Cerebellar tDCS: A Novel Approach to Augment Language Treatment Post-stroke
- 1Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 2Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 3Department of Otolaryngology—Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 4Department of Biomedical Engineering, The City College of New York of CUNY, New York, NY, USA
- 5Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 6Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 7Department of Cognitive Science, Johns Hopkins University, Baltimore, MD, USA
People with post-stroke aphasia may have some degree of chronic deficit for which current rehabilitative treatments are variably effective. Accumulating evidence suggests that transcranial direct current stimulation (tDCS) may be useful for enhancing the effects of behavioral aphasia treatment. However, it remains unclear which brain regions should be stimulated to optimize effects on language recovery. Here, we report on the therapeutic potential of right cerebellar tDCS in augmenting language recovery in SMY, who sustained bilateral MCA infarct resulting in aphasia and anarthria. We investigated the effects of 15 sessions of anodal cerebellar tDCS coupled with spelling therapy using a randomized, double-blind, sham controlled within-subject crossover trial. We also investigated changes in functional connectivity using resting state functional magnetic resonance imaging before and 2 months post-treatment. Both anodal and sham treatments resulted in improved spelling to dictation for trained and untrained words immediately after and 2 months post-treatment. However, there was greater improvement with tDCS than with sham, especially for untrained words. Further, generalization to written picture naming was only noted during tDCS but not with sham. The resting state functional connectivity data indicate that improvement in spelling was accompanied by an increase in cerebro-cerebellar network connectivity. These results highlight the therapeutic potential of right cerebellar tDCS to augment spelling therapy in an individual with large bilateral chronic strokes.
Introduction
Aphasia is a leading cause of disability following stroke and can affect every aspect of daily life, including interpersonal relationships, work, and community interactions. Speech-language therapy is the mainstay of treatment. Therapy is beneficial for language recovery; however, gains in therapy are variable and progress may be slow, especially after large, chronic left hemisphere lesions (Brady et al., 2016). Recently, neuromodulation with tDCS has been introduced to increase the efficiency of speech and language therapy (for recent reviews see de Aguiar et al., 2015; Sebastian et al., 2016b). Studies indicate that anodal tDCS over peri-lesional left hemisphere (LH) language regions has the potential to augment language outcomes in individuals with chronic aphasia (e.g., Baker et al., 2010; Fiori et al., 2011; Fridriksson et al., 2011; Vestito et al., 2014). However, large LH stroke impedes improvement of language functions that are dependent on LH networks. In such cases, enhancing the function of non-damaged hemisphere with the goal of facilitating compensation has been investigated. However, some data suggest that recruitment of right hemisphere (RH) regions can be maladaptive in the chronic stage. Also, several studies have shown benefit of RH inhibitory (cathodal) tDCS or combined LH anodal tDCS + RH cathodal tDCS (e.g., Marangolo et al., 2014; Manenti et al., 2015). However, inhibition of the RH might have detrimental effects on cognitive functions that normally rely on the RH. Previous studies have not evaluated the effect of tDCS in individuals with large, bilateral chronic stroke.
This case study illustrates the potential usefulness of a novel electrode placement for tDCS augmentation of language therapy in chronic post-stroke aphasia: the right cerebellum.
Evidence from functional neuroimaging and neuroanatomical investigations indicate that the right cerebellum is important for language and cognitive functions (e.g., Leiner et al., 1989; Schmahmann, 1991, 2001; Middleton and Strick, 1994; Stoodley and Schmahmann, 2009; Murdoch, 2010; Stoodley et al., 2012; Marien et al., 2014; for recent reviews see De Smet et al., 2013; Keren-Happuch et al., 2014). Damage to the right cerebellum has been associated with deficits in a variety of language tasks (e.g., Hassid, 1995; Marien et al., 1996, 2000; Gómez Beldarrain et al., 1997; Fabbro et al., 2004; Baillieux et al., 2010). In addition, cerebellar tDCS studies in healthy individuals provide evidence that right cerebellar tDCS modulates cognitive and language functions such as verb generation (Pope and Miall, 2012), verbal fluency (Turkeltaub et al., 2016), working memory (Boehringer et al., 2013; Macher et al., 2014), and implicit learning (Ferrucci et al., 2013). See Grimaldi et al. (2016) for a recent review. Beneficial cognitive effects from right cerebellar tDCS have been found for both anodal and cathodal stimulation.
Given the role of the cognitive and language functions of the cerebellum and the ability of cerebellar tDCS to modify behavior in healthy individuals, cerebellar tDCS may have a uniquely valuable therapeutic role for individuals with aphasia. Furthermore, cerebellum can be stimulated even in patients with aphasia associated with bilateral hemispheric strokes. In addition, the cerebellum is regarded as an important region involved in skill learning (Morton and Bastian, 2006; Galea et al., 2011). Therefore, cerebellar tDCS could also augment response to language therapy by enhancing learning skills.
