Impaired Communication Between the Dorsal and Ventral Stream: Indications from Apraxia
- 1Faculty of Health and Life Sciences, Department of Psychology, Northumbria University, Newcastle upon Tyne, UK
- 2Institute of Research in the Psychological Sciences, Université catholique de Louvain, Louvain-le-Neuve, Belgium
- 3Psychology, School of Natural Sciences, University of Stirling, Stirling, UK
Patients with apraxia perform poorly when demonstrating how an object is used, particularly when pantomiming the action. However, these patients are able to accurately identify, and to pick up and move objects, demonstrating intact ventral and dorsal stream visuomotor processing. Appropriate object manipulation for skilled use is thought to rely on integration of known and visible object properties associated with “ventro-dorsal” stream neural processes. In apraxia, it has been suggested that stored object knowledge from the ventral stream may be less readily available to incorporate into the action plan, leading to an over-reliance on the objects’ visual affordances in object-directed motor behavior. The current study examined grasping performance in left hemisphere stroke patients with (N = 3) and without (N = 9) apraxia, and in age-matched healthy control participants (N = 14), where participants repeatedly grasped novel cylindrical objects of varying weight distribution. Across two conditions, object weight distribution was indicated by either a memory-associated cue (object color) or visual-spatial cue (visible dot over the weighted end). Participants were required to incorporate object-weight associations to effectively grasp and balance each object. Control groups appropriately adjusted their grasp according to each object’s weight distribution across each condition, whereas throughout the task two of the three apraxic patients performed poorly on both the memory-associated and visual-spatial cue conditions. A third apraxic patient seemed to compensate for these difficulties but still performed differently to control groups. Patients with apraxia performed normally on the neutral control condition when grasping the evenly weighted version. The pattern of behavior in apraxic patients suggests impaired integration of visible and known object properties attributed to the ventro-dorsal stream: in learning to grasp the weighted object accurately, apraxic patients applied neither pure knowledge-based information (the memory-associated condition) nor higher-level information given in the visual-spatial cue condition. Disruption to ventro-dorsal stream predicts that apraxic patients will have difficulty learning to manipulate new objects on the basis of information other than low-level visual cues such as shape and size.
Introduction
Apraxia is a high-level movement disorder that commonly occurs after lesions to the left frontoparietal motor network. In addition to impaired gesture imitation, apraxia is recognized by performance errors when demonstrating how objects are used (Goldenberg, 1995; Buxbaum, 2001). Although these errors are most apparent when pantomiming the use of objects, with a marked improvement during actual object-use, both pantomime and actual use can be affected (De Renzi and Lucchelli, 1988; Buxbaum and Saffran, 2002; Sunderland and Shinner, 2007; Goldenberg, 2009). Skilful manipulation of objects requires the integration of stored information about the object’s typical use and action processes enabling the object to be grasped appropriately based on the object’s visual affordances and spatial location. In the case of apraxia, it is believed that this integrative process is disturbed. However it is currently not clear whether these deficits affect apraxic patients’ ability to learn to manipulate new objects.
Close examination of object knowledge in apraxic patients confirms that performance errors cannot be attributed to impaired ventral (vision-for-perception) or dorsal (vision-for-action) streams of the visual pathways model (Goodale and Milner, 1992; Milner and Goodale, 2006). Apraxic patients can identify visually presented objects (Daprati and Sirigu, 2006) and order familiar objects in weight order (Dawson et al., 2010; Li et al., 2011). These patients also use structural properties to appropriately reach and grasp familiar objects, infer the use of novel objects based on their affordances, and apply appropriate grip force using recent sensorimotor feedback (Gordon et al., 1993; Sirigu et al., 1995; Goldenberg and Hagmann, 1998; Ietswaart et al., 2006; Frey, 2007; Hermsdörfer et al., 2011; Randerath et al., 2011; Sunderland et al., 2013; Eidenmüller et al., 2014). However, patients with apraxia produce incorrect hand postures attributed to functional use of objects and disturbed anticipatory grip force control for familiar objects (Buxbaum et al., 2003). These results confirm that different mechanisms of the visual pathways model are important depending on the goal of the motor act and support recent evidence suggesting that a “ventro-dorsal” sub-stream of the traditional dorsal pathway may be necessary when processing sensorimotor information based on long-term action representations of how objects are functionally used (Buxbaum and Kalénine, 2010; Binkofski and Buxbaum, 2013). It could be that this sub-stream may be implicated in apraxia.
Unlike the dorsal pathway that extends bilaterally from occipital to superior parietal and dorsal pre-motor areas, the ventro-dorsal sub-stream is left lateralized, projecting medially from occipital to left inferior parietal lobe (IPL) and ventral pre-motor regions. Through a mutual connection with the ventral stream via the left IPL, perceptual information can be incorporated into action plans (Rizzolatti and Matelli, 2003; Buxbaum and Kalénine, 2010; Rizzolatti et al., 2011; Binkofski and Buxbaum, 2013; Vingerhoets, 2014) enabling objects to be grasped for use by applying stored knowledge of how objects are functionally manipulated to the physical properties of the objects presented (Frey, 2007; Almeida et al., 2013; Garcea and Mahon, 2014). In support of object-use errors observed in apraxia, there is an established relationship between apraxic symptoms and damage to regions implicated in the ventro-dorsal stream, in particular inferior parietal regions that suggest this pathway may indeed be disrupted (Haaland et al., 2000; Buxbaum, 2001; Buxbaum et al., 2006, 2007; Frey, 2007; Goldenberg, 2009; Garcea and Mahon, 2014). The subsequent failure to effectively access and implement information from the ventral stream into the action plan results in an over-reliance on the intact dorsal stream. Consequently, objects are manipulated based on what is visually afforded irrespective of the goal of the action (Randerath et al., 2011).
That said, apraxic patients have shown equivalent performance to controls when making memory-driven reach and grasp movements also reliant on the integration of ventral and dorsal processes (Ietswaart et al., 2001; Dawson et al., 2010). Although these findings suggest that apraxic patients can successfully utilize stored representations, it remains possible that the visuo-motor transformation involved in simple reach and grasp movements may not be difficult enough to place sufficient demand on high-level perceptual processes. The proposal of ventro-dorsal disturbance in apraxia has also been argued to place too much importance on different components of object knowledge; in particular, retrieval of knowledge of an objects prototypical use that is dependent on previous experience, which cannot account for apraxic errors during novel object-use (Goldenberg and Hagmann, 1998; Goldenberg, 2014). Yet such knowledge retrieval furthermore assumes that skilled object-use relies on the retrieval of information from “storehouses” as opposed to the convergence of short- and long-term visual representations depending on the goal of the motor act.
While the research outlined suggests apraxic patients have difficulties accessing and incorporating stored knowledge of actions related to skilled use of familiar objects, it remains unclear how these patients learn to manipulate new objects. Of the few studies that have assessed this issue, Barde et al. (2007) trained patients to match novel gestures to novel object pictures that were high or low afforded by associated objects. Apraxic patients demonstrated a greater ability to correctly match gestures to object shape for the high than low afforded gestures during action recognition, but were consistently poor compared to controls during action production regardless of affordance. This may be due to the use of two-dimensional objects during training reducing the affordance bias during action production. Retrieval of the appropriate action associated with the object may also have been more difficult when the goal was simply to produce the correct action, as there is no clear feedback as to whether the action goal was achieved in a comparable manner to appropriately grasping an object to fulfil a function.
The current study explored the impact of affordance on object manipulation by requiring participants to repeatedly lift and balance novel objects of differing weight distribution. Over two conditions, the weight distribution of different cylindrical objects was indicated using different object-weight associations, either by a symbolic memory-association between the color of the object and its weight distribution or by a visual-spatial cue of a “dot” over the weighted end of the object. Change in object manipulation over repeated lifts determined whether apraxic patients successfully used object knowledge obtained through experience to inform their grasp, or whether they continually relied on the visual cues to guide action.
Specifically, this study examined participants’ point of grasp along the object depending on weight distribution. When grasping unbalanced objects, healthy adults intuitively choose a grasp close to the center of mass in order to minimize the energy required by grip force to compensate for load torque (Salimi et al., 2003; Duemmler et al., 2008; Endo et al., 2011). This is said to be estimated visually prior to initial object grasping, which is reflected in accurate grasping of unfamiliar objects for the first time (Lederman and Wing, 2003) or when asked to visually point to the center of mass (Baud-Bovy and Soechting, 2001; Duemmler et al., 2008). Action execution was used throughout the study rather than perceptual task learning. This enabled apraxic patients to get strong visual feedback as to whether the action goal of balancing each object had been achieved during each trial. It was anticipated that apraxic patients would show greater performance accuracy when the object afforded the correct gesture with increased contextual information provided (akin to findings by Barde et al. (2007) in the recognition task).
During the memory-associated condition, when each object’s weight distribution was indicated symbolically by the color of the object, apraxic patients were expected to be impaired. Due to the symmetrical shape of the object, apraxic patients were expected to be biased towards more central grasp points and require a greater number of trials to accurately balance the object. In the visual-spatial cue condition, when the center of mass is indicated by a “dot” over the weighted end, apraxic patients may benefit from this meaningful visible cue over time to prompt a more accurate grasp-point over each trial. An alternative prediction was that apraxic patients might continue to use low-level affordance cues of object structure to indicate weight distribution, resulting in more central grasps rather than to the left or right of the object. Inappropriate manipulation of memory-associated and visual-spatial cued objects would confirm that apraxics over-rely on visual information processed by the dorsal visual stream due to ventral, stored knowledge, being unsuccessfully incorporated into the action plan via the ventro-dorsal sub-stream. Such behavior would add insight into what information apraxic patients can effectively utilize during goal directed action.
