Forearm Flexor Muscles in Children with Cerebral Palsy Are Weak, Thin and Stiff
- 1Department of Women's and Children's Health, Karolinska Institute, Stockholm, Sweden
- 2Division of Biokinesiology and Physical Therapy, University of Southern California, Los Angeles, CA, USA
- 3Department of Neurology, Karolinska Hospital Huddinge, Stockholm, Sweden
- 4Karolinska Institute, Stockholm, Sweden
- 5Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, USA
- 6Department of Pediatric orthopedic Surgery, Astrid Lindgren's Children Hospital, Stockholm, Sweden
Children with cerebral palsy (CP) often develop reduced passive range of motion with age. The determining factor underlying this process is believed to be progressive development of contracture in skeletal muscle that likely changes the biomechanics of the joints. Consequently, to identify the underlying mechanisms, we modeled the mechanical characteristics of the forearm flexors acting across the wrist joint. We investigated skeletal muscle strength (Grippit®) and passive stiffness and viscosity of the forearm flexors in 15 typically developing (TD) children (10 boys/5 girls, mean age 12 years, range 8–18 yrs) and nine children with CP Nine children (6 boys/3 girls, mean age 11 ± 3 years (yrs), range 7–15 yrs) using the NeuroFlexor® apparatus. The muscle stiffness we estimate and report is the instantaneous mechanical response of the tissue that is independent of reflex activity. Furthermore, we assessed cross-sectional area of the flexor carpi radialis (FCR) muscle using ultrasound. Age and body weight did not differ significantly between the two groups. Children with CP had a significantly weaker (−65%, p < 0.01) grip and had smaller cross-sectional area (−43%, p < 0.01) of the FCR muscle. Passive stiffness of the forearm muscles in children with CP was increased 2-fold (p < 0.05) whereas viscosity did not differ significantly between CP and TD children. FCR cross-sectional area correlated to age (R2 = 0.58, p < 0.01), body weight (R2 = 0.92, p < 0.0001) and grip strength (R2 = 0.82, p < 0.0001) in TD children but only to grip strength (R2 = 0.60, p < 0.05) in children with CP. We conclude that children with CP have weaker, thinner, and stiffer forearm flexors as compared to typically developing children.
Introduction
An insult to the immature, developing brain before the age of two results in a condition clinically referred to as cerebral palsy (CP), characterized by motor impairment (Rosenbaum et al., 2007). Despite that the brain injury is non-progressive, motor function commonly deteriorates over time and a progressive contracture formation, i.e., a decrease in passive range of motion (pROM), is common (Hagglund and Wagner, 2011). For many children, surgical treatment is needed to restore and preserve musculoskeletal function. Clinical experience suggests that contracture formation is due to shortening/stiffening of the musculotendinous complex, as the range of motion of the joint often is practically normal when tendons of contracted muscles are cut during surgery. What causes the increased stiffness and shortness of the muscle is not known, but clinically we know that it affects joint biomechanics.
Children with CP generally have less skeletal muscle mass as compared to typically developing (TD) children (Barrett and Lichtwark, 2010). Recent reports have highlighted stunted growth in the lower limb as a contributing factor to contracture development (Gough and Shortland, 2012) and slowed growth rate has been detected as early as 15-months of age (Herskind et al., 2015). Several investigators have described increased passive stiffness of the calf muscle in children with CP and different tests have shown that the calf muscle is 22–120% stiffer in CP children compared to TD (Ross et al., 2011; de Gooijer-van de Groep et al., 2013; Geertsen et al., 2015). The process starts early and increased whole muscle passive stiffness in the calf has been described as early as 3 years of age (Willerslev-Olsen et al., 2013). Biochemical studies of muscle in CP have shown an increased content of intramuscular collagen (Booth et al., 2001), with an increased amount of connective tissue around fiber bundles i.e., a thickening of the perimysial extracellular matrix (de Bruin et al., 2014). The perimysium is considered to be in a physical continuum with the tendon (Passerieux et al., 2007). Thus, marked collagen deposition in the perimysium could potentially offer an increased resistance to passive stretch.
Less information is available on how skeletal muscle size correlates to joint biomechanics and muscle function for the upper limb. This is unfortunate as the clinical importance of accurate information on skeletal muscle function and biomechanical properties prior to for example tendon transfer surgery of the wrist is of great importance and a guide in clinical decision making. Therefore, we aimed to model the mechanical characteristics of the forearm flexors acting across the wrist joint in children with CP during passive stretch. Second, we aimed to correlate skeletal muscle size and biomechanics to age, body weight and strength.
