Corrigendum: Clinical Characteristics and Predictors of Outcome for Onconeural Antibody-Associated Disorders: A Retrospective Analysis
- 1Department of Neurology, Graduate School of the Second Military Medical University, Shanghai, China
- 2Department of Neurology, General Hospital of Jinan Military Command of PLA, Jinan, China
- 3Department of Neurology, People’s Liberation Army 152 Hospital, Pingdingshan, China
Objective: To describe and analyze the clinical characteristics, laboratory data, management, and outcome of patients with onconeural antibody-associated disorders (OAAD) and identify predictors for poor outcome.
Methods: This was a retrospective review of all patients with potential OAAD, who were hospitalized in Jinan General Hospital between September 2009 and July 2017. We clarified the diagnosis, collected comprehensive information and categorized patients into three groups: paraneoplastic neurological disorders (PNDs), autoimmune encephalitis (AE), and possible OAAD. Within the three groups, we analyzed a range of clinical and laboratory parameters and used univariate and multivariate regression analysis to identify predictors for poor outcome [modified Rankin Scale (mRS) = 3–6].
Results: From 158 patients, we identified 70 who fulfilled the criteria for OAAD, including 44 men (62.9%) and 26 women (37.1%). There were 38 patients (54.3%) in the PNDs group, 14 patients (20%) in the AE group, and 18 patients (25.7%) in the possible OAAD group. After the last follow-up, 14 (36.8%), 9 (64.2%), and 12 (66.7%) had a good outcome (mRS = 0–2). However, 6 (15.8%), 2 (14.3%), and 3 (16.7%) died, respectively. Univariate analysis showed that duration prior to the hospital (p = 0.0224) and urinary incontinence/retention (p = 0.0043) were associated with poor outcome (mRS = 3–6). After multivariate regression analysis, urinary incontinence/retention (p = 0.0388) and an immunocompromised state (p = 0.0247) remained as significant factors for poor outcome.
Conclusion: Urinary incontinence/retention and an immunocompromised state represent significant predictors of a worse prognosis for patients with OAAD. By contrast, cerebrospinal fluid analysis showed that serum autoantibodies and tumor markers, the function of crucial organs, electrophysiology, and radiological findings were not associated with a poor outcome.
Introduction
Onconeural antibody-associated disorders (OAADs) are a type of heteroplasmic neurological syndrome and are drawing increasing attention from neurologists. OAADs are usually associated with some form of neuronal autoantibody, part of which is known to be associated with systemic cancer. The target antigens include nuclear or cytoplasmic proteins, such as Hu, Yo, Ri, or intracellular synaptic proteins, such as 65 kDa glutamic acid decarboxylase, amphiphysin, or cell-surface or synaptic proteins, such as the N-methyl-d-aspartate receptor (NMDAR), the α-amino-3-hydroxy-5 -methyl-4-isoxazolepropionic acid receptor, and the γ-aminobutyric acid receptor-B (GABABR) (1–3). Furthermore, OAADs can be broadly divided into two clinical categories: classic paraneoplastic neurological syndromes (PNDs) and autoimmune encephalitis (AE) (4).
Paraneoplastic neurological disorders are mostly accepted as neurological syndromes that are immune mediated and triggered when systemic tumors express certain neuronal antigens (3). PND syndromes can manifest in the central nervous system, peripheral system, neuromuscular junction and muscle (5). However, AE generally refers to a group of disease processes that share analogical clinical features and neuroimaging findings and are largely differentiated by characteristic antibodies which mediate immune attacks on different neural structures (6). The presentations of AE include memory or behavioral deficits, new focal findings, seizures, cerebrospinal fluid (CSF) pleocytosis, and abnormalities upon magnetic resonance imaging (MRI) (7). Previous reports have indicated that approximately 1 in 10,000 patients with cancer eventually develop PNDs (8), thus showing that this disease is associated with a low prevalence. In other research, patients were preselected using clinical criteria and serological screening; these data showed that 163/649 (25%) cases were serologically positive for antibodies associated with PNDs during a 23-month period (4). In terms of AE, the annual incidence of encephalitis, of any etiology, is 2–3 cases per 100,000 patients in northern Europe (9), of which, at least 20% are immune mediated. The predominant cohort of patients for AE includes those with anti-NMDA-receptor encephalitis (4%) and voltage-gated potassium channels (VGKC)-complex antibody positive encephalitis (3%) (9).
In the present study, we analyzed the medical records from a series of patients with OAAD, investigated the spectrum of clinical characteristics and management strategies associated with this disease and evaluated potential predictors which could be used to influence outcome.
Materials and Methods
Standard Protocol Approvals and Patient Consent
The study was approved by the ethics committee of the General Hospital of Jinan Military Command (NO. 201611). All patients provided informed written consent to allow their medical records to be used in this study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Study Design
We conducted a retrospective review of all patients with potential PNDs or AE between September 2009 and July 2017, and who were tested for classical paraneoplastic antibodies and autoimmune synaptic antibodies. Records were manually reviewed and patients with a final diagnosis of PNDs, AE, or possible OAAD were recruited into this study.
