How emotions change time
- Department of Psychology, National University of Singapore, Singapore
Experimental evidence suggests that emotions can both speed-up and slow-down the internal clock. Speeding up has been observed for to-be-timed emotional stimuli that have the capacity to sustain attention, whereas slowing down has been observed for to-be-timed neutral stimuli that are presented in the context of emotional distractors. These effects have been explained by mechanisms that involve changes in bodily arousal, attention, or sentience. A review of these mechanisms suggests both merits and difficulties in the explanation of the emotion-timing link. Therefore, a hybrid mechanism involving stimulus-specific sentient representations is proposed as a candidate for mediating emotional influences on time. According to this proposal, emotional events enhance sentient representations, which in turn support temporal estimates. Emotional stimuli with a larger share in ones sentience are then perceived as longer than neutral stimuli with a smaller share.
Introduction
Being able to time is essential for a range of tasks including driving a car, cooking a meal, or having a conversation (Schirmer, 2004; Buhusi and Meck, 2005). Although we typically learn and complete these tasks with little effort, there are instances in which cars collide, meals overcook, and conversations desynchronize. Thus, it seems that, although accurate on average, our sense of time is not fixed but varies within and across individuals.
Prominent among the factors that influence our sense of time are emotions (for reviews see Droit-Volet and Meck, 2007; Craig, 2009a; Droit-Volet and Gil, 2009). Evidence for this comes from a range of paradigms some of which will be shortly reviewed here. One paradigm entails the presentation of stimuli for which participants subsequently estimate or reproduce the duration. With a few exceptions (e.g., Noulhiane et al., 2007), to-be-timed emotional stimuli elicit longer estimates and reproduction times than to-be-timed neutral stimuli (Angrilli et al., 1997; Noulhiane et al., 2007; Doi and Shinohara, 2009). A second popular paradigm is called duration bisection. In the training phase of this paradigm, participants learn to discriminate two anchor durations, one short and one long. In the test phase, participants perceive probe stimuli ranging in duration from the short to the long anchor and judge whether these stimuli are more similar to the short or the long anchor. Again, with a few exceptions (Gil et al., 2009; Droit-Volet et al., 2010), emotional test stimuli are judged more similar to the long anchor than neutral stimuli (Effron et al., 2006; Gil et al., 2007; Grommet et al., 2011). Finally, researchers have examined the relationship between emotion and subjective time using a duration discrimination paradigm with intermediate emotional or neutral distractors (Lui et al., 2011). Here participants are presented with two successive stimuli (i.e., S1/S2) and indicate whether the second stimulus was shorter or longer than the first. Participants are more likely to judge S2 as shorter when it was preceded by an emotional as compared to neutral distractor.
Together, this literature confirms the popular perception that emotions both speed-up and slow-down our sense of time. Moreover, it suggests that emotional events are generally perceived as longer than they really are, which may be conceptualized as a speeding up of subjective temporal pulses. It also suggests that neutral events occurring in the context of a distracting, emotional one are perceived as shorter then they really are, which may be conceptualized as a slowing down of subjective temporal pulses. Different mechanisms have been proposed to explain these effects and the remainder of this paper will focus on reviewing and contrasting them.
Arousal Modulates Time
The first mechanism draws on a popular timing model derived from scalar expectancy theory (SET; Gibbon et al., 1984). This model entails a clock stage comprising a pacemaker and an accumulator, both of which are connected by a switch. The pacemaker emits pulses that are collected by the accumulator when the switch is in a closed state. Delayed closing or flickering of the switch causes pulses to be lost (Penney, 2003). The clock stage is followed by a memory stage. During this stage, pulses from the accumulator enter working memory and are compared with previously stored durations. The result of this comparison informs temporal decisions and associated behavioral responses.
