Opposing Cholinergic and Serotonergic Modulation of Layer 6 in Prefrontal Cortex
- 1Department of Physiology, University of Toronto, Toronto, ON, Canada
- 2Department of Pharmacology and Toxicology, University of Toronto, Toronto, ON, Canada
- 3Department of Obstetrics and Gynecology, University of Toronto, Toronto, ON, Canada
- 4Department of Psychiatry, University of Toronto, Toronto, ON, Canada
Prefrontal cortex is a hub for attention processing and receives abundant innervation from cholinergic and serotonergic afferents. A growing body of evidence suggests that acetylcholine (ACh) and serotonin (5-HT) have opposing influences on tasks requiring attention, but the underlying neurophysiology of their opposition is unclear. One candidate target population is medial prefrontal layer 6 pyramidal neurons, which provide feedback modulation of the thalamus, as well as feed-forward excitation of cortical interneurons. Here, we assess the response of these neurons to ACh and 5-HT using whole cell recordings in acute brain slices from mouse cortex. With application of exogenous agonists, we show that individual layer 6 pyramidal neurons are bidirectionally-modulated, with ACh and 5-HT exerting opposite effects on excitability across a number of concentrations. Next, we tested the responses of layer 6 pyramidal neurons to optogenetic release of endogenous ACh or 5-HT. These experiments were performed in brain slices from transgenic mice expressing channelrhodopsin in either ChAT-expressing cholinergic neurons or Pet1-expressing serotonergic neurons. Light-evoked endogenous neuromodulation recapitulated the effects of exogenous neurotransmitters, showing opposing modulation of layer 6 pyramidal neurons by ACh and 5-HT. Lastly, the addition of 5-HT to either endogenous or exogenous ACh significantly suppressed the excitation of pyramidal neurons in prefrontal layer 6. Taken together, this work suggests that the major corticothalamic layer of prefrontal cortex is a substrate for opposing modulatory influences on neuronal activity that could have implications for regulation of attention.
Introduction
The medial prefrontal cortex is essential for higher cognitive functions such as attention (Miller and Cohen, 2001; Knudsen, 2007; Logue and Gould, 2014). Although debate exists concerning the relationship of frontal brain areas between species in respect to structure and function, there is general agreement that the medial frontal portion of the brain is essential for executive functions, such as attention (Uylings et al., 2003; Bicks et al., 2015; Carlen, 2017). This is supported by the fact that lesions to this region impair performance on attention tasks across species, demonstrated by work in humans (Szczepanski and Knight, 2014), nonhuman primates (Rossi et al., 2009) and rodents (Muir et al., 1996; Granon et al., 1998). The deepest layer of prefrontal cortex, layer 6, is emerging as a key player in attentional control (Zikopoulos and Barbas, 2006; Béhuret et al., 2015; Wimmer et al., 2015). This layer sends extensive projections to thalamic nuclei (Mercer et al., 2005; Watts and Thomson, 2005; West et al., 2006; Zikopoulos and Barbas, 2006; Thomson, 2010) and to local circuit interneurons in cerebral cortex (Olsen et al., 2012; Tian et al., 2016). The corticothalamic connections are critical for top-down control of attentional processes (Miller and Cohen, 2001; Alitto and Usrey, 2003; Sherman, 2007; Wallace and Bertrand, 2013) and the corticocortical connections mediate cortical gain control (Olsen et al., 2012; Kim et al., 2016). The combination of these functions place layer 6 of prefrontal cortex as a hub connecting two important networks in control of attention. The importance of understanding the complexities of layer 6 modulation is emphasized by recent work showing that the frequency of corticothalamic neuronal firing determines whether it will inhibit or excite the thalamus (Crandall et al., 2015).
Deep layers of medial prefrontal cortex receive input from a number of neurotransmitter systems that modulate attentional processes (Logue and Gould, 2014; Meunier et al., 2017), with two prominent ones being acetylcholine (Ach; Robbins and Roberts, 2007; Bentley et al., 2011; Hasselmo and Sarter, 2011) and serotonin (5-HT; Amargós-Bosch et al., 2004; de Almeida and Mengod, 2008; Mengod et al., 2015; Muzerelle et al., 2016). Cholinergic projections to the mPFC arise from the nucleus basalis of the basal forebrain (Dunnett et al., 1991; Muir et al., 1993; Pang et al., 1993; McGaughy et al., 2002; Logue and Gould, 2014) and are associated with enhanced performance on attentional tasks (Himmelheber et al., 2000; Passetti et al., 2000; Sarter et al., 2001; Wallace and Bertrand, 2013). Disruptions to the cholinergic system interfere with attention (Robbins et al., 1989; Muir et al., 1992, 1993; Pang et al., 1993; Voytko et al., 1994; McGaughy et al., 2002; Dalley et al., 2004; Newman and McGaughy, 2008; Hasselmo and Sarter, 2011). Prefrontal cortex also receives 5-HT projections from the midbrain dorsal raphe nucleus (Amargós-Bosch et al., 2004; Celada et al., 2013; Logue and Gould, 2014; Leiser et al., 2015; Mengod et al., 2015; Muzerelle et al., 2016). Brain serotonergic activity has the opposite effect on attention than ACh. Increased 5-HT levels negatively affect performance on attention tasks (Ramaekers et al., 1995; Riedel et al., 1999, 2005; Schmitt et al., 2002; Wingen et al., 2008; Graf et al., 2013; Golub et al., 2017), while reductions in 5-HT enhance attention (Schmitt et al., 2000; Gallagher et al., 2003; Wingen et al., 2007). While it has been suggested that ACh and 5-HT can alter performance in the same attentional task (Jäkälä et al., 1992), it is not yet clear that there is a direct cortical interaction between these two modulatory systems in attentional processes (Steckler and Sahgal, 1995).
If ACh and 5-HT engage in a “tug-of-war” over attention, layer 6 of prefrontal cortex is an excellent candidate location for this interaction. Layer 6 pyramidal neurons are excited by ACh (Kassam et al., 2008; Bailey et al., 2010; Guillem et al., 2011; Tian et al., 2011; Poorthuis et al., 2013) and inhibited by 5-HT (Tian et al., 2016), with effects persisting in the presence of synaptic blockers, suggesting that ACh and 5-HT act directly on postsynaptic receptors on the same neurons. In prefrontal layer 6, there is abundant expression of the α4 and β2 subunits of high affinity nicotinic ACh receptors, as well as the α5 accessory subunit (Wada et al., 1989). Similarly, there is prominent expression of 5-HT1A receptors (Amargós-Bosch et al., 2004; Santana et al., 2004). However, it is not known whether the same neurons are bidirectionally modulated, nor how ACh and 5-HT in combination would affect the physiology of individual layer 6 pyramidal neurons. These questions are important because the cholinergic and serotonergic modulatory systems are both active during waking (Buzsaki et al., 1988; Jacobs and Azmitia, 1992) and are dynamically regulated in response to environmental stimuli (5-HT: Ranade and Mainen, 2009; Cohen et al., 2015; ACh: Buzsaki et al., 1988; Détári and Vanderwolf, 1987; Parikh et al., 2007; Sarter et al., 2016). Prefrontal 5-HT is increased by acute stressors (Fujino et al., 2002; Bland et al., 2003), which are known to impair attentional performance (Sänger et al., 2014). Yet rodent cognitive testing may underestimate the role of prefrontal 5-HT on attention, as typical training and testing paradigms (Winstanley et al., 2003; Bari et al., 2008) employ chronic stress conditions that would reduce levels of prefrontal 5-HT, including food restriction followed by rewards (Chandler-Laney et al., 2007; Fallon et al., 2007) and prolonged periods of single housing (Sargin et al., 2016), leading to an underestimation of potential effects of 5-HT on attention during acute stressors. Of note, human work suggests that social and emotional context will increase 5-HT effects on cognition (Osinsky et al., 2008; Daly et al., 2010; Beacher et al., 2011; Elliott et al., 2011; Frodl et al., 2015).
Here, we examined how the cholinergic and serotonergic systems interact in prefrontal cortex at the level of layer 6 neuronal physiology. Initial experiments examined whether individual layer 6 neurons are modulated by both ACh and 5-HT. Next, we investigated the effects of endogenous neurotransmitter release from cholinergic or serotonergic terminals. Finally, we studied the interaction between these modulators by assessing changes in neuronal responses to ACh during exposure to 5-HT. Taken together, this work begins to assess the outcome of combined cholinergic and serotonergic regulation of a prefrontal layer at the heart of attention. This could have significant implications for understanding how attentional processes in prefrontal cortex are modulated during times of heightened stress or emotion.
Materials and Methods
Experimental Animals/Brain Slice Preparation
Guidelines of the Canadian Council on Animal Care were followed, and all experimental procedures were approved by the Faculty of Medicine Animal Care Committee at the University of Toronto. Wild-type mice on a C57BL/6 background were used for experiments assessing the effects of exogenous ACh and 5-HT on neuronal activity. To study the effects of endogenous ACh release on neural activity, mice that heterozygously express blue-light sensitive channelrhodopsin in ChAT-containing cholinergic projections neurons (ChAT-ChR2-YFP BAC) on a C57BL/6 background were used (Zhao et al., 2011; Ivanova et al., 2016), permitting blue light stimulation to trigger the release of ACh from presynaptic terminals. Additional experiments were performed in mice heterozygous for ChATcre (ChAT-IRES-Cre, Rossi et al., 2011; Chen et al., 2016) and for Ai32 (RCL-ChR2(H134R)/EYFP, Madisen et al., 2012; O’Neill et al., 2017), a second mouse line that led to expression of blue-light sensitive channelrhodopsin in ChAT-containing cholinergic projections neurons. To assess the effects of endogenous 5-HT release on neural activity, mice heterozygous for Pet1cre (Tg(Fev-cre)1Esd, Scott et al., 2005) and homozygous for Ai32 (RCL-ChR2(H134R)/EYFP) on a C57BL/6 background were used, permitting blue light stimulation to release 5-HT from presynaptic terminals. A small subset of Pet1cre mice heterozygous for Ai32 were also tested. As layer 6 neurons in these mice did not detect an effect of light stimulation, we followed up with recording from 5-HT neurons in dorsal raphe in these mice. At 3 weeks of age mice were weaned, separated based on sex, and group housed (2–4 mice per cage) and given ad libitum access to food and water on a 12-h light/dark cycle with lights on at 7 AM.
