Lower Functional Connectivity of the Periaqueductal Gray Is Related to Negative Affect and Clinical Manifestations of Fibromyalgia
- 1Division of Brain, Imaging and Behaviour-Systems Neuroscience, Krembil Research Institute, Toronto Western Hospital, University Health Network, Toronto, ON, Canada
- 2Lawson Health Research Institute, London, ON, Canada
- 3Department of Medical Biophysics, University of Western Ontario, London, ON, Canada
- 4Departments of Clinical Neurosciences and Oncology, University of Western Ontario, London, ON, Canada
- 5Department of Anesthesia and Perioperative Medicine, University of Western Ontario, London, ON, Canada
- 6Department of Psychology, University of Western Ontario, London, ON, Canada
- 7Department of Surgery and Institute of Medical Science, University of Toronto, London, ON, Canada
Fibromyalgia (FM) syndrome is characterized by chronic widespread pain, muscle tenderness and emotional distress. Previous studies found reduced endogenous pain modulation in FM. This deficiency of pain modulation may be related to the attributes of chronic pain and other clinical symptoms experienced in patients with FM. Thus, we tested whether there is a link between the clinical symptoms of FM and functional connectivity (FC) of the periaqueductal gray (PAG), a key node of pain modulation. We acquired resting state 3T functional MRI (rsfMRI) data from 23 female patients with FM and 16 age- and sex- matched healthy controls (HC) and assessed FM symptoms with the Brief Pain Inventory (BPI), Fibromyalgia Impact Questionnaire (FIQ), Hospital Anxiety and Depression Scale (HADS) and Pain Catastrophizing Scale (PCS). We found that patients with FM exhibit statistically significant disruptions in PAG FC, particularly with brain regions implicated in negative affect, self-awareness and saliency. Specifically, we found that, compared to HCs, the FM patients had stronger PAG FC with the lingual gyrus and hippocampus but weaker PAG FC with regions associated with motor/executive functions, the salience (SN) and default mode networks (DMN). The attenuated PAG FC was also negatively correlated with FIQ scores, and positively correlated with the magnification subscale of the PCS. These alterations were correlated with emotional and behavioral symptoms of FM. Our study implicates the PAG as a site of dysfunction contributing to the clinical manifestations and pain in FM.
Introduction
Fibromyalgia (FM) is a chronic pain condition present in 2%–8% of the population with a higher prevalence in women (Clauw, 2014). Patients with FM suffer tremendously not only from chronic widespread pain but also from muscle tenderness, persistent fatigue, sleep disturbances, mood and cognitive changes, the cause of which are not understood (Wolfe et al., 1990, 2010; Clauw, 2014).
The peripheral and central mechanisms underlying FM are not fully known. Recent studies of skin biopsies (Levine and Saperstein, 2015), found that patients with small fiber polyneuropathy (SFPN) had reduced small fiber diameters compared to healthy subjects (Doppler et al., 2015), and this in consistent with decreased C fiber nociceptors conduction velocity by Serra et al. (2014). However, SFPN was only observed in 30%–50% of patient previously diagnosed with FM and has also been found in some healthy controls (HC; Oaklander et al., 2013; Giannoccaro et al., 2014; Serra et al., 2014). Furthermore, decreased intraepidermal nerve fiber density can be found in other conditions (Devigili et al., 2008) and there is no correlations between small fiber diameters and symptoms severity (Kosmidis et al., 2014; Doppler et al., 2015). Therefore, the role of the peripheral and central nervous systems in FM is still not understood. Given that objective measures of small fibers does not necessarily correlate with perceived pain in FM, the central nervous system and its neuroplasticity is likely to play some role in FM (Clauw, 2015). In support of a central contribution to FM, several psychophysical studies point to abnormal central processing of nociceptive inputs and ineffective descending modulation of nociceptive signals. For example, patients with FM have lower pain thresholds, enhanced temporal summation (Price et al., 2002; Staud and Smitherman, 2002; Staud et al., 2003; Serra et al., 2014), and deficient conditioned pain modulation (Lautenbacher and Rollman, 1997; Julien et al., 2005). Furthermore, a functional MRI (fMRI) study reported brain regions involved in descending pain modulation such as the periaqueductal gray (PAG) have attenuated responses to painful stimuli in FM patients (Jensen et al., 2012).
The PAG is known for its role in both acute and chronic pain and analgesia (Reynolds, 1969; Dostrovsky and Deakin, 1977; Lovick, 1985; Keay and Bandler, 1993; Hemington and Coulombe, 2015), but it is also involved in fear, anxiety and cardiovascular responses (Bandler et al., 1985, 2000; Bandler and Shipley, 1994; Linnman et al., 2012). These functions are particularly important for emotional and behavioral responses to stress and pain. Previous studies suggested that the PAG is an integration center that generates an appropriate behavioral and autonomic response to stress and pain.
We recently studied functional connectivity (FC), i.e., synchronous slow frequency oscillation between brain areas (Davis and Moayedi, 2013), of the PAG. We reported that subregions of the PAG has FC not only with brain regions involved in descending pain modulation (rACC, aMCC, medulla), but also regions related to executive functions, such as the prefrontal cortex (PFC), striatum and the hippocampus (Coulombe et al., 2016). This information allows us to infer that these brain regions are working as a network and its dysfunction could lead to clinical signs and symptoms. The PAG shows abnormal FC in many chronic pain diseases, including FM (Cifre et al., 2012; Pujol et al., 2014). Considering these observations, and previous animal research, the PAG likely is involved not only in shaping pain perception, but also in coping behavior related to an unavoidable pain, extended to social interactions worrying and catastrophizing under conditions of chronic pain (Hassett et al., 2000; Cifre et al., 2012; Pujol et al., 2014; Coulombe et al., 2016).
Therefore, the aim of this study was to measure resting state FC of the PAG in FM and HC and link abnormalities with FM clinical symptoms (measured using standardized questionnaires (Fibromyalgia Impact Questionnaire (FIQ), Hospital Anxiety and Depression Scale (HADS), Pain Catastrophization Scale). We tested the hypothesis that there is abnormal PAG FC in patients with FM that are related to their chronic pain symptoms and affect-related clinical symptoms. Patients with FM not only suffer from chronic pain but often report depression, anxiety, catastrophization and cognition impairment which can diminish their quality of life (Hassett et al., 2000). Because hypervigilance has been suggested in this pathology (Crombez et al., 2004; Eccleston and Crombez, 2007), we expected FC with brain region such as the default mode and salience networks (DMN, SN) to be related to FM clinical symptoms. These networks are known to be disrupted in many chronic pain diseases, including in FM (Baliki et al., 2008; Napadow et al., 2010; Loggia et al., 2013; Kucyi et al., 2014).
Materials and Methods
Participants
Twenty-three female FM patients and 16 age- and sex- matched HC were recruited. Patients were recruited through the Rheumatology Clinic, St. Joseph’s Health Care London, Canada, and were diagnosed with FM using the criteria of the American College of Rheumatology (Wolfe et al., 1990). They were also screened for any exclusion criteria, i.e., a concurrent treatment with antidepressants, an active psychosis, or any recent change in their pain medication (any dose alternation within the preceding month). The HC subjects were recruited from the community. They were screened and excluded if they were pregnant or breastfeeding, suffering from chronic illness including neurological/psychiatric disorders. All subjects provided informed written consent to procedures approved by the Health Sciences Research Ethics Board of the University of Western Ontario. As previously described (Shokouhi et al., 2016). FM patients and HC did not differ in terms of age (FM: 50.6 ± 8.1 years; HC: 49.8 ± 11.0 years; Mann-Whitney U statistic = 177.5, p = 0.864). Arterial spin labeling (ASL) data from these study participants have been previously reported (Shokouhi et al., 2016).