Methods
Participants
Twenty-seven right-handed participants were recruited, 13 of which had suffered a stroke (Mage 68 ± 14, 8 male) within 27 months (Mmonths 15 ± 10) and 14 age-matched healthy control participants (Mage 70 ± 9, 5 male). In the patient group, and at the time of testing, three patients displayed symptoms of apraxia and 10 patients did not show signs of apraxia. The ethics committee within Northumbria University’s Department of Psychology and a local NHS ethics committee approved the project.
On the basis of CT, MRI scans and clinical notes, patients who had a brain hemeorrhage or an infarct involving the left hemisphere were recruited from rehabilitation centers and National Health Hospitals within the North East of England. Patients presented with degrees of aphasia, right-sided weakness, or sensory loss. Table 1 describes each patient’s lesion and the Brodmann areas implicated. Lesions were mapped using MRIcron software package (Rorden et al., 2007)1 based on the radiologist’s MRI and/or CT clinical scans of each patient. The areas of damage for each patient were mapped using MRIcron software package; lesions were determined based on the radiologist’s scan reports and the digital brain image. Scans were then normalized to a common stereotaxic space using Clinical Tool box software through SPM and applied to the Brodmann Atlas included in MRIcron (Rorden et al., 2012)2. Lesions for the three apraxic patients are visually documented in Figure 1.
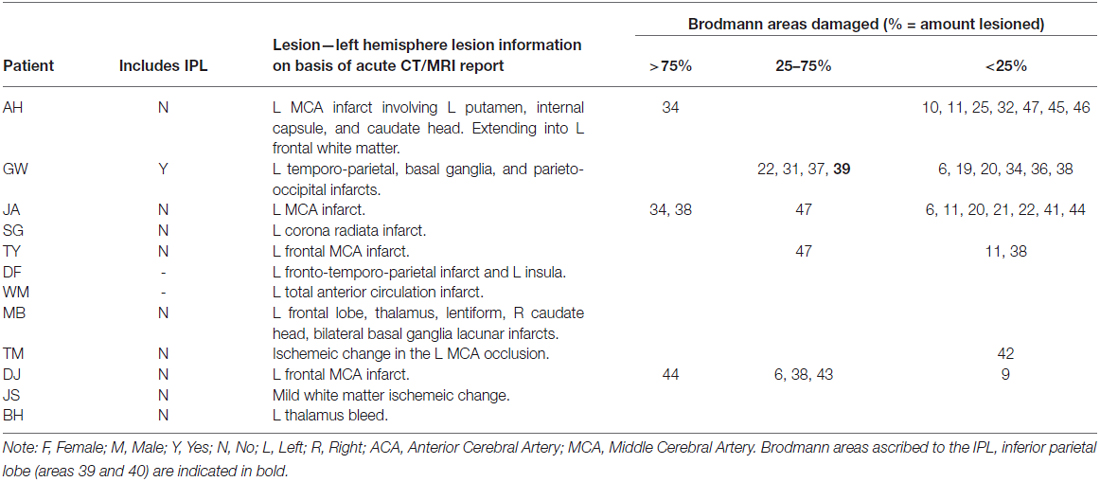
Table 1. Description of each apraxic (top) and non-apraxic (bottom) patient’s lesion as described in the radiologist’s CT and/or MRI reports and when mapped onto the Brodmann atlas.
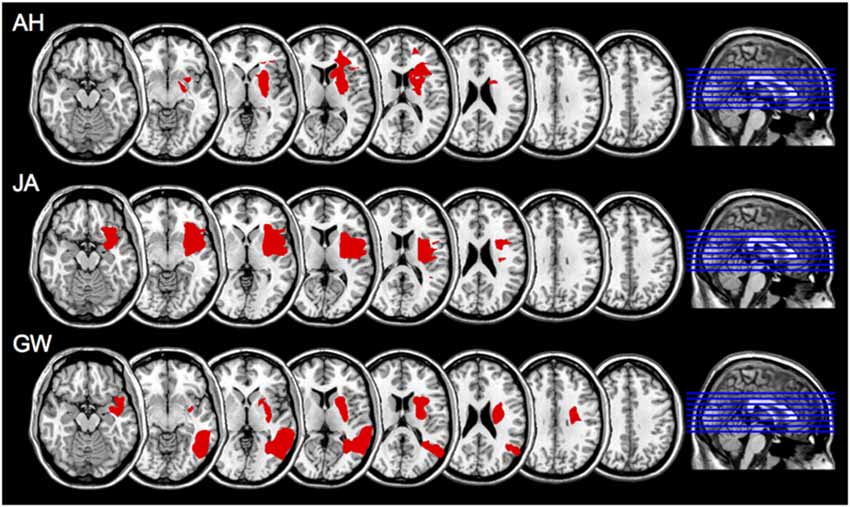
Figure 1. Scan slices for apraxic patients AH, JA, and GW; lesioned areas were applied to a template scan allowing clear visualization of the anatomical landmarks. The lesion area(s) are in red. Left is right as per neurological convention.
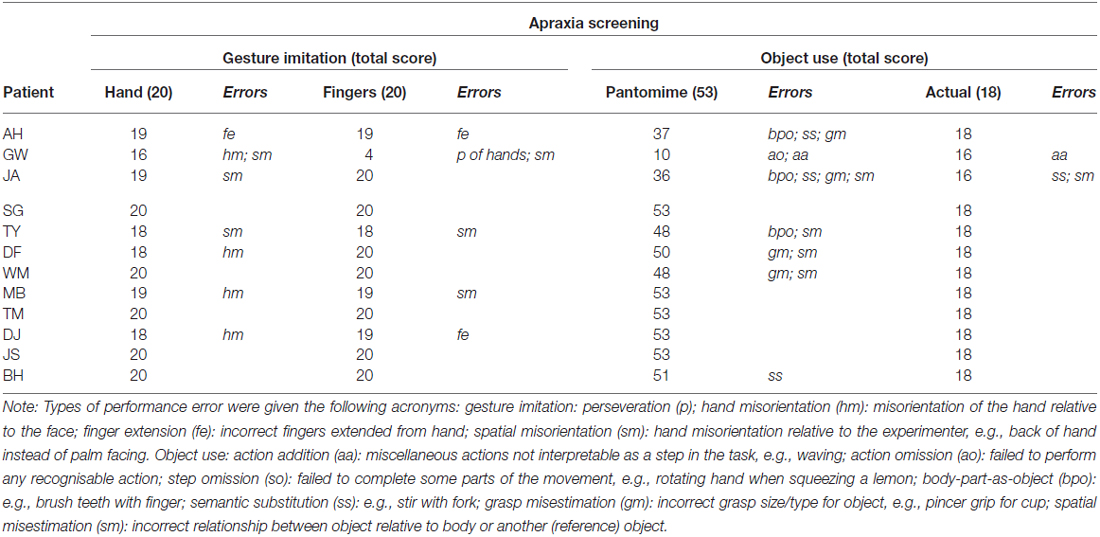
The presence of apraxia was classified on the basis of abnormal performance in one or more of the apraxia screening tools assessing gesture imitation and familiar object-use (pantomime and actual use). Further test batteries and clinical notes were used to exclude any patient presenting with global cognitive deficits or known dementia, severe receptive aphasia or failure to follow one-stage commands (according to the language comprehension token test by De Renzi and Faglioni, 1978), or significant signs of visuospatial neglect (according to the Apples Test by Bickerton et al., 2011). One non-apraxic patient was later excluded (FR) as he was diagnosed with early onset of vascular dementia. Patient details are described in Table 2 and apraxia screening performance in Table 3.
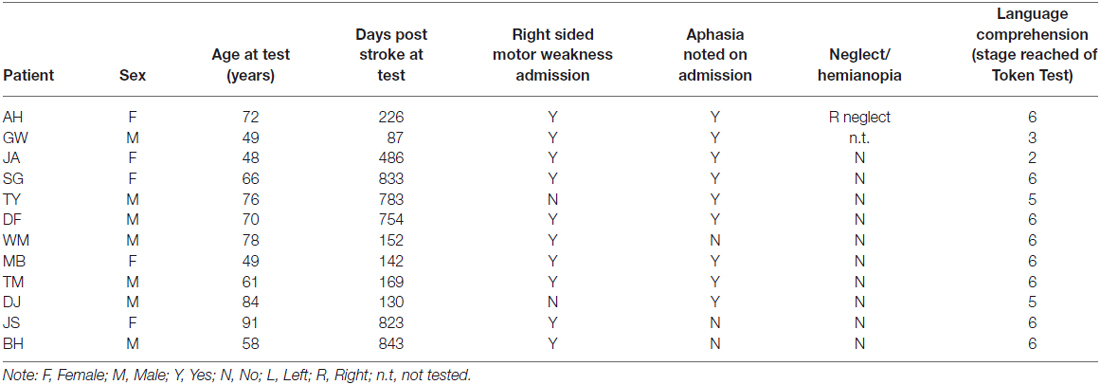
Table 2. Screening performance of patient groups, including apraxics (top) and non-apraxics (bottom); includes FR who was excluded due to early onset vascular dementia.
Healthy age-matched control participants did not have a history of brain damage or stroke. These participants were recruited from the Psychology Department’s participant database and were given monetary compensation for their time.
Materials
Apraxia Screening
Gesture imitation of hand and finger postures (Goldenberg, 1996)
The experimenter demonstrated different hand postures relative to the head and finger postures irrespective of the hands position in relation to the body. Gestures were performed “like a mirror”; the experimenter sat opposite the patient, performing each posture with their right hand to be imitated by the patients’ left hand after the demonstration had ended. Successful imitation of each gesture on the first trial was awarded two points; one point was given if the patient was successful after a further demonstration; zero points if the gesture was not imitated correctly. A total score of 20 could be achieved by imitating 10 gestures of each kind.
