The Number of Pulses Needed to Measure Corticospinal Excitability by Navigated Transcranial Magnetic Stimulation: Eyes Open vs. Close Condition
- 1Department of Physiology, Faculty of Medicine, King Saud University, Riyadh, Saudi Arabia
- 2Berenson-Allen Center for Noninvasive Brain Stimulation, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
- 3Department of Physical Medicine and Rehabilitation Medicine, Hallym University Sacred Heart Hospital, Seoul, South Korea
- 4Hallym Institute for Translational Genomics and Bioinformatics, Hallym University College of Medicine, Seoul, South Korea
- 5Department of Physical Medicine and Rehabilitation, National Health Insurance Ilsan Hospital, Seoul, South Korea
- 6Neuromodulation Program, Division of Epilepsy and Clinical Neurophysiology, Department of Neurology and the F.M. Kirby Neurobiology Center, Boston Children’s Hospital, Boston, MA, USA
- 7Department of Radiology and Medical Imaging, College of Medicine, King Saud University, Riyadh, Saudi Arabia
Objective: Motor evoked potentials (MEPs) obtained by transcranial magnetic stimulation (TMS) enable measures of the corticospinal excitability (CSE). However the reliability of TMS-derived CSE measures is suboptimal due to appreciable pulse-to-pulse MEP amplitude variability. We thus calculated how many TMS–derived MEPs will be needed to obtain a reliable CSE measure in awake adult subjects, in the eyes open (EO) and eyes closed (EC) conditions.
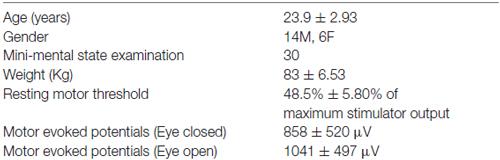
Methods: Twenty healthy adults (70% male) received 40 consecutive navigated TMS pulses (120% resting motor threshold, RMT) in the EO or EC conditions on two separate days in randomized order.
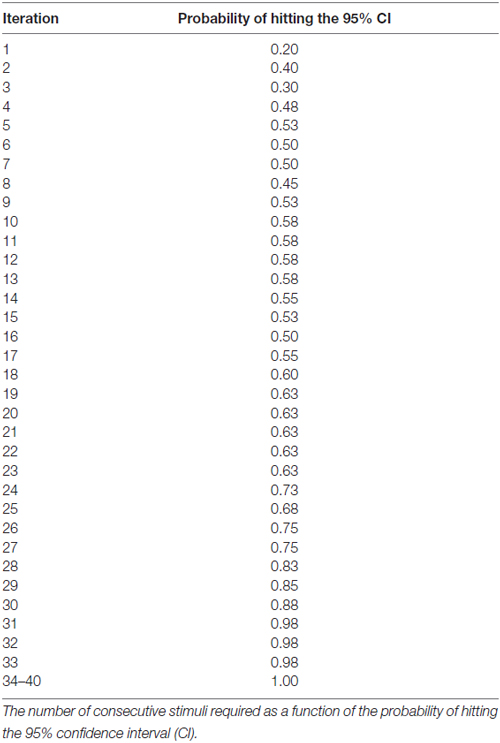
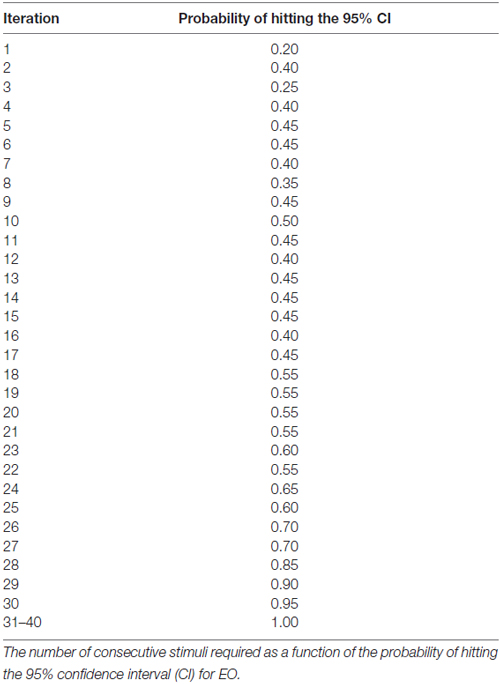
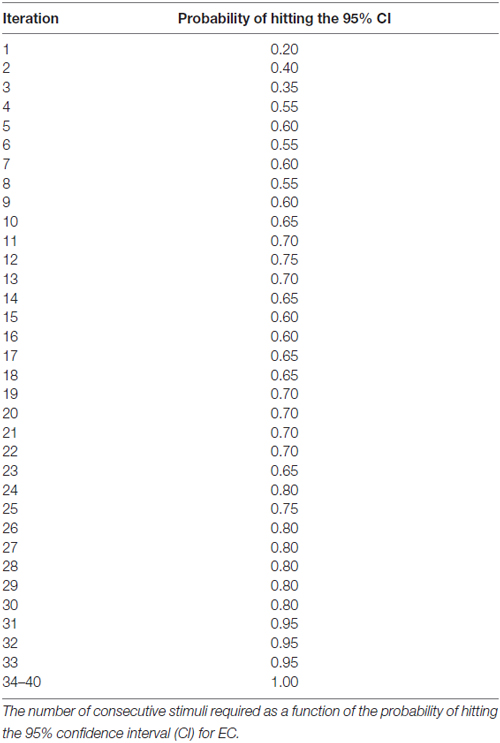
Results: For either the EO or EC condition, the probability that the 95% confidence interval (CI) derived from consecutive MEP amplitude measured included the true CSE, increased when the number of consecutive stimuli increased (EO: p = 0.05; EC: p = 0.001). No significant effect of RMT, Mini-Mental State Examination (MMSE) score, or gender on the CSE estimates was identified. At least 34 consecutive stimuli were required to obtain a most reliable CSE estimate in the EO condition and 31 in the EC condition.
Conclusion: Our findings indicate that >30 consecutive MEPs may be necessary in order to obtain a CSE measure in healthy adults.
