The Brain of Binge Drinkers at Rest: Alterations in Theta and Beta Oscillations in First-Year College Students with a Binge Drinking Pattern
- 1Neuropsychophysiology Lab, Research Center in Psychology (CIPsi), School of Psychology, University of Minho, Braga, Portugal
- 2Department of Clinical Psychology and Psychobiology, University of Santiago de Compostela, Santiago de Compostela, Spain
- 3Laboratory of Cognitive and Computational Neuroscience, Center for Biomedical Technology, Madrid, Spain
- 4Department of Basic Psychology II, Complutense University of Madrid, Madrid, Spain
Background: Previous studies have reported anomalous resting brain activity in the electroencephalogram (EEG) of alcoholics, often reflected as increased power in the beta and theta frequency bands. The effects of binge drinking, the most common pattern of excessive alcohol consumption during adolescence and youth, on brain activity at rest is still poorly known. In this study, we sought to assess the pattern of resting-state EEG oscillations in college-aged binge drinkers (BDs).
Methods: Resting-state brain activity during eyes-open and eyes-closed conditions was recorded from 60 channels in 80 first-year undergraduate students (40 controls and 40 BDs). Cortical sources activity of EEG rhythms was estimated using exact Low-Resolution Electromagnetic Tomography (eLORETA) analysis.
Results: EEG-source localization analysis revealed that BDs showed, in comparison with controls, significantly higher intracranial current density in the beta frequency band over the right temporal lobe (parahippocampal and fusiform gyri) during eyes-open resting state as well as higher intracranial current density in the theta band over the bilateral occipital cortex (cuneus and lingual gyrus) during eyes-closed resting condition.
Conclusions: These findings are in line with previous results observing increased beta and/or theta power following chronic or heavy alcohol drinking in alcohol-dependent subjects and BDs. Increased tonic beta and theta oscillations are suggestive of an augmented cortical excitability and of potential difficulties in the information processing capacity in young BDs. Furthermore, enhanced EEG power in these frequency bands may respond to a neuromaturational delay as a result of excessive alcohol consumption during this critical brain developmental period.
Introduction
According to the National Institute of Alcohol Abuse and Alcoholism, a binge is a pattern of drinking alcohol that brings blood alcohol concentration (BAC) to 0.08 g percent or higher, which corresponds to consuming five or more drinks for men and four or more for women within a 2-h interval (National Institute of Alcohol, Abuse and Alcoholism, 2004). This type of excessive alcohol use is a highly prevalent pattern, mostly among high school and college students (Courtney and Polich, 2009). Recent reports show that around one third of young Europeans and North Americans are binge drinkers (BDs; Kraus et al., 2016; SAMHSA, 2016).
This prevalent pattern has been related to an array of negative consequences, including traffic collisions, poor academic performance, risky sexual behavior and neurocognitive deficits (White and Hingson, 2013; Carbia and López-Caneda, 2016). Concerning the latter, studies using neuropsychological batteries have demonstrated that BDs exhibit poor performance on tasks involving a number of cognitive processes such as, verbal and prospective memory (Heffernan et al., 2010; Carbia et al., 2017), inhibitory control (Czapla et al., 2015) or decision making (Moreno et al., 2012).
Electrophysiological measures of brain activity have also proved to be efficient methods for the comprehension of neurocognitive function as well as for the understanding of dysfunctional processes linked to various psychiatric disorders, including alcoholism (Parvaz et al., 2011; Kamarajan and Porjesz, 2015). Impairments in a variety of neurophysiological parameters have been consistently observed in alcoholic patients both during task execution and during resting state conditions (Campanella et al., 2009). In the resting electroencephalogram (EEG)—i.e., the electrophysiological recording of oscillatory brain activity while the person is relaxed- increased power in the beta and theta frequency bands has been reported extensively. In this sense, findings from several laboratories have repeatedly showed that alcoholics manifest higher resting beta power than non-dependent subjects (Costa and Bauer, 1997; Rangaswamy et al., 2002, 2003; Coutin-Churchman and Moreno, 2008; Ehlers et al., 2010; Mumtaz et al., 2016; Meyers et al., 2017). Furthermore, increases in the beta band in relapsers in comparison with alcoholics who remained abstinent (Bauer, 2001; Saletu-Zyhlarz et al., 2004) and in non-alcoholic individuals compared to alcoholic relatives (Pollock et al., 1995; Rangaswamy et al., 2004), appears to strength the relationship between the alcoholic spectrum and the beta power.
As with beta rhythms (although perhaps less consistently), elevated theta activity at rest has been reported from resting-state EEG studies in alcohol abusers (Pollock et al., 1992; Rangaswamy et al., 2003; Mumtaz et al., 2016; but see Saletu-Zyhlarz et al., 2004; Coutin-Churchman et al., 2006 for different findings).
Despite the functional meaning of these higher beta and theta rhythms in alcoholics is still under discussion, the increased power in this frequency bands has often been interpreted as reflecting cortical hyperexcitability—for beta rhythms- and reduced-cognitive processing capacity—for theta frequencies (Rangaswamy et al., 2002, 2003; Porjesz and Begleiter, 2003; Campanella et al., 2009).
Although comparatively much less studied, electrophysiological measures of brain activity also appear to be sensitive to the BD pattern (López-Caneda et al., 2014a; Petit et al., 2014b). As such, anomalous brain responses have been documented in BD youths during cognitive tasks using event-related potentials (ERPs) and event-related oscillations (EROs; Crego et al., 2009; Maurage et al., 2012; Smith and Mattick, 2013; Petit et al., 2014a; Watson et al., 2014; López-Caneda et al., 2017). Despite its well documented potential for detecting neurofunctional anomalies in chronic alcoholics, brain activity during task-free resting states has been virtually unaddressed in the BD population. As such, to the best of our knowledge only two studies have directly assessed the effects of BD on the frequency spectrum of the whole brain at rest (Courtney and Polich, 2010; Correas et al., 2015). Specifically, the EEG study conducted by Courtney and Polich (2009) showed increased delta and beta power in high-BDs during passive viewing, which was considered a potential biomarker for future alcoholism. Correas et al. (2015), using magnetoencephalography (MEG), reported increased theta and decreased alpha power in the occipital region of young BDs during eyes-closed resting state, which was considered an initial sign of anomalous neural activity caused by BD in youth. Although both studies point to anomalies in neural activity at rest in young BDs, the neurofunctional effects of this pattern of binge alcohol intake on the resting brain are far from well-studied.
The current study is the first to use source localization analysis to examine multichannel EEG data to detect the active brain regions during eyes-open and eyes-closed resting state in young BDs. To this end, we used the exact Low Resolution Electromagnetic Tomography Analysis (eLORETA), a well-validated source reconstruction model for mapping source activations of scalp-recorded EEG (Pascual-Marqui, 2007; Pascual-Marqui et al., 2011). We aimed to identify potentially abnormal intracortical EEG patterns in young BDs as compared to age-matched controls in specific brain regions to provide new neurophysiological evidence that might prove useful for early detection of brain damage associated with the BD pattern. We hypothesized that young BDs will display anomalous patterns of brain activity compared to control subjects, specifically in those frequency spectra typically impaired in alcohol abusers, namely, theta, and beta frequencies.
