Long-term Potentiation Decay and Poor Long-lasting Memory Process in the Wild Rodents Proechimys from Brazil’s Amazon Rainforest
- 1Disciplina de Neurociência, Departamento de Neurologia e Neurocirurgia, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
- 2Departamento de Ciencias Morfológicas, Facultad de Ciencias Médicas, Universidad Nacional Autónoma de Honduras, Tegucigalpa, Honduras
- 3Université de Strasbourg—INSERM U-1114—Neuropsychologie Cognitive, Physiopathologie de la Schizophrénie, Strasbourg, France
- 4Departamento de Farmacologia, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
- 5Departamento de Psicobiologia, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
Proechimys are small terrestrial rodents from Amazon rainforest. Each animal species is adapted to a specific environment in which the animal evolved therefore without comparative approaches unique characteristics of distinct species cannot be fully recognized. Laboratory rodents are exceedingly inbred strains dissociated from their native habitats and their fundamental ecological aspects are abstracted. Thus, the employment of exotic non-model species can be informative and complement conventional animal models. With the aim of promoting comparative studies between the exotic wildlife populations in the laboratory and traditional rodent model, we surveyed a type of synaptic plasticity intimately related to memory encoding in animals. Using theta-burst paradigm, in vitro long-term potentiation (LTP) in the CA1 subfield of hippocampal slices was assessed in the Amazon rodents Proechimys and Wistar rats. Memory, learning and anxiety were investigated through the plus-maze discriminative avoidance task (PM-DAT) and object recognition test. In PM-DAT, both animal species were submitted to two test sessions (3-h and 24-h) after the conditioning training. Proechimys exhibited higher anxiety-like behavior in the training session but during test sessions both species exhibited similar patterns of anxiety-related behavior. After 3-h of the training, Proechimys and Wistar spent significantly less time in the aversive enclosed arm than in the non-aversive arm. But, at 24-h after training, Wistar rats remained less time in the aversive closed arm in comparison with the non-aversive one, while Proechimys rodents spent the same amount of time in both enclosed arms. In the object recognition test, both species were evaluated at 24-h after the acquisition session and similar findings than those of the PM-DAT (24-h) were obtained, suggesting that long-term memory duration did not persist for 24-h in the Amazon rodent. Field excitatory post-synaptic potentials recordings revealed that LTP decays rapidly over time reaching basal levels at 90 min after theta-burst stimulation in Proechimys, contrasting to the stable LTP found in the Wistar rats which was observed throughout 3-h recording period. These findings suggest a link between the LTP decay and the lack of 24-h long-lasting memory process in Proechimys. Nevertheless, why early-phase LTP in Proechimys decays very rapidly remains to be elucidated.
Introduction
Proechimys (Rodentia-Echimyidae) are small terrestrial rodents from Amazon rainforest that play a critical role in the forest dynamics acting as seed predators and seed dispersers (Rojas-Robles et al., 2012; Amaral et al., 2013). They are generalists regarding the occupation of the forest territory and their existence is very well predicted by variables that qualify young or devastated forested areas (Lambert and Adler, 2000). These spiny rats are important contributors in woodland maintenance and regeneration. In the last years, studies have shown that Proechimys rodents exhibit unique structural and functional brain characteristics (Fabene et al., 2001, 2004; Arida et al., 2005; Scorza et al., 2010, 2011). Brains, as well as all animal traits, are extremely distinct and adjusted to the habitats in which animals evolved and developed (Carlson, 2012). Laboratory rodents are exceedingly inbred strains dissociated from their native habitats for generations thus lacking genetic and behavior diversity (Klaus and Amrein, 2012; Keifer and Summers, 2016). Fundamental ecological aspects are entirely abstracted in these laboratory animals. In principle, every species can add something to the comprehension and progress of science (Manger et al., 2008). By taking account of similarities and differences among species, researches can appreciate unique features and general principles of the nervous system (Keifer and Summers, 2016). Therefore, wild non-model species can complement conventional animal models and have something to offer to our comprehension of the brain (Brenowitz and Zakon, 2015).
Memory endows animals with the ability to learn and adjust their behavior based on past experiences and is one of the most puzzling processes of the brain. Ramón y Cajal (1894) was the first to speculate that memory relies on strengthening of synapses between neuronal cells. In addition to propose the value of environmental complexity and its influences on animals (Hebb, 1947), generating interest in how the environment impacts both the brain and behavior, Hebb (1949) also inaugurated the ideas of activity-dependent synaptic plasticity and cell-assembly formation in an attempt to explain the processes of learning and memory (Mayford et al., 2012; Poo et al., 2016). In the hippocampal formation, the “Hebbian” mechanisms gained great attention after the description of long-term potentiation (LTP) by Bliss and Lømo (1973). They showed that a brief high-frequency stimulation at excitatory synapses in the brain led to long-lasting enhancement of synaptic transmission efficiency (Bliss and Lømo, 1973), a discovery that revealed the plasticity of synapses, an essential property of learning and memory (Lømo, 2016). Notwithstanding, LTP has been cautiously acknowledged in the initial articles as the physiological foundation of learning and memory (Bliss and Gardner-Medwin, 1973; Bliss and Lømo, 1973), it is still currently debated whether LTP is related to these brain processes, although investigations on animals deliver robust evidence that it is indeed necessary (Morris et al., 1986; McHugh et al., 1996; Frey and Morris, 1997; Whitlock et al., 2006; Shema et al., 2007; Cooke and Bear, 2010; Madroñal et al., 2010; Sacktor, 2011; Glanzman, 2013; Nabavi et al., 2014).
Taken together, this work was conceived with the goal of promoting comparative studies for memory process and synaptic plasticity between the exotic wildlife populations in the laboratory and traditional rodent model. We surveyed a type of synaptic plasticity intimately related to memory encoding in animals. Using theta-burst paradigm that imitates in vivo firing patterns of hippocampal neurons, in vitro LTP in the CA1 subfield of hippocampal slices was assessed in the Amazon rodents Proechimys and Wistar rats. Memory, learning and anxiety were investigated through the plus-maze discriminative avoidance task (PM-DAT) and object recognition test.
