Modulation of Adult Hippocampal Neurogenesis by Sleep: Impact on Mental Health
- 1Department of Basic and Clinical Neuroscience, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom
- 2Surrey Sleep Research Centre, Department of Clinical and Experimental Medicine, Faculty of Health and Medical Science, University of Surrey, Guildford, United Kingdom
The process of neurogenesis has been demonstrated to occur throughout life in the subgranular zone (SGZ) of the hippocampal dentate gyrus of several mammals, including humans. The basal rate of adult hippocampal neurogenesis can be altered by lifestyle and environmental factors. In this perspective review, the evidence for sleep as a modulator of adult hippocampal neurogenesis is first summarized. Following this, the impacts of sleep and sleep disturbances on hippocampal-dependent functions, including learning and memory, and depression are critically evaluated. Finally, we postulate that the effects of sleep on hippocampal-dependent functions may possibly be mediated by a change in adult hippocampal neurogenesis. This could provide a route to new treatments for cognitive impairments and psychiatric disorders.
Introduction
Neurogenesis is the process by which new neurons arise from neural stem and progenitor cells, mature, specialize and become integrated and functional within the neuronal network. Although originally believed to occur solely during the early stages of development, it was later demonstrated to be present in adult birds (Goldman and Nottebohm, 1983), rodents (Altman and Das, 1965; van Praag et al., 1999), monkeys (Kornack and Rakic, 1999), and even humans (Eriksson et al., 1998; Spalding et al., 2013). Neurogenic niches have been recorded in various specific areas of the central nervous system in adult rodents, namely the subventricular zone (SVZ) lining the lateral ventricles, where neuroblasts originate and newborn neurons eventually migrate to the olfactory bulb (Altman, 1969), and the subgranular zone (SGZ) of the hippocampal dentate gyrus where new neurons migrate a short distance through the granule cell layer (Altman and Das, 1965). In adult humans, evidence supports the existence of a similar neurogenic niche in the hippocampal SGZ (Spalding et al., 2013) but neuroblasts originating from the human SVZ appear to migrate into the striatum (Ernst et al., 2014).
In this review, we focus on the hippocampal neurogenic niche. The hippocampus has many interesting higher level functions which these newly formed neurons could potentially become part of, such as mood and emotion (Miller and Hen, 2015) and learning and memory (Aimone et al., 2014). Therefore, a change in neurogenesis could affect these hippocampal-dependent functions. For example, there is experimental evidence linking decreased neurogenesis to impaired learning, especially in the spatial domain (Shors et al., 2001; Thuret et al., 2009), decreased memory retention (Deng et al., 2010), and pattern separation (Clelland et al., 2009). On the other hand, hippocampal-dependent learning tasks appear to increase neurogenesis (Dobrossy et al., 2003). Likewise, computational modeling of the effects of adult neurogenesis on hippocampal function has generated different theories for the role of newborn neurons. These include encoding of temporal information into memories, avoidance of memory interference and cognitive flexibility during learning of new tasks and balancing pattern separation/integration (reviewed in Gonçalves et al., 2016). A reduction in neurogenesis has also been associated with depressive symptoms (Egeland et al., 2017), and antidepressant treatments with an increase in neurogenesis (Grassi Zucconi et al., 2006; Sahay and Hen, 2007; Anacker et al., 2011). Overall, new theories are emerging linking regulation of dentate gyrus functions by adult neurogenesis affecting the cognitive processes which have important implications for both memory and mood (Anacker and Hen, 2017).
Neurogenesis in adults does not occur at a constant rate and is affected by many endogenous and exogenous factors, including hormones (Tanapat et al., 1999), physical and psychosocial stress (Gould et al., 1999), physical activity (Brown et al., 2003), diet (Murphy et al., 2014), enriched environments (Brown et al., 2003), and age (Kuhn et al., 1996). Sleep- and circadian rhythms have also been suggested to be key modulators of plasticity in neural networks (Abel et al., 2013; Frank and Cantera, 2014), resulting in alterations in learning and memory, cognitive performance, as well as emotion regulation (Lo et al., 2012; Krause et al., 2017). In this context, a role for sleep specifically in adult neurogenesis has been investigated in the past decades (Guzman-Marin et al., 2003; Hairston et al., 2005).
This review will summarize and critically evaluate the evidence of the role of sleep as a modulator of adult hippocampal neurogenesis, which primarily emerges from studies of acute and chronic sleep disruption in rodents. We will then discuss the effects of sleep disruption on hippocampal-dependent cognitive functions in rodents and humans. Lastly, we will hypothesize that the negative effects of sleep disturbances on hippocampal-dependent cognitive functions in humans may possibly occur due to a decrease in adult hippocampal neurogenesis. If this hypothesis is ascertained, it could open new avenues for research and treatments of cognitive disorders and psychiatric diseases.
Modulation of Adult Hippocampal Neurogenesis by Sleep in Rodents
Sleep and the Hippocampus
Sleep is divided into two types, i.e., rapid-eye-movement (REM) sleep, also known as paradoxical sleep, and non-REM (NREM) sleep, which encompass deep slow wave sleep. NREM and REM sleep can be measured and identified using electroencephalogram (EEG) recordings (Dijk, 2009). REM and NREM sleep are characterized by specific oscillatory rhythms in the EEG, such as theta rhythms in REM sleep, while slow waves, spindles and sharp wave ripples are observed during NREM sleep. Some of these EEG rhythms are associated with the hippocampus, such as theta oscillations in REM sleep and high frequency sharp wave ripples in NREM sleep which involve 10 to 18% of hippocampal neurons (Watson and Buzsaki, 2015). A close temporal association between hippocampal sharp wave ripples and sleep spindle oscillations arising from the thalamo-cortical networks has been established (Siapas and Wilson, 1998; Sirota et al., 2003). Furthermore, a temporal coupling has also been observed with cortical slow oscillations (Sirota et al., 2003; Molle et al., 2006; Sullivan et al., 2011). These EEG rhythms have been proposed to contribute to memory consolidation (Buzsaki et al., 1987; Buzsaki, 1989; Wilson and McNaughton, 1994; Girardeau et al., 2009; Karlsson and Frank, 2009; Buzsaki and Silva, 2012), transmitting information from the hippocampus to the cortex and other regions (Buzsaki, 1989; Sirota and Buzsaki, 2005; Hahn et al., 2007). The different sub-regions of the hippocampus (i.e., CA1, CA3, DG) contribute to memory consolidation, and are characterized by specific population activity patterns that can be temporally coupled (Daumas et al., 2005; Abel et al., 2013; Aton et al., 2014; Josselyn et al., 2015). In the DG, dentate spikes are triggered by the entorhinal cortex and associated with hilar interneuron activity, while they suppress the CA3-CA1 population activity during SWS (Bragin et al., 1995). A recent study identified that CA1 parvalbumin-expressing interneurons show enhanced firing coherence with CA1 theta activity and mediate hippocampal network oscillations involved in memory consolidation (Ognjanovski et al., 2017).
Chronic Sleep Disruption
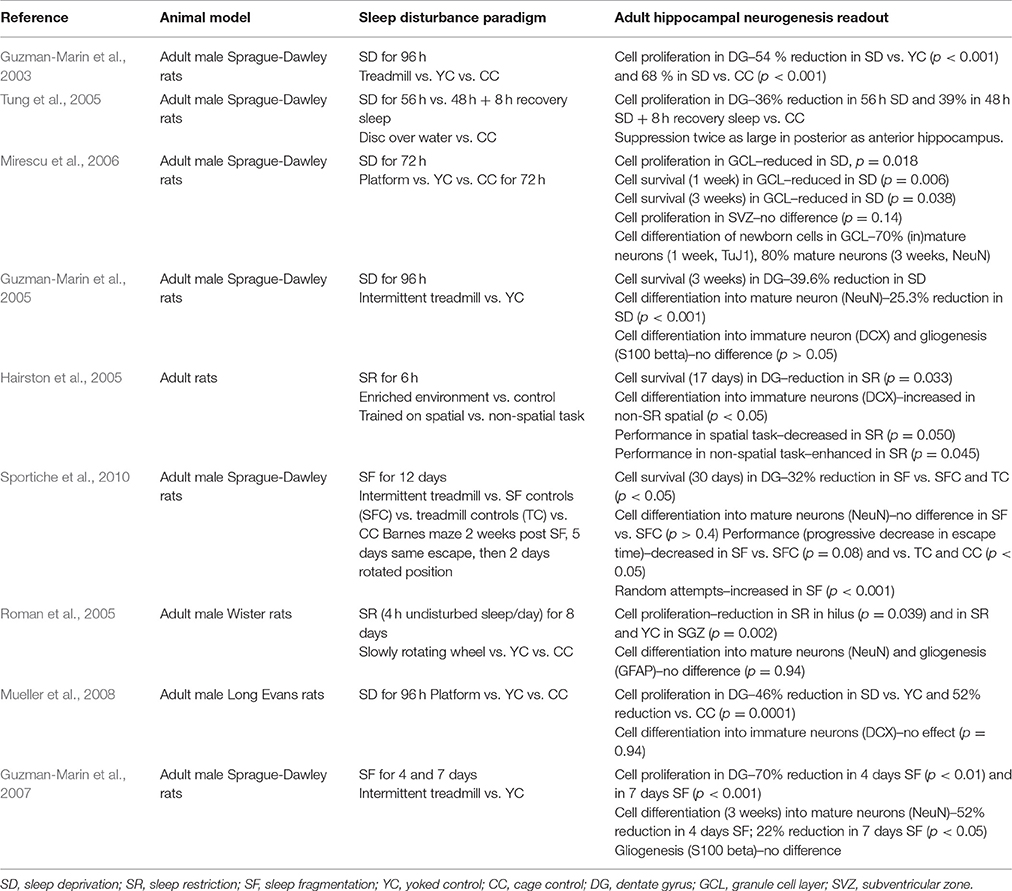
Many studies over the past decades have investigated a potential role of sleep on neurogenesis, primarily by using chronic sleep disruption over a large range of durations in rodents (Guzman-Marin et al., 2003, 2005, 2007; Hairston et al., 2005; Roman et al., 2005; Tung et al., 2005; Mirescu et al., 2006; Sportiche et al., 2010; Mueller et al., 2015), as summarized in Table 1. Sleep disruption have included total sleep deprivation, sleep restriction, sleep fragmentation, as well as relatively selective REM sleep deprivation. Thus, methods to induce deprivation/disruption of sleep are numerous, including treadmill running (Hairston et al., 2005; Roman et al., 2005; Mirescu et al., 2006; Sportiche et al., 2010), slow rotating wheels (Guzman-Marin et al., 2005), and over the water platforms (Guzman-Marin et al., 2003; Tung et al., 2005). These methodologies are all associated with potential confounding effects (e.g., stress, imposed locomotion) but appropriate controls can address some of these confounders (e.g., locomotion). Another aspect that may also contribute to discrepancies between studies is the time of day at which experiments are performed, as many physiological mechanisms display daily circadian rhythms. Studies addressing this caveat have not led to a consensus on whether aspects of neurogenesis exhibit daily rhythms (reviewed in Meerlo et al., 2009; Fernandes et al., 2015; Mueller et al., 2015), requiring thus further experiments. Despite this, some coherent results have been obtained across methodologies, suggesting that sleep disturbances per se might be responsible for the changes in neurogenesis.
