- 1University of Cincinnati College of Medicine, Cincinnati, OH, United States
- 2Department of Neurology, James J and Joan A. Gardner Center for Parkinson’s Disease and Movement Disorders, University of Cincinnati, Cincinnati, OH, United States
- 3Tikvah for Parkinson, Jerusalem, Israel
- 4Departments of Internal Medicine and Neurology, Massachusetts General Hospital, Boston, MA, United States
- 5Harvard Medical School, Boston, MA, United States
- 6Columbia University Medical Center, New York, NY, United States
- 7Department of Neurosurgery, Shaare Zedek Medical Center, Jerusalem, Israel
- 8Department of Neuropsychogeriatrics, Herzog Hospital, Jerusalem, Israel
- 9Faculty of Medicine, Hebrew University, Hadassah Medical Center, Jerusalem, Israel
An Increasing Problem with Multiple Challenges: Parkinson’s Disease (PD)
With a prevalence of 1% in the population older than 65 years old, PD is recognized as the second most common neurodegenerative disorder after Alzheimer disease. PD affects approximately eight million people worldwide (1), more than the combined number of patients diagnosed with multiple sclerosis, muscular dystrophy, and amyotrophic lateral sclerosis (2).
Clinically, PD represents a complex and multifaceted syndrome characterized by a variable combination of motor and non-motor symptoms (3). Motor symptoms include tremor, rigidity, and bradykinesia, frequently associated with alteration of postural stability. Non-motor symptoms include cognitive dysfunctions (frontal dysexecutive syndrome, eventually resulting in cognitive impairment), mood–behavioral disorders (impulsivity, anxiety, and depression), cardiovascular alterations (orthostatic hypotension and supine hypertension), fatigue, sleep abnormalities, and gastrointestinal and urinary dysfunctions.
For over two decades, care for chronic, neurological disorders, including PD, has been fragmented (4). Lack of integration in care delivery has potentiated numerous misconceptions among patients and providers (5, 6), including erroneous understandings of the natural course of PD and of the availability and utility of various treatment modalities (6–8). In addition, blossoming time constraints in many settings frequently limit the possibility to address the crucial roles of physical therapy, dietary therapy (9–12), and fall-prevention programs which can reduce the morbidity and the cost burden of the disease (13) and often thwart the first cause of hospitalization for PD patients.
A Parkinson Care Advocate (PCA) to Promote Continuity of Care
According to Freeman and colleagues’ notion of continuity of care (4), several elements converge to promote the highest quality of care, including relationships, management, information, societal context, and personal agency (volition). Initial qualitative exploration (14) that elicited desires from PD patients and caregivers demonstrated alignment with Freeman’s attributes. Patients’ articulated desires to receive assistance with diagnostic acceptance and prognostication; to obtain accurate information surrounding the disease; and to experience integrated care and periodic follow-up as modalities of treatment evolve (14). As in other neurodegenerative diseases, the therapeutic importance of fortifying a patient’s sense of agency in a phenomenological context that powerfully challenges the sense of self is immense (15, 16).
We, therefore, hypothesize that embodiment of Freeman’s continuity of care model through development and implementation of the PCA may decrease the prevalence of misconceptions about PD among patients and their family members (Figure 1) in addition to promoting coordination and integration of PD care delivery. Similar to the ParkinsonNet (17, 18) model, which involved standardization of physiotherapy for PD patients (17), this model emphasizes that PCAs undertake integrative (19, 20), educational roles within specific key cohorts, including those with poor treatment compliance, low health literacy (21), or advanced-stage disease. Akin to the diabetes educator (22) and other educational providers, implementation of PCAs portend improvements in clinical outcomes through fostering continuity of patient care, surmounting barriers in health literacy, coordinating tailored exercise sessions, and promoting cost-effective programs targeted at prevention (23).