Introduction
Transcranial magnetic stimulation (TMS) is a non-invasive, painless and safe method for focal cortical stimulation induced by generating high-intensity magnetic field by passing a brief intracranial electrical current through a magnetic coil (Barker et al., 1985; Kobayashi and Pascual-Leone, 2003). When applied to the primary motor cortex (M1) and coupled with hand surface electromyography (EMG), TMS is often used to measure corticospinal excitability (CSE) as reflected in the amplitude or area under the curve of a hand muscle TMS-induced motor evoked potential (MEP). CSE is generally equated to the average MEP amplitude, resulting from multiple stimuli to the same region at identical intensities (Kiers et al., 1993; Magistris et al., 1998; Rösler et al., 2002; Pitcher et al., 2003; Darling et al., 2006; Mars et al., 2007; Bashir et al., 2011, 2014; Roy Choudhury et al., 2011; Lewis et al., 2014; Cakar et al., 2016). Yet MEP amplitudes vary considerably from pulse to pulse in individual subjects (Magistris et al., 1998; Rösler et al., 2002; Pitcher et al., 2003; Roy Choudhury et al., 2011), and this leads to compromising reliability of CSE metrics. In most protocols, 6–10 MEPs are averaged for the CSE estimate (Magistris et al., 1998; Rösler et al., 2002; Pitcher et al., 2003; Bashir et al., 2011, 2014; Roy Choudhury et al., 2011; Lewis et al., 2014). Yet number of TMS pulses and MEPs that are necessary to obtain a reliable CSE estimate is unknown? We therefore stimulated the motor cortex in healthy volunteers, and measured the number of intrinsic hand muscle MEP amplitudes that would be needed to obtain a reliable CSE estimate.
Since the instantaneous state of cortical motor neuron and cortical interneuron activity likely plays a role in MEP amplitude variability in TMS experiments, we obtained these measures in two conditions: awake with eyes open (EO) and eyes closed (EC), which previously have been demonstrated to modulate cortical and/or spinal motoneural output (Rossini et al., 1991).
In the present experiment, we hypothesized to test whether the number of pulses needed to establish a CSE metric from the first dorsal interosseous (FDI) muscle representation is mediated by factor for which studies may not reliably control: activation of the visual system as defined by the EO and EC condition.