Here, we report behavioral and neural effects of right cerebellar tDCS with behavioral spelling treatment in a participant who sustained bilateral MCA infarct resulting in aphasia and complete anarthria. Participant SMY is mute following his second stroke but has retained some ability to write and type. Because he depends on writing to communicate, recognizable spelling is critical for effective social function. Therefore, cerebellar tDCS plus behavioral spelling treatment could improve spelling recovery through its roles in language and learning. We sought to evaluate the following hypotheses: (1) Improvement in spelling to dictation (in treated and untreated words) will be greater with tDCS + spelling treatment than with sham + spelling treatment; (2) Improvement will last longer after tDCS treatment than sham treatment at 2 months post-treatment; (3) Improvement in other language tasks (written picture naming) will be greater after tDCS than sham; (4) Functional connectivity between the right cerebellum and the residual left and right hemisphere language regions of interest will be greater post-treatment compared to pre-treatment.
Case Report
Patient History
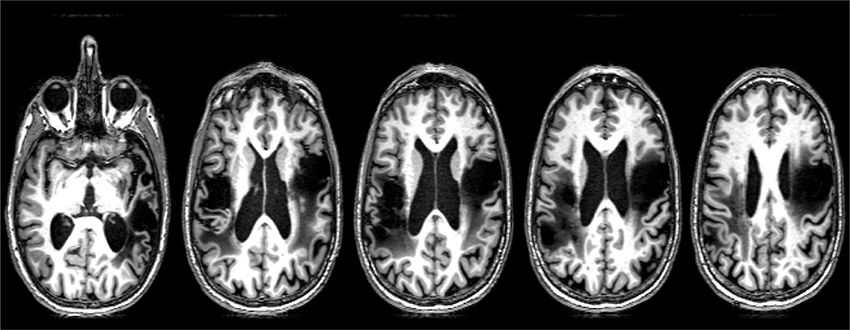
SMY is a 57-year-old, right-handed man with a master's degree, employed as an architect until he had an ischemic stroke due to carotid dissection. MRI revealed left MCA territory infarct, involving frontal, temporal, and insular cortex. This stroke resulted in right hemiparesis and aphasia. He underwent extensive inpatient and outpatient rehabilitation, and showed resolution of hemiparesis and substantial improvement in language. He survived a second (right hemisphere) stroke due to carotid dissection 4 years later. MRI revealed acute infarct of right MCA territory, involving the fronto-parietal and insular cortex (Figure 1). His second stroke resulted in left hemiparesis, dysphagia necessitating PEG placement, aphasia, and no speech production due to anarthria. Please see Figure 1 for lesion location.
He was enrolled in the study in 2015, 5 years after his second stroke. At the time of his enrollment, SMY was independent in activities of daily living and resided with his wife. SMY scored 26/28 on the auditory comprehension subtest on the Aphasia Diagnostic Profile test (Helm-Estabrooks, 1992). Participant SMY is mute and communicates by writing in a book or on an iPad, augmented with a variety of gestures. This study was carried out in accordance with the recommendations of the ‘Johns Hopkins Medicine Institutional Review Boards’ with written informed consent from all subjects. SMY gave written informed consent in accordance with the Declaration of Helsinki.
Spelling and Naming Performance
Given that SMY is mute and the focus of treatment was on spelling, only written language was assessed in detail. SMY's narrative writing consisted of simple sentences with frequent phonologically implausible nonword errors (center → cect), semantic errors (garage → house), and letter omissions (piano → pian). SMY was able to write simple and common 3-letter words and some 4-letter words without difficulty. He was not able to identify his errors in writing. The Johns Hopkins Dysgraphia Battery (Goodman and Caramazza, 1985) was administered (See Supplementary Material).
Spelling to dictation of words and pseudowords was very impaired (12% accurate on words and 0% accurate on pseudowords). On words, he showed a significant effect of grammatical word class (e.g., verbs vs. nouns 36 vs. 21% accurate; χ2 = 4.8; p < 0.05), and concreteness (concrete vs. abstract 57 vs. 33% accurate; χ2 = 6.8; p < 0.05). Spelling was significantly influenced by word-length: 78% correct on 4-letter words, 57% on 5-letter words, 50% on 6-letter words, 36% on 7-letter words, and 28% on 8-letter words (e.g., 4-letter vs. 5, 6, 7, 8 letters; p < 0.05). There was no effect of regularity.
