Evidence for Tonic Control by the GABAA Receptor of Extracellular D-Serine Concentrations in the Medial Prefrontal Cortex of Rodents
- 1Department of Psychiatry and Behavioral Sciences, Tokyo Medical and Dental University, Tokyo, Japan
- 2Center for Brain Integration Research, Tokyo Medical and Dental University, Tokyo, Japan
Endogenous D-serine is a putative dominant co-agonist for the N-methyl-D-aspartate glutamate receptor (NMDAR) in the mammalian forebrain. Although the NMDAR regulates the higher order brain functions by interacting with various neurotransmitter systems, the possible interactions between D-serine and an extra-glutamatergic system largely remain elusive. For the first time, we show in the rat and mouse using an in vivo microdialysis technique that the extracellular D-serine concentrations are under tonic increasing control by a major inhibitory transmitter, GABA, via the GABAA (GABAAR) in the medial prefrontal cortex (mPFC). Thus, an intra-mPFC infusion of a selective GABAAR antagonist, bicuculline (BIC), caused a concentration-dependent and reversible decrease in the extracellular levels of D-serine in the rat mPFC without affecting those of another intrinsic NMDAR coagonist, glycine and an NMDAR agonist, L-glutamate. The decreasing effects of BIC were eliminated by co-infusion of a selective GABAA agonist, muscimol (MUS) and were mimicked by a GABAA antagonist, gabazine (GBZ). In contrast, selective blockade of the GABAB or homomeric ρGABAA (formerly GABAC) receptor by saclofen or (1,2,5,6-tetrahydropyridin-4-yl)-methylphosphinic acid (TPMPA), respectively, failed to downregulate the prefrontal extracellular D-serine levels. Moreover, the local BIC application attenuated the ability of NMDA given to the mPFC to increase the cortical extracellular concentrations of taurine, indicating the hypofunction of the NMDAR. Finally, in the mouse mPFC, the reduction of the extracellular D-serine levels by a local injection of BIC into the prefrontal portion was replicated, and was precluded by inhibition of the neuronal or glial activity by co-local injection with tetrodotoxin (TTX) or fluorocitrate (Fluo), respectively. These findings suggest that the GABAAR-mediated regulation of the D-serine signaling may exert fine-tuning of the NMDAR function and require both neuronal and glial activities in the mammalian mPFC.
Introduction
A body of evidence has been accumulated indicating that D-serine in mammalian brains is an intrinsic coagonist for the N-methyl-D-aspartate type glutamate receptor (NMDAR) that plays a pivotal role in the expression and control of higher order brain functions (for a review see Nishikawa, 2011). The significance of D-serine is also designated by its neuroanatomical features such as the presence at high concentrations with a brain-predominant and NMDAR-like distribution throughout life and the enrichment in the cerebral cortical regions during the adult period (Hashimoto et al., 1993, 1995; Kumashiro et al., 1995). Importantly, D-serine is shown to be essential for the NMDAR activation based on observations that the selective degradation of D-serine by D-amino acid oxidase or D-serine deaminase without affecting the levels of another NMDA receptor coagonist, glycine, elicits a marked attenuation of the NMDAR channel-mediated inward current, calcium influx and cGMP formation in the forebrain areas in in vitro experiments (Matsui et al., 1995; Mothet et al., 2000). Disturbances in the D-serine signaling at the NMDAR has been considered to be involved in the pathophysiology of brain disorders because the D-serine depleted mice by genetic destruction of serine racemase, a D-serine synthesizing enzyme, display the NMDAR hypofunction (Benneyworth et al., 2012; Ishiwata et al., 2015) and various patterns of abnormal behavior and neuroanatomical changes including models of schizophrenia (Basu et al., 2009; Labrie et al., 2009).
The coagonist property of D-serine appears to need a specific regulatory system of the extracellular concentrations of D-serine to maintain the appropriate NMDAR activity. Although the D-serine metabolism-related molecules, such as serine racemase, D-amino acid oxidase, some neutral amino acid transporters and ionotropic glutamate receptors (Nishikawa, 2011; Ishiwata et al., 2013a,b, 2015) are found to modify the extracellular D-serine concentrations in discrete brain areas, our knowledge about the exact cellular and molecular mechanisms of control of the extracellular D-serine signaling is still limited. Especially, no studies have reported the control of the extracellular D-serine signaling by GABA neurotransmission that is a major inhibitory system in the nervous system whereas there are numerous articles on the D-serine modulation by glutamatergic transmission (Nishikawa, 2011; Ishiwata et al., 2013a). In terms of the significance of the GABA system and its balance with the excitatory glutamate system in the brain (Nestler et al., 2009), to explore the possible GABAergic control and its molecular basis, we investigated in the rat and mouse the influence of pharmacological manipulation of the various GABA receptor (GABAR) subtypes on the extracellular D-serine levels by using their respective selective antagonists and agonists in the medial prefrontal cortex (mPFC). The mPFC was chosen according to the accumulating data that this region is one of the brain areas with the highest tissue and extracellular concentration of D-serine and enriched by glutamate and GABA synapses (Nestler et al., 2009; Nishikawa, 2011).
To further clarify the role of the potential GABAergic modifications of the D-serine levels in control of the NMDAR function, we evaluated the responses of the extracellular taurine release to activation of the NMDAR (Del Arco and Mora, 1999; Oja and Saransaari, 2000; Scheller et al., 2000; Gobert et al., 2011). Because glycine and L-glutamate also augment the NMDAR function, we compared the effects of the GABAergic agents on these NMDAR-binding amino acids (Nishikawa, 2011).
Finally, to gain an insight into the cellular mechanisms of the probable GABAergic control of D-serine, we analyzed the influences of the local inhibition of the neuronal or glial activities on the interaction in the mPFC. These analyses were conducted on the basis of our previous observations pointing out that both the neurons and glia participate in the modulation of the extracellular D-serine concentrations, i.e., (1) depolarization stimuli generated by veratrine caused a prominent decrease in the extracellular contents of D-serine (Hashimoto et al., 1995); and (2) the glial activity inhibition by a reversible gliotoxin, fluorocitrate (Fluo), reduced those of D-serine (Kanematsu et al., 2006).
To achieve these objectives, we employed an in vivo microdialysis technique, which can locally infuse various chemicals into any brain portion, in freely moving animals combined with a concurrent quantitative assay of the chiral and non-chiral amino acids in the dialysates by high-performance liquid chromatography (HPLC) with fluorometric detection (Hashimoto et al., 1995; Ishiwata et al., 2013a,b, 2015), since monitoring the dynamics of the extracellular D-serine seems to demand the conditions preserving the interrelationships among various neurons and glia under no anesthesia (Nishikawa, 2011; Fossat et al., 2012; Rosenberg et al., 2013).
Materials and Methods
Animals
The present animal experiments were performed in strict accordance with the guidance of the Tokyo Medical and Dental University and were approved by the Animal Investigation Committee of the institute and the university. Male Wistar rats (Clea Japan, Japan) weighing 200–250 g or male C57BL/6J mice (Clea Japan, Inc., Japan) weighing 20–25 g between postnatal days 50 and 56 were used. The animals were housed at 23.0 ± 0.5°C in a humidity-controlled room under a 12-h light-dark cycle (lights on at 8 a.m.) and were allowed food and water ad libitum.
Chemicals
(-)-Bicuculline methioide (BIC), muscimol (MUS), NMDA, cis-4-[phosphomethyl]-piperidine-2-carboxylic acid (CGS 19755: CGS), isoguvacine hydrochloride (IGU), gabazine (SR 95531) hydrobromide (GBZ), (R)-bacrofen (BAC), sacrofen (SAC), (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA) and tetrodotoxin citrate (TTX) were purchased from Tocris Bioscience (USA), and DL-fluorocitrate barium salt from Sigma-Aldrich. All other chemicals were of ultrapure grade and commercially available. The doses always refer to the free bases.
The concentration ranges from 10 μM to 250 μM of the locally applied BIC into the mPFC via the dialysis probe were selected based on the following reasons: (1) Bicuculline is usually used at the concentrations ranging from 1 μM to 100 μM in in vitro experiments such as bath application for the brain slices; (2) from molecular weight (MW) dependence of dialysis tube permeability and the permeability rate of D-serine or (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropioinic acid ((S)-AMPA) with a MW 105 or 186 across the dialysis membrane, which has been estimated as approximately 14 or 10%, respectively, by HPLC analysis in our lab (at the flow rate of 2 μl/min used in this study: Hashimoto et al., 1995; Ishiwata et al., 2013b), the rate of BIC with a higher MW of 367 could be expected to be less than 10% although we could not directly detect BIC in our HPLC system; and (3) the points of (1) and (2) suggest that, in the present study, 10–250 μM of BIC in the tube could provide less than 1–25 μM outside the dialysis probe, which is within the generally used levels.
In Vivo Microdialysis
The in vivo microdialysis was achieved using rats or mice in most or some experiments, respectively, as previously reported (Hashimoto et al., 1995; Ishiwata et al., 2013a,b, 2015). The use of two species was aimed to not only compare the possible GABAergic control of D-serine between them but also to expand the investigations on the interaction in the genetically-modified mice in the future. Rats or mice were anesthetized with pentobarbital (40 mg/kg, intraperitoneally) and mounted on a stereotaxic frame. A straight-shaped cellulose dialysis tubing (3.0 mm (rat) or 2.0 mm (mouse) in length, 0.16 mm internal diameter, MW cutoff 50,000, EICOM Co., Ltd., Japan) was then implanted into the mPFC (rat, A +3.2 mm, V +5.2 mm, L −0.7 mm; mouse, A +1.5 mm, V +3.0 mm, L −0.35 mm) according to the atlas of Paxinos and Watson (2005) for the rat or Paxinos and Franklin (2004) for the mouse. Two days after surgery, the dialysis probe was perfused with a Ringer solution (NaCl, 147 mM; KCl, 4 mM; CaCl2, 1.3 mM; pH 7.4) at the flow rate of 2 μl/min. After stabilizing for at least 80 min, the dialysate samples were collected every 20 min. The first three samples were used to determine the basal concentration of each amino acid. The collected samples were stored at −80°C until derivatization following the addition of D-homocysteic acid as the internal standard. After termination of the experiments, the location of the dialysis probe was macroscopically verified in each case from 150-μm-thick serial coronal slices.
