- 1Department of Neurosurgery, Yale School of Medicine, New Haven, CT, United States
- 2Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT, United States
- 3Division of Neurology, Children’s Hospital of Philadelphia, Philadelphia, PA, United States
- 4Department of Neurology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
- 5Yale School of Medicine, New Haven, CT, United States
- 6Department of Pediatrics, Yale School of Medicine, New Haven, CT, United States
- 7Department of Cellular and Molecular Physiology, Yale School of Medicine, New Haven, CT, United States
We present two recent cases of toddlers who developed malignant cerebellar edema subsequent to accidental ingestion of prescription opioids. Both children presented acute neurological decline, hydrocephalus, and tonsillar herniation requiring emergent ventricular drain placement, suboccipital craniectomy, and partial cerebellectomy. Together with several other reports, these cases suggest the existence of an uncommon yet severe syndrome of acute opioid-induced malignant cerebellar edema. We hypothesize that the condition results from a combination of primary opioid receptor-mediated changes in neuronal metabolism that are exacerbated by secondary hypoxic insult. If recognized promptly, this syndrome can be treated with emergent neurosurgical intervention with good clinical outcomes. These cases also illustrate the unintended consequences and innocent victims of the spiraling prescription opioid epidemic, which will likely increase in prevalence. Recognition of this syndrome by clinicians is thus critical.
Introduction
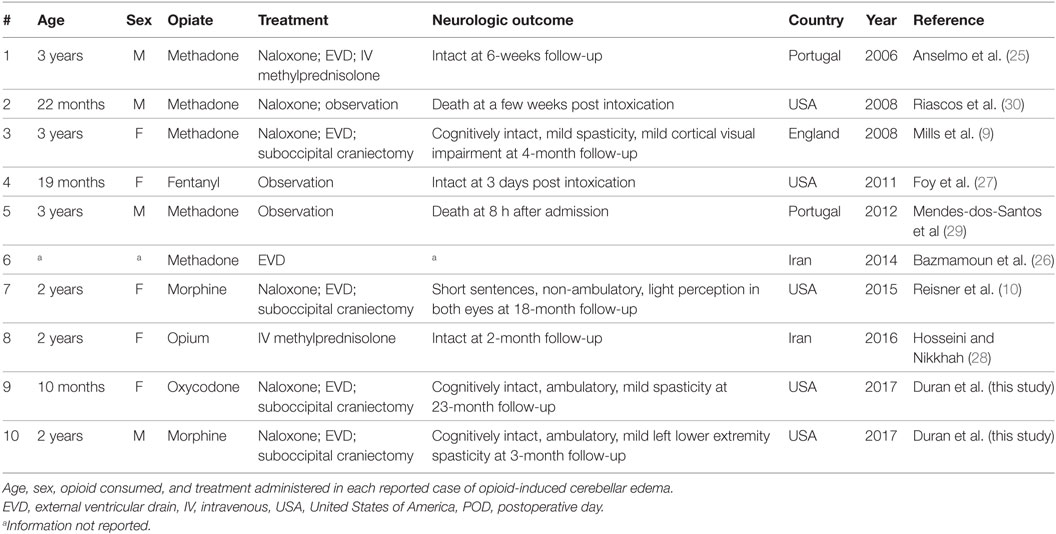
The United States (US) is in the midst of a nation-wide drug overdose epidemic (1). Data from the Centers for Disease Control and Prevention reveals that drug overdoses increased by close to threefold between 1999 and 2014. In 2015, the death toll from drug overdose in the US was 52,404. Over 60% of these deaths were opioid-related (1). It has been well-established that legal opioid prescription patterns in the US correlate with opioid-related deaths (2). Nearly 50% of all opioid-related deaths in the US involve a prescription opioid (3), with methadone, oxycodone, morphine, and hydrocodone, the most commonly abused drugs in this category (4).
Opioid-induced neurotoxicity is a multifactorial syndrome, with a wide spectrum of symptoms, including confusion, hallucinations, delirium, and seizures, which have been well described in adults (5). In contrast, the effects of opioid intoxication in children are poorly understood and have been described in only a handful of cases (6). A better understanding of the clinical presentation, radiographic findings, and pathophysiological mechanisms of accidental prescription opioid intoxication in children is important given the persistently elevated opioid prescription rates across the US (7).