Materials and Methods
Animals
Male Proechimys rodents (n = 18), originally from the Amazon rainforest, were bred in a colony established at the Neuroscience Laboratory’s facility (Federal Register-IBAMA number 1561643) of Escola Paulista de Medicina/Universidade Federal de São Paulo (EPM/UNIFESP). Male Wistar (n = 18) rats were acquired from CEDEME/UNIFESP. Throughout the study, the animals were maintained at a constant temperature of 22 ± 1°C, 12-h light-dark cycle and with free access to food and water. All animal procedures were carried out in accordance with the Ethical and Practical Principles of the Use of Laboratory Animals with the approval of the Ethical Committee of UNIFESP (CEUA 8615110915). Precautions were taken to minimize the number of rodents utilized in the experiments.
Plus-Maze Discriminative Avoidance Task (PM-DAT)
The PM-DAT is an useful test for investigating hippocampal-dependent memory involving emotionality and conveniently allows the simultaneous study of the learning, memory and anxiety (Silva and Frussa-Filho, 2000; Rachetti et al., 2013; Frussa-Filho et al., 2016; Leão et al., 2016b). The device used was a modified elevated plus-maze, 52 cm above the floor, composed of two closed arms (48 × 16 × 50 cm) opposite to two open arms (48 × 16 cm). Wistar rats (n = 10) and Proechimys rodents (n = 9) were conditioned to decide between the two enclosed arms (in one of them, a 100-W lamp and a 2200 W hair drier, as aversive stimuli, were both placed above the end of the arm) whilst avoiding the open arms of the device (Figure 1A). During the training session, which lasted 10 min, each rodent was positioned in the center of the device and each time the animal penetrated the aversive enclosed arm, the aversive stimulus was generated until the rodent left this arm. Then, the animals were submitted to two test sessions of 3 min each, which occurred 3 h and 24 h after the conditioning training session. In the test sessions, the rodents were put on device but aversive stimuli were not actioned, although the hair-dryer and the lamp stayed positioned. Rodents were randomly tested and, after each session, a 5% alcohol solution was used to clean the device. Learning was assessed by time the animal remained in the aversive enclosed arm during training session (Silva et al., 2004; Carvalho et al., 2006; Patti et al., 2006). In turn, memory was assessed by time spent in the aversive enclosed arm during the test sessions (Silva and Frussa-Filho, 2000; Rachetti et al., 2013; Leão et al., 2016b). Anxiety-like behavior was given by the percent time spent in the open arms (amount of time the animal remained in the open arms/total time spent in both open and closed arms) of the device (Silva et al., 2000; Calzavara et al., 2004; Leão et al., 2016a).
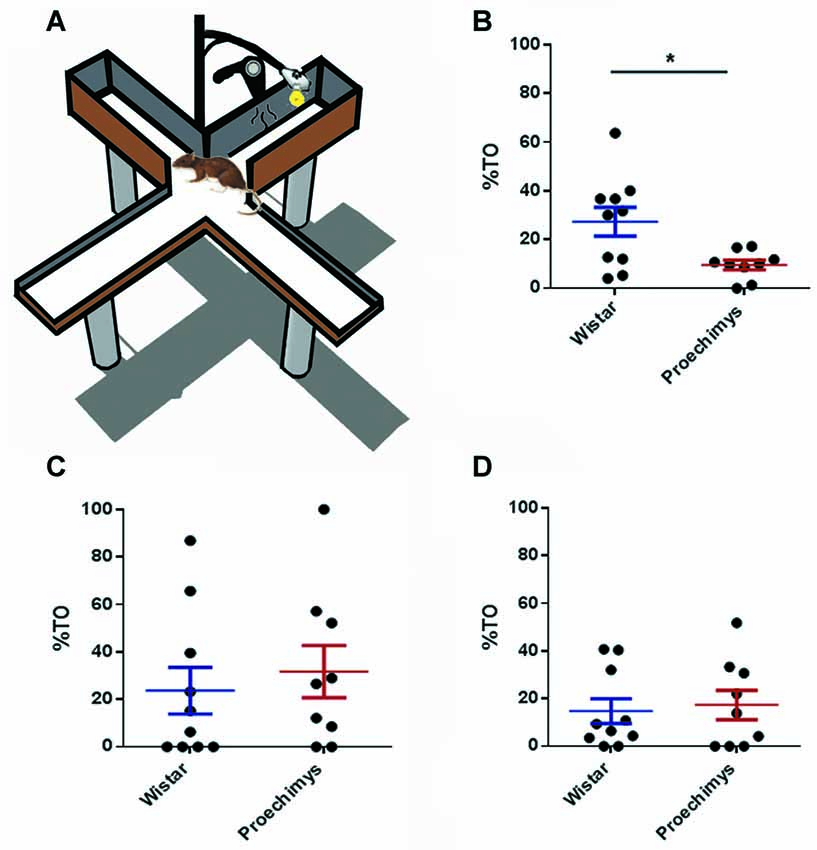
Figure 1. (A) Plus-maze discriminative avoidance task (PM-DAT). (B) Percent time (%TO) spent by Wistar and Proechimys in the open arms 10 min-exposure training session. (C) Three-minutes exposure test session after 3 h of the training session. (D) Three-minutes exposure test session after 24 h of the training session (*p < 0.05).
Object Recognition Task
A rectangular acrylic box (66 × 40 × 30 cm) was used in the experiments. The objects to be recognized were made of glass and presented different shapes and textures, they were tested in advance and animals showed no preferential exploration. Exploration was established when rodents sniffed or touched the object with the nose and/or forepaws. Before the test, the Wistar (n = 8) and Proechimys (n = 9) were habituated to the experimental box by allowing them to freely explore it during 10-min every day for consecutive 5 days. No stimulus object was placed inside the arena during habituation. The rodents were individually inserted into the box with two distinct objects (A and B) and left to freely explore them for 5-min. Twenty four hours later, animals were placed again in the same experimental box for a 5-min, but now they were presented to the familiar object (A) and to a novel object (C).
Brain Slice Preparation
Brain slice preparation was produced as described previously (Salar et al., 2016). Briefly, rodents were anesthetized with 1% isoflurane dissolved in 70% N2O and 30% O2. Then, adult Proechimys (n = 4) and Wistar rats (n = 4) were decapitated and brains quickly removed. Horizontal hippocampal slices were made in ice-cold artificial cerebrospinal fluid (aCSF) at 4 ± 0.5°C temperature containing (in mM): NaCl 129, NaHCO3 21, KCl 3, CaCl2 1.6, MgSO4 1.8, NaH2PO4 1.25, and glucose 10, saturated with 95% O2 and 5% CO2. Using a vibratome (LEICA VT 1200S), 400 μm thick brain slices were generated and promptly moved to an interface chamber perfused with aCSF at 36 ± 0.5°C (flow rate: 1.5–2 ml/min, pH 7.4, osmolarity: 295–300 mOsmol/L) where they were allowed to recover for 2–3 h.