The present in vivo microdialysis technique and its experimental conditions are able to detect the neural and glial modifications of the extracellular concentrations of the neurotransmitters, neuromodulators and their metabolite in the mPFC. Thus, our previous studies, in agreement with the results reported by other research groups (Korf and Venema, 1985; Westerink and Tuinte, 1986; Paulsen et al., 1987), ascertained that the aforementioned experimental conditions enable us to observe the following: (1) a terodotoxin-sensitive depolarization-provoked increase in the extracellular liberation of the classical amino acid neurotransmitters, L-glutamate and glycine (Hashimoto et al., 1995); (2) a remarkable diminution or complete elimination of the extracellular release of a classical neurotransmitter, dopamine, by cessation of the nerve impulse traffic or calcium chelation (Nishijima et al., 1996); and (3) alterations in the glial functioning by monitoring the extracellular L-glutamine levels (Kanematsu et al., 2006).
High-Performance Liquid Chromatography (HPLC) Analysis
The simultaneous determination of the free amino acid enantiomers and non-chiral amino acids in the dialysate was accomplished by our previously described method using HPLC and fluorometric detection (Hashimoto et al., 1995; Ishiwata et al., 2013a,b, 2015). Briefly, the dialysate sample was derivatized with N-tert-butyloxycarbonyl-L-cysteine (Boc-L-Cys) and o-phthaldialdehyde for 2 min at room temperature. The amino acid derivative was immediately applied to the HPLC system and separated on a 4-μm Nova-Pak C18 column (300 × 3.9 mm, I.D., Waters, Japan). The column was operated at the constant flow-rate of 0.8 ml/min at 30°C. Mobile phase A was 0.1 M acetate buffer (pH 6.0) containing 7% acetonitrile and 3% tetrahydrofuran, and mobile phase B was the acetate buffer containing 22% acetonitrile and 3% tetrahydrofuran. The separation of the amino acid derivatives was performed using a linear gradient from mobile phase A to B in 53 min. The fluorescent amino acid derivatives were detected using a Waters 2475 Multi l fluorescence detector spectrofluorometer (Waters Co., Ltd.). The excitation and emission wavelengths were 344 and 443 nm, respectively.
The basal levels of the cortical dialysate D-serine, L-serine, glycine, L-glutamate, L-glutamine and taurine determined in the respective experimental groups in the present study (Table 1) are similar to each other and in good agreement with those of our previous reports (Hashimoto et al., 1995; Ishiwata et al., 2013a,b, 2015).
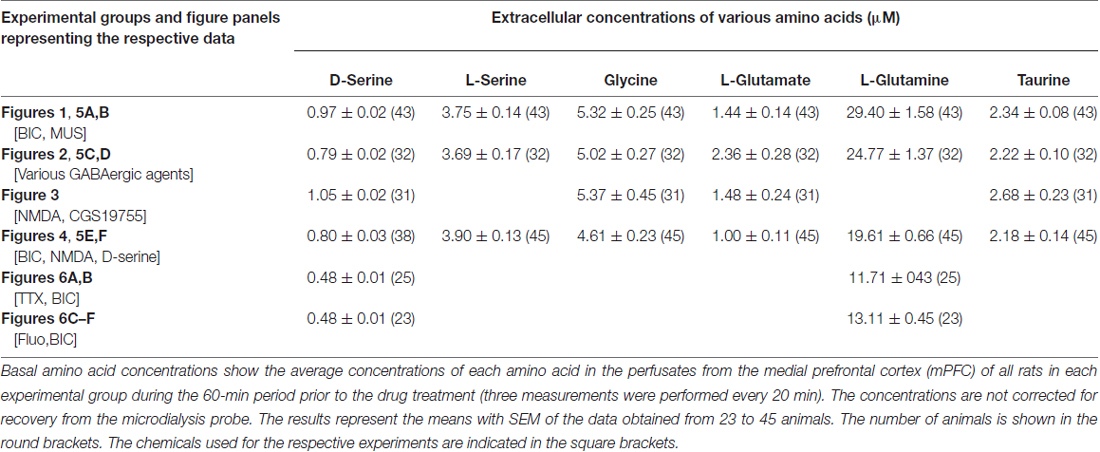
Table 1. The uncorrected absolute values of the basal extracellular concentrations of D-serine, L-serine, glycine, L-glutamate, L-glutamine and taurine in the medial frontal cortex of the rat or mouse.
Data Analysis
The average concentration of each substance during the period preceding the drug treatment (three measurements were performed every 20 min) was used as the baseline control value (=100%). The individual data are expressed as percentages of this baseline period. The means with SEM of the results obtained from 4 to 13 animals were calculated using the corresponding periods. The areas under the curves (AUC) of the concentration vs. time plots for the dialysate amino acids at 0–60 min of infusion from time 0 in the rat experiments (see the above “In Vivo Microdialysis” Section; Figures 1–5) or for 60 min after the start of the BIC (Figure 6) infusion in the mouse experiments were calculated and used as the overall measures of the treatment effects (Matthews et al., 1990).
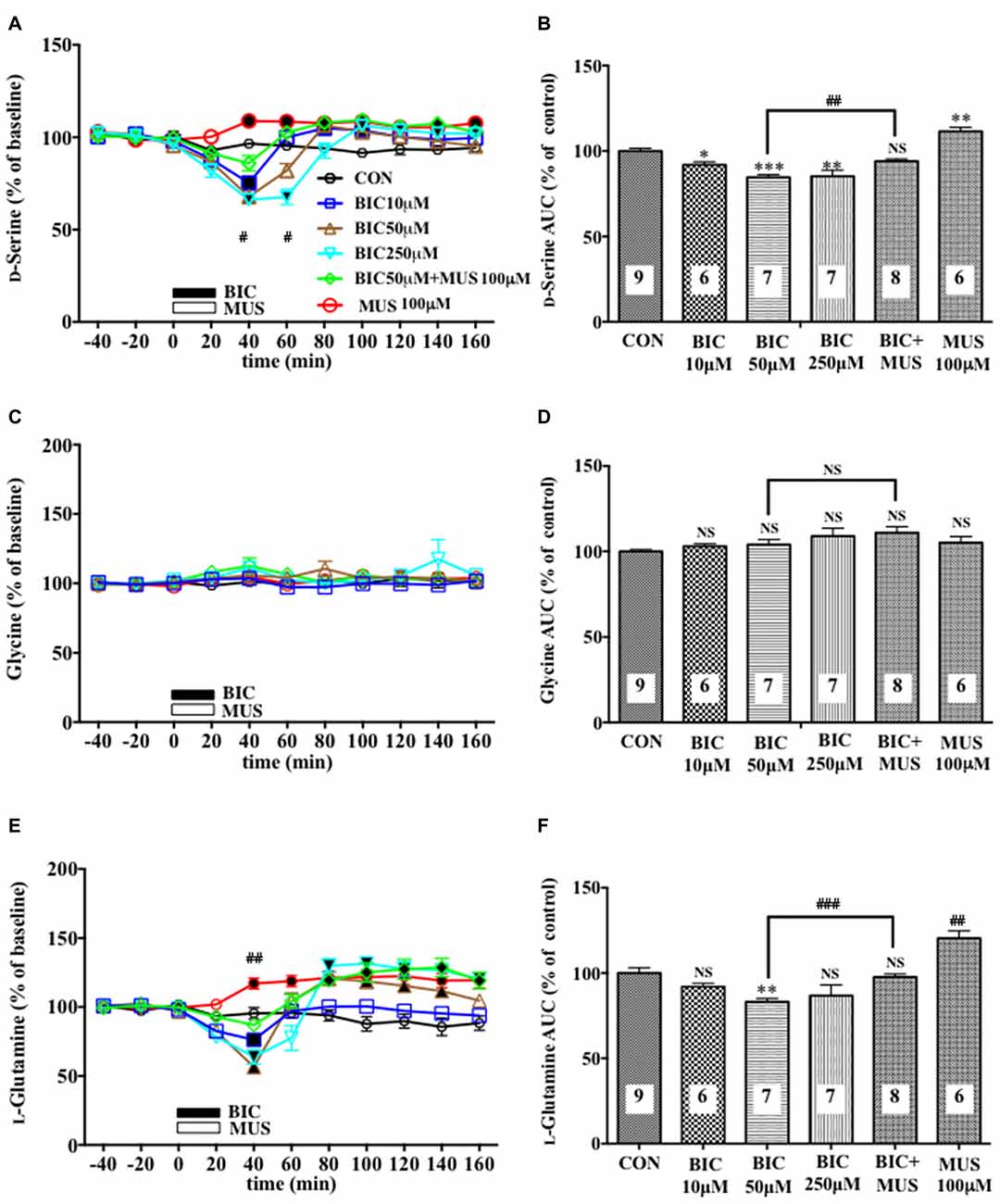
Figure 1. Effects of local infusion of a GABAAR antagonist or agonist or in combination on the extracellular concentrations of various amino acids in the rat medial prefrontal cortex (mPFC). In (A,C,E), each point represents the mean with the SEM of data obtained from six to nine animals and expressed as a percentage of the basal extracellular levels of D-serine (A), glycine (C) and L-glutamine (E). The filled symbols indicate the statistically significant differences in the time point data at P < 0.05, 0.01 or 0.001 as compared to the Ringer solution alone-infused controls: open symbols, not significant (A,C,E) (D-serine (A): bicuculline (BIC) 10 μM, a significant decrease (↓) at 40 min, a significant increase (↑) at 80 and 100 min; bicuculline (BIC) 50 μM, (↓) at 40 min, (↑) at 80 min; BIC 250 μM; (↓) at 40 and 60 min, (↑) at 100 and 140 min; muscimol (MUS) 100 μM, (↑) at 40–160 min; L-glutamine (E): BIC 10 μM, (↓) at 40 min; BIC 50 μM, (↓) at 20 and 40 min, (↑) at 80–140 min; BIC 250 μM, (↓) at 40 min, (↑) at 80–160 min; MUS100 μM, (↑) at 40–160 min). #p < 0.05 or ##p < 0.01 between the BIC (50 μM)-infused and the BIC (50 μM) + MUS (100 μM)-infused animals (A,E). An open or filled bar followed by a drug name indicates the continuous local infusion of the Ringer solution of each drug. The area under the curve (AUC) data were calculated by cumulating each amino acid concentration for every 20-min consecutive observation from 0 min to 60 min of treatment (B,D,F). *p < 0.05, **p < 0.01 or ***p < 0.001 as compared to the Ringer solution alone-infused controls. ##p < 0.01 or ###p < 0.001 between the BIC (50 μM)-infused and the BIC (50 μM) + MUS (100 μM)-infused animals. NS, not significant. The number of animals in each group is shown at the bottom of each column.