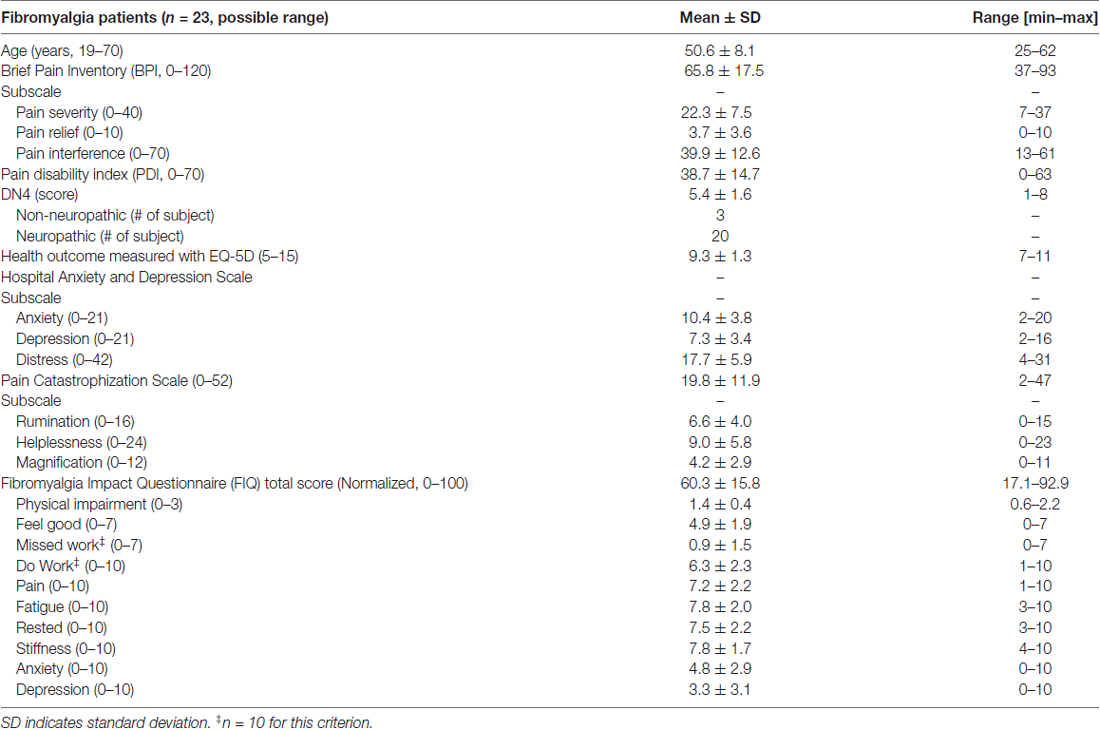
We used standardized questionnaires in order to evaluate their pain and other collateral symptoms, such as anxiety, depression, catastrophization and impact of the disease on their life. Clinical assessment was performed only in FM subjects and included validated and commonly used metrics: the severity of symptoms in FM was evaluated using the self-administered FIQ, which is a well-known measure of functional disabilities such as pain, stiffness, fatigue, anxiety, depression, physical functioning, work status and well-being in FM (Wolfe et al., 1997; Perrot et al., 2003; Bennett, 2005). Depressive mood and anxiety disorders were assessed using the HADS (Zigmond and Snaith, 1983). Pain severity and impact of pain on daily functions were assessed using the self-administered Brief Pain Inventory (BPI; Cleeland and Ryan, 1994; Atkinson et al., 2011). The Pain catastrophizing scale (PCS) evaluates three dimensions of catastrophic thinking related to pain: rumination, magnification, and helplessness (Sullivan et al., 1995). The pain disability index (PDI) measures the impact of pain on a person’s essential life activities such as family/home responsibilities, recreation, social activity, occupation, sexual behavior, self-care and life-supporting activities (Tait et al., 1987). Each area is rated on an 11-point scale (0 = no disability to 10 = total disability) for a maximum score of 70 (34). All questionnaires have good internal consistency and construct validity and for all of them a higher score indicates a worse condition.
Image Acquisition
Each participant underwent neuroimaging on a 3T MRI system (Biograph mMR, Siemens, Erlanger, Germany) at the Lawson Health Research Institute with an 32-element receive-only head coil, including a high-resolution T1-weighted whole-brain anatomical scan (176 axial slices; 256 × 256 matrix; 1 × 1 × 1 mm voxels) using a 3-D magnetization-prepared rapid gradient-echo imaging sequence (flip angle = 9°; TE = 2.98 ms; TR = 2000 ms; TI = 900 ms), and a 10.5-min resting state fMRI T2* weighted gradient echo echo-planar imaging sequence (GRE-EPI) scan (42 slices; 200 × 200 matrix; 2 × 2 × 3 mm voxels; TE = 30 ms; TR = 3000 ms; 210 volumes). Subjects were instructed to stay awake with their eyes closed (Shokouhi et al., 2016).
Images Pre-Processing
All images were preprocessed using standard methods previously published, using freely available FSL software (FMRIB‘s software library1), Matlab customized scripts, and fMRISTAT toolbox. Our preprocessing steps included the deletion of the first four volumes, the removal of non-brain tissue (FSL’s brain extraction tool), and head motion correction (MCFLIRT, 6 motion parameters). Images were all linearly realigned to the first T1-weighted image (FLIRT, 6 DOF), registered to the Montreal Neurological Institute 152 (MNI 152) 2-mm standard space (FLIRT, 12 DOF), and corrected for head motion (MCFLIRT, 6 parameters).
Artifacts created by spurious noise and motion can occur in brain imaging, particularly with patients who have difficulty lying still in the scanner for a long period of time. Therefore, in order to limit confounding effect of physiological noises such as cardiac pulsation and modulation associated with the respiration, we used the aCompCor method, which was developed to identify specific patterns of structured noise, and remove them (Behzadi et al., 2007; Kucyi et al., 2013, 2014; Muschelli et al., 2014). The first step of this method segments brain tissue into a component that includes only white matter (WM) and cerebrospinal fluid (CSF), and uses it to model physiological noise (see below). The images were segmented using FSLs segmentation tool (FAST). The WM and CSF components were thresholded to keep only voxels with high probability of being WM and CSF (top 198 cm3 and top 20 cm3; Chai et al., 2012; Kucyi et al., 2013). Then, a principal component analysis (PCA) was used to model the time series of the noise component with the first five WM and CSF components. This noise component, in addition to the six motion parameters obtained earlier, were then regressed out through its addition in the general linear model as a nuisance component of blood oxygen level dependent (BOLD) time series. Finally, all functional data were spatially smoothed using a 4-mm kernel full-width at half maximum kernel (FWHM) and a 0.005–0.05 Hz band-pass was applied (Rogachov et al., 2016).
Seed-to-Voxels Whole Brain Analysis
We carefully considered the issue of choosing the PAG seed architecture. As mentioned earlier, the PAG is involved in both pain perception and emotional reaction to a pain and/or stressful stimuli. It has been suggested that the autonomic nervous system is linked to pain modulation deficiency in FM (Chalaye et al., 2012, 2014) and anxiety, hypervigilance and descending pain modulation is likely deficient in FM patients. We chose to seed the whole PAG as has been done in previous studies (Eippert et al., 2009; Kucyi et al., 2013) because: (1) we were interest in the clinical profile of FM; (2) considering the correlation between FC and clinical symptoms, a whole PAG seed was more appropriate than separate left and right PAG seeds (Cifre et al., 2012; Schmidt-Wilcke et al., 2014); (3) this minimized the number of statistical comparisons; and (4) we had no specific hypothesis about the left or right PAG (Kucyi et al., 2014). We are aware of the limitations of this seed considering our previous publication of the columnar organization of the PAG (Coulombe et al., 2016), however, this was best seed to use to answer the aim of the current study.
The location of the PAG seed was chosen based on a previous study from our lab (MNI coordinates: 0, −32, −12; size: 6-mm radius sphere; Kucyi et al., 2013; Figure 1). For each subject, the seed was registered to their 2-mm native space (trilinear interpolation to the previously pre-processed image), binarized and the time series of each voxel of the seed was extracted. The BOLD time series was put in relation with the time series of every other voxel of the brain. FMRIB’s FMRI Expert Analysis Tool with FILM (FMRIB’s Improved Linear Model) general linear model using nonlinear registration with a 10-mm wrap was used to analyze each subject. The higher-level analysis used FMRIB’s mixed effects thresholded at Z = 2.3 and a cluster-based P = 0.05 (Flame 1 + 2).
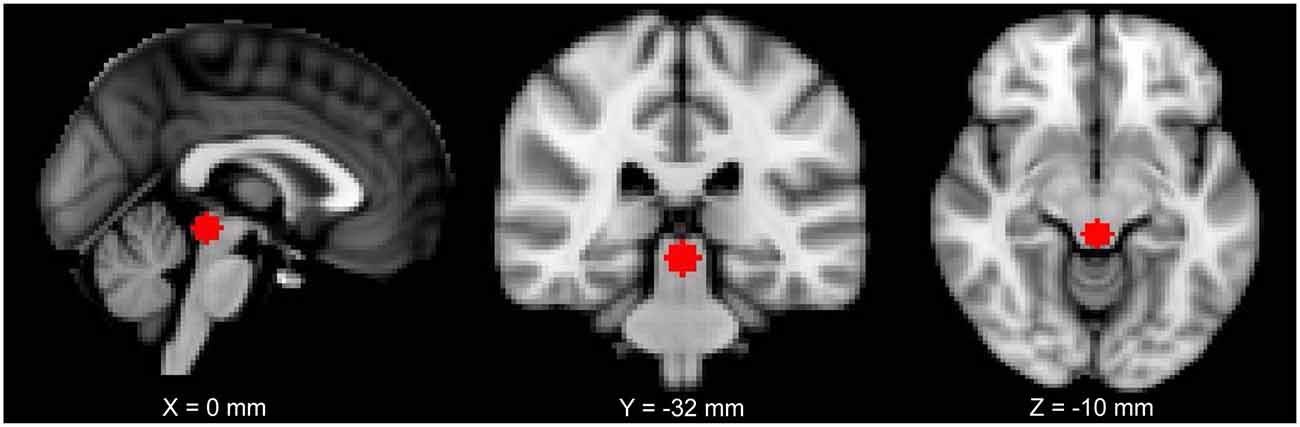
Figure 1. The periaqueductal gray (PAG) seed used in this study shown on a 2 mm Montreal Neurological Institute (MNI) standard space template.