In Vitro Electrophysiology—Stimulation and Recording
Extracellular field potentials (FPs) were recorded from the stratum radiatum of cornu Ammonis 1 (CA1) area. The recording electrode consisted of a chlorinated silver wire inserted into a glass pipette filled with 154 mM NaCl (5–10 MΩ). Schaffer collateral axons received the orthodromic stimuli through a bipolar twisted electrode (Ni-Cr wire with 50 μm diameter tip) positioned in the stratum radiatum of the CA1 hippocampal subfield. All data were low-pass filtered at 3 kHz, digitized at 10 KHz and stored on computer disk using a CED 1401 interface for off-line analysis using Spike 2 v6.09 (CED-1401, Cambridge, UK). Before starting the LTP protocol, input-output (I/O) relationships of stimulus intensity vs. field excitatory postsynaptic potentials (fEPSP) magnitude were performed in order to determine the maximal response after constant increments of stimulus intensity until no further increase in the fEPSP amplitude. From that, stimulus intensities were adapted to generate a fEPSP slope of 50%–60% of the maximum obtained from the I/O. Field EPSP slope values were accessed after the fiber volley in order to avoid the influence of other sources of current flow. Twenty minutes of stable baseline recording period was established before application of the high frequency stimulation, in which paired pulses were delivered with an inter-stimulus interval of 50 ms. LTP was induced by Theta Burst Stimulation (TBS) protocol (Liu et al., 2016), which consists in 10 bursts repeated at 200 ms intervals, with four pulses at 100 Hz for each burst, at the same stimulus intensity as the baseline, delivered to the Schaffer collaterals and the responses were recorded during 180 min.
Statistical Analysis
Electrophysiological data were analyzed using custom-made scripts (© Jan-Oliver Hollnagel, MATLAB R2013b; Salar et al., 2016). The fEPSP slope was measured between 20%–80% of its maximal amplitude. In order to verify the potentiation after TBS, field EPSP slopes were normalized relative to the averaged baseline response. Statistical analysis of behavioral and electrophysiological data was reported as mean ± standard error of the mean (SEM). Shapiro–Wilk test was used to determine normality. On behavioral tests, the statistical significance was assessed by non-parametric Mann-Whitney test, since less than 30 animals were used in the study, therefore the sample is not reliable to perform parametric approach (Ghasemi and Zahediasl, 2012). Statistical analysis for LTP was performed using two-way ANOVA (fEPSP slope at different times) followed by Bonferroni post hoc test. p < 0.05 (*) was considered to indicate a significant difference. Statistical analysis performed using Prism 5.00 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Plus-Maze Discriminative Avoidance Task (PM-DAT)
The wild Amazon rodents exhibited high anxiety-like behavior in the training session. The Wistar group spent more time on the open arms of the equipment than the Proechimys group (U = 18.00; p < 0.05; Figure 1B), throughout the training session. However, during test sessions, both species of animals exhibited similar patterns of anxiety-related behavior. No differences, between Wistar e Proechimys, were observed in the amount of time spent in the open arms of the device during the test sessions that occurred 3 h (U = 36.00; p > 0.05) and 24 h (U = 45.00; p > 0.05) after the training session (Figures 1C,D).
In the training session both species of rodents spent less time in the aversive closed arm in comparison to the amount of time spent in the non-aversive closed arm. Wistar (U = 0.00; p > 0.0001) and Proechimys (U = 0.00; p > 0.0001; Figures 2A,B).
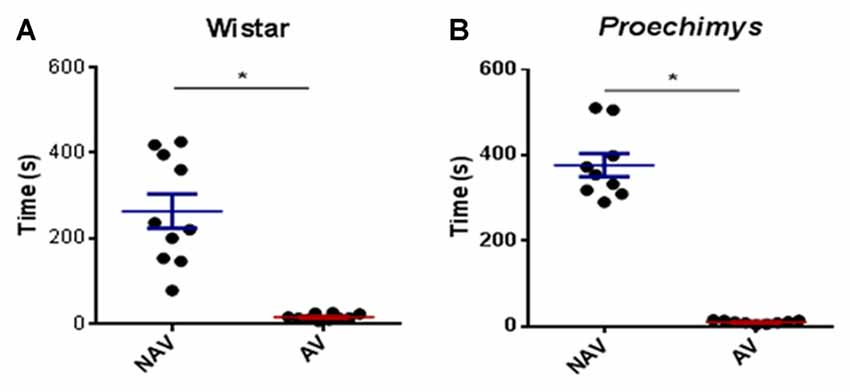
Figure 2. Training session in the plus-maze discriminative avoidance. Time spent by Wistar (A) and Proechimys (B) in aversive (AV) and non-aversive (NAV) enclosedarms (*p < 0.05).
In the test session, after 3 h of the training, both groups spent significantly less time in the aversive enclosed arm when compared to the time spent in the non-aversive enclosed arm. Wistar (U = 23.00; p < 0.05) and Proechimys (U = 17.50; p < 0.05; Figures 3A,B), suggesting that both animal species remembered the aversive situation.
