Therapeutic outcomes of transplantation of amniotic fluid-derived stem cells in experimental ischemic stroke
- 1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA
- 2Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China
- 3Department of Neurological Surgery, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
- 4Laboratory of Molecular Genetics, DISPUTer, School of Medicine and Health Sciences, “G. d ’Annunzio” University, Chieti-Pescara, Italy
Accumulating preclinical evidence suggests the use of amnion as a source of stem cells for investigations of basic science concepts related to developmental cell biology, but also for stem cells’ therapeutic applications in treating human disorders. We previously reported isolation of viable rat amniotic fluid-derived stem (AFS) cells. Subsequently, we recently reported the therapeutic benefits of intravenous transplantation of AFS cells in a rodent model of ischemic stroke. Parallel lines of investigations have provided safety and efficacy of stem cell therapy for treating stroke and other neurological disorders. This review article highlights the need for investigations of mechanisms underlying AFS cells’ therapeutic benefits and discusses lab-to-clinic translational gating items in an effort to optimize the clinical application of the cell transplantation for stroke.
Why is There a Need for Novel Treatments in Stroke?
Stroke, the fourthleading cause of death and the leading cause of disability in the United States (Roger et al., 2012), has only one FDA-approved drug, namely tissue plasminogen activator (tPA). Due to tPA limitations and complications, which include a limited therapeutic window (4.5 h from disease onset to tPA administration) and adverse effects associated with delayed treatment (i.e., hemorrhagic transformation), only a mere 3 percent of ischemic stroke patients actually benefit from the tPA treatment (Graham, 2003; Yip and Demaerschalk, 2007). This significant unmet clinical need for stroke has prompted investigations to increase the therapeutic window with innovative treatment strategies specifically targeting the restorative phase, which begins days to weeks post-stroke (Matsukawa et al., 2009; Yu et al., 2009; Antonucci et al., 2011; Kaneko et al., 2011; Manuelpillai et al., 2011).
Do Stem Cells Exist in the Amnion?
Stem cells have emerged as a prospective restorative agent for stroke due to their ability to abrogate sub-acute and chronic secondary cell death associated with the disease (Borlongan et al., 1997; Nishino and Borlongan, 2000). We previously reported the isolation of viable rat amniotic fluid-derived stem (AFS) cells (Tajiri et al., 2012). Together with other research teams, the grafted stem cells’ production of trophic factors and cytokines, as well as the increase in levels of neurotrophic factors and reduced inflammatory response in the ischemic stroke region, have been directly attributed to the positive effects by transplantation of AFS cells (Yu et al., 2009; Antonucci et al., 2011; Kaneko et al., 2011; Manuelpillai et al., 2011). Furthermore, the inhibition of apoptosis and oxidative stress, in tandem with stimulation of angiogenesis, neurogenesis, and synaptogenesis, may be linked as a benefit of AFS cells against stroke deficits (Yu et al., 2009; Antonucci et al., 2011; Kaneko et al., 2011; Manuelpillai et al., 2011). Even though stem cells can be harvested from various sources (Borlongan et al., 2004, 2005; Hematti et al., 2004; Clavel and Verfaillie, 2008; Burns et al., 2009; Ou et al., 2010), including bone marrow, fetal and embryonic tissues, amnion-derived stem cells are an appealing choice because of many logistical and ethical advantages including the ease of isolation of the stem cells from amnion tissue and the fluid (Yu et al., 2009; Antonucci et al., 2011; Kaneko et al., 2011; Manuelpillai et al., 2011). Similar to amniotic-tissue derived cells, the harvest of AFS cells poses negligible risk of injury to the fetus. These cells are isolated from amniotic fluid collected from amniocentesis, a pre-natal exam performed at around 15–20 weeks of gestation. Accordingly, since AFS cells can be isolated much earlier, compared to amniotic tissue-derived cells, AFS cells possess properties that closely resemble embryonic and mesenchymal cell markers, which could be more beneficial at treating diseases. The low immunogenicity, low tumorgenicity, high proliferative capacity and anti-inflammatory characteristics, are phenotypic features of transplantable cells (Newman et al., 2005) that support AFS cells to be a safe and effective donor cell for stroke (Yu et al., 2009; Antonucci et al., 2011; Kaneko et al., 2011; Manuelpillai et al., 2011). Another distinguishing feature of AFS cells when compared to amniotic tissue-derived cells is the sterility involved. AFS cells are harvested via amniocentesis under aseptic condition, but the sterility may be compromised when stem cells are extracted from amnion tissue during child delivery.
AFS cells can phenotypically commit to various lineages (Prusa and Hengstschlager, 2002; In’t Anker et al., 2003; Prusa et al., 2003; Fauza, 2004; Tsai et al., 2004, 2006; McLaughlin et al., 2006). A misleading concept is the term “fluid” associated with AFS cells because cells isolated during amniocentesis are comprised of multiple stem cells originating from extra-embryonic and embryonic tissues (Prusa and Hengstschlager, 2002) and their properties differ with gestational age (Yu et al., 2009; Antonucci et al., 2011; Kaneko et al., 2011; Manuelpillai et al., 2011). Accordingly, phenotypic characterization of AFS cells reveal a plethora of stem cell subtypes, from pluripotent embryonic stem cells to multipotent adult stem cells owing likely to the age-dependent tissue plasticity potential (De Coppi et al., 2007; Mauro et al., 2010). Indeed, AFS cells from second trimester amniotic fluid display the ability to differentiate into all three germ layers and express Oct-4, Nanog, and SSEA-4 (Roubelakis et al., 2007), which are pluripotent embryonic stem cell markers (Yu et al., 2009; Antonucci et al., 2011; Kaneko et al., 2011; Manuelpillai et al., 2011). Although considered as “adult stem cells”, the doubling time for the AFS cells population is approximately 30–36 h with a high regeneration capacity that can be extended for over 250 doublings without any measurable loss of chromosomal telomere length (De Coppi et al., 2007). Altogether, these studies support the amnion as a potent source of stem cells for investigations of basic science concepts related to developmental cell biology, but also for therapeutic purposes such as AFS cell transplantation for treating human disorders, especially stroke.