Materials and Methods
Participants
Eighty students from the Complutense University of Madrid (Spain) participated in the study. This study is framed within a research project aimed to assess brain damage associated with BD. In this project, neuropsychological, neurostructural and MEG measurements were also taken. Thus, the present study shares part of the sample with the recent MEG study from Correas et al. (2015). Participants were selected on the basis of their responses to a questionnaire that included the Spanish validated version of the Alcohol Use Disorder Identification Test (AUDIT; Guillamón et al., 1999), the question 10 of the Alcohol Use Questionnaire (“When you drink, how fast do you drink? 1 drink in 3 or more h; 1 drink in 2 h; Drinks per hour: 1, 2, 3, 4, 5, 6, 7, or more”; Townshend and Duka, 2002), and other information about alcohol and drugs consumption gathered through a semi-structured interview. According to NIAAA's BD definition, participants reaching BAC ≥ 0.08 g/dL at least once during the last month were classified as BDs. On the other hand, the control group consisted of students who have never reached an alcohol concentration of 0.08 g/dL. BAC was calculated based on the information of the drinking episodes of the last 6 months according to the following formula:
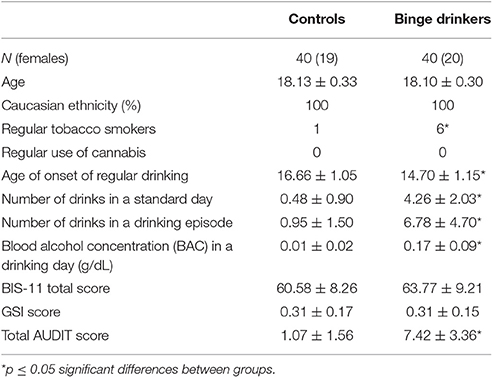
where G corresponds to grams of alcohol consumed on one occasion (the occasion of greatest consumption in the last month); W is body weight (kilograms); bw is a constant related to the water content of the human body, with value 0.68 for males and 0.55 for females; mr is the metabolization rate with a value of 0.15 for males and 0.18 for females; and DP is the drinking period (hours). Consequently, 40 participants were classified as BDs (20 females) and 40 as controls (19 females).
Impulsivity was assessed by the Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995) and psychopathological symptoms were measured by the Symptom Checklist-90 revised questionnaire (SCL-90-R; Derogatis, 1983). Exclusion criteria were: non-corrected sensory deficits, any episode of loss of consciousness for more than 20 min, history of traumatic brain injury or neurological disorder, personal history of psychopathological disorders (according to DSM-IV-TR criteria), family history of alcoholism or substance abuse in first degree relatives, consumption of medical drugs with psychoactive effects (e.g., sedatives or anxiolytics) during the week previous to the assessment, AUDIT scores ≥ 20, and use of illegal drugs except cannabis.
Participants were asked to refrain from consuming alcohol at least 24 h before the EEG session. They were submitted to a Breathalyzer test, and the assessment was only performed after verifying 0% breath alcohol level. Additionally, subjects were instructed not to smoke, or drink tea or coffee for at least 3 h before the assessment. All participants provided written informed consent prior to assessment. The study was approved by the Ethics Committee of the Complutense University of Madrid and the procedure was undertaken in accordance with the Code of Ethical Principles for Medical Research Involving Humans Subjects outlined in the Declaration of Helsinki.
EEG Acquisition
As part of a more extensive EEG study, 3 and 4 min of electroencephalographic signal were acquired during eyes-open and eyes-closed resting state, respectively. Each subject was seated in a comfortable armchair located in a light- and sound-attenuated electrically shielded room. The electroencephalogram (EEG) was recorded using a 64-channel ActiCap system (Brain Products, Munich, Germany). Electrodes were located on the scalp according to the 10/10 system. All active electrodes were referred to the nose tip and grounded with an electrode placed at Fpz. Vertical and horizontal electrooculogram activity was recorded to control for potentials evoked by eye movements and blinks. Electrode impedances were kept below 20 kΩ. EEG signals were continuously amplified and digitized at a rate of 500 Hz, and filtered on-line with a 0.01–100 Hz band pass filter.
Data Analysis
Demographic data
Demographic variables were analyzed by a Student's t-test or chi-square test for independent samples.
EEG Processing
EEG data were processed with BrainVision Analyser software (Version 2.1). The signal was digitally filtered off-line with a 0.1–70 Hz band-pass filter and then corrected for ocular artifacts by the procedure developed by Gratton et al. (1983). It was then segmented into epochs of 4,000 ms for both eyes-open and eyes-closed resting state. Epochs exceeding ± 100 μV at any scalp electrode were rejected. The number of surviving epochs did not differ significantly between groups in the eyes-open condition (Control group: 42.2 ± 4.7; BD group: 42.2 ± 4.9) nor in the eyes-closed condition (Control group: 54.5 ± 6.6; BD group: 56.1 ± 5.4).
EEG-Source Localization Analysis
For the estimation of cortical sources activity, EEG data from 60 channels were analyzed using eLORETA; free academic software available at http://www.uzh.ch/keyinst/loreta.htm (Pascual-Marqui et al., 2011). The eLORETA is a three-dimensional, discrete and linear weighted minimal norm inverse solution method. The weights endow the tomography with the property of exact localization to test point sources, yielding images of current density with exact localization (spatial resolution 5 mm; Pascual-Marqui, 2007, 2009). eLORETA images represent the electrical activity at each of the 6,239 voxels (spatial resolution 5 mm) in the neuroanatomic Montreal Neurological Institute (MNI) space (Mazziotta et al., 2001).
EEG data were re-referenced to the common average before fast Fourier transformation. Mean power for each of the four classical frequency bands was computed and averaged across epochs for each subject in eyes-open and eyes-closed conditions separately: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz).
Voxel-by-voxel between-group comparisons of the spectral amplitude in source space were conducted using nonparametric statistical mapping method (SnPM; Nichols and Holmes, 2002) implemented in the eLORETA software package. For each single voxel, a t-test comparing amplitude estimates for groups at defined frequency bands was performed. Then, 5,000 randomizations with the SnPM procedure were applied to determine a statistical threshold (p < 0.05) corrected for multiple comparisons. Significant voxels were attributed to the corresponding Brodmann areas using the MNI space.