Materials and Methods
Subjects
Twenty right-handed control subjects (age 19–31 years; 70% males) participated in this study (Table 1). The Edinburgh Handedness Inventory (EHI; Oldfield, 1971) was administered to verify that all subjects were right-handed (right-handedness 1.93 ± 0.27). The participants had normal cognitive scores (normal range: 28–30) as indexed by Mini-Mental State Examination (MMSE; Folstein et al., 2001).
Participants underwent two experimental sessions separated by 1 week using a within-subjects, a crossover randomized design, during which they underwent M1 TMS with MEP monitoring in either the EO or EC condition.
Participants had no contraindications to receive TMS and were not taking any medication known to affect motor cortical excitability at the time of the study (Rossi et al., 2009). Furthermore, neurological examination of the subjects revealed no abnormal signs to suggest any underlying neurological or psychological condition. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki and was approved by the local Review Board (King Khalid University Hospital). All participants gave their written informed consent prior to enrollment in the study.
Experimental Set-Up
The stimulation setup consisted of a frameless stereotaxic system for navigation (Visor2 ANT; Netherlands) connected to TMS system (Mag Pro X100 (MagVenture, Denmark)). All subjects had a high-resolution anatomical head magnetic resonance image (MRI) that was used to ensure stimulation accuracy during and across sessions. In each follow-up session co-registration errors to the MRI’s surface landmarks were matched to ≤3 mm.
Surface EMG signals were recorded from FDI muscle in which active electrodes were attached and reference electrodes were placed over the metacarpophalangeal joints. These EMG signals were band-pass filtered (8−500 Hz), amplified, displayed and stored for off-line analysis. The TMS system delivered trigger pulses that synchronized with EMG systems.
During the sessions participants sat on a comfortable chair with their hands in a supine position on their laps, wearing earplugs, and remaining silent to avoid speech-induced modulation of cortical (Bashir et al., 2011, 2014).
TMS Protocol
All subjects had undergone a high-resolution T1-weighted structural MRI scan before TMS session. Imaging data were fed to the navigation software (Visor2, ANT, Netherlands) for automatic 3D brain reconstruction that was used to guide navigation and deliver TMS over M1 (“hot spot”). In each TMS session, the motor cortical output was mapped carefully for the optimal representation of the FDI muscle on left hemisphere (dominant hemisphere) as previously described (Bashir et al., 2011, 2014).
The TMS coil was held tangentially to the skull with the coil handle pointed 45° posterior-laterally to the sagittal plane, which the coil and the induced current on the cortex orientated perpendicularly to the anatomically defined central sulcus and induces a posterior-to-anterior current direction (Bashir et al., 2011, 2014).
Each individual’s resting motor threshold (RMT) was determined as the minimum %maximal stimulation output (MSO) TMS intensity that produced at least 5 out of 10 MEPs ≥50 μV, that was obtained at that location. The site which evoked MEPs with highest amplitude (henceforth, the hot spot) was then marked on each subject’s MRI. Nearly always, the hotspot did not change; sometimes, the coil position needed a minor adjustment in roll or pitch. Forty single stimuli 4–8 s apart were delivered to the hot spot at an intensity of 120% of RMT to determine baseline MEP peak to peak amplitude for the EO and EC conditions.
MEP Data Analysis
To minimize the variability of TMS-induced individual MEPs, they were screened for artifact and for signal indicating voluntary contraction, and excluded (<1%) if the root mean square EMG exceeded 5 mV during the 50-ms period immediately preceding the onset of the TMS pulse as previously described (Cuypers et al., 2014). All subjects data were analyzed for 2 blocks (EO and EC) of 40 consecutive TMS stimuli at 120% RMT. The order of these two conditions was randomized and counterbalanced across participants. For each subject, the average MEPs of EO or EC was calculated for subsets of consecutive stimuli:
The generalized estimating equation (GEE; SAS Institute, Cary, NC, USA) was used to determine the contribution of the EO and EC condition, as well as gender and MMSE score on the . To evaluate the accuracy of the MEP estimates, a 95% confidence interval (CI) was calculated using all 40 stimuli for each subject, per condition as previously described (Cuypers et al., 2014). Based on both the MEPn values and the CI, it is possible to determine whether MEPn is included in CI, yielding a binary variable (0 = not included in the CI, 1 = included in the CI) as previously described by Cuypers et al. (2014). The number of consecutive stimuli required as a function of the probability of hitting the 95% CI.