As neurogenesis depends on cell proliferation and cell survival, maturation and differentiation, it is important to categorize and scrutinize the readouts of these reports separately to better understand the impact of sleep on the neurogenic process. Cell proliferation and survival are generally quantified using bromodeoxyuridine (BrdU), while the identification of neurons is achieved using in addition a neural marker such as NeuN (Mullen et al., 1992). Hence, cells co-expressing these factors are identified as newly formed neurons. Studies evaluating sleep disruption on adult neurogenesis have primarily focused on cell proliferation, survival and differentiation, while the integration of newborn neurons in existing neural circuits has received less attention.
Total Sleep Deprivation and Effects on Cell Proliferation
Total sleep deprivation over a period of 48 h or more has consistently been reported to decrease the basal rate of cell proliferation in the dentate gyrus from 30 to 80% (Table 1). Guzman-Marin and colleagues (Guzman-Marin et al., 2003) sleep-deprived adult Sprague-Dawley rats for 96 h by subjecting them to forced locomotion in a treadmill. Rats exhibited a 54% reduction in proliferation in the dorsal hippocampus when compared to treadmill controls (i.e., displaying similar amount of locomotor activity) and a 68% reduction when compared to home-cage controls. However, rats were euthanized 48 h after BrdU administration, which allowed time for further mitotic cycles and/or apoptosis. A similar experiment by Tung and colleagues (Tung et al., 2005) used a disc-over-water paradigm to induce REM sleep deprivation for 56 h. BrdU was injected 2 h before tissue collection, thus ensuring only cells in the S phase were labeled. The study showed a 36% decrease in cell proliferation, confirming the effect across sleep disruption methodologies. In addition, this reduction lasted after 8 h of sleep recovery following deprivation, showing that the effects of sleep deprivation on neurogenesis were not normalized within this 8 h sleep recovery period. This specific decrease was mostly observed in the posterior dentate gyrus. Later studies have confirmed the specificity of sleep deprivation to dentate gyrus neurogenesis, while neurogenesis in the SVZ was not altered (Mirescu et al., 2006).
Total Sleep Deprivation and Effects on Cell Survival, Maturation and Differentiation
Other studies investigated the effects of sleep deprivation on cell survival and cell differentiation. Guzman-Marin et al. (2005) sleep-deprived rats for 96 h using an intermittent treadmill and administered BrdU 3 weeks before neurogenesis assessment (Table 1). They observed a 39.6% decrease in BrdU-positive cells in sleep-deprived rats compared to treadmill controls, and significant differences in the percentage of cells co-labeling BrdU and NeuN (46.6 vs. 71.9% respectively) (Table 1).
Other studies investigating cell survival, maturation and differentiation used other sleep disruption approaches, such as sleep fragmentation and sleep restriction are discussed below.
Sleep Fragmentation and Sleep Restriction
While total sleep deprivation is a well-established and frequently used methodology to assess the contribution of sleep to physiological processes, other types of disruptions, such as sleep fragmentation or sleep restriction, are closer to sleep disturbances in humans comorbid with many psychiatric disorders (Baglioni et al., 2016). Hairston et al. (2005) reduced the amount of sleep by 50% for 4 days using an enriched environment while rats were being trained on spatial (hippocampal-dependent) and non-spatial (non-hippocampal dependent) tasks. A decrease in cell proliferation and cell survival was observed in sleep-restricted rats, as well as a decrease in neural differentiation in sleep-restricted rats which were trained on the spatial task. Sleep fragmentation induced by enforced locomotion, using an intermittent treadmill, for 4 or 7 days also induced a 70% reduction of BrdU- and Ki67-labeled cells (Ki67, marker of cell proliferation) (Guzman-Marin et al., 2007). The same study showed that the number of cells expressing a neuronal phenotype 3 weeks after BrdU injection also decreased by 52% and 22% respectively. Sportiche et al. (2010) also reported a 32% decrease in BrdU-labeled cells after 12 days of sleep fragmentation, while Guzman-Marin et al. (2007) examined cell differentiation 3 weeks after a 1-, 4-, and 7-day sleep fragmentation paradigm, and reported a 30, 52, and 22% reduction of cells expressing a neuronal phenotype respectively. Rats subjected to 7-day sleep fragmentation also showed less suppression of REM sleep (presumably due to an increased homeostatic drive for REM sleep) which could account for the reduced suppression of cell differentiation (Table 1).
REM Sleep Deprivation
Studies further investigated whether a specific sleep stage may be primarily associated with neurogenesis, using different methodologies to induce sleep disruptions. Several studies used the platform over water method (Mirescu et al., 2006; Mueller et al., 2008) and showed reduced cell proliferation. Of note, while this paradigm primarily suppresses REM sleep, it also decreases the amount of NREM sleep. Guzman-Marin et al. (2008) used an alternative approach to attempt to selectively disrupt REM sleep during 4 days, with a treadmill being triggered when rats enter REM sleep assessed by online EEG recordings. Cell proliferation was 63% lower in REM sleep-deprived (REMD) rats compared to yoked controls (YC) and 82% lower than observed in home-cage controls. Strikingly, all groups showed a positive correlation between cell proliferation and the percentage of REM sleep. These results suggest that lack of REM sleep plays a key role in reduced neurogenesis induced by sleep deprivation, irrespective of the methodology used and their potential confounding effects (e.g., stress). Although REMD and YC rats did not differ significantly regarding amount of NREM sleep or EEG-derived slow wave activity which quantifies the slow wave oscillations (1–4 Hz) characteristic of deep NREM sleep, it cannot be ruled out that the REMD procedure may induce other changes, including in waking behaviors, essential for the proliferative process. In contrast, a selective effect of REM sleep on cell maturation remains unclear, with some studies showing no significant differences (Table 1), while neuronal maturation was lower in REM sleep-deprived rats using a more selective REM sleep deprivation method (Guzman-Marin et al., 2008).
Potential Mechanisms Contributing to the Effects of Sleep Disruption: Stress, Circadian Rhythmicity or Sleep Disruption Per se?
Even though there is a consensus on the negative effects of chronic sleep disturbances on neurogenesis, the mechanisms by which it occurs remain controversial. As acute sleep deprivation has not consistently been shown to decrease neurogenesis (see section below) and recovery is not immediate (Tung et al., 2005), the effects of sleep disruption may be indirect.
Stress and glucocorticoids
It has been established that stress affects neurogenesis (Mirescu and Gould, 2006) and sleep deprivation methods have been associated with an activation of the hypothalamo-pituitary-adrenal axis, widely assessed by increased levels of stress hormones such as corticosterone and adrenocorticotropic hormone (Tartar et al., 2009; Mongrain et al., 2010). Hence, the observed effects of sleep disruption on neurogenesis may be partly mediated by an increase in glucocorticoid levels and not a lack of sleep per se. Mirescu et al. (2006) reported that the reduction of neurogenesis observed in rats sleep deprived for 72 h was associated with elevated corticosterone levels. Importantly, using adrenalectomy combined with corticosterone supplementation in drinking water (to prevent corticosterone increase) abolished this reduction in cell proliferation. A further study by Guzman-Marin et al. (2007) reported that adrenalectomised rats with corticosterone replacement subjected to sleep fragmentation showed 55% fewer BrdU-positive cells when compared with adrenalectomised controls.
Circadian regulation
A plethora of functions in the hippocampus show circadian rhythmicity. For instance, a recent study demonstrated using fMRI scheduled throughout the 24 h cycle that the response of the hippocampus during a sustained attention task exhibited 24 h rhythmicity (Muto et al., 2016). Neural progenitor cells have a cell cycle of 24.7 h (Cameron and McKay, 2001), suggesting the possibility of circadian control. Hippocampal neurogenesis was suggested to be circadian-dependent under certain conditions (Goergen et al., 2002). Furthermore, a mouse study showed that exercise significantly increased neurogenesis only when administered at specific times during the day (Holmes et al., 2004). This could suggest that neurogenesis might be regulated by hippocampal clock genes. For instance, Per1, Cry, or Clock genes alter cell growth and cell cycle progression in mice (Matsuo et al., 2003; Gery et al., 2006; Miller et al., 2007). Corticosterone (CORT), brain-derived neuropathic factor (BDNF) (Sairanen et al., 2005; Rossi et al., 2006), and melatonin have also been proposed as mediators in the effect of circadian rhythms on hippocampal function (Ramirez-Rodriguez et al., 2011). At the molecular level, a circadian-controlled gene expression has been observed in the hippocampus in both rodents and humans (Jilg et al., 2010). In addition, sleep disruption methods alter the 24 h rest-activity rhythms and related waking behaviors, such as drinking and eating patterns, or nesting behavior. However, the contribution of circadian rhythms to different aspects of neurogenesis has been relatively unexplored. Circadian disruption imposed with a repeated “jet lag” protocol (i.e., twice-weekly phase advanced for 4 weeks) induced a reduction in cell proliferation and neurogenesis in hamsters, associated with impaired hippocampal-dependent learning and independent of glucocorticoids alterations (Gibson et al., 2010). Another study using chronic jet lag in Wistar rats suggests that the effects of circadian disruption are dependent on the type and duration of shifts, with phase advance (i.e., “traveling eastbound”) being associated with a greater decrease in the number of immature neurons compared to phase delay (“traveling westbound”) (Kott et al., 2012). Constant bright light conditions in rats, another approach to induce short- and long-term disruption of circadian rhythms did not affect cell proliferation and survival (Mueller et al., 2011). Moreover, several core circadian clock genes have also been implicated in adult hippocampal neurogenesis. Bmal1 knockout mice, characterized by arrhythmic wheel-running activity and loss of circadian rhythmicity for clock gene expression, showed no significant difference in cell proliferation levels compared to wild-type controls (Rakai et al., 2014). However, the number of pyknotic cells, a proxy of cell death, was reduced and cell survival was enhanced, suggesting a possible role of the Bmal1 clock gene and circadian rhythmicity in several aspects of neurogenesis. Another clock gene, Period2, was proposed to contribute to cell proliferation, generation of immature newborn neurons and cell survival in the dentate gyrus (Borgs et al., 2009). Interestingly, a study showed a diurnal rhythms of neurogenesis in the olfactory bulb in crustaceans, with the highest rate occurring at the most active time, i.e., dusk (Goergen et al., 2002). This raises the possibility that hormones and exercise influence neurogenesis within a circadian control pathway. Further investigation is needed to further disentangle the contribution of sleep per se from circadian rhythmicity in neurogenesis.