Pantomime of object use (based on Goldenberg et al., 2007)
Participants were required to demonstrate the use of 19 objects. The experimenter presented a drawn image of each object (taken from Cycowicz et al., 1997) and named the action to be pantomimed. Points were given for the presence of predefined movement features (Goldenberg et al., 2007 details these). With exception to demonstrating the use of scissors, body-part-as-object errors were marked as incorrect. A total of 53 points could be obtained, with less than 43 measured as pathological.
Actual object use (based on De Renzi and Lucchelli, 1988)
Participants were given the same verbal description of the action to be demonstrated as in the pantomime task. Eighteen of the pantomimed objects were presented; one point was given if used correctly and zero if incorrect. The incorrect use of two or more objects was considered pathological.
Object Grasping Task
Object stimuli
Five cardboard cylinder tubes (length: 24.5 cm, diameter: 3.7 cm) were used, each containing a 17 g weight (length: 2 cm, diameter: 1.5 cm) in one or both ends. The five cylindrical objects comprised of two experimental conditions: “memory-associated” and “visual-spatial cue”, and one screening condition: “neutral-control”. The “memory-associated” condition consisted of one green and one blue cylinder; when presented to the participant, the green object was weighted on the left, whereas the blue object was weighted on the right. Participants were required to remember the color-weight associations when lifting the object without a visual cue indicating weight distribution on either end of the cylinder. The visual-spatial cue condition consisted of two gray objects that were unevenly weighted, containing a weight in either the left or right end of the object. The heavier end of each object was marked with a red “dot” (1 cm diameter), which acted as a visual cue of the weight distribution when acting upon the object. Finally, the neutral-control condition consisted of one gray object that was evenly weighted with one weight in each end of the cylinder. This screened for any confounds such as visuospatial neglect or comprehension issues that would impact task performance. In addition to the main objects, two white practice cylinders were used when giving task instructions: one evenly-weighted (length: 42 cm, diameter: 1.5 cm) and one unevenly-weighted object (length: 46, diameter 1.7 cm, 34 g weight on the right side). The practice cylinders did not resemble test objects in size and weight to minimize priming effects of grasping these objects prior to the main experiment.
A horizontal bar (length: 30 cm, diameter: 0.5 cm) was positioned perpendicular to the participant, 35 cm in front of the participant and 24 cm above the table. Both the experimenter and participant used the bar to indicate the extent to which the object was balanced. For the duration of testing a video camera was placed behind the horizontal bar and recorded each trial. A schematic representation of the experimental setup can be seen in Figure 2.
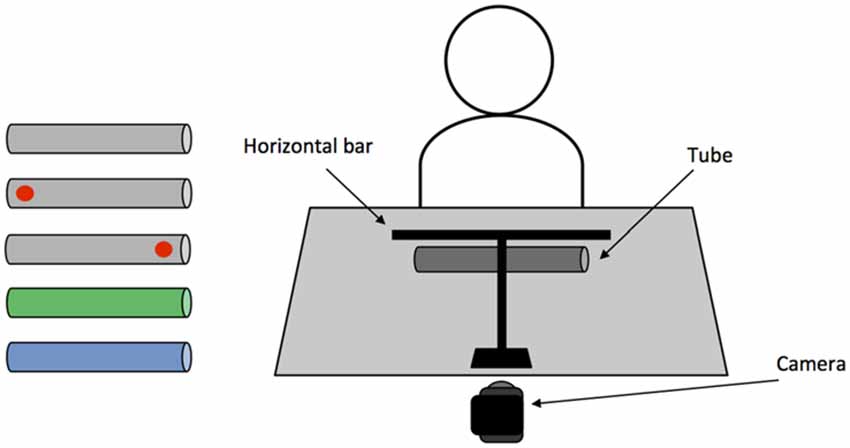
Figure 2. (Left) Objects used in the main task. From top: neutral-control evenly weighted; left and right weighted visual-spatial “dot” cue; left weighted/green and right weighted/blue memory-associated. (Right) Schematic representation of the experimental setup.
Procedure
Each participant was seated at the workspace where the objects were presented. Using the horizontal bar as a guide, participants were instructed to lift and balance each object using a pincer grip with the index and thumb of their left hand. After the object was lifted to the horizontal bar, participants returned the object to the table and removed their hand from it before another trial began. It was emphasized that if the object was imbalanced, they should not compensate by tightly pinching the object or rotating their wrist during or at the end of each lift. Task instructions were demonstrated using the evenly weighted practice cylinder. Participants were then requested to practice the task procedure using the same cylinder. Once participants successfully completed the movement they were presented the unevenly weighted practice cylinder and repeated the process. After it was evident that participants understood the procedure, the main task was started. During the main task, to ensure each participant had the same experience with the object, they were asked to lift and balance each object five times before being presented the next object. In each block, objects were presented in a random order. Overall, there were five testing blocks in which participants saw each object once; including each individual trial, participants lifted each object 25 times, totalling 125 trials. The video camera recorded participants completing each trial.
Data Analysis
Task performance across each condition was initially compared between each control group (healthy and non-apraxics) using a two-way mixed model ANOVA exploring OBJECT (memory-associated; visual-spatial cue; neutral-control) × GROUP (Healthy vs. Non-apraxic controls) to rule out differences across control groups. Each apraxic patient was then compared to the control groups separately using modified t-tests recommended when estimating the abnormality of an individual patient’s score against a control sample that is modest in size (Crawford and Garthwaite, 2002; Crawford et al., 2010). In order to purely assess whether object-weight associations were learnt as opposed to participants relying on semantic labelling (e.g., green is left weighted) to complete the task, object-weight associations were not explicitly described to the participants during the study. This also accommodated for any language deficits. Instead, learning of object-weight associations was determined by assessing participants’ change in performance accuracy over trials (TC) and change in performance accuracy over blocks (BC). The former would indicate whether apraxic patients’ performance improved with repeated lifts of the same object and the latter would confirm whether apraxic patients applied what they had learned in previous blocks when each object was reintroduced. The points at which the object was grasped were used as a guide to evaluate grasp behavior.
Firstly, in order to analyze the video footage, photo snapshots were created when participants were at the maximal point of object lift. From each snapshot, the “point of grasp” was measured based on the midpoint position of the index finger along the object (from right to left). Grasps were considered accurate depending on whether the object was successfully balanced and an appropriate point of grasp was applied to compensate for the objects weight distribution. This ensured that participants were accurate due to adjusting their grasp-point along the object, as opposed to applying greater grip force or by rotating their wrist during each lift. If the location of an individual’s grasp was greater than two standard deviations from the “optimum” point of grasp (OP) to compensate for weight distribution, it was marked as inaccurate. The optimum point of grasp was measured for each object based on healthy control participants mean point of grasp for the fifth trial across all blocks.
Accuracy Change Over Trials (TC)
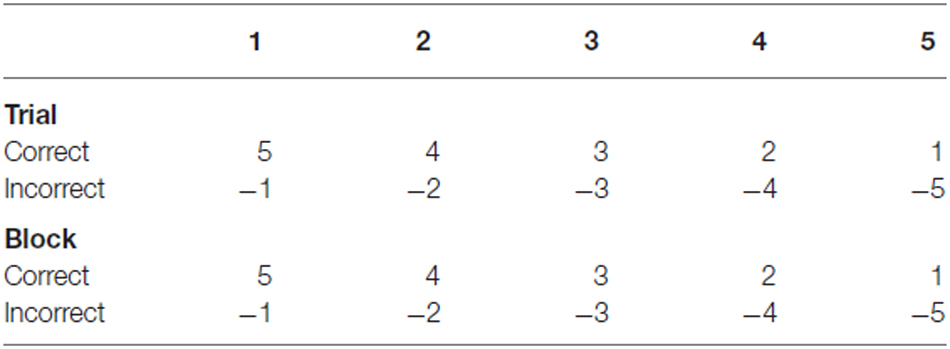
Grasp accuracy was compared between Trial 1 and Trial 5 across blocks. Performance change across trials would indicate whether apraxic patients’ performance improved with repeated grasps of the same object. To compare performance, accuracy was first weighted; accurate grasps in early trials (e.g., Trial 1) received a greater weighting compared to accurate grasps in later trials (e.g., Trial 5). This reflected the extent to which performance was driven by trial-and-error or learning each objects weight distribution. Inaccurate grasps were given a negative score: fewer points were deducted when grasps were inaccurate in early trials and greater points deducted when performing inaccurately in later trials. These reflected the extent to which participants failed to adapt their grasp based on each objects’ weight distribution with repeated grasps of the same object (see Table 4 for weighted scores). As a greater score could be achieved in Trial 1 compared to Trial 5, these scores were then calculated as proportions of the maximum score achievable in that trial, across all 5 blocks. For example, in Trial 1 an accurate grasp scores 5 points, over 5 blocks a maximum score of 25 can be achieved, whereas for Trial 5 an accurate grasp scores 1 point, over 5 blocks a maximum score of 5 can be achieved. Once participants’ scores in Trial 1 and Trial 5 were transformed into proportions, accuracy in Trial 5 was deducted from Trial 1 (as outlined in the equation below). Based on this calculation, a greater negative score signifies improved accuracy across trials, a positive score signifies reduced or consistently poor performance across trials, and a score of zero indicates that the participant achieved the highest accuracy across trials.
Accuracy change (TC) = (block 1–5 average scoretrial 1/maximum scoretrial 1) − (block 1–5 average scoretrial 5/maximum scoretrial 5).