Herein, we present two recent cases of toddlers who ingested prescription opioids resulting in acute neurological decline, with associated malignant cerebellar edema followed by hydrocephalus, tonsillar herniation, and the need for emergent neurosurgical intervention for life-saving treatment. Together with other recent reports depicting a nearly identical clinical-radiographic presentation, these cases suggest the existence of an uncommon yet severe syndrome of malignant cerebellar edema of probable multifactorial origin. An increased awareness of this condition is of paramount importance given the rising prevalence of accidental prescription opioid intoxications among the pediatric population in the context of the ongoing adult opioid epidemic (8).
Background
All procedures in this study comply with Yale University’s Human Investigation Committee and Human Research Protection Program. Oral and written informed consent was obtained from the parents of both patients whose cases are herein reported.
Case 1
A previously healthy 10-month-old female was found by her mother after waking in the morning to have slow, labored breathing. She was unresponsive to voice and tactile stimulation. This prompted a call to 911. On arrival, emergency medical services personnel noted that the infant was non-arousable, bradypneic, and stridorous, with a blood oxygen saturation of 80% on room air. She was transported to the emergency department of a regional hospital, where orotracheal intubation was immediately performed. Her blood pressure on arrival was 93/53 mmHg (mean arterial pressure 66 mmHg). Neurologic examination revealed 1 mm hyporeactive pupils bilaterally, global hypertonia, and hyperreflexia. A non-contrast computed tomography (CT) scan of the head was obtained. Arterial blood gas demonstrated a combined metabolic and respiratory acidosis (pH 7.15, PaCO2 29, PaO2 103, 10). Additional laboratory evaluation revealed mild transaminitis (aspartate aminotransferase 100 IU/L, normal = 8–50 IU/L; alanine aminotransferase 55 IU/L, normal = 7–45 IU/L) and leukocytosis (31,000/mm3, normal = 6,000–11,000/mm3). A broad differential diagnosis of respiratory failure of unknown origin was proposed, and treatment was pursued for possible allergic reaction or meningitis with two doses of epinephrine (0.085 mg), methylprednisolone (2 mg/kg), diphenhydramine (2 mg/kg), and a meningitic dose of ceftriaxone. The patient was then transferred to our tertiary care center.
On admission, workup was expanded with a full toxicology panel, which was positive for oxycodone and its metabolite, oxymorphone. Of note, elevated transaminases raised concern for acetaminophen toxicity; however this was ruled out by a negative result on the toxicology panel. A diagnosis of respiratory failure secondary to opiate ingestion and overdose was established. A total of four doses of naloxone (0.1 mg/kg i.v. push each) were administered with improvement in level of consciousness and reduction of ventilator support that allowed extubation. Continuous naloxone infusion was administered for 5 h thereafter. Results of initial neuroimaging were reviewed and revealed extensive symmetric bilateral cerebellar hypoattenuation (Figure 1A). The patient was admitted to the pediatric intensive care unit and was placed under close observation with frequent neurological exams.
