- 1Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom
- 2Emergency Department, Edinburgh Royal Infirmary, Edinburgh, United Kingdom
Delayed cerebral ischemia (DCI) is a life-threatening complication after subarachnoid hemorrhage. There is a strong association between cerebral vessel narrowing and DCI. Alpha calcitonin gene-related peptide (αCGRP) is a potent vasodilator, which may be effective at reducing cerebral vessel narrowing after subarachnoid hemorrhage (SAH). Here, we report a meta-analysis of data from nine in vivo animal studies identified in a systematic review in which αCGRP was administered in SAH models. Our primary outcome was change in cerebral vessel diameter and the secondary outcome was change in neurobehavioral scores. There was a 40.8 ± 8.2% increase in cerebral vessel diameter in those animals treated with αCGRP compared with controls (p < 0.0005, 95% CI 23.7–57.9). Neurobehavioral scores were reported in four publications and showed a standardized mean difference of 1.31 in favor of αCGRP (CI −0.49 to 3.12). We conclude that αCGRP reduces cerebral vessel narrowing seen after SAH in animal studies but note that there is insufficient evidence to determine its effect on functional outcomes.
Introduction
Aneurysmal subarachnoid hemorrhage is a significant cause of morbidity and mortality worldwide with an annual incidence of 9 per 100,000 person years (1–3). 27% of all stroke-related potential years of life lost before the age of 65 years are attributable to subarachnoid hemorrhage (SAH), and the average age at first onset of SAH is 55 years (4–6). Of the 85–90% of patients who survive to reach hospital, up to 42% will die within 1 month of the SAH and 20% remain dependent on others for activities of daily living (6, 7).
Despite successful treatment of the ruptured aneurysm and reduction of the risk of rebleeding, approximately 30% of treated patients develop focal neurological or cognitive deficits conventionally attributed to delayed cerebral ischemia (DCI) (8–11). A strong association exists between cerebral vessel narrowing and DCI, and it has been suggested that treating vessel narrowing after SAH may improve outcomes (12).
Alpha calcitonin gene-related peptide (αCGRP) is an endogenous neuropeptide and powerful vasodilator. αCGRP exerts its relaxing properties through nitric oxide and endothelium-dependent or endothelin-independent pathways (13). Endogenous αCGRP appears to be released and subsequently depleted in response to cerebral vasoconstriction after SAH, leading to the suggestion that exogenous αCGRP may be beneficial in managing DCI (14–16). Several animal studies and three human trials have investigated the effect of αCGRP on cerebral arteries after SAH with a view to administering αCGRP as a treatment for DCI (17–40). However, there has been no systematic summary assessing the efficacy of αCGRP in reducing cerebral vasospasm in animal models.
Here, we use this approach to summarize data from publications reporting in vivo animal studies that investigated the effects of αCGRP after experimental SAH.
Methods
The study protocol has been published elsewhere and is available open access but a brief description is given below (41).
Search Strategy and Study Selection
In January 2015, we searched two electronic databases (MEDLINE via PubMed Central, and EMBASE via OvidSP) using the key words “alpha calcitonin gene-related peptide,” “αCGRP,” and “subarachnoid hemorrhage” in combination using the Boolean operator [AND]. The search was restricted to “other animals.” Two investigators (Liam M. C. Flynn and Caroline J. Begg) independently screened the abstracts and titles to identify those that met our inclusion criteria. Any differences were resolved by discussion with a third reviewer (Peter J. D. Andrews). We included in vivo animal studies describing the effect of αCGRP in animal models of SAH where outcome was reported as a change in arterial diameter and the articles were published in English. We also included studies which examined in vivo SAH and αCGRP administration with postmortem in vitro measurements of artery diameter.
The above search was repeated in July 2017 using the same methods. No further studies meeting eligibility criteria were identified.
Data Extraction
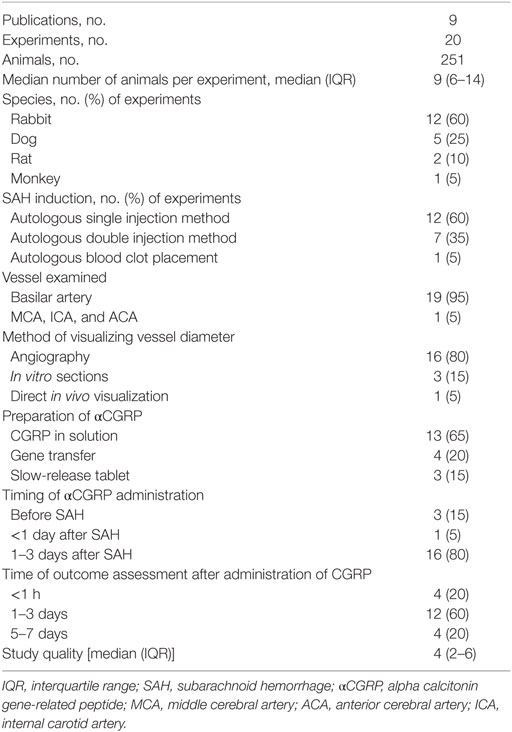
Two investigators independently extracted data relating to species of animal and weight; method of inducing SAH (single injection, double injection, or clot placement); whether the basilar artery, middle cerebral artery, anterior cerebral artery, or internal carotid artery were measured; the method of measurement (angiography, in vitro measurement or direct in vivo visualization); anesthetic agent used; dose of αCGRP and time of administration from SAH; reporting and method of randomization; reporting and method of blinding; animal welfare guideline statement; statement of sample size calculation; whether there was a statement of potential conflicts of interest and the use of animals with comorbidities. Study quality was assessed using the CAMARADES 10-item quality checklist (42). One point was awarded for each of (1) publication in a peer-reviewed journal, (2) statement of control temperature, (3) randomized intervention allocation, (4) intervention allocation concealment, (5) blinded assessment of outcome, (6) avoidance of anesthetics with marked intrinsic neuroprotective properties (ketamine), (7) statement of a priori sample size collection, (8) statement of compliance with regulatory requirements, (9) conflicts of interest statement, and (10) use of animals with comorbidities.