Errors in spelling to dictation were mostly phonologically implausible nonword errors (e.g., parent → parpe) and some unrelated word errors (palace → pea). His 0% accuracy on nonword errors indicates impairment at the level of sublexical mechanisms for phoneme-grapheme conversion (PGC), although he was also impaired in access to written word forms. His word length effect can be explained by greater opportunity to err on longer words in his attempts to rely on (impaired) PGC. We decided to treat sublexical spelling—PGC—as a first step, to give him some rules he could rely on to at least produce a plausible spelling.
SMY's written picture naming performance was assessed using the Philadelphia Naming Test (PNT, Roach et al., 1996) to examine generalization from spelling-dictation to written picture naming. SMY's performance on the written picture-naming task was impaired. He scored 121/175 on the PNT prior to treatment. Errors in written naming were predominantly phonologically implausible nonword errors: 63% (candle → calc), some semantic errors: 13% (hose → cable), some unrelated word errors: 13% (mustache → mustang), and some no responses: 11%.
Procedure
tDCS Treatment
We used a double-blind, within-subject crossover trial design, with random order of treatments. There were two experimental conditions: “right cerebellar tDCS + behavioral (spelling) treatment” and “sham tDCS + behavioral treatment.” Each condition consisted of 15 consecutive training sessions, 3–5 per week, separated by 2 months. Evaluation took place before, immediately after, and 2 months post-treatment for each condition (Tsapkini et al., 2014). SMY was randomized to the “sham” condition first followed by the “tDCS” condition. tDCS was administered for the first 20 min of the 45-min treatment session of behavioral spelling treatment. tDCS was delivered at a constant current of 2 mA for 20 min via two 25 cm2 saline soaked sponge electrodes using a ActivaDose II stimulator (ActiveTec Inc., Salt Lake City, Utah). The anode was centered on the right cerebellum (1 cm under, and 4 cm lateral to the inion: Pope and Miall, 2012) and the cathode was placed on the right deltoid muscle. Sham tDCS was applied using the same electrode configuration, but current intensity was ramped down to zero after 30 s (Gandiga et al., 2006).
Behavioral Spelling Treatment
We employed a spelling treatment protocol previously described (Tsapkini et al., 2014) that specifically targeted PGC in spelling to dictation. We selected 80 words from the Johns Hopkins Dysgraphia Battery that SMY misspelled. Words were divided into two sets: trained words (n = 40), practiced during treatment (sham and tDCS), and untrained words (n = 40), only tested prior to the start of treatment, end of treatment, and 2 months post-treatment. Stimuli in both sets were matched for lexical frequency, letter length, and concreteness. Words were 4–8 letters long and consisted of nouns, verbs, and adjectives. For each treatment condition (sham and tDCS), we compared the correct responses (1) pre-treatment and immediately after treatment, (2) pre-treatment and 2 months post-treatment on each stimulus type (trained words, untrained words, or written picture naming) with McNemar's test for correlated responses (see Table 1).
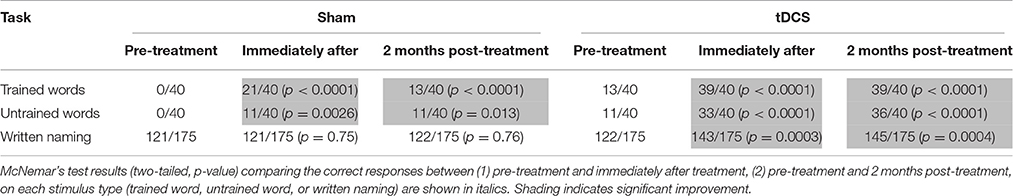
Table 1. Raw scores for trained words, untrained words, and written picture naming prior to the start of treatment, immediately after treatment and 2 months post-treatment for each condition.
The behavioral spelling treatment consisted of training PGC in the context of each dictated word practiced. For each trained word, SMY was asked to point to the letter corresponding to each phoneme (from a set of letters). If he was correct, he was reinforced. If he was incorrect, the clinician pointed to the correct letter. Then SMY was asked to write the letter(s) corresponding to a particular phoneme for the trained word. If he was incorrect, the clinician wrote the correct letter. Finally, SMY was explicitly instructed in PGC for all letter-sounds of the word. Each session consisted of teaching the PGC of five trained words; when SMY met criteria (90% accuracy across three sessions), new words were introduced. It should be noted that SMY did not receive any other therapy except for support groups, including during follow-up periods.
