Disparate Effects of Lithium and a GSK-3 Inhibitor on Neuronal Oscillatory Activity in Prefrontal Cortex and Hippocampus
- 1Department of Pharmacology and Toxicology, University of Toronto, Toronto, ON, Canada
- 2Department of Medicine, University of Toronto, Toronto, ON, Canada
Glycogen synthase kinase-3 (GSK-3) plays a critical role in cognitive dysfunction associated with Alzheimer’s disease (AD), yet the mechanism by which GSK-3 alters cognitive processes in other disorders, such as schizophrenia, remains unknown. In the present study, we demonstrated a role for GSK-3 in the direct regulation of neuronal oscillations in hippocampus (HIP) and prelimbic cortex (PL). A comparison of the GSK-3 inhibitors SB 216763 and lithium demonstrated disparate effects of the drugs on spatial memory and neural oscillatory activity in HIP and PL. SB 216763 administration improved spatial memory whereas lithium treatment had no effect. Analysis of neuronal local field potentials in anesthetized animals revealed that whereas both repeated SB 216763 (2.5 mg/kg) and lithium (100 mg/kg) induced a theta frequency spike in HIP at approximately 10 Hz, only SB 216763 treatment induced an overall increase in theta power (4–12 Hz) compared to vehicle. Acute administration of either drug suppressed slow (32–59 Hz) and fast (61–100 Hz) gamma power. In PL, both drugs induced an increase in theta power. Repeated SB 216763 increased HIP–PL coherence across all frequencies except delta, whereas lithium selectively suppressed delta coherence. These findings demonstrate that GSK-3 plays a direct role in the regulation of theta oscillations in regions critically involved in cognition, and highlight a potential mechanism by which GSK-3 may contribute to cognitive decline in disorders of cognitive dysfunction.
Introduction
Glycogen synthase kinase-3 (GSK-3) is a constitutively active protein kinase with an array of physiological functions including mediating intracellular signaling, as well as regulating neuronal plasticity, gene expression, and cell survival (Grimes and Jope, 2001). GSK-3 exists as two isoforms, GSK-3α and GSK-3β, but the major research focus has been on GSK-3β as a result of its involvement in neuropsychiatric and neurodegenerative disease. Indeed, in recent years GSK-3 has been shown to be fundamental to the processes underlying cognitive function and evidence suggests that increased GSK-3 activity may represent a common biochemical mediator of impaired cognitive function in many central nervous system diseases (Emamian et al., 2004; Takashima, 2006; Ances et al., 2008; King et al., 2013).
A role for overactive GSK-3 as a critical mediator of cognitive decline in Alzheimer’s disease (AD) has been widely documented (Balaraman et al., 2006; Huang and Klein, 2006; Martinez and Perez, 2008; Muyllaert et al., 2008; Medina and Avila, 2010; Maqbool et al., 2016). In AD, overactivation of GSK-3 induces hyperphosphorylation of tau proteins resulting in the development of neurofibrillary tangles (NFTs) (Balaraman et al., 2006; Llorens-Martin et al., 2014) which are thought to contribute directly to cognitive dysfunction (Bloom, 2014). In schizophrenia, a disorder associated with cognitive dysfunction, a role for elevated GSK-3 activity in the neuropathology of this disorder is provided by postmortem findings, as well as genetic and behavioral studies. For example, reduced phosphorylation levels of GSK-3 (which correlate with increased activity) in postmortem frontal cortex samples of patients with schizophrenia has been documented (Emamian et al., 2004). In addition, associations between genetic variation in the GSK-3 gene and schizophrenia (SZ) have been reported (Li et al., 2011; Blasi et al., 2013; Tang et al., 2013) and a number of other susceptibility genes for SZ, including the genes encoding for the proteins disrupted in SZ (DISC1), neuregulin 1 (NRG-1), and dysbindin converge on GSK-3 signaling (Numakawa et al., 2004; Clapcote et al., 2007; Keri et al., 2009; Mao et al., 2009; Guo et al., 2010; Seres et al., 2010; Lipina et al., 2011, 2012). More recently, developmental inhibition of GSK-3 was shown to alleviate spatial memory deficiencies in a mouse model of schizophrenia predisposition (Tamura et al., 2016) indicative of a direct link between GSK-3 and the development of processes critical to aberrant cognitive functioning in this disorder. In line with these findings GSK-3 has been shown to be involved in mediating reductions in cognitive performance associated with diabetes mellitus (King et al., 2013; Wang and Zhao, 2016), Fragile-X syndrome (Guo et al., 2012; Franklin et al., 2014; Pardo et al., 2017), and human immunodeficiency virus (Ances et al., 2008), and in a model of traumatic brain injury improvements in spatial memory induced by valproate were associated with inhibition of GSK-3 in hippocampus (Dash et al., 2010).
A critical role for GSK-3 in inducing cognitive dysfunction via neurofibrillary tangle formation in AD is known. Yet, the involvement of this protein in other disorders of cognitive decline raises the possibility of an additional, and more direct, role for this kinase in mediating the processes underlying learning and memory not only in disease, but in healthy organisms as well. This idea is supported by studies in healthy animals showing that increased GSK-3 expression, within whole brain or in cortical and hippocampal neurons, could negatively affect cognitive function in both rats and mice (Hernandez et al., 2002; Liu et al., 2003). However, behavioral studies examining the effects of GSK-3 inhibitors on learning and memory under normal conditions are conflicting, with some reports showing positive (Nocjar et al., 2007; Lipina et al., 2013), negative (Al Banchaabouchi et al., 2004; Hu et al., 2009), or no effects of the drugs (Guo et al., 2012; Franklin et al., 2014). Despite these disparate findings, the large number of studies showing a role for GSK-3 in mediating cognitive performance in neurodegenerative disease, neuropsychiatric disorders and in injury, suggest that while a number of neuropathological processes may converge on GSK-3 signaling, the downstream effects of its increased activation are likely similar across many disease processes.
One possible consequence of increased GSK-3 activation is through the disruption of brain electrophysiological rhythms. Neuronal oscillatory rhythms, which are derived from macroscopic oscillations of neuronal populations, have patterns that are highly conserved across species (Buzsaki et al., 2013) and are critical to cognitive functioning (Igarashi, 2015; Helfrich and Knight, 2016). Neuronal oscillations in prefrontal cortex (PFC) and hippocampus (HIP) specifically have been shown to be of significant importance, having been linked with working memory in both humans (Cohen, 2011; Roux and Uhlhaas, 2014) and in animals (Pesaran et al., 2002; Benchenane et al., 2010; O’Neill et al., 2013), and dysregulated neuronal oscillatory activity in and between HIP and PFC have been widely documented in disease states such as AD and schizophrenia (Kwon et al., 1999; Herrmann and Demiralp, 2005; Schmiedt et al., 2005; Basar-Eroglu et al., 2007; Uhlhaas et al., 2008; Missonnier et al., 2010; Uhlhaas and Singer, 2010; Basar et al., 2013, 2016). For example, in humans or animal models reduced event-related increases in cortical gamma activity, or gamma-theta frequency coupling, in both AD and schizophrenia have been demonstrated (Kwon et al., 1999; Basar-Eroglu et al., 2007; Lodge et al., 2009; Missonnier et al., 2010; Zhang et al., 2016; Nakazono et al., 2017). Additionally in HIP, rodent models have demonstrated reduced elicited theta power, and/or gamma-theta coupling, in both disorders (Howlett et al., 2004; Villette et al., 2010; Scott et al., 2012; Mesbah-Oskui et al., 2015; Kalweit et al., 2017), with the augmentation of elicited theta power demonstrated to be characteristic of drugs used in clinical treatment of AD such as acetylcholinesterase inhibitors and memantine (Kinney et al., 1999; Guadagna et al., 2012).
Further evidence of a role for GSK-3 in neuronal oscillations comes from reports showing the regulation of ion channel function by this protein kinase (Wildburger and Laezza, 2012). Of relevance to the proposed study, GSK-3 has been shown to control the function of the N-methyl-D-aspartate (NMDA) receptor (Chen et al., 2007), a receptor also implicated in the regulation of oscillatory activity within the PFC and HIP (Lee et al., 2017; Lemercier et al., 2017; Miller et al., 2017). Specifically, Chen et al. (2007) demonstrated that the pharmacological inhibition or silencing of GSK-3 induced a long-lasting reduction of NMDA receptor-mediated ionic synaptic current in cortical pyramidal neurons. Interestingly, the effect of GSK-3 was specific for the GluN2B-containing NMDA receptors (Chen et al., 2007), receptors that are involved in the induction of long-term depression (LTD) (Liu et al., 2004; Massey et al., 2004). In addition to regulating NMDA receptor function, GSK-3 is also involved in voltage-gated sodium (Nav) channel function, both through regulation of surface expression (Yanagita et al., 2009; James et al., 2015) or functional activity via phosphorylation of fibroblast growth factor 14 (FGF14) (Shavkunov et al., 2013), a protein that interacts with the C terminus of Nav channels to regulate both the functional properties and subcellular distribution of the channels (Goldfarb et al., 2007; Shavkunov et al., 2013). Nav channels have been recently shown to play a role in the regulation of PFC and HIP gamma oscillations (Verret et al., 2012; Rama et al., 2015), and have been implicated as playing a key role in the neuropathophysiology of AD (Verret et al., 2012).
Lithium has been shown to inhibit GSK-3β directly in vitro, albeit with high Ki (∼2.0 for GSK-3β, Klein and Melton), and in vivo through activation of Akt (Beaulieu et al., 2004). However, lithium has also been demonstrated to inhibit other enzymes including inositol monophosphatases (IMPAs) (Berridge et al., 1989), bisphosphate 3′-nucleotidase (BPNT1) (Spiegelberg et al., 2005), and cyclooxygenase (COX) (Klein and Melton, 1996; Stambolic et al., 1996). Furthermore, lithium has been shown to influence numerous neurotransmitter systems including, serotonin, dopamine, and glutamate (Malhi et al., 2013). Despite the known role of GSK-3 in learning and memory, the effects of lithium on cognition are conflicting, with studies showing positive effects (Letendre et al., 2006; Nunes et al., 2013; Matsunaga et al., 2015; Daglas et al., 2016; Forlenza et al., 2016), little to no effect (Joffe et al., 1988; Schifitto et al., 2009; Bourne et al., 2013; Pfennig et al., 2014; Decloedt et al., 2016), or negative effects (Shaw et al., 1987; Monks et al., 2004; Senturk et al., 2007) of treatment on cognitive function. In the present study, we therefore sought to evaluate and compare the effects of a direct GSK-3 inhibitor, SB 216763, with lithium on the regulation of neuronal oscillatory activity within, and between, the HIP and PFC and the impact of these drugs on cognitive performance in a water maze test of spatial memory and reversal learning, tests that require HIP and PFC function, respectively (Broersen, 2000; Graybeal et al., 2011). Animals were administered five daily drug or vehicle injections with recordings taken from anesthetized rats at baseline, prior to behavioral testing on day 1, and following behavioral testing on day 1 and day 5.
Materials and Methods
Animals
Twenty-four adult male Wistar rats weighing approximately 350–400 g at the start of the experiments were used. Rats were housed up to three rats per cage in polyethylene cages in a colony room maintained on a 12-h light–dark cycle with free access to food and water. Rats were handled for 2 min daily for 5 days before the start of experiments. All treatments were performed during the light phase of the day–night cycle. All procedures involving animals complied with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care, 1993), and were approved by the Animal Care Ethics Committee of the University of Toronto.
Drugs
The GSK-3 inhibitor SB 216763 (Tocris Bioscience) was dissolved in a solution of DMSO, polyethylene glycol and sterile water, and administered at a dose of 2.5 mg/kg (i.p.) (Zhao et al., 2016; Wickens et al., 2017). Lithium chloride (lithium) was dissolved in 0.9% saline and administered at a dose of 100 mg/kg (i.p.). This dose was chosen as it was shown to increase phosphorylation of Akt (Zheng et al., 2013), an upstream negative regulator of GSK-3. For non-drug injections, an equivalent volume of vehicle (50% of the control animals received saline and 50% received DMSO, polyethylene glycol, sterile water) was administered. All injections were administered at a volume of 1.0 ml/kg.
Behavior
Behavioral tests took place 10 min post-injection for SB 216763 and 30 min post-injection for lithium. Vehicle-treated animals were divided into two groups that underwent testing 10 or 30 min post-injection. For this group the data was pooled as no intra-group variation was evident. Animals were trained to locate a submerged platform in the Morris water maze using an allocentric task (i.e., using distal cues to find the platform) (Broersen, 2000). The maze consisted of a circular pool (2 m diameter) filled with water maintained at 23 ± 1°C. The pool contained a transparent circular platform (10 cm diameter) in one quadrant with the surface 3 cm below the water surface, and placed approximately 20 cm from the wall.