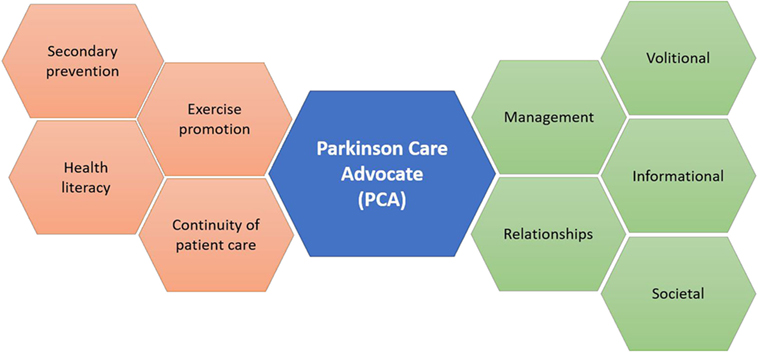
Figure 1. The Parkinson Care Advocate (PCA) will serve to integrate and coordinate multiple dimensions of Parkinson’s disease care, in tune with themes from Freeman’s model.
Education—Internet, Groups, and Health Literacy
Disease education is vital to treatment success (11, 24). PD patients obtain knowledge about their condition from their neurologists (20), whose abilities to engage in comprehensive disease counseling may be limited by time and resource constraints (4); from non-specialized consultants who patients may sparingly visit (13); and from the Internet, often a source of misinformation (24). Regardless of modality, acquiring accurate and actionable information about the disease is crucial. This is especially pressing among patients with reduced health literacy (24) who may experience higher rates of medical non-adherence as a result (24). Community-based studies found that patients feel that inadequate time with their health-care teams is devoted to education (4, 23). While a multitude of Internet resources exist, past research (24) has suggested that only 30% of those over 60 years old use the Internet for health-related information. Moreover, much of the information that may be encountered online may be inaccurate, vague, or outdated (25–27).
If PD patients do seek information via the Internet (a 2017 Google search for “Parkinson’s disease” returned approximately 15,400,000 results), is the available information evidence-based and comprehensible to those who require it (7)? The US Department of Health and Human Services (USDHHS) recommends that health literature be composed at a reading level between the United States equivalent of fourth- and sixth- grade levels (24). This recommendation is at odds with a recent assessment (24) of the top 100 PD web-pages returned from a search, which revealed that most consumer-focused PD web-pages were written at a college undergraduate level, with only 0–4% of these pages satisfying the USDHHS recommendations. Although this specific disease group’s literacy rates have not been empirically explored, PD is primarily a disease of the elderly (aged 65 and older), 61% of whom have a basic or below basic-reading level (24).
To surmount these barriers to comprehensive PD education, initial evidence suggests a role for a PCA-like provider in group (6, 28) and individual (23) settings. For newly diagnosed patients (n = 24) and their caregivers, a 3-h educational session provided information and psychological support (6). Around 87% of respondents believed the session contributed to their ability to explain their illnesses to family and friends; 68% stated that the session aided in their acquaintance with staff; and 78% replied that it made them feel welcome.
Of those with low-health literacy, many will not have achieved a college-level education and will have limited command of the English language. PCAs will, therefore, be charged with translating and formatting written and oral materials into the appropriate dialect at the appropriate literacy level in accordance with USDHHS recommendations. Tailoring the delivery of this knowledge to its intended audience will increase comfort among PD patients with their diagnosis and may reduce the incidence of hospitalizations which are secondary to non-adherence vis-à-vis poor education. Third, as caregivers and family members are often neglected during disease planning, an active and involved PCA may prove salutary for the social, psychological, spiritual (29, 30), and physical well-being of patients’ caregivers and family members (31).
Tailored Care through Exercise
Several mechanisms (9) explain the motor- and non-motor- benefits PD patients derive from various types of exercise (Table 1), including but not limited to the prevention of secondary complications, such as falls (13, 32). This notion might be even more salient within certain ethnicities, as data, for example, suggest that PD-carriers for pathogenic variants in LRRK2 or GBA portend greater risk for freezing of gait and a higher risk for falls (33). Customized plans, through PCAs, thus, should be designed (10), that are personalized to both disease severity (5) and PD phenotype, including postural instability and cognitive dysfunction (33).

Table 1. Mechanisms and types of exercisea for Parkinson’s disease (PD) (9).
Under the supervision of physical therapists and neurologists, PCAs might be charged with coordinating exercise regimens during the most opportune times, consistent with the ON-therapeutic window [“flexibility continuity” (4)] but also with set days and times [“longitudinal continuity” (4)]. Additionally, with the advent of PD wearables that may inform providers of functional status (34), PCAs might serve as human adjuncts [“relational continuity” (4)], sensitive to non-motor features undetected by wearables and promoting the early activation of programs to preserve mobility.