Definitions Used in This Study
We used specific diagnostic criteria to define PNDs and AE (5). Definite PNDs were defined as follows: (1) a classical syndrome and cancer that develops within 5 years of the diagnosis of neurological disorder; (2) a non-classical syndrome that resolves or significantly improves after cancer treatment without concomitant immunotherapy, provided that the syndrome was not susceptible to spontaneous remission; (3) a non-classical syndrome with onconeural antibodies (well characterized or not) and cancer that develops within 5 years of the diagnosis of neurological disorder; (4) A neurological syndrome (classical or not) with well characterized onconeural antibodies (anti-Hu, Yo, CV2, Ri, Ma2, or amphiphysin), but without cancer. Possible PNDs were defined as described previously (5): (1) a classical syndrome, no onconeural antibodies, no cancer but at high risk of an underlying tumor; (2) a neurological syndrome (classical or not) with partially characterized onconeural antibodies but without cancer; and (3) a non-classical syndrome, no onconeural antibodies, and the presence of cancer within 2 years of diagnosis.
Definite AE was defined, as described previously (7): (1) sub-acute onset (rapid progression of less than 3 months) of working memory deficits (short-term memory loss), altered mental status, or psychiatric symptoms; (2) at least one of the following: new focal CNS findings; seizures which could not be explained by a previously known seizure disorder; CSF pleocytosis (white blood cell count of more than five cells per mm3); MRI features suggestive of encephalitis; (3) positive antibody testing in the serum or CSF, mainly targeting cell-surface or synaptic proteins; and (4) reasonable exclusion of other disorders. Possible AE was defined as an absence of positive antibody testing in the serum or CSF.
The “duration prior to the hospital” was defined as the number of days from the onset of symptoms to hospitalization in our hospital. A “good outcome” was defined as a grade of 0–2 on the modified Rankin Scale (mRS), while a “poor outcome” was defined as a mRS of 3–6. An “immunocompromised state” was defined as when patients received chemotherapy, chronic immunosuppressants or steroids (for >3 months). IgG index was calculated according to the following formula: [CSF IgG (mg/mL)/serum IgG (mg/mL)]/[CSF albumin (mg/mL)/serum albumin (mg/mL)]. MRI fluid-attenuated inversion recovery (FLAIR)/T2 abnormality was defined as high signal intensity in images of the brain parenchyma. “Total tumors” refers to the total number of tumors for a specific patient, including those which were newly identified, and those recorded in historical records.
Inclusion/Exclusion Criteria
Patients were included in the study if they concurred with criteria outlined above, and were under treatment in our hospital. We excluded cases if there was any evidence of infectious or post-infectious encephalitis, autoimmune disease-associated encephalitis, toxic-metabolic encephalopathy, brain tumor or metastasis, vitamin deficiency or alcohol-related encephalopathy, side-effects of drugs (chemotherapeutics or others), primary angiitis of the CNS, or other diseases (e.g., acquired immune deficiency syndrome and Creutzfeldt–Jakob disease).
Categorization of Patients
Patients were categorized into three groups: those with PNDs, AE, or possible OAAD. Patients were categorized as definite PNDs or AE when they concurred with the appropriate definitions. Patients who were classified as possible PNDs or AE were all categorized into the possible OAAD group.
Clinical Characteristics and Investigations
Basic demographic information (age, gender) were collated for each patient, along with the duration prior to the hospital, and whether there was an immunocompromised state or not. All patients underwent consultation and a neurological examination by an attending neurologist, who ascertained clinical symptoms, neurological signs, and mRS upon admission. The same information was collected just prior to discharge. mRS data were collected by telephone follow-up on a semi-annual basis. During the period of hospital care, intensive care unit (ICU) admission, mechanical ventilation, and therapy were recorded.
In this study, we analyzed a range of laboratory data, including CSF analysis (glucose, protein, white blood cells, IgG index), serum autoantibodies [anti-nuclear antibodies (ANA), onconeural antibodies] and tumor markers [neuron-specific endase (NSE), carcinoembryonic antigen (CEA)]. Data were also acquired so that we could evaluate the function of crucial organs, such as the heart, liver, and kidney and also the relative state of hemopoiesis. Such data included creatine phosphokinase isoenzyme (CK-MB), glutamic oxalacetic transaminase/glutamic-pyruvic transaminase (AST/ALT), urea nitrogen/creatinine (BUN/Cr), the counts of leukocyte, polymorphonuclear and thrombocyte. Electroencephalograms (EEGs) and electromyography (EMG) were also carried out in order to assess electrophysiology. Neuroimaging examinations, including brain MRI FLAIR/T2 abnormality and computed tomography (CT), were also used to investigate for underlying tumors. All of the results were studied from the first time in our hospital.