Results
Demographic Results
Demographic and alcohol consumption data are summarized in Table 1. There were no significant differences between groups regarding age, handedness, regular use of cannabis (one or more times a week), general severity index (GSI) of the SCL-R, or BIS-11 scores. Groups differed significantly in age of onset of regular drinking, number of drinks in a standard day, number of drinks in a drinking episode, total AUDIT score, regular use of tobacco (one or more times a week) and BAC (p < 0.001 for all comparisons).
eLORETA Results
eLORETA analysis revealed significant differences between controls and BDs at p < 0.05 after correction for multiple testing during eyes-open and eyes-closed conditions. Specifically, the current density in the beta frequency band during eyes-open resting state was significantly greater in the BD group in comparison with the Control group (t threshold required for significance: t = 3.115) in a large cluster involving the right parahippocampal and the fusiform gyri, as specified in Table 2 and illustrated in Figure 1.
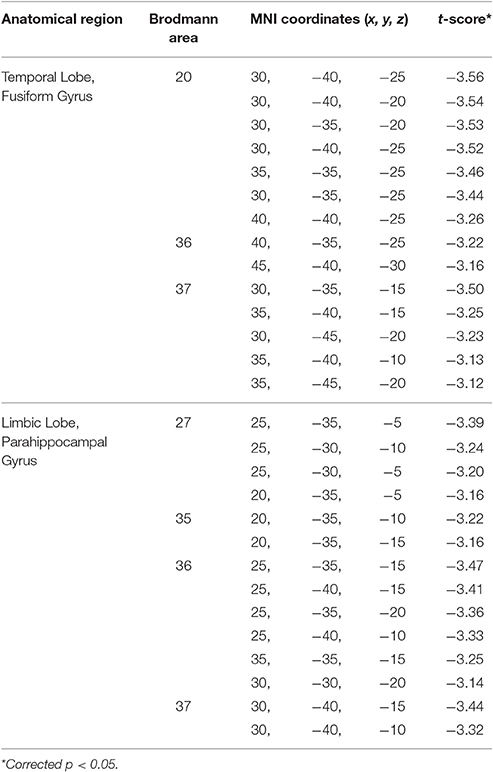
Table 2. Summary of the brain areas with significantly higher current density in the beta frequency band during eyes-open resting state in the binge drinking group relative to the control group.
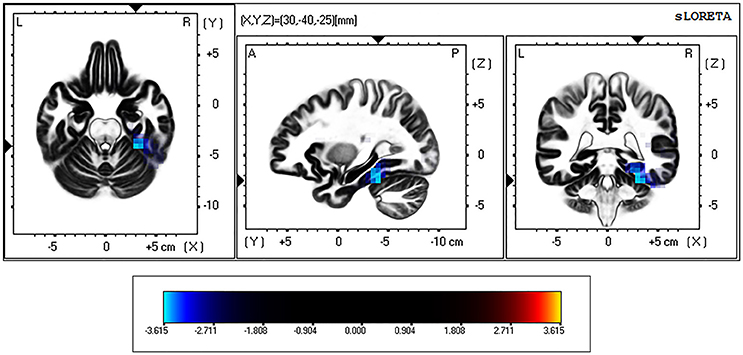
Figure 1. eLORETA-based statistical non-parametric maps (SnPM), comparing the exact current density values between control and binge drinking subjects during eyes-open resting state. Significantly higher current density (corrected p < 0.05) in the beta (12–30 Hz) frequency band in the binge drinking group relative to control group is shown in blue. L, Left; R, right; A, anterior; P, posterior.
On the other hand, during the eyes-closed condition, the current density in the theta frequency band was significantly greater in the BD group in comparison with the Control group (t threshold required for significance: t = 3.194) in a large bilateral cluster involving the occipital cortex, specifically the cuneus and the lingual gyrus (see Table 3 and Figure 2).
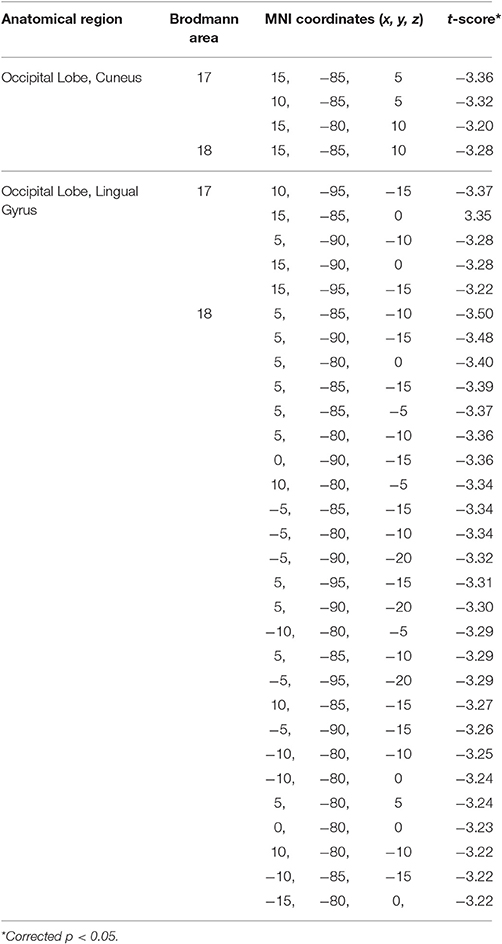
Table 3. Summary of the brain areas with significantly higher current density in the theta frequency band during eyes-closed resting state in the binge drinking group relative to the control group.
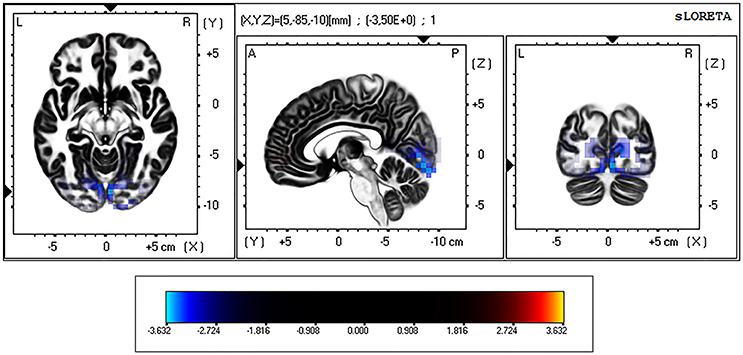
Figure 2. eLORETA-based statistical non-parametric maps (SnPM), comparing the exact current density values between control and binge drinking subjects during eyes-closed resting state. Significantly higher current density (corrected p < 0.05) in the theta (4–8 Hz) frequency band in the binge drinking group relative to control group is shown in blue. L, Left; R, right; A, anterior; P, posterior.
Discussion
The major finding of the present study is that young university students reporting a BD pattern displayed, in comparison with age-matched control subjects, a significantly higher intracranial current density in the beta frequency band in the right temporal lobe (parahippocampal and fusiform gyri) during eyes-open resting state as well as higher intracranial current density in the theta band in the bilateral occipital cortex (cuneus and lingual gyrus) during eyes-closed resting condition (see Figure 3).