Are AFS Cells Transplantable and Do They Exert Functional Benefits?
The following protocol allows the isolation of AFS cells from isolated amniotic fluid samples obtained from timed pregnant Sprague-Dawley rats at gestation age 16–18 weeks (Tajiri et al., 2012). For each sample, 2–3 ml of amniotic fluid, corresponding to a cell number ranging from 2 × 103 to 2 × 106 are centrifuged for 10 min at 1800 rpm. The high variance in the amount of cells which can be isolated from amniotic fluid is influenced by factors such as child birth, genetics, and fluid cell isolation. It is expected, however, that these discrepancies can be overcome when technology is able to be standardized with appropriate quality control and assurance. This will yield a more accurate and efficient cell isolation in the near future. Pellets are then resuspended in Iscove’s modified Dulbecco’s medium supplemented with 20% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma), 2 mM L-glutamine, 5 ng/ml basic fibroblast growth factor (FGF2) and incubated at 37°C with 5% humidified CO2. After 7 days, non-adherent cells are removed and the adherent cells are allowed to grow in the same medium, which is changed every 4 days. When the cell culture reaches confluency (about 20 days after the primary culture), cells are treated with 0.05% trypsin and 0.02% EDTA, then counted and replaced in 25 cm2 culture flasks. For routine AFS cell procedure, an approximate yield of about 22 million stem cells per amnion fluid aspirate is anticipated. The viability yield of AFS cells is about 70%, which would be an estimated 15 million stem cells per amnion fluid aspirate. An estimated range of several million AFS cells per milliliter of amnion fluid are therefore expected to be obtained.
This protocol allows ample AFS cells for transplantation studies. Indeed, accumulating preclinical data have demonstrated the potential of transplantation of AFS cells for treating experimental models of brain diseases (Yu et al., 2009; Antonucci et al., 2011; Kaneko et al., 2011; Manuelpillai et al., 2011). For example, AFS cells are a potentially valuable source of stem cells to treat Parkinson’s disease because under standard neuronal induction protocols for stem cells, AFS cells preferentially differentiate into a dopaminergic phenotype (Pisani et al., 2005). In parallel, transplantation of AFS cells has been examined in stroke, with AFS transplanted ischemic stroke mice exhibiting reduced short-term memory impairment and improved sensorimotor ability, somatosensory functions, and motor coordination (Rehni et al., 2007). Although this study shows the beneficial effects of AFS cell transplantation in experimental stroke, the mechanism underlying the observed therapeutic benefits remained underexplored. Perhaps AFS cells are mediating specific neurotransmitter release to restore cellular function in an injured brain (Broughton et al., 2013). This may explain a reversal of disease-induced impairment of memory following AFS cell transplantation in an ischemic model of stroke when transfer latency time (TLT) is used as a parameter of memory. If administration of AFS cells following middle cerebral artery occlusion and reperfusion significantly attenuated ischemia-reperfusion induced increase in day 7 TLT, it is likely that the AFS cells are secreting the neurotransmitters necessary to ameliorate memory and subsequently, cognition in general (Rehni et al., 2007).
How do AFS Cells Afford Repair of the Stroke Brain?
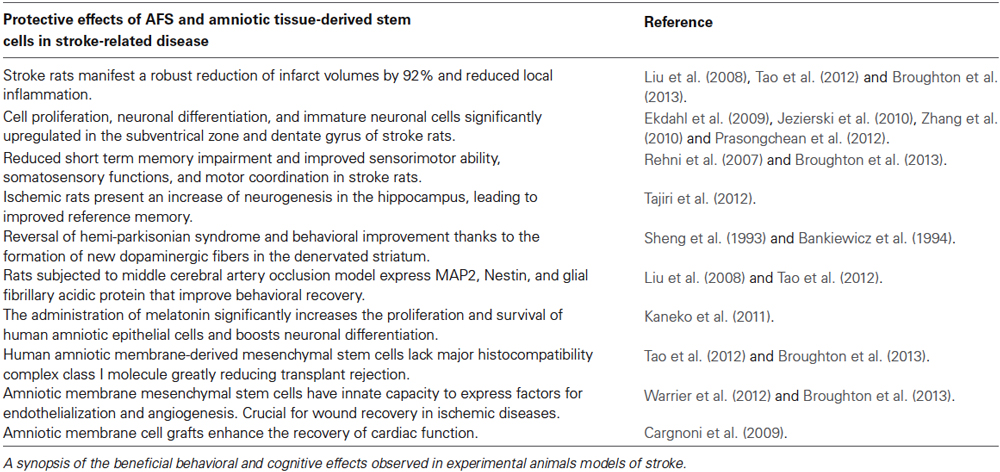
Our group transplanted AFS cells in experimental stroke animals (via occlusion of the middle cerebral artery; Borlongan et al., 1998) using motor and cognitive tests, and subsequent histological analysis of the brain to assess the mechanism of action associated with AFS cell therapy for stroke. Although still impaired compared to sham-operated animals (Acosta et al., 2010), intravenous transplantation of AFS cells (1 million viable cells) in adult Sprague-Dawley rats at 30 days after experimental stroke revealed that motor and cognitive deficits were reduced at 60 days post-MCAo compared to vehicle-infused stroke animals (Tajiri et al., 2012). Moreover, histological analyses revealed that the AFS cell-transplanted stroke animals significantly decreased infarct volumes by 92% compared to the vehicle-infused stroke animals, which likely facilitated the recovery of motor and cognitive functions. In addition, cell proliferation was significantly upregulated in the neurogenic subventrical zone of the AFS cell-transplanted stroke animals compared to the vehicle-infused stroke animals. In tandem, immature neuronal cells also increased in the subventrical zone of AFS cell-transplanted stroke animals compared to the vehicle-infused stroke animals. The increased cell proliferation and reduced neuronal loss in the subventrical zone was similarly observed in the dentate gyrus, another major neurogenic niche. That AFS cell transplantation attenuated stroke-induced behavioral and histological deficits coincided with increased cell proliferation and neuronal differentiation in the two neurogenic sites, namely subventricular zone and dentate gyrus, implicates a major role of graft-induced host tissue repair in the brain remodeling process following stroke (Table 1).