Modeling Current Flow in the Right Cerebellum
To understand the electric field distribution of right cerebellar tDCS, we completed a modeling study. One high resolution T1 MRI-scan (1 mm3 voxels) of a healthy control extending between the c7 vertebra and the vertex was segmented into 11 tissue compartments using automated algorithm and manual segmentation techniques using ScanIP (Simpleware) as previously described (Datta et al., 2009). Specific conductivity values were assigned to the individual tissue compartments. The MRI-based Finite Element Method (FEM) models were generated using COMSOL Multiphysics to predict current flow in volume conductor physics studies involving two 5 × 5 cm sponge pad electrodes. A 2 mA stimulation boundary condition was applied to the anode (right cerebellar cortex, 1 cm under, and 4 cm lateral to the inion) and a ground condition was applied to the cathode (right deltoid muscle). An electric isolation condition was applied to the remaining boundaries. Plots of the electric field profile (0–1.2 V/m) were displayed in a false color scale (blue-red) on a 3D rendering of the brain. The results indicate that the maximum electric field amplitude was generated in the right cerebellum with some spread to the left cerebellum but without spread to adjacent occipital cortex or other cortical areas (see Figure 2).
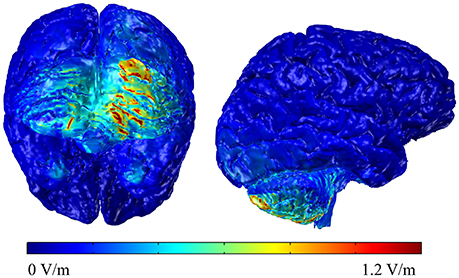
Figure 2. Back and lateral views of the modeling data of the electric field distributions below the stimulating electrode on the right cerebellum.
MRI Acquisition
Resting state fMRI was acquired twice: prior to the start of the study and 2 months after the completion of the study (6 month interval between scans). Scans were acquired on a 3-T Philips Achieva MRI scanner with a 32-channel head coil. Resting state images were acquired using EPI and the following scan parameters: TR = 2000 ms, TE = 30 ms, flip-angle = 90, matrix = 64 × 64, FOV = 240 × 240 mm, 35 3 mm parallel axial slices covering the whole brain, 210 volumes. High resolution 3D MPRAGE was acquired in the sagittal plan utilizing a multishot, turbo field echo pulse sequence and the following scan parameters: TR = 6800 ms, TE = 31 ms, matrix = 256 × 256, FOV = 256 × 240 × 240 mm, 170 1 mm slices covering the whole brain. One normal control (59/Female) was scanned longitudinally using the same scan interval as SMY.
Changes in connectivity were examined between the right cerebellum and peri-lesional language regions of interest (ROIs) in the LH and RH. Only non-lesioned regions were included in the ROI. The ROI included: superior frontal gyrus (SFG), superior frontal gyrus_prefrontal cortex (SFG_PFC), middle frontal gyrus/dorsolateral prefrontal cortex (MFG_DLPC), middle temporal gyrus pole (MTG_pole), inferior temporal gyrus (ITG), fusiform gyrus (FG), left and right cerebellum. For a full description of resting state-processing steps see Sebastian et al. (2016a). Briefly, the structural scan was segmented using the multi-atlas mapping and parcellation approach, co-registered to the motion and slice timing (SPM8) corrected resting state dynamics. Time courses were extracted from the specific ROIs, which were regressed for physiological nuisance by applying CompCor (Behzadi et al., 2007). From the “nuisance-corrected” time courses we obtained the parcel-by-parcel correlation matrices, z-transformed by Fisher's method. The whole procedure was performed automatically in BrainGPS (Li et al., 2015).
Results
Treatment Data
SMY showed an overall improvement in spelling with a notable increase in writing speed. Trained words were the same for the sham and tDCS conditions. However, out of the 40 trained words, SMY reached criterion for only 17 trained words during the sham condition. On an average, he required 4.5 (range: 3–7) sessions to reach criterion for each trained word. SMY reached criterion more quickly during the tDCS condition. He was able to reach criterion in 3.2 sessions (range: 3–4).
SMY correctly spelled to dictation 21/40 trained words and 11/40 untrained words after sham treatment, whereas he correctly spelled to dictation 39/40 trained words and 33/40 untrained words after tDCS treatment. Therefore, both sham and tDCS treatments were effective for trained and untrained words immediately post-treatment, but there was significantly greater improvement with tDCS than with sham for both trained and untrained words (trained words: χ2 = 14.77, p = 0.0001; untrained words: χ2 = 15.758, p < 0.0001). At 2 months post-treatment, SMY showed maintenance of learned PGC rules in spelling to dictation on trained and untrained words in both tDCS and sham conditions; however, significantly greater maintenance was noted in the tDCS condition compared to the sham condition (trained words: χ2 = 26.97, p < 0.0001; untrained words: χ2 = 23.22, p < 0.0001). In addition, generalization from spelling to dictation to written picture naming was noted only in the tDCS condition.