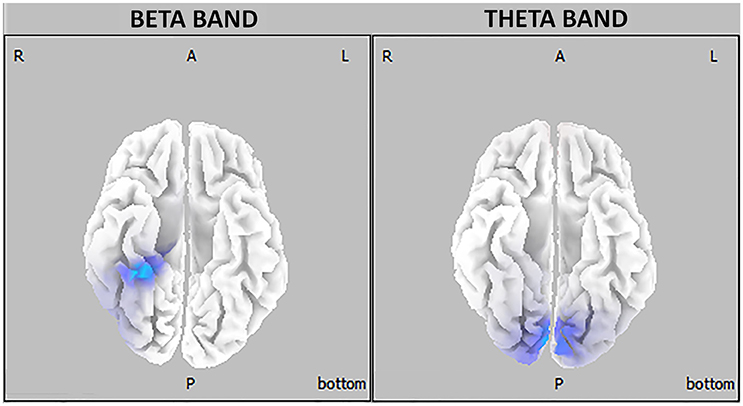
Figure 3. Three-dimensional eLORETA statistical maps of EEG oscillations showing between-groups differences in beta (right) and theta (left) frequency bands (blue-coded for p < 0.05, corrected for multiple testing).
As noted in the introduction, increased beta frequency rhythms in the EEG of alcoholics, mostly during rest, has been widely documented (Rangaswamy and Porjesz, 2014). Although it is difficult to associate oscillatory activity of a single frequency rhythm with a unique brain function, it has been proposed that tonic beta rhythms indicate states of enhanced arousal (Steriade et al., 1993; Engel and Fries, 2010). Thus, an imbalance in these oscillations may affect the overall level of neural excitation, so that an increment in the beta band may reflect a state of cortical hyperexcitability (Edenberg et al., 2004). In this sense, the increased beta power commonly observed in chronic alcoholics has been associated with a neural disinhibition resulting from an imbalance between excitatory and inhibitory neurons (Begleiter and Porjesz, 1999; Porjesz and Begleiter, 2003; Coutin-Churchman et al., 2006).
Our data extend the notion of abnormally increased cortical excitability seen in alcoholics to youths with a BD pattern, who also show higher beta power at rest than age-matched controls. In the same vein, the only previous study that, to our knowledge, has assessed the resting EEG of young BDs also observed increased beta- and delta-power in high-BDs during passive viewing (Courtney and Polich, 2010). According to these authors and to our results, this increment in beta power would be suggestive of brain overactivity in young BDs.
The results of the present study also revealed that the BD group exhibited higher theta power compared to the control group during eyes-closed resting state. As stated early, increased resting theta activity has been reported from several studies in alcohol abusers (Pollock et al., 1992; Rasgaswamy et al., 2003; Mumtaz et al., 2016).
In addition to alcoholics, enhanced tonic theta power has been observed in a variety of neurological disorders including Alzheimer's disease (Dauwels et al., 2010), vascular dementia (Babiloni et al., 2004), or attention deficit hyperactivity disorder (Burke and Edge, 2013). Likewise, increases in the tonic theta band have been remarked in altered neurophysiological states of the brain such as, drowsy and sleep states (Makeig et al., 2000; Tanaka et al., 2000) and after consumption of anesthetic drugs (Voss and Sleigh, 2007) or depressant substances such as, alcohol (Stenberg et al., 1994; Ehlers et al., 1998; Ilan and Gevins, 2001), which appears to suggest that when the ability to respond to external stimuli decreases, spontaneous theta activity increases (Klimesch, 1999).
It is important to note here that tonic activity differs from phasic or event-related changes in their origin and in their functional implications. While phasic changes are more or less under volitional control and take place at a rapid rate, tonic changes are (usually) not deliberate and occur over the lifetime in response to circadian rhythms, fatigue, distress, etc. (Klimesch, 1999). Regarding theta frequency band, tonic and phasic theta oscillations seem to be dissociated with respect to cognitive performance. In this sense, it has been reported that phasic theta power is enhanced as a function of task difficulty, memory load or attentional and cognitive control demands, and that increments in this phasic oscillation are positively associated with increasing cognitive performance (Klimesch et al., 2008; Nigbur et al., 2011; Cavanagh and Shackman, 2015; Clayton et al., 2015). Meanwhile, the opposite holds true for tonic theta activity, i.e., a pronounced suppression of tonic theta power is observed with increasing cognitive activity, whereas increments are associated with impaired global cognitive function (Jeong, 2004; Niedermeyer, 2005; Dubbelink et al., 2014). Consequently, large power in the range of tonic theta frequency has been considered an index of reduced cognitive processing capacity (Klimesch, 1999), and this is the reason why the higher resting theta activity present in the EEG of alcoholics has been suggested to reflect a deficit in information processing capacity (Rangaswamy et al., 2003; Campanella et al., 2009). Accordingly, the increased tonic theta power observed in the BD group of the present study might indicate a poorer information processing capacity compared to the control group.
Supporting the notion that BD may lead to similar spontaneous oscillatory activity as that identified in alcoholics, the only study examining whole brain activity measured by MEG in young BDs and conducted from our research group also reported higher theta power in the occipital region in BDs in comparison with controls during eyes-closed resting state (Correas et al., 2015). According to other BD studies assessing potential neural anomalies at the functional and structural level (Squeglia et al., 2012; Howell et al., 2013; Doallo et al., 2014; López-Caneda et al., 2014b; Correas et al., 2016), studies suggest that abnormal increases in the theta frequency band might correspond with a neuromaturational delay induced by a binge pattern of alcohol consumption during the adolescent period.
Adolescence is a key developmental stage with profound changes in brain morphology, generally reflected in an increase in global white matter volumes and a decrease in global gray matter structures (Lenroot and Giedd, 2006; Fuhrmann et al., 2015). Mirroring these neuromaturational changes, the oscillatory activity in the brain at rest is reduced in a broad frequency range from low-delta to high-beta between late childhood and early adulthood (Dustman et al., 1999; Boord et al., 2007; Lüchinger et al., 2012; Rodríguez-Martínez et al., 2017). Specifically, Whitford et al. (2007) and Buchmann et al. (2011) reported a reduction in gray matter volumes with age (ranging from 10 to 30 years and from 8 to 19 years, respectively) that was accompanied by a significant decrease in spectral power—most prominent in low frequencies—during awake and sleep EEG recordings. This juxtaposed decline in EEG power and gray matter has led to the suggestion that the synaptic pruning that typically takes place during the transition from childhood to adulthood is responsible for the observed reduction in EEG frequencies (Boord et al., 2007; Whitford et al., 2007; Feinberg and Campbell, 2010; Buchmann et al., 2011; Gómez et al., 2017).