Electrophysiology
Electrophysiology experiments were performed in acute brain slices obtained from adult male mice (mean ± SE; postnatal day 110 ± 5, n = 36 mice). After deep anesthesia with chloral hydrate (400 mg/kg), mice were decapitated and their brains were quickly extracted and chilled in 4°C sucrose ACSF (254 mM sucrose, 10 mM D-glucose, 24 mM NaHCO3, 2 mM CaCl2, 2 mM MgSO4, 3 mM KCl, 1.25 mM NaH2PO4; pH 7.4). A Dosaka linear slicer (SciMedia, Costa Mesa, CA, USA) was used to obtain 400 μM thick coronal brain slices of prefrontal cortex (range 2.34–0.74 from Bregma; Paxinos and Franklin, 2001), and for a small subset of mice coronal slices of dorsal raphe (range −4.48 to −4.84 from Bregma; Paxinos and Franklin, 2001), which recovered for ~2 h in regular ACSF (128 mM NaCl, 10 mM D-glucose, 26 mM NaHCO3, 2 mM CaCl2, 2 mM MgSO44, 3 mM KCl, 1.25 mM NaH2PO4; pH 7.4). To maintain synthesis of 5-HT (Liu et al., 2005), brain slices from Pet1cre/Ai32+/− and Pet1cre/Ai32+/+ were recovered and recorded in the presence of L-tryptophan (2.5 μM for dorsal raphe slices; 30 μM for prefrontal slices).
For whole cell patch clamp recording, brain slices were placed in a perfusion chamber on the stage of a BX50W1 microscope (Olympus, Tokyo, Japan), perfused with oxygenated ACSF (95%O2, 5%CO2) at a rate of 3–4 ml/minute at 30°C for cortical slices and room temperature for dorsal raphe slices. Layer 6 pyramidal neurons in the prelimbic and cingulate regions of the medial prefrontal cortex were identified based on morphological characteristics (pyramidal shape, large cell body, orientation of apical dendrite; see Contreras, 2004; Andjelic et al., 2009; van Aerde and Feldmeyer, 2015). Recording electrodes (2–4 MΩ) filled with 5 mM KCl, 2 mM MgCl2, 4 mM K2-ATP, 0.4 mM Na2-GTP, 10 mM Na2-phosphocreatine and 10 mM HEPES buffer and with pH adjusted to 7.3 using KOH were used to patch layer 6 pyramidal neurons. Recordings were obtained using an EPC10 (HEKA Electronik, Lambrecht/Pfalz, Germany). All data were acquired at 20 kHz and low pass filtered at 3 kHz using pClamp software (Molecular Devices, Palo Alto, CA, USA) and corrected for the liquid junction potential (14 mV). For the small subset of recordings in the dorsal raphe, the electrophysiological properties of the GFP-positive 5-HT neurons we observed were: resting membrane potential (RMP; −64 ± 2 mV), input resistance (750 ± 81 MΩ), and action potential amplitude (94 ± 5 mV). The electrophysiological properties of prefrontal layer 6 pyramidal neurons from the different mouse lines are illustrated in Table 1.
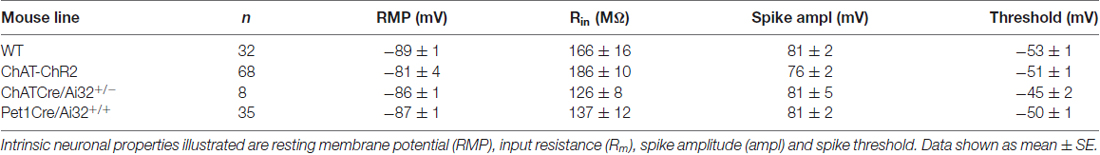
Table 1. Electrophysiological properties of prefrontal layer 6 pyramidal neurons in each mouse line.
We employed several different electrophysiological strategies to examine the co-modulation of layer 6 pyramidal neurons by ACh and 5-HT. Initial experiments probed the sensitivity of individual neurons to ACh and 5-HT using relatively strong exogenous stimulation (1 mM ACh for 15 s and 10 μM 5-HT for 30 s). Resulting currents were measured in voltage-clamp at a holding potential of −75 mV. Next, we tested the sensitivity of layer 6 neurons to modulation that is potentially more physiologically-relevant, applying lower concentrations of exogenous ACh and 5-HT (10 μM ACh or 1 μM 5-HT, 30 s). For these experiments, we aimed to simulate how activation of cholinergic or serotonergic inputs to the prefrontal cortex might affect the firing rate of layer 6 neurons. To this end, we recorded from layer 6 pyramidal neurons in current clamp and delivered trains of brief depolarizing stimuli (5 ms pulses at 5 Hz for 4 s; repeated every 10 s). Stimulation intensity was selected to be “peri-threshold,” yielding ~50% success at eliciting action potentials in a replicable pattern across baseline trials. Changes in firing frequency were then recorded following application of either ACh or 5-HT under these conditions.
To test the effects of endogenously-released ACh or 5-HT, we used optogenetic experiments in which channelrhodopsin-expressing terminals of either ChAT or Pet1-expressing neurons were stimulated with blue (473 nm; 2–6 mW) LED light using an optic fiber (Thorlabs, Newton, NJ, USA) on a mechanical manipulator (Narishige International, East Meadow, NY, USA) targeted onto layer 6 pyramidal neurons or a microscope-mounted collimated LED (Thorlabs). In brain slices of ChAT/ChR2 and ChATcre/Ai32+/− mice, neurons were held at subthreshold membrane potentials (−70 to −65 mV) and light (5 ms pulses at 10 Hz for 3 s) was used to induce ACh release. In Pet1Cre/Ai32+/+ mice, neurons were depolarized to supra-threshold levels to elicit action potential firing (2–3 Hz) and light (5 ms pulses, 10 Hz, 5 s) used to assess the suppression of action potential firing by endogenous 5-HT release. Additional experiments applied peri-threshold trains of depolarizing stimuli to layer 6 neurons in either ChAT/ChR2 or Pet1Cre/Ai32 mice to assess the effects of endogenous neurotransmitter release on neuronal firing rate. Finally, the inhibitory effects of low levels of 5-HT were tested against the depolarization elicited by the strongest level of light-evoked ACh release (5 ms pulses, 10 Hz), as well as the interaction between higher levels of exogenous 5-HT and ACh.
Pharmacology
For the exogenous cholinergic and serotonergic stimulation, neuronal current responses were assessed by bath application of ACh (acetylcholine chloride, Sigma-Aldrich, Oakville, ON, Canada) and 5-HT (serotonin creatinine sulfate, Sigma-Aldrich, St. Louis, MO) in ACSF. Neurons were tested with strong stimulation (1 mM ACh for 15 s; 10 μM 5-HT for 30 s) or milder stimulation (10 μM ACh for 30 s; 1 μM 5-HT for 30 s) to approximate endogenous conditions more closely. For the optogenetic experiments, the role of different cholinergic receptor subtypes in mediating the light-evoked ACh response was assessed using the α4β2 nicotinic ACh receptor antagonist DHβE (Sigma-Aldrich, 3 μM), and the muscarinic receptor antagonist atropine (Sigma-Aldrich, 200 nM). The role of different 5-HT receptor subtypes in the light-evoked 5-HT response was assessed using the 5-HT1A receptor antagonist WAY100635 (Tocris, Bristol, UK; 30 nM).
Statistical Analysis
All data are expressed as mean ± SE. Recordings were analyzed using Clampfit software (Molecular Devices) and statistically analyzed using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA, USA) using paired or unpaired Student’s t-tests with a significance level of p < 0.05.
Results
Opposite Cholinergic and Serotonergic Modulation of Prefrontal Layer 6 Neurons
First, we investigated whether the same prefrontal layer 6 pyramidal neurons respond both to ACh and to 5-HT, using bath application of these neurotransmitters and measuring current responses in voltage clamp. As illustrated in Figures 1A,B, ACh (1 mM) elicited large inward currents (−101 ± 9 pA) and, after a 5 min washout period, 5-HT (10 μM) elicited moderate outward currents (57 ± 7 pA). These responses were significantly different (t9 = 13.7, p < 0.0001), but there was no significant correlation between the size of ACh and 5-HT responses in the same neurons (r = 0.18, p = 0.6, n = 10). A lower concentration of ACh (10 μM), elicited smaller inward currents (−7.6 ± 2 pA, n = 13), while a lower concentration of 5-HT (1 μM), elicited smaller outward currents (18.0 ± 4 pA, n = 8). The statistically significant difference between responses to ACh and 5-HT was maintained (t19 = 6.9, p < 0.0001).
