The hippocampus and imagining the future: where do we stand?
- 1 Australian Research Council Centre of Excellence in Cognition and its Disorders, Sydney, NSW, Australia
- 2 Centre for Brain Research and Department of Psychology, The University of Auckland, Auckland, New Zealand
- 3 Department of Psychology, Harvard University, Cambridge, MA, USA
Recent neuroimaging work has demonstrated that the hippocampus is engaged when imagining the future, in some cases more than when remembering the past. It is possible that this hippocampal activation reflects recombining details into coherent scenarios and/or the encoding of these scenarios into memory for later use. However, inconsistent findings have emerged from recent studies of future simulation in patients with memory loss and hippocampal damage. Thus, it remains an open question as to whether the hippocampus is necessary for future simulation. In this review, we consider the findings from patient studies and the neuroimaging literature with respect to a new framework that highlights three component processes of simulation: accessing episodic details, recombining details, and encoding simulations. We attempt to reconcile these discrepancies between neuroimaging and patient studies by suggesting that different component processes of future simulation may be differentially affected by hippocampal damage.
Introduction
In daily life, particularly during the unoccupied moments, we often revert to our inner mental world and engage with our aspects of our lives outside of the present. Mentally projecting ourselves back into the past or forward into the future can take make forms – a cursory thought, a vague image, or a vivid and consuming scenario. There has been increasing interest in understanding the ways in which remembering and future thinking are similar or different, both in terms of cognitive and neural processes, and whether such characteristics are evident for various forms of past and future thinking (for recent reviews, see Schacter et al., 2008; Szpunar, 2010). These studies have been informed by a closely related line of neuroimaging research showing that when people are consumed by various forms of thoughts and images, these internally directed cognitive activities are accompanied by a characteristic pattern of neural activity – known as the default network (Buckner et al., 2008; Spreng et al., 2009).
This network, which includes many regions traditionally associated with memory, such as the hippocampus, is also up-regulated by tasks that specifically require a focus on remembering and imagining personal experiences (Buckner and Carroll, 2007; Schacter et al., 2007; Spreng et al., 2009). Motivated by findings that remembering and imagining engage the same “common core network,” we advanced the constructive episodic simulation hypothesis, which holds that the common neural activity for past and future reflects a reliance on memory to provide the details comprising both remembered and imagined event representations (Schacter and Addis, 2007). In that theory, as well as in this review, we focus on a particularly vivid form of future thinking: the imaginative construction or simulation of scenarios that might occur in one’s future. We hypothesized that the flexible use of episodic details from memory during imaginative simulations of the future can help to understand constructive aspects of memory, such as its susceptibility to distortion (see also Schacter et al., 2011b). Like autobiographical memories of past experiences, these simulations are considered “episodic” in nature because they represent the self engaging in a specific event in a particular spatiotemporal context. And although the emphasis here is primarily on simulations located in the imagined future, because of the adaptive value of such simulations for maximizing future success (Ingvar, 1985; Suddendorf and Corballis, 1997, 2007; Schacter and Addis, 2007; Szpunar, 2010), simulations can also focus on present or past events; indeed, we have argued that many of the same processes discussed here are likely also applicable under those conditions (Addis et al., 2009a).
One of the more compelling and even unexpected findings from research on the neural underpinnings of episodic simulations is that the hippocampus, a region traditionally thought of as a “memory region,” can be engaged to a greater degree when imagining than remembering (e.g., Addis et al., 2007b; for reviews, see Schacter and Addis, 2009; Buckner, 2010). Such findings raise the question of what is unique about episodic simulation or future thinking that recruits the hippocampus. In very general terms, it would appear that more intensive processing is required when imagining future events relative to retrieving past events, because the former requires construction of a novel event, whereas the latter involves retrieval of an already established event. However, determining what specific component processes underlie this “more intensive processing,” and which such processes rely on the hippocampus, is necessary to better understand this future > past effect. A number of candidate cognitive processes exist. Although both remembering and imagining typically involve the reactivation of memories and episodic details comprising these memories, only imagining requires the additional step of recombining such details into a new arrangement – the imagined scenario. It is plausible that this recombination process would engage the hippocampus, given its role in relational memory processes that link together disparate bits of information (Eichenbaum, 2001). Also, if these newly constructed scenarios are ever to be accessed in future, they need to be encoded and stored in memory (Ingvar, 1985). In this review, we will discuss the conditions under which a hippocampal future > past effect emerges, and also consider recent work investigating whether hippocampal activation during future thinking reflects access to episodic details, recombining these details to construct specific scenarios, and/or the encoding of these scenarios into memory.
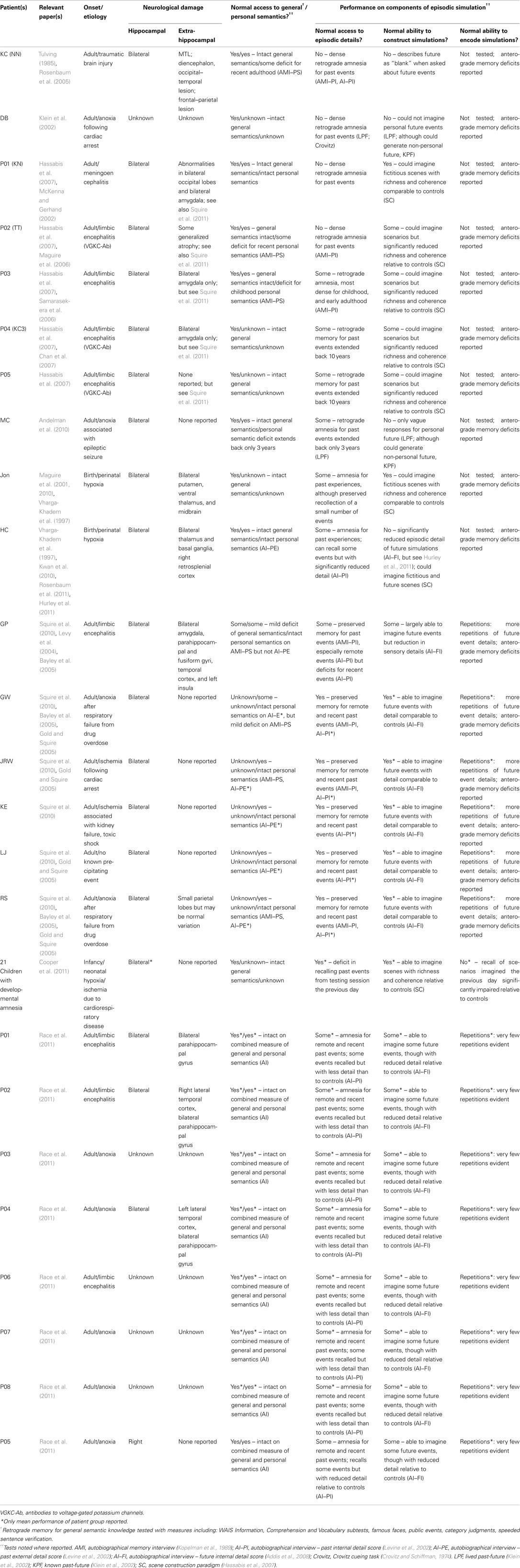
A related line of enquiry is to determine not only whether the hippocampus is active during future simulation but whether it makes a critical and necessary contribution. While it has been long established that a functioning hippocampus is necessary for the retrieval of detailed autobiographical memories (for a review, see Moscovitch et al., 2005), it is less clear whether this is the case for future simulation (see Table 1 for a summary of patient cases discussed herein). While some patients with hippocampal damage and impaired episodic memory also exhibit difficulties in imagining detailed and coherent future events (Hassabis et al., 2007; Andelman et al., 2010; Race et al., 2011), other studies do not report imagination deficits in such patients. Spared simulation abilities in the context of hippocampal damage and memory loss have been reported in an adult developmental amnesic patient (Maguire et al., 2010), a group of developmental amnesic school-aged children (Cooper et al., 2011; see also, Hurley et al., 2011), and a group of adult patients with bilateral hippocampal damage (Squire et al., 2010).
