Cognitive Function and Brain Atrophy Predict Non-pharmacological Efficacy in Dementia: The Mihama-Kiho Scan Project2
- 1Department of Dementia Prevention and Therapeutics, Graduate School of Medicine, Mie University, Tsu, Japan
- 2Department of Neurology, Graduate School of Medicine, Mie University, Tsu, Japan
- 3Yamaha Music Foundation, Tokyo, Japan
- 4Department of Health and Welfare, Mihama Town Hall, Mihama, Japan
- 5Department of Health and Welfare, Kiho Town Hall, Kiho, Japan
- 6Department of Neurosurgery, Kinan Hospital, Mihama, Japan
We aimed to determine whether neuropsychological deficits and brain atrophy could predict the efficacy of non-pharmacological interventions. Forty-six participants with mild-to-moderate dementia were monitored for 6 months; 25 underwent an intervention involving physical exercise with music, and 21 performed cognitive stimulation tasks. Participants were categorized into improvement (IMP) and no-IMP subgroups. In the exercise-with-music group, the no-IMP subgroup performed worse than the IMP subgroup on the Rivermead Behavioural Memory Test at baseline. In the cognitive-stimulation group, the no-IMP subgroup performed worse than the IMP subgroup on Raven’s Colored Progressive Matrices and the cognitive functional independence measure at baseline. In the no-IMP subgroup, voxel-based morphometric analysis at baseline revealed more extensive gray matter loss in the anterior cingulate gyrus and left middle frontal gyrus in the exercise-with-music and cognitive-stimulation groups, respectively. Participants with mild-to-moderate dementia with cognitive decline and extensive cortical atrophy are less likely to show improved cognitive function after non-pharmaceutical therapy.
Introduction
The World Alzheimer Report has estimated that there were 46.8 million people with dementia worldwide in 2015, and that this number will reach 131.5 million by 2050 (Prince et al., 2015). However, pharmacological treatments are relatively ineffective in halting the progression of dementia. The lack of pharmacological options (e.g., vaccines or disease-modifying agents) has shifted the focus of researchers toward non-pharmacological treatments for dementia, such as cognitive training, aerobic physical exercise, and music therapy (Gates and Sachdev, 2014; Groot et al., 2016; Zhang et al., 2017).
Accumulating evidence suggests that non-pharmacological treatment may maintain or decrease the rate of cognitive decline in adults with mild cognitive impairment and early stage dementia (Rodakowski et al., 2015). Additionally, the protective effects of aerobic physical exercise against the development and/or progression of dementia have been well documented (Laurin et al., 2001; Abbott et al., 2004; Ravaglia et al., 2008; Erickson et al., 2011). Additional studies have suggested that physical exercise combined with cognitive training exerts a greater positive impact on cognitive function than physical exercise alone (Fabre et al., 2002; Oswald et al., 2006; Shatil, 2013; Satoh et al., 2014, 2017; Tabei et al., 2017). In older adults, physical ExM induces greater positive effects on visuospatial function and leads to more extensive neuroanatomical changes (Tabei et al., 2017) than exercise alone. In participants with mild-to-moderate dementia, ExM also exerts greater positive effects on cognitive function and activities of daily living than CS using portable game consoles or drills (e.g., easy calculations, mazes, and mistake-searching in pictures) (Satoh et al., 2017).
Despite recent advancements, few studies have focused on predicting outcomes following non-pharmacological treatment. Knowledge of such predictors may allow clinicians to modify factors that are likely to attenuate the effects of the intervention and to provide more targeted treatment (Hsu et al., 2017). Indeed, previous studies have demonstrated that clinical and demographic factors can influence the effect of non-pharmacological activities on cognition and on the behavioral and psychological symptoms of dementia (Särkämö et al., 2016; Hsu et al., 2017). However, the neuropsychological factors influencing non-pharmacological treatment, as well as the neural basis of its efficacy, remain unknown. Furthermore, no randomized controlled trials have compared such effects among different non-pharmacological treatment options.
Therefore, the purpose of the present study was to determine whether neuropsychological factors and regional brain atrophy [as measured via voxel-based morphometry (VBM)] can predict the rate of improvement following non-pharmacological treatment in participants with mild-to-moderate dementia and to compare the effects of different non-pharmacological interventions.
Materials and Methods
Participants
The present study included participants with mild-to-moderate dementia who had utilized nursing services, such as day care or group homes, in the towns of Mihama and Kiho, which are situated at the southern end of the Kii peninsula in Japan. This study received approval from the Kinan Hospital Research Ethics Committee, and conformed to the tenets of the Declaration of Helsinki, and all participants provided written informed consent. This study was registered at UMIN-CTR (UMIN000017066) on April 7, 2015. The present study included the same participants as a previous study, which investigated the effect of non-pharmacological interventions in participants with mild-to-moderate dementia (Satoh et al., 2017). The inclusion criteria were as follows: (a) current diagnosis of intractable dementia by neurological specialists, based on ICD-10 diagnostic criteria (World Health Organization, 1993); (b) score between 16 and 26 on the MMSE; (c) stable physical and psychological condition; and (d) preserved hearing, visual acuity, and physical movement sufficient to enable participation in the intervention. Participants were excluded if they met any of the following criteria: (a) presence of chronic debilitating disease, such as malignancy or infection; (b) presence of severe cardiac, respiratory, and/or orthopedic disabilities that would prevent participation in the intervention; (c) presence of paresis or coordination disturbances that would prevent participation in the intervention; and (d) diagnosis of treatable dementia.