The allocentric task consisted of four trials per day with an inter-trial interval of 2 min for four consecutive days. Each trial began with placing the rat into the pool in one of the three quadrants that did not contain the platform (the starting quadrant was similar for all rats tested). Rats were allowed to swim for 2 min after which, if the rat did not find the platform, it was placed on the platform where it remained for a period of 30 s. For each subsequent trial the release point of the rat was shifted, however, the platform always remained in the same position. During the reversal, on day 5, the platform was moved to the quadrant opposite, and animals were allocated up to 15 trials to locate the platform under 20 s three times consecutively. Swimming trajectories were recorded and analyzed with Any-maze software (Stoelting). The escape latencies and distance traveled were obtained from each rat. The mean swimming distance and the mean daily latencies from the four daily trials were compared.
Surgeries
Rats were anesthetized with isoflurane (induction 5%, maintenance 2%), administered the analgesic ketoprofen (5 mg/kg, s.c.) and secured in a stereotaxic frame. Body temperature was maintained at 37°C by a warming pad. Two custom length (25 mm) polyimide-insulated stainless steel wire twisted electrodes (Plastics One: E363/3-2TW, 0.125 mm) were implanted unilaterally into the dorsal CA1 region of the HIP (AP -3.6, ML -1.8, DV -2.9) and two into the prelimbic (PL) region of the PFC (AP +3.0, L -0.7, DV -4.0; Paxinos and Watson, 2005). Two screws attached to Teflon-insulated 20 mm length stainless steel wire (Plastics One, E363/20, 0.56 mm) were fixed into the skull above lambda as ground and the opposite hemisphere from electrode placement as reference. Additional anchor screws were attached to the skull and electrodes secured with dental cement to the anchor screws. The animals received an additional injection of ketoprofen 24 h following surgery and were allowed to recover individually in their home cage for a minimum of 10 days before the experiments were performed. Electrode placement was validated post-mortem.
Electrophysiology
All LFP oscillatory recordings (Intan Technologies) were performed in anesthetized animals (isoflurane 2%) under a heating lamp. Breathing and heart rate were monitored while animals were under anesthesia. Baseline recordings were performed 3 days prior to the beginning of the behavioral experiment. During the behavioral experiment recordings were taken post-drug injection following (and not prior to) the last trial on Injection Day 1 (acute drug administration) or Injection Day 5 (repeated drug administration). We therefore chose to anesthetize the animals prior to recording to evaluate drug-evoked responses and at the same time eliminate the contribution of arousal state that may occur as a result of variations in overall swimming duration. LFP oscillatory data was collected for a 5 min period and sampled at a rate of 5000 samples/s. The spectral power of LFP oscillations in each region and coherence between regions were analyzed using routines from the Chronux software package for MATLAB (MathWorks). Recordings were downsampled, segmented, detrended, and low-pass filtered to remove high frequencies greater than 100 Hz. Continuous multitaper spectral power and coherence (tapers = [5 9]) between regions was calculated for each segment in the following frequency bands: delta (1–4 Hz), theta (>4–12 Hz), beta (>12–32 Hz), slow gamma (>32–59 Hz), and fast gamma (>61–100 Hz).
Data Analysis
For the behavioral data, trials were averaged and the statistical significance of each dependent measure evaluated using a repeated measures ANOVA with a within subjects factor of Trial Day, and Drug as the between-subjects factors, and followed by Bonferroni post hoc tests. All data are expressed as means ± SEM. LFP spectral power was normalized to total spectral power. LFP power curves are reported as mean power for the slower frequencies and log-transformed for the gamma frequencies (>40 Hz) to better exhibit group differences. Quantification of the LFP power data at each frequency measure is reported as means ± SEM for between group comparisons, or as percent change from baseline for within group comparisons. For the within subjects analysis, a repeated measures ANOVA was performed for each frequency, with a within subjects factor of Injection # (baseline, 1 or 5 days), and followed by paired Student’s t-tests for post hoc comparisons to baseline. Some planned comparisons were performed to evaluate within subject changes between acute or repeated drug treatments with baseline. For the between subjects analysis, an ANOVA was performed for each frequency at each Injection # (baseline, 1, 5 days), with Drug as the between-subjects factor and followed by Bonferroni post hoc comparisons. Computations were performed using the SPSS/PC+ statistical package (IBM, Armonk, NY, United States).
Results
Effect of GSK-3 Inhibition on Spatial and Reversal Learning
The experimental timeline is depicted in Figure 1A. To evaluate whether GSK-3 inhibition could enhance spatial learning and memory the effects of selective GSK-3 inhibitor SB 216763, and the non-selective inhibitor lithium, on allocentric and reversal learning in the Morris water maze were examined. Significant differences between drug treatments in latency to find the platform and the distance traveled in the maze were evaluated. A comparison of all three groups across injections revealed a significant Drug × Trial Day interaction [F(6,60) = 2.5, p = 0.030]. SB 216763 resulted in improved spatial learning such that by Injection 4 animals that received SB 216763, but not lithium, exhibited a latency to escape significantly faster than the vehicle-treated controls (SB vs. Veh, p = 0.037, Bonferroni post hoc, Figure 1B) [Trial Day 4: Drug F(2,21) = 3.8, p = 0.041]. No significant differences in the total swimming distance were observed between groups (Figure 1C). Analysis of the swim trajectories revealed significant Drug × Trial Day interactions when Center Time [F(6,60) = 2.3, p = 0.038] and Center Distance [F(6,60) = 2.2, p = 0.04] were analyzed. By Trial Day 4, SB 216763 treatment resulted in the rats spending significantly more time in the center of the pool (SB vs. Veh, p = 0.023, Bonferroni post hoc, Figures 1D,E) which may reflect an anxiolytic effect of the drug (Crofton et al., 2017).
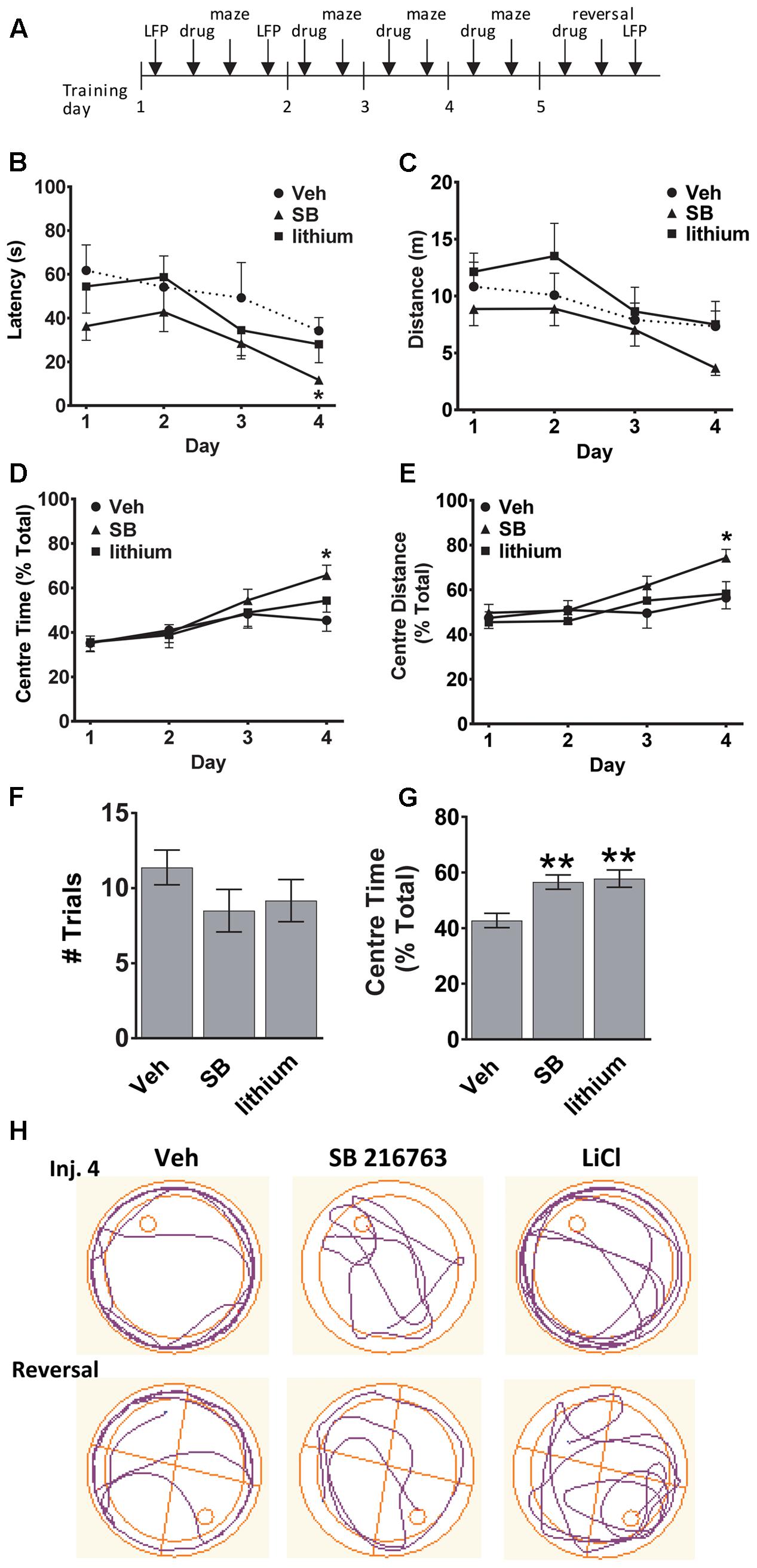
FIGURE 1. Effect of GSK-3 inhibition on learning and memory in the water maze. (A) Experimental timeline. (B,C) SB 216763 (2.5 mg/kg, i.p.), but not lithium (100 mg/kg, i.p.), reduced the latency to find the platform compared to vehicle controls by Injection Day 4, with no significant effects on the total distance traveled. (D,E) Rats treated with SB 216763 spent significantly more time, and traveled greater distance, in the center of the maze whereas vehicle-treated rats spent more time in the periphery. (F) There were no differences between and treatments on the number of trials required to find the platform during the reversal. (G) SB 216763 or lithium treatment resulted in rats spending more time in the center of the maze compared to the periphery during the reversal. (H) Representative swim plots from Injection 4 and the reversal test following drug treatment. N = 8 rats/group. Data are expressed as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, compared to vehicle-treated rats, Bonferroni post hoc test.
To assess cognitive flexibility, which requires PFC function (Graybeal et al., 2011), we next evaluated the animals during a reversal learning task by shifting the platform to the opposite quadrant and evaluating the number of trials it took to learn the new location of the platform and escape. No significant differences were evident between the three treatment groups (Figure 1F). Across all trials, both SB 216763- and lithium-treated animals spent a higher proportion of time searching the center of the maze for the platform during the reversal task (SB: 56.6 ± 2.6%, lithium: 57.8 ± 3.1%, Veh: 42.7 ± 2.6%, Figure 1G) [ANOVA: F(2,201) = 10.1, p = 0.0001]. Representative swim trajectories for Injection 4 and the reversal task are displayed in Figure 1H.
Temporal Effects of GSK-3 Inhibition on HIP Oscillatory Activity
Before the first swim on trial day 1, and following the final swim trial on days 1 and 5 LFP neuronal activity was evaluated in anesthetized animals to assess temporal changes in baseline, acute and repeated drug administration effects on oscillatory activity and coherence in and between dorsal HIP and PL (Figure 2A). LFPs are thought to represent the summed synchronous activity of relatively large groups of neurons (Mitzdorf, 1985; Logothetis, 2003), and can provide vital information on synchronous network activity both within and between regions. Evaluation of the oscillatory changes in HIP and PL of vehicle-treated rats (Figures 2B,C) during water maze training revealed no significant within subject Injection effects in HIP (Figures 2B,C). Recordings taken from SB 216763-treated animals (Figures 2D,E) showed that, in contrast to the vehicle-treated rats, SB 216763 treatment induced significant changes in HIP theta oscillations as revealed by a significant within subject effect of Injection [theta: F(2,22) = 4.6, p = 0.023]. Repeated, but not acute, SB 216763 increased theta power compared to baseline (p = 0.029, Figures 2D,E). There were no significant Injection effects of lithium treatment and planned comparisons to baseline revealed no significant effects on overall theta power (Figures 2F,G), however, both repeated SB 216763 or lithium administration induced a peak in the theta band at approximately 10 Hz compared to baseline, an effect also not evident in the vehicle-treated rats (Figure 2B). Acute lithium treatment suppressed both slow (p = 0.029 vs. baseline) and fast (p = 0.042 vs. baseline) gamma power in HIP, however, these effects were lost with repeated drug treatment (Figures 2F,G).