Can We Translate AFS Cell Graft-Mediated Functional Recovery in Experimental Stroke to the Clinic?
Many stroke victims present with symptoms characterized by sensorimotor and cognitive functions (Grefkes et al., 2008; Rush et al., 2010; Lin et al., 2011). Up to now, most stroke animal models have focused on motor impairments, disregarding the cognitive declines that proceed after the brain offense (Borlongan, 2009; Chopp et al., 2009; STEPS, 2009). Findings on the pathology of ischemic stroke support that secondary cell death may extend beyond the routine cortical damage towards the hippocampus, which is the key brain structure for learning and memory consolidation (Scoville and Milner, 1957; Shors et al., 2002; Saxe et al., 2006; Dupret et al., 2008). A decline in adult neurogenesis is magnified during aging and correlates with worsening of cognitive functions (McDonald and Wojtowicz, 2005; Drapeau and Nora Abrous, 2008; Encinas et al., 2011). Interestingly, the impaired neurogenesis and the cognitive decline in the aging hippocampus (Freret et al., 2009; Dhawan et al., 2010; Zvejniece et al., 2012) also accompany stroke (Dhawan et al., 2010; Wattanathorn et al., 2011; Zvejniece et al., 2012).
Whereas our cognitive test results did not show any difference in learning performance between the transplanted and vehicle-infused stroke animals, the AFS cell-transplanted stroke animals demonstrated a significantly improved reference memory compared to the vehicle-infused stroke animals. This improvement in the memory task directly correlates with increased neurogenesis in the hippocampus of AFS cell-transplanted stroke animals relative to vehicle-infused stroke animals. In order to increase the clinical relevance of cell therapy for stroke, especially when evaluating cognitive deficits, is to employ a hippocampal dependent task for spatial memory and reference memory (Gallagher et al., 1993; Duva et al., 1997; Clarke et al., 1999; Anisman and McIntyre, 2002; Broadbent et al., 2004; Clark et al., 2007; Gillani et al., 2010; Bergado et al., 2011; Jurgens et al., 2012), and long-term potentiation (Bliss and Collingridge, 1993; Norris and Foster, 1999; Gusev and Alkon, 2001; Richardson et al., 2002; Vorhees and Williams, 2006; Pisu et al., 2011; Xu et al., 2012). Our data advanced the notion that transplanted AFS cells selectively aid in the recovery of the reference memory, but not task acquisition. Cognitive improvements in stem/progenitor cell transplanted stroke animals have been linked previously with the decline in cerebral infarct volumes (Nishino et al., 1993; Fukunaga et al., 1999, 2003; Mimura et al., 2005), as well as reduced secondary cell death loss in brain areas known to modulate motor and cognitive functions (Ebrahimi et al., 1992; Pisani et al., 2005; Takahashi et al., 2008; Tabuse et al., 2010; Miyoshi et al., 2012). That the AFS cell transplanted stroke animals displayed improved motor and cognitive performance, reduced the brain infarcts and the secondary cell death, and enhanced the level of neurogenesis provide insights on possible mechanisms of action underlying the functional benefits afforded by AFS cell transplantation. Our observed transplant-mediated recovery of motor and cognitive functions confirms a recent report of AFS therapeutic effects in stroke (Rehni et al., 2007), but our study expanded the short timeline of 7 days from the previous study to 63 days.
The transplantation of AFS cells shows extensive therapeutic benefits such as reduction in infarct volume, enhanced cell proliferation, increased neuronal differentiation, and improved memory and motor skills, seen with other stem cells (Yasuhara et al., 2006a,b, 2008; Hara et al., 2007). The exact underlying mechanism of these benefits is still unknown and understanding therapeutic pathways could potentially yield promising insights into optimizing AFS cell transplantation towards the clinical trial stage. Clinical trials that closely reflect the preclinical data on safety and efficacy of AFS cells in animal models of stroke will enhance the functional outcome of this cell therapy in stroke patients. Based on preclinical data demonstrating AFS cell safety and efficacy, patients who suffer a stroke and show significant inflammation of the brain or who display short-term memory loss due to the accompanying injury to the hippocampus seem to be the best candidates for future clinical trials of AFS cells. Moreover, AFS cell therapy trials could be extended to patients who have suffered from cardiac stroke, as animal models show promising improvement in cardiac functions following AFS cell transplantation (Bollini et al., 2011). In addition, a better understanding of the mechanism(s) involved in the functional improvement seen in transplanted stroke animals will likely further optimize AFS cell therapy.
When contemplating with clinical applications of AFS cells, the route of transplantation is key to successful outcomes of targeted stroke patient population. Intracerebral (Rehni et al., 2007; Liu et al., 2008), intraperitoneal (Ghionzoli et al., 2010), and intravenous (Tajiri et al., 2012) have been explored in AFS transplantation in experimental models of cerebral ischemia. The intravenous route of transplantation is a minimally invasive procedure and poses less risk to the patient compared to intracerebral transplantation. A peripheral injection method may be favored over the direct transplantation route so that the stem cells can be administered quickly after the onset of a stroke. A limited FDA-approved clinical trial for intravenous transplantation of placenta/amnion-derived stem cells in sub-acute stroke patients was terminated with no available safety and efficacy results (ClinicalTrials.gov., 2014).