Accuracy Change Over Blocks (BC)
Using the same calculation, performance across blocks was assessed by comparing the average accuracy across trials between Block 1 and Block 5. Performance change across blocks would confirm whether apraxic patients applied what they had learned in previous blocks when each object was reintroduced. As with trial data, performance across blocks was weighted using positive and negative scores. In early blocks, participants received greater points for accurate grasps and fewer points were deducted for inaccurate grasps, whereas in later blocks participants received fewer points for accurate grasps and more points were deducted for inaccurate grasps. Scores were transformed into proportions of the maximum score before accuracy in Block 5 was deducted from accuracy in Block 1.
Notably during testing, non-apraxic patients BH and JS completed only four testing blocks due to experiencing fatigue when lifting the objects several times. The same calculation applied to the final block was instead applied to Block 4 for these patients.
Results
In order to confirm whether apraxic patients utilized memory-associations or visual-spatial cues regarding weight distribution when balancing each object, performance change across trials and across blocks were assessed. Points of grasp for each object were used as a guide to evaluate grasp behavior.
Accuracy Change Across Trials (TC)
Healthy Controls vs. Non-Apraxics
An initial two-way mixed model ANOVA exploring OBJECT (memory-associated; visual-spatial cue; neutral-control) × GROUP ruled out differences in performance change across Trials in healthy and non-apraxic controls. Non-significant main effects confirmed that performance was comparable across control groups (GROUP: F(1,21) = 0.139, p = 0.713, = 0.007) and between objects (OBJECT: F(1.357,28.504) = 3.583, p = 0.058, = 0.145). However, a significant interaction OBJECT × GROUP (F(1.357,28.504) = 8.479, p = 0.004, = 0.288) was identified. Independent samples t-test did not reveal significant differences in performance for all conditions (p > 0.05) except the neutral-control condition (t(21) = 2.353, p = 0.028). Non-apraxics showed greater improvement in task performance from Trial 1–5 (TC = −0.333 ± 0.280) on the evenly weighted object compared to healthy controls whose performance reduced (TC = 0.257 ± 0.714). Notably, differences easily arise on the evenly-weighted neutral-control object, because the point scoring system works with difference from the mean and standard deviation on this condition in normal performance is very small (and differences are therefore of limited interest).
Despite variances in performance change for the neutral-control object, healthy and non-apraxic controls consistently grasped the object close to the optimum grasp-point (OP = 13.18 cm). Examining grasp-point behavior of controls across all three conditions, both groups initially grasped closer to the center of each object in Trial 1, but by Trial 5 were ≤1.32 cm from the optimum grasp-point for each object. Observing individual scores for performance change over trials (TC) confirms that each control participant appropriately adapted their grasp-point over repeated lifts to account for the weight distribution of each object. Of note, non-apraxic control participant JS did not perform as efficiently as the other non-apraxic patients in the memory-associated and visual-spatial cue conditions. However, she was still markedly more accurate than AH and GW. Patient JS also performed at ceiling during the language comprehension test and apraxia screening indicating that her performance was not applicable to poor comprehension or apraxia. Instead, her performance may be more attributable to her age; JS was the oldest participant (91) and testing had to be terminated after the fourth test block as she became fatigued. Together, these findings indicate that healthy and non-apraxic controls effectively utilize both memory-associated and visual-spatial cued information to improve performance when repeatedly lifting each object (see Table 5 for performance change over trials, Table 6 for participants’ average points of grasp, and Figure 3 for accuracy change across trials).
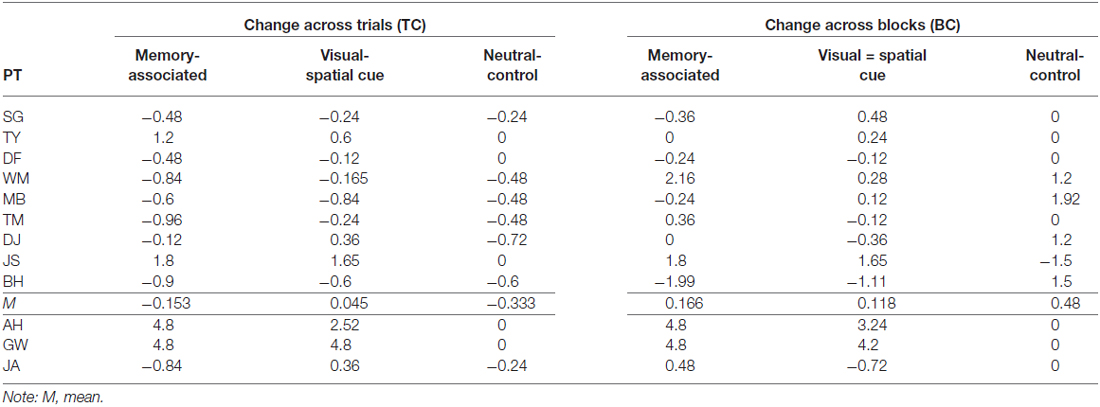
Table 5. Performance change over trials (TC) and blocks (BC) in non-apraxic (top) and apraxic (bottom) patients.
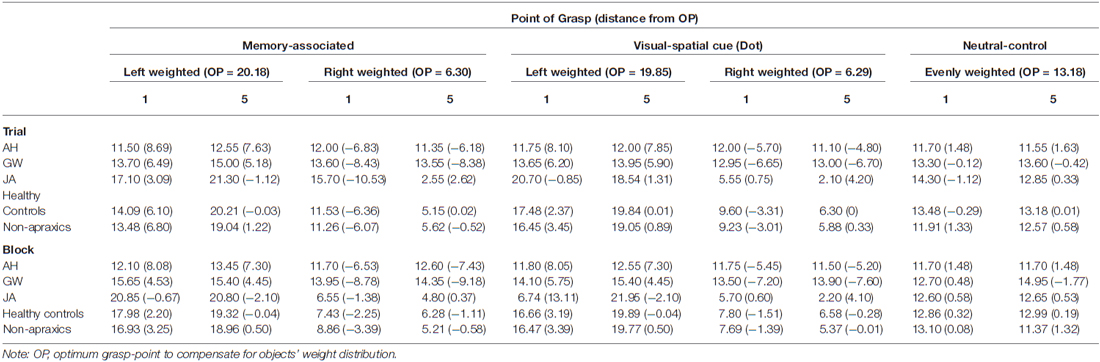
Table 6. Point of grasp (cm). Top: Trial 1 and 5 across blocks, including the overall average point of grasp and standard deviation across every trial for each object. Bottom: Block 1 and 5 across trials, including the overall average point of grasp and standard deviation across every block for each object.
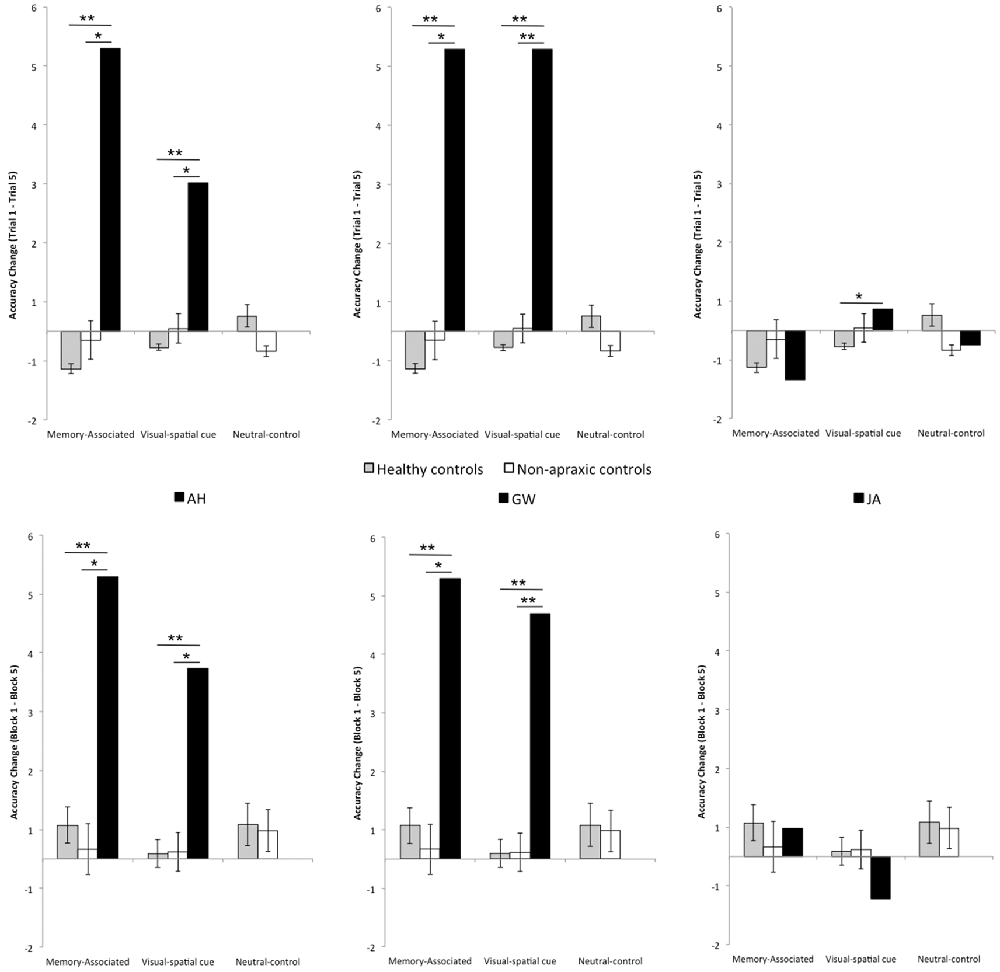
Figure 3. (Top) Change in grasp accuracy between Trial 1 and Trial 5 across blocks, including standard error bars. (Bottom) Change in grasp accuracy between Block 1 and Block 5 across trials, including standard error bars. For both Trial and Block analyses a negative score indicates an improvement in performance across trials; a positive score indicates a reduced or consistently poor performance. Scores close to zero reflect consistent high accuracy across trials. The black bars at the top of the graphs indicate significant relationships: two asterisks denotes a p value < 0.001, and a single asterisk denotes a p value < 0.05.
