- 1Department of Statistics, Texas A&M University, College Station, TX, United States
- 2Department of Agricultural Economics, Texas A&M University, College Station, TX, United States
In order to reduce the noise of brain signals, neuroeconomic experiments typically aggregate data from hundreds of trials collected from a few individuals. This contrasts with the principle of simple and controlled designs in experimental and behavioral economics. We use a frequency domain variant of the stationary subspace analysis (SSA) technique, denoted as DSSA, to filter out the noise (nonstationary sources) in EEG brain signals. The nonstationary sources in the brain signal are associated with variations in the mental state that are unrelated to the experimental task. DSSA is a powerful tool for reducing the number of trials needed from each participant in neuroeconomic experiments and also for improving the prediction performance of an economic choice task. For a single trial, when DSSA is used as a noise reduction technique, the prediction model in a food snack choice experiment has an increase in overall accuracy by around 10% and in sensitivity and specificity by around 20% and in AUC by around 30%, respectively.
1. Introduction
The interest of economists and other social scientists to integrate neurophysiological data to study human behavior has dramatically increased. Neuroeconomics has opened the door to research aiming to explain behavioral models of decision making (Camerer et al., 2004). However, neuroeconomics has only had limited reception into mainstream economics, perhaps due to the limitation of brain processes for improving the prediction of economic behavior (see Harrison, 2008; Bernheim, 2009; Konovalov and Krajbich, 2016). After all, economists are ultimately interested in predicting behavior (Gul and Pesendorfer, 2008; Fehr and Rangel, 2011).
Behavioral and experimental economics seem to be very much interrelated with neuroeconomics. However, behavioral and experimental economics rely on simple and controlled experiments to infer causality. Brain data, by nature, are very noisy. This is due to the fact that subjects react to the presented stimuli and process it in their brain visually (i.e., colors, shapes, etc.), physically (i.e., moving their eyes and muscles), emotionally, engaging in memory and other processes that simultaneously activate different regions of the brain. The data collected from an individual on a single trial measures the activity of the brain for the stimuli, but it also captures the noise from all other activity unrelated to the task of the experiment. Brain experiments typically implement hundreds of trials that when aggregated filter out noisy signals (Plassmann et al., 2007; Hare et al., 2009; Milosavljevic et al., 2010). There is a trade-off between the experimental economics principle of designing simple experiments to assess causality and the neuroeconomics need for a large number of trials to reduce the noise in brain signals.
An emerging literature in neuroeconomics uses brain signals to directly explain choice behavior. One of the models used to explain decision making is the Neural Random Utility Model (NRUM; Webb et al., 2013). EEG data have been used to predict purchase decisions (Ravaja et al., 2013), consumer's future choices (Telpaz et al., 2015), predict preferences (Khushaba et al., 2012, 2013) and response to advertisements (Boksem and Smidts, 2015; Venkatraman et al., 2015).
EEG signals from different electrodes measuring brain activity have, in the past, been regarded as a multi-dimensional nonstationary time series; see Ombao et al. (2005) and von Bünau et al. (2010) for examples. Kaplan et al. (2005) regard the nonstationarity as the “unavoidable noise” in the brain signal. Here the nonstationary sources in the brain signal contributes to the noise in the EEG data and removing this nonstationarity is extremely useful for prediction purposes in brain related experiments. We use the words noise and nonstationarity interchangeably because in our setup the nonstationary sources contribute to parts of the signal that are unrelated to the task related activity in the experiment. Hence eliminating nonstationarity reduces noise in the brain signal. See section 3.2 and Figures 1, 2 for illustrations of the signal before and after noise reduction. von Bünau et al. (2009) and von Bünau et al. (2010) associate alpha oscillations in the data as a nonstationary source. These oscillations appear usually in the range of 8–12 Hz and are associated with blinking, fatigue or tiredness. Over the course of the experiment such changes in the EEG time series are unrelated to the experimental task and corrupt the signal.
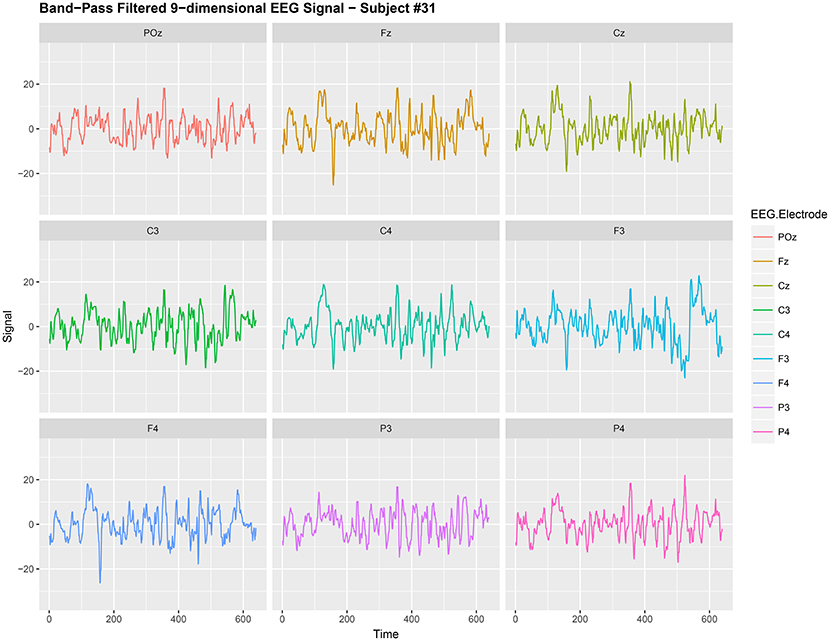
Figure 1. Band-Pass filtered 9-dimensional EEG signal {Xt,j:t = 1, 2, …, 640} (before noise reduction) gathered from subject #31 while responsing to food-choice question number 9.