Results from a 2016 study (13) from 231 Australian PD patients, evaluating the cost-effectiveness of a 6-month secondary prevention program for PD patients, found that fewer participants in the intervention (exercise), as compared to the control (no exercise) group, experienced declines in mobility, and the intervention saved 574 AUD and 9,570 AUD for each fall prevented and for each participant who staved off further deterioration in mobility, respectively. Overall, the intervention yielded an 80% chance of cost-effectiveness and participants in the intervention group demonstrated marked economic benefits among all clinical measures, including total falls, frequency of those avoiding severe impairments in mobility, and quality of life years.
Conclusion
Parkinson care advocates will follow their patient base continuously, in tune with Freeman’s model of care. Basic knowledge of PD personalized to patients’ literacies will be provided and community resources offered via meetings within the home or group settings. Exercise regimens aimed at secondary prevention can also be planned under appropriate guidance. The PCA can foreseeably serve as a central point of contact to assess and relay progress to the treatment team, answer questions, coordinates referrals, and offer encouragement. The program’s cost-effectiveness foreseeably offsets investment costs through maintaining or improving QoL, preventing secondary complications, and delaying the need for skilled nursing facility placement. To these ends, this initiative will be instrumental in promoting a more comprehensive, patient-centered, and cost-effective approach to Parkinson care.
Author Contributions
LS conceived of the idea, wrote the first draft of the manuscript, and revised subsequent drafts for intellectual content. DS conceived of the idea and revised the manuscript for intellectual content. MY added and revised the manuscript for important intellectual content. AW added and revised the manuscript for important intellectual content. UH contributed to the intellectual concepts and added and revised the manuscript for important intellectual content. YK, AE, and AM added and revised the manuscript for important intellectual content.
Conflict of Interest Statement
LS has served on the executive editorial board for Carnegie Mellon University’s Triple Helix Journal for Science, Society, and Law, and has received remuneration from Yahoo!, Johnson & Johnson, and Tablet Magazine. He is an ad hoc consultant for Tikvah for Parkinson. DS is founder of Tikvah for Parkinson. She has served as managing editor for BreslovWorld. She has received publishing royalties from Feldheim; Breslev Research Institute; Israel Book Shop; Yafeh Nof; Jerusalem Publications; Art Scroll Publishers; http://Aish.com; Bina; http://www.breslev.co.il; http://Chabad.org; Hamodia; Horizons; Inspiraion; Jerusalem Post; Jerusalem Report; Jewish Homemaker; Jewish Lifestyles; Jewish Observer; Lakewood Shopper; http://OU.org; Voice of Lakewood; Yated Ne’eman American Edition; Yated Ne’eman Israeli Edition; and YeshivaWorld News. MY and AW have nothing to disclose. UH is an ad hoc consultant for Tikvah for Parkinson. YK is a medical advisor to Tikvah for Parkinson. AE has received grant support from NIH, Great Lakes Neurotechnologies, and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, TEVA, Impax, Merz, Acadia, Cynapsus, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from Abbvie, UCB, USWorldMeds, Lundbeck, Acadia, the American Academy of Neurology, and the Movement Disorders Society. He serves as Associate Editor of the Journal of Clinical Movement Disorders and on the editorial board of Parkinsonism and Related Disorders. AM has received grant support from UCB Pharma and speaker honoraria from CSL Behring, UCB Pharma and Teva Pharmaceuticals. He has received personal compensation from Edge Consulting S.r.l., MediK S.r.l., and Sthetos S.r.l.
Abbreviations
PD, Parkinson’s disease; PCA, Parkinson care advocate; QoL, quality of life; USDHHS, US Department and Health and Human Services; UPDRS, Unified Parkinson disease Rating Scale; PT, physical therapist.