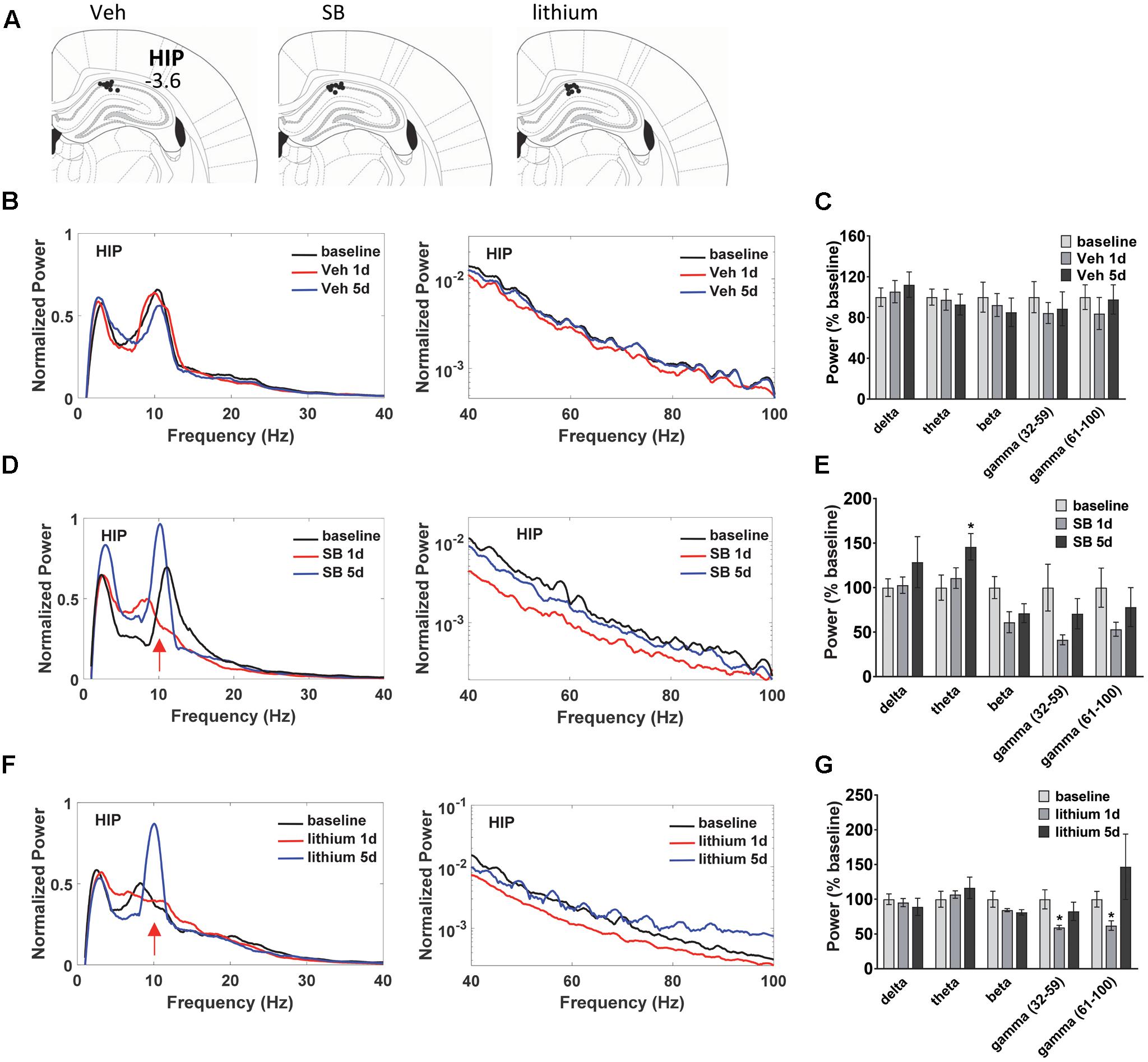
FIGURE 2. Temporal effects of GSK-3 inhibition on hippocampus (HIP) oscillations. (A) Electrode placements in HIP from each of the three treatment groups. (B,C) Power spectrums and quantification showing no deviations from baseline in HIP oscillatory power with vehicle administration. (D) Repeated GSK-3 inhibition by SB 216763 (2.5 mg/kg) induced a theta spike in HIP at approximately 10 Hz (red arrow) and induced an overall increased in theta power in this region. (E) Quantification of the HIP power spectrum at each frequency with SB 216763 treatment following water maze training on days 1 and 5, and at baseline. (F,G) Repeated lithium (100 mg/kg) induced a 10 Hz theta spike (red arrow) but did not induce an overall increase in theta power (4–12 Hz). Reduced slow and fast gamma powers were evident after acute treatment. N = 8 rats/group with 1–2 recordings/region/rat. Curves represent group means following acute (red) and repeated (blue) drug administration. Bars represent means ± SEM and are expressed as percent of baseline. ∗p < 0.05 compared to baseline, paired Student’s t-test.
Temporal Effects of GSK-3 Inhibition on PL Oscillatory Activity
Evaluation of drug effects in PL (Figure 3A) revealed no Injection effects following vehicle treatment (Figures 3B,C). Following SB 216763 administration an Injection effect was evident for the slow gamma band [F(2,24) = 9.325, p = 0.0001], with a reduction in slow gamma power following both acute and repeated drug treatment (Figures 3D,E). Similar to that observed in HIP, an increase in theta power was evident following repeated SB 216763 treatment (p = 0.033 vs. baseline). Animals treated with lithium exhibited significant Injection effects in the delta, theta, beta, and fast gamma bands [delta: F(2,30) = 7.6, p = 0.002; theta: F(2,30) = 9.4, p = 0.001; beta: F(2,30) = 6.2, p = 0.006; fast gamma: F(2,30) = 9.0, p = 0.001]. Compared to baseline acute lithium increased delta power (p = 0.001), and suppressed theta (p = 0.001), and slow gamma (p = 0.008) power, effects lost with repeated treatment. Following 5 days of lithium treatment animals exhibited reduced beta power compared to baseline (p = 0.011) and an increased in fast gamma power (p = 0.008, Figures 3F,G).
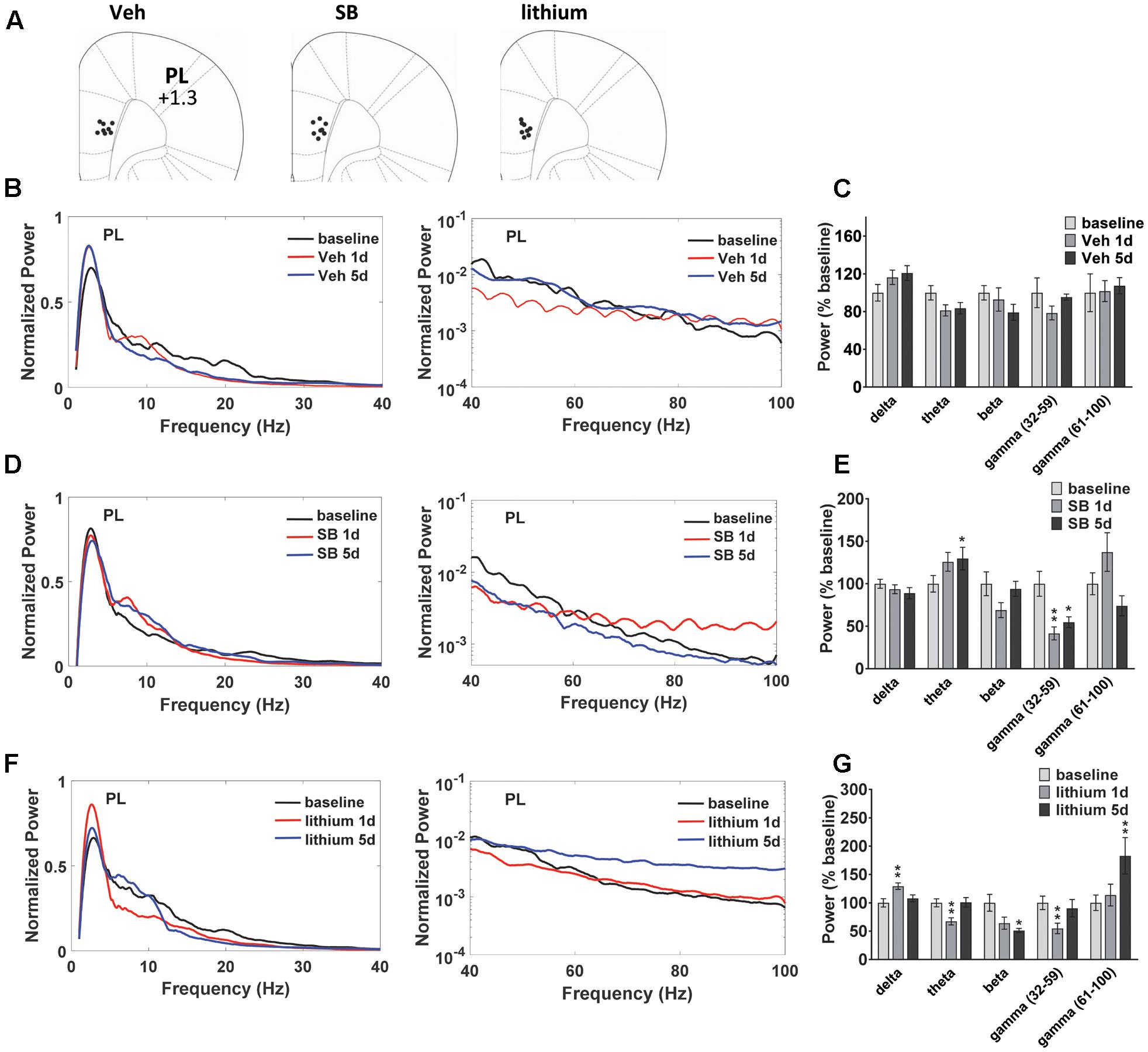
FIGURE 3. Temporal effects of GSK-3 inhibition on prelimbic cortex (PL) oscillations. (A) Electrode placements in PL from each of the three treatment groups. (B,C) Power spectrums and quantification showing no deviations from baseline in PL oscillatory power with vehicle administration. (D) Compared to baseline repeated SB 216763 (2.5 mg/kg) increased theta power in PL. Acute or repeated SB 216763 suppressed slow gamma power. (E) Quantification of the PL power spectrum at each frequency at baseline and following SB 216763 administration on days 1 and 5. (F,G) Changes in the power spectrum and quantification following acute or repeated lithium (100 mg/kg) treatment. N = 8 rats/group with 1–2 recordings/region/rat. Curves represent group means following acute (red) and repeated (blue) drug administration. Bars represent absolute value means ± SEM and are expressed as percent of baseline. ∗p < 0.05, ∗∗p < 0.01 compared to baseline, paired Student’s t-test.
Temporal Effects of GSK-3 Inhibition on HIP–PL Coherence
Oscillatory coherence between HIP and PL in vehicle-treated rats and following GSK-3 inhibition was next examined (Figure 4). In vehicle-treated rats (Figure 4A) the repeated measures ANOVA revealed an Injection effect for all frequencies except delta [theta: F(2,22) = 5.3, p = 0.016; beta: F(2,22) = 3.6, p = 0.049; slow gamma: F(2,22) = 8.5, p = 0.002; fast gamma: F(2,22) = 13.9, p = 0.0001]. At the completion of the 5 days of water maze testing significant decreases in HIP–PL gamma coherence were observed for all frequencies compared to baseline (Figure 4A). Acute or repeated administration of SB 216763 increased HIP–PL coherence in the delta, theta and slow gamma bands (Figure 4B) [Injection Effects, delta: F(2,22) = 4.8, p = 0.018; theta: F(2,22) = 3.5, p = 0.048; slow gamma: F(2,22) = 3.5, p = 0.048]. Repeated, but not acute, lithium enhanced theta band power compared to baseline with no effects on the other frequencies (Figure 4C) [Injection Effects, theta: F(2,30) = 34.7, p = 0.0001].
