- Department of Health and Exercise Science, Colorado State University, Fort Collins, CO, USA
Fatigue is one of the most disabling side effects in people with multiple sclerosis. While this fact is well known, there has been a remarkable lack of progress in determining the pathophysiological mechanisms behind fatigue and the establishment of effective treatments. The main barrier has been the lack of a unified definition of fatigue that can be objectively tested with validated experimental models. In this “perspective article” we propose the use of the following model and definition of fatigue: the decrease in physical and/or mental performance that results from changes in central, psychological, and/or peripheral factors. These changes depend on the task being performed, the environmental conditions it is performed in, and the physical and mental capacity of the individual. Our definition and model of fatigue outlines specific causes of fatigue and how it affects task performance. We also outline the strengths and weaknesses of commonly used measures of fatigue and suggest, based on our model and definition, new research strategies, which should include multiple measures. These studies should be mechanistic with validated experimental models to determine changes in central, psychological, and/or peripheral factors that explain fatigue. The proposed new research strategies may lead to the identification of the origins of MS related fatigue and the development of new, more effective treatments.
Fatigue is the most common and disabling symptom experience by people with multiple sclerosis (PwMS). Up to 92% of PwMS are affected by fatigue, which strongly influences quality of life (1). However, fatigue remains poorly understood and PwMS continue to suffer from a lack of effective fatigue treatments. Despite significant effort to elucidate the pathogenic mechanisms of fatigue, current knowledge is limited. Several factors contribute to the lack of progress in fatigue research, but the most important factor is that “fatigue” is often not clearly defined or is used without meaningful measurements in clinical and research settings (2). Kluger et al. (3) states: “Current treatments are non-specifically targeted to a vaguely defined symptom with unsatisfactory outcomes.” Providing further support to these statements, a recent Cochrane Review (4) on exercise therapy for fatigue in MS concluded there are important methodological issues to overcome. Heine et al. (4) reported most studies did not: explicitly include PwMS who experienced fatigue, use a validated measure of fatigue as the primary outcome, or target fatigue specifically. Berger (5) questions whether MS related fatigue can be treated and improved with current disease-modifying drugs, e.g., amantadine, methylphenidate, and modafinil, without having a precise definition of fatigue.
In this “perspective paper” we propose a model of fatigue designed to give clinicians and researchers a better understanding of fatigue, critically review current fatigue measures used in MS, and provide suggestions for new research strategies to better understand fatigue in MS.
Definitions of Fatigue
Many studies investigating fatigue have failed to objectively define fatigue, and those that did, have used varying definitions. Furthermore, the origins of fatigue vary between conditions and research in some diseases, such as MS, has failed to fully understand the difference between fatigue and related phenomena, such as depressed mood or sleep disorders (3). As a result, Kluger and colleagues (3) recently proposed a unified taxonomy for fatigue in neurological disorders that classified fatigue into two major domains: performance fatigability and perceptions of fatigue. Performance fatigability was defined as the magnitude or rate of change in a performance criterion relative to a reference value over a given time of task performance. Perceptions of fatigue was defined as a subjective sensation of weariness, increasing sense of effort, mismatch between effort expended and actual performance, or exhaustion.
We propose fatigue should be defined as: the decrease in physical and/or mental performance that results from changes in central, psychological, and/or peripheral factors. Indeed these factors all have conditional dependency in that the changes in central, psychological, and peripheral factors of fatigue depend on the task being performed, the environmental conditions it is performed in, and the physical and mental capacity of the individual (Figure 1). Central factors of fatigue are related to changes within the function of the central nervous system, such as neurotransmitter levels and intrinsic neuronal excitability, while psychological factors of fatigue include mood disorders, perceptions of effort, motivation, temporal and performance feedback, and arousal. Finally, peripheral factors of fatigue refer to physiological changes, such as pH, muscle contractility and excitability, and substrate availability. Importantly, the phrase factors of fatigue is used because there are many changes from a variety of locations that can all interact to contribute to fatigue. As a result, terminology that refers to a specific location of the fatigue, such as central, peripheral, and muscle, should be avoided as fatigue is almost never focused to a specific location.