Within the heterogeneous pattern of neurodevelopmental trajectories, the temporal cortex is the region where gray matter matures last, peaking generally around 17 years of age (Giedd, 2004; Gogtay et al., 2004), although some studies have extended this peak to around 30 years of age (Sowell et al., 2003). Taking into account the relationship between EEG power and gray matter changes, the increased beta power recorded over the right temporal cortex (parahippocampal and fusiform gyri) in the young BDs—aged 18 years—of our study might be related to neuroanatomical immaturity in these subjects compared to controls. As mentioned above, this would be in accordance with several neurostructural studies indicating enlarged gray matter (in some cases gender- and region-specific) in the BD population (Squeglia et al., 2012; Howell et al., 2013; Doallo et al., 2014; Kvamme et al., 2016; Morris et al., 2017). In particular, Kvamme et al. (2016) found greater gray matter volumes in female BDs in the bilateral fusiform gyrus and the right parahippocampal gyrus, showing that these regions may be affected—i.e., may undergo delayed synaptic pruning—due to BD. However, the lack of studies directly comparing electrophysiological and structural data in young BDs coupled with the fact that other studies have reported reduced instead increased cortical volumes in this population (Mashhoon et al., 2014; Squeglia et al., 2015) exhort us to be cautious in this interpretation.
Besides that, a key feature of EEG maturation is the progressive replacement of tonic theta power in the occipital region in favor of faster rhythms from childhood to adolescence and early adulthood, continuing until the third decade of life (Niedermeyer, 2005; Segalowitz et al., 2010). Indeed, despite resting theta activity having its maximum in occipital regions, this is not prevalent in normal adult waking EEG (Puligheddu et al., 2005; Kamarajan and Porjesz, 2015; Gómez et al., 2017). Thus, the higher occipital theta power observed in young BDs relative to age-matched controls may be linked to a slowing down in the reduction of theta rhythms that naturally occurs during development. However, further studies examining the development of theta activity in young BDs are warranted to confirm this hypothesis.
In summary, the present study used EEG-source localization analysis in order to identify potentially anomalous resting EEG frequency patterns in young BDs. Results showed a significantly higher intracranial current density in the beta frequency band over the right medial temporal cortex (parahippocampal and fusiform gyri) during eyes-open rest, and in the theta band over the bilateral occipital cortex (cuneus and lingual gyrus) during eyes-closed, in 18 year-old BDs compared to age-matched controls. These findings seem to be in line with previous results in alcohol-dependent subjects and BDs which observed increased beta and/or theta power following chronic or heavy alcohol drinking. As in these studies, the augmented tonic beta and theta oscillations obtained in our study suggest cortical hyperexcitability and potential difficulties in the information processing capacity of young BDs. The presence of this anomalous EEG pattern in young non-dependent BDs with a relatively short history of consumption could be due to more deleterious effects of alcohol on still-in-development brains, perhaps by means of a delay on neuromaturational processes. Finally, although additional research involving the study of spontaneous EEG rhythms in BDs is required, this abnormally elevated tonic neural activity might act as a potential marker of early brain damage associated with BD.
Author Contributions
EL and AnC collected the data. EL and AlC analyzed the data. SR, FC, and FM designed the study. EL wrote the first draft of the manuscript and all authors read, revised, and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the projects SPI/2010/134 and SPI/2010/051 from the Spanish Ministry of Health and Social Politics (National Plan on Drugs), and the project PSI2015-70525-P from the Spanish Ministry of Science and Innovation co-financed by European Regional Development Found. EL and AlC were supported by the Postdoctoral Fellowship of the Portuguese Foundation for Science and Technology SFRH/BPD/109750/2015 and SFRH/BPD/91440/2012 respectively, as well as by the Psychology Research Centre (UID/PSI/01662/2013), co-financed by FEDER through COMPETE2020 under the PT2020 Partnership Agreement (POCI-01-0145-FEDER-007653).
References
Babiloni, C., Binetti, G., Cassetta, E., Cerboneschi, D., Dal Forno, G., Del Percio, C., et al. (2004). Mapping distributed sources of cortical rhythms in mild Alzheimer's disease. A multicentric EEG study. Neuroimage 22, 57–67. doi: 10.1016/j.neuroimage.2003.09.028
Bauer, L. O. (2001). Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology 25, 332–340. doi: 10.1016/S0893-133X(01)00236-6
Begleiter, H., and Porjesz, B. (1999). What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol. Clin. Exp. Res. 23, 1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x
Boord, P. R., Rennie, C. J., and Williams, L. M. (2007). Integrating brain and body measures: correlations between EEG and metabolic changes over the human lifespan. J. Integr. Neurosci. 6, 205–218. doi: 10.1142/S0219635207001416
Buchmann, A., Ringli, M., Kurth, S., Schaerer, M., Geiger, A., Jenni, O. G., et al. (2011). EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb. Cortex. 21, 607–615. doi: 10.1093/cercor/bhq129
Burke, A., and Edge, A. (2013). “Neurodevelopmental pathways of childhood ADHD into adulthood: maturational lag, deviation, or both,” in Attention Deficit Hyperactivity Disorder in Children and Adolescents, ed S. Banerjee (Rijeka: InTech), 21–49.
Campanella, S., Petit, G., Maurage, P., Kornreich, C., Verbanck, P., and Noël, X. (2009). Chronic alcoholism: insights from neurophysiology. Clin. Neurophysiol. 39, 191–207. doi: 10.1016/j.neucli.2009.08.002
Carbia, C., Cadaveira, F., Caama-o-Isorna, F., Rodríguez-Holguín, S., and Corral, M. (2017). Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PLoS ONE 12:e0171393. doi: 10.1371/journal.pone.0171393
Carbia, C., and López-Caneda, E. (2016). “Who is at risk for binge drinking? risk factors involved in excessive alcohol drinking in adolescents and young adults,” in Alcohol Consumption: Patterns, Influences and Health Effects, ed W. Gutierrez (New York, NY: Nova Science Publishers), 23–64.
