- 1Otorhinolaryngology Department, ENT Institute, Affiliated Eye and ENT Hospital of Fudan University, Shanghai, China
- 2Institutes of Biomedical Sciences, Fudan University, Shanghai, China
Background: Sudden sensorineural hearing loss (SSHL) not only involves cochlear function but might also be accompanied by vestibular disturbances. The assessment of vestibular function could be of great relevance in SSHL.
Objective: To investigate the prevalence of vestibulocochlear lesions in SSHL and the correlation of specific vestibular organs with hearing prognosis.
Data sources: A complete literature search of eligible studies in the PubMed and EMBASE databases was performed.
Study selection: For our aim, studies that focused on vestibular examination in the case of SSHL were retrieved, including caloric tests, cervical vestibular-evoked myogenic potential (cVEMP) tests, or ocular vestibular-evoked myogenic potential (oVEMP) tests.
Results: Of the 18 studies included, a caloric test was performed in 16 studies, cVEMP in 13 studies, and oVEMP in 5 studies, and together the studies included a total population of 1,468 subjects. The scores on the Agency for Healthcare Research and Quality (AHRQ) questionnaire ranged from 6 to 11. These results indicated that the most commonly damaged vestibular organ in SSHL was the utricle and superior vestibular pathway (U + S) followed by the lateral semicircular canal and superior vestibular pathway (LSC + S), the saccule and inferior vestibular pathway (S + I), and the cochlea only (C only). The meta-analysis indicated that SSHL patients with vertigo have a statistically increased risk of vestibular organ lesions compared with those without vertigo, including the LSC + S subgroup (OR = 4.89, 95% CI = 1.20–19.93, I2 = 80%, p = 0.03) and the S + I subgroup (OR = 3.58, 95% CI = 1.61–7.95, I2 = 0%, p = 0.002). The pooled possibility of hearing recovery within the LSC + S lesion group was less than half that of the non-LSC + S lesion group (OR = 0.24, 95% CI = 0.11–0.52, I2 = 68%, p = 0.0003).
Conclusion: This study shows the relevance of vestibular damage concomitant with SSHL and that SSHL patients with vertigo are at an increased risk of vestibular organ lesions compared with patients without vertigo. LSC + S lesions thus appear to be a critical variable that influence the possibility of hearing improvement in SSHL.
Introduction
Sudden sensorineural hearing loss (SSHL) is clinically characterized by a rapid onset sensorineural hearing loss of more than 30 dB for at least three contiguous audiometric frequencies within a period of 3 days (1–3). Approximately 5–20 per 100,000 persons are afflicted with SSHL annually, and nearly 20–60% of SSHL patients complain of simultaneous vertigo (4–6). Because of the close correlation between the cochlea and the vestibular organs both anatomically and phylogenetically, impairment of cochlear function, which can result in SSHL, might be accompanied by vestibular disturbances (7, 8). Thus, the pathology of SSHL involves not only the cochlea but also, at least to some extent, the vestibular organs (7–9).
Recently, some vestibular diagnostic methods have been included in the general evaluation of patients suffering from SSHL, such as the caloric test, cervical vestibular-evoked myogenic potential (cVEMP), and ocular vestibular-evoked myogenic potential (oVEMP). The caloric test is a method for investigating lateral semicircular canal function and superior vestibular integrity, the cVEMP can be used for assessing saccular function and the inferior vestibular pathway, and oVEMP can be used for evaluating utricular function and the superior vestibular pathway (10–13). Some clinical studies have been performed to identify the vestibular dysfunction in patients with SSHL. Abnormal caloric test results have been reported in 39–74% of patients with SSHL (14–16), and the results of Korres et al. and Niu et al. indicated that abnormal vestibular examinations are associated with profound hearing loss in SSHL patients (7, 17). Iwasaki et al. found that the saccule is more often involved than the semicircular canal in SSHL patients with vertigo (18); however, recent results by Lee et al. do not support the hypothesis above, and they found higher rates of lesions in the semicircular canal of SSHL patients (19). Thus, the vestibulocochlear lesion patterns, the clinical relevance of vertigo in relation to vestibulocochlear lesion location, and the correlation of vestibular organ damage with the prognosis of hearing loss in SSHL have not yet achieved consensus. To address this issue, we performed a systematic review and meta-analysis to shed light on the presumed pathomechanisms that are involved in the clinical manifestation and prognosis of SSHL.
Materials and Methods
Data Sources and Search Strategy
The initial literature review of relevant studies for assessing the correlation of lesion pattern with the clinical manifestation of SSHL, with or without the presence of vertigo, was carried out in the PubMed and EMBASE databases using a combination of keywords, including “sudden hearing loss,” “sudden deafness,” “caloric test,” “canal paresis,” “VEMP,” and “vestibular-evoked myogenic potential.” All pertinent articles or abstracts that were published in English were retrieved with no restrictions on the date of publication. Additional papers were searched for in the reference lists of the identified papers.
Eligibility Criteria for Study Selection
The eligibility criteria for inclusion in our analysis were as follows: retrospective or prospective original research containing the routine examinations of vestibular function in SSHL patients; information on whether vestibular symptoms were involved in patients with SSHL; and a homogenous examination regime (caloric test, cVEMP, or oVEMP) in each group. Excluded papers included comments, reviews, case reports, practice guidelines, and editorials. Those studies that failed to provide sufficient information to calculate these variables were also excluded in our analysis.
Data Abstraction
The required information and data were independently extracted from the selected studies and quantified by the two authors in a standardized manner. The characteristics of each study included year of publication, research design, age and number of participants, presence of vertigo, presence of canal paresis, examination regime (caloric test, cVEMP, or oVEMP), and hearing improvement, including the number of subjects with good or poor hearing recovery in subgroups of abnormal vs. normal caloric response, abnormal vs. normal cVEMP response, and abnormal vs. normal oVEMP response. The retrieved data were compared and revised in mutual agreement between the two authors.
Quality Assessment and Sensitivity Analysis
The checklist of the Agency for Healthcare Research and Quality (AHRQ) was used to assess the quality and bias risk of the included studies (20). This scale has 11 evaluation criteria, including source of information, inclusion and exclusion criteria, time period, consecutive subjects, mask of subjective components, quality assurance, explanation of exclusions, control of confounding factors, method of handling missing data, completeness of data collection, and follow-up. An item is scored as 1 if included in the article and 0 if not. A score of 8 or more is indicative of a high-quality study.
A sensitivity analysis was further performed to investigate the heterogeneity of the included data and to estimate the impacts of each study on pooled outcomes based on the rule of omission. It also was used to assess and correct any publication bias. A study was confirmed as a source of heterogeneity if the I2 value decreased significantly.
