- Laboratory for the Molecular Biology of Neural Systems, Advanced Medical Research Center, Nara Medical University, Kashihara, Japan
Inhibitory interneurons in the olfactory bulb are generated continuously throughout life in the subventricular zone and differentiate into periglomerular and granule cells. Neural circuits that undergo reorganization by newborn olfactory bulb interneurons are necessary for odor detection, odor discrimination, olfactory memory, and innate olfactory responses. Although sensory experience has been shown to regulate development in a variety of species and in various structures, including the retina, cortex, and hippocampus, little is known about how sensory experience regulates the dendritic development of newborn olfactory bulb interneurons. Recent studies revealed that the 5T4 oncofetal trophoblast glycoprotein and the neuronal Per/Arnt/Sim domain protein 4 (Npas4) transcription factor regulate dendritic branching and dendritic spine formation, respectively, in olfactory bulb interneurons. Here, we summarize the molecular mechanisms that underlie the sensory input-dependent development of newborn interneurons and the formation of functional neural circuitry in the olfactory bulb.
Introduction
Sensory experience is recognized as a critical factor in the development and plastic modification of neural circuits in vertebrates (Katz and Shatz, 1996; Sanes and Lichtman, 2001; Nithianantharajah and Hannan, 2006; Lepousez et al., 2013). As well as newborn hippocampal neurons (Vadodaria and Jessberger, 2013), newborn olfactory bulb (OB) interneurons are a good model for studying the postnatal modification of neural circuits by sensory inputs from the external world. Specific odorants activate olfactory sensory neurons that express the corresponding odorant receptors (Mori and Sakano, 2011; Takeuchi and Sakano, 2014). The olfactory sensory neurons project their axons to specific glomeruli in the OB and can subsequently activate a specific neural circuit locally, facilitating the dendritic development of OB interneurons via interactions with excitatory projection neurons, such as mitral and tufted cells (Figure 1A; Mori and Sakano, 2011; Lepousez et al., 2013; Figueres-Oñate et al., 2014; Imai, 2014). Precursors for OB interneurons are generated throughout life in the subventricular zone of the lateral ventricle, migrate along the rostral migratory stream (RMS) and differentiate into γ-aminobutyric acid (GABA)-releasing inhibitory interneurons, such as periglomerular cells (PGCs) and granule cells (GCs; Figure 1A; Chazal et al., 2000; Alvarez-Buylla et al., 2008; Lledo et al., 2008; Whitman and Greer, 2009; Adam and Mizrahi, 2010; Kaneko et al., 2010; Sakamoto et al., 2011; Sequerra, 2014). Neural circuits that undergo reorganization by newborn OB interneurons are assumed to be essential for odor detection, odor discrimination, olfactory memory, and innate olfactory responses (Alonso et al., 2012; Sakamoto et al., 2014; Gschwend et al., 2015). It is well known that odor-evoked neural activity affects the survival and integration of newborn OB interneurons (Petreanu and Alvarez-Buylla, 2002; Rochefort et al., 2002; Yamaguchi and Mori, 2005; Bastien-Dionne et al., 2010; Lin et al., 2010; Sawada et al., 2011). In addition, elimination of GCs via cell death is promoted by top-down inputs from the olfactory cortex to the OB during the postprandial period (Yokoyama et al., 2011; Komano-Inoue et al., 2014). Moreover, odor deprivation and odor-enriched environments suppress and facilitate, respectively, dendritogenesis and spinogenesis in newborn OB interneurons (Saghatelyan et al., 2005; Kelsch et al., 2009; Livneh et al., 2009; Breton-Provencher et al., 2014; Lepousez et al., 2014). However, molecular mechanisms regulating the sensory experience-dependent dendritogenesis and spinogenesis in OB newborn interneurons remain unknown. Recent studies revealed that the 5T4 oncofetal trophoblast glycoprotein regulates the dendritic arborization of OB GCs in a sensory input-dependent manner (Yoshihara et al., 2012), whereas the neuronal Per/Arnt/Sim domain protein 4 (Npas4) transcription factor controls the sensory input-dependent dendritic spine formation of OB GCs (Yoshihara et al., 2014).
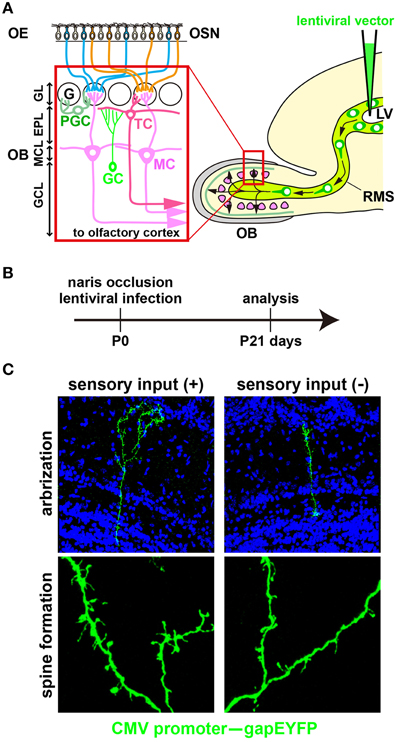
Figure 1. Development of newborn OB interneurons is regulated by odor-evoked neural activity. (A) Schematic representation of the olfactory bulb neural circuitry. Olfactory bulb (OB) interneurons are generated throughout life in the subventricular zone of the lateral ventricle (LV), migrate along the rostral migratory stream (RMS), and differentiate into γ-aminobutyric acid (GABA)-releasing inhibitory interneurons such as granule cells (GCs) and periglomerular cells (PGCs) in the OB. GCs and PGCs modulate the neural activity of excitatory projection neurons, including mitral and tufted cells (MC and TC) through dendrodendritic synapses between inhibitory and excitatory neurons. EPL, external plexiform layer; G, glomerulus; GCL, granule cell layer; GL, glomerular layer; MCL, mitral cell layer; OE, olfactory epithelium; OSN, olfactory sensory neuron. (B) A lentiviral vector (CMV promoter–gapEYFP gene) was injected into the lateral ventricle (LV) of wild-type mice at postnatal day 0 (P0). After 3 weeks (P21), YFP+ interneurons can be visualized in the olfactory bulb (OB). The activity-dependent development of newborn interneurons was analyzed by injecting a lentivirus into the LV of unilaterally naris-occluded mice. (C) Newborn granule cells (GCs) were analyzed in the open and closed sides of the OB from P21 naris-occluded mice. GC dendrites are less branched and have lower spine density in the closed than in the open side of the OB (modified from Yoshihara et al., 2012, 2014).
Development of Newborn OB Interneurons is Regulated by Odor-induced Neural Activity
Neurogenesis arises continuously throughout life in two areas of the mouse brain, such as the subgranular zone of the dentate gyrus (Vadodaria and Gage, 2014) and the subventricular zone of the lateral ventricle (Tong and Alvarez-Buylla, 2014). In the latter, interneuronal neuroblasts migrate along the RMS to the OB (Figure 1A). After neuroblasts arrive at the OB, dendritogenesis and spinogenesis occur in those cells, which then differentiate into mature GABAergic inhibitory interneurons (GCs and PGCs) and incorporate into pre-existing neural circuits in the OB (Alvarez-Buylla et al., 2008; Lledo et al., 2008; Whitman and Greer, 2009; Adam and Mizrahi, 2010; Kaneko et al., 2010; Sakamoto et al., 2011; Sequerra, 2014). Previous studies showed that odor-evoked neural activity is required for the development of newborn OB interneurons at the following four steps.