The recommendations of Stem cell Therapeutics as an Emerging Paradigm for Stroke (STEPS; Borlongan, 2009; Chopp et al., 2009; STEPS, 2009) are likely to facilitate the translational research on AFS cell grafts for stroke. Such translational blueprint for cell therapy in stroke provides important guidance in experimental stroke testing and stem cell therapy, including the need for multiple strains, both genders, and age groups to evaluate safety and efficacy of AFS cells (Borlongan, 2009; Chopp et al., 2009; STEPS, 2009). Equally important is the phenotypic characterization of AFS cells in order to allow replications of the results, but also to maintain quality control and quality assurance of the cells for transplantation therapy in the clinic. With this in mind, another key component of translating laboratory studies into clinical applications is to carefully and critically analyze the type of transplantation procedure to be employed in the clinic. The efficient isolation of AFS cells (i.e., during amniocentesis) (Kalogiannidis et al., 2011) may prove applicable to allogenic grafts in the stroke patients. The animal model shows the efficacy of AFS cells 30 days after stroke, which suggests its beneficial application to chronic stroke patients.
The collection of the AFS cells will likely require banking of cells associated with storage costs that may not be covered by health insurance. This will in turn require the donor to shoulder the costs ranging to thousands of dollars. The studies conducted so far exploring the immunogenic properties of human amniotic cells have revealed that these cells inhibit the function of different immune cells of the innate and adaptive immune response (Borlongan et al., 1996; Insausti et al., 2014); however, it is also documented that even autologous grafts have the potential to elicit an immune response. Therefore, further investigations to determine whether AFS cells are truly immune-free need to be conducted. In addition, the heterogenic population of AFS cells contains many cell phenotypes. While such variety of cell lineages suggests differentiation capability of AFS cells to multiple cell phenotypes and thus allowing many disease indications for AFS cell therapy, the stability of phenotypic expression and functional effects of AFS cells remain to be fully investigated. Altogether, these challenges may seem to hinder translational potential of AFS cells, but they pose as open avenues of research waiting to be discovered towards realizing the full potential of AFS cells for transplant therapy.
It is also important to discuss the practical issues associated with stroke therapy in adults. The unpredictable occurrence of a stroke event can be addressed by the ready availability of AFS cells. However, the route of stem cell administration may be dictated by the stroke disease phase. For example, acute stroke will require rapid delivery of cells via minimally invasive route, while chronic stroke may be amenable to intracerebral delivery of the cells. In addition, the ready availability of the amnion cells and timing of transplantation for both acute and chronic phases of stroke will require cryopreserved cells, thereby requiring high viability of cells following freezing and thawing storage. In this case, a panel of cell release criteria monitoring high viability of cells, normal karyotyping, and phenotypic stability (among other cell quality assurance issues) need to be in place prior to transplantation of AFS cells as these are common cell release criteria in place for other donor stem cell types. Ultimately, the maintenance of therapeutic efficacy due to scarce data to date providing “efficacy” readout for cells prior to transplantation is a challenge. A simple assay of neurotransmitter release or growth factor secretion after thawing may need to be developed as an efficacy release criterion which should enhance the therapeutic outcome of AFS cell transplantation.
Transplanted AFS cells-induced cell proliferation, in tandem with a decrease in neuronal loss, produced robust reduction in cerebral infarcts (Tajiri et al., 2012). The subventricular zone and dentate gyrus, two neurogenic niches in the brain, are critical to the repair of damaged brain tissue (Ekdahl et al., 2009; Jezierski et al., 2010; Zhang et al., 2010; Prasongchean et al., 2012). The results imply that AFS cell transplantation may have enhanced endogenous repair mechanisms by maximizing the potential of these two neurogenic sites to confer a host brain remodeling process. Intravenous AFS cell transplantation in the chronic stage of experimental stroke decreased motor and cognitive impairments, maintained cell proliferation, and increased cell differentiation in the host brain (Tajiri et al., 2012). The mechanism of action appears to involve AFS grafted cells’ capacity to solicit endogenous stem cells for brain repair. These observations offer insights into therapeutic pathways of brain repair, and guide the design of clinical trials of cell therapy in stroke.
Conflict of Interest Statement
CVB holds patents and has pending patents in stem cell biology and applications.
Acknowledgments
Cesar V. Borlongan is funded by the National Institutes of Health 1R01NS071956-01A1, the Department of Defense W81XWH-11-1-0634, and VA Merit Review.
References
Acosta, S., Jernberg, J., Sanberg, C. D., Sanberg, P. R., Small, B. J., Gemma, C., et al. (2010). NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res. 13, 581–588. doi: 10.1089/rej.2009.1011
Anisman, H., and McIntyre, D. C. (2002). Conceptual, spatial and cue learning in the Morris water maze in fast or slow kindling rats: attention deficit comorbidity. J. Neurosci. 22, 7809–7817.
Antonucci, I., Stuppia, L., Kaneko, Y., Yu, S., Tajiri, N., Bae, E., et al. (2011). Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant. 20, 789–795. doi: 10.3727/096368910x539074
Bankiewicz, K. S., Palmatier, M., Plunkett, R. J., Cummins, A., and Oldfield, E. H. (1994). Reversal of hemiparkinsonian syndrome in nonhumans primates by amnion implantation into caudate nucleus. J. Neurosurg. 81, 869–876. doi: 10.3171/jns.1994.81.6.0869
Bergado, J. A., Almaguer, W., Rojas, Y., Capdevila, V., and Frey, J. U. (2011). Spatial and emotional memory in aged rats: a behavioral-statistical analysis. Neuroscience 172, 256–269. doi: 10.1016/j.neuroscience.2010.10.064
Bliss, T. V., and Collingridge, G. L. (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39. doi: 10.1038/361031a0
Bollini, S., Cheung, K. K., Riegler, J., Dong, X., Smart, N., Ghionzoli, M., et al. (2011). Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev. 20, 1985–1994. doi: 10.1089/scd.2010.0424
Borlongan, C. V. (2009). Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke 40, S146–S148. doi: 10.1161/STROKEAHA.108.533091
Borlongan, C. V., Evans, A., Yu, G., and Hess, D. C. (2005). Limitations of intravenous human bone marrow CD133+ cell grafts in stroke rats. Brain Res. 1048, 116–122. doi: 10.1016/j.brainres.2005.04.087
Borlongan, C. V., Hadman, M., Sanberg, C. D., and Sanberg, P. R. (2004). Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 35, 2385–2389. doi: 10.1161/01.str.0000141680.49960.d7
Borlongan, C. V., Hida, H., and Nishino, H. (1998). Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport 9, 3615–3621. doi: 10.1097/00001756-199811160-00012
Borlongan, C. V., Koutouzis, T. K., Jorden, J. R., Martinez, R., Rodriguez, A. I., Poulos, S. G., et al. (1997). Neural transplantation as an experimental treatment modality for cerebral ischemia. Neurosci. Biobehav. Rev. 21, 79–90. doi: 10.1016/0149-7634(95)00063-1
Borlongan, C. V., Stahl, C. E., Cameron, D. F., Saporta, S., Freeman, T. B., Cahill, D. W., et al. (1996). CNS immunological modulation of neural graft rejection and survival. Neurol. Res. 18, 297–304.