Statistical Analysis
The various studies included non-comparative data and comparative data in a dichotomous pattern. Non-comparative analyses were carried out using the R version 3.4.1 software (R Foundation for Statistical Computing). We estimated the cumulative prevalence (event rate) from each study and the corresponding 95% confidence interval (CI) for each outcome in the non-comparative data. Event rates for each investigation were pooled in the meta-analysis across eligible studies using a fixed-effect model (p > 0.1) or a random-effect model (p ≤ 0.1). Comparative analyses were performed using Review Manager (RevMan) software (Version 5.3; Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014). The prognosis outcomes were displayed using 95% CI in forest plots. The pooled association between the presence of vertigo and vestibular lesions was quantitatively analyzed using the Mantel–Haenszel method with a combined estimate of the odds ratio (OR) (21). The heterogeneity of the included studies was statistically assessed using a p-value (heterogeneity was statistically significant when p ≤ 0.05) and an I2 statistic (a derivative of the Cochran Q statistic, I2 < 50% indicates low heterogeneity and I2 > 50% indicates high heterogeneity) (22, 23). A fixed-effect model was adopted when I2 < 50%; otherwise, a random-effect model was adopted. The result was considered to be statistically significant when p ≤ 0.05, and the potential asymmetry and publication bias was visually estimated from funnel plots.
Results
The Characteristics and Quality Assessments of the Included Studies
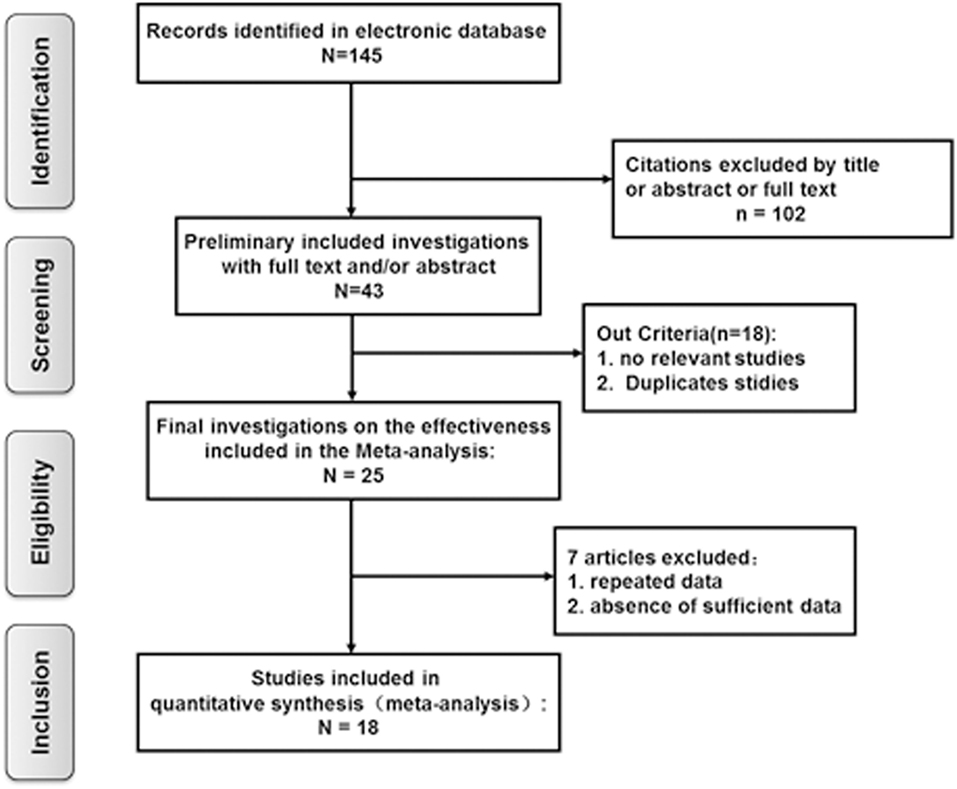
Based on the criteria mentioned above, 145 potentially relevant references were initially identified. Of these, 102 studies were excluded after screening by the title, abstract, or full text. A further 18 studies were removed because they lacked vestibular function examination in SSHL with or without vestibular symptoms. Of the remaining 25 investigations, 7 articles were excluded from the final analysis due to duplicated data containing the same participants or to not providing sufficient information to calculate these variables. Finally, a total of 18 eligible articles met our inclusion criteria and were included in the final meta-analysis (7, 8, 14, 17–19, 24–35) (Figure 1).
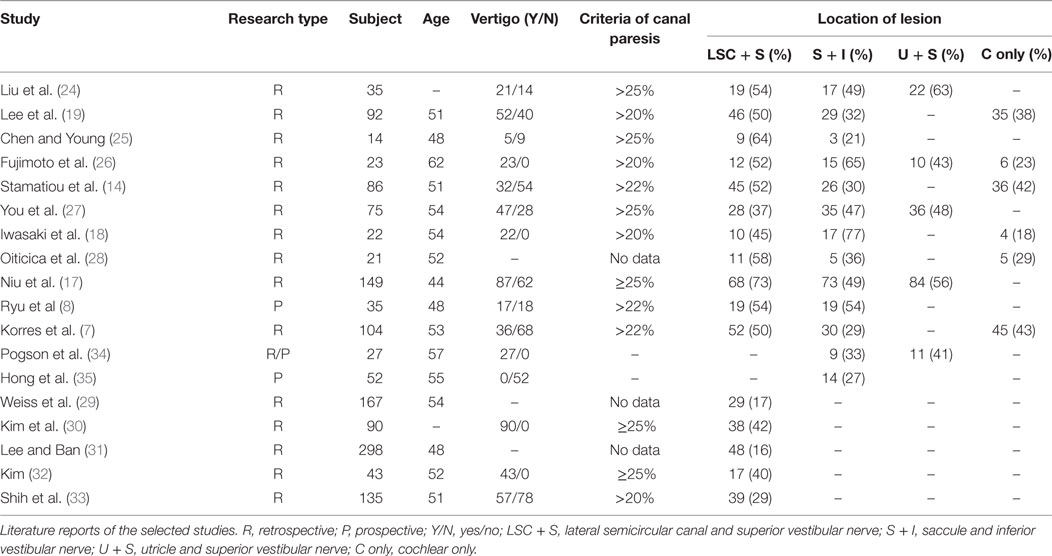
The main demographic features and clinical characteristics of the patients are listed in Table 1. Of the 18 studies included, 15 were retrospective studies (7, 14, 17–19, 24–33), 2 were prospective studies (8, 35), and 1 was a prospective/retrospective study (34), and the studies covered a total population of 1,468 subjects. The mean age ranged from 44 to 62 years, with the proportion of females ranging from 35 to 74%. The occurrence rate of vertigo was also summarized as a list. Concerning the types of vestibular examinations, the caloric test was performed in 16 studies (7, 8, 14, 17–19, 24–33), the cVEMP test was performedin 13 studies (7, 8, 14, 17–19, 24–28, 34, 35), and the oVEMP test was performed in 5 studies (17, 24, 26, 27, 34). In all 18 investigations, hearing recovery for abnormal and normal caloric response was provided in 7 studies (18, 19, 27, 29–31, 33), the number of patients with hearing improvement with abnormal and normal cVEMP response was provided in 4 studies (18, 19, 25, 27), and 1 study provided hearing outcomes for abnormal and normal oVEMP responses (27). The AHRQ scores ranged from 6 to 11, including 15 high-quality studies and 3 medium-quality studies (Table 2).