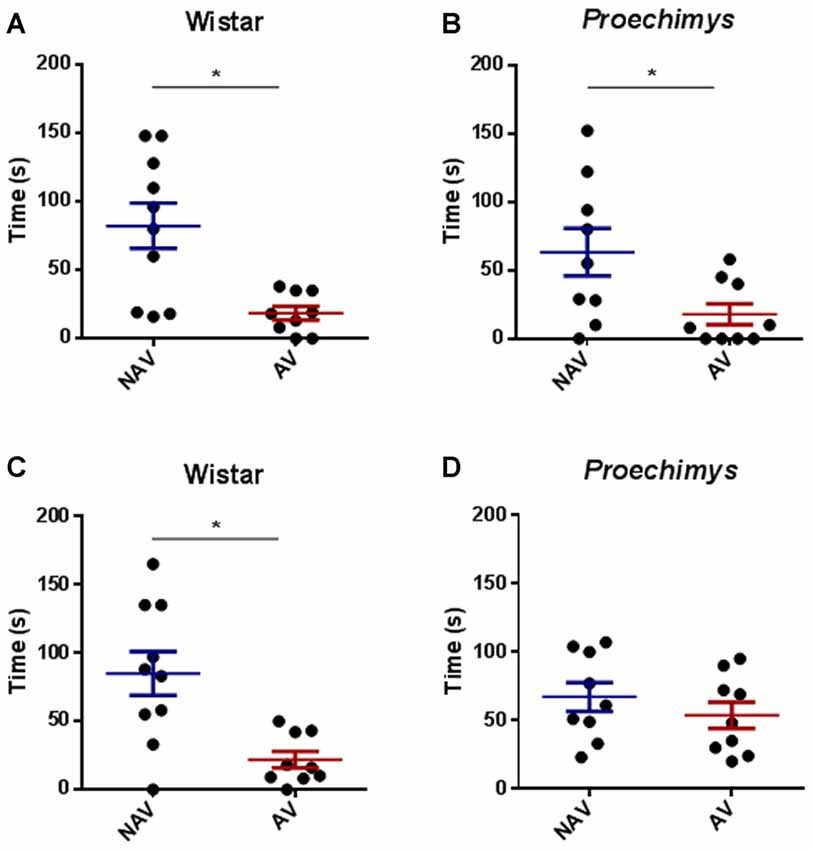
Figure 3. Test session in the plus-maze discriminative avoidance. Test session after 3 h of training session, time spent by Wistar (A) and Proechimys (B) in aversive (AV) and non-aversive (NAV) enclosed arms. Test session after 24 h of the training session, time spent by Wistar (C) and Proechimys (D) in aversive (AV) and non-aversive (NAV) enclosed arms (*p < 0.05).
In the test session, 24 h after training, Wistar rats remained less time in the aversive closed arm in comparison with the non-aversive arm (U = 21.50; p < 0.05; Figure 3C), although Proechimys rodents exhibited no difference in the amount of time spent in both enclosed arms (U = 19.00; p > 0.05; Figure 3D), suggesting that long term memory duration did not persist for 24 h in Proechimys.
Object Recognition Task
The comparison between the time spent to explore both objects (A and B) revealed an equivalent exploration time between the different groups of rodents in the acquisition trial, Wistar (U = 27.50 and p > 0.05) and Proechimys (U = 31.50 and p > 0.05; Figures 4A,B). Wistar rats, 24 h later of the acquisition phase, spent significantly more time exploring the novel object compared with the familiar object (U = 12.50 and p < 0.05; Figure 4C). In contrast there was no significant difference in exploration of the novel object when compared to familiar object in Proechimys rodents (U = 33.00 and p > 0.05; Figure 4D), suggesting that long term memory duration did not persist for 24 h in Proechimys.
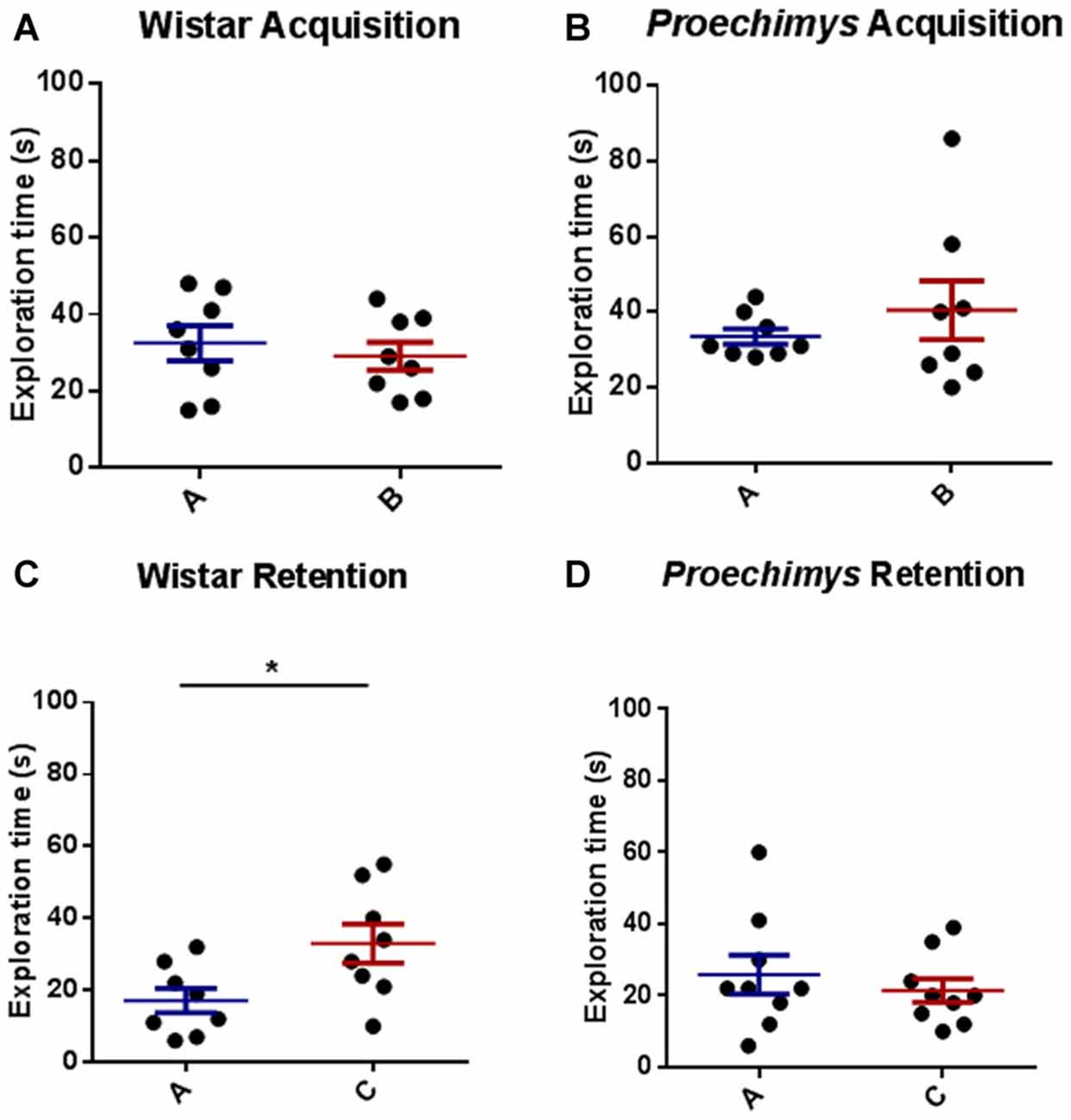
Figure 4. Object recognition task. Acquisition trial, exploration time spent by Wistar (A) and Proechimys (B) in object A and B. Retention trial, exploration time spent by Wistar (C) and Proechimys (D) in familiar and novel object (*p < 0.05).