Other underlying mechanisms have been proposed to contribute to the effects of sleep disruption on neurogenesis, among which alterations in neurotransmitter systems and growth factors (Meerlo et al., 2009; Garcia-Garcia et al., 2011; Mueller et al., 2015).
Acute Sleep Disruption
The effect of acute sleep deprivation on cell proliferation and neurogenesis remains elusive. An increase in cell proliferation following 12 h gentle handling in rats resulting in 80% wakefulness has been reported in the dentate gyrus, while no effects were observed in the SVZ (Grassi Zucconi et al., 2006). By contrast, Guzman-Marin et al. (2007) reported no difference in cell proliferation following 1-day of sleep fragmentation induced by automated intermittent treadmill activation, while the percentage of new cells expressing a neuronal phenotype 3 weeks after BrdU injection was reduced. One-day sleep restriction allowing 4 h of undisturbed sleep decreased cell proliferation in the hilus (Roman et al., 2005). Several studies reported no significant difference after 1 day of sleep deprivation using various paradigm, e.g., small platform (Mirescu et al., 2006), intermittent treadmill (Guzman-Marin et al., 2008) or deprivation by gentle procedures (van der Borght et al., 2006).
While several studies showing differences regarding species, age, strains, methods and/or durations led to consistent results in chronic sleep disturbances (highlighting a role of sleep disturbance per se), the effects of acute sleep disruption on neurogenesis remain unclear. This may be primarily due to differences in methodologies (e.g., duration of acute sleep disruption, strains, age), but sleep disturbances per se may directly affect neurogenesis levels. Another confounding effect could arise from different assessments of neurogenesis and its dynamics. For instance, Junek et al. (2010) combined BrdU (incorporated in the S-phase of the cell cycle only) with two intrinsic cell proliferation markers [proliferating cell neuronal antigen (PCNA) and Ki67 (identifying cells in all phases of the cell cycle)] and showed that although the number of BrdU-labeled cells increased after an acute 12 h sleep deprivation, there was no difference in PCNA- and Ki67-labeled cells compared to controls. This suggested that the apparent rise in cell proliferation could be due to a cell cycle acceleration and not an actual rise in the number of proliferating cells.
It is worth to note that preclinical studies investigating the contribution of sleep to adult hippocampal neurogenesis have been primarily using males. Hence, it would be important to evaluate putative sex differences, especially in the context of neurogenesis-dependent behaviors and the close association of neurogenesis with mood and psychiatric disorders.
Effect of Sleep on Adult Hippocampal Neurogenesis-Dependent Behaviors
In the previous sections, it has been established that chronic sleep disruption reduces basal levels of neurogenesis in the adult hippocampus. The hippocampus is part of the limbic system and has reciprocal connections with the amygdala and prefrontal cortex, and is thus important in regulating cognitive functions and mood (Pitkanen et al., 2000). It is interesting to consider not only the potential impact of sleep on basal rates of neurogenesis but also on the mediation of changes in neurogenesis associated with hippocampal plasticity, cognition and mood.
The function of adult newborn DG neurons has been involved in many aspects of learning and memory consolidation, such as contextual fear conditioning and long-term spatial memory retention, but discrepancies remain. Importantly, these discrepancies might arise from different environmental regulation and thereby different experiences during the maturation of newborn neurons which play a key role in their integration in hippocampal neural structure (for review Gonçalves et al., 2016).
Hippocampal-Dependent Cognitive Processes and Synaptic Plasticity
Sleep has long been thought to play a key role in learning and memory, partly due to the re-activation of neural networks involved in information acquisition, which facilitates consolidation and integration (Walker, 2009; Diekelmann and Born, 2010). A large body of literature describes in humans and animals the importance of sleep before learning and for the consolidation of hippocampal-dependent memory (reviewed in Abel et al., 2013; Vorster and Born, 2015; Krause et al., 2017).
The time of learning and the timing of sleep after learning was shown to be important for performance in hippocampal-dependent episodic-like memory and contextual fear memory in rodents and humans (Palchykova et al., 2006, 2009; Vorster and Born, 2015). Sleep deprivation prior learning also affects the encoding of new hippocampal-dependent memories in rodents and humans (Yoo et al., 2007; Hagewoud et al., 2010). Such effects have been reported using other hippocampal-dependent tasks, such as fear conditioning and episodic memory, but not for non-hippocampal-dependent memories (McDermott et al., 2003; Yoo et al., 2007; Tiba et al., 2008; Van Der Werf et al., 2009). Another study further demonstrated that sleep continuity, with a minimal unit of uninterrupted sleep, was crucial for memory consolidation (Rolls et al., 2011). The authors suggested that this effect was associated with the replay-related events taking place during sleep. A selective role of NREM sleep and REM sleep has also been extensively investigated (Diekelmann and Born, 2010). A recent study demonstrated a causal role of REM sleep theta oscillations by showing that specific inhibition of hippocampal theta oscillations during REM sleep disrupts memory consolidation (Boyce et al., 2016).
The role of sleep for molecular and cellular aspects of hippocampal synaptic plasticity and consolidation has also been extensively studied (for review, e.g., Abel et al., 2013; Kreutzmann et al., 2015; Havekes and Abel, 2017). For instance, recent studies identified some of the molecular and structural mechanisms underlying the detrimental effects of acute short sleep deprivation on memory. These include the attenuation of mTORC1-dependent protein synthesis, reduction of dendritic spine density mediated by the increase activity of the actin-binding protein cofilin, known to play a crucial function in synaptic structure, and the spatial cAMP degradation (Havekes et al., 2016; Tudor et al., 2016).
Overall, the detrimental effects of sleep disruption on neurogenesis, described above, are likely to contribute to the vulnerability of hippocampal networks associated with cognitive impairments.
Mood, Emotion Regulation, and Depressive Disorder
The hippocampus is a limbic structure and contributes to emotional processing and mood disorders, while alterations in adult neurogenesis have been reported in major depression (Miller and Hen, 2015). A growing body of evidence suggests that sleep modulates the regulation of emotions, and sleep disruption has a pervasive effect and is particularly associated with negative emotional experience (Walker, 2009; Harvey, 2011; Palmer and Alfano, 2017).
It is well accepted that sleep disturbances are comorbid with many neurological and psychiatric disorders. This association has been studied for mood disorders, and primarily major depression. Most individuals suffering from depression (i.e., up to 90%) complain about sleep disturbances (Nutt et al., 2008) with a large number experiencing insomnia. Moreover, sleep disturbances are a risk factor to depression, relapse and suicides (Hamilton, 1989; Perlis et al., 1997), and a meta-analysis found that sleep disturbances represent an important risk factor for depression in the elderly (Cole and Dendukuri, 2003). Recent meta-analyses confirmed the occurrence of sleep disturbances by polysomnography recordings in depressed patients, pointing to REM sleep alterations and disturbed sleep continuity (Pillai et al., 2011; Baglioni et al., 2016). Interestingly, REM sleep has been shown to be suppressed by most antidepressant approaches, including antidepressants, electroconvulsive therapy or sleep deprivation (Riemann et al., 2001).
These results suggest that sleep disturbances may mediate the development of depression, and hypnotics (i.e., sleep-promoting drugs) may have beneficial effects. A double-blind placebo-controlled trial reported that co-administration of an antidepressant (fluoxetine; selective serotonin reuptake inhibitor, SSRI) and a hypnotic (eszopiclone; non-benzodiazepine compound acting at GABAA receptors) induced greater benefit in both sleep (i.e., reduced sleep latency and wake after sleep onset and increased total sleep time) and depression assessed by the improved Physician-assessed Clinical Global Impression and HAM-D17 scores, than fluoxetine alone. These beneficial effects were maintained 2 weeks after eszopiclone discontinuation (Krystal et al., 2007), while improvement was no longer observed after 3 months of co-administration of fluoxetine and clonazepam, a benzodiazepine drug (Smith et al., 2002). Zolpidem, the most commonly used hypnotic, also improved sleep in patients with major depressive disorder and persistent insomnia while under antidepressant treatment. Subjective sleep, daytime functioning and well-being were improved by zolpidem (Asnis et al., 1999). In addition, non-pharmacological treatments of insomnia, such as cognitive behavioral therapy, improved the depression and insomnia outcomes (i.e., remission), when provided to patients suffering of insomnia comorbid with depression, with escitalopram as antidepressant treatment (Manber et al., 2008).
These studies suggest that sleep disturbances are a core symptoms of major depression and may have a role in the development of depression. Addressing sleep disruption in mood disorders would improve the treatment of depression.
Bridging the Gap: Effects of Sleep on Cognition and Mood Through Adult Hippocampal Neurogenesis
As discussed in previous sections, both neurogenesis and hippocampal-dependent functions are sensitive to sleep. Thus, we hypothesize that the detrimental effects of sleep on cognition and mood might be mediated by a decrease in neurogenesis (Figure 1).
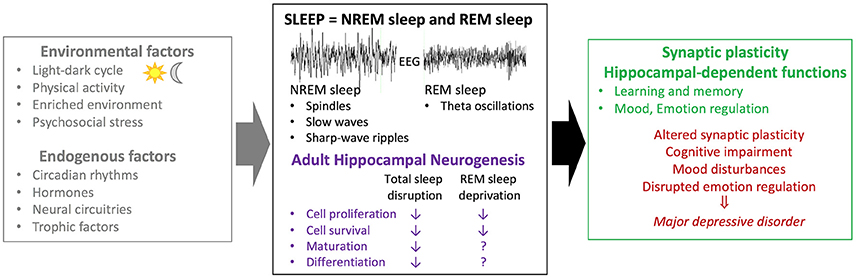
Figure 1. Association between sleep and adult hippocampal neurogenesis in physiological conditions and mood disorders. Several factors including environmental factors (e.g., exercise, physical/psychosocial stress) and endogenous factors (e.g., trophic factors) have been suggested to modulate adult hippocampal neurogenesis. While studies investigating the effects of circadian rhythms are sparse, several lines of evidence suggest that sleep acts as a modulator of adult hippocampal neurogenesis. Sleep disruption (i.e., sleep deprivation; fragmentation; selective REM sleep deprivation) leads to decreased basal rates of cell proliferation and survival in rodents. A selective role for REM sleep on cell maturation and differentiation remain to be clarified. Moreover, sleep disruption has a negative impact on synaptic plasticity and hippocampal-dependant functions. Both sleep disturbances and alterations in adult hippocampal neurogenesis have been associated with major depressive disorders. Based on these different lines of evidence, it can be postulated that sleep effects on hippocampal-dependent functions could be mediated, in part, by altered hippocampal neurogenesis. EEG, electroencephalogram; NREM sleep, non rapid-eye-movement sleep; REM sleep, rapid-eye-movement sleep.