Cavanagh, J. F., and Shackman, A. J. (2015). Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. J. Physiol. Paris. 109, 3–15. doi: 10.1016/j.jphysparis.2014.04.003
Clayton, M. S., Yeung, N., and Kadosh, R. C. (2015). The roles of cortical oscillations in sustained attention. Trends Cogn. Sci. 19, 188–195. doi: 10.1016/j.tics.2015.02.004
Correas, A., Cuesta, P., López-Caneda, E., Holguín, S. R., García-Moreno, L. M., Pineda-Pardo, J. A., et al. (2016). Functional and structural brain connectivity of young binge drinkers: a follow-up study. Sci. Rep. 6:31293. doi: 10.1038/srep31293
Correas, A., Rodriguez Holguín, S., Cuesta, P., López-Caneda, E., García-Moreno, L. M., Cadaveira, F., et al. (2015). Exploratory analysis of power spectrum and functional connectivity during resting state in young binge drinkers: a MEG study. Int. J. Neural Syst. 25:1550008. doi: 10.1142/S0129065715500082
Costa, L., and Bauer, L. (1997). Quantitative electroencephalographic differences associated with alcohol, cocaine, heroin and dual-substance dependence. Drug Alcohol. Depend. 46, 87–93. doi: 10.1016/S0376-8716(97)00058-6
Courtney, K. E., and Polich, J. (2009). Binge drinking in young adults: data, definitions, and determinants. Psychol. Bull. 135, 142–156. doi: 10.1037/a0014414
Courtney, K. E., and Polich, J. (2010). Binge drinking effects on EEG in young adult humans. Int. J. Environ. Res. Public Health 7, 2325–2336. doi: 10.3390/ijerph7052325
Coutin-Churchman, P., and Moreno, R. (2008). Intracranial current density (LORETA) differences in QEEG frequency bands between depressed and non-depressed alcoholic patients. Clin. Neurophysiol. 119, 948–958. doi: 10.1016/j.clinph.2007.12.013
Coutin-Churchman, P., Moreno, R., A-ez, Y., and Vergara, F. (2006). Clinical correlates of quantitative EEG alterations in alcoholic patients. Clin. Neurophysiol. 117, 740–751. doi: 10.1016/j.clinph.2005.12.021
Crego, A., Holguín, S. R., Parada, M., Mota, N., Corral, M., and Cadaveira, F. (2009). Binge drinking affects attentional and visual working memory processing in young university students. Alcohol. Clin. Exp. Res. 33, 1870–1879. doi: 10.1111/j.1530-0277.2009.01025.x
Czapla, M., Simon, J. J., Friederich, H. C., Herpertz, S. C., Zimmermann, P., and Loeber, S. (2015). Is binge drinking in young adults associated with an alcohol-specific impairment of response inhibition? Eur. Addict. Res. 21, 105–113. doi: 10.1159/000367939
Dauwels, J., Vialatte, F., and Cichocki, A. (2010). Diagnosis of Alzheimer's disease from EEG signals: where are we standing? Curr. Alzheimer Res. 7, 487–505. doi: 10.2174/156720510792231720
Derogatis, L. R. (1983). Administration, Scoring and Procedures Manual II for the Revised Version of the SCL-90. Baltimore, MD: Johns Hopkins University Press.
Doallo, S., Cadaveira, F., Corral, M., Mota, N., López-Caneda, E., and Holguín, S. R. (2014). Larger mid-dorsolateral prefrontal gray matter volume in young binge drinkers revealed by voxel-based morphometry. PLoS ONE 9:e96380. doi: 10.1371/journal.pone.0096380
Dubbelink, K. T. O., Hillebrand, A., Twisk, J. W., Deijen, J. B., Stoffers, D., Schmand, B. A., et al. (2014). Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology 82, 263–270. doi: 10.1212/WNL.0000000000000034
Dustman, R. E., Shearer, D. E., and Emmerson, R. Y. (1999). Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clin. Neurophysiol. 110, 1399–1409. doi: 10.1016/S1388-2457(99)00102-9
Edenberg, H. J., Dick, D. M., Xuei, X., Tian, H., Almasy, L., Bauer, L. O., et al. (2004). Variations in GABRA2, encoding the α2 subunit of the GABA A receptor, are associated with alcohol dependence and with brain oscillations. Am. J. Hum. Genet. 74, 705–714. doi: 10.1086/383283
Ehlers, C. L., Havstad, J., Prichard, D., and Theiler, J. (1998). Low doses of ethanol reduce evidence for nonlinear structure in brain activity. J. Neurosci. 18, 7474–7486.
Ehlers, C. L., Phillips, E., Gizer, I. R., Gilder, D. A., and Wilhelmsen, K. C. (2010). EEG spectral phenotypes: heritability and association with marijuana and alcohol dependence in an American Indian community study. Drug Alcohol. Depend. 106, 101–110. doi: 10.1016/j.drugalcdep.2009.07.024
Engel, A. K., and Fries, P. (2010). Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165. doi: 10.1016/j.conb.2010.02.015
Feinberg, I., and Campbell, I. G. (2010). Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 72, 56–65. doi: 10.1016/j.bandc.2009.09.008
Fuhrmann, D., Knoll, L. J., and Blakemore, S. J. (2015). Adolescence as a sensitive period of brain development. Trends Cogn. Sci. 19, 558–566. doi: 10.1016/j.tics.2015.07.008
Giedd, J. N. (2004). Structural magnetic resonance imaging of the adolescent brain. Ann. N.Y. Acad. Sci. 1021, 77–85. doi: 10.1196/annals.1308.009
Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 101, 8174–8179. doi: 10.1073/pnas.0402680101
Gómez, C. M., Rodríguez-Martínez, E. I., Fernández, A., Maestú, F., Poza, J., and Gómez, C. (2017). Absolute power spectral density changes in the magnetoencephalographic activity during the transition from childhood to adulthood. Brain Topogr. 30, 87–97. doi: 10.1007/s10548-016-0532-0
Gratton, G., Coles, M. G., and Donchin, E. (1983). A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 55, 468–484. doi: 10.1016/0013-4694(83)90135-9
Guillamón, M. C., Solé, A. G., and Farran, J. C. (1999). Test para la identificación de transtornos por uso de alcohol (audit): traducción y validación del audit al catalán y castellano. Adicciones 11, 337–347. doi: 10.20882/adicciones.613
Heffernan, T., Clark, R., Bartholomew, J., Ling, J., and Stephens, S. (2010). Does binge drinking in teenagers affect their everyday prospective memory? Drug Alcohol. Depend. 109, 73–78. doi: 10.1016/j.drugalcdep.2009.12.013
Howell, N. A., Worbe, Y., Lange, I., Tait, R., Irvine, M., Banca, P., et al. (2013). Increased ventral striatal volume in college-aged binge drinkers. PLoS ONE 8:e74164. doi: 10.1371/journal.pone.0074164
Ilan, A. B., and Gevins, A. (2001). Prolonged neurophysiological effects of cumulative wine drinking. Alcohol 25, 137–152. doi: 10.1016/S0741-8329(01)00191-4
Jeong, J. (2004). EEG dynamics in patients with Alzheimer's disease. Clin. Neurophysiol. 115, 1490–1505. doi: 10.1016/j.clinph.2004.01.001
Kamarajan, C., and Porjesz, B. (2015). Advances in electrophysiological research. Alcohol Res. Curr. Rev. 37, 53–87.
