Aversive emotional interference impacts behavior and prefronto-striatal activity during increasing attentional control
- 1Department of Basic Medical Sciences, Neuroscience and Sense Organs, University of Bari “Aldo Moro”, Bari, Italy
- 2IRCCS “Casa Sollievo della Sofferenza”, S. Giovanni Rotondo, Italy
- 3Cognitive Brain Research Unit, Institute of Behavioral Sciences, University of Helsinki, Helsinki, Finland
- 4Azienda Ospedaliero-Universitaria Consorziale, Policlinico di Bari, Bari, Italy
- 5pRED, NORD DTA Neuroscience, Hoffman-La Roche Ltd, Basel, Switzerland
Earlier studies have demonstrated that emotional stimulation modulates attentional processing during goal-directed behavior and related activity of a brain network including the inferior frontal gyrus (IFG) and the caudate nucleus. However, it is not clear how emotional interference modulates behavior and brain physiology during variation in attentional control, a relevant question for everyday life situations in which both emotional stimuli and cognitive load vary. The aim of this study was to investigate the impact of negative emotions on behavior and activity in IFG and caudate nucleus during increasing levels of attentional control. Twenty two healthy subjects underwent event-related functional magnetic resonance imaging while performing a task in which neutral or fearful facial expressions were displayed before stimuli eliciting increasing levels of attentional control processing. Results indicated slower reaction time (RT) and greater right IFG activity when fearful compared with neutral facial expressions preceded the low level of attentional control. On the other hand, fearful facial expressions preceding the intermediate level of attentional control elicited faster behavioral responses and greater activity in the right and left sides of the caudate. Finally, correlation analysis indicated a relationship between behavioral correlates of attentional control after emotional interference and right IFG activity. All together, these results suggest that the impact of negative emotions on attentional processing is differentially elicited at the behavioral and physiological levels as a function of cognitive load.
Introduction
In everyday living, we perceive a complex visual environment and fully analyze many items and events at one time. Attentional control allows the flexible allocation of attentional resources to relevant stimuli while suppressing stimuli that are less relevant. This cognitive process provides a top-down bias for analysis and representation of relevant information in the face of concurrent and non-relevant information (Desimone and Duncan, 1995).
According to the load theory of attention, modulation of attentional processes by distractors during goal-directed behavior depends on the cognitive load (Lavie et al., 2004; Lavie, 2005). More specifically, processing of task-irrelevant information is dependent upon the perceptual attentional load in such a way that task-irrelevant information is processed only under low attentional conditions and is suppressed by high attentional loads. In other words, an increase in cognitive demands by active attentional processing may lead to greater inhibition of elaboration of distracting stimuli and to a reduced impact of these stimuli on behavior. This top-down regulation during goal-directed behavior may therefore be seen as a mechanism of hierarchical integration to ensure maintenance of performance at higher cognitive loads in the presence of potentially interfering stimuli (Gray et al., 2002; Serrien et al., 2006). However, the attentional load concept does not fully explain the divergent results reported in the literature, and it may be more flexible than initially thought. For example, facilitation effects on behavioral performance by task-irrelevant stimuli have been reported (Xu et al., 2011; Ziaei et al., 2014).
Emotional cues are critical during social interactions and can modulate cognitive processes, including attention. According to the “salience hypothesis,” modulation of attentional processes may also depend on emotional salience of task-irrelevant stimuli (Eltiti et al., 2005; Gupta and Srinivasan, 2015). For example, previous studies have indicated that negative, relative to neutral, emotional stimuli are associated with impairment of accuracy and reaction time (RT) during goal-directed attentional control processes (Simpson et al., 2000; Hare et al., 2005; Blair et al., 2007; Hindi Attar and Müller, 2012; Jasinska et al., 2012). On the other hand, some studies have reported facilitation of attentional processes during emotional interference (Geng and Diquattro, 2010; Swallow and Jiang, 2010; Kanske and Kotz, 2011; Lindström and Bohlin, 2011; Ziaei et al., 2014). Emotional and attentional paradigms in these studies varied both in terms of salience and attentional load, possibly explaining some of these inconsistencies. At the physiological level, the interaction between emotion and cognition is based on dynamic coordination between various brain areas that often participate in both emotion elaboration and cognition (Pessoa, 2008; Kellermann et al., 2012; Dolcos et al., 2013). For example, cognitive processes involving differentiation between targets and distractors and selection of appropriate responses have been associated with activity in prefronto-striatal circuits (McNab and Klingberg, 2008; Xu et al., 2011; Jarcho et al., 2014; Langeslag et al., 2014). Interestingly, in some recent studies, both inferior frontal gyrus (IFG) and caudate nucleus have been involved in the physiology of emotional interference during goal-directed attentional control processes (Langeslag et al., 2014; Ziaei et al., 2014). In particular, the IFG has been specifically associated with sustained attentional control, coding of behavioral significance of emotional stimuli, inhibition of distracting negative emotions, and regulation of motor responses (Aron et al., 2004; Ochsner et al., 2004; Phan et al., 2005; Beer et al., 2006; Dolcos and McCarthy, 2006; Erk et al., 2007; Sakagami and Pan, 2007; Mitchell et al., 2008; Sommer et al., 2008; Schulz et al., 2009; Urry et al., 2009; Hampshire et al., 2010; Mincic, 2010; Munakata et al., 2011; Shafer and Dolcos, 2012; Depue et al., 2015). In more detail, the orbital and triangular parts of the IFG are distinctly involved in resolution of emotional interference (Schulz et al., 2009; Levens and Phelps, 2010). The orbital part is the main area of the IFG to interface between sensory events and cognitive control (Sakagami and Pan, 2007; Kret et al., 2011). The opercular and triangular parts of the IFG are both involved in executive function and emotional interference (Schulz et al., 2009; Barber et al., 2013). However, these two areas are cytoarchitectonically different and functionally dissociable (Heim et al., 2009; Katzev et al., 2013). Furthermore, the caudate nucleus has been related to attentional processing, elaboration of negative stimuli, and related arousal (Crofts et al., 2001; Herwig et al., 2007; Roiser et al., 2007; Scholes et al., 2007; Carretié et al., 2009; Levita et al., 2009; Gerdes et al., 2010; Geliebter et al., 2013; Hart et al., 2013; Jarcho et al., 2014), as well as to integration of emotional and cognitive processing and suppression of emotional interference (Langeslag et al., 2014; Ziaei et al., 2014). Interestingly, IFG activation has been associated with emotional interference during processing of stimuli requiring low levels of attention, and caudate activation has been associated with emotional interference when high levels of attentional processing are required (Blair et al., 2007; Ali et al., 2010; Xu et al., 2011; Ziaei et al., 2014). However, there are no studies exploring how emotional interference modulates activity in these areas and related behavior during increasing levels of attentional control processing.
The aim of this study was to investigate the potential for emotional task-irrelevant items to modulate behavior and brain activity during various levels of attentional control. With this purpose, we used stimuli from a recently developed cognitive paradigm, the Variable Attentional Control (VAC) task, which requires increasing levels of attentional control processing associated with a physiological linear increase in prefrontal activity (Blasi et al., 2005, 2007, 2010, 2013; Zhang et al., 2007). The three levels of attentional control generated by the stimuli of this task were manipulated to study the relationship between varying cognitive load and emotional processing. Fearful and neutral facial expressions were used to add emotional interference for each level of attentional processing. Based on previous studies, we predicted that cognitive performance as well as activity in the IFG and in the caudate nucleus would vary as a function of the interaction between attentional load and emotional interference. According to recent findings, we also predicted that the IFG would be involved in the physiology of emotional interference at low levels of attentional control and the caudate nucleus at higher levels (Blair et al., 2007; Ali et al., 2010; Xu et al., 2011; Ziaei et al., 2014). Consistent with the load theory of attention, and given the greater potential for emotional interference of aversive, relative to neutral, stimuli, we also hypothesized that brain activity in these areas would be preferentially sensitive to negative emotional interference during lower loads of attentional control.
Methods
Subjects
Twenty-two healthy subjects were enrolled in this study (13 males; mean age ± SD, 25.5 ± 4.5 years). Inclusion criteria were absence of any psychiatric disorder, as evaluated using the Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV, of any significant neurological or medical condition revealed by clinical and magnetic resonance imaging evaluation, of history of head trauma with loss of consciousness, and of pharmacological treatment that could influence cerebral metabolism or blood flow, or drug abuse in the past year. The Wechsler Intelligence Scale—Revised was used to evaluate the intelligent quotient (IQ) (mean ± SD, 103.2 ± 14.5). Socio-economic status (Hollingshead and Redlich, 1955) (mean ± SD, 36.3 ± 16.2) and handedness (Oldfield, 1971) were also measured (mean ± SD, 0.74 ± 0.4). All subjects underwent fMRI while performing the Emotional VAC task (see below).
