- 1Department of Neurosurgery, Yijishan Hospital, Wannan Medical College, Wuhu, China
- 2Department of Neurosurgery, The First People’s Hospital of Kunshan, Jiangsu University, Suzhou, China
- 3Department of Neurosurgery, The Affiliated Brain Hospital, Nanjing Medical University, Nanjing, China
Objective: Traumatic brain injury (TBI) occurs commonly in children. Repeat computed tomography (CT) follow up of TBI patients is often scheduled to identify progressive hemorrhagic injury (PHI). However, the utility of repeated CT scans, especially in children with mild TBI [Glasgow Coma Scale (GCS) scores of 13–15], has been debated. The purposes of the present study were to identify clinical predictors of PHI in children with mild TBI and to clarify relevant clinical factors via radiological examination.
Methods: From 2014 to 2016, we retrospectively enrolled children <15 years of age with mild TBI. We recorded age, sex, GCS scores on admission, causes of head injury, timing of initial CT, any loss of consciousness, vomiting and seizure data, and type of TBI. Based on repeat CT findings, patients were dichotomized into either a PHI group or a non-PHI group. Also, clinical data were comparatively reviewed. Multivariate logistic regression analysis was used to identify clinical predictors of PHI.
Results: Of the 175 enrolled children, 15 (8.6%) experienced PHI. Univariate analysis revealed that GCS score on admission, cause of head injury, vomiting, seizure, and TBI type were associated with PHI. Multivariate logistic regression analysis showed that a GCS score of 13 and epidural hemorrhage (EDH) were independently associated with PHI (hazard ratio = 0.131, P = 0.018; hazard ratio = 6.612, P = 0.027, respectively).
Conclusion: A GCS score of 13 and EDH were associated with PHI. These factors should be considered when deciding whether to repeat CT on children with mild TBI.
Introduction
Traumatic brain injury (TBI) is common in children and is an important cause of disability and morbidity (1). The incidence of TBI in children is estimated to be 250 per 100,000 per year. TBI accounts for >7,000 deaths and 600,000 emergency department visits annually among children in the United States (2). The vast majority of TBIs in children are mild, requiring no specific therapy, and associated with no sequelae (1). However, a small proportion of patients presenting with mild TBI develop progressive hemorrhagic injury (PHI). Therefore, it is important to recognize and intervene early in PHI to improve prognosis. Computed tomography (CT) scanning for initial evaluation of TBI is well-established. Repeat CT is also frequently performed. However, neither the indications for, nor the timing of, repeat CT scans in children are well-established. Most centers routinely schedule CT for patients with moderate or severe TBI, but the utility of such an approach remains debatable in patients with mild TBI, especially children. Studies advocating routine repeat CT suggest early medical intervention via osmotic therapy or surgical intervention featuring placement of an intracranial pressure monitor or craniotomy before neurological deterioration is evident (3–5). However, several recent studies have questioned this practice, rather advocating the use of repeat CT only for non-examinable patients and those exhibiting no improvement on neurological examination (6, 7). Children are more vulnerable to the carcinogenic effects of radiation than are adults because of their higher cell replication rates and their longer expected lifespans. It is well-known that the younger the age at the time of radiation exposure, the higher the risk of cancer induction (8, 9). Minimizing the number of CT scans is of great importance in children, because of the increased risk of lethal malignancies induced by exposure to ionizing radiation (10, 11). No present guideline addresses the utility of repeat CT in terms of the follow up of mild TBI in children. The purposes of this study were to assess the predictive role of clinical factors on admission in children with mild TBI using sequential radiological examination.
Materials and Methods
This retrospective study was approved by the Institutional Ethics Board of the Yijishan Hospital of Wannan Medical College. All medical records of children <15 years of age who had been hospitalized for mild TBI between January 1, 2014 and December 30, 2016 were reviewed. Data collected included age, sex, Glasgow Coma Scale (GCS) score on admission, causes of head injury, timing of initial CT, any loss of consciousness (LOC), vomiting and seizure status, and type of TBI. Inclusion criteria were as follows: (1) GCS score on admission ≥13; (2) initial head CT within 24 h after trauma; and (3) at least one repeat CT scan within 72 h of admission. Exclusion criteria were as follows: (1) any known coagulation disorder; (2) severe thoracic or abdominal injury; (3) diagnosis of a skull-base fracture based on clinical symptoms; and (4) initial cranial CT scan revealing the need for emergency surgery. PHI was diagnosed if the repeat CT scan showed worsening of the condition because of development of a new lesion or an increase ≥25% of the original lesional volume as shown on the first post-injury CT scan (4). We divided all patients into a PHI group and a non-PHI group.