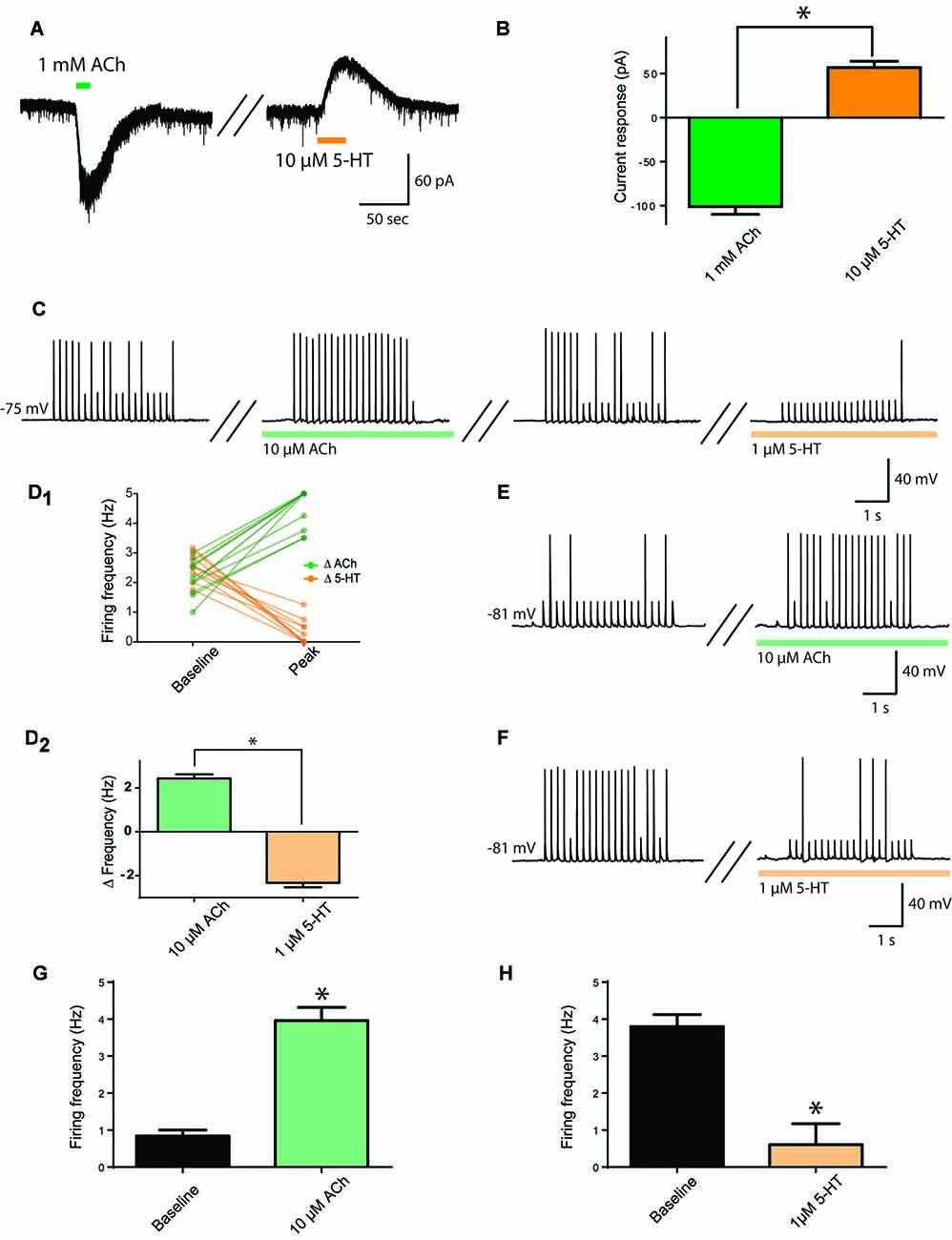
Figure 1. Opposing cholinergic and serotonergic modulation of prefrontal layer 6 neurons. (A) Example voltage-clamp recording showing inward current elicited by strong acetylcholine (ACh) stimulation (1 mM) and outward current elicited by strong serotonin (5-HT; 10 μM) stimulation in the same layer 6 neuron. (B) Bar graph summarizing inward and outward currents elicited by ACh and 5-HT respectively in layer 6 pyramidal neurons (t9 = 13.7, p < 0.0001). (C) To assess the effects of lower stimulation by ACh and 5-HT on firing frequency, a train of peri-threshold current pulses was delivered to layer 6 pyramidal neurons. Example neuron shows the frequency of action potentials elicited compared across baseline, ACh (10 μM), washout, and 5-HT (1 μM) conditions. (D) Linked-data plot (D1) and bar graph (D2) summarize the change in frequency of action potentials fired in response to ACh and 5-HT respectively in layer 6 pyramidal neurons (t11 = 15.3, p < 0.0001). The strength of this modulation raises the possibility of ceiling and floor effects. (E) Therefore, to probe further the ability of ACh (10 μM) to enhance action potential firing, a train of current pulses with lower spike probability was used, as shown in this example. (F) Furthermore, to probe further the ability of 5-HT (1 μM) to suppress action potential firing, a train of current pulses with greater spike probability was used, as shown in this example. (G) Bar graph summarizing the spike frequency enhancing effects of a low level of exogenous ACh (t18 = 7.57, p < 0.0001). (H) Bar graph showing the spike frequency suppressing effects of a low level of 5-HT (t6 = 5.4, p = 0.0016). *Denotes p < 0.05. Darker green and orange indicate higher concentration of ACh and 5-HT (1 mM and 10 μM, respectively), while lighter green and orange indicate lower concentration of ACh and 5-HT (10 μM and 1 μM, respectively).
With the ultimate goal of assessing how endogenous ACh and 5-HT modulate layer 6 neuronal activity, we developed an experimental paradigm more sensitive to lower neurotransmitter levels. To accomplish this, we determined the peri-threshold current amplitude experimentally and administered trains of current injection in current clamp from rest (5 ms pulses at 5 Hz for 4 s, average baseline firing frequency: 2.4 ± 0.1 Hz). This level would permit bidirectional modification of action potential firing. As illustrated in Figures 1C,D1,2, ACh and 5-HT had opposing and significantly different effects on action potential firing in response to the current train in the same neurons (t11 = 15.3, p < 0.0001, n = 12), with ACh (10 μM) significantly increasing action potential firing rate by 2.4 ± 0.2 Hz and 5-HT (1 μM) significantly decreasing action potential firing rate by 2.3 ± 0.2 Hz. The strength of this modulation raised the possibility of ceiling and floor effects. Therefore, as illustrated in Figures 1E–H, we tested cholinergic excitation using a lower initial spike success level, finding a significant increase in action potential firing frequency (baseline, 0.8 ± 0.2; ACh, 4.0 ± 0.4 Hz; t18 = 7.57, p < 0.0001, n = 19) and serotonergic inhibition using a higher initial spike success level, finding a significant decrease in action potential firing frequency (baseline, 3.8 ± 0.3; 5-HT, 0.6 ± 0.6 Hz; t6 = 5.4, p = 0.002, n = 7). These results illustrate that ACh and 5-HT have opposing effects on the excitability of layer 6 prefrontal neurons.
Endogenous Modulation of Action Potential Firing in Prefrontal Layer 6 Neurons
To test the effects of endogenous ACh or 5-HT release on the firing activity of layer 6 pyramidal neurons, we employed transgenic mouse models that allowed us to use light to stimulate ACh or 5-HT release onto layer 6 pyramidal neurons (Figure 2A). Note, levels of channelrhodopsin expressed in afferents correspond to the different cholinergic and serotonergic mouse lines used. As such, response to light stimulation shows that modulation is possible, but does not set an upper bound on its strength, and lack of response does not rule out a modulatory role for those afferents.