Materials and Methods
Participants
Nine children [6 boys/3 girls, mean age 11 ± 3 years (yrs), range 7–15 yrs] with CP (3 bilateral/6 unilateral) scheduled for upper limb surgery at Karolinska University Hospital, Stockholm, Sweden were consecutively included in the study. Inclusion criteria were confirmed CP diagnosis, age between 7 and 18 yrs, and cognitive ability to follow instructions. The children with CP were classified according to the Gross Motor Function Classification Scale (GMFCS) and the Manual ability classification system (MACS). GMFCS I means that the child can walk indoors and outdoors without limitations, and children in GMFCS V has no means of independent mobility. The GMFCS classification has been shown to be the best predictor of treatment results in CP (Shore et al., 2012). Eight of the children were classified as GMFCS I and one child as GMFCS III. The GMFCS III child needed a walking aid indoors and a wheel chair outdoors. MACS I means that the child handles objects easily and successfully with minimal limitation in speed and accuracy, and children in MACS V do not handle objects and has limited ability to perform even simple actions (requires total assistance). The children in the current study were classified as follows; MACS I three children, MACS II four children and MACS III two children.
As controls, a convenience sample (n = 15) of TD children was recruited (10 boys/5 girls, mean age 12 yrs, range 8–18 yrs). This study was carried out in accordance with the recommendations of the Regional Ethical Review Board in Stockholm with written informed consent obtained from all subjects and minimum one parent per subject in accordance with the Declaration of Helsinki. The protocol was approved by the Regional Ethical Review Board in Stockholm.
Clinical Assessment and Strength Measurements
Passive range of motion (pROM) of the wrist was assessed by a physiotherapist or trained medical student using a goniometer. All children were thereafter tested for maximal grip strength using a dedicated device, Grippit® (AB Detektor, Göteborg, Sweden). The arm of the subject was placed in a neutral position (thumb upwards) with the ulnar side resting in a padded semicircular plastic tube. All subjects received verbal encouragement and were instructed to grip the handle as hard as they could and maintain the same intensity for a 10 s period. The handle size was adjustable and each child was allowed to independently choose the handle size.
Neuroflexor Measurements and Data Processing
For wrist stiffness measurements, we used the NeuroFlexor® (NeuroFlexor® Scientific, Release 0.0.6, Aggero MedTech AB, Solna, Sweden). With the elbow in 90°, the hand was placed on the NeuroFlexor® platform, so that the axes of rotation of the wrist and the platform were aligned. Subjects were instructed to remain relaxed throughout the experiments and the device applied ramp-and-hold perturbations with a velocity of 5°/s, by extending the wrist from 20° palmar flexion to 30° extension. It recorded the angle of the wrist and the forces exerted by the limb and device. For each subject, we recorded 5 trials. We also recorded a trial following removal of the subject's hand from the device (no-hand trial) to account for forces generated by the mechanical properties of the apparatus only.
We subtracted the no-hand force from the with-hand forces and used a linear least-squares technique to identify the biomechanical model (Schouten et al., 2008; Meskers et al., 2015):
where FWH(t) and FNH(t) are the recorded forces in the with-hand and no-hand trials, m is the mass of the hand (point mass), l is the length of the hand from the wrist joint, θ(t) is the recorded wrist angle and θ′(t), θ″(t) are its first and second derivatives (angular velocity and acceleration) computed numerically by differentiating the recorded joint angle, g is the gravitational acceleration and finally K and B are the joint stiffness and viscous parameters, respectively. The second and third-order power of joint angles (θ2(t), θ3(t)) were also included in the model to account for nonlinear changes of joint biomechanics as a function of joint angle (Sobhani Tehrani et al., 2013). The main assumption of the model is that the neural component of the force is negligible, which is a fair assumption as the velocity of perturbation was low enough to avoid evoking reflex responses (Jalaleddini et al., 2016). Thus, the muscle stiffness we estimate and report is the instantaneous mechanical response of the tissue that is independent of reflex activity. From this model, we quantified and reported the mean and standard deviation of the stiffness and viscous parameters across the trials. Figure 1 shows typical measured and predicted forces using the biomechanical model. The biomechanical model accounted for 95.5 ± 3.6% of the variance in measured force, on average.
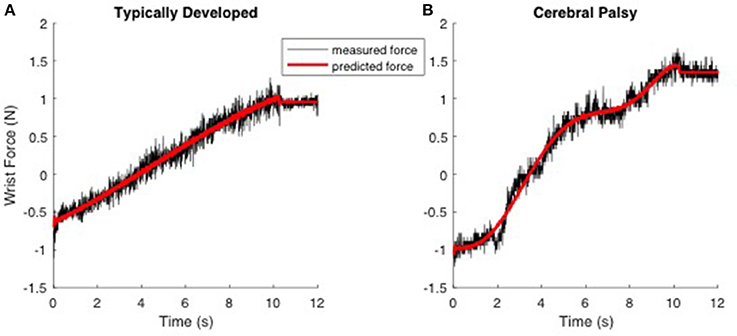
Figure 1. Typical trials demonstrating the prediction of the measured force using the biomechanical model in response to ramp-and-hold perturbation of 50 degrees with a low velocity (5°/s) experiment. Left; (A) TD. Right; (B) CP. The hand is moving from time 0 to 10 s. Data was recorded for another 2 s (time 10–12 s). The black and red line represents measured and predicted forces, respectively.