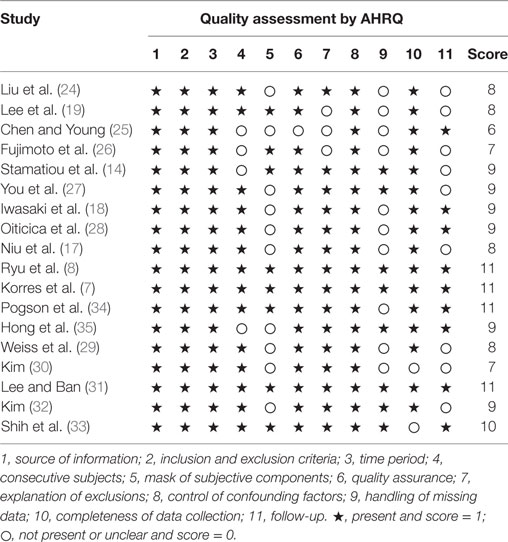
Table 2. Quality control of the selected studies according to the criteria of the Agency for Healthcare Research and Quality (AHRQ).
The Prevalence of Vestibulocochlear Lesion Patterns in Sudden Sensorineural Hearing Loss (SSHL)
As summarized in Figure 2, lesions of the lateral semicircular canal and superior vestibular pathway (LSC + S) were evaluated by the caloric test; lesions of the saccule and inferior vestibular pathway (S + I) were evaluated by the cVEMP test; lesions of the utricle and/or superior vestibular pathway (U + S) were evaluated by the oVEMP test, and the remaining lesions were assigned as cochlear lesions only (C only). We performed a meta-analysis of the prevalence of inner ear damage within the LSC + S, S + I, U + S, and C only subgroups of SSHL independently. There was a combined proportion of 0.44 (95% CI, 0.34–0.55, p < 0.01) with a random-effect model (I2 = 91%) in the mean occurrence rate of LSC + S; a combined proportion of 0.41 (95% CI, 0.34–0.49, p < 0.01) with a random-effect model (I2 = 74%) in the mean occurrence rate of S + I; a combined proportion of 0.53 (95% CI, 0.47–0.58, p = 0.27) with a fixed-effect model (I2 = 23%) in the mean occurrence rate of U + S; and a combined proportion of 0.39 (95% CI, 0.34–0.44, p = 0.22) with a fixed-effect model (I2 = 39%) in the mean occurrence rate of C only. Forest plots of the success rates are shown in Figure 3. Asymmetry was observed in the pool of data from the included investigations, indicating that there was some publication bias, as demonstrated by a funnel plot (Figure 4). The results indicated that the prevalence of inner ear organ damage was greatest in the U + S subgroup in SSHL, followed by LSC + S, S + I, and C only.
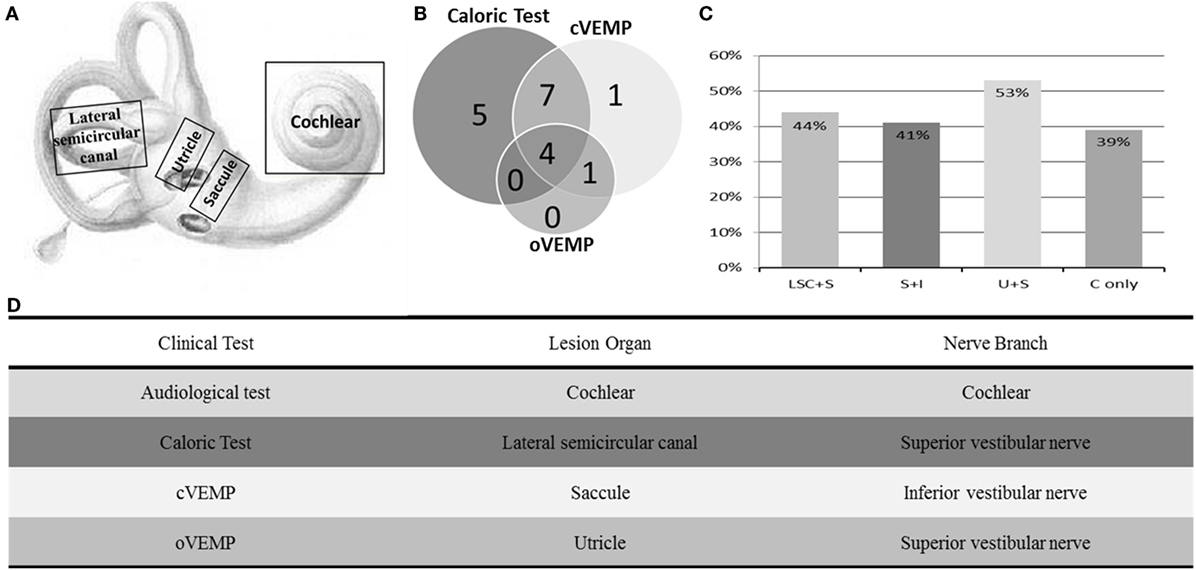
Figure 2. Summary of audio-vestibular abnormalities in SSHL. (A) Schematic diagram of the vestibulocochlear lesion patterns, including the cochlea, saccule, utricle, and lateral semicircular canal. (B) Distributions of vestibular test abnormalities of all 18 studies, including 16 by caloric test, 13 by cervical vestibular-evoked myogenic potential test (cVEMP), and 5 by ocular vestibular-evoked myogenic potential test (oVEMP). (C) Percentage of inner ear lesion locations in the included studies––LSC + S, abnormal caloric test; S + I, abnormal cVEMP test; U + S, abnormal oVEMP test; and C only, cochlear impairment only. (D) Table of clinical tests performed, organs involved, and their corresponding innervation.