Correlation between FC of Identified Clusters with Symptom Severity Metrics
We next further examined the FC of each cluster found to differ between the FM and HC groups (see in the contrast analysis above, and numbered in Table 1). We extracted the values of FC using FSL function “meants”, i.e., the average of the timeseries each set of voxels. These data were correlated, in FM subjects, with the major metrics of negative affect parameters assessed (anxiety, depression, distress, rumination, helplessness, magnification or global catastrophizing score) and the major clinical indices of FM assessed (pain severity, FIQ score normalized). To account for multiple comparisons, we did a Bonferroni correction (α/number of comparison) and, therefore, set our p-value at <0.007 for psychological symptoms (7 symptoms to compare) and <0.025 for pain severity/interference (2 scores to compare).
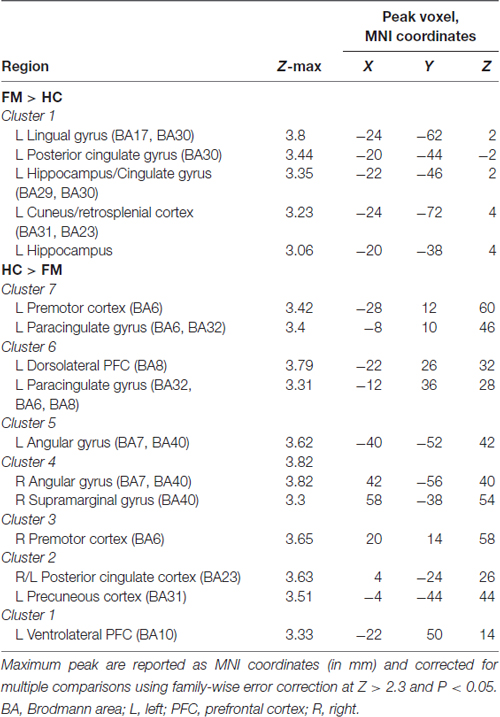
Table 1. Peak Montreal Neurological Institute (MNI) coordinates of contrast comparison of periaqueductal gray (PAG) seed functional connectivity (FC) between fibromyalgia (FM) and healthy controls (HC).
Results
Description of the FM Population
The demographic profile and symptomology of the FM patients are shown in Table 2. Based on available normative data of the questionnaires used, our patients reported symptoms of moderate severity (Bennett et al., 2009), mild anxiety and depression (normal 0–7, mild 8–10, moderate 11–14 and severe 15–21; Zigmond and Snaith, 1983). All patients had chronic pain and most (20/23) also reported sensory descriptors that are typically associated with neuropathic pain (Bouhassira et al., 2005). The patient PCS scores fell within the 50th percentile, and the subscores of rumination (44th percentile), magnification (63th percentile) and helplessness (55th percentile) place them at moderate risk for development of pain chronicity (Sullivan, 2009). Only four of the 23 FM subjects had PCS scores ≥30 that indicate clinically relevant pain catastrophizing (Sullivan, 2009).
PAG Functional Connectivity in FM and HC
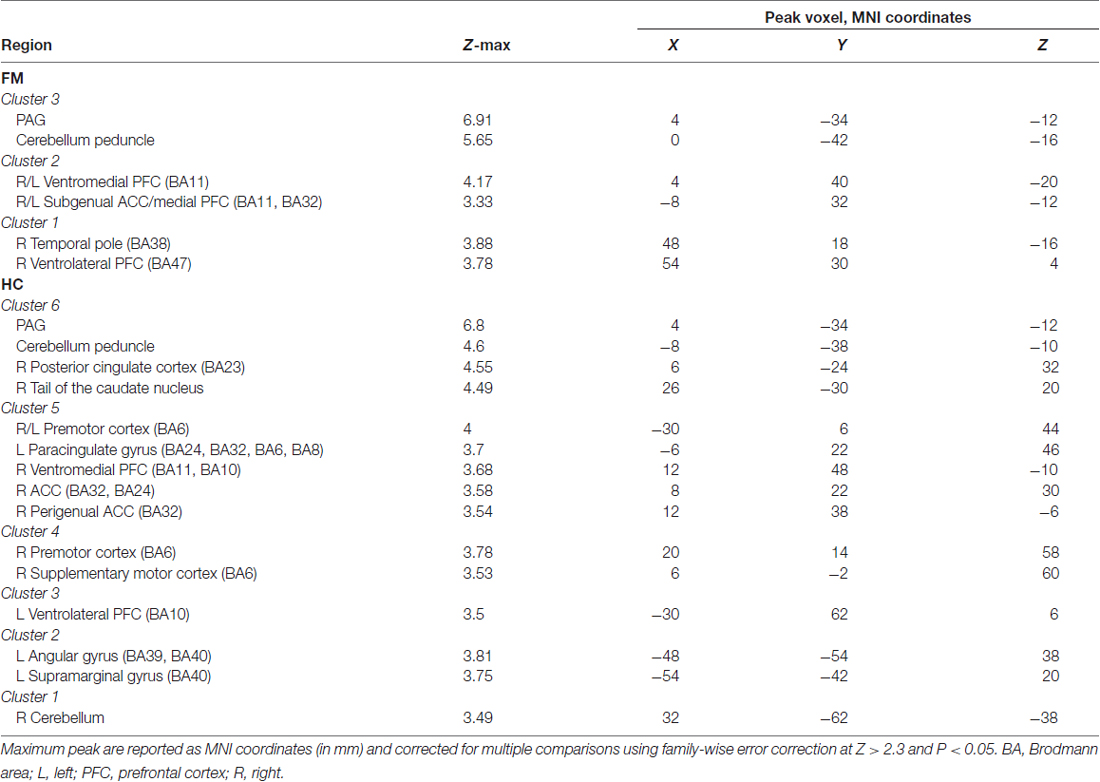
We first evaluated the FC of the PAG in both HC and FM groups (Figure 2, Table 3). Results are expressed as significant clusters of voxels, i.e., few adjacent voxels. In the HC group, we found that the PAG showed significant positive FC with six clusters of brain areas (see Table 3). These clusters included the brainstem, cerebellum, the anterior cingulate cortex (ACC), and the ventrolateral (vlPFC) and ventromedial (vmPFC) parts of the PFC, premotor cortex (PMC), and also to regions in the parietal lobe including the precuneus, angular gyrus (AG) and the posterior cingulate cortex (PCC). There were no significant regions found to have anticorrelated FC to the PAG in the HC group.