Statistics
The data were analyzed using Excel 2016. Normality was assessed using the Shapiro–Wilk Normality Test on R software. Continuous variables, which were normally distributed, were compared between groups with the Student’s t-test. For continuous variables that did not follow normal distributions, we used the Mann–Whitney U test (Wilcoxon rank-sum test). The Chi-square test or Fisher’s exact test, as appropriate, was used to compare categorical variables between groups, and a binomial logistic regression model was used to evaluate the association between variables and outcome. All statistical analyses were performed in R (the R Project: http://www.R-project.org). Odds ratios (ORs) and their 95% confidence intervals (CIs) were also calculated. The predefined level of significance was 0.05.
Results
Demographics and Clinical Characteristics
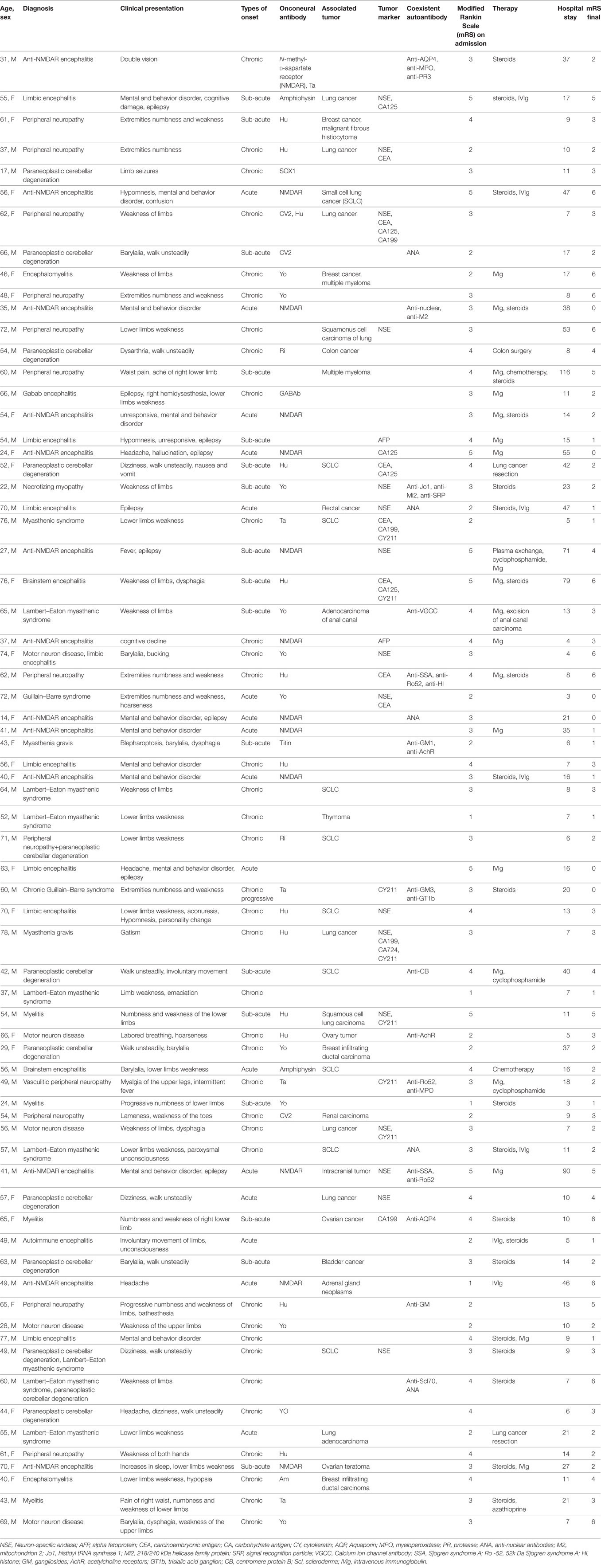
From a total of 158 patients, we identified 70 patients who fulfilled the criteria for OAAD, including 44 men (62.9%) and 26 women (37.1%) with a median age of 55 years (range: 14–78 years). The median duration prior to the hospital was 74 (range: 7–1,090). The median number of days in hospital was 37 (range: 3–116). A summary of the patient demographics and characteristics is given in Table 1.