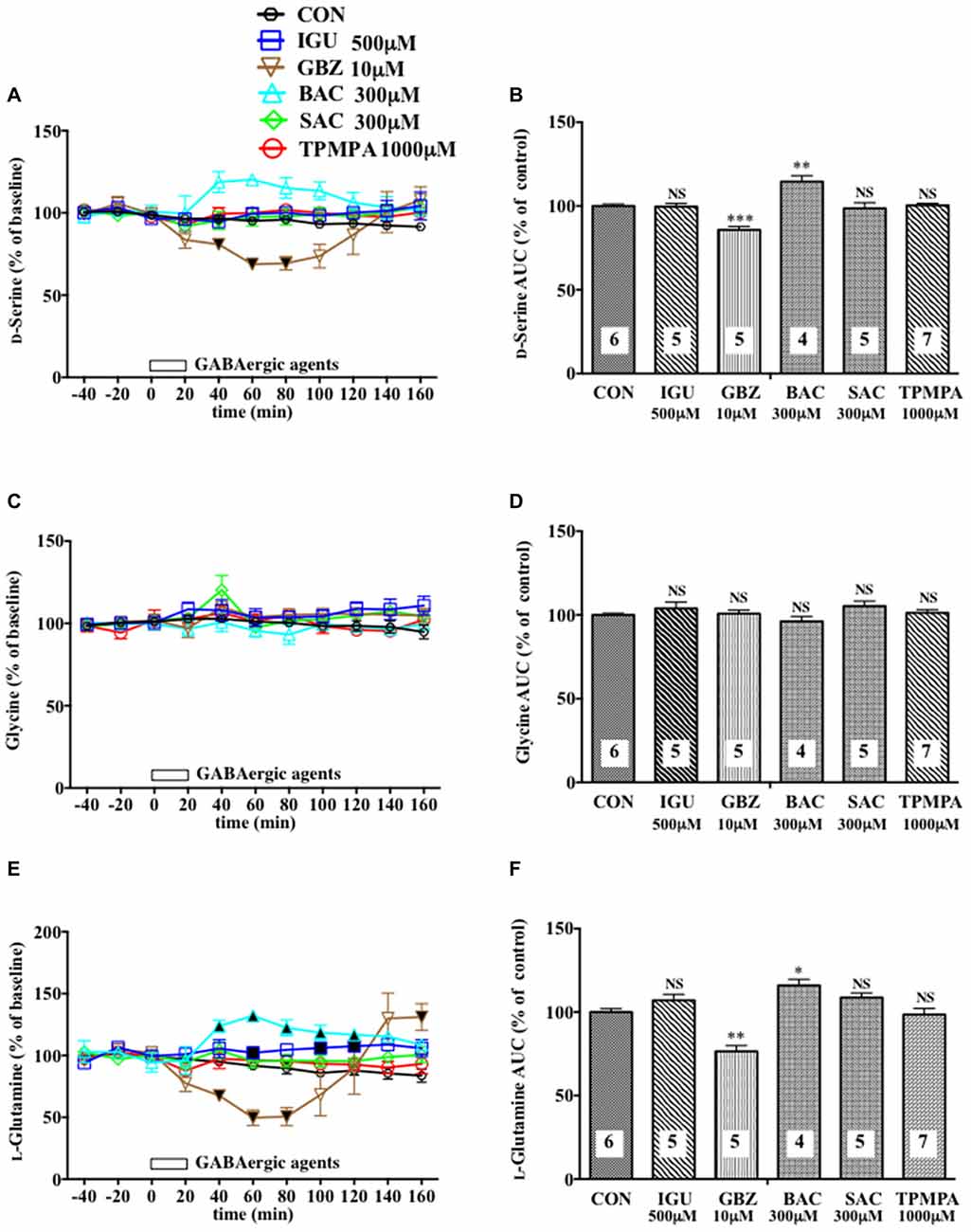
Figure 2. Effects of local infusion of diverse GABAergic agents on the extracellular concentrations of various amino acids in the rat mPFC. In (A,C,E), each point represents the mean with the SEM of data obtained from four to seven animals and expressed as a percentage of the basal D-serine (A), glycine (C) and L-glutamine (E). The filled and open symbols indicate statistical comparisons in the same manner as described in Figure 1 (D-serine (A): gabazine (GBZ) 10 μM, a significant decrease (↓) at 40–80 min; L-glutamine (E) isoguvacine hydrochloride (IGU) 500 μM, a significant increase (↑) at 60, 100 and 120 min; GBZ 10 μM, (↓) at 40–80 min; bacrofen (BAC) 300 μM, (↑) at 40–120 min). An open bar followed by a drug name indicates the continuous local infusion of Ringer solution of each drug. The AUC data (B,D,F): *p < 0.05, **p < 0.01 or ***p < 0.001 as compared to the Ringer solution alone-infused controls. NS, not significant. The number of animals in each group is shown at the bottom of each column.
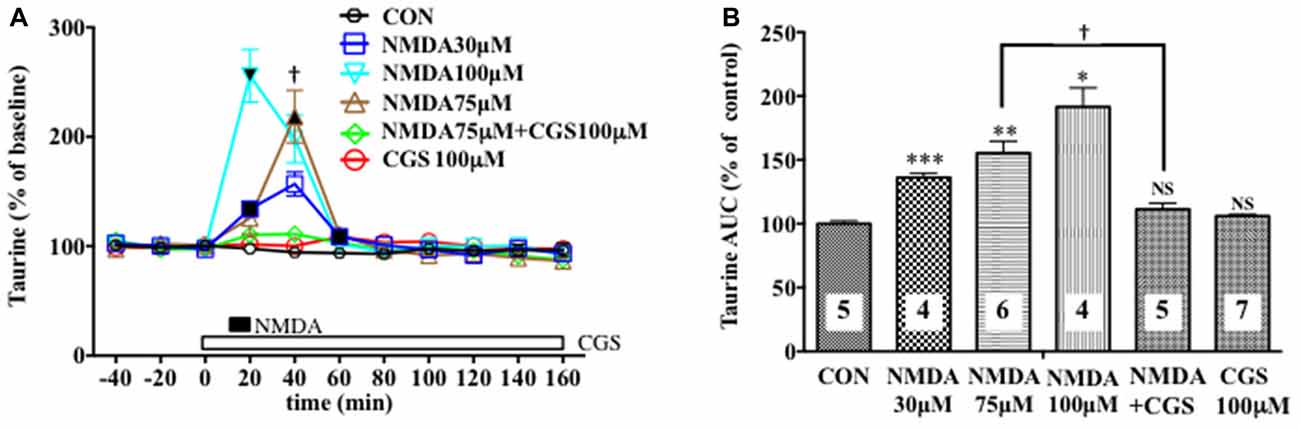
Figure 3. Effects of local infusion of N-methyl-D-aspartate glutamate (NMDA), CGS 19755, or in combination on the extracellular concentrations of various amino acids in the rat mPFC. In (A), each point represents the mean with the SEM of data obtained from four to seven animals and expressed as a percentage of the basal taurine. The filled and open symbols indicate statistical comparisons in the same manner as described in Figure 1 (taurine: NMDA 30 μM, a significant increase (↑) at 20 and 60 min; NMDA 75 μM, (↑) at 40 min; NMDA 100 μM, (↑) at 20 min). †p < 0.05 between the NMDA- and CGS 19755 (CGS)-infused animals (A). An open, shaded or filled bar followed by a drug name indicates the continuous local infusion of Ringer solution of each drug. The AUC data (B): *p < 0.05, **p < 0.01 or ***p < 0.001 as compared to the Ringer solution alone-infused controls. †p < 0.05 between the NMDA- and CGS 19755 (CGS)-infused animals. NS, not significant. The number of animals in each group is shown at the bottom of each column.