Cognition–Learning and Memory
Several studies have reported that learning a hippocampal-dependent task leads to an increase in neurogenesis, while learning deficits are often associated with a decrease in neurogenesis (Gould et al., 1999; Shors et al., 2001; Dobrossy et al., 2003). Blocking neurogenesis also results in decreasing performance in some hippocampal-dependent tasks, such as formation of trace memories (Shors et al., 2002), trace fear conditioning (Madsen et al., 2003), place recognition (Raber et al., 2004), spatial learning in the Morris water maze (Dupret et al., 2007), and pattern separation (Clelland et al., 2009). However, Epp and colleagues recently showed that increasing hippocampal neurogenesis weakens existing memories and facilitates the encoding of new, conflicting (but not non-conflicting) information in mice. Conversely, decreasing neurogenesis stabilizes existing memories, and impedes the encoding of new, conflicting information (Epp et al., 2016). It is also worth noting that learning affects newborn neurons differently depending on their relative ages. For instance, survival is enhanced in slightly mature neurons whereas apoptosis is induced in immature cells (Lemaire et al., 2000). These two processes are inter-linked, and blocking one pathway inhibits the other one, while also impairing memory. Subsequently, memory consolidation requires both addition and removal of neurons depending on their age and functional relevance.
Conditions enhancing or reducing neurogenesis are often associated, respectively, with enhanced (Kempermann et al., 1997; van Praag et al., 1999; Leuner et al., 2004; Mirescu and Gould, 2006) or disrupted (Kuhn et al., 1996; Diamond et al., 1999; Eisch et al., 2000; Gross, 2000; Lemaire et al., 2000; Drapeau et al., 2003; Scerri et al., 2006) hippocampal-dependent learning and memory. Since both hippocampal-dependent learning/memory and adult hippocampal neurogenesis are sensitive to sleep disruptions, the negative effects of sleep loss or sleep fragmentation on hippocampal-dependent learning and memory could be partly due to reduced neurogenesis, contributing to the vulnerability of hippocampal circuitries.
One study (Sportiche et al., 2010) investigated the impact of 4-days sleep deprivation on neurogenesis and spatial vs. non-spatial learning using a water maze paradigm in rats. Subjects that had learned the hippocampal-dependent task showed enhanced cell survival, which was abolished following sleep deprivation. Unexpectedly, sleep-deprived rats showed impaired performance in the spatial task, but enhanced performance in the non-spatial task. In another study (Backhaus et al., 2006), rats subjected to sleep fragmentation for 12 days showed a 32% reduction of BrdU labeled cells compared to yoked and home-cage controls. These subjects were also trained in a Barnes maze paradigm, and the progressive decline in escape time observed in control animals was slower in sleep-fragmented rats which also showed more random, non-spatial search strategies. These results reinforce the hypothesis that the detrimental effects of sleep disruption on cognitive performance might be mediated through a decrease in neurogenesis which has also been proposed to be implicated in cognitive flexibility (Gonçalves et al., 2016; Anacker and Hen, 2017).
Theories of memory formation acknowledge the importance of remodeling and alteration of synaptic strength in neuronal networks, but also the role of sleep in architecting these plasticity processes (Schmidt-Hieber et al., 2004; Stickgold, 2005; Stickgold and Walker, 2005). Therefore, the integration, the maturation and the synaptic strengthening of new neurons to an existing neuronal circuitry could be a complementary form of plasticity. Newborn neurons display robust long-term potentiation and show a lower threshold for their induction (Ramirez-Amaya et al., 2006; Curtis et al., 2007) making them strong candidates to be recruited by behavioral activation (Videbech and Ravnkilde, 2004; Kee et al., 2007).
An increasing interest in the role of glia in neurogenesis has recently emerged. For instance, microglia has been shown to regulate the proliferation and differentiation of neural progenitor cells as well as to control the resulting number of newborn neurons by phagocytosis (Sato, 2015). Astrocytes and gliotransmission have also been implicated in the regulation of sleep homeostasis and working memory deficits induced by sleep deprivation via the adenosine A1 receptor (Halassa et al., 2009; Frank, 2013).
Mood–Depression
There is strong evidence regarding the involvement of the hippocampus in depression. fMRI studies have reported reduced hippocampal volume in depressed patients (MacQueen et al., 2003; Campbell et al., 2004), and the magnitude of this volume reduction was correlated with the frequency, as well as the severity of the pathology (Sheline, 2000). Structural changes such as gray matter alterations (Frodl et al., 2002) might be reversed during remission (Bremner and Vermetten, 2004), and then subsequently associated with fluctuating levels of adult neurogenesis. Many depressed patients show hippocampal-dependent cognitive deficits (Brooke et al., 1994) which suggest hippocampal dysfunction (Brooke et al., 1994; Beason-Held et al., 1999). Antidepressants have been reported to act at the level of the hippocampus, such as SSRIs and the multimodal antidepressant vortioxetine (Dale et al., 2016), and to restrain the hippocampal volume reduction (Sheline et al., 2003) while improving cognition (Bremner and Vermetten, 2004).
Post-mortem studies investigating the difference in neurogenesis in depressed versus healthy subjects are inconsistent. For instance, a study reported an increase in neurogenesis in patients treated with antidepressants, whereas no difference between healthy and depressed subjects was observed (Boldrini et al., 2009). By contrast, other studies showed either fewer progenitor cells in depressed patients and no difference following antidepressant treatment (Lucassen et al., 2010) or no change at all compared to healthy patients (Reif et al., 2006). These discrepancies may be due to variations in medication dose and duration, age, length of the disorder, and/or to focusing on the proliferation stage of neurogenesis only.
However, it does not seem that changes in neurogenesis are involved in the etiology of depression. Indeed, suppression of neurogenesis alone in rats did not lead to depressive or anxious phenotypes (Santarelli et al., 2003; Wang et al., 2008; Jayatissa et al., 2009; Fuss et al., 2010) while mice did show neither enhanced depressive-like behaviors (Eisch and Petrik, 2012). However, mice showing lower levels of neurogenesis are more susceptible to stress and display depressive-like phenotypes (Egeland et al., 2017). That could support the hypothesis that a decrease in neurogenesis would need to be associated with genetic predisposition or an environmental trigger such as stress in adulthood to lead to depression. For instance, adult neurogenesis has been proposed to play a role in stress resilience (reviewed in Levone et al., 2015; Besnard and Sahay, 2016).
On the other hand, neurogenesis seems to be related to the behavioral symptoms of depression and their treatment. An increase in neurogenesis was observed following several antidepressant administrations in rodents, including SSRIs, monoamine-oxidase inhibitors, serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, all of them serotonin enhancers (Malberg et al., 2000; Czeh et al., 2001; Li et al., 2004; Xu et al., 2006), as well as in non-human primates (i.e., fluoxetine) (Perera et al., 2007). Furthermore, the mechanisms of action of these compounds have been shown to be specific to the SGZ (Malberg et al., 2000; Santarelli et al., 2003; Perera et al., 2007). Studies also reported that blocking neurogenesis in mice abolished the positive effect of antidepressant treatment (Santarelli et al., 2003; Surget et al., 2008). A specific role of neurogenesis in mediating antidepressant treatment action would also explain the delayed onset of therapeutic action. For example, it has been reported that neurogenesis increases following 14- or 28-days of fluoxetine administration, which coincides with the time required for therapeutic action to start, but such effects were not observed following 1- or 5 days of treatment (Malberg et al., 2000). Similar effects regarding neurogenesis modulation have been observed by using psychotropic medications reporting antidepressant effects (Chen et al., 2000; Malberg et al., 2000; Santarelli et al., 2003; Kodama et al., 2004; Nixon and Crews, 2004), as well as non-pharmacological treatments such as electroconvulsive therapy (Scott et al., 2000; Perera et al., 2007) or exercise (van Praag et al., 2005). In addition, predisposing factors to depression, such as chronic stress (Czeh et al., 2001; Coe et al., 2003; Pham et al., 2003), alcohol abuse (Nixon and Crews, 2004), opioid use (Eisch et al., 2000; Krystal, 2012; Scherrer et al., 2016), and hypothyroidism (Madeira et al., 1991) also alter neurogenesis. However, it is worth noting that antidepressants might show both neurogenesis- dependent and -independent effects (Sahay and Hen, 2007; David et al., 2009). For example, in some but not all behavioral paradigms, an inhibition of neurogenesis leads to the effects of fluoxetine being blocked (David et al., 2009), suggesting that other brain regions such as the cingulate cortex could also mediate antidepressant action (Hajek et al., 2008).
As both neurogenesis and depression are linked to sleep, it would be reasonable to assume that sleep could partly affect depression through neurogenesis, and subsequently that the antidepressant-like effects of hypnotics could be linked to increased neurogenesis. Twice daily administration of eszoplicone during 2 weeks increased cell survival by 46% in the SGZ/granule cell layer in rats while having no effect on cell proliferation. Most of these cells showed BrdU-NeuN double-labeling confirming their neuronal identity (Methippara et al., 2010). Yet, another study (Su et al., 2009) reported that chronic co-treatment with eszoplicone and fluoxetine increased cell survival by 50% but again had no effect on cell proliferation; the observed effects were significantly higher when drugs were co-administered compared to single drug treatment. However, acute and chronic administration of zolpidem did not show similar results (Takase et al., 2009). Nevertheless, that could be explained by the fact that newborn neurons do not express GABAA receptor alpha 1 subunit to which binds zolpidem.
How newly born neurons contribute to mood or to symptoms of depression is still not fully understood. The cognitive impairments observed in depression which can be modulated by neurogenesis are also observed in other psychiatric disorders. Decreased hippocampal volumes are reported in patients with schizophrenia, addiction, dementia and anxiety (Kempermann et al., 2008; Thompson et al., 2008; Revest et al., 2009), suggesting that neurogenesis might be an important mediating factor in the pathogenesis. A relevant neuronal mechanism could thus be a decrease in neurogenesis altering the mean age and overall characteristics of neurons in the dentate gyrus, subsequently influencing hippocampal networks' properties and function (Lucassen et al., 2010). Although new adult-born granule cells are in minority amongst cells generated during development, they have been reported to induce an overall increase in dentate gyrus activity and to have a disproportionate influence on hippocampal circuitry and behavior. The way these scarce newborn neurons have an impact on global brain function could be due to their capability to serve both as encoding units and modulators of the patterns and timings of more mature neurons (Ming and Song, 2011).