Such findings imply that a fully intact hippocampus may not be required for future simulation. However, the inconsistent results yielded from these studies raise a number of important questions. Does the temporal extent of amnesia influence the degree to which imagined scenarios can be constructed? Does the age of onset of hippocampal damage affect the degree of impairment? Does the location of the damage within the hippocampus influence the pattern of spared and impaired abilities? Can residual hippocampal tissue support future simulation? Are particular simulation tasks better able to detect deficits? In considering the findings from patient studies in conjunction with those from neuroimaging literature, we will attempt to reconcile these discrepant results by suggesting that different component processes of future simulation may be differentially affected by hippocampal damage: although the processes of accessing and recombining details to construct and encode a future event are inherently related processes in healthy individuals, it is possible that in the damaged brain these processes are, to some extent, dissociable.
Access to Memory Details: The Episodic Fodder for Future Simulations
In recent years, neuroimaging has provided evidence to suggest that imagining the future relies on much of the same neural machinery as remembering the past. One hypothesis that such findings motivate is that memories must be reactivated in order to extract the information needed to “flesh out” detailed simulations. Indeed, if simulations involve the projection of the self in time beyond the present (Buckner and Carroll, 2007) and are to be meaningful for that individual, then personally relevant episodic details from memory are needed. Such elements include the major components of an episode, including people, places, and objects previously encountered by the individual. In their scene construction hypothesis, Hassabis and Maguire (2007) argue that spatial information is particularly important. A spatial framework provides a platform upon which to build the scenario, and without this, an imagined event would likely lack a sense of coherence.
Although common hippocampal activity for past and future events is suggestive of access to mnemonic information during both tasks, it is not conclusive. Addis and Schacter (2008) examined whether hippocampal responses during remembering and imagining were modulated by subjective ratings of the detail comprising these events. Activity in the posterior hippocampus correlated with detail ratings for both past and future events, consistent with the idea that both tasks require access to episodic details. Moreover, Weiler et al. (2010b) found activity in the posterior hippocampus was associated with both past and future events, though the responses had differing timecourses. Nevertheless, the location of this neural response dovetails with studies implicating the posterior hippocampus in retrieval as opposed to encoding (Lepage et al., 1998; Schacter and Wagner, 1999; Prince et al., 2005), in the reinstatement of previous conditions (Preston et al., 2004; Giovanello et al., 2009), and in the amount of detail comprising autobiographical memories (Addis et al., 2004b).
However, the most convincing evidence that access to episodic details may be necessary for future simulations comes from studies of patients with memory loss (see Table 1). One of the early observations of a link between past and future thinking came from Tulving (1985). In a discussion of K.C., a patient with dense autobiographical amnesia resulting from a head injury, it was also noted that K.C. exhibited difficulties in imagining specific episodes in his personal future. Similarly, amnesic patient D.B., who sustained brain damage as a result of cardiac arrest and anoxia, cannot remember or imagine personal events (Klein et al., 2002). In both cases, the neuroanatomical damage is not restricted to the hippocampus (patient K.C. has damage in and beyond the hippocampus, including extensive prefrontal damage, Rosenbaum et al., 2005; no neuroanatomical findings have been reported for patient D.B.). Nevertheless, these reports raised the possibility that there is a link between remembering and imagining – that being able to access details from episodic memory may be an important and perhaps necessary condition of the successful construction of episodic simulations.
Similar results have been reported in patients with damage reported to be limited to the hippocampus. Hassabis et al. (2007) found that four out of five patients with hippocampal amnesia could not construct imaginary scenarios of everyday scenes: their constructions contained significantly less content that those of controls, and the details that were generated were not well integrated. Although the authors also found that providing patients with details did not improve their performance, the provided information was semantic in nature and therefore may not have been sufficient to support imaginings that have an episodic basis. One critical issue is whether these patients have damage circumscribed to the hippocampus. Although Maguire and Hassabis (2011) state these patients were “specifically selected” for damaged restricted to the hippocampus, Squire et al. (2011) disagree with this assessment. They argue that aspects of the clinical profiles of these patients (e.g., generalized atrophy, seizures, personality change) suggest the presence of damage outside of the hippocampus. They also note that the one patient in the Hassabis et al. (2007) study who did not exhibit imagination deficits had a different etiology (meningoencephalitis and recurrent meningitis, vs. limbic encephalitis in the four other patients), as well as residual hippocampal tissue and function (Hassabis et al., 2007).
Race et al. (2011) examined the ability to remember and imagine in a group of eight amnesic patients with medial temporal damage. This study is important for two reasons. First, the paradigm included a condition in which participants were required to construct narratives when the details did not have to be retrieved from memory but were presented as pictures (also see Gaesser et al., 2011). When completing the past and future tasks, amnesic patients generated significantly fewer episodic details than did controls, and the number of episodic details for past and future narratives was correlated. Critically, hippocampal damage did not disrupt the ability to construct a narrative in the picture condition, where access to episodic memory was not required. Moreover, performance on the picture narrative task was not correlated with performance on the future task. Second, although the etiology and extent of damage varied across the eight patients, there was one patient in whom damage was confirmed as being limited to the hippocampus. Importantly, the performance of this patient mirrored that of the other patients who had some degree of extra-hippocampal temporal damage, suggesting that damage to the hippocampus alone is sufficient to disrupt future simulation. Together, the observations from this study further support the notion that in the context of hippocampal damage, it is an inability to access details in episodic memory, and not more general deficits in narrative ability, that underlies deficient episodic simulation performance.