Long-term Potentiation
In order to evaluate LTP under physiological condition, animals that underwent behavioral tests were not used. Eight slices per animal group (n = 16) were used in the experiments (Figure 5A). There was no significant effect of interaction between groups (F(2,42) = 0.5335; p > 0.05). Bonferroni’s post hoc test showed that the potentiation in the slope of fEPSP was significantly different (F(1,42) = 64.84; p < 0.0001) between, Proechimys (17.2 ± 8.4%) and Wistar (54.4 ± 1.2%) at 60 min after TBS (Figure 5C). In Proechimys, fEPSP slope potentiation decayed over time reaching basal levels at 90 min after TBS, contrasting to Wistar rats (10.1 ± 7.1% vs. 40.1 ± 4.1%, respectively) in which potentiation was stable during recordings (e.g., fEPSP slope potentiation at 180 min after TBS, Proechimys 1.5 ± 3.1%, Wistar 33.3 ± 6.0%; Figures 5B,D). The results revealed differences associated with the maintenance phase of LTP between Proechimys and Wistar, since LTP was successfully induced in both animal species after TBS protocol.
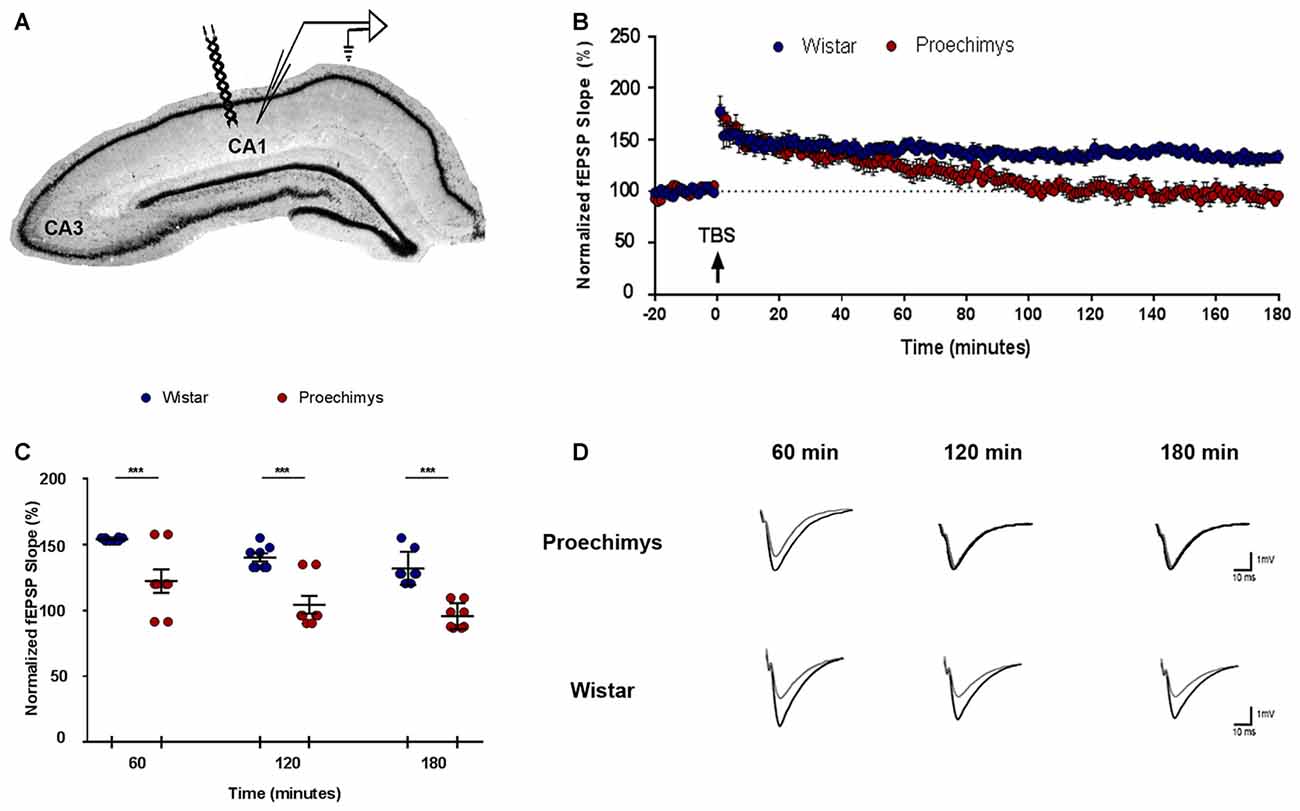
Figure 5. Long-term potentiation (LTP) in Proechimys and Wistar rodents. (A) Illustration shows the position of both electrodes recording and stimulation placed in CA1 hippocampal subfield. (B) Time evolution of fEPSP slopes of Proechimys and Wistar. Arrow indicates when theta burst stimulation (TBS) protocol was delivered. (C) The graph represents the average of fEPSP slope at 60, 120 and 180 min after TBS. (D) Representative superimposed recordings of averaged fEPSP before (gray trace) and 60, 120 and 180 min after (black trace) TBS in Proechimys and Wistar (***p < 0.05).
Discussion
In the PM-DAT we aimed to concomitantly assess learning, memory and anxiety (Silva and Frussa-Filho, 2000). At 3 h following training session, the animals returned to the apparatus for a memory retention test and both Proechimys and Wistar rodents were successful in remembering the task. However, at 24 h after training session, divergent long-term memory abilities were found and only Wistar rats exhibited memory retention. The results obtained in Wistar rats are in accordance with similar studies in the literature (Silva et al., 2000; Rachetti et al., 2013) but this is the first investigation of memory in the Proechimys rodents.
Studies using the PM-DAT have suggested that the 3-min test session, in comparison to the 10-min training session, is not long enough for animals to learn that although aversive devices are still present in the apparatus, they are not activated (Silva et al., 2000; Frussa-Filho et al., 2016). However, it could be different for Proechimys, since previous studies were performed in Wistar rats and PM-DAT was not validated for these wild rodents. Hence, through the object recognition test, one of the most commonly used behavioral tests in rodents (Antunes and Biala, 2012; Leger et al., 2013), 24-h long-term memory of Proechimys was assessed and the same results as those of PM-DAT were found. The findings obtained using Wistar rats are in accordance with previous studies (da Silveira et al., 2013; Furini et al., 2015).