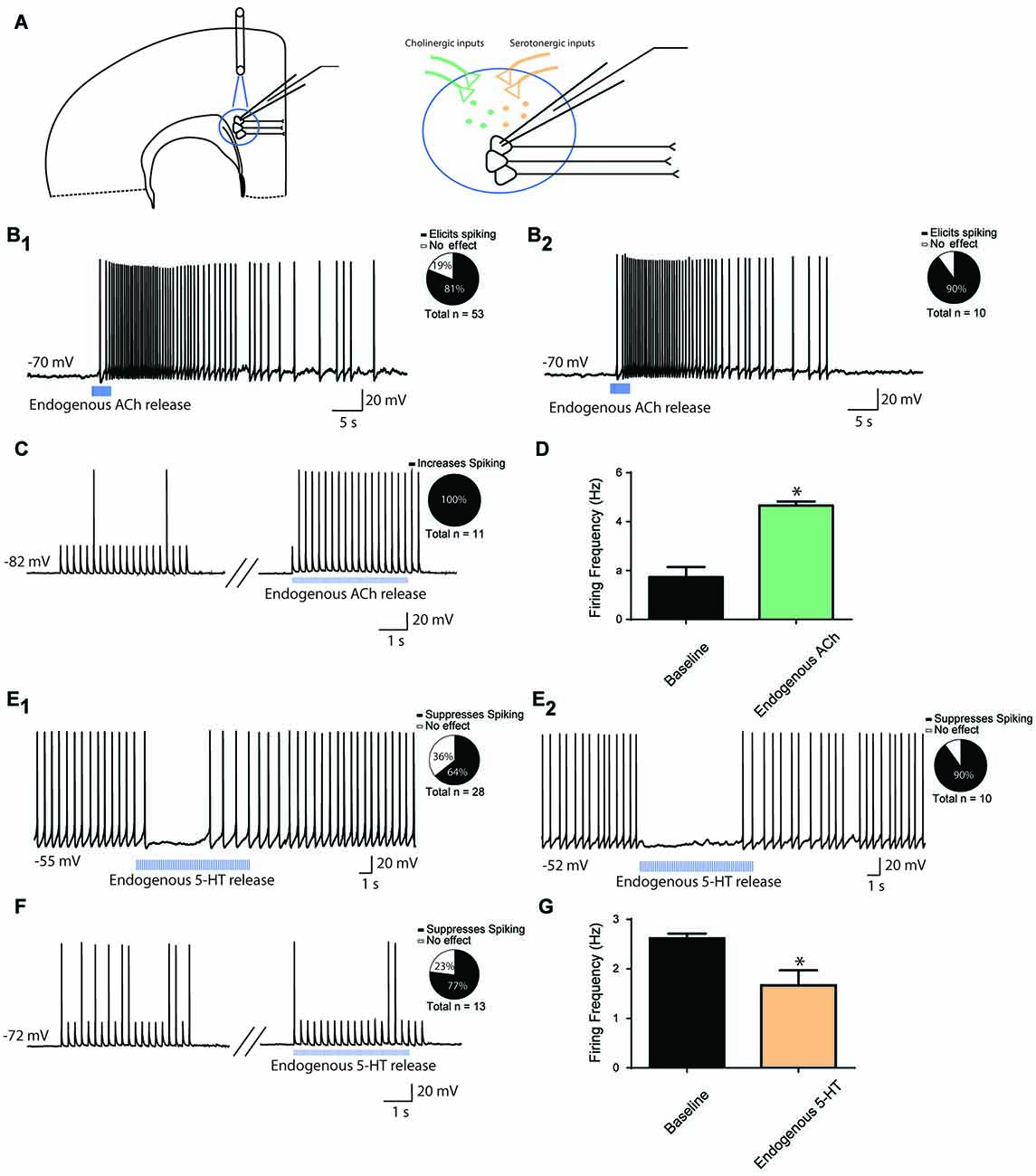
Figure 2. Endogenous modulation of action potential firing in prefrontal layer 6 neurons. (A) Schematic diagram illustrating light-evoked ACh or 5-HT release onto layer 6 pyramidal neurons from presynaptic terminals. (B) A train of optogenetic stimuli (5 ms pulses, 10 Hz) to release ACh can elicit depolarization and action potential spiking in layer 6 neurons in cortical slices of mice with channelrhodopsin in axonal fibers from ChAT-expressing ACh neurons. For this experiment, neurons were injected with constant electrical current to bring them to a membrane potential of −70 mV (B1). Pie chart illustrates that action potentials were elicited in 43/53 neurons. ACh-elicited action potential firing persisted in the presence of synaptic blockers CNQX, APV and picrotoxin (B2). Pie chart illustrates that action potentials were evoked in in 9/10 neurons. (C) In combination with a train of peri-threshold electrical pulses, optogenetic release of ACh increases the probability of action potential firing. Pie chart illustrates that action potential frequency was increased in 11/11 neurons. (D) Bar graph showing that spike frequency was significantly increased by optogenetic release of endogenous ACh (t10 = 6.9, p < 0.0001). (E) A train of optogenetic stimuli (5 ms pulses, 10 Hz) to release 5-HT can reduce action potential firing in layer 6 neurons in cortical slices of mice with channelrhodopsin in axonal fibers from Pet1cre-expressing 5-HT neurons (E1). For this experiment, neurons were injected with constant electrical current to bring them to spiking. Pie chart illustrates that action potentials were suppressed in 18/28 neurons. The 5-HT elicited suppression persisted in the presence of synaptic blockers CNQX, APV and picrotoxin (E2). Pie chart illustrates that action potentials were suppressed in 9/10 neurons. (F) In combination with a train of peri-threshold electrical pulses, optogenetic stimuli to release 5-HT decreases the probability of action potential firing. Pie chart illustrates that action potential frequency was decreased in 10/13 neurons. (G) Bar graph showing a small but significant decrease in spike frequency upon optogenetic release of 5-HT (t12 = 3.3, p = 0.007). *Denotes p < 0.05.
As illustrated in Figure 2B1, light stimulation of cholinergic terminals in brain slices of ChAT-ChR2 mice led to action potential firing in 43/53 (n = 53) layer 6 pyramidal neurons held at subthreshold membrane potentials. Of note, optogenetic stimulation of cholinergic afferents in a different transgenic line (ChATcre/Ai32+/−) had similar effects, with light stimulation inducing action potential firing in 6/8 neurons (n = 8, not shown). This effect persisted in the presence of synaptic blockers CNQX, APV and picrotoxin in 9/10 neurons (n = 10, Figure 2B2). Pharmacological manipulation of nicotinic and muscarinic ACh receptors, respectively with DHβE (3 μM) and/or atropine (200 nM), either individually or in tandem, both contributed to endogenous ACh-induced spiking. DHβE blocked spiking in 5/7 neurons, while atropine blocked spiking in 5/7 neurons. Co-application of DHβE and atropine blocked spiking in 5/5 neurons tested. These findings are consistent with a role for both subtypes of ACh receptors in mediating the response of layer 6 pyramidal neurons to exogenous ACh (Kassam et al., 2008; Bailey et al., 2010; Tian et al., 2011). Next, we examined how light-evoked endogenous release of ACh affects firing activity during trains of depolarizing current injection, in order to assess the consequences of endogenous ACh for prefrontal neurons to follow stimuli. Optogenetic stimulation of cholinergic afferents led to a significant increase in neuronal firing rate following trains of current injection in neurons that were sitting at RMP (baseline, 1.7 ± 0.4; endogenous ACh, 4.7 ± 0.2 Hz; t10 = 6.9, p < 0.0001), with an increase in firing rate occurring in 11/11 neurons tested (n = 11, Figures 2C,D).
Although 5-HT terminals are found in abundance in the prefrontal cortex, early experiments in Pet1cre/Ai32+/− mice did not detect a response to light in the cortex (n = 6), despite robust light-elicited inward currents in dorsal raphe 5-HT cells (1.4 ± 0.4 nA, n = 11; response to a single 5 ms flash of light). Therefore, we bred this line to be homozygous for Ai32 (Pet1cre/Ai32+/+) with the goal of increasing the channelrhodopsin expression in serotonergic afferents. In these Pet1cre/Ai32+/+ mice, optogenetic stimulation of serotonergic afferents in prefrontal slices had detectable electrophysiological effects. We injected depolarizing current to elicit stable baseline action potential firing (2–3 Hz) in order to measure the degree of inhibition mediated by endogenous 5-HT release. Using this paradigm, light stimulation of serotonergic afferents inhibited action potential firing in 18/28 neurons (n = 28, Figure 2E1). This effect persisted in the presence of synaptic blockers CNQX, APV and picrotoxin in 9/10 neurons (n = 10, Figure 2E2). Consistent with exogenous serotonergic modulation (Tian et al., 2016), the light evoked responses in brain slices from Pet1cre/Ai32+/+ mice were sensitive to suppression by an antagonist of 5-HT receptors. Pharmacological manipulation of 5-HT1A receptors with WAY100635 (30 nM) completely suppressed the light-induced inhibition in 5/6 neurons tested. Finally, we examined how endogenous release of 5-HT affects firing activity in response to current injection at RMP, simulating how 5-HT may modulate prefrontal layer 6 neuronal activity in response to inputs. Optogenetic stimulation of serotonergic afferents led to a significant decrease in neuronal firing rate (baseline, 2.6 ± 0.1 Hz; endogenous 5-HT, 1.7 ± 0.3 Hz; t12 = 3.3, p = 0.007), with a decrease in firing rate occurring in 10/13 neurons tested (n = 13, Figures 2F,G). These results suggest that endogenous ACh and 5-HT modulate layer 6 prefrontal neuron excitability in similar directions to exogenous bath application, with endogenous ACh increasing and endogenous 5-HT decreasing neuronal excitability.
Interaction of Cholinergic and Serotonergic Influences on Layer 6 Neuronal Activity
To observe more directly the interaction between ACh and 5-HT and their opposing influences on neuronal activity in layer 6 pyramidal neurons, we next optogenetically stimulated cholinergic afferents (Figure 3A) before and during bath application of 5-HT (1 μM) to simulate the coincident activity of ACh and 5-HT on layer 6 neurons. The presence of 5-HT inhibited the excitability normally induced by ACh light stimulation (n = 25, Figures 3B,C). There was a significant reduction in ACh-elicited depolarization (baseline, 12.0 ± 0.9 mV; 5-HT, 7.0 ± 0.8 mV; t24 = 4.7, p < 0.0001), a significant decrease in the number of action potentials evoked (baseline, 23 ± 4 spikes; 5-HT, 8 ± 3 spikes; t23 = 5.2, p < 0.0001), a significant decrease in peak action potential firing frequency (baseline, 4.0 ± 0.5 Hz, 5-HT, 1.9 ± 0.5 Hz; t22 = 6.0, p < 0.0001), and a significant decrease in the duration of action potential firing (baseline, 6.3 ± 0.9 s; 5-HT, 2.1 ± 0.6 s; t24 = 6.3, p < 0.0001). These results show that 5-HT can impair the ability of endogenous ACh to stimulate layer 6 pyramidal neurons effectively.