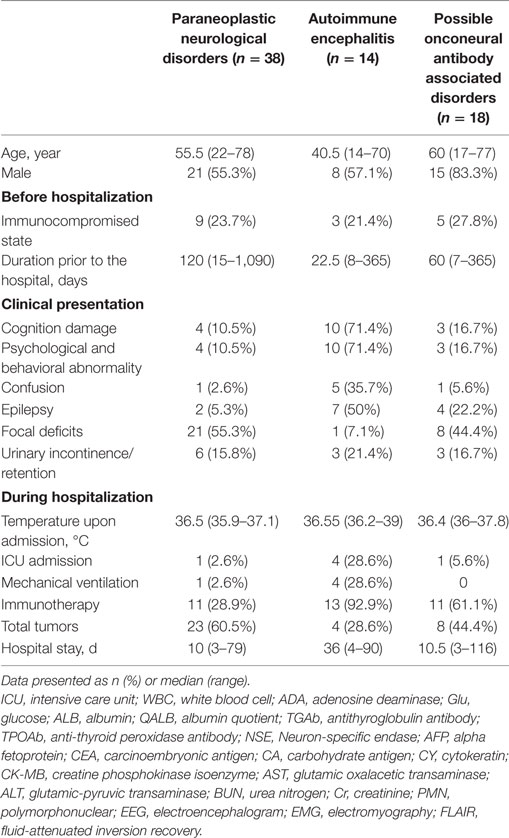
In this study, 38 patients (54.3%) were classified into the PNDs group, including 17 women (44.7%) and 21 men (55.3%). There were 14 patients (20%) diagnosed with AE, including 6 women (42.9%) and 8 men (57.1%). The other 18 patients (25.7%) were classified as having possible OAAD, including 3 women (16.7%) and 15 men (83.3%). There were 17 patients (24.3%) with mental and behavioral abnormalities, 13 (18.6%) with epilepsy, and 12 (17.1%) with autonomic neuropathic lesions. Detailed statistical analysis of patient demographics, clinical presentation, and hospital course, in each of the three groups, is given in Table 2.
Investigations
During hospitalization, 60 patients (85.7%) underwent CNS examination by MRI and 18 patients (30%) were identified as having FLAIR/T2 abnormalities. Furthermore, 27 patients (38.6%) received EEG examinations, 19 of which (70.4%) showed an abnormal slow wave or a sharp and slow wave. EMG was performed in 41 patients (58.6%) and identified 37 positive cases (90.2%). Selected patients underwent CSF examination to determine cell count, protein concentration, and immunoglobulin (Ig) concentration. We also tested characterized onconeural antibodies (anti-Hu, Yo, Ri, Ma2, CV2, and amphiphysin) in 67 patients (95.7%) and NMDAR antibody in 27 patients (38.6%). A further cohort of 59 patients (84.3%) were screened for serum tumor markers, including NSE, AFP, CEA, CA125, CA153, CA199, CA724, and CY211; 26 positive cases (44.1%) were identified. Coexistent autoantibodies were also examined in 37 patients (54.3%), including anti-nuclear/myositis/ganglioside antibody profiles, anti-myeloperoxidase (MPO)/protease 3 (PR3)/acetylcholine receptors (AchR)/aquaporin 4 (AQP4) antibodies, and 19 patients (51.4%) were shown to exhibit the coexistence of other autoantibodies.
We also collected a range of other data to allow us to evaluate the status of primary organ function. All patients were tested for AST/ALT, BUN/Cr, the counts of leukocyte, polymorphonuclear, and thrombocyte, and CK-MB was tested in 44 patients (62.9%). The results were analyzed to identify potential predictors of outcome and are presented in Table 3.
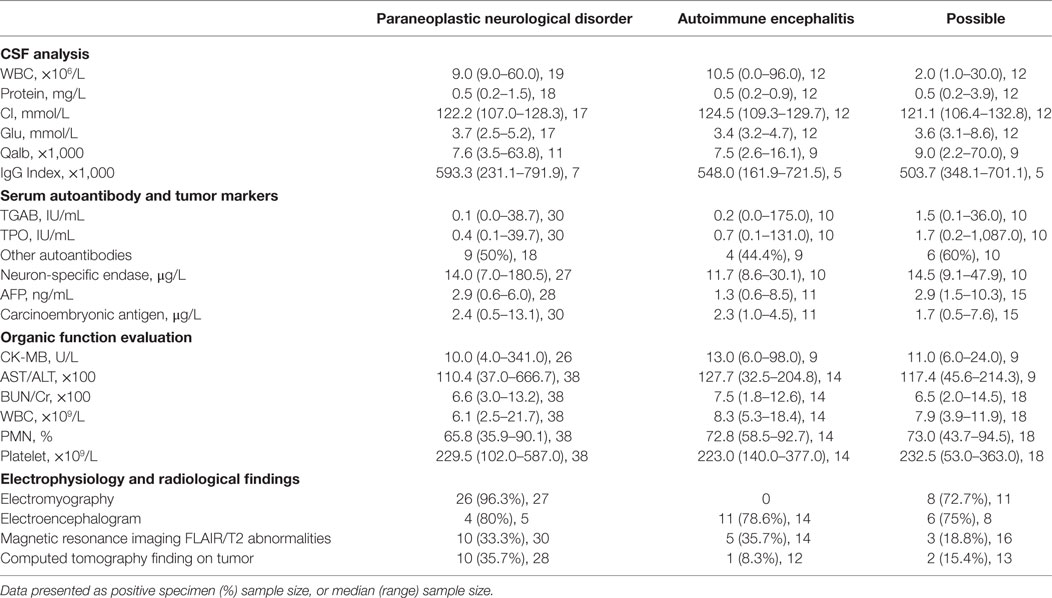
Table 3. Cerebrospinal fluid (CSF), other test results, electrophysiology, and radiological findings.