Written informed consent was obtained from all participants prior to enrolling them in the study, which received approval from the Independent Ethical Committee of “Azienda Ospedaliero-Universitaria Consorziale, Policlinico di Bari”. All experimental procedures were carried out in accordance with the 2013 WMA Declaration of Helsinki.
Emotional Variable Attentional Control (EVAC) Task
Subjects performed a task (Figure 1) that was specifically designed to obtain emotional interference preceding various demands of attentional control processing. Attentional stimuli used in this task were identical to those used in previous studies (Blasi et al., 2005, 2007, 2010, 2011, 2013; Zhang et al., 2007). These stimuli consisted of arrows of three different sizes (1 large, 6 medium, 42 small) pointing either to the right or to the left; seven small arrows were embedded in each medium-sized arrow; and six medium-sized arrows were embedded in one large arrow. The direction of the arrows of each size was always the same. To increase the level of attentional control required, the direction of the stimuli of each size was congruent or incongruent relative to those of other sizes. This resulted in the following conditions:
• Low level of attentional control (LOW): All three sizes of arrows were congruent in direction with each other. The cue was the word BIG.
• Intermediate level of attentional control (INT): The big arrow was incongruent in direction to the small and the medium arrows; the cue was the word SMALL.
• High level of attentional control (HIGH): The medium sized-arrows were incongruent in direction to the big and the small arrows; the cue was the word SMALL.
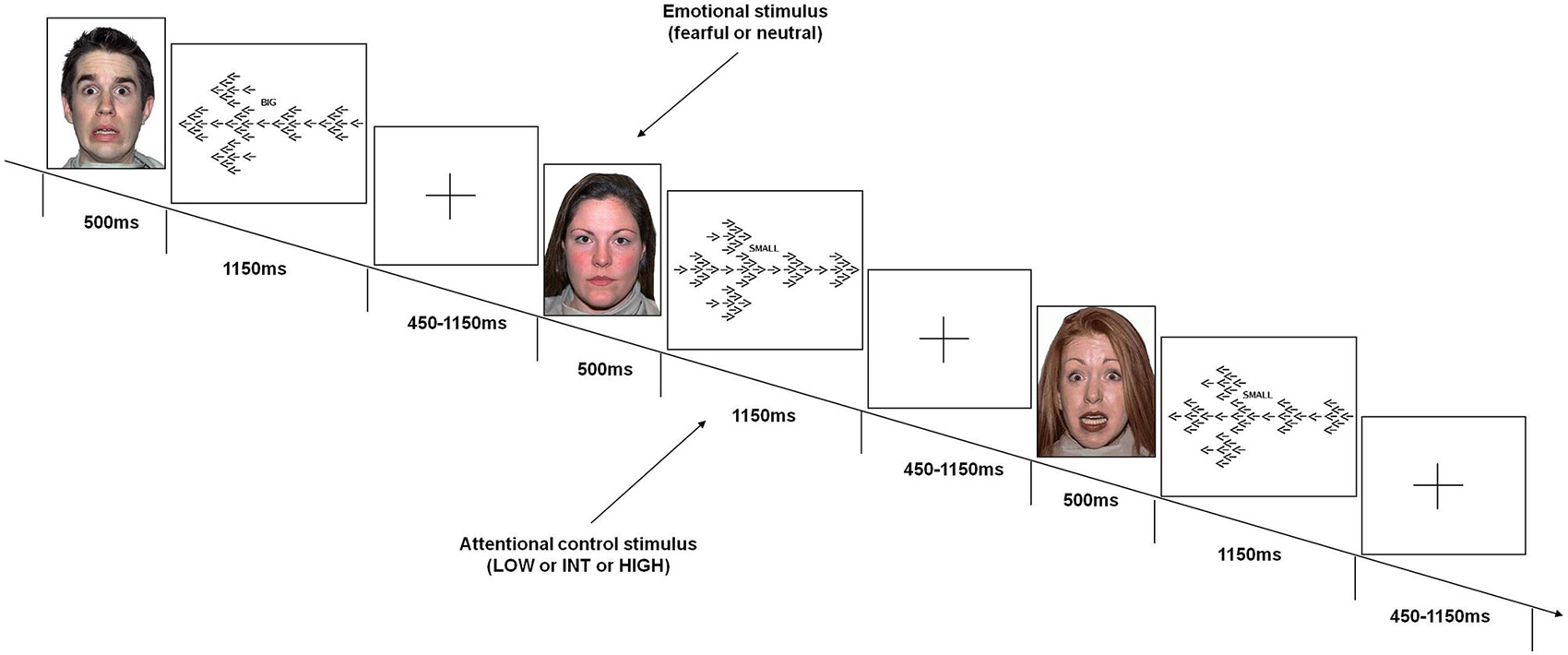
Figure 1. Emotional variable attentional control (EVAC) task. Events included emotional stimuli (fearful or neutral faces) before stimuli requiring three levels of attentional control load (LOW or INT or HIGH).
Emotional interference was obtained using unfamiliar faces with either fearful or neutral facial expressions derived from a validated set of facial pictures (NimStim; Tottenham et al., 2009).1 These emotional stimuli were displayed just before the attentional processing stimuli without any time interval.
Subjects were instructed by a cue word (BIG or SMALL) displayed above each attentional stimulus to press a button corresponding to the direction of the large or small arrows (either right or left). They were instructed to respond to the attentional stimuli with the thumb of their right hand using a button box (right button for “right” response, left button for “left” response), and to press the response button as fast and accurately as possible. Furthermore, they were asked to move their thumb to a small plastic knob placed between the buttons after each response. All subjects were trained on attentional stimuli of the task prior to the fMRI session to stabilize performance. More specifically, just before fMRI scanning, subjects were instructed on task rules and performed the task outside the fMRI environment until the average behavioral performance did not grossly vary across trials in terms of behavioral accuracy (±10 percentage points across trials). No instructions related to emotional stimuli were administered.
A total of 192 attentional stimuli was preceded by the respective 192 stimuli providing emotional interference, which were 96 fearful and 96 neutral facial expressions. There were 64 stimuli for each level of attentional control. Thirty two stimuli of each attentional level were preceded by facial stimuli with fearful expression, and 32 by facial stimuli with neutral expression. This design resulted in 6 conditions: fearful-low attentional control; fearful-intermediate attentional control; fearful-high attentional control; neutral-low attentional control; neutral-intermediate attentional control; neutral-high attentional control. The order of the stimuli was randomly distributed across the session (Friston et al., 1999). Each emotional stimulus was presented for 500 ms, while each attentional stimulus was displayed for 1150 ms. The total duration of the task was 8 min. A fixation cross-hair was presented during the interstimulus interval (before emotional stimuli), which ranged from 450–1150 ms (mean ISI = 700 ms).
Stimuli in the fMRI setting were presented via a back-projection system and responses were recorded through a fiber optic response box, which allowed measurement of RT for each trial. We report behavioral performance of the task performed in the scanner during the fMRI experiment.
Blood Oxygen Level-Dependent fMRI
We performed BOLD fMRI using a GE Signa 3T scanner (gradient-echo-planar-imaging sequence, time repetition/time echo = 2000/30 ms; 26 interleaved slices, thickness = 4 mm, gap = 1 mm; voxel size = 3.75 × 3.75 × 5 mm; scans = 260; flip angle = 90°; field of view = 24 cm; and matrix = 64 × 64) while subjects performed the emotional variable attentional control (EVAC) task. The first 4 scans were discarded to allow for signal saturation.