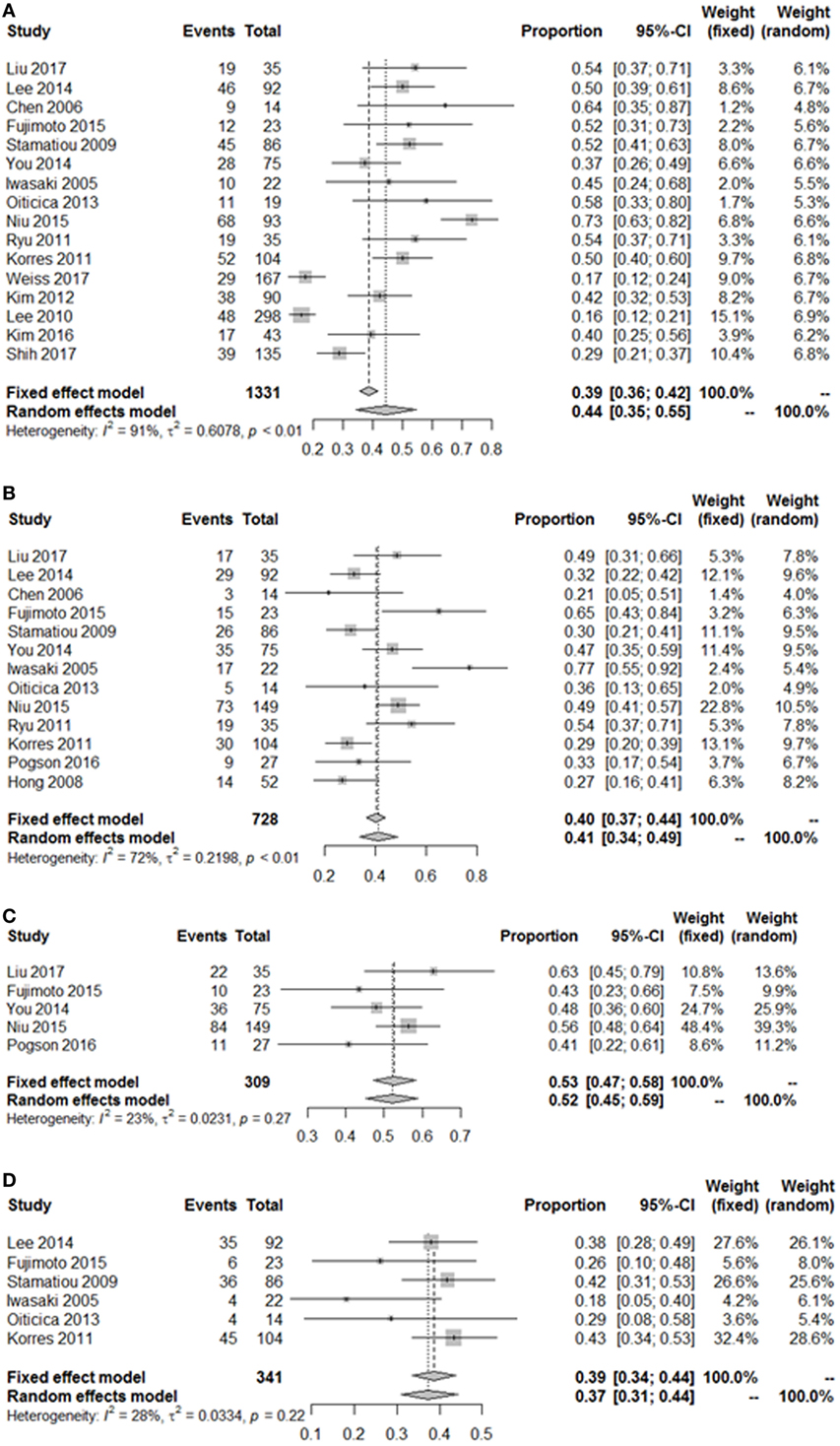
Figure 3. Pooled event rate of inner ear organ lesion locations. (A) Pooled occurrence rate of lateral semicircular canal lesion and superior vestibular nerve (LSC + S). (B) Pooled occurrence rate of saccule and inferior vestibular nerve lesion (S + I). (C) Pooled occurrence rate of utricle and superior vestibular nerve lesion (U + S). (D) Pooled occurrence rate of cochlea-only lesion (C only). Random, random-effect method; fixed, fixed-effect method.
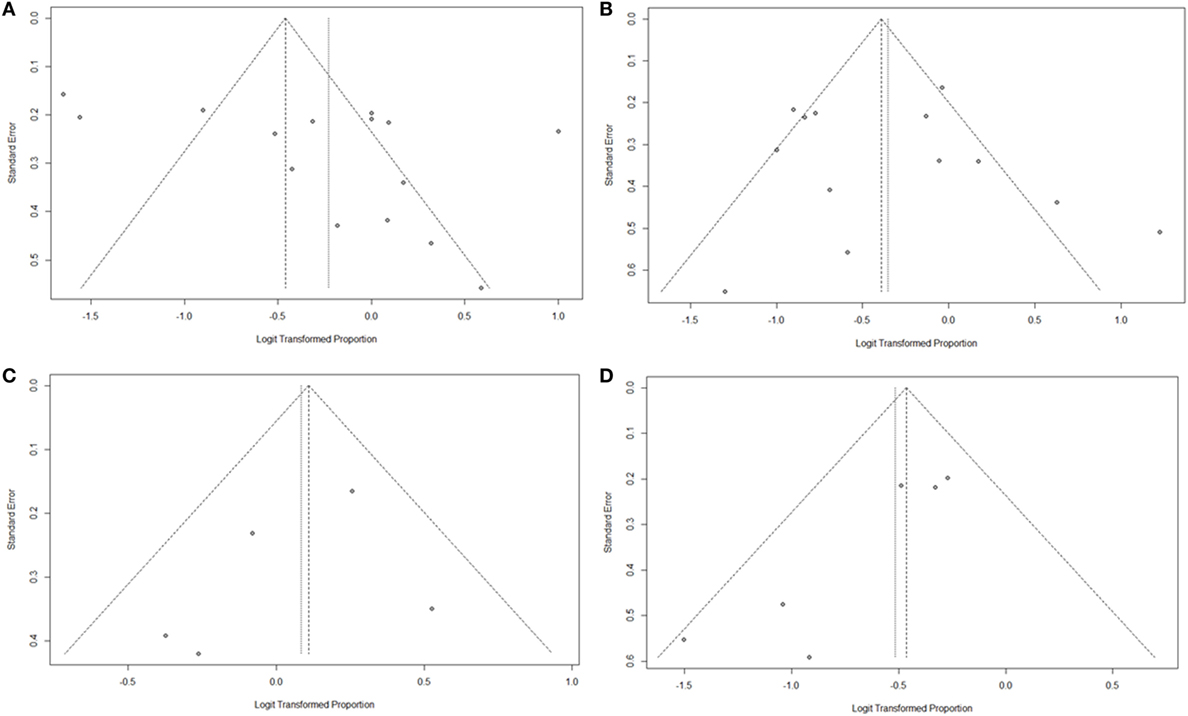
Figure 4. Funnel plots for the evaluation of publication bias. (A) Funnel plot of the included studies in the meta-analysis of pooled proportions within the LSC + S subgroup. (B) Funnel plot of the included studies in the meta-analysis of pooled proportions within the S + I subgroup. (C) Funnel plot of the included studies in the meta-analysis of pooled proportions within the U + S subgroup. (D) Funnel plot of the included studies in the meta-analysis of pooled proportions within the C only subgroup.
Considering the bias of vestibular balance in the studies, we performed further meta-analyses within the vertigo subgroup and the non-vertigo subgroup (Table 3). The pooled proportions for LSC + S, S + I, U + S, and C only within the vertigo subgroup were 0.50 (95% CI, 0.44–0.56, I2 = 44%, p = 0.1), 0.57 (95% CI, 0.41–0.72, I2 = 70%, p = 0.01), 0.54 (95% CI, 0.31–0.77, I2 = 77%, p = 0.01), and 0.25 (95% CI, 0.16–0.35, I2 = 0%, p = 0.53), respectively. The mean occurrence rates of LSC + S and S + I in the non-vertigo subgroup were 0.29 (95% CI, 0.00–0.58, I2 = 93%, p < 0.01) and 0.23 (95% CI, 0.16–0.31, I2 = 5%, p = 0.35), respectively. We were unable to obtain the pooled proportion in the U + S and C only subgroups because there was only one study including these subgroups. From the data above, we found that more than half of the patients suffered from some form of vestibular organ lesion in SSHL with vertigo, while only a quarter of the patients had damage of the vestibular organ in SSHL without vertigo.
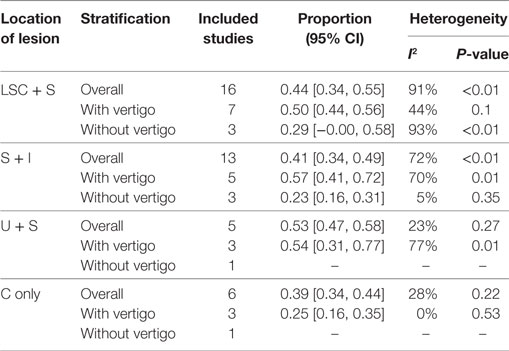
Table 3. Summary of the meta-analysis for the prevalence of the vestibulocochlear lesion patterns in SSHL.