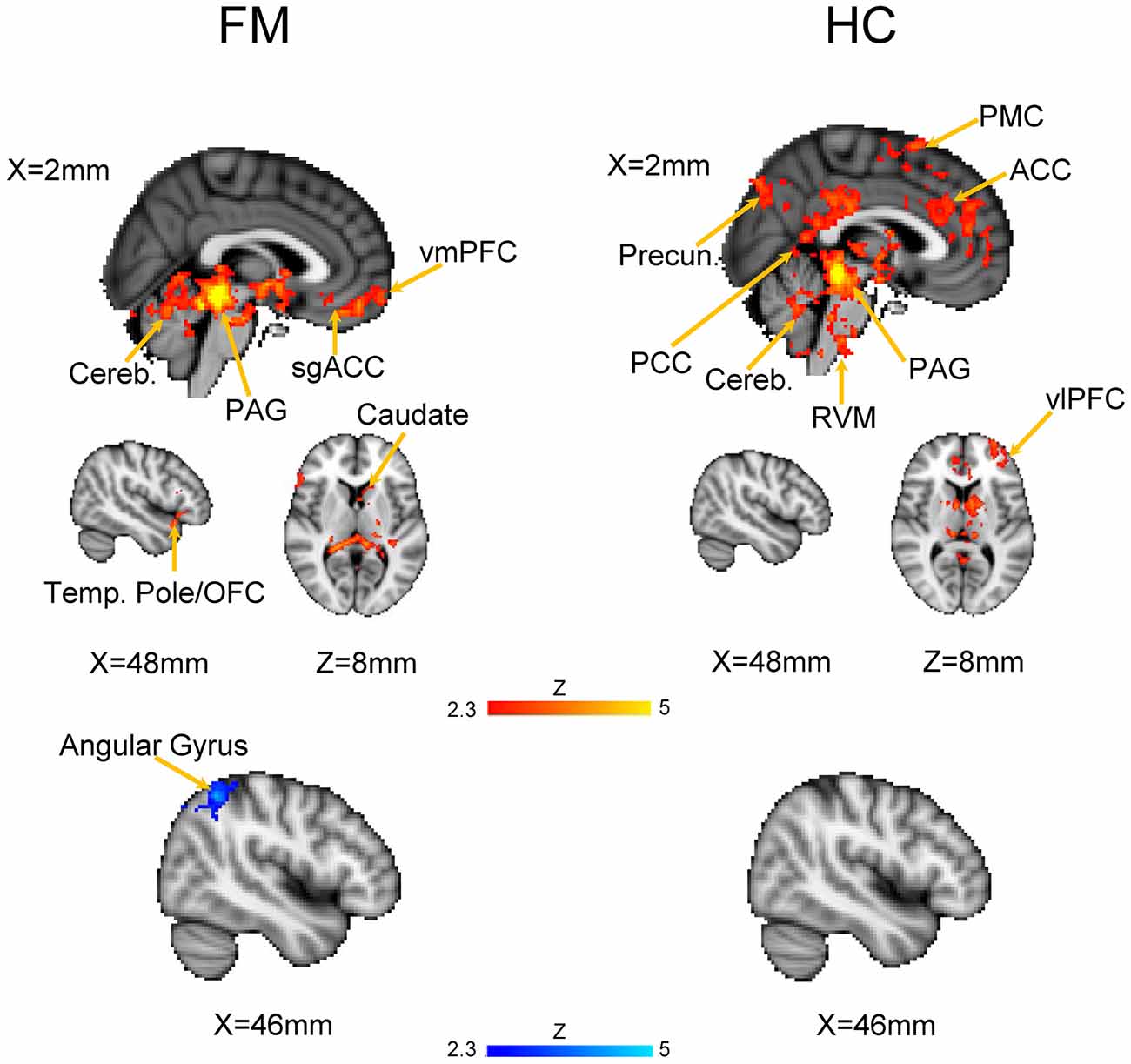
Figure 2. Functional connectivity (FC) of a PAG seed in patients with Fibromyalgia (FM; n = 23) and in healthy controls (HC; n = 16). Images are thresholded at a whole-brain family wise error rate-corrected Z > 2.3; cluster-based p < 0.05. Warm colors represent positive correlation, cold colors represent negative correlation. Cereb., cerebellum; vmPFC, ventromedial prefrontal cortex; sgACC, subgenual anterior cinculate cortex; PAG, periaqueducatal gray; OFC, orbitofrontal cortex; Temp. Pole, temporal pole; Precun., precuneus; PCC, posterior cingulate cortex; ACC, anterior cingulate cortex; PMC, premotor cortex; RVM, rostroventral medulla; vlPFC, ventrolateral PFC.
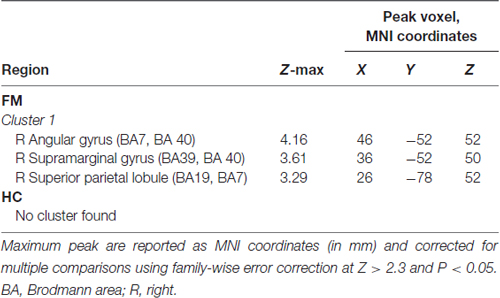
In the FM group, there were three clusters with positive FC to the PAG. These clusters encompassed the regions surrounding the PAG such as the mesencephalic reticular formation, subgenual ACC (sgACC), ventromedial and ventrolateral parts of the PFC, and the temporal pole (Figure 2, Table 3). Additionally, the PAG had negative (i.e., anticorrelations) FC with the right AG, supramarginal gyrus and the superior parietal lobule (Figure 2, Table 4).
We next performed a contrast analysis to determine differences in FC between the HC and FM groups (see Figure 3, Table 1). This analysis revealed a single cluster that had greater PAG FC in the FM group compared to the HC group. This cluster encompassed the left lingual gyrus, PCC, cuneus, retrosplenial cortex and hippocampus. Conversely, compared to the HC group, the FM group had reduced PAG FC with seven clusters of voxels (Table 1) located in the AG/lateral occipital cortex (LOC; bilaterally), PCC, PMC/supplementary motor area (SMA), dorsolateral (dlPFC)- dorsomedial (dmPFC) and vlPFC.
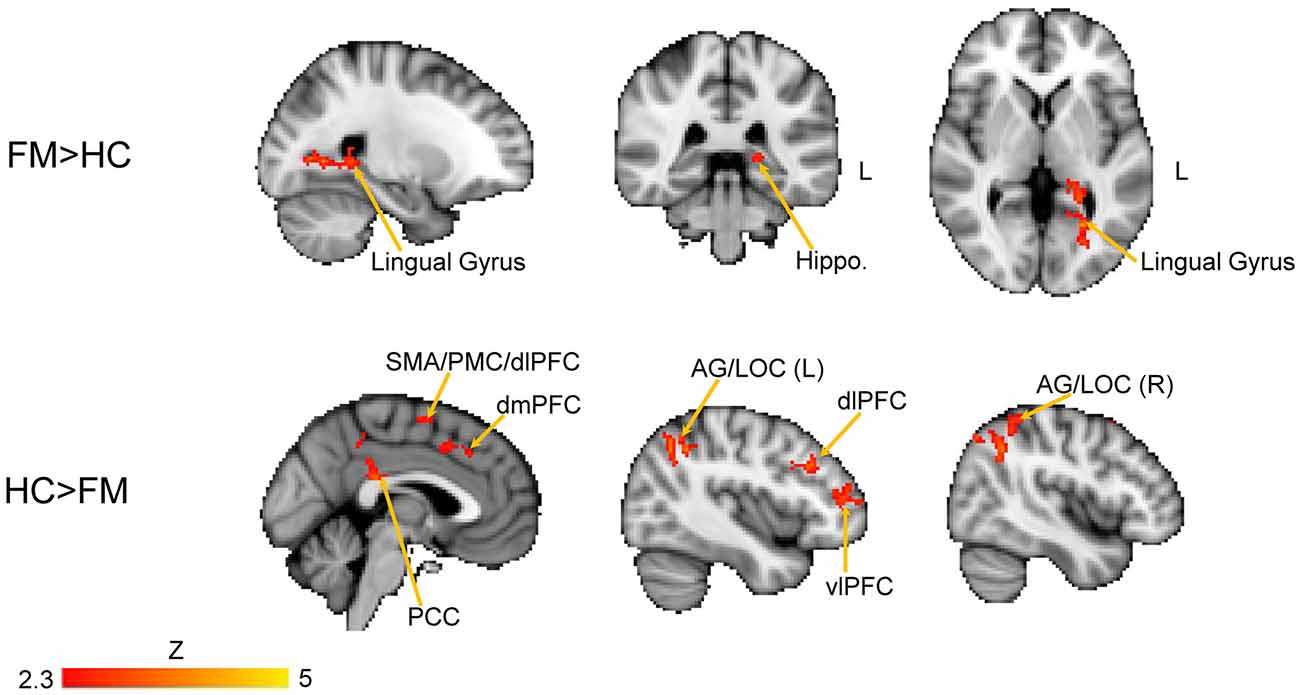
Figure 3. Contrast comparisons between FM and HC. Brain regions with enhanced resting state FC with the PAG in FM (n = 23) compared to HC (n = 16); FEW-corrected Z > 2.3; cluster-based p < 0.05. Coordinates for FM > HC, X = −24; Y = −38, Z = 2 (mm). HC > FM, X = −2, Y = −40, Z = 44 (mm). Hippo, hippocampus; PCC, posterior cingulate cortex; PMC, PFC; vlPFC, ventrolateral PFC; dlPFC, dorsolateral PFC; dmPFC, dorsomedial PFC; SMA, supplementary motor area; AG, angular gyrus; LOC, lateral occipital cortex.
Correlation between PAG FC and Clinical Manifestation of FM
The contrast comparison between FM and HC (FM > HC and HC > FM) yielded respectively 1 and 7 different clusters (Figure 3, Table 1). These clusters were correlated with pain symptoms and affect in FM subject (Figure 4). The single cluster that showed greater PAG FC in FM compared to HCs (FM > HC, i.e., lingual gyrus, PCC, cuneus, retrosplenial cortex and hippocampus) did not show statistically significant correlations with any of our affective or pain-related parameters. However, for HC > FM, cluster 3 (PMC and SMA) and cluster six dlPFC that showed reduced PAG FC in the FM group both were negatively correlated with the FIQ normalized score (cluster 3: r = −0.63, p < 0.002; cluster 6: r = −0.56, p < 0.006)). In addition, cluster 3 (PMC and SMA) and cluster 7 (dmPFC) were both correlated with the magnification subscale of the PCS (cluster 3: r = −0.56, p < 0.006; cluster 7: r = −0.65; p < 0.0009). Thus, a greater decrease in FC of these regions was associated with greater magnification of the pain.