Data Analysis
Behavioral Data
Analysis of variance (ANOVA) was used to compare behavioral data (% correct responses and RT of correct responses). We included only subjects who had an average accuracy above 50% (forced choice between two options). Bonferroni correction was used for post hoc analysis to correct for multiple comparisons. Because of our priori hypothesis, post hoc analysis of the interaction between the level of attentional control and emotional interference was performed for each level of attentional control between fearful and neutral faces.
fMRI Data
Analysis was completed using the event-related module within Statistical Parametric Mapping 5 (SPM5).2 Images of each subject were realigned, spatially normalized into the Montreal Neurologic Institute (MNI) template (a 12-parameter affine model), and spatially smoothed (10-mm Gaussian filter). After realignment, data sets were also screened for high quality (scan stability) as demonstrated by small motion correction (less than 2.5 mm translation and less than 2° rotation). The fMRI responses were modeled using a canonical hemodynamic function and temporally filtered using a high-pass filter of 128 Hz and an HRF-shape low-pass filter. Vectors were created for each condition using the timing of correct responses for each stimulus type. The timing of incorrect responses and residual movement were also modeled as regressors of no interest. A t statistic was then used to produce a statistical image for BOLD responses relative to brain processing of stimuli associated with correct responses for each condition. A random effects ANOVA was used at the group level to investigate the main effect of emotional interference, of attentional control stimuli, and their interaction. For all analyses on activity during the EVAC task, we focused our attention on the orbital and triangular parts of IFG and on the caudate nucleus because of their central role in the regulation of cognitive and emotional processes as well as in their integration. Thus, we used a statistical threshold of p < 0.05, k ≥ 5, Family Wise Error (FWE)-corrected for multiple comparisons within brain regions of interest (i.e., orbital and triangular parts of IFG, left and right caudate), as defined by the WFU pickatlas software, version 1.04 (Functional MRI Laboratory at the Wake Forest University School of Medicine).3 The orbital and the triangular parts of the IFG were used as two distinct regions of interest (ROIs) because of their different cytoarchitectonic features, connections with different brain areas, and different functional contributions (Petrides and Pandya, 2002; Wimber et al., 2008). Although both areas are involved in coding of behavioral significance of emotional stimuli and both exert control over attentional and emotional function regulation needed to perform the task (Sakagami and Pan, 2007; Schulz et al., 2009), only the triangular part appears to be involved in complex sensory guided motor acts, possibly by storing motor representation of goal-directed hand actions (Iacoboni and Wilson, 2006).
To further investigate directionality of the interaction between emotional interference and attentional load, post hoc analysis was performed on parameter estimates extracted from clusters crossing the statistical threshold of p < 0.05 (FWE corrected) within the predefined ROIs using MarsBar.4 Finally, to investigate the relationship between brain activity and behavior, Spearman’s correlation analysis was performed between parameter estimates extracted with the same threshold from the above-mentioned ROIs and both accuracy and RT during the EVAC task.
Results
Behavioral Data
ANOVA on accuracy data indicated a main effect of increasing level of attentional control (F(2,42) = 31.25; p < 0.000001; average number and % of correct responses: HIGH 26.55, 82.9%; INT 29.52, 92.3%; LOW 31.32, 97.9%), no effect of emotional interference (F(1,21) = 0.98; p = 0.3), and no interaction between level of attentional control and emotional interference (F(2,42) = 1.37; p = 0.2). Post hoc analysis of the main effect of increasing level of attentional control showed a significant difference across all three levels (HIGH > INT, p < 0.00004; HIGH > LOW, p < 0.0000001; INT > LOW, p < 0.02).
Furthermore, analysis of RTs revealed a main effect of increasing level of attentional control (F(2,42) = 103.25; p < 0.000001), no effect of emotional interference (F(1,21) = 0.07; p = 0.7), and an interaction between level of attentional control and emotional interference (F(2,42) = 14.23; p < 0.00002) (Figure 2). Post hoc analysis of the main effect of increasing level of attentional control showed a significant difference across all three levels (HIGH > INT, p < 0.0000001; HIGH > LOW, p < 0.0000001; INT > LOW, p < 0.0000001). Post hoc analysis of the interaction between the level of attentional control and emotional interference indicated a statistically significant difference between RTs at the lower level of attentional control when preceded by presentation of fearful facial expressions (fearful > neutral, p < 0.002). An effect of emotional interference was also present at the intermediate level of attentional control, but in the opposite direction (neutral > fearful; p < 0.002). At the higher level of attentional control, a statistical trend in the same direction of the lower level was found (fearful > neutral; p = 0.06).
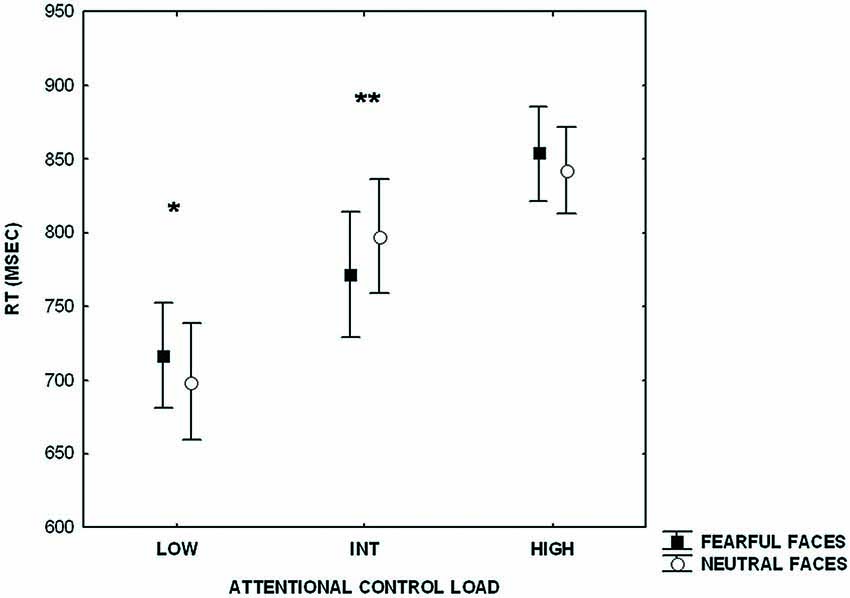
Figure 2. Plot showing reaction time (RT)s during processing of attentional control stimuli (LOW, INT, HIGH) after emotional stimuli (fearful and neutral faces). Measures presented are mean ± standard deviations. *p < 0.002, **p < 0.002.
Imaging Data
Consistent with previous studies (Blasi et al., 2005, 2007, 2010), ANOVA indicated a main effect of attentional control load in the right side of the IFG (BA9 and BA47) (Table 1). No effect of emotional interference was found on brain activity. However, there was a significant interaction between the level of attentional control and emotional interference in the right side of the IFG (BA47) and in both sides of the caudate (Figure 3; Table 1). Post hoc analysis of parameter estimates extracted from IFG revealed that interference by fearful facial expressions at the low level of attentional control was associated with greater activity relative to interference with neutral facial expressions (p = 0.004) (Figure 3A). Similar post hoc analyses for the left and right sides of the caudate again indicated an effect of emotional interference in the same direction, but at the intermediate level of attentional control (left: p = 0.001; right: p = 0.01) (Figures 3B,C). A statistical trend in the opposite direction was found for the right side of the caudate at the higher level of attentional control (p = 0.06).
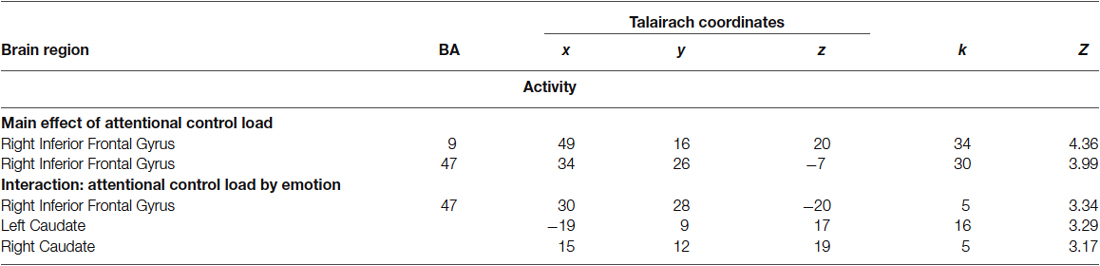
Table 1. Local maxima of brain activity associated with a main effect of attentional control and with an interaction of attentional control by emotion.