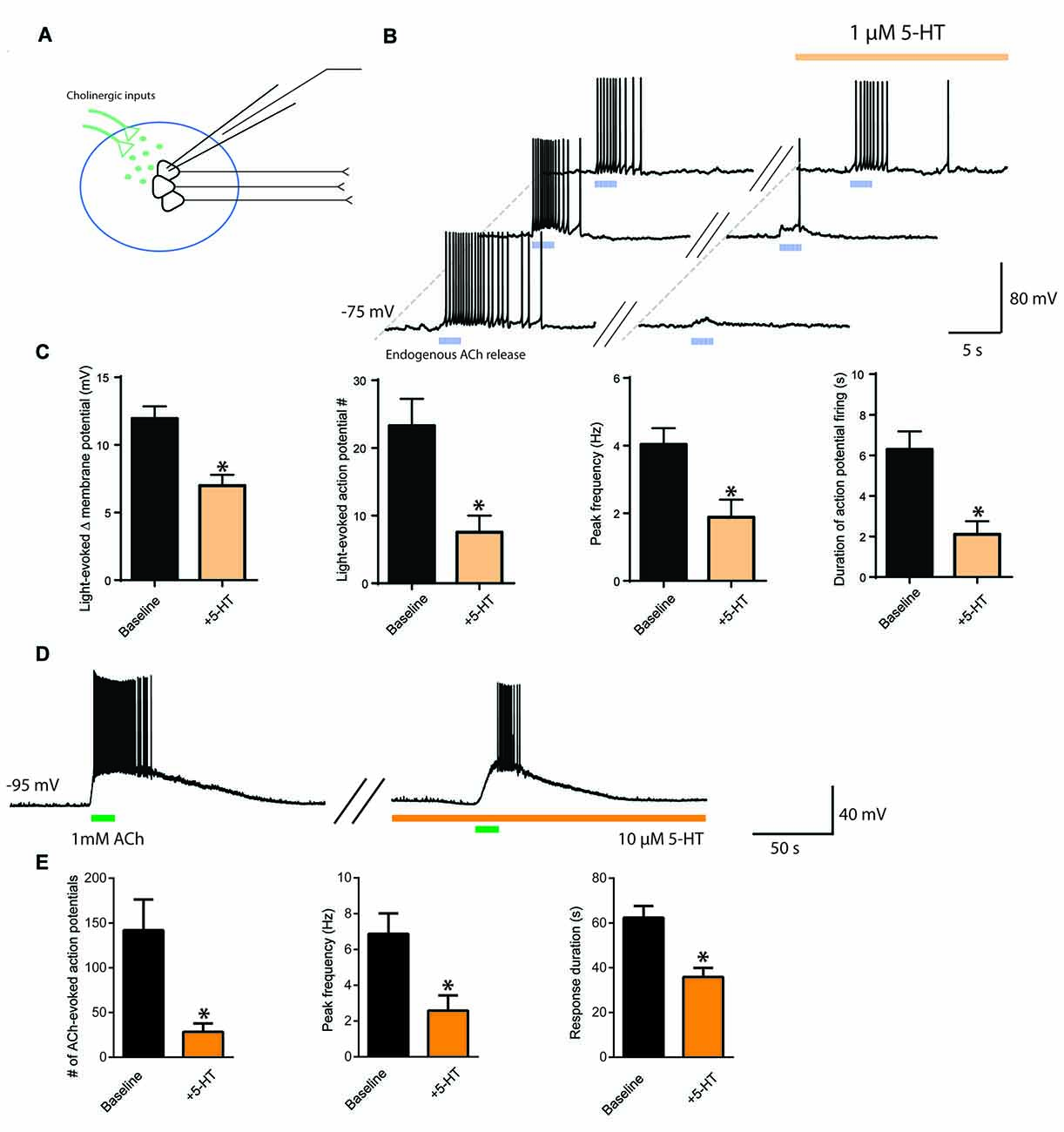
Figure 3. Interaction of cholinergic and serotonergic influences on layer 6 neuronal activity. 5-HT inhibits ACh-induced facilitation of action potential firing. (A) Schematic illustrating light-evoked stimulation of cholinergic fibers synapsing onto layer 6 pyramidal neurons in medial prefrontal cortex. (B) Three different example layer 6 pyramidal neurons demonstrating that the action potential firing evoked by endogenous ACh (5 ms pulses, 10 Hz) is inhibited by application of a low level of exogenous 5-HT (1 μM). (C) Bar charts show the significant reductions exerted by 5-HT on the following parameters of the layer 6 excitation elicited by optogenetic release of endogenous ACh: change in membrane potential (t24 = 4.7, p < 0.0001), number of action potentials (t23 = 5.2, p < 0.0001), peak spike frequency (t22 = 6.0, p < 0.0001), and duration of action potential firing (t24 = 6.3, p < 0.0001). (D) Example neuron showing that the stronger excitation of layer 6 pyramidal neurons through application of exogenous ACh remains susceptible to attenuation by 5-HT. (E) Bar charts show the significant reductions exerted by 5-HT on the following parameters of layer 6 excitation is inhibited by 5-HT: number of action potentials (t11 = 4.0, p = 0.0021), peak frequency (t11 = 7.4, p < 0.0001) and response duration (t12 = 5.27, p = 0.0003). *Denotes p < 0.05. Darker orange indicates a higher concentration of 5-HT (10 μM), while lighter orange indicates a lower concentration of 5-HT (1 μM).
To probe further the interaction between cholinergic and serotonergic stimulation of layer 6, we investigated how the effects of strong exogenous cholinergic stimulation would be affected by strong exogenous serotonergic stimulation. Bath application of ACh (1 mM) strongly depolarized layer 6 pyramidal neurons, evoking action potential firing from rest. This cholinergic modulation was significantly suppressed by bath application of 5-HT (10 μM). Significant differences were observed in the number of action potentials evoked (baseline, 142 ± 34 spikes, 5-HT, 28 ± 9 spikes; t11 = 4.0, p = 0.0021), in peak firing frequency (baseline, 6.9 ± 1.1 Hz; 5-HT, 2.6 ± 0.9 Hz; t11 = 7.4, p < 0.0001), and in response duration (baseline, 62.4 ± 5.2 s; 5-HT, 35.9 ± 4.0 s; t12 = 5.27, p = 0.0003; Figures 3D,E, n = 13). This provides evidence that even the excitation elicited in layer 6 pyramidal neurons by strong cholinergic stimulation can be reduced by serotonergic stimulation, which may be relevant for understanding interactions that may occur when both neuromodulators are increased in prefrontal cortex during highly stressful situations (Mark et al., 1996; Bland et al., 2003).
Discussion
Here, we probed the opposition between cholinergic and serotonergic modulation of layer 6 pyramidal neurons of prefrontal cortex. First, we examined the effects of ACh and 5-HT on individual pyramidal neurons, then we examined how the excitability of layer 6 neurons is altered by optogenetic release of ACh and 5-HT, and finally we tested the effects of ACh and 5-HT in combination. We used a number of experimental paradigms in this work, including a new paradigm to assess the ability of neuromodulators to enhance or suppress action potential firing in response to a train of brief depolarizing stimuli. Overall, this work shows that individual layer 6 pyramidal neurons in prefrontal cortex are sensitive to direct modulation by both ACh and to 5-HT. This cholinergic excitation and serotonergic inhibition is recapitulated by release of the endogenous neuromodulators. Endogenous excitation of layer 6 pyramidal neurons by ACh was sensitive to both nicotinic and muscarinic receptor blockade, while suppression of endogenously released 5-HT was largely eliminated by blockade of 5-HT1A receptors. We have also shown that the ability of ACh to drive layer 6 pyramidal neurons in prefrontal cortex is constrained by 5-HT. Since cholinergic modulation of deep prefrontal cortex is essential for optimal attentional performance (Bailey et al., 2010; Guillem et al., 2011), these results point to a cellular mechanism to explain how increasing 5-HT release disrupts task attention.
We probed layer 6 pyramidal neurons with lower concentrations of exogenous agonists than typically used in previous work (Kassam et al., 2008; Bailey et al., 2010; Tian et al., 2011, 2016), and used optogenetic stimulation to investigate the consequences of endogenous release of ACh or 5-HT. In working with brain slices from transgenic mice expressing channelrhodopsin in either cholinergic or serotonergic neurons, however, it becomes evident that the expression level of channelrhodopsin in these modulatory afferents determines the maximal effects that can be detected. In short, failure to observe modulation does not mean that it does not happen in vivo, just that there may be insufficient potential for the light in the terminal field to stimulate release. This occurs with the Pet1cre/Ai32+/− mouse and may account for the relatively modest effects of optogenetic stimulation in the brain slices of Pet1cre/Ai32+/+ mice. We see more powerful effects of optogenetic stimulation in both the ChAT-ChR2 and ChATcre/Ai32+/− mouse lines, which we used to observe the effects of endogenous release of ACh. Our work is a first demonstration of the effects of endogenous serotonergic modulation of prefrontal layer 6, and it extends earlier cholinergic work (Hedrick and Waters, 2015) with experiments assessing the impact of endogenous cholinergic modulation on action potential frequency in response to trains of stimuli delivered to neurons otherwise at rest.
In vivo, the attention-enhancing effects of prefrontal ACh release have been extensively studied (Arnold et al., 2002; Parikh et al., 2007; Gritton et al., 2016), but the adverse behavioral effects of 5-HT on attention tasks are less well understood (Ramaekers et al., 1995; Riedel et al., 1999, 2005; Schmitt et al., 2002; Wingen et al., 2008; Golub et al., 2017). Recent work raises the concept that 5-HT may subvert top-down cortical signaling in favor of sensory processing (Lottem et al., 2016). This hypothesis is consistent with the inhibitory effects of 5-HT on layer 6 neurons known for their top-down feedback to the thalamus (Alitto and Usrey, 2003; Zikopoulos and Barbas, 2006; Thomson, 2010) and role in cortical gain control (Olsen et al., 2012; Tian et al., 2016). While both the cholinergic and serotonergic systems are active during waking (Buzsaki et al., 1988; Jacobs and Azmitia, 1992) and the cholinergic system is certainly active during attention (Arnold et al., 2002; Parikh et al., 2007; Gritton et al., 2016), the cholinergic modulation of prefrontal cortex may be opposed by 5-HT to differing extents depending on the environmental circumstances (Ranade and Mainen, 2009), such as the presence of stressors (Fujino et al., 2002; Bland et al., 2003) or potentially the emotional content of an attention task (Osinsky et al., 2008; Daly et al., 2010; Beacher et al., 2011; Elliott et al., 2011; Frodl et al., 2015).