SMY showed a notable change in spelling error types post-treatment, especially after the tDCS condition. Before treatment, SMY's spelling to dictation was 0% correct (40/40 errors) for both trained and untrained words (trained words: 33/40 or 82.5% phonologically implausible nonword errors; 3/40 or 7.5% phonologically plausible nonword errors; 4/40 or 10% unrelated word errors, untrained words: 35/40 or 87.5% phonologically implausible nonword errors; 2/40 or 5% phonologically plausible nonword errors; 3/40 or 7.5% unrelated word errors). After sham treatment, SMY made 19 errors on the 40 trained words (15/19 or 79% phonologically implausible nonword errors; 1/19 or 5% phonologically plausible nonword errors; 3/19 or 16% unrelated word errors) and 29 errors on the 40 untrained words (26/29 or 90% phonologically implausible nonword errors; 1/29 or 3% phonologically plausible nonword errors; 2/29 or 7% unrelated word errors). After tDCS treatment, he made one error on the trained words and seven errors on the untrained words. All errors were phonologically plausible nonword errors (e.g., pigeon → peigon), and were thus more functional than pretreatment errors.
Resting State Data
Resting state functional connectivity analysis data for SMY and the normal control are shown in Figure 3. For SMY, pre-treatment, weak correlations (connectivity) were noted between the right cerebellum and the left and right hemisphere ROIs, and also between the LH and RH ROIs (indicated by dark blue on the graph in Figure 3). For example, SMY showed weak connectivity (low z scores) between the right cerebellum and the left MFG_DLPC before treatment (dark blue color, 1st column 5th cell from bottom left). At 2 months post completion of treatment, connectivity was higher between the right cerebellum and ROIs in the LH and RH and also between the LH and RH ROIs (light blue and green colors). The difference map between pre-treatment resting state connectivity and 2 months post-treatment connectivity shows stronger correlations between the right cerebellum and the LH and RH ROIs (red, orange, and yellow). The control participant showed very similar connectivity at both the time points between (1) the right cerebellum and the LH and RH ROIs and (2) LH and RH ROIs. Difference map did not indicate a marked difference in z scores between time point 1 and 2.
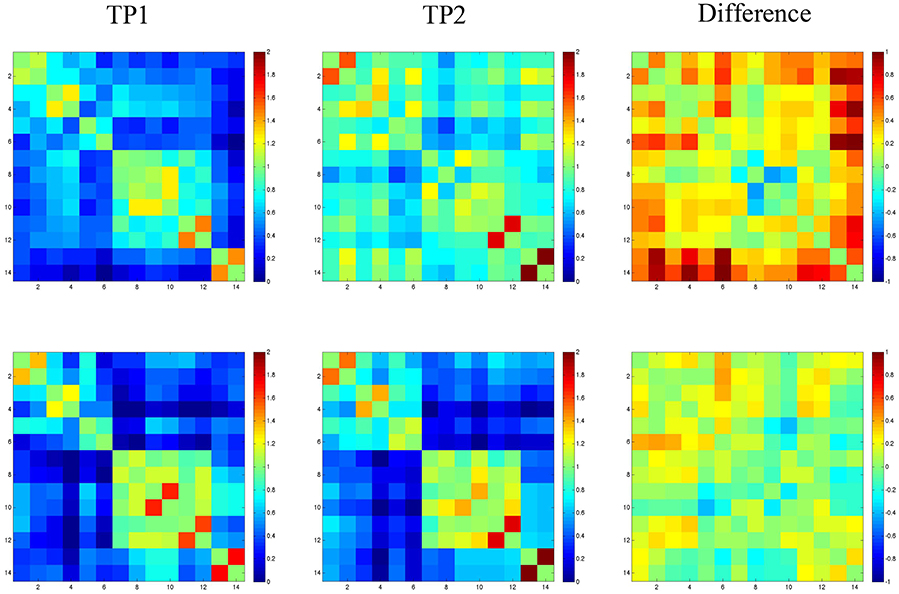
Figure 3. Fisher-transformed correlation matrix for the resting state data for SMY (top panel) at time point 1 (TP1: prior to the start of treatment) and time point 2 (TP2: 2-months follow up time point). Control participant's data is shown in the bottom panel. Difference map shows the difference in correlation between the scan for the resting state data. Correlations were assessed across 14 ROIs. Regions are labeled as numbers corresponding to the left and right superior frontal gyrus (SFG; region 1 and 2), superior frontal gyrus_prefrontal cortex (SFG_PFC; region 3 and 4), middle frontal gyrus dorsolateral prefrontal cortex (MFG_DLPC; region 5 and 6), middle temporal gyrus pole (MTG_pole; region 7 and 8), inferior temporal gyrus (ITG; region 9 and 10), fusiform gyrus (FG; region 11 and 12), and cerebellum (region 13 and 14).