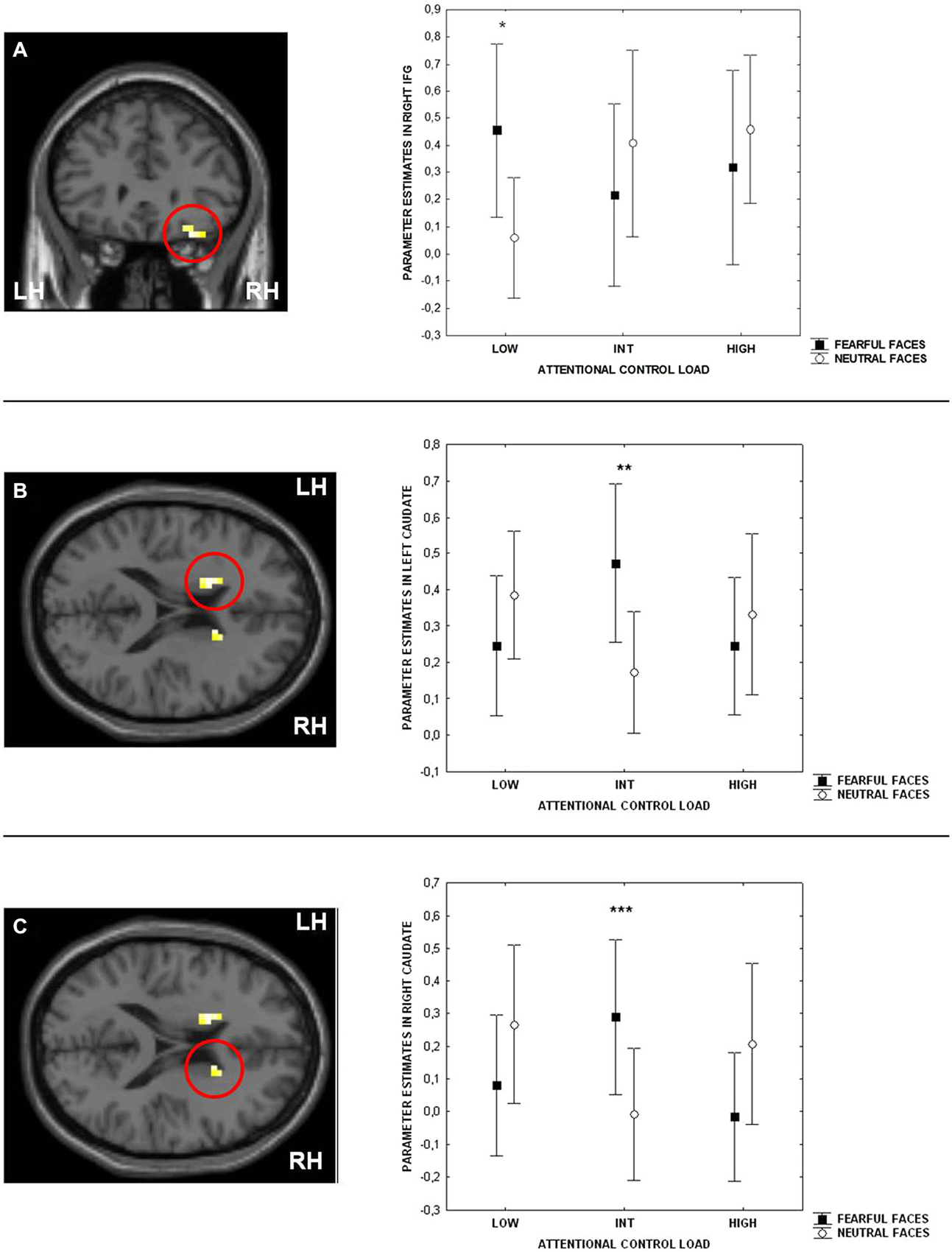
Figure 3. Sections of the brain showing the interaction between attentional control load and emotion in right Inferior Frontal Gyrus (IFG) (BA47) (A), left (B) and right (C) caudate. Graphs represent parameter estimates extracted from these clusters. Measures presented are mean ± standard deviations. *p = 0.004, **p = 0.001, ***p = 0.01. For more statistics, see Section Results.
Correlation Analysis
No significant correlations were found between behavioral accuracy data and brain activity in clusters showing an emotional interference by attentional load interaction (all p > 0.2). However, there was a significant negative correlation between parameter estimates in right IFG and RTs at the low level of attentional control after neutral facial expressions were shown (r = −0.5; p = 0.009) (Figure 4). No significant correlation was found at the same level of attentional control after fearful facial expressions were shown (r = −0.33; p > 0.1). Finally, no significant brain activity-RT correlations were found at the intermediate and high levels of attentional control (p > 0.15).
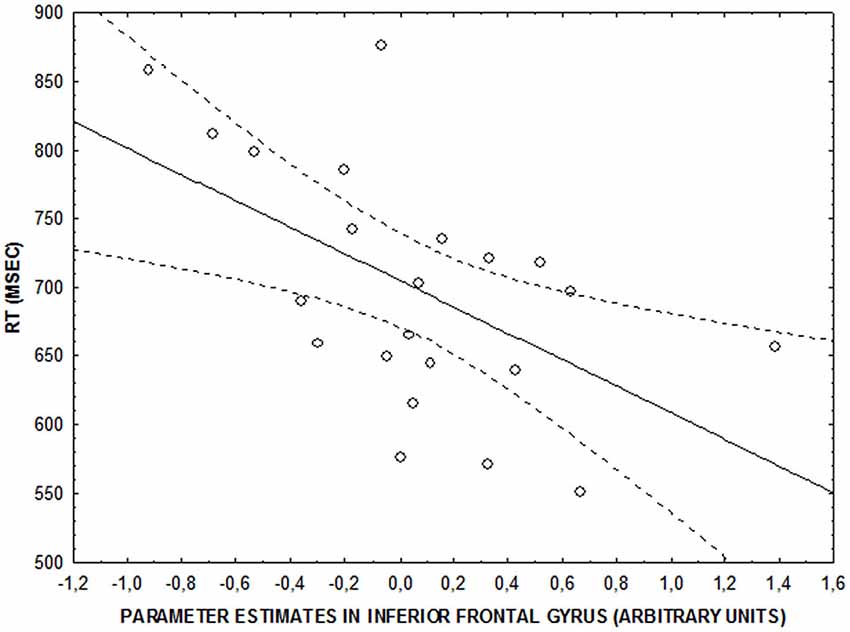
Figure 4. Scatterplot showing the correlation between activity in right IFG and RT (ms) at the lower level of attentional control after neutral faces (r = −0.5; p = 0.009).
Discussion
Our results indicated differential effects of emotional interference on behavior and activity in prefronto-striatal brain areas during attentional control as a function of cognitive load. In particular, stimuli eliciting the lower level of attentional control were associated with slower RTs as well as greater activity in the right side of the IFG when anticipated by fearful, relative to neutral, facial expressions. However, the interaction between emotional stimuli and the intermediate level of attentional control was associated with the opposite behavioral pattern but with a similar effect on brain activity, this time taking place in both sides of the caudate. Finally, no statistically significant behavioral or imaging effects of emotional interference were found at the higher level of attentional control. Together, these findings suggest that negative emotions modulate goal-directed behavior and related brain activity when low and intermediate levels of attentional control are required, but they do not impact attentional processing when high levels of attentional control are required. More generally, these results suggest that negative emotions may modulate cognitive efficiency during attentional control processing.
At the lower level of attentional control, the presentation of fearful, relative to neutral, faces before attentional stimuli was associated with longer RTs. Slower performances of attentional processes by negative emotions has been previously reported (Vuilleumier et al., 2001; Mather et al., 2006; Blair et al., 2007; Jasinska et al., 2012). Moreover, our data support that this slowing is related to the emotional salience of interference and the level of attentional control required for goal-directed behavior. In more detail, slowing of cognitive processes takes place when negative emotional stimuli anticipate goal-directed behavior requiring a low level of attentional control. This result coincides with the load theory of attention and the “salience hypothesis.” According to the first model, when lower attentional control levels are directed to task-relevant items, task-irrelevant stimuli result in high interference because sufficient cognitive resources are available to process all information. Furthermore, the “salience hypothesis” suggests that this interference depends on the emotional salience of task-irrelevant stimuli. We found that task-irrelevant negative emotions have a detrimental effect on RTs when low levels of attentional control were directed to task-relevant items. Moreover, our fMRI data are also consistent with earlier reports, indicating greater activation in the right side of the IFG after fearful facial expressions were presented at the lower level of attentional control only (Blair et al., 2007; Xu et al., 2011). In particular, we found greater engagement of activity on the right side of the IFG for slower responses, suggesting a greater need for neuronal resources when negative emotional interference is present. Moreover, correlation analysis revealed that greater responses in this brain area after neutral facial expressions were presented during low levels of attentional control were correlated with faster RTs, while this relationship is not as tight after fearful facial expressions were presented. A possible interpretation of these results is that the physiological relationship between activity in right IFG and RT during attentional processing partially breaks down after presentation of stimuli with high emotional salience, such as viewing fearful expressions. Therefore, our results may support greater engagement of the IFG to control the impact of aversive facial expressions only when a low attentional level is required and that at the intermediate level, this interference is no longer regulated by cortical areas, but by subcortical ones.