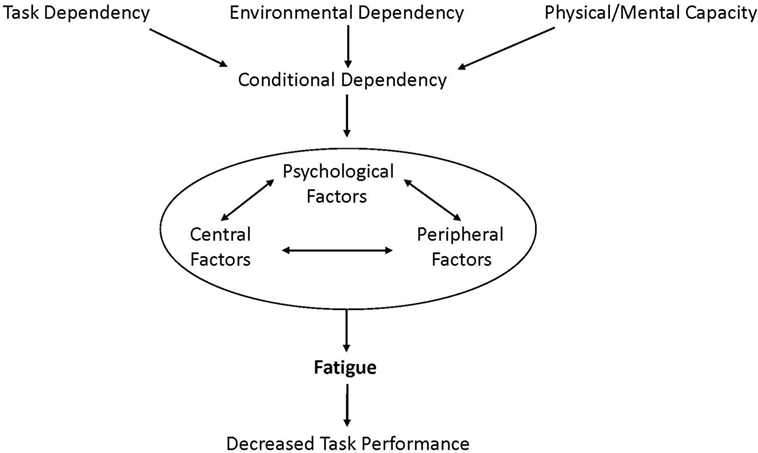
Figure 1. Fatigue is defined as the decrease in physical and/or mental performance that results from changes in central, psychological, and/or peripheral factors. These depend on the task being performed, the environmental conditions it is performed in, and the physical and mental capacity of the individual (conditional dependency). Importantly, fatigue is greatly affected by the factors of conditional dependency and the interactive changes in central, psychological, and/or peripheral factors that cause fatigue.
Task dependency has been identified and discussed as a component of fatigue in healthy individuals for several decades (6–9). A variety of human studies indicate that fatigue is not caused exclusively by any common set of factors, but depends on the type of cognitive or motor task that is being performed. However, many conditions beyond the specific task being performed can affect fatigue. One distinct difference between our model and previous models (3, 10) is the concept of conditional dependency, which considers not only task dependency but also how the condition it is performed in and the physical and mental capacity of the individual affect fatigue. Environmental dependency considers how factors of the environment affect fatigue, such as temperature and comfort level of the individual in the space. This can be of particular importance in MS, as warm environments can greatly affect physical abilities of PwMS (11). Finally, fatigue depends on a person’s physical and mental capacity, which includes the consideration of differences in fatigue before and after the onset of a disease or day-to-day variation in disease status due to disease progression or temporary relapse. These different conditions each interact with each other, as well as the central, psychological, and peripheral factors of fatigue discussed above. Our model not only displays how the varying conditions affect the interactive/factors but also the importance of considering the impact of factors, such as depression, general lack of motivation, and altered states of arousal, when measuring fatigue. This is most easily seen in the reported interactions between depression and fatigue in PwMS, where studies have shown that reported fatigue levels often decrease once depression is accounted for (12, 13). Therefore, what is being reported as “fatigue” is often a manifestation of an underlying condition, which may or may not be related to an individual’s ability to perform a task.
Assessment of Fatigue in MS
The two interactive subtypes (perception and performance) of fatigue proposed by Kluger and colleagues (3) are each important when considering the impact of fatigue on PwMS. Increased perceptions of fatigue can have a significant impact on activities of daily living, mood, and likelihood to engage in social activities, resulting in a reduced quality of life. While performance fatigability may only impact PwMS during a task, it has meaningful impact on an individual’s ability to perform activities of daily living and to live independently. Furthermore, performance fatigability impacts an individual’s exercise capacity, which is important in managing the symptoms of MS (14).
Subjective Measurements of Fatigue
A remarkable number of questionnaires have been developed to assess fatigue. They range in length from single-item scales [e.g., visual analog scale (VAS)], to multidimensional scales claiming to assess various dimensions of fatigue, such as physical vs. mental [e.g., modified fatigue impact scale (MFIS)]. Most of the items on these assessments are similar and correlate very well with each other. One major issue with fatigue questionnaires is the construct contamination that mars the validity and specificity of such scales (2). For example, fatigue questionnaires often include questions about tiredness and cognition, which are not always associated with fatigue (2).
Self-report questionnaires can be influenced by other symptoms of MS, require patients to make difficult reflective assessments, and are completely subjective. Despite these limitations, the fatigue severity scale (FSS) (15) and the MFIS (16) are commonly the only measures of fatigue in many studies [e.g., Ref. (17–19)]. Most of these studies were incapable of demonstrating responsiveness to changes over time to therapeutic interventions, likely because of a lack of specificity in the questions or underlying factors of MS that may be reported as fatigue. However, questionnaires will continue to have value, especially for measuring perceptions of fatigue, until quick and easy objective clinical assessments are available.
