Modulating Spatial Processes and Navigation via Transcranial Electrical Stimulation: A Mini Review
- 1Center for Applied Brain and Cognitive Sciences, School of Engineering, Tufts University, Medford, MA, United States
- 2Cognitive Science Team, U.S. Army Natick Soldier Research, Development and Engineering Center, Natick, MA, United States
- 3Department of Psychology, Tufts University, Medford, MA, United States
Transcranial electrical stimulation (tES) uses low intensity current to alter neuronal activity in superficial cortical regions, and has gained popularity as a tool for modulating several aspects of perception and cognition. This mini-review article provides an overview of tES and its potential for modulating spatial processes underlying successful navigation, including spatial attention, spatial perception, mental rotation and visualization. Also considered are recent advances in empirical research and computational modeling elucidating several stable cortical-subcortical networks with dynamic involvement in spatial processing and navigation. Leveraging these advances may prove valuable for using tES, particularly transcranial direct and alternating current stimulation (tDCS/tACS), to indirectly target subcortical brain regions by altering neuronal activity in distant yet functionally connected cortical areas. We propose future research directions to leverage these advances in human neuroscience.
Introduction
Decades of empirical research have demonstrated involvement of diverse lateral and medial brain regions in spatial processing and navigation, including parietal, prefrontal and medial temporal areas (Spiers and Maguire, 2006; Iaria et al., 2007; Whitlock et al., 2008). The reliable involvement of these regions has made them of interest as targets for electrical neuromodulation, attempting to alter the acquisition of spatial knowledge and skills (Brunyé et al., 2014; Wright and Krekelberg, 2014; Oldrati et al., 2018). However, noninvasive electrical neuromodulation is largely limited to superficial cortical layers, limiting the ability to directly target deeper brain structures such as the retrosplenial cortex, posterior cingulate cortex (PCC), or the medial temporal lobes (de Berker et al., 2013). Recent advances in functional connectivity analyses have revealed stable functional networks (Kravitz et al., 2011; Sherrill et al., 2015; Boccia et al., 2017), suggesting that the modulation of superficial brain regions such as the inferior parietal lobule and lateral prefrontal cortex may carry powerful downstream consequences for deeper brain systems involved in spatial processing and real-world navigation. The present mini-review article provides an overview of existing literature using Transcranial electrical stimulation (tES) to modulate several spatial processes underlying navigation behavior, and then proposes that continuing electrical neuromodulation research leverages recent advances in functional connectivity to afford indirect targeting of deep brain areas of critical importance to spatial processing (Keeser et al., 2011; Weber et al., 2014).
Transcranial Electrical Stimulation
tES is a neuroscientific method for inducing transient alterations in neuronal membrane potential by administering electrical current via electrodes positioned on the scalp (Nitsche et al., 2008; Silva et al., 2008; Woods et al., 2016). Evidence from animal models and computational modeling demonstrates that tES can induce a subthreshold depolarization of pyramidal and possibly glial cells (Ruohonen and Karhu, 2012; Molaee-Ardekani et al., 2013; Rahman et al., 2013), and a growing body of literature demonstrates behavioral impacts of tES on a range of perceptual, cognitive, social and emotional tasks (Jacobson et al., 2012; Santiesteban et al., 2012; Berlim et al., 2013; Brunoni and Vanderhasselt, 2014; Dedoncker et al., 2016). While there are several techniques for administering tES, the present mini-review focuses on the most commonly-used method, transcranial direct current stimulation (tDCS), and incorporates some recent innovations in transcranial alternating current stimulation (tACS; Paulus, 2011; Woods et al., 2016).
With tDCS, low intensity direct current is delivered via electrodes arranged on the scalp. Traditionally, tDCS is delivered in a so-called bipolar montage, involving one anodal and one cathodal electrode, typically positioned directly over a cortical target (Paulus, 2011). For instance, one popular bipolar montage involves placing an anodal electrode over the left dorsolateral prefrontal cortex (dlPFC; 10/20 site F3), with the cathode placed on the right supraorbital area (Brunoni and Vanderhasselt, 2014; Dedoncker et al., 2016). This montage is thought to increase neuronal excitability in the left dlPFC, inducing behavioral effects on working memory and executive control (Tremblay et al., 2014). More recently, multi-electrode montages are being used in an effort to administer relatively focalized stimulation to cortical targets, typically involving 4–5 electrodes arranged in an optimal manner (i.e., higher density at target) according to finite element electrical field models of the human head (Datta et al., 2009; Ruffini et al., 2014).
With tACS, low intensity alternating current is delivered via electrodes arranged on the scalp, in a similar manner to tDCS. However, tACS typically administers sine-wave stimulation waveforms that specifically target frequency bands of cortical oscillations (Herrmann et al., 2013). tACS can thus administer current that is frequency-matched to an intrinsic frequency of a cortical area or network. Computational modeling suggests that tACS may thus be capable of promoting specific activity frequencies in brain areas or networks, perhaps via entrainment (Ali et al., 2013), or plasticity alterations (Vossen et al., 2015). If oscillatory brain activity is fundamental to information processing and behavior, then modulating oscillations with tACS should selectively alter such functions; some recent studies have found promise in this technique (Sejnowski and Paulsen, 2006; Herrmann et al., 2013; Neuling et al., 2013; Chander et al., 2016).
tES and Spatial Processing
Whereas many tDCS and tACS studies focus on modulating working memory task performance (e.g., Jaušovec et al., 2014; Martin et al., 2014), an emerging body of empirical research has demonstrated some impacts on the spatial processes underlying navigation behavior, including spatial perception and attention, mental rotation, and spatial visualization. This typology of spatial processes generally follows that of Linn and Petersen (1985). Below we report the results of a literature review examining tDCS and tACS influences on spatial processing and navigation, with papers identified via Google Scholar and PubMed, using the terms tDCS, tACS, spatial cognition, spatial perception, spatial attention, mental rotation, spatial visualization, wayfinding and/or navigation.