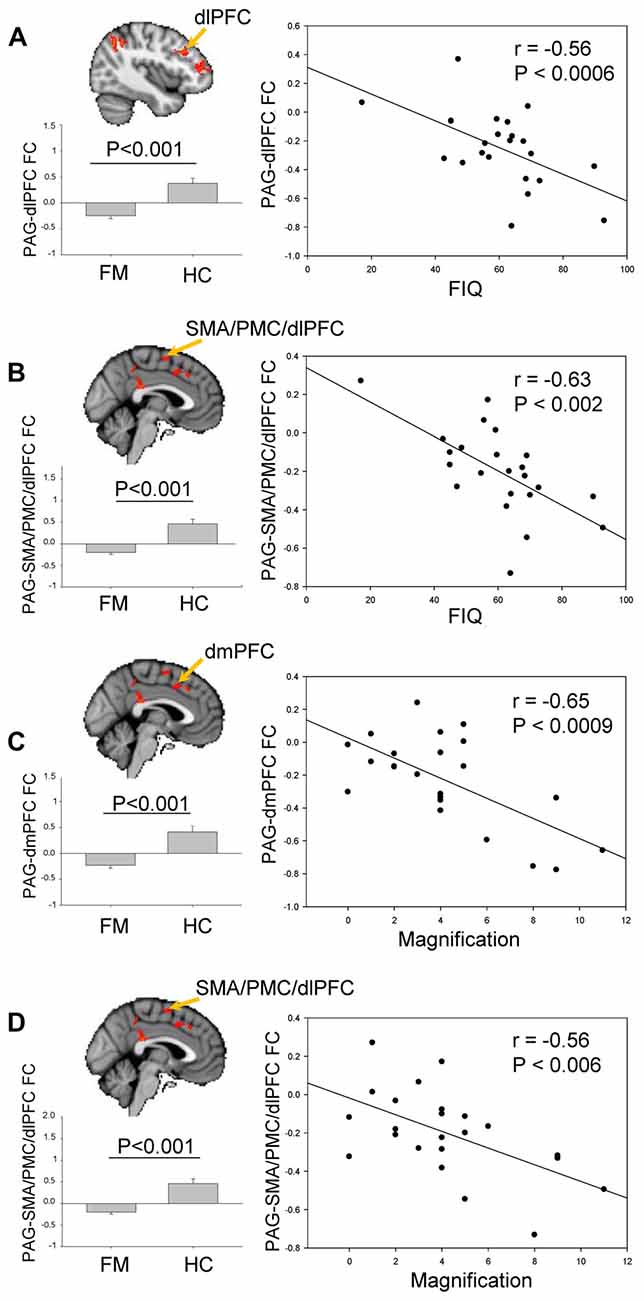
Figure 4. Brain regions exhibiting significant differences of PAG FC between FM and HC (see Figure 3). (A) Comparison of PAG-dlPFC FC (cluster 3) between HC and FM, and its correlation with Fibromyalgia Impact questionnaire (FIQ) scores. (B) Comparison of PAG-SMA/PMC/dlPFC FC (cluster 6) between HC and FM, and its correlation with FIQ scores. (C) Comparison of PAG-dmPFC FC (cluster 7) between HC and FM, and its correlation with the magnification subscale of the Pain Catastrophizing Scale (PCS). (D) Comparison of PAG- SMA/PMC/dlPFC FC (cluster 3) between HC and FM, and its correlation with the magnification subscale of the PCS. To control for multiple comparisons, statistical significance was set at p < 0.007 because of the seven psychological factors (anxiety, depression, distress, magnification, helplessness, rumination, global PCS score) and at p < 0.025 because of the two pain parameters (pain severity, FIQ normalized). PMC, PFC; dlPFC, dorsolateral PFC; dmPFC, dorsomedial PFC; SMA, supplementary motor area.
Discussion
In this study, we have demonstrated that FC of the PAG is disrupted in patients with FM compared to HCs, and that weakened connectivity is related to patients’ individual traits and the clinical manifestations of FM. The key findings of our study are compared to HCs, the FM patients had: (1) stronger PAG FC in a single brain cluster that encompasses the lingual gyrus, PCC, cuneus, retrosplenial cortex and hippocampus; (2) weaker PAG FC with regions associated with motor/executive functions, SN and DMN; and (3) decreased PAG FC in the PMC, SMA and PFC, is negatively connected with FM symptoms severity measured with the FIQ and with the magnification sub-score of the PCS. These results are discussed below as potential sites of dysfunction not only in FM, but possibly in other chronic pain diseases.
The various functions of the PAG, and subsequently its dysfunction in chronic conditions, can be understood through its connections to other brain areas and networks. For example, ongoing pain in FM patients has been shown to be negatively correlated with the resting state FC between the PAG and the executive attention network and insula (Pujol et al., 2014). Decreased FC has also been reported between the PAG and the ACC, thalamus and caudate nucleus in FM (Cifre et al., 2012). Furthermore, the (DMN, SN) show dysfunction across many chronic pain diseases, including in FM (Baliki et al., 2008; Napadow et al., 2010; Loggia et al., 2013; Kucyi et al., 2014). These networks may be particularly important in the pathology of FM because of their role in the hypervigilance present in FM (Crombez et al., 2004; Eccleston and Crombez, 2007). Many studies have shown dysfunction along the endocrine axis. For example, low melatonin secretion in FM has been suggested to play a role in the lack of restorative sleep (Wikner et al., 1998), and dysregulation of cortisol (Crofford et al., 1996, 2004; Crofford, 1998) perhaps leading to an allostatic load (sympathetic nervous system dysfunction and a general inability of the system to adapt to changes; Martinez-Lavin and Vargas, 2009; Martinez-Lavin, 2012; Martínez-Martínez et al., 2014). This concept is in line with the proposition that FM might be triggered by stress or altered saliency or interpretation of stimuli (Buskila et al., 2008; Häuser et al., 2011, 2013).
Previous brain imaging studies of FM have focused on pain-related brain abnormalities, evoked brain responses, and on localized regional cerebral blood flow abnormalities (Shokouhi et al., 2016). However, given the wide-range of clinical symptoms in FM, and the likely involvement of brain networks, here we used fMRI to identify variations in whole brain resting state FC of the PAG, and their association with the symptoms prevalent in FM.
It is important to consider the normal connectivity of the PAG to appreciate the dysfunctions found in the FM patients. We found greater FC in the left hippocampal formation, retrosplenial cortex and lingual gyrus in the FM patients. The retrosplenial cortex is adjacent to the caudal part of the corpus callosum (Broadmann area 29–30), and it has connection with the hippocampus formation, the PCC, the precuneus and the lingual gyrus. These regions are involved mostly in spatial memory, orientation and navigation, but some studies associate them to episodic/working memory and emotion-related functions (Sutherland et al., 1988; Maguire, 2001; Warburton et al., 2001; Harker and Whishaw, 2004; Spreng et al., 2009). Interestingly, the retrosplenial cortex has been found to be disrupted across several chronic pain states. For example, Wik et al. (2003) found reduced resting-state cerebral blood flow (rCBF) in the higher retrosplenial cortex of FM patients compared to controls (Wik et al., 2003), and showed that patients had lower rCBF only in the left retrosplenial cortex during acute (“barely tolerable”) pressure pain on a tender-point located above their right elbow for 1 min (Wik et al., 2006). Moreover, another team showed group effects between migraine patients and HC using resting-state FC and a seed located in the anterior insula (Hubbard et al., 2014). They reported increased rs-FC between the anterior insula and a region which appears to be the anterior part of the lingual gyrus, and increased gray matter volume in the left hippocampus in migraineurs. There is also support from animal models for a role of the retrosplenial cortex in chronic pain. For example, decreased metabolism in the retrosplenial cortex was found to predict neuropathic pain in a spinal nerve ligation rat model (Kim et al., 2014). Other animal studies have identified the retrosplenial cortex as being involved in emotional behavior, particularly associated with response to unfamiliar stimuli. Indeed, lesions of the retrosplenial cortex of rats results in increased anxiety and an impaired active avoidance response (Lukoyanov and Lukoyanova, 2006). Thus, in light of these previous studies, our findings suggest that the retrosplenial cortex has a role in a broader range of chronic pain syndromes. Further, given the lack of correlation of this regions with clinical manifestation of FM, we suggest that the role of retrosplenial cortex in chronic pain may be more fundamental to pain itself rather than to a specific negative affect or symptom severity.