The Clinical Value of Vertigo as a Prognostic Indicator of Lesion Location in SSHL
The clinical value of vertigo in inner ear organ damage was assessed by meta-analysis. Three studies described the occurrence rates of LSC + S lesions in a dichotomous pattern (vertigo group vs. non-vertigo group). We adopted a random-effect model with a high heterogeneity (I2 = 80%) to evaluate whether LSC + S in the vertigo group was more likely to be damaged than in the non-vertigo group. The weighted mean OR was 4.89, and the 95% CI was 1.20–19.93 (Figure 5A) with statistical significance (p = 0.03).
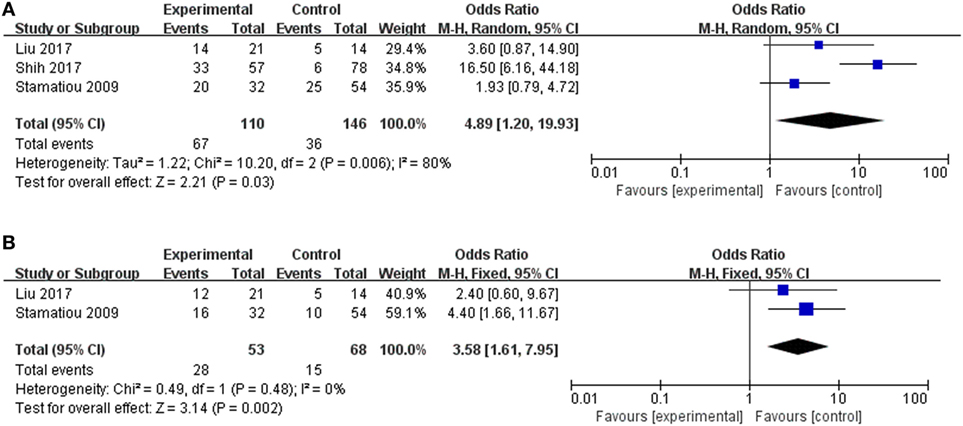
Figure 5. Forest plots of the synthesized data from the selected studies. (A) Comparison of the occurrence rate of lateral semicircular canal and superior vestibular nerve lesion (LSC + S) between the vertigo and non-vertigo groups. (B) Comparison of the occurrence rate of saccule and inferior vestibular nerve lesion (S + I) between the vertigo and non-vertigo groups. OR, odds ratio; M–H, Mantel–Haenszel method; random, random-effect method; fixed, fixed-effect method.
Only two studies were assessed in the S + I subgroup, and there was a combined OR of 3.58 (95% CI 1.61–7.95, p = 0.002) with a fixed-effect model (I2 = 0%) (Figure 5B). There was a statistically significant difference in the occurrence rates of LSC + S and S + I lesions between the vertigo and non-vertigo groups, which suggests that the presence of vertigo is indicative of a positive prognosis of greater vestibular dysfunction. Asymmetry was demonstrated by a funnel plot (Figure 6).
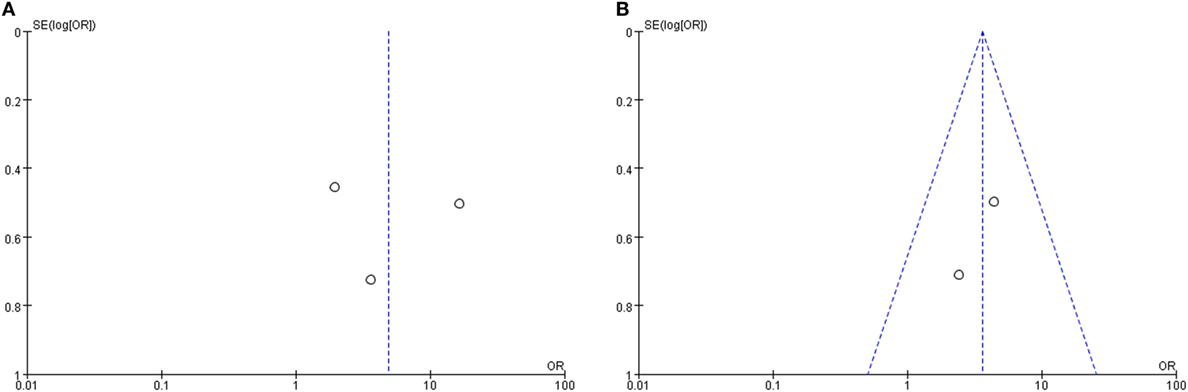
Figure 6. Funnel plots for the evaluation of publication bias. (A) Funnel plot of the included studies in the meta-analysis of the occurrence rate of lateral semicircular canal and superior vestibular nerve lesion (LSC + S). (B) Funnel plot of the included studies in the meta-analysis of the occurrence rate of saccule and inferior vestibular nerve lesion (S + I). MD, mean difference; OR, odds ratio.
The Correlation of Vestibular Organ Damage with Prognosis of Hearing Variables in SSHL
We next evaluated the hearing recovery of SSHL with or without LSC + S lesions by the caloric test in a total of 7 studies. We adopted a random-effect model with a high heterogeneity (I2 = 68%) to evaluate whether LSC + S lesions were more likely to be associated with poor hearing recovery compared with the group without LSC + S lesions. The weighted mean OR was 0.24, and the 95% CI was 0.11–0.52 (Figure 7A) with statistical significance (p = 0.0003).
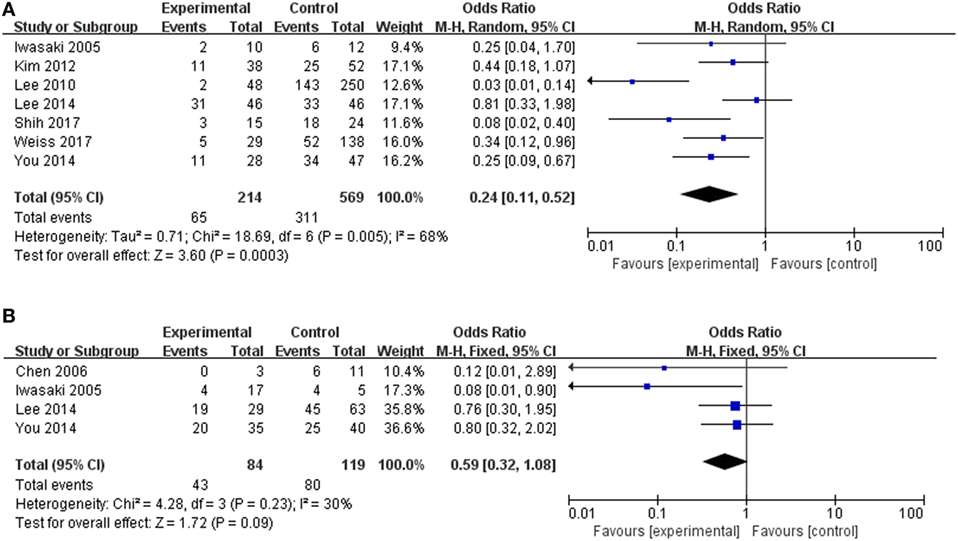
Figure 7. Forest plots of synthesized data from the selected studies. (A) Comparison of hearing improvement between the abnormal caloric response and normal caloric response groups. (B) Comparison of hearing improvement between the abnormal cVEMP response and normal cVEMP response groups. OR, odds ratio; M–H, Mantel–Haenszel method; random, random-effect method; fixed, fixed-effect method.