The ability to remember and learn is absolutely fundamental for survival and every animal has its own physiological attribute, so one cannot truly compare the memory of two different species. Additionally, one can argue that under standard laboratory housing conditions, animals are exposed to potentially stressful and anxiogenic environmental challenges (Morgan and Tromborg, 2007) which may unfavorably impact the behavioral assessments, especially in the case of a wild-derived animal such as Proechimys. However, that does not seem to be the case here because higher anxiety-like behavior exhibited by Proechimys’s during one trial training session, when animals could be affected by the novelty-induced stress (File et al., 1990; Schneider et al., 2011), disappeared when they were tested at 3 h and 24 h after training. These findings obtained through PM-DAT suggested that Proechimys were not prone to higher anxiety levels than Wistar rats. Nevertheless, it is not possible exclude that the higher level of stress in Proechimys during acquisition task may have affected long-term maintenance of memory. Similarly, it is unlikely that higher levels of anxiety could explain the poor performance of Proechimys rodents in the novel object recognition test since both species showed similar total exploration time in both acquisition (when an object is introduced for the first time) and training sessions. The 5% alcohol solution is perhaps a limitation of our study since it may be not sufficient to avoid olfactory cues in the case of interspecies experiments. However, we cannot totally rule out that poor performance could be related to novelty avoidance due to higher emotionality.
Thereby, learning and memory are required for all animals to adapt and survive in their environment. Recent investigations fulfill several of the criteria that are required and sufficient to link learning/memory and LTP of synaptic strength between hippocampal neurons (Pastalkova et al., 2006; Whitlock et al., 2006). Current evidence has showed a possible relation between the maintenance of memory and the maintenance of LTP (Pastalkova et al., 2006; Sacktor, 2008).
Using TBS, here we found that LTP was induced in both Proechimys and Wistar rats but LTP of the excitatory postsynaptic potentials of the CA1 cells of Proechimys failed to be maintained over the course of 180 min of recording. Proechimys’s LTP potentiation decayed over time reaching baseline levels at 90 min after TBS, in contrast to Wistar rats in which potentiation could be observed throughout 3-h recording period. As shown in Figure 5C, fEPSP recorded in Proechimys’s brain slices showed that TBS could only produce a rapid decaying LTP. The literature reports that even weak protocols of induction can usually induce LTP that can last several hours in rodents (Lu et al., 2011; Dong et al., 2015). Theta-burst and tetanic stimulation have been the most frequently used experimental paradigms to induce LTP (Cao and Harris, 2014). Maybe, theta stimulation used in our work could be a very weak stimulation for Proechimys. For this reason, we also assessed LTP using different tetanic stimulation protocols, even multiple tetani in quick succession, but with similar results as those found with TBS (data not shown). Nevertheless, why early-phase LTP in Proechimys decays very rapidly remains an open question. It will be necessary to examine the adaptive function of cognitive processes in Proechimys thus allowing to predict what properties this cognitive system needs to have. Still, in vivo studies in Proechimys addressing the mechanisms underlying LTP maintenance and how this process is linked to memory are necessary.
Author Contributions
MJGM, JEM-C and LBL-S: performed and analyzed the behavioral experiments. SZR-G: performed and analyzed the in vitro electrophysiology experiments. MLA and EAC: critical revision of the manuscript. MJGM: interpretation and wrote the manuscript. FAS: design the work and data analysis interpretation. CAS: conception and design the work, perform data analysis, interpretation and wrote the manuscript.
Funding
This research was supported by CEPID/FAPESP (2013/08028-1) and FAPESP/CNPq/MCT-Instituto Nacional de Neurociência Translacional (573604/2008-8).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript is dedicated to the memory of the remarkable scientist and dear friend Professor Roberto Frussa-Filho, who developed the plus-maze discriminative avoidance model used in this work.
References
Amaral, P. J. S., Nagamachi, C. Y., Noronha, R. C. R., Costa, M. J. R., Pereira, A. L., Rossi, R. V., et al. (2013). Proechimys (Rodentia, Echimyidae): characterization and taxonomic considerations of a form with a very low diploid number and a multiple sex chromosome system. BMC Genet. 14:21. doi: 10.1186/1471-2156-14-21
Antunes, M., and Biala, G. (2012). The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13, 93–110. doi: 10.1007/s10339-011-0430-z
Arida, R. M., Scorza, F. A., de Amorim Carvalho, R., and Cavalheiro, E. A. (2005). Proechimys guyannensis: an animal model of resistance to epilepsy. Epilepsia 46, 189–197. doi: 10.1111/j.1528-1167.2005.01065.x
Bliss, T. V. P., and Gardner-Medwin, A. R. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the unanaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 357–374. doi: 10.1113/jphysiol.1973.sp010274
Bliss, T. V. P., and Lømo, T. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356. doi: 10.1113/jphysiol.1973.sp010273
Brenowitz, E. A., and Zakon, H. H. (2015). Emerging from the bottleneck: benefits of the comparative approach to modern neuroscience. Trends Neurosci. 38, 273–278. doi: 10.1016/j.tins.2015.02.008