Broadbent, N. J., Squire, L. R., and Clark, R. E. (2004). Spatial memory, recognition memory and the hippocampus. Proc. Natl. Acad. Sci. U S A 101, 14515–14520. doi: 10.1073/pnas.0406344101
Broughton, B. R., Lim, R., Arumugam, T. V., Drummond, G. R., Wallace, E. M., and Sobey, C. G. (2013). Post-stroke inflammation and the potential efficacy of novel stem cell therapies: focus on amnion epithelial cells. Front. Cell. Neurosci. 6:66. doi: 10.3389/fncel.2012.00066
Burns, T. C., Verfaillie, C. M., and Low, W. C. (2009). Stem cells for ischemic brain injury: a critical review. J. Comp. Neurol. 515, 125–144. doi: 10.1002/cne.22038
Cargnoni, A., Di Marcello, M., Campagnol, M., Nassuato, C., Albertini, A., and Parolini, O. (2009). Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant. 18, 1147–1159. doi: 10.3727/096368909x12483162196764
Chopp, M., Steinberg, G. K., Kondziolka, D., Lu, M., Bliss, T. M., Li, Y., et al. (2009). Who’s in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant. 18, 691–693. doi: 10.3727/096368909X470883
Clark, R. E., Broadbent, N. J., and Squire, L. R. (2007). The hippocampus and spatial memory: findings with a novel modification of the water maze. J. Neurosci. 27, 6647–6654. doi: 10.1523/jneurosci.0913-07.2007
Clarke, M. S., Prendergast, M. A., and Terry, A. V. Jr. (1999). Plasma membrane ordering agent pluronic F-68 (PF-68) reduces neurotransmitter uptake and release and produces learning and memory deficits in rats. Learn. Mem. 6, 634–649. doi: 10.1101/lm.6.6.634
Clavel, C., and Verfaillie, C. M. (2008). Bone-marrow-derived cells and heart repair. Curr. Opin. Organ Transplan. 13, 36–43. doi: 10.1097/MOT.0b013e3282f428d1
ClinicalTrials.gov. (2014). A Phase 2A, Prospective, Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Study to Evaluate the Safety of Intravenous Infusion of Human Placenta-Derived Cells (PDA001) for the Treatment of Adults Following Ischemic Stroke. Available online at: http://clinicaltrials.gov/ct2/show/NCT01310114
De Coppi, P., Bartsch, G. Jr., Siddiqui, M. M., Xu, T., Santos, C. C., Perin, L., et al. (2007). Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25, 100–106. doi: 10.1038/nbt1274
Dhawan, J., Benveniste, H., Nawrocky, M., Smith, S. D., and Biegon, A. (2010). Transient focal ischemia results in persistent and widespread neuroinflammation and loss of glutamate NMDA receptors. Neuroimage 51, 599–605. doi: 10.1016/j.neuroimage.2010.02.073
Drapeau, E., and Nora Abrous, D. (2008). Stem cell review series: role of neurogenesis in age-related memory disorders. Aging Cell. 7, 569–589. doi: 10.1111/j.1474-9726.2008.00369.x
Dupret, D., Revest, J. M., Koehl, M., Ichas, F., De Giorgi, F., Costet, P., et al. (2008). Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3:e1959. doi: 10.1371/journal.pone.0001959
Duva, C. A., Floresco, S. B., Wunderlich, G. R., Lao, T. L., Pinel, J. P., and Phillips, A. G. (1997). Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behav. Neurosci. 111, 1184–1196. doi: 10.1037/0735-7044.111.6.1184
Ebrahimi, A., Pochet, R., and Roger, M. (1992). Topographical organization of the projections from physiologically identified areas of the motor cortex to the striatum in the rat. Neurosci. Res. 14, 39–60. doi: 10.1016/s0168-0102(05)80005-7
Ekdahl, C. T., Kokaia, Z., and Lindvall, O. (2009). Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158, 1021–1029. doi: 10.1016/j.neuroscience.2008.06.052
Encinas, J. M., Michurina, T. V., Peunova, N., Park, J. H., Tordo, J., Peterson, D. A., et al. (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566–579. doi: 10.1016/j.stem.2011.03.010
Fauza, D. (2004). Amniotic fluid and placental stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 18, 877–891. doi: 10.1016/j.bpobgyn.2004.07.001
Freret, T., Bouet, V., Leconte, C., Roussel, S., Chazalviel, L., Divoux, D., et al. (2009). Behavioral deficits after distal focal cerebral ischemia in mice: usefulness of adhesive removal test. Behav. Neurosci. 123, 224–230. doi: 10.1037/a0014157
Fukunaga, A., Kawase, T., and Uchida, K. (2003). Functional recovery after simultaneous transplantation with neuro-epithelial stem cells and adjacent mesenchymal tissues into infarcted rat brain. Acta Neurochir. (Wien) 145, 473–480; discussion 480–471.