The clinical value of S + I damage according to the cVEMP test was assessed by meta-analysis. Four studies described the hearing outcomes of SSHL with or without S + I lesion. There was a combined OR of 0.59 (95% CI 0.32–1.08, p = 0.09) with a fixed-effect model (I2 = 30%) (Figure 7B). Asymmetry was demonstrated by a funnel plot (Figure 8). Due to a lack of data, we were unable to estimate the role of U + S lesions by oVEMP in the prognosis of hearing outcome in SSHL.
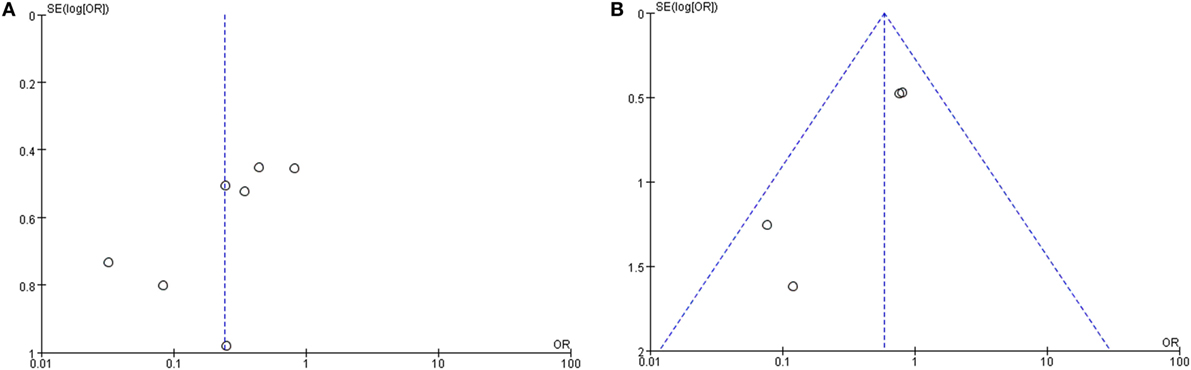
Figure 8. Funnel plots for the evaluation of publication bias. (A) Funnel plot of the included studies in the meta-analysis of hearing improvement between the abnormal caloric response and normal caloric response groups. (B) Funnel plot of the included studies of hearing recovery rate between the abnormal cVEMP response and normal cVEMP response groups. MD, mean difference; OR, odds ratio.
Sensitivity Assessment and Publication Bias
Finally, a sensitivity analysis was performed to assess and correct the publication bias based on the rule of omission. When each study was excluded sequentially to assess the stability of the final results, we found that no investigation affected the pooled risk estimate (Figure 9). However, we did find that the heterogeneity was significantly reduced if the investigation by Shih et al. was omitted in our study. In that case, the funnel plot was symmetrical in general and the heterogeneity test changed from I2 = 80%, p = 0.03 to I2 = 0%, p = 0.03.
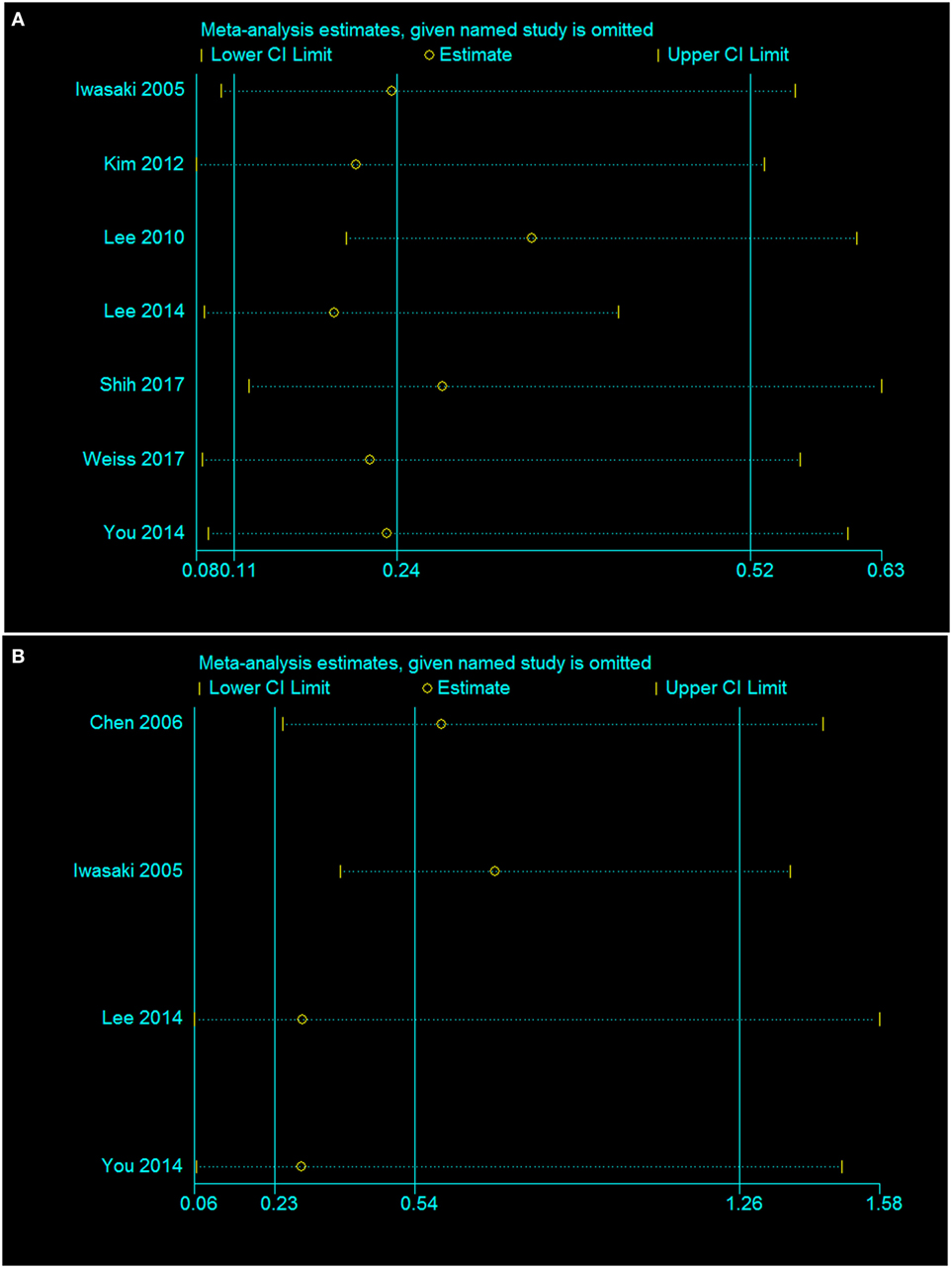
Figure 9. Sensitivity analysis of the association of the presence or absence of caloric response (A) and cVEMP response (B) in the hearing recovery rate in SSHL.