Discussion
There is accumulating evidence supporting a role for sleep as a mediator of neurogenesis and its effects on cognition and mood. However, there are certain limitations which must be acknowledged. Regarding cognition, different hippocampal-dependent memories are sensitive to different sleep stages, not necessarily REM sleep primarily associated with a decrease in neurogenesis. In addition, many animal and human studies investigating the impact of sleep on cognition only disturb sleep acutely whereas chronic sleep disruptions have been consistently associated with decreased neurogenesis. This could come from the fact that learning-associated neurogenesis is more sensitive than basal neurogenesis. With respect to mood, contradictory results have been obtained when using different hypnotics or looking at post-mortem studies while the variability in subjects as well as in methodologies makes comparisons difficult. It is worth noting there is also some evidences of an improvement of depressive symptoms after acute sleep deprivation (Giedke and Schwarzler, 2002) but this does not rule out a possible opposing effect of chronic sleep disruption on both depression and neurogenesis. Furthermore, patients suffering from mood disorders could be “reset” following a one night sleep deprivation procedure, questioning again the complex relationship of sleep disturbances with neurogenesis (Krystal, 2012).
The “neurogenic interactome” (Eisch and Petrik, 2012) aims to explain some of these discrepancies by considering diverse neurogenesis altering factors, reciprocal connections of the hippocampus and other areas and behavioral consequences. This makes the link between the effect of changes in neurogenesis on cognition with those observed on mood. Decreased neurogenesis may be detrimental for pattern separation (i.e., the differentiation of similar memories with overlapping hippocampal inputs into different representations; McClelland et al., 1995) and thus lead not only to impaired learning and memory, but also to the inability to adequately discern danger or stress signals. Mueller and colleagues (Mueller et al., 2015) also alluded to pattern separation, suggesting de-correlation of entorhinal cortex inputs leading to formation of separate memory representations is involved in antidepressant action. Kempermann and colleagues (Kempermann, 2008) proposed adult hippocampal neurogenesis enables adaptation of this network to novelty and complexity encountered throughout life which would improve its functioning by separating units of information. The emotional role of the hippocampus would be a consequence of memories being linked to emotional information.
The relationship between neurogenesis and sleep is still a relatively recent research field and it will greatly benefit from further research. Studies comparing mice and rats, as well as different strains and sex, could clarify discrepancies. A key experiment would involve subjecting rodents to sleep disruption while selectively pharmacologically preserving hippocampal neurogenesis to observe whether the cognitive and behavioral deficits are still observed. It would also be of interest to investigate the following: whether the reduction in neurogenesis induced by sleep disruption is normalized by antidepressants, the differential effects of neurogenesis in the ventral and dorsal dentate gyrus, the underlying biochemical and molecular mechanisms of sleep disturbances on neurogenesis, and the effects of disrupted neurogenesis in structures downstream of the hippocampus. Technological advances (e.g., pharmacogenetic ablation of neurogenesis) could potentially allow for the reversible block of neurogenesis in rodents to investigate its relationship with sleep in specific stages of learning and memory or specific timings in mood disorders. It would also be useful to establish standardized labeling paradigms as the effects on younger and older newborn neurons may be different.
This may lead to a better understanding of the role of sleep and neurogenesis in the adult hippocampus and the potential clinical benefits in preventing and treating cognitive disorders and psychiatric diseases.
Author Contributors
CN carried out the literature review research and wrote the article. OB reviewed and revised the literature and the article. RW revised the literature review and contributed to drafting the manuscript. ST conceived, supervised and revised the literature review.
Funding
OB was supported by BBSRC funding (Grant BB/L020068/1). ST lab is supported by the Medical Research Council UK (MR/N030087/1). RW lab is supported by the British Biotechnology and Biological Sciences Research Council (BB/I008926/1) and the Leverhulme Trust (RPG-2014-267).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abel, T., Havekes, R., Saletin, J. M., and Walker, M. P. (2013). Sleep, plasticity and memory from molecules to whole-brain networks. Curr. Biol. 23, R774–R788. doi: 10.1016/j.cub.2013.07.025
Aimone, J. B., Li, Y., Lee, S. W., Clemenson, G. D., Deng, W., and Gage, F. H. (2014). Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev. 94, 991–1026. doi: 10.1152/physrev.00004.2014
Altman, J. (1969). Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 137, 433–457.
Altman, J., and Das, G. D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335. doi: 10.1002/cne.901240303
Anacker, C., and Hen, R. (2017). Adult hippocampal neurogenesis and cognitive flexibility [mdash] linking memory and mood. Nat. Rev. Neurosci. 18, 335–346. doi: 10.1038/nrn.2017.45
Anacker, C., Zunszain, P. A., Cattaneo, A., Carvalho, L. A., Garabedian, M. J., Thuret, S., et al. (2011). Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatry 16, 738–750. doi: 10.1038/mp.2011.26
Asnis, G. M., Chakraburtty, A., DuBoff, E. A., Krystal, A., Londborg, P. D., Rosenberg, R., et al. (1999). Zolpidem for persistent insomnia in SSRI-treated depressed patients. J. Clin. Psychiatry 60, 668–676. doi: 10.4088/JCP.v60n1005
Aton, S. J., Suresh, A., Broussard, C., and Frank, M. G. (2014). Sleep promotes cortical response potentiation following visual experience. Sleep 37, 1163–1170. doi: 10.5665/sleep.3830
Backhaus, J., Junghanns, K., Born, J., Hohaus, K., Faasch, F., and Hohagen, F. (2006). Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol. Psychiatry 60, 1324–1330. doi: 10.1016/j.biopsych.2006.03.051
Baglioni, C., Nanovska, S., Regen, W., Spiegelhalder, K., Feige, B., Nissen, C., et al. (2016). Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol. Bull. 142, 969–990. doi: 10.1037/bul0000053
Beason-Held, L. L., Rosene, D. L., Killiany, R. J., and Moss, M. B. (1999). Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus 9, 562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X
Besnard, A., and Sahay, A. (2016). Adult hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology 41, 24–44. doi: 10.1038/npp.2015.167
Boldrini, M., Underwood, M. D., Hen, R., Rosoklija, G. B., Dwork, A. J., John Mann, J., et al. (2009). Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34, 2376–2389. doi: 10.1038/npp.2009.75
Borgs, L., Beukelaers, P., Vandenbosch, R., Nguyen, L., Moonen, G., Maquet, P., et al. (2009). Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci. 10:30. doi: 10.1186/1471-2202-10-30
Boyce, R., Glasgow, S. D., Williams, S., and Adamantidis, A. (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816. doi: 10.1126/science.aad5252
Bragin, A., Jando, G., Nadasdy, Z., van Landeghem, M., and Buzsaki, G. (1995). Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J. Neurophysiol. 73, 1691–1705.
Bremner, J. D., and Vermetten, E. (2004). Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 1032, 154–157. doi: 10.1196/annals.1314.012
Brooke, S. M., de Haas-Johnson, A. M., Kaplan, J. R., Manuck, S. B., and Sapolsky, R. M. (1994). Dexamethasone resistance among nonhuman primates associated with a selective decrease of glucocorticoid receptors in the hippocampus and a history of social instability. Neuroendocrinology 60, 134–140. doi: 10.1159/000126743
Brown, J., Cooper-Kuhn, C. M., Kempermann, G., Van Praag, H., Winkler, J., Gage, F. H., et al. (2003). Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 17, 2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x
Buzsaki, G. (1989). Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31, 551–570. doi: 10.1016/0306-4522(89)90423-5
Buzsaki, G., Haas, H. L., and Anderson, E. G. (1987). Long-term potentiation induced by physiologically relevant stimulus patterns. Brain Res. 435, 331–333. doi: 10.1016/0006-8993(87)91618-0
Buzsaki, G., and Silva, F. L. (2012). High frequency oscillations in the intact brain. Progr. Neurobiol. 98, 241–249. doi: 10.1016/j.pneurobio.2012.02.004
Cameron, H. A., and McKay, R. D. (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 435, 406–417. doi: 10.1002/cne.1040
Campbell, S., Marriott, M., Nahmias, C., and MacQueen, G. M. (2004). Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am. J. Psychiatry 161, 598–607. doi: 10.1176/appi.ajp.161.4.598
Chen, G., Rajkowska, G., Du, F., Seraji-Bozorgzad, N., and Manji, H. K. (2000). Enhancement of hippocampal neurogenesis by lithium. J. Neurochem. 75, 1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x
Clelland, C. D., Choi, M., Romberg, C., Clemenson, G. D. Jr., Fragniere, A., Tyers, P., et al. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213. doi: 10.1126/science.1173215
Coe, C. L., Kramer, M., Czeh, B., Gould, E., Reeves, A. J., Kirschbaum, C., et al. (2003). Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol. Psychiatry 54, 1025–1034. doi: 10.1016/S0006-3223(03)00698-X
Cole, M. G., and Dendukuri, N. (2003). Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am. J. Psychiatry 160, 1147–1156. doi: 10.1176/appi.ajp.160.6.1147
Curtis, M. A., Kam, M., Nannmark, U., Anderson, M. F., Axell, M. Z., Wikkelso, C., et al. (2007). Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 315, 1243–1249. doi: 10.1126/science.1136281
Czeh, B., Michaelis, T., Watanabe, T., Frahm, J., de Biurrun, G., van Kampen, M., et al. (2001). Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. U.S.A. 98, 12796–12801. doi: 10.1073/pnas.211427898
Dale, E., Pehrson, A. L., Jeyarajah, T., Li, Y., Leiser, S. C., Smagin, G., et al. (2016). Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS Spectr. 21, 143–161. doi: 10.1017/S1092852915000425
Daumas, S., Halley, H., Frances, B., and Lassalle, J. M. (2005). Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn. Mem. 12, 375–382. doi: 10.1101/lm.81905
David, D. J., Samuels, B. A., Rainer, Q., Wang, J. W., Marsteller, D., Mendez, I., et al. (2009). Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62, 479–493. doi: 10.1016/j.neuron.2009.04.017
Deng, W., Aimone, J. B., and Gage, F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350. doi: 10.1038/nrn2822
Diamond, D. M., Park, C. R., Heman, K. L., and Rose, G. M. (1999). Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 9, 542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N
Diekelmann, S., and Born, J. (2010). The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126. doi: 10.1038/nrn2762
Dijk, D. J. (2009). Regulation and functional correlates of slow wave sleep. J. Clin. Sleep Med. 5, S6–15.