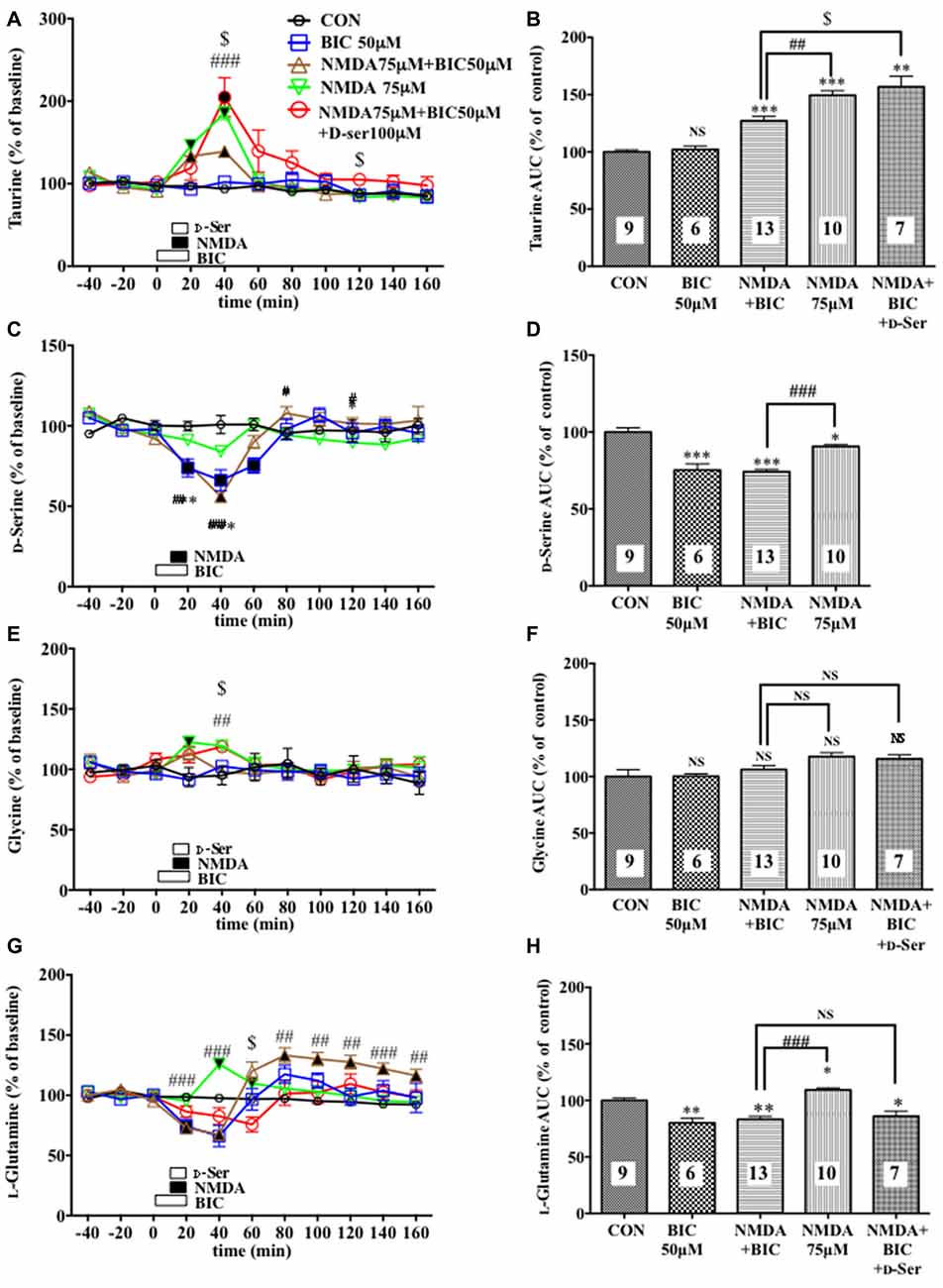
Figure 4. Effects of local infusion of BIC, NMDA, BIC + NMDA, or BIC + NMDA + D-serine on the extracellular concentrations of various amino acids in the rat mPFC. In (A,C,E,G), each point represents the mean with the SEM of data obtained from 7 to 13 animals and expressed as a percentage of the basal taurine (A), D-serine (C), glycine (E) and L-glutamine (G). The filled and open symbols indicate statistical comparisons in the same manner as described in Figure 1 (taurine (A): NMDA 75 μM + BIC 50 μM, a significant increase (↑) at 20 and 40 min; NMDA 75 μM, (↑) at 20 and 40 min; NMDA 75 μM + BIC 50 μM + D-Ser, (↑) at 20 and 40 min; D-serine (C): BIC 50 μM, a significant decrease (↓) at 20–60 min; NMDA 75 μM + BIC 50 μM, (↓) at 20 and 40 min; glycine (E): NMDA 75 μM, (↑)at 20 min; L-glutamine: BIC 50 μM, (↓) at 20 min; NMDA 75 μM + BIC 50 μM, (↓) at 20 and 40 min, (↑) at 80–160 min; NMDA 75 μM, (↑) at 40 and 60 min). #p < 0.05, ##p < 0.01 or ###p < 0.001 between the NMDA + BIC- and NMDA-infused animals. $p < 0.05 between the NMDA + BIC- and NMDA + BIC + D-serine (D-Ser)-infused animals. An open, shaded or filled bar followed by a drug name indicates the continuous local infusion of Ringer solution of each drug. The AUC data (B,D,F,H): *p < 0.05, **p < 0.01 or ***p < 0.001 as compared to the Ringer solution alone-infused controls. #p < 0.05, ##p < 0.01 or ###p < 0.001 between the NMDA + BIC- and NMDA-infused animals. $p < 0.05 between the NMDA + BIC- and NMDA + BIC + D-Ser-infused animals. NS, not significant. The number of animals in each group is shown at the bottom of each column.
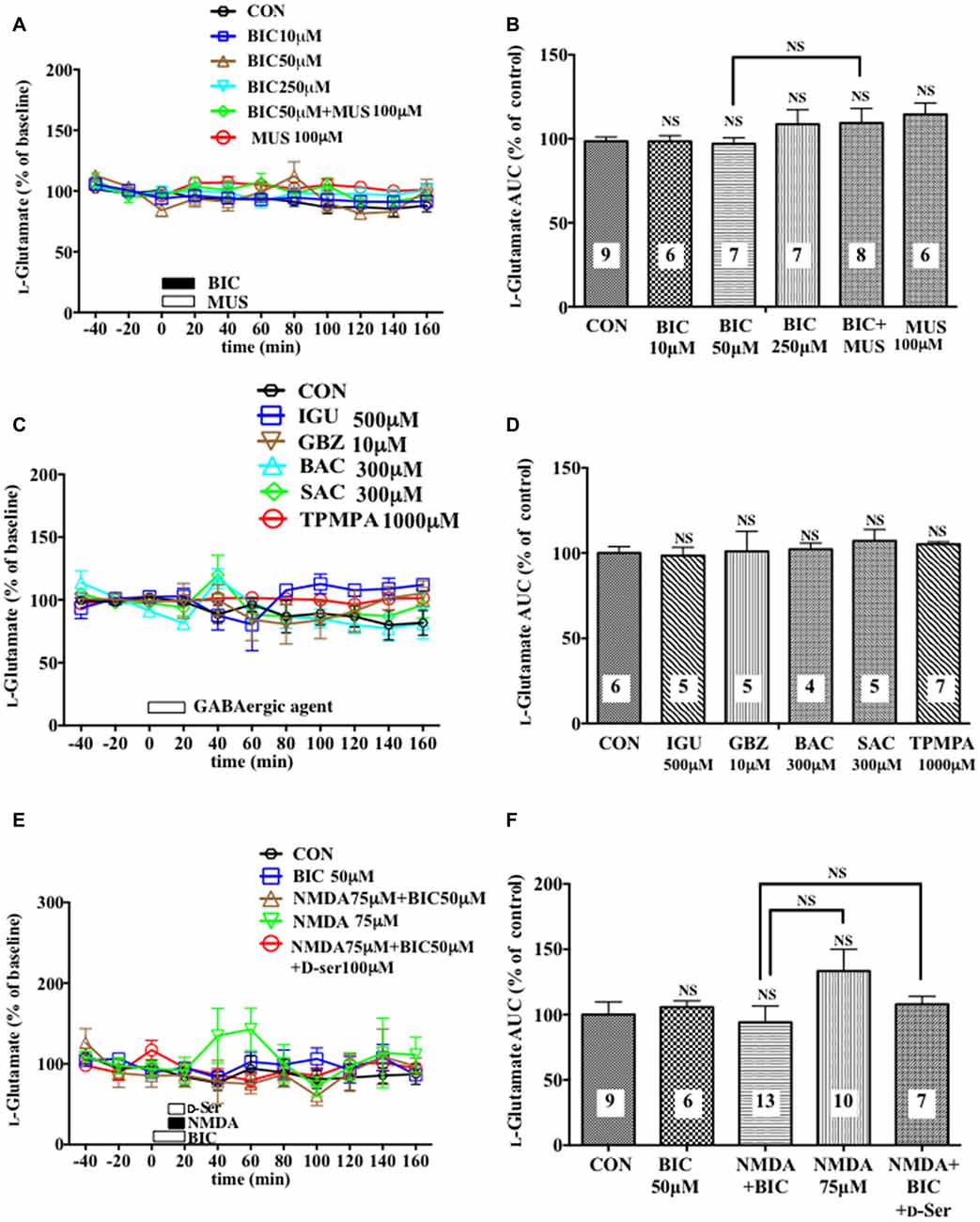
Figure 5. Effects of local infusion of various GABAergic agents, NMDA, D-seine or in combination on the extracellular concentrations of L-glutamate in the rat mPFC. In (A,C,E), each point represents the mean with the SEM of data obtained from 4 to 13 animals and expressed as a percentage of the basal extracellular levels of L-glutamate. The open symbols indicate no statistically significant differences in the time point data at P > 0.05 (A,C,E). An open or filled bar followed by a drug name indicates the continuous local infusion of Ringer solution of each drug. The AUC data were calculated by cumulating each amino acid concentration for every 20-min consecutive observation from 0 min to 60 min of treatment (B,D,F). NS, not significant as compared to the Ringer solution alone-infused controls or between the two groups linked by the solid line. The number of animals in each group is shown at the bottom of each column.