Discussion
This report is, to our knowledge, the first to use cerebellar neuromodulation to augment spelling recovery in an individual with large bilateral chronic strokes. This case study illustrated the potential usefulness of novel electrode placement for tDCS augmentation of language therapy in chronic post-stroke aphasia. Results suggest that anodal tDCS of the right cerebellum coupled with behavioral therapy is more effective than behavioral therapy alone in improving spelling to dictation. Furthermore, generalization to written picture naming was facilitated by tDCS, not sham. Finally, the resting state functional connectivity data indicate that improvement in spelling is accompanied by an increase in cerebro-cerebellar network connectivity.
We found robust improvement in SMY's spelling skills for both trained and untrained words especially after tDCS. Robust effects could be due to the duration and intensity of treatment (15 sessions, 3–5 per week). Alternatively, long-term stimulation may have induced long-term potentiation of neurons that may have lowered the threshold of neuronal excitability and subsequent modification in synaptic connectivity in the areas applied (e.g., Fritsch et al., 2010). In addition, cerebellum is a critical region involved in skill learning and repeated tDCS along with behavioral therapy might have facilitated the learning of writing skills and/or compensatory strategies. Facilitation of skill learning by cerebellar tDCS has been reported earlier. For example, Galea et al. (2011) showed that anodal tDCS applied over the cerebellum during reaching adaptation task facilitated learning. Similarly, Ferrucci et al. (2013) showed that anodal tDCS applied over the cerebellum during a procedural learning task facilitated implicit learning.
Although, right cerebellum is not traditionally considered to be associated with spelling, we show that cerebellar tDCS along with spelling therapy resulted in significant improvement in SMY's spelling abilities. Given that SMY sustained two large strokes involving the left and right hemispheres, tDCS may have enhanced changes in neuroplasticity resulting in modification of networks underlying spelling.
Conclusions
This study, although preliminary, yielded interesting findings and promising avenues for further studies on cerebellar tDCS in post-stroke aphasia. Limitations include that this was a case study. In addition, tDCS followed sham in this experimental design and thus any extra benefits of tDCS might in fact be the benefits of having a second treatment period after already having had the first treatment period and time for consolidation. Cerebellar tDCS may not have comparable beneficial effects in patients with unilateral LH lesions. Also, resting state data were not acquired before and after completion of each treatment condition (i.e., sham and tDCS). Therefore, it is not possible to disentangle the network connectivity changes associated with tDCS vs. behavioral treatment.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication. AH, RS, KT, and PC were involved in conception and design, analysis and interpretation of data. RS, SS, CL, AM, CD, and DT performed language evaluation, tDCS treatment, and or neuroimaging data acquisition. AM and MB performed computational modeling and interpretation. AF performed neuroimaging analysis and interpretation. RS, AH, KT, DT, and PC drafted the article and revised it critically for important intellectual content.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Julius Fridriksson, Dr. Chris Rorden, and Taylor Hanayik for providing the tDCS device and computer related assistance. This research was supported by NIDCD K99 DC015554, R01 DC05375, and P50 DC014664. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fnhum.2016.00695/full#supplementary-material
References
Baillieux, H., De Smet, H. J., Dobbeleir, A., Paquier, P. F., De Deyn, P. P., and Mariën, P. (2010). Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex 46, 869–879. doi: 10.1016/j.cortex.2009.09.002