Diagnosis and Treatment
Patients included in this study were divided into three groups: those with PNDs (38 patients, 54.3%), AE (14 patients, 20%) and possible OAAD (18, 25.7%). The main diagnoses were anti-NMDAR encephalitis (13/70 patients, 18.6%), peripheral neuropathy (12/70 patients, 17.1%), paraneoplastic cerebellar degeneration (12/70 patients, 17.1%) and Lambert-Eaton myasthenic syndrome (8/70 patients, 11.4%). The main onconeural antibodies detected were anti-Hu (13/70 patients, 18.6%), anti-NMDAR (13/70 patients, 18.6%), and anti-Yo (11/70 patients, 15.7%). Overall, 36 patients (51.4%) were found to possess associated tumors, the most common tumor being lung cancer (19/36 patients, 52.8%), particularly small cell lung cancer (SCLC) (10/19 patients, 52.6%).
In total, 39 patients (55.7%) underwent anti-tumor or immunotherapy and 4 patients (10.3%) received tumor surgery, 22 patients (56.4%) were administered with steroids, 26 patients (66.7%) were treated with IVIg, and 13 patients (33.3%) received both steroids and IVIg. Some patients were admitted into the ICU (6/70 patients, 8.6%), and five patients (7.1%) required mechanical ventilation.
Outcomes
At discharge, there were 17 patients (44.7%) with PNDs, 9 (64.3%) with AE and 10 (55.6%) with possible OAAD who had a good outcome. By contrast, there were 21 (55.3%) patients with PNDs, 5 (35.7) with AE and 8 (44.4%) with possible OAAD who had a poor outcome. After the last follow-up (median: 23 months; range: 0–84), 14 patients (36.8%), 9 patients (64.2%), and 12 patients (66.7%) had a good outcome. However, six patients (15.8%), two patients (14.3%), and three patients (16.7%) died in the three cohorts, respectively.
Predictors of Outcome
After the last follow-up, the entire patient cohort was classified into groups of good (mRS = 0–2) or poor (mRS = 3–6) outcome after the last follow-up. Univariate analysis was then used to assess variables which were potentially associated with the functional outcome of patients (Table 4). Univariate analysis showed that duration prior to the hospital (p = 0.0224) and urinary incontinence/retention (p = 0.0043) were factors associated with poor outcome.
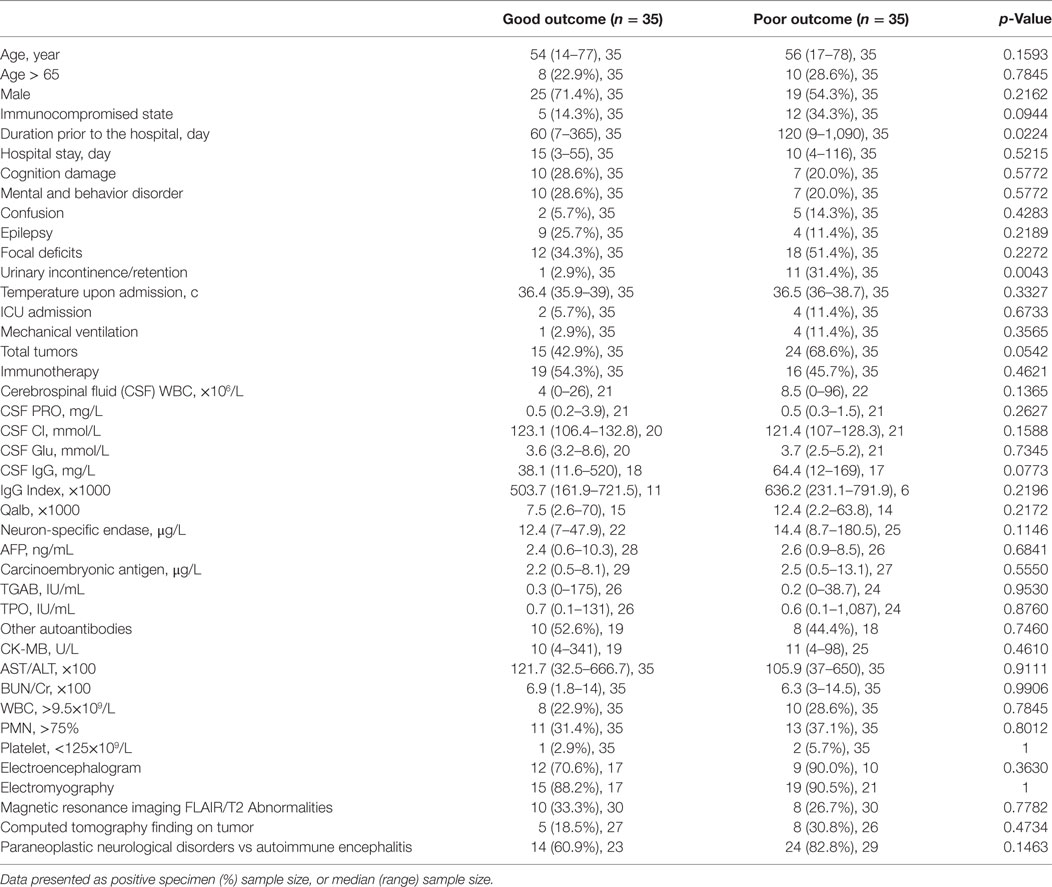
Table 4. Variables associated with functional outcome in onconeural antibody associated disorders patients: univariate analysis.