Research in both non-human animals and humans suggests that bodily arousal affects timing at the clock stage. For example, rats injected with the psychostimulant methamphetamine, a drug which enhances dopaminergic activity, treat a given duration as longer than do rats injected with saline (Meck, 1983). In contrast, rats injected with the dopamine antagonist haloperidol treat the duration as shorter than do the control rats (Meck, 1983). In humans, changes in physiological arousal as assessed by self-report and psychophysiological markers have been shown to concur with changes in perceived time. Relative overestimations have been found in conjunction with a putative increase in bodily arousal (Angrilli et al., 1997; Droit-Volet et al., 2011; Mella et al., 2011), whereas relative underestimations have been found with a putative decrease in bodily arousal (Wearden et al., 1999). As these effects typically represent a simple shift in the timing function, researchers assume that arousal positively correlates with pacemaker rate. Moreover, because emotions frequently increase physiological arousal, emotion effects on timing have been proposed to be mediated by the arousal/pacemaker link (Droit-Volet and Meck, 2007; Droit-Volet and Gil, 2009).
Although compelling, this proposal is not without problems. First, the concept of arousal and its relationship to an internal clock are poorly defined. Implicit in the published work is the understanding that arousal equates with sympathetic activation as determined by increased heart-rate, breathing, or skin conductance (e.g., Droit-Volet et al., 2011). However, this and other possible conceptualizations are not clearly spelled out. Moreover, it is assumed that this arousal somehow feeds back to the internal clock supported by the dopaminergic system. Yet, how this feedback occurs is still unclear. Second, available evidence for a relationship between emotional arousal and duration estimates is at best indirect. Although studies have shown corresponding differences in arousal measures and duration judgments elicited by emotional versus neutral stimuli, they never actually correlated the two at an individual or trial level. Moreover, even if such a correlation were found, effects of arousal on attention and working memory (Brennan and Arnsten, 2008; Advokat, 2010) offer an alternative causal link. Third, there appears to be a mismatch between the time-course of emotion-induced arousal and timing effects reported in the literature. The latter have been observed primarily for short stimuli (∼2–3 s) and seem to dissipate for longer stimuli (Noulhiane et al., 2007; Mella et al., 2011). In comparison, emotion-induced increases in physiological arousal are relatively sluggish with heart-rate and skin conductance taking between 3 and 6 s to peak (Bradley et al., 2001; Schirmer and Escoffier, 2010). Moreover, heart-rate for example typically decreases for the first 3 s through parasympathetic activation (Bradley et al., 2001). During this time, one should observe a slow-down in time keeping if heart-rate or sympathetic activation translate into pacemaker rate. Finally, the arousal model fails to fully accommodate existing data (Droit-Volet and Meck, 2007; Droit-Volet and Gil, 2009). Not all emotions lead to an increase in physiological parameters linked to arousal (e.g., sadness; Levenson et al., 1990) but may nevertheless elicit temporal overestimation (Lee et al., 2011). Furthermore, situations in which the timing stimulus is neutral, but presented in the context of an emotionally arousing distractor, fail to produce temporal overestimations (Lui et al., 2011). Given these problems, the arousal/pacemaker link may not or may not fully explain the relationship between emotions and time.
Attention Modulates Time
A second putative mechanism by which emotions may shape our experience of time is linked to the cognitive resources allocated to stimulus processing. Two possibilities of how stimulus processing may affect timing will be discussed here. The first concerns the amount of attention individuals dedicate to time. In reference to the SET timing model, mentioned above, it assumes that the clock stage (Macar et al., 1994; Droit-Volet, 2003) and/or memory stage (Buhusi and Meck, 2009) share attentional resources with other ongoing mental processes such that greater resource allocation to the temporal dimension of a stimulus results in fewer temporal pulses being lost. Support for this notion comes from studies that manipulated participant attention to time. For example, Macar et al. (1994) presented participants with stimuli for which they had to subsequently judge both duration and intensity. On some trials, participants were cued to focus all or most of their attention on the duration task, whereas on other trials, they were cued to focus all or most of their attention on the intensity task. Compared to the former, the latter trials were more likely to result in temporal underestimations of the target suggesting that pulses from a putative pacemaker were lost.