Skeletal Muscle Ultra Sound Assessment and Image Processing
The cross sectional area of the flexor carpi radialis muscle (FCR) was measured with B-mode ultrasonographic equipment with a linear probe (BK medical Flex focus 1202, 8670, scanning frequency 12 MHz Herlev, Denmark). The subjects were instructed to place their elbow on a height adjustable table with the dorsum of the hand toward the examiner and the fingers extended. If this position was difficult for the child to maintain, the caretaker was instructed to assist and hold the arm in the above-described position. Then, the distance from the olecranon to the styloid processes of the ulna was determined using a measuring tape. Skin markings were made at the transition between the proximal and middle third of the forearm, using a black permanent marker, to ensure correct placement of the ultrasound probe. During the ultrasound examination the subject was sitting in a standardized position with the dorsum of the forearm resting flat on a height adjustable table, the elbow at approximately 60° of flexion, arm supinated and fingers extended. Following generous application of water-soluble gel (Gurò Medigel, Gurò s.a.s.—Catenanuova (En)—Italy) the head of the ultrasound probe was placed proximal and parallel to the line marking, and at a right angle to the radius and ulna. The interfaces between subcutaneous adipose tissue and muscle tissue, between muscle tissue and fascia and between muscle tissue and bone were identified on the ultrasonic image. The border of the muscle was outlined by free hand, and the supplied software in the ultrasonograph was used to calculate the cross-sectional area.
Statistical Analysis
Values are reported as means ± SD. Differences in age, body weight, isometric grip strength, skeletal muscle cross sectional area, muscle passive stiffness and viscosity were investigated by the Mann-Whitney-Wilcoxon test. Linear regression was used to investigate correlations between FCR area and age, body weight, grip strength and passive stiffness and viscosity, respectively. Significance level was set at p < 0.05 for all statistical comparisons.
Results
No difference was seen between the groups with respect to age (CP 11.3 yrs ± 3.1 vs. TD 12.3 yrs ± 3.7, ns) and body weight (CP 46.8 ± 26.1 kg vs. TD 44.7 ± 14.9 kg, ns). FCR cross sectional area was 43% smaller in children with CP as compared to TD children (CP 0.84 cm2 ± 0.38 vs. TD 1.47 cm2 ± 0.75, p < 0.01). As expected, grip strength (10 s isometric contraction) was also significantly lower, −65% in CP as compared to TD children (CP 58.3N ± 32.1 vs. TD 167.5N ± 93.5, p < 0.01, Figure 2).
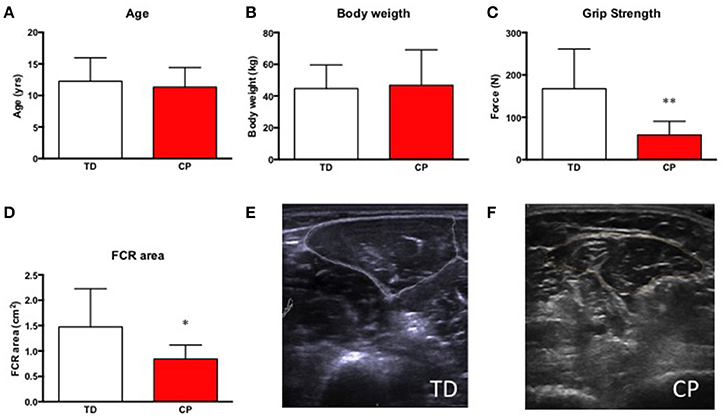
Figure 2. (A) Age in years of included research subjects, typically developed children (TD) and children with cerebral palsy (CP). (B) Body weight in kg in TD and CP. (C) Grip strength (N) in TD and CP. **Denotes significantly different from TD, p < 0.01. (D) Cross-sectional area of the flexor carpi radialis muscle (FCR) in cm2 in TD and CP. *Denotes significantly different from TD, p < 0.05. For all bar graphs, TD (white) & CP (red). (E,F) Representative ultrasound images of forearm muscles in TD (E) and CP (F) children. Encircled areas represent FCR.
Skeletal muscle passive stiffness was increased 2-fold in CP as compared to TD (CP 4.65 ± 3.42 vs. TD 1.96 ± 0.62, p < 0.05) whereas skeletal muscle viscosity didn't differ significantly between the groups (CP 1.71 ± 1.22 vs. 1.27 ± 0.86, Figure 3).