Results
The CSE estimates did not differ between the EO and EC conditions. The probability that MEPn fell within the CI40 CSE estimate increased with successive TMS pulses (Figure 1). The GEE analysis showed that the estimate of the CI increased when the number of consecutive stimuli increased (p = 0.05 for EO and p = 0.001 for EC).
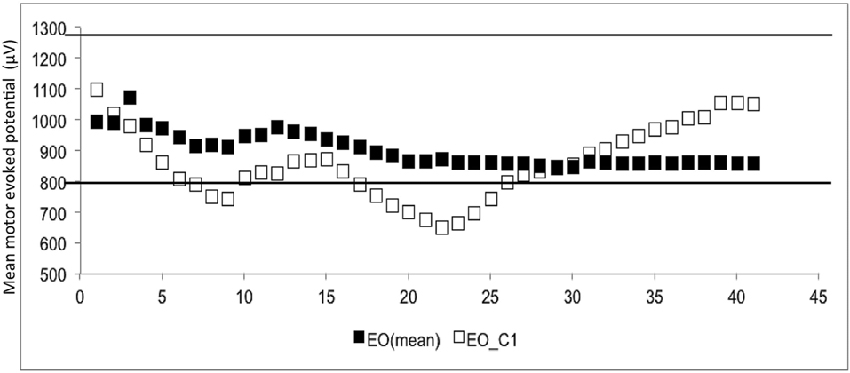
Figure 1. The mean and 1st subject data of eye open (▪ = mean, □ = the data of 1st subject). The Y-axis shows the motor evoked potential (MEP) amplitude (μV), while the number of transcranial magnetic stimulation (TMS) stimuli (n) is shown on the X-axis. White dots represent the individual (raw) MEPs, whereas the black dots represent the average of consecutive MEPs (MEPn). Dashed lines represent the 95% confidence interval (CI), which is based upon all 40 stimuli.
At least 31 consecutive stimuli were required in EO and 34 in EC condition to reach a 100% probability that the average of MEP fell within the 95% CI40 (Figure 2, Tables 2, 3). The GEE analysis showed a not significant effect of RMT, MMSE and gender on CSE estimates (all, p < 0.05). The interaction effect of RMT was not significant. But the age was significant with EC condition. As the age increased, the probability of 95% CI also increased. Female with EO: 29th, with EC: 24th and the male with EO: 31th, with EC condition: 34th (Table 4).
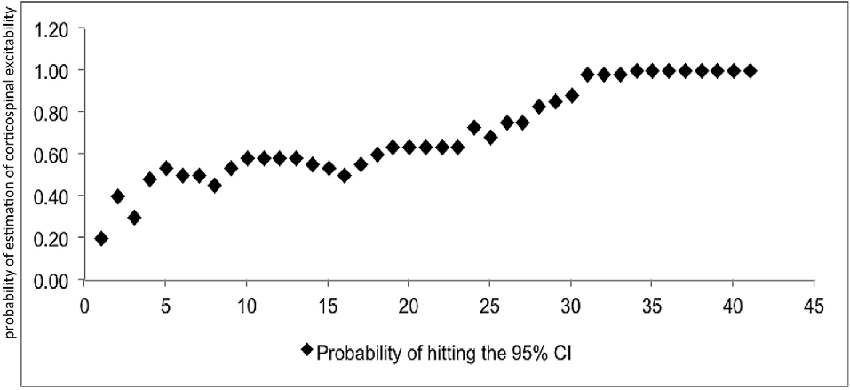
Figure 2. Mean probability in 95% CI. The Y-axis shows probability of inclusion in the 95% confidence interval (CI), while the number of transcranial magnetic stimulation (TMS) stimuli (n) is shown on the X-axis.
Discussion
We demonstrate that >30 stimuli are required to obtain an accurate CSE estimate when MEP measures are obtained from the FDI muscle in healthy adults. Notably, this measure is independent of RMT, and gender. This basic practical information might play a crucial role for obtaining a reliable CSE in the TMS experiments. This study showed the intra-rater reliability, as every part of the data were collected by the same rater.