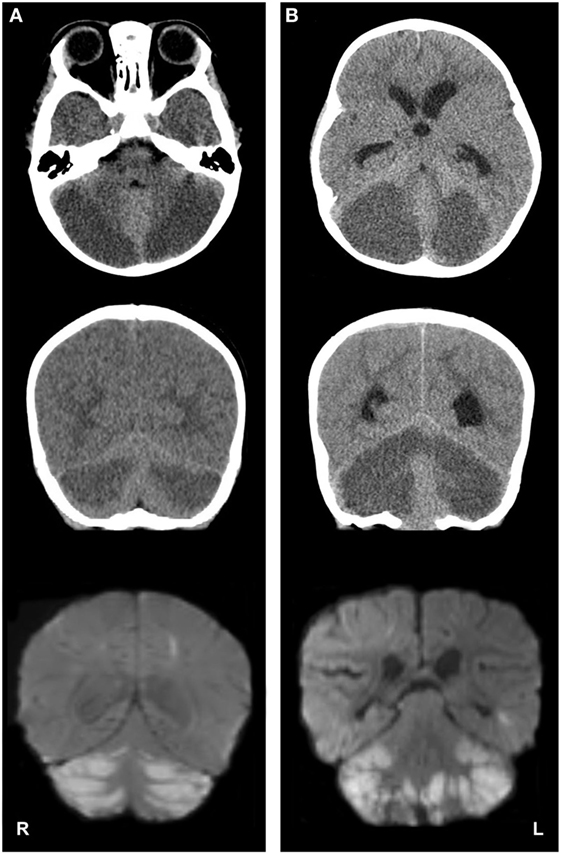
Figure 1. Imaging studies depicting cerebellar edema and restricted water diffusion after accidental opioid overdose in two infants. Panel (A), case 1; panel (B), case 2. Top row: axial computed tomography (CT) images show severe, bilateral cerebellar hypoattenuation, with ventral displacement of the cerebellar vermis and compression of the fourth ventricles. Middle row: coronal CT images demonstrate bilateral cerebellar hypoattenuation and upward displacement of the tectal plate. Bottom row: coronal magnetic resonance diffusion-weighted imaging (DWI) depicts bilateral, symmetric cerebellar diffusion restriction [note: DWI images on panel (B) are postoperative].
On the third day of hospitalization, she became obtunded, with hyporeactive pupillary reflexes, hypertonia, hypertension, and ocular bobbing. Emergent magnetic resonance imaging (MRI) of the brain was obtained. The MRI demonstrated a pattern of symmetric restricted diffusion in the cerebellar hemispheres (Figure 1A), right parietal lobule, the heads of the caudate nuclei bilaterally and the right putamen. Additionally, hydrocephalus, elevation of the tectum, cerebellar tonsillar herniation, and an area of increased susceptibility weighted imaging signal in the left perirolandic area were present which suggested hemorrhagic transformation of an infarct.
The patient was taken emergently to the operating room for placement of an external ventricular drain, suboccipital craniectomy, and a C1 laminectomy. A significant portion of the edematous and ischemic cerebellar hemispheres and tonsils were resected bilaterally, thereby decompressing the posterior fossa and cervicomedullary junction.
A postoperative magnetic resonance angiography (MRA) of the head and neck showed normal anterior and posterior circulations. A full coagulopathy workup and echocardiography were unremarkable. No other visceral or musculoskeletal injuries were noted, and an investigation by the child abuse team provided no evidence for physical abuse. After an uneventful postoperative course, the patient was discharged on postoperative day 11.
Follow-up at 33 months of age (23 months after presentation) demonstrated an active, engaged child who spoke in full sentences. She was able to ambulate, jump, and climb stairs with alternating feet but had mild spasticity and internal rotation of her left leg with gait and occasional dystonic posturing of the left upper extremity.
Case 2
A 25-month-old male with a history of mild reactive airway disease and sickle cell trait was noted by his mother to be breathing slowly and erratically 2 h after being laid down for a nap. Emergency medical services were called and paramedics encountered a pulseless, apneic patient. Cardiopulmonary resuscitation was immediately initiated. Return of spontaneous circulation was achieved in the ambulance on route to our institution’s emergency department.
On arrival, physical examination revealed an afebrile toddler, unresponsive to voice or noxious stimulation with agonal respirations, a blood oxygen saturation of 93% with bag-mask-assisted ventilation, and a blood pressure of 99/65 mmHg (mean arterial pressure 76 mmHg). Neurologic examination demonstrated 3 mm, non-reactive pupils and global hyperreflexia. Orotracheal intubation was performed emergently. Initial laboratory evaluation revealed normal electrolytes (Na+ 138 mEq/L, K+ 4.9 mEq/L, Cl− 104 mEq/L, Ionized Ca2+ 5.2 mEq/L), leukocyte count (11,100/mm3), venous blood gases (pH 7.37, PvCO2 44, PvO2 47, 25.7), and serum lactate (0.9 mmol/L). Upon further interrogation, the patient’s mother reported the presence of prescription opiates (extended release morphine) in the household, which she uses for pain control secondary to frequent sickle cell anemia crises. A total of two doses of naloxone (0.1 mg/kg i.v. push each) were administered, after which the patient regained pupillary reactivity and began moving all four extremities spontaneously. A urine toxicology panel was obtained, which was positive for morphine.