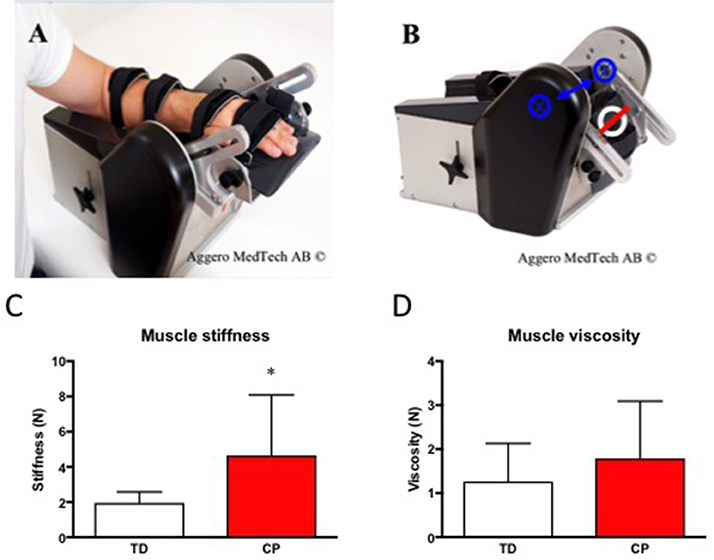
Figure 3. (A) The NeuroFlexor® with hand at 30° extension. (B) The NeuroFlexor® at 20° Flexion. Blue arrow and crossed rings highlight the axis of rotation. White ring highlights the position of the force sensor. The red line was used to localize the center of the force sensor. (C) Skeletal muscle passive stiffness (N) in TD and CP. *Denotes significantly different from TD, p < 0.05. (D) Skeletal muscle viscosity (N) in TD and CP. For all bar graphs, TD (white) & CP (red). Photos in (A,B) were used with permission from Aggero Medtech AB.
Skeletal muscle size correlated to age (R2 = 0.58, p < 0.01), body weight (R2 = 0.92, p < 0.0001) and strength (R2 = 0.58, p < 0.01) in TD children. Interestingly, the same relationship between skeletal muscle size did not correlate to age and body weight in children with CP, however still showing a significant correlation to strength (R2 = 0.60, p < 0.05). Skeletal muscle size did not correlate to passive stiffness or viscosity in CP or in TD children (Figure 4).
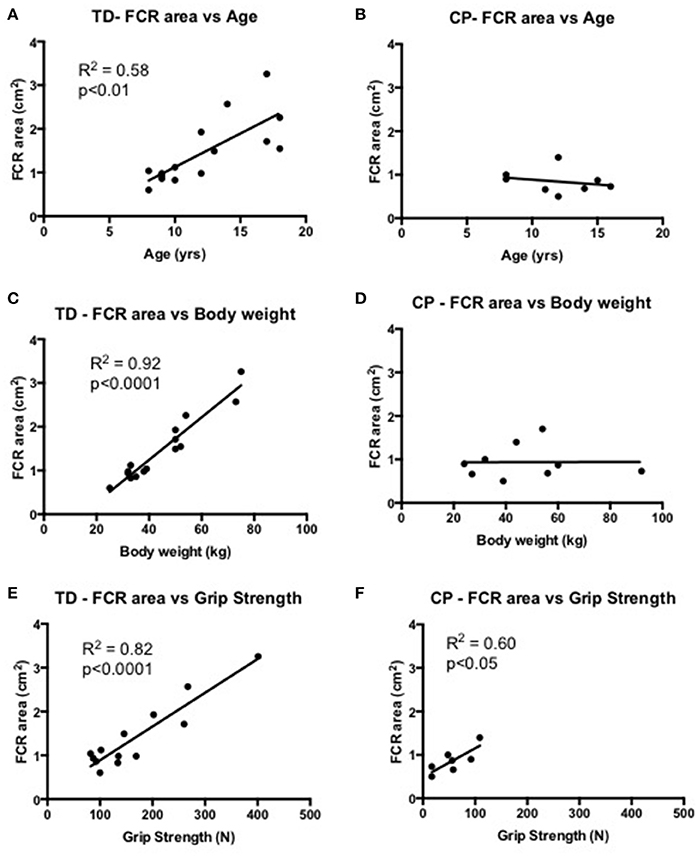
Figure 4. (A,B) Correlational analysis of the flexor carpi radialis muscle (FCR) cross-sectional area (cm2) and age (yrs) in TD (graph A) and CP (graph B). (C,D) Correlational analysis of FCR cross-sectional area (cm2) and body weight (kg) in TD (graph C) and CP (graph D). (E,F) Correlational analysis of FCR cross-sectional area (cm2) and grip strength (N) in TD (graph E) and CP (graph F). R2 and significance level indicated in each graph when relevant.