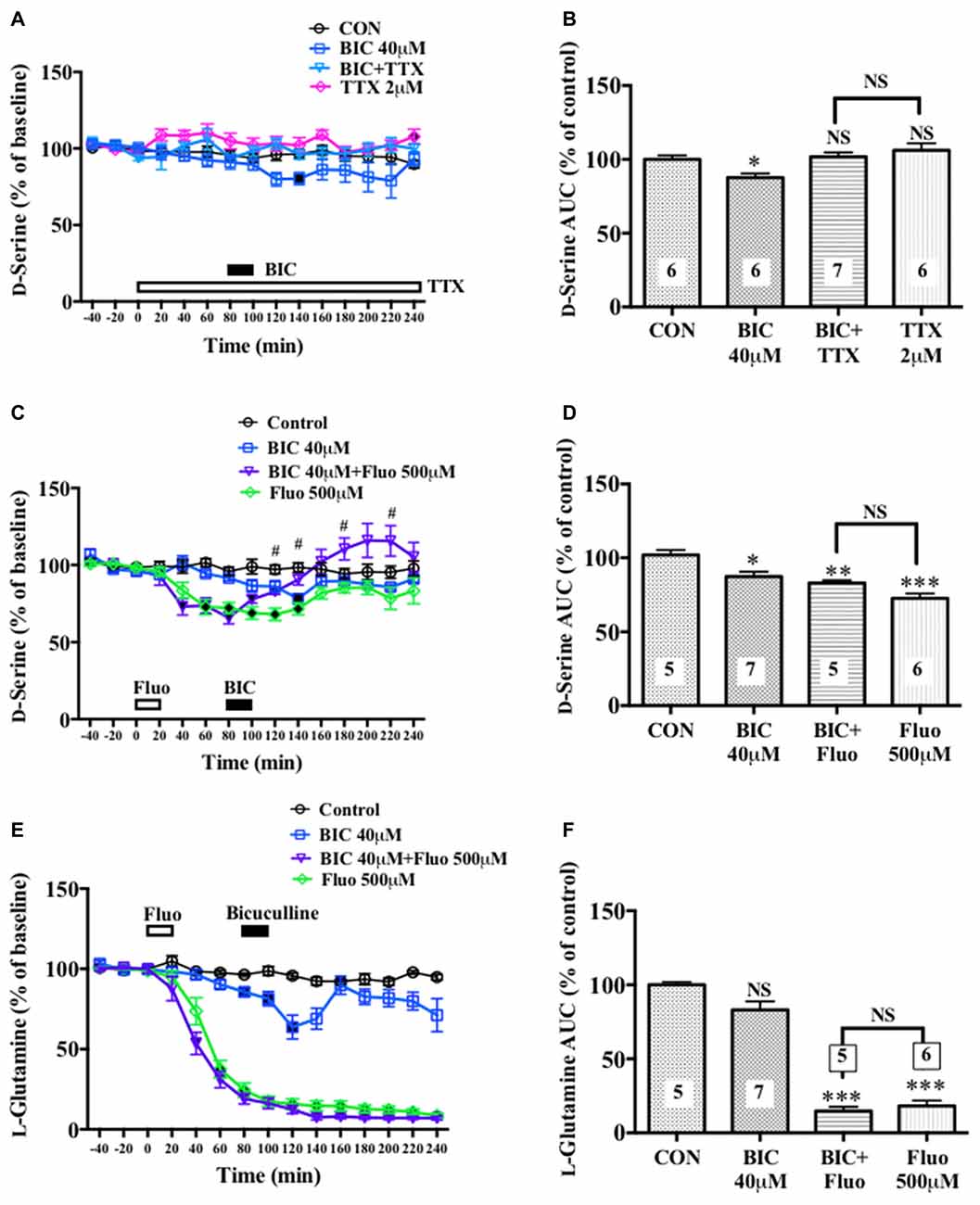
Figure 6. Effects of local infusion of BIC, fluorocitrate (Fluo), tetrodotoxin (TTX) or in combination on the extracellular concentrations of D-serine in the mouse mPFC. In panels (A,C,E), each point represents the mean with the SEM of data obtained from five to seven animals and expressed as a percentage of the basal D-serine. The filled symbols indicate the statistically significant differences in the time point data at P < 0.05, 0.01 or 0.001 as compared to the Ringer solution alone-infused controls: open symbols, not significant (A,C,E) (D-serine (A): BIC 40 μM, a significant decrease (↓) at 140 min; D-serine (C): BIC 40 μM, (↓) at 140 min; BIC 40 μM + Fluo 500 μM, (↓) at 40–120 min; Fluo 500 μM, (↓) at 40–140 min; L-glutamine (E): BIC 40 μM, (↓) at 80–120 min; BIC 40 μM + Fluo 500 μM, (↓) at 40–240 min; Fluo 500 μM, (↓) at 40–240 min). #p < 0.05 between the BIC+fluorocitrate (Fluo)- and Fluo-infused animals. An open or filled bar with a drug name indicates the continuous local infusion of Ringer solution of each drug. The AUC data for 60 min after the start of the BIC infusion (B,D,F): *p < 0.05, **p < 0.01 or ***p < 0.001 as compared to the Ringer solution alone-infused controls. NS, not significant. The number of animals are shown at the bottom of or above each column. It should be noted that the decreasing effects of the locally applied BIC on the extracellular concentrations of D-serine in the rat mPFC (Figures 1A,B, 4E,F) were reproduced in the mouse mPFC.
For comparison between the two groups, statistical evaluations were performed using the unpaired two-tailed Student’s t test (the homogeneous variance for each experimental group) or Aspin-Welch’s t test (the homogeneous and heterogeneous variance for each experimental group). Statistical differences among more than three groups were estimated by Bonferroni’s method (Wallenstein et al., 1980). These statistical analyses were applied to the AUC data to clarify the exact effects of various drug treatments and their mutual differences during the specific period of each experiment (Ishiwata et al., 2013a,b). In addition, in contrast to the longitudinal view, the data of different treatment groups at each time point were similarly compared to get crosscutting view of the results of the dialysis experiments (Ishiwata et al., 2013a,b).
Results
Effects of Local Injection of GABA Receptor Agents on the Extracellular Concentrations of D-Serine, Glycine, L-Glutamate and L-Glutamine in the Rat Medial Prefrontal Cortex
A 20-min intra-mPFC infusion of bicuculline (BIC), a selective antagonist for the GABAA receptor (GABAAR; Enna and McCarson, 2013), via the dialysis tubing, produced in the mPFC a significant reduction in the extracellular concentrations of D-serine in a concentration-dependent and reversible manner (Figures 1A,B) during a 0–60 min post-injection without effects on those of another NMDAR coagonist, glycine (Figures 1C,D) and an NMDAR agonist, L-glutamate (Figures 5A,B; Ogden and Traynelis, 2011). The extracellular levels of L-glutamine that have been considered to reflect the activity of glia (Hertz, 2004; Kanematsu et al., 2006) were decreased by the local BIC injection in a dose-related fashion. The reductions in the contents of the extracellular D-serine and L-glutamine were significantly inhibited by the local infusion of a selective GABAAR agonist, MUS (Enna and McCarson, 2013), which alone slightly increased the D-serine (Figures 1A,B) and L-glutamine (Figures 1E,F) concentrations.
Another selective GABAAR antagonist, gabazine (GBZ; Katzner et al., 2011; Enna and McCarson, 2013), mimicked the reducing effects of BIC on the extracellular D-serine and L-glutamine contents (Figures 2A,B,E,F) with no significant influence on the glycine (Figures 2C,D) and L-glutamate (Figures 5C,D) levels in the mPFC. However, a high effective concentration of a selective GABAAR agonist, isoguvacine (IGU), a GABABR antagonist, sacrofen (SAC), or a selective antagonist for the ρGABAAR (formerly GABACR), (1,2,5,6-tetrahydropyridin-4-yl)-methylphosphinic acid (TPMPA), failed to modify the frontal extracellular levels of D-serine, glycine, L-glutamine (Figures 2A–F) and L-glutamate (Figures 5C,D; Katzner et al., 2011; Ng et al., 2011; Enna and McCarson, 2013). A GABABR selective agonist, bacrofen (BAC), produced a modest, but significant increase in the extracellular D-serine and L-glutamine in the cortical portion (Figures 2A,B,E,F).
Evaluation of NMDA Receptor Function by Monitoring the Effects of the Local Injection of NMDA, an NMDA Receptor Antagonist, or in Combination on the Extracellular Concentrations of Taurine in the Rat Medial Prefrontal Cortex
We evaluated the NMDAR function under the BIC-induced reduction in the cortical extracellular D-serine contents by estimating the NMDA-evoked increase in the extracellular taurine concentrations as a well-documented index of the NMDAR responses (Del Arco and Mora, 1999; Oja and Saransaari, 2000; Scheller et al., 2000; Gobert et al., 2011). As shown in Figures 3A,B, we confirmed its reliability in that a 10-min intra-mPFC application of NMDA induced a concentration-related and reversible increase in the frontal extracellular taurine levels in a fashion sensitive to the selective NMDAR antagonist, cis-4-[phosphomethyl]-piperidine-2- carboxylic acid (CGS19755 (CGS), Ogden and Traynelis, 2011) given via the dialysis probe. This consequence is the first demonstration of the exact relationship between the NMDAR function and extracellular taurine levels in the local brain portion, the mPFC, which enabled us to validate the influence of the modifications of the extracellular D-serine levels on the NMDAR function by simultaneously monitoring the time course of changes in the extracellular contents of D-serine and in the NMDA-elicited upregulation of extracellular taurine in the prefrontal portion.
Effects of a Selective GABAA Receptor Antagonist on the NMDA-Induced Elevation of the Extracellular Concentrations of Taurine in the Rat Medial Prefrontal Cortex
A 20-min local infusion of BIC into the mPFC again reduced the extracellular concentrations of D-serine (Figures 4C,D), but not glycine (Figures 4E,F) and L-glutamate (Figures 5E,F), and significantly attenuated the ability of NMDA to augment the cortical extracellular taurine contents without affecting their basal levels (Figures 4A,B). This attenuation indicated hypofunction of the NMDAR by the diminished levels of the extracellular D-serine and was reversed by the intra-cortical co-infusion of D-serine (Figures 4A,B). The D-serine co-infusion did not cancel the BIC-induction of the diminution in the extracellular concentrations of L-glutamine (Figures 4G,H).
Effects of Attenuation of Glial or Neuronal Activity on Bicuculline-Induced Decrease in the Extracellular D-Serine Concentrations in the Mouse Medial Prefrontal Cortex
A significant increase or decrease in the extracellular D-serine concentrations in the mouse mPFC was observed after neuronal activity inhibition by TTX (Figures 6A,B) or glial activity attenuation by Fluo (Figures 6C,D), respectively. The similar effects of TTX (Hashimoto et al., 1995) and Fluo (Kanematsu et al., 2006) on the extracellular D-serine levels were found in the rat mPFC in our previous studies. In addition, a locally applied BIC-induced decline in the extracellular D-serine levels seen in the rat mPFC (Figures 1A,B, 4A,B) was reproduced in the mouse (Figures 6A–D). These results suggest that rats and mice share the same control mechanisms of the extracellular D-serine signaling.
We further found that an intra-mPFC infusion of TTX (Figures 6A,B) completely abolished the diminishing effects of BIC on the frontal extracellular D-serine contents. When Fluo was locally administered for 20 min in advance (Figures 6C,D), the local BIC infusion did not downregulate, but tended to upregulate, the AUC of the D-serine levels for 60 min from the start of the BIC infusion (time points of 80–140 min) in the mPFC (Figure 6D). Under the condition of pretreatment with Fluo, there was a significant increase in the dialysate D-serine contents in the BIC + Fluo-perfused animals as compared to the Fluo alone-applied animals at time points of 120, 140, 180 and 220 min (Figure 6C). The results from the Fluo infusion experiments indicate that turning down the glial activity by Fluo not only eliminates the ability of BIC to reduce the extracellular D-serine contents but also confer the elevating effects on the contents to BIC.
Discussion
By using an in vivo dialysis technique, we have for the first time provided evidence for the GABAergic control of D-serine signaling by revealing that an intra-mPFC infusion of a selective GABAAR antagonist, BIC, causes a concentration-dependent, reversible and a GABAA agonist-sensitive decrease in the extracellular concentrations of D-serine, but not glycine or L-glutamate, in the cortical area of rats and mice. The decreasing effects are mimicked by another GABAAR antagonist, GBZ, but not by the GABABR or ρGABAAR agents. The reduction in the extracellular D-serine contents following the BIC infusion leads to diminution of an NMDA-evoked increase in the extracellular taurine concentrations, indicating hypofunction of the NMDAR. The GABAAR agonist, MUS or GBZ, produces a slight increase or no change in the extracellular D-serine levels, respectively. These data suggest that the GABAAR may be implicated in a tonic and weaker phasic accelerating regulation of the extracellular release of D-serine in the mPFC.