The interpretation of our results about CSE estimates by number of consecutive stimuli might be required different for other age groups and in disease populations, because our data was obtained in healthy young subjects. Second, all subjects were informed with respect to the experimental procedures due to uncertainty about their first TMS experience; which likely caused changes in attention, and this variable may differ from study to study. Furthermore, experimental set-up (navigated TMS, different coil types and shapes), EMG hardware and signal analysis for noise elimination can affect variability and reliability of the CSE measurements. Last our measures were obtained from the CI muscle (FDI) and a different numerical result may be seen in other muscle groups.
It is paramount that any reliability studies, such as ours, clearly outline the extent to which its results can be generalized. We thus proposed to extrapolate from our results with qualification. Reliability and reproducibility of protocol depends on the sample, the paradigm, the TMS setup and the operator (Beckerman et al., 2001).
The variability related to the TMS method should be similar across the laboratories, when one considers that we used stimulator and recording devices that is commonly available. This is the first TMS reliability study showing spatial stimulation stability within and between sessions using neuronavigation (Schönfeldt-Lecuona et al., 2005). It is noteworthy to emphasize that the lack of controlling in pitch and roll of TMS coil just by using marked scalps or swimming caps can cause measurement error as it allows spatial drift (Julkunen et al., 2009) and reduces the constancy and strength of intracranial electrical currents conveyed to a target (Cincotta et al., 2010).
The design of TMS experiments can be improved by the present study outcome.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
SB was supported by grants from Deanship of Scientific Research (RGP 216) from King Saud University, Saudi Arabia. AR receives support form NIH NIMH R01 100186, NIH NINDS R01NS088583, The Boston Children’s Hospital Translational Research Program (TRP), Autism Speaks, The Assimon Family, Sage Pharmaceuticals, Eisai Pharmaceuticals, Massachusetts Life, Sciences, Neuroelectrics, and Brainsway.
References
Barker, A. T., Jalinous, R., and Freeston, I. L. (1985). Non-invasive magnetic stimulation of human motor cortex. Lancet 1, 1106–1107. doi: 10.1016/s0140-6736(85)92413-4
Bashir, S., Edwards, D., and Pascual-Leone, A. (2011). Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr. 24, 54–64. doi: 10.1007/s10548-010-0165-7
Bashir, S., Perez, J. M., Horvath, J. C., Pena-Gomez, C., Vernet, M., Capia, A., et al. (2014). Differential effects of motor cortical excitability and plasticity in young and old individuals: a Transcranial Magnetic Stimulation (TMS) study. Front. Aging Neurosci. 6:111. doi: 10.3389/fnagi.2014.00111
Beckerman, H., Roebroeck, M. E., Lankhorst, G. J., Becher, J. G., Bezemer, P. D., and Verbeek, A. L. (2001). Smallest real difference, a link between reproducibility and responsiveness. Qual. Life Res. 10, 571–578. doi: 10.1023/A:1013138911638
Cakar, E., Akyuz, G., Durmus, O., Bayman, L., Yagci, I., Karadag-Saygi, E., et al. (2016). The relationships of motor-evoked potentials to hand dexterity, motor function, and spasticity in chronic stroke patients: a transcranial magnetic stimulation study. Acta Neurol. Belg. 116, 481–487. doi: 10.1007/s13760-016-0633-2
Cincotta, M., Giovannelli, F., Borgheresi, A., Balestrieri, F., Toscani, L., Zaccara, G., et al. (2010). Optically tracked neuronavigation increases the stability of hand-held focal coil positioning: evidence from “transcranial” magnetic stimulation-induced electrical field measurements. Brain Stimul. 3, 119–123. doi: 10.1016/j.brs.2010.01.001
Cuypers, K., Thijs, H., and Meesen, R. L. J. (2014). Optimization of the transcranial magnetic stimulation protocol by defining a reliable estimate for corticospinal excitability. PLoS One 9:e86380. doi: 10.1371/journal.pone.0086380
Darling, W. G., Wolf, S. L., and Butler, A. J. (2006). Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp. Brain Res. 174, 376–385. doi: 10.1007/s00221-006-0468-9
Folstein, M. F., Folstein, S. E., McHugh, P. R., and Fanjiang, G. (2001). Mini-Mental State Examination User’s Guide. Odessa, FL: Psychological Assessment Resources.