Diagnoses of AD were performed in accordance with criteria established by the National Institute of Neurologic Disorders and Stroke/Alzheimer Disease and Related Disorders Association (McKhann et al., 1984). Diagnoses of VaD were performed in accordance with criteria established by the National Institute of Neurological Disorders and Stroke/Association Internationale pour la Recherche et l’Enseignement en Neurosciences (Román et al., 1993).
All participants received treatment with anti-dementia drugs, the most common of which was donepezil hydrochloride. Pharmacological and non-pharmacological activities performed in the nursing-care facilities remained unchanged during the 6-months intervention period. Neuropsychological assessments and brain MRI were performed at baseline and following the intervention.
Procedures
Detailed descriptions of the procedures for each non-pharmacological intervention and neuropsychological assessment can be found in our previous report (Satoh et al., 2017).
Physical Exercise With Music (ExM)
Participants participated in 40-min physical exercise sessions once per week over the 6-months intervention period (total number of sessions: 24). The exercise program was developed by the Yamaha Music Foundation based on a program used in our previous study, which investigated the efficacy of physical exercise combined with music in older adults with normal cognitive function (Satoh et al., 2014; Tabei et al., 2017). The program consisted of muscle training for the upper and lower extremities, hand clapping to music, breath and voice training, and singing. The exercise trainers were professional musicians who also held private licenses as physical trainers with the Yamaha Music Foundation.
Cognitive Stimulation (CS)
Participants trained using a program called “Yawaraka Atama Juku (flexible thinking club)” developed by Nintendo Co., Ltd., using a portable game console (Nintendo DS LL, Kyoto, Japan) and performed drills consisting of easy calculations (Kawashima, 2003), mazes, and mistake-searching in pictures. Each session was 40 min long and were conducted once per week over the 6-months intervention period (total sessions: 24). Each session was moderated by nurses, certified care workers, or psychiatric social workers employed at the respective nursing facility.
Neuropsychological Assessment
The MMSE (Folstein et al., 1975) and RCPM (Raven, 1947) were used to quantify cognitive function. In addition to an overall score, the RCPM task measures performance time, which reflects the psychomotor speed of the participants. Memory was evaluated using the LM-I/-II subtests of the RBMT (Wilson et al., 1985), which requires immediate and delayed recall of a short story. The RBMT contains four stories of varying difficulty and different word counts. We used different stories for the pre- and post-test periods to avoid the influence of familiarity with story content.
Visuospatial ability was assessed using methods described by Strub and Black (2001). Participants were shown images of cubes and Necker cubes, and were then asked to draw a picture of each. Each drawing was scored by assigning one of four possible grades (0: poor, 1: fair, 2: good, and 3: excellent).
Frontal lobe function was assessed using two tasks: WF and Trail Making Test -A/-B (Partington and Leiter, 1949). The WF test consists of category and letter domains. In the categorical WF task, participants were asked to name as many animals as possible in 1 min. In the letter WF task, participants were asked to name as many objects as possible in 1 min beginning with each of the following four phonemes: ka, sa, ta, and te (Dohi et al., 1992). The average scores for these four phonemes were used for statistical analyses.
The Functional Independence Measure was used to evaluate functional performance regarding activities of daily living. The Functional Independence Measure consists of both motor and cognitive function domains. Motor function is assessed on thirteen items, including eating, dressing, evacuation, urination, and walking. Cognitive function is assessed on five items associated with understanding, expression, and memory. The maximum motor function, cognitive function, and overall Functional Independence Measure scores are 91, 35, and 126, respectively, with higher scores indicating better function. These neuropsychological assessments were administered to participants in both groups before and after the 6-month intervention period.
Improvement and No-Improvement Groups
Participants were dichotomized into an IMP or no-IMP subgroup based on MMSE scores following the intervention. Participants with an increased MMSE score of 2 points or more were included in the IMP subgroup, while the remaining participants were included in the no-IMP subgroup. The cut-off of 2 points was determined based on the findings of previous studies, which had shown that changes in MMSE scores of 2 points are beyond the threshold of chance (Morris et al., 1993; Bowie et al., 1999; Ballard et al., 2003).
MRI Acquisition
T1-weighted gradient echo MR images were obtained using a 1.5-T MR scanner (Echelon Oval, Hitachi Medical Corporation, Tokyo, Japan). Scan parameters were as follows: repetition time = Shortest (Automatic); echo time = 11 ms; flip angle = 90°; field of view = 230 mm × 230 mm; slice thickness = 4 mm; gapless; in-plane resolution = 0.45 mm × 0.45 mm. Scans were obtained both before and after the 6-month intervention period.