Discussion
This study shows that forearm flexor muscles in children with CP are stiffer as compared to TD children. Similar findings have been described for the lower limb, specifically the ankle flexors. Previous reports have estimated the passive tension to be 22–120% higher in CP for the ankle flexor muscles (Ross et al., 2011; de Gooijer-van de Groep et al., 2013; Geertsen et al., 2015). Thus, our finding of a 2-fold increase in skeletal muscle stiffness of the forearm flexors is comparable to data on weight bearing muscles of the lower limb. Information on the current status of the muscle is of great clinical importance when planning treatments, such as stretching, use of splints and surgical interventions e.g., tendon transfer surgery. Tendon lengthening, rerouting and transfer in the upper limb aim to balance the muscles of the wrist, so that the wrist can be maintained in a functional neutral position. All these surgical techniques have a component of dosing—how tight should the tendon transfer be, how long the tendon lengthening? This dosing is highly dependent on how stiff the transferred or lengthened muscle is. Long-term results are difficult to predict, as there often is a progression of contracture formation and stiffness. Abnormalities such as contracture and stiffness in even a few muscles will add yet another obstacle to smooth and efficient movements (Keenan et al., 2009; Kutch and Valero-Cuevas, 2011; Valero-Cuevas et al., 2015) which can progress very quickly in these children.
Spastic CP is characterized by a hyperexcitibility of the stretch reflex (Lance, 1980). Due to the fact that many children with spastic CP eventually develop skeletal muscle contractures, spasticity has been believed to be causative and clinical practice has up until recently been focused on spasticity reducing therapies for the preservation of motor function. However, recent reports have clearly demonstrated that despite good control of spasticity and reduction of muscle tone, by intramuscular botulinum toxin injections (chemical denervation) or selective dorsal rhizotomy that disrupts the reflex loop, skeletal muscle contracture formation progresses (Alhusaini et al., 2011; Tedroff et al., 2014). The poor effect of spasticity reduction on contracture development has raised the question of whether other factors such as growth disturbances and/or alterations in muscle composition are of greater mechanistic importance. Furthermore, recent findings suggest an overestimation of the contribution of spasticity to increased passive tension in muscle of young children with CP and at the same time an underestimation of the presence of contractures (Willerslev-Olsen et al., 2013; Herskind et al., 2015). In a large population study of children with CP, spasticity has been shown to peak around 4 years of age and thereafter decreases and level out at 12 years of age, whereas reductions in passive range of motion i.e., skeletal muscle contractures is progressive (Hagglund and Wagner, 2011). Therefore, we have chosen to focus on the instantaneous mechanical response of the muscle that is independent of reflex activity.
Muscle fiber size increases from 10–12 μm2 at birth to 40–60 μm2 after puberty (Oertel, 1988). Lexell et al have shown that skeletal muscle fiber size continuously increases with age up until and shortly after puberty. The same study also found a close relationship between fiber size and muscle cross sectional area (Lexell et al., 1992). Children with CP have reduced growth in general (Day et al., 2007). Skeletal muscle growth rate is reduced, evident as early as at 15-months of age (Herskind et al., 2015) and without showing any tendency to catch up later in life (Malaiya et al., 2007; Barber et al., 2011, 2016; Noble et al., 2014b). Just as for passive stiffness of skeletal muscles, deficit in skeletal muscle size in children with CP has so far only been described for the lower limb. The cross sectional area of the FCR muscle was significantly smaller in children with CP as compared to TD children. We have shown in previous studies that wrist flexors in children with CP have a greater fiber size variability and altered myosin composition compared to TD children (Ponten and Stal, 2007). In addition, the sarcomere, the smallest contractile unit of the muscle, has altered properties in CP (Ponten et al., 2007). By using laser diffraction methodology, we have measured sarcomere lengths of wrist flexors intra-operatively during tendon transfer surgeries. With the wrist held in neutral position, the sarcomeres were longer in CP compared to control, and sarcomeres were also longer—i.e., more stretched out- the worse the wrist contracture was (Ponten et al., 2007). This means that the wrist extension movement by the NeuroFlexor® likely results in extreme stress of the sarcomeres, with less overlap of the actin and myosin filaments and more stress on the perimyseal collagen surrounding the fiber bundles (de Bruin et al., 2014). In our study population the cross sectional area of the wrist flexor FCR, as determined by ultrasound, was significantly smaller in CP as compared to TD children and did not correlate to age or body weight. This implies that the differing brain damage the children with CP have, has a greater impact on muscle growth than age and body size. This further supports the impression of great heterogeneity within CP previously reported (Handsfield et al., 2016) and underscores the need for structured, individualized longitudinal follow-up.