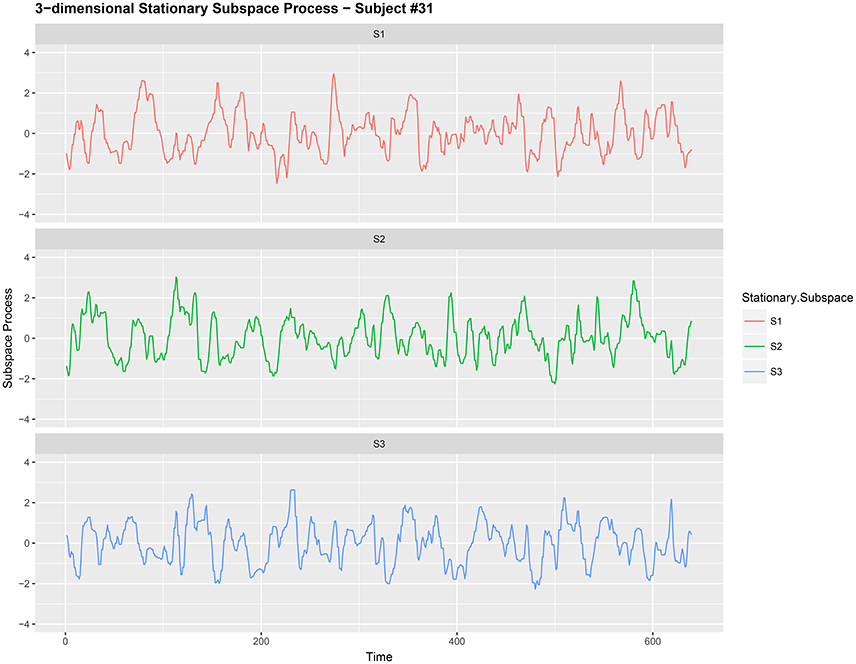
Figure 2. Three-dimensional stationary subspace process {Yt,j:t = 1, 2, …, 640} (after noise reduction) gathered from subject #31 while responsing to food-choice question number 9.
Transforming a multi-dimensional nonstationary time series into stationarity through linear transformations is a nontrivial problem of fundamental importance in many application areas. Stationary subspace analysis (SSA) (von Bünau et al., 2009) is a recent technique that attempts to find stationary linear transformations, in lower dimensions, of multi-dimensional nonstationary time series, where nonstationarity means independent heterogenous data with mean and variance smoothly varying across time.
In this paper, we apply dependent SSA (DSSA, for short) method proposed in Sundararajan and Pourahmadi (2017) for the general class of multi-dimensional nonstationary time series to data from a neuroeconomics case study. DSSA relies on the asymptotic uncorrelatedness of the discrete Fourier transform (DFT) of a second-order stationary time series at different Fourier frequencies and unlike the well-known cointegration theory that is restricted to parametric models such as VAR, DSSA avoids parametric model assumptions.
We employ the DSSA technique, as a noise reduction step to separate stationary (useful signal) and nonstationary sources to reduce noise in the EEG brain signal. This is important because using this process may move neurophysiological responses to become more aligned with the design of traditional economics experiments. In other words the nonstationary sources in the brain signal are associated with variations in the mental state that are unrelated to the experimental task at hand. Hence the DSSA technique can be useful in reducing the number of trials needed from each participant in neuroeconomic experiments. More importantly, the technique greatly improves the prediction performance of an incentivized economic food choice task. In addition, the DSSA technique performs a formal test of stationarity that ensures there is a statistically significant reduction in nonstationarity (noise) in the observed signal. The ability of DSSA and ISSA in detecting the true dimension of the stationary subspace process is illustrated for different sample sizes and dimensions and it was observed that in most cases DSSA performs better than ISSA. See section 3 of Sundararajan and Pourahmadi (2017) for more details.
The rest of the paper has the following content: Section 2 introduces the SSA model setup, describes the existing SSA technique and then the DSSA approach. Section 3 discusses an empirical application of decisions for purchasing food snacks. Subjects were presented with 10 food snack choice questions and the observed EEG signal from 9 electrodes is treated as a 9-dimensional time series. The various steps taken for noise reduction are described in section 3.2 and the “noise reduced” series is then analyzed. Finally, prediction models (section 3.2.2) of the decision made by the subjects regarding various food choices are constructed and their performance is illustrated.
2. Stationary Subspace Analysis (SSA)
We begin with the SSA model setup and the method in von Bünau et al. (2009) which deals with independent and heteroscedastic data and is denoted by ISSA. Then a SSA method for dependent data, denoted by DSSA, for finding the stationary subspace process (noise reduced signal) of the observed second-order nonstationary process (observed EEG signal) is described.
2.1. ISSA
Let {Xt} be the observed p-dimensional nonstationary time series that is linearly generated by d stationary sources and p − d nonstationary sources . More precisely,
where A is the unknown p × p (invertible) mixing matrix, As and An are p × d and p × (p − d) matrices, respectively. The dimension d is unknown and needs to be estimated. In ISSA, the notion of stationarity is with respect to the first two moments (i.e., mean and lag-0 covariance).
The goal is to estimate the demixing matrix B = A−1 so that Yt = BXt is separated into stationary and nonstationary sources. The nonstationary source contributes to the noise (for example, fatigue and tiredness of participants during the experiment) in the observed EEG signal and separating it from the stationary source is useful in improving performance in prediction models.
The matrix B is assumed to be an orthogonal matrix. The matrix B is estimated first by dividing the time series data into N epochs and then minimizing, as a function of B, the Kullback-Leibler (KL) divergence between Gaussian distributions across these segments. Let , , i = 1, 2, …, N, be the estimated mean and covariance of the data for the ith segment, respectively. Let the d × p matrix B1 be the first d rows of B. It follows that the mean and variance of on the ith segment are
The matrix B is then chosen so that the means and covariances vary the least across all epochs. This leads to en estimate B1 such that B1Xt is the target stationary source. The objective function is the sum of the Kullback-Leibler (KL) divergences between the on each segment and a normal distribution with their grand averages as its parameters, namely where and :
The matrix B is estimated by minimizing L(B); see von Bünau et al. (2009) for more details. In the above methodology, the partitioning of the time series into N segments comes with some disadvantages. In addition to the independence assumption, ISSA works under the assumption that nonstationarity in the data is only with respect to the first two moments (mean and variance) which can be restrictive.
2.2. Dependent SSA (DSSA)
Here we describe the DSSA approach to finding a stationary subspace process of given multi-dimensional second-order nonstationary processes satisfying Equation (1) using properties from the frequency domain. This method does not require dividing the time series data into several segments and utilizes a test of stationarity for determining whether the estimated source is stationary. This would be useful in not only finding the stationary subspace process (noise reduced signal) but to also ensure there is a statistically significant reduction in the nonstationarity (noise) in the observed EEG signal.