For meta-analysis, we recorded arterial diameter for intervention and control groups as a percentage from baseline (mean values and a measure of variance with the number of animals per group). Where a single control group was used for multiple treatment groups, we adjusted the size of the control group entered into the meta-analysis by dividing the size of the control group by the number of treatment groups served (43).
Two of the nine publications did not report quantitative data in their text, only presenting graphed data (26, 32). Mean values with SEM were estimated from the graphs of these studies using Universal Desktop Ruler for Windows.
Statistical Analysis
We performed meta-analysis using normalized mean difference with a random effects model (43). We used univariate meta-regression to explore associations of animal species, sex, strain, and quality issues. We used meta-regression of transformed data using a three-component cubic spline to assess dose–response. Where multiple experiments were performed in the same publication we treated these as separate studies (43). All in vivo experiments investigating the effect of αCGRP in animal models of SAH where outcome was reported as a change in arterial diameter were included. We also included experiments which examined in vivo SAH and αCGRP administration with postmortem in vitro measurements of artery diameter. Where one study administered different doses of αCGRP to separate groups of animals but had a shared control group we treated these as separate experiments and divided the number of controls between the experiments as per Vesterinen et al. (43). Results are presented as the mean ± SE unless otherwise stated. Statistical analysis was performed with STATA 14 (StataCorp LP).
Results
We identified 142 publications from our initial search. After combining MEDLINE and EMBASE results and deleting duplicates, 57 publications remained. Titles and abstracts were then screened for eligibility by two authors resulting in 21 publications investigating αCGRP and cerebral vessel narrowing (17–37). After examining the full papers of these abstracts and removing those with in vitro αCGRP administration (n = 12) and those which lacked SAH models, nine eligible publications remained (17, 21, 22, 26, 27, 32, 35–37). The nine publications included in the review were published between 1989 and 2013 (median year 1996). From the 9 publications, 20 experiments were included in meta-analysis (Figure 1).
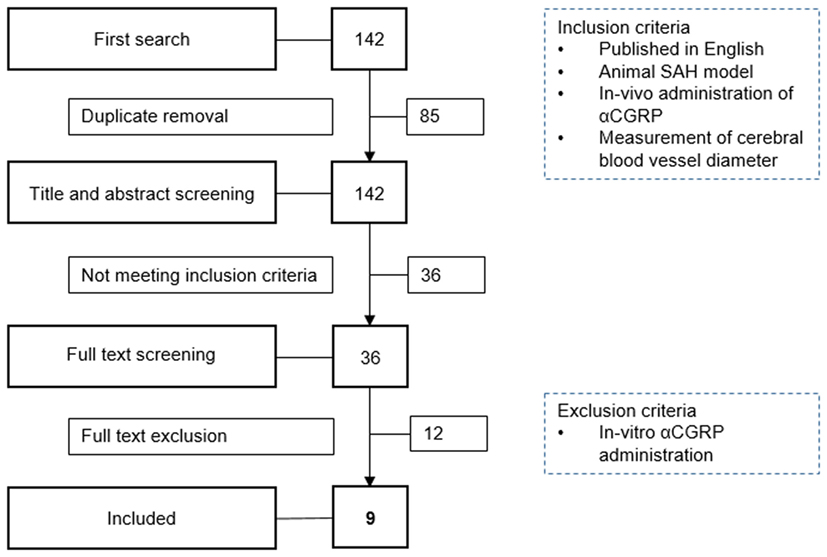
Figure 1. Flowchart of study selection. SAH, subarachnoid hemorrhage; αCGRP, alpha calcitonin gene-related peptide.