Spatial Attention
Spatial attention involves the dynamic and selective prioritization and sustainment of attention toward locations in space (Posner, 1980). A number of distributed brain areas have been implicated in spatial attention, most notably the posterior parietal cortex (PPC) in a primarily right-lateralized frontoparietal visuospatial network (Rafal and Posner, 1987; Corbetta et al., 1995; Constantinidis and Steinmetz, 1996, 2005; Corbetta, 1998; Thiebaut de Schotten et al., 2011). Several studies have targeted the PPC with tDCS, and assessed behavioral impacts on tasks demanding spatial attention. In one study, anodal tDCS administered to the right PPC improved change detection in individuals with lower than ceiling performance, presumably due to enhanced spatial attention toward relevant areas of the task (Tseng et al., 2012). Another study demonstrated that anodal tDCS administered to the right PPC can ameliorate some spatial attention deficits shown by patients with left visuospatial neglect (Sparing et al., 2009). Additional studies demonstrate impacts of tDCS over the PPC on spatial orienting (Bolognini et al., 2010), spatial reorienting (Roy et al., 2015), and tests of lateralized spatial attention bias (de Tommaso et al., 2014). In one tACS study, gamma frequency stimulation over V1 was not shown to modulate spatial attention (though it did alter contrast perception; Laczó et al., 2012). In another, anti-phase gamma frequency stimulation over the left temporal and parietal cortex enhanced visual working memory, suggesting an impact on spatial attention (Tseng et al., 2016). Additional regions implicated in spatial attention, including the superior temporal sulcus, frontal eye fields, anterior cingulate and thalamic nuclei, have not been directly targeted by tDCS or tACS, or have been targeted but not in a manner related to spatial attention.
Spatial Perception
Spatial perception involves perceiving and comprehending spatial information, particularly with regard to the body’s orientation (Loomis and Philbeck, 2008). This includes perceiving spatial relationships among objects, and your position relative to those relationships. Several studies have demonstrated that spatial perception engages areas of the PPC, most notably the right inferior parietal lobule, the middle and inferior frontal gyri, and the superior temporal gyrus (Andersen et al., 1985; Andersen, 1987; Woldorff et al., 1999; Ellison et al., 2004; Straube and Chatterjee, 2010). Very few studies have examined tES influences on spatial perception. In one, tDCS of the PPC altered the perception of object location, with mislocalization biased in the direction contralateral to stimulated hemisphere (Wright and Krekelberg, 2014). Stimulating the right PPC also induces polarity-specific modulation of spatial information reliance during causality inferencing (Straube et al., 2011). Research using tDCS or tACS to target the middle and inferior frontal gyri, and superior temporal gyrus, has not examined influences on spatial perception tasks.
Mental Rotation
Mental rotation involves mental spatial transformations of objects around one or more axes of rotation. The seminal mental rotation task involves comparing two three-dimensional objects, mentally rotating one object to match or mismatch a reference object (Shepard and Metzler, 1971). An abundance of lesioning and functional neuroimaging studies has demonstrated the importance of the parietal cortex in mental rotation, with some studies suggesting a relatively right-lateralized mechanism (Ratcliff, 1979; Deutsch et al., 1988; Cohen et al., 1996; Tagaris et al., 1996, 1997; Richter et al., 1997; Gauthier et al., 2002; Jordan et al., 2002). Given the more general engagement of the prefrontal cortex in working memory and executive control tasks (D’Esposito et al., 1998), perhaps not surprisingly this region has been implicated in maintaining goals and monitoring and updating spatial relations during mental rotation (Cohen et al., 1996). Indeed targeting the dorsolateral prefrontal cortex with tDCS modulates performance on spatial working memory tasks in general (Alencastro et al., 2017), though it does not appear to specifically influence mental rotation performance (Oldrati et al., 2018). To date, no studies have specifically examined tDCS or tACS targeting the parietal cortex and measuring behavioral outcomes on a mental rotation task, however, three related studies are worth mentioning. One study leveraged an implanted array of electrodes over the parietal cortex, demonstrating that high intensity (7–12 mA) superior parietal stimulation dramatically and selectively impaired mental rotation ability (Zacks et al., 2003). Second, online and offline transcranial alternating current stimulation (tACS) was recently found to benefit mental rotation performance, though the electrode montage used did not specifically target parietal areas (Kasten and Herrmann, 2017).
Spatial Visualization
Spatial visualization involves complex, sequential manipulations of spatial information (Linn and Petersen, 1985; Kozhevnikov et al., 2007). It involves spatial attention and perception, and sometimes mental rotation, but extends these processes to multi-step spatial procedures (e.g., Rubik’s cube, paper folding) with multiple analytic strategies that can be adopted to develop a solution. A broad network of functionally connected brain regions is implicated in spatial visualization, particularly executive control and working memory regions (e.g., dlPFC, anterior cingulate), and regions implicated in spatial perception, attention, and mental rotation (e.g., posterior and superior parietal cortices; Sack et al., 2007; Watson and Chatterjee, 2012). Very few studies have examined tDCS or tACS influences on spatial visualization. In one study, tDCS centered over the dlPFC enhanced training of a mental paper folding task; however, this pattern only emerged when tDCS was administered online (rather than offline, before the task; Oldrati et al., 2018).
tES and Navigation
Wayfinding involves deliberate navigation between waypoints in large-scale environments, and is one of the most complex and frequent tasks undertaken by humans. It is often distinguished from the motoric sequences underlying the navigation of well-learned routes, for instance from home to a workplace, in that it also involves developing and using spatial representations to support movement (Hartley et al., 2003). Successful wayfinding generally involves recognizing places, learning sequences, identifying decision points and making decisions and behavioral responses, developing associations among environmental features, transforming perspectives, and constantly relating the directly perceived environment with environmental knowledge and goal representations (Allen, 1999; Klippel, 2003; Montello, 2005; Wiener et al., 2009; Dudchenko, 2010).