Patient AH
Single case t-tests confirmed that when grasping memory-associated objects, patient AH was significantly worse than healthy (p < 0.001, t = 17.100) and non-apraxic controls (p = 0.001, t = 4.775) with at least a minimum of 99.93% of controls falling below AH’s score. During the visual-spatial cue condition, patient AH also performed significantly worse than both healthy controls (p < 0.001, t = 13.363) and non-apraxics (p = 0.007, t = 3.160) with at least a minimum of 99.33% of controls falling below AH’s score. For both memory-associated and visual-spatial cue conditions, AH’s accuracy was consistently poor (TC ≥ 2.52) whereas control groups generally improved performance across trials (TC from 0.045 to −0.274).
Observing the average grasp-points for both the memory-associated and visual spatial cue conditions, patient AH maintained a point of grasp towards the center of each object (from 11.10 cm to 13.45 cm). These grasps were at least 4.8 cm from the optimum grasp-point to compensate for weight distribution of each object. Unlike control groups, patient AH did not adjust her grasp towards the weighted end of across trials.
As this patient did not adjust her grasp away from the midpoint, when grasping the neutral-control object AH’s performance change was comparable to both healthy controls (p = 0.367, t = −0.348; an estimated 36.68% falling below AH’s score) and non-apraxics (p = 0.271 t = 1.128; an estimated 85.40% falling below AH’s score). AH’s use of midpoint grasps confirms that her symptoms of right-sided visual neglect identified in the cancellation task did not affect grasp performance.
Patient GW
Performance of patient GW mirrored that of patient AH. Performance change over trials was worse than healthy and non-apraxic controls when grasping unevenly weighted objects in both the memory-associated and visual-spatial cue conditions: for all comparisons p ≤ 0.001, with at least an estimated 99.93% of controls falling below GW’s score. Patient GW was consistently unsuccessful in balancing these objects (TC = 4.8 for each), with average points of grasp ranging from 13.46 cm to 14.76 cm across all four objects, and at least 5.18 cm from the optimum grasp-point. Overall, GW’s average grasp was consistently close to or slightly to the left of each object’s center regardless of their weight distribution, with minimal variance in grasp-points across conditions. However when grasping the neutral-control object, GW’s performance was comparable to both healthy (p = 0.367; an estimated 36.68% falling below GW’s score) and non-apraxic controls (p = 0.146; an estimated 85.40% falling below GW’s score). Patient GW’s average grasp-points were close to the optimum point of grasp.
Patient JA
Apraxic patient JA’s performance change across trials was comparable to both healthy and non-apraxic controls for the memory-associated and neutral-control conditions (p > 0.05; an estimated 25.65% to 61.96% of controls falling below JA’s score). During the visual-spatial cue condition, although JA was comparable to non-apraxics (p = 0.349, t = 0.402; an estimated 65.10% of controls falling below JA’s score), performance change was significantly different to healthy controls (p = 0.005, t = 3.032; an estimated 99.52% of controls falling below JA’s score). It was evident in this condition that JA did not greatly improve grasp accuracy between Trial 1–5 (TC = 0.360) and continued to make errors by the final trial. Although JA achieved largely normal performance on this measure of accuracy change across trials, her qualitative behavior did not look normal. She was slow and deliberate in her reach movements, apparently in an attempt to compensate for her difficulty performing this task. This prompted a closer look at grasp-point and grasp-point variance, in an attempt to quantify her unusual behavior in performing the task. Average grasp-points in Trial 1 and 5 suggests JA typically reorients her grasp towards the weighted end of the object, grasping ≤1.31 cm from the optimum grasp-point. When grasping the right-weighted object, JA deviated to a more extreme rightward grasp; average grasp-point was 4.20 cm further right than the optimum point (6.29 cm) by Trial 5, whereas grasp-points of healthy controls were less than half a centimetre from the optimum point. Observing the grasp-points of JA in relation to the optimum grasp point to compensate for object weight distribution, her point of grasp was further from the optimum point in Trial 5 compared to Trial 1 in the visual-spatial cue condition for both the left and right weighted objects, showing that she continues to adapt her grasp-point even if they were more accurate in previous trials. Similarly, patient JA’s grasps are much more varied suggesting that she does not confidently learn the object-weight associations but may continue to exercise a trial-and-error procedure throughout.
Statistically this behavior was not so much apparent in the average grasp-point variance itself but in the standard deviation of her grasp-point variance. On the average grasp-point variance JA showed marginally significant differences on the memory associated condition (M = 20.69 cm) compared to healthy controls (M = 12.78 cm, p = 0.057, t = 1.691; an estimated 94.26% falling below JA’s score) and non-apraxic controls (M = 12.92 cm, p = 0.055, t = 1.798; an estimated 94.50% falling below JA’s score) controls. In the visual-spatial cue condition JA’s grasp-point variance was not different from control participants (healthy controls: p = 0.435, t = 0.168; non-apraxics: p = 0.453, t = 0.122). But critically JA did differ in both conditions on the standard deviation of her grasp-point variance. On the memory associated condition JA’s variance standard deviation at 20.20 cm was significantly larger than healthy controls (M = 4.52 cm, p = 0.018, t = 2.333; an estimated 98.18% falling below JA’s score), and non-apraxics (M = 4.10 cm, p = 0.001, t = 4.504; an estimated 99.9% falling below JA’s score). This is similarly evidenced by the standard deviation of patient JA’s grasp-point variance in the visual-spatial cue condition. JA’s grasp-point variance standard deviation at 19.74 cm was significantly greater than healthy controls (M = 6.28 cm, p = 0.02, t = 2.279; an estimated 97.99% falling below JA’s score), and non-apraxic participants (M = 5.23 cm, p = 0.014, t = 2.667; an estimated 98.58% falling below JA’s score). Of course on the neutral-control condition neither JA’s grasp-point variance (M = 2.92 cm) nor the standard deviation of patient JA’s grasp-point variance (M = 5.80 cm) was different from healthy controls (both not significantly different to JA at M = 9.22 cm and M = 6.27 cm subsequently) or non-apraxics (both not significantly different to JA at M = 11.29 cm and M = 3.37 cm subsequently).
Accuracy Change Across Blocks (BC)
Healthy Controls vs. Non-Apraxics
Non-significant main effects and interactions from the two-way mixed model ANOVA confirmed that performance change across Blocks was comparable between control groups: OBJECT, F(1.288,27.045) = 0.986, p = 0.381, = 0.045, GROUP F(1,21) = 0.385, p = 0.542, = 0.018, OBJECT × GROUP F(1.288,27.045) = 0.264, p = 0.671, = 0.012. Both healthy and non-apraxic controls adjusted their point of grasp across blocks depending on the weight distribution of each object; individual scores for performance change over blocks confirms that all healthy and non-apraxic control participants successfully adapted their grasp-point to accommodate for the weight distribution when the objects were reintroduced in later blocks (see Table 5 for performance change over trials, Table 6 for average grasp-points and Figure 3 for accuracy change across blocks); grasps were ≤1.32 cm from the optimum grasp-point by the final block. Accuracy was also maintained across blocks (BC ranged from 0.094 to 0.583).
Patient AH
Accuracy change was worse than both healthy and non-apraxic controls during the memory-associated and visual-spatial cue conditions (for all comparisons p < 0.05, with at least an estimated 99.65% of controls falling below AH’s score). Patient AH’s score for accuracy change across blocks (BC ≥ 3.24) was indicative of consistently inaccurate object grasps compared to both control groups (BC ≤ 0.583). Average grasp-points confirm that AH did not adjust her grasp according to the weight distribution of each object but maintained a more central grasp; across both Block 1 and Block 5, AH’s grasp-point ranged between 11.50 and 13.45 cm, at least 5.20 cm from the optimum point of grasp. This suggested that AH failed to utilize stored knowledge of weight distribution when the object was reintroduced.
As before, patient AH’s performance change was comparable to healthy (p = 0.344, t = −0.411; an estimate of 34.38% of controls falling below AH’s score) and non-apraxic controls (p = 0.339, t = −0.430; an estimate of 33.94% of controls falling below AH’s score) when grasping the neutral-control object. Patient AH’s accuracy was consistently high (BC = 0) and maintained a central grasp-point within 1.48 cm from the optimum point of grasp.
Patient GW
Similarly, during the memory-associated and visual-spatial cue conditions patient GW performed worse than healthy controls and non-apraxics; for all comparisons p < 0.05, with at least an estimated 96.76% of controls falling below GW’s score. Patient GW grasped each object centrally at least 5.18 cm from the optimum grasp-point resulting in a consistently poor accuracy change across blocks (BC ≥ 4.20).
Mirroring patient AH, when grasping the neutral-control object, GW’s performance change was equivalent to healthy (p = 0.344, t = −0.411) and non-apraxic controls (p = 0.339, t = −0.430). Patient GW maintained a central point of grasp within 1.77 cm from the optimum grasp-point confirming that grasps were consistently accurate across blocks (BC = 0).