Characteristics of Studies
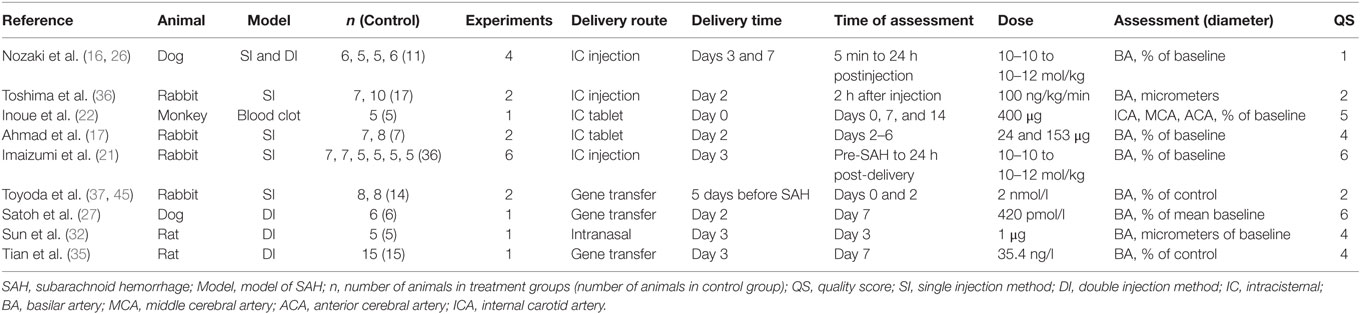
The total number of animals included in analysis was 251, and the median number of animals used per experiment was 9 [interquartile range (IQR) 6–14]. Twelve experiments used rabbits (n = 156, 62% of all animals). Four experiments used New Zealand White, and eight experiments used Japanese White. Five experiments used mongrel dogs (n = 45, 18% of all animals). Two experiments used rats (n = 40, 16% of all animals). One used Wistar rats, and the other used Sprague Dawley rats. The remaining experiment used Macaca fascicularis (n = 10, 4% of all animals). The median number of study quality checklist items scored was 4 (IQR 2–6). No studies used animals with comorbidities; reported a statement of potential conflicts of interest or stated an a priori sample size calculation. 40% of studies reported control of body temperature. 20% described a randomized treatment allocation, and 45% reported allocation concealment. Half of the experiments used a blinded assessment of outcome, and half used an anesthetic agent other than ketamine. All studies were published in peer-reviewed journals, and 70% reported compliance with local animal welfare guidelines. In 16/20 (80%) of the experiments, SAH was induced by autologous blood injection by either single or double injection methods, the remainder were induced with blood clot placement. Further study characteristics are presented in Tables 1 and 2.
Treatment Effect
All 21 publications reporting in vivo and in vitro experiments demonstrated a dilation of cerebral arteries after αCGRP administration. Of the 20 eligible in vivo experiments (taken from the nine eligible publications) included in meta-analysis, there was a 40.8 ± 8.2% increase in cerebral vessel diameter in the αCGRP group compared with controls (p < 0.0005, 95% CI 23.7–57.9, I2 96%, Figure 2). There was also a significant dose–response to αCGRP in the 10 experiments, which administered a single dose into the cerebroventricular system (Figure 3).
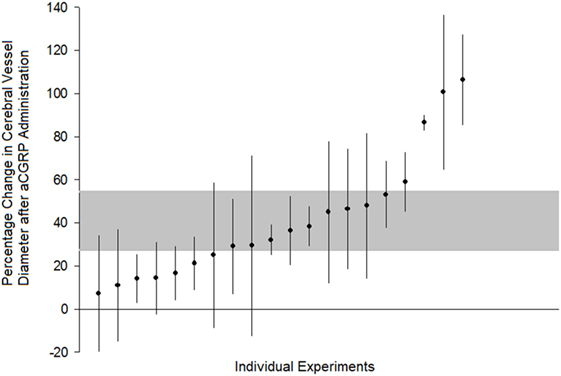
Figure 2. Comparison of individual experiments ranked according to the effect of alpha calcitonin gene-related peptide on change in cerebral vessel diameter. Vertical error bars represent 95% confidence intervals for the individual estimates while the gray area represents the 95% interval for the grouped (all studies) estimate. Percentage change is in comparison to post-subarachnoid hemorrhage diameter or control values, depending upon how the original publication reported results.
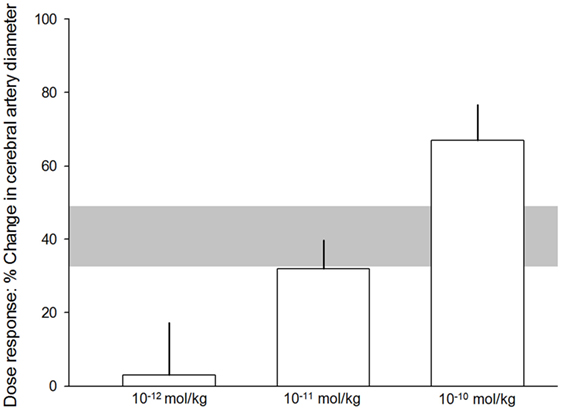
Figure 3. Dose–response relationship. 10−12 mol/kg of alpha calcitonin gene-related peptide (αCGRP), 3.1 ± 14.2%. 10−11 mol/kg αCGRP, 32 ± 7.8%. 10−10 mol/kg αCGRP, 67 ± 9.7%. The error bars represent SE while the shaded gray area represents the SE of the grouped (all experiments with single doses) estimate. Differences were statistically significant (p < 0.05).