(1) Proliferation of neural stem cells and migration of neuroblasts. An odor-enriched environment enhances proliferation of neural stem cells in both the RMS and subventricular zone, although chemical lesion of olfactory sensory neurons increases cell proliferation in the RMS alone (Alonso et al., 2008). When neuroblasts arrive at the OB, the direction of migration changes from rostral to radial (Hack et al., 2002; Saghatelyan et al., 2004; Belvindrah et al., 2011; Saha et al., 2012). Radial migration of neuroblasts in the OB is controlled by the secreted glycoprotein reelin (Hack et al., 2002) and the extracellular matrix glycoprotein tenascin-R (Saghatelyan et al., 2004; David et al., 2013). Because tenascin-R is produced by pre-existing GCs and expressed in a sensory input-dependent manner, its lack decreases the radial migration of neuroblasts as well as spine development of newborn GCs (Saghatelyan et al., 2004; David et al., 2013).
(2) Differentiation, survival, and death of newborn interneurons. When immature interneurons reach a given layer in the OB, they differentiate into mature PGCs and GCs. The production levels of the GABA synthetic enzyme (glutamic acid decarboxylase 67: GAD67) and GABA in OB interneurons are regulated in an activity-dependent manner (Parrish-Aungst et al., 2011; Lau and Murthy, 2012). A PGC subtype expresses tyrosine hydroxylase (TH) gene, encoding a rate-limiting enzyme for dopamine synthesis, in an odor input-dependent manner (Bastien-Dionne et al., 2010; Bovetti et al., 2013; Lazarini et al., 2014). The TH expression in PGCs is controlled by transcription factors such as Er81 (Cave et al., 2010) and COUP-TFI (Bovetti et al., 2013; Zhou et al., 2015) in an activity-dependent manner, whereas the transcription factor Pax6 is upregulated in TH-positive PGCs in odor-deprived mice (Bastien-Dionne et al., 2010). In unilaterally naris-occluded mice, the apoptotic rate of newborn GCs is increased on the closed side of the OB (Rochefort et al., 2002; Yamaguchi and Mori, 2005; Bastien-Dionne et al., 2010; Lin et al., 2010; Sawada et al., 2011), whereas their survival rate is increased in odor-enriched environments (Rochefort et al., 2002; Rochefort and Lledo, 2005). The survival and death of newborn PGCs are also regulated by sensory inputs. For example, newborn PGC death is induced by the connective tissue growth factor (CTGF) secreted from external tufted cells in the OB (Khodosevich et al., 2013). In odor-stimulated glomeruli, external tufted cells secrete more CTGF protein, enhancing death of newborn PGCs through transforming growth factor-β (TGF-β) receptor signaling downstream of CTGF (Khodosevich et al., 2013). In addition, olfactory deprivation negatively affects the survival of newborn calretinin-positive PGCs (Kato et al., 2012), whereas odor enrichment increases the cell number of TH-positive PGCs (Bonzano et al., 2014).
(3) Dendritic morphogenesis of newborn interneurons. In odor deprivation, the length and branching number of GC dendrites are decreased; by contrast, they are increased in an odor-enriched environment (Figures 1B,C; Saghatelyan et al., 2005; Yoshihara et al., 2012). GABAA receptor mutant mice exhibit impaired dendritic branching and spine formation in OB GCs, suggesting that GABAergic synaptic transmission is important for proper dendritogenesis and spinogenesis (Pallotto et al., 2012). Furthermore, fragile X mental retardation protein (FMRP), which is an mRNA-binding protein essential for multiple aspects of neuronal mRNA metabolism, downregulates dendritic spine formation in OB GCs and is necessary for activity-dependent dendritic remodeling (Scotto-Lomassese et al., 2011).
(4) Dendritic spine formation of newborn interneurons. Through their dendritic spines, OB interneurons connect to projection neurons (mitral and tufted cells) to modulate activity (Lledo et al., 2008; Adam and Mizrahi, 2010; Imai, 2014). In odor-deprived mice, the dendritic spine formation of OB interneurons is suppressed in the distal dendritic domain and accelerated in the proximal dendritic domain; by contrast, dendritic spine formation is increased in the distal dendritic domain in odor-enriched environments (Figure 1C; Saghatelyan et al., 2005; Kelsch et al., 2009, 2012b; Livneh et al., 2009; Breton-Provencher et al., 2014; Lepousez et al., 2014). It is suggested that synapses of neonatal-born GCs retain a higher level of plasticity in response to changes in neural activity than those of adult-born GCs (Kelsch et al., 2012b). Odor input-dependent neural activity induces the formation and retraction of filopodia in the distal dendritic domain of GCs via NMDA receptor signaling (Kelsch et al., 2012a; Breton-Provencher et al., 2014). In OB GCs, odor-discrimination learning increases spine density in proximal dendritic domains, which receive top-down inputs from the olfactory cortex (Yokoyama et al., 2011; Komano-Inoue et al., 2014; Lepousez et al., 2014). Furthermore, corticotropin-releasing hormone (CRH) is produced in a subtype of OB interneurons at the external plexiform layer in an activity-dependent manner. When CRH is received by newly generated OB GCs, synaptogenesis in the GC dendrites is accelerated via CRH receptor signaling (Garcia et al., 2014).
5T4 Glycoprotein Regulates Dendritic Branching of Newborn OB Granule Cells in a Sensory Input-dependent Manner
Although odor-evoked neural activity is required for proper dendritic development of OB interneurons, its regulatory mechanisms remain unexplained. DNA microarray and in situ hybridization screenings in the unilaterally naris-occluded OB identified the oncofetal trophoblast glycoprotein gene, 5T4, which is expressed in a specific subtype of OB interneurons following sensory experience (Imamura et al., 2006; Yoshihara et al., 2012). 5T4 is a type I membrane protein with an extracellular domain containing seven leucine-rich repeats bordered by characteristic leucine-rich repeat N- and C-flanking regions and a cytoplasmic domain containing a PDZ interaction motif (King et al., 1999). The 5T4 protein was first identified while searching for molecules with invasive properties shared by placental trophoblasts and cancer cells (Hole and Stern, 1990). The 5T4 gene expression is upregulated in many different carcinomas, while showing only low levels in most normal tissues (Southall et al., 1990) except for high levels in the brain and ovary (King et al., 1999; Barrow et al., 2005).
In the OB, the 5T4 gene is expressed not only in a subtype of PGCs at the glomerular layer, but also in a subtype of GCs (5T4-positive GCs) at the mitral-cell and superficial-GC layers (Imamura et al., 2006; Yoshihara et al., 2012). In the odor-deprived OB, the number of 5T4-positive GCs is decreased in the mitral-cell, and superficial-GC layers, indicating that the expression of 5T4 in 5T4-positive GCs is dependent on sensory inputs (Imamura et al., 2006; Yoshihara et al., 2012). Overexpression of 5T4 in newborn GCs by injecting lentiviral vectors into the lateral ventricle gives rise to more branched dendrites than those observed in control GCs (Yoshihara et al., 2012). In addition, 5T4-overexpressing GCs have more branched dendrites even under sensory deprivation, whereas both 5T4 knockdown and knockout (KO) significantly reduce the dendritic branching of GCs in the OB (Yoshihara et al., 2012). Thus, 5T4 protein appears to be necessary for dendritic branching in OB interneurons.