A non-contrast head CT was obtained (Figure 1B), which revealed acute hydrocephalus and extensive, symmetric bilateral cerebellar hemispheric hypoattenuation consistent with severe cerebellar edema that resulted in compression of the fourth ventricle and displacement of the midbrain and pons superiorly. Cervicomedullary junction compression secondary to cerebellar tonsillar herniation was also noted. Neurosurgery was consulted and the patient was emergently taken to the operating room for the placement of an external ventricular drain, suboccipital craniectomy, and a C1 laminectomy. A significant portion of the edematous and ischemic cerebellar hemispheres and tonsils were resected bilaterally, thereby decompressing the posterior fossa and cervicomedullary junction.
A brain MRI obtained on postoperative day 1 demonstrated cerebellar and cervicomedullary junction decompression, with extensive restricted diffusion in the remaining cerebellar parenchyma (Figure 1B). Multifocal areas of diffusion restriction were also noted bilaterally in the anterior cerebral artery-middle cerebral artery and middle cerebral artery-posterior cerebral artery watershed territories involving portions of the frontal, occipital, and temporal lobe white matter. An MRA/MR venogram demonstrated no craniocervical or intracranial arterial abnormalities or evidence of cortical or dural venous sinus thrombosis. The patient was extubated on postoperative day 5. Assessment at postoperative day 18 demonstrated an easily arousable child, with spontaneous eye opening, equally round and reactive pupils, and with purposeful movements of all four extremities.
Outpatient follow-up at 29 months of age (3 months after presentation) demonstrated an active child speaking in full sentences. He was able to ambulate independently, albeit with a slightly widened base of support, and mild left lower extremity spasticity which did not limit his ability to climb or descend stairs with rail support.
Discussion
We describe two toddlers with malignant cerebellar edema after accidental prescription opioid ingestion. Both children developed acute neurological decline, hydrocephalus, and tonsillar herniation that required emergent cerebrospinal fluid diversion and surgical decompression of their posterior fosse. In both instances, patients had good clinical outcomes though with some deficits considering the life-threatening nature of their presentations. To our knowledge, there are only two other reported cases of children requiring surgical posterior fossa decompression for malignant cerebellar edema after accidental prescription opioid ingestion (9, 10). Both cases had nearly identical clinical courses and radiographic findings when compared to our patients. Together, these four cases suggest the existence of an uncommon but severe syndrome of predominantly cerebellar malignant edema. If recognized promptly, this syndrome can be treated with emergent neurosurgical intervention with good clinical outcomes. These cases also illustrate the “trickle-down” effect of the current prescription opioid epidemic, which will likely increase the prevalence of this syndrome.
Direct neurotoxicity is the hallmark of multiple narcotic as well as non-narcotic drugs and toxins. Several molecules with neurotoxic effects exhibit predilection for white matter, which over the years has received the blanket term “toxic leukoencephalopathy” (11). This white matter-predominant pattern of injury has been reported after exposure to various other non-opioid substances including toluene, cocaine, methamphetamine, and chemotherapeutic agents such as 5-fluorouracil and methotrexate, among others (12–17).
The neurologic sequelae of opiate intoxication and overdose are multiple, including brain injury from primary neurotoxicity and secondary hypoxia/anoxia (18–20). Numerous reports and anecdotal descriptions of the direct neurotoxic effects of opiates are present in the medical literature (18, 21, 22). These effects are well documented in adults. Clinically, presentations are heterogeneous and include a wide range of symptoms from decreased alertness to more severe obtundation, delirium, and seizures (5, 23). Histopathologic examination of white matter samples collected from cases of opioid-induced leukoencephalopathy reveal spongiform degeneration, oligodendroglial vacuolization, and fluid entrapment between myelin lamellae, without demyelination (21). Radiologically, on head CT, a symmetric, diffuse hypoattenuation of both supratentorial and infratentorial white matter is often recognized. On MRI, diffuse cortical swelling and cerebellar hyperintensity on T2-weighted and diffusion-weighted imaging (DWI) are present. Interestingly, in adult cases of inhalation exposure to non-prescription opioids, supra and infratentorial leukoencephalopathic changes, hallmarked by cerebellitis is relatively common, and has been labeled “chasing the dragon” syndrome (24).