The effect size tended to be lower in studies that reported randomization, blinded assessment of outcome, blinded induction of SAH, and use of an anesthetic agent without intrinsic neuroprotective properties. However, none of these observations reached statistical significance. There was also a trend toward lower effect size in studies reporting compliance with more quality checklist items. This ranged from 57.3 ± 10.7% (p < 0.05) from experiments with a quality score (QS) of 1 to 28.1 ± 9.1% (p < 0.01) from experiments with a QS of 6 (see Figure 4). There was no statistically significant difference in treatment effect between species.
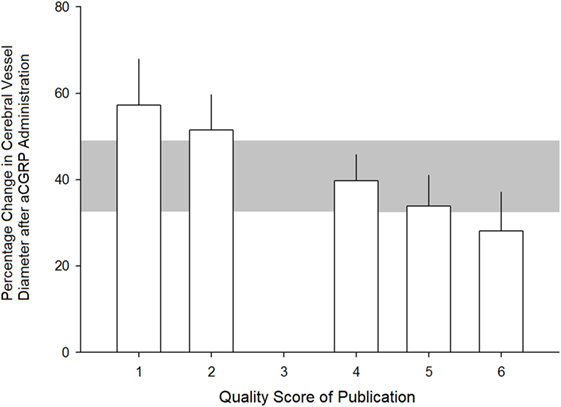
Figure 4. Relationship between quality score of studies and estimated efficacy of alpha calcitonin gene-related peptide at dilating cerebral arteries. See Section “Methods” for quality scoring criteria. Error bars represent SE while the gray area represents the grouped (all studies) estimate. Differences were not statistically significant (p > 0.05).
Neurological Outcome and Adverse Effects
Four studies reported an effect on neurological outcome after αCGRP administration (17, 21, 22, 35). The standardized mean difference was 1.31 (95% CI −0.49 to 3.12, Q 40.5, n = 65 animals) in favor of αCGRP. Tian et al. reported neurological outcome based on a comprehensive scoring system (0–48, 0 = best score, 48 = worst score) measured three times daily and based upon the assessment of four functions, which has been used elsewhere (35, 44). The mean neurological outcome on day 7 for the αCGRP group was significantly better than for the control group (10.67 ± 1.16 versus 22.33 ± 2.08, respectively, p < 0.001). Imaizumi et al. assessed neurological outcome based on food intake and a slope tolerance test on days 2, 3, and 4 post-SAH (21). No significant difference was found between the αCGRP and control groups for either assessment. Inoue et al. reported no significant difference in food intake, observable hemiparesis, consciousness disturbance or response to stimulation between the αCGRP and control groups (22). Ahmad et al. reported neurological outcome from grade I (normal) to grade III (unable to stand and presented abnormal posture) in addition to performing a slope tolerance test. Two rabbits in the control group were grade II (slow in response but able to walk) and III, respectively, all other rabbits were normal, and there was no statistical difference between the groups in the slope tolerance test. In all studies where it was measured, food intake was decreased after SAH, but there was no significant difference between the αCGRP and control groups. Inoue et al. noted a significant decrease in weight in their αCGRP group compared with the control group at day 14, but note no other adverse effects and were unable to explain this change in weight.
Eight publications reported physiological parameters that might be associated with adverse events. There was no significant difference in systemic arterial pressures or arterial blood gas results between the aCGRP or control groups. Imaizumi et al. found that all animals tended to have an increased respiratory rate for approximately 6 h after intrathecal injection of either αCGRP or vehicle and demonstrated a high blood pH and low pCO2, but again no difference between the groups (21). Both Nozaki et al. (16) and Toshima et al. (36) demonstrated a decrease in mean arterial blood pressure when αCGRP was administered intravenously, which was not seen by intrathecal administration. The study by Toshima et al. demonstrates a marked decrease in mean arterial blood pressure following intravenous administration of αCGRP which is not seen with intracisternal administration (~70 versus ~40 mmHg at 30 min after αCGRP administration).
Discussion
Intrathecal administration of αCGRP dilates cerebral arteries in a dose-dependent manner in animal models and appears to be associated with fewer systemic effects than intravenous administration, chiefly the avoidance of hypotension. Furthermore, the effect of αCGRP on cerebral arteries appears to be more pronounced in the context of SAH, possibly because sensitivity of the artery to αCGRP may be greater owing to the depletion of endogenous αCGRP after SAH (30, 45). Alternatively, it may be that these arteries have a higher capacity for dilation after being constricted following SAH. In addition to the decreased systemic side effects seen by intrathecal administration, this route exposes αCGRP to the abluminal side of the blood vessel wall in a way more akin to its endogenous action. αCGRP is able to dilate cerebral arteries independently of endothelial cells, which are morphologically damaged after SAH and so avoids a problem associated with endothelin antagonists (46, 47).