Recall that the discrete Fourier transform (DFT) of any d-variate series {Zt}, 1 ≤ t ≤ T, is given by
where , k = 1, 2, …, T. Treating the DFT as a time series indexed by k, its lag-r autocovariance is the d × d complex valued matrix given by
where denotes the complex conjugate transpose and r = 0, 1, …. It is well known (Theorem 4.3.1 of Brillinger, 2001) that for a second-order stationary time series , its DFTs are asymptotically uncorrelated when ωi ≠ ωj, i.e.,
where denotes the DFT of of . Thus, for a given positive integer m, based on the magnitudes of the first few autocovariances of the DFTs of , we construct the following objective function as a measure of departure from stationarity:
where for a matrix A ∈ ℝd × d, denotes its Frobenius norm. A solution is obtained by minimizing DY(B) subject to the orthonormality assumption , see Sundararajan and Pourahmadi (2017) for more details. In section 2.2.5 of the previous work a sequential technique for estimating the unknown dimension d of the stationary subspace is described.
The previous work includes theoretical justifications for DSSA to correctly identify the dimension d of the stationary source. Also, numerous simulation examples with different types of stationary and nonstationary sources are simulated and the ability of DSSA and ISSA to identify a stationary subspace process and its dimension d has been discussed. In these examples, for the nonstationary sources in Equation (1), independent Gaussian components with changing variances and dependent Gaussian components with changing variances are simulated. The dimensions were allowed to vary from 1 to 5. Other simulation examples include stationary vector autoregressive processes (VAR) with no nonstationary sources (d = p), time-varying vector moving average processes (VMA) with no stationary sources (d = 0) and nonstationary unit-root VAR processes with the number of nonstationary sources varying from 1 to p − 1.
3. Experiment of Economic Choices: A Case Study
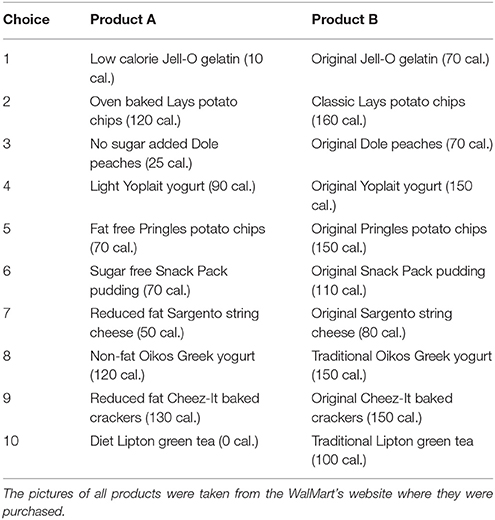
A total of 181 right-handed students participated in a food snack choice decision experiment conducted in the Texas A&M Human Behavior Laboratory. The sample consisted of about 50% females and 50% males. The subjects were presented with 10 food choice task questions (10 trials). Each choice consisted of two food products, product A and product B. The two products within each alternative had the same features relative to brand, price, packaging and flavor. The only difference between each pair of products was that one of them had fewer calories, making it a healthier choice (original strawberry Jello −70 calories- vs. sugar-free strawberry Jello −10 calories)1. Subjects were asked to fast for 3 h prior to the experiment, and received a compensation fee of $20 in exchange for their participation. In order to incentivize and make the food choice task real, one of the 10 tasks was randomly selected to be binding and participants had to eat the food snack before being paid and leaving the laboratory. The displayed picture of each item was the actual photo available for purchase in Walmart's website; however, the participants were not aware that the products were purchased in Walmart.
The experimental design proceeds as follow. At the beginning of the experiment, a blank slide with a fixation point in the middle of the computer screen was presented for 2 s. Then, for each food choice task, the actual product images were presented in the following screen for 8 s. A separate decision slide asked participants which of the two food snacks they prefer to eat. After each decision, an inter-stimulus slide was presented for 0.75 s. The order of the products was randomized across trials in the experimental design; however, all subjects completed the task in the same order.
3.1. Data Acquisition
The participant was fitted with a proper size EEG headset (B-Alert X10, Advanced Brain Monitoring, Inc.) with 9 electrodes to record brain activity from the pre-frontal (F3, F4, FZ), central (C3, C4, CZ), and parietal (P3, P4, POZ) cortices and a linked mastoid reference. An electrode impedance test was performed to ensure proper conductivity of the electrodes. The impedance level threshold was 20 kΩ. An EEG calibration procedure was implemented before the data collection. The EEG calibration incorporated choice tasks (unrelated to the study), psychomotor, and auditory psychomotor vigilance tasks. The EEG data was collected at a sampling rate of 256 Hz. The experiment was presented using the iMotions software platform.
3.2. Data Analysis
For any given food choice task, say product A vs. product B, we gathered the 9-dimensional EEG signal from the 9 electrodes from the start of the stimuli when the product images are shown to 2.5 seconds after the start. On the digital signal scale, this constitutes 640 observations (2.5 × 256). More precisely, for each subject j = 1, 2, …, 181, the data comprises of 640 observations across time.
Given the raw 9-dimensional EEG time series obtained in this case study, we proceed according to the following algorithm to obtain the prediction results:
In Step 2 we pre-whiten {Xt,j} before further analysis by computing the 9 × 9 sample covariance matrix Sj and then transform the data to . This standardization reduces the cross-sectional correlation in {Xt,j}.
3.2.1. Noise Reduction via SSA
It is common to treat data like {Xt,j} as a nonstationary time series (Ombao et al., 2005; Park et al., 2014). The words noise and nonstationarity are used interchangeably because in our setup the nonstationary sources contribute to parts of the signal that are unrelated to the food choice task. Hence eliminating nonstationarity reduces noise in the brain signal. As an illustration, we make a plot of the 9-dimensional EEG signal Xt,j (before noise reduction) in Figure 1. In Figure 2, we then plot a 3-dimensional stationary subspace process obtained after application of DSSA. The presence of nonstationarity (noise) in Xt,j was confirmed by carrying out formal tests of stationarity (Jentsch and Subba Rao, 2015). Hence we resort to the SSA technique for removing this nonstationarity from the signal and this is described in this section.
As a pre-processing technique to reduce noise, we apply DSSA and ISSA described in section 2 to obtain a d dimensional stationary subspace process where d < 9, denoted by {Yt,j}. Since the actual dimension d is unknown, we present the results for d = 4, 5, 6, 7, 8. We also applied the sequential technique in Sundararajan and Pourahmadi (2017) to detect d for each subject and each food choice task. Here we obtained a mode of d = 8 as an estimate of the dimension of the stationary subspace.