In contrast, there is a paucity of information regarding the neurological effects of prescription opioid overdose in children. Our comprehensive review of the literature revealed 8 additional cases of acute pediatric opioid-induced leukoencephalopathy with cerebellar edema (Table 1) (9, 10, 25–30). Patients were toddlers, who accidentally consumed their caregiver’s prescription opiates orally with the exception of one case in which administration occurred through a transdermal fentanyl patch (27). In a single instance, the exposure was to raw opium with no mention of the route of administration (28). All children developed obtundation, often severe enough to require orotracheal intubation. Abnormal neurologic examination findings were universally described, and included altered consciousness ranging from obtundation to coma, bradypnea, miotic or hyporesponsive pupils, hyperreflexia and/or hypertonia, and ataxia. Interestingly, in all reported cases, there are several common neuroradiologic characteristics. Often, the first study obtained in the emergency department was a non-contrast head CT, which revealed bilateral and symmetric cerebellar hypoattenuation, with a variable element of cisternal effacement and hydrocephalus, dependent on the magnitude of mass effect exerted by parenchymal edema. On MRI, cerebellar diffusion restriction on DWI, and hyperintensity on T2-weighted and fluid attenuation inversion recovery sequences were frequently described (9, 10, 25, 28). Restricted diffusion, albeit most marked in the cerebellum, was also present in watershed areas of the deep white matter, where circulatory redundancy between the anterior and posterior circulation is minimal. This draws an interesting parallel between opioid-induced acute cerebellar edema in toddlers and “chasing the dragon” syndrome in adults.
Typical areas of selective brain vulnerability to insult vary with age and mechanism. In the pediatric population, these areas are characterized by elevated metabolic activity, such as gray matter, or areas of active myelination (31). In neonates, infants, and toddlers, cerebellar injury secondary to hypoxia or neuroinflammation is uncommon, especially in the setting of hypoxic insult (32). During times of mild to moderate hypoxic–ischemic insult, autoregulatory mechanisms are able maintain perfusion to vital structures such as the brainstem, thalami, basal ganglia, hippocampi, and cerebellum by shunting blood from less metabolically active structures, such as the cerebral cortex and white matter (32). In severe hypoxic–ischemic encephalopathy, the cerebellum is often the last structure affected, leading it to appear bright on head CT (“bright cerebellar sign”). The pattern of primary hypoxic–ischemic encephalopathy is thus different from our cases in which the cerebellum was most severely affected. Furthermore, non-accidental injury was considered in both of our cases but was unlikely given the lack of typical imaging or physical findings to support this diagnosis like signs of external trauma, subdural hemorrhage, or hemorrhage in the high cervical cord or other cervical spine injury (33, 34).
Predominantly cerebellar injury, along with more discrete areas of supratentorial leukoencephalopathy appears to be a distinct pattern as a response to opioid neurotoxicity in the pediatric population. Of note, the distribution and predominance of opiate receptors differs between the adult and developing human cerebellum (35), providing a plausible explanation for the differential presentation of opioid-induce neurotoxicity observed across age groups. Unfortunately, no histopathologic examination of excised cerebellar tissue has been performed in pediatric patients, including the two cases we present above.