Other effects of αCGRP were also reported. Sun et al. observed a reduction in cortical cell death, decreased endothelial death and upregulated vascular endothelial growth factor with evidence of angiogenesis after αCGRP administration (32).
There is robust evidence that αCGRP dilates cerebral blood vessels after SAH in animal models, and there is a clear association between DCI and cerebral vessel narrowing in humans. However, it remains unclear whether the association between cerebral vessel narrowing and DCI is causative. Etminan et al. noted in their systematic review and meta-analysis (4,235 patients) that pharmaceutical interventions have decreased the incidence of radiographic vasospasm (decreased cerebral artery diameter on angiography or increased flow velocity on transcranial Doppler) without decreasing poor clinical outcomes (48). The authors note that methodological problems, inadequate sample sizes, insensitivity of clinical outcome measures and mechanisms other than vasospasm that also contribute to poor outcomes could explain the dissociation between vasospasm and clinical outcome in their review. In contrast to these findings, Kimball et al. in a systematic review found that 24 of 27 publications (1,028 patients) reporting the use of transluminal balloon angioplasty noted an improvement in vessel diameter and neurological deficits (49). This review also included mostly small, low-quality studies (based upon the GRADE classification system) (50).
The CONSCIOUS trials investigated the effect of the endothelin-receptor antagonist (ERA), Clazosentan, in the treatment of DCI after SAH (51, 52). Clazosentan was found to produce a dose-dependent reduction in angiographic vasospasm but no significant effect on morbidity or mortality. Endothelin is involved in the regulation of a large variety of organ functions apart from its vasoconstrictor function. It may be that Clazosentan successfully reduced arterial narrowing but also inhibited endothelin’s organ regulatory functions masking any improvement due to improved cerebral diameter. Laban et al., in their systematic review of experimental SAH studies of ERAs, demonstrated a 54% improvement in vessel diameter after administration but no significant effect on mortality and no studies reported effects on functional outcomes (53). The authors concluded that there was no neurobehavioral data to support progression from preclinical to clinical trials for ERAs. In contrast to ERAs, some of the experiments in this review did examine neurobehavioral scores. While there is not a large amount of data, there is a positive signal consistent with a substantial effect. Similarly, a non-statistically significant improvement in outcome for the CGRP group was seen in the European CGRP in SAH trial (RR 0.88, CI 0.60–1.28). Johnston et al. did observe a statistically significant 88.9% treatment preference for CGRP versus placebo in their small study (38, 39). Therefore, although we do not think there is sufficient evidence to support progression directly to a Phase III trial we do think progression to a Phase I trial is appropriate.
Limitations of Studies
There were no female-only experiments and the majority of experiments in this systematic review used the single hemorrhage model of SAH (60%). The other forms were double injection and clot placement. Animals rarely develop a vessel narrowing-related ischemic neurological deficit from any of these methods. Megyesi et al. note that this is probably because animal brains have a plentiful collateral blood supply (54). While this is probably irrelevant for measuring the effect of αCGRP on vessel diameter, it becomes more problematic when trying to assess neurological outcomes. Another translational problem arises from the times of administration of αCGRP and assessment of neurobehavioral outcomes. In humans, DCI is said to occur most commonly between days 3 and 10 and cerebral vessel narrowing is maximal between days 6 and 10 after ictus (12). The experiments assessed in this review administered αCGRP before and up to 3 days after SAH and assessed the response within hours to one week after administration (Table 1). The authors also note that the best model of vasospasm seems to be the primate model in which a blood clot is placed around a large cerebral vessel (54). Only one of the studies we analyzed uses this model. More recently, Titova et al. in their systematic review state that dog models of SAH are considered superior and that the ability of murine models to reflect human vasospasm is disputed (55).
One of the challenges of translating these animal data and methods to human trials is the invasive nature of the intrathecal route. Previous human trials with intravenous administration of αCGRP have used a continuous infusion owing to the short half-life of αCGPR in the systemic circulation (approximately 7–10 min) (56). However, when administered into the cerebrospinal fluid the effects of αCGRP have continued for 4–6 h (21, 26). Therefore, a continuous infusion of αCGRP into the cerebral spinal fluid may not be necessary. Furthermore, Toyoda et al. (37) and Sun et al. (32) demonstrate novel approaches to administering αCGRP, one via gene transfer and the other by intranasal delivery. If intraventricular administration of αCGRP in humans ameliorates cerebral vessel narrowing and avoids the adverse effects seen with intravenous delivery, both gene transfer and intranasal delivery offer potential alternatives without the difficulties associated with an intraventricular drain.
Conclusion
We demonstrate a significant dilation of cerebral arteries after αCGRP administration in animal models of SAH. However, there is insufficient animal data to determine the effect of αCGRP on neurobehavioral outcomes after SAH. We recommend that any future experimental studies investigating the effect of αCGRP in SAH include neurobehavioral scores as an outcome measure. The dilatory effect of αCGRP appears augmented after SAH, and there is some evidence that systemic effects of αCGRP are lessened by intrathecal administration compared with intravenous administration.