3.2.2. The Prediction Models
We discuss three prediction models based on logistic regression with different derived features. The aim of the prediction models discussed below is to fit a model to predict product choice (A or B) based on the input signal. While building prediction models M1 and M2, only Step 2 of the algorithm is used, for prediction model M3 both Steps 2 and 3 are needed. Note that model M2 assumes that {Xt,j} is stationary whereas model M3 assumes that {Xt,j} is nonstationary and applies SSA before extracting features and estimating the prediction model.
3.2.2.1. Model M1
A standard model similar to Telpaz et al. (2015) is based on the importance of the pre-frontal EEG channels in explaining choice behavior in individuals. Following their aggregation technique to reduce the noise when computing preference scores for products, we take average of the signals from the 3 pre-frontal channels (F3, F4, FZ) over the 2.5 s. The signal here is a 3-dimensional band-pass filtered signal that was pre-whitened. The average is taken per subject per food choice question (say product A vs. product B). For subject j, j = 1, 2, …, 181 , this average denoted by the scalar is used as a feature in the following logistic regression model:
for j = 1, 2, …, 181. In the model above we have denoted 1 for product A and 0 for product B and the model predicts the class (0 or 1) based on the derived feature .
3.2.2.2. Model M2
In this approach, to distinguish between the two classes denoted as 1 for product A and 0 for product B, we take {Xt,j} the pre-whitened 9-dimensional band-pass filtered signal. We then focus on the covariance structure of Xt,j for each of the two classes (0 and 1). The aim is to derive features that bring out the differences between the two classes based on the covariance structure of the signal. This is achieved by computing the average spectral density matrices for the two classes over the Fourier frequencies:
where gj(ωk) is the estimated 9 × 9 spectral matrix for subject j using observations {Xt,j}, ni for i = 0, 1 is the number of subjects in the two classes and , k = 1, 2, …, 640, are the fundamental Fourier frequencies. The spectral matrix was estimated using a Daniell kernel with smoothing window length 25 (approximately ); see Example 10.4.1 in Brockwell and Davis (1991).
In order to train the classifier, for every subject j ∈ {1, 2, …, 181}, a distance vector pj,AB = (p0,j,AB, p1,j,AB) is computed where
and ||·||F is the Frobenius norm of a matrix. It measures the distance to the center of each of the two classes and serves as our two-dimensional feature vector used in constructing the following logistic regression model (prediction model):
for j = 1, 2, …, 181 and cj, AB is the class indicator (1 for product A or 0 for product B) for subject j.
3.2.2.3. Model M3
Here we apply Step 2 on the raw 9-dimensional EEG signal to obtain {Xt,j}. Then we obtain on the d-variate stationary subspace processes, {Yt,j}, using DSSA/ISSA (Step 3). Similar to the approach in model M2, we aim to capture the differences between the two classes based on the covariance structure of the signal. Unlike model M2, we apply DSSA and ISSA described in section 2 to obtain a d-dimensional stationary subspace process where d < 9, denoted by {Yt,j}. Features to be fed into the prediction model will be based on {Yt,j} as opposed to model M2 wherein {Xt,j} was used. Then, proceeding as in model M2, we compute the average spectral density matrices for the two classes over the Fourier frequencies:
where fj(ωk) is the estimated d × d spectral matrix for subject j using observations {Yt,j}, ni for i = 0, 1 is the number of subjects in the two classes and , k = 1, 2, …, 640 are the fundamental Fourier frequencies. The spectral matrix was estimated using a Daniell kernel with smoothing window length 25 (approximately ).
In order to train the classifier, for every subject j ∈ {1, 2, …, 181}, a distance vector dj,AB = (d0,j,AB, d1,j,AB) is computed where
and ||·||F is the Frobenius norm of a matrix. It measures the distance to the center of each of the two classes and serves as our two-dimensional feature vector used in constructing the following logistic regression model (prediction model):
for j = 1, 2, …, 181 and cj, AB is the class indicator (1 for product A or 0 for product B) for subject j.
3.2.3. Prediction Performance
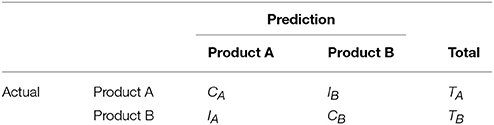
We asses the performance by computing the overall prediction accuracy and the average sensitivity and specificity. Using the confusion matrix given in Table 1, we compute two prediction accuracy measures given by
where A1 is the overall prediction accuracy of the model and A2, in a binary classification context, is the average of sensitivity (true positive rate) and specificity (true negative rate) of the prediction models. Finally, we present an estimate of the AUC: area under the ROC curve (LeDell et al., 2015) for the 10 food choice questions for each of the 3 models and this measure is denoted as A3. The ROC curve plots the true positive rate against the false positive rate and is a useful measure of model performance. The area under the ROC curve (known as AUC) varies between 0 and 100% with a value of 50% as baseline (uninformative classifier).
In Table 2, we shuffle the class labels randomly and fit the prediction models and assess the performance measures. The shuffling of labels is done 500 times, each time fitting the prediction models, and the average performance measure over the 500 runs across the 10 food choice questions is presented. This enables us to identify a baseline for the 3 performance measures (70% for performance measure A1 and 50% for performance measures A2 and A3).
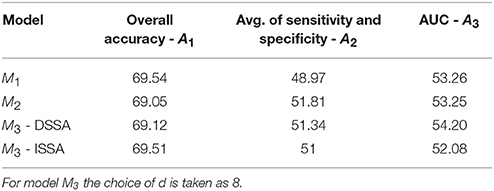
Table 2. Prediction performance of the 3 models with shuffled labels: the average of the 3 performance measures A1, A2, and A3 (AUC) taken across the 10 food choice questions for the three competing models M1, M2, and M3.
These accuracy rates are computed using a 10-fold cross-validation technique where the data is randomly divided into 10 nearly equal parts. Each part is removed, in turn, while the remaining data are used to fit the prediction models M1, M2, M3 and the predictions are carried out for the left out part. More precisely, the computed accuracy rates are the out-of sample estimates wherein for any given pair of products A and B, the prediction model is fit based on roughly 90% of the subjects and the predictions are carried out for the remaining subjects.