Elements of spatial attention, perception, mental rotation, visualization and working memory are critical for supporting wayfinding, and people differ dramatically in their ability to find their way through complex environments (Hegarty and Waller, 2005). The diverse engagement of cognitive processes in wayfinding is reflected in the diversity of brain regions implicated in supporting these processes (Maguire et al., 1999; Burgess et al., 2002; Shelton and Gabrieli, 2002; Schinazi and Epstein, 2010), and the diversity of taxonomies devoted specifically to understanding the processes engaged during wayfinding (Siegel and White, 1975; Wiener et al., 2009; Chrastil, 2013). Only one study has examined tES influences on wayfinding (Brunyé et al., 2014). In that study, the authors targeted the right medial temporal lobe with a multielectrode tDCS montage and demonstrated no main effect of tDCS on virtual wayfinding performance. Targeting deep brain structures in medial temporal areas may not be feasible with tES; instead, researchers may find value in indirectly targeting these areas by stimulating nodes in functional neural networks.
Functional Networks in Navigation
In the above typology of spatial processes, we focused primarily on focal brain regions underlying spatial performance, though stable functional networks have also been identified supporting several aspects of spatial processing. Byrne et al. (2007) described a dynamic neural model to characterize interactions among brain regions implicated in human navigation. The model distinguishes between an egocentric system (posterior parietal), allocentric system (medial temporal), and transformational (retrosplenial) system. Functional connectivity analyses have identified at least three functional pathways involved in communicating among these systems (Kravitz et al., 2011):
1. The parieto-prefrontal pathway connects lateral and ventral intraparietal, and middle and medial superior temporal lobe areas, to prefrontal regions, and is involved in spatial attention including the initiation and control of eye movements, and top-down executive control of visuospatial working memory processing (Xu and Chun, 2006).
2. The parieto-premotor pathway consists of two parallel projections, one connecting the ventral intraparietal and dorsal premotor cortices, and the other connecting the medial intraparietal and ventral premotor cortices, both engaged in initiating and controlling several visually-directed actions (e.g., reaching for and grasping objects) using parietal object representations (Blangero et al., 2009; Reichenbach et al., 2014).
3. Finally, a parieto-medial temporal pathway connects the caudal inferior parietal lobe (cIPL) and a range of areas including retrosplenial cortex (RSC) and PCC, and secondarily to the hippocampus and parahippocampus (Rushworth et al., 2006). Inferior parietal areas have been implicated in a range of navigation-relevant functions, including representing distant space in world- and object-centered frame of reference, egocentric heading direction, and egocentric distance (Brotchie et al., 1995; Crowe et al., 2005; Chafee et al., 2007; Harvey et al., 2012), suggesting importance for real-world navigation. Concordant with intermediary roles between posterior parietal and medial temporal regions, the PCC and RSC have been implicated in translating between egocentric (parietal) and allocentric (medial temporal) representations of space, and in relating optic flow to heading direction and movement toward goals (Vogt et al., 1992; Burgess, 2008; Epstein, 2008; Sherrill et al., 2015; Boccia et al., 2016; Wiener et al., 2016).
Research continues to better define the anatomical and functional links between brain regions implicated in spatial processing and navigation. One outcome of this research is affording better understandings of how tES may prove tractable for modulating brain circuits engaged in spatial processing.
Leveraging Functional Connectivity with tES
Few studies have examined the influence of modulating superficial tES targets on more distributed neural networks. In two such studies, tDCS of the left dlPFC altered resting-state connectivity in several functional networks, including the default mode network, frontal-parietal network, and self-referential network (Keeser et al., 2011; Peña-Gómez et al., 2012). In the spatial processing domain, two related studies administered tDCS over the parietal cortex and found altered functional connectivity between this region and the prefrontal cortices and several subcortical regions, both during a virtual navigation task (Hampstead et al., 2014) and during resting state (Krishnamurthy et al., 2015). No reliable impacts on behavior were found, however, in this research the stimulated electrode site (Pz) sits over bilateral superior parietal regions rather than a lateral inferior parietal lobule.
Continuing research may find value in specifically targeting the parieto-medial temporal and parieto-prefrontal pathways. High fidelity head models that predict current propagation can be used to maximize current density at relatively superficial cortical targets, such as the right inferior parietal lobule. For instance, using the HD Targets finite element model developed by Soterix Medical Incorporation (New York, NY, USA), Figure 1 demonstrates predicted current density with a multielectrode array targeting the cIPL (i.e., angular gyrus). This montage uses two anodes at locations P6 (1.0 mA) and P4 (1.0 mA), and three cathodes at locations CP2, CP4, CP6, and PO4 (0.5 mA each). With 2.0 mA total current, maximum electrical field intensity at target is approximately 0.22 V/m. Given functional connectivity between the cIPL and the RSC, PCC, hippocampus and parahippocampus, targeted neuromodulation of the relatively lateral cIPL region might be expected to modulate several aspects of spatial processing with implications for large-scale navigation performance, for instance egocentric and allocentric perspective switching and integration, and maintaining orientation relative to goal locations.