Author Contributions
LF and CB conducted independent literature searches and performed dual data entry. LF wrote the manuscript. PA and MM edited the manuscript and provided input into review design. MM performed statistical analysis.
Conflict of Interest Statement
LF and PA have previously applied to the Medical Research Council for funding for a clinical trial involving the administration of αCGRP to patients who have suffered an aSAH.
References
1. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry (2007) 78:1365–72. doi:10.1136/jnnp.2007.117655
2. Anderson C, Anderson N, Bonita R, Dunbabin D, Hankey G, Jamrozik K, et al. Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian cooperative research on subarachnoid hemorrhage study (ACROSS). Stroke (2000) 31:1843–50. doi:10.1161/01.STR.31.8.1843
3. Linn FH, Rinkel GJ, Algra A, van Gijn J. Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta-analysis. Stroke (1996) 27:625–9. doi:10.1161/01.STR.27.4.625
4. Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology (1998) 50:1413–8. doi:10.1212/WNL.50.5.1413
5. Rivero-Arias O, Gray A, Wolstenholme J. Burden of disease and costs of aneurysmal subarachnoid haemorrhage (aSAH) in the United Kingdom. Cost Eff Resour Alloc (2010) 8:6. doi:10.1186/1478-7547-8-6
6. Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke (1997) 28:660–4. doi:10.1161/01.STR.28.3.660
7. Ingall T, Asplund K, Mahonen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke (2000) 31:1054–61. doi:10.1161/01.STR.31.5.1054
8. Brilstra EH, Rinkel GJ, Algra A, van Gijn J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology (2000) 55:1656–60. doi:10.1212/WNL.55.11.1656
9. Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage Part I: incidence and effects. J Clin Neurosci (1994) 1:19–26. doi:10.1016/0967-5868(94)90021-3
10. Roos YB, de Haan RJ, Beenen LF, Groen RJ, Albrecht KW, Vermeulen M. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: a prospective hospital based cohort study in the Netherlands. J Neurol Neurosurg Psychiatry (2000) 68:337–41. doi:10.1136/jnnp.68.3.337
11. Hijdra A, Van Gijn J, Stefanko S, Van Dongen KJ, Vermeulen M, Van Crevel H. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: clinicoanatomic correlations. Neurology (1986) 36:329. doi:10.1212/wnl.36.3.329
12. Flynn L, Andrews P. Advances in the understanding of delayed cerebral ischaemia after aneurysmal subarachnoid haemorrhage. F1000Res (2015) 4:F1000 Faculty Rev–1200. doi:10.12688/f1000research.6635.1
13. Kokkoris S, Andrews P, Webb DJ. Role of calcitonin gene-related peptide in cerebral vasospasm, and as a therapeutic approach to subarachnoid hemorrhage. Front Endocrinol (2012) 3:135. doi:10.3389/fendo.2012.00135
14. Arienta C, Balbi S, Caroli M, Fumagalli G. Depletion of calcitonin gene-related peptide in perivascular nerves during acute phase of posthemorrhagic vasospasm in the rabbit. Brain Res Bull (1991) 27:605–9. doi:10.1016/0361-9230(91)90034-H
15. Juul R, Hara H, Gisvold SE, Brubakk AO, Fredriksen TA, Waldemar G, et al. Alterations in perivascular dilatory neuropeptides (CGRP, SP, VIP) in the external jugular vein and in the cerebrospinal fluid following subarachnoid haemorrhage in man. Acta Neurochir (Wien) (1995) 132:32–41. doi:10.1007/BF01404845
16. Nozaki K, Kikuchi H, Mizuno N. Changes of calcitonin gene-related peptide-like immunoreactivity in cerebrovascular nerve fibers in the dog after experimentally produced subarachnoid hemorrhage. Neurosci Lett (1989) 102:27–32. doi:10.1016/0304-3940(89)90302-9
17. Ahmad I, Imaizumi S, Shimizu H, Kaminuma T, Ochiai N, Tajima M, et al. Development of calcitonin gene-related peptide slow-release tablet implanted in CSF space for prevention of cerebral vasospasm after experimental subarachnoid haemorrhage. Acta Neurochir (Wien) (1996) 138:1230–40. doi:10.1007/BF01809753
18. Feng W, Tian XH, Zhuang ZW, Chen E, Kang JL, Sun J, et al. The treatment of cerebral vasospasm after subarachnoid hemorrhage with calcitonin gene-related peptide gene. [Chinese]. Zhonghua Shen Jing Ge Za Zhi (2013) 46:614–9.