Dobrossy, M. D., Drapeau, E., Aurousseau, C., Le Moal, M., Piazza, P. V., and Abrous, D. N. (2003). Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry 8, 974–982. doi: 10.1038/sj.mp.4001419
Drapeau, E., Mayo, W., Aurousseau, C., Le Moal, M., Piazza, P. V., and Abrous, D. N. (2003). Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 100, 14385–14390. doi: 10.1073/pnas.2334169100
Dupret, D., Fabre, A., Dobrossy, M. D., Panatier, A., Rodriguez, J. J., Lamarque, S., et al. (2007). Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 5:e214. doi: 10.1371/journal.pbio.0050214
Egeland, M., Guinaudie, C., Du Preez, A., Musaelyan, K., Zunszain, P. A., Fernandes, C., et al. (2017). Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Transl. Psychiatry 7:e1101. doi: 10.1038/tp.2017.68
Eisch, A. J., Barrot, M., Schad, C. A., Self, D. W., and Nestler, E. J. (2000). Opiates inhibit neurogenesis in the adult rat hippocampus. Proc. Natl. Acad. Sci. U.S.A. 97, 7579–7584. doi: 10.1073/pnas.120552597
Eisch, A. J., and Petrik, D. (2012). Depression and hippocampal neurogenesis: a road to remission? Science 338, 72–75. doi: 10.1126/science.1222941
Epp, J. R., Silva Mera, R., Kohler, S., Josselyn, S. A., and Frankland, P. W. (2016). Neurogenesis-mediated forgetting minimizes proactive interference. Nat. Commun. 7:10838. doi: 10.1038/ncomms10838
Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A., et al. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317. doi: 10.1038/3305
Ernst, A., Alkass, K., Bernard, S., Salehpour, M., Perl, S., Tisdale, J., et al. (2014). Neurogenesis in the striatum of the adult human brain. Cell 156, 1072–1083. doi: 10.1016/j.cell.2014.01.044
Fernandes, C., Rocha, N. B., Rocha, S., Herrera-Solis, A., Salas-Pacheco, J., Garcia-Garcia, F., et al. (2015). Detrimental role of prolonged sleep deprivation on adult neurogenesis. Front. Cell. Neurosci. 9:140. doi: 10.3389/fncel.2015.00140
Frank, M. G. (2013). “Astroglial regulation of sleep,” in The Genetic Basis of Sleep and Sleep Disorders, eds M. T. Paul Shaw and J. Michael Thorpy (Cambridge, UK: Cambridge University Press), 84–91.
Frank, M. G., and Cantera, R. (2014). Sleep, clocks, and synaptic plasticity. Trends Neurosci. 37, 491–501. doi: 10.1016/j.tins.2014.06.005
Frodl, T., Meisenzahl, E. M., Zetzsche, T., Born, C., Groll, C., Jager, M., et al. (2002). Hippocampal changes in patients with a first episode of major depression. Am. J. Psychiatry 159, 1112–1118. doi: 10.1176/appi.ajp.159.7.1112
Fuss, J., Ben Abdallah, N. M., Hensley, F. W., Weber, K. J., Hellweg, R., and Gass, P. (2010). Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS ONE 5:e12769. doi: 10.1371/journal.pone.0012769
Garcia-Garcia, F., De la Herran-Arita, A. K., Juarez-Aguilar, E., Regalado-Santiago, C., Millan-Aldaco, D., Blanco-Centurion, C., et al. (2011). Growth hormone improves hippocampal adult cell survival and counteracts the inhibitory effect of prolonged sleep deprivation on cell proliferation. Brain Res. Bull. 84, 252–257. doi: 10.1016/j.brainresbull.2011.01.003
Gery, S., Komatsu, N., Baldjyan, L., Yu, A., Koo, D., and Koeffler, H. P. (2006). The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 22, 375–382. doi: 10.1016/j.molcel.2006.03.038
Gibson, E. M., Wang, C., Tjho, S., Khattar, N., and Kriegsfeld, L. J. (2010). Experimental ‘jet lag’ inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS ONE 5:e15267. doi: 10.1371/journal.pone.0015267
Giedke, H., and Schwarzler, F. (2002). Therapeutic use of sleep deprivation in depression. Sleep Med. Rev. 6, 361–377. doi: 10.1053/smrv.2002.0235
Girardeau, G., Benchenane, K., Wiener, S. I., Buzsaki, G., and Zugaro, M. B. (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223. doi: 10.1038/nn.2384
Goergen, E. M., Bagay, L. A., Rehm, K., Benton, J. L., and Beltz, B. S. (2002). Circadian control of neurogenesis. J. Neurobiol. 53, 90–95. doi: 10.1002/neu.10095
Goldman, S. A., and Nottebohm, F. (1983). Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc. Natl. Acad. Sci. U.S.A. 80, 2390–2394. doi: 10.1073/pnas.80.8.2390
Gonçalves, J. T., Schafer, S. T., and Gage, F. H. (2016). Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167, 897–914. doi: 10.1016/j.cell.2016.10.021
Gould, E., Tanapat, P., Hastings, N. B., and Shors, T. J. (1999). Neurogenesis in adulthood: a possible role in learning. Trends Cogn. Sci. 3, 186–192. doi: 10.1016/S1364-6613(99)01310-8
Grassi Zucconi, G., Cipriani, S., Balgkouranidou, I., and Scattoni, R. (2006). ‘One night’ sleep deprivation stimulates hippocampal neurogenesis. Brain Res. Bull. 69, 375–381. doi: 10.1016/j.brainresbull.2006.01.009
Gross, C. G. (2000). Neurogenesis in the adult brain: death of a dogma. Nat. Rev. Neurosci. 1, 67–73. doi: 10.1038/35036235
Guzman-Marin, R., Bashir, T., Suntsova, N., Szymusiak, R., and McGinty, D. (2007). Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience 148, 325–333. doi: 10.1016/j.neuroscience.2007.05.030
Guzman-Marin, R., Suntsova, N., Bashir, T., Nienhuis, R., Szymusiak, R., and McGinty, D. (2008). Rapid eye movement sleep deprivation contributes to reduction of neurogenesis in the hippocampal dentate gyrus of the adult rat. Sleep 31, 167–175. doi: 10.1093/sleep/31.2.167
Guzman-Marin, R., Suntsova, N., Methippara, M., Greiffenstein, R., Szymusiak, R., and McGinty, D. (2005). Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur. J. Neurosci. 22, 2111–2116. doi: 10.1111/j.1460-9568.2005.04376.x
Guzman-Marin, R., Suntsova, N., Stewart, D. R., Gong, H., Szymusiak, R., and McGinty, D. (2003). Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J. Physiol. 549, 563–571. doi: 10.1113/jphysiol.2003.041665
Hagewoud, R., Havekes, R., Novati, A., Keijser, J. N., Van der Zee, E. A., and Meerlo, P. (2010). Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J. Sleep Res. 19, 280–288. doi: 10.1111/j.1365-2869.2009.00799.x
Hahn, T. T., Sakmann, B., and Mehta, M. R. (2007). Differential responses of hippocampal subfields to cortical up-down states. Proc. Natl. Acad. Sci. U.S.A. 104, 5169–5174. doi: 10.1073/pnas.0700222104
Hairston, I. S., Little, M. T., Scanlon, M. D., Barakat, M. T., Palmer, T. D., Sapolsky, R. M., et al. (2005). Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J. Neurophysiol. 94, 4224–4233. doi: 10.1152/jn.00218.2005
Hajek, T., Kozeny, J., Kopecek, M., Alda, M., and Hoschl, C. (2008). Reduced subgenual cingulate volumes in mood disorders: a meta-analysis. J. Psychiatry Neurosci. 33, 91–99.
Halassa, M. M., Florian, C., Fellin, T., Munoz, J. R., Lee, S. Y., Abel, T., et al. (2009). Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219. doi: 10.1016/j.neuron.2008.11.024
Hamilton, M. (1989). Frequency of symptoms in melancholia (depressive illness). Br. J. Psychiatry 154, 201–206. doi: 10.1192/bjp.154.2.201
Harvey, A. G. (2011). Sleep and circadian functioning: critical mechanisms in the mood disorders? Annu. Rev. Clin. Psychol. 7, 297–319. doi: 10.1146/annurev-clinpsy-032210-104550
Havekes, R., Park, A. J., Tolentino, R. E., Bruinenberg, V. M., Tudor, J. C., Lee, Y., et al. (2016). Compartmentalized PDE4A5 signaling impairs hippocampal synaptic plasticity and long-term memory. J. Neurosci. 36, 8936–8946. doi: 10.1523/JNEUROSCI.0248-16.2016
Havekes, R., and Abel, T. (2017). The tired hippocampus: the molecular impact of sleep deprivation on hippocampal function. Curr. Opin. Neurobiol. 44, 13–19. doi: 10.1016/j.conb.2017.02.005
Holmes, M. M., Galea, L. A., Mistlberger, R. E., and Kempermann, G. (2004). Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J. Neurosci. Res. 76, 216–222. doi: 10.1002/jnr.20039
Jayatissa, M. N., Henningsen, K., West, M. J., and Wiborg, O. (2009). Decreased cell proliferation in the dentate gyrus does not associate with development of anhedonic-like symptoms in rats. Brain Res. 1290, 133–141. doi: 10.1016/j.brainres.2009.07.001
Jilg, A., Lesny, S., Peruzki, N., Schwegler, H., Selbach, O., Dehghani, F., et al. (2010). Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus 20, 377–388. doi: 10.1002/hipo.20637
Josselyn, S. A., Kohler, S., and Frankland, P. W. (2015). Finding the engram. Nat. Rev. Neurosci. 16, 521–534. doi: 10.1038/nrn4000
Junek, A., Rusak, B., and Semba, K. (2010). Short-term sleep deprivation may alter the dynamics of hippocampal cell proliferation in adult rats. Neuroscience 170, 1140–1152. doi: 10.1016/j.neuroscience.2010.08.018
Karlsson, M. P., and Frank, L. M. (2009). Awake replay of remote experiences in the hippocampus. Nat. Neurosci. 12, 913–918. doi: 10.1038/nn.2344
Kee, N., Teixeira, C. M., Wang, A. H., and Frankland, P. W. (2007). Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 10, 355–362. doi: 10.1038/nn1847
Kempermann, G. (2008). The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 31, 163–169. doi: 10.1016/j.tins.2008.01.002
Kempermann, G., Krebs, J., and Fabel, K. (2008). The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr. Opin. Psychiatry 21, 290–295. doi: 10.1097/YCO.0b013e3282fad375
Kempermann, G., Kuhn, H. G., and Gage, F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. doi: 10.1038/386493a0
Kodama, M., Fujioka, T., and Duman, R. S. (2004). Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol. Psychiatry 56, 570–580. doi: 10.1016/j.biopsych.2004.07.008
Kornack, D. R., and Rakic, P. (1999). Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. U.S.A. 96, 5768–5773. doi: 10.1073/pnas.96.10.5768
Kott, J., Leach, G., and Yan, L. (2012). Direction-dependent effects of chronic “jet-lag” on hippocampal neurogenesis. Neurosci. Lett. 515, 177–180. doi: 10.1016/j.neulet.2012.03.048
Krause, A. J., Simon, E. B., Mander, B. A., Greer, S. M., Saletin, J. M., Goldstein-Piekarski, A. N., et al. (2017). The sleep-deprived human brain. Nat. Revi. Neurosci. 8, 404–418. doi: 10.1038/nrn.2017.55
Kreutzmann, J. C., Havekes, R., Abel, T., and Meerlo, P. (2015). Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience 309, 173–190. doi: 10.1016/j.neuroscience.2015.04.053
Krystal, A. D. (2012). Psychiatric disorders and sleep. Neurol. Clin. 30, 1389–1413. doi: 10.1016/j.ncl.2012.08.018
Krystal, A., Fava, M., Rubens, R., Wessel, T., Caron, J., Wilson, P., et al. (2007). Evaluation of eszopiclone discontinuation after cotherapy with fluoxetine for insomnia with coexisting depression. J. Clin. Sleep Med. 3, 48–55.