Our results showed that the disrupted FCs between the left dlPFC and the right motor associative cortex (SMA/PMC/dlPFC) of FM was negatively correlated with FIQ score, which represent FM symptom severity. dlPFC is a main region of the frontal associative cortex, involved in shifting cognitive set following integration of information of many regions including the limbic system. Also, the dlPFC is particularly known for its central role in working memory holding information, mediating awareness in order to process chronological sequences and self-monitoring. Finally, dlPFC is known to be involved in pain modulation (Lorenz et al., 2003) through its connection with the ACC, the thalamus and the insula, which, when activated, decreased the connectivity between the midbrain and the thalamus resulting in descending pain modulation (Lorenz et al., 2003). Indeed, left dlPFC stimulation using repetitive transcranial magnetic stimulation (rTMS) decreased pain rating (Brighina et al., 2011). This relation between disrupted connectivity between the PAG and dlPFC and increased FIQ score is important because it sheds light on an impaired circuit in FM, which correlates with FM symptomatology. In fact, the involvement of this region does not seem to be specific to FM since a study showed abnormal activation and thinner gray matter of the left DLPFC in chronic low back pain patients compared to HC (Seminowicz et al., 2011). Those results are all in line with our findings, and once again suggest broader implication of this region. The disruption of this region seems to be generalized to multiple forms of chronic pain, and does not seem to be specific to FM or migraines.
Our results showed increased FC between the PAG and many brain regions involved in heteromodal integration of stimuli, which is predominant in the attention and SN, particularly the frontal and parietal association cortex and other regions of the DMN. The connectivity of these regions was not only decreased in FM, it was also correlated with the FIQ score and with the Magnification sub-scale of the PCS. The frontal association cortex plays an important role in sustained and selective attention and allows the brain to engage and disengage when relevant stimuli happen. The PMC and supplementary area are involved in the planning of self-initiated movements and motor learning, but has also been shown to play a role in anticipation of pain, possibly associated with avoidance behavior and catastrophizing (Gracely et al., 2004; Nachev et al., 2008; Kano et al., 2013). In relation to our results, some studies found a role of motor and pre-motor areas in depression. Exner et al. (2009) showed decreased gray matter volume in the right pre-supplementary areas of patients with depressive disorders with melancholic features. This result was associated with psychomotor retardation in these patients. Not only is GMV altered in depression, but so is the WM integrity of pathways connecting SMA, pre-SMA and M1 (Bracht et al., 2012). It has been shown that pain catastrophizing is correlated with development of depression in chronic pain (Keefe et al., 1989). Catastrophization is a maladaptive coping strategy, and it impacts a number of behavioral and neuronal processes involved in the shaping of chronic pain and the disabilities resulting of it.
In FM, disrupted connectivity between the DMN and pre-motor areas could be associated with fear of movement, psychomotor retardation, or maybe the study participants were simply more diligent about the instruction about not to move in the scanner. Furthermore, a recent study showed altered connectivity of the DMN in FM, in relation to the duration of the symptoms and cognitive and emotional processing (Fallon et al., 2016). A relationship between impaired FC of the DMN and the Rumination subscore of the PCS was also observed in temporomandibular syndrome (Kucyi et al., 2014). Interestingly, in our study, rumination was not correlated with any PAG disruption of FC. Disruption of the DMN seems to be present in many chronic pain disorders (Loggia et al., 2013; Kucyi et al., 2014; Hemington et al., 2016), and the involvement of the DMN in the efficiency of descending pain modulation system, as suggested by Kucyi et al. (2014), is very likely.
We note some limitations of our study relate to our choice of the PAG seed. This decision point impacts the results due to the heterogeneity of the PAG as shown in our previous study of the columnar organization of the PAG in HC (Coulombe et al., 2016). However, in our current study, we had a different goal, which was to determine the global involvement of the PAG with symptom severity in FM in general. As such, we did not study certain specifics in detail (e.g., laterality). In the future, it would be interesting to expand upon the current study to specifically detail each PAG column to examine hypotheses about dysfunction of the autonomic nervous system and allostasis in FM. We also note a technical limitation of the current study is the small size of the brainstem and studied regions. Thus, even small seeds and the technical issues of pre-processing are unlikely to be absolutely confined to subdivisions of the PAG and other areas. This issue also motivated our choice of a more global PAG seed.
Conclusion
Our findings assert the general multi-functionality of the PAG, an important hub that relays information about somatosensory inputs to provide a conscious and emotional perspective, and modulates nociceptive information. Both affective and somatosensory information are important and could influence the pain perception of FM patients. A novel finding was the increased FC in FM between the PAG and the retrosplenial cortex. This region is under-appreciated in chronic pain studies and further studies are needed to understand its specific role in chronic pain conditions. Future studies using a larger sample size could build on our current findings to investigate the columnar organization of the PAG in FM and its involvement in autonomic nervous system which are of interest to understand the pathophysiology of FM.
Author Contributions
The study was designed by KDD, KStL, DEM and PM-F. DEM and PM-F recruited the patients and did the clinical data assessment. KSL, MS and WRN acquired the imaging data and did some preliminary processing of the data. M-AC created the specific study questions, did the main data analysis and wrote the article with KDD. All authors participated in the editing of the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Kasey Hemington, Joshua Cheng and Aaron Kucyi for insights and technical assistance with data analysis and for providing the preprocessing pipeline. This work was funded by the Canadian Institutes of Health Research (KDD and KStL). M-AC had a fellowship from University of Toronto Centre for the Study of Pain.
Footnotes
References
Atkinson, T. M., Rosenfeld, B. D., Sit, L., Mendoza, T. R., Fruscione, M., Lavene, D., et al. (2011). Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J. Pain Symptom Manage. 41, 558–565. doi: 10.1016/j.jpainsymman.2010.05.008
Baliki, M. N., Geha, P. Y., Apkarian, A. V., and Chialvo, D. R. (2008). Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 28, 1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008
Bandler, R., Depaulis, A., and Vergnes, M. (1985). Identification of midbrain neurones mediating defensive behaviour in the rat by microinjections of excitatory amino acids. Behav. Brain Res. 15, 107–119. doi: 10.1016/0166-4328(85)90058-0
Bandler, R., Keay, K. A., Floyd, N., and Price, J. (2000). Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res. Bull. 53, 95–104. doi: 10.1016/s0361-9230(00)00313-0
Bandler, R., and Shipley, M. T. (1994). Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 17, 379–389. doi: 10.1016/0166-2236(94)90047-7
Behzadi, Y., Restom, K., Liau, J., and Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. doi: 10.1016/j.neuroimage.2007.04.042
Bennett, R. (2005). The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin. Exp. Rheumatol. 23, S154–S162. Available online at: http://www.myalgia.com/Bennett%20FIQ%20review%202005.pdf
Bennett, R. M., Bushmakin, A. G., Cappelleri, J. C., Zlateva, G., and Sadosky, A. B. (2009). Minimal clinically important difference in the fibromyalgia impact questionnaire. J. Rheumatol. 36, 1304–1311. doi: 10.3899/jrheum.081090
Bouhassira, D., Attal, N., Alchaar, H., Boureau, F., Brochet, B., Bruxelle, J., et al. (2005). Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 114, 29–36. doi: 10.1016/j.pain.2004.12.010
Bracht, T., Federspiel, A., Schnell, S., Horn, H., Höfle, O., Wiest, R., et al. (2012). Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS One 7:e52238. doi: 10.1371/journal.pone.0052238
Brighina, F., De Tommaso, M., Giglia, F., Scalia, S., Cosentino, G., Puma, A., et al. (2011). Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J. Headache Pain 12, 185–191. doi: 10.1007/s10194-011-0322-8
Buskila, D., Atzeni, F., and Sarzi-Puttini, P. (2008). Etiology of fibromyalgia: the possible role of infection and vaccination. Autoimmun. Rev. 8, 41–43. doi: 10.1016/j.autrev.2008.07.023
Chai, X. J., Castañón, A. N., Ongür, D., and Whitfield-Gabrieli, S. (2012). Anticorrelations in resting state networks without global signal regression. Neuroimage 59, 1420–1428. doi: 10.1016/j.neuroimage.2011.08.048
Chalaye, P., Goffaux, P., Bourgault, P., Lafrenaye, S., Devroede, G., Watier, A., et al. (2012). Comparing pain modulation and autonomic responses in fibromyalgia and irritable bowel syndrome patients. Clin. J. Pain 28, 519–526. doi: 10.1097/AJP.0b013e31823ae69e
Chalaye, P., Lafrenaye, S., Goffaux, P., and Marchand, S. (2014). The role of cardiovascular activity in fibromyalgia and conditioned pain modulation. Pain 155, 1064–1069. doi: 10.1016/j.pain.2013.12.023
Cifre, I., Sitges, C., Fraiman, D., Muñoz, M. Á., Balenzuela, P., González-Roldán, A., et al. (2012). Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom. Med. 74, 55–62. doi: 10.1097/PSY.0b013e3182408f04
Clauw, D. J. (2014). Fibromyalgia: a clinical review. JAMA 311, 1547–1555. doi: 10.1001/jama.2014.3266
Clauw, D. J. (2015). What is the meaning of “small fiber neuropathy” in fibromyalgia? Pain 156, 2115–2116. doi: 10.1097/j.pain.0000000000000311
Cleeland, C. S., and Ryan, K. M. (1994). Pain assessment: global use of the Brief Pain Inventory. Ann. Acad. Med. Singap. 23, 129–138.