It was recently shown that 5T4 is both induced by and negatively regulates the Wnt canonical pathway, which then facilitates the response to the noncanonical pathway (Figure 2; Kagermeier-Schenk et al., 2011; Zhao et al., 2014). Thus, neural activity may induce the production of a canonical Wnt ligand to upregulate 5T4, which subsequently blocks the canonical pathway and favors the noncanonical pathway in OB interneurons by inhibiting the internalization of a Wnt-coreceptor, low-density lipoprotein receptor-related protein 6 (LPR6) (Kagermeier-Schenk et al., 2011; Zhao et al., 2014). In fact, compared with the wild type, disruption of the Wnt5a gene, which encodes a noncanonical Wnt ligand expressed in a subtype of OB interneurons, gives rise to reduced dendritic extension in GCs (Pino et al., 2011). It is possible that Wnt5a production regulates the noncanonical planar cell polarity pathway, leading to facilitation of dendritic arborization (van Amerongen and Nusse, 2009; Hirota et al., 2012). Recently, 5T4 deletion and domain-swap experiments established that the 5T4 intracellular domain without a PDZ-binding motif is necessary and sufficient for the dendritic branching of OB GCs, but the 5T4 extracellular leucine-rich repeat domain is not necessary for dendritic branching (Yoshihara et al., 2012). However, the 5T4 extracellular domain is reportedly essential for inhibition of Wnt/β-catenin signaling (Kagermeier-Schenk et al., 2011; Zhao et al., 2014). Therefore, it is likely that 5T4 regulates dendritic branching in a Wnt signaling-independent manner. Because the 5T4 intracellular region may interact with cytoskeletal proteins to regulate the dendritic arborization of GCs, the identification of 5T4-associating proteins will enable us to understand the mechanisms regulating the dendritic development of OB interneurons in an activity-dependent manner.
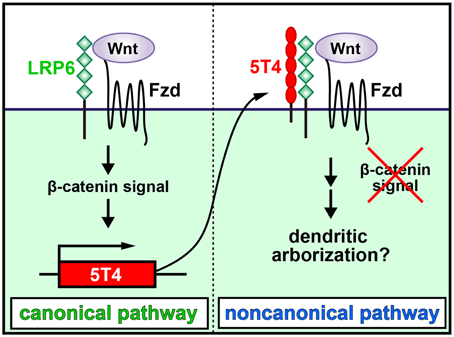
Figure 2. Schematic diagram of dendritic development in newborn OB granule cells regulated by odor inputs. 5T4 leucine-rich repeat (LRR)-containing transmembrane protein is induced by and downregulates the Wnt canonical pathway. Neural activity may induce the production of a canonical Wnt ligand to upregulate 5T4, which subsequently blocks the canonical pathway and favors the noncanonical pathway in OB interneurons by inhibiting the internalization of a Wnt-coreceptor, low-density lipoprotein receptor-related protein 6 (LPR6). This leads to facilitation of dendritic arborization in OB GCs (modified from Kagermeier-Schenk et al., 2011; Yoshihara et al., 2012).
Npas4 Transcription Factor Regulates Dendritic Spine Formation of Newborn OB Granule Cells in a Sensory Input-dependent Manner
Although odor-induced neural activity is required for spine formation in OB interneurons, its regulatory mechanism remains unresolved. DNA microarray and in situ hybridization screenings identified a transcription factor gene, Npas4, which is expressed in a subtype of OB GCs following sensory experience (Bepari et al., 2012; Yoshihara et al., 2014). Npas4 is an immediate early gene induced by neural activity via a calcium-dependent signaling pathway (Lin et al., 2008; Pruunsild et al., 2011; Ramamoorthi et al., 2011; Bloodgood et al., 2013). In addition, Npas4 promotes the formation of inhibitory synapses in the developing visual system (Lin et al., 2008; Bloodgood et al., 2013) and adjusts the homeostatic inhibitory/excitatory balance in excitatory neurons to induce visual cortical plasticity (Maya-Vetencourt et al., 2012). Moreover, the Npas4 protein interacts with several promoters regulated by neural activity and mediates brain-derived neurotrophic factor (BDNF) gene expression in cortical pyramidal and hippocampal CA3 neurons (Lin et al., 2008; Pruunsild et al., 2011; Ramamoorthi et al., 2011; Bloodgood et al., 2013).
Overexpression of Npas4 in newborn OB GCs by injecting lentiviruses into the lateral ventricle gives rise to an increase in spine density even under sensory deprivation conditions (Yoshihara et al., 2014). Furthermore, Npas4 overexpression increases the number of puncta stained by either the postsynaptic marker gephyrin or pre-synaptic marker synaptoporin at the distal region of GC dendrites. By contrast, both Npas4 knockdown and KO cause a significant reduction in the spine density of GC dendrites (Yoshihara et al., 2014). Thus, Npas4 is necessary and sufficient for increasing sensory input-dependent synaptogenesis in OB GCs. Interestingly, Npas4 is also required for activity-dependent spine development of adult-born GCs in the hippocampal dentate gyrus (Sim et al., 2013).
The mechanism for the Npas4 regulation of synaptogenesis in OB interneurons was explored using chromatin immunoprecipitation sequencing (ChIP-Seq) to search for Npas4 target genes in homogenized OB tissues with an Npas4 antibody associating with the promoter regions that bind Npas4. A novel target of Npas4, the oncogenic E3 ubiquitin ligase gene, murine double minute 2 (Mdm2), is expressed at low levels in the wild-type OB but at higher levels in the Npas4 KO OB (Yoshihara et al., 2014). Lateral ventricle injections of lentiviruses to either overexpress or knockdown Mdm2 showed reduction and enhancement, respectively, in the spine density of GC dendrites compared with those in controls (Yoshihara et al., 2014), demonstrating that Mdm2 is a bona fide target gene of Npas4 and that Mdm2 expression is suppressed by Npas4 (Figure 3).
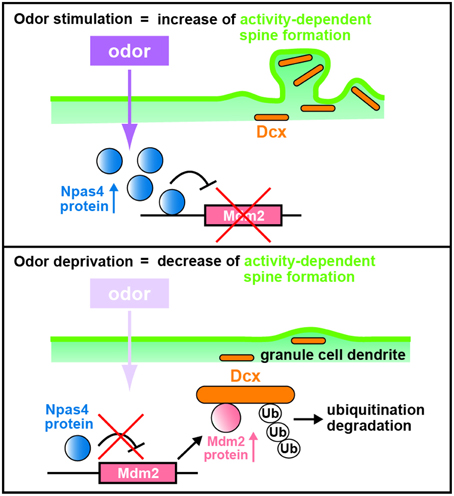
Figure 3. Schematic diagram of dendritic spine formation in newborn OB granule cells regulated by odor inputs. In wild-type olfactory bulb (OB) granule cells (GCs), odor stimulation induces Npas4 expression to suppress Mdm2 expression. The suppression of Mdm2 promotes the spine formation of GC dendrites mediated by doublecortin (Dcx). By contrast, odor deprivation decreases Npas4 expression in OB GCs to upregulate the expression of Mdm2, leading to ubiquitination of Dcx. This results in degradation of Dcx and reduced dendritic spine density in OB GCs, as in the case of Npas4-knockout OB GCs (modified from Yoshihara et al., 2014).