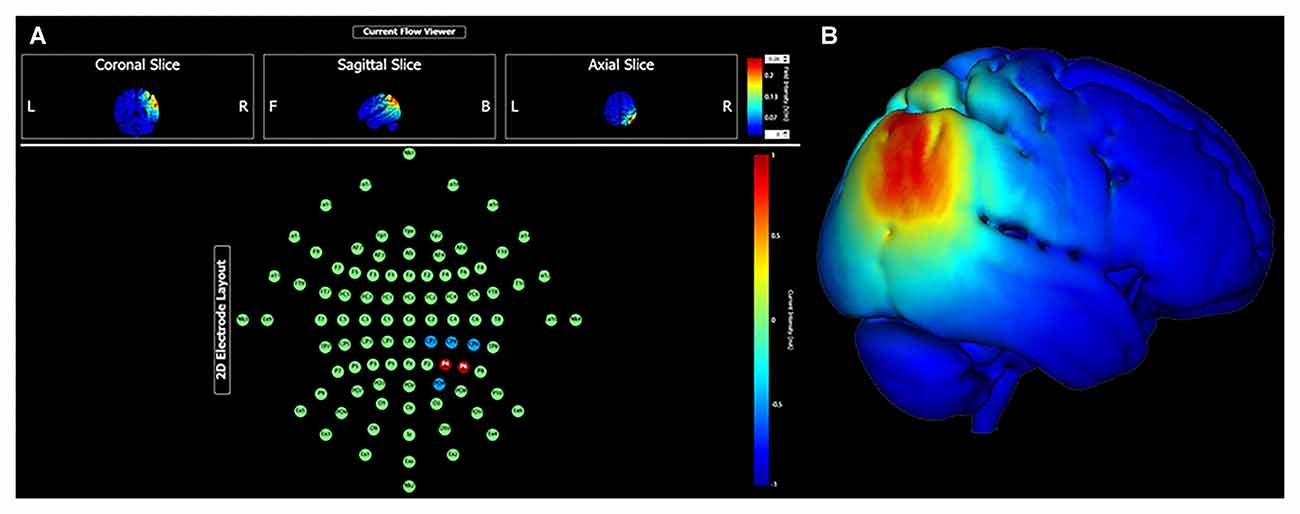
Figure 1. Transcranial direct current stimulation (tDCS) targeting the right caudal inferior parietal lobule (angular gyrus) with 2.0 mA current intensity. Panel (A) shows electrode montage and current flow in coronal, sagittal and axial views. Panel (B) shows electrical field intensity overlaid onto a standard MNI head model (MNI 152).
Behavioral outcomes related to parieto-medial temporal pathway might be dissociated with outcomes of targeting the parieto-prefrontal pathway. Specifically, targeting lateral and ventral intraparietal areas might be expected to impact visuospatial spatial working memory performance, whereas targeting the cIPL may not. These types of dissociations between stimulation locations, stimulation conditions (e.g., tDCS, tACS), and behavioral outcomes can help elucidate behavioral influences of each pathway, and reveal methods for altering spatial performance. Furthermore, as research reveals the oscillatory dynamics of the parieto-medial temporal pathway, frequency-specific tACS might also prove valuable for modulating network resonance and behavioral outcomes (Ali et al., 2013; Marshall and Binder, 2013).
Conclusion
People differ dramatically in spatial abilities (Hegarty and Waller, 2005), and identifying reliable methods for enhancing or accelerating spatial skills education and training may prove valuable in both healthy and clinical populations. For instance, body- and world-centered spatial visualization skills are fundamental to many work-related domains, especially science, technology, engineering and mathematics (STEM) disciplines (Sorby, 1999; Titus and Horsman, 2009; Taylor and Hutton, 2013; Uttal et al., 2013; Burte et al., 2017). Future research might find value in using tES to selectively upregulate networks engaged in successful spatial thinking. This research will be enabled by at least three specific research areas. First, better defining functional connectivity between cortical and subcortical brain regions during spatial processing and navigation, and how these networks might vary in structure and function across individuals. Second, identifying how tES modulates cortical and network activity, and how these dynamics might vary over time and across individuals (Krause et al., 2013). Third, advances in finite element modeling that include customized (i.e., individualized) cortical targets will afford specificity and reliability of stimulation protocols (Radman et al., 2009), and possibly enhance real-world behavioral outcomes.
Author Contributions
TTB conceived the review and prepared the manuscript.
Funding
This review was supported by applied research funding from the U.S. Army Natick Soldier Research, Development and Engineering Center (NSRDEC; #18-109).
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alencastro, A. S., Borigato, E. M., Rios, I. B., Santos, M. O., Melo, R. C. A., Torres, R. E., et al. (2017). Impairment of the visuo-spatial sketch pad by left prefrontal transcranial direct current stimulation. Brain Stimul. 10, 336–337. doi: 10.1016/j.brs.2016.12.010
Ali, M. M., Sellers, K. K., and Fröhlich, F. (2013). Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J. Neurosci. 33, 11262–11275. doi: 10.1523/jneurosci.5867-12.2013
Allen, G. L. (1999). “Spatial abilities, cognitive maps and wayfinding: bases for individual differences in spatial cognition and behavior,” in Wayfinding Behavior: Cognitive Mapping and Other Spatial Processes, ed. R. G. Golledge (Baltimore, MD: The Johns Hopkins University Press), 46–80.
Andersen, R. A. (1987). “Inferior parietal lobule function in spatial perception and visuomotor integration,” in Handbook of Physiology Section 1: The Nervous System. Volume V: Higher Functions of the Brain, Part 2, eds V. B. Mountcastle, F. Plum and S. R. Geiger (Bethesda, MD: American Physiology Association), 483–518.