In line with this, some researchers reported underestimation of emotional relative to neutral stimuli in timing tasks. For example, participants underestimated the duration of appetizing and disgusting food pictures relative to neutral food pictures and this was interpreted to reflect a diversion of attention from the temporal dimension of the pictures to their emotional dimension (Gil et al., 2009). As such, the first attentional mechanism introduced here has some explanatory power. Moreover, unlike the arousal mechanism, it makes clear statements as to the underlying processes and has the advantage that temporal distortions can be explained by processes occurring during rather than after temporal encoding. Yet, its problem is that it discords with the overwhelming number of studies that find overestimation for emotional as compared to neutral stimuli. Given the greater significance of emotional relative to neutral events, the former should always, not just in the case of disgust, divert attention away from time and produce temporal underestimations.
A second variant of the cognitive resource mechanism addresses this problem. It assumes that it is the stimulus processing itself that contributes to timing and that attentional resources directed toward or away from the stimulus determine timing accuracy (Hicks et al., 1977; Ornstein, 1997). This proposal fits most over- and underestimation effects reported in the literature. Overestimations of emotional relative to neutral stimuli could be explained by the former recruiting more cognitive resources and having greater ability to sustain attention than the latter. Underestimations of neutral stimuli presented in an emotional as compared to neutral context could be explained by the former being more distracting than the latter, thereby leaving fewer resources for the processing of the timing stimulus (Lui et al., 2011). Nevertheless, this proposal is not without problems. For example, it cannot explain why presentation durations of appetizing foods are underestimated relative to neutral foods (Gil et al., 2009) and, more importantly, it conflicts with the evidence that attention to non-temporal stimulus dimensions typically impairs timing (Macar et al., 1994).
Sentient Processing Modulates Time
A third proposal for how emotions influence time evokes the concept of sentience – often equated with being aware or conscious of one’s self (Craig, 2009a; Wittmann, 2009). Specifically, it holds that external and internal sensory signals including information from the muscles, skeletal system, and internal organs are integrated in the brain and enable self-awareness. As we experience ourselves across moments or time, the proposal makes sentience the basis for temporal perception and the mediator in the relationship between emotions and time. Emotion-induced bodily changes, such as heart-rate decelerations or accelerations, presumably increase sentience and thereby sensitivity to the passage of time. Different lines of theoretical and experimental work substantiate this possibility.
Vierordt, a nineteenth century pioneer of timing research, raised the “self” as a potential origin for the emergence of time (Vierordt, 1868). Furthermore, James (1981) argued for a reliance of temporal perception on sensation and noted that perceived durations tend to have an “emotional feeling.” He also famously speculated about a critical role of bodily feedback for emotion. Although heavily criticized at the time, recent emotion theory has turned back to James and leveraged on his ideas. Important with respect to the present paper is Damasio’s somatic marker hypothesis, which holds that an emotional event triggers bodily changes whose feedback to the brain critically contributes, not just to the emotional experience, but also to the processing of the event and event-related decision making (Damasio, 1996). In extension of this, some researchers interested in the processing of time now hold that there is a relationship between sentient processing and subjective time (Effron et al., 2006; Craig, 2009a; Wittmann, 2009).
Experimental support for this assertion is steadily accumulating. For example, researchers have identified a correlation between self-awareness and temporal estimates. Individuals who are experimentally deprived of visual, tactile, and/or auditory stimulation and whose primary sensory experiences therefore come from within report that the hours pass more slowly than usual (Schulman et al., 1967). Furthermore, it was demonstrated that both bodily change and awareness of one’s current bodily state positively predict temporal estimates in interval-timing tasks (Meissner and Wittmann, 2011). Individuals with a more pronounced decrease in heart-rate during temporal encoding and a better accuracy at guessing their own heart-rate were less likely to underestimate a given temporal duration.
Neuroimaging studies further substantiate the link between sentient processing and time. Sentient processing has been associated with the insular cortex. This structure receives input from all senses including the somatosensory sense, which apart from its role in touch informs about visceral, skeletal, and muscular states. The insula has therefore been proposed as the seat for self-awareness or consciousness (Craig, 2009a,b). Although, temporal processing has been largely linked to striatal and prefrontal structures (Buhusi and Meck, 2005), the insula has been implicated as well (Pouthas et al., 2005; Livesey et al., 2007; Wittmann et al., 2010; for a review see Wittmann, 2009). For example, Pouthas et al. (2005) isolated insula activation both when comparing a timing task with a control task and when comparing the timing of long with short durations. Wittmann et al. (2010) further tested the relationship between insular activation and time. They found that this activation increased linearly with the encoded stimulus duration. Based on this and the intricate connections between insula and striatum (Chikama et al., 1997), one may venture that the insula’s sentient computations support temporal perception and potentially mediate the relationship between emotions and time.