MRI Analysis
MRI data were analyzed using SPM12 (Wellcome Trust Centre for Neuroimaging, University College London, London, United Kingdom) implemented in MATLAB R2012a (MathWorks, Natick, MA, United States). In the pre-processing phase, images were set to match the anterior to posterior commissure line using an automated MATLAB script. The images were then visually inspected to check for possible scan issues such as field distortion and movement artifacts. Reoriented images were corrected for intensity inhomogeneity and segmented into GM, white matter, cerebrospinal fluid, and other tissues outside the brain using SPM12 tissue probability maps. The images were registered to the East Asian Brains International Consortium for Brain Mapping space template via affine regularization. We created a population-specific template using the SPM12 DARTEL template procedure to directly compare data between the IMP and no-IMP subgroups. We investigated group differences in GM volume, as well as the relationship between neuropsychological assessment results and GM at the whole-brain level. High-dimensional DARTEL was used to create non-linear, modulated-normalized GM and white matter images, which were smoothed using a Gaussian kernel of 8 mm FWHM (full-width at half-maximum). For whole-brain and multiple regression analyses, we assessed the statistical significance at a voxel threshold of p < 0.005 (uncorrected), within contiguous clusters of at least 20 voxels. We obtained both MNI and Talairach coordinates to detect the anatomical regions of the clusters. We used a transform from Matthew Brett1 to convert MNI coordinates to Talairach coordinates, and Talairach Client 2.4.3 (Lancaster et al., 2000) was used to identify the anatomical regions corresponding to Talairach coordinates.
Statistical Analyses
Differences in demographic variables and neuropsychological assessment results between the IMP and no-IMP subgroups were analyzed using independent t-tests for continuous data, chi-square tests for dichotomous data, and Mann–Whitney U tests for non-parametric data. Differences of p < 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics software version 20 (IBM Corp., Armonk, NY, United States).
Results
Participant Characteristics
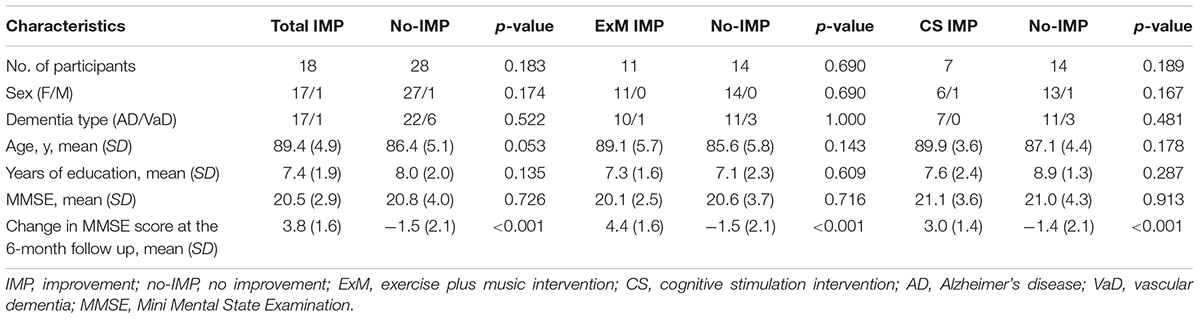
Participants who participated in more than 75% of all sessions (more than 18) and completed the neuropsychological and MRI assessments before and after the intervention were included in the final analysis. Following exclusion of the remaining participants, the ExM and CS groups included 25 and 21 participants, respectively (ExM: AD = 21; VaD = 4; CS: AD = 18, VaD = 3) (Table 1). Prior to the intervention, there were no significant differences in sex ratio, dementia type, age, years of education, or MMSE scores between the ExM and CS groups. However, among participants in both ExM and CS groups, MMSE scores were significantly worse in the no-IMP subgroup than in the IMP subgroup at the 6-month follow-up (p < 0.001) (Table 1).
Neuropsychological Assessments
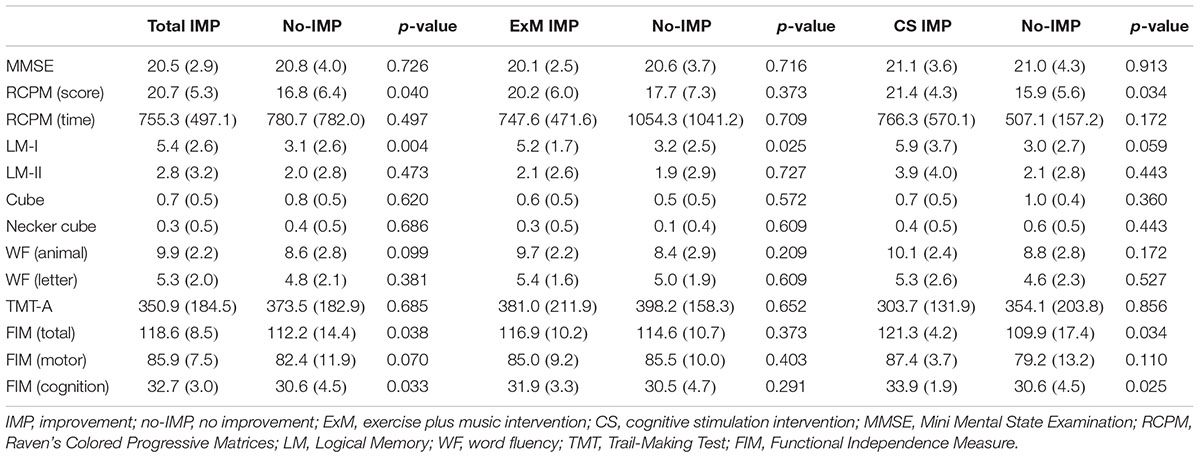
Baseline cube and Necker cube scores were significantly worse in the ExM group than in the CS group (p = 0.022, 0.01), although no significant differences were observed in the other neuropsychological assessments.