19. Holland JP, Sydserff SG, Taylor WA, Bell BA. Calcitonin gene-related peptide reduces brain injury in a rat model of focal cerebral ischemia. Stroke (1994) 25:2055–8; discussion 2058–2059. doi:10.1161/01.STR.25.10.2055
20. Hongo K, Tsukahara T, Kassell NF, Ogawa H. Effect of subarachnoid hemorrhage on calcitonin gene-related peptide-induced relaxation in rabbit basilar artery. Stroke (1989) 20:100–4. doi:10.1161/01.STR.20.1.100
21. Imaizumi S, Shimizu H, Ahmad I, Kaminuma T, Tajima M, Yoshimoto T. Effect of calcitonin gene-related peptide on delayed cerebral vasospasm after experimental subarachnoid hemorrhage in rabbits. Surg Neurol (1996) 46:263–71. doi:10.1016/0090-3019(96)00048-1
22. Inoue T, Shimizu H, Kaminuma T, Tajima M, Watabe K, Yoshimoto T. Prevention of cerebral vasospasm by calcitonin gene-related peptide slow-release tablet after subarachnoid hemorrhage in monkeys. Neurosurgery (1996) 39:984–90. doi:10.1227/00006123-199611000-00020
23. Locatelli M. The importance of substance P and calcitonin gene related peptide as vasodilator neuropeptide during acute phase of experimental posthemorrhagic vasospasm. J Neurosurg Sci (2000) 44:186–91.
24. Nozaki K. [Reversal of experimental cerebral vasospasm by neuropeptides]. Nihon Geka Hokan (1990) 59:55–67.
25. Nozaki K, Okamoto S, Uemura Y, Kikuchi H, Mizuno N. Vascular relaxation properties of calcitonin gene-related peptide and vasoactive intestinal polypeptide in subarachnoid hemorrhage. J Neurosurg (1990) 72:792–7. doi:10.3171/jns.1990.72.5.0792
26. Nozaki K, Uemura Y, Okamoto S, Kikuchi H, Mizuno N. Relaxant effect of calcitonin gene-related peptide on cerebral arterial spasm induced by experimental subarachnoid hemorrhage in dogs. J Neurosurg (1989) 71:558–64. doi:10.3171/jns.1989.71.4.0558
27. Satoh M, Perkins E, Kimura H, Tang J, Chun Y, Heistad DD, et al. Posttreatment with adenovirus-mediated gene transfer of calcitonin gene-related peptide to reverse cerebral vasospasm in dogs. J Neurosurg (2002) 97:136–42. doi:10.3171/jns.2002.97.1.0136
28. Shimizu H, Imaizumi S, Ishtiaq A, Kaminuma T, Tajima M, Yoshimoto T. Effect of calcitonin gene-related peptide and vasoactive intestinal polypeptide on delayed cerebral vasospasm studied after experimental subarachnoid hemorrhage in rabbits. [Japanese]. Neurol Surg (1994) 22:131–9.
29. Shiokawa Y, Holst JJ, Torben J, Rasmussen N, Schmidt P, Svendgaard NA. Cerebrovascular changes following administration of gammaglobulins against substance P or calcitonin gene related peptide in monkey with subarachnoid haemorrhage. Br J Neurosurg (1993) 7:507–18. doi:10.3109/02688699308995073
30. Sobey CG, Heistad DD, Faraci FM. Effect of subarachnoid hemorrhage on dilatation of rat basilar artery in vivo. Am J Physiol Heart Circ Physiol (1996) 271:H126–32.
31. Song J, Zhang M, Liang Q, Sui L, Xi L, Wang W. Correlation of calcitonin gene-related peptide and endothelin receptor A with subarachnoid hemorrhage. Neural Regen Res (2011) 6:47–54.
32. Sun BL, Shen FP, Wu QJ, Chi SM, Yang MF, Yuan H, et al. Intranasal delivery of calcitonin gene-related peptide reduces cerebral vasospasm in rats. Front Biosci (Elite Ed) (2010) 2:1502–13. doi:10.2741/e209
33. Sutter B, Suzuki S, Arthur AS, Kassell NF, Lee KS. Effects of subarachnoid hemorrhage on vascular responses to calcitonin gene-related peptide and its related second messengers. J Neurosurg (1995) 83:516–21. doi:10.3171/jns.1995.83.3.0516
34. Sutter B, Suzuki S, Kassell NF, Lee KS. Characteristics of relaxation induced by calcitonin gene-related peptide in contracted rabbit basilar artery. J Neurosurg (1995) 82:91–6. doi:10.3171/jns.1995.82.1.0091
35. Tian XH, Wang ZG, Meng H, Wang YH, Feng W, Wei F, et al. Tat peptide-decorated gelatin-siloxane nanoparticles for delivery of CGRP transgene in treatment of cerebral vasospasm. Int J Nanomedicine (2013) 8:865–76. doi:10.2147/ijn.s39951
36. Toshima M, Kassell NF, Tanaka Y, Dougherty DA. Effect of intracisternal and intravenous calcitonin gene-related peptide on experimental cerebral vasospasm in rabbits. Acta Neurochir (Wien) (1992) 119:134–8. doi:10.1007/BF01541797
37. Toyoda K, Faraci FM, Watanabe Y, Ueda T, Andresen JJ, Chu Y, et al. Gene transfer of calcitonin gene-related peptide prevents vasoconstriction after subarachnoid hemorrhage. Circ Res (2000) 87:818–24. doi:10.1161/01.RES.87.9.818
38. Bailey IC, Lyttle JA, Matthew B, Braadvert G, Nelson RJ, Stranjalis G, et al. Effect of calcitonin-gene-related peptide in patients with delayed postoperative cerebral ischaemia after aneurysmal subarachnoid haemorrhage. European CGRP in subarachnoid haemorrhage study group. Lancet (1992) 339:831–4.