One such possible mediation could entail an influence of emotion-induced bodily changes on sentient processing. Specifically, such changes might heighten sentient processing and self-awareness thereby contributing to an increased sensitivity to the passage of time (Wittmann, 2009). Evidence in support of this possibility is currently only indirect but nevertheless promising. First, researchers have shown that both bodily change and sentience positively predict the duration of temporal estimates. But more importantly, there seems to be a correlation between both predictors. Individuals with a greater reduction in heart-rate during timing trials tended to be better at guessing their own heart-rate prior to the timing task (Meissner and Wittmann, personal communication). A second line of support, for a link between bodily changes and sentient processing comes from the emotion literature. As demonstrated repeatedly, emotional stimuli elicit greater bodily changes than neutral stimuli (Bradley et al., 2001) such that bodily changes may well account for associated differences in the perception of time. Furthermore, meta-analyses find the insula more strongly activated for emotional as compared to neutral events, particularly when individuals focus on how they feel (Phan et al., 2004; Lee and Siegle, 2009). Thus, one may infer that emotion-induced bodily changes enhance sentient representations in the insula and that these representations are further enhanced if bodily changes become the focus of attention. Lastly, a study by Effron et al. (2006) speaks to the relationship between bodily states, sentience, and time. These authors asked participants to perform a temporal bisection task with emotional and neutral facial expressions in the test phase. In one condition, participants were prevented from automatically embodying the facial expression by holding a pen in their mouth. In another condition, participants could move their face freely. Relative overestimations for emotional as compared to neutral expressions were only found in the latter condition suggesting that bodily representations are critical for emotion effects on time.
Like the attentional proposal, the sentient proposal has a few advantages over the arousal proposal. First, it outlines a concrete mechanism and brain substrate by which emotions influence time. As such, the proposal can be tested relatively easily. Second, it ties emotion-induced temporal distortions to processes that occur while, not after, participants encode time. Specifically, while the arousal proposal evokes sympathetic activations that often peak after the timing stimulus has lapsed, the sentient proposal allows for any bodily changes to affect time. Hence, also parasympathetic changes, such as heart-rate decelerations that occur after the onset of a timing stimulus, can affect temporal estimates. Nevertheless, the sentient proposal is not without problems. Specifically, if one assumes that emotional stimuli lead to bodily changes and thus potentially heighten sentient processing and temporal awareness, such stimuli should consistently produce longer temporal estimates than neutral stimuli. However, emotional events may both speed-up and slow-down subjective time relative to neutral events and thus, by itself, this proposal also fails as an adequate explanation.
Model Integration
As usual, where there is conflict, reconciliation may be attempted through a compromise of existing proposals. Such a compromise could take different forms. One possibility is that the various mechanisms identified here all support the relationship between emotions and time and that their respective contributions vary across situations and, perhaps, individuals. For example, their contributions may depend on the particular emotion that is evoked by a timing stimulus or distractor. Fear relevant stimuli might accelerate an internal clock by increasing bodily arousal, capturing an individual’s attention (Pourtois et al., 2006), and enhancing sentient processing. Disgust relevant stimuli, on the other hand, may decelerate an internal clock by biasing our attention away from both the stimulus and its temporal dimension (Curtis et al., 2004) in-spite of up-regulating sentient processing (Wicker et al., 2003). Likewise different mechanisms may be at play depending on whether emotional stimuli are the context or focus of the timing task and depending on stimulus duration. With respect to the latter factor, one could envision emotion effects on attention to be more acute during initial as compared to continued stimulus processing thus leading to a drop-off of emotion effects for the timing of longer stimuli.