Patient JA
Across all three conditions (memory-associated/visual-spatial cue/neutral-control) patient JA’s performance change was comparable to controls (p > 0.05; an estimated 12.60% to 67.27% of controls falling below JA’s score). However, as discussed when examining grasp-point behavior across trials, patient JA makes slow and deliberate movements as if she struggles with the task, evident in a sub-analysis showing abnormal grasp-point variance across trials. The same sub-analysis is also applied here to show that JA exercises a trial-and-error procedure until the final experimental block. When grasping the left weighted object in the memory-associated condition and the right weighted object in the visual-spatial cue condition, grasp-points moved further away from the optimum point of grasp to compensate for weight distribution in Block 5 compared to Block 1 (Table 6). Additionally, the average point of grasp of the left weighted visual-spatial cue condition in Block 1 was on the opposite side of the object from the optimum grasp-point indicating that she did not utilize the dot cue to indicate weight distribution. Therefore, although performance change appears comparable to control groups, patient JA’s grasp behavior demonstrates performance deficits that differentiate her from control groups and may be indicative of more subtle deficits in the integration of visible and known object properties.
Discussion
To assess whether apraxic patients successfully integrate stored knowledge of objects into action plans, participants were required to learn different weight distributions when lifting and balancing objects using a pincer grip. Over two conditions, each objects’ weight distribution was indicated by either a memory-associated cue (object color) or visual-spatial cue (visible dot over the weighted end). If apraxic patients fail to incorporate stored information into their grasp, we expected that patients might disregard the location of the objects’ center of mass and instead over-rely on visual information, resulting in more centrally oriented grasps based on object structure. The experiment was designed to examine whether patients could learn to grasp the weighted objects accurately when given a meaningful visual-spatial cue indicating the object weight distribution, which would result in increasingly accurate grasps over time if this higher-level information was successfully integrated.
Performance change across trials (TC) and across blocks (BC) in the neutral-control screening condition confirmed that all apraxic patients (AH, GW, and JA) successfully grasped and balanced the evenly weighted object, eliminating the possibility any confounds such as hemispatial neglect or impaired task comprehension might be impacting their performance in the experimental conditions. Comparable to healthy and non-apraxic controls, during consecutive grasps of the neutral-control object (TC) and when grasping the object as it was reintroduced in later blocks (BC), apraxic patients’ central grasp-points remained close to the optimum point of grasp to compensate for weight distribution. Accurate grasping performance during the neutral-control condition indicates that apraxic patients can successfully manipulate objects when the weight distribution is indicated by the objects’ structure (symmetrical cylinder).
Although patient JA’s performance change was within the normal range (see below for a discussion of JA’s pattern of results) during a majority of the memory-associated and visual-spatial cue conditions, patients AH and GW failed to update their grasp-point when the objects were unevenly weighted in both conditions. For both the memory-associated and visual-spatial cue conditions, patient AH and GW maintained a central grasp-point during recurrent trials with the same object (TC) or when the objects were reintroduced in later blocks (BC). Failure to compensate for load torque by reorienting grasps towards the center of mass suggests that these apraxic patients failed to integrate acquired knowledge regarding objects into action plans. Inaccurate grasp-points persisting into the final test block was particularly representative of this. Paired with unimpaired behavior in the neutral-control condition, grasp performance of patients AH and GW suggests an over-reliance on the structural properties afforded by the object. Maintained central grasp-points in the memory-associated and visual-spatial cue conditions perhaps indicate that AH and GW continually referred to structural properties afforded by the object to guide their grasp behavior and did not benefit from either a meaningful visual-spatial cue or symbolic cue of weight distribution.
Patient AH and GW’s performance is compatible with previous research indicating that in addition to impaired perception of skilled object-use (Buxbaum and Saffran, 2002; Buxbaum et al., 2003; Myung et al., 2010), apraxic patients frequently choose inappropriate non-functional grasps (Randerath et al., 2009, 2010; Sunderland et al., 2011) or demonstrate impaired grip force for familiar objects (Gordon et al., 1993; Dawson et al., 2010; Hermsdörfer et al., 2011; Eidenmüller et al., 2014). The performance of patient AH and GW across all three conditions support the proposal that the ventro-dorsal stream is compromised in these patients, resulting in impaired performance when grasping asymmetrically weighted objects. Confirmation that the impairment lies at the ventro-dorsal level comes from the fact that processing of object structure remains intact. Therefore ventro-dorsal disruption appears to impair skilled use of familiar objects, but also when learning to manipulate novel objects.
Interestingly, both patients AH and GW did not appear to benefit at all from the “dot” cue in the visual-spatial cue condition, and there was no evidence of learning. In healthy populations when an object is asymmetrically weighted, grasp-points typically migrate towards the weighted end, particularly when visual cues indicate where the center of mass is located (Endo et al., 2011). Apraxics use of familiar objects also improves from pantomime to actual-use with increased affordance or contextual cues (De Renzi and Lucchelli, 1988; Buxbaum and Saffran, 2002; Sunderland and Shinner, 2007; Goldenberg, 2009; Randerath et al., 2011). Although apraxic patients would not use the visual-spatial cue as effectively as control participants, it was hypothesized that the presence of increased visual information in the form of a visible dot over the weighted end might prompt more appropriate grasps in later trials or when the object was reintroduced.
It is possible that a visual cue, such as a dot, is not ecologically meaningful and subsequently requires more explicit learning. This differs from implicit visual geometric cues of shape and size that are ecologically meaningful (Gentile, 2000; Salimi et al., 2003). Consequently the explicit learning of a visual dot-weight association may also be reliant on higher order perceptual processes to conceptualize the meaning of the dot cue. If this is the case, comparable performance in the memory-associated and visual-spatial cue conditions may be due to both requiring integration of stored and visible information via the ventro-dorsal stream. Therefore, it is reasonable that apraxic patients AH and GW did not benefit from the high-level visual cue. Studies showing improved apraxic performance with increased contextual information may be attributed to an increased presence of low-level affordance cues regarding the objects’ size and structure. Yet, it remains that apraxic patients may be able to register and utilize these memory-associated and visual-spatial cues but that low-level affordance cues are more dominant. According to the affordance competition hypothesis (Cisek, 2007), potential motor actions are generated simultaneously and selected on the basis of the action goal. Therefore, if object affordances compete for selection, the more symbolic memory-associated or visual-spatial cues may be overpowered by more salient low-level cues of object structure. Although it is not certain why these apraxic patients did not benefit from the visual-spatial cue, this observation is interesting when trying to understand what information, be it visual or symbolic, individuals use when manipulating objects to achieve action goals. If apraxic patients are more reliant on low-level affordance cues, this could have a substantial impact on their ability to learn to use new objects or appropriately use familiar objects when these cues are ambiguous. However, as very few studies have assessed learning of skilled movement in apraxia this can only be speculated, and emphasizes the need to explore learning in apraxia to determine the types of cues these patients can successfully utilize to inform their grasp.
Additionally, it was somewhat surprising that patients’ AH and GW did not benefit from short-term sensorimotor feedback to improve grasp performance during subsequent trials within a block (TC). Attributed to the bilateral dorsal stream, rapidly decaying sensorimotor memory is formed and updated with repeated grasps of the same object (Bursztyn and Flanagan, 2008; Buxbaum and Kalénine, 2010). Apraxic patients apply appropriate fingertip force when repeatedly lifting novel objects, suggesting sensorimotor memories can be formed and applied (Gordon et al., 1993; Ietswaart et al., 2001; Dawson et al., 2010; Hermsdörfer et al., 2011; Li et al., 2011; Randerath et al., 2011; Eidenmüller et al., 2014). However, more central grasp-points remained fairly constant between the first and last trial in the current study. AH and GW may fail to update their-grasp points with repeated lifts due to visible structural information and short-term sensorimotor feedback being in conflict; object shape suggests a central weight distribution whereas sensorimotor feedback indicates it is either to the left or the right of the object. In grip force studies, the novel objects were typically symmetrical with a central weight distribution; the shape of the novel object corroborates sensorimotor feedback of object weight, resulting in improved fingertip force with repeated lifts (for examples see Gordon et al., 1993; Dawson et al., 2010; Li et al., 2011). Consequently it is argued that failure to use short-term sensorimotor feedback by patient AH and GW is not because this process is disrupted, but that the design of the current task causes an impediment between visual and sensorimotor information leading to low-level visual affordance cues to be favored. Taken together, the performance of patient AH and GW in memory-associated and visual-spatial cue conditions confirms that they fail to incorporate stored knowledge into action plans even in the presence of certain visible cues.
Interestingly, patient JA’s performance change was comparable to control groups in all conditions, except when compared to healthy controls during repeated grasps (TC) of the visual-spatial cue objects. However, further analyses of grasp-point indicate that patient JA did indeed struggle to apply knowledge-based information or visual-spatial cues in learning to grasp the weighted objects. Exploring JA’s behavior when grasping visual-spatial cued objects, a positive score for accuracy change over trials indicates that JA continued to make errors to the final trial. Although these errors were only minor in contrast to patient AH and GW who consistently failed to adjust their grasp-point according to weight distribution, when examining individual participants’ performance change none of the non-apraxic patients or healthy controls failed to adapt their grasp-point over repeated lifts (TC) and when the objects were reintroduced (BC). Therefore it is possible that apraxic patient JA used compensatory mechanisms to improve performance. Patient JA’s variable grasp behavior also suggests that she may be maintaining a trial-and-error procedure throughout the experiment. In particular, when grasping specific objects within the memory-associated and visual-spatial cue conditions, patient JA’s grasp-point deviated further from the optimum point of grasp to compensate for object weight distribution in later trials and when the objects were reintroduced, whereas control participants grasps moved closer to the optimum grasp-point. Likewise, patient JA’s point of grasp was grossly variable from Block 1–5; JA adjusted her grasp-point by almost 20 cm in both the memory-associated and visual-spatial cue conditions. This behavior seemed to demonstrate a more subtle manifestation of the deficit in the integration of visible and known object properties that results in more changeable grasp accuracy.