Increased skeletal muscle echo intensity as measured by ultrasound has previously been used to infer altered skeletal muscle composition (Heckmatt et al., 1982; Nielsen et al., 2006). Pitcher et al. recently investigated the gastrocnemius muscle of 40 children with cerebral palsy and compared their findings to typically developed age matched controls (n = 12) (Pitcher et al., 2015). They show increased skeletal muscle echo intensity in CP and suggest that the underlying cause is due to increased content of non-contractile tissue e.g., collagen. Biochemical analysis of hydroxyproline content, a measure of intramuscular collagen content, correlates to spasticity severity (muscle tone as assessed by Modified Ashworth Scale, MAS) in children with CP (Booth et al., 2001). Staining muscle collagen using Sirius red has in CP shown increased amount of perimyseal collagen surrounding muscle fiber bundles (de Bruin et al., 2014). Similarity, MRI assessment of the lower limb indicates that young adults with CP have higher intramuscular fat content as compared to healthy controls (Noble et al., 2014a). Thus, it's without a doubt that skeletal muscle in individuals with CP with time contains an increased amount of non-contractile material. We interpret this as an indicator of the progressive remodeling in CP muscle. Despite that CP is defined as a non-progressive disease, skeletal muscle pathophysiology seems to continuously worsen over time.
We conclude that children with CP have weaker, thinner and stiffer forearm flexors as compared to typically developed children.
Author Contributions
FvW: Performed experiments, analyzed data, drafted manuscript (MS), designed figures, finalized MS, approved final version of MS. KJ: Analyzed data, drafted MS, designed figures, finalized MS, approved final version of MS. BE: Performed experiments, designed figures, finalized MS, approved final version of MS. JF: Performed experiments, finalized MS, approved final version of MS. FJVC: Conception of study, analyzed data, approved final version of MS. EP: Conception of study, drafted MS, finalized MS, approved final version of MS.
Funding
The research reported herein was supported by grants from Sunnerdahls handikapp Stiftelse, Norrbacka-Eugeniastiftelsen and Stiftelsen Samariten to FvW; Linnea and Josef Carlssons Stiftelse, Stiftelsen Promobilia and by grants provided by the Stockholm County Council (ALF project) to EP. This material is in part based upon work supported by NIH Grant R01-052345, NIH Grant R01-050520 to FJVC. This project is also supported by the Postdoctoral Research Scholarship from Fonds de Recherche du Québec- Nature et Technologies to KJ.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to Johan Gäverth, Påvel Lindberg, Jeanette Plantin and Ruoli Wang for their valuable comments and advice.
References
Alhusaini, A. A., Crosbie, J., Shepherd, R. B., Dean, C. M., and Scheinberg, A. (2011). No change in calf muscle passive stiffness after botulinum toxin injection in children with cerebral palsy. Dev. Med. Child Neurol. 53, 553–558. doi: 10.1111/j.1469-8749.2011.03930.x
Barber, L. A., Read, F., Lovatt Stern, J., Lichtwark, G., and Boyd, R. N. (2016). Medial gastrocnemius muscle volume in ambulant children with unilateral and bilateral cerebral palsy aged 2 to 9 years. Dev. Med. Child Neurol. 58, 1146–1152. doi: 10.1111/dmcn.13132
Barber, L., Hastings-Ison, T., Baker, R., Barrett, R., and Lichtwark, G. (2011). Medial gastrocnemius muscle volume and fascicle length in children aged 2 to 5 years with cerebral palsy. Dev. Med. Child Neurol. 53, 543–548. doi: 10.1111/j.1469-8749.2011.03913.x
Barrett, R. S., and Lichtwark, G. A. (2010). Gross muscle morphology and structure in spastic cerebral palsy: a systematic review. Dev. Med. Child Neurol. 52, 794–804. doi: 10.1111/j.1469-8749.2010.03686.x
Booth, C. M., Cortina-Borja, M. J., and Theologis, T. N. (2001). Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev. Med. Child Neurol. 43, 314–320. doi: 10.1017/S0012162201000597
Day, S. M., Strauss, D. J., Vachon, P. J., Rosenbloom, L., Shavelle, R. M., and Wu, Y. W. (2007). Growth patterns in a population of children and adolescents with cerebral palsy. Dev. Med. Child Neurol. 49, 167–171. doi: 10.1111/j.1469-8749.2007.00167.x