Andersen, R. A., Essick, G. K., and Siegel, R. M. (1985). Encoding of spatial location by posterior parietal neurons. Science 230, 456–458. doi: 10.1126/science.4048942
Berlim, M. T., Van den Eynde, F., and Daskalakis, Z. J. (2013). Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J. Psychiatr. Res. 47, 1–7. doi: 10.1016/j.jpsychires.2012.09.025
Blangero, A., Menz, M. M., McNamara, A., and Binkofski, F. (2009). Parietal modules for reaching. Neuropsychologia 47, 1500–1507. doi: 10.1016/j.neuropsychologia.2008.11.030
Boccia, M., Guariglia, C., Sabatini, U., and Nemmi, F. (2016). Navigating toward a novel environment from a route or survey perspective: neural correlates and context-dependent connectivity. Brain Struct. Funct. 221, 2005–2021. doi: 10.1007/s00429-015-1021-z
Boccia, M., Sulpizio, V., Nemmi, F., Guariglia, C., and Galati, G. (2017). Direct and indirect parieto-medial temporal pathways for spatial navigation in humans: evidence from resting-state functional connectivity. Brain Struct. Funct. 222, 1945–1957. doi: 10.1007/s00429-016-1318-6
Bolognini, N., Olgiati, E., Rossetti, A., and Maravita, A. (2010). Enhancing multisensory spatial orienting by brain polarization of the parietal cortex. Eur. J. Neurosci. 31, 1800–1806. doi: 10.1111/j.1460-9568.2010.07211.x
Brotchie, P. R., Andersen, R. A., Snyder, L. H., and Goodman, S. J. (1995). Head position signals used by parietal neurons to encode locations of visual stimuli. Nature 375, 232–235. doi: 10.1038/375232a0
Brunoni, A. R., and Vanderhasselt, M.-A. (2014). Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn. 86, 1–9. doi: 10.1016/j.bandc.2014.01.008
Brunyé, T. T., Holmes, A., Cantelon, J., Eddy, M. D., Gardony, A. L., Mahoney, C. R., et al. (2014). Direct current brain stimulation enhances navigation efficiency in individuals with low sense of direction. Neuroreport 25, 1175–1179. doi: 10.1097/wnr.0000000000000214
Burgess, N. (2008). Spatial cognition and the brain. Ann. N Y Acad. Sci. 1124, 77–97. doi: 10.1196/annals.1440.002
Burgess, N., Maguire, E. A., and O’Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron 35, 625–641. doi: 10.1016/s0896-6273(02)00830-9
Burte, H., Gardony, A. L., Hutton, A., and Taylor, H. A. (2017). Think3d!: improving mathematics learning through embodied spatial training. Cogn. Res. Princ. Implic. 2:13. doi: 10.1186/s41235-017-0052-9
Byrne, P., Becker, S., and Burgess, N. (2007). Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev. 114, 340–375. doi: 10.1037/0033-295x.114.2.340
Chafee, M. V., Averbeck, B. B., and Crowe, D. A. (2007). Representing spatial relationships in posterior parietal cortex: single neurons code object-referenced position. Cereb. Cortex 17, 2914–2932. doi: 10.1093/cercor/bhm017
Chander, B. S., Witkowski, M., Braun, C., Robinson, S. E., Born, J., Cohen, L. G., et al. (2016). tACS phase locking of frontal midline theta oscillations disrupts working memory performance. Front. Cell. Neurosci. 10:120. doi: 10.3389/fncel.2016.00120
Chrastil, E. R. (2013). Neural evidence supports a novel framework for spatial navigation. Psychon. Bull. Rev. 20, 208–227. doi: 10.3758/s13423-012-0351-6
Cohen, M. S., Kosslyn, S. M., Breiter, H. C., DiGirolamo, G. J., Thompson, W. L., Anderson, A. K., et al. (1996). Changes in cortical activity during mental rotation A mapping study using functional MRI. Brain 119, 89–100. doi: 10.1093/brain/119.1.89
Constantinidis, C., and Steinmetz, M. A. (1996). Neuronal activity in posterior parietal area 7a during the delay periods of a spatial memory task. J. Neurophysiol. 76, 1352–1355.
Constantinidis, C., and Steinmetz, M. A. (2005). Posterior parietal cortex automatically encodes the location of salient stimuli. J. Neurosci. 25, 233–238. doi: 10.1523/jneurosci.3379-04.2005
Corbetta, M. (1998). Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc. Natl. Acad. Sci. U S A 95, 831–838. doi: 10.1073/pnas.95.3.831
Corbetta, M., Shulman, G. L., Miezin, F. M., and Petersen, S. E. (1995). Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science 270, 802–805. doi: 10.1126/science.270.5237.802
Crowe, D. A., Averbeck, B. B., Chafee, M. V., and Georgopoulos, A. P. (2005). Dynamics of parietal neural activity during spatial cognitive processing. Neuron 47, 885–891. doi: 10.1016/j.neuron.2005.08.005
Datta, A., Bansal, V., Diaz, J., Patel, J., Reato, D., and Bikson, M. (2009). Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2, 201.e1–207.e1. doi: 10.1016/j.brs.2009.03.005
de Berker, A. O., Bikson, M., and Bestmann, S. (2013). Predicting the behavioral impact of transcranial direct current stimulation: issues and limitations. Front. Hum. Neurosci. 7:613. doi: 10.3389/fnhum.2013.00613
Dedoncker, J., Brunoni, A. R., Baeken, C., and Vanderhasselt, M.-A. (2016). A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: influence of stimulation parameters. Brain Stimul. 9, 501–517. doi: 10.1016/j.brs.2016.04.006
D’Esposito, M., Aguirre, G. K., Zarahn, E., Ballard, D., Shin, R. K., and Lease, J. (1998). Functional MRI studies of spatial and nonspatial working memory. Cogn. Brain Res. 7, 1–13. doi: 10.1016/s0926-6410(98)00004-4
de Tommaso, M., Invitto, S., Ricci, K., Lucchese, V., Delussi, M., Quattromini, P., et al. (2014). Effects of anodal TDCS stimulation of left parietal cortex on visual spatial attention tasks in men and women across menstrual cycle. Neurosci. Lett. 574, 21–25. doi: 10.1016/j.neulet.2014.05.014
Deutsch, G., Bourbon, W. T., Papanicolaou, A. C., and Eisenberg, H. M. (1988). Visuospatial tasks compared via activation of regional cerebral blood flow. Neuropsychologia 26, 445–452. doi: 10.1016/0028-3932(88)90097-8
Dudchenko, P. A. (2010). Why People Get Lost: The Psychology and Neuroscience of Spatial Cognition. Oxford: Oxford University Press.