The striking resemblance in presentation, patient characteristics, neuroradiologic findings, and triggering mechanism between these cases leads us to believe we are facing a variant of opioid-induced encephalopathy, primarily affecting the pediatric population. We hypothesize this condition is the result of a multifactorial insult primarily affecting white matter, most striking in the cerebellum, due to the combined influence of direct opioid receptor-mediated phenomena altering neuronal and/or glial metabolism, aggravated by the influence of the anoxic injury secondary to respiratory depression or arrest in the context of opioid overdose. Formal pathologic examination of excised tissue in these cases will aid in determining the magnitude and type of acute cellular response in similar cases, allowing to better adjudicate a differential burden of responsibility to primary, opioid-induced neuroinflammation versus hypoxia/anoxia in cases of pediatric opioid-induced neurotoxicity.
Ideal management strategies for pediatric opioid-induced malignant cerebellar edema are yet to be defined, as the natural history and progression of this condition remains poorly described. In acute cerebellar edema of other etiologies (frequently secondary to inflammation—i.e., cerebellitis), there is marked clinical variability; the condition can have a relatively benign and self-limited progression, or result in fulminant swelling leading to tonsillar herniation and death (36). Careful neurologic evaluation and early neuroimaging should be routinely performed for pediatric patients in the setting of opioid intoxication or overdose. Medical management for acute cerebellitis of other etiologies, and in specific cases of opioid-induced pediatric acute malignant cerebellar edema, focused on amelioration of cerebral and cerebellar inflammation with steroids has been attempted (25, 28, 37). The role of surgical intervention in cases of severe pediatric cerebellitis has been previously described (38). Neurosurgical intervention, aimed at posterior fossa and cervicomedullary junction decompression should be considered in cases in which herniation is imminent or in progress and unlikely to be ameliorated with medical therapy alone.
Pediatric opioid overdose remains a relatively rare occurrence in the US (39). However, epidemiologic data and published reports suggest a disturbingly increasing trend, which mirrors the sharp increase in opioid overdose cases in adults (40). As prescription and illegal opiates continue to make their way to households with children, the risk of exposure will continue to increase.
Concluding Remarks
In summary, we describe two cases of pediatric acute toxic cerebellar edema associated with oral prescription opiate ingestion and incorporate these into a comprehensive review of existing literature. Opioid-induced malignant cerebellar edema should be included in the differential diagnosis when a pediatric patient presents with signs and symptoms of opiate intoxication and an abnormal neurologic examination. Raising awareness of this condition is of paramount importance, given the increasing prevalence of pediatric opioid intoxication. Future directions should include microscopic examination of excised cerebellar tissue in this context, to clarify the pathophysiology of this emerging condition.
Ethics Statement
All procedures in this study comply with Yale University’s Human Investigation Committee (HIC) and Human Research Protection Program. Oral and written informed consent was obtained from the parents of both patients whose cases are herein reported.
Author Contributions
DD and RM were the principal authors of this manuscript with guidance from both KK and LB. JM, JK, CF, AS, and YY were significantly involved in the research portion of the paper and contributed to portions of the write up as well. The paper was reviewed, revised, and critically analyzed by GS, MD, and KK for imaging interpretation in the case of GS and the surgical content by MD and KK who performed the surgical procedures on case 1 and case 2, respectively. All throughout the process, LB was instrumental in her guidance and critical evaluation of the manuscript for content and accuracy. LB was the principle editor of the pediatric neurology aspect, RM of the neuroradiology aspect, and KK from the neurosurgical aspect. LB and RM were the principal editors throughout the process and were under the guidance of KK.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the families of their patients for entrusting them with their care.
References
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths – United States, 2010-2015. MMWR Morb Mortal Wkly Rep (2016) 66:1445–52. doi:10.15585/mmwr.mm655051e1
2. Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA (2011) 305:1315–21. doi:10.1001/jama.2011.370
3. Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 199-2015 on CDC WONDER Online Database. CDC WONDER (2016).