Multivariate regression analysis was then performed for all patients. Urinary incontinence/retention (p = 0.0388) and an immunocompromised state (p = 0.0247) were significant factors associated with a poor outcome. Nevertheless, duration prior to the hospital was not statistically associated with a poor outcome (p = 0.0674) (Table 5).
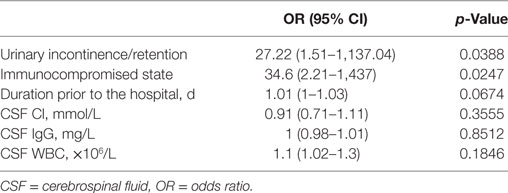
Table 5. Poor outcome factors in onconeural antibody associated disorders patients: multivariate analysis.
Discussion
In this study, we integrated PNDs and AE as a category named OAAD for the similarity of underlying pathogenesis and clinical management as follows. Most cases of OAAD are probably immune mediated, as demonstrated by the fact that anti-neuronal antibodies can be found in the CSF and serum (4). For paraneoplastic disorders, the ectopic expression of neuronal antigens within the tumor appears to contribute to breaking the immune tolerance and activating the immune response. For non-paraneoplastic disorders, molecular mimicry may initiate an immune response against neuronal antigens during the process of virus infection (10). The manifestation of OAAD can appear as encephalitis, encephalopathy, peripheral/autonomic neuropathy, or syndromes involving the neuromuscular junction. The clinical approach to diagnosing OAAD is to identify the neurological syndrome, detect specific antibodies in the serum or CSF, and verify the underlying cancer in some cases. In terms of clinical management, immunotherapy and tumor removal are the primary course of treatment.
Our study investigated the clinical presentation and management, CSF analysis, serum autoantibodies and tumor markers, the function of crucial organs, electrophysiology, and radiological findings in patients with OAAD. Furthermore, we used these data to identify potential predictors of outcome. In univariate analysis, duration prior to the hospital (p = 0.0224) and urinary incontinence/retention (p = 0.0043) were associated with a poor outcome. Furthermore, an immunocompromised state (p = 0.0944), the total number of tumors (p = 0.0542) and CSF IgG concentration (p = 0.0773) may also be associated with outcome. After multivariate regression analysis, urinary incontinence/retention and an immunocompromised state were shown to be significantly related to a poor outcome.
Urinary incontinence/retention is the main manifestation of damage to the autonomic nerve and its nerve center, including the lobulus paracentralis, hypothalamus, and parasympathetic center in the spinal cord. Our findings verified that this was the presentation of a serious injury to the nervous center and indicated a poor outcome. These results have not been reported previously for PNDs and AE. Further research is now required to explore the underlying pathogenesis of this condition. An immunocompromised state was confirmed as a factor associated with a poor outcome, as in a previous study of encephalitis (11). However, in the previous study of anti-NMDAR encephalitis, the predictors associated with a good outcome included early treatment, the lack of ICU requirement and a longer follow-up period (12), and therefore did not incorporate the factors utilized in the present study. More clinical research is now needed to identify the specific factors associated with outcome for patients with OAAD.
In terms of clinical presentation, we selected cognitive damage, mental and behavioral abnormality, confusion, epilepsy, focal deficits, urinary incontinence/retention, and temperature upon admission as influential factors with which to investigate outcome. Univariate and multivariate regression analysis confirmed that urinary incontinence/retention was a significant factor associated with poor outcome. Therapeutic measures, the total number of tumors and the duration prior to the hospital or during the hospital stay were also evaluated in the present study but did not show any significant relationship with outcome. Furthermore, ICU admission and mechanical ventilation did not show any relationship with outcome, which contradicted the results of a previous report (12). Univariate analysis showed that duration prior to the hospital was significantly associated with poor outcome (p = 0.0224), although this relationship was not significant when tested by multivariate regression analysis (p = 0.0674). In our cohort, duration prior to the hospital ranged from 1 week to 3 years. A longer time before the hospital visit always related to the progression of a potential tumor, the functional decline of major organs and a delay in the administration of appropriate treatments. This factor was verified as an indicator of poor outcome in the cohort study reported by Maarten (12).