Mdm2 is localized at synapses, ubiquitinates and degrades postsynaptic density protein-95 (PSD-95) in rat hippocampal neurons (Colledge et al., 2003). However, according to the data of western blot analysis, the amount of PSD-95 protein is similar in wild-type and Npas4 KO OBs (Yoshihara et al., 2014). Thus, to reveal the mechanism by which Mdm2 regulates synaptogenesis in OB interneurons, Mdm2 target proteins that are produced differentially between wild-type and Npas4 KO OBs were searched using isobaric tags for relative and absolute quantitation (iTRAQ) proteomics (Yoshihara et al., 2014). Proteomic and cell-line analyses revealed that Mdm2 ubiquitinates and leads to the degradation of a microtubule-associated protein, doublecortin (Dcx). Dcx is generally used as a marker for immature neurons in the adult neurogenic lineage (Brown et al., 2003; Saaltink et al., 2012). Dcx regulates the migration and dendritic development of migrating neurons in the OB core region, including the RMS and the deep GC layer (Ocbina et al., 2006; Belvindrah et al., 2011). Immunohistochemical analysis of OB sections with a Dcx antibody indicates that the intensity of immunofluorescent signals in GC dendrites at the external-plexiform and GC layers are two-fold lower in the Npas4 KO OB than in the wild-type OB (Yoshihara et al., 2014). Furthermore, overexpression and knockdown of Dcx achieved by injecting lentiviruses into the lateral ventricle enhance and reduce, respectively, spine density in GC dendrites (Yoshihara et al., 2014). Thus, Dcx plays an important role in increasing the dendritic spine density of OB GCs: Npas4 protein inhibits Mdm2 expression, which prevents ubiquitination and degradation of Dcx, thereby promoting dendritic spine development in OB GCs following sensory experience (Figure 3).
Interestingly, two Dcx homologs, Dcx-like kinases 1 and 2 (Dclk1/Dclk2), reportedly regulate dendritic spine formation in hippocampal neurons (Shin et al., 2013). It was also recently demonstrated that Dcx is necessary for synapse formation in proper neuromuscular junction in the mouse and human (Bourgeois et al., 2015). Dcx and Dclk1/Dclk2 bind to an actin-binding protein, spinophilin, which is known to regulate spine morphology (Tsukada et al., 2003). Dcx also induces the bundling and cross-linking of microtubules and F-actin (Tsukada et al., 2005). Thus, the microtubule-binding protein Dcx family may play a crucial role in dendritic spine development of OB GCs.
Perspectives
In the cerebral cortex and hippocampus, neural activity regulates a program of gene transcription to affect synaptic development and plasticity (Ebert and Greenberg, 2013). Elevation of intracellular calcium levels induced by neural activity leads to activation of genes for multiple signaling molecules, including calmodulin kinase II (CaMK II), protein kinase A, cyclic AMP-responsive element-binding protein (CREB), and calcineurin (Ebert and Greenberg, 2013; Kawashima et al., 2014). CREB signaling is required for the survival, migration, and dendritic development of OB interneurons (Herold et al., 2011). Activation of the multiple signaling cascades facilitates the expression of neural activity-dependent genes, including the immediate early genes c-fos, egr1, and Arc (Ebert and Greenberg, 2013; Kawashima et al., 2014). A recent study found that neuronal activity induces DNA breaks in the promoters of immediate early genes and facilitates their expression (Madabhushi et al., 2015). Among the immediate early genes, c-fos and egr1 transcription factors activate genes for BDNF and Arc to regulate synaptic development (Ebert and Greenberg, 2013). In newborn OB interneurons, several immediate early genes (c-fos, egr1, and Arc) are expressed in a sensory input-dependent manner (Guthrie et al., 1993; Inaki et al., 2002; Busto et al., 2009; Bepari et al., 2012). Because the expression of the 5T4 gene is at a basal level in OB GCs after exiting the RMS, it is important to explore how the odor input-dependent expression is regulated by transcription factors, including those immediate early genes described above. It was recently reported that BDNF overexpression increases dendritic spine density of GCs (McDole et al., 2015). Therefore, future studies that identify the target genes of these immediate early genes, including Npas4, will allow us to understand the molecular mechanisms underlying activity-dependent development in newborn OB interneurons.
The functional significance of sensory input-dependent development in newborn OB interneurons has been explored. Olfactory experiences, such as odor-enrichment and odor-discrimination learning, regulate the maturation and survival of adult-born OB interneurons (Alonso et al., 2006; Livneh et al., 2009; Breton-Provencher et al., 2014; Lepousez et al., 2014). Newborn OB interneurons possess specific properties that are different from those of pre-existing interneurons, such as enhanced synaptic plasticity during a critical time window (Carleton et al., 2003; Panzanelli et al., 2009; Pallotto et al., 2012; Livneh et al., 2014), suggesting that these newborn interneurons uniquely contribute to odor processing. Consistent with this suggestion, odor detection and odor-discrimination learning are reportedly impaired in mice with diminished adult neurogenesis in the OB (Gheusi et al., 2000; Enwere et al., 2004; Breton-Provencher et al., 2009). However, the genetic ablation of adult-born interneurons causes deficits in innate olfactory responses, including predator avoidance and sexual behaviors (Sakamoto et al., 2011), but not in other normal olfactory abilities, such as odor detection and simple odor discrimination (Imayoshi et al., 2008; Sakamoto et al., 2011). This incongruence could be attributable to differences in the subtypes and numbers of OB interneurons that were manipulated in the individual studies. However, none of the methodologies used in these studies, including physically or genetically eliminating newborn cells, block the birth of adult-born OB interneurons in a spatially and temporally specific manner (Gheusi et al., 2000; Enwere et al., 2004; Imayoshi et al., 2008; Breton-Provencher et al., 2009; Sultan et al., 2010; Lazarini et al., 2014). A genetic activation study in which channelrhodopsin-2 was selectively expressed in newborn GCs showed that photostimulation of adult-born neurons (2 months old) facilitates difficult odor-discrimination learning and improves odor memory, whereas photostimulation of postnatal day 6-born neurons does not (Alonso et al., 2012; Gschwend et al., 2015). On the other hand, genetic inhibition of synaptic transmission in postnatal-born neurons impairs difficult odor-discrimination learning (Sakamoto et al., 2014). Because Dcx is produced in younger mature GCs, but is absent in older mature GCs (Brown et al., 2003), Npas4 may regulate dendritic spine formation in the younger but not the older mature GCs (Yoshihara et al., 2014). Interestingly, conditional KO of Npas4 function in OB neurons impairs difficult odor-discrimination learning without affecting the ability to detect odors (Yoshihara et al., 2014), suggesting that spine development in newborn younger GCs is required in part for olfactory behaviors. By regulating the activity-dependent synaptic development of newborn OB GCs, Npas4 may play a role in shaping the functional neural circuitry involved in olfactory discrimination learning. Collectively, these studies provide important evidence that newborn OB interneurons, in which spine formation is regulated by Npas4 in a sensory experience-dependent manner, play a functional role in the OB circuitry and thus in its behavioral manifestation. Because GCs can be divided into several subtypes (Merkle et al., 2014), it is assumed that each GC subtype forms a distinct local circuit in the OB (Mori et al., 1983; Orona et al., 1983; Shepherd et al., 2004). Thus, future studies that individually manipulate the subtypes of OB interneurons in a spatially and temporally specific manner will help us to understand their functions in the OB circuitry.