While studies of amnesia give insight into the ability to simulate when there is little, or no, access to episodic details, studies of aging – where deficits in accessing past events are present but comparatively milder – have also provided relevant evidence. In a series of studies, we have examined the ability to remember and imagine in healthy and also in pathological aging (i.e., patients in the early stages of Alzheimer’s disease), in which autobiographical memory is typically affected (Levine et al., 2002), and hippocampal atrophy and dysfunction are also evident (Hedden and Gabrieli, 2004). In these studies, we had participants generate memories of past events and simulations of future events in response to word cues and found that the number of episodic details comprising events in older or demented adults was reduced relative to appropriate control groups (for a review, see Schacter et al., 2011a). Moreover, the number of episodic details for past events is strongly correlated with the number of details comprising future events. These correlations are consistently evident across old and young (Addis et al., 2008, 2010), and across demented and healthy older adults (Addis et al., 2009b), and exist even when controlling other factors that may more generally influence the detail of narratives, such as cognitive decline and verbal fluency (Addis et al., 2009b). The deficits in episodic remembering and imagining that we have documented in older adults do also extend to a picture description task that does not require episodic memory (Gaesser et al., 2011). Nonetheless, we also found that the age deficits in remembering and imagining were still observed after controlling for general narrative abilities, as measured by this picture description task. Neuroimaging evidence suggests that the reduction in episodic detail when older adults describe past and future events may be related to dysfunction in the regions supporting episodic detail, including the hippocampus (Addis et al., 2011b).
What is to be made, then, of patients with memory loss who can still imagine the future? Such findings appear to speak against the idea that access to memories is a critical precursor to future simulation. Squire et al. (2010) reported that a group of patients with damage to the hippocampus showed an intact ability to create detailed imaginary future events. However, although these patients have hippocampal damage, it is notable that their degree of retrograde amnesia is minimal: these patients can retrieve events from the remote past, and only exhibit a mild (and non-significant) deficit for retrieving memories from the recent past. Thus, the results of this study could also be interpreted as supporting the notion that access to the past – even in the context of hippocampal damage – can provide a basis for imagining the future.
However, there are reported cases of hippocampal damage that has differentially affected remembering but not imagining. For instance, Maguire and colleagues reported that developmentally amnesic patients who sustained hippocampal damage early in life can construct imaginary scenarios (Maguire et al., 2010; Hurley et al., 2011; but see, Kwan et al., 2010). Moreover, as noted earlier, one of the patients from the Hassabis et al. (2007) study could also complete their scene construction task. Interestingly, some of these patients have been noted to have residual hippocampal tissue that appears to be functional, in that it is activated during memory tasks (Maguire et al., 2010), although such activation has not yet been shown during future simulation. These researchers also report normal imagination abilities in a group of children with hippocampal damage and amnesia (Cooper et al., 2011), further suggesting that the time of onset of the amnesia may be an important consideration. It is possible that with early damage, these patients develop other strategies or rely either on residual episodic memories or detailed semantic information to construct scenarios (Cooper et al., 2011).
It is also notable that these findings have emerged using the scene construction task. Hassabis et al. (2007) mention that this task was designed to “increase the dependence of constructions on generalized semantic memory representations.” On each trial, a sentence cue (e.g., “Imagine you are lying on a white sandy beach”) is provided to take participants into a generic scene; it is very likely that this scene can then be fleshed out with semantic detail. Thus, it is possible that these patients are able to complete this particular imagination task using detailed yet semantic representations of how certain scenes or episodes unfold, rather than extracting information from their own experiences. However, when the task requires creation of a specific and novel episode, similar patients (e.g., with developmental amnesia) show simulation deficits – particularly in the amount of episodic detail generated (Kwan et al., 2010). Although amnesics may generate fewer episodic details relative to controls, they sometimes show little or no reduction in the number of semantic details comprising their event narratives (Race et al., 2011). It has also been shown in other studies that patients with episodic, but not semantic, memory deficits can successfully complete future thinking tasks that are based primarily on general knowledge (e.g., non-personal future tasks; Klein et al., 2002).
When faced with reduced or no access to episodic memory, it may be a natural compensation strategy to rely on semantic information to aid in describing autobiographical events. Using a scoring technique that specifically parses episodic from non-episodic information (Levine et al., 2002), we have also found that although older adults show a decline in the amount of episodic detail comprising their past and future events, they show a corresponding increase in the amount of non-episodic, conceptual information (Addis et al., 2008, 2010; note also that this pattern extends to picture description; Gaesser et al., 2011). In line with this finding, older adults also show an increase, relative to young, in their recruitment of lateral temporal regions during autobiographical tasks (Addis et al., 2011b); these regions are thought to mediate semantic and conceptual autobiographical information (Graham et al., 2003; Addis et al., 2004a).
Another key question is whether access to episodic details is sufficient for future simulation to occur. It is likely that this ability is only a starting point; once episodic details are extracted, they still have to be used in a meaningful way, which we have argued requires additional processes such as detail recombination (e.g., Addis and Schacter, 2008; Schacter and Addis, 2009). Nonetheless, the findings discussed earlier of intact future simulation performance in hippocampal amnesics with relatively preserved autobiographical memories (Squire et al., 2010) suggests that access to episodic details may be sufficient for future simulation. By contrast, Andelman et al. (2010) reported a case study of a patient, M.C., with a bilateral hippocampal lesion and loss of autobiographical memory restricted only to the past 3 years. Thus, at 27 years of age, she still had approximately 20 years of episodic memories to draw upon when completing a future simulation task. M.C. was, however, unable to do so: when asked to describe her personal future, her responses were vague and general, or she reported that she simply did not know. Because there was no quantitative assessment of future simulation performance in this case, the results must be interpreted cautiously. Still, they raise the possibility that while access to episodic details may be necessary in order to construct episodic simulations, it may not be sufficient.
Detail Recombination: Constructing a Coherent Scenario
As we have reviewed above, being able to access details from episodic memory can be conceptualized as an initial stage in the process of episodic simulation. Of course, having a jumble of details is useless if they cannot be recombined and integrated appropriately. We have argued that “detail recombination” is critical to imagining coherent scenarios – the kinds of simulations one creates when thinking about experiences relevant in their daily lives. Given the role of the hippocampus, particularly the anterior hippocampus, in relational processing, we have argued that this region is likely critical in the ability to form coherent scenarios (e.g., Addis and Schacter, 2008; Schacter and Addis, 2009).
This proposal is based on an integration of findings from various neuroimaging studies. An early meta-analysis of medial temporal activity during memory tasks reported that the anterior portion of the hippocampus appears to be particularly responsive to tasks with relational demands (Schacter and Wagner, 1999); subsequent work has further supported this anterior localization of relational memory processes (e.g., Giovanello et al., 2004; Jackson and Schacter, 2004; Kirwan and Stark, 2004; Chua et al., 2007; Staresina and Davachi, 2008, 2009). The role of this region within the realm of relational memory may be further refined, based on findings from Preston et al., 2004; see also Heckers et al., 2004). This work suggests that the anterior hippocampus may be particularly involved in the recombination of details extracted from various memories. Using a transitive inference paradigm, participants first learned to associate one set of items (faces, A) with another set of items (houses, B). They then learned to associate those same houses (B) with a new set of items (novel faces, C). During the scanning session, seeing items (A, B, or C) taken from any of the memories (A–B, B–C) resulted in posterior hippocampal activity, further implicating the posterior hippocampus in retrieval or reinstatement. However, seeing novel rearrangements of such details (A–C) resulted in selective anterior hippocampal activity. This recombination process can be considered analogous to future simulation, where we argue details extracted from different memories that may have not been encountered together in reality, are rearranged in imagination – and similarly, this recombination process should also engage the anterior hippocampus.