The overall accuracy rate (A1) for models M1 and M2 computed and plotted in Figure 3 shows that it varies between 69 and 72% for both models. Next, we look at the performance measure A2 as an average of the sensitivity and specificity of models M1 and M2. We notice from Figure 4 that both methods perform poorly with accuracy rates around 50%. Note that as opposed to averaging over the signal across the 3 channels in model (Equation 8), we also assessed the performance of the logistic regression models fitted individually with each of the pre-frontal channels. We obtained rates (not presented here) similar to that seen in Figures 3, 4 in terms of overall prediction accuracy and average of sensitivity and specificity.
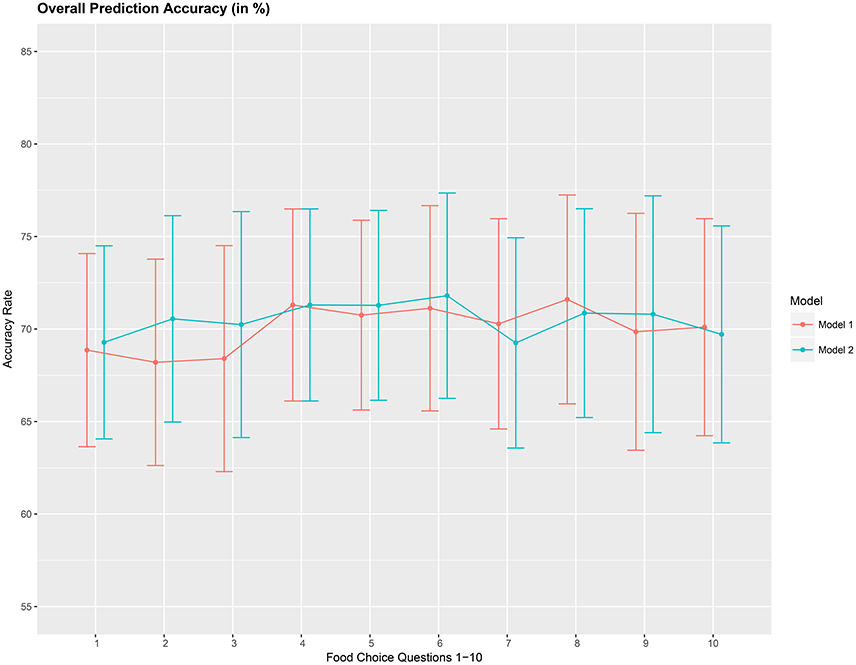
Figure 3. Overall prediction accuracy rate (A1), in %, based on a 10-fold cross-validation for the 10 food choice tasks for the two mdoels M1 and M2. Approximate 95% confidence intervals included for each accuracy estimate.
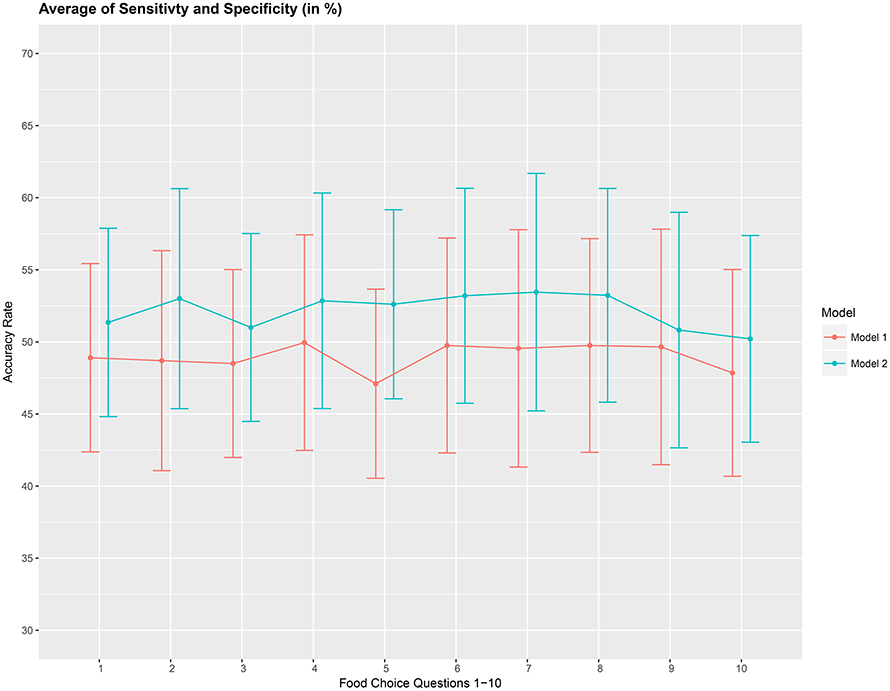
Figure 4. Prediction accuracy rate as average of sensitivity and specificity (A2), in %, based on a 10-fold cross-validation for the 10 food choice questions for the two models M1 and M2. Approximate 95% confidence intervals included for each accuracy estimate.
Next, we study the overall prediction accuracy (A1) after applying the pre-processing techniques DSSA and ISSA and removing the nonstationarity (noise) in the EEG signal, and fitting the prediction model (Equation 12 of model M3). Since the actual dimension of the stationary subspace is unknown, in Table 3 we present the results for dimensions d = 4, 5, 6, 7, 8, which show that DSSA performs better than ISSA in most cases. The average overall accuracy rate based on the 10 food choice tasks for each value of d is given in Figure 5. It is seen that the 10-fold cross-validation accuracy rate is around 80% for each of the 10 tasks when the dimension d = 8. This rate is roughly 10% more than the accuracy rate from Figure 3 wherein no SSA-type pre-processing technique is applied. We also notice that as the dimension of the stationary subspace d increases, the accuracy rate also increases. This phenomenon was also observed in von Bünau et al. (2010) and confirms the improvements in prediction accuracy when there are fewer nonstationary sources (noise) in model (Equation 1). The DSSA/ISSA turns out to be a very useful tool for reducing the noise (nonstationarity) in the EEG signal.