Baker, J. M., Rorden, C., and Fridriksson, J. (2010). Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke 41, 1229–1236. doi: 10.1161/STROKEAHA.109.576785
Behzadi, Y., Restom, K., Liau, J., and Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. doi: 10.1016/j.neuroimage.2007.04.042
Boehringer, A., Macher, K., Dukart, J., Villringer, A., and Pleger, B. (2013). Cerebellar transcranial direct current stimulation modulates verbal working memory. Brain Stimul. 6, 649–653. doi: 10.1016/j.brs.2012.10.001
Brady, M. C., Kelly, H., Godwin, J., and Enderby, P. (2016). Speech and language therapy for aphasia following stroke. Cochrane Database Syst. Rev. CD000425. doi: 10.1002/14651858.CD000425.pub3
Datta, A., Bansal, V., Diaz, J., Patel, J., Reato, D., and Bikson, M. (2009). Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2, 201–207. doi: 10.1016/j.brs.2009.03.005
de Aguiar, V., Paolazzi, C. L., and Miceli, G. (2015). tDCS in post-stroke aphasia: the role of stimulation parameters, behavioral treatment and patient characteristics. Cortex 63, 296–316. doi: 10.1016/j.cortex.2014.08.015
De Smet, H. J., Paquier, P., Verhoeven, J., and Mariën, P. (2013). The cerebellum: its role in language and related cognitive and affective functions. Brain Lang. 127, 334–342. doi: 10.1016/j.bandl.2012.11.001
Fabbro, F., Tavano, A., Corti, S., Bresolin, N., De Fabritiis, P., and Borgatti, R. (2004). Long-term neuropsychological deficits after cerebellar infarctions in two young adult twins. Neuropsychologia 42, 536–545. doi: 10.1016/j.neuropsychologia.2003.09.006
Ferrucci, R., Brunoni, A. R., Parazzini, M., Vergari, M., Rossi, E., Fumagalli, M., et al. (2013). Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum 12, 485–492. doi: 10.1007/s12311-012-0436-9
Fiori, V., Coccia, M., Marinelli, C. V., Vecchi, V., Bonifazi, S., Ceravolo, M. G., et al. (2011). Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. J. Cogn. Neurosci. 23, 2309–2323. doi: 10.1162/jocn.2010.21579
Fridriksson, J., Richardson, J. D., Baker, J. M., and Rorden, C. (2011). Transcranial direct current stimulation improves naming reaction time in fluent aphasia a double-blind, sham-controlled study. Stroke 42, 819–821. doi: 10.1161/STROKEAHA.110.600288
Fritsch, B., Reis, J., Martinowich, K., Schambra, H. M., Ji, Y., Cohen, L. G., et al. (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66, 198–204. doi: 10.1016/j.neuron.2010.03.035
Galea, J. M., Vazquez, A., Pasricha, N., de Xivry, J. J., and Celnik, P. (2011). Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb. Cortex 21, 1761–1770. doi: 10.1093/cercor/bhq246
Gandiga, P. C., Hummel, F. C., and Cohen, L. G. (2006). Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 117, 845–850. doi: 10.1016/j.clinph.2005.12.003
Gómez Beldarrain, M., Garcia-Monco, J. C., Quintana, J. M., Llorens, V., and Rodeno, E. (1997). Diaschisis and neuropsychological performance after cerebellar stroke. Eur. Neurol. 37, 82–89. doi: 10.1159/000117415
Goodman, R. A., and Caramazza, A. (1985). The Johns Hopkins University Dysgraphia Battery. Baltimore, MD: Johns Hopkins University.
Grimaldi, G., Argyropoulos, G. P., Bastian, A., Cortes, M., Davis, N. J., Edwards, D. J., et al. (2016). Cerebellar Transcranial Direct Current Stimulation (ctDCS) a novel approach to understanding cerebellar function in health and disease. Neuroscientist 22, 83–97. doi: 10.1177/1073858414559409
Hassid, E. I. (1995). A case of language dysfunction associated with cerebellar infarction. Neurorehabil. Neural Repair 9, 157–160. doi: 10.1177/154596839500900304
Keren-Happuch, E., Chen, S. H. A., Ho, M. H. R., and Desmond, J. E. (2014). A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum. Brain Mapp. 35, 593–615. doi: 10.1002/hbm.22194
Leiner, H. C., Leiner, A. L., and Dow, R. S. (1989). Reappraising the cerebellum: what does the hindbrain contribute to the forebrain? Behav. Neurosci. 103:998. doi: 10.1037/0735-7044.103.5.998
Li, Y., Ceritoglu, C., Jiang, H., Kolsany, A. E., Brown, T. J. A., Tang, X., et al. (2015). “BrainGPS: a cloud-based platform for neuroimage analysis and neuroradiological studies,” Paper Presented at the 23th ISMRM Meeting (Toronto, ON).