More recently, Staresina and Davachi (2009) investigated hippocampal responses to the process of integrating details across time and space. They identified a region in the anterior hippocampus that was more responsive when details were presented in a spatiotemporally discontiguous manner (i.e., separated across time and space) and required integration, relative to when details were presented in a contiguous, integrated form. Conceptually, we suggest that this process again maps onto the kind of recombination thought to occur during simulation: an integration of details from memories formed in different spatiotemporal contexts.
The findings of Preston et al. (2004) and Staresina and Davachi (2009) dovetail with those from a neuroimaging study of past and future detail. In that study, we (Addis and Schacter, 2008) found common responses to detail of past and future events in posterior hippocampus, but the anterior hippocampus was responsive only to the amount of detail comprising future events – which are presumably recombined across spatiotemporally distinct experiences. Interestingly, we have replicated the finding of differential future activity within the anterior hippocampus across a number of studies using autobiographical cuing (e.g., Addis et al., 2007b; adapted from Crovitz and Schiffman, 1974) and experimental recombination paradigms (Addis et al., 2009a). While the cueing task requires an individual to generate future events from generic cues (nouns), the experimental recombination paradigm uses random rearrangements of episodic details (persons, places, objects) taken from the individual’s own memories, thus ensuring that detail recombination occurs. Moreover, these paradigms enable examination of activity during the initial construction of the future event when the cue is presented, and the subsequent elaboration of the event once it is in mind. With this approach, we have found that over the course of a simulation trial, this differential hippocampal activity typically emerges during the initial construction phase rather than being evident throughout the duration of a simulation trial (Addis et al., 2007b, 2009a, 2011a; Martin et al., 2011). This temporal pattern suggests that the differential future-related activity is associated with processes occurring early in the construction of future events, when detail recombination would be expected to occur. Other labs have also reported similar future-related effects in the anterior hippocampus. For instance, Weiler et al. (2010a) found that imagining future events that had a low probability of occurring during the upcoming holidays was associated with more anterior hippocampal activity than events with a higher probability of occurring. The authors suggested that perhaps low probability events place a higher demand on the binding of disparate event features relative to high probability events that may be already planned.
Determining the boundary conditions of the future > past effect will provide a better understanding of whether detail recombination is important for engaging the anterior aspect of the hippocampus. Importantly, we have recently shown that this effect is limited to certain types of future events. We examined hippocampal activity when imagining specific (unique) and general (routine) future events, hypothesizing that constructing a specific future event should place greater demand on recombining details and hippocampal resources relative to constructing a generic future event that more closely relies on conceptual knowledge about routines (Addis et al., 2011a). Indeed, our analysis supported this hypothesis, demonstrating that hippocampal activity was strongest when imagining specific future events relative to more generic and routinized ones. Participant ratings confirmed that specific future events were more detailed and novel than general future events, further suggesting that the process of constructing an event that is both detailed and novel engages the anterior hippocampal region. Additionally, because these findings suggest that the hippocampus is not strongly engaged by constructing generic future events, it may not be surprising that patients with hippocampal damage can imagine the future in a gist-like, conceptual manner.
These observations from neuroimaging studies suggest that dysfunction in the hippocampus may result in deficits in recombining details. Several findings suggest the presence of such difficulties. Hassabis et al. (2007) found that not only did the events constructed by hippocampal amnesics lack content overall, but the details they did generate were not well integrated and lacked a spatial coherence. In healthy older adults who show some degree of structural and functional dysfunction in the hippocampus (Hedden and Gabrieli, 2004), we found that the integration of memory details into simulations was reduced relative to young adults (Addis et al., 2010). Using the experimental recombination paradigm, we experimentally “extracted” person, place, and object details from different past events; random recombinations of a participant’s memory details were later presented during a future simulation task. Importantly, each future simulation was required to include the person, place, and object details presented. While both groups were able to include all three details in the simulations, the young group was better able to integrate these three details into the same imagined spatiotemporal context. In contrast, older adults integrated on average two of the three details into the same spatiotemporal context, and then often touched on the third detail in a separate context, essentially resulting in a series of “mini-events.” These findings suggest that even with experimental support to access details from various episodic memories, the ability to integrate these details into a coherent scenario with a specific temporal and spatial context may be reduced in populations with compromised hippocampal function.
Again, one might raise the question that if the hippocampus is necessary for detail recombination, how is it that some patients with hippocampal damage can imagine seemingly coherent future events? One issue is that not every study of future simulation in patients includes a measure of detail integration or spatial coherence and thus in instances where hippocampal patients can successfully imagine, it can be difficult to determine whether the scenarios constructed were in fact coherent. Maguire and Hassabis (2011) argue that the number of spatial references produced by the patients studied by Squire et al. (2010) appear reduced relative to the typical level of controls, suggesting that these patients may have been creating primarily semantic representations. Moreover, it is possible to imagine a future event with minimal, if any, detail recombination: one can “recast” past events into the future. It is possible that paradigms using single cues may elicit recasting. For instance, if shown the cue word “car,” one might recall a relevant experience (“my car breaking down and my husband picking me up”) and then imagine that experience unfolding in the same way in future. In many protocols, it is ensured that participants are generated novel scenarios (e.g., Addis et al., 2007b, 2008; Hassabis et al., 2007), but this is not always done or reported. In order to circumvent this possibility, we designed an experimental recombination paradigm in which participants are required to recombine details extracted from their own past events (Addis et al., 2009a). Although this paradigm has been employed with older adults (Addis et al., 2010), replicating our findings using the cue word paradigm, it has not yet been used to assess recombination abilities in patients with circumscribed hippocampal damage. The results of such a study would be of considerable interest.
Memory for the Future: Encoding Future Simulations
Differential engagement of the anterior hippocampus may also reflect the process of encoding newly imagined scenarios. Indeed, the anterior portion of the hippocampus has been implicated in encoding (Schacter and Wagner, 1999; Spaniol et al., 2009), particularly for relational (e.g., Jackson and Schacter, 2004; Kirwan and Stark, 2004; Chua et al., 2007; Staresina and Davachi, 2008, 2009) and novel (Kohler et al., 2005) information. If the adaptive significance of simulating several alternative “behavioral modes” is to maximize success in anticipated situations (Ingvar, 1985) and flexible planning (Boyer, 2008), then retaining this “fitness-relevant” information in memory for future reference is a necessary step. Nairne et al. (2007) investigated whether information relevant to survival is remembered better than survival-irrelevant information. In that study, participants judged whether items were relevant to survival (having provisions and protection) or to non-survival activities moving (moving to a foreign country) or judged the items for pleasantness. In line with the idea that we are tuned to remember fitness-relevant information, subsequent memory performance was boosted for items rated as survival-relevant. Interestingly, more recent work using a variant of the paradigm developed by Nairne and colleagues suggests that the much of the benefit of “survival processing” may be attributable to the engagement of encoding processes that support planning for the future (Klein et al., 2010).