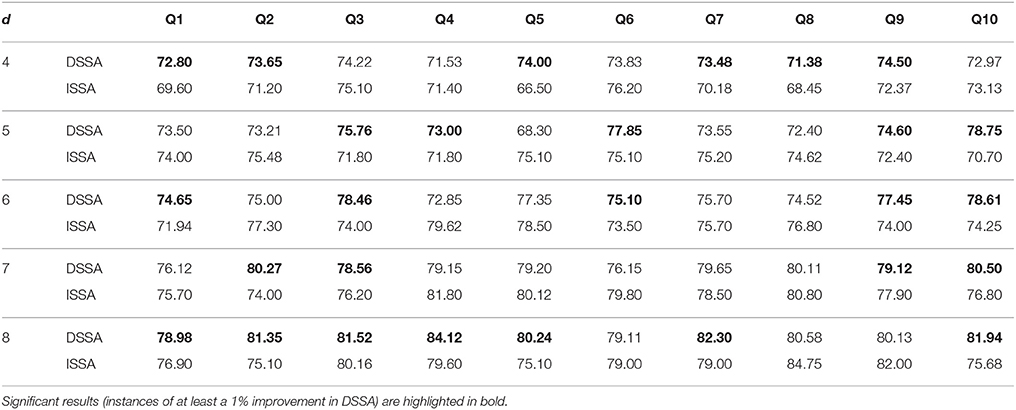
Table 3. Ten-fold cross-validation overall prediction accuracy (in %) for the 10 questions Q1–Q10 corresponding to d = 4, 5, 6, 7, 8 for DSSA and ISSA (model M3).
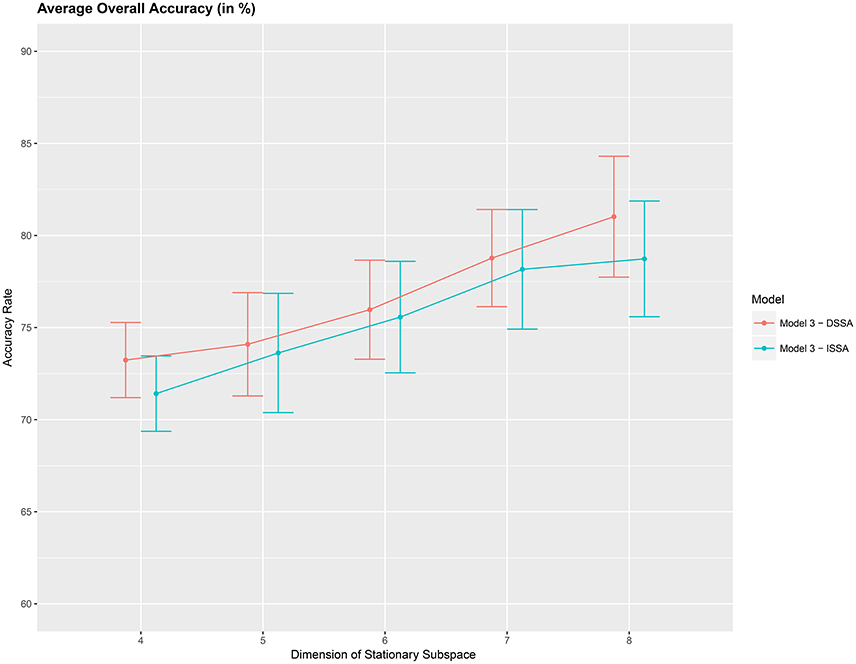
Figure 5. Average 10-fold cross-validation overall accuracy rate (in %) for the 10 food choice questions (y-axis) vs. dimension of the stationary subspace (x-axis). Approximate 95% confidence intervals included for each accuracy estimate.
We then asses the performance measure A2 which is an average of the sensitivity and specificity for model M3. We set the dimension of the stationary subspace at d = 8. Figure 6 shows that DSSA performs slightly better than ISSA in most cases. More importantly, we note that in comparison to Figure 4, DSSA has roughly a 20% increase in the performance measure A2.
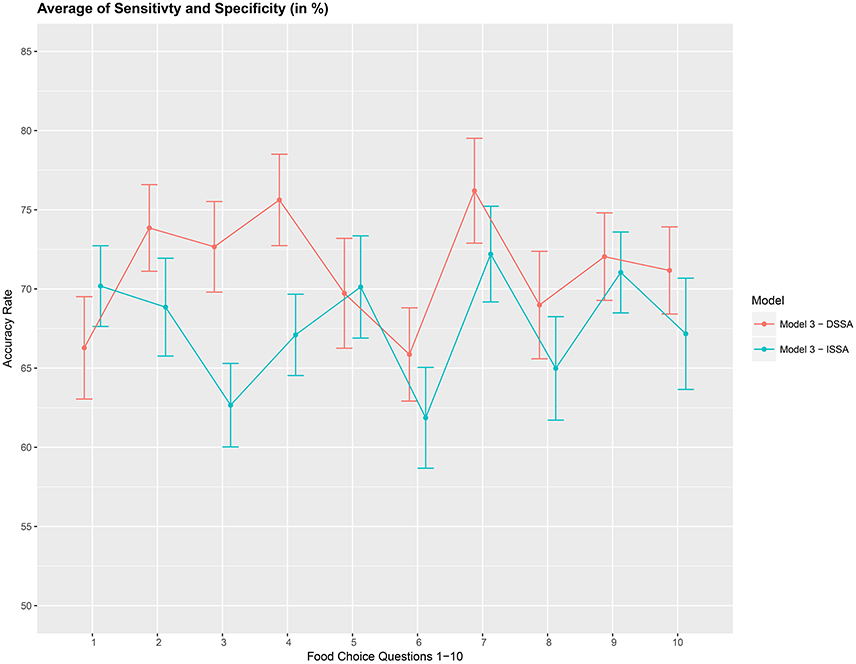
Figure 6. Prediction accuracy rate: average of sensitivity and specificity (A2), in %, based on a 10-fold cross-validation for the 10 food choice questions. Model M3 was used with d = 8. Approximate 95% confidence intervals included for each accuracy estimate.
Finally, we present a cross-validation estimate of the AUC for the 3 competing models in Figure 7. We again notice roughly a 20% increase when using DSSA/ISSA (Model M3) as a noise reduction technique before constructing the prediction model.