What would be the benefit of 5-HT attenuating the cholinergic enhancement of task attention? While our society rewards strong task attention, interference by 5-HT appears consistent with the growing understanding of serotonergic modulation of cognitive and behavioral flexibility (Nonkes et al., 2012; Matias et al., 2017). Clinically, this phenomenon appears relevant to attention abnormalities seen in neurological and psychiatric disorders that are accompanied by serotonergic disruption. For example, some types of focused task attention can be difficult to disrupt in people with autism, a condition associated with low 5-HT levels in brain (Dougherty et al., 2013; Adamsen et al., 2014; Muller et al., 2016). Conversely, increases in 5-HT may contribute to adverse consequences of selective 5-HT reuptake inhibitors on attention (Ramaekers et al., 1995; Riedel et al., 2005; Graf et al., 2013; Golub et al., 2017). Taken together, our electrophysiological and optogenetic results suggest a potential cellular mechanism underlying the opposing influences of ACh and 5-HT on attention: these modulators exert opposite neurophysiological effects on the excitability of layer 6 pyramidal neurons in prefrontal cortex.
Author Contributions
DWS, MKT, DS, SV, KI and EKL designed the experiments; contributed to the revision of the article. DWS, MKT, DS, KI and SV performed the experiments and analyzed the data. DWS, MKT and EKL wrote the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Discovery Grant to EKL from the Natural Sciences and Engineering Research Council of Canada, the Canadian Institute for Health Research (MOP-89825), the Canada Research Chair in Developmental Cortical Physiology and the Canadian Foundation for Innovation. DWS was in part supported by a ASRP postdoctoral fellowship from the Alzheimer Society of Canada and MKT by a Canada Graduate Scholarship Banting and Best Doctoral Award from the Canadian Institutes of Health Research. We thank Ms. Duong “Izzy” Chu and Ms. Janice McNabb for expert technical assistance, as well as acknowledge the kind gift of ChAT-IRES-cre+/+ mice from Dr. Eric Turner of Seattle Children’s Research Institute Center for Integrative Brain Research and the University of Washington, Seattle, WA, USA.
References
Adamsen, D., Ramaekers, V., Ho, H. T., Britschgi, C., Rüfenacht, V., Meili, D., et al. (2014). Autism spectrum disorder associated with low serotonin in CSF and mutations in the SLC29A4 plasma membrane monoamine transporter (PMAT) gene. Mol. Autism 5:43. doi: 10.1186/2040-2392-5-43
Alitto, H. J., and Usrey, W. M. (2003). Corticothalamic feedback and sensory processing. Curr. Opin. Neurobiol. 13, 440–445. doi: 10.1016/s0959-4388(03)00096-5
Amargós-Bosch, M., Bortolozzi, A., Puig, M. V., Serrats, J., Adell, A., Celada, P., et al. (2004). Co-expression and in vivo interaction of serotonin1A and Serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb. Cortex 14, 281–299. doi: 10.1093/cercor/bhg128
Andjelic, S., Gallopin, T., Cauli, B., Hill, E. L., Roux, L., Badr, S., et al. (2009). Glutamatergic nonpyramidal neurons from neocortical layer VI and their comparison with pyramidal and spiny stellate neurons. J. Neurophysiol. 101, 641–654. doi: 10.1152/jn.91094.2008
Arnold, H. M., Burk, J. A., Hodgson, E. M., Sarter, M. J., and Bruno, J. P. (2002). Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience 114, 451–460. doi: 10.1016/s0306-4522(02)00292-0
Bailey, C. D., De Biasi, M., Fletcher, P. J., and Lambe, E. K. (2010). The nicotinic acetylcholine receptor α5 subunit plays a key role in attention circuitry and accuracy. J. Neurosci. 30, 9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010
Bari, A., Dalley, J. W., and Robbins, T. W. (2008). The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat. Protoc. 3, 759–767. doi: 10.1038/nprot.2008.41
Beacher, F. D., Gray, M. A., Minati, L., Whale, R., Harrison, N. A., and Critchley, H. D. (2011). Acute tryptophan depletion attenuates conscious appraisal of social emotional signals in healthy female volunteers. Psychopharmacology 213, 603–613. doi: 10.1007/s00213-010-1897-5
Béhuret, S., Deleuze, C., and Bal, T. (2015). Corticothalamic synaptic noise as a mechanism for selective attention in thalamic neurons. Front. Neural Circuits 9:80. doi: 10.3389/fncir.2015.00080
Bentley, P., Driver, J., and Dolan, R. J. (2011). Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog. Neurobiol. 94, 360–388. doi: 10.1016/j.pneurobio.2011.06.002
Bicks, L. K., Koike, H., Akbarian, S., and Morishita, H. (2015). Prefrontal cortex and social cognition in mouse and man. Front. Psychol. 6:1805. doi: 10.3389/fpsyg.2015.01805
Bland, S. T., Hargrave, D., Pepin, J. L., Amat, J., Watkins, L. R., and Maier, S. F. (2003). Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology 28, 1589–1596. doi: 10.1038/sj.npp.1300206
Buzsaki, G., Bickford, R. G., Ponomareff, G., Thal, L. J., Mandel, R., and Gage, F. H. (1988). Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J. Neurosci. 8, 4007–4026.
Carlen, M. (2017). What constitutes the prefrontal cortex? Science 358, 478–482. doi: 10.1126/science.aan8868
Celada, P., Puig, M. V., and Artigas, F. (2013). Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 7:25. doi: 10.3389/fnint.2013.00025
Chandler-Laney, P. C., Castaneda, E., Pritchett, C. E., Smith, M. L., Giddings, M., Artiga, A. I., et al. (2007). A history of caloric restriction induces neurochemical and behavioral changes in rats consistent with models of depression. Pharmacol. Biochem. Behav. 87, 104–114. doi: 10.1016/j.pbb.2007.04.005
Chen, L., Yin, D., Wang, T. X., Guo, W., Dong, H., Xu, Q., et al. (2016). Basal forebrain cholinergic neurons primarily contribute to inhibition of electroencephalogram delta activity, rather than inducing behavioral wakefulness in mice. Neuropsychopharmacology 41, 2133–2146. doi: 10.1038/npp.2016.13
Cohen, J. Y., Amoroso, M. W., and Uchida, N. (2015). Serotonergic neurons signal reward and punishment on multiple timescales. Elife 4:e06346. doi: 10.7554/eLife.06346
Contreras, D. (2004). Electrophysiological classes of neocortical neurons. Neural. Netw. 17, 633–646. doi: 10.1016/j.neunet.2004.04.003
Crandall, S. R., Cruikshank, S. J., and Connors, B. W. (2015). A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron 86, 768–782. doi: 10.1016/j.neuron.2015.03.040
Dalley, J. W., Theobald, D. E., Bouger, P., Chudasama, Y., Cardinal, R. N., and Robbins, T. W. (2004). Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-Saporin-induced lesions of the medial prefrontal cortex. Cereb. Cortex 14, 922–932. doi: 10.1093/cercor/bhh052
Daly, E., Deeley, Q., Hallahan, B., Craig, M., Brammer, M., Lamar, M., et al. (2010). Effects of acute tryptophan depletion on neural processing of facial expressions of emotions in humans. Psychopharmacology 210, 499–510. doi: 10.1007/s00213-010-1850-7
de Almeida, J., and Mengod, G. (2008). Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: implications for schizophrenia and its treatment. J. Neurochem. 107, 488–496. doi: 10.1111/j.1471-4159.2008.05649.x
Détári, L., and Vanderwolf, C. H. (1987). Activity of identified cortically projecting and other basal forebrain neurons during large slow waves and cortical activation in anaesthetized rats. Brain Res. 437, 1–8. doi: 10.1016/0006-8993(87)91521-6
Dougherty, J. D., Maloney, S. E., Wozniak, D. F., Rieger, M. A., Sonnenblick, L., Coppola, G., et al. (2013). The disruption of Celf6, a gene identified by translational profiling of serotonergic neurons, results in autism-related behaviors. J. Neurosci. 33, 2732–2753. doi: 10.1523/JNEUROSCI.4762-12.2013
Dunnett, S. B., Everitt, B. J., and Robbins, T. W. (1991). The basal forebrain-cortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trends. Neurosci. 14, 494–501. doi: 10.1016/0166-2236(91)90061-x
Elliott, E., Zahn, R., Deakin, J. F., and Anderson, I. M. (2011). Affective cognition and its disruption in mood disorders. Neuropsychopharmacology 36, 153–182. doi: 10.1038/npp.2010.77
Fallon, S., Shearman, E., Sershen, H., and Lajtha, A. (2007). Food reward-induced neurotransmitter changes in cognitive brain regions. Neurochem. Res. 32, 1772–1782. doi: 10.1007/s11064-007-9343-8
Frodl, T., Szyf, M., Carballedo, A., Ly, V., Dymov, S., Vaisheva, F., et al. (2015). DNA methylation of the serotonin transporter gene (SLC6A4) is associated with brain function involved in processing emotional stimuli. J. Psychiatry Neurosci. 40, 296–305. doi: 10.1503/jpn.140180
Fujino, K., Yoshitake, T., Inoue, O., Ibii, N., Kehr, J., Ishida, J., et al. (2002). Increased serotonin release in mice frontal cortex and hippocampus induced by acute physiological stressors. Neurosci. Lett. 320, 91–95. doi: 10.1016/s0304-3940(02)00029-0
Gallagher, P., Massey, A. E., Young, A. H., and McAllister-Williams, R. H. (2003). Effects of acute-tryptophan depletion on executive function in healthy male volunteers. BMC Psychiatry 3:10. doi: 10.1186/1471-244x-3-10
Golub, M. S., Hackett, E. P., Hogrefe, C. E., Leranth, C., Elsworth, J. D., and Roth, R. H. (2017). Cognitive performance of juvenile monkeys after chronic fluoxetine treatment. Dev. Cogn. Neurosci. 26, 52–61. doi: 10.1016/j.dcn.2017.04.008
Graf, H., Abler, B., Hartmann, A., Metzger, C. D., and Walter, M. (2013). Modulation of attention network activation under antidepressant agents in healthy subjects. Int. J. Neuropsychopharmacol. 16, 1219–1230. doi: 10.1017/s1461145712001368
Granon, S., Hardouin, J., Courtièr, A., and Poucet, B. (1998). Evidence for the involvement of the rat prefrontal cortex in sustained attention. Q. J. Exp. Psychol. B 51, 219–233.