Three kinds of evidence demonstrate the adaptive value of simulations. First, it is well established that simulations play an important role in psychological well-being. Being able to generate specific and detailed simulations of future events can enhance one’s ability to cope with upcoming situations (Taylor and Schneider, 1989; Taylor et al., 1998; Brown et al., 2002). For instance, creating simulations about positive future outcomes can improve emotion regulation, resulting in decreased amounts of worry related to upcoming future events (Brown et al., 2002). In addition to helping one cope with the prospect of an upcoming event, mentally simulating appropriate actions for future stressful situations can enhance one’s ability to cope if and when those situations arise (Taylor and Schneider, 1989).
Second, simulations are used when attempting to solve open-ended or ill-defined problems, where different possible solution paths need to be mentally evaluated. Using the Means-Ends Problem Solving Test, Sheldon et al. (2011) examined the ability of older adults and patients with temporal lobe epilepsy to solve open-ended social problems. Both of these groups are known to have some degree of impairment on tasks of autobiographical memory (Levine et al., 2002; Addis et al., 2007a; St-Laurent et al., 2009); older adults are also known to show reduced performance on episodic simulation tasks (Addis et al., 2008). It was found that when simulating solutions to ill-defined problems, both groups generated fewer relevant steps than controls. This finding suggests that without full access to episodic memory and the ability to generate detailed simulations, the effectiveness of problem solving is reduced (for relevant neuroimaging evidence, see Spreng et al., 2010; Gerlach et al., 2011).
Third, recent studies have demonstrated that episodic simulation has a significant impact on temporal discounting of future rewards: when people imagine experiencing a reward in the future, they show an increased tendency to favor rewards that produce greater long-term payoffs, thereby countering the normal tendency to devalue delayed rewards (Peters and Büchel, 2010; Benoit et al., 2011). Interestingly, fMRI data reveal that these effects of episodic simulation on temporal discounting are associated with increased coupling between activity in the hippocampus and prefrontal regions involved in reward representation (Peters and Büchel, 2010; Benoit et al., 2011). Related studies have shown that varying the manner in which memory is queried can also influence temporal discounting toward long-term payoffs when memory queries emphasize the production of patient (vs. impatient) thoughts (Weber et al., 2007). It would be interesting to approach effects of episodic simulation on temporal discounting from the theoretical perspective of query theory (Johnson et al., 2007) and to determine whether the memory-based effects on temporal discounting have a similar neural basis to those shown for episodic simulation.
In order to influence future behaviors and realize these adaptive benefits of simulation, it is important that simulations are encoded and maintained in memory (Ingvar, 1985; Szpunar et al., in press). There is indirect evidence to support this idea. For instance, individuals tend to act in a way that is consistent with or constrained by how they have imagined themselves in those situations (Johnson and Sherman, 1990), implying that some record of that simulation influences later behavior. There is typically a high correspondence of stated intentions and subsequent behavior (Fishbein and Ajzen, 1980). Consider also prospective memory, where an intention is encoded into memory and later accessed and implemented when triggered by a target event or time cue. It is likely that the intentions involved in prospective memory range in the degree to which they draw upon simulations. Particularly relevant to the idea of episodic simulation is the process of forming “implementation intentions” (Gollwitzer, 1999) which involve imagining and rehearsing a plan with reference to the specific future context in which it will be executed. Research has shown that creating implementation intentions significantly increases the likelihood of carrying out that intention (Orbell et al., 1997; Chasteen et al., 2001), again suggesting that these simulations are not only stored in memory but do influence future behavior. Poppenk et al. (2010) directly investigated the process of encoding intentions, using fMRI to see whether later memory for intentions was associated with hippocampal activity during encoding. They found that successful encoding of intentions engaged the hippocampus, as did the encoding of other forms of information, such as present actions. But unique to the prospective task was the recruitment of frontopolar cortex, consistent with finding that damage to this region results in deficits of prospective memory (e.g., Burgess et al., 2000).
If the involvement of the hippocampus in future simulation is only to encode imagined scenarios, then hippocampal damage would not necessarily result in an inability to construct simulations – just an inability to encode and retain them. There are some data to suggest that this might be the case (see Table 1). For instance, although children with hippocampal damage can imagine scenarios, when asked to recall them the following day, they do so with less accuracy and consistency than healthy controls (Cooper et al., 2011). Additionally, adults with hippocampal damage appear to repeat themselves more than controls when describing future events, possibly indicative of a failure to sufficiently encode the scenario as it is constructed (Squire et al., 2010).
We conducted an fMRI study (Martin et al., 2011) to investigate whether hippocampal activity during future simulation is indeed related to successful encoding by incorporating the experimental recombination (Addis et al., 2009a) and subsequent memory (e.g., Wagner et al., 1998) paradigms. During scanning, participants were presented with random recombinations of person, location, and object details taken from their own memories and for each set of details, they imagined a novel future event involving all three details. After scanning, participants completed an unexpected cued recall test, in which they were showed two details and had to recall the third. By this design, we had an objective measure of whether the critical details comprising each simulation were successfully encoded. As predicted, successfully encoded simulations were associated with greater activity in the anterior right hippocampus than simulations that were later-forgotten. Moreover, the posterior right hippocampus was also modulated by encoding success. A functional connectivity analysis revealed that both the anterior and posterior hippocampus exhibited connectivity with each other and a wider brain network (including medial prefrontal and medial parietal regions) during successful encoding. When encoding was not successful, the posterior hippocampus did not show this pattern of connectivity. However, it is interesting to note that during unsuccessful encoding, the anterior region still exhibited connectivity with the wider core network. It is possible that this neural pattern reflects the attempt to construct a simulation, even if it is ultimately not encoded sufficiently to be recalled later. We also found that the imagined events that were later-remembered were on average more detailed that later-forgotten ones, and activity in regions exhibiting an encoding effect was also modulated by the level of detail. Together, these observations suggest that constructing a memorable scenario may be related, at least in part, to how well the composite details were retrieved from memory and recombined.
Future Directions: Mapping Component Processes to Hippocampal Regions
Considering together the patient and neuroimaging data reviewed here, there appears to be evidence supporting the idea that there are three important component processes involved in the simulation of episodic future events. First, details stored in episodic memory with which to furnish the simulation must be accessed. Second, the details extracted from various memories need to be recombined and integrated into a spatiotemporal context in order imbue a simulation with a sense of coherence. Third, if a simulation is to influence and guide future behaviors, it needs to be successfully encoded into memory. The evidence reviewed herein suggests that these different processes all rely, to some extent, on the hippocampus. It remains an open and important question as to whether different subregions of the hippocampus are specifically associated with specific component processes. While the posterior hippocampus likely supports the retrieval of previously experienced details, particularly those spatial in nature, the anterior hippocampus supports the recombination of extracted details into a coherent scenario, and both regions support successful encoding.
This framework may be able to inform the debate on whether hippocampal damage disrupts the ability to imagine the future (Squire et al., 2010; Maguire and Hassabis, 2011). It is critical that future research on patients with hippocampal damage employ more refined experimental designs to probe whether detail access, detail recombination and/or encoding of simulations is disrupted. The case study approach may be particularly important here. There is considerable variance of performance across patients with hippocampal damage, and it will be important to understand the specific patterns of spared and impaired sub-processes within each case. Moreover, it is likely that the nature and location of damage to the hippocampus is critical. Differential impairments of the construction and/or encoding of future simulations may emerge depending on the nature of the hippocampal damage: whether it is confined to the anterior and/or posterior aspects, affects primarily the right hippocampus, affects the entirety of the structure, or extends beyond its boundaries. Moreover, it will be critical in future studies to ascertain whether damage in amnesic patients is restricted to the hippocampus or extends more broadly.