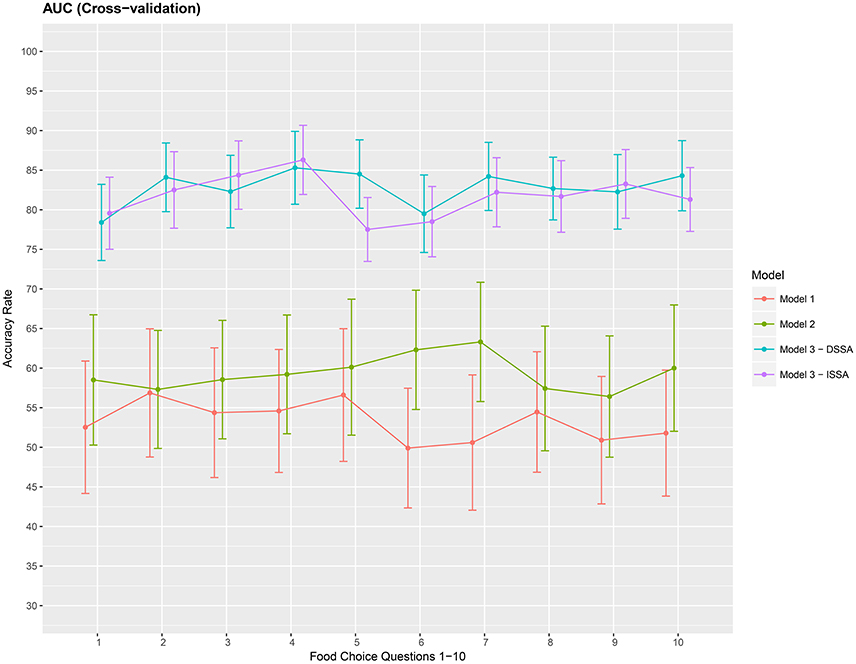
Figure 7. Cross-validation estimate of the AUC in % (Area under the ROC curve) for the 3 models M1 M2 and M3. Approximate 95% confidence intervals included for each accuracy estimate. For model M3 we take d = 8.
The average of the 3 performance measures A1, A2, and A3(AUC) taken across the 10 food choice questions for the three competing models M1, M2, and M3 is reported in Table 4. We note that for models M1 and M2 the overall prediction accuracy (A1) is roughly 70% which is treated as a baseline for this measure. However, the performance measures A2 and A3 (AUC) are only around 50% which suggests a poor performance. In contrast for model M3, overall accuracy rate increased by roughly 10%, the measure A2 is higher by around 20% and measure A3 (AUC) is significantly higher (increase of roughly 30%) than models M1 and M2.
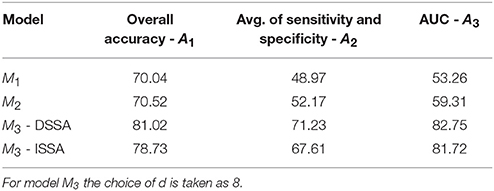
Table 4. The average of the 3 performance measures A1, A2, and A3 (AUC) taken across the 10 food choice questions for the three competing models M1, M2, and M3.
4. Concluding Remarks
EEG records the electrical activity of the brain directly in the scalp. EEG signals have high temporal resolution, thus providing rich time series data of brain activity. We concentrate on EEG because it is a less expensive method to obtain brain data, making it more accessible. Brain data, however, is inherently noisy, because it captures the brain activity for the stimuli, along with other activity unrelated to the task of the experiment. In order to filter out the noise, neuroeconomic experiments typically aggregate data from hundreds of trials from each participant. In addition to potential fatigue effects, we point out a tradeoff between the basic experimental economics principle of simplicity with the neuroeconomic need for a large number of trials to reduce brain signal noise. We apply a new statistical technique to a food choice task and show its potential for reducing the noise and hence the number of trials needed for EEG experiments. Based on the results presented in section 3.2.3, we notice that the overall accuracy rate is around 80% for each of the 10 trials when noise reduction is carried out through SSA (model M3). More importantly, the overall prediction accuracy from a single trial increased by around 10%, the average of sensitivity and specificity increased by around 20% and the AUC increased by roughly 30% when the DSSA/ISSA technique was used to reduce signal noise.
The improvement in the prediction results in this case study by implementing noise reduction via DSSA/ISSA indicates the dynamic behavior of the brain processes. This time-varying behavior leads to nonstationarity in the observed multi-dimensional EEG signal. This phenomenon has been observed and studied in other works (Ombao et al., 2005; Demiralp et al., 2007; von Bünau et al., 2009; Sundararajan and Pourahmadi, 2017, to name a few) wherein the observed EEG signal is treated as a multi-dimensional nonstationary time series. Hence removing nonstationarity from the EEG signal is seen to improve prediction performance in the above cited works and also in the current case study. The improvement in the prediction performance of EEG brain data shown in this case study is encouraging. Future work can build upon our procedure to develop other post-processing techniques to further refine neurophysiological predictors of choice behavior.
Ethics Statement
The Study was approved by the IRB protocol number IRB2016-0059D.
Author Contributions
The research problem and data came from the second author (Human Behavior Laboratory at Texas A&M). The methodology and computations were performed by the first and third authors. Contributions toward presentation of the results and connecting the research problem to the methodology came from all three authors. All authors have made direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was carried out in accordance with the recommendations of the Texas A&M Institutional Review Board with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Texas A&M IRB board protocol IRB2016-0059D. The first author was supported by the National Science Foundation DMS-1513647. The work of the third author was supported by the National Science Foundation DMS-1612984. We thank Michelle Segovia for assistance with data collection.
Footnotes
1. ^The product list and amount of calories is listed in section A.1 in Appendix. The focus of this paper is reducing EEG noise to improve the prediction of which of the two food snacks participants would choose, irrespective of the product's identity.
References
Bernheim, B. D. (2009). On the potential of neuroeconomics: a critical (but hopeful) appraisal. Am. Econ. J. Microecon. 1, 1–41. doi: 10.1257/mic.1.2.1
Boksem, M. A., and Smidts, A. (2015). Brain responses to movie trailers predict individual preferences for movies and their population-wide commercial success. J. Market. Res. 52, 482–492. doi: 10.1509/jmr.13.0572
Brillinger, D. (2001). Time Series: Data Analysis and Theory. Society for Industrial and Applied Mathematics. doi: 10.1137/1.9780898719246
Brockwell, P. J., and Davis, R. A. (1991). Time Series: Theory and Methods. Berlin: Springer-Verlag.
Camerer, C. F., Loewenstein, G., and Prelec, D. (2004). Neuroeconomics: why economics needs brains. Scand. J. Econ. 106, 555–579. doi: 10.1111/j.0347-0520.2004.00377.x
Demiralp, T., Bayraktaroglu, Z., Lenz, D., Junge, S., Busch, N. A., Maess, B., et al. (2007). Gamma amplitudes are coupled to theta phase in human EEG during visual perception. Int. J. Psychophysiol. 64, 24–30. doi: 10.1016/j.ijpsycho.2006.07.005
Fehr, E., and Rangel, A. (2011). Neuroeconomic foundations of economic choice—recent advances. J. Econ. Perspect. 25, 3–30. doi: 10.1257/jep.25.4.3
Gul, F., and Pesendorfer, W. (2008). “The case for mindless economics,” in The Foundations of Positive and Normative Economics: A Handbook, Vol. 1, eds A. Caplin and A. Schotter (Oxford University Press), 3–42.