These subtle effects in JA were in line with the behavior she displayed. JA, a young and highly motivated patient, performed the task slowly and deliberately. She appeared more aware of her deficit than the other patients. Perhaps this due to the fact that she was aware of her apraxic symptoms that included actual object-use (evident in standard apraxia screening). If this is the case, JA is more likely to compensate for her impairment resulting in improved grasping performance compared to the other apraxic patients. Although patient AH has a similar lesion to JA, she inevitably will have been less aware of her apraxic symptoms that did not include actual object-use. Likewise, GW demonstrated more severe apraxic errors across the screening tasks and may be less able to effectively compensate for his impairment. No compensative strategies in performance of the experimental task were apparent in AH or GW who performed the task very quickly, immediately reaching for the object at the start of each trial and rapidly lifting each object before returning it to the table. In contrast, JA showed awareness of difficulty with the task, commenting on completion that she tried to apply strategies: she said that when the object was placed in the testing area, she observed whether one end of the object landed on the table first as a potential clue to its weight distribution. Although the availability of such cues were avoided through careful placement of each object, it may be beneficial to occlude participants’ view when objects are placed on the table. However, it was felt that the presence of each object during testing ensured that participants were aware that each object reintroduced in later blocks was the same as those seen previously. Finally, the less gross errors of patient JA on the grasping task compared to AH and GW cannot be attributed to better comprehension, as JA scored the least in the language comprehension test. Likewise, JA did not suffer from milder apraxic symptoms; as described, patient GW demonstrated the more severe apraxic symptoms whereas JA’s apraxic behavior was comparable to AH.
Rather than ventro-dorsal processing remaining intact in patient JA, it is believed that through her careful performance, she managed to assemble compensatory strategies, even when weight distribution was afforded by a high-level visual-spatial cue. Appropriate performance when behavior is delayed in apraxic patients suggests that stored knowledge is maintained, but difficult to access. As described, accurate memory-driven reach and grasp performance is observed when apraxic patients pick up basic blocks based on simple size and distance information (Ietswaart et al., 2001). Myung et al. (2010) also confirmed that during semantic judgements, apraxic patients also showed greater fixations on object pictures that were manipulation-related to the target word (e.g., “typewriter” and “piano”) when the manipulation relationship was not task relevant; the fixation position was comparable to their non-apraxic control group but the effect emerged later, again indicating that stored representations are preserved but not easily accessible. The magnitude of delayed activation of manipulation related action information in apraxia is predicted by poorer object-use pantomime performance and the extent to which inferior parietal and posterior temporal regions were compromised (Lee et al., 2014). Therefore, the extended delay between reach and grasp movements used by JA in her slow and deliberate performance (compared to patient AH and GW who initiated grasps immediately) may have enabled her to incorporate stored knowledge into action plans. Further, the variable nature of her points of grasp along each object may be indicative of when her compensatory strategies were less effective. This may also indicate why JA continued to make grasping errors by the final trial when grasping the visual-spatial cued objects.
Although the design of the current study delayed reach-to-grasp action between trials by requiring participants to return their hand to the table before beginning another grasp movement, the duration of this delay was not controlled. Further investigation is required to confirm whether delay between reaching and grasping can reduce performance errors when balancing novel objects. It is probable that such compensatory strategies may rely on critical brain structures being intact; JA presented with frontal lesions that implicate white matter whilst parietal regions remain undamaged (as was the case in AH). In contrast, GW’s lesion implicates temporal and parietal regions of the left hemisphere suggesting that the critical juncture between the ventral and dorsal pathways may be compromised (Rizzolatti and Matelli, 2003; Buxbaum and Kalénine, 2010; Rizzolatti et al., 2011; Binkofski and Buxbaum, 2013; Vingerhoets, 2014). This corresponds with patient GW’s markedly poor performance across all apraxic tests. Based on research showing a strong association between impaired object-use and temporal and parietal damage (Goldenberg, 2009; Vingerhoets, 2014), impaired use of memory-associated and visual-spatial cued information is expected in this patient.
In conclusion, apraxia was associated with a disrupted ability to utilize memory-associated or visual-spatial cued information indicating weight distribution. Specifically, patient AH and GW failed to successfully incorporate memory-associated information where weight distribution was indicated by the objects color, and visual-spatial cued information in the form of a dot cue over the objects weighted. Grasps were inaccurate during repeated lifts and when the objects were reintroduced. A third apraxic patient (JA) seemed to compensate for these difficulties but still showed performance errors that may be attributable to a more subtle impairment. These results indicate that apraxia impairs the ability to utilize meaningful visual-spatial cue or symbolic memory-associated cues when grasping objects to achieve specific action goals. Crucially, the abnormal grasping behavior in these apraxic patients suggests that integration of visible and known object properties attributed to the ventro-dorsal stream is impaired. Not only does disruption to ventro-dorsal processing impair use of familiar objects, but also these results would predict that apraxia is associated with difficulty learning to manipulate new objects.
Author Contributions
CE, conception and design of the research task; acquisition, analysis and interpretation of the data; drafting the manuscript and final editing. MGE, contribution to the conception of the task, critically revising and editing the manuscript, and final approval of the manuscript. LJT, contribution to the conception of the task, final approval of the manuscript version to be published. MI, substantial contribution to the conception and design of the research task, interpretation of the data and critically revising and editing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was funded by Northumbria University. We would like to thank the individuals who took part in the study, the local NHS hospitals and rehabilitation centres in the North East of England where many were recruited, and in particular the responsible clinicians Dr. David Bruce, Dr. Tim Cassidy, Dr. Akif Gani, and Dr. Chris Price and their teams.
Footnotes
References
Almeida, J., Fintzi, A. R., and Mahon, B. Z. (2013). Tool manipulation knowledge is retrieved by way of the ventral visual object processing pathway. Cortex 49, 2334–2344. doi: 10.1016/j.cortex.2013.05.004
Barde, L. H. F., Buxbaum, L. J., and Moll, A. D. (2007). Abnormal reliance on object structure in apraxics’ learning of novel object-related actions. J. Int. Neuropsychol. Soc. 13, 997–1008. doi: 10.1017/s1355617707070981
Baud-Bovy, G., and Soechting, J. (2001). Visual localization of the center of mass of compact, asymmetric, two-dimensional shapes. J. Exp. Psychol. Hum. Percept. Perform. 27, 692–706. doi: 10.1037/0096-1523.27.3.692
Bickerton, W.-L., Samson, D., and Humphreys, G. W. (2011). Separating forms of neglect using the apples test: validation and functional prediction in chronic and acute stroke. Neuropsychology 25, 567–580. doi: 10.1037/a0023501
Binkofski, F., and Buxbaum, L. J. (2013). Two action systems in the human brain. Brain Lang. 127, 222–229. doi: 10.1016/j.bandl.2012.07.007
Bursztyn, L. L. C. D., and Flanagan, J. R. (2008). Sensorimotor memory of weight asymmetry in object manipulation. Exp. Brain Res. 184, 127–133. doi: 10.1007/s00221-007-1173-z
Buxbaum, L. J. (2001). Ideomotor apraxia: a call to action. Neurocase 7, 445–458. doi: 10.1093/neucas/7.6.445
Buxbaum, L. J., and Kalénine, S. (2010). Action knowledge, visuomotor activation and embodiment in the two action systems. Ann. N Y Acad. Sci. 1191, 201–218. doi: 10.1111/j.1749-6632.2010.05447.x
Buxbaum, L. J., Kyle, K., Grossman, M., and Coslett, H. B. (2007). Left inferior parietal representations for skilled hand-object interactions: evidence from stroke and corticobasal degeneration. Cortex 43, 411–423. doi: 10.1016/s0010-9452(08)70466-0
Buxbaum, L. J., Kyle, K. M., Tang, K., and Detre, J. A. (2006). Neural substrates of knowledge of hand postures for object grasping and functional object use: evidence from fMRI. Brain Res. 1117, 175–185. doi: 10.1016/j.brainres.2006.08.010
Buxbaum, L. J., and Saffran, E. M. (2002). Knowledge of object manipulation and object function: dissociations in apraxic and nonapraxic subjects. Brain Lang. 82, 179–199. doi: 10.1016/s0093-934x(02)00014-7
Buxbaum, L. J., Sirigu, A., Schwartz, M. F., and Klatzky, R. (2003). Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia 41, 1091–1113. doi: 10.1016/s0028-3932(02)00314-7
Cisek, P. (2007). Cortical mechanisms of action selection: the affordance competition hypothesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 1585–1599. doi: 10.1098/rstb.2007.2054
Crawford, J. R., and Garthwaite, P. H. (2002). Investigation of the single case in neuropsychology: confidence limits on the abnormality of test scores and test score differences. Neuropsychologia 40, 1196–1208. doi: 10.1016/s0028-3932(01)00224-x
Crawford, J. R., Garthwaite, P. H., and Porter, S. (2010). Point and interval estimates of effect sizes for the case-controls design in neuropsychology: rationale, methods, implementations and proposed reporting standards. Cogn. Neuropsychol. 27, 245–260. doi: 10.1080/02643294.2010.513967
Cycowicz, Y. M., Friedman, D., Rothstein, M., and Snodgrass, J. G. (1997). Picture naming by young children: norms for name agreement, familiarity and visual complexity. J. Exp. Child Psychol. 65, 171–237. doi: 10.1006/jecp.1996.2356
Daprati, E., and Sirigu, A. (2006). How we interact with objects: learning from brain lesions. Trends Cogn. Sci. 10, 265–270. doi: 10.1016/j.tics.2006.04.005
Dawson, A. M., Buxbaum, L. J., and Duff, S. V. (2010). The impact of left hemisphere stroke on force control with familiar and novel objects: neuroanatomic substrates and relationship to apraxia. Brain Res. 1317, 124–136. doi: 10.1016/j.brainres.2009.11.034
De Renzi, E., and Faglioni, P. (1978). Normative data and screening power of a shortened version of the token test. Cortex 14, 41–49. doi: 10.1016/s0010-9452(78)80006-9
De Renzi, E., and Lucchelli, F. (1988). Ideational apraxia. Brain 111, 1173–1185. doi: 10.1093/brain/111.5.1173
Duemmler, T., Schoeberl, P., and Schwarzer, G. (2008). Development of visual center of mass localization for grasp point selection. Cogn. Dev. 23, 370–384. doi: 10.1016/j.cogdev.2008.06.002
Eidenmüller, S., Randerath, J., Goldenberg, G., Li, Y., and Hermsdörfer, J. (2014). The impact of unilateral brain damage on anticipatory grip force scaling when lifting everyday objects. Neuropsychologia 61, 222–234. doi: 10.1016/j.neuropsychologia.2014.06.026
Endo, S., Wing, A. M., and Bracewell, R. M. (2011). Haptic and visual influences on grasp point selection. J. Mot. Behav. 43, 427–431. doi: 10.1080/00222895.2011.621996
Frey, S. H. (2007). What puts the how in where? Tool use and the divided visual streams hypothesis. Cortex 43, 368–375. doi: 10.1016/s0010-9452(08)70462-3
Garcea, F. E., and Mahon, B. Z. (2014). Parcellation of left parietal tool representations by functional connectivity. Neuropsychologia 60, 131–143. doi: 10.1016/j.neuropsychologia.2014.05.018
Gentile, A. M. (2000). “Skill acquisition: action, movement and neuromotor processes,” in Movement Science: Foundations for Physical Therapy in Rehabilitation, eds J. H. Carr and R. B. Shepherd (Gaithersburg, MD: Aspen Publishers), 111–187.