Julkunen, P., Säisänen, L., Danner, N., Niskanen, E., Hukkanen, T., Mervaala, E., et al. (2009). Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. Neuroimage 44, 790–795. doi: 10.1016/j.neuroimage.2008.09.040
Kiers, L., Cros, D., Chiappa, K. H., and Fang, J. (1993). Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 89, 415–423. doi: 10.1016/0168-5597(93)90115-6
Kobayashi, M., and Pascual-Leone, A. (2003). Transcranial magnetic stimulation in neurology. Lancet Neurol. 2, 145–156. doi: 10.1016/S1474-4422(03)00321-1
Lewis, G. N., Signal, N., and Taylor, D. (2014). Reliability of lower limb motor evoked potentials in stroke and healthy populations: how many responses are needed? Clin. Neurophysiol. 125, 748–754. doi: 10.1016/j.clinph.2013.07.029
Magistris, M. R., Rösler, K. M., Truffert, A., and Myers, J. P. (1998). Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain 121, 437–450. doi: 10.1093/brain/121.3.437
Mars, R. B., Bestmann, S., Rothwell, J. C., and Haggard, P. (2007). Effects of motor preparation and spatial attention on corticospinal excitability in a delayedresponse paradigm. Exp. Brain Res. 182, 125–129. doi: 10.1007/s00221-007-1055-4
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
Pitcher, J. B., Ogston, K. M., and Miles, T. S. (2003). Age and sex differences in human motor cortex input-output characteristics. J. Physiol. 546, 605–613. doi: 10.1113/jphysiol.2002.029454
Rösler, K. M., Petrow, E., Mathis, J., Aranyi, Z., Hess, C. W., and Magistris, M. R. (2002). Effect of discharge desynchronization on the size of motor evoked potentials: an analysis. Clin. Neurophysiol. 113, 1680–1687. doi: 10.1016/s1388-2457(02)00263-8
Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., and Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 120, 2008–2039. doi: 10.1016/j.clinph.2009.08.016
Rossini, P. M., Desiato, M. T., Lavaroni, F., and Caramia, M. D. (1991). Brain excitability and electroencephalographic activation: non-invasive evaluation in healthy humans via transcranial magnetic stimulation. Brain Res. 567, 111–119. doi: 10.1016/0006-8993(91)91442-4
Roy Choudhury, K., Boyle, L., Burke, M., Lombard, W., Ryan, S., and McNamara, B. (2011). Intra subject variation and correlation of motor potentials evoked by transcranial magnetic stimulation. Ir. J. Med. Sci. 180, 873–880. doi: 10.1007/s11845-011-0722-4
Keywords: motor evoked potential, electromyography, muscles, transcranial magnetic stimulation
Citation: Bashir S, Yoo W-K, Kim HS, Lim HS, Rotenberg A and Abu Jamea A (2017) The Number of Pulses Needed to Measure Corticospinal Excitability by Navigated Transcranial Magnetic Stimulation: Eyes Open vs. Close Condition. Front. Hum. Neurosci. 11:121. doi: 10.3389/fnhum.2017.00121
Received: 22 June 2016; Accepted: 02 March 2017;
Published: 21 March 2017.
Edited by:
Mikhail Lebedev, Duke University, USAReviewed by:
Sandro M. Krieg, Technische Universität München, GermanyNeil Gerald Muggleton, National Central University, Taiwan
Selene Schintu, National Institute of Neurological Disorders and Stroke, USA
John Thomas Gale, Emory University School of Medicine, USA
Copyright © 2017 Bashir, Yoo, Kim, Lim, Rotenberg and Abu Jamea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shahid Bashir, sbashir10@gmail.com
 Shahid Bashir
Shahid Bashir Woo-Kyoung Yoo
Woo-Kyoung Yoo Hyoung Seop Kim5
Hyoung Seop Kim5  Alexander Rotenberg
Alexander Rotenberg