Hare, T. A., Camerer, C. F., and Rangel, A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324, 646–648. doi: 10.1126/science.1168450
Harrison, G. W. (2008). Neuroeconomics: a critical reconsideration. Econ. Philos. 24, 303–344. doi: 10.1017/S0266267108002009
Jentsch, C., and Subba Rao, S. (2015). A test for second order stationarity of a multivariate time series. J. Econ. 185, 124–161. doi: 10.1016/j.jeconom.2014.09.010
Kaplan, A. Y., Fingelkurts, A. A., Fingelkurts, A. A., Borisov, S. V., and Darkhovsky, B. S. (2005). Nonstationary nature of the brain activity as revealed by EEG/MEG: methodological, practical and conceptual challenges. Signal Process. 85, 2190–2212. doi: 10.1016/j.sigpro.2005.07.010
Khushaba, R. N., Greenacre, L., Kodagoda, S., Louviere, J., Burke, S., and Dissanayake, G. (2012). Choice modeling and the brain: a study on the electroencephalogram (eeg) of preferences. Exp. Syst. Appl. 39, 12378–12388. doi: 10.1016/j.eswa.2012.04.084
Khushaba, R. N., Wise, C., Kodagoda, S., Louviere, J., Kahn, B. E., and Townsend, C. (2013). Consumer neuroscience: assessing the brain response to marketing stimuli using electroencephalogram (EEG) and eye tracking. Exp. Syst. Appl. 40, 3803–3812. doi: 10.1016/j.eswa.2012.12.095
Konovalov, A., and Krajbich, I. (2016). Over a decade of neuroeconomics: what have we learned? Organ. Res. Methods. doi: 10.1177/1094428116644502
LeDell, E., Petersen, M., and van der Laan, M. (2015). Computationally efficient confidence intervals for cross-validated area under the ROC curve estimates. Electron. J. Statist. 9, 1583–1607. doi: 10.1214/15-EJS1035
Milosavljevic, M., Malmaud, J., Huth, A., Koch, C., and Rangel, A. (2010). The drift diffusion model can account for the accuracy and reaction time of value-based choices under high and low time pressure. Judgm. Decis. Making 5, 437–449. doi: 10.2139/ssrn.1901533
Ombao, H., von Sachs, R., and Guo, W. (2005). Slex analysis of multivariate nonstationary time series. J. Am. Stat. Assoc. 100, 519–531. doi: 10.1198/016214504000001448
Park, T., Eckley, I. A., and Ombao, H. C. (2014). Estimating time-evolving partial coherence between signals via multivariate locally stationary wavelet processes. IEEE Trans. Signal Process. 62, 5240–5250. doi: 10.1109/TSP.2014.2343937
Plassmann, H., O'Doherty, J., and Rangel, A. (2007). Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J. Neurosci. 27, 9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007
Ravaja, N., Somervuori, O., and Salminen, M. (2013). Predicting purchase decision: the role of hemispheric asymmetry over the frontal cortex. J. Neurosci. Psychol. Econ. 6:1. doi: 10.1037/a0029949
Sundararajan, R. R., and Pourahmadi, M. (2017). Stationary subspace analysis of nonstationary processes. J. Time Series Anal. doi: 10.1111/jtsa.12274. [Epub ahead of print].
Telpaz, A., Webb, R., and Levy, D. J. (2015). Using EEG to predict consumers' future choices. J. Market. Res. 52, 511–529. doi: 10.1509/jmr.13.0564
Venkatraman, V., Dimoka, A., Pavlou, P. A., Vo, K., Hampton, W., Bollinger, B., et al. (2015). Predicting advertising success beyond traditional measures: new insights from neurophysiological methods and market response modeling. J. Market. Res. 52, 436–452. doi: 10.1509/jmr.13.0593
von Bünau, P., Meinecke, F. C., Scholler, S., and Müller, K. R. (2010). “Finding stationary brain sources in EEG data,” in 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (Buenos Aires), 2810–2813. doi: 10.1109/IEMBS.2010.5626537
von Bünau, P., Meinecke, F. C., Király, F. C., and Müller, K.-R. (2009). Finding stationary subspaces in multivariate time series. Phys. Rev. Lett. 103:214101. doi: 10.1103/PhysRevLett.103.214101
Webb, R., Glimcher, P. W., Levy, I., Lazzaro, S. C., and Rutledge, R. B. (2013). Neural random utility: relating cardinal neural observables to stochastic choice Behaviour'. SSRN. doi: 10.2139/ssrn.2143215
A. Appendix
A.1. List of Food Snack Products Used in the Experiment
A.1.1. Experimental Instructions
The Food Choice Task will proceed as follows:
1. This stage consists of 10 choice situations.
2. In each trial, you will be presented with two food products.
3. You need to choose which of the two products you would prefer to eat.
4. Your decisions are real. At the conclusion of the experiment, one decision will be randomly selected to be binding.
5. You will receive one single unit of the food product you chose and will have to eat it at the end of today's session.
Keywords: choice behavior, neuroeconomics, EEG data, multi-dimensional time series, stationary subspace analysis
JEL Codes: C32, D87
Citation: Sundararajan RR, Palma MA and Pourahmadi M (2017) Reducing Brain Signal Noise in the Prediction of Economic Choices: A Case Study in Neuroeconomics. Front. Neurosci. 11:704. doi: 10.3389/fnins.2017.00704
Received: 19 July 2017; Accepted: 30 November 2017;
Published: 14 December 2017.
Edited by:
Paul E. M. Phillips, University of Washington, United StatesReviewed by:
Dino J. Levy, Tel Aviv University, IsraelVijay Mohan K. Namboodiri, University of North Carolina at Chapel Hill, United States
Copyright © 2017 Sundararajan, Palma and Pourahmadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco A. Palma, mapalma@tamu.edu
 Raanju R. Sundararajan
Raanju R. Sundararajan Marco A. Palma
Marco A. Palma Mohsen Pourahmadi1
Mohsen Pourahmadi1