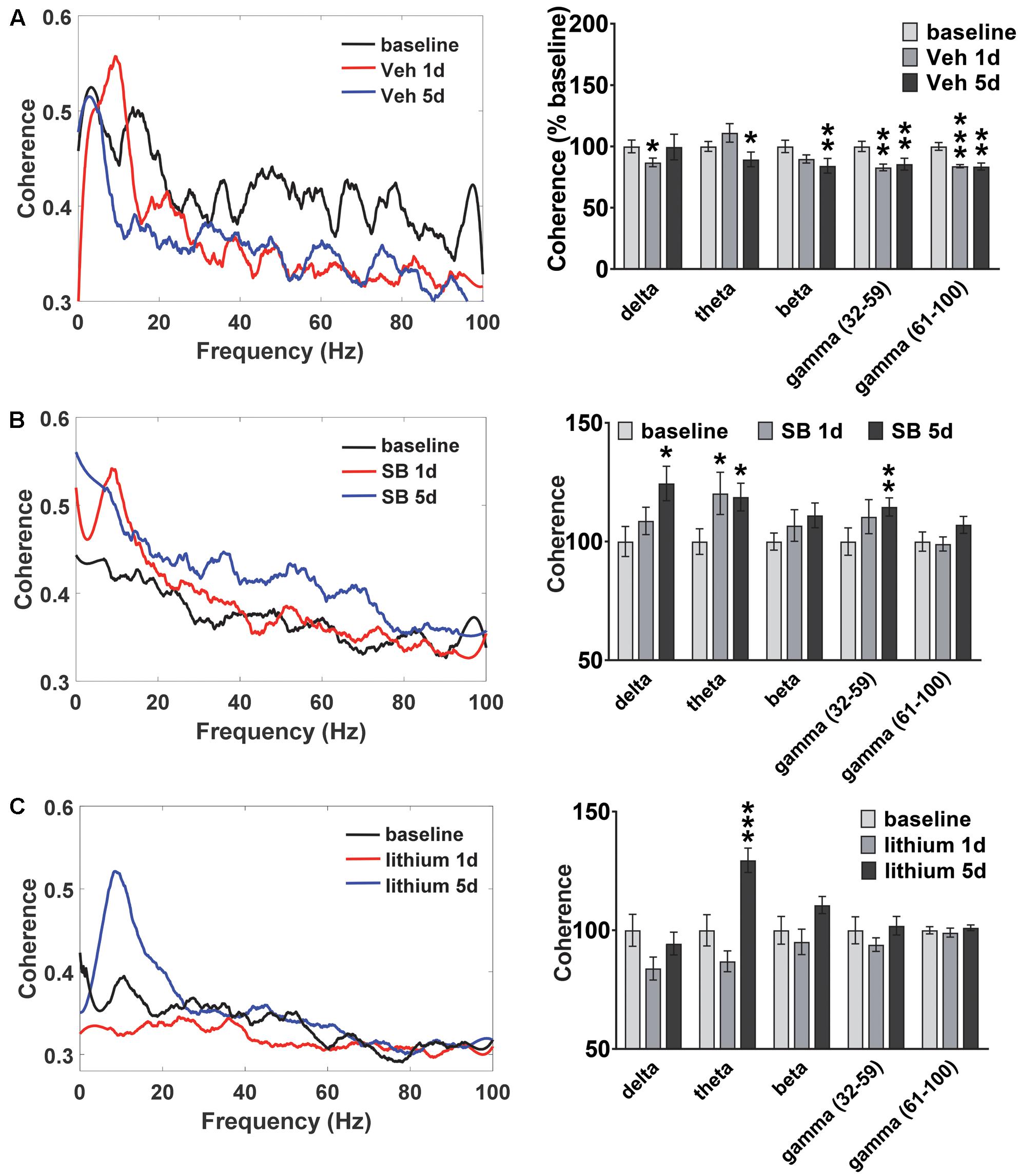
FIGURE 4. Temporal effect of GSK-3 inhibition on HIP-PL coherence. (A) Repeated vehicle administration suppressed HIP–PL coherence. (B) Acute or repeated SB 216763 (2.5 mg/kg) increased theta coherence compared baseline, with increased delta and low gamma coherence with repeated treatment. (C) Repeated lithium (100 mg/kg) increased theta power compared to baseline. N = 8 rats, 1–2 recordings/region/rat. Curves represent group means following acute (red) and repeated (blue) drug administration. Bars represent means ± SEM and are expressed as percent of baseline. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 compared to baseline, paired Student’s t-test.
Effects of Direct GSK-3 Inhibition versus Lithium on HIP and PL Oscillations and Coherence
In addition to evaluating temporal changes in neural oscillatory power and coherence following drug treatment (within subjects comparisons), between group comparisons were also performed (Figures 5, 6). In both HIP and PL, spectral power did not differ between groups prior to drug administration, nor did HIP–PL oscillatory coherence (Supplementary Figure S1). After a single injection of vehicle, SB 216763 or lithium, ANOVA analysis of spectral power revealed a significant effect of Drug in the beta, slow and fast gamma bands for HIP (Figures 6A,B) [beta: F(2,25) = 6.2, p = 0.005; slow gamma: F(2,25) = 15.8, p = 0.0001; fast gamma: F(2,25) = 8.5, p = 0.001]. Acute administration of either SB 216763 or lithium induced robust decreases in both slow and fast gamma compared to vehicle (Figures 5A,B). With repeated drug treatment significant Drug effects were evident in the theta and fast gamma bands [theta: F(2,25) = 3.5, p = 0.041; slow gamma: F(2,25) = 3.5, p = 0.039]. Compared to vehicle, only SB 216763 exhibited increased overall theta power following repeated treatment (Figures 5C,D). However, a significant increase in theta power (9–11 Hz) was evident following either SB 216763 (p = 0.005) or lithium (p = 0.028, Figures 5C,D) compared to the controls [ANOVA: theta (9–11), F(2,25) = 6.9, p = 0.003].
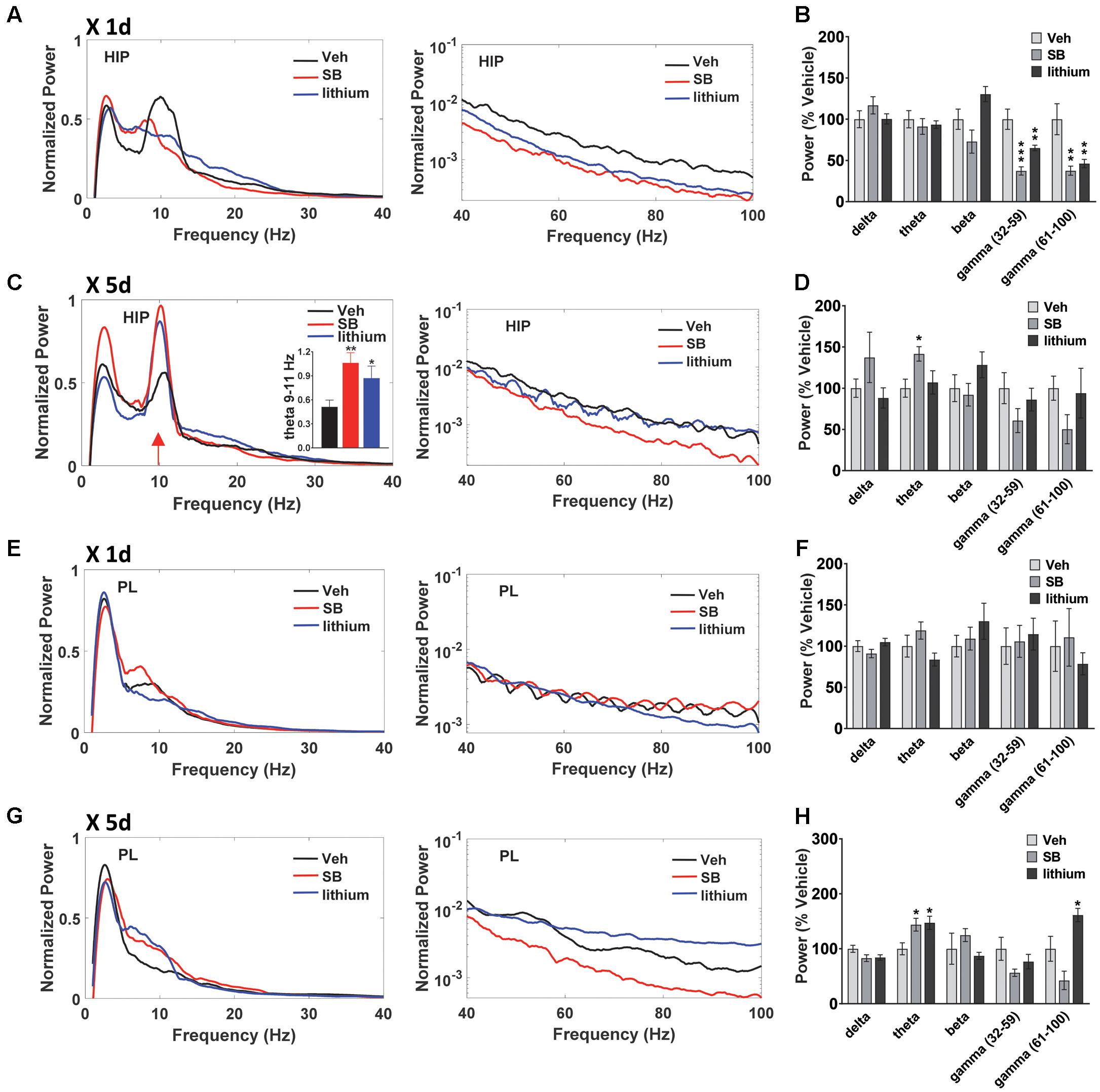
FIGURE 5. Effect of GSK-3 inhibition on HIP and PL oscillations. (A,B) Acute administration of SB 216763 (2.5 mg/kg) or lithium (100 mg/kg) reduced slow and fast gamma power in HIP. (C) Repeated SB 216763 or lithium induced a theta spike at approximately 10 Hz. (D) Quantification of the power spectrum showing increased overall theta power following SB 216763, but not lithium, compared to vehicle. (E,F) Acute SB 216763 or lithium did not alter PL oscillatory power. (G,H) Repeated SB 216763 or lithium increased theta power in PL compared to vehicle-treated rats. Lithium also induced an increase in fast gamma power. N = 8 rats/group with 1–2 recordings/region/rat. Curves represent group means following acute (red) and repeated (blue) drug administration. Bars represent means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 compared to vehicle, Bonferroni post hoc test.
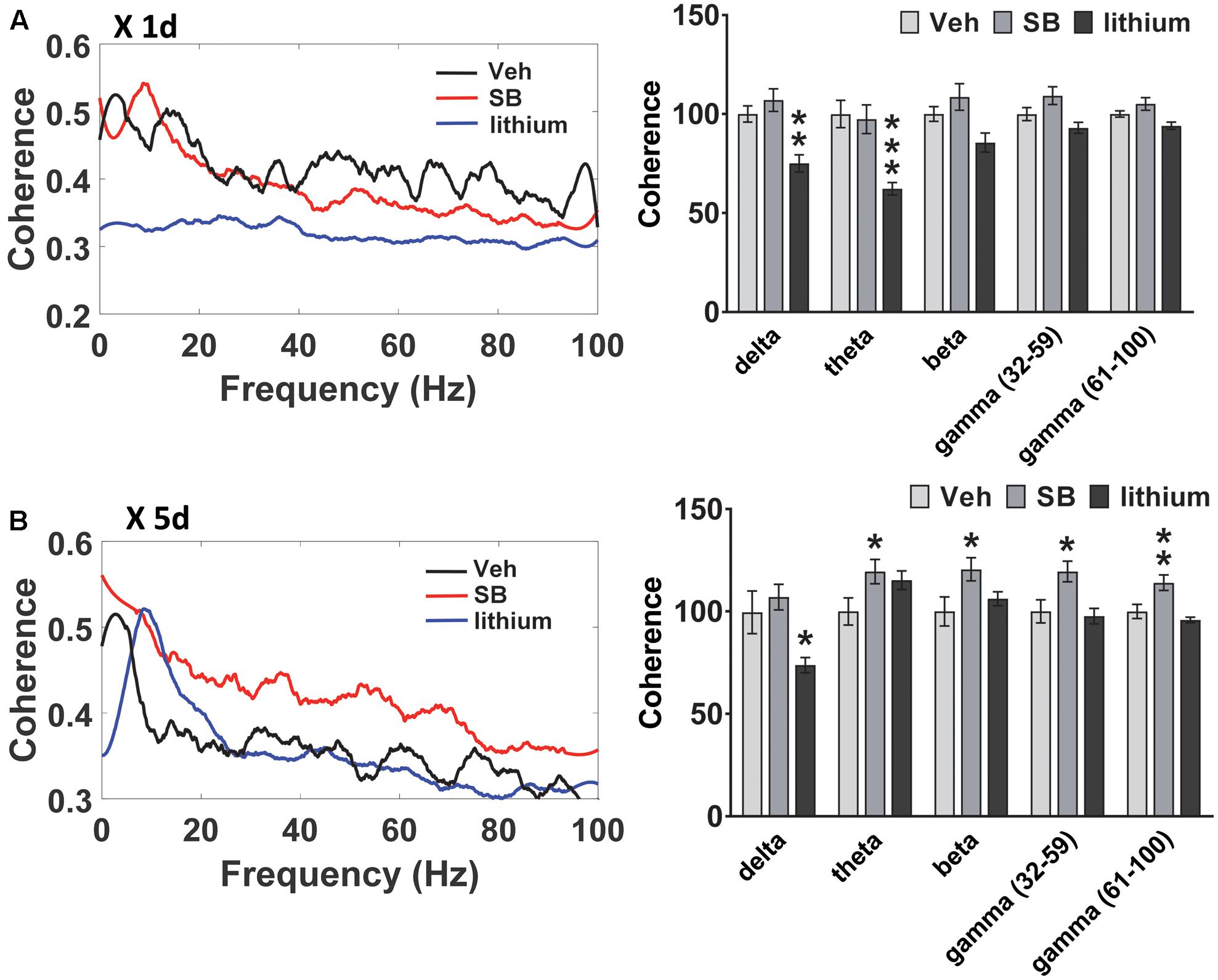
FIGURE 6. Effect of GSK-3 inhibition on HIP–PL coherence in rats. (A) No effect of acute SB 216763 (2.5 mg/kg) on HIP–PL coherence whereas lithium (100 mg/kg) suppressed delta and theta coherence. (B) Repeated SB 216763 increased HIP–PL coherence at all frequencies except delta. Lithium selectively suppressed delta coherence. N = 8 rats/group with 1–2 recordings/region/rat. Curves represent group means following acute (red) and repeated (blue) drug administration. Bars represent means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 compared to vehicle, Bonferroni post hoc test.