Objective Measurements of Fatigue
Objective measures of fatigue are limited to variables obtained during physical or mental tasks, and measured pre-, during-, and post-task. The measures of these tasks can be objectively quantified in research and clinical settings. Fatigue during motor tasks is usually characterized by the decline in peak force, power, accuracy, or speed from pre- to post-task. During cognitive tasks, fatigue is often measured as declines in reaction time or accuracy-over-time on continuous tasks. While several studies have attempted to objectively quantify MS related factors of fatigue using the neuroimaging techniques (described below), they have failed to measure factors of performance fatigability. Separating fatigue into the motor and cognitive tasks or domains provides objective assessments that are less likely to be contaminated by other symptoms of MS and help distinguish fatigue from related factors, such as reduced cognitive processing speed, sleep disorders, depressive symptoms, and anxiety (20, 21). Therefore, it is suggested that measures, such as sleepiness and mood be included as covariates, when investigating MS related fatigue. Depression, mood, anxiety, cognitive–behavioral factors, motivation, sleep disorders, and low sense of control contribute to fatigue (22–24). How people then react to these underlying conditions may serve to prolong or worsen fatigue. Specifically, depression affects a significant proportion of PwMS during their life span (25). Accordingly, Bakshi et al. (26) found that depression is associated with MS related fatigue, indicating that depression should be controlled for.
Once fatigue is defined, valid tasks and indices may be employed to measure the contributing factors of fatigue. Several technologies are available to investigate the changes in these factors during tasks, including: electromyography, metabolic measures, transcranial magnetic stimulation, magnetic resonance imaging (MRI), near infrared spectroscopy, and positron emission tomography (PET). Since MS related fatigue is a multifactorial problem, it is important to use multiple instruments to investigate fatigue, and identify the central, psychological, and peripheral factors contributing to the decrease in task performance.
Factors Contributing to Fatigue in MS
Central Factors
Central factors contributing to fatigue include neurotransmitter levels, inflammation, neuronal excitability, substrate utilization/transport, axonal conduction velocity, and many others. CNS inflammation is a hallmark of MS and has been suggested to play a role in MS related fatigue, although the current literature reports conflicting findings (27). For example, some researchers have shown associations between cytokine levels and fatigue questionnaire scores (28–30), while others have shown no association between cytokine or c-reactive protein levels with fatigue questionnaires (31, 32). Therefore, future research is needed to determine whether CNS inflammation, in addition to many other factors, is a potential central factor of fatigue in MS.
Neuroimaging studies have started to provide direct evidence of the central factors of fatigue in MS. Roelcke et al. (33) used 18F-fluorodeoxyglucose-PET to measure cerebral glucose metabolism in PwMS. They found significant hypometabolism throughout the brain in fatigued PwMS, based on FSS scores, suggesting dysfunctional cerebral activity might be responsible in MS related fatigue. However, it cannot be ruled out that cerebral hypometabolism is caused by other symptoms, such as depression, which were not specified in the fatigue questionnaire.
Functional-magnetic resonance imaging (fMRI) is a prominent neuroimaging technique used to investigate cerebral activity. Although this technique provides the opportunity to detect brain regions involved with motor or cognitive tasks, the interpretations and conclusions resulting from fMRI studies are often misleading. A good example is a recent fMRI study that examined cognitive fatigue in PwMS (34). A cognitive task was performed within the MRI scanner and the VAS was used during the task to measure “state” cognitive fatigue (35). The authors concluded that PwMS had increased brain activity in the caudate, compared to healthy controls, resulting in greater VAS scores. However, there was no significant group x time interaction, indicating the task elicited the same change in task performance (fatigue) in both the MS and healthy groups. Furthermore, performances on the neurophysiological tests were not different between groups. Similar findings were seen in Rocca et al. (36), where fMRI during a motor task was obtained from PwMS with and without “fatigue” and matched healthy controls. Brain activation strategies were different between the groups during the motor tasks without differences or changes in task performance. Task performance was similar between the investigated groups in both studies, which suggests that the findings only help explain underlying factors that contributed to the initial difference in fatigue between the groups (perceptions of fatigue), not performance fatigability. If altered brain activation strategies lead to decreased task performance, then conclusions about performance fatigability could have been made.