Another challenge will be to find ways in which to differentiate the process of recombining details to construct a simulation and the encoding of those simulations. These processes are closely related in two ways: cognitively, with more detailed simulations being more successfully encoded; and neurally, with both processes engaging the anterior right hippocampus. As such, they may be difficult to disentangle. One fruitful avenue may be to investigate whether detail recombination and successful encoding are mediated by specific hippocampal subfields. The hippocampal formation is a circuit comprised of several anatomically distinct subregions, including the dentate gyrus, three cornu ammonis (CA1/CA2/CA3) areas, and the subiculum. Recent work suggests a functional distinction between the input structures into the hippocampus (dentate gyrus/CA2/CA3) and the output (subiculum/CA1). Specifically, while the input structures appear to be involved in encoding, the output structures may be more involved in binding (Carr et al., 2010). Moreover, the finding that the dentate gyrus is involved in encoding is consistent with the hypothesis that the ability to form temporal associations among new experiences that happen close together in time is ultimately dependent upon the continuous production of new-born granule cells in the dentate gyrus (Aimone et al., 2006; Deng et al., 2010). Extrapolating these findings to the realm of future simulation, it is possible that detail recombination during future simulation may be differentially associated with CA1/subiculum, and successful encoding with dentate gyrus/CA2/CA3. Recent developments in ultra-high-field 7T MRI to obtain exceptionally high resolution images of hippocampal subfield anatomy – including distinct layers within subfields (e.g., Kerchner et al., 2010) – will no doubt facilitate more detailed investigations of the roles of different hippocampal subfields.
Neuroimaging studies to date suggest there may also be lateralization effects in the hippocampal activity that is differentially associated with future thinking. Specifically, we initially reported that hippocampal activity common to past and future events was evident in the left hippocampus, but that the future > past effect was specific to the right hippocampus (Addis et al., 2007b). A number of other studies finding future-related activity also report a right lateralization (Weiler et al., 2010a,b; Addis et al., 2011a; Martin et al., 2011), although some studies report such activity is bilateral (Addis et al., 2009a). Interestingly, a patient with damage that affected only the right hippocampus exhibited difficulties in generating detailed future simulations (Race et al., 2011), suggesting the right hippocampus may indeed be critical to this ability. However, it remains to be determined what specific contribution the right hippocampus might be making to future simulation.
The research considered here is in an early stage of development. It is only during the past few years that studies examining the contribution of the hippocampus to imagining the future have begun in earnest, and it is clear that much remains to be learned. Further integration of this new line of work with more firmly established research on hippocampal contributions to memory encoding and retrieval will be critical to advancing our understanding, as will integration with animal studies of such related phenomena as prospective coding in the hippocampus (e.g., Ferbinteanu and Shapiro, 2003; Foster and Wilson, 2006; Johnson and Redish, 2007; for discussion, see Buckner, 2010). We are hopeful that these kinds of studies will help to increase our understanding of the neural and cognitive processes that link memory and imagination, and in so doing, provide new insights into how the future depends on the past.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Preparation of this manuscript was supported in part by the Australian Research Council Centre of Excellence in Cognition and its Disorders (CE110001021), a Royal Society of New Zealand Marsden Grant (UOA0810), and a Grant-in-Aid for Research and Study Leave from The University of Auckland awarded to Donna Rose Addis, and a National Institute of Mental Health grant (MH060941) awarded to Daniel L. Schacter.
References
Addis, D. R., Cheng, T., Roberts, R. P., and Schacter, D. L. (2011a). Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus 21, 1045–1052.
Addis, D. R., Roberts, R. P., and Schacter, D. L. (2011b). Age-related neural changes in autobiographical remembering and imagining. Neuropsychologia 49, 3656–3669.
Addis, D. R., McIntosh, A. R., Moscovitch, M., Crawley, A. P., and McAndrews, M. P. (2004a). Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage 23, 1460–1471.
Addis, D. R., Moscovitch, M., Crawley, A. P., and McAndrews, M. P. (2004b). Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus 14, 752–762.
Addis, D. R., Moscovitch, M., and McAndrews, M. P. (2007a). Consequences of hippocampal damage across the autobiographical memory retrieval network in patients with left temporal lobe epilepsy. Brain 130, 2327–2342.
Addis, D. R., Wong, A. T., and Schacter, D. L. (2007b). Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45, 1363–1377.
Addis, D. R., Musicaro, R., Pan, L., and Schacter, D. L. (2010). Episodic simulation of past and future events in older adults: evidence from an experimental recombination task. Psychol. Aging 25, 369–376.
Addis, D. R., Pan, L., Vu, M.-A., Laiser, N., and Schacter, D. L. (2009a). Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia 47, 2222–2238.
Addis, D. R., Sacchetti, D. C., Ally, B. A., Budson, A. E., and Schacter, D. L. (2009b). Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia 47, 2660–2671.
Addis, D. R., and Schacter, D. L. (2008). Effects of detail and temporal distance of past and future events on the engagement of a common neural network. Hippocampus 18, 227–237.
Addis, D. R., Wong, A. T., and Schacter, D. L. (2008). Age-related changes in the episodic simulation of future events. Psychol. Sci. 19, 33–41.
Aimone, J. B., Wiles, J., and Gage, F. H. (2006). Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci. 9, 723–727.
Andelman, F., Hoofien, D., Goldberg, I., Aizenstein, O., and Neufeld, M. Y. (2010). Bilateral hippocampal lesion and a selective impairment of the ability for mental time travel. Neurocase 16, 426–435.
Bayley, P. J., Gold, J. J., Hopkins, R. O., and Squire, L. R. (2005). The neuroanatomy of remote memory. Neuron 46, 799–810.
Benoit, R. G., Gilbert, S. J., and Burgress, P. W. (2011). A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J. Neurosci. 31, 6771–6779.
Boyer, P. (2008). Evolutionary economics of mental time travel? Trends Cogn. Sci. (Regul. Ed.) 12, 219–224.
Brown, G. P., MacLeod, A. K., Tata, P., and Goddard, L. (2002). Worry and the simulation of future outcomes. Anxiety Stress Coping 15, 1–17.
Buckner, R. L. (2010). The role of the hippocampus in prediction and imagination. Annu. Rev. Psychol. 61, 27–48.
Buckner, R. L., Andrews-Hanna, J. R., and Schacter, D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38.
Buckner, R. L., and Carroll, D. C. (2007). Self-projection and the brain. Trends Cogn. Sci. (Regul. Ed.) 11, 49–57.
Burgess, P. W., Veitch, E., de Lacy Costello, A., and Shallice, T. (2000). The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia 38, 848–863.
Carr, V. A., Rissman, J., and Wagner, A. D. (2010). Imaging the human medial temporal lobe with high-resolution fMRI. Neuron 65, 298–308.