Author Contributions
SY, HT, and AT wrote the paper.
Funding
This work was supported by Grants-in-Aid for Scientific Research on (B) (AT), (C) (HT and SY), and Innovative Areas (Adaptive circuit shift) (AT) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. AT was supported by grants from the Smoking Research Foundation, Japanese Applied Enzymology Foundation, Senshin Medical Research Foundation, and Ono Medical Research Foundation, and a Nara Medical University Grant-in-Aid for Collaborative Research Projects in Japan. SY and HT were supported by grants from Takeda Science Foundation and Astellas Foundation for Research on Metabolic Disorders, Japan. SY was supported by a grant from Terumo Foundation for Life Sciences and Arts. HT was supported by grants from the Salt Science Research Foundation (no. 14C3), Mishima Kaiun Memorial Foundation, and Banyu Life Science International Foundation in Japan.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all members of the Tsuboi laboratory and the animal facility staff of Nara Medical University for their assistance.
References
Adam, Y., and Mizrahi, A. (2010). Circuit formation and maintenance - perspectives from the mammalian olfactory bulb. Curr. Opin. Neurobiol. 20, 134–140. doi: 10.1016/j.conb.2009.11.001
Alonso, M., Lepousez, G., Wagner, S., Bardy, C., Gabellec, M. M., Torquet, N., et al. (2012). Activation of adult-born neurons facilitates learning and memory. Nat. Neurosci. 15, 897–904. doi: 10.1038/nn.3108
Alonso, M., Ortega-Pérez, I., Grubb, M. S., Bourgeois, J. P., Charneau, P., and Lledo, P. M. (2008). Turning astrocytes from the rostral migratory stream into neurons: a role for the olfactory sensory organ. J. Neurosci. 28, 11089–11102. doi: 10.1523/jneurosci.3713-08.2008
Alonso, M., Viollet, C., Gabellec, M. M., Meas-Yedid, V., Olivo-Marin, J. C., and Lledo, P. M. (2006). Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J. Neurosci. 26, 10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006
Alvarez-Buylla, A., Kohwi, M., Nguyen, T. M., and Merkle, F. T. (2008). The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb. Symp. Quant. Biol. 73, 357–365. doi: 10.1101/sqb.2008.73.019
Barrow, K. M., Ward, C. M., Rutter, J., Ali, S., and Stern, P. L. (2005). Embryonic expression of murine 5T4 oncofoetal antigen is associated with morphogenetic events at implantation and in developing epithelia. Dev. Dyn. 233, 1535–1545. doi: 10.1002/dvdy.20482
Bastien-Dionne, P. O., David, L. S., Parent, A., and Saghatelyan, A. (2010). Role of sensory activity on chemospecific populations of interneurons in the adult olfactory bulb. J. Comp. Neurol. 518, 1847–1861. doi: 10.1002/cne.22307
Belvindrah, R., Nissant, A., and Lledo, P. M. (2011). Abnormal neuronal migration changes the fate of developing neurons in the postnatal olfactory bulb. J. Neurosci. 31, 7551–7562. doi: 10.1523/JNEUROSCI.6716-10.2011
Bepari, A. K., Watanabe, K., Yamaguchi, M., Tamamaki, N., and Takebayashi, H. (2012). Visualization of odor-induced neuronal activity by immediate early gene expression. BMC Neurosci. 13:140. doi: 10.1186/1471-2202-13-140
Bloodgood, B. L., Sharma, N., Browne, H. A., Trepman, A. Z., and Greenberg, M. E. (2013). The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 503, 121–125. doi: 10.1038/nature12743
Bonzano, S., Bovetti, S., Fasolo, A., Peretto, P., and De Marchis, S. (2014). Odour enrichment increases adult-born dopaminergic neurons in the mouse olfactory bulb. Eur. J. Neurosci. 40, 3450–3457. doi: 10.1111/ejn.12724
Bourgeois, F., Messéant, J., Kordeli, E., Petit, J. M., Delers, P., Bahi-Buisson, N., et al. (2015). A critical and previously unsuspected role for doublecortin at the neuromuscular junction in mouse and human. Neuromuscul. Disord. 25, 461–473. doi: 10.1016/j.nmd.2015.01.012
Bovetti, S., Bonzano, S., Garzotto, D., Giannelli, S. G., Iannielli, A., Armentano, M., et al. (2013). COUP-TFI controls activity-dependent tyrosine hydroxylase expression in adult dopaminergic olfactory bulb interneurons. Development 140, 4850–4859. doi: 10.1242/dev.089961
Breton-Provencher, V., Coté, D., and Saghatelyan, A. (2014). Activity of the principal cells of the olfactory bulb promotes a structural dynamic on the distal dendrites of immature adult-born granule cells via activation of NMDA receptors. J. Neurosci. 34, 1748–1759. doi: 10.1523/jneurosci.3013-13.2014
Breton-Provencher, V., Lemasson, M., Peralta, M. R. III., and Saghatelyan, A. (2009). Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J. Neurosci. 29, 15245–15257. doi: 10.1523/jneurosci.3606-09.2009
Brown, J. P., Couillard-Després, S., Cooper-Kuhn, C. M., Winkler, J., Aigner, L., and Kuhn, H. G. (2003). Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10. doi: 10.1002/cne.10874
Busto, G. U., Elie, J. E., Kermen, F., Garcia, S., Sacquet, J., Jourdan, F., et al. (2009). Expression of Zif268 in the granule cell layer of the adult mouse olfactory bulb is modulated by experience. Eur. J. Neurosci. 29, 1431–1439. doi: 10.1111/j.1460-9568.2009.06689.x
Carleton, A., Petreanu, L. T., Lansford, R., Alvarez-Buylla, A., and Lledo, P. M. (2003). Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 6, 507–518. doi: 10.1038/nn1048
Cave, J. W., Akiba, Y., Banerjee, K., Bhosle, S., Berlin, R., and Baker, H. (2010). Differential regulation of dopaminergic gene expression by Er81. J. Neurosci. 30, 4717–4724. doi: 10.1523/JNEUROSCI.0419-10.2010
Chazal, G., Durbec, P., Jankovski, A., Rougon, G., and Cremer, H. (2000). Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mouse. J. Neurosci. 20, 1446–1457.