It has been suggested that an imbalance of dopamine in the CNS and immune system plays an important role in fatigue (37). Neuroimaging studies have shown that brain regions with impaired structure and function are heavily innervated by dopaminergic neurons (38, 39). Rönnbäck and Hansson (40) stated that “mental fatigue” is also associated with impaired glutamate neurotransmission and hypothesized that there might be a genetic failure preventing astroglial glutamate transporters from upregulating. In our model, the aforementioned findings refer to perceptions which are modulated by central and psychological factors, and if they do not change during the performance of a task they should only be classified as a factor of perceptions of fatigue.
Some studies showed associations between fatigue and lesion load/location and the degree of gray matter atrophy (41, 42). They found that MS related fatigue, assessed by the MFIS, FSS, and MRI, is at least partially associated with disruption of frontal and parietal pathways and cortical areas involved in cognitive/attentional processing. However, based on our proposed model of fatigue, it remains unknown whether structural brain changes, such as atrophy and lesion load, are factors of performance fatigability since they have not been associated with an objective measure of performance fatigue.
Together, these studies show that central factors of fatigue undoubtedly contribute to perceptions of fatigue in PwMS. However, the necessary measures of performance fatigability have not been performed in these studies, and therefore it is still unknown whether these central factors contribute to performance fatigability in PwMS.
Psychological Factors
Psychological factors, such as perceived effort, subjective sense of worsening performance over time, motivation, and cognitive impairment are contributors to fatigue (43). Serotonin (44) and dopamine (45) are just two examples of central factors that influence psychological factors and play an important role in fatigue. Engström et al. (46) showed that PwMS who have high fatigue demonstrate reduced mesocorticolimbic connectivity compared to healthy adults during a complex working memory task. Finke et al. (47) showed that high fatigue scores in PwMS were negatively correlated with resting-state mesocorticolimbic connectivity. These findings suggest that MS related fatigue is associated with reduced connectivity between the regions innervated with dopamine, possibly due to reduced dopamine levels.
Peripheral Factors
Fatigue in PwMS can also arise from one or several of the peripheral factors that were described above. Slowing of muscle contractile properties (48–51), decreased muscle oxidative capacity (48, 52), impaired excitation–contraction coupling (50, 53), and altered muscle metabolic response to exercise (50, 53, 54) may contribute to fatigue in MS. Sharma et al. (50) showed that intramuscular components contribute to fatigue in MS by demonstrating that greater decreases in phosphocreatine and intracellular pH was associated with greater force reduction (performance fatigability). In this context, it is important to mention muscle afferent feedback, which includes the possibility that metabolites can alter CNS motor output (55).
Final Thoughts and Future Research Directions
When compared to advances made in other domains of disease status and disability in PwMS, fatigue continues to lag behind. The lack of progress is largely due to the varying subjectivity in the definition and assessment of fatigue between research groups. Our proposed theoretical model provides specific areas of objective fatigue assessment that can be applied in research and intervention settings. Because of the complexity of fatigue in PwMS, it is important that future studies should not only account for covariates, including depression and sleepiness, but also require integration of multiple measures directed at the different factors that influence fatigue. Currently, several techniques are available to measure many of the factors that could contribute to fatigue in PwMS. Central factors can be examined via neuroimaging techniques, such as PET and MRI, psychological factors via the Brief Repeatable Battery of Neuropsychological Tests (56), and peripheral factors with electromyography and MR spectroscopy to name a few.
In this “perspective paper” we proposed a standardized definition of fatigue and identified factors that contribute to fatigue. However, this list is not exhaustive; our model is hypothetical and further research is needed to elucidate all mechanisms of MS related fatigue and to validate our proposed model. Research studies should focus on clearly defined outcome variables, which contribute to fatigue and not primarily on the location of fatigue. Future research studies on MS related fatigue should be extended to include psychological screening to determine underlying conditions, which may or may not impair task performance but should be distinguished from fatigue. We propose fatigue should be defined as: the decrease in physical and/or mental performance that results from changes in central, psychological, and/or peripheral factors. Importantly, these changes depend on the task being performed, the environmental conditions it is performed in, and the disease status of the individual.
An example for the objective measurement of fatigue in PwMS with defined outcome variables is a study by Sharma et al. (50). Fatigability of the anterior tibialis muscle was quantified in PwMS and controls during intermittent electrical stimulation. During stimulation, the decline in tetanic force, phosphocreatine, and intracellular pH was greater in PwMS than in controls, indicating an abnormal intramuscular component of fatigue in MS. Importantly, this study eliminated the influence of perceptions of fatigue since it did not involve voluntary muscle activity.