Goldenberg, G. (1995). Imitating gestures and manipulating a manikin - the representation of the human body in ideomotor apraxia. Neuropsychologia 33, 63–72. doi: 10.1016/0028-3932(94)00104-w
Goldenberg, G. (1996). Defective imitation of gestures in patients with damage in the left or right hemispheres. J. Neurol. Neurosurg. Psychiatry 61, 176–180. doi: 10.1136/jnnp.61.2.176
Goldenberg, G. (2009). Apraxia and the parietal lobes. Neuropsychologia 47, 1449–1459. doi: 10.1016/j.neuropsychologia.2008.07.014
Goldenberg, G. (2014). Apraxia–the cognitive side of motor control. Cortex 57, 270–274. doi: 10.1016/j.cortex.2013.07.016
Goldenberg, G., and Hagmann, S. (1998). Tool use and mechanical problem solving in apraxia. Neuropsychologia 36, 581–589. doi: 10.1016/s0028-3932(97)00165-6
Goldenberg, G., Hermsdörfer, J., Glindemann, R., Rorden, C., and Karnath, H.-O. (2007). Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb. Cortex 17, 2769–2776. doi: 10.1093/cercor/bhm004
Goodale, M. A., and Milner, A. D. (1992). Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25. doi: 10.1016/0166-2236(92)90344-8
Gordon, A. M., Westling, G., Cole, K. J., and Johansson, R. S. (1993). Memory representations underlying motor commands used during manipulation of common and novel objects. J. Neurophysiol. 69, 1789–1796.
Haaland, K. Y., Harrington, D. L., and Knight, R. T. (2000). Neural representations of skilled movement. Brain 123, 2306–2313. doi: 10.1093/brain/123.11.2306
Hermsdörfer, J., Li, Y., Randerath, J., Goldenberg, G., and Eidenmüller, S. (2011). Anticipatory scaling of grip forces when lifting objects of everyday life. Exp. Brain Res. 212, 19–31. doi: 10.1007/s00221-011-2695-y
Ietswaart, M., Carey, D. P., and Della Sala, S. (2006). Tapping, grasping and aiming in ideomotor apraxia. Neuropsychologia 44, 1175–1184. doi: 10.1016/j.neuropsychologia.2005.10.003
Ietswaart, M., Carey, D. P., Della Sala, S., and Dijkhuizen, R. S. (2001). Memory-driven movements in limb apraxia: is there evidence for impaired communication between the dorsal and the ventral streams? Neuropsychologia 39, 950–961. doi: 10.1016/s0028-3932(01)00027-6
Lederman, S. J., and Wing, A. M. (2003). Perceptual judgement, grasp point selection and object symmetry. Exp. Brain Res. 152, 156–165. doi: 10.1007/s00221-003-1522-5
Lee, C.-I., Mirman, D., and Buxbaum, L. J. (2014). Abnormal dynamics of activation of object use information in apraxia: evidence from eyetracking. Neuropsychologia 59, 13–26. doi: 10.1016/j.neuropsychologia.2014.04.004
Li, Y., Randerath, J., Goldenberg, G., and Hermsdörfer, J. (2011). Size-weight illusion and anticipatory grip force scaling following unilateral cortical brain lesion. Neuropsychologia 49, 914–923. doi: 10.1016/j.neuropsychologia.2011.02.018
Milner, A. D., and Goodale, M. A. (2006). The Visual Brain in Action. 2nd Edn. Oxford: Oxford University Press.
Myung, J., Blumstein, S., Yee, E., Sedivy, J. C., Thompson-Schill, S. L., and Buxbaum, L. J. (2010). Impaired access to manipulation features in apraxia: evidence from eyetracking and semantic judgment tasks. Brain Lang. 112, 101–112. doi: 10.1016/j.bandl.2009.12.003
Randerath, J., Goldenberg, G., Spijkers, W., Li, Y., and Hermsdörfer, J. (2010). Different left brain regions are essential for grasping a tool compared with its subsequent use. Neuroimage 53, 171–180. doi: 10.1016/j.neuroimage.2010.06.038
Randerath, J., Goldenberg, G., Spijkers, W., Li, Y., and Hermsdorfer, J. (2011). From pantomime to actual use: how affordances can facilitate actual tool-use. Neuropsychologia 49, 2410–2416. doi: 10.1016/j.neuropsychologia.2011.04.017
Randerath, J., Li, Y., Goldenberg, G., and Hermsdörfer, J. (2009). Grasping tools: effects of task and apraxia. Neuropsychologia 47, 497–505. doi: 10.1016/j.neuropsychologia.2008.10.005
Rizzolatti, G., Fogassi, L., and Luppino, G. (2011). “The two dorsal visual streams and their role in perception,” in Cerebral Plasticity: New Perspectives, 1st Edn, eds L. M. Chalupa, N. Berardi, M. Caleo, L. Galli-Resta, and T. Pizzorusso (Cambridge, MA: MIT Press), 259–273.
Rizzolatti, G., and Matelli, M. (2003). Two different streams form the dorsal visual system: anatomy and functions. Exp. Brain Res. 153, 146–157. doi: 10.1007/s00221-003-1588-0
Rorden, C., Bonilha, L., Fridriksson, J., Bender, B., and Karnath, H.-O. (2012). Age-specific CT and MRI templates for spatial normalization. Neuroimage 61, 957–965. doi: 10.1016/j.neuroimage.2012.03.020
Rorden, C., Karnath, H.-O., and Bonilha, L. (2007). Improving lesion-mapping. J. Cogn. Neurosci. 19, 1081–1088. doi: 10.1162/jocn.2007.19.7.1081
Salimi, I., Frazier, W., Reilmann, R., and Gordon, A. M. (2003). Selective use of visual information signaling objects’ center of mass for anticipatory control of manipulative fingertip forces. Exp. Brain Res. 150, 9–18. doi: 10.1007/s00221-003-1394-8
Sirigu, A., Cohen, L., Duhamel, J.-R., Pillon, B., Dubois, B., and Agid, Y. (1995). A selective impairment of hand posture for object utilization in apraxia. Cortex 31, 41–55. doi: 10.1016/s0010-9452(13)80104-9
Sunderland, A., and Shinner, C. (2007). Ideomotor apraxia and functional ability. Cortex 43, 359–367. doi: 10.1016/s0010-9452(08)70461-1
Sunderland, A., Wilkins, L., and Dineen, R. (2011). Tool use and action planning in apraxia. Neuropsychologia 49, 1275–1286. doi: 10.1016/j.neuropsychologia.2011.01.020
Sunderland, A., Wilkins, L., Dineen, R., and Dawson, S. E. (2013). Tool-use and the left hemisphere: what is lost in ideomotor apraxia? Brain Cogn. 81, 183–192. doi: 10.1016/j.bandc.2012.10.008
Keywords: apraxia, visual affordance, ventro-dorsal stream, visual pathways model, grasping
Citation: Evans C, Edwards MG, Taylor LJ and Ietswaart M (2016) Impaired Communication Between the Dorsal and Ventral Stream: Indications from Apraxia. Front. Hum. Neurosci. 10:8. doi: 10.3389/fnhum.2016.00008
Received: 05 September 2015; Accepted: 11 January 2016;
Published: 01 February 2016.
Edited by:
Ferdinand Binkofski, RWTH Aachen University, GermanyReviewed by:
Annalisa Setti, University College Cork, IrelandMarc Himmelbach, University Hospital Tuebingen, Germany
Copyright © 2016 Evans, Edwards, Taylor and Ietswaart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carys Evans, carysemilyevans@gmail.com
 Carys Evans
Carys Evans Martin G. Edwards
Martin G. Edwards Lawrence J. Taylor
Lawrence J. Taylor Magdalena Ietswaart
Magdalena Ietswaart