The differential influences of the GABAAR antagonists on the D-serine, glycine and L-glutamate contents and the GABAAR antagonist-selective decreasing effects on the concentrations of D-serine in the frontal extracellular fluid exclude the possibility that the GABAergic control of D-serine seen in our experiments might be a nonspecific phenomenon. GABAAR antagonists, such as picrotoxin, BIC and gabazine at high doses have been reported to act on the strychnine-sensitive inhibitory glycine receptors in the retina and artificially expressed in HEK cells, depending on the composition of their subunits (Wang and Slaughter, 2005). However, the results that the reducing effects of BIC on the extracellular D-serine contents are reversed by a selective GABAAR agonist, MUS, that lacks the substantial effects on the inhibitory glycine receptor (Lee et al., 2011; Cinelli et al., 2016) strongly support the GABAAR-mediated nature of the effects of BIC. This view is also consonant with the data obtained from the mammalian cerebral cortex that BIC has been shown to inhibit the strychnine-insensitive GABA-induced CI- flux, but failed to affect the strychnine-sensitive glycine-induced CI- flux (Engblom et al., 1996).
The fact that the diminished extracellular levels of D-serine by the GABAAR blockade without changes in those of another NMDAR coagonist, glycine and of an NMDAR agonist, L-glutamate, elicited the NMDAR hypofunction supports the concept that D-serine may play a key role in the activation of the NMDAR as a major intrinsic coagonist for the glutamate receptor in the rodent forebrain (Nishikawa, 2011; Ogden and Traynelis, 2011). These data also deny the assumption that the NMDAR hypofunction might chiefly be caused by reduced stimulation of the glutamate site of the NMDAR. This link between the GABAergic control of D-serine and NMDAR function found in the mPFC of freely moving animals extrapolates the view that the GABAergic regulation of D-serine signaling could constitute an essential system for the physiological fine-tuning of the NMDAR activity.
It has been suggested that D-serine and glycine differentially act at the synaptic and extrasynaptic NMDARs, respectively (Papouin et al., 2012). Because the attenuated NMDAR function resulted from the selective reduction in the D-serine, but not glycine, levels after BIC infusion in the mPFC, and because NMDA activates synaptic and extrasynaptic NMDARs, the extracellular D-serine controlled by GABAergic transmission could centrally act on the synaptic NMDARs.
The exact molecular and cellular mechanisms underlying the tonic facilitatory control by the GABAAR of the extracellular D-serine levels await further elucidation. The activation of GABAARs could elicit D-serine release from certain cortical neurons by exocytosis because: (1) GABA causes noradrenaline exocytosis from noradrenergic nerve terminals in the hippocampus (Fassio et al., 1999); and (2) some cortical neurons in the rat are shown to be immunostained with a D-serine specific antibody (Ding et al., 2011; Balu et al., 2014). Alternatively, the excitatory influence of the GABAergic axo-axonic cells that has been reported in the rat and human cortex (Szabadics et al., 2006) might be implicated in the presently identified GABAergic control of D-serine. Furthermore, the facilitatory influence can be produced by GABAAR-mediated attenuation of the non-GABAergic inhibitory neurons. It cannot be excepted that the blockade of the GABAAR would result in reduction of the D-serine release by the increasing extracellular L-glutamate contents through liberating certain glutamatergic neurons from inhibitory regulation via their GABAAR because, we found the downregulating effects of an AMPA glutamate receptor agonist on the prefrontal extracellular D-serine levels (Ishiwata et al., 2013b). However, this mechanism is negated by the absence of upregulation of the extracellular L-glutamate contents following local application of the GABAAR antagonists (Figure 5).
The decrease in the frontal extracellular D-serine levels by the GABAA antagonists is more likely to be associated with the reduced activity of a group of glial cells because these GABAA antagonists also produced a parallel drop in the extracellular L-glutamine concentration (Figures 1E,F, 2E,F, 4G,H) that is a marker for the glial activity (Kanematsu et al., 2006). In agreement with this presumption, we have observed that attenuation of the glial activity by a local infusion of Fluo decreases the basal levels of the extracellular D-serine and L-glutamine (Kanematsu et al., 2006; Figures 6C–F), and blocked the ability of BIC to decrease the D-serine contents (Figures 6C,D) in the mPFC. The reducing effects of the GABAAR antagonists on the L-glutamine levels favor the view that the glia controlling the extracellular D-serine may be under tonic facilitation by the GABAAR in the mPFC. Consequently, it is possible that D-serine could be released from a population of the frontal GABAAR-expressing glial cells. The GABAARs on astroglia, oligodendroglia and their precursor cells have indeed been shown to be excitatory in nature as stimulation of the GABAARs of these glia depolarize these cells by the efflux of Cl− ions due to the status that glial cells maintain much higher concentrations of the Cl− ion in the cytoplasm than in the extracellular fluid (Verkhratsky and Steinhäuser, 2000; Lin and Bergles, 2004). In this postulated cellular setup, the recovery of NMDAR functioning (Figures 4A,B) with no significant changes in the extracellular L-glutamine levels (Figures 4G,H) after the addition of D-serine in the presence of BIC appears to agree with the idea that the exogenous D-serine might restore the NMDAR response (Figures 4A,B) by direct stimulation of its glycine site without reinstating the activity of the assumed glial cells possessing the GABAARs.
The contrasting increased extracellular contents of L-glutamine after a local application of MUS (Figure 1E), BAC (Figure 2E), and NMDA (Figure 4G) may mirror activation of certain glial cells, and are consistent with the previous observations that local or systemic application of GABA (Meier et al., 2008), GABABR agonists including BAC (Gobert et al., 2011; Gould et al., 2014) and L-glutamate (Rao et al., 2003) augmented glial activity and/or the extracellular L-glutamine release in in vitro or in vivo experiments. No change or a slight decrease in the extracellular D-serine concentrations subsequent to an infusion of the GABABR antagonist, SAC (Figure 2A) or NMDA (Figure 4B), repectively, suggest that GABABR and NMDAR could control the glial cells that are not under influence of the GABAAR.
The frontal GABAergic interneurons could thus impinge on the hypothetical GABAAR-equipped glia and be required for the putative tonic facilitatory control over these glial cells. In support of this cellular connection, cessation of the nerve impulse flow by TTX eliminates the decreasing effects of BIC on the extracellular D-serine contents (Figures 5A,B). However, unlike the BIC perfusion, the inhibition of the GABAergic transmission by TTX failed to decrease the extracellular levels of D-serine. Although the precise reason for this inconsistency is still unclear, the non-decreasing effects of TTX could be explained by the simultaneous blockade by TTX of the unknown cortical neurons that bring a sustained inhibitory influence on the D-serine-regulating glia.
On the basis of the data discussed above, we schematically summarize the hypothetical cellular and molecular compositions and their interrelationships involved in the control by GABAAR of the extracellular D-serine in Figure 7. D-Serine could be released from certain neurons or glial cells because: (1) D-serine-like immunoreactivity has been shown in neurons (Benneyworth et al., 2012; Balu et al., 2014); (2) the cerebral white matter enriched with glial cells contains high concentrations of D-serine (Kumashiro et al., 1995); and (3) Fluo-induced inhibition of glial activity reduces the extracellular D-serine contents (Kanematsu et al., 2006). Together with these observations, the neuronal and glial activity-dependent nature of the GABAAR–mediated control of the extracellular D-serine levels suggests that undefined D-serine release machineries (Figure 7: yellow-green cylinders) in the glia or neurons might receive facilitatory signals (Figure 7: magenta arrows) under regulation of the excitatory GABAAR-expressing glia that are targets of the GABA neurons in the mPFC. Considering the differential expressions of various GABAAR subunits in astrocytes, oligodendrocytes and NG2 glia (Renzel et al., 2013; Balia et al., 2015; Arellano et al., 2016), and the localization of serine racemase in neurons (Balu et al., 2014), further investigations by using selective agents and specific antibodies for the respective GABAAR subunits, mice with cell type-specific conditional modifications of GABA- and D-serine-related genes, measures of the extracellular GABA concentrations or in combinations would clarify the accurate cell setups for the GABAergic regulation of D-serine release. Since recent studies have revealed that serine racemase, a D-serine synthese, is exclusively localized in neurons (Benneyworth et al., 2012; Balu et al., 2014), the putative glial D-serine could be supplied from neurons by some transfer systems that are indicated by the dotted green arrows in Figure 7.