Fukunaga, A., Uchida, K., Hara, K., Kuroshima, Y., and Kawase, T. (1999). Differentiation and angiogenesis of central nervous system stem cells implanted with mesenchyme into ischemic rat brain. Cell Transplant. 8, 435–441.
Gallagher, M., Burwell, R., and Burchinal, M. (1993). Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 107, 618–626. doi: 10.1037/0735-7044.107.4.618
Ghionzoli, M., Cananzi, M., Zani, A., Rossi, C. A., Leon, F. F., Pierro, A., et al. (2010). Amniotic fluid stem cell migration after intraperitoneal injection in pup rats: implication for therapy. Pediatr. Surg. Int. 26, 79–84. doi: 10.1007/s00383-009-2504-x
Gillani, R. L., Tsai, S. Y., Wallace, D. G., O’Brien, T. E., Arhebamen, E., Tole, M., et al. (2010). Cognitive recovery in the aged rat after stroke and anti-Nogo-A immunotherapy. Behav. Brain Res. 208, 415–424. doi: 10.1016/j.bbr.2009.12.015
Graham, G. D. (2003). Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta-analysis of safety data. Stroke 34, 2847–2850. doi: 10.1161/01.str.0000101752.23813.c3
Grefkes, C., Nowak, D. A., Eickhoff, S. B., Dafotakis, M., Kust, J., Karbe, H., et al. (2008). Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann. Neurol. 63, 236–246. doi: 10.1002/ana.21228
Gusev, P. A., and Alkon, D. L. (2001). Intracellular correlates of spatial memory acquisition in hippocampal slices: long-term disinhibition of CA1 pyramidal cells. J. Neurophysiol. 86, 881–899.
Hara, K., Matsukawa, N., Yasuhara, T., Xu, L., Yu, G., Maki, M., et al. (2007). Transplantation of post-mitotic human neuroteratocarcinoma-overexpressing Nurr1 cells provides therapeutic benefits in experimental stroke: in vitro evidence of expedited neuronal differentiation and GDNF secretion. J. Neurosci. Res. 85, 1240–1251. doi: 10.1002/jnr.21234
Hematti, P., Hong, B. K., Ferguson, C., Adler, R., Hanawa, H., Sellers, S., et al. (2004). Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS Biol. 2:e423. doi: 10.1371/journal.pbio.0020423
Insausti, C. L., Blanquer, M., Garcia-Hernandez, A. M., Castellanos, G., and Moraleda, J. M. (2014). Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning 7, 53–63. doi: 10.2147/SCCAA.S58696
In’t Anker, P. S., Scherjon, S. A., Kleijburg-van der Keur, C., Noort, W. A., Claas, F. H., Willemze, R., et al. (2003). Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102, 1548–1549. doi: 10.1182/blood-2003-04-1291
Jezierski, A., Gruslin, A., Tremblay, R., Ly, D., Smith, C., Turksen, K., et al. (2010). Probing stemness and neural commitment in human amniotic fluid cells. Stem Cell Rev. 6, 199–214. doi: 10.1007/s12015-010-9116-7
Jurgens, H. A., Amancherla, K., and Johnson, R. W. (2012). Influenza infection induces neuroinflammation, alters hippocampal neuron morphology and impairs cognition in adult mice. J. Neurosci. 32, 3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012
Kalogiannidis, I., Prapa, S., Dagklis, T., Karkanaki, A., Petousis, S., Prapas, Y., et al. (2011). Amniocentesis-related adverse outcomes according to placental location and risk factors for fetal loss after midtrimester amniocentesis. Clin. Exp. Obstet. Gynecol. 38, 239–242.
Kaneko, Y., Hayashi, T., Yu, S., Tajiri, N., Bae, E. C., Solomita, M. A., et al. (2011). Human amniotic epithelial cells express melatonin receptor MT1, but not melatonin receptor MT2: a new perspective to neuroprotection. J. Pineal Res. 50, 272–280. doi: 10.1111/j.1600-079x.2010.00837.x
Lin, M. S., Chiu, M. J., Wu, Y. W., Huang, C. C., Chao, C. C., Chen, Y. H., et al. (2011). Neurocognitive improvement after carotid artery stenting in patients with chronic internal carotid artery occlusion and cerebral ischemia. Stroke 42, 2850–2854. doi: 10.1161/STROKEAHA.111.613133
Liu, T., Wu, J., Huang, Q., Hou, Y., Jiang, Z., Zang, S., et al. (2008). Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock 29, 603–611. doi: 10.1097/SHK.0b013e318157e845
Manuelpillai, U., Moodley, Y., Borlongan, C. V., and Parolini, O. (2011). Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta 32(Suppl. 4), S320–S325. doi: 10.1016/j.placenta.2011.04.010
Matsukawa, N., Yasuhara, T., Hara, K., Xu, L., Maki, M., Yu, G., et al. (2009). Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 10:126. doi: 10.1186/1471-2202-10-126
Mauro, A., Turriani, M., Loannoni, A., Russo, V., Martelli, A., Di Giacinto, O., et al. (2010). Isolation, characterization and in vitro differentiation of ovine amniotic stem cells. Vet. Res. Commun. 34(Suppl. 1), S25–S28. doi: 10.1007/s11259-010-9393-2
McDonald, H. Y., and Wojtowicz, J. M. (2005). Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci. Lett. 385, 70–75. doi: 10.1016/j.neulet.2005.05.022
McLaughlin, D., Tsirimonaki, E., Vallianatos, G., Sakellaridis, N., Chatzistamatiou, T., Stavropoulos-Gioka, C., et al. (2006). Stable expression of a neuronal dopaminergic progenitor phenotype in cell lines derived from human amniotic fluid cells. J. Neurosci. Res. 83, 1190–1200. doi: 10.1002/jnr.20828