Macher, K., Böhringer, A., Villringer, A., and Pleger, B. (2014). Cerebellar-parietal connections underpin phonological storage. J. Neurosci. 34, 5029–5037. doi: 10.1523/JNEUROSCI.0106-14.2014
Manenti, R., Petesi, M., Brambilla, M., Rosini, S., Miozzo, A., Padovani, A., et al. (2015). Efficacy of semantic–phonological treatment combined with tDCS for verb retrieval in a patient with aphasia. Neurocase 21, 109–119. doi: 10.1080/13554794.2013.873062
Marangolo, M., Fiori, V., Gelfo, F., Shofany, J., Razzano, C., Caltagirone, C., et al. (2014). Bihemispheric tDCS enhances language recovery but does not alter BDNF levels in chronic aphasic patients. Restor. Neurol. Neurosci. 32, 367–379. doi: 10.3233/RNN-130323
Marien, P., Ackermann, H., Adamaszek, M., Barwood, C. H., Beaton, A., Desmond, J., et al. (2014). Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum 13, 386–410. doi: 10.1007/s12311-013-0540-5
Marien, P., Engelborghs, S., Pickut, B. A., and De Deyn, P. P. (2000). Aphasia following cerebellar damage: fact or fallacy? J. Neurolinguist. 13, 145–171. doi: 10.1016/S0911-6044(00)00009-9
Marien, P., Saerens, J., Nanhoe, R., Moens, E., Nagels, G., Pickut, B. A., et al. (1996). Cerebellar induced aphasia: case report of cerebellar induced prefrontal aphasic language phenomena supported by SPECT findings. J. Neurol. Sci. 144, 34–43. doi: 10.1016/S0022-510X(96)00059-7
Middleton, F. A., and Strick, P. L. (1994). Anatomical evidence for cerebellar and basal ganglia nvolvement in higher cognitive function. Science 266, 458–461. doi: 10.1126/science.7939688
Morton, S. M., and Bastian, A. J. (2006). Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J. Neurosci. 26, 9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006
Murdoch, B. E. (2010). The cerebellum and language: historical perspective and review. Cortex 46, 858–868. doi: 10.1016/j.cortex.2009.07.018
Pope, P. A., and Miall, R. C. (2012). Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul. 5, 84–94. doi: 10.1016/j.brs.2012.03.006
Roach, A., Schwartz, M. F., Martin, N., Grewal, R. S., and Brecher, A. (1996). The Philadelphia naming test: scoring and rationale. Clin. Aphasiol. 24, 121–134.
Schmahmann, J. D. (1991). An emerging concept: the cerebellar contribution to higher function. Arch. Neurol. 48, 1178–1187. doi: 10.1001/archneur.1991.00530230086029
Schmahmann, J. D. (2001). The cerebrocerebellar system: anatomic substrates of the cerebellar contribution to cognition and emotion. Int. Rev. Psychiatry 13, 247–260. doi: 10.1080/09540260120082092
Sebastian, R., Long, C., Purcell, J. J., Faria, A. V., Lindquist, M., Jarso, S., et al. (2016a). Imaging network level language recovery after left PCA stroke. Restor. Neurol. Neurosci. 34, 473–489. doi: 10.3233/RNN-150621
Sebastian, R., Tsapkini, K., and Tippett, D. C. (2016b). Transcranial direct current stimulation in post stroke aphasia and primary progressive aphasia: current knowledge and future clinical applications. Neurorehabilitation 39, 141–152. doi: 10.3233/NRE-161346
Stoodley, C. J., and Schmahmann, J. D. (2009). Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44, 489–501. doi: 10.1016/j.neuroimage.2008.08.039
Stoodley, C. J., Valera, E. M., and Schmahmann, J. D. (2012). Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59, 1560–1570. doi: 10.1016/j.neuroimage.2011.08.065
Tsapkini, K., Frangakis, C., Gomez, Y., Davis, C., and Hillis, A. E. (2014). Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: preliminary results and challenges. Aphasiology 28, 1112–1130. doi: 10.1080/02687038.2014.930410
Turkeltaub, P. E., Swears, M. K., D'Mello, A. M., and Stoodley, C. J. (2016). Cerebellar tDCS as a novel treatment for aphasia? Evidence from behavioral and resting-state functional connectivity data in healthy adults. Restor. Neurol. Neurosci. 34, 491–505. doi: 10.3233/RNN-150633
Keywords: cerebellar tDCS, stroke, aphasia, spelling therapy, resting state fMRI
Citation: Sebastian R, Saxena S, Tsapkini K, Faria AV, Long C, Wright A, Davis C, Tippett DC, Mourdoukoutas AP, Bikson M, Celnik P and Hillis AE (2017) Cerebellar tDCS: A Novel Approach to Augment Language Treatment Post-stroke. Front. Hum. Neurosci. 10:695. doi: 10.3389/fnhum.2016.00695
Received: 16 August 2016; Accepted: 29 December 2016;
Published: 12 January 2017.
Edited by:
David Copland, University of Queensland, AustraliaReviewed by:
Giancarlo Zito, Fatebenefratelli Hospital, ItalySvetlana Malyutina, National Research University Higher School of Economics, Russia
Copyright © 2017 Sebastian, Saxena, Tsapkini, Faria, Long, Wright, Davis, Tippett, Mourdoukoutas, Bikson, Celnik and Hillis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Argye E. Hillis, argye@jhmi.edu
 Rajani Sebastian
Rajani Sebastian Sadhvi Saxena
Sadhvi Saxena Kyrana Tsapkini1
Kyrana Tsapkini1  Andreia V. Faria
Andreia V. Faria Charltien Long
Charltien Long Donna C. Tippett
Donna C. Tippett Marom Bikson
Marom Bikson Argye E. Hillis
Argye E. Hillis