Calzavara, M. B., Lopez, G. B., Abílio, V. C., Silva, R. H., and Frussa-Filho, R. (2004). Role of anxiety levels in memory performance of spontaneously hypertensive rats. Behav. Pharmacol. 15, 545–553. doi: 10.1097/00008877-200412000-00003
Cao, G., and Harris, K. M. (2014). Augmenting saturated LTP by broadly spaced episodes of theta-burst stimulation in hippocampal area CA1 of adult rats and mice. J. Neurophysiol. 112, 1916–1924. doi: 10.1152/jn.00297.2014
Carlson, B. A. (2012). Diversity matters: the importance of comparative studies and the potential for synergy between neuroscience and evolutionary biology. Arch. Neurol. 69, 987–993. doi: 10.1001/archneurol.2012.77
Carvalho, R. C., Patti, C. C., Takatsu-Coleman, A. L., Kameda, S. R., Souza, C. F., Garcez-do-Carmo, L., et al. (2006). Effects of reserpine on the plus-maze discriminative avoidance task: dissociation between memory and motor impairments. Brain Res. 1122, 179–183. doi: 10.1016/j.brainres.2006.09.008
Cooke, S. F., and Bear, M. F. (2010). Visual experience induces long-term potentiation (LTP) in the primary visual cortex. J. Neurosci. 30, 16304–16313. doi: 10.1523/JNEUROSCI.4333-10.2010
da Silveira, C. K. B., Furini, C. R. G., Benetti, F., Monteiro Sda, C., and Izquierdo, I. (2013). The role of histamine receptors in the consolidation of object recognition memory. Neurobiol. Learn. Mem. 103, 64–71. doi: 10.1016/j.nlm.2013.04.001
Dong, Z., Han, H., Li, H., Bai, Y., Wang, W., Tu, M., et al. (2015). Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis. J. Clin. Invest. 125, 234–247. doi: 10.1172/JCI77888
Fabene, P. F., Andrioli, A., Priel, M. R., Cavalheiro, E. A., and Bentivoglio, M. (2004). Fos induction and persistence, neurodegeneration, and interneuron activation in the hippocampus of epilepsy-resistant versus epilepsy-prone rats after pilocarpine-induced seizures. Hippocampus 14, 895–907. doi: 10.1002/hipo.20003
Fabene, P. F., Correia, L., Carvalho, R. A., Cavalheiro, E. A., and Bentivoglio, M. (2001). The spiny rat Proechimys guyannensis as model of resistance to epilepsy: chemical characterization of hippocampal cell populations and pilocarpine-induced changes. Neuroscience 104, 979–1002. doi: 10.1016/s0306-4522(01)00138-5
File, S. E., Mabbutt, P. S., and Hitchcott, P. K. (1990). Characterisation of the phenomenon of “one-trial tolerance” to the anxiolytic effect of chlordiazepoxide in the elevated plus-maze. Psychopharmacology 102, 98–101. doi: 10.1007/bf02245751
Frey, U., and Morris, R. G. M. (1997). Synaptic tagging and long-term potentiation. Nature 385, 533–536. doi: 10.1038/385533a0
Frussa-Filho, R., de Lima Patti, C., Fukushiro, D. F., Ribeiro, L. T. C., Kameda, S. R., and de Cassia Carvalho, R. (2016). “The plus-maze discriminative avoidance task: an ethical rodent model for concomitant evaluation of learning, memory, anxiety, motor activity and their interactions,” in Rodent Model as Tools in Ethical Biomedical Research, eds M. Andersen and S. Tufik (Cham: Springer), 327–344.
Furini, C. R. G., Myskiw Jde, C., Schmidt, B. E., Zinn, C. G., Peixoto, P. B., Pereira, L. D., et al. (2015). The relationship between protein synthesis and protein degradation in object recognition memory. Behav. Brain Res. 294, 17–24. doi: 10.1016/j.bbr.2015.07.038
Ghasemi, A., and Zahediasl, S. (2012). Normality tests for statistical analysis: a guide for non-statisticians. Int. J. Endocrinol. Metab. 10, 486–489. doi: 10.5812/ijem.3505
Hebb, D. O. (1947). The effects of early experience on problem solving at maturity. Am. Psychol. 2, 306–307.
Hebb, D. O. (1949). The Organization of Behavior: A Neuropsychological Theory. New York, NY: Wiley & Sons.
Keifer, J., and Summers, C. H. (2016). Putting the “biology” back into “neurobiology”: the strength of diversity in animal model systems for neuroscience research. Front. Syst. Neurosci. 10:69. doi: 10.3389/fnsys.2016.00069
Klaus, F., and Amrein, I. (2012). Running in laboratory and wild rodents: differences in context sensitivity and plasticity of hippocampal neurogenesis. Behav. Brain Res. 227, 363–370. doi: 10.1016/j.bbr.2011.04.027
Lambert, T. D., and Adler, G. H. (2000). Microhabitat use by a tropical forest rodent, Proechimys semispinosus, in central Panama. J. Mammal. 81, 70–76. doi: 10.1093/jmammal/81.1.70
Leão, A. H. F. F., Cabral, A., Izídio, G. S., Ribeiro, A. M., and Silva, R. H. (2016a). Diazepam effects on aversive memory retrieval and extinction: role of anxiety levels. Pharmacol. Biochem. Behav. 141, 42–49. doi: 10.1016/j.pbb.2015.11.012
Leão, A. H. F. F., Medeiros, A. M., Apolinário, G. K. S., Cabral, A., Ribeiro, A. M., Barbosa, F. F., et al. (2016b). Hippocampal-dependent memory in the plus-maze discriminative avoidance task: the role of spatial cues and CA1 activity. Behav. Brain Res. 304, 24–33. doi: 10.1016/j.bbr.2016.02.012
Leger, M., Quiedeville, A., Bouet, V., Haelewyn, B., Boulouard, M., Schumann-Bard, P., et al. (2013). Object recognition test in mice. Nat. Protoc. 8, 2531–2537. doi: 10.1038/nprot.2013.155
Liu, M.-G., Li, H.-S., Li, W.-G., Wu, Y.-J., Deng, S.-N., Huang, C., et al. (2016). Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms. Sci. Rep. 6:23350. doi: 10.1038/srep23350
Lømo, T. (2016). Scientific discoveries: what is required for lasting impact. Annu. Rev. Physiol 78, 1–21. doi: 10.1146/annurev-physiol-021115-105257
Lu, Y., Ji, Y., Ganesan, S., Schloesser, R., Martinowich, K., Sun, M., et al. (2011). TrkB as a potential synaptic and behavioral tag. J. Neurosci. 31, 11762–11771. doi: 10.1523/JNEUROSCI.2707-11.2011
Madroñal, N., Gruart, A. S., Sacktor, T. C., and Delgado-García, J. M. (2010). PKMf inhibition reverses learning-induced increases in hippocampal synaptic strength and memory during trace eyeblink conditioning. PLoS One 5:e10400. doi: 10.1371/journal.pone.0010400