Colledge, M., Snyder, E. M., Crozier, R. A., Soderling, J. A., Jin, Y., Langeberg, L. K., et al. (2003). Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron 40, 595–607. doi: 10.1016/S0896-6273(03)00687-1
David, L. S., Schachner, M., and Saghatelyan, A. (2013). The extracellular matrix glycoprotein tenascin-R affects adult but not developmental neurogenesis in the olfactory bulb. J. Neurosci. 33, 10324–10339. doi: 10.1523/JNEUROSCI.5728-12.2013
Ebert, D. H., and Greenberg, M. E. (2013). Activity-dependent neuronal signalling and autism spectrum disorder. Nature 493, 327–337. doi: 10.1038/nature11860
Enwere, E., Shingo, T., Gregg, C., Fujikawa, H., Ohta, S., and Weiss, S. (2004). Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 24, 8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004
Figueres-Oñate, M., Gutiérrez, Y., and López-Mascaraque, L. (2014). Unraveling Cajal's view of the olfactory system. Front. Neuroanat. 8:55. doi: 10.3389/fnana.2014.00055
Garcia, I., Quast, K. B., Huang, L., Herman, A. M., Selever, J., Deussing, J. M., et al. (2014). Local CRH signaling promotes synaptogenesis and circuit integration of adult-born neurons. Dev. Cell 30, 645–659. doi: 10.1016/j.devcel.2014.07.001
Gheusi, G., Cremer, H., McLean, H., Chazal, G., Vincent, J. D., and Lledo, P. M. (2000). Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc. Natl. Acad. Sci. U.S.A. 97, 1823–1828. doi: 10.1073/pnas.97.4.1823
Gschwend, O., Abraham, N. M., Lagier, S., Begnaud, F., Rodriguez, I., and Carleton, A. (2015). Neuronal pattern separation in the olfactory bulb improves odor discrimination learning. Nat. Neurosci. 18, 1474–1482. doi: 10.1038/nn.4089
Guthrie, K. M., Anderson, A. J., Leon, M., and Gall, C. (1993). Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc. Natl. Acad. Sci. U.S.A. 90, 3329–3333. doi: 10.1073/pnas.90.8.3329
Hack, I., Bancila, M., Loulier, K., Carroll, P., and Cremer, H. (2002). Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat. Neurosci. 5, 939–945. doi: 10.1038/nn923
Herold, S., Jagasia, R., Merz, K., Wassmer, K., and Lie, D. C. (2011). CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol. Cell. Neurosci. 46, 79–88. doi: 10.1016/j.mcn.2010.08.008
Hirota, Y., Sawada, M., Kida, Y. S., Huang, S. H., Yamada, O., Sakaguchi, M., et al. (2012). Roles of planar cell polarity signaling in maturation of neuronal precursor cells in the postnatal mouse olfactory bulb. Stem Cells 30, 1726–1733. doi: 10.1002/stem.1137
Hole, N., and Stern, P. L. (1990). Isolation and characterization of 5T4, a tumour-associated antigen. Int. J. Cancer 45, 179–184. doi: 10.1002/ijc.2910450132
Imai, T. (2014). Construction of functional neuronal circuitry in the olfactory bulb. Semin. Cell Dev. Biol. 35, 180–188. doi: 10.1016/j.semcdb.2014.07.012
Imamura, F., Nagao, H., Naritsuka, H., Murata, Y., Taniguchi, H., and Mori, K. (2006). A leucine-rich repeat membrane protein, 5T4, is expressed by a subtype of granule cells with dendritic arbors in specific strata of the mouse olfactory bulb. J. Comp. Neurol. 495, 754–768. doi: 10.1002/cne.20896
Imayoshi, I., Sakamoto, M., Ohtsuka, T., Takao, K., Miyakawa, T., Yamaguchi, M., et al. (2008). Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 11, 1153–1161. doi: 10.1038/nn.2185
Inaki, K., Takahashi, Y. K., Nagayama, S., and Mori, K. (2002). Molecular-feature domains with posterodorsal-anteroventral polarity in the symmetrical sensory maps of the mouse olfactory bulb: mapping of odourant-induced Zif268 expression. Eur. J. Neurosci. 15, 1563–1574. doi: 10.1046/j.1460-9568.2002.01991.x
Kagermeier-Schenk, B., Wehner, D., Ozhan-Kizil, G., Yamamoto, H., Li, J., Kirchner, K., et al. (2011). The transmembrane protein Waif1/5T4 inhibits Wnt/β-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Dev. Cell 21, 1129–1143. doi: 10.1016/j.devcel.2011.10.015
Kaneko, N., Marín, O., Koike, M., Hirota, Y., Uchiyama, Y., Wu, J. Y., et al. (2010). New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron 67, 213–223. doi: 10.1016/j.neuron.2010.06.018
Kato, Y., Kaneko, N., Sawada, M., Ito, K., Arakawa, S., Murakami, S., et al. (2012). A subtype-specific critical period for neurogenesis in the postnatal development of mouse olfactory glomeruli. PLoS ONE 7:e48431. doi: 10.1371/journal.pone.0048431
Katz, L. C., and Shatz, C. J. (1996). Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138. doi: 10.1126/science.274.5290.1133
Kawashima, T., Okuno, H., and Bito, H. (2014). A new era for functional labeling of neurons: activity-dependent promoters have come of age. Front. Neural Circuits. 8:37. doi: 10.3389/fncir.2014.00037
Kelsch, W., Li, Z., Eliava, M., Goengrich, C., and Monyer, H. (2012a). GluN2B-containing NMDA receptors promote wiring of adult-born neurons into olfactory bulb circuits. J. Neurosci. 32, 12603–12611. doi: 10.1523/JNEUROSCI.1459-12.2012
Kelsch, W., Lin, C. W., Mosley, C. P., and Lois, C. (2009). A critical period for activity-dependent synaptic development during olfactory bulb adult neurogenesis. J. Neurosci. 29, 11852–11858. doi: 10.1523/JNEUROSCI.2406-09.2009
Kelsch, W., Sim, S., and Lois, C. (2012b). Increasing heterogeneity in the organization of synaptic inputs of mature olfactory bulb neurons generated in newborn rats. J. Comp. Neurol. 520, 1327–1338. doi: 10.1002/cne.22799
Khodosevich, K., Lazarini, F., von Engelhardt, J., Kaneko, H., Lledo, P. M., and Monyer, H. (2013). Connective tissue growth factor regulates interneuron survival and information processing in the olfactory bulb. Neuron 79, 1136–1151. doi: 10.1016/j.neuron.2013.07.011