Chan, D., Henley, S. M. D, Rossor, M. N., and Warrington, E. K. (2007). Extensive and temporally ungraded retrograde amnesia in encephalitis associated with antibodies to voltage-gated potassium channels. Arch. Neurol. 64, 404–410.
Chasteen, A. L., Park, D. C., and Schwarz, N. (2001). Implementation intentions and facilitation of prospective memory. Psychol. Sci. 12, 457–461.
Chua, E., Schacter, D. L., Rand-Giovannetti, E., and Sperling, R. A. (2007). Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus 17, 1071–1080.
Cooper, J. M., Vargha-Khadem, F., Gadian, D. G., and Maguire, E. A. (2011). The effect of hippocampal damage in children on recalling the past and imagining new experiences. Neuropsychologia 49, 1843–1850.
Crovitz, H. F., and Schiffman, H. (1974). Frequency of episodic memories as a function of their age. Psychon. Bull. Rev. 4, 517–518.
Deng, W., Aimone, J. B., and Gage, F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350.
Eichenbaum, H. (2001). The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav. Brain Res. 127, 199–207.
Ferbinteanu, J., and Shapiro, M. L. (2003). Prospective and retrospective memory coding in the hippocampus. Neuron 40, 1227–1239.
Fishbein, M., and Ajzen, I. (1980). Belief, Attitude, Intention, and Behavior. Reading, MA: Addison-Wesley.
Foster, D. J., and Wilson, M. A. (2006). Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683.
Gaesser, D., Sacchetti, D. C., Addis, D. R., and Schacter, D. L. (2011). Characterizing age-related changes in remembering the past and imagining the future. Psychol. Aging 26, 80–84.
Gerlach, K. D., Spreng, R. N., Gilmore, A. W., and Schacter, D. L. (2011). Solving future problems: default network and executive activity associated with goal-directed mental simulations. Neuroimage 55, 1816–1824.
Giovanello, K. S., Schnyer, D., and Verfaellie, M. (2004). A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus 14, 5–8.
Giovanello, K. S., Schnyer, D., and Verfaellie, M. (2009). Distinct hippocampal regions make unique contributions to relational memory. Hippocampus 19, 111–117.
Gold, J. J., and Squire, L. R. (2005). Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus 15, 79–85.
Gollwitzer, P. M. (1999). Implementation intentions: strong effects of simple plans. Am. Psychol. 54, 493–503.
Graham, K. S., Lee, A. C., Brett, M., and Patterson, K. (2003). The neural basis of autobiographical and semantic memory: new evidence from three PET studies. Cogn. Affect. Behav. Neurosci. 3, 234–254.
Hassabis, D., Kumaran, D., Vann, S. D., and Maguire, E. A. (2007). Patients with hippocampal amnesia cannot imagine new experiences. Proc. Natl. Acad. Sci. U.S.A. 104, 1726–1731.
Hassabis, D., and Maguire, E. A. (2007). Deconstructing episodic memory with construction. Trends Cogn. Sci. (Regul. Ed.) 11, 299–306.
Heckers, S., Zalesak, M., Weiss, A. P., Ditman, T., and Titone, D. (2004). Hippocampal activation during transitive inference in humans. Hippocampus 14, 153–162.
Hedden, T., and Gabrieli, J. D. (2004). Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 5, 87–96.
Hurley, N. C., Maguire, E. A., and Vargha-Khadem, F. (2011). Patient HC with developmental amnesia can construct future scenarios. Neuropsychologia 49, 3620–3628.
Ingvar, D. H. (1985). “Memory of the future”: an essay on the temporal organization of conscious awareness. Hum. Neurobiol. 4, 127–136.
Jackson, O. III, and Schacter, D. L. (2004). Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage 21, 456–462.
Johnson, A., and Redish, A. D. (2007). Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J. Neurosci. 27, 12176–12189.
Johnson, E. J., Häubl, G., and Keinan, A. (2007). Aspects of endowment: a query theory of value construction. J. Exp. Psychol. Learn Mem. Cogn. 33, 461–474.
Johnson, M. K., and Sherman, S. J. (1990). “Constructing and reconstructing the past and the future in the present,” in Handbook of Motivation and Cognition: Foundations of Social Behavior, Vol. 2, eds E. T. Higgins, and R. M. Sorrentino (New York: The Guilford Press), 482–526.
Kerchner, G. A., Hess, C. P., Hammond-Rosenbluth, K. E., Xu, D., Rabinovici, G. D., Kelley, D. A. C., Vigneron, D. B., Nelson, S. J., and Miller, B. L. (2010). Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology 75, 1381–1387.
Kirwan, C. B., and Stark, C. E. (2004). Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus 14, 919–930.
Klein, S. B., Loftus, J., and Kihlstrom, J. F. (2002). Memory and temporal experience: the effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Soc. Cogn. 20, 353–379.
Klein, S. B., Robertson, T. E., and Delton, A. W. (2010). Facing the future: memory as an evolved system for planning future acts. Mem. Cognit. 38, 13–22.
Kohler, S., Danckert, S., Gati, J. S., and Menon, R. S. (2005). Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: a comparison based on event-related fMRI. Hippocampus 15, 763–774.
Kopelman, M. D., Wilson, B. A., and Baddeley, A. D. (1989). The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. J. Clin. Exp. Neuropsychol. 11, 724–744.
Kwan, D., Carson, N., Addis, D. R., and Rosenbaum, R. S. (2010). Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychologia 48, 3179–3186.
Lepage, M., Habib, R., and Tulving, E. (1998). Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus 8, 313–322.
Levine, B., Svoboda, E., Hay, J. F., Winocur, G., and Moscovitch, M. (2002). Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol. Aging 17, 677–689.
Levy, D. A., Bayley, P. J., and Squire, L. R. (2004). The anatomy of semantic knowledge: medial vs. lateral temporal lobe. Proc. Natl. Acad. Sci. U.S.A. 101, 6710–6715.
Maguire, E. A., and Hassabis, D. (2011). Role of the hippocampus in imagination and future thinking. Proc. Natl. Acad. Sci. U.S.A. 108, E39.
Maguire, E. A., Nannery, R., and Spiers, H. J. (2006). Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain 129, 2894–2907.
Maguire, E. A., Vargha-Khadem, F., and Hassabis, D. (2010). Imagining fictitious and future experiences: evidence from developmental amnesia. Neuropsychologia 48, 3187–3192.
Maguire, E. A., Vharga-Khadem, F., and Mishkin, M. (2001). The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain 124, 1156–1170.
Martin, V. C., Schacter, D. L., Corballis, M. C., and Addis, D. R. (2011). A role for the hippocampus in encoding simulations of future events. Proc. Natl. Acad. Sci. U.S.A. 108, 13858–13863.
McKenna, P., and Gerhand, S. (2002). Preserved semantic learning in an amnesic patient. Cortex 38, 37–58.
Moscovitch, M., Rosenbaum, R. S., Gilboa, A., Addis, D. R., Westmacott, R., Grady, C., and Nadel, L. (2005). Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J. Anat. 207, 35–66.