References
1. Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurol Clin (1996) 14:317–35. doi:10.1016/S0733-8619(05)70259-0
2. Borlongan CV, Burns J, Tajiri N, Stahl CE, Weinbren NL, Shojo H, et al. Epidemiological survey-based formulae to approximate incidence and prevalence of neurological disorders in the United States: a meta-analysis. PLoS One (2013) 8:e78490. doi:10.1371/journal.pone.0078490
3. Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry (2008) 79:368–76. doi:10.1136/jnnp.2007.131045
4. Aspinal F, Gridley K, Bernard S, Parker G. Promoting continuity of care for people with long-term neurological conditions: the role of the neurology nurse specialist. J Adv Nurs (2012) 68:2309–19. doi:10.1111/j.1365-2648.2011.05928.x
5. Giladi N, Balash Y. The clinical approach to gait disturbances in Parkinson’s disease; maintaining independent mobility. J Neural Transm Suppl (2006) 327–32. doi:10.1007/978-3-211-45295-0_49.
6. Geva N, Hilel A, Manor Y, Arad S, Ezra A, Giladi N, et al. Group meetings for newly diagnosed Parkinson’s disease patients and their spouses: a preliminary experience [abstract]. in (mov disord.). (2016). Available from: http://www.mdsabstracts.org/abstract/group-meetings-for-newly-diagnosed-parkinsons-disease-patients-and-their-spouses-a-preliminary-experience/
7. Robledo I, Jankovic J. Media hype: patient and scientific perspectives on misleading medical news. Mov Disord (2017). doi:10.1002/mds.26993
8. Espay AJ, Lang AE. Common myths in the use of levodopa in Parkinson disease: when clinical trials misinform clinical practice. JAMA Neurol (2017) 74(6):633–4. doi:10.1001/jamaneurol.2017.0348
9. Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol (2013) 12:716–26. doi:10.1016/S1474-4422(13)70123-6
10. Giladi N. Mobility and exercise in movement disorders. Parkinsonism Relat Disord (2009) 15(Suppl 3):S46–8. doi:10.1016/S1353-8020(09)70779-5
11. Sokol LL, Young MJ, Jankovic J. Counseling at-risk Parkinson’s disease cohorts: integrating emerging evidence. Curr Genet Med Rep (2017) 5(2):100–7. doi:10.1007/s40142-017-0116-7
12. Sokol LL, Young MJ, Espay AJ, Postuma RB. Cautionary optimism: caffeine and Parkinson’s disease risk. J Clin Mov Disord (2016) 3:7. doi:10.1186/s40734-016-0037-8
13. Farag I, Sherrington C, Hayes A, Canning CG, Lord SR, Close JCT, et al. Economic evaluation of a falls prevention exercise program among people with Parkinson’s disease. Mov Disord (2016) 31:53–61. doi:10.1002/mds.26420
14. Van Der Eijk M, Nijhuis FAP, Faber MJ, Bloem BR. Moving from physician-centered care towards patient-centered care for Parkinson’s disease patients. Parkinsonism Relat Disord (2013) 19:923–7. doi:10.1016/j.parkreldis.2013.04.022
15. Bramley N, Eatough V. The experience of living with Parkinson’s disease: an interpretative phenomenological analysis case study. Psychol Health (2005) 20:223–35. doi:10.1080/08870440412331296053
16. Young MJ, Bursztajn HJ. Narrative, identity and the therapeutic encounter. Ethics Med Public Health (2016) 2:523–34. doi:10.1016/j.jemep.2016.10.009
17. Munneke M, Nijkrake MJ, Keus SH, Kwakkel G, Berendse HW, Roos RA, et al. Efficacy of community-based physiotherapy networks for patients with Parkinson’s disease: a cluster-randomised trial. Lancet Neurol (2010) 9:46–54. doi:10.1016/S1474-4422(09)70327-8
18. Bloem BR, Munneke M. Revolutionising management of chronic disease: the ParkinsonNet approach. BMJ (2014) 348:g1838. doi:10.1136/bmj.g1838
19. Young MJ, Pham J. Improving the electronic nexus between generalists and specialists: a public health imperative? Healthc (2016) 4:302–6. doi:10.1016/j.hjdsi.2016.10.002
20. Lim SY, Tan AH, Fox SH, Evans AH, Low SC. Integrating patient concerns into Parkinson’s disease management. Curr Neurol Neurosci Rep (2017) 17:3. doi:10.1007/s11910-017-0717-2