Ellison, A., Schindler, I., Pattison, L. L., and Milner, A. D. (2004). An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain 127, 2307–2315. doi: 10.1093/brain/awh244
Epstein, R. A. (2008). Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn. Sci. 12, 388–396. doi: 10.1016/j.tics.2008.07.004
Gauthier, I., Hayward, W. G., Tarr, M. J., Anderson, A. W., Skudlarski, P., and Gore, J. C. (2002). BOLD activity during mental rotation and viewpoint-dependent object recognition. Neuron 34, 161–171. doi: 10.1016/s0896-6273(02)00622-0
Hampstead, B. M., Brown, G. S., and Hartley, J. F. (2014). Transcranial direct current stimulation modulates activation and effective connectivity during spatial navigation. Brain Stimul. 7, 314–324. doi: 10.1016/j.brs.2013.12.006
Hartley, T., Maguire, E. A., Spiers, H. J., and Burgess, N. (2003). The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron 37, 877–888. doi: 10.1016/S0896-6273(03)00095-3
Harvey, C. D., Coen, P., and Tank, D. W. (2012). Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484, 62–68. doi: 10.1038/nature10918
Hegarty, M., and Waller, D. A. (2005). “Individual differences in spatial abilities,” in The Cambridge Handbook of Visuospatial Thinking, eds P. Shah and A. Miyake (New York, NY: Cambridge University Press), 121–169.
Herrmann, C. S., Rach, S., Neuling, T., and Strüber, D. (2013). Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci. 7:279. doi: 10.3389/fnhum.2013.00279
Iaria, G., Chen, J. K., Guariglia, C., Ptito, A., and Petrides, M. (2007). Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. Eur. J. Neurosci. 25, 890–899. doi: 10.1111/j.1460-9568.2007.05371.x
Jacobson, L., Koslowsky, M., and Lavidor, M. (2012). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp. Brain Res. 216, 1–10. doi: 10.1007/s00221-011-2891-9
Jaušovec, N., Jaušovec, K., and Pahor, A. (2014). The influence of theta transcranial alternating current stimulation (tACS) on working memory storage and processing functions. Acta Psychol. 146, 1–6. doi: 10.1016/j.actpsy.2013.11.011
Jordan, K., Wüstenberg, T., Heinze, H. J., Peters, M., and Jäncke, L. (2002). Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia 40, 2397–2408. doi: 10.1016/s0028-3932(02)00076-3
Kasten, F. H., and Herrmann, C. S. (2017). Transcranial alternating current stimulation (tACS) enhances mental rotation performance during and after stimulation. Front. Hum. Neurosci. 11:2. doi: 10.3389/fnhum.2017.00002
Keeser, D., Meindl, T., Bor, J., Palm, U., Pogarell, O., Mulert, C., et al. (2011). Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 31, 15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011
Klippel, A. (2003). “Wayfinding choremes,” in Spatial Information Theory: Foundations of Geographic Information Science. Conference on Spatial Information Theory (COSIT) 2003, eds W. Kuhn, M. F. Worboys and S. Timpf (Berlin: Springer), 320–334.
Kozhevnikov, M., Motes, M. A., and Hegarty, M. (2007). Spatial visualization in physics problem solving. Cogn. Sci. 31, 549–579. doi: 10.1080/15326900701399897
Krause, B., Márquez-Ruiz, J., and Cohen Kadosh, R. (2013). The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Front. Hum. Neurosci. 7:602. doi: 10.3389/fnhum.2013.00602
Kravitz, D., Saleem, K., Baker, C., and Mishkin, M. (2011). A new neural framework for visuospatial processing. J. Vis. 11:319. doi: 10.1167/11.11.923
Krishnamurthy, V., Gopinath, K., Brown, G. S., and Hampstead, B. M. (2015). Resting-state fMRI reveals enhanced functional connectivity in spatial navigation networks after transcranial direct current stimulation. Neurosci. Lett. 604, 80–85. doi: 10.1016/j.neulet.2015.07.042
Laczó, B., Antal, A., Niebergall, R., Treue, S., and Paulus, W. (2012). Transcranial alternating stimulation in a high γ frequency range applied over V1 improves contrast perception but does not modulate spatial attention. Brain Stimul. 5, 484–491. doi: 10.1016/j.brs.2011.08.008
Linn, M. C., and Petersen, A. C. (1985). Emergence and characterization of sex differences in spatial ability: a meta-analysis. Child Dev. 56, 1479–1498. doi: 10.1111/j.1467-8624.1985.tb00213.x
Loomis, J. M., and Philbeck, J. W. (2008). “Measuring perception with spatial updating and action,” in Embodiment, Ego-Space, and Action, eds R. L. Klatzky, M. Behrmann and B. MacWhinney (Mahwah, NJ: Erlbaum), 1–43.
Maguire, E. A., Burgess, N., and O’Keefe, J. (1999). Human spatial navigation: cognitive maps, sexual dimorphism, and neural substrates. Curr. Opin. Neurobiol. 9, 171–177. doi: 10.1016/s0959-4388(99)80023-3
Marshall, L., and Binder, S. (2013). Contribution of transcranial oscillatory stimulation to research on neural networks: an emphasis on hippocampo-neocortical rhythms. Front. Hum. Neurosci. 7:614. doi: 10.3389/fnhum.2013.00614
Martin, D. M., Liu, R., Alonzo, A., Green, M., and Loo, C. K. (2014). Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: effect of timing of stimulation. Exp. Brain Res. 232, 3345–3351. doi: 10.1007/s00221-014-4022-x
Molaee-Ardekani, B., Márquez-Ruiz, J., Merlet, I., Leal-Campanario, R., Gruart, A., Sánchez-Campusano, R., et al. (2013). Effects of transcranial direct current stimulation (tDCS) on cortical activity: a computational modeling study. Brain Stimul. 6, 25–39. doi: 10.1016/j.brs.2011.12.006
Montello, D. R. (2005). “Navigation,” in The Cambridge Handbook of Visuospatial Thinking, eds P. Shah and A. Miyake (New York, NY: Cambridge University Press), 257–294.