Kuhn, H. G., Dickinson-Anson, H., and Gage, F. H. (1996). Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16, 2027–2033.
Lemaire, V., Koehl, M., Le Moal, M., and Abrous, D. N. (2000). Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 97, 11032–11037. doi: 10.1073/pnas.97.20.11032
Leuner, B., Mendolia-Loffredo, S., Kozorovitskiy, Y., Samburg, D., Gould, E., and Shors, T. J. (2004). Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 24, 7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004
Levone, B. R., Cryan, J. F., and O'Leary, O. F. (2015). Role of adult hippocampal neurogenesis in stress resilience. Neurobiol. Stress 1, 147–155. doi: 10.1016/j.ynstr.2014.11.003
Li, Y. F., Zhang, Y. Z., Liu, Y. Q., Wang, H. L., Yuan, L., and Luo, Z. P. (2004). Moclobemide up-regulates proliferation of hippocampal progenitor cells in chronically stressed mice. Acta Pharmacol. Sin. 25, 1408–1412.
Lo, J. C., Groeger, J. A., Santhi, N., Arbon, E. L., Lazar, A. S., Hasan, S., et al. (2012). Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS ONE 7:e45987. doi: 10.1371/journal.pone.0045987
Lucassen, P. J., Stumpel, M. W., Wang, Q., and Aronica, E. (2010). Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology 58, 940–949. doi: 10.1016/j.neuropharm.2010.01.012
MacQueen, G. M., Campbell, S., McEwen, B. S., Macdonald, K., Amano, S., Joffe, R. T., et al. (2003). Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. U.S.A. 100, 1387–1392. doi: 10.1073/pnas.0337481100
Madeira, M. D., Cadete-Leite, A., Andrade, J. P., and Paula-Barbosa, M. M. (1991). Effects of hypothyroidism upon the granular layer of the dentate gyrus in male and female adult rats: a morphometric study. J. Comp. Neurol. 314, 171–186. doi: 10.1002/cne.903140116
Madsen, T. M., Kristjansen, P. E., Bolwig, T. G., and Wortwein, G. (2003). Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience 119, 635–642. doi: 10.1016/S0306-4522(03)00199-4
Malberg, J. E., Eisch, A. J., Nestler, E. J., and Duman, R. S. (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110.
Manber, R., Edinger, J. D., Gress, J. L., San Pedro-Salcedo, M. G., Kuo, T. F., and Kalista, T. (2008). Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep 31, 489–495. doi: 10.1093/sleep/31.4.489
Matsuo, T., Yamaguchi, S., Mitsui, S., Emi, A., Shimoda, F., and Okamura, H. (2003). Control mechanism of the circadian clock for timing of cell division in vivo. Science 302, 255–259. doi: 10.1126/science.1086271
McClelland, J. L., McNaughton, B. L., and O'Reilly, R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457. doi: 10.1037/0033-295X.102.3.419
McDermott, C. M., LaHoste, G. J., Chen, C., Musto, A., Bazan, N. G., and Magee, J. C. (2003). Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J. Neurosci. 23, 9687–9695.
Meerlo, P., Mistlberger, R. E., Jacobs, B. L., Heller, H. C., and McGinty, D. (2009). New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med. Rev. 13, 187–194. doi: 10.1016/j.smrv.2008.07.004
Methippara, M., Bashir, T., Suntsova, N., Szymusiak, R., and McGinty, D. (2010). Hippocampal adult neurogenesis is enhanced by chronic eszopiclone treatment in rats. J. Sleep Res. 19, 384–393. doi: 10.1111/j.1365-2869.2010.00833.x
Miller, B. H., McDearmon, E. L., Panda, S., Hayes, K. R., Zhang, J., Andrews, J. L., et al. (2007). Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 104, 3342–3347. doi: 10.1073/pnas.0611724104
Miller, B. R., and Hen, R. (2015). The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 30, 51–58. doi: 10.1016/j.conb.2014.08.012
Ming, G. L., and Song, H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702. doi: 10.1016/j.neuron.2011.05.001
Mirescu, C., and Gould, E. (2006). Stress and adult neurogenesis. Hippocampus 16, 233–238. doi: 10.1002/hipo.20155
Mirescu, C., Peters, J. D., Noiman, L., and Gould, E. (2006). Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc. Natl. Acad. Sci. U.S.A. 103, 19170–19175. doi: 10.1073/pnas.0608644103
Molle, M., Yeshenko, O., Marshall, L., Sara, S. J., and Born, J. (2006). Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J. Neurophysiol. 96, 62–70. doi: 10.1152/jn.00014.2006
Mongrain, V., Hernandez, S. A., Pradervand, S., Dorsaz, S., Curie, T., Hagiwara, G., et al. (2010). Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep 33, 1147–1157. doi: 10.1093/sleep/33.9.1147
Mueller, A. D., Mear, R. J., and Mistlberger, R. E. (2011). Inhibition of hippocampal neurogenesis by sleep deprivation is independent of circadian disruption and melatonin suppression. Neuroscience 193, 170–181. doi: 10.1016/j.neuroscience.2011.07.019
Mueller, A. D., Meerlo, P., McGinty, D., and Mistlberger, R. E. (2015). Sleep and adult neurogenesis: implications for cognition and mood. Curr. Top. Behav. Neurosci. 25, 151–181. doi: 10.1007/7854_2013_251
Mueller, A. D., Pollock, M. S., Lieblich, S. E., Epp, J. R., Galea, L. A., and Mistlberger, R. E. (2008). Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am. J. Physiol. Regul. Integr. Compar. Physiol. 294, R1693–R1703. doi: 10.1152/ajpregu.00858.2007
Mullen, R. J., Buck, C. R., and Smith, A. M. (1992). NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201–211.
Murphy, T., Dias, G. P., and Thuret, S. (2014). Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural Plast. 2014:563160. doi: 10.1155/2014/563160
Muto, V., Jaspar, M., Meyer, C., Kussé, C., Chellappa, S. L., Degueldre, C., et al. (2016). Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science 353, 687–690. doi: 10.1126/science.aad2993
Nixon, K., and Crews, F. T. (2004). Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J. Neurosci. 24, 9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004
Nutt, D., Wilson, S., and Paterson, L. (2008). Sleep disorders as core symptoms of depression. Dialogues Clin. Neurosci. 10, 329–336.