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 29, 169–195. doi: 10.1016/S0165-0173(98)00056-3
Klimesch, W., Freunberger, R., Sauseng, P., and Gruber, W. (2008). A short review of slow phase synchronization and memory: evidence for control processes in different memory systems? Brain Res. 1235, 31–44. doi: 10.1016/j.brainres.2008.06.049
Kraus, L., Guttormsson, U., Leifman, H., Arpa, S., Molinaro, S., Monshouwer, K., et al. (2016). ESPAD Report 2015. Results from the European School Survey Project on Alcohol and Other Drugs. Lisbon: European Monitoring Centre for Drugs and Drug Addiction and the European School Survey Project on Alcohol and Other Drugs. Available online at: http://www.espad.org/sites/espad.org/files/ESPAD_report_2015.pdf
Kvamme, T. L., Schmidt, C., Strelchuk, D., Chang-Webb, Y. C., Baek, K., and Voon, V. (2016). Sexually dimorphic brain volume interaction in college-aged binge drinkers. Neuroimage Clin. 10, 310–317. doi: 10.1016/j.nicl.2015.12.004
Lenroot, R. K., and Giedd, J. N. (2006). Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 30, 718–729. doi: 10.1016/j.neubiorev.2006.06.001
López-Caneda, E., Mota, N., Crego, A., Velasquez, T., Corral, M., Holguín, S. R., et al. (2014a). Anomalías neurocognitivas asociadas al consumo intensivo de alcohol (binge drinking) en jóvenes y adolescentes: Una revisión. Adicciones 26, 334–359. doi: 10.20882/adicciones.39
López-Caneda, E., Rodríguez Holguín, S., Corral, M., Doallo, S., and Cadaveira, F. (2014b). Evolution of the binge drinking pattern in college students: neurophysiological correlates. Alcohol 48, 407–418. doi: 10.1016/j.alcohol.2014.01.009
López-Caneda, E., Rodríguez Holguín, S., Correas, Á., Carbia, C., González-Villar, A., Maestú, F., et al. (2017). Binge drinking affects brain oscillations linked to motor inhibition and execution. J. Psychopharmacol. 31, 873–882. doi: 10.1177/0269881116689258
Lüchinger, R., Michels, L., Martin, E., and Brandeis, D. (2012). Brain state regulation during normal development: intrinsic activity fluctuations in simultaneous EEG–fMRI. Neuroimage 60, 1426–1439. doi: 10.1016/j.neuroimage.2012.01.031
Makeig, S., Jung, T. P., and Sejnowski, T. J. (2000). Awareness during drowsiness: dynamics and electrophysiological correlates. Can. J. Exp. Psychol. 54, 266–273. doi: 10.1037/h0087346
Mashhoon, Y., Czerkawski, C., Crowley, D. J., Cohen-Gilbert, J. E., Sneider, J. T., and Silveri, M. M. (2014). Binge alcohol consumption in emerging adults: anterior cingulate cortical “thinness” is associated with alcohol use patterns. Alcohol. Clin. Exp. Res. 38, 1955–1964. doi: 10.1111/acer.12475
Maurage, P., Joassin, F., Speth, A., Modave, J., Philippot, P., and Campanella, S. (2012). Cerebral effects of binge drinking: respective influences of global alcohol intake and consumption pattern. Clin. Neurophysiol. 123, 892–901. doi: 10.1016/j.clinph.2011.09.018
Mazziotta, J., Toga, A., Evans, A., Fox, P., Lancaster, J., Zilles, K., et al. (2001). A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 1293–1322. doi: 10.1098/rstb.2001.0915
Meyers, J. L., Zhang, J., Wang, J. C., Su, J., Kuo, S. I., Kapoor, M., et al. (2017). An endophenotype approach to the genetics of alcohol dependence: a genome wide association study of fast beta EEG in families of African ancestry. Mol. Psychiatry. doi: 10.1038/mp.2016.239. [Epub ahead of print].
Moreno, M., Estevez, A. F., Zaldivar, F., Montes, J. M. G., Gutiérrez-Ferre, V. E., Esteban, L., et al. (2012). Impulsivity differences in recreational cannabis users and binge drinkers in a university population. Drug Alcohol. Depend. 124, 355–362. doi: 10.1016/j.drugalcdep.2012.02.011
Morris, L. S., Dowell, N. G., Cercignani, M., Harrison, N. A., and Voon, V. (2017). Binge drinking differentially affects cortical and subcortical microstructure. Addict. Biol. doi: 10.1111/adb.12493. [Epub ahead of print].
Mumtaz, W., Vuong, P. L., Xia, L., Malik, A. S., and Rashid, R. B. A. (2016). An EEG-based machine learning method to screen alcohol use disorder. Cogn. Neurodyn. 11, 161–171. doi: 10.1007/s11571-016-9416-y
National Institute of Alcohol, Abuse Alcoholism (2004). NIAAA council approves definition of binge drinking. NIAAA Newsletter 3. Available online at: http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf
Nichols, T. E., and Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15, 1–25. doi: 10.1002/hbm.1058
Niedermeyer, E. (2005). “The normal EEG of the waking adult,” in Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, eds E. Niedermeyer and F. Lopes da Silva (Philadelphia, PA: Williams and Wilkins), 155–164.
Nigbur, R., Ivanova, G., and Stürmer, B. (2011). Theta power as a marker for cognitive interference. Clin. Neurophysiol. 122, 2185–2194. doi: 10.1016/j.clinph.2011.03.030
Parvaz, M. A., Alia-Klein, N., Woicik, P. A., Volkow, N. D., and Goldstein, R. Z. (2011). Neuroimaging for drug addiction and related behaviors. Rev. Neurosci. 22, 609–624. doi: 10.1515/RNS.2011.055
Pascual-Marqui, R. D. (2007). Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: exact, zero error localization. arXiv:0710.3341.
Pascual-Marqui, R. D. (2009). “Theory of the EEG inverse problem,” in Quantitative EEG Analysis: Methods and Clinical Applications, eds S. Tong and N. V. Thakor (Boston, MA: Artech House), 121–140.
Pascual-Marqui, R. D., Lehmann, D., Koukkou, M., Kochi, K., Anderer, P., Saletu, B., et al. (2011). Assesing interactions in the brain with exact low-resolution electromagnetic tomography. Phil. Trans. A Math. Phys. Eng. Sci. 369, 3768–3784. doi: 10.1098/rsta.2011.0081
Patton, J. H., Stanford, M. S., and Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1
Petit, G., Kornreich, C., Dan, B., Verbanck, P., and Campanella, S. (2014a). Electrophysiological correlates of alcohol-and non-alcohol-related stimuli processing in binge drinkers: a follow-up study. J. Psychopharmacol. 28, 1041–1052. doi: 10.1177/0269881114545663
Petit, G., Maurage, P., Kornreich, C., Verbanck, P., and Campanella, S. (2014b). Binge drinking in adolescents: a review of neurophysiological and neuroimaging research. Alcohol Alcohol. 49, 198–206. doi: 10.1093/alcalc/agt172
Pollock, V. E., Earleywine, M., and Gabrielli, W. F. (1995). Personality and EEG β in older adults with alcoholic relatives. Alcohol. Clin. Exp. Res. 19, 37–43. doi: 10.1111/j.1530-0277.1995.tb01470.x
Pollock, V. E., Schneider, L. S., Zemansky, M. F., Gleason, R. P., and Pawluczyk, S. (1992). Topographic quantitative EEG amplitude in recovered alcoholics. Psychiat. Res. Neuroim. 45, 25–32. doi: 10.1016/0925-4927(92)90011-R
Porjesz, B., and Begleiter, H. (2003). Alcoholism and human electrophysiology. Alcohol Res. Health 27, 153–160.