All data were analyzed using SPSS (SPSS, Inc., Chicago, IL, USA) software. The χ2 test was employed to compare differences in categorical outcome variables between the two groups. We used the independent-samples t-test to compare differences between continuous parametric variables and the Mann–Whitney U test to compare differences between non-parametric continuous variables. Continuous parameters are shown as mean ± SD. After identifying risk factors for PHI via single-factor analysis, we further performed logistic regression analysis to determine predictors of PHI. Such predictors were deemed significant if the probabilities that they were insignificant were less than 0.05.
Results
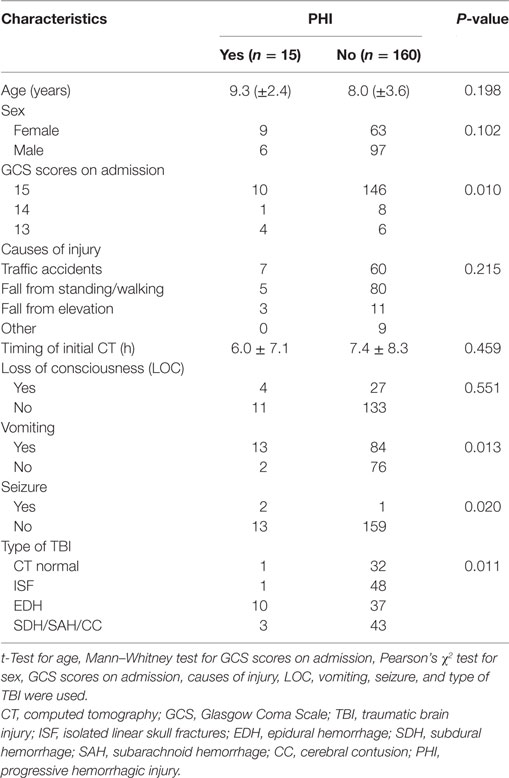
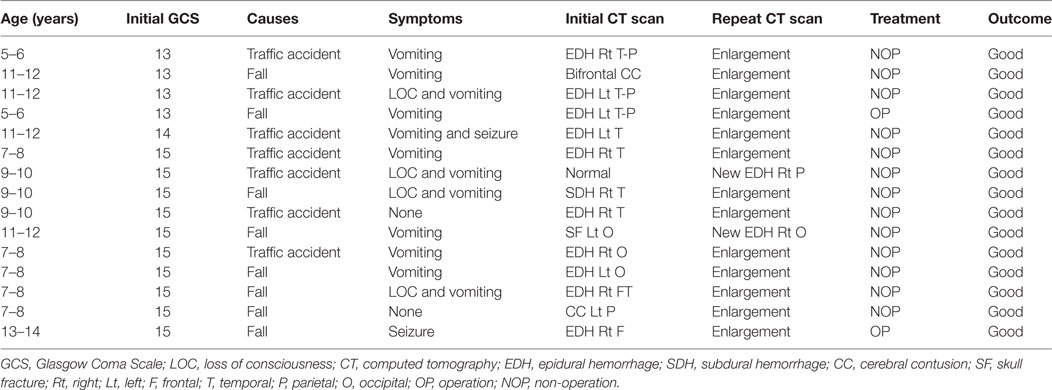
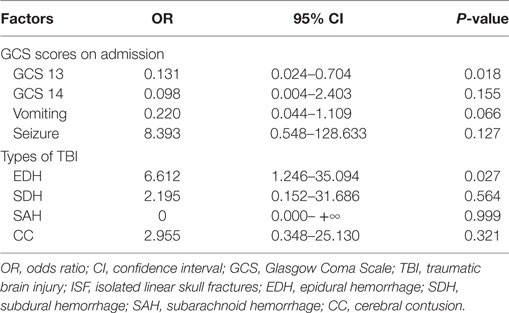
From January 1, 2014 to December 30, 2016, 175 children <15 years of age who presented to Yijishan Hospital of Wannan Medical College with mild TBI met the inclusion criteria. Of these, 103 (58.9%) were males; the mean age was 8.16 ± 3.49 years. Patient demographic characteristics are shown in Table 1. Of the 175 children with mild TBI, 15 patients (8.6%) experienced PHI (Table 2). Falling when standing/walking (n = 85, 48.6%) was the leading cause of injury, followed by traffic accidents (n = 67, 38.3%). In total, 31 patients (17.7%) had documented LOC, 97 (55.4%) had a history of vomiting, and 3 (1.7%) had a history of post-traumatic seizure. Head CT findings on admission were normal in 33 patients; they identified isolated linear skull fractures (ISFs) in 49, epidural hemorrhage (EDH) in 47, subdural hemorrhage in 11, subarachnoid hemorrhage in 16, and cerebral contusion in 19. The PHI and non-PHI group were composed of 15 and 160 patients, respectively. When the two groups were compared, univariate analysis showed that PHI was significantly correlated with the GCS score on admission, causes of head injury, vomiting, seizures, and type of TBI (all P < 0.05) (Table 1). timing of initial CT were 6.0 ± 7.1 and 7.4 ± 8.3 h in the PHI and non-PHI group, respectively; thus, it was shorter in the PHI group, but the difference was not significant (P = 0.459). We found no significant difference in the causes of injury between the PHI group and non-PHI group (P = 0.215), but traffic accidents were the leading cause of PHI (7 of 67, 10.4%). Moreover, neither sex nor LOC was significantly associated with PHI. Of the various potential risk factors, logistic regression identified four predictors (Table 3). Multivariate logistic regression analysis revealed that GCS scores ≥13 and EDH during the early phase of injury were independently associated with PHI (hazard ratio = 0.131, P = 0.018; hazard ratio = 6.612, P = 0.027, respectively).
Discussion