Despite its ability to accommodate the data, the possibility of situation-specific timing mechanisms is unsatisfying. It seems too flexible to have true explanatory power and thus one may attempt to reconcile the literature by proposing a fourth, hybrid mechanism with a more general utility. In light of the preceding discussion it seems that the stimulus and the sentient processing mechanisms are well suited for this. Specifically, all one needs to do is accept the idea that sentient processing is stimulus-specific; that we experience ourselves not independently from our environment but in relationship to the various stimuli and events we encounter. Thus, we create stimulus-specific sentient and ultimately temporal representations.
Although speculative, this notion is not too far fetched because there is already evidence that we can assign different temporal values to equally long and temporarily overlapping stimuli (Penney et al., 2000). Specifically, two concurrent stimuli of equivalent duration, but differing in modality may be perceived as of different duration, thereby raising the possibility of different (i.e., stimulus-specific) sentient representations. In the context of emotions, such representations could account for both relative over and underestimations observed in interval-timing studies. As mentioned before, overestimations would result for emotional as compared to neutral timing stimuli due to an increase in sentient processing. Underestimations would result for neutral timing stimuli presented in the context of emotional as compared to neutral distractors. Here, sentient representations emerging from the timing stimulus would be relatively weaker than the sentient representations emerging from the emotional distractor, making the former seem shorter than it really is.
The hybrid model proposed here (Figure 1) fairs better than the arousal, attention, or sentient model alone in accommodating the existing data. Moreover, by making emotions relevant for timing it solves the issue that attention to non-temporal neutral information (e.g., intensity) impairs timing, whereas attention to non-temporal emotional information does not. It holds that attending to emotions is similar to attending to time. Nevertheless, the model is at present highly speculative. It rests on a few assumptions that have not yet been tested or for which existing evidence is still equivocal. For example, the assumption that emotion-induced bodily changes enhance self-awareness awaits investigation. This could be done by relating objective physiological measures to self-reports of cardiac or respiratory activity and to temporal judgments. Specifically, one could test whether self-reports mediate the relationship between bodily change and subjective time. Furthermore, the idea of stimulus-specific sentient representations needs further investigation. The evidence cited above merely suggests that we can perceive concurrently presented same-duration stimuli as differently long. Whether and in what way this is linked to different sentient representations and whether emotions could differently moderate those are still open issues.
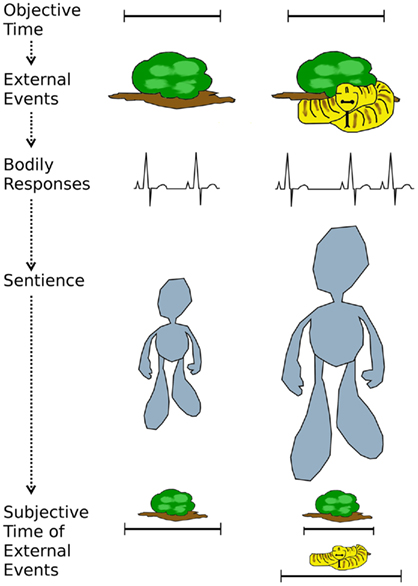
Figure 1. Illustrated is a potential mechanism by which emotions may influence the perceived duration of external events. Depending on their emotional significance, environmental stimuli may trigger bodily changes, which in turn may increase self-awareness or sentience – a potential contributor to the perception of time. Depending on their contribution to bodily and sentient changes, concurrent environmental stimuli may be perceived as relatively longer (e.g., snake) or shorter (e.g., shrub) than their objective duration.
Conclusion
Emotion-induced subjective changes in time are a ubiquitous everyday phenomenon that is well documented in the literature. Different proposals have been put forth to explain how emotions impact time. Of these proposals, a hybrid that combines sentient and stimulus processing seems to best accommodate existing experimental data as it can account for why we sometimes experience events to last longer and shorter than they really are. According to this proposal, such events trigger bodily changes that increase our sense of “being” across time. The subjective duration of objects that concur with such events then depends on how much they share in sentience or awareness. Salient objects that capture and hold attention would be overestimated relative to other, less salient objects. Thus, differences in perceptual salience would translate into differences in mnemonic salience as the more important stimuli would receive greater temporal weights. As such these objects may more readily influence present and future behaviors.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I would like to thank Trevor Penney and Marc Wittmann for commenting on an earlier version of this manuscript. This work was supported by the NUS Yong Investigator Award (WBS R581-000-066-101) and the A*Star SERC grant (0921570130).