Discussion
Vestibular involvement in SSHL was first reported in 1949, and the role of vertigo in the clinical manifestation and prognosis of SSHL has been extensively studied since then (36). A number of studies have revealed the prevalence of inner ear organ lesion location in SSHL with or without vertigo (14–16, 18). However, some findings about the exact role of vestibular dysfunctions and lesion locations in SSHL have been unclear or contradictory, which led us to perform the present meta-analysis in order to draw proper conclusions with regard to the available evidence.
In the present systematic review and meta-analysis, we first found that vestibular organs were involved in SSHL regardless of the presence of vertigo, which indicated that vertigo might not be an independent and determining factor in SSHL. This phenomenon might instead be the result of the close correlation of the cochlear and vestibular organs both anatomically and genetically (7, 8).
To further evaluate the extent to which the entire inner ear, especially the vestibular organs, is involved in SSHL, we quantified the prevalence of the vestibulocochlear lesion patterns in our meta-analysis. The pooled event rates in our review showed that the U + S were the most susceptible to damage in SSHL, followed by the LSC + S and the S + I. Anatomically, the longer and narrower bony canal makes the superior vestibular nerve more susceptible to possible ischemic labyrinthine changes or other entrapments compared with the inferior vestibular nerve or singular nerves (37, 38). Manzari et al. also found that impairment of the utricle alone, as demonstrated by the results of the oVEMP test, was commonly associated with vestibular symptoms in the SSHL patients (39). Similarly, a significantly declining trend of inner ear deficits from the utricle to the saccule, and semicircular canals was identified after exposure to organic solvents (40). Peng-Chieh et al. explained that it was a combined result of peripheral and central parts of auditory pathways (40). Thus, the pathogenesis and pathological changes involved in otolith organ dysfunction in SSHL should be further explored in future studies.
In addition, our analysis also suggested that SSHL patients with vertigo are at an increased risk of vestibular organ lesions compared with patients who are not affected by vertigo. This result indicated that vertigo might be an important prognostic indicator in vestibulocochlear lesion patterns in SSHL. Furthermore, consistent results were confirmed by the statistical significance in the comparative dichotomous data in the LSC + S and S + I subgroups, which supports the above conclusions.
To determine the role of damage to the vestibular apparatus in the prognosis of hearing outcomes in SSHL, we quantified the occurrence of hearing improvement in our meta-analysis. We found that the likelihood of hearing recovery in the abnormal caloric response group was significantly worse than in the normal caloric response group, which might indicate that the abnormal caloric response is a negative prognostic marker in SSHL. In support of the hypothesis of Kim et al. the presence of concurrent canal dysfunction was associated with poor hearing outcome in SSHL (30). However, the pooled hearing recovery in the abnormal cVEMP response group was nearly half that of the recovery in the normal cVEMP response group, but this difference was still not statistically significant. This suggests that S + I lesions are not a critical variable in determining the extent of hearing improvement in SSHL. Further clinical data on the hearing outcomes of S + I lesions (by cVEMP tests) and U + S lesions (by oVEMP tests) are needed in order to draw more robust conclusions.
To the best of our knowledge, this is the first systematic review and meta-analysis to quantitatively summarize the prevalence of vestibulocochlear lesion patterns and to explore the prognostic role of vertigo in inner ear organ lesion patterns in SSHL. The results were extensive enough to be valid, including both pooled event rates within non-comparative studies and dichotomous data within comparative studies. In addition, the rule of omission was applied to assess and correct for publication bias in the sensitivity analysis.
There are several limitations that must be considered in the current systematic review. First, most of included studies were generally retrospective observational studies, which introduced publication bias and might not necessarily infer causation in our analysis. There was also a wide variety in the type of hearing loss, the degree of vertigo, and other accompany symptoms, as described in each study, which might reduce the reliability of our analysis. Third, the inter-study heterogeneity based on population differences was not evaluated using a meta-regression analysis. Therefore, the results should be interpreted with caution, and future well-designed studies are needed to investigate the causation of vestibular dysfunction in SSHL.
Conclusion
Our overall associations indicated that patients are at a substantially increased risk of vestibular organ lesions following a diagnosis of SSHL, especially in the presence of vertigo. Nearly half of the patients suffered from vestibular dysfunction in SSHL, and LSC + S lesions might be a critical variable in determining the extent of hearing improvement in SSHL. Thus, we conclude that the focus should not be just on the cochlea but also on the diagnosis and treatment of the vestibular system in SSHL regardless of the presence of vertigo. Emphasis on vestibular function might also be of important significance in the clinical manifestation and prognosis of SSHL.
Author Contributions
The literature was screened and methodological quality was assessed independently by HY and HL. HL drafted the manuscript. HY and HL performed the meta-analysis. All authors approved the final version and agree to be accountable for this work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Xiaofei Ye for kind advice on the statistical analysis. This work was supported by the National Key R&D Program of China (grant number 2017YFA0103900, grant number 2017YFA0103904, and grant number 2016YFC0905200) and the National Natural Science Foundation of China (grant number 81300825, grant number 81620108005, grant number 81230019, and grant number 81470687).
References
2. Saunders JE, Luxford WM, Devgan KK, Fetterman BL. Sudden hearing loss in acoustic neuroma patients. Otolaryngol Head Neck Surg (1995) 113(1):23–31. doi:10.1016/S0194-59989570140-0
3. Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg (2012) 146(3 Suppl):S1–35. doi:10.1177/0194599812436449
4. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med (2008) 359(8):833–40. doi:10.1056/NEJMcp0802129
5. Park HM, Jung SW, Rhee CK. Vestibular diagnosis as prognostic indicator in sudden hearing loss with vertigo. Acta Otolaryngol Suppl (2001) 545:80–3. doi:10.1080/000164801750388171
6. Stokroos RJ, Albers FW. The etiology of idiopathic sudden sensorineural hearing loss. A review of the literature. Acta Otorhinolaryngol Belg (1996) 50(1):69–76.