4. Ossiander EM. Using textual cause-of-death data to study drug poisoning deaths. Am J Epidemiol (2014) 179:884–94. doi:10.1093/aje/kwt333
6. Ostwal S, Salins N, Deodhar J, Muckaden MA. Fentanyl-induced neurotoxicity in children. J Pain Palliat Care Pharmacother (2015) 29:385–7. doi:10.3109/15360288.2015.1101639
7. Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med (2015) 372:241–8. doi:10.1056/NEJMsa1406143
8. Palmiere C, Staub C, La Harpe R, Mangin P. Parental substance abuse and accidental death in children. J Forensic Sci (2010) 55:819–21. doi:10.1111/j.1556-4029.2010.01349.x
9. Mills F, MacLennan SC, Devile CJ, Saunders DE. Severe cerebellitis following methadone poisoning. Pediatr Radiol (2008) 38:227–9. doi:10.1007/s00247-007-0635-6
10. Reisner A, Hayes LL, Holland CM, Wrubel DM, Kebriaei MA, Geller RJ, et al. Opioid overdose in a child: case report and discussion with emphasis on neurosurgical implications. J Neurosurg (2015) 16:752–7. doi:10.3171/2015.4.PEDS14667
11. Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med (2001) 345:425–32. doi:10.1056/NEJM200108093450606
12. Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol (2014) 32:949–59. doi:10.1200/JCO.2013.53.0808
13. Lin C-M, Liu C-K. Reversible cerebral periventricular white matter changes with corpus callosum involvement in acute toluene-poisoning. J Neuroimaging (2015) 25:497–500. doi:10.1111/jon.12155
14. Narayana PA, Herrera JJ, Bockhorst KH, Esparza-Coss E, Xia Y, Steinberg JL, et al. Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res (2014) 221:220–30. doi:10.1016/j.pscychresns.2014.01.005
15. Sierra S, Luquin N, Carrato C. Lethal leukoencephalopathy secondary to Tegafur, a 5-fluorouracil prodrug. J Neurol Sci (2015) 357:326–8. doi:10.1016/j.jns.2015.07.040
16. Sprague JE, Everman SL, Nichols DE. An integrated hypothesis for the serotonergic axonal loss induced by 3.4-methylenedioxymethamphetamine. Neurotoxicology (1998) 19:427–41.
17. Vosoughi R, Schmidt BJ. Multifocal leukoencephalopathy in cocaine users: a report of two cases and review of the literature. BMC Neurol (2015) 15:208. doi:10.1186/s12883-015-0467-1
18. Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev (2008) 58:192–208. doi:10.1016/j.brainresrev.2008.03.002
19. Monárrez-Espino J, Laflamme L, Rausch C, Elling B, Möller J. New opioid analgesic use and the risk of injurious single-vehicle crashes in drivers aged 50–80 years: a population-based matched case–control study. Age Ageing (2016) 45:628–34. doi:10.1093/ageing/afw115
20. Pattinson KTS. Opioids and the control of respiration. Br J Anaesth (2008) 100:747–58. doi:10.1093/bja/aen094
21. Büttner A, Mall G, Penning R, Weis S. The neuropathology of heroin abuse. Forensic Sci Int (2000) 113:435–42. doi:10.1016/S0379-0738(00)00204-8
22. Singh R, Saini M. Toxic leucoencephalopathy after ‘chasing the dragon’. Singapore Med J (2015) 56:e102–4. doi:10.11622/smedj.2015094
23. Wolters EC, Stam FC, Lousberg RJ, Wijngaarden GKV, Rengelink H, Schipper MEI, et al. Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet (1982) 320:1233–7. doi:10.1016/S0140-6736(82)90101-5
24. Bartlett E, Mikulis DJ. Chasing “chasing the dragon” with MRI: leukoencephalopathy in drug abuse. Br J Radiol (2005) 78:997–1004. doi:10.1259/bjr/61535842
25. Anselmo M, Rainho AC, do Carmo Vale M, Estrada J, Valente R, Correia M, et al. Methadone intoxication in a child: toxic encephalopathy? J Child Neurol (2006) 21:618–20. doi:10.1177/08830738060210071101
26. Bazmamoun H, Fayyazi A, Khajeh A, Sabzehei MK, Khezrian F. A study of methadone-poisoned children referred to Hamadan’s Besat Hospital/Iran. Iran J Child Neurol (2014) 8:34–7.