Participants in the no-IMP subgroup had significantly poorer baseline scores on the RCPM, LM-I section of the RBMT, and cognitive functional independence than those in the IMP subgroup (p = 0.04, 0.004, 0.033) (Table 2). Furthermore, baseline scores on the LM-I subtest were significantly worse in the no-IMP subgroup than the IMP subgroup (p = 0.025) of the ExM participants. In the CS group, baseline scores on the RCPM test and cognitive functional independence measure were significantly worse in the no-IMP subgroup than in the IMP subgroup (p = 0.034, 0.025, respectively) (Table 2).
MRI Assessments
Cube and Necker cube scores were used as covariates. Subtraction analysis revealed more extensive loss of GM in the left middle frontal gyrus in the no-IMP subgroup at baseline, relative to the IMP subgroup (Figures 1A,B and Table 3). Analysis of MMSE scores at the 6-month follow up revealed that changes in MMSE scores were positively correlated with volume in the left middle frontal gyrus (Figure 1C and Table 3). Subtraction analysis revealed more extensive GM loss in the anterior cingulate gyrus and left middle frontal gyrus in no-IMP participants in both the ExM (Figure 2A and Table 3) and CS (Figure 2B and Table 3) groups at baseline, respectively.
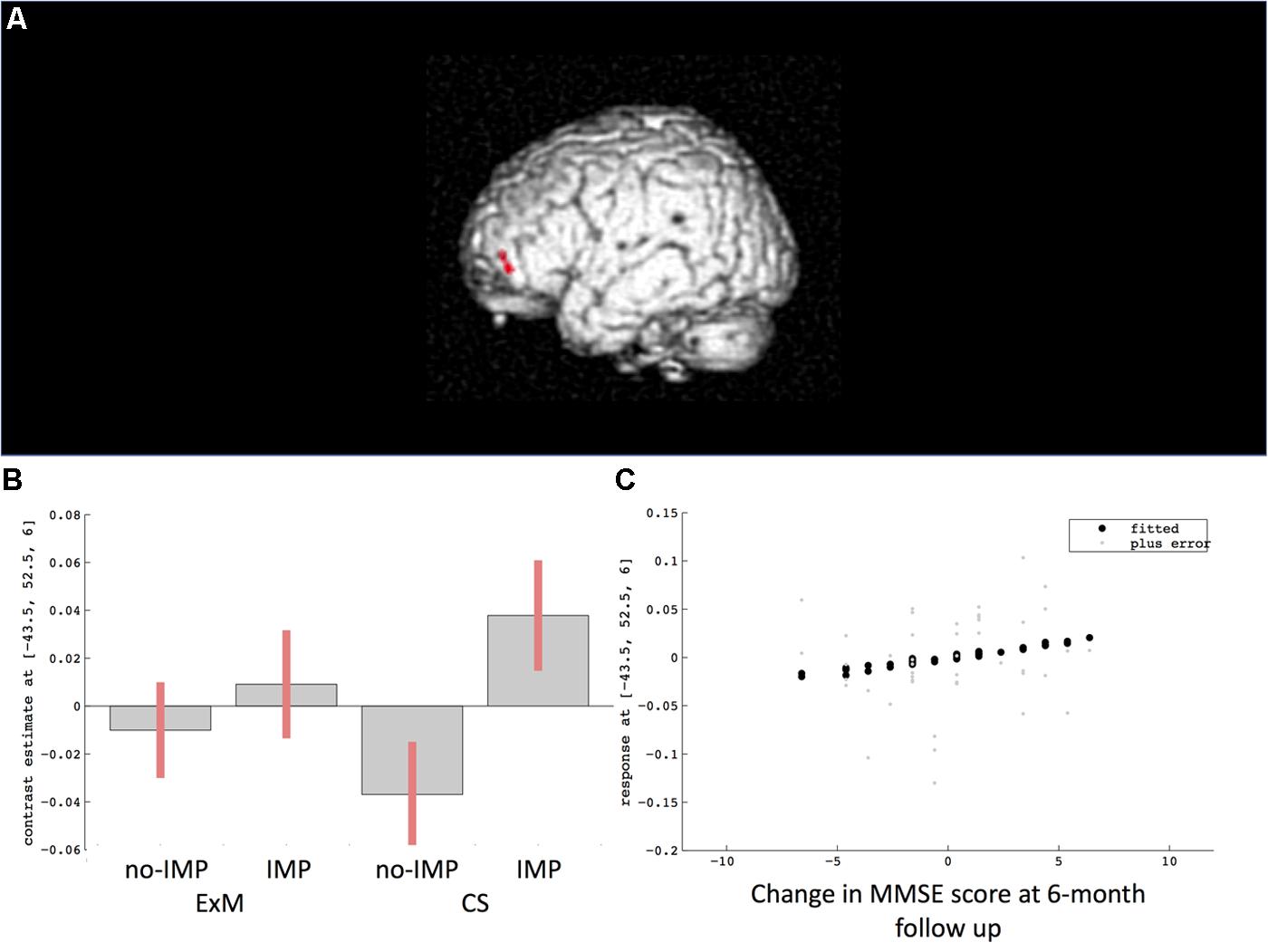
FIGURE 1. Results of subtraction analysis between the IMP and non-IMP subgroups of the whole cohort (A,B). Correlation between changes in MMSE scores and gray matter (GM) volume (C). IMP, improvement; no-IMP, no-improvement; MMSE, Mini Mental State Examination.