In PL no significant effects were evident following acute drug administration (Figures 5E,F), with significant theta and fast gamma Drug effects following repeated treatment [theta: F(2,25) = 5.4, p = 0.008; fast gamma: F(2,25) = 10.2, p = 0.0001]. Repeated SB 216763 or lithium each increased theta power compared to vehicle (p = 0.020 and p = 0.028, respectively, Figures 5G,H). Repeated lithium also increased fast gamma power in PL compared to vehicle-treated rats (p = 0.012).
A comparison of HIP–PL oscillatory coherence (Figure 6) between drug treatments revealed significant Drug effects at all frequencies [delta: F(2,25) = 8.3, p = 0.001; theta: F(2,25) = 2.9, p = 0.049; beta: F(2,25) = 4.0, p = 0.024; slow gamma: F(2,25) = 3.9, p = 0.024; slow gamma: F(2,25) = 3.4, p = 0.035]. Compared to vehicle, direct inhibition of GSK-3 by SB 216763 increased coherence at all frequencies except delta following repeated, but not acute treatment (Figure 6). Conversely, lithium suppressed HIP–PL delta and theta coherence with acute treatment, with reduced delta coherence with repeated treatment.
Discussion
A pivotal role for GSK-3 in the regulation of learning and memory is widely accepted. Yet, except for its involvement in tau hyperphosphorylation and NFT formation in AD, little is known regarding the mechanisms by which GSK-3 regulates cognitive function. In the present study, we showed in rats that systemic inhibition of GSK-3 activity had direct effects on neuronal oscillatory activity in HIP and PL, and further, that direct GSK-3 inhibition induced oscillatory changes that were distinct from the non-selective GSK-3 inhibitor lithium. Selective inhibition of GSK-3 by SB 216763 significantly improved spatial memory and altered the animals’ learning strategy, increasing goal-directed exploratory behavior within the center of the maze. At the completion of behavioral testing, analysis of LFPs revealed there was a 10 Hz theta frequency spike in HIP following either SB 216763 or lithium. However, whereas selective GSK-3 inhibition increased overall theta power in both HIP and PL, lithium induced a theta power increase selectively in PL coincident with an increase in fast gamma power. Furthermore, whereas repeated SB 216763 increased HIP–PL coherence across all frequencies, lithium suppressed delta coherence, with no effect at the other frequencies.
Preclinical studies in animal models of disease have provided promising evidence of cognitive improvement as a result of treatment with GSK-3 inhibitors, with beneficial effects of GSK-3 inhibition, either by lithium or selective inhibitors, having been demonstrated in model systems of schizophrenia (Beaulieu et al., 2004; Chan et al., 2012; Lipina et al., 2013; Maksimovic et al., 2014), Fragile-X syndrome (Yuskaitis et al., 2010; Guo et al., 2012; Franklin et al., 2014; Pardo et al., 2017) as well as traumatic brain injury (Yu et al., 2012a,b). In addition, the large number of reports that have focused on AD, as a result of the known role of GSK-3 in AD neuropathology, are also positive showing significant improvements not only in cognitive performance but also in AD-related pathologies (Hu et al., 2009; Sereno et al., 2009; Gong et al., 2011; Onishi et al., 2011; Zhang et al., 2011; Avrahami et al., 2013; Noh et al., 2013; Nunes et al., 2015). In humans, however, the clinical efficacy of these compounds remains less clear, partly because of a lack of studies evaluating the impact of selective GSK-3 inhibitors on cognition and partly due to the large number of conflicting reports from studies evaluating the therapeutic effects of lithium. For example, clinical studies for the most part have demonstrated beneficial effects of lithium in improving cognition or preventing further cognitive decline in AD (Nunes et al., 2013; Matsunaga et al., 2015; Forlenza et al., 2016). Yet, positive effects (Letendre et al., 2006), or no effect (Schifitto et al., 2009; Decloedt et al., 2016) of lithium treatment have been shown for HIV-associated cognitive decline, and in bipolar disorder the findings are even more conflicting with reports showing positive effects (Daglas et al., 2016), little to no effects (Joffe et al., 1988; Bourne et al., 2013; Pfennig et al., 2014), or negative effects (Shaw et al., 1987; Monks et al., 2004; Senturk et al., 2007) of treatment on cognitive function.
In contrast to the numerous studies that have evaluated the effects of GSK-3 inhibition on cognition in neuropsychiatric and neurodegenerative disease, fewer studies have examined the effects of these compounds in healthy animals and humans. In animals, conflicting results due to differing dosing and treatment regimens make their impact on cognitive performance more difficult to define. For example, in mice acute treatment with the GSK-3 inhibitors TDZD-8 or VP0.7, or repeated treatment with SB 216763 every other day for 2 weeks, did not alter spatial learning (Guo et al., 2012; Franklin et al., 2014) whereas treatment with the dual phosphodiesterase-7 and GSK-3 inhibitor VP1.15 improved performance in the spatial object recognition test, the Y-maze task, and cued fear memory (Lipina et al., 2013). In rats, inhibition of GSK-3 following icv infusion of SB 216763 (20 ng/μl) did not affect performance in the Morris water maze (Tian et al., 2012), in contrast to the present findings which clearly showed a cognitive enhancing effect of the drug when repeatedly administered systemically at a dose of 2.5 mg/kg. Similar discrepancies have been reported with lithium with one study showing reduced spatial memory with intra-HIP administration (Parsaei et al., 2016), other studies showing enhanced cognitive performance with lithium treatment (Nocjar et al., 2007; Tsaltas et al., 2007), and other reports showing little or no effect of this compound in cognitive tasks (Vasconcellos et al., 2003; de Vasconcellos et al., 2005; Watase et al., 2007; Dai et al., 2012; Contestabile et al., 2013; King and Jope, 2013). The findings reported herein also showed a lack of effect of lithium in spatial learning using a dose previously shown to increase Akt activity (Zheng et al., 2013), a kinase known to phosphorylate and inhibit GSK-3, although positive trends were observed in some of the parameters evaluated. This lack of an effect of lithium on cognitive performance has also been consistently demonstrated in healthy humans, with some studies also showing lithium-induced mild impairments in various cognitive domains (Weingartner et al., 1985; Linnoila et al., 1986; Stip et al., 2000; Wingo et al., 2009).
The large number of conflicting reports evaluating the effects of lithium on cognition in both healthy and diseased states indicate that any of the observed therapeutic effects of lithium on cognition may not only reflect a combination of different dosing regimens but may be dependent on the disease under investigation. Although one mechanism of action of lithium is the inhibition of GSK-3, it is necessary to keep in mind that lithium has an array of biological substrates which would undoubtedly contribute to the drug’s effects. It is therefore logical that the functional effects of lithium could induce differing behavioral responses a result of interactions with the various neuropathologies under study. Lithium has been shown to inhibit other enzymes such as IMPAs (Berridge et al., 1989), BPNT1 (Spiegelberg et al., 2005), and cyclooxygenase (COX) (Klein and Melton, 1996; Stambolic et al., 1996). Therefore, even if lithium could have positive effects on cognitive processes through GSK-3 inhibition, some of these effects may be offset through its actions at other targets.
Although further research is required to determine the precise mechanisms underlying GSK-3 effects on cognition, one possible mediator is the NMDA receptor. In primary cortical pyramidal neurons or cortical slices GSK-3 was shown to phosphorylate NR2B-containing NMDA receptors, resulting in increased receptor internalization, and leading to a reduction in synaptic current (Chen et al., 2007; Li et al., 2009). Another result of increased GSK-3 activity is the phosphorylation of β-catenin and its subsequent degradation, leading to inhibition of GluN2B gene transcription (Li et al., 2009). Reports indicate that increased GSK-3 activity in might predispose synapses to LTD, suppress long-term potentiation (LTP), and/or shift the threshold of LTP induction through alteration of AMPA and/or NMDA receptor trafficking (Peineau et al., 2007, 2008; Bradley et al., 2012). Interestingly, it has been proposed that inositol depletion by lithium may disrupt LTP through its effects at IMPAs (Berridge et al., 1989), a hypothesis supported by evidence showing a lithium-induced reduction of cellular levels of IP3 (Berridge et al., 1989; Sarkar et al., 2005), a process critical to cytosolic calcium influx and LTP. In addition, inactivation of NMDA receptors of the dorsal hippocampus were proposed to be involved in lithium-induced spatial learning impairment (Parsaei et al., 2016). On the other hand, COX inhibitors have been demonstrated to have positive effects on HIP and cortex-related cognitive functioning in mice (Syed et al., 2015), but inhibit spatial memory retention in rats (Sharifzadeh et al., 2005) suggesting that the interplay between these different lithium targets are complex and make the resulting behavioral outcomes difficult to predict.
One notable similarity between SB 216763 and lithium was that administration of either drug increased HIP and PL theta oscillations, although only SB 216763 increased HIP–PL theta coherence. Theta rhythms are critically associated with movement (Vanderwolf, 1969), are present during behaviors associated with salient stimuli (Tesche and Karhu, 1999), and have been linked to behaviors related to decision-making processes, such as spatial memory in rodents (Jones and Wilson, 2005) or working memory in humans (Kahana et al., 1999; Tesche and Karhu, 1999; Raghavachari et al., 2001). Both repeated SB 216763 or lithium induced a 10 Hz HIP theta spike, although an overall increase in theta power was evident only following SB 216763 administration. The theta rhythm in HIP specifically is known to play a pivotal role in learning and memory function and to impact directly on performance in specific cognitive tasks (see reviews, Harris and Gordon, 2015; Korotkova et al., 2017). For example, disruption of the HIP theta rhythm in rats suppressed initial learning in the water maze (McNaughton et al., 2006), and reduced both working and reference memory performance in a continuous conditional discrimination task (Givens and Olton, 1994). Repeated SB 216763 and lithium administration were also shown to result in increased theta oscillatory activity in PL. Although less is known regarding the role of PFC theta in learning and memory, in human patients mid-frontal cortical theta activity was reported to serve as temporal context for the coordination of networks during learning, specifically within the medial PFC (Mas-Herrero and Marco-Pallares, 2016), and was also suggested to be integral in the regulation of ‘cognitive control’ (Cavanagh and Frank, 2014). SB 216763 increased HIP-PL theta coherence, although a similar change was not observed with lithium treatment. Studies have demonstrated links between HIP–PFC theta coupling with performance in a variety of spatial memory tasks (Jones and Wilson, 2005; Benchenane et al., 2010; Hyman et al., 2010).
A note on methodology: a caveat of the present study was that recordings were taken following water maze training with the rats anesthetized at the time of recording. The study was designed in this manner for two reasons. Firstly, the numerous conflicting reports in evaluating the impact of GSK-3 inhibition on learning and memory in healthy animals indicated we utilize a test in which the animals would be very motivated to complete and at the same time minimize variables that would contribute to alterations in neuronal oscillations, such as food restriction. In line with this, anesthetizing the animals would minimize contributions of neuronal oscillatory changes resulting from stress, fatigue, and consequently movement and alertness, and thus the focus would be predominantly on the exploration of direct drug effects. Clearly further characterization of GSK-3 effects on oscillations and their correlation to behavioral output would require recordings to occur in awake freely behaving animals. In addition, it is important to keep in mind that normalization of the spectra conserves the total power across the entire spectrum. Thus, what is represented are not changes in absolute values, but rather changes in the shape of the curve following drug treatment. Yet, despite these limitations, these findings lay the groundwork for a potential new avenue of research evaluating in depth the relationship between GSK-3 activity and neuronal oscillations in specific regions of the brain and the importance of this relationship in central nervous system disorders.
Conclusion
The present findings point to a previously unknown role for GSK-3 in the regulation of neuronal HIP and PL theta oscillations in brain that could provide a lead into what could happen if GSK-3 activity became abnormally increased in a pathophysiologic state. Although conflicting reports of the therapeutic effects of lithium on cognition currently raise caution as to the therapeutic benefit of this drug in cognitive dysfunction disorders, furthering our understanding of the exact mechanisms that underlie the disparate effects of lithium on learning and memory would clearly be beneficial given its widespread use in the treatment of neuropsychiatric disorders. This idea is strengthened by the present findings which showed that direct inhibition of GSK-3 improved spatial memory and enhanced theta oscillations in both HIP and PL, two regions integral to cognitive function.
Author Contributions
MP assisted with the surgeries, designed and performed the experiments, analyzed the data, prepared the figures and wrote the manuscript. TN and TF performed the surgeries and the experiments. SG contributed to the writing of the manuscript.