de Bruin, M., Smeulders, M. J., Kreulen, M., Huijing, P. A., and Jaspers, R. T. (2014). Intramuscular connective tissue differences in spastic and control muscle: a mechanical and histological study. PLoS ONE 9:e101038. doi: 10.1371/journal.pone.0101038
de Gooijer-van de Groep, K. L., de Vlugt, E., de Groot, J. H., van der Heijden-Maessen, H. C., Wielheesen, D. H., van Wijlen-Hempel, R. M., et al. (2013). Differentiation between non-neural and neural contributors to ankle joint stiffness in cerebral palsy. J. Neuroeng. Rehabil. 10:81. doi: 10.1186/1743-0003-10-81
Geertsen, S. S., Kirk, H., Lorentzen, J., Jorsal, M., Johansson, C. B., and Nielsen, J. B. (2015). Impaired gait function in adults with cerebral palsy is associated with reduced rapid force generation and increased passive stiffness. Clin. Neurophysiol. 126, 2320–2329. doi: 10.1016/j.clinph.2015.02.005
Gough, M., and Shortland, A. P. (2012). Could muscle deformity in children with spastic cerebral palsy be related to an impairment of muscle growth and altered adaptation? Dev. Med. Child Neurol. 54, 495–499. doi: 10.1111/j.1469-8749.2012.04229.x
Hagglund, G., and Wagner, P. (2011). Spasticity of the gastrosoleus muscle is related to the development of reduced passive dorsiflexion of the ankle in children with cerebral palsy: a registry analysis of 2,796 examinations in 355 children. Acta Orthop. 82, 744–748. doi: 10.3109/17453674.2011.618917
Handsfield, G. G., Meyer, C. H., Abel, M. F., and Blemker, S. S. (2016). Heterogeneity of muscle sizes in the lower limbs of children with cerebral palsy. Muscle Nerve 53, 933–945. doi: 10.1002/mus.24972
Heckmatt, J. Z., Leeman, S., and Dubowitz, V. (1982). Ultrasound imaging in the diagnosis of muscle disease. J. Pediatr. 101, 656–660. doi: 10.1016/S0022-3476(82)80286-2
Herskind, A., Ritterband-Rosenbaum, A., Willerslev-Olsen, M., Lorentzen, J., Hanson, L., Lichtwark, G., et al. (2015). Muscle growth is reduced in 15-month-old children with cerebral palsy. Dev Med. Child Neurol. 58, 485–491. doi: 10.1111/dmcn.12950
Jalaleddini, S. K., Sobhani Tehrani, E., and Kearney, R. (2016). A subspace approach to the structural decomposition and identification of ankle joint dynamic stiffness. IEEE Trans. Biomed. Eng. doi: 10.1109/TBME.2016.2604293
Keenan, K. G., Santos, V. J., Venkadesan, M., and Valero-Cuevas, F. J. (2009). Maximal voluntary fingertip force production is not limited by movement speed in combined motion and force tasks. J. Neurosci. 29, 8784–8789. doi: 10.1523/JNEUROSCI.0853-09.2009
Kutch, J. J., and Valero-Cuevas, F. J. (2011). Muscle redundancy does not imply robustness to muscle dysfunction. J. Biomech. 44, 1264–1270. doi: 10.1016/j.jbiomech.2011.02.014
Lance, J. W. (1980). “Symposium synopsis,” in Spasticity: Disordered Motor Control, eds R. G. Feldman, W. P. Koella, and R. R. Young (Chicago, IL: Year Book Medical Publishers), 485–494.
Lexell, J., Sjostrom, M., Nordlund, A. S., and Taylor, C. C. (1992). Growth and development of human muscle: a quantitative morphological study of whole vastus lateralis from childhood to adult age. Muscle Nerve 15, 404–409. doi: 10.1002/mus.880150323
Malaiya, R., McNee, A. E., Fry, N. R., Eve, L. C., Gough, M., and Shortland, A. P. (2007). The morphology of the medial gastrocnemius in typically developing children and children with spastic hemiplegic cerebral palsy. J. Electromyogr. Kinesiol. 17, 657–663. doi: 10.1016/j.jelekin.2007.02.009
Meskers, C. G., de Groot, J. H., de Vlugt, E., and Schouten, A. C. (2015). NeuroControl of movement: system identification approach for clinical benefit. Front. Integr. Neurosci. 9:48. doi: 10.3389/fnint.2015.00048
Nielsen, P. K., Jensen, B. R., Darvann, T., Jorgensen, K., and Bakke, M. (2006). Quantitative ultrasound tissue characterization in shoulder and thigh muscles–a new approach. BMC Musculoskelet. Disord. 7:2. doi: 10.1186/1471-2474-7-2
Noble, J. J., Charles-Edwards, G. D., Keevil, S. F., Lewis, A. P., Gough, M., and Shortland, A. P. (2014a). Intramuscular fat in ambulant young adults with bilateral spastic cerebral palsy. BMC Musculoskelet. Disord. 15:236. doi: 10.1186/1471-2474-15-236