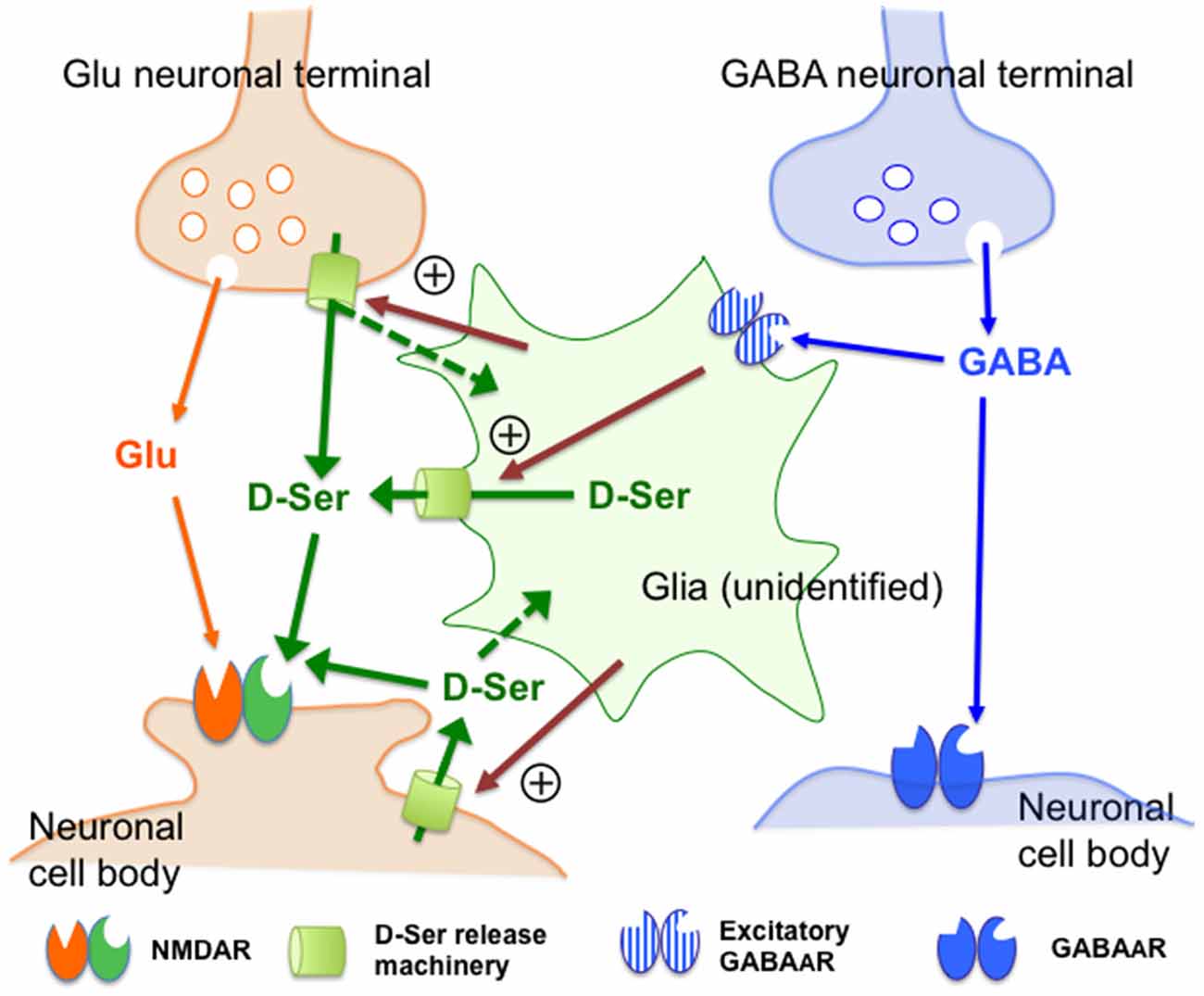
Figure 7. Schematic representation of the possible mechanisms of the control of the extracellular D-serine by the GABAA receptor. This figure visualizes the views regarding how GABAA receptors regulate D-serine release (see the explanations and arguments in the (“Discussion” Section). Together with the observations indicating that D-serine could be released from certain types of neurons or glia, the neuronal and glial activity-dependent nature of the GABAAR-mediated control of D-serine suggests that undefined D-serine release machineries (D-Ser release machinery: yellow-green cylinders) in the glia or neurons might be stimulated by facilitatory signals (magenta arrows) under regulation of the excitatory GABAAR-expressing glia that are targets of the GABA neurons in the mPFC. The dotted green arrows indicate the hypothetical transfer systems of D-serine from neurons to glia. Abbreviations: Glu, glutamate; GABA, γ-aminobutyric acid; D-Ser, D-serine. The “+” sign surrounded by circle indicates facilitatory control. The assumed excitatory GABAAR-expressing glia, molecular machineries and facilitatory signals for D-serine release have not yet been identified.
From the viewpoint of the indispensable role of D-serine in the activation of the NMDAR by L-glutamate, the GABAergic control of the extracellular D-serine signal could work as an indirect regulatory equipment for the NMDAR and constitute a way to harmonize the glutamatergic excitatory and GABAergic inhibitory transmission. Based on this hypothesis, the reduced GABAergic tone can prevent the resulting over-acceleration of the NMDAR function, which causes pathological changes in the brain, e.g., neuronal cell death and degeneration (Inoue et al., 2008) and pain stimulation (Ren et al., 2006), by diminishing the extracellular D-serine concentrations at the glycine site of the NMDAR. On the other hand, the lack of effects of the GABAergic agents used in this study on the cortical extracellular concentrations of L-glutamate and glycine (Figures 1C,D, 2C,D, 4E,F, 5A–F) indicate that the initial step of the regulation of the glutamate transmission by GABARs could be the newly uncovered GABAergic control of D-serine.
It is also noted that the NMDA perfusion into the mPFC evoked a small, but significant decrease in the prefrontal extracellular D-serine levels. This phenomenon appears to be in line with the D-serine reduction subsequent to the AMPA type glutamate receptor stimulation (Ishiwata et al., 2013b) and the GABAAR blockade (Figures 1A,B, 2A,B, 4C,D), suggesting that these D-serine modulations through distinct receptors may assemble a process to prevent overactivation of the NMDAR.
In conclusion, the present findings demonstrate that the GABAAR exerts a tonic and weaker phasic facilitatory control of the extracellular D-serine levels in a neuronal and glial activity-dependent fashion in the mammalian mPFC. We propose the following mechanisms underlying the GABAAR-mediated regulation of D-serine: (1) GABA derived from the prefrontal cortical GABAergic neurons may continuously impinge on the excitatory GABAAR expressed on a group of glia that liberate D-serine; and (2) interruption of this tonic GABAergic transmission at the GABAAR would induce a decline in the extracellular release of D-serine by disfacilitation of these glial activities. The GABAergic control of D-serine could be composed of an unreported type of feedback system that can compensate for the deficit in the GABAergic inhibitory neurotransmission by attenuation of the excitatory input by the NMDAR. This hypothesis could explain that the GABAergic deficits may lead to hypofunction of the NMDAR in certain neuropsychiatric disorders such as schizophrenia. Therefore, advancing the research to explain the exact molecular and cellular components of the GABA-D-serine interaction should contribute to a deeper understanding of the biological basis of the neurological and mental functions and dysfunctions and to the development of novel strategies for their diagnosis and treatment.
Author Contributions
AU and TN established the methods for the in vivo microdialysis technique and the semi-automatic quantitative analysis of the chiral and non-chiral amino acids by HPLC with fluorometric detection, which were used in the present study. AU, SI and HI performed the animal experiments and HPLC measurements of various amino acids, wrote their protocols and parts of the preliminary draft of the manuscript, figures and a table and undertook the statistical analysis under the guidance of TN. TN conceived, designed and directed this project, and wrote the final version of the manuscript. All authors contributed to and have approved the final manuscript.
Funding
This study was supported by the Core Research for Evolutional Science and Technology (CREST) program funded by the Ministry of Education, Culture, Sports, Science and Technology of Japan. In addition, a part of this study was supported by Tokyo Medical and Dental University funds.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms. Koyuki Kaneda for her assistance in the present in vivo dialysis experiments and Drs. Katsunobu Takahashi and Naoki Yamamoto for their helpful comments on this study.
References
Arellano, R. O., Sánchez-Gómez, M. V., Alberdi, E., Canedo-Antelo, M., Chara, J. C., Palomino, A., et al. (2016). Axon-to-glia interaction regulates GABAA receptor expression in oligodendrocytes. Mol. Pharmacol. 89, 63–74. doi: 10.1124/mol.115.100594
Balia, M., Vélez-Fort, M., Passlick, S., Schäfer, C., Audinat, E., Steinhäuser, C., et al. (2015). Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb. Cortex 25, 1114–1123. doi: 10.1093/cercor/bht309
Balu, D. T., Takagi, S., Puhl, M. D., Benneyworth, M. A., and Coyle, J. T. (2014). D-Serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell. Mol. Neurobiol. 34, 419–435. doi: 10.1007/s10571-014-0027-z
Basu, A. C., Tsai, G. E., Ma, C. L., Ehmsen, J. T., Mustafa, A. K., Han, L., et al. (2009). Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry 14, 719–727. doi: 10.1038/mp.2008.130
Benneyworth, M. A., Li, Y., Basu, A. C., Bolshakov, V. Y., and Coyle, J. T. (2012). Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell. Mol. Neurobiol. 32, 613–624. doi: 10.1007/s10571-012-9808-4
Cinelli, E., Iovino, L., Bongianni, F., Pantaleo, T., and Mutolo, D. (2016). GABAA- and glycine-mediated inhibitory modulation of the cough reflex in the caudal nucleus tractus solitarii of the rabbit. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L570–L580. doi: 10.1152/ajplung.00205.2016
Del Arco, A., and Mora, F. (1999). Effects of endogenous glutamate on extracellular concentrations of GABA, dopamine and dopamine metabolites in the prefrontal cortex of the freely moving rat: involvement of NMDA and AMPA/KA receptors. Neurochem. Res. 24, 1027–1035. doi: 10.1023/A:1021056826829
Ding, X., Ma, N., Nagahama, M., Yamada, K., and Semba, R. (2011). Localization of D-serine and serine racemase in neurons and neuroglias in mouse brain. Neurol. Sci. 32, 263–267. doi: 10.1007/s10072-010-0422-2
Engblom, A. C., Eriksson, K. S., and Akerman, K. E. (1996). Glycine and GABAA receptor-mediated chloride fluxes in synaptoneurosomes from different parts of the rat brain. Brain Res. 712, 74–83. doi: 10.1016/0006-8993(95)01484-5
Enna, S. J., and McCarson, K. E. (2013). Characterization of GABA receptors. Curr. Protoc. Pharmacol. 63, 1.7.1–1.7.20. doi: 10.1002/0471141755.ph0107s63
Fassio, A., Rossi, F., Bonanno, G., and Raiteri, M. (1999). GABA induces norepinephrine exocytosis from hippocampal noradrenergic axon terminals by a dual mechanism involving different voltage-sensitive calcium channels. J. Neurosci. Res. 57, 324–331. doi: 10.1002/(SICI)1097-4547(19990801)57:3<324::AID-JNR4>3.0.CO;2-Z
Fossat, P., Turpin, F. R., Sacchi, S., Dulong, J., Shi, T., Rivet, J.-M., et al. (2012). Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb. Cortex 22, 595–606. doi: 10.1093/cercor/bhr130
Gobert, A., Rivet, J. M., Billiras, R., Parsons, F., and Millan, M. J. (2011). Simultaneous quantification of D- vs. L-serine, taurine, kynurenate, phosphoethanolamine and diverse amino acids in frontocortical dialysates of freely-moving rats: differential modulation by N-methyl-D-aspartate (NMDA) and other pharmacological agents. J. Neurosci. Methods 202, 143–157. doi: 10.1016/j.jneumeth.2011.08.040
Gould, T., Chen, L., Emri, Z., Pirttimaki, T., Errington, A. C., Crunelli, V., et al. (2014). GABAB receptor-mediated activation of astrocytes by gamma-hydroxybutyric acid. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20130607. doi: 10.1098/rstb.2013.0607