Coulombe, M. A., Erpelding, N., Kucyi, A., and Davis, K. D. (2016). Intrinsic functional connectivity of periaqueductal gray subregions in humans. Hum. Brain Mapp. 37, 1514–1530. doi: 10.1002/hbm.23117
Crofford, L. J. (1998). The hypothalamic-pituitary-adrenal stress axis in fibromyalgia and chronic fatigue syndrome. Z. Rheumatol. 57, 67–71. doi: 10.1007/s003930050239
Crofford, L. J., Engleberg, N. C., and Demitrack, M. A. (1996). Neurohormonal perturbations in fibromyalgia. Baillieres Clin. Rheumatol. 10, 365–378. doi: 10.1016/s1521-6942(06)80047-1
Crofford, L. J., Young, E. A., Engleberg, N. C., Korszun, A., Brucksch, C. B., McClure, L. A., et al. (2004). Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav. Immun. 18, 314–325. doi: 10.1016/j.bbi.2003.12.011
Crombez, G., Eccleston, C., Van den Broeck, A., Goubert, L., and Van Houdenhove, B. (2004). Hypervigilance to pain in fibromyalgia: the mediating role of pain intensity and catastrophic thinking about pain. Clin. J. Pain 20, 98–102. doi: 10.1097/00002508-200403000-00006
Davis, K. D., and Moayedi, M. (2013). Central mechanisms of pain revealed through functional and structural MRI. J. Neuroimmune Pharmacol. 8, 518–534. doi: 10.1007/s11481-012-9386-8
Devigili, G., Tugnoli, V., Penza, P., Camozzi, F., Lombardi, R., Melli, G., et al. (2008). The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 131, 1912–1925. doi: 10.1093/brain/awn093
Doppler, K., Rittner, H. L., Deckart, M., and Sommer, C. (2015). Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. Pain 156, 2319–2325. doi: 10.1097/j.pain.0000000000000285
Dostrovsky, J. O., and Deakin, J. F. (1977). Periaqueductal grey lesions reduce morphine analgesia in the rat. Neurosci. Lett. 4, 99–103. doi: 10.1016/0304-3940(77)90151-3
Eccleston, C., and Crombez, G. (2007). Worry and chronic pain: a misdirected problem solving model. Pain 132, 233–236. doi: 10.1016/j.pain.2007.09.014
Eippert, F., Bingel, U., Schoell, E. D., Yacubian, J., Klinger, R., Lorenz, J., et al. (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543. doi: 10.1016/j.neuron.2009.07.014
Exner, C., Lange, C., and Irle, E. (2009). Impaired implicit learning and reduced pre-supplementary motor cortex size in early-onset major depression with melancholic features. J. Affect. Disord. 119, 156–162. doi: 10.1016/j.jad.2009.03.015
Fallon, N., Chiu, Y., Nurmikko, T., and Stancak, A. (2016). Functional connectivity with the default mode network is altered in fibromyalgia patients. PLoS One 11:e0159198. doi: 10.1371/journal.pone.0159198
Giannoccaro, M. P., Donadio, V., Incensi, A., Avoni, P., and Liguori, R. (2014). Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve 49, 757–759. doi: 10.1002/mus.24156
Gracely, R. H., Geisser, M. E., Giesecke, T., Grant, M. A., Petzke, F., Williams, D. A., et al. (2004). Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 127, 835–843. doi: 10.1093/brain/awh098
Harker, K. T., and Whishaw, I. Q. (2004). Impaired place navigation in place and matching-to-place swimming pool tasks follows both retrosplenial cortex lesions and cingulum bundle lesions in rats. Hippocampus 14, 224–231. doi: 10.1002/hipo.10159
Hassett, A. L., Cone, J. D., Patella, S. J., and Sigal, L. H. (2000). The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis Rheum. 43, 2493–2500. doi: 10.1002/1529-0131(200011)43:11<2493::AID-ANR17>3.0.CO;2-W
Häuser, W., Galek, A., Erbslöh-Möller, B., Köllner, V., Kühn-Becker, H., Langhorst, J., et al. (2013). Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 154, 1216–1223. doi: 10.1016/j.pain.2013.03.034
Häuser, W., Kosseva, M., Üceyler, N., Klose, P., and Sommer, C. (2011). Emotional, physical, and sexual abuse in fibromyalgia syndrome: a systematic review with meta-analysis. Arthritis Care Res. 63, 808–820. doi: 10.1002/acr.20328
Hemington, K. S., and Coulombe, M. A. (2015). The periaqueductal gray and descending pain modulation: why should we study them and what role do they play in chronic pain? J. Neurophysiol. 114, 2080–2083. doi: 10.1152/jn.00998.2014
Hemington, K. S., Wu, Q., Kucyi, A., Inman, R. D., and Davis, K. D. (2016). Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct. Funct. 221, 4203–4219. doi: 10.1007/s00429-015-1161-1
Hubbard, C. S., Khan, S. A., Keaser, M. L., Mathur, V. A., Goyal, M., and Seminowicz, D. A. (2014). Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 1:e20.14. doi: 10.1523/eneuro.0006-14.2014
Jensen, K. B., Loitoile, R., Kosek, E., Petzke, F., Carville, S., Fransson, P., et al. (2012). Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol. Pain 8:32. doi: 10.1186/1744-8069-8-32
Julien, N., Goffaux, P., Arsenault, P., and Marchand, S. (2005). Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 114, 295–302. doi: 10.1016/j.pain.2004.12.032
Kano, M., Farmer, A. D., Aziz, Q., Giampietro, V. P., Brammer, M. J., Williams, S. C., et al. (2013). Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G687–G699. doi: 10.1152/ajpgi.00385.2012
Keay, K. A., and Bandler, R. (1993). Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal grey of the rat. Neurosci. Lett. 154, 23–26. doi: 10.1016/0304-3940(93)90162-e
Keefe, F. J., Brown, G. K., Wallston, K. A., and Caldwell, D. S. (1989). Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain 37, 51–56. doi: 10.1016/0304-3959(89)90152-8
Kim, C. E., Kim, Y. K., Chung, G., Im, H. J., Lee, D. S., Kim, J., et al. (2014). Identifying neuropathic pain using 18F-FDG micro-PET: a multivariate pattern analysis. Neuroimage 86, 311–316. doi: 10.1016/j.neuroimage.2013.10.001
Kosmidis, M. L., Koutsogeorgopoulou, L., Alexopoulos, H., Mamali, I., Vlachoyiannopoulos, P. G., Voulgarelis, M., et al. (2014). Reduction of Intraepidermal Nerve Fiber Density (IENFD) in the skin biopsies of patients with fibromyalgia: a controlled study. J. Neurol. Sci. 347, 143–147. doi: 10.1016/j.jns.2014.09.035
Kucyi, A., Moayedi, M., Weissman-Fogel, I., Goldberg, M. B., Freeman, B. V., Tenenbaum, H. C., et al. (2014). Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J. Neurosci. 34, 3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014
Kucyi, A., Salomons, T. V., and Davis, K. D. (2013). Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc. Natl. Acad. Sci. U S A 110, 18692–18697. doi: 10.1073/pnas.1312902110
Lautenbacher, S., and Rollman, G. B. (1997). Possible deficiencies of pain modulation in fibromyalgia. Clin. J. Pain 13, 189–196. doi: 10.1097/00002508-199709000-00003
Levine, T. D., and Saperstein, D. S. (2015). Routine use of punch biopsy to diagnose small fiber neuropathy in fibromyalgia patients. Clin. Rheumatol. 34, 413–417. doi: 10.1007/s10067-014-2850-5
Linnman, C., Moulton, E. A., Barmettler, G., Becerra, L., and Borsook, D. (2012). Neuroimaging of the periaqueductal gray: state of the field. Neuroimage 60, 505–522. doi: 10.1016/j.neuroimage.2011.11.095
Loggia, M. L., Kim, J., Gollub, R. L., Vangel, M. G., Kirsch, I., Kong, J., et al. (2013). Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain 154, 24–33. doi: 10.1016/j.pain.2012.07.029
Lorenz, J., Minoshima, S., and Casey, K. L. (2003). Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126, 1079–1091. doi: 10.1093/brain/awg102
Lovick, T. A. (1985). Ventrolateral medullary lesions block the antinociceptive and cardiovascular responses elicited by stimulating the dorsal periaqueductal grey matter in rats. Pain 21, 241–252. doi: 10.1016/0304-3959(85)90088-0