The primary concern when treating patients with TBI is the development of a clinically significant brain injury that must be rapidly identified and treated in a timely manner. As is true of all injuries, prevention is the most powerful tool when combating TBI. Once TBI occurs, however, management is centered on prevention of secondary damage to the brain (1). PHI, a secondary process, is one of the most important and devastating complications after TBI. Because of different evaluation and enrollment criteria, the reported incidences of PHI at different research centers ranged from 8 to 67% (4, 5, 12–14). In the present study, we found that PHI occurred in 15 patients (8.6%), and 2 (1.1%) underwent delayed surgical interventions. Many studies have sought to use PHI-related parameters to define threshold values for predicting PHI in patients with TBI. Several factors have been implicated as PHI determinants, including a medical history of hypertension (13), older age (14, 15), longer time from injury to the first CT scan (16), trauma severity (17), volume of the initial hematoma (17), and coagulopathy (15, 16). These studies focused principally on moderate-to-severe traumatic brain injuries in adults. However, to the best of our knowledge, few efforts have been made to predict PHI after children are admitted with mild TBI. The traumatic pathology of children is completely different from that of adults. This is because child TBI exhibits several distinctive characteristics that differ from those of adults; these differences are attributable to age-related anatomical and physiological differences, the pattern of injury based on the physical activities of children, and the difficulties associated with neurological evaluation in children. Thus, extrapolating results from adult head trauma studies to child populations may not be appropriate from the viewpoint of clinical decision-making. Therefore, the ultimate goal of our study was to assess the utility of admission data in predicting PHI in children with acute mild TBI.
We focused on possible risk factors for PHI, including causes of injury, type of TBI, and GCS scores on admission. Univariate analysis showed that causes of injury exhibited no association with PHI. However, we had only limited data on the severity of trauma, such as the heights of falls and the velocities of vehicles involved in accidents. To obtain more precise data, such factors should be considered.
Previous studies focused on patients with moderate and severe TBI (GCS < 13) and found that a lower GCS score was a risk factor for PHI, which is consistent with our study (12, 18). We found that a GCS score of 13 (hazard ratio = 0.131, P = 0.018) was independently associated with PHI in children with mild TBI.
As shown above, PHI was strongly associated with the type of TBI. Similar to previous studies, we found that children with mild TBI yielding normal cranial CT scans (19, 20) or exhibiting ISFs (6, 21, 22) were at very low risk for PHI and at an extremely low risk of neurosurgical intervention. In our present study, 33 patients with initial normal cranial CT scans and 49 with ISFs underwent repeat CT scans, and 1 patient in each group developed PHI. Neither required neurosurgical intervention, and neither developed sequelae. According to the literature, when initial head CT reveals no intracranial hemorrhage, the recent trend has been to monitor the child clinically. Repeat imaging is not necessary for many children, who can be safely discharged (after the emergency department evaluates the reliability of caretakers) into a safe environment with strict discharge instructions, including indications for a return to hospital.