Gritton, H. J., Howe, W. M., Mallory, C. S., Hetrick, V. L., Berke, J. D., and Sarter, M. (2016). Cortical cholinergic signaling controls the detection of cues. Proc. Natl. Acad. Sci. U S A 113, 1089–1097. doi: 10.1073/pnas.1516134113
Guillem, K., Bloem, B., Poorthuis, R. B., Loos, M., Smit, A. B., Maskos, U., et al. (2011). Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science 333, 888–891. doi: 10.1126/science.1207079
Hasselmo, M. E., and Sarter, M. (2011). Modes and models of forebrain cholinergic neuromodulation and cognition. Neuropsychopharmacology 36, 52–73. doi: 10.1038/npp.2010.104
Hedrick, T., and Waters, J. (2015). Acetylcholine excites neocortical pyramidal neurons via nicotinic receptors. J. Neurophysiol. 113, 2195–2209. doi: 10.1152/jn.00716.2014
Himmelheber, A. M., Sarter, M., and Bruno, J. P. (2000). Increases in cortical acetylcholine release during sustained attention performance in rats. Cogn. Brain Res. 9, 313–325. doi: 10.1016/s0926-6410(00)00012-4
Ivanova, E., Yee, W. C., and Sagdullaev, B. T. (2016). Leveraging optogenetic-based neurovascular circuit characterization for repair. Neurotherapeutics 13, 341–347. doi: 10.1007/s13311-015-0419-x
Jacobs, B. L., and Azmitia, E. C. (1992). Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229. doi: 10.1152/physrev.1992.72.1.165
Jäkälä, P., Sirviö, J., Jolkkonen, J., and Riekkinen, P. Jr., Acsady, L., Riekkinen, P. (1992). The effects of p-chlorophenylalanine-induced serotonin synthesis inhibition and muscarinic blockade on the performance of rats in a 5-choice serial reaction time task. Behav. Brain Res. 51, 29–40. doi: 10.1016/s0166-4328(05)80309-2
Kassam, S. M., Herman, P. M., Goodfellow, N. M., Alves, N. C., and Lambe, E. K. (2008). Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors. J. Neurosci. 28, 8756–8764. doi: 10.1523/JNEUROSCI.2645-08.2008
Kim, H., Ährlund-Richter, S., Wang, X., Deisseroth, K., and Carlén, M. (2016). Prefrontal parvalbumin neurons in control of attention. Cell 164, 208–218. doi: 10.1016/j.cell.2015.11.038
Knudsen, E. I. (2007). Fundamental components of attention. Annu. Rev. Neurosci. 30, 57–78. doi: 10.1146/annurev.neuro.30.051606.094256
Leiser, S. C., Li, Y., Pehrson, A. L., Dale, E., Smagin, G., and Sanchez, C. (2015). Serotonergic regulation of prefrontal cortex cortical circuitries involved in cognitive processing: a review of individual 5-HT receptor mechanisms and concerted effects of 5-HT receptors exemplified by the multimodal antidepressant Vortioxetine. ACS Chem. Neurosci. 6, 970–986. doi: 10.1021/cn500340j
Liu, R. J., Lambe, E. K., and Aghajanian, G. K. (2005). Somatodendritic autoreceptor regulation of serotonergic neurons: dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur. J. Neurosci. 21, 945–958. doi: 10.1111/j.1460-9568.2005.03930.x
Logue, S. F., and Gould, T. J. (2014). The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol. Biochem. Behav. 123, 45–54. doi: 10.1016/j.pbb.2013.08.007
Lottem, E., Lörincz, M. I., and Mainen, Z. F. (2016). Optogenetic activation of dorsal raphe serotonin neurons rapidly inhibits spontaneous but not odor-evoked activity in olfactory cortex. J. Neurosci. 36, 7–18. doi: 10.1523/JNEUROSCI.3008-15.2016
Madisen, L., Mao, T., Koch, H., Zhuo, J. M., Berenyi, A., Fujisawa, S., et al. (2012). A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802. doi: 10.1038/nn.3078
Mark, G. P., Rada, P. V., and Shors, T. J. (1996). Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Nat. Commun. 74, 767–774. doi: 10.1016/0306-4522(96)00211-4
Matias, S., Lottem, E., Dugue, G. P., and Mainen, Z. F. (2017). Activity patterns of serotonin neurons underlying cognitive flexibility. Elife 6:e20552. doi: 10.7554/eLife.20552
McGaughy, J., Dalley, J. W., Morrison, C. H., Everitt, B. J., and Robbins, T. W. (2002). Selective behavioral and neurochemical effect of cholinergic lesions produced by intrabasalis infusions of 192 IgG-Saporin on attentional performance in a five-choice serial reaction time task. J. Neurosci. 22, 1905–1913.
Mengod, G., Palacios, J. M., and Cortés, R. (2015). Cartography of 5-HT1A and 5-HT2A receptor subtypes in prefrontal cortex and its projections. ACS Chem. Neurosci. 6, 1089–1098. doi: 10.1021/acschemneuro.5b00023
Mercer, A., West, D. C., Morris, O. T., Kirchhecker, S., Kerkhoff, J. E., and Thomson, A. M. (2005). Excitatory connections made by presynaptic cortico-cortical pyramidal neurons in layer 6 of the neocortex. Cereb. Cortex 15, 1485–1496. doi: 10.1093/cercor/bhi027
Meunier, C. N., Chameau, P., and Fossier, P. M. (2017). Modulation of synaptic plasticity in the cortex needs to understand all the players. Front. Synaptic. Neurosci. 9:2. doi: 10.3389/fnsyn.2017.00002
Miller, E. K., and Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167
Muir, J. L., Dunnett, S. B., Robbins, T. W., and Everitt, B. J. (1992). Attentional functions of the forebrain cholinergic systems: effects of intraventricular hemicholinium, physostigmine, basal forebrain lesions and intracortical grafts on a multiple-choice serial reaction time task. Exp. Brain Res. 89, 611–622. doi: 10.1007/bf00229886
Muir, J. L., Everitt, B. J., and Robbins, T. W. (1996). The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb. Cortex 6, 470–481. doi: 10.1093/cercor/6.3.470
Muir, J. L., Page, K. J., Sirinathsinghji, D. J., Robbins, T. W., and Everitt, B. J. (1993). Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory, and attention. Behav. Brain Res. 57, 123–131. doi: 10.1016/0166-4328(93)90128-D
Muller, C. L., Anacker, A. M. J., and Veenstra-VanderWeele, J. (2016). The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience 321, 24–41. doi: 10.1016/j.neuroscience.2015.11.010
Muzerelle, A., Scotto-Lomassese, S., Bernard, J. F., Soiza-Reilly, M., and Gaspar, P. (2016). Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct. Funct. 221, 535–561. doi: 10.1007/s00429-014-0924-4
Newman, L. A., and McGaughy, J. (2008). Cholinergic deafferentation of prefrontal cortex increases sensitivity to cross-modal distractors during a sustained attention task. J. Neurosci. 28, 2642–2650. doi: 10.1523/JNEUROSCI.5112-07.2008
Nonkes, L. J., van de Vondervoort, I. I., de Leeuw, M. J., Wilaars, L. P., Maes, J. H., and Homberg, J. R. (2012). Serotontin transporter knockout rats show improved strategy set-shifting and reduced latent inhibition. Learn. Mem. 19, 190–193. doi: 10.1101/lm.025908.112
O’Neill, B., Patel, J. C., and Rice, M. E. (2017). Characterization of optically and electrically evoked dopamine release in striatal slices from digenic knock-in mice with DAT-driven expression of channelrhodopsin. ACS Chem. Neurosci. 8, 310–319. doi: 10.1021/acschemneuro.6b00300
Olsen, S. R., Bortone, D. S., Adesnik, H., and Scanziani, M. (2012). Gain control by layer six in cortical circuits of vision. Nature 483, 47–54. doi: 10.1038/nature10835
Osinsky, R., Reuter, M., Küpper, Y., Schmitz, A., Kozyra, E., Alexander, N., et al. (2008). Variation in the serotonin transporter gene modulates selective attention to threat. Emotion 8, 584–588. doi: 10.1037/a0012826
Pang, K., Williams, M. J., Egeth, H., and Olton, D. S. (1993). Nucleus basalis magnocellularis and attention: effects of muscimol infusions. Behav. Neurosci. 107, 1031–1038. doi: 10.1037/0735-7044.107.6.1031
Parikh, V., Kozak, R., Martinez, V., and Sarter, M. (2007). Prefrontal acetylcholine release controls cue detection on multiple time scales. Neuron 56, 141–154. doi: 10.1016/j.neuron.2007.08.025
Passetti, F., Dalley, J. W., O’Connell, M. T., Everitt, B. J., and Robbins, T. W. (2000). Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur. J. Neurosci. 12, 3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x
Paxinos, G., and Franklin, K. B. J. (2001). The Mouse Brain in Stereotaxic Coordinates. 2nd Edn. San Diego, CA: Academic Press.