While the study design described above was ideal to identify several peripheral factors of performance fatigability, future studies must include voluntary muscle activity, which incorporates central and psychological factors, to fully understand fatigue. This can be accomplished by measuring changes in peripheral factors (muscle strength and activity, pH, glycogen, etc.), as well as measures of central and psychological factors (dopamine, motivation, perceived effort, etc.). For example, the design used by Sharma et al. (50) could be expanded to include voluntary muscle activity, and neuroimaging techniques (fMRI and PET) could be applied to measure changes in central and psychological factors. Perceptions of fatigue should be monitored in this example using the techniques, such as the Borg scale of perceived exertion. The associations of these measures then may provide insights to the origins and mechanisms of MS related fatigue.
By using a uniformed understanding and measurement of fatigue, progress may finally be made in effectively treating the symptoms of fatigue and improving quality of life in PwMS.
Author Contributions
TR, JK, and NK contributed to drafting the article and revising it critically for important intellectual content. All authors approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by the Colorado State University Libraries Open Access Research/Scholarship Fund.
References
1. Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C. Treatments for fatigue in multiple sclerosis: a rapid and systematic review. Health Technol Assess (2000) 4:1–61. doi: 10.3310/hta4270
2. DeLuca J, Genova HM, Capili EJ, Wylie GR. Functional neuroimaging of fatigue. Phys Med Rehabil Clin N Am (2009) 20:325–37. doi:10.1016/j.pmr.2008.12.007
3. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illness: proposal for a unified taxonomy. Neurology (2013) 80:409–16. doi:10.1212/WNL.0b013e31827f07be
4. Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev (2015) 9:1. doi:10.1002/14651858.CD009956.pub2
5. Berger JR. Functional improvements and symptom management in multiple sclerosis: clinical efficacy and current therapies. Am J Manag Care (2011) 17:S146–53.
6. Bigland-Ritchie B. EMG/force relations and fatigue of human voluntary contractions. Exerc Sport Sci Rev (1981) 9:75–117. doi:10.1249/00003677-198101000-00002
7. Bigland-Ritchie B, Rice CL, Garland SJ, Walsh MML. Task-dependent factors in fatigue of human voluntary contractions. In: Gandevia SC, Enoka RM, McComas AJ, Stuart DG, Thomas CK, editors. Fatigue: Neural and Muscular Mechanisms. New York: Plenum (1995). p. 361–80.
9. Enoka RM, Baudry S, Rudroff T, Farina D, Klass M, Duchateau J. Unraveling the neurophysiology of muscle fatigue. J Electromyogr Kinesiol (2011) 21:208–19. doi:10.1016/j.jelekin.2010.10.006
10. Strober LB, Arnett PA. An examination of four models predicting fatigue in multiple sclerosis. Arch Clin Neuropsychol (2005) 20:631–46. doi:10.1016/j.acn.2005.04.002
11. Noronha MJ, Vas CJ, Aziz H. Autonomic dysfunction (sweating responses) in multiple sclerosis. J Neurol Neurosurg Psychiatry (1968) 31:19–22. doi:10.1136/jnnp.31.1.19