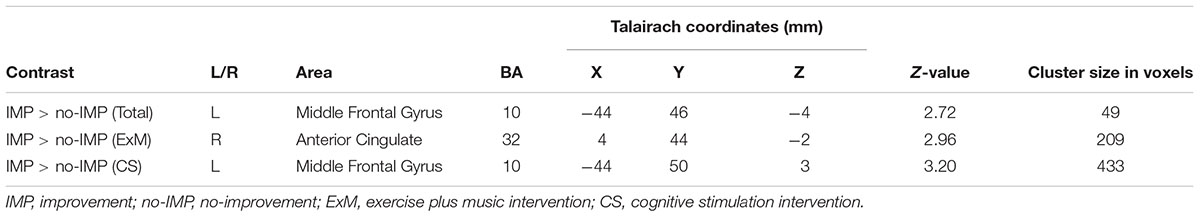
TABLE 3. Cluster sizes, peak locations, and statistical values for regions showing significant pre-intervention differences between the IMP and no-IMP subgroups.
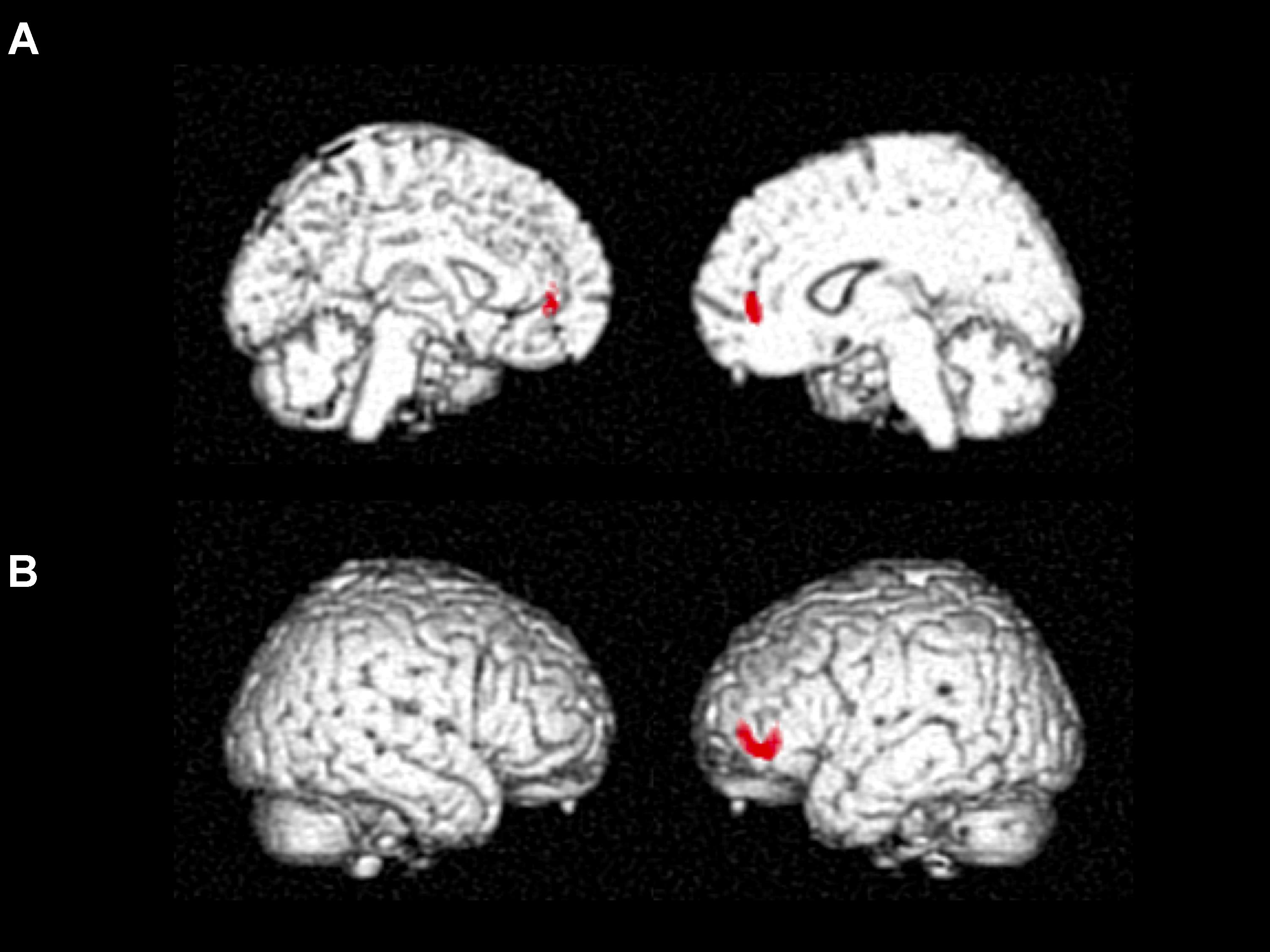
FIGURE 2. Results of subtraction analysis between the IMP and non-IMP subgroups of the ExM (A) and CS groups (B). IMP, improvement; no-IMP, no-improvement; ExM, exercise plus music intervention; CS, cognitive stimulation intervention.