7. Korres S, Stamatiou GA, Gkoritsa E, Riga M, Xenelis J. Prognosis of patients with idiopathic sudden hearing loss: role of vestibular assessment. J Laryngol Otol (2011) 125(3):251–7. doi:10.1017/S0022215110002082
8. Ryu IS, Yoon TH, Ahn JH, Kang WS, Choi BS, Lee JH, et al. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging in sudden sensorineural hearing loss: correlations with audiologic and vestibular testing. Otol Neurotol (2011) 32(8):1205–9. doi:10.1097/MAO.0b013e31822e969f
9. Shinjo Y, Jin Y, Kaga K. Assessment of vestibular function of infants and children with congenital and acquired deafness using the ice-water caloric test, rotational chair test and vestibular-evoked myogenic potential recording. Acta Otolaryngol (2007) 127(7):736–47. doi:10.1080/00016480601002039
10. Shepard NT, Jacobson GP. The caloric irrigation test. Handb Clin Neurol (2016) 137:119–31. doi:10.1016/B978-0-444-63437-5.00009-1
11. Colebatch JG, Rosengren SM, Welgampola MS. Vestibular-evoked myogenic potentials. Handb Clin Neurol (2016) 137:133–55. doi:10.1016/B978-0-444-63437-5.00010-8
12. Pelosi S, Schuster D, Jacobson GP, Carlson ML, Haynes DS, Bennett ML, et al. Clinical characteristics associated with isolated unilateral utricular dysfunction. Am J Otolaryngol (2013) 34(5):490–5. doi:10.1016/j.amjoto.2013.04.008
13. Kantner C, Gurkov R. Characteristics and clinical applications of ocular vestibular evoked myogenic potentials. Hear Res (2012) 294(1–2):55–63. doi:10.1016/j.heares.2012.10.008
14. Stamatiou G, Gkoritsa E, Xenellis J, Riga M, Korres S. Semicircular canal versus otolithic involvement in idiopathic sudden hearing loss. J Laryngol Otol (2009) 123(12):1325–30. doi:10.1017/S0022215109990715
15. Wilson WR, Laird N, Kavesh DA. Electronystagmographic findings in idiopathic sudden hearing loss. Am J Otolaryngol (1982) 3(4):279–85. doi:10.1016/S0196-0709(82)80067-7
16. Shaia FT, Sheehy JL. Sudden sensori-neural hearing impairment: a report of 1,220 cases. Laryngoscope (1976) 86(3):389–98. doi:10.1288/00005537-197603000-00008
17. Niu X, Zhang Y, Zhang Q, Xu X, Han P, Cheng Y, et al. The relationship between hearing loss and vestibular dysfunction in patients with sudden sensorineural hearing loss. Acta Otolaryngol (2016) 136(3):225–31. doi:10.3109/00016489.2015.1110750
18. Iwasaki S, Takai Y, Ozeki H, Ito K, Karino S, Murofushi T. Extent of lesions in idiopathic sudden hearing loss with vertigo: study using click and galvanic vestibular evoked myogenic potentials. Arch Otolaryngol Head Neck Surg (2005) 131(10):857–62. doi:10.1001/archotol.131.10.857
19. Lee HS, Song JN, Park JM, Park KH, Kim HB, Seo JH. Association between vestibular function and hearing outcome in idiopathic sudden sensorineural hearing loss. Korean J Audiol (2014) 18(3):131–6. doi:10.7874/kja.2014.18.3.131
20. Guise JM, Butler M, Chang C, Viswanathan M, Pigott T, Tugwell P, et al. AHRQ series on complex intervention systematic reviews-paper 7: PRISMA-CI elaboration and explanation. J Clin Epidemiol (2017) 90:51–8. doi:10.1016/j.jclinepi.2017.06.017
21. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst (1959) 22(4):719–48.
22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi:10.1002/sim.1186
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi:10.1136/bmj.327.7414.557
24. Liu J, Zhou RH, Liu B, Leng YM, Liu JJ, Liu DD, et al. Assessment of balance and vestibular functions in patients with idiopathic sudden sensorineural hearing loss. J Huazhong Univ Sci Technolog Med Sci (2017) 37(2):264–70. doi:10.1007/s11596-017-1726-8
25. Chen CN, Young YH. Differentiating the cause of acute sensorineural hearing loss between Meniere’s disease and sudden deafness. Acta Otolaryngol (2006) 126(1):25–31. doi:10.1080/00016480510012363
26. Fujimoto C, Egami N, Kinoshita M, Sugasawa K, Yamasoba T, Iwasaki S. Involvement of vestibular organs in idiopathic sudden hearing loss with vertigo: an analysis using oVEMP and cVEMP testing. Clin Neurophysiol (2015) 126(5):1033–8. doi:10.1016/j.clinph.2014.07.028
27. You TZ, Wang SJ, Young YH. Registering grades of sudden deafness to predict the hearing outcome via an inner-ear test battery. Int J Audiol (2014) 53(3):153–8. doi:10.3109/14992027.2013.851798
28. Oiticica J, Bittar RS, Castro CC, Grasel S, Pereira LV, Bastos SL, et al. Contribution of audiovestibular tests to the topographic diagnosis of sudden deafness. Int Arch Otorhinolaryngol (2013) 17(3):305–14. doi:10.7162/S1809-977720130003000011
29. Weiss D, Bocker AJ, Koopmann M, Savvas E, Borowski M, Rudack C. Predictors of hearing recovery in patients with severe sudden sensorineural hearing loss. J Otolaryngol Head Neck Surg (2017) 46(1):27. doi:10.1186/s40463-017-0207-1
30. Kim YH, Kim KS, Choi H, Choi JS, Han CD. Benign paroxysmal positional vertigo is not a prognostic factor in sudden sensorineural hearing loss. Otolaryngol Head Neck Surg (2012) 146(2):279–82. doi:10.1177/0194599811421733
31. Lee NH, Ban JH. Is BPPV a prognostic factor in idiopathic sudden sensory hearing loss? Clin Exp Otorhinolaryngol (2010) 3(4):199–202. doi:10.3342/ceo.2010.3.4.199
32. Kim CH, Shin JE, Yang YS, Im D. Sudden sensorineural hearing loss with positional vertigo: Initial findings of positional nystagmus and hearing outcomes. Int J Audiol (2016) 55(10):541–46. doi:10.1080/14992027.2016.1194532
33. Shih CP, Chou YC, Chen HC, Lee JC, Chu YH, Wang CH. Analysis of caloric test responses in sudden hearing loss. Ear Nose Throat J (2017) 96(2):59–64.
34. Pogson JM, Taylor RL, Young AS, McGarvie LA, Flanagan S, Halmagyi GM, et al. Vertigo with sudden hearing loss: audio-vestibular characteristics. J Neurol (2016) 263(10):2086–96. doi:10.1007/s00415-016-8214-0
35. Hong SM, Byun JY, Park CH, Lee JH, Park MS, Cha CI. Saccular damage in patients with idiopathic sudden sensorineural hearing loss without vertigo. Otolaryngol Head Neck Surg (2008) 139(4):541–5. doi:10.1016/j.otohns.2008.07.003
37. Goebel JA, O’Mara W, Gianoli G. Anatomic considerations in vestibular neuritis. Otol Neurotol (2001) 22(4):512–8. doi:10.1097/00129492-200107000-00018
38. Gianoli G, Goebel J, Mowry S, Poomipannit P. Anatomic differences in the lateral vestibular nerve channels and their implications in vestibular neuritis. Otol Neurotol (2005) 26(3):489–94. doi:10.1097/01.mao.0000169787.99835.9f
39. Manzari L, Burgess AM, Curthoys IS. Does unilateral utricular dysfunction cause horizontal spontaneous nystagmus? Eur Arch Otorhinolaryngol (2012) 269(11):2441–5. doi:10.1007/s00405-012-2127-z
Keywords: vestibular dysfunction, vertigo, sudden sensorineural hearing loss, caloric test, cervical vestibular-evoked myogenic potential, ocular vestibular-evoked myogenic potential, meta-analysis
Citation: Yu H and Li H (2018) Vestibular Dysfunctions in Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-analysis. Front. Neurol. 9:45. doi: 10.3389/fneur.2018.00045
Received: 18 November 2017; Accepted: 18 January 2018;
Published: 05 February 2018
Edited by:
Toshihisa Murofushi, Teikyo University, JapanReviewed by:
Maurizio Barbara, Sapienza Università di Roma, ItalyYasuhiro Chihara, Raffles Japanese Clinic, Japan
Copyright: © 2018 Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huawei Li, hwli@shmu.edu.cn
 Huiqian Yu1
Huiqian Yu1 Huawei Li
Huawei Li