Nairne, J. S., Thompson, S. R., and Pandeirada, J. N. S. (2007). Adaptive memory: survival processing enhances retention. J. Exp. Psychol. Gen. 33, 263–273.
Orbell, S., Hodgkins, S., and Sheeran, P. (1997). Implementation intentions and the theory of planned behavior. Pers. Soc. Psychol. Bull. 23, 945–954.
Peters, J., and Büchel, C. (2010). Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron 66, 138–148.
Poppenk, J., Moscovitch, M., McIntosh, A. R., Ozcelik, E., and Craik, F. I. M. (2010). Encoding the future: successful processing of intentions engages predictive brain networks. Neuroimage 49, 905–913.
Preston, A. R., Shrager, Y., Dudukovic, N. M., and Gabrieli, J. D. (2004). Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus 14, 148–152.
Prince, S. E., Daselaar, S. M., and Cabeza, R. (2005). Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J. Neurosci. 25, 1203–1210.
Race, E., Keane, M. M., and Verfaellie, M. (2011). Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J. Neurosci. 31, 10262–10269.
Rosenbaum, R. S., Carson, N., Abraham, N., Bowles, B., Kwan, D., Köhler, S., Svoboda, E., Levine, B., and Richards, B. (2011). Impaired event memory and recollection in a case of developmental amnesia. Neurocase 17, 394–409.
Rosenbaum, R. S., Köhler, S., Schacter, D. L., Moscovitch, M., Westmacott, R., Black, S. E., and Tulving, E. (2005). The case of K.C.: contributions of a memory-impaired person to memory theory. Neuropsychologia 43, 989–1021.
Samarasekera, S. R., Vincent, A., Welch, J. L., Jackson, M., Nichols, P., and Griffiths, T. D. (2006). Course and outcome of acute limbic encephalitis with negative voltage-gated potassium channel antibodies. J. Neurol. Neurosurg. Psychiatr. 78, 391–394.
Schacter, D. L., and Addis, D. R. (2007). The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 773–786.
Schacter, D. L., and Addis, D. R. (2009). On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1245–1253.
Schacter, D. L., Addis, D. R., and Buckner, R. L. (2007). The prospective brain: remembering the past to imagine the future. Nat. Rev. Neurosci. 8, 657–661.
Schacter, D. L., Addis, D. R., and Buckner, R. L. (2008). Episodic simulation of future events: concepts, data, and applications. Ann. N. Y. Acad. Sci. 1124, 39–60.
Schacter, D. L., Gaesser, B., and Addis, D. R. (2011a). “Age-related changes in the episodic simulation of past and future events,” in Successful Remembering and Successful Forgetting: A Festschrift in Honor of Robert A. Bjork, ed. A. S. Benjamin (New York: Psychology Press), 505–525.
Schacter, D. L., Guerin, S. A., and St. Jacques, P. L. (2011b). Memory distortion: an adaptive perspective. Trends Cogn. Sci. (Regul. Ed.) 15, 467–474.
Schacter, D. L., and Wagner, A. D. (1999). Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9, 7–24.
Sheldon, S., McAndrews, M. P., and Moscovitch, M. (2011). Episodic memory processes mediated by the medial temporal lobes contribute to open-ended problem solving. Neuropsychologia 49, 2439–2447.
Spaniol, J., Davidson, P. S., Kim, A. S., Han, H., Moscovitch, M., and Grady, C. L. (2009). Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 47, 1765–1779.
Spreng, R. N., Mar, R. A., and Kim, A. S. N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510.
Spreng, R. N., Stevens, W. D., Chamberlain, J. P., Gilmore, A. W., and Schacter, D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53, 303–317.
Squire, L. R., McDuff, S. G., and Frascino, J. C. (2011). Reply to Maguire and Hassabis: autobiographical memory and future imagining. Proc. Natl. Acad. Sci. U.S.A. 108, E40.
Squire, L. R., van der Horst, A. S., McDuff, S. G. R., Frascino, J. C., Hopkinse, R. O., and Mauldin, K. N. (2010). Role of the hippocampus in remembering the past and imagining the future. Proc. Natl. Acad. Sci. U.S.A. 107, 19044–19048.
Staresina, B. P., and Davachi, L. (2008). Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J. Cogn. Neurosci. 20, 1478–1489.
Staresina, B. P., and Davachi, L. (2009). Mind the gap: binding experiences across space and time in the human hippocampus. Neuron 63, 267–276.
St-Laurent, M., Moscovitch, M., Levine, B., and McAndrews, M. P. (2009). Determinants of autobiographical memory in patients with unilateral temporal lobe epilepsy or excisions. Neuropsychologia 47, 2211–2221.
Suddendorf, T., and Corballis, M. C. (1997). Mental time travel and the evolution of the human mind. Genet. Soc. Gen. Psychol. Monogr. 123, 133–167.
Suddendorf, T., and Corballis, M. C. (2007). The evolution of foresight: what is mental time travel, and is it unique to humans? Behav. Brain Sci. 30, 299–351.
Szpunar, K. K. (2010). Episodic future thought: an emerging concept. Perspect. Psychol. Sci. 5, 142–162.
Szpunar, K. K., Addis, D. R., and Schacter, D. L. (in press). Memory for emotional simulations: remembering a rosy future. Psychol. Sci.
Taylor, S. E., Pham, L. B., Rivkin, I. D., and Armor, D. A. (1998). Harnessing the imagination: mental simulation, self-regulation and coping. Am. Psychol. 53, 429–439.
Taylor, S. E., and Schneider, S. K. (1989). Coping and the simulation of events. Soc. Cogn. 7, 174–194.
Vharga-Khadem, F., Gadian, D. G., Watkins, K. E., Connelly, A., Van Paesschen, W., and Mishkin, M. (1997). Differential effects of early hippocampal pathology on episodic and semantic memory. Science 277, 376–380.
Wagner, A. D., Schacter, D. L., Rotte, M., Koutstaal, W., Maril, A., Dale, A. M., and Buckner, R. L. (1998). Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 281, 1188–1191.
Weber, E. U., Johnson, E. J., Milch, K. F., Chang, H., Brodscholl, J. C., and Goldstein, D. G. (2007). Asymmetrical discounting in intertemporal choice. Psychol. Sci. 18, 516–523.
Weiler, J. A., Suchan, B., and Daum, I. (2010a). Foreseeing the future: occurrence probability of imagined future events modulates hippocampal activation. Hippocampus 20, 685–690.
Keywords: hippocampus, future, imagination, simulation, episodic, autobiographical
Citation: Addis DR and Schacter DL (2012) The hippocampus and imagining the future: where do we stand? Front. Hum. Neurosci. 5:173. doi: 10.3389/fnhum.2011.00173
Received: 02 October 2011;
Paper pending published: 07 November 2011;
Accepted: 12 December 2011;
Published online: 04 January 2012.
Edited by:
Srikantan S. Nagarajan, University of California San Francisco, USAReviewed by:
Bernd Weber, Rheinische-Friedrich-Wilhelms Universität, GermanyDoris Tsao, University of Bremen, Germany
Copyright: © 2012 Addis and Schacter. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Donna Rose Addis, Department of Psychology, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand. e-mail: d.addis@auckland.ac.nz