Discussion
The present study aimed to determine whether neuropsychological factors and regional brain atrophy can predict the rate of IMP in participants with mild-to-moderate dementia following non-pharmacological intervention. Among ExM participants, the no-IMP subgroup exhibited poorer baseline performance on the LM-I subtest of the RBMT than participants in the IMP subgroup. Among CS participants, the no-IMP subgroup exhibited poorer baseline RCPM and cognitive functional independence measure scores than participants in the IMP subgroup. Such differences may be associated with differences in cognitive resources required to execute each intervention. Proper execution of the ExM protocol requires the participant to remember the exercise patterns from week to week, while proper execution of the CS protocol requires spatial and mathematical abilities that allow understanding of geometric designs and missing pieces, as well as expressive abilities and memory function to complete calculations, mazes, and search for mistakes in images. Although previous studies have indicated that exercise and ExM tasks enhance memory in participants with dementia (Radak et al., 2010; Intlekofer and Cotman, 2013; Satoh et al., 2017), our results suggest that execution of the intervention requires a certain amount of memory function reserve. These findings highlight the importance of early intervention, when cognitive function may be somewhat preserved (Dubois et al., 2016).
Voxel-based morphometry analysis revealed more extensive loss of GM in the anterior cingulate gyrus in no-IMP participants of the ExM group, and in the left middle frontal gyrus in the CS group at baseline relative to that observed in the IMP subgroup. These findings may also have been associated with the execution of each intervention. Previous studies have demonstrated that the anterior cingulate gyrus plays a key role in music processing in participants with dementia (Omar et al., 2011; Fletcher et al., 2015; Jacobsen et al., 2015), while the left middle frontal gyrus is associated with calculation (Maurer et al., 2016) and visual search (Weidner et al., 2009) in CS tasks. Therefore, our results indicate that the extent and location of brain atrophy at baseline may represent the neural basis of task execution for each intervention. In addition, these parameters may aid in predicting the efficacy of non-pharmacological treatment.
Previous studies have indicated that characteristic impairments across cognitive domains (Mori et al., 2016), Barthel Index (Formiga et al., 2010), and lower pre-treatment regional cerebral blood flow in the right orbitofrontal cortex (Hongo et al., 2008) can be used to predict better responses to cholinesterase inhibitors treatment in older adults with dementia. Our findings suggest that the efficacy of non-pharmacological treatment can also be predicted based on neural characteristics and the extent of cognitive decline.
The present study has some limitations of note. First, as we did not include healthy controls, further studies are required to investigate differences in neuropsychological factors and regional brain atrophy between healthy individuals and participants with mild-to-moderate dementia. Such findings may allow clinicians to predict the most appropriate non-pharmacological treatment for each participant. Second, the intervention period of the present study lasted only 6 months. Explicit differences in neuropsychological factors and regional brain atrophy may be more evident following a longer intervention period. Future studies should also aim to include a larger number of participants to enhance the accuracy of prediction.
Conclusion
Our findings suggest that participants with mild-to-moderate dementia who have experienced cognitive decline, reduced ability to perform activities of daily living, and extensive cortical atrophy are less likely to exhibit IMPs in cognitive function following non-pharmacological treatment. Thus, some characteristics of pre-treatment cognitive dysfunction and regional brain atrophy may aid clinicians in determining the most appropriate non-pharmacological intervention for each participant.
Author Contributions
MS: conceived and designed the experiments. TT, NN, and KN: conducted the experiments. KT: analyzed the data. KT and MS: wrote the paper. JO: contributed materials. HK: analyzed and interpreted the data. HT: supervised and interpreted the data. All authors read and approved the final version of the paper.
Funding
This study was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (Grant No: 15K08909) and Grant-in-Aid for Young Scientists (B) (Grant Nos: 25870325 and 17K17811).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Keisuke Okamoto who works in the laboratory, Isamu Nonomura in radiology, and Hideshi Kanai in the community cooperation room at Kinan Hospital, and Takayuki Yotsuji at the Yamaha Music Foundation, for their kind contributions to this study.
Abbreviations
AD, Alzheimer’s disease; CS, cognitive stimulation; ExM, exercise with music; GM, gray matter; IMP, improvement; LM, logical memory; MMSE, Mini-Mental State Examination; no-IMP, no-improvement; RBMT, Rivermead Behavioural Memory Test; RCPM, Raven’s Colored Progressive Matrices; VaD, vascular dementia; WF, word fluency.