Neuling, T., Rach, S., and Herrmann, C. S. (2013). Orchestrating neuronal networks: sustained after-effects of transcranial alternating current stimulation depend upon brain states. Front. Hum. Neurosci. 7:161. doi: 10.3389/fnhum.2013.00161
Nitsche, M. A., Cohen, L. G., Wassermann, E. M., Priori, A., Lang, N., Antal, A., et al. (2008). Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 1, 206–223. doi: 10.1016/j.brs.2008.06.004
Oldrati, V., Colombo, B., and Antonietti, A. (2018). Combination of a short cognitive training and tDCS to enhance visuospatial skills: a comparison between online and offline neuromodulation. Brain Res. 1678, 32–39. doi: 10.1016/j.brainres.2017.10.002
Paulus, W. (2011). Transcranial electrical stimulation (tES’tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 21, 602–617. doi: 10.1080/09602011.2011.557292
Peña-Gómez, C., Sala-Lonch, R., Junqué, C., Clemente, I. C., Vidal, D., Bargalló, N., et al. (2012). Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimul. 5, 252–263. doi: 10.1016/j.brs.2011.08.006
Posner, M. I. (1980). Orienting of attention. Q. J. Exp. Psychol. 32, 3–25. doi: 10.1080/00335558008248231
Radman, T., Ramos, R. L., Brumberg, J. C., and Bikson, M. (2009). Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2, 215.e3–228.e3. doi: 10.1016/j.brs.2009.03.007
Rafal, R. D., and Posner, M. I. (1987). Deficits in human visual spatial attention following thalamic lesions. Proc. Natl. Acad. Sci. U S A 84, 7349–7353. doi: 10.1073/pnas.84.20.7349
Rahman, A., Reato, D., Arlotti, M., Gasca, F., Datta, A., Parra, L. C., et al. (2013). Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J. Physiol. 591, 2563–2578. doi: 10.1113/jphysiol.2012.247171
Ratcliff, G. (1979). Spatial thought, mental rotation and the right cerebral hemisphere. Neuropsychologia 17, 49–54. doi: 10.1016/0028-3932(79)90021-6
Reichenbach, A., Thielscher, A., Peer, A., Bülthoff, H. H., and Bresciani, J. P. (2014). A key region in the human parietal cortex for processing proprioceptive hand feedback during reaching movements. Neuroimage 84, 615–625. doi: 10.1016/j.neuroimage.2013.09.024
Richter, W., Ugurbil, K., Georgopoulos, A., and Kim, S.-G. (1997). Time-resolved fMRI of mental rotation. Neuroreport 8, 3697–3702. doi: 10.1097/00001756-199712010-00008
Roy, L. B., Sparing, R., Fink, G. R., and Hesse, M. D. (2015). Modulation of attention functions by anodal tDCS on right PPC. Neuropsychologia 74, 96–107. doi: 10.1016/j.neuropsychologia.2015.02.028
Ruffini, G., Fox, M. D., Ripolles, O., Miranda, P. C., and Pascual-Leone, A. (2014). Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage 89, 216–225. doi: 10.1016/j.neuroimage.2013.12.002
Ruohonen, J., and Karhu, J. (2012). TDCS possibly stimulates glial cells. Clin. Neurophysiol. 123, 2006–2009. doi: 10.1016/j.clinph.2012.02.082
Rushworth, M. F. S., Behrens, T. E. J., and Johansen-Berg, H. (2006). Connection patterns distinguish 3 regions of human parietal cortex. Cereb. Cortex 16, 1418–1430. doi: 10.1093/cercor/bhj079
Sack, A. T., Kohler, A., Bestmann, S., Linden, D. E. J., Dechent, P., Goebel, R., et al. (2007). Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous fMRI, TMS, and behavioral studies. Cereb. Cortex 17, 2841–2852. doi: 10.1093/cercor/bhm013
Santiesteban, I., Banissy, M. J., Catmur, C., and Bird, G. (2012). Enhancing social ability by stimulating right temporoparietal junction. Curr. Biol. 22, 2274–2277. doi: 10.1016/j.cub.2012.10.018
Schinazi, V. R., and Epstein, R. A. (2010). Neural correlates of real-world route learning. Neuroimage 53, 725–735. doi: 10.1016/j.neuroimage.2010.06.065
Sejnowski, T. J., and Paulsen, O. (2006). Network oscillations: emerging computational principles. J. Neurosci. 26, 1673–1676. doi: 10.1523/JNEUROSCI.3737-05d.2006
Shelton, A. L., and Gabrieli, J. D. E. (2002). Neural correlates of encoding space from route and survey perspectives. J. Neurosci. 22, 2711–2717.