Ognjanovski, N., Schaeffer, S., Wu, J., Mofakham, S., Maruyama, D., Zochowski, M., et al. (2017). Parvalbumin-expressing interneurons coordinate hippocampal network dynamics required for memory consolidation. Nat. Commun. 8:15039. doi: 10.1038/ncomms15039
Palchykova, S., Winsky-Sommerer, R., Meerlo, P., Durr, R., and Tobler, I. (2006). Sleep deprivation impairs object recognition in mice. Neurobiol. Learn. Mem. 85, 263–271. doi: 10.1016/j.nlm.2005.11.005
Palchykova, S., Winsky-Sommerer, R., and Tobler, I. (2009). Sleep deprivation in the dark period does not impair memory in OF1 mice. Chronobiol. Int. 26, 682–696. doi: 10.1080/07420520902926025
Palmer, C. A., and Alfano, C. A. (2017). Sleep and emotion regulation: an organizing, integrative review. Sleep Med. Rev. 31, 6–16. doi: 10.1016/j.smrv.2015.12.006
Perera, T. D., Coplan, J. D., Lisanby, S. H., Lipira, C. M., Arif, M., Carpio, C., et al. (2007). Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 27, 4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007
Perlis, M. L., Giles, D. E., Buysse, D. J., Tu, X., and Kupfer, D. J. (1997). Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J. Affect. Disord. 42, 209–212. doi: 10.1016/S0165-0327(96)01411-5
Pham, K., Nacher, J., Hof, P. R., and McEwen, B. S. (2003). Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 17, 879–886. doi: 10.1046/j.1460-9568.2003.02513.x
Pillai, V., Kalmbach, D. A., and Ciesla, J. A. (2011). A meta-analysis of electroencephalographic sleep in depression: evidence for genetic biomarkers. Biol. Psychiatry 70, 912–919. doi: 10.1016/j.biopsych.2011.07.016
Pitkanen, A., Pikkarainen, M., Nurminen, N., and Ylinen, A. (2000). Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A Review. Ann. N. Y. Acad. Sci. 911, 369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x
Raber, J., Rola, R., LeFevour, A., Morhardt, D., Curley, J., Mizumatsu, S., et al. (2004). Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 162, 39–47. doi: 10.1667/RR3206
Rakai, B. D., Chrusch, M. J., Spanswick, S. C., Dyck, R. H., and Antle, M. C. (2014). Survival of adult generated hippocampal neurons is altered in circadian arrhythmic mice. PLoS ONE 9:e99527. doi: 10.1371/journal.pone.0099527
Ramirez-Amaya, V., Marrone, D. F., Gage, F. H., Worley, P. F., and Barnes, C. A. (2006). Integration of new neurons into functional neural networks. J. Neurosci. 26, 12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006
Ramirez-Rodriguez, G., Ortiz-Lopez, L., Dominguez-Alonso, A., Benitez-King, G. A., and Kempermann, G. (2011). Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 50, 29–37. doi: 10.1111/j.1600-079X.2010.00802.x
Reif, A., Fritzen, S., Finger, M., Strobel, A., Lauer, M., Schmitt, A., et al. (2006). Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry 11, 514–522. doi: 10.1038/sj.mp.4001791
Revest, J. M., Dupret, D., Koehl, M., Funk-Reiter, C., Grosjean, N., Piazza, P. V., et al. (2009). Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry 14, 959–967. doi: 10.1038/mp.2009.15
Riemann, D., Berger, M., and Voderholzer, U. (2001). Sleep and depression–results from psychobiological studies: an overview. Biol. Psychol. 57, 67–103. doi: 10.1016/S0301-0511(01)00090-4
Rolls, A., Colas, D., Adamantidis, A., Carter, M., Lanre-Amos, T., Heller, H. C., et al. (2011). Optogenetic disruption of sleep continuity impairs memory consolidation. Proc. Natl. Acad. Sci. U.S.A. 108, 13305–13310. doi: 10.1073/pnas.1015633108
Roman, V., Van der Borght, K., Leemburg, S. A., Van der Zee, E. A., and Meerlo, P. (2005). Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res. 1065, 53–59. doi: 10.1016/j.brainres.2005.10.020
Rossi, C., Angelucci, A., Costantin, L., Braschi, C., Mazzantini, M., Babbini, F., et al. (2006). Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 24, 1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x
Sahay, A., and Hen, R. (2007). Adult hippocampal neurogenesis in depression. Nat. Neurosci. 10, 1110–1115. doi: 10.1038/nn1969
Sairanen, M., Lucas, G., Ernfors, P., Castren, M., and Castren, E. (2005). Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 25, 1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005
Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., et al. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809. doi: 10.1126/science.1083328
Scerri, C., Stewart, C. A., Breen, K. C., and Balfour, D. J. (2006). The effects of chronic nicotine on spatial learning and bromodeoxyuridine incorporation into the dentate gyrus of the rat. Psychopharmacology (Berl). 184, 540–546. doi: 10.1007/s00213-005-0086-4
Scherrer, J. F., Salas, J., Copeland, L. A., Stock, E. M., Ahmedani, B. K., Sullivan, M. D., et al. (2016). Prescription opioid duration, dose, and increased risk of depression in 3 large patient populations. Ann. Fam. Med. 14, 54–62. doi: 10.1370/afm.1885
Schmidt-Hieber, C., Jonas, P., and Bischofberger, J. (2004). Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187. doi: 10.1038/nature02553
Scott, B. W., Wojtowicz, J. M., and Burnham, W. M. (2000). Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp. Neurol. 165, 231–236. doi: 10.1006/exnr.2000.7458
Sheline, Y. I. (2000). 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol. Psychiatry 48, 791–800. doi: 10.1016/S0006-3223(00)00994-X
Sheline, Y. I., Gado, M. H., and Kraemer, H. C. (2003). Untreated depression and hippocampal volume loss. Am. J. Psychiatry 160, 1516–1518. doi: 10.1176/appi.ajp.160.8.1516
Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., and Gould, E. (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376. doi: 10.1038/35066584
Shors, T. J., Townsend, D. A., Zhao, M., Kozorovitskiy, Y., and Gould, E. (2002). Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–584. doi: 10.1002/hipo.10103
Siapas, A. G., and Wilson, M. A. (1998). Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128. doi: 10.1016/S0896-6273(00)80629-7
Sirota, A., and Buzsaki, G. (2005). Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat. Syst. 3, 245–259. doi: 10.1017/S1472928807000258
Sirota, A., Csicsvari, J., Buhl, D., and Buzsaki, G. (2003). Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. U.S.A. 100, 2065–2069. doi: 10.1073/pnas.0437938100
Smith, W. T., Londborg, P. D., Glaudin, V., Painter, J. R., and Summit Research, N. (2002). Is extended clonazepam cotherapy of fluoxetine effective for outpatients with major depression? J. Affect. Disord. 70, 251–259.
Spalding, K. L., Bergmann, O., Alkass, K., Bernard, S., Salehpour, M., Huttner, H. B., et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. doi: 10.1016/j.cell.2013.05.002
Sportiche, N., Suntsova, N., Methippara, M., Bashir, T., Mitrani, B., Szymusiak, R., et al. (2010). Sustained sleep fragmentation results in delayed changes in hippocampal-dependent cognitive function associated with reduced dentate gyrus neurogenesis. Neuroscience 170, 247–258. doi: 10.1016/j.neuroscience.2010.06.038
Stickgold, R. (2005). Sleep-dependent memory consolidation. Nature 437, 1272–1278. doi: 10.1038/nature04286
Stickgold, R., and Walker, M. P. (2005). Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 28, 408–415.
Su, X. W., Li, X. Y., Banasr, M., and Duman, R. S. (2009). Eszopiclone and fluoxetine enhance the survival of newborn neurons in the adult rat hippocampus. Int. J. Neuropsychopharmacol. 12, 1421–1428. doi: 10.1017/S1461145709990629
Sullivan, D., Csicsvari, J., Mizuseki, K., Montgomery, S., Diba, K., and Buzsaki, G. (2011). Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J. Neurosci. 31, 8605–8616. doi: 10.1523/JNEUROSCI.0294-11.2011
Surget, A., Saxe, M., Leman, S., Ibarguen-Vargas, Y., Chalon, S., Griebel, G., et al. (2008). Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry 64, 293–301. doi: 10.1016/j.biopsych.2008.02.022
Takase, L. F., Fornal, C. A., and Jacobs, B. L. (2009). Effects of the hypnotic drug zolpidem on cell proliferation and survival in the dentate gyrus of young and old rats. Brain Res. 1259, 26–31. doi: 10.1016/j.brainres.2008.12.049
Tanapat, P., Hastings, N. B., Reeves, A. J., and Gould, E. (1999). Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 19, 5792–5801.
Tartar, J. L., Ward, C. P., Cordeira, J. W., Legare, S. L., Blanchette, A. J., McCarley, R. W., et al. (2009). Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels. Behav. Brain Res. 197, 450–453. doi: 10.1016/j.bbr.2008.08.035
Thompson, A., Boekhoorn, K., Van Dam, A. M., and Lucassen, P. J. (2008). Changes in adult neurogenesis in neurodegenerative diseases: cause or consequence? Genes Brain Behav. 7(Suppl. 1), 28–42. doi: 10.1111/j.1601-183X.2007.00379.x
Thuret, S., Toni, N., Aigner, S., Yeo, G. W., and Gage, F. H. (2009). Hippocampus-dependent learning is associated with adult neurogenesis in MRL/MpJ mice. Hippocampus 19, 658–669. doi: 10.1002/hipo.20550
Tiba, P. A., Oliveira, M. G., Rossi, V. C., Tufik, S., and Suchecki, D. (2008). Glucocorticoids are not responsible for paradoxical sleep deprivation-induced memory impairments. Sleep 31, 505–515. doi: 10.1093/sleep/31.4.505
Tudor, J. C., Davis, E. J., Peixoto, L., Wimmer, M. E., van Tilborg, E., Park, A. J., et al. (2016). Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Sci. Signal. 9:ra41. doi: 10.1126/scisignal.aad4949
Tung, A., Takase, L., Fornal, C., and Jacobs, B. (2005). Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience 134, 721–723. doi: 10.1016/j.neuroscience.2005.06.008
van der Borght, K., Ferrari, F., Klauke, K., Roman, V., Havekes, R., Sgoifo, A., et al. (2006). Hippocampal cell proliferation across the day: increase by running wheel activity, but no effect of sleep and wakefulness. Behav. Brain Res. 167, 36–41. doi: 10.1016/j.bbr.2005.08.012
Van Der Werf, Y. D., Van Der Helm, E., Schoonheim, M. M., Ridderikhoff, A., and Van Someren, E. J. (2009). Learning by observation requires an early sleep window. Proc. Natl. Acad. Sci. U.S.A. 106, 18926–18930. doi: 10.1073/pnas.0901320106
van Praag, H., Kempermann, G., and Gage, F. H. (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270.
van Praag, H., Shubert, T., Zhao, C., and Gage, F. H. (2005). Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005
Videbech, P., and Ravnkilde, B. (2004). Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry 161, 1957–1966. doi: 10.1176/appi.ajp.161.11.1957
Vorster, A. P., and Born, J. (2015). Sleep and memory in mammals, birds and invertebrates. Neurosci. Biobehav. Rev. 50, 103–119. doi: 10.1016/j.neubiorev.2014.09.020
Walker, M. P. (2009). The role of sleep in cognition and emotion. Ann. N. Y. Acad. Sci. 1156, 168–197. doi: 10.1111/j.1749-6632.2009.04416.x
Wang, J. W., David, D. J., Monckton, J. E., Battaglia, F., and Hen, R. (2008). Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci. 28, 1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008
Watson, B. O., and Buzsaki, G. (2015). Sleep, Memory & Brain Rhythms. Daedalus 144, 67–82. doi: 10.1162/DAED_a_00318
Wilson, M. A., and McNaughton, B. L. (1994). Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679. doi: 10.1126/science.8036517
Xu, H., Chen, Z., He, J., Haimanot, S., Li, X., Dyck, L., et al. (2006). Synergetic effects of quetiapine and venlafaxine in preventing the chronic restraint stress-induced decrease in cell proliferation and BDNF expression in rat hippocampus. Hippocampus 16, 551–559. doi: 10.1002/hipo.20184
Keywords: neurogenic niche, plasticity, cognition, sleep disruption, memory, psychiatric disorders, mood, depression
Citation: Navarro-Sanchis C, Brock O, Winsky-Sommerer R and Thuret S (2017) Modulation of Adult Hippocampal Neurogenesis by Sleep: Impact on Mental Health. Front. Neural Circuits 11:74. doi: 10.3389/fncir.2017.00074
Received: 31 May 2017; Accepted: 26 September 2017;
Published: 12 October 2017.
Edited by:
Marcos G. Frank, Washington State University Health Sciences Spokane, United StatesReviewed by:
Alex Dranovsky, Columbia University, United StatesMatthew Jones, University of Bristol, United Kingdom
Copyright © 2017 Navarro-Sanchis, Brock, Winsky-Sommerer and Thuret. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandrine Thuret, sandrine.1.thuret@kcl.ac.uk
 Cristina Navarro-Sanchis1
Cristina Navarro-Sanchis1  Olivier Brock
Olivier Brock Raphaelle Winsky-Sommerer
Raphaelle Winsky-Sommerer Sandrine Thuret
Sandrine Thuret