At the intermediate level of attentional control, anticipation of attentional stimuli by fearful, relative to neutral, faces was associated with faster RTs. Facilitation of attentional processes by negative emotional items has been reported previously (Erk et al., 2007; Geng and Diquattro, 2010; Swallow and Jiang, 2010; Kanske and Kotz, 2011; Lindström and Bohlin, 2011; Ziaei et al., 2014). Moreover, our data suggest that this facilitation is related to the emotional salience of distracters and the level of attentional control required for goal-directed behavior. In particular, cognitive processes were facilitated when negative emotional stimuli anticipated goal-directed behavior requiring intermediate levels of attentional control. A possible interpretation of this result is that when intermediate levels of attentional control are required during goal-directed behavior, sufficient cognitive resources are still available to process task-irrelevant information. In line with recent studies, arousal elicited by the processing of task-irrelevant negative emotions may increase attentional control efficiency during goal-directed behavior (Lindström and Bohlin, 2011; Sutherland and Mather, 2012). We found that task-irrelevant negative emotions had a beneficial effect on behavior when the intermediate level of attentional control was directed to task-relevant items. Also, our fMRI data indicated greater activity in the right and left sides of the caudate after fearful facial expressions were presented at intermediate level of attentional control. These data coincide with a recent study suggesting the key role of this brain area when irrelevant stimuli interfere at higher levels of attentional control (Ali et al., 2010). Moreover, caudate activity has been associated with automaticity during cognitive processes and higher behavioral efficiency (Raichle et al., 1994; Delazer et al., 2003; Floyer-Lea and Matthews, 2004; Poldrack et al., 2005; Saling and Phillips, 2007; Harsay et al., 2011; Ofen et al., 2012). However, we found that a signal change in right and left sides of the caudate failed to correlate with behavioral responses during the task. Still, in a recent study, activation in the caudate nucleus has been correlated with better behavioral performance after negative emotional interference at higher levels of attentional processing (Ziaei et al., 2014). Therefore, our data may suggest additional recruitment of neuronal resources in the right and left sides of the caudate to filter interference of aversive facial expressions during intermediate levels of attentional control. Another, more speculative, interpretation is that filtering out of emotional interference by caudate activation leads to faster RTs. These explanations are speculative and should be treated with caution.
Finally, at the higher level of attentional control there were no significant differences between fearful and neutral interference in behavioral and imaging data, even though statistical trends were present. This lack of any interference by task-irrelevant stimuli only at higher levels of attentional control has been previously reported (Sadeh and Bredemeier, 2011). Our data suggest that goal-directed behavior requiring high levels of attentional control is relatively independent of any emotional interference especially if compared with the effects found at the lower and intermediate levels. A possible interpretation of this result, based on the load theory of attention, is that at higher attentional levels, task-irrelevant information does not achieve significant interference because of possible saturation of all available resources during processing of task-relevant information.
Another possible speculation from our results may take into account putative arousing effects associated with aversive stimuli (Erk et al., 2007; Gotoh et al., 2010). Some previous studies have demonstrated that the greater the arousal elicited by emotional stimuli, the slower the RTs achieved during cognitive processing (Hartikainen et al., 2007; Kuhbandner and Zehetleitner, 2011; Ossowski et al., 2011). However, in other studies arousal has been associated with the opposite behavioral pattern or no impact on RTs (Lindström and Bohlin, 2011; van Steenbergen et al., 2011). Taken together, our results could suggest that greater arousal elicited by negative emotional stimuli rather than neutral ones is able to modulate goal-directed behavior. Furthermore, this modulation depends on the level of attentional control required for processing of task-relevant items with detrimental and beneficial effects at the lower and the intermediate levels respectively, and no interference at the higher levels.
Finally, our data may suggest that the signaling function (alertness) induced by fearful facial expressions modulates detection of other environmental cues. Similar results have been reported recently (Phelps et al., 2006; Bocanegra and Zeelenberg, 2009; Gable and Harmon-Jones, 2010; Lv et al., 2011). According to our results, fearful faces may reduce the speed of perception and inhibit detection of other environmental cues when low loads of attentional control are required. On the other hand, when intermediate levels of attentional control are required, fearful facial expressions could increase speed of perception and facilitate detection of other environmental cues. These observations fit well with the view that the adaptive function of emotional states is to rapidly and flexibly switch between different modes of response to best meet the current challenges of the environment (Gray, 2004; Sutherland and Mather, 2012).
A limitation of this study may be the number of trials used at the high level of attentional control, which could explain the statistical trend we found in behavioral and imaging data between fearful and neutral interference. According to a previous study, 25 trials are sufficient to provide stable activation maps (Murphy and Garavan, 2005). We found more than 25 correct trials for each condition, on average, at the high level of attentional control (26.6 or 83.1% with fearful interference and 26.5 or 82.8% with neutral interference). This is the reason why we did not expect any influence from the number of trials on our results.
In conclusion, this study suggests that emotionally irrelevant stimuli during attentional control processing modulate behavior and brain activity in right IFG and both sides of the caudate as a function of cognitive load. These findings add knowledge to some aspects of the complex functional architecture underlying the integration of emotion and attention in the human brain and could have potential clinical implications. Impairment of emotional and cognitive functions have long been regarded as characterizing several major psychiatric disorders such as schizophrenia, bipolar disorder, depression, and anxiety disorders (Bertolino et al., 2006, 2009; Matsuo et al., 2007; Siegle et al., 2007; Pauly et al., 2008; Sailer et al., 2008; Blasi et al., 2009, 2010; Etkin et al., 2010; Lee et al., 2010; Sadeh and Bredemeier, 2011; van Wingen et al., 2011; Brotman et al., 2014; Diwadkar et al., 2014). However, little is known about the interaction between emotion and cognition in patients. As such, our findings could provide a basis for formulating and experimenting hypotheses in future research aimed at investigating the nature of neural deficits in these disorders and their clinical correlates.
Conflict of Interest Statement
Dr. Bertolino is a full time employee of Hoffman-La Roche, Ltd. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
IFG, Inferior Frontal Gyrus; EVAC, Emotional Variable Attentional Control.