References
Advokat, C. (2010). What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD). Neurosci. Biobehav. Rev. 34, 1256–1266.
Angrilli, A., Cherubini, P., Pavese, A., and Mantredini, S. (1997). The influence of affective factors on time perception. Percept. Psychophys. 59, 972–982.
Bradley, M. M., Codispoti, M., Cuthbert, B. N., and Lang, P. J. (2001). Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion 1, 276–298.
Brennan, A. R., and Arnsten, A. F. T. (2008). Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann. N. Y. Acad. Sci. 1129, 236–245.
Buhusi, C. V., and Meck, W. H. (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765.
Buhusi, C. V., and Meck, W. H. (2009). Relative time sharing: new findings and an extension of the resource allocation model of temporal processing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1875–1885.
Chikama, M., McFarland, N. R., Amaral, D. G., and Haber, S. N. (1997). Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J. Neurosci. 17, 9686–9705.
Craig, A. D. B. (2009a). Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1933–1942.
Craig, A. D. B. (2009b). How do you feel – now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70.
Curtis, V., Aunger, R., and Rabie, T. (2004). Evidence that disgust evolved to protect from risk of disease. Proc. R. Soc. B Biol. Sci. 271, S131–S133.
Damasio, A. R. (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 1413–1420.
Doi, H., and Shinohara, K. (2009). The perceived duration of emotional face is influenced by the gaze direction. Neurosci. Lett. 457, 97–100.
Droit-Volet, S. (2003). Alerting attention and time perception in children. J. Exp. Child. Psychol. 85, 372–384.
Droit-Volet, S., Bigand, E., Ramos, D., and Bueno, J. L. O. (2010). Time flies with music whatever its emotional valence. Acta Psychol. (Amst.) 135, 226–232.
Droit-Volet, S., Fayolle, S. L., and Gil, S. (2011). Emotion and time perception: effects of film-induced mood. Front. Integr. Neurosci. 5:33. doi: 10.3389/fnint.2011.00033
Droit-Volet, S., and Gil, S. (2009). The time-emotion paradox. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1943–1953.
Droit-Volet, S., and Meck, W. H. (2007). How emotions colour our perception of time. Trends Cogn. Sci. (Regul. Ed.) 11, 504–513.
Effron, D. A., Niedenthal, P. M., Gil, S., and Droit-Volet, S. (2006). Embodied temporal perception of emotion. Emotion 6, 1–9.
Gibbon, J., Church, R. M., and Meck, W. H. (1984). Scalar timing in memory. Ann. N. Y. Acad. Sci. 423, 52–77.
Gil, S., Niedenthal, P. M., and Droit-Volet, S. (2007). Anger and time perception in children. Emotion 7, 219–225.
Gil, S., Rousset, S., and Droit-Volet, S. (2009). How liked and disliked foods affect time perception. Emotion 9, 457–463.
Grommet, E. K., Droit-Volet, S., Gil, S., Hemmes, N. S., Baker, A. H., and Brown, B. L. (2011). Time estimation of fear cues in human observers. Behav. Processes 86, 88–93.
Hicks, R. E., Miller, G. W., Gaes, G., and Bierman, K. (1977). Concurrent processing demands and the experience of time-in-passing. Am. J. Psychol. 90, 431–446.
Lee, K.-H., Seelam, K., and O’Brien, T. (2011). The relativity of time perception produced by facial emotion stimuli. Cogn. Emot. PMID: 21432628. [Epub ahead of print].
Lee, K. H., and Siegle, G. J. (2009). Common and distinct brain networks underlying explicit emotional evaluation: a meta-analytic study. Soc. Cogn. Affect. Neurosci. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19270039 [Accessed July 14, 2011].
Levenson, R. W., Ekman, P., and Friesen, W. V. (1990). Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology 27, 363–384.
Livesey, A. C., Wall, M. B., and Smith, A. T. (2007). Time perception: manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia 45, 321–331.