King, K. W., Sheppard, F. C., Westwater, C., Stern, P. L., and Myers, K. A. (1999). Organisation of the mouse and human 5T4 oncofoetal leucine-rich glycoprotein genes and expression in foetal and adult murine tissues. Biochim. Biophys. Acta 1445, 257–270. doi: 10.1016/S0167-4781(99)00055-X
Komano-Inoue, S., Manabe, H., Ota, M., Kusumoto-Yoshida, I., Yokoyama, T. K., Mori, K., et al. (2014). Top-down inputs from the olfactory cortex in the postprandial period promote elimination of granule cells in the olfactory bulb. Eur. J. Neurosci. 40, 2724–2733. doi: 10.1111/ejn.12679
Lau, C. G., and Murthy, V. N. (2012). Activity-dependent regulation of inhibition via GAD67. J. Neurosci. 32, 8521–8531. doi: 10.1523/JNEUROSCI.1245-12.2012
Lazarini, F., Gabellec, M. M., Moigneu, C., de Chaumont, F., Olivo-Marin, J. C., and Lledo, P. M. (2014). Adult neurogenesis restores dopaminergic neuronal loss in the olfactory bulb. J. Neurosci. 34, 14430–14442. doi: 10.1523/JNEUROSCI.5366-13.2014
Lepousez, G., Nissant, A., Bryant, A. K., Gheusi, G., Greer, C. A., and Lledo, P. M. (2014). Olfactory learning promotes input-specific synaptic plasticity in adult-born neurons. Proc. Natl. Acad. Sci. U.S.A. 111, 13984–13989. doi: 10.1073/pnas.1404991111
Lepousez, G., Valley, M. T., and Lledo, P. M. (2013). The impact of adult neurogenesis on olfactory bulb circuits and computations. Annu. Rev. Physiol. 75, 339–363. doi: 10.1146/annurev-physiol-030212-183731
Lin, C. W., Sim, S., Ainsworth, A., Okada, M., Kelsch, W., and Lois, C. (2010). Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron 65, 32–39. doi: 10.1016/j.neuron.2009.12.001
Lin, Y., Bloodgood, B. L., Hauser, J. L., Lapan, A. D., Koon, A. C., Kim, T. K., et al. (2008). Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455, 1198–1204. doi: 10.1038/nature07319
Livneh, Y., Adam, Y., and Mizrahi, A. (2014). Odor processing by adult-born neurons. Neuron 81, 1097–1110. doi: 10.1016/j.neuron.2014.01.007
Livneh, Y., Feinstein, N., Klein, M., and Mizrahi, A. (2009). Sensory input enhances synaptogenesis of adult-born neurons. J. Neurosci. 29, 86–97. doi: 10.1523/JNEUROSCI.4105-08.2009
Lledo, P. M., Merkle, F. T., and Alvarez-Buylla, A. (2008). Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 31, 392–400. doi: 10.1016/j.tins.2008.05.006
Madabhushi, R., Gao, F., Pfenning, A. R., Pan, L., Yamakawa, S., Seo, J., et al. (2015). Activity-induced DNA breaks govern the expression of neuronal early-response genes. Cell 161, 1592–1605. doi: 10.1016/j.cell.2015.05.032
Maya-Vetencourt, J. F., Tiraboschi, E., Greco, D., Restani, L., Cerri, C., Auvinen, P., et al. (2012). Experience-dependent expression of NPAS4 regulates plasticity in adult visual cortex. J. Physiol. (Lond). 590, 4777–4787. doi: 10.1113/jphysiol.2012.234237
McDole, B., Isgor, C., Pare, C., and Guthrie, K. (2015). BDNF over-expression increases olfactory bulb granule cell dendritic spine density in vivo. Neuroscience 304, 146–160. doi: 10.1016/j.neuroscience.2015.07.056
Merkle, F. T., Fuentealba, L. C., Sanders, T. A., Magno, L., Kessaris, N., and Alvarez-Buylla, A. (2014). Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci. 17, 207–214. doi: 10.1038/nn.3610
Mori, K., Kishi, K., and Ojima, H. (1983). Distribution of dendrites of mitral, displaced mitral, tufted, and granule cells in the rabbit olfactory bulb. J. Comp. Neurol. 219, 339–355. doi: 10.1002/cne.902190308
Mori, K., and Sakano, H. (2011). How is the olfactory map formed and interpreted in the mammalian brain? Annu. Rev. Neurosci. 34, 467–499. doi: 10.1146/annurev-neuro-112210-112917
Nithianantharajah, J., and Hannan, A. J. (2006). Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709. doi: 10.1038/nrn1970
Ocbina, P. J., Dizon, M. L., Shin, L., and Szele, F. G. (2006). Doublecortin is necessary for the migration of adult subventricular zone cells from neurospheres. Mol. Cell. Neurosci. 33, 126–135. doi: 10.1016/j.mcn.2006.06.014
Orona, E., Scott, J. W., and Rainer, E. C. (1983). Different granule cell populations innervate superficial and deep regions of the external plexiform layer in rat olfactory bulb. J. Comp. Neurol. 217, 227–237. doi: 10.1002/cne.902170209
Pallotto, M., Nissant, A., Fritschy, J. M., Rudolph, U., Sassoè-Pognetto, M., Panzanelli, P., et al. (2012). Early formation of GABAergic synapses governs the development of adult-born neurons in the olfactory bulb. J. Neurosci. 32, 9103–9115. doi: 10.1523/JNEUROSCI.0214-12.2012
Panzanelli, P., Bardy, C., Nissant, A., Pallotto, M., Sassoè-Pognetto, M., Lledo, P. M., et al. (2009). Early synapse formation in developing interneurons of the adult olfactory bulb. J. Neurosci. 29, 15039–15052. doi: 10.1523/JNEUROSCI.3034-09.2009
Parrish-Aungst, S., Kiyokage, E., Szabo, G., Yanagawa, Y., Shipley, M. T., and Puche, A. C. (2011). Sensory experience selectively regulates transmitter synthesis enzymes in interglomerular circuits. Brain Res. 1382, 70–76. doi: 10.1016/j.brainres.2011.01.068
Petreanu, L., and Alvarez-Buylla, A. (2002). Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 22, 6106–6113.
Pino, D., Choe, Y., and Pleasure, S. J. (2011). Wnt5a controls neurite development in olfactory bulb interneurons. ASN Neuro. 3:e00059. doi: 10.1042/AN20100038
Pruunsild, P., Sepp, M., Orav, E., Koppel, I., and Timmusk, T. (2011). Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J. Neurosci. 31, 3295–3308. doi: 10.1523/JNEUROSCI.4540-10.2011
Ramamoorthi, K., Fropf, R., Belfort, G. M., Fitzmaurice, H. L., McKinney, R. M., Neve, R. L., et al. (2011). Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science 334, 1669–1675. doi: 10.1126/science.1208049
Rochefort, C., Gheusi, G., Vincent, J. D., and Lledo, P. M. (2002). Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 22, 2679–2689.