Footnotes
- ^ http://www.macbrain.org/resources.htm
- ^ http://www.fil.ion.ucl.ac.uk/spm
- ^ http://rad.wfubmc.edu
- ^ http://marsbar.sourceforge.net/
References
Ali, N., Green, D. W., Kherif, F., Devlin, J. T., and Price, C. J. (2010). The role of the left head of caudate in suppressing irrelevant words. J. Cogn. Neurosci. 22, 2369–2386. doi: 10.1162/jocn.2009.21352
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Aron, A. R., Robbins, T. W., and Poldrack, R. A. (2004). Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 8, 170–177. doi: 10.1016/j.tics.2004.02.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barber, A. D., Caffo, B. S., Pekar, J. J., and Mostofsky, S. H. (2013). Effects of working memory demand on neural mechanisms of motor response selection and control. J. Cogn. Neurosci. 25, 1235–1248. doi: 10.1162/jocn_a_00394
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Beer, J. S., Knight, R. T., and D’Esposito, M. (2006). Controlling the integration of emotion and cognition: the role of frontal cortex in distinguishing helpful from hurtful emotional information. Psychol. Sci. 17, 448–453. doi: 10.1111/j.1467-9280.2006.01726.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bertolino, A., Caforio, G., Petruzzella, V., Latorre, V., Rubino, V., Dimalta, S., et al. (2006). Prefrontal dysfunction in schizophrenia controlling for COMT Val158Met genotype and working memory performance. Psychiatry Res. 147, 221–226. doi: 10.1016/j.pscychresns.2006.04.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bertolino, A., Fazio, L., Caforio, G., Blasi, G., Rampino, A., Romano, R., et al. (2009). Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain 132, 417–425. doi: 10.1093/brain/awn248
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blair, K. S., Smith, B. W., Mitchell, D. G. V., Morton, J., Vythilingam, M., Pessoa, L., et al. (2007). Modulation of emotion by cognition and cognition by emotion. Neuroimage 35, 430–440. doi: 10.1016/j.neuroimage.2006.11.048
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blasi, G., Goldberg, T. E., Elvevåg, B., Rasetti, R., Bertolino, A., Cohen, J., et al. (2007). Differentiating allocation of resources and conflict detection within attentional control processing. Eur. J. Neurosci. 25, 594–602. doi: 10.1111/j.1460-9568.2007.05283.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blasi, G., Mattay, V. S., Bertolino, A., Elvevag, B., Callicott, J. H., Das, S., et al. (2005). Effect of catechol-O-methyltransferase val158met genotype on attentional control. J. Neurosci. 25, 5038–5045. doi: 10.1523/jneurosci.0476-05.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blasi, G., Napolitano, F., Ursini, G., Di Giorgio, A., Caforio, G., Taurisano, P., et al. (2013). Association of GSK-3β genetic variation with GSK-3β expression, prefrontal cortical thickness, prefrontal physiology and schizophrenia. Am. J. Psychiatry 170, 868–876. doi: 10.1176/appi.ajp.2012.12070908
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blasi, G., Napolitano, F., Ursini, G., Taurisano, P., Romano, R., Caforio, G., et al. (2011). DRD2/AKT1 interaction on D2 c-AMP independent signaling, attentional processing and response to olanzapine treatment in schizophrenia. Proc. Natl. Acad. Sci. U S A 108, 1158–1163. doi: 10.1073/pnas.1013535108
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blasi, G., Popolizio, T., Taurisano, P., Caforio, G., Romano, R., Di Giorgio, A., et al. (2009). Changes in prefrontal and amygdala activity during olanzapine treatment in schizophrenia. Psychiatry Res. 173, 31–38. doi: 10.1016/j.pscychresns.2008.09.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blasi, G., Taurisano, P., Papazacharias, A., Caforio, G., Romano, R., Lobianco, L., et al. (2010). Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb. Cortex 20, 837–845. doi: 10.1093/cercor/bhp146
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bocanegra, B. R., and Zeelenberg, R. (2009). Emotion improves and impairs early vision. Psychol. Sci. 20, 707–713. doi: 10.1111/j.1467-9280.2009.02354.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brotman, M. A., Tseng, W. L., Olsavsky, A. K., Fromm, S. J., Muhrer, E. J., Rutenberg, J. G., et al. (2014). Fronto-limbic-striatal dysfunction in pediatric and adult patients with bipolar disorder: impact of face emotion and attentional demands. Psychol. Med. 44, 1639–1651. doi: 10.1017/s003329171300202x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carretié, L., Ríos, M., de la Gándara, B. S., Tapia, M., Albert, J., López-Martín, S., et al. (2009). The striatum beyond reward: caudate responds intensely to unpleasant pictures. Neuroscience 164, 1615–1622. doi: 10.1016/j.neuroscience.2009.09.031
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Crofts, H. S., Dalley, J. W., Collins, P., Van Denderen, J. C., Everitt, B. J., Robbins, T. W., et al. (2001). Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb. Cortex 11, 1015–1026. doi: 10.1093/cercor/11.11.1015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Delazer, M., Domahs, F., Bartha, L., Brenneis, C., Lochy, A., Trieb, T., et al. (2003). Learning complex arithmetic–an fMRI study. Brain Res. Cogn. Brain Res. 18, 76–88. doi: 10.1016/j.cogbrainres.2003.09.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Depue, B. E., Orr, J. M., Smolker, H. R., Naaz, F., and Banich, M. T. (2015). The organization of right prefrontal networks reveals common mechanisms of inhibitory regulation across cognitive, emotional and motor processes. Cereb. Cortex doi: 10.1093/cercor/bhu324. [Epub ahead of print].
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Desimone, R., and Duncan, J. (1995). Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222. doi: 10.1146/annurev.neuro.18.1.193
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Diwadkar, V. A., Bakshi, N., Gupta, G., Pruitt, P., White, R., and Eickhoff, S. B. (2014). Dysfunction and Dysconnection in cortical-striatal networks during sustained attention: genetic risk for schizophrenia or bipolar disorder and its impact on brain network function. Front. Psychiatry 5:50. doi: 10.3389/fpsyt.2014.00050
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dolcos, F., Iordan, A. D., Kragel, J., Stokes, J., Campbell, R., McCarthy, G., et al. (2013). Neural correlates of opposing effects of emotional distraction on working memory and episodic memory: an event-related FMRI investigation. Front. Psychol. 4:293. doi: 10.3389/fpsyg.2013.00293
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dolcos, F., and McCarthy, G. (2006). Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 26, 2072–2079. doi: 10.1523/jneurosci.5042-05.2006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Eltiti, S., Wallace, D., and Fox, E. (2005). Selective target processing: perceptual load or distractor salience? Percept. Psychophys. 67, 876–885. doi: 10.3758/bf03193540
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Erk, S., Kleczar, A., and Walter, H. (2007). Valence-specific regulation effects in a working memory task with emotional context. Neuroimage 37, 623–632. doi: 10.1016/j.neuroimage.2007.05.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Etkin, A., Prater, K. E., Hoeft, F., Menon, V., and Schatzberg, A. F. (2010). Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am. J. Psychiatry 167, 545–554. doi: 10.1176/appi.ajp.2009.09070931
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Floyer-Lea, A., and Matthews, P. M. (2004). Changing brain networks for visuomotor control with increased movement automaticity. J. Neurophysiol. 92, 2405–2412. doi: 10.1152/jn.01092.2003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Friston, K. J., Zarahn, E., Josephs, O., Henson, R. N., and Dale, A. M. (1999). Stochastic designs in event-related fMRI. Neuroimage 10, 607–619. doi: 10.1006/nimg.1999.0498
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gable, P., and Harmon-Jones, E. (2010). The blues broaden, but the nasty narrows: attentional consequences of negative affects low and high in motivational intensity. Psychol. Sci. 21, 211–215. doi: 10.1177/0956797609359622
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Geliebter, A., Pantazatos, S. P., McOuatt, H., Puma, L., Gibson, C. D., and Atalayer, D. (2013). Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav. Brain Res. 243, 91–96. doi: 10.1016/j.bbr.2012.12.023