Puligheddu, M., de Munck, J. C., Stam, C. J., Verbunt, J., de Jongh, A., van Dijk, B. W., et al. (2005). Age distribution of MEG spontaneous theta activity in healthy subjects. Brain Topogr. 17, 165–175. doi: 10.1007/s10548-005-4449-2
Rangaswamy, M., and Porjesz, B. (2014). “Understanding alcohol use disorders with neuroelectrophysiology,” in Handbook of Clinical Neurology, eds V Pfefferbaum and E. V. Sullivan (New York, NY: Elsevier), 383–414.
Rangaswamy, M., Porjesz, B., Chorlian, D. B., Choi, K., Jones, K. A., Wang, K., et al. (2003). Theta power in the EEG of alcoholics. Alcohol. Clin. Exp. Res. 27, 607–615. doi: 10.1111/j.1530-0277.2003.tb04397.x
Rangaswamy, M., Porjesz, B., Chorlian, D. B., Wang, K., Jones, K. A., Bauer, L. O., et al. (2002). Beta power in the EEG of alcoholics. Biol. Psychiatry 52, 831–842. doi: 10.1016/S0006-3223(02)01362-8
Rangaswamy, M., Porjesz, B., Chorlian, D. B., Wang, K., Jones, K. A., Kuperman, S., et al. (2004). Resting EEG in offspring of male alcoholics: beta frequencies. Int. J. Psychophysiol. 51, 239–251. doi: 10.1016/j.ijpsycho.2003.09.003
Rodríguez-Martínez, E. I., Ruiz-Martínez, F. J., Barriga Paulino, C., and Gómez, C. M. (2017). Frequency shift in topography of spontaneous brain rhythms from childhood to adulthood. Cogn. Neurodyn. 11:23. doi: 10.1007/s11571-016-9402-4
Saletu-Zyhlarz, G. M., Arnold, O., Anderer, P., Oberndorfer, S., Walter, H., Lesch, O. M., et al. (2004). Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol Alcohol. 39, 233–240. doi: 10.1093/alcalc/agh041
Segalowitz, S. J., Santesso, D. L., and Jetha, M. K. (2010). Electrophysiological changes during adolescence: a review. Brain Cogn. 72, 86–100. doi: 10.1016/j.bandc.2009.10.003
Smith, J. L., and Mattick, R. P. (2013). Evidence of deficits in behavioural inhibition and performance monitoring in young female heavy drinkers. Drug Alcohol. Depend. 133, 398–404. doi: 10.1016/j.drugalcdep.2013.06.020
Sowell, E. R., Peterson, B. S., Thompson, P. M., Welcome, S. E., Henkenius, A. L., and Toga, A. W. (2003). Mapping cortical change across the human life span. Nat. Neurosci. 6, 309–315. doi: 10.1038/nn1008
Substance Abuse Mental Health Services Administration (SAMHSA). (2016). Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health (HHS Publication No. SMA 16-4984, NSDUH Series H-51). Available online at: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2015Rev1/NSDUH-FFR1-2015Rev1/NSDUH-FFR1-2015Rev1/NSDUH-National%20Findings-REVISED-2015.htm
Squeglia, L. M., Sorg, S. F., Schweinsburg, A. D., Wetherill, R. R., Pulido, C., and Tapert, S. F. (2012). Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology 220, 529–539. doi: 10.1007/s00213-011-2500-4
Squeglia, L. M., Tapert, S. F., Sullivan, E. V., Jacobus, J., Meloy, M. J., Rohlfing, T., et al. (2015). Brain development in heavy-drinking adolescents. Am. J. Psychiatry 172, 531–542. doi: 10.1176/appi.ajp.2015.14101249
Stenberg, G., Sano, M., Rosén, I., and Ingvar, D. H. (1994). EEG topography of acute ethanol effects in resting and activated normals. J. Stud. Alcohol. 55, 645–656. doi: 10.15288/jsa.1994.55.645
Steriade, M., McCormick, D. A., and Sejnowski, T. J. (1993). Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679–685. doi: 10.1126/science.8235588
Tanaka, H., Hayashi, M., and Hori, T. (2000). Topographical characteristics of slow wave activities during the transition from wakefulness to sleep. Clin. Neurophysiol. 111, 417–427. doi: 10.1016/S1388-2457(99)00253-9
Townshend, J. M., and Duka, T. (2002). Patterns of alcohol drinking in a population of young social drinkers: a comparison of questionnaire and diary measures. Alcohol Alcohol. 37, 187–192. doi: 10.1093/alcalc/37.2.187
Voss, L., and Sleigh, J. (2007). Monitoring consciousness: the current status of EEG-based depth of anaesthesia monitors. Best Pract. Res. Clin. Anaesthesiol. 21, 313–325. doi: 10.1016/j.bpa.2007.04.003
Watson, T. D., Sweeney, J. F., and Louis, H. (2014). Neurocognitive, psychological and behavioral correlates of binge drinking and use of alcohol with caffeinated beverages in college-aged adults. Am. J. Drug Alcohol Abuse 40, 58–66. doi: 10.3109/00952990.2013.843005
White, A., and Hingson, R. (2013). The burden of alcohol use: excessive alcohol consumption and related consequences among college students. Alcohol Res. 35, 201–218.
Keywords: alcohol, binge drinking, adolescence, EEG, resting state, eLORETA
Citation: López-Caneda E, Cadaveira F, Correas A, Crego A, Maestú F and Rodríguez Holguín S (2017) The Brain of Binge Drinkers at Rest: Alterations in Theta and Beta Oscillations in First-Year College Students with a Binge Drinking Pattern. Front. Behav. Neurosci. 11:168. doi: 10.3389/fnbeh.2017.00168
Received: 23 May 2017; Accepted: 23 August 2017;
Published: 14 September 2017.
Edited by:
Bahar Güntekin, School of International Medicine, Istanbul Medipol University, TurkeyReviewed by:
Ricardo Marcos Pautassi, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaAna-Maria Cebolla, Free University of Brussels, Belgium
Janette Louise Smith, University of New South Wales, Australia
Copyright © 2017 López-Caneda, Cadaveira, Correas, Crego, Maestú and Rodríguez Holguín. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduardo López-Caneda, eduardo.lopez@usc.es; eduardo.lopez@psi.uminho.pt
 Eduardo López-Caneda
Eduardo López-Caneda Fernando Cadaveira
Fernando Cadaveira Angeles Correas3
Angeles Correas3  Fernando Maestú
Fernando Maestú Socorro Rodríguez Holguín
Socorro Rodríguez Holguín