Mimura, T., Dezawa, M., Kanno, H., and Yamamoto, I. (2005). Behavioral and histological evaluation of a focal cerebral infarction rat model transplanted with neurons induced from bone marrow stromal cells. J. Neuropathol. Exp. Neurol. 64, 1108–1117. doi: 10.1097/01.jnen.0000190068.03009.b5
Miyoshi, E., Wietzikoski, E. C., Bortolanza, M., Boschen, S. L., Canteras, N. S., Izquierdo, I., et al. (2012). Both the dorsal hippocampus and the dorsolateral striatum are needed for rat navigation in the Morris water maze. Behav. Brain Res. 226, 171–178. doi: 10.1016/j.bbr.2011.09.011
Newman, M. B., Misiuta, I., Willing, A. E., Zigova, T., Karl, R. C., Borlongan, C. V., et al. (2005). Tumorigenicity issues of embryonic carcinoma-derived stem cells: relevance to surgical trials using NT2 and hNT neural cells. Stem Cells Dev. 14, 29–43. doi: 10.1089/scd.2005.14.29
Nishino, H., and Borlongan, C. V. (2000). Restoration of function by neural transplantation in the ischemic brain. Prog. Brain Res. 127, 461–476. doi: 10.1016/s0079-6123(00)27022-2
Nishino, H., Koide, K., Aihara, N., Kumazaki, M., Sakurai, T., and Nagai, H. (1993). Striatal grafts in the ischemic striatum improve pallidal GABA release and passive avoidance. Brain Res. Bull. 32, 517–520. doi: 10.1016/0361-9230(93)90300-z
Norris, C. M., and Foster, T. C. (1999). MK-801 improves retention in aged rats: implications for altered neural plasticity in age-related memory deficits. Neurobiol. Learn. Mem. 71, 194–206. doi: 10.1006/nlme.1998.3864
Ou, Y., Yu, S., Kaneko, Y., Tajiri, N., Bae, E. C., Chheda, S. H., et al. (2010). Intravenous infusion of GDNF gene-modified human umbilical cord blood CD34+ cells protects against cerebral ischemic injury in spontaneously hypertensive rats. Brain Res. 1366, 217–225. doi: 10.1016/j.brainres.2010.09.098
Pisani, A., Centonze, D., Bernardi, G., and Calabresi, P. (2005). Striatal synaptic plasticity: implications for motor learning and Parkinson’s disease. Mov. Disord. 20, 395–402. doi: 10.1002/mds.20394
Pisu, M. G., Dore, R., Mostallino, M. C., Loi, M., Pibiri, F., Mameli, R., et al. (2011). Down-regulation of hippocampal BDNF and Arc associated with improvement in aversive spatial memory performance in socially isolated rats. Behav. Brain Res. 222, 73–80. doi: 10.1016/j.bbr.2011.03.021
Prasongchean, W., Bagni, M., Calzarossa, C., De Coppi, P., and Ferretti, P. (2012). Amniotic fluid stem cells increase embryo survival following injury. Stem Cells Dev. 21, 675–688. doi: 10.1089/scd.2011.0281
Prusa, A. R., and Hengstschlager, M. (2002). Amniotic fluid cells and human stem cell research: a new connection. Med. Sci. Monit. 8, RA253–RA257.
Prusa, A. R., Marton, E., Rosner, M., Bernaschek, G., and Hengstschlager, M. (2003). Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum. Reprod. 18, 1489–1493. doi: 10.1093/humrep/deg279
Rehni, A. K., Singh, N., Jaggi, A. S., and Singh, M. (2007). Amniotic fluid derived stem cells ameliorate focal cerebral ischaemia-reperfusion injury induced behavioural deficits in mice. Behav. Brain Res. 183, 95–100. doi: 10.1016/j.bbr.2007.05.028
Richardson, D. P., Byrnes, M. L., Brien, J. F., Reynolds, J. N., and Dringenberg, H. C. (2002). Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig. Eur. J. Neurosci. 16, 1593–1598. doi: 10.1046/j.1460-9568.2002.02214.x
Roger, V. L., Go, A. S., Lloyd-Jones, D. M., Benjamin, E. J., Berry, J. D., Borden, W. B., et al. (2012). Executive summary: heart disease and stroke statistics—2012 update: a report from the american heart association. Circulation 125, 188–197. doi: 10.1161/CIR.0b013e3182456d46
Roubelakis, M. G., Pappa, K. I., Bitsika, V., Zagoura, D., Vlahou, A., Papadaki, H. A., et al. (2007). Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 16, 931–952. doi: 10.1089/scd.2007.0036
Rush, B. K., McNeil, R. B., Gamble, D. M., Luke, S. H., Richie, A. N., Albers, C. S., et al. (2010). Behavioral symptoms in long-term survivors of ischemic stroke. J. Stroke Cerebrovasc. Dis. 19, 326–332. doi: 10.1016/j.jstrokecerebrovasdis.2009.09.009
Saxe, M. D., Battaglia, F., Wang, J. W., Malleret, G., David, D. J., Monckton, J. E., et al. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U S A 103, 17501–17506. doi: 10.1073/pnas.0607207103
Scoville, W. B., and Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21. doi: 10.1136/jnnp.20.1.11
Sheng, J. G., McShane, L. M., Plunkett, R. J., Cummins, A. C., Oldfield, E. H., Kopin, I. J., et al. (1993). Dopaminergic neuronal sprouting and behavioral recovery in hemi-parkinsonian rats after implantation of amnion cells. Exp. Neurol. 123, 192–203. doi: 10.1006/exnr.1993.1152
Shors, T. J., Townsend, D. A., Zhao, M., Kozorovitskiy, Y., and Gould, E. (2002). Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–584. doi: 10.1002/hipo.10103
STEPS Participants. (2009). Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 40, 510–515. doi: 10.1161/STROKEAHA.108.526863
Tabuse, M., Yaguchi, M., Ohta, S., Kawase, T., and Toda, M. (2010). A simple behavioral test for locomotor function after brain injury in mice. J. Clin. Neurosci. 17, 1412–1416. doi: 10.1016/j.jocn.2010.01.056