Lui, M. A., Penney, T. B., and Schirmer, A. (2011). Emotion effects on timing: attention versus pacemaker accounts. PLoS ONE 6, e21829. doi: 10.1371/journal.pone.0021829
Macar, F., Grondin, S., and Casini, L. (1994). Controlled attention sharing influences time estimation. Mem. Cognit. 22, 673–686.
Meck, W. H. (1983). Selective adjustment of the speed of internal clock and memory processes. J. Exp. Psychol. Anim. Behav. Process. 9, 171–201.
Meissner, K., and Wittmann, M. (2011). Body signals, cardiac awareness, and the perception of time. Biol. Psychol. 86, 289–297.
Mella, N., Conty, L., and Pouthas, V. (2011). The role of physiological arousal in time perception: psychophysiological evidence from an emotion regulation paradigm. Brain Cogn. 75, 182–187.
Noulhiane, M., Mella, N., Samson, S., Ragot, R., and Pouthas, V. (2007). How emotional auditory stimuli modulate time perception. Emotion 7, 697–704.
Penney, T. B. (2003). “Modality differences in interval timing: attention, clock speed, and memory,” in Functional and Neural Mechanisms of Interval Timing, ed. W. Meck (Boca Raton, FL: CRC Press), 209–234.
Penney, T. B., Gibbon, J., and Meck, W. H. (2000). Differential effects of auditory and visual signals on clock speed and temporal memory. J. Exp. Psychol. Hum. Percept. Perform. 26, 1770–1787.
Phan, K. L., Wager, T. D., Taylor, S. F., and Liberzon, I. (2004). Functional neuroimaging studies of human emotions. CNS Spectr. 9, 258–266.
Pourtois, G., Schwartz, S., Seghier, M. L., Lazeyras, F., and Vuilleumier, P. (2006). Neural systems for orienting attention to the location of threat signals: an event-related fMRI study. Neuroimage 31, 920–933.
Pouthas, V., George, N., Poline, J.-B., Pfeuty, M., Vandemoorteele, P.-F., Hugueville, L., Ferrandez, A.-M., Lehéricy, S., Lebihan, D., and Renault, B. (2005). Neural network involved in time perception: an fMRI study comparing long and short interval estimation. Hum. Brain Mapp. 25, 433–441.
Schirmer, A. (2004). Timing speech: a review of lesion and neuroimaging findings. Brain Res. Cogn. Brain Res. 21, 269–287.
Schirmer, A., and Escoffier, N. (2010). Emotional MMN: anxiety and heart rate correlate with the ERP signature for auditory change detection. Clin. Neurophysiol. 121, 53–59.
Schulman, C. A., Richlin, M., and Weinstein, S. (1967). Hallucinations and disturbances of affect, cognition, and physical state as a function of sensory deprivation. Percept. Mot. Skills 25, 1001–1024.
Wearden, J. H., Pilkington, R., and Carter, E. (1999). “Subjective lengthening” during repeated testing of a simple temporal discrimination. Behav. Processes 46, 25–38.
Wicker, B., Keysers, C., Plailly, J., Royet, J. P., Gallese, V., and Rizzolatti, G. (2003). Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron 40, 655–664.
Wittmann, M. (2009). The inner experience of time. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1955–1967.
Keywords: interval-timing, insula, affective, temporal, speed
Citation: Schirmer A (2011) How emotions change time. Front. Integr. Neurosci. 5:58. doi: 10.3389/fnint.2011.00058
Received: 21 July 2011;
Paper pending published: 09 August 2011;
Accepted: 14 September 2011;
Published online: 05 October 2011.
Edited by:
Warren H. Meck, Duke University, USAReviewed by:
Antonio Pereira, Federal University of Rio Grande do Norte, BrazilJason Tipples, University of Hull, UK
Jessica Lake, Duke University, USA
Copyright: © 2011 Schirmer. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Annett Schirmer, Department of Psychology, Faculty of Arts and Social Sciences, National University of Singapore, 9 Arts Link, Block AS4, Level 2, Singapore 117570. e-mail: schirmer@nus.edu.sg