21. Hemming JP, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ, et al. Racial and socioeconomic disparities in parkinsonism. Arch Neurol (2011) 68:498–503. doi:10.1001/archneurol.2010.326
22. Heald AH, Anderson SG, Khan A, Stocker J, Davies S, Bliss K, et al. Success rates in a diabetes specialist nurse-led education programme: re-setting the glucostat. Exp Clin Endocrinol Diabetes (2017) 125:297–300. doi:10.1055/s-0042-108055
23. Jarman B, Hurwitz B, Cook A, Bajekal M, Lee A. Effects of community based nurses specialising in Parkinson’s disease on health outcome and costs: randomised controlled trial. BMJ (2002) 324:1072–5. doi:10.1136/bmj.324.7345.1072
24. Fitzsimmons PR, Michael BD, Hulley JL, Scott GO. A readability assessment of online Parkinson’s disease information. J R Coll Physicians Edinb (2010) 40:292–6. doi:10.4997/JRCPE.2010.401
25. Gill HK, Gill N, Young SD. Online technologies for health information and education: a literature review. J Consum Health Internet (2013) 17:139–50. doi:10.1080/15398285.2013.780542
26. Purcell GP, Wilson P, Delamothe T. The quality of health information on the internet. BMJ (2002) 324:557. doi:10.1136/bmj.324.7337.557
27. Zhang Y. Beyond quality and accessibility: source selection in consumer health information searching. J Assoc Inf Sci Technol (2014) 65:911–27. doi:10.1002/asi.23023
28. Peled R, Shpunt D, Manor Y, Brozgol M, Ezra A, Hezi N, et al. Multidisciplinary group program integrating voice and dance movement therapy for Parkinson’s disease patients: preliminary experience. Mov Disord. (2016). Available from: http://www.mdsabstracts.org/abstract/multidisciplinary-group-program-integrating-voice-and-dance-movement-therapy-for-parkinsons-disease-patients-preliminary-experience/
29. Kaufman Y, Anaki D, Binns M, Freedman M. Cognitive decline in Alzheimer disease: impact of spirituality, religiosity, and QOL. Neurology (2007) 68:1509–14. doi:10.1212/01.wnl.0000260697.66617.59
30. Giaquinto S, Bruti L, Dall’Armi V, Palma E, Spiridigliozzi C. Religious and spiritual beliefs in outpatients suffering from Parkinson disease. Int J Geriatr Psychiatry (2011) 26:916–22. doi:10.1002/gps.2624
31. O’Reilly F, Finnan F, Allwright S, Smith GD, Ben-Shlomo Y. The effects of caring for a spouse with Parkinson’s disease on social, psychological and physical well-being. Br J Gen Pract (1996) 46:507–12.
32. Woodford H, Walker R. Emergency hospital admissions in idiopathic’s Parkinson’s disease. Mov Disord (2005) 20:1104–8. doi:10.1002/mds.20485
33. Giladi N, Mirelman A, Thaler A, Orr-Urtreger A. A personalized approach to Parkinson’s disease patients based on founder mutation analysis. Front Neurol (2016) 7:1–5. doi:10.3389/fneur.2016.00071
Keywords: Parkinson, patient-centered, public health, secondary prevention, cost–benefit
Citation: Sokol LL, Shapiro D, Young MJ, Wise AH, Hadelsberg UP, Kaufman Y, Espay AJ and Merola A (2017) The Parkinson Care Advocate: Integrating Care Delivery. Front. Neurol. 8:364. doi: 10.3389/fneur.2017.00364
Received: 17 March 2017; Accepted: 10 July 2017;
Published: 27 July 2017
Edited by:
Pille Taba, University of Tartu, EstoniaReviewed by:
Maurizio Zibetti, University of Turin, ItalyWalter Maetzler, University of Kiel, Germany (Janet Maria Theresia Van Uem contributed to the review of Walter Maetzler)
Copyright: © 2017 Sokol, Shapiro, Young, Wise, Hadelsberg, Kaufman, Espay and Merola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leonard L. Sokol, lsokol@alumni.cmu.edu
 Leonard L. Sokol
Leonard L. Sokol Debbie Shapiro
Debbie Shapiro Michael J. Young
Michael J. Young Adina H. Wise
Adina H. Wise Uri P. Hadelsberg7
Uri P. Hadelsberg7 Alberto J. Espay
Alberto J. Espay Aristide Merola
Aristide Merola