Poorthuis, R. B., Bloem, B., Schak, B., Wester, J., de Kock, C. P., and Mansvelder, H. D.. (2013). Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb. Cortex 23, 148–161. doi: 10.1093/cercor/bhr390
Ramaekers, J. G., Muntjewerff, N. D., and O’Hanlon, J. F. (1995). A comparative study of acute and subchronic effects of dothiepin, fluoxetine and placebo on psychomotor and actual driving performance. Br. J. Clin. Pharmac. 39, 397–404. doi: 10.1111/j.1365-2125.1995.tb04468.x
Ranade, S. P., and Mainen, Z. F. (2009). Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor and reward events. J. Neurophysiol. 102, 3026–3037. doi: 10.1152/jn.00507.2009
Riedel, W. J., Eikmans, K., Heldens, A., and Schmitt, J. A. (2005). Specific serotonergic reuptake inhibition impairs vigilance performance acutely and after subchronic treatment. J. Psychopharmacol. 19, 12–20. doi: 10.1177/0269881105048887
Riedel, W. J., Klaassen, T., Deutz, N. E., van Someren, A., and van Praag, H. M. (1999). Tryptophan depletion in normal volunteers produces selective impairment in memory consolidation. Psychopharmacology 141, 362–369. doi: 10.1007/s002130050845
Robbins, T. W., Everitt, B. J., Marston, H. M., Wilkinson, J., Jones, G. H., and Page, K. J. (1989). Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behav. Brain Res. 25, 221–240. doi: 10.1016/s0166-4328(89)80143-3
Robbins, T. W., and Roberts, A. C. (2007). Differential regulation of fronto-executive function by monoamines and acetylcholine. Cereb. Cortex 17, 1151–1160. doi: 10.1093/cercor/bhm066
Rossi, J., Balthasar, N., Olson, D., Scott, M., Berglund, E., Lee, C. E., et al. (2011). Melanocortin-4-receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204. doi: 10.1016/j.cmet.2011.01.010
Rossi, A. F., Pessoa, L., Desimone, R., and Ungerleider, L. G. (2009). The prefrontal cortex and the executive control of attention. Exp. Brain Res. 192, 489–497. doi: 10.1007/s00221-008-1642-z
Sänger, J., Bechtold, L., Schoofs, D., Blaszkewicz, M., and Wascher, E. (2014). The influence of acute stress on attention mechanisms and its electrophysiological correlates. Front. Behav. Neurosci. 8:353. doi: 10.3389/fnbeh.2014.00353
Santana, N., Bortolozzi, A., Serrats, J., Mengod, G., and Artigas, F. (2004). Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex 14, 1100–1109. doi: 10.1093/cercor/bhh070
Sargin, D., Oliver, D. K., and Lambe, E. K. (2016). Chronic social isolation reduces 5-HT neuronal activity via upregulated SK3 calcium-activated potassium channels. Elife 5:e21416. doi: 10.7554/eLife.21416
Sarter, M., Givens, B., and Bruno, J. P. (2001). The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res. Rev. 35, 146–160. doi: 10.1016/s0165-0173(01)00044-3
Sarter, M., Lustig, C., Berry, A. S., Gritton, H., Howe, W. M., and Parikh, V. (2016). What do phasic cholinergic signals do? Neurobiol. Learn. Mem. 130, 135–141. doi: 10.1016/j.nlm.2016.02.008
Schmitt, J. A., Jorissen, B. L., Sobczak, S., van Boxtel, M. P., Hogervorst, E., Deutz, N. E., et al. (2000). Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J. Psychopharmacol. 14, 21–29. doi: 10.1177/026988110001400102
Schmitt, J. A., Ramaekers, J. G., Kruizinga, M. J., van Boxtel, M. P., Vuurman, E. F., and Riedel, W. J. (2002). Additional dopamine reuptake inhibition attenuates vigilance impairment induced by serotonin reuptake inhibition in man. J. Psychopharmacol. 16, 207–214. doi: 10.1177/026988110201600303
Scott, M. M., Wylie, C. J., Lerch, J. K., Murphy, R., Lobur, K., Herlitze, S., et al. (2005). A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc. Natl. Acad. Sci. U S A 102, 16472–16477. doi: 10.1073/pnas.0504510102
Sherman, S. M. (2007). The thalamus is more than just a relay. Curr. Opin. Neurobiol. 17, 417–422. doi: 10.1016/j.conb.2007.07.003
Steckler, T., and Sahgal, A. (1995). The role of serotonergic-cholinergic interactions in the mediation of cognitive behaviour. Behav. Brain Res. 67, 165–199. doi: 10.1016/0166-4328(94)00157-b
Szczepanski, S. M., and Knight, R. T. (2014). Insights into human behavior from lesions to the prefrontal cortex. Neuron 83, 1002–1018. doi: 10.1016/j.neuron.2014.08.011
Thomson, A. M. (2010). Neocortical layer 6, a review. Front. Neuroanat. 4:13. doi: 10.3389/fnana.2010.00013
Tian, M. K., Bailey, C. D., De Biasi, M., Picciotto, M. R., and Lambe, E. K. (2011). Plasticity of prefrontal attention circuitry: upregulated muscarinic excitability in response to decreased nicotinic signaling following deletion of α5 or β2 subunits. J. Neurosci. 31, 16458–16463. doi: 10.1523/JNEUROSCI.3600-11.2011
Tian, M. K., Schmidt, E. F., and Lambe, E. K. (2016). Serotonergic suppression of mouse prefrontal circuits implicated in task attention. eNeuro 3:ENEURO.0269-16.2016. doi: 10.1523/ENEURO.0269-16.2016
Uylings, H. B., Groenewegen, H. J., and Kolb, B. (2003). Do rats have a prefrontal cortex? Behav. Brain Res. 146, 3–17. doi: 10.1016/j.bbr.2003.09.028
van Aerde, K. I., and Feldmeyer, D. (2015). Morphological and physiological characterization of pyramidal neuron subtypes in rat medial prefrontal cortex. Cereb. Cortex 25, 788–805. doi: 10.1093/cercor/bht278
Voytko, M. L., Olton, D. S., Richardson, R. T., Gorman, L. K., Tobin, J. R., and Price, D. L. (1994). Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J. Neurosci. 14, 167–186.
Wada, E., Wada, K., Boulter, J., Deneris, E., Heinemann, S., Patrick, J., et al. (1989). Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J. Comp. Neurol. 284, 314–335. doi: 10.1002/cne.902840212
Wallace, T. L., and Bertrand, D. (2013). Importance of nicotinic acetylcholine receptor system in the perfrontal cortex. Biochem. Pharmacol. 85, 1713–1720. doi: 10.1016/j.bcp.2013.04.001
Watts, J., and Thomson, A. M. (2005). Excitatory and inhibitory connections show selectivity in the neocortex. J. Physiol. 562, 89–97. doi: 10.1113/jphysiol.2004.076984
West, D. C., Mercer, A., Kirchhecker, S., Morris, O. T., and Thomson, A. M. (2006). Layer 6 cortico-thalamic pyramidal cells preferentially innervate interneurons and generate facilitations EPSPs. Cereb. Cortex 16, 200–211. doi: 10.1093/cercor/bhi098
Wimmer, R. D., Schmitt, L. I., Davidson, T. J., Nakajima, M., Deisseroth, K., and Halassa, M. M. (2015). Thalamic control of sensory selection in divided attention. Nature 526, 705–709. doi: 10.1038/nature15398
Wingen, M., Kuypers, K. P., and Ramaekers, J. G. (2007). The role of 5-HT1A and 5-HT2A receptors in attention and motor control: a mechanistic study in healthy volunteers. Psychopharmacology 190, 391–400. doi: 10.1007/s00213-006-0614-x
Wingen, M., Kuypers, K. P., van de Ven, V., Formisano, E., and Ramaekers, J. G. (2008). Sustained attention and serotonin: a pharmaco-fMRI study. Hum. Psychopharmacol. 23, 221–230. doi: 10.1002/hup.923
Winstanley, C. A., Chudasama, Y., Dalley, J. W., Theobald, D. E., Glennon, J. C., and Robbins, T. W. (2003). Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Pyschopharmacology 167, 304–314. doi: 10.1007/s00213-003-1398-x
Zhao, S., Ting, J. T., Atallah, H. E., Qiu, L., Tan, J., Gloss, B., et al. (2011). Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat. Methods 8, 745–752. doi: 10.1038/nmeth.1668
Keywords: acetylcholine, serotonin, attention, stress, prefrontal, corticothalamic, optogenetics, electrophysiology
Citation: Sparks DW, Tian MK, Sargin D, Venkatesan S, Intson K and Lambe EK (2018) Opposing Cholinergic and Serotonergic Modulation of Layer 6 in Prefrontal Cortex. Front. Neural Circuits 11:107. doi: 10.3389/fncir.2017.00107
Received: 30 September 2017; Accepted: 13 December 2017;
Published: 04 January 2018.
Edited by:
Amy F. T. Arnsten, Yale School of Medicine, Yale University, United StatesReviewed by:
Anita Disney, Vanderbilt University, United StatesLi Jiang, Stony Brook University, United States
Angela Roberts, University of Cambridge, United Kingdom
Copyright © 2018 Sparks, Tian, Sargin, Venkatesan, Intson and Lambe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Evelyn K. Lambe, evelyn.lambe@utoronto.ca
† These authors have contributed equally to this work as co-first authors.
‡ Present address: Derya Sargin, Hotchkiss Brain Institute and the Department of Physiology and Pharmacology, University of Calgary, Calgary, AB, Canada
 Daniel W. Sparks
Daniel W. Sparks Michael K. Tian
Michael K. Tian Derya Sargin
Derya Sargin Sridevi Venkatesan
Sridevi Venkatesan Katheron Intson
Katheron Intson Evelyn K. Lambe
Evelyn K. Lambe