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Geng, J. J., and Diquattro, N. E. (2010). Attentional capture by a perceptually salient non-target facilitates target processing through inhibition and rapid rejection. J. Vis. 10:5. doi: 10.1167/10.6.5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gerdes, A. B., Wieser, M. J., Mühlberger, A., Weyers, P., Alpers, G. W., Plichta, M. M., et al. (2010). Brain activations to emotional pictures are differentially associated with valence and arousal ratings. Front. Hum. Neurosci. 4:175. doi: 10.3389/fnhum.2010.00175
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gotoh, F., Kikuchi, T., and Olofsson, U. (2010). A facilitative effect of negative affective valence on working memory. Scand. J. Psychol. 51, 185–191. doi: 10.1111/j.1467-9450.2009.00766.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gray, J. R. (2004). Integration of emotion and cognitive control. Curr. Dir. Psychol. Sci. 13, 46–48. doi: 10.1111/j.0963-7214.2004.00272.x
Gray, J. R., Braver, T. S., and Raichle, M. E. (2002). Integration of emotion and cognition in the lateral prefrontal cortex. Proc. Natl. Acad. Sci. U S A 99, 4115–4120. doi: 10.1073/pnas.062381899
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, R., and Srinivasan, N. (2015). Only irrelevant sad but not happy faces are inhibited under high perceptual load. Cogn. Emot. 29, 747–754. doi: 10.1080/02699931.2014.933735
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hampshire, A., Chamberlain, S. R., Monti, M. M., Duncan, J., and Owen, A. M. (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50, 1313–1319. doi: 10.1016/j.neuroimage.2009.12.109
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hare, T. A., Tottenham, N., Davidson, M. C., Glover, G. H., and Casey, B. J. (2005). Contributions of amygdala and striatal activity in emotion regulation. Biol. Psychiatry 57, 624–632. doi: 10.1016/j.biopsych.2004.12.038
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Harsay, H. A., Cohen, M. X., Oosterhof, N. N., Forstmann, B. U., Mars, R. B., and Ridderinkhof, K. R. (2011). Functional connectivity of the striatum links motivation to action control in humans. J. Neurosci. 31, 10701–10711. doi: 10.1523/JNEUROSCI.5415-10.2011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hart, S. J., Bizzell, J., McMahon, M. A., Gu, H., Perkins, D. O., and Belger, A. (2013). Altered fronto-limbic activity in children and adolescents with familial high risk for schizophrenia. Psychiatry Res. 212, 19–27. doi: 10.1016/j.pscychresns.2012.12.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hartikainen, K. M., Ogawa, K. H., Soltani, M., and Knight, R. T. (2007). Emotionally arousing stimuli compete for attention with left hemispace. Neuroreport 18, 1929–1933. doi: 10.1097/wnr.0b013e3282f1ca18
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heim, S., Eickhoff, S. B., and Amunts, K. (2009). Different roles of cytoarchitectonic BA 44 and BA 45 in phonological and semantic verbal fluency as revealed by dynamic causal modelling. Neuroimage 48, 616–624. doi: 10.1016/j.neuroimage.2009.06.044
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Herwig, U., Abler, B., Walter, H., and Erk, S. (2007). Expecting unpleasant stimuli–an fMRI study. Psychiatry Res. 154, 1–12. doi: 10.1016/j.pscychresns.2006.02.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hindi Attar, C., and Müller, M. M. (2012). Selective attention to task-irrelevant emotional distractors is unaffected by the perceptual load associated with a foreground task. PLoS One 7:e37186. doi: 10.1371/journal.pone.0037186
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hollingshead, A. B., and Redlich, F. C. (1955). Social mobility and mental illness. Am. J. Psychiatry 112, 179–185. doi: 10.1176/ajp.112.3.179
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Iacoboni, M., and Wilson, S. M. (2006). Beyond a single area: motor control and language within a neural architecture encompassing Broca’s area. Cortex 42, 503–506. doi: 10.1016/s0010-9452(08)70387-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jarcho, J. M., Fox, N. A., Pine, D. S., Leibenluft, E., Shechner, T., Degnan, K. A., et al. (2014). Enduring influence of early temperament on neural mechanisms mediating attention-emotion conflict in adults. Depress. Anxiety 31, 53–62. doi: 10.1002/da.22140
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jasinska, A. J., Yasuda, M., Rhodes, R. E., Wang, C., and Polk, T. A. (2012). Task difficulty modulates the impact of emotional stimuli on neural response in cognitive-control regions. Front. Psychol. 3:345. doi: 10.3389/fpsyg.2012.00345
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kanske, P., and Kotz, S. A. (2011). Emotion triggers executive attention: anterior cingulate cortex and amygdala responses to emotional words in a conflict task. Hum. Brain Mapp. 32, 198–208. doi: 10.1002/hbm.21012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Katzev, M., Tüscher, O., Hennig, J., Weiller, C., and Kaller, C. P. (2013). Revisiting the functional specialization of left inferior frontal gyrus in phonological and semantic fluency: the crucial role of task demands and individual ability. J. Neurosci. 33, 7837–7845. doi: 10.1523/JNEUROSCI.3147-12.2013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kellermann, T. S., Sternkopf, M. A., Schneider, F., Habel, U., Turetsky, B. I., Zilles, K., et al. (2012). Modulating the processing of emotional stimuli by cognitive demand. Soc. Cogn. Affect. Neurosci. 7, 263–273. doi: 10.1093/scan/nsq104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kret, M. E., Denollet, J., Grèzes, J., and de Gelder, B. (2011). The role of negative affectivity and social inhibition in perceiving social threat: an fMRI study. Neuropsychologia 49, 1187–1193. doi: 10.1016/j.neuropsychologia.2011.02.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kuhbandner, C., and Zehetleitner, M. (2011). Dissociable effects of valence and arousal in adaptive executive control. PLoS One 6:e29287. doi: 10.1371/journal.pone.0029287
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Langeslag, S. J. E., van der Veen, F. M., and Röder, C. H. (2014). Attention modulates the dorsal striatum response to love stimuli. Hum. Brain Mapp. 35, 503–512. doi: 10.1002/hbm.22197
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lavie, N. (2005). Distracted and confused?: selective attention under load. Trends Cogn. Sci. 9, 75–82. doi: 10.1016/j.tics.2004.12.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lavie, N., Hirst, A., de Fockert, J. W., and Viding, E. (2004). Load theory of selective attention and cognitive control. J. Exp. Psychol. Gen. 133, 339–354. doi: 10.1037/0096-3445.133.3.339
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, S. J., Lee, H. K., Kweon, Y. S., Lee, C. T., and Lee, K. U. (2010). Deficits in facial emotion recognition in schizophrenia: a replication study with korean subjects. Psychiatry Investig. 7, 291–297. doi: 10.4306/pi.2010.7.4.291
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Levens, S. M., and Phelps, E. A. (2010). Insula and orbital frontal cortex activity underlying emotion interference resolution in working memory. J. Cogn. Neurosci. 22, 2790–2803. doi: 10.1162/jocn.2010.21428
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Levita, L., Hare, T. A., Voss, H. U., Glover, G., Ballon, D. J., and Casey, B. J. (2009). The bivalent side of the nucleus accumbens. Neuroimage 44, 1178–1187. doi: 10.1016/j.neuroimage.2008.09.039
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lindström, B. R., and Bohlin, G. (2011). Emotion processing facilitates working memory performance. Cogn. Emot. 25, 1196–1204. doi: 10.1080/02699931.2010.527703
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lv, J. Y., Wang, T., Tu, S., Zheng, F., and Qiu, J. (2011). The effect of different negative emotional context on involuntary attention: an ERP study. Brain Res. Bull. 86, 106–109. doi: 10.1016/j.brainresbull.2011.06.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mather, M., Mitchell, K. J., Raye, C. L., Novak, D. L., Greene, E. J., and Johnson, M. K. (2006). Emotional arousal can impair feature binding in working memory. J. Cogn. Neurosci. 18, 614–625. doi: 10.1162/jocn.2006.18.4.614
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Matsuo, K., Glahn, D. C., Peluso, M. A., Hatch, J. P., Monkul, E. S., Najt, P., et al. (2007). Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol. Psychiatry 12, 158–166. doi: 10.1038/sj.mp.4001894
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McNab, F., and Klingberg, T. (2008). Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 11, 103–107. doi: 10.1038/nn2024
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mincic, A. M. (2010). Neural substrate of the cognitive and emotional interference processing in healthy adolescents. Acta Neurobiol. Exp. (Wars) 70, 406–422.