12. Marin H, Menza MA. Specific treatment of residual fatigue in depressed patients. Psychiatry (Edgmont) (2004) 1:12–8.
13. Dzurec LC. Experiences of fatigue and depression before and after low-dose 1-thyroxine supplementation in essentially euthyroid individuals. Res Nurs Health (1997) 20(5):389–98. doi:10.1002/(SICI)1098-240X(199710)20:5<389::AID-NUR3>3.0.CO;2-K
14. Motl RW, Sandroff BM. Benefits of exercise training in multiple sclerosis. Curr Neurol Neurosci Rep (2015) 15:62. doi:10.1007/s11910-015-0585-6
15. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol (1989) 46:1121–3. doi:10.1001/archneur.1989.00520460115022
16. Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci (1994) 21:9–14. doi:10.1017/S0317167100048691
17. Gobbi C, Rocca MA, Riccitelli G, Pagani E, Messini R, Preziosa P, et al. Influence of the topography of brain damage on depression and fatigue in patients with multiple sclerosis. Mult Scler (2014) 20:192–201. doi:10.1177/1352458513493684
18. Pellicano C, Gallo A, Li X, Ikonomidou VN, Evangelou IE, Ohayon JM, et al. Relationship of cortical atrophy to fatigue in patients with multiple sclerosis. Arch Neurol (2010) 67:447–53. doi:10.1001/archneurol.2010.48
19. Pardini M, Bonzano L, Bergamino M, Bommarito G, Feraco P, Murugavel A, et al. Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Mult Scler (2015) 21:442–7. doi:10.1177/1352458514546791
20. Andreasen AK, Spliid PE, Andersen H, Jakobsen J. Fatigue and processing speed are related in multiple sclerosis. Eur J Neurol (2010) 17:212–8. doi:10.1111/j.1468-1331.2009.02776.x
21. Labuz-Roszak B, Kubicka-Baczyk K, Pierzchała K, Machowska-Majchrzak A, Skrzypek M. Fatigue and its associations with sleep disorders, depressive symptoms and anxiety in patients with multiple sclerosis. Neurol Neurochir Pol (2012) 46:309–17. doi:10.5114/ninp.2012.30261
22. Smith RL, Lucaccini LF, Epstein MH. Effects of monetary rewards and punishment on vigilance performance. J Appl Psychol (1967) 51:411–6. doi:10.1037/h0025045
23. Ansley L, Robson PJ, St Clair Gibson A, Noakes TD. Anticipatory pacing strategies during supramaximal exercise lasting longer than 30 s. Med Sci Sports Exerc (2004) 36:209–14. doi:10.1249/01.MSS.0000113474.31529.C6
24. Marcora S. Counterpoint: afferent feedback from fatigued locomotor muscles is not an important determinant of endurance exercise performance. J Appl Physiol (1985) (2010) 108:454–6. doi:10.1152/japplphysiol.01393.2009
25. Feinstein A, Magalhaes S, Richard JF, Audet B, Moore C. The link between multiple sclerosis and depression. Nat Rev Neurol (2014) 10:507–17. doi:10.1038/nrneurol.2014.139
26. Bakshi R, Shaikh ZA, Miletich RS, Czarnecki D, Dmochowski J, Henschel K, et al. Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler (2000) 6:181–5. doi:10.1191/135245800701566052
27. Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev (2016) 15(3):210–20. doi:10.1016/j.autrev.2015.11.005
28. Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P. Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult Scler (2004) 10:165–9. doi:10.1191/1352458504ms991oa
29. Heesen C, Nawrath L, Reich C, Bauer N, Schulz K, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behavior? J Neurol Neurosurg Psychiatry (2006) 77:34–9. doi:10.1136/jnnp.2005.065805
30. Malekzadeh A, Van de Geer-Peeters W, De Groot V, Teunissen CE, Beckerman H, TREFAMS-ACE Study Group. Fatigue in patients with multiple sclerosis: is it related to pro- and anti-inflammatory cytokines? Dis Markers (2015) 2015(2015):758314. doi:10.1155/2015/758314
31. Giovannoni G, Thompson AJ, Miller DH, Thompson EJ. Fatigue is not associated with raised inflammatory markers in multiple sclerosis. Neurology (2001) 57:676–81. doi:10.1212/WNL.57.4.676
32. Heesen C, Koehler G, Gross R, Tessmer W, Schulz K, Gold SM. Altered cytokine mRNA expression in multiple sclerosis patients with fatigue. Mult Scler (2005) 11:51–7. doi:10.1191/1352458505ms1129oa
33. Roelcke U, Kappos L, Lechner-Scott J, Brunnschweiler H, Huber S, Ammann W, et al. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology (1997) 48:1566–71. doi:10.1212/WNL.48.6.1566
34. Genova HM, Rajagopalan V, DeLuca J, Das A, Binder A, Arjunan A, et al. Examination of cognitive fatigue in multiple sclerosis, using functional magnetic resonance imaging and diffusion tensor imaging. PLoS One (2013) 8:e78811. doi:10.1371/journal.pone.0078811
35. Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation (2001).
36. Rocca MA, Meani A, Riccitelli GC, Colombo B, Rodegher M, Falini A, et al. Abnormal adaptation over time of motor network recruitment in multiple sclerosis patients with fatigue. Mult Scler (2015). doi:10.1177/1352458515614407
37. Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol (2015) 6:52. doi:10.3389/fneur.2015.00052
38. Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol (2004) 74:1–58. doi:10.1016/j.pneurobio.2004.10.002
39. Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci (2000) 20:2369–82.