Footnotes
References
Abbott, R. D., White, L. R., Ross, G. W., Masaki, K. H., Curb, J. D., and Petrovitch, H. (2004). Walking and dementia in physically capable elderly men. J. Am. Med. Assoc. 292, 1447–1453. doi: 10.1001/jama.292.12.1447
Ballard, C., Rowan, E., Stephens, S., Kalaria, R., and Kenny, R. A. (2003). Prospective follow-up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia-free stroke survivors >75 years of age. Stroke 34, 2440–2444. doi: 10.1161/01.STR.0000089923.29724.CE
Bowie, P., Branton, T., and Holmes, J. (1999). Should the mini mental state examination be used to monitor dementia treatments? Lancet 354, 1527–1528. doi: 10.1016/S0140-6736(99)03486-8
Dohi, N., Iwaya, T., and Kayamori, R. (1992). Seishin-kinou Hyouka, the Evaluation of Mental Function. Tokyo: Ishiyaku Publishers, Inc.
Dubois, B., Hampel, H., Feldman, H. H., Scheltens, P., Aisen, P., Andrieu, S., et al. (2016). Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 12, 292–323. doi: 10.1016/j.jalz.2016.02.002
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U.S.A. 108, 3017–3022. doi: 10.1073/pnas.1015950108
Fabre, C., Chamari, K., Mucci, P., Masse-Biron, J., and Prefaut, C. (2002). Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int. J. Sports Med. 23, 415–421. doi: 10.1055/s-2002-33735
Fletcher, P. D., Downey, L. E., Golden, H. L., Clark, C. N., Slattery, C. F., Paterson, R. W., et al. (2015). Auditory hedonic phenotypes in dementia: a behavioural and neuroanatomical analysis. Cortex 67, 95–105. doi: 10.1016/j.cortex.2015.03.021
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Formiga, F., Fort, I., Robles, M. J., Rodriguez, D., and Regalado, P. (2010). Lower Barthel Index scores predict less prescription of pharmacological therapy in elderly patients with Alzheimer disease. Dement. Geriatr. Cogn. 29, 198–203. doi: 10.1159/000278348
Gates, N. J., and Sachdev, P. (2014). Is cognitive training an effective treatment for preclinical and early Alzheimer’s disease? J. Alzheimers Dis. 42(Suppl. 4), S551–S559. doi: 10.3233/JAD-141302
Groot, C., Hooghiemstra, A. M., Raijmakers, P. G. H. M., van Berckel, B. N. M., Scheltens, P., Scherder, E. J. A., et al. (2016). The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res. Rev. 25, 13–23. doi: 10.1016/j.arr.2015.11.005
Hongo, J., Nakaaki, S., Shinagawa, Y., Murata, Y., Sato, J., Tatsumi, H., et al. (2008). SPECT-identified neuroanatomical predictor of the cognitive effects of donepezil treatment in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. 26, 556–566. doi: 10.1159/000181148
Hsu, T. J., Tsai, H. T., Hwang, A. C., Chen, L. Y., and Chen, L. K. (2017). Predictors of non-pharmacological intervention effect on cognitive function and behavioral and psychological symptoms of older people with dementia. Geriatr. Gerontol. Int. 17(Suppl. 1), 28–35. doi: 10.1111/ggi.13037
Intlekofer, K. A., and Cotman, C. W. (2013). Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol. Dis. 57, 47–55. doi: 10.1016/j.nbd.2012.06.011
Jacobsen, J. H., Stelzer, J., Fritz, T. H., Chételat, G., La Joie, R., and Turner, R. (2015). Why musical memory can be preserved in advanced Alzheimer’s disease. Brain 138, 2438–2450. doi: 10.1093/brain/awv135
Kawashima, R. (2003). Calculation Drill for Adults Which Improves Brain Function. Tokyo: Kumon Publication.
Lancaster, J. L., Woldorff, M. G., Parsons, L. M., Liotti, M., Freitas, C. S., Rainey, L., et al. (2000). Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 10, 120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8
Laurin, D., Verreault, R., Lindsay, J., MacPherson, K., and Rockwood, K. (2001). Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 58, 498–504. doi: 10.1001/archneur.58.3.498
Maurer, S., Tanigawa, N., Sollmann, N., Hauck, T., Ille, S., Boeckh-Behrens, T., et al. (2016). Non-invasive mapping of calculation function by repetitive navigated transcranial magnetic stimulation. Brain Struct. Funct. 221, 3927–3947. doi: 10.1007/s00429-015-1136-2
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34, 939–944. doi: 10.1212/WNL.34.7.939
Mori, E., Ikeda, M., Nakagawa, M., Miyagishi, H., and Kosaka, K. (2016). Pretreatment cognitive profile likely to benefit from donepezil treatment in dementia with Lewy bodies: pooled analyses of two randomized controlled trials. Dement. Geriatr. Cogn. 42, 58–68. doi: 10.1159/000447586
Morris, J. C., Edland, S., Clark, C., Galasko, D., Koss, E., Mohs, R., et al. (1993). The consortium to establish a registry for Alzheimer’s disease (CERAD). Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology 43, 2457–2465. doi: 10.1212/WNL.43.12.2457
Omar, R., Henley, S. M., Bartlett, J. W., Hailstone, J. C., Gordon, E., Sauter, D. A., et al. (2011). The structural neuroanatomy of music emotion recognition: evidence from frontotemporal lobar degeneration. Neuroimage 56, 1814–1821. doi: 10.1016/j.neuroimage.2011.03.002
Oswald, W. D., Gunzelmann, T., Rupprecht, R., and Hagen, B. (2006). Differential effects of single versus combined cognitive and physical training with older adults: the SimA study in a 5-year perspective. Eur. J. Ageing 3, 179–192. doi: 10.1007/s10433-006-0035-z
Partington, J. E., and Leiter, R. G. (1949). Partington’s pathway test. Psychol. Serv. Center Bull. 1, 9–20.