Mitchell, D. G., Luo, Q., Mondillo, K., Vythilingam, M., Finger, E. C., and Blair, R. J. (2008). The interference of operant task performance by emotional distracters: an antagonistic relationship between the amygdala and frontoparietal cortices. Neuroimage 40, 859–868. doi: 10.1016/j.neuroimage.2007.08.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Munakata, Y., Herd, S. A., Chatham, C. H., Depue, B. E., Banich, M. T., and O’Reilly, R. C. (2011). A unified framework for inhibitory control. Trends Cogn. Sci. 15, 453–459. doi: 10.1016/j.tics.2011.07.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Murphy, K., and Garavan, H. (2005). Deriving the optimal number of events for an event-related fMRI study based on the spatial extent of activation. Neuroimage 27, 771–777. doi: 10.1016/j.neuroimage.2005.05.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ochsner, K. N., Ray, R. D., Cooper, J. C., Robertson, E. R., Chopra, S., Gabrieli, J. D., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 23, 483–499. doi: 10.1016/j.neuroimage.2004.06.030
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ofen, N., Chai, X. J., Schuil, K. D., Whitfield-Gabrieli, S., and Gabrieli, J. D. (2012). The development of brain systems associated with successful memory retrieval of scenes. J. Neurosci. 32, 10012–10020. doi: 10.1523/jneurosci.1082-11.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ossowski, U., Malinen, S., and Helton, W. S. (2011). The effects of emotional stimuli on target detection: indirect and direct resource costs. Conscious. Cogn. 20, 1649–1658. doi: 10.1016/j.concog.2011.08.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pauly, K., Seiferth, N. Y., Kellermann, T., Backes, V., Vloet, T. D., Shah, N. J., et al. (2008). Cerebral dysfunctions of emotion-cognition interactions in adolescent-onset schizophrenia. J. Am. Acad. Child Adolesc. Psychiatry 47, 1299–1310. doi: 10.1097/chi.0b013e318184ff16
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pessoa, L. (2008). On the relationship between emotion and cognition. Nat. Rev. Neurosci. 9, 148–158. doi: 10.1038/nrn2317
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Petrides, M., and Pandya, D. N. (2002). Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 16, 291–310. doi: 10.1046/j.1460-9568.2001.02090.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Phan, K. L., Fitzgerald, D. A., Nathan, P. J., Moore, G. J., Uhde, T. W., and Tancer, M. E. (2005). Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry 57, 210–219. doi: 10.1016/j.biopsych.2004.10.030
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Phelps, E. A., Ling, S., and Carrasco, M. (2006). Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol. Sci. 17, 292–299. doi: 10.1111/j.1467-9280.2006.01701.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Poldrack, R. A., Sabb, F. W., Foerde, K., Tom, S. M., Asarnow, R. F., Bookheimer, S. Y., et al. (2005). The neural correlates of motor skill automaticity. J. Neurosci. 25, 5356–5364. doi: 10.1523/jneurosci.3880-04.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Raichle, M. E., Fiez, J. A., Videen, T. O., Macleod, A. M., Pardo, J. V., Fox, P. T., et al. (1994). Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb. Cortex 4, 8–26. doi: 10.1093/cercor/4.1.8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Roiser, J. P., Muller, U., Clark, L., and Sahakian, B. J. (2007). The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. Int. J. Neuropsychopharmacol. 10, 449–461. doi: 10.1017/s146114570600705x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sadeh, N., and Bredemeier, K. (2011). Individual differences at high perceptual load: the relation between trait anxiety and selective attention. Cogn. Emot. 25, 747–755. doi: 10.1080/02699931.2010.500566
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sailer, U., Robinson, S., Fischmeister, F. P., Konig, D., Oppenauer, C., Lueger-Schuster, B., et al. (2008). Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia 46, 2836–2844. doi: 10.1016/j.neuropsychologia.2008.05.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sakagami, M., and Pan, X. (2007). Functional role of the ventrolateral prefrontal cortex in decision making. Curr. Opin. Neurobiol. 17, 228–233. doi: 10.1016/j.conb.2007.02.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Saling, L. L., and Phillips, J. G. (2007). Automatic behaviour: efficient not mindless. Brain Res. Bull. 73, 1–20. doi: 10.1016/j.brainresbull.2007.02.009
Scholes, K. E., Harrison, B. J., O’neill, B. V., Leung, S., Croft, R. J., Pipingas, A., et al. (2007). Acute serotonin and dopamine depletion improves attentional control: findings from the stroop task. Neuropsychopharmacology 32, 1600–1610. doi: 10.1038/sj.npp.1301262
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schulz, K. P., Clerkin, S. M., Halperin, J. M., Newcorn, J. H., Tang, C. Y., and Fan, J. (2009). Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Hum. Brain Mapp. 30, 2821–2833. doi: 10.1002/hbm.20706
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Serrien, D. J., Ivry, R. B., and Swinnen, S. P. (2006). Dynamics of hemispheric specialization and integration in the context of motor control. Nat. Rev. Neurosci. 7, 160–166. doi: 10.1038/nrn1849
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shafer, A. T., and Dolcos, F. (2012). Neural correlates of opposing effects of emotional distraction on perception and episodic memory: an event-related FMRI investigation. Front. Integr. Neurosci. 6:70. doi: 10.3389/fnint.2012.00070
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Siegle, G. J., Thompson, W., Carter, C. S., Steinhauer, S. R., and Thase, M. E. (2007). Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol. Psychiatry 61, 198–209. doi: 10.1016/j.biopsych.2006.05.048
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Simpson, J. R., Ongur, D., Akbudak, E., Conturo, T. E., Ollinger, J. M., Snyder, A. Z., et al. (2000). The emotional modulation of cognitive processing: an fMRI study. J. Cogn. Neurosci. 12(Suppl. 2), 157–170. doi: 10.1162/089892900564019
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sommer, M., Hajak, G., Dohnel, K., Meinhardt, J., and Müller, J. L. (2008). Emotion-dependent modulation of interference processes: an fMRI study. Acta Neurobiol. Exp. (Wars) 68, 193–203.
Sutherland, M. R., and Mather, M. (2012). Negative arousal amplifies the effects of saliency in short-term memory. Emotion 12, 1367–1372. doi: 10.1037/a0027860
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Swallow, K. M., and Jiang, Y. V. (2010). The attentional boost effect: transient increases in attention to one task enhance performance in a second task. Cognition 115, 118–132. doi: 10.1016/j.cognition.2009.12.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tottenham, N., Tanaka, J. W., Leon, A. C., Mccarry, T., Nurse, M., Hare, T. A., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 168, 242–249. doi: 10.1016/j.psychres.2008.05.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Urry, H. L., Van Reekum, C. M., Johnstone, T., and Davidson, R. J. (2009). Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage 47, 852–863. doi: 10.1016/j.neuroimage.2009.05.069
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
van Steenbergen, H., Band, G. P., and Hommel, B. (2011). Threat but not arousal narrows attention: evidence from pupil dilation and saccade control. Front. Psychol. 2:281. doi: 10.3389/fpsyg.2011.00281
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
van Wingen, G. A., Van Eijndhoven, P., Tendolkar, I., Buitelaar, J., Verkes, R. J., and Fernandez, G. (2011). Neural basis of emotion recognition deficits in first-episode major depression. Psychol. Med. 41, 1397–1405. doi: 10.1017/s0033291710002084
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vuilleumier, P., Armony, J. L., Driver, J., and Dolan, R. J. (2001). Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 30, 829–841. doi: 10.1016/s0896-6273(01)00328-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wimber, M., Bauml, K.-H., Bergstrom, Z., Markopoulos, G., Heinze, H.-J., and Richardson-Klavehn, A. (2008). Neural markers of inhibition in human memory retrieval. J. Neurosci. 28, 13419–13427. doi: 10.1523/JNEUROSCI.1916-08.2008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xu, J., Monterosso, J., Kober, H., Balodis, I. M., and Potenza, M. N. (2011). Perceptual load-dependent neural correlates of distractor interference inhibition. PLoS One 6:e14552. doi: 10.1371/journal.pone.0014552
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Y., Bertolino, A., Fazio, L., Blasi, G., Rampino, A., Romano, R., et al. (2007). Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing and neuronal activity during working memory. Proc. Natl. Acad. Sci. U S A 104, 20552–20557. doi: 10.1073/pnas.0707106104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ziaei, M., Peira, N., and Persson, J. (2014). Brain systems underlying attentional control and emotional distraction during working memory encoding. Neuroimage 87, 276–286. doi: 10.1016/j.neuroimage.2013.10.048
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: attentional control, emotion, inferior frontal gyrus, caudate nucleus, fMRI
Citation: Papazacharias A, Taurisano P, Fazio L, Gelao B, Di Giorgio A, Lo Bianco L, Quarto T, Mancini M, Porcelli A, Romano R, Caforio G, Todarello O, Popolizio T, Blasi G and Bertolino A (2015) Aversive emotional interference impacts behavior and prefronto-striatal activity during increasing attentional control. Front. Behav. Neurosci. 9:97. doi: 10.3389/fnbeh.2015.00097
Received: 22 December 2014; Accepted: 03 April 2015;
Published online: 21 April 2015.
Edited by:
Lars Schwabe, University of Hamburg, GermanyReviewed by:
Katarina Dedovic, Douglas Institute Research Centre, CanadaAlexandru Iordan, University of Illinois at Urbana-Champaign, USA
Copyright © 2015 Papazacharias, Taurisano, Fazio, Gelao, Di Giorgio, Lo Bianco, Quarto, Mancini, Porcelli, Romano, Caforio, Todarello, Popolizio, Blasi and Bertolino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Bertolino, pRED, NORD DTA Neuroscience, Hoffman-La Roche Ltd, Grenzacherstrasse 74/3W204B, Basel, 4021, Switzerland alessandro.bertolino@uniba.it
† These authors have contributed equally to this work.
 Apostolos Papazacharias
Apostolos Papazacharias Paolo Taurisano
Paolo Taurisano Leonardo Fazio
Leonardo Fazio Barbara Gelao
Barbara Gelao Annabella Di Giorgio
Annabella Di Giorgio Luciana Lo Bianco
Luciana Lo Bianco Tiziana Quarto
Tiziana Quarto Marina Mancini
Marina Mancini Annamaria Porcelli
Annamaria Porcelli Raffaella Romano
Raffaella Romano Grazia Caforio
Grazia Caforio Orlando Todarello
Orlando Todarello Teresa Popolizio2
Teresa Popolizio2  Giuseppe Blasi
Giuseppe Blasi Alessandro Bertolino
Alessandro Bertolino