Funding
This work was supported by a grant from the W. Garfield Weston Foundation (to MP and SG) (grant #: RR150081).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
SG holds a Canada Research Chair in Molecular Neuroscience.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2017.00434/full#supplementary-material
References
Al Banchaabouchi, M., Peña de Ortiz, S., Menendez, R., Ren, K., and Maldonado-Vlaar, C. S. (2004). Chronic lithium decreases Nurr1 expression in the rat brain and impairs spatial discrimination. Pharmacol. Biochem. Behav. 79, 607–621. doi: 10.1016/j.pbb.2004.09.015
Ances, B. M., Letendre, S. L., Alexander, T., and Ellis, R. J. (2008). Role of psychiatric medications as adjunct therapy in the treatment of HIV associated neurocognitive disorders. Int. Rev. Psychiatry 20, 89–93. doi: 10.1080/09540260701877670
Avrahami, L., Farfara, D., Shaham-Kol, M., Vassar, R., Frenkel, D., and Eldar-Finkelman, H. (2013). Inhibition of glycogen synthase kinase-3 ameliorates beta-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J. Biol. Chem. 288, 1295–1306. doi: 10.1074/jbc.M112.409250
Balaraman, Y., Limaye, A. R., Levey, A. I., and Srinivasan, S. (2006). Glycogen synthase kinase 3beta and Alzheimer’s disease: pathophysiological and therapeutic significance. Cell. Mol. Life Sci. 63, 1226–1235. doi: 10.1007/s00018-005-5597-y
Basar, E., Basar-Eroglu, C., Guntekin, B., and Yener, G. G. (2013). Brain’s alpha, beta, gamma, delta, and theta oscillations in neuropsychiatric diseases: proposal for biomarker strategies. Suppl. Clin. Neurophysiol. 62, 19–54. doi: 10.1016/B978-0-7020-5307-8.00002-8
Basar, E., Schmiedt-Fehr, C., Mathes, B., Femir, B., Emek-Savas, D. D., Tulay, E., et al. (2016). What does the broken brain say to the neuroscientist? Oscillations and connectivity in schizophrenia, Alzheimer’s disease, and bipolar disorder. Int. J. Psychophysiol. 103, 135–148. doi: 10.1016/j.ijpsycho.2015.02.004
Basar-Eroglu, C., Brand, A., Hildebrandt, H., Karolina Kedzior, K., Mathes, B., and Schmiedt, C. (2007). Working memory related gamma oscillations in schizophrenia patients. Int. J. Psychophysiol. 64, 39–45. doi: 10.1016/j.ijpsycho.2006.07.007
Beaulieu, J. M., Sotnikova, T. D., Yao, W. D., Kockeritz, L., Woodgett, J. R., Gainetdinov, R. R., et al. (2004). Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. U.S.A. 101, 5099–5104. doi: 10.1073/pnas.0307921101
Benchenane, K., Peyrache, A., Khamassi, M., Tierney, P. L., Gioanni, Y., Battaglia, F. P., et al. (2010). Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66, 921–936. doi: 10.1016/j.neuron.2010.05.013
Berridge, M. J., Downes, C. P., and Hanley, M. R. (1989). Neural and developmental actions of lithium: a unifying hypothesis. Cell 59, 411–419. doi: 10.1016/0092-8674(89)90026-3
Blasi, G., Napolitano, F., Ursini, G., Di Giorgio, A., Caforio, G., Taurisano, P., et al. (2013). Association of GSK-3beta genetic variation with GSK-3beta expression, prefrontal cortical thickness, prefrontal physiology, and schizophrenia. Am. J. Psychiatry 170, 868–876. doi: 10.1176/appi.ajp.2012.12070908
Bloom, G. S. (2014). Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 71, 505–508. doi: 10.1001/jamaneurol.2013.5847
Bourne, C., Aydemir, O., Balanza-Martinez, V., Bora, E., Brissos, S., Cavanagh, J. T., et al. (2013). Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr. Scand. 128, 149–162. doi: 10.1111/acps.12133
Bradley, C. A., Peineau, S., Taghibiglou, C., Nicolas, C. S., Whitcomb, D. J., Bortolotto, Z. A., et al. (2012). A pivotal role of GSK-3 in synaptic plasticity. Front. Mol. Neurosci. 5:13. doi: 10.3389/fnmol.2012.00013
Broersen, L. M. (2000). Attentional processes and learning and memory in rats: the prefrontal cortex and hippocampus compared. Prog. Brain Res. 126, 79–94. doi: 10.1016/S0079-6123(00)26008-1
Buzsaki, G., Logothetis, N., and Singer, W. (2013). Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 80, 751–764. doi: 10.1016/j.neuron.2013.10.002
Cavanagh, J. F., and Frank, M. J. (2014). Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 18, 414–421. doi: 10.1016/j.tics.2014.04.012
Chan, M. H., Chiu, P. H., Lin, C. Y., and Chen, H. H. (2012). Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine. Schizophr. Res. 136, 96–103. doi: 10.1016/j.schres.2012.01.024
Chen, P., Gu, Z., Liu, W., and Yan, Z. (2007). Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Mol. Pharmacol. 72, 40–51. doi: 10.1124/mol.107.034942
Clapcote, S. J., Lipina, T. V., Millar, J. K., Mackie, S., Christie, S., Ogawa, F., et al. (2007). Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 54, 387–402. doi: 10.1016/j.neuron.2007.04.015
Cohen, M. X. (2011). Hippocampal-prefrontal connectivity predicts midfrontal oscillations and long-term memory performance. Curr. Biol. 21, 1900–1905. doi: 10.1016/j.cub.2011.09.036
Contestabile, A., Greco, B., Ghezzi, D., Tucci, V., Benfenati, F., and Gasparini, L. (2013). Lithium rescues synaptic plasticity and memory in Down syndrome mice. J. Clin. Invest. 123, 348–361. doi: 10.1172/JCI64650
Crofton, E. J., Nenov, M. N., Zhang, Y., Scala, F., Page, S. A., McCue, D. L., et al. (2017). Glycogen synthase kinase 3 beta alters anxiety-, depression-, and addiction-related behaviors and neuronal activity in the nucleus accumbens shell. Neuropharmacology 117, 49–60. doi: 10.1016/j.neuropharm.2017.01.020
Daglas, R., Cotton, S. M., Allott, K., Yucel, M., Macneil, C. A., Hasty, M. K., et al. (2016). A single-blind, randomised controlled trial on the effects of lithium and quetiapine monotherapy on the trajectory of cognitive functioning in first episode mania: a 12-month follow-up study. Eur. Psychiatry 31, 20–28. doi: 10.1016/j.eurpsy.2015.09.460
Dai, M., Freeman, B., Shikani, H. J., Bruno, F. P., Collado, J. E., Macias, R., et al. (2012). Altered regulation of Akt signaling with murine cerebral malaria, effects on long-term neuro-cognitive function, restoration with lithium treatment. PLOS ONE 7:e44117. doi: 10.1371/journal.pone.0044117
Dash, P. K., Orsi, S. A., Zhang, M., Grill, R. J., Pati, S., Zhao, J., et al. (2010). Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLOS ONE 5:e11383. doi: 10.1371/journal.pone.0011383
de Vasconcellos, A. P., Zugno, A. I., Dos Santos, A. H., Nietto, F. B., Crema, L. M., Goncalves, M., et al. (2005). Na+,K+-ATPase activity is reduced in hippocampus of rats submitted to an experimental model of depression: effect of chronic lithium treatment and possible involvement in learning deficits. Neurobiol. Learn. Mem. 84, 102–110. doi: 10.1016/j.nlm.2005.05.002
Decloedt, E. H., Freeman, C., Howells, F., Casson-Crook, M., Lesosky, M., Koutsilieri, E., et al. (2016). Moderate to severe HIV-associated neurocognitive impairment: a randomized placebo-controlled trial of lithium. Medicine 95:e5401. doi: 10.1097/MD.0000000000005401
Emamian, E. S., Hall, D., Birnbaum, M. J., Karayiorgou, M., and Gogos, J. A. (2004). Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat. Genet. 36, 131–137. doi: 10.1038/ng1296
Forlenza, O. V., Aprahamian, I., de Paula, V. J., and Hajek, T. (2016). Lithium, a therapy for AD: current evidence from clinical trials of neurodegenerative disorders. Curr. Alzheimer Res. 13, 879–886. doi: 10.2174/1567205013666160219112854
Franklin, A. V., King, M. K., Palomo, V., Martinez, A., McMahon, L. L., and Jope, R. S. (2014). Glycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragile X mice. Biol. Psychiatry 75, 198–206. doi: 10.1016/j.biopsych.2013.08.003
Givens, B., and Olton, D. S. (1994). Local modulation of basal forebrain: effects on working and reference memory. J. Neurosci. 14, 3578–3587.
Goldfarb, M., Schoorlemmer, J., Williams, A., Diwakar, S., Wang, Q., Huang, X., et al. (2007). Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron 55, 449–463. doi: 10.1016/j.neuron.2007.07.006
Gong, E. J., Park, H. R., Kim, M. E., Piao, S., Lee, E., Jo, D. G., et al. (2011). Morin attenuates tau hyperphosphorylation by inhibiting GSK3beta. Neurobiol. Dis. 44, 223–230. doi: 10.1016/j.nbd.2011.07.005
Graybeal, C., Feyder, M., Schulman, E., Saksida, L. M., Bussey, T. J., Brigman, J. L., et al. (2011). Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat. Neurosci. 14, 1507–1509. doi: 10.1038/nn.2954
Grimes, C. A., and Jope, R. S. (2001). The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 65, 391–426. doi: 10.1016/S0301-0082(01)00011-9
Guadagna, S., Bundgaard, C., Hovelso, N., Volbracht, C., Francis, P. T., Egebjerg, J., et al. (2012). Memantine potentiates hippocampal theta oscillations at a therapeutic dose in anesthetized mice: a mechanistic link to its cognitive-enhancing properties. Neuropharmacology 62, 2208–2218. doi: 10.1016/j.neuropharm.2012.01.014
Guo, W., Murthy, A. C., Zhang, L., Johnson, E. B., Schaller, E. G., Allan, A. M., et al. (2012). Inhibition of GSK3beta improves hippocampus-dependent learning and rescues neurogenesis in a mouse model of fragile X syndrome. Hum. Mol. Genet. 21, 681–691. doi: 10.1093/hmg/ddr501
Guo, W. P., Fu, X. G., Jiang, S. M., and Wu, J. Z. (2010). Neuregulin-1 regulates the expression of Akt, Bcl-2, and Bad signaling after focal cerebral ischemia in rats. Biochem. Cell Biol. 88, 649–654. doi: 10.1139/O09-189
Harris, A. Z., and Gordon, J. A. (2015). Long-range neural synchrony in behavior. Annu. Rev. Neurosci. 38, 171–194. doi: 10.1146/annurev-neuro-071714-034111
Helfrich, R. F., and Knight, R. T. (2016). Oscillatory dynamics of prefrontal cognitive control. Trends Cogn. Sci. 20, 916–930. doi: 10.1016/j.tics.2016.09.007
Hernandez, F., Borrell, J., Guaza, C., Avila, J., and Lucas, J. J. (2002). Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J. Neurochem. 83, 1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x
Herrmann, C. S., and Demiralp, T. (2005). Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 116, 2719–2733. doi: 10.1016/j.clinph.2005.07.007
Howlett, D. R., Richardson, J. C., Austin, A., Parsons, A. A., Bate, S. T., Davies, D. C., et al. (2004). Cognitive correlates of Abeta deposition in male and female mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. Brain Res. 1017, 130–136. doi: 10.1016/j.brainres.2004.05.029
Hu, S., Begum, A. N., Jones, M. R., Oh, M. S., Beech, W. K., Beech, B. H., et al. (2009). GSK3 inhibitors show benefits in an Alzheimer’s disease (AD) model of neurodegeneration but adverse effects in control animals. Neurobiol. Dis. 33, 193–206. doi: 10.1016/j.nbd.2008.10.007
Huang, H. C., and Klein, P. S. (2006). Multiple roles for glycogen synthase kinase-3 as a drug target in Alzheimer’s disease. Curr. Drug Targets 7, 1389–1397. doi: 10.2174/1389450110607011389
Hyman, J. M., Zilli, E. A., Paley, A. M., and Hasselmo, M. E. (2010). Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Front. Integr. Neurosci. 4:2. doi: 10.3389/neuro.07.002.2010
Igarashi, K. M. (2015). Plasticity in oscillatory coupling between hippocampus and cortex. Curr. Opin. Neurobiol. 35, 163–168. doi: 10.1016/j.conb.2015.09.005
James, T. F., Nenov, M. N., Wildburger, N. C., Lichti, C. F., Luisi, J., Vergara, F., et al. (2015). The Nav1.2 channel is regulated by GSK3. Biochim. Biophys. Acta 1850, 832–844. doi: 10.1016/j.bbagen.2015.01.011
Joffe, R. T., MacDonald, C., and Kutcher, S. P. (1988). Lack of differential cognitive effects of lithium and carbamazepine in bipolar affective disorder. J. Clin. Psychopharmacol. 8, 425–428. doi: 10.1097/00004714-198812000-00008
Jones, M. W., and Wilson, M. A. (2005). Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLOS Biol. 3:e402. doi: 10.1371/journal.pbio.0030402
Kahana, M. J., Sekuler, R., Caplan, J. B., Kirschen, M., and Madsen, J. R. (1999). Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399, 781–784. doi: 10.1038/21645
Kalweit, A. N., Amanpour-Gharaei, B., Colitti-Klausnitzer, J., and Manahan-Vaughan, D. (2017). Changes in neuronal oscillations accompany the loss of hippocampal LTP that occurs in an animal model of psychosis. Front. Behav. Neurosci. 11:36. doi: 10.3389/fnbeh.2017.00036
Keri, S., Seres, I., Kelemen, O., and Benedek, G. (2009). Neuregulin 1-stimulated phosphorylation of AKT in psychotic disorders and its relationship with neurocognitive functions. Neurochem. Int. 55, 606–609. doi: 10.1016/j.neuint.2009.06.002
King, M. K., and Jope, R. S. (2013). Lithium treatment alleviates impaired cognition in a mouse model of fragile X syndrome. Genes Brain Behav. 12, 723–731. doi: 10.1111/gbb.12071
King, M. R., Anderson, N. J., Guernsey, L. S., and Jolivalt, C. G. (2013). Glycogen synthase kinase-3 inhibition prevents learning deficits in diabetic mice. J. Neurosci. Res. 91, 506–514. doi: 10.1002/jnr.23192
Kinney, G. G., Patino, P., Mermet-Bouvier, Y., Starrett, J. E. Jr., and Gribkoff, V. K. (1999). Cognition-enhancing drugs increase stimulated hippocampal theta rhythm amplitude in the urethane-anesthetized rat. J. Pharmacol. Exp. Ther. 291, 99–106.