Rochefort, C., and Lledo, P. M. (2005). Short-term survival of newborn neurons in the adult olfactory bulb after exposure to a complex odor environment. Eur. J. Neurosci. 22, 2863–2870. doi: 10.1111/j.1460-9568.2005.04486.x
Saaltink, D. J., Håvik, B., Verissimo, C. S., Lucassen, P. J., and Vreugdenhil, E. (2012). Doublecortin and doublecortin-like are expressed in overlapping and non-overlapping neuronal cell population: implications for neurogenesis. J. Comp. Neurol. 520, 2805–2823. doi: 10.1002/cne.23144
Saghatelyan, A., de Chevigny, A., Schachner, M., and Lledo, P. M. (2004). Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat. Neurosci. 7, 347–356. doi: 10.1038/nn1211
Saghatelyan, A., Roux, P., Migliore, M., Rochefort, C., Desmaisons, D., Charneau, P., et al. (2005). Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron 46, 103–116. doi: 10.1016/j.neuron.2005.02.016
Saha, B., Ypsilanti, A. R., Boutin, C., Cremer, H., and Chédotal, A. (2012). Plexin-B2 regulates the proliferation and migration of neuroblasts in the postnatal and adult subventricular zone. J. Neurosci. 32, 16892–16905. doi: 10.1523/JNEUROSCI.0344-12.2012
Sakamoto, M., Ieki, N., Miyoshi, G., Mochimaru, D., Miyachi, H., Imura, T., et al. (2014). Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning. J. Neurosci. 34, 5788–5799. doi: 10.1523/JNEUROSCI.0674-14.2014
Sakamoto, M., Imayoshi, I., Ohtsuka, T., Yamaguchi, M., Mori, K., and Kageyama, R. (2011). Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc. Natl. Acad. Sci. U.S.A. 108, 8479–8484. doi: 10.1073/pnas.1018782108
Sanes, J. R., and Lichtman, J. W. (2001). Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805. doi: 10.1038/35097557
Sawada, M., Kaneko, N., Inada, H., Wake, H., Kato, Y., Yanagawa, Y., et al. (2011). Sensory input regulates spatial and subtype-specific patterns of neuronal turnover in the adult olfactory bulb. J. Neurosci. 31, 11587–11596. doi: 10.1523/JNEUROSCI.0614-11.2011
Scotto-Lomassese, S., Nissant, A., Mota, T., Néant-Féry, M., Oostra, B. A., Greer, C. A., et al. (2011). Fragile X mental retardation protein regulates new neuron differentiation in the adult olfactory bulb. J. Neurosci. 31, 2205–2215. doi: 10.1523/JNEUROSCI.5514-10.2011
Sequerra, E. B. (2014). Subventricular zone progenitors in time and space: generating neuronal diversity. Front. Cell. Neurosci. 8:434. doi: 10.3389/fncel.2014.00434
Shepherd, G. M., Chen, W. R., and Greer, C. A. (2004). “Olfactory bulb,” in The Synaptic Organization of the Brain, ed G. M. Shepherd (Oxford: Oxford University Press), 165–216.
Shin, E., Kashiwagi, Y., Kuriu, T., Iwasaki, H., Tanaka, T., Koizumi, H., et al. (2013). Doublecortin-like kinase enhances dendritic remodelling and negatively regulates synapse maturation. Nat. Commun. 4, 1440. doi: 10.1038/ncomms2443
Sim, S., Antolin, S., Lin, C. W., Lin, Y., and Lois, C. (2013). Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4. J. Neurosci. 33, 7928–7940. doi: 10.1523/JNEUROSCI.1571-12.2013
Southall, P. J., Boxer, G. M., Bagshawe, K. D., Hole, N., Bromley, M., and Stern, P. L. (1990). Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br. J. Cancer 61, 89–95. doi: 10.1038/bjc.1990.20
Sultan, S., Mandairon, N., Kermen, F., Garcia, S., Sacquet, J., and Didier, A. (2010). Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J. 24, 2355–2363. doi: 10.1096/fj.09-151456
Takeuchi, H., and Sakano, H. (2014). Neural map formation in the mouse olfactory system. Cell. Mol. Life Sci. 71, 3049–3057. doi: 10.1007/s00018-014-1597-0
Tong, C. K., and Alvarez-Buylla, A. (2014). SnapShot: adult neurogenesis in the V-SVZ. Neuron 81, 220–220.e1. doi: 10.1016/j.neuron.2013.12.004
Tsukada, M., Prokscha, A., Oldekamp, J., and Eichele, G. (2003). Identification of neurabin II as a novel doublecortin interacting protein. Mech. Dev. 120, 1033–1043. doi: 10.1016/S0925-4773(03)00177-1
Tsukada, M., Prokscha, A., Ungewickell, E., and Eichele, G. (2005). Doublecortin association with actin filaments is regulated by neurabin II. J. Biol. Chem. 280, 11361–11368. doi: 10.1074/jbc.m405525200
Vadodaria, K. C., and Gage, F. H. (2014). SnapShot: adult hippocampal neurogenesis. Cell 156, 1114–1114.e1. doi: 10.1016/j.cell.2014.02.029
Vadodaria, K. C., and Jessberger, S. (2013). Maturation and integration of adult born hippocampal neurons: signal convergence onto small Rho GTPases. Front. Synaptic Neurosci. 5:4. doi: 10.3389/fnsyn.2013.00004
van Amerongen, R., and Nusse, R. (2009). Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214. doi: 10.1242/dev.033910
Whitman, M. C., and Greer, C. A. (2009). Adult neurogenesis and the olfactory system. Prog Neurobiol. 89, 162–175. doi: 10.1016/j.pneurobio.2009.07.003
Yamaguchi, M., and Mori, K. (2005). Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc. Natl. Acad. Sci. U.S.A. 102, 9697–9702 doi: 10.1073/pnas.0406082102
Yokoyama, T. K., Mochimaru, D., Murata, K., Manabe, H., Kobayakawa, K., Kobayakawa, R., et al. (2011). Elimination of adult-born neurons in the olfactory bulb is promoted during the postprandial period. Neuron 71, 883–897. doi: 10.1016/j.neuron.2011.05.046
Yoshihara, S., Takahashi, H., Nishimura, N., Kinoshita, M., Asahina, R., Kitsuki, M., et al. (2014). Npas4 regulates Mdm2 and thus Dcx in experience-dependent olfactory bulb interneuron dendritic spine development. Cell Rep. 8, 843–857. doi: 10.1016/j.celrep.2014.06.056
Yoshihara, S., Takahashi, H., Nishimura, N., Naritsuka, H., Shirao, T., Hirai, H., et al. (2012). 5T4 glycoprotein regulates the sensory input-dependent development of a specific subtype of newborn interneurons in the mouse olfactory bulb. J. Neurosci. 32, 2217–2226. doi: 10.1523/JNEUROSCI.5907-11.2012
Zhao, Y., Malinauskas, T., Harlos, K., and Jones, E. Y. (2014). Structural insights into the inhibition of Wnt signaling by cancer antigen 5T4/Wnt-activated inhibitory factor 1. Structure 22, 612–620. doi: 10.1016/j.str.2014.01.009
Keywords: adult neurogenesis, olfactory bulb interneuron, neural activity-dependent, dendritogenesis, spinogenesis, 5T4, Npas4
Citation: Yoshihara S-i, Takahashi H and Tsuboi A (2016) Molecular Mechanisms Regulating the Dendritic Development of Newborn Olfactory Bulb Interneurons in a Sensory Experience-Dependent Manner. Front. Neurosci. 9:514. doi: 10.3389/fnins.2015.00514
Received: 15 October 2015; Accepted: 22 December 2015;
Published: 12 January 2016.
Edited by:
Laura López-Mascaraque, Instituto Cajal (CSIC), SpainReviewed by:
Harold Cremer, Centre National de La Recherche Scientifique, FranceKrishna Vadodaria, Salk Institute for Biological Science, USA
Copyright © 2016 Yoshihara, Takahashi and Tsuboi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akio Tsuboi, atsuboi@naramed-u.ac.jp
 Sei-ichi Yoshihara
Sei-ichi Yoshihara Hiroo Takahashi
Hiroo Takahashi Akio Tsuboi
Akio Tsuboi