In line with previous studies, we found that patients with EDHs (hazard ratio = 6.612, P = 0.027) were more prone to develop PHI than were patients with other types of lesions. Howe et al. (23) argued that repeat CT in children not exhibiting clinical deterioration should be avoided, whereas those with EDH were more likely to progress and thus required routine repeat CT, which was indicated in such cases. Kim et al. (18) found that children with EDH were at risk of PHI. A trend indicating the need for repeat CT surveillance in those with EDHs is evident due to the possibility of progression without symptoms and sudden, rapid neurological decline (23). In our present study, 15 patients experienced PHI; 10 of these patients developed acute EDH; and 2 underwent surgical treatment despite the fact that they were clinically stable. Given the high incidence of PHI caused by EDH in this population, potential predictors of a poor outcome, and early intervention may improve prognosis. EDH may represent a unique form of posttraumatic intracranial hematoma in such populations. At our institution, we schedule second routine CT scans for this subgroup of injured children.
Conclusion
We found that a GCS score of 13 and EDH significantly increased the risk of PHI in children <15 years of age with mild TBI. These factors should be considered as indications for repeat CT examination.
Ethics Statement
This retrospective study was approved by the Institutional Ethical Board of the Yijishan Hospital of Wannan Medical College.
Author Contributions
Hongyi Liu and XJ designed the experiments; SC, YD and ZW performed data analysis; Hua Liu provided scientific expertise; GD wrote the manuscript. Because GD and Hua Liu contributed equally to this work, they are considered as co-first authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Guanglan Di for the English revision of the text.
Funding
This study was supported by Medical science and technology development fund of Jiangsu University (JLY20160047).
References
1. Caskey RC, Nance ML. Management of pediatric mild traumatic brain injury. Adv Pediatr (2014) 61(1):271–86. doi:10.1016/j.yapd.2014.03.006
2. Schneier AJ, Shields BJ, Hostetler SG, Xiang H, Smith GA. Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics (2006) 118(2):483–92. doi:10.1542/peds.2005-2588
3. Thomas BW, Mejia VA, Maxwell RA, Dart BW, Smith PW, Gallagher MR, et al. Scheduled repeat CT scanning for traumatic brain injury remains important in assessing head injury progression. J Am Coll Surg (2010) 210(5):824–30. doi:10.1016/j.jamcollsurg.2009.12.039
4. Tong WS, Zheng P, Xu JF, Guo YJ, Zeng JS, Yang WJ, et al. Early CT signs of progressive hemorrhagic injury following acute traumatic brain injury. Neuroradiology (2011) 53(5):305–9. doi:10.1007/s00234-010-0659-8
5. Thorson CM, Van Haren RM, Otero CA, Guarch GA, Curia E, Barrera JM, et al. Repeat head computed tomography after minimal brain injury identifies the need for craniotomy in the absence of neurologic change. J Trauma Acute Care Surg (2013) 74(4):967–73; discussion 973–965. doi:10.1097/TA.0b013e3182877fed
6. Hentzen AS, Helmer SD, Nold RJ, Grundmeyer RW III, Haan JM. Necessity of repeat head computed tomography after isolated skull fracture in the pediatric population. Am J Surg (2015) 210(2):322–5. doi:10.1016/j.amjsurg.2014.11.011
7. Joseph B, Aziz H, Pandit V, Kulvatunyou N, Hashmi A, Tang A, et al. A three-year prospective study of repeat head computed tomography in patients with traumatic brain injury. J Am Coll Surg (2014) 219(1):45–51. doi:10.1016/j.jamcollsurg.2013.12.062
8. Brenner DJ. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol (2002) 32(4):228–1; discussion 242–224. doi:10.1007/s00247-002-0671-1