Noble, J. J., Fry, N. R., Lewis, A. P., Keevil, S. F., Gough, M., and Shortland, A. P. (2014b). Lower limb muscle volumes in bilateral spastic cerebral palsy. Brain Dev. 36, 294–300. doi: 10.1016/j.braindev.2013.05.008
Oertel, G. (1988). Morphometric analysis of normal skeletal muscles in infancy, childhood and adolescence. An autopsy study. J. Neurol. Sci. 88, 303–313. doi: 10.1016/0022-510x(88)90227-4
Passerieux, E., Rossignol, R., Letellier, T., and Delage, J. P. (2007). Physical continuity of the perimysium from myofibers to tendons: involvement in lateral force transmission in skeletal muscle. J. Struct. Biol. 159, 19–28. doi: 10.1016/j.jsb.2007.01.022
Pitcher, C. A., Elliott, C. M., Panizzolo, F. A., Valentine, J. P., Stannage, K., and Reid, S. L. (2015). Ultrasound characterization of medial gastrocnemius tissue composition in children with spastic cerebral palsy. Muscle Nerve 52, 397–403. doi: 10.1002/mus.24549
Ponten, E., Gantelius, S., and Lieber, R. L. (2007). Intraoperative muscle measurements reveal a relationship between contracture formation and muscle remodeling. Muscle Nerve 36, 47–54. doi: 10.1002/mus.20780
Ponten, E. M., and Stal, P. S. (2007). Decreased capillarization and a shift to fast myosin heavy chain IIx in the biceps brachii muscle from young adults with spastic paresis. J. Neurol. Sci. 253, 25–33. doi: 10.1016/j.jns.2006.11.006
Rosenbaum, P., Paneth, N., Leviton, A., Goldstein, M., Bax, M., Damiano, D., et al. (2007). A report: the definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 49, 8–14. doi: 10.1111/j.1469-8749.2007.tb12610.x
Ross, S. A., Foreman, M., and Engsberg, J. R. (2011). Comparison of 3 different methods to analyze ankle plantarflexor stiffness in children with spastic diplegia cerebral palsy. Arch. Phys. Med. Rehabil. 92, 2034–2040. doi: 10.1016/j.apmr.2011.06.025
Schouten, A. C., Mugge, W., and van der Helm, F. C. (2008). NMClab, a model to assess the contributions of muscle visco-elasticity and afferent feedback to joint dynamics. J. Biomech. 41, 1659–1667. doi: 10.1016/j.jbiomech.2008.03.014
Shore, B. J., Yu, X., Desai, S., Selber, P., Wolfe, R., and Graham, H. K. (2012). Adductor surgery to prevent hip displacement in children with cerebral palsy: the predictive role of the Gross Motor Function Classification System. J. Bone Joint Surg. Am. 94, 326–334. doi: 10.2106/JBJS.J.02003
Sobhani Tehrani, E., Jalaleddini, K., and Kearney, R. E. (2013). Linear parameter varying identification of ankle joint intrinsic stiffness during imposed walking movements. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 4923–4927. doi: 10.1109/embc.2013.6610652
Tedroff, K., Lowing, K., and Astrom, E. (2014). A prospective cohort study investigating gross motor function, pain, and health-related quality of life 17 years after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol. 57, 484–490. doi: 10.1111/dmcn.12665
Valero-Cuevas, F. J., Cohn, B. A., Yngvason, H. F., and Lawrence, E. L. (2015). Exploring the high-dimensional structure of muscle redundancy via subject-specific and generic musculoskeletal models. J. Biomech. 48, 2887–2896. doi: 10.1016/j.jbiomech.2015.04.026
Keywords: cerebral palsy, skeletal muscle, muscle stiffness, muscle size, upper limb
Citation: von Walden F, Jalaleddini K, Evertsson B, Friberg J, Valero-Cuevas FJ and Pontén E (2017) Forearm Flexor Muscles in Children with Cerebral Palsy Are Weak, Thin and Stiff. Front. Comput. Neurosci. 11:30. doi: 10.3389/fncom.2017.00030
Received: 30 December 2016; Accepted: 07 April 2017;
Published: 25 April 2017.
Edited by:
Xiaoli Li, Beijing Normal University, ChinaReviewed by:
Ming Li, Peking University First Hospital, ChinaGeoffrey Handsfield, University of Auckland, New Zealand
Copyright © 2017 von Walden, Jalaleddini, Evertsson, Friberg, Valero-Cuevas and Pontén. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eva Pontén, eva.ponten@ki.se
 Ferdinand von Walden
Ferdinand von Walden Kian Jalaleddini
Kian Jalaleddini Björn Evertsson3,4
Björn Evertsson3,4  Francisco J. Valero-Cuevas
Francisco J. Valero-Cuevas Eva Pontén
Eva Pontén