In our CSF analysis, as well as investigating CSF components such as WBC, protein, Glu, and IgG, we also calculated the albumin quotient (Qalb) as a marker of the integrity of the blood–brain barrier, and IgG index as an estimate of intrathecal IgG synthesis (Tables 3 and 4). Univariate analysis showed that only CSF IgG may be associated with a poor outcome, although there was no statistical significance when tested by multivariate regression analysis. High IgG index, pleocytosis and increased protein levels usually appear within the first few days after neurological symptoms (4, 13–15). The dynamic changes of CSF components may be one of the factors associated with the lack of statistical significance in our present study, as might the low numbers of CSF tested.
In the present study, we also investigated tumor markers, onconeural antibodies and coexistent anti-thyroid autoantibodies, anti-nuclear/myositis/ganglioside antibody profiles, and anti-MPO/PR3/AchR/AQP4 antibodies. Our testing showed that 16/47 patients (34%) were positive for tumor markers and NSE, and that the next highest positive marker was CEA (9/56 patients, 16.1%). There were 51/70 patients (72.9%) who possessed onconeural antibodies, including Hu (13 patients), NMDAR (13 patients), and Yo (11 patients) (see Table 1). A total of 25 patients (35.7%) tested positive for serum autoantibodies. The coexistent autoantibody mainly included antithyroglobulin antibody (TGAb) (13 patients), anti-thyroid peroxidase antibody (TPOAb) (5 patients), ANA (5 patients). Neither univariate nor multivariate regression analysis revealed any significant relationship between antibody level and poor outcome. A previous study evaluated the coexistence of autoantibodies in cases of myasthenia gravis; 52% (39/75) patients tested positive for autoantibodies, including 27 cases with TPOAb, 17 cases with TGAb, and 17 cases of ANA (16). The pathogenesis underlying the coexistence of autoantibodies in the same patient remains unclear. Gene phenotypes, environmental factors, infection, immune dysfunction, and other factors may be connected with this phenomenon. The relationship between autoantibodies and the potential risks of autoimmune diseases, however, remains uncertain. Whether and how coexistent autoantibodies influence the therapeutic approaches and the prognosis of OAAD requires further research.
Clearly, the failure of crucial organs may be related to a poor outcome, at least to some extent. In the present study, we evaluated the function of important organs including heart, liver, kidney, and the hemopoiesis system. We tested AST/ALT, BUN/Cr, leukocytes, PMN, and thrombocytes in all patients, and CK-MB in 44 patients, in order to assess organic function. The number of patients with leukocytosis (WBC > 9.5 × 109/L) was 10 (28.6%) in the group of poor outcome and 8 (22.9%) in the group of good outcome; for patients with thrombocytopenia (Platelets < 125 × 109/L), the number was 1 (2.9%) and 2 (5.7%); for patients with PMN ratio rise (>75%), the number was 11 (31.4%) and 13 (37.1%), respectively. Other indicators of organ function were rarely abnormal in our cohort. All results were analyzed by both univariate and multivariate regression analysis but none of these tests revealed a relationship with poor outcome. The function of the main organs is seldom evaluated as an indicator of outcome in a cohort study. While we obtained a negative result in this study, we plan to perfect the protocol and increase sample size in order to create a more robust study.
Our present study also considered electrophysiology and radiological findings in our diagnosis and in the prediction of outcome. The number of patients with abnormalities in EEG was 12 (70.6%) in the good outcome group and 9 (90%) in the poor outcome group; for patients with abnormalities in EMG, the number was 15 (88.2%) and 19 (90.5%); for MRI FLAIR/T2, the number was 10 (33.3%) and 8 (26.7%); for CT finding on tumor, the number was 5 (18.5%) and 8 (30.8%), respectively (Table 4). The detailed data in the PNDs, AE, and possible OAAD groups are presented in Table 3. Further statistical analysis failed to reveal any significant differences in terms of indicators of outcome. As reported previously, few patients with anti-NMDAR encephalitis showed FLAIR/T2 abnormalities in the cerebral parenchymal (12–15). In another study, abnormalities were observed in brain MRI and EEG in 33 and 90% of all patients, respectively (12), which concurred with our own cohort.
Overall, there was a 15.7% mortality rate (11/70 patients) in our current study, which was higher than the rates of 9.5 and 9% reported in previous studies (11, 12). The number of dead patients distributed in PNDs was 54.5% (6/11), in AE 18.2% (2/11), in possible OAAD 27.3% (3/11). In the PNDs group, 24 patients (63.2%, 24/38) had a poor outcome; this compared to 35.7% (5/14) and 33.3% (6/18) in the AE and possible OAAD groups. In terms of treatment, there were 35 patients who were administered with immunotherapy, predominantly steroids or IVIg, or both. Of the patients receiving treatments, 19 patients were classified as having a good outcome while 16 were classified as having a poor outcome; univariate analysis showed that there was no difference between these two groups of patients (p = 0.4621).