Hashimoto, A., Nishikawa, T., Oka, T., and Takahashi, K. (1993). Endogenous D-serine in rat brain: N-methyl-D-aspartate receptor-related distribution and aging. J. Neurochem. 60, 783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x
Hashimoto, A., Oka, T., and Nishikawa, T. (1995). Extracellular concentration of endogenous free D-serine in the rat brain as revealed by in vivo microdialysis. Neuroscience 66, 635–643. doi: 10.1016/0306-4522(94)00597-x
Hertz, L. (2004). Intercellular metabolic compartmentation in the brain: past, present and future. Neurochem. Int. 45, 285–296. doi: 10.1016/s0197-0186(03)00293-6
Inoue, R., Hashimoto, K., Harai, T., and Mori, H. (2008). NMDA- and β-amyloid1–42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J. Neurosci. 28, 14486–14491. doi: 10.1523/JNEUROSCI.5034-08.2008
Ishiwata, S., Ogata, S., Umino, A., Shiraku, H., Ohashi, Y., Kajii, Y., et al. (2013a). Increasing effects of S-methyl-L-cysteine on the extracellular D-serine concentrations in the rat medial frontal cortex. Amino Acids 44, 1391–1395. doi: 10.1007/s00726-013-1464-6
Ishiwata, S., Umino, A., Umino, M., Yorita, K., Fukui, K., and Nishikawa, T. (2013b). Modulation of extracellular d-serine content by calcium permeable AMPA receptors in rat medial prefrontal cortex as revealed by in vivo microdialysis. Int. J. Neuropsychopharmacol. 16, 1395–1406. doi: 10.1017/S1461145712001484
Ishiwata, S., Umino, A., Balu, D. T., Coyle, J. T., and Nishikawa, T. (2015). Neuronal serine racemase regulates extracellular D-serine levels in the adult mouse hippocampus. J. Neural Transm. 122, 1099–1103. doi: 10.1007/s00702-015-1388-2
Kanematsu, S., Ishii, S., Umino, A., Fujihira, T., Kashiwa, A., Yamamoto, N., et al. (2006). Evidence for involvement of glial cell activity in the control of extracellular D-serine contents in the rat brain. J. Neural Transm. 113, 1717–1721. doi: 10.1007/s00702-006-0517-3
Katzner, S., Busse, L., and Carandini, M. (2011). GABAA inhibition controls response gain in visual cortex. J. Neurosci. 31, 5931–5941. doi: 10.1523/JNEUROSCI.5753-10.2011
Korf, J., and Venema, K. (1985). Amino acids in rat striatal dialysates: methodological aspects and changes after electroconvulsive shock. J. Neurochem. 45, 1341–1348. doi: 10.1111/j.1471-4159.1985.tb07198.x
Kumashiro, S., Hashimoto, A., and Nishikawa, T. (1995). Free D-serine in post-mortem brains and spinal cords of individuals with and without neuropsychiatric diseases. Brain Res. 681, 117–125. doi: 10.1016/0006-8993(95)00307-c
Labrie, V., Fukumura, R., Rastogi, A., Fick, L. J., Wang, W., Boutros, P. C., et al. (2009). Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum. Mol. Genet. 18, 3227–3243. doi: 10.1093/hmg/ddp261
Lee, I. O., Son, J. K., Lim, E. S., and Kim, Y. S. (2011). Pharmacology of intracisternal or intrathecal glycine, muscimol and baclofen in strychnine-induced thermal hyperalgesia of mice. J. Korean Med. Sci. 26, 1371–1377. doi: 10.3346/jkms.2011.26.10.1371
Lin, S. C., and Bergles, D. E. (2004). Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 7, 24–32. doi: 10.1038/nn1162
Matsui, T., Sekiguchi, M., Hashimoto, A., Tomita, U., Nishikawa, T., and Wada, K. (1995). Functional comparison of D-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J. Neurochem. 65, 454–458. doi: 10.1046/j.1471-4159.1995.65010454.x
Matthews, J. N., Altman, D. G., Campbell, M. J., and Royston, P. (1990). Analysis of serial measurements in medical research. BMJ 300, 230–235. doi: 10.1136/bmj.300.6725.680
Meier, S. D., Kafitz, K. W., and Rose, C. R. (2008). Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 56, 1127–1137. doi: 10.1002/glia.20684
Mothet, J. P., Parent, A. T., Wolosker, H., Brady, R. O. Jr., Linden, D. J., Ferris, C. D., et al. (2000). D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. U S A 97, 4926–4931. doi: 10.1073/pnas.97.9.4926
Nestler, E. J., Hyman, S. E., and Malenka, R. G. (Eds). (2009). “Excitatory and inhibitory amino acids,” in Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, 2nd Edn. (New York, NY: The McGraw-Hill Companies, Inc.), 117–144.
Ng, C. K., Kim, H.-L., Gavande, N., Yamamoto, I., Kumar, R. J., Mewett, K. N., et al. (2011). Medicinal chemistry of ρGABAC receptors. Future Med. Chem. 3, 197–209. doi: 10.4155/fmc.10.286
Nishijima, K., Kashiwa, A., Hashimoto, A., Iwama, H., Umino, A., and Nishikawa, T. (1996). Differential effects of phencyclidine and methamphetamine on dopamine metabolism in rat frontal cortex and striatum as revealed by in vivo dialysis. Synapse 2, 304–312. doi: 10.1002/(sici)1098-2396(199604)22:4<304::aid-syn2>3.0.co;2-f
Nishikawa, T. (2011). Analysis of free D-serine in mammals and its biological relevance. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 3169–3183. doi: 10.1016/j.jchromb.2011.08.030
Ogden, K. K., and Traynelis, S. F. (2011). New advances in NMDA receptor pharmacology. Trends Pharmacol. Sci. 32, 726–733. doi: 10.1016/j.tips.2011.08.003
Oja, S. S., and Saransaari, P. (2000). Modulation of taurine release by glutamate receptors and nitric oxide. Prog. Neurobiol. 62, 407–425. doi: 10.1016/s0301-0082(00)00005-8
Papouin, T., Ladépêche, L., Ruel, J., Sacchi, S., Labasque, M., Hanini, M., et al. (2012). Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150, 633–646. doi: 10.1016/j.cell.2012.06.029
Paulsen, R. E., Contestabile, A., Villani, L., and Fonnum, F. (1987). An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J. Neurochem. 48, 1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x
Paxinos, G., and Franklin, K. (2004). The Mouse Brain in Stereotaxic Coordinates. 2nd Edn. Amsterdam: Elsevier Academic Press.
Paxinos, G., and Watson, C. (2005). The Rat Brain in Stereotaxic Coordinates. 5th Edn. Amsterdam: Elsevier Academic Press.
Rao, T. S., Lariosa-Willingham, K. D., and Yu, N. (2003). Glutamate-dependent glutamine, aspartate and serine release from rat cortical glial cell cultures. Brain Res. 78, 213–222. doi: 10.1016/s0006-8993(03)02841-5
Ren, W. H., Guo, J. D., Cao, H., Wang, H., Wang, P. F., Sha, H., et al. (2006). Is endogenous D-serine in the rostral anterior cingulate cortex necessary for pain-related negative affect? J. Neurochem. 96, 1636–1647. doi: 10.1111/j.1471-4159.2006.04046.x
Renzel, R., Sadek, A. R., Chang, C. H., Gray, W. P., Seifert, G., and Steinhäuser, C. (2013). Polarized distribution of AMPA, but not GABAA , receptors in radial glia-like cells of the adult dentate gyrus. Glia 61, 1146–1154. doi: 10.1002/glia.22505
Rosenberg, D., Artoul, S., Segal, A. C., Kolodney, G., Radzishevsky, I., Dikopoltsev, E., et al. (2013). Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J. Neurosci. 33, 3533–3544. doi: 10.1523/JNEUROSCI.3836-12.2013
Scheller, D., Szathmary, S., Kolb, J., and Tegtmeier, F. (2000). Observations on the relationship between the extracellular changes of taurine and glutamate during cortical spreading depression, during ischemia, and within the area surrounding a thrombotic infarct. Amino Acids 19, 571–583. doi: 10.1007/s007260070007
Szabadics, J., Varga, C., Molnár, G., Oláh, S., Barzó, P., and Tamás, G. (2006). Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311, 233–235. doi: 10.1126/science.1121325
Verkhratsky, A., and Steinhäuser, C. (2000). Ion channels in glial cells. Brain Res. Rev. 32, 380–412. doi: 10.1016/s0165-0173(99)00093-4
Wallenstein, S., Zucker, C. L., and Fleiss, J. L. (1980). Some statistical methods useful in circulation research. Circ. Res. 47, 1–9. doi: 10.1161/01.res.47.1.1
Wang, P., and Slaughter, M. M. (2005). Effects of GABA receptor antagonists on retinal glycine receptors and on homomeric glycine receptor alpha subunits. J. Neurophysiol. 93, 3120–3126. doi: 10.1152/jn.01228.2004
Keywords: N-methyl-D-aspartate glutamate receptor, GABAA receptor, glia, in vivo microdialysis, medial prefrontal cortex, neuron, D-serine
Citation: Umino A, Ishiwata S, Iwama H and Nishikawa T (2017) Evidence for Tonic Control by the GABAA Receptor of Extracellular D-Serine Concentrations in the Medial Prefrontal Cortex of Rodents. Front. Mol. Neurosci. 10:240. doi: 10.3389/fnmol.2017.00240
Received: 01 April 2017; Accepted: 17 July 2017;
Published: 02 August 2017.
Edited by:
Hiroyuki Okuno, Kyoto University, JapanReviewed by:
Hisashi Mori, University of Toyama, JapanJohn J. Woodward, Medical University of South Carolina, United States
Copyright © 2017 Umino, Ishiwata, Iwama and Nishikawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toru Nishikawa, tnis.psyc@tmd.ac.jp
† These authors have contributed equally to this work.
‡Present address: Hisayuki Iwama, Kanagawa Psychiatric Center, Yokohama, Japan
 Asami Umino1,2†
Asami Umino1,2†  Sayuri Ishiwata
Sayuri Ishiwata Toru Nishikawa
Toru Nishikawa