27. Foy L, Seeyave DM, Bradin SA. Toxic leukoencephalopathy due to transdermal fentanyl overdose. Pediatr Emerg Care (2011) 27:854–6. doi:10.1097/PEC.0b013e31822c281f
28. Hosseini F, Nikkhah A. Acute cerebellitis following opium intoxication: a case report and literature review. J Pediatr Rev (2017) 5:e8803. doi:10.17795/jpr-8803
29. Mendes-dos-Santos C, Geraldo AF, Tavares JB, Neto L, Campos JG. Acute cerebellitis in children: regarding different etiologies. Acta Med Port (2012) 25:38–41. Portuguese.
30. Riascos R, Kumfa P, Rojas R, Cuellar H, Descartes F. Fatal methadone intoxication in a child. Emerg Radiol (2008) 15:67–70. doi:10.1007/s10140-007-0627-8
31. Rocha-Ferreira E, Hristova M. Plasticity in the neonatal brain following hypoxic-ischaemic injury. Neural Plast (2016) 2016:16. doi:10.1155/2016/4901014
32. Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics (2008) 28:417–39. doi:10.1148/rg.282075066
33. Duhaime AC, Gennarelli TA, Thibault LE, Bruce DA, Margulies SS, Wiser R. The shaken baby syndrome. J Neurosurg (1987) 66:409–15. doi:10.3171/jns.1987.66.3.0409
34. Hadley MN, Sonntag VKH, Rekate HL, Murphy A. The infant whiplash-shake injury syndrome: a clinical and pathological study. Neurosurgery (1989) 24:536–40. doi:10.1227/00006123-198904000-00008
35. Zagon IS, Gibo DM, McLaughlin PJ. Adult and developing human cerebella exhibit different profiles of opioid binding sites. Brain Res (1990) 523:62–8. doi:10.1016/0006-8993(90)91635-T
36. Kornreich L, Shkalim-Zemer V, Levinsky Y, Abdallah W, Ganelin-Cohen E, Straussberg R. Acute cerebellitis in children. J Child Neurol (2016) 31:991–7. doi:10.1177/0883073816634860
37. Bozzola E, Bozzola M, Tozzi AE, Calcaterra V, Longo D, Krzystofiak A, et al. Acute cerebellitis in varicella: a ten year case series and systematic review of the literature. Ital J Pediatr (2014) 40:57–57. doi:10.1186/1824-7288-40-57
38. de Ribaupierre S, Meagher-Villemure K, Villemure JG, Cotting J, Jeannet PY, Porchet F, et al. The role of posterior fossa decompression in acute cerebellitis. Child Nerv Syst (2005) 21:970–4. doi:10.1007/s00381-005-1176-7
39. Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr (2016) 170:1195–201. doi:10.1001/jamapediatrics.2016.2154
Keywords: cerebellar edema, opioid intoxication, pediatric critical care, opiates, suboccipital craniectomy
Citation: Duran D, Messina RD, Beslow LA, Montejo JD, Karimy JK, Gavankar Furey C, Sheridan AD, Sze G, Yarman Y, DiLuna ML and Kahle KT (2017) Malignant Cerebellar Edema Subsequent to Accidental Prescription Opioid Intoxication in Children. Front. Neurol. 8:362. doi: 10.3389/fneur.2017.00362
Received: 25 May 2017; Accepted: 07 July 2017;
Published: 25 July 2017
Edited by:
John R. Mytinger, The Research Institute at Nationwide Children’s Hospital, United StatesReviewed by:
Gouri Rao Passi, Choithram Hospital and Research Centre, IndiaChristopher Mario Inglese, Medical College of Wisconsin, United States
Copyright: © 2017 Duran, Messina, Beslow, Montejo, Karimy, Gavankar Furey, Sheridan, Sze, Yarman, DiLuna and Kahle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert D. Messina, robert.messina@yale.edu
†These authors have contributed equally to this work.
 Daniel Duran
Daniel Duran Robert D. Messina
Robert D. Messina Lauren A. Beslow
Lauren A. Beslow Julio D. Montejo
Julio D. Montejo Jason K. Karimy1
Jason K. Karimy1 Charuta Gavankar Furey
Charuta Gavankar Furey Alison D. Sheridan
Alison D. Sheridan