274471
Yoshida, S., Sugahara, K., Hashimoto, M., Hirose, Y., Shimogori, H., and Yamashita, H. (2015). The minimum peptides of IGF-1 and substance P protect vestibular hair cells against neomycin ototoxicity. Acta Otolaryngol. 135, 411–415. doi: 10.3109/00016489.2014.979438
Keywords: ARHL, GH, IGF system, presbycusis, rare diseases
Citation: Rodríguez-de la Rosa L, Lassaletta L, Calvino M, Murillo-Cuesta S and Varela-Nieto I (2017) The Role of Insulin-Like Growth Factor 1 in the Progression of Age-Related Hearing Loss. Front. Aging Neurosci. 9:411. doi: 10.3389/fnagi.2017.00411
Received: 13 October 2017; Accepted: 27 November 2017;
Published: 12 December 2017.
Edited by:
Filippo Tempia, Università degli Studi di Torino, ItalyReviewed by:
Fabio Mammano, Università degli Studi di Padova, ItalyJacques Epelbaum, Institut National de la Santé et de la Recherche Médicale, France
Copyright © 2017 Rodríguez-de la Rosa, Lassaletta, Calvino, Murillo-Cuesta and Varela-Nieto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Murillo-Cuesta, smurillo@iib.uam.es
†These authors have contributed equally to this work and are both senior authors.
 Lourdes Rodríguez-de la Rosa
Lourdes Rodríguez-de la Rosa Luis Lassaletta1,2,3,4
Luis Lassaletta1,2,3,4  Miryam Calvino
Miryam Calvino Silvia Murillo-Cuesta
Silvia Murillo-Cuesta Isabel Varela-Nieto
Isabel Varela-Nieto