Prince, M., Wimo, A., Guerchet, M., Ali, G. C., Wu, Y. T., and Prina, M. (2015). World Alzheimer Report 2015 - The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer’s Disease International.
Radak, Z., Hart, N., Sarga, L., Koltai, E., Atalay, M., Ohno, H., et al. (2010). Exercise plays a preventive role against Alzheimer’s disease. J. Alzheimers Dis. 20, 777–783. doi: 10.3233/JAD-2010-091531
Ravaglia, G., Forti, P., Lucicesare, A., Pisacane, N., Rietti, E., Bianchin, M., et al. (2008). Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology 70, 1786–1794. doi: 10.1212/01.wnl.0000296276.50595.86
Raven, J. C. (1947). Coloured Progressive Matrices Sets A, AB, B. Manual Sections A and 2. Oxford: Oxford Psychologists Press.
Rodakowski, J., Saghafi, E., Butters, M. A., and Skidmore, E. R. (2015). Non-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: an updated scoping review. Mol. Aspects Med. 43, 38–53. doi: 10.1016/j.mam.2015.06.003
Román, G. C., Tatemichi, T. K., Erkinjuntti, T., Cummings, J. L., Masdeu, J. C., Garcia, J. A., et al. (1993). Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43, 250–260. doi: 10.1212/WNL.43.2.250
Särkämö, T., Laitinen, S., Numminen, A., Kurki, M., Johnson, J. K., and Rantanen, P. (2016). Clinical and demographic factors associated with the cognitive and emotional efficacy of regular musical activities in dementia. J. Alzheimers Dis. 49, 767–781. doi: 10.3233/JAD-150453
Satoh, M., Ogawa, J. I., Tokita, T., Nakaguchi, N., Nakao, K., Kida, H., et al. (2014). The effects of physical exercise with music on cognitive function of elderly people: Mihama-Kiho project. PLoS One 9:e95230. doi: 10.1371/journal.pone.0095230
Satoh, M., Ogawa, J. I., Tokita, T., Nakaguchi, N., Nakao, K., Kida, H., et al. (2017). Physical exercise with music maintains activities of daily living in patients with dementia: Mihama-Kiho project part 21. J. Alzheimers Dis. 57, 85–96. doi: 10.3233/JAD-161217
Shatil, E. (2013). Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front. Aging Neurosci. 5:8. doi: 10.3389/fnagi.2013.00008
Strub, R. L., and Black, F. W. (2001). The Mental Status Examination in Neurology, 4th Edn. Philadelphia, PA: F.A. Davis Company.
Tabei, K. I., Satoh, M., Ogawa, J. I., Tokita, T., Nakaguchi, N., Nakao, K., et al. (2017). Physical exercise with music reduces gray and white matter loss in the frontal cortex of elderly people: the Mihama-Kiho scan project. Front. Aging Neurosci. 9:174. doi: 10.3389/fnagi.2017.00174
Weidner, R., Krummenacher, J., Reimann, B., Müller, H. J., and Fink, G. R. (2009). Sources of top-down control in visual search. J. Cogn. Neurosci. 21, 2100–2113. doi: 10.1162/jocn.2008.21173
Wilson, B., Cockburn, J., and Baddeley, A. (1985). The Rivermead Behavioural Memory Test. Bury St Edmunds: Thames Valley Test Company.
World Health Organization (1993). International Statistical Classification of Diseases and Related Health Problems, 10th Rev. Geneva: World Health Organization.
Keywords: dementia, exercise with music, cognitive dysfunction, voxel-based morphometry, atrophy, frontal lobe
Citation: Tabei K, Satoh M, Ogawa J, Tokita T, Nakaguchi N, Nakao K, Kida H and Tomimoto H (2018) Cognitive Function and Brain Atrophy Predict Non-pharmacological Efficacy in Dementia: The Mihama-Kiho Scan Project2. Front. Aging Neurosci. 10:87. doi: 10.3389/fnagi.2018.00087
Received: 26 September 2017; Accepted: 14 March 2018;
Published: 12 April 2018.
Edited by:
Athanasios Alexiou, Novel Global Community Educational Foundation (NGCEF), AustraliaReviewed by:
Sadaf Jahan, Council of Scientific and Industrial Research (CSIR), IndiaElizabeta Blagoja Mukaetova-Ladinska, Newcastle University, United Kingdom
Copyright © 2018 Tabei, Satoh, Ogawa, Tokita, Nakaguchi, Nakao, Kida and Tomimoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ken-ichi Tabei, tabei@clin.medic.mie-u.ac.jp; kenichi.tabei@gmail.com Masayuki Satoh, bruckner@clin.medic.mie-u.ac.jp
 Ken-ichi Tabei
Ken-ichi Tabei Masayuki Satoh
Masayuki Satoh Jun-ichi Ogawa
Jun-ichi Ogawa Tomoko Tokita4
Tomoko Tokita4