The present study strengthens our holistic evaluation of crucial organs and analyzes the coexistence of antoantibodies and tumor markers in patients with OAAD, while also considering clinical presentation and management, CSF analysis, electrophysiology, and radiological findings. The present study was limited in several ways. First, there was a shortage of exhaustive and complete data. Second, the study was retrospective in design, which led to the shortage of data. Finally, due to the long period of time before hospital attendance (mean: 146.4 days; range: 7–1090 days), the pathophysiological status of patients changed and appropriate treatments, including tumor surgery and immunotherapy, were delayed. Collectively, these factors would have influenced the clinical presentation, radiological findings, and test results, and may also have influenced the outcome, at least to some extent.
Conclusion
In the present study, 15.7% of our patients died and 50% of our patient cohort experienced a good or poor outcome, respectively. The distribution of mortality and outcome was not significantly different when compared across the PNDs, AE, and possible OAAD groups. In terms of indicators of prognosis, we identified that urinary incontinence/retention and an immunocompromised state represent significant factors that are associated with outcome (OR = 27.22 and 34.6, respectively) and portend a worse outcome.
Ethics Statement
This study was carried out in accordance with the recommendations of the ethics committee of General Hospital of Jinan Military Command with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the ethics committee of General Hospital of Jinan Military Command.
Author Contributions
BC and SL designed the study. SL and YQ contributed equally to this work and should be considered co-first authors. They analyzed the data and performed the writting of the manuscript. HH, HG, XW, and SM collected and sorted the clinical data. CL implemented laboratory technique and checked the testing result. BN accomplished the data statistics.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81671631).
References
1. Lancaster E, Dalmau J. Neuronal autoantigens – pathogenesis, associated disorders and antibody testing. Nat Rev Neurol (2012) 8(7):380–90. doi:10.1038/nrneurol.2012.99
2. Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol (2008) 7(4):327–40. doi:10.1016/s1474-4422(08)70060-7
3. Hoftberger R, Rosenfeld MR, Dalmau J. Update on neurological paraneoplastic syndromes. Curr Opin Oncol (2015) 27(6):489–95. doi:10.1097/CCO.0000000000000222
4. Dalmau J, Rosenfeld MR. Autoimmune encephalitis update. Neuro Oncol (2014) 16(6):771–8. doi:10.1093/neuonc/nou030
5. Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry (2004) 75(8):1135–40. doi:10.1136/jnnp.2003.034447
6. Kelley BP, Patel SC, Marin HL, Corrigan JJ, Mitsias PD, Griffith B. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol (2017) 38(6):1070–8. doi:10.3174/ajnr.A5086
7. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol (2016) 15(4):391–404. doi:10.1016/S1474-4422(15)00401-9
8. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med (2003) 349(16):1543–54. doi:10.1056/NEJMra023009
9. Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis (2010) 10(12):835–44. doi:10.1016/S1473-3099(10)70222-X
10. Leypoldt F, Wandinger KP, Bien CG, Dalmau J. Autoimmune encephalitis. Eur Neurol Rev (2013) 8(1):31–7. doi:10.17925/ENR.2013.08.01.31
11. Singh TD, Fugate JE, Rabinstein AA. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology (2015) 84(4):359–66. doi:10.1212/WNL.0000000000001190
12. Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol (2013) 12(2):157–65. doi:10.1016/S1474-4422(12)70310-1
13. Armangue T, Titulaer MJ, Malaga I, Bataller L, Gabilondo I, Graus F, et al. Spanish anti: pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr (2013) 162(4):850–6.e2. doi:10.1016/j.jpeds.2012.10.011
14. Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol (2008) 7(12):1091–8. doi:10.1016/S1474-4422(08)70224-2
15. Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain (2010) 133(Pt 6):1655–67. doi:10.1093/brain/awq113
Keywords: onconeural antibody, clinical characteristics, management, outcome, predictors
Citation: Liao S, Qian Y, Hu H, Niu B, Guo H, Wang X, Miao S, Li C and Cao B (2017) Clinical Characteristics and Predictors of Outcome for Onconeural Antibody-Associated Disorders: A Retrospective Analysis. Front. Neurol. 8:584. doi: 10.3389/fneur.2017.00584
Received: 31 August 2017; Accepted: 18 October 2017;
Published: 13 November 2017
Edited by:
Sandro M. Krieg, Technische Universität München, GermanyReviewed by:
Nico Sollmann, Klinikum rechts der Isar der TU München, GermanyBrad E. Zacharia, Penn State Milton S. Hershey Medical Center, United States
Copyright: © 2017 Liao, Qian, Hu, Niu, Guo, Wang, Miao, Li and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingzhen Cao, cbzxia2011@163.com
†These authors have contributed equally to this work and should be considered co-first authors.
 Shaohua Liao
Shaohua Liao Ying Qian
Ying Qian Huaiqiang Hu
Huaiqiang Hu Bing Niu2
Bing Niu2 Bingzhen Cao
Bingzhen Cao