Klein, P. S., and Melton, D. A. (1996). A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U.S.A. 93, 8455–8459. doi: 10.1073/pnas.93.16.8455
Korotkova, T., Ponomarenko, A., Monaghan, C. K., Poulter, S. L., Cacucci, F., Wills, T., et al. (2017). Reconciling the different faces of hippocampal theta: the role of theta oscillations in cognitive, emotional and innate behaviors. Neurosci. Biobehav. Rev. doi: 10.1016/j.neubiorev.2017.09.004 [Epub ahead of print].
Kwon, J. S., O’Donnell, B. F., Wallenstein, G. V., Greene, R. W., Hirayasu, Y., Nestor, P. G., et al. (1999). Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch. Gen. Psychiatry 56, 1001–1005. doi: 10.1001/archpsyc.56.11.1001
Lee, J., Hudson, M. R., O’Brien, T. J., Nithianantharajah, J., and Jones, N. C. (2017). Local NMDA receptor hypofunction evokes generalized effects on gamma and high-frequency oscillations and behavior. Neuroscience 358, 124–136. doi: 10.1016/j.neuroscience.2017.06.039
Lemercier, C. E., Holman, C., and Gerevich, Z. (2017). Aberrant alpha and gamma oscillations ex vivo after single application of the NMDA receptor antagonist MK-801. Schizophr. Res. 188, 118–124. doi: 10.1016/j.schres.2017.01.017
Letendre, S. L., Woods, S. P., Ellis, R. J., Atkinson, J. H., Masliah, E., van den Brande, G., et al. (2006). Lithium improves HIV-associated neurocognitive impairment. AIDS 20, 1885–1888. doi: 10.1097/01.aids.0000244208.49123.1b
Li, M., Mo, Y., Luo, X. J., Xiao, X., Shi, L., Peng, Y. M., et al. (2011). Genetic association and identification of a functional SNP at GSK3beta for schizophrenia susceptibility. Schizophr. Res. 133, 165–171. doi: 10.1016/j.schres.2011.09.013
Li, Y. C., Xi, D., Roman, J., Huang, Y. Q., and Gao, W. J. (2009). Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J. Neurosci. 29, 15551–15563. doi: 10.1523/JNEUROSCI.3336-09.2009
Linnoila, M., Rudorfer, M. V., Dubyoski, K. V., Rawlings, R. R., and Eckardt, M. J. (1986). Effects of one-week lithium treatment on skilled performance, information processing, and mood in healthy volunteers. J. Clin. Psychopharmacol. 6, 356–359. doi: 10.1097/00004714-198612000-00006
Lipina, T. V., Kaidanovich-Beilin, O., Patel, S., Wang, M., Clapcote, S. J., Liu, F., et al. (2011). Genetic and pharmacological evidence for schizophrenia-related Disc1 interaction with GSK-3. Synapse 65, 234–248. doi: 10.1002/syn.20839
Lipina, T. V., Palomo, V., Gil, C., Martinez, A., and Roder, J. C. (2013). Dual inhibitor of PDE7 and GSK-3-VP1.15 acts as antipsychotic and cognitive enhancer in C57BL/6J mice. Neuropharmacology 64, 205–214. doi: 10.1016/j.neuropharm.2012.06.032
Lipina, T. V., Wang, M., Liu, F., and Roder, J. C. (2012). Synergistic interactions between PDE4B and GSK-3: DISC1 mutant mice. Neuropharmacology 62, 1252–1262. doi: 10.1016/j.neuropharm.2011.02.020
Liu, L., Wong, T. P., Pozza, M. F., Lingenhoehl, K., Wang, Y., Sheng, M., et al. (2004). Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304, 1021–1024. doi: 10.1126/science.1096615
Liu, S. J., Zhang, A. H., Li, H. L., Wang, Q., Deng, H. M., Netzer, W. J., et al. (2003). Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J. Neurochem. 87, 1333–1344. doi: 10.1046/j.1471-4159.2003.02070.x
Llorens-Martin, M., Jurado, J., Hernandez, F., and Avila, J. (2014). GSK-3beta, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 7:46. doi: 10.3389/fnmol.2014.00046
Lodge, D. J., Behrens, M. M., and Grace, A. A. (2009). A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J. Neurosci. 29, 2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009
Logothetis, N. K. (2003). The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci. 23, 3963–3971.
Maksimovic, M., Vekovischeva, O. Y., Aitta-aho, T., and Korpi, E. R. (2014). Chronic treatment with mood-stabilizers attenuates abnormal hyperlocomotion of GluA1-subunit deficient mice. PLOS ONE 9:e100188. doi: 10.1371/journal.pone.0100188
Malhi, G. S., Tanious, M., Das, P., Coulston, C. M., and Berk, M. (2013). Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs 27, 135–153. doi: 10.1007/s40263-013-0039-0
Mao, Y., Ge, X., Frank, C. L., Madison, J. M., Koehler, A. N., Doud, M. K., et al. (2009). Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136, 1017–1031. doi: 10.1016/j.cell.2008.12.044
Maqbool, M., Mobashir, M., and Hoda, N. (2016). Pivotal role of glycogen synthase kinase-3: a therapeutic target for Alzheimer’s disease. Eur. J. Med. Chem. 107, 63–81. doi: 10.1016/j.ejmech.2015.10.018
Martinez, A., and Perez, D. I. (2008). GSK-3 inhibitors: a ray of hope for the treatment of Alzheimer’s disease? J. Alzheimers Dis. 15, 181–191. doi: 10.3233/JAD-2008-15204
Mas-Herrero, E., and Marco-Pallares, J. (2016). Theta oscillations integrate functionally segregated sub-regions of the medial prefrontal cortex. Neuroimage 143, 166–174. doi: 10.1016/j.neuroimage.2016.08.024
Massey, P. V., Johnson, B. E., Moult, P. R., Auberson, Y. P., Brown, M. W., Molnar, E., et al. (2004). Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J. Neurosci. 24, 7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004
Matsunaga, S., Kishi, T., Annas, P., Basun, H., Hampel, H., and Iwata, N. (2015). Lithium as a treatment for Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis. 48, 403–410. doi: 10.3233/JAD-150437
McNaughton, N., Ruan, M., and Woodnorth, M. A. (2006). Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus 16, 1102–1110. doi: 10.1002/hipo.20235
Medina, M., and Avila, J. (2010). Glycogen synthase kinase-3 (GSK-3) inhibitors for the treatment of Alzheimer’s disease. Curr. Pharm. Des. 16, 2790–2798. doi: 10.2174/138161210793176581
Mesbah-Oskui, L., Georgiou, J., and Roder, J. C. (2015). Hippocampal place cell and inhibitory neuron activity in disrupted-in-schizophrenia-1 mutant mice: implications for working memory deficits. NPJ Schizophr. 1:15011. doi: 10.1038/npjschz.2015.11
Miller, A. M. P., Frick, B. J., Smith, D. M., Radulovic, J., and Corcoran, K. A. (2017). Network oscillatory activity driven by context memory processing is differently regulated by glutamatergic and cholinergic neurotransmission. Neurobiol. Learn. Mem. 145, 59–66. doi: 10.1016/j.nlm.2017.08.010
Missonnier, P., Herrmann, F. R., Michon, A., Fazio-Costa, L., Gold, G., and Giannakopoulos, P. (2010). Early disturbances of gamma band dynamics in mild cognitive impairment. J. Neural Transm. 117, 489–498. doi: 10.1007/s00702-010-0384-9
Mitzdorf, U. (1985). Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev. 65, 37–100.
Monks, P. J., Thompson, J. M., Bullmore, E. T., Suckling, J., Brammer, M. J., Williams, S. C., et al. (2004). A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord. 6, 550–564. doi: 10.1111/j.1399-5618.2004.00147.x
Muyllaert, D., Kremer, A., Jaworski, T., Borghgraef, P., Devijver, H., Croes, S., et al. (2008). Glycogen synthase kinase-3beta, or a link between amyloid and tau pathology? Genes Brain Behav. 7(Suppl. 1), 57–66. doi: 10.1111/j.1601-183X.2007.00376.x
Nakazono, T., Lam, T. N., Patel, A. Y., Kitazawa, M., Saito, T., Saido, T. C., et al. (2017). Impaired in vivo gamma oscillations in the medial entorhinal cortex of knock-in Alzheimer model. Front. Syst. Neurosci. 11:48. doi: 10.3389/fnsys.2017.00048
Nocjar, C., Hammonds, M. D., and Shim, S. S. (2007). Chronic lithium treatment magnifies learning in rats. Neuroscience 150, 774–788. doi: 10.1016/j.neuroscience.2007.09.063
Noh, M. Y., Chun, K., Kang, B. Y., Kim, H., Park, J. S., Lee, H. C., et al. (2013). Newly developed glycogen synthase kinase-3 (GSK-3) inhibitors protect neuronal cells death in amyloid-beta induced cell model and in a transgenic mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 435, 274–281. doi: 10.1016/j.bbrc.2013.04.065
Numakawa, T., Yagasaki, Y., Ishimoto, T., Okada, T., Suzuki, T., Iwata, N., et al. (2004). Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum. Mol. Genet. 13, 2699–2708. doi: 10.1093/hmg/ddh280
Nunes, M. A., Schowe, N. M., Monteiro-Silva, K. C., Baraldi-Tornisielo, T., Souza, S. I., Balthazar, J., et al. (2015). Chronic microdose lithium treatment prevented memory loss and neurohistopathological changes in a transgenic mouse model of Alzheimer’s disease. PLOS ONE 10:e0142267. doi: 10.1371/journal.pone.0142267
Nunes, M. A., Viel, T. A., and Buck, H. S. (2013). Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr. Alzheimer Res. 10, 104–107.
O’Neill, P. K., Gordon, J. A., and Sigurdsson, T. (2013). Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J. Neurosci. 33, 14211–14224. doi: 10.1523/JNEUROSCI.2378-13.2013
Onishi, T., Iwashita, H., Uno, Y., Kunitomo, J., Saitoh, M., Kimura, E., et al. (2011). A novel glycogen synthase kinase-3 inhibitor 2-methyl-5-(3-{4-[(S)-methylsulfinyl]phenyl}-1-benzofuran-5-yl)-1,3,4-oxadiazole decreases tau phosphorylation and ameliorates cognitive deficits in a transgenic model of Alzheimer’s disease. J. Neurochem. 119, 1330–1340. doi: 10.1111/j.1471-4159.2011.07532.x
Pardo, M., Beurel, E., and Jope, R. S. (2017). Cotinine administration improves impaired cognition in the mouse model of Fragile X syndrome. Eur. J. Neurosci. 45, 490–498. doi: 10.1111/ejn.13446
Parsaei, L., Torkaman-Boutorabi, A., Asadi, F., and Zarrindast, M. R. (2016). Interaction between dorsal hippocampal NMDA receptors and lithium on spatial learning consolidation in rats. Brain Res. Bull. 127, 1–10. doi: 10.1016/j.brainresbull.2016.07.007
Paxinos, G., and Watson, C. (2005). The Rat Brain in Stereotaxic Coordinates, 5th Edn. New York, NY: Academic Press.