Manger, P. R., Cort, J., Ebrahim, N., Goodman, A., Henning, J., Karolia, M., et al. (2008). Is 21st century neuroscience too focussed on the rat/mouse model of brain function and dysfunction? Front. Neuroanat. 2:5. doi: 10.3389/neuro.05.005.2008
Mayford, M., Siegelbaum, S. A., and Kandel, E. R. (2012). Synapses and memory storage. Cold Spring Harb. Perspect. Biol. 4:a005751. doi: 10.1101/cshperspect.a005751
McHugh, T. J., Blum, K. I., Tsien, J. Z., Tonegawa, S., and Wilson, M. A. (1996). Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87, 1339–1349. doi: 10.1016/s0092-8674(00)81828-0
Morgan, K. N., and Tromborg, C. T. (2007). Sources of stress in captivity. Appl. Anim. Behav. Sci. 102, 262–302. doi: 10.1016/j.applanim.2006.05.032
Morris, R. G., Anderson, E., Lynch, G. S., and Baudry, M. (1986). Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319, 774–776. doi: 10.1038/319774a0
Nabavi, S., Fox, R., Proulx, C. D., Lin, J. Y., Tsien, R. Y., and Malinow, R. (2014). Engineering a memory with LTD and LTP. Nature 511, 348–352. doi: 10.1038/nature13294
Pastalkova, E., Serrano, P., Pinkhasova, D., Wallace, E., Fenton, A. A., and Sacktor, T. C. (2006). Storage of spatial information by the maintenance mechanism of LTP. Science 313, 1141–1144. doi: 10.1126/science.1128657
Patti, C. L., Kameda, S. R., Carvalho, R. C., Takatsu-Coleman, A. L., Lopez, G. B., Niigaki, S. T., et al. (2006). Effects of morphine on the plus-maze discriminative avoidance task: role of state-dependent learning. Psychopharmacology 184, 1–12. doi: 10.1007/s00213-005-0238-6
Poo, M.-M., Pignatelli, M., Ryan, T. J., Tonegawa, S., Bonhoeffer, T., Martin, K. C., et al. (2016). What is memory? The present state of the engram. BMC Biol. 14:40. doi: 10.1186/s12915-016-0261-6
Rachetti, A. L. F., Arida, R. M., Patti, C. L., Zanin, K. A., Fernades-Santos, L., Frussa-Filho, R., et al. (2013). Fish oil supplementation and physical exercise program: distinct effects on different memory tasks. Behav. Brain Res. 237, 283–289. doi: 10.1016/j.bbr.2012.09.048
Ramón y Cajal, S. (1894). The croonian lecture: la fine structure des centres nerveux. Proc. R. Soc. London 44, 444–468. doi: 10.1098/rspl.1894.0063
Rojas-Robles, R., Stiles, F. G., and Muñoz-Saba, Y. (2012). Frugivory and seed dispersal Oenocarpus bataua palm (Arecaceae) in a forest from the Colombian Andes. Rev. Biol. Trop. 60, 1445–1461. doi: 10.15517/rbt.v60i4.2054
Sacktor, T. C. (2008). PKMζ, LTP maintenance, and the dynamic molecular biology of memory storage. Prog. Brain Res. 169, 27–40. doi: 10.1016/S0079-6123(07)00002-7
Sacktor, T. C. (2011). How does PKMζ maintain long-term memory? Nat. Rev. Neurosci. 12, 9–15. doi: 10.1038/nrn2949
Salar, S., Lapilover, E., Müller, J., Hollnagel, J.-O., Lippmann, K., Friedman, A., et al. (2016). Synaptic plasticity in area CA1 of rat hippocampal slices following intraventricular application of albumin. Neurobiol. Dis. 91, 155–165. doi: 10.1016/j.nbd.2016.03.008
Schneider, P., Ho, Y.-J., Spanagel, R., and Pawlak, C. R. (2011). A novel elevated plus-maze procedure to avoid the one-trial tolerance problem. Front. Behav. Neurosci. 5:43. doi: 10.3389/fnbeh.2011.00043
Scorza, C. A., Araujo, B. H. S., Arida, R. M., Scorza, F. A., Torres, L. B., Amorim, H. A., et al. (2010). Distinctive hippocampal CA2 subfield of the Amazon rodent Proechimys. Neuroscience 169, 965–973. doi: 10.1016/j.neuroscience.2010.05.079
Scorza, C. A., Araujo, B. H. S., Leite, L. A., Torres, L. B., Otalora, L. F. P., Oliveira, M. S., et al. (2011). Morphological and electrophysiological properties of pyramidal-like neurons in the stratum oriens of Cornu ammonis 1 and Cornu ammonis 2 area of Proechimys. Neuroscience 177, 252–268. doi: 10.1016/j.neuroscience.2010.12.054
Shema, R., Sacktor, T. C., and Dudai, Y. (2007). Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM. Science 317, 951–953. doi: 10.1126/science.1144334
Silva, R. H., Abílio, V. C., Takatsu, A. L., Kameda, S. R., Grassl, C., Chehin, A. B., et al. (2004). Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology 46, 895–903. doi: 10.1016/j.neuropharm.2003.11.032
Silva, R. H., Bergamo, M., and Frussa-Filho, R. (2000). Effects of neonatal ganglioside GM1 administration on memory in adult and old rats. Pharmacol. Toxicol. 87, 120–125. doi: 10.1034/j.1600-0773.2000.pto870304.x
Silva, R. H., and Frussa-Filho, R. (2000). The plus-maze discriminative avoidance task: a new model to study memory—anxiety interactions. Effects of chlordiazepoxide and caffeine. J. Neurosci. Methods 102, 117–125. doi: 10.1016/s0165-0270(00)00289-2
Keywords: memory, long-term potentiation, learning, anxiety, hippocampus, plus-maze discriminative avoidance task
Citation: Guimarães Marques MJ, Reyes-Garcia SZ, Marques-Carneiro JE, Lopes-Silva LB, Andersen ML, Cavalheiro EA, Scorza FA and Scorza CA (2018) Long-term Potentiation Decay and Poor Long-lasting Memory Process in the Wild Rodents Proechimys from Brazil’s Amazon Rainforest. Front. Behav. Neurosci. 12:2. doi: 10.3389/fnbeh.2018.00002
Received: 03 October 2017; Accepted: 09 January 2018;
Published: 23 January 2018.
Edited by:
Nadine Ravel, UMR5292 Centre de Recherche en Neurosciences de Lyon (CRNL), FranceReviewed by:
Chantal Mathis, Centre National de la Recherche Scientifique (CNRS), FranceIngrid Bethus, University of Sophia Antipolis, France
Copyright © 2018 Guimarães Marques, Reyes-Garcia, Marques-Carneiro, Lopes-Silva, Andersen, Cavalheiro, Scorza and Scorza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fulvio A. Scorza, scorza@unifesp.br
Carla A. Scorza, carla.scorza@unifesp.br
 Marcia J. Guimarães Marques
Marcia J. Guimarães Marques Selvin Z. Reyes-Garcia
Selvin Z. Reyes-Garcia José E. Marques-Carneiro
José E. Marques-Carneiro Leonardo B. Lopes-Silva
Leonardo B. Lopes-Silva Monica L. Andersen
Monica L. Andersen Esper A. Cavalheiro
Esper A. Cavalheiro Fulvio A. Scorza
Fulvio A. Scorza Carla A. Scorza
Carla A. Scorza