40. Rönnbäck L, Hansson E. On the potential role of glutamate transport in mental fatigue. J Neuroinflammation (2004) 1:22. doi:10.1186/1742-2094-1-22
41. Tedeschi G, Dinacci D, Lavorgna L, Prinster A, Savettieri G, Quattrone A, et al. Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J Neurol Sci (2007) 263:15–9. doi:10.1016/j.jns.2007.07.004
42. Sepulcre J, Masdeu JC, Goñi J, Arrondo G, Vélez de Mendizábal N, Bejarano B, et al. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler (2009) 15:337–44. doi:10.1177/1352458508098373
43. Bol Y, Duits AA, Hupperts RMM, Vlaeyen JWS, Verhey FRJ. The psychology of fatigue in patients with multiple sclerosis: a review. J Psychosom Res (2009) 66:3–11. doi:10.1016/j.jpsychores.2008.05.003
44. Meussen R, Watson P, Hasegawa H, Roelands B, Piacentini MF. Central fatigue: the serotonin hypothesis and beyond. Sports Med (2006) 36:881–909. doi:10.2165/00007256-200636100-00006
45. Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med (2008) 10:67–80. doi:10.1007/s12017-008-8032-3
46. Engström M, Flensner G, Landtblom AM, Ek AC, Karlsson T. Thalamo-striato-cortical determinants to fatigue in multiple sclerosis. Brain Behav (2013) 3:715–28. doi:10.1002/brb3.181
47. Finke C, Schlichting J, Papazoglou S, Scheel M, Freing A, Soemmer C, et al. Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler (2015) 21:925–34. doi:10.1177/1352458514555784
48. Kent-Braun JA, Sharma KR, Miller RG, Weiner MW. Postexercise phosphocreatine resynthesis is slowed in multiple sclerosis. Muscle Nerve (1994) 17:835–41. doi:10.1002/mus.880170802
49. Rice CL, Vollmer TL, Bigland-Ritchie B. Neuromuscular responses of patients with multiple sclerosis. Muscle Nerve (1992) 15:1123–32. doi:10.1002/mus.880151011
50. Sharma KR, Kent-Braun J, Mynhier MA, Weiner MW, Miller RG. Evidence of an abnormal intramuscular component of fatigue in multiple sclerosis. Muscle Nerve (1995) 18:1403–11. doi:10.1002/mus.880181210
51. Sheean GL, Murray NM, Rothwell JC, Miller DH, Thompson AJ. An electrophysiological study of the mechanism of fatigue in multiple sclerosis. Brain (1997) 120:299–315. doi:10.1093/brain/120.2.299
52. Kent-Braun JA, Ng AV, Castro M, Weiner MW, Gelinas D, Dudley GA, et al. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J Appl Physiol (1985) (1997) 83:1998–2004.
53. Kent-Braun JA, Sharma KR, Weiner MW, Miller RG. Effects of exercise on muscle activation and metabolism in multiple sclerosis. Muscle Nerve (1994) 17:1162–9. doi:10.1002/mus.880171006
54. Ng AV, Dao HT, Miller RG, Gelinas DF, Kent-Braun JA. Blunted pressor and intramuscular metabolic responses to voluntary isometric exercise in multiple sclerosis. J Appl Physiol (1985) (2000) 88:871–80.
55. Noakes TD, Clair Gibson A, Lambert EV. From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans: summary and conclusions. Br J Sports Med (2005) 39:120–4. doi:10.1136/bjsm.2003.010330
Keywords: multiple sclerosis, perceptions, performance fatigability, neuroimaging, questionnaires
Citation: Rudroff T, Kindred JH and Ketelhut NB (2016) Fatigue in Multiple Sclerosis: Misconceptions and Future Research Directions. Front. Neurol. 7:122. doi: 10.3389/fneur.2016.00122
Received: 13 November 2015; Accepted: 20 July 2016;
Published: 02 August 2016
Edited by:
Björn Tackenberg, University of Marburg, GermanyReviewed by:
Jörg Kraus, Public Hospital Zell am See, AustriaSven G. Meuth, Universitätsklinikum Münster, Germany
Copyright: © 2016 Rudroff, Kindred and Ketelhut. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thorsten Rudroff, thorsten.rudroff@colostate.edu
 Thorsten Rudroff
Thorsten Rudroff John H. Kindred
John H. Kindred Nathaniel B. Ketelhut
Nathaniel B. Ketelhut