Peineau, S., Bradley, C., Taghibiglou, C., Doherty, A., Bortolotto, Z. A., Wang, Y. T., et al. (2008). The role of GSK-3 in synaptic plasticity. Br. J. Pharmacol. 153(Suppl. 1), S428–S437. doi: 10.1038/bjp.2008.2
Peineau, S., Taghibiglou, C., Bradley, C., Wong, T. P., Liu, L., Lu, J., et al. (2007). LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53, 703–717. doi: 10.1016/j.neuron.2007.01.029
Pesaran, B., Pezaris, J. S., Sahani, M., Mitra, P. P., and Andersen, R. A. (2002). Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat. Neurosci. 5, 805–811. doi: 10.1038/nn890
Pfennig, A., Alda, M., Young, T., MacQueen, G., Rybakowski, J., Suwalska, A., et al. (2014). Prophylactic lithium treatment and cognitive performance in patients with a long history of bipolar illness: no simple answers in complex disease-treatment interplay. Int. J. Bipolar Disord. 2:16. doi: 10.1186/s40345-014-0016-7
Raghavachari, S., Kahana, M. J., Rizzuto, D. S., Caplan, J. B., Kirschen, M. P., Bourgeois, B., et al. (2001). Gating of human theta oscillations by a working memory task. J. Neurosci. 21, 3175–3183.
Rama, S., Zbili, M., Bialowas, A., Fronzaroli-Molinieres, L., Ankri, N., Carlier, E., et al. (2015). Presynaptic hyperpolarization induces a fast analogue modulation of spike-evoked transmission mediated by axonal sodium channels. Nat. Commun. 6:10163. doi: 10.1038/ncomms10163
Roux, F., and Uhlhaas, P. J. (2014). Working memory and neural oscillations: alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cogn. Sci. 18, 16–25. doi: 10.1016/j.tics.2013.10.010
Sarkar, S., Floto, R. A., Berger, Z., Imarisio, S., Cordenier, A., Pasco, M., et al. (2005). Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 170, 1101–1111. doi: 10.1083/jcb.200504035
Schifitto, G., Zhong, J., Gill, D., Peterson, D. R., Gaugh, M. D., Zhu, T., et al. (2009). Lithium therapy for human immunodeficiency virus type 1-associated neurocognitive impairment. J. Neurovirol. 15, 176–186. doi: 10.1080/13550280902758973
Schmiedt, C., Brand, A., Hildebrandt, H., and Basar-Eroglu, C. (2005). Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res. Cogn. Brain Res. 25, 936–947. doi: 10.1016/j.cogbrainres.2005.09.015
Scott, L., Feng, J., Kiss, T., Needle, E., Atchison, K., Kawabe, T. T., et al. (2012). Age-dependent disruption in hippocampal theta oscillation in amyloid-beta overproducing transgenic mice. Neurobiol. Aging 33, 481.e13–e23. doi: 10.1016/j.neurobiolaging.2011.12.010
Senturk, V., Goker, C., Bilgic, A., Olmez, S., Tugcu, H., Oncu, B., et al. (2007). Impaired verbal memory and otherwise spared cognition in remitted bipolar patients on monotherapy with lithium or valproate. Bipolar Disord. 9(Suppl. 1), 136–144. doi: 10.1111/j.1399-5618.2007.00481.x
Sereno, L., Coma, M., Rodriguez, M., Sanchez-Ferrer, P., Sanchez, M. B., Gich, I., et al. (2009). A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol. Dis. 35, 359–367. doi: 10.1016/j.nbd.2009.05.025
Seres, I., Kelemen, O., Benedek, G., and Keri, S. (2010). Neuregulin 1-induced AKT phosphorylation in monozygotic twins discordant for schizophrenia. Neurochem. Int. 56, 906–910. doi: 10.1016/j.neuint.2010.03.018
Sharifzadeh, M., Naghdi, N., Khosrovani, S., Ostad, S. N., Sharifzadeh, K., and Roghani, A. (2005). Post-training intrahippocampal infusion of the COX-2 inhibitor celecoxib impaired spatial memory retention in rats. Eur. J. Pharmacol. 511, 159–166. doi: 10.1016/j.ejphar.2005.01.041
Shavkunov, A. S., Wildburger, N. C., Nenov, M. N., James, T. F., Buzhdygan, T. P., Panova-Elektronova, N. I., et al. (2013). The fibroblast growth factor 14.voltage-gated sodium channel complex is a new target of glycogen synthase kinase 3 (GSK3). J. Biol. Chem. 288, 19370–19385. doi: 10.1074/jbc.M112.445924
Shaw, E. D., Stokes, P. E., Mann, J. J., and Manevitz, A. Z. (1987). Effects of lithium carbonate on the memory and motor speed of bipolar outpatients. J. Abnorm. Psychol. 96, 64–69. doi: 10.1037/0021-843X.96.1.64
Spiegelberg, B. D., Dela Cruz, J., Law, T. H., and York, J. D. (2005). Alteration of lithium pharmacology through manipulation of phosphoadenosine phosphate metabolism. J. Biol. Chem. 280, 5400–5405. doi: 10.1074/jbc.M407890200
Stambolic, V., Ruel, L., and Woodgett, J. R. (1996). Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6, 1664–1668. doi: 10.1016/S0960-9822(02)70790-2
Stip, E., Dufresne, J., Lussier, I., and Yatham, L. (2000). A double-blind, placebo-controlled study of the effects of lithium on cognition in healthy subjects: mild and selective effects on learning. J. Affect. Disord. 60, 147–157. doi: 10.1016/S0165-0327(99)00178-0
Syed, H., Ikram, M. F., Yaqinuddin, A., and Ahmed, T. (2015). Cyclooxygenase I and II inhibitors distinctly enhance hippocampal- and cortex-dependent cognitive functions in mice. Mol. Med. Rep. 12, 7649–7656. doi: 10.3892/mmr.2015.4351
Takashima, A. (2006). GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J. Alzheimers Dis. 9(Suppl. 3), 309–317. doi: 10.3233/JAD-2006-9S335
Tamura, M., Mukai, J., Gordon, J. A., and Gogos, J. A. (2016). Developmental inhibition of Gsk3 rescues behavioral and neurophysiological deficits in a mouse model of schizophrenia predisposition. Neuron 89, 1100–1109. doi: 10.1016/j.neuron.2016.01.025
Tang, H., Shen, N., Jin, H., Liu, D., Miao, X., and Zhu, L. Q. (2013). GSK-3beta polymorphism discriminates bipolar disorder and schizophrenia: a systematic meta-analysis. Mol. Neurobiol. 48, 404–411. doi: 10.1007/s12035-013-8414-x
Tesche, C. D., and Karhu, J. (1999). Interactive processing of sensory input and motor output in the human hippocampus. J. Cogn. Neurosci. 11, 424–436. doi: 10.1162/089892999563517
Tian, M., Zhu, D., Xie, W., and Shi, J. (2012). Central angiotensin II-induced Alzheimer-like tau phosphorylation in normal rat brains. FEBS Lett. 586, 3737–3745. doi: 10.1016/j.febslet.2012.09.004
Tsaltas, E., Kontis, D., Boulougouris, V., Papakosta, V. M., Giannou, H., Poulopoulou, C., et al. (2007). Enhancing effects of chronic lithium on memory in the rat. Behav. Brain Res. 177, 51–60. doi: 10.1016/j.bbr.2006.11.003
Uhlhaas, P. J., Haenschel, C., Nikolic, D., and Singer, W. (2008). The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 34, 927–943. doi: 10.1093/schbul/sbn062
Uhlhaas, P. J., and Singer, W. (2010). Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 11, 100–113. doi: 10.1038/nrn2774
Vanderwolf, C. H. (1969). Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 26, 407–418. doi: 10.1016/0013-4694(69)90092-3
Vasconcellos, A. P., Tabajara, A. S., Ferrari, C., Rocha, E., and Dalmaz, C. (2003). Effect of chronic stress on spatial memory in rats is attenuated by lithium treatment. Physiol. Behav. 79, 143–149. doi: 10.1016/S0031-9384(03)00113-6
Verret, L., Mann, E. O., Hang, G. B., Barth, A. M., Cobos, I., Ho, K., et al. (2012). Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721. doi: 10.1016/j.cell.2012.02.046
Villette, V., Poindessous-Jazat, F., Simon, A., Lena, C., Roullot, E., Bellessort, B., et al. (2010). Decreased rhythmic GABAergic septal activity and memory-associated theta oscillations after hippocampal amyloid-beta pathology in the rat. J. Neurosci. 30, 10991–11003. doi: 10.1523/JNEUROSCI.6284-09.2010
Wang, X., and Zhao, L. (2016). Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3beta signaling pathway. Biochem. Biophys. Res. Commun. 473, 428–434. doi: 10.1016/j.bbrc.2016.03.024
Watase, K., Gatchel, J. R., Sun, Y., Emamian, E., Atkinson, R., Richman, R., et al. (2007). Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLOS Med. 4:e182. doi: 10.1371/journal.pmed.0040182
Weingartner, H., Rudorfer, M. V., and Linnoila, M. (1985). Cognitive effects of lithium treatment in normal volunteers. Psychopharmacology 86, 472–474. doi: 10.1007/BF00427911
Wickens, R. H., Quartarone, S. E., and Beninger, R. J. (2017). Inhibition of glycogen synthase kinase-3 by SB 216763 affects acquisition at lower doses than expression of amphetamine-conditioned place preference in rats. Behav. Pharmacol. 28, 262–271. doi: 10.1097/FBP.0000000000000283
Wildburger, N. C., and Laezza, F. (2012). Control of neuronal ion channel function by glycogen synthase kinase-3: new prospective for an old kinase. Front. Mol. Neurosci. 5:80. doi: 10.3389/fnmol.2012.00080
Wingo, A. P., Wingo, T. S., Harvey, P. D., and Baldessarini, R. J. (2009). Effects of lithium on cognitive performance: a meta-analysis. J. Clin. Psychiatry 70, 1588–1597. doi: 10.4088/JCP.08r04972
Yanagita, T., Maruta, T., Nemoto, T., Uezono, Y., Matsuo, K., Satoh, S., et al. (2009). Chronic lithium treatment up-regulates cell surface NaV1.7 sodium channels via inhibition of glycogen synthase kinase-3 in adrenal chromaffin cells: enhancement of Na+ influx, Ca2+ influx and catecholamine secretion after lithium withdrawal. Neuropharmacology 57, 311–321. doi: 10.1016/j.neuropharm.2009.05.006
Yu, F., Wang, Z., Tchantchou, F., Chiu, C. T., Zhang, Y., and Chuang, D. M. (2012a). Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J. Neurotrauma 29, 362–374. doi: 10.1089/neu.2011.1942
Yu, F., Zhang, Y., and Chuang, D. M. (2012b). Lithium reduces BACE1 overexpression, beta amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. J. Neurotrauma 29, 2342–2351. doi: 10.1089/neu.2012.2449
Yuskaitis, C. J., Mines, M. A., King, M. K., Sweatt, J. D., Miller, C. A., and Jope, R. S. (2010). Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem. Pharmacol. 79, 632–646. doi: 10.1016/j.bcp.2009.09.023
Zhang, X., Heng, X., Li, T., Li, L., Yang, D., Zhang, X., et al. (2011). Long-term treatment with lithium alleviates memory deficits and reduces amyloid-beta production in an aged Alzheimer’s disease transgenic mouse model. J. Alzheimers Dis. 24, 739–749. doi: 10.3233/JAD-2011-101875
Zhang, X., Zhong, W., Brankack, J., Weyer, S. W., Muller, U. C., Tort, A. B., et al. (2016). Impaired theta-gamma coupling in APP-deficient mice. Sci. Rep. 6:21948. doi: 10.1038/srep21948
Zhao, R., Chen, J., Ren, Z., Shen, H., and Zhen, X. (2016). GSK-3beta inhibitors reverse cocaine-induced synaptic transmission dysfunction in the nucleus accumbens. Synapse 70, 461–470. doi: 10.1002/syn.21922
Keywords: GSK-3, lithium, neuronal oscillations, coherence, hippocampus, prefrontal cortex, spatial memory
Citation: Nguyen T, Fan T, George SR and Perreault ML (2018) Disparate Effects of Lithium and a GSK-3 Inhibitor on Neuronal Oscillatory Activity in Prefrontal Cortex and Hippocampus. Front. Aging Neurosci. 9:434. doi: 10.3389/fnagi.2017.00434
Received: 25 August 2017; Accepted: 15 December 2017;
Published: 12 January 2018.
Edited by:
Cheng-Xin Gong, Institute for Basic Research in Developmental Disabilities (IBR), United StatesCopyright © 2018 Nguyen, Fan, George and Perreault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melissa L. Perreault, perreaum@uoguelph.ca
†Present address: Melissa L. Perreault, Department of Molecular and Cellular Biology, University of Guelph, Guelph, ON, Canada
 Tuan Nguyen1
Tuan Nguyen1  Theresa Fan
Theresa Fan Susan R. George
Susan R. George Melissa L. Perreault
Melissa L. Perreault