Tajiri, N., Acosta, S., Glover, L. E., Bickford, P. C., Jacotte Simancas, A., Yasuhara, T., et al. (2012). Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats. PLoS One 7:e43779. doi: 10.1371/journal.pone.0043779
Takahashi, K., Yasuhara, T., Shingo, T., Muraoka, K., Kameda, M., Takeuchi, A., et al. (2008). Embryonic neural stem cells transplanted in middle cerebral artery occlusion model of rats demonstrated potent therapeutic effects, compared to adult neural stem cells. Brain Res. 1234, 172–182. doi: 10.1016/j.brainres.2008.07.086
Tao, J., Ji, F., Liu, B., Wang, F., Dong, F., and Zhu, Y. (2012). Improvement of deficits by transplantation of lentiviral vector-modified human amniotic mesenchymal cells after cerebral ischemia in rats. Brain Res. 1448, 1–10. doi: 10.1016/j.brainres.2012.01.069
Tsai, M. S., Hwang, S. M., Tsai, Y. L., Cheng, F. C., Lee, J. L., Chang, Y. G., et al. (2006). Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol. Reprod. 74, 545–551. doi: 10.1095/biolreprod.105.046029
Tsai, M. S., Lee, J. L., Chang, Y. J., and Hwang, S. M. (2004). Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 19, 1450–1456. doi: 10.1093/humrep/deh279
Vorhees, C. V., and Williams, M. T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858. doi: 10.1038/nprot.2006.116
Warrier, S., Haridas, N., and Bhonde, R. (2012). Inherent propensity of amnion-derived mesenchymal stem cells towards endothelial lineage: vascularization from an avascular tissue. Placenta 33, 850–858. doi: 10.1016/j.placenta.2012.07.001
Wattanathorn, J., Jittiwat, J., Tongun, T., Muchimapura, S., and Ingkaninan, K. (2011). Zingiber officinale mitigates brain damage and improves memory impairment in focal cerebral ischemic rat. Evid. Based Complement. Alternat. Med. 2011:429505. doi: 10.1155/2011/429505
Xu, J., Wang, Y., Li, N., Xu, L., Yang, H., and Yang, Z. (2012). L-3-n-butylphthalide improves cognitive deficits in rats with chronic cerebral ischemia. Neuropharmacology 62, 2424–2429. doi: 10.1016/j.neuropharm.2012.02.014
Yasuhara, T., Borlongan, C. V., and Date, I. (2006a). Ex vivo gene therapy: transplantation of neurotrophic factor-secreting cells for cerebral ischemia. Front. Biosci. 11, 760–775. doi: 10.2741/1834
Yasuhara, T., Hara, K., Maki, M., Mays, R. W., Deans, R. J., Hess, D. C., et al. (2008). Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats. J. Cereb. Blood Flow Metab. 28, 1804–1810. doi: 10.1038/jcbfm.2008.68
Yasuhara, T., Matsukawa, N., Yu, G., Xu, L., Mays, R. W., Kovach, J., et al. (2006b). Behavioral and histological characterization of intrahippocampal grafts of human bone marrow-derived multipotent progenitor cells in neonatal rats with hypoxic-ischemic injury. Cell Transplant. 15, 231–238. doi: 10.3727/000000006783982034
Yip, T. R., and Demaerschalk, B. M. (2007). Estimated cost savings of increased use of intravenous tissue plasminogen activator for acute ischemic stroke in Canada. Stroke 38, 1952–1955. doi: 10.1161/strokeaha.106.479477
Yu, S. J., Soncini, M., Kaneko, Y., Hess, D. C., Parolini, O., Borlongan, C. V., et al. (2009). Amnion: a potent graft source for cell therapy in stroke. Cell Transplant. 18, 111–118. doi: 10.3727/096368909788341243
Zhang, C., Chopp, M., Cui, Y., Wang, L., Zhang, R., Zhang, L., et al. (2010). Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J. Neurosci. Res. 88, 3275–3281. doi: 10.1002/jnr.22495
Keywords: cerebral ischemia, neural stem/progenitor cells, regenerative medicine, neurogenesis, neurotrophic factors
Citation: Tajiri N, Acosta S, Portillo-Gonzales GS, Aguirre D, Reyes S, Lozano D, Pabon M, Peña ID, Ji X, Yasuhara T, Date I, Solomita MA, Antonucci I, Stuppia L, Kaneko Y and Borlongan CV (2014) Therapeutic outcomes of transplantation of amniotic fluid-derived stem cells in experimental ischemic stroke. Front. Cell. Neurosci. 8:227. doi: 10.3389/fncel.2014.00227
Received: 31 May 2014; Accepted: 23 July 2014;
Published online: 13 August 2014.
Edited by:
Thorsten Doeppner, University of Duisburg-Essen, GermanyReviewed by:
Josephine Herz, University Clinic Essen, University of Duisburg-Essen, GermanySeongjin Yu, NHRI, Taiwan
Copyright © 2014 Tajiri, Acosta, Portillo-Gonzales, Aguirre, Reyes, Lozano, Pabon, Peña, Ji, Yasuhara, Date, Solomita, Antonucci, Stuppia, Kaneko and Borlongan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cesar V. Borlongan, Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair,University of South Florida Morsani College of Medicine, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA e-mail: cborlong@health.usf.edu
 Naoki Tajiri
Naoki Tajiri Sandra Acosta
Sandra Acosta Gabriel S. Portillo-Gonzales1
Gabriel S. Portillo-Gonzales1  Daniela Aguirre
Daniela Aguirre Stephanny Reyes
Stephanny Reyes Diego Lozano
Diego Lozano Mibel Pabon
Mibel Pabon Ike Dela Peña
Ike Dela Peña Takao Yasuhara
Takao Yasuhara Isao Date
Isao Date Marianna A. Solomita
Marianna A. Solomita Ivana Antonucci
Ivana Antonucci Liborio Stuppia
Liborio Stuppia Yuji Kaneko
Yuji Kaneko Cesar V. Borlongan
Cesar V. Borlongan