39. Johnston FG, Bell BA, Robertson IJ, Miller JD, Haliburn C, O’Shaughnessy D, et al. Effect of calcitonin-gene-related peptide on postoperative neurological deficits after subarachnoid haemorrhage. Lancet (1990) 335:869–72. doi:10.1016/0140-6736(90)90473-I
40. Juul R, Aakhus S, Björnstad K, Gisvold SE, Brubakk AO, Edvinsson L. Calcitonin gene-related peptide (human alpha-CGRP) counteracts vasoconstriction in human subarachnoid haemorrhage. Neurosci Lett (1994) 170:67–70. doi:10.1016/0304-3940(94)90240-2
41. Flynn LMC, Macleod MR, Begg CJ, Andrews PJ. Efficacy of alpha-calcitonin gene-related peptide in dilating cerebral arteries: protocol for a systematic review and meta-analysis of in vivo animal studies. Evid Based Preclin Med (2016) 3:1–3. doi:10.1002/ebm2.13
42. Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci (2007) 30:433–9. doi:10.1016/j.tins.2007.06.009
43. Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, et al. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods (2014) 221:92–102. doi:10.1016/j.jneumeth.2013.09.010
44. Yokoo N, Sheng H, Mixco J, Homi HM, Pearlstein RD, Warner DS. Intraischemic nitrous oxide alters neither neurologic nor histologic outcome: a comparison with dizocilpine. Anesth Analg (2004) 99:896–903, table of contents. doi:10.1213/01.ane.0000132973.32387.8b
45. Toyoda K, Faraci FM, Russo AF, Davidson BL, Heistad DD. Gene transfer of calcitonin gene-related peptide to cerebral arteries. Am J Physiol Heart Circ Physiol (2000) 278:H586–94.
46. Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab (1987) 7:720–8. doi:10.1038/jcbfm.1987.126
47. Findlay JM, Weir BK, Kanamaru K, Espinosa F. Arterial wall changes in cerebral vasospasm. Neurosurgery (1989) 25:736–45; discussion 745–736. doi:10.1227/00006123-198911000-00008
48. Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab (2011) 31:1443–51. doi:10.1038/jcbfm.2011.7
49. Kimball MM, Velat GJ, Hoh BL; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Critical care guidelines on the endovascular management of cerebral vasospasm. Neurocrit Care (2011) 15:336–41. doi:10.1007/s12028-011-9600-1
50. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ (2004) 328:1490. doi:10.1136/bmj.328.7454.1490
51. Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol (2011) 10:618–25. doi:10.1016/s1474-4422(11)70108-9
52. Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke (2008) 39:3015–21. doi:10.1161/strokeaha.108.519942
53. Laban KG, Vergouwen MD, Dijkhuizen RM, Sena ES, Macleod MR, Rinkel GJ, et al. Effect of endothelin receptor antagonists on clinically relevant outcomes after experimental subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab (2015) 35(7):1085–9. doi:10.1038/jcbfm.2015.89
54. Megyesi JF, Vollrath B, Cook DA, Findlay JM. In vivo animal models of cerebral vasospasm: a review. Neurosurgery (2000) 46:448–60; discussion 460–441. doi:10.1097/00006123-200002000-00035
55. Titova E, Ostrowski RP, Zhang JH, Tang J. Experimental models of subarachnoid hemorrhage for studies of cerebral vasospasm. Neurol Res (2009) 31:568–81. doi:10.1179/174313209x382412
Keywords: calcitonin gene-related peptide, CGRP, cerebral vasospasm, delayed cerebral ischemia, subarachnoid hemorrhage
Citation: Flynn LMC, Begg CJ, Macleod MR and Andrews PJD (2017) Alpha Calcitonin Gene-Related Peptide Increases Cerebral Vessel Diameter in Animal Models of Subarachnoid Hemorrhage: A Systematic Review and Meta-analysis. Front. Neurol. 8:357. doi: 10.3389/fneur.2017.00357
Received: 08 May 2017; Accepted: 06 July 2017;
Published: 25 July 2017
Edited by:
Nikolaus Plesnila, Institute for Stroke and Dementia Research, GermanyReviewed by:
George C. Wellman, University of Vermont, United StatesW. Taylor Kimberly, Massachusetts General Hospital, United States
Copyright: © 2017 Flynn, Begg, Macleod and Andrews. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liam M. C. Flynn, lmc.flynn@gmail.com
 Liam M. C. Flynn
Liam M. C. Flynn Caroline J. Begg2
Caroline J. Begg2 Peter J. D. Andrews
Peter J. D. Andrews