Lukoyanov, N. V., and Lukoyanova, E. A. (2006). Retrosplenial cortex lesions impair acquisition of active avoidance while sparing fear-based emotional memory. Behav. Brain Res. 173, 229–236. doi: 10.1016/j.bbr.2006.06.026
Maguire, E. A. (2001). The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand. J. Psychol. 42, 225–238. doi: 10.1111/1467-9450.00233
Martinez-Lavin, M. (2012). Fibromyalgia: when distress becomes (Un)sympathetic pain. Pain Res. Treat. 2012:981565. doi: 10.1155/2012/981565
Martinez-Lavin, M., and Vargas, A. (2009). Complex adaptive systems allostasis in fibromyalgia. Rheum. Dis. Clin. North Am. 35, 285–298. doi: 10.1016/j.rdc.2009.05.005
Martínez-Martínez, L. A., Mora, T., Vargas, A., Fuentes-Iniestra, M., and Martínez-Lavín, M. (2014). Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studies. J. Clin. Rheumatol. 20, 146–150. doi: 10.1097/RHU.0000000000000089
Muschelli, J., Nebel, M. B., Caffo, B. S., Barber, A. D., Pekar, J. J., and Mostofsky, S. H. (2014). Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 96C, 22–35. doi: 10.1016/j.neuroimage.2014.03.028
Nachev, P., Kennard, C., and Husain, M. (2008). Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 9, 856–869. doi: 10.1038/nrn2478
Napadow, V., LaCount, L., Park, K., As-Sanie, S., Clauw, D. J., and Harris, R. E. (2010). Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 62, 2545–2555. doi: 10.1002/art.27497
Oaklander, A. L., Herzog, Z. D., Downs, H. M., and Klein, M. M. (2013). Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 154, 2310–2316. doi: 10.1016/j.pain.2013.06.001
Perrot, S., Dumont, D., Guillemin, F., Pouchot, J., Coste, J., and French Group for Quality of Life Research. (2003). Quality of life in women with fibromyalgia syndrome: validation of the QIF, the French version of the fibromyalgia impact questionnaire. J. Rheumatol. 30, 1054–1059. Available online at: http://www.jrheum.org/content/30/5/1054
Price, D. D., Staud, R., Robinson, M. E., Mauderli, A. P., Cannon, R., and Vierck, C. J. (2002). Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 99, 49–59. doi: 10.1016/s0304-3959(02)00053-2
Pujol, J., Macià, D., Garcia-Fontanals, A., Blanco-Hinojo, L., Lãpez-Solà, M., Garcia-Blanco, S., et al. (2014). The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain 155, 1492–1503. doi: 10.1016/j.pain.2014.04.028
Reynolds, D. V. (1969). Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164, 444–445. doi: 10.1126/science.164.3878.444
Rogachov, A., Cheng, J. C., Erpelding, N., Hemington, K. S., Crawley, A. P., and Davis, K. D. (2016). Regional brain signal variability: a novel indicator of pain sensitivity and coping. Pain 157, 2483–2492. doi: 10.1097/j.pain.0000000000000665
Schmidt-Wilcke, T., Ichesco, E., Hampson, J. P., Kairys, A., Peltier, S., Harte, S., et al. (2014). Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin. 6, 252–261. doi: 10.1016/j.nicl.2014.09.007
Seminowicz, D. A., Wideman, T. H., Naso, L., Hatami-Khoroushahi, Z., Fallatah, S., Ware, M. A., et al. (2011). Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 31, 7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011
Serra, J., Collado, A., Solà, R., Antonelli, F., Torres, X., Salgueiro, M., et al. (2014). Hyperexcitable C nociceptors in fibromyalgia. Ann. Neurol. 75, 196–208. doi: 10.1002/ana.24065
Shokouhi, M., Davis, K. D., Moulin, D. E., Morley-Forster, P., Nielson, W. R., Bureau, Y., et al. (2016). Basal ganglia perfusion in fibromyalgia is related to pain disability and disease impact: an arterial spin labeling study. Clin. J. Pain 32, 495–505. doi: 10.1097/AJP.0000000000000295
Spreng, R. N., Mar, R. A., and Kim, A. S. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510. doi: 10.1162/jocn.2008.21029
Staud, R., Cannon, R. C., Mauderli, A. P., Robinson, M. E., Price, D. D., and Vierck, C. J. Jr. (2003). Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain 102, 87–95. doi: 10.1016/s0304-3959(02)00344-5
Staud, R., and Smitherman, M. L. (2002). Peripheral and central sensitization in fibromyalgia: pathogenetic role. Curr. Pain Headache Rep. 6, 259–266. doi: 10.1007/s11916-002-0046-1
Sullivan, M. J. L., Bishop, S. R., and Pivik, J. (1995). The pain catastrophizing scale: development and validation. Psychol. Assess. 7, 524–532. doi: 10.1037/1040-3590.7.4.524
Sutherland, R. J., Whishaw, I. Q., and Kolb, B. (1988). Contributions of cingulate cortex to two forms of spatial learning and memory. J. Neurosci. 8, 1863–1872.
Tait, R. C., Pollard, C. A., Margolis, R. B., Duckro, P. N., and Krause, S. J. (1987). The pain disability index: psychometric and validity data. Arch. Phys. Med. Rehabil. 68, 438–441.
Warburton, E. C., Baird, A., Morgan, A., Muir, J. L., and Aggleton, J. P. (2001). The conjoint importance of the hippocampus and anterior thalamic nuclei for allocentric spatial learning: evidence from a disconnection study in the rat. J. Neurosci. 21, 7323–7330.
Wik, G., Fischer, H., Bragée, B., Kristianson, M., and Fredrikson, M. (2003). Retrosplenial cortical activation in the fibromyalgia syndrome. Neuroreport 14, 619–621. doi: 10.1097/01.wnr.0000063662.48571.ea
Wik, G., Fischer, H., Finer, B., Bragee, B., Kristianson, M., and Fredrikson, M. (2006). Retrospenial cortical deactivation during painful stimulation of fibromyalgic patients. Int. J. Neurosci. 116, 1–8. doi: 10.1080/00207450690962208
Wikner, J., Hirsch, U., Wetterberg, L., and Röjdmark, S. (1998). Fibromyalgia—a syndrome associated with decreased nocturnal melatonin secretion. Clin. Endocrinol. 49, 179–183. doi: 10.1046/j.1365-2265.1998.00503.x
Wolfe, F., Anderson, J., Harkness, D., Bennett, R. M., Caro, X. J., Goldenberg, D. L., et al. (1997). Health status and disease severity in fibromyalgia: results of a six-center longitudinal study. Arthritis Rheum. 40, 1571–1579. doi: 10.1002/1529-0131(199709)40:9<1571::AID-ART5>3.0.CO;2-0
Wolfe, F., Clauw, D. J., Fitzcharles, M. A., Goldenberg, D. L., Katz, R. S., Mease, P., et al. (2010). The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 62, 600–610. doi: 10.1002/acr.20140
Wolfe, F., Smythe, H. A., Yunus, M. B., Bennett, R. M., Bombardier, C., Goldenberg, D. L., et al. (1990). The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 33, 160–172. doi: 10.1002/art.1780330203
Keywords: functional magnetic resonance imaging, periaqueductal gray, resting-state fMRI, functional connectivity, descending pain modulation, fibromyalgia
Citation: Coulombe M-A, St. Lawrence K, Moulin DE, Morley-Forster P, Sohokouhi M, Nielson WR and Davis KD (2017) Lower Functional Connectivity of the Periaqueductal Gray Is Related to Negative Affect and Clinical Manifestations of Fibromyalgia. Front. Neuroanat. 11:47. doi: 10.3389/fnana.2017.00047
Received: 27 March 2017; Accepted: 15 May 2017;
Published: 08 June 2017.
Edited by:
Yun-Qing Li, Fourth Military Medical University, ChinaReviewed by:
Christoph Schmitz, Ludwig-Maximilians-Universität München, GermanyAdelaida María A. M. Castro Sánchez, University of Almería, Spain
Copyright © 2017 Coulombe, St. Lawrence, Moulin, Morley-Forster, Sohokouhi, Nielson and Davis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen D. Davis, kdavis@uhnres.utoronto.ca
 Marie-Andrée Coulombe
Marie-Andrée Coulombe Keith St. Lawrence
Keith St. Lawrence Dwight E. Moulin4
Dwight E. Moulin4  Karen D. Davis
Karen D. Davis