9. Huang WY, Muo CH, Lin CY, Jen YM, Yang MH, Lin JC, et al. Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: a nation-wide population-based cohort study. Br J Cancer (2014) 110(9):2354–60. doi:10.1038/bjc.2014.103
10. Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet (2012) 380(9840):499–505. doi:10.1016/S0140-6736(12)60815-0
11. Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr (2013) 167(8):700–7. doi:10.1001/jamapediatrics.2013.311
12. Hu GW, Lang HL, Guo H, Wu L, Zhang P, Kuang W, et al. A risk score based on admission characteristics to predict progressive hemorrhagic injury from traumatic brain injury in children. Eur J Pediatr (2017) 176(6):689–96. doi:10.1007/s00431-017-2897-9
13. Wan X, Fan T, Wang S, Zhang S, Liu S, Yang H, et al. Progressive hemorrhagic injury in patients with traumatic intracerebral hemorrhage: characteristics, risk factors and impact on management. Acta Neurochir (2017) 159(2):227–35. doi:10.1007/s00701-016-3043-6
14. Folkerson LE, Sloan D, Cotton BA, Holcomb JB, Tomasek JS, Wade CE. Predicting progressive hemorrhagic injury from isolated traumatic brain injury and coagulation. Surgery (2015) 158(3):655–61. doi:10.1016/j.surg.2015.02.029
15. Yuan F, Ding J, Chen H, Guo Y, Wang G, Gao WW, et al. Predicting progressive hemorrhagic injury after traumatic brain injury: derivation and validation of a risk score based on admission characteristics. J Neurotrauma (2012) 29(12):2137–42. doi:10.1089/neu.2011.2233
16. Oertel M, Kelly DF, Mcarthur D, Boscardin WJ, Glenn TC, Lee JH, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg (2002) 96(1):109–16. doi:10.3171/jns.2002.96.1.0109
17. Cepeda S, Gómez PA, Castaño-Leon AM, Martínez-Pérez R, Munarriz PM, Lagares A. Traumatic intracerebral hemorrhage: risk factors associated with progression. J Neurotrauma (2015) 32(16):1246–53. doi:10.1089/neu.2014.3808
18. Kim WH, Lim DJ, Kim SH, Ha SK, Choi JI, Kim SD. Is routine repeated head CT necessary for all pediatric traumatic brain injury? J Korean Neurosurg Soc (2015) 58(2):125–30. doi:10.3340/jkns.2015.58.2.125
19. Chern JJ, Sarda S, Howard BM, Jea A, Tubbs RS, Brahma B, et al. Utility of surveillance imaging after minor blunt head trauma. J Neurosurg Pediatr (2014) 14(3):306–10. doi:10.3171/2014.6
20. Holmes JF, Borgialli DA, Nadel FM, Quayle KS, Schambam N, Cooper A, et al. Do children with blunt head trauma and normal cranial computed tomography scan results require hospitalization for neurologic observation? Ann Emerg Med (2011) 58(4):315–22. doi:10.1016/j.annemergmed.2011.03.060
21. Powell EC, Atabaki SM, Wootton-Gorges S, Wisner D, Mahajan P, Glass T, et al. Isolated linear skull fractures in children with blunt head trauma. Pediatrics (2015) 135(4):e851–7. doi:10.1542/peds.2014-2858
22. Arrey EN, Kerr ML, Fletcher S, Cox CS Jr, Sandberg DI. Linear nondisplaced skull fractures in children: who should be observed or admitted? J Neurosurg Pediatr (2015) 16(6):703–8. doi:10.3171/2015.4.PEDS1545
Keywords: mild traumatic brain injury, children, progressive hemorrhagic injury, computed tomography, clinical predictors
Citation: Di G, Liu H, Jiang X, Dai Y, Chen S, Wang Z and Liu H (2017) Clinical Predictors of Progressive Hemorrhagic Injury in Children with Mild Traumatic Brain Injury. Front. Neurol. 8:560. doi: 10.3389/fneur.2017.00560
Received: 10 July 2017; Accepted: 05 October 2017;
Published: 13 November 2017
Edited by:
Vassilis E. Koliatsos, Johns Hopkins School of Medicine, United StatesReviewed by:
Ralph George Depalma, Department of Veterans Affairs Office of Research and Development, United StatesKarim A. Sarhane, University of Toledo, United States
Copyright: © 2017 Di, Liu, Jiang, Dai, Chen, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyi Liu, hyliu18@126.com
†These authors have contributed equally to this work.
 Guangfu Di
Guangfu Di Hua Liu2†
Hua Liu2†