Shepard, R. N., and Metzler, J. (1971). Mental rotation of three-dimensional objects. Science 171, 701–703. doi: 10.1126/science.171.3972.701
Sherrill, K. R., Chrastil, E. R., Ross, R. S., Erdem, U. M., Hasselmo, M. E., and Stern, C. E. (2015). Functional connections between optic flow areas and navigationally responsive brain regions during goal-directed navigation. Neuroimage 118, 386–396. doi: 10.1016/j.neuroimage.2015.06.009
Siegel, A. W., and White, S. H. (1975). The development of spatial representations of large-scale environments. Adv. Child Dev. Behav. 10, 9–55. doi: 10.1016/s0065-2407(08)60007-5
Silva, S., Basser, P. J., and Miranda, P. C. (2008). Elucidating the mechanisms and loci of neuronal excitation by transcranial magnetic stimulation using a finite element model of a cortical sulcus. Clin. Neurophysiol. 119, 2405–2413. doi: 10.1016/j.clinph.2008.07.248
Sparing, R., Thimm, M., Hesse, M. D., Küst, J., Karbe, H., and Fink, G. R. (2009). Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain 132, 3011–3020. doi: 10.1093/brain/awp154
Spiers, H. J., and Maguire, E. A. (2006). Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage 31, 1826–1840. doi: 10.1016/j.neuroimage.2006.01.037
Straube, B., and Chatterjee, A. (2010). Space and time in perceptual causality. Front. Hum. Neurosci. 4:28. doi: 10.3389/fnhum.2010.00028
Straube, B., Wolk, D., and Chatterjee, A. (2011). The role of the right parietal lobe in the perception of causality: a tDCS study. Exp. Brain Res. 215, 315–325. doi: 10.1007/s00221-011-2899-1
Tagaris, G. A., Kim, S.-G., Strupp, J. P., Andersen, P., Uğurbil, K., and Georgopoulos, A. P. (1996). Quantitative relations between parietal activation and performance in mental rotation. Neuroreport 7, 773–776. doi: 10.1097/00001756-199602290-00022
Tagaris, G. A., Kim, S.-G., Strupp, J. P., Andersen, P., Uğurbil, K., and Georgopoulos, A. P. (1997). Mental rotation studied by functional magnetic resonance imaging at high field (4 tesla): performance and cortical activation. J. Cogn. Neurosci. 9, 419–432. doi: 10.1162/jocn.1997.9.4.419
Taylor, H. A., and Hutton, A. (2013). Think3d!: training spatial thinking fundamental to stem education. Cogn. Instr. 31, 434–455. doi: 10.1080/07370008.2013.828727
Thiebaut de Schotten, M. T., Dell’Acqua, F., Forkel, S. J., Simmons, A., Vergani, F., Murphy, D. G. M., et al. (2011). A lateralized brain network for visuospatial attention. Nat. Neurosci. 14, 1245–1246. doi: 10.1038/nn.2905
Titus, S., and Horsman, E. (2009). Characterizing and improving spatial visualization skills. J. Geosci. Edu. 57, 242–254. doi: 10.5408/1.3559671
Tremblay, S., Lepage, J. F., Latulipe-Loiselle, A., Fregni, F., Pascual-Leone, A., and Théoret, H. (2014). The uncertain outcome of prefrontal tDCS. Brain Stimul. 7, 773–783. doi: 10.1016/j.brs.2014.10.003
Tseng, P., Chang, Y. T., Chang, C. F., Liang, W. K., and Juan, C. H. (2016). The critical role of phase difference in γ oscillation within the temporoparietal network for binding visual working memory. Sci. Rep. 6:32138. doi: 10.1038/srep32138
Tseng, P., Hsu, T.-Y., Chang, C.-F., Tzeng, O. J. L., Hung, D. L., Muggleton, N. G., et al. (2012). Unleashing potential: transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low-performing individuals. J. Neurosci. 32, 10554–10561. doi: 10.1523/JNEUROSCI.0362-12.2012
Uttal, D. H., Meadow, N. G., Tipton, E., Hand, L. L., Alden, A. R., Warren, C., et al. (2013). The malleability of spatial skills: a meta-analysis of training studies. Psychol. Bull. 139, 352–402. doi: 10.1037/a0028446
Vogt, B. A., Finch, D. M., and Olson, C. R. (1992). Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb. Cortex 2, 435–443. doi: 10.1093/cercor/2.6.435-a
Vossen, A., Gross, J., and Thut, G. (2015). α power increase after transcranial alternating current stimulation at α frequency (a-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 8, 499–508. doi: 10.1016/j.brs.2014.12.004
Watson, C. E., and Chatterjee, A. (2012). A bilateral frontoparietal network underlies visuospatial analogical reasoning. Neuroimage 59, 2831–2838. doi: 10.1016/j.neuroimage.2011.09.030
Weber, M. J., Messing, S. B., Rao, H., Detre, J. A., and Thompson-Schill, S. L. (2014). Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: a tDCS-fMRI study. Hum. Brain Mapp. 35, 3673–3686. doi: 10.1002/hbm.22429
Whitlock, J. R., Sutherland, R. J., Witter, M. P., Moser, M.-B., and Moser, E. I. (2008). Navigating from hippocampus to parietal cortex. Proc. Natl. Acad. Sci. U S A 105, 14755–14762. doi: 10.1073/pnas.0804216105
Wiener, J. M., Büchner, S. J., and Hölscher, C. (2009). Taxonomy of human wayfinding tasks: a knowledge-based approach. Spat. Cogn. Comput. 9, 152–165. doi: 10.1080/13875860902906496
Wiener, M., Michaelis, K., and Thompson, J. C. (2016). Functional correlates of likelihood and prior representations in a virtual distance task. Hum. Brain Mapp. 37, 3172–3187. doi: 10.1002/hbm.23232
Woldorff, M. G., Tempelmann, C., Fell, J., Tegeler, C., Gaschler-Markefski, B., Hinrichs, H., et al. (1999). Lateralized auditory spatial perception and the contralaterality of cortical processing as studied with functional magnetic resonance imaging and magnetoencephalography. Hum. Brain Mapp. 7, 49–66. doi: 10.1002/(sici)1097-0193(1999)7:1<49::aid-hbm5>3.0.co;2-j
Woods, A. J., Antal, A., Bikson, M., Boggio, P. S., Brunoni, A. R., Celnik, P., et al. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 127, 1031–1104. doi: 10.1016/j.clinph.2015.11.012
Wright, J. M. J. M., and Krekelberg, B. (2014). Transcranial direct current stimulation over posterior parietal cortex modulates visuospatial localization. J. Vis. 14:5. doi: 10.1167/14.9.5
Xu, Y., and Chun, M. M. (2006). Dissociable neural mechanisms supporting visual short-term memory for objects. Nature 440, 91–95. doi: 10.1038/nature04262
Keywords: transcranial direct current stimulation, transcranial alternating current stimulation, spatial cognition, visualization, navigation, functional connectivity
Citation: Brunyé TT (2018) Modulating Spatial Processes and Navigation via Transcranial Electrical Stimulation: A Mini Review. Front. Hum. Neurosci. 11:649. doi: 10.3389/fnhum.2017.00649
Received: 01 November 2017; Accepted: 19 December 2017;
Published: 09 January 2018.
Edited by:
Thackery Ian Brown, Georgia Institute of Technology, United StatesReviewed by:
Barbara Colombo, Champlain College, United StatesPhilip Tseng, Taipei Medical University, Taiwan
Copyright © 2018 Brunyé. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tad T. Brunyé, tbruny01@tufts.